Synthetic method for Dtena modified fragment of apratoxin marine natural product
A natural product and synthetic method technology, applied in the direction of organic chemistry, can solve the problems of long reaction steps and unsuitability for industrial production, and achieve the effects of fewer reaction steps, high total yield and good product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
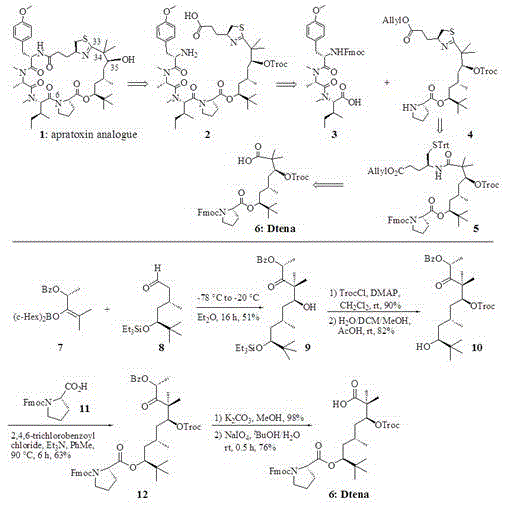
[0018] Embodiment 1: the synthesis of beta-hydroxy ketone (9, see accompanying drawing)
[0019] Add 20 ml of anhydrous diethyl ether to the reaction flask, cool to -78°C, add enol boroether 7 (see attached drawing, prepared according to the reference document Synthesis, 1998,639, 10 mmol) and aldehyde 8 (see attached drawing, according to Reference Org. Lett., 2003, 5, 3503 preparation, 10mmol) was added into the reaction flask, naturally raised to -20°C, stirred at -20°C for 16 hours, TLC detected the end of the esterification reaction. Add 20 ml of water to quench, shake and separate the liquids, extract the aqueous phase with ethyl acetate (20mLx3), combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain beta-hydroxyketone (9, See accompanying drawing), yield is 51%.
Embodiment 2
[0020] Embodiment 2: the synthesis of O-Troc alcohol (10, see accompanying drawing)
[0021] Beta-hydroxyketone (10, 10.0 mmol) and 20 ml of dichloromethane were added to the reaction flask, and TrocCl (12.0 mmol) and DMAP (13.0 mmol) were added at room temperature, stirred at room temperature, and detected by TLC. After the reaction (about 6 hours), add 20 ml of water to quench, shake and separate the liquids, extract the aqueous phase with dichloromethane (20mLx3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate to obtain beta-OTroc Ketones (90%). Take the beta-OTroc ketone (5.0mmol) and dissolve it in 10ml of H 2 In O / DCM / MeOH (1:1:1) mixed solvent, acetic acid (AcOH, 5.5 mmol) was added at zero temperature, stirred at room temperature, and detected by TLC. After the reaction (about 8 hours), add 20 ml of water to quench, shake and separate the liquids, extract the aqueous phase with dichloromethane (20mLx3), combine the organic phas...
Embodiment 3
[0022] Example 3: Synthesis of o-O-Bz-based ketones (12, see accompanying drawing) containing proline structural units
[0023] N-Fmoc proline (11, see attached figure, 2.0 mmol) with 2,4,6-trichlorobenzoyl chloride (2.0 mmol) and Et 3 N (2.0 mmol) was dissolved in 10 ml of toluene (PhMe), and stirred at room temperature for 2 hours to generate mixed anhydride. O-Troc alcohol (10, see figure, 2.0 mmol) was added to the mixed acid anhydride reaction system, and the reaction was stirred for 6 hours at a reaction temperature of 90°C. TLC detected the end of the reaction, quenched by adding 20 ml of saturated aqueous sodium bicarbonate solution, separated after shaking, and extracted the aqueous phase with dichloromethane (20mLx3), combined the organic phases, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography , and the o-O-Bz-based ketone (12, see attached figure) containing the proline structural unit was obtained with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com