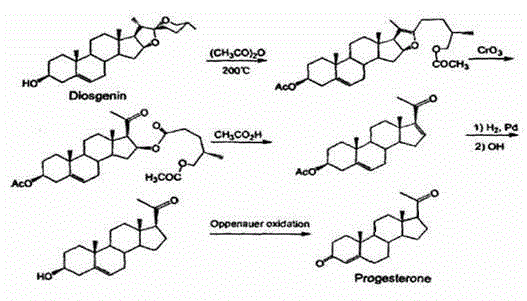
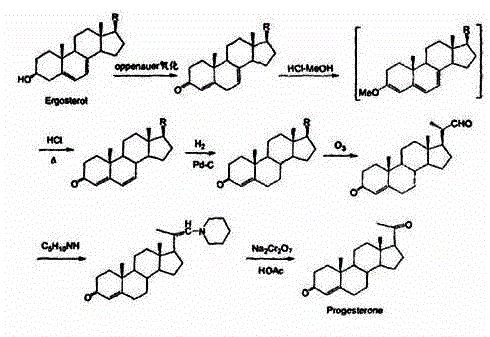
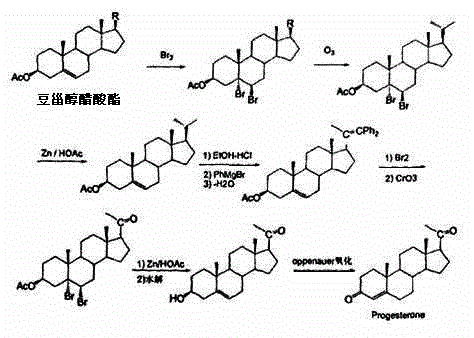
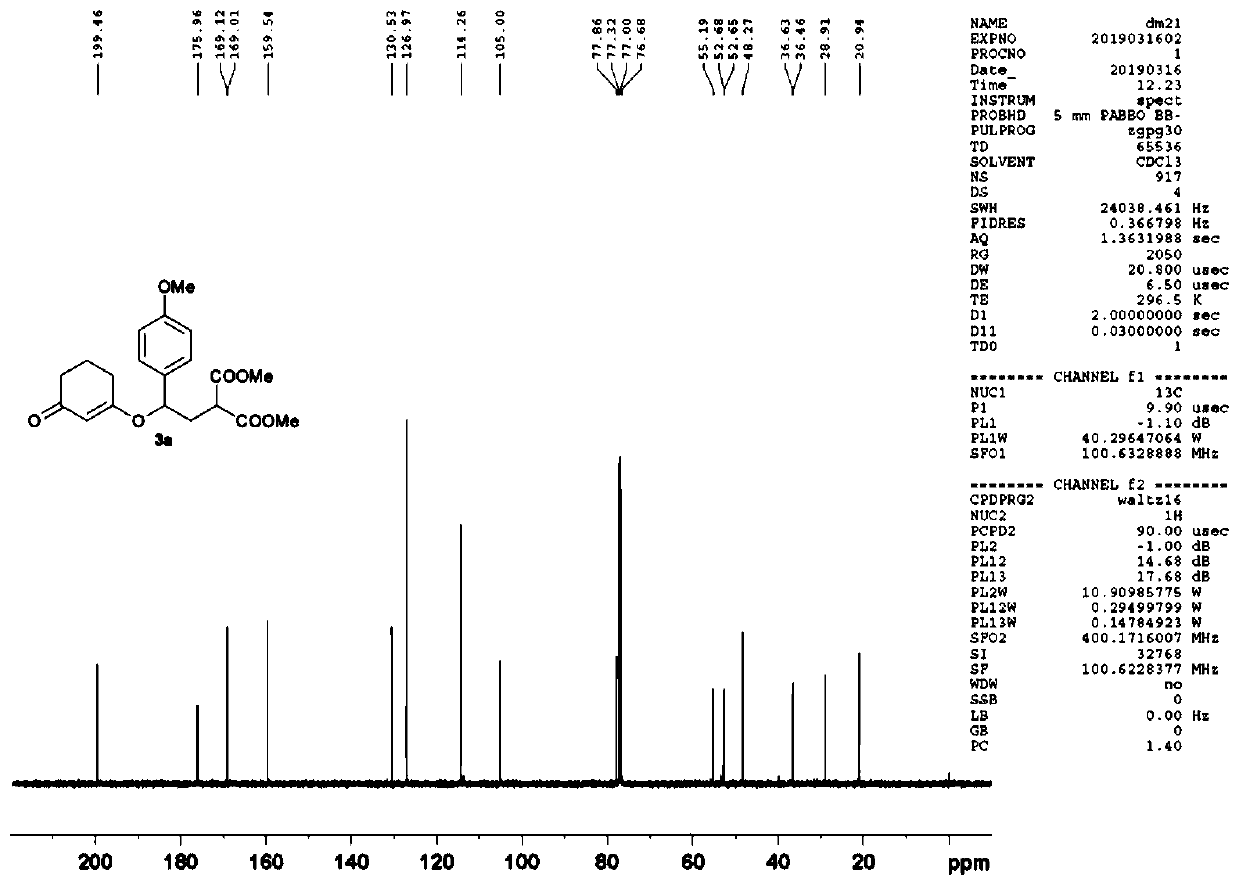
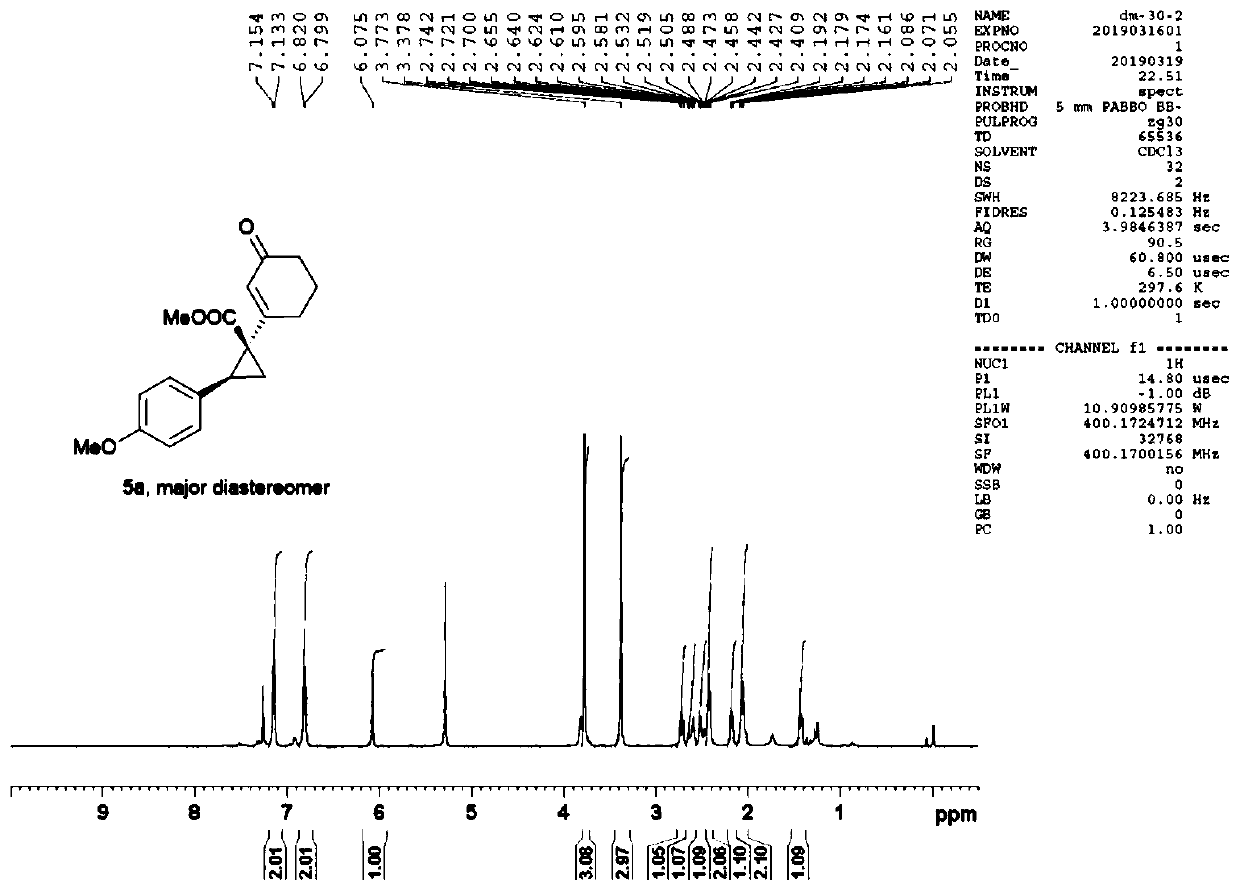
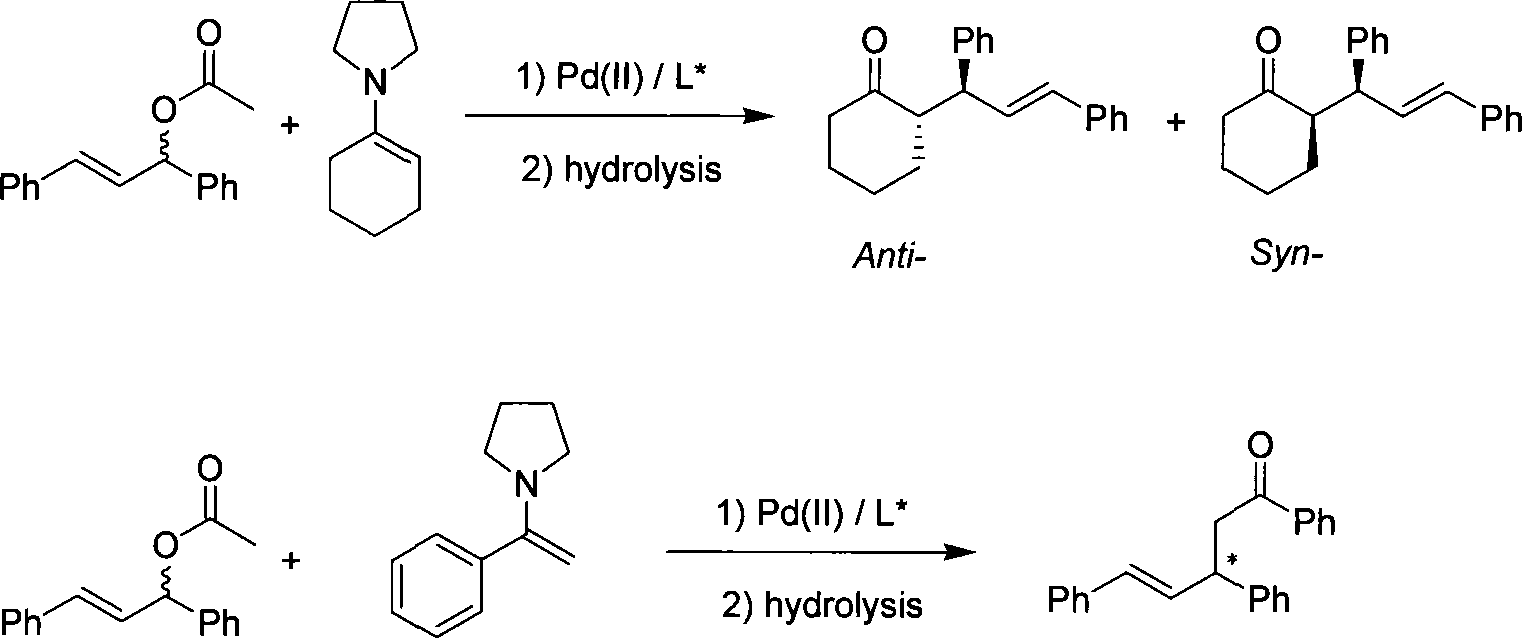
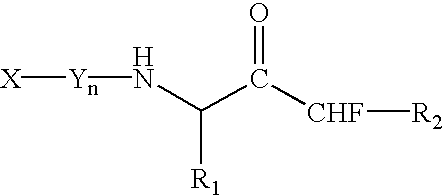
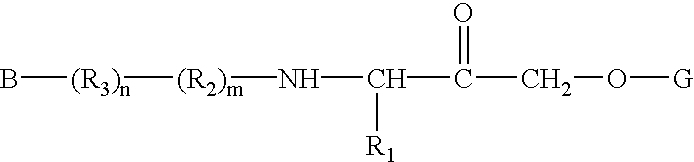
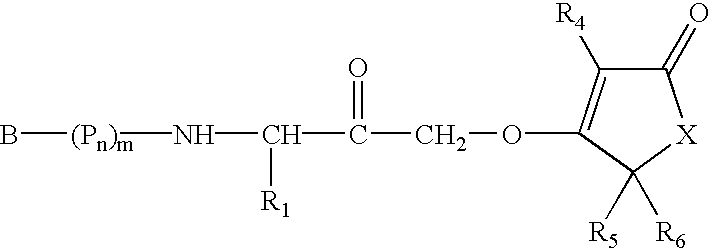
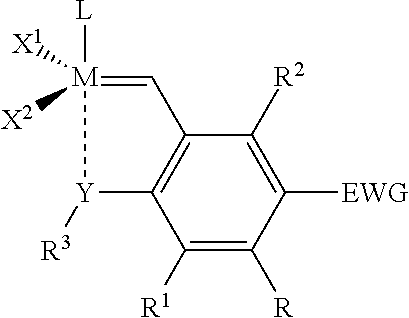
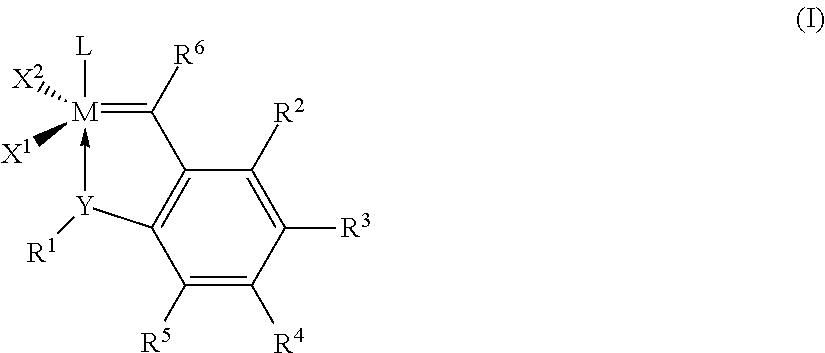
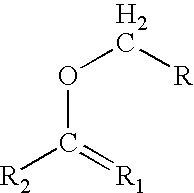
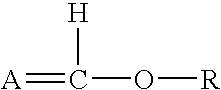
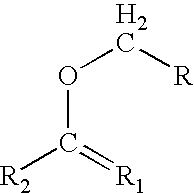
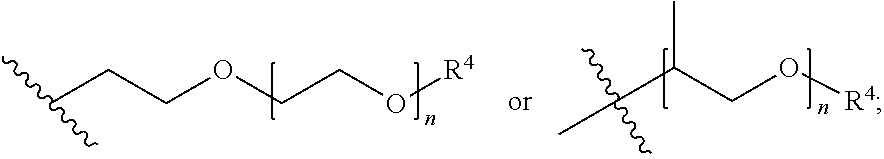
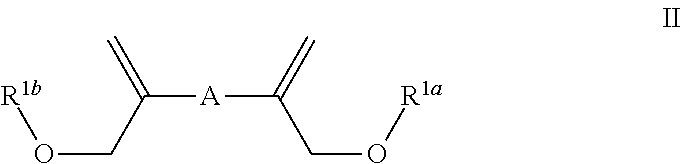
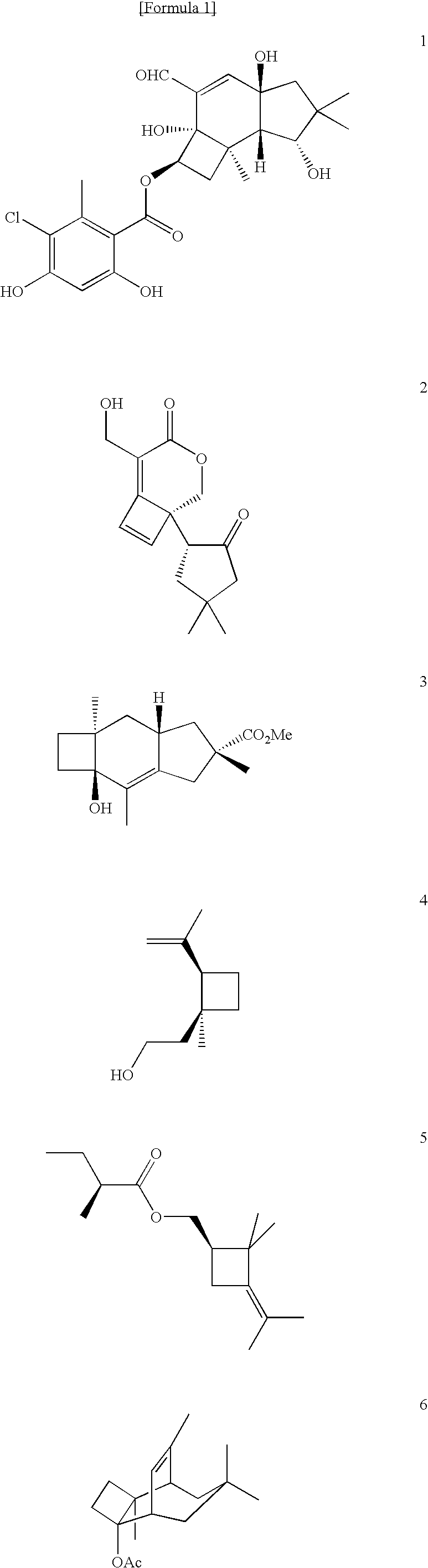
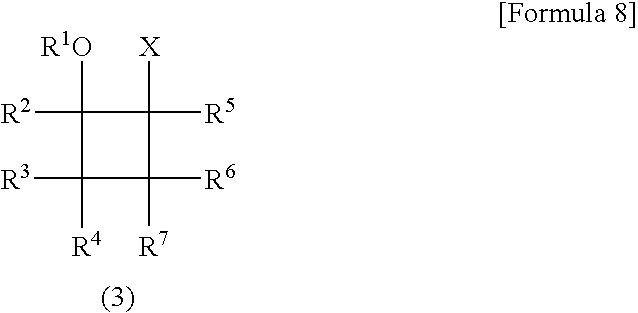
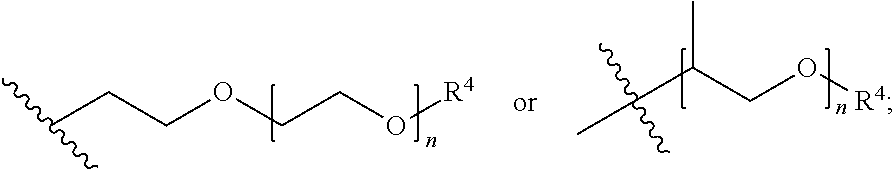
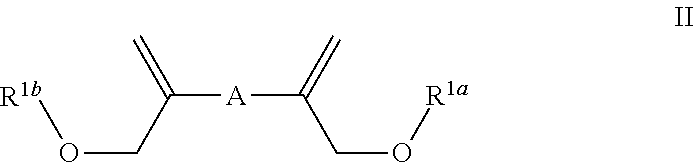
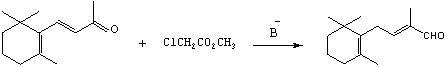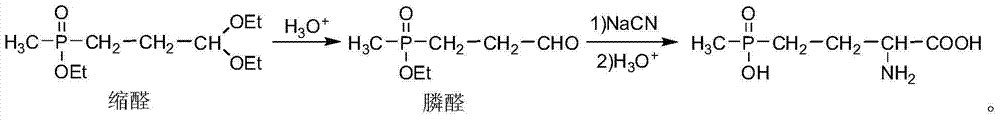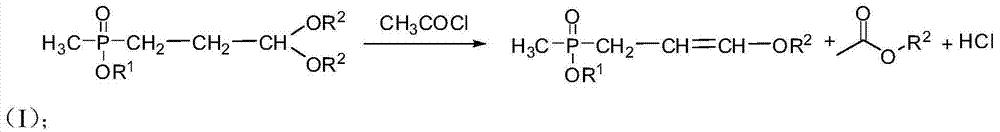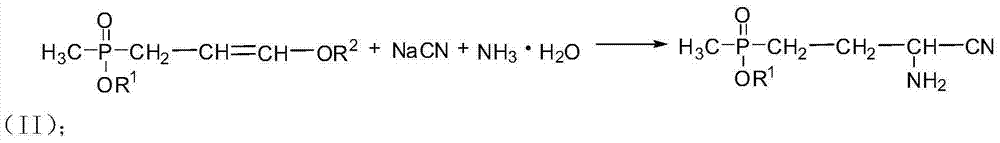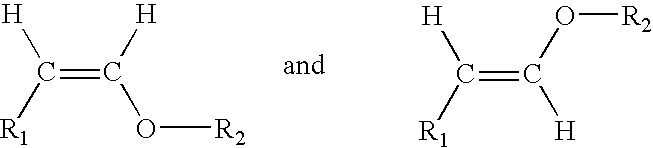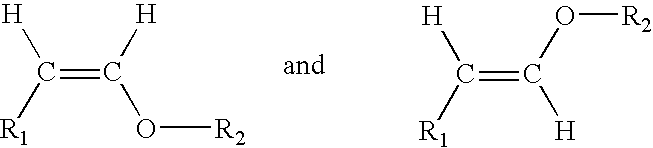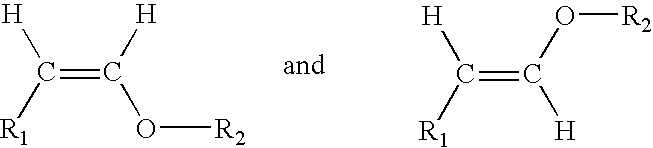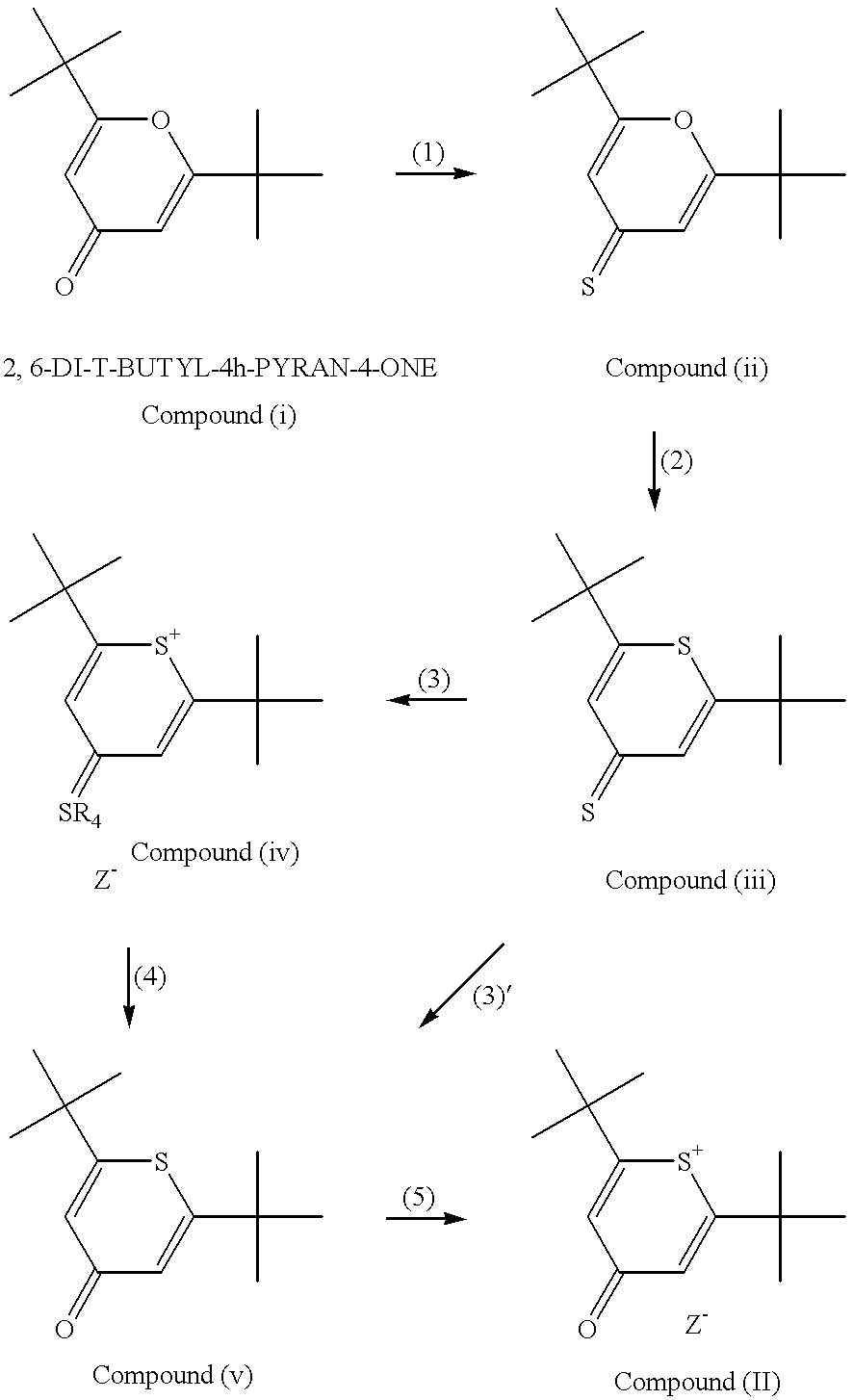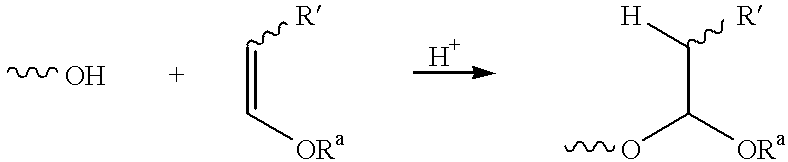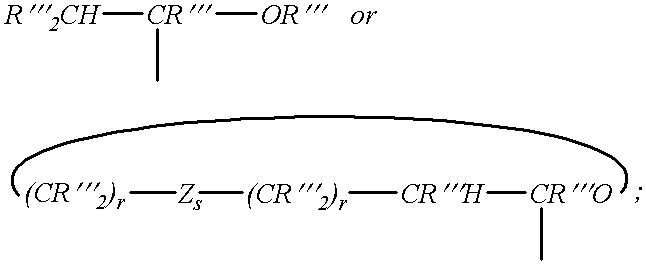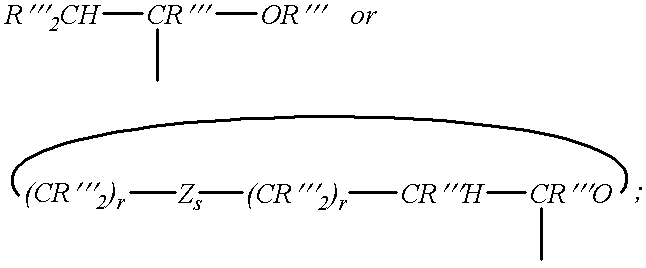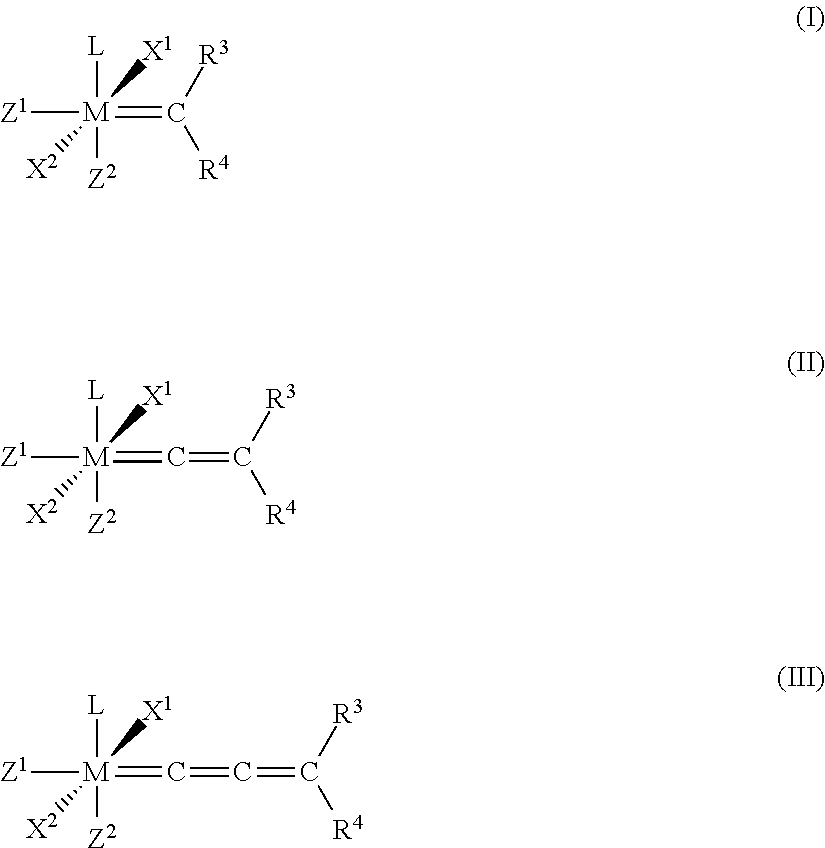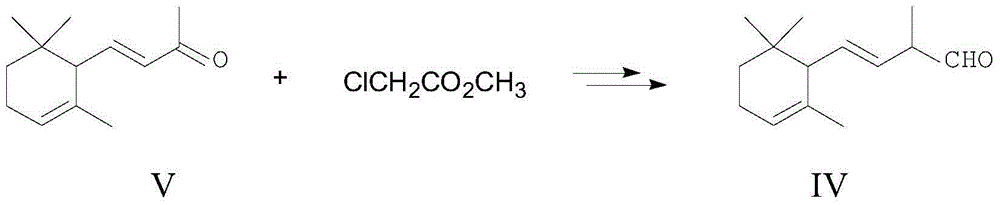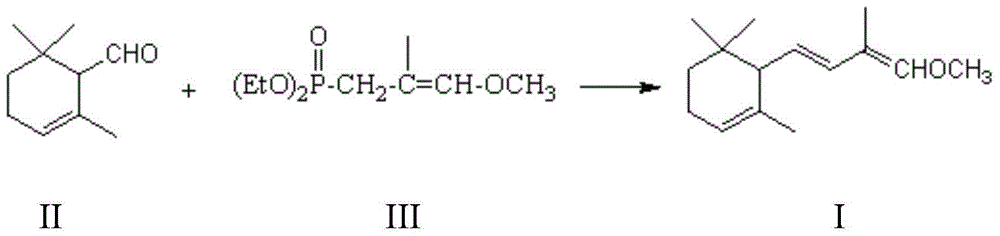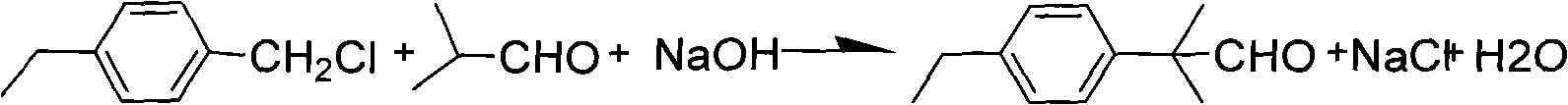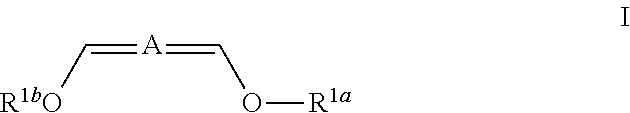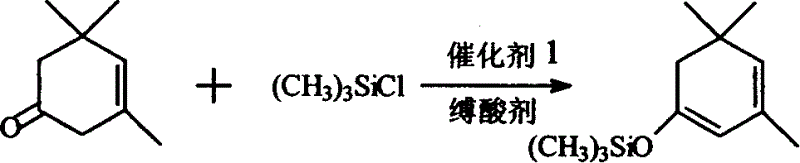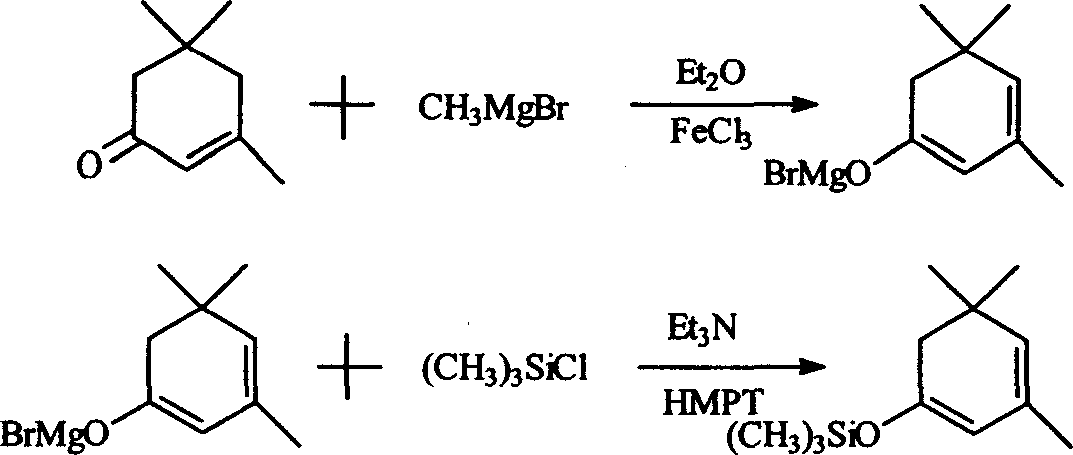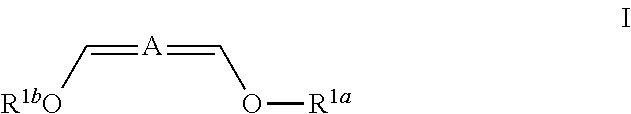Patents
Literature
77 results about "Enol ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
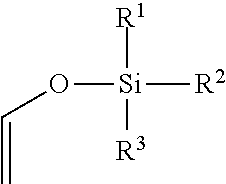
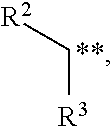
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R₂C=CR-OR where R = H, alkyl, or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH₂. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.
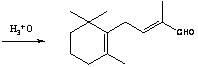
Method for preparing intermediate of vitamin A, namely tetradecanal
InactiveCN102190565AOrganic compound preparationCarbonyl compound preparation by hydrolysisEnol etherDissociation reaction
The invention provides a method for preparing an intermediate of vitamin A, namely C14-aldehyde, and an intermediate of the C14-aldehyde, namely C14 enol ether, which comprises the following steps of: (1) under the protection of inert gas, performing rearrangement dissociation reaction on C4 phosphonate at the temperature of between -40 and 30DEG C in the presence of alkali in an ether solvent ora dipolar aprotic solvent; (2) adding beta-cyclocitral, and performing Wittig-Homer condensation reaction at the temperature of between -40 and 30DEG C in the presence of alkali in the ether solvent or the dipolar aprotic solvent to obtain the C14 enol ether; and (3) under the protection of inert gas, mixing the C14 enol ether, an acid catalyst, water and a homogenous phase solvent, and performing hydrolysis reaction at the temperature of between 10 and 35DEG C with stirring to obtain the C14-aldehyde. The method has the advantages of simple process, readily available raw materials and low cost, and has great industrial value.
Owner:SHAOXING UNIVERSITY +1
Preparation method of C-14 enol ether
InactiveCN102180774AThe reaction route is simpleRaw materials are easy to obtainEther preparationDimethyl methylphosphonateDiethyl methylphosphonate
The invention relates to a preparation method of C-14 enol ether. The C-14 enol ether is an intermediate of vitamin A, and the chemical name of the C-14 enol ether is 1-methoxy-2-methyl-4-(2,6,6-trimehtyl-1-cyclohexene-1-ly)-1,3-butadiene. The preparation method comprises the step of carrying out Wittig-Horner condensation reaction on beta-irisone as shown in a formula 4 and diethyl methylphosphonate as shown in a formula 8 at the temperature of minus 40-30 DEG C in an ether solvent or dipolar aprotic solvent in the presence of alkali so as to prepare the C-14 enol ether as shown in a formula7. According to the preparation method, reaction route is simple, and raw materials are simple and available.
Owner:SHAOXING UNIVERSITY
Use of cyclic enol ether terpenoid for producing promotive neurocyte proliferate and differentiation medicament
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Method for preparing progesterone by taking 1,4-androstenedione as raw material
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
Preparation method of amino-nitrile and intermediate for preparing glufosinate-ammonium
ActiveCN104497039AHigh yieldSatisfy productivityGroup 5/15 element organic compoundsGlufosinate-ammoniumSodium cyanide
The invention discloses a preparation method of amino-nitrile and an intermediate for preparing glufosinate-ammonium. The preparation method disclosed by the invention aims at solving the problem of low glufosinate-ammonium yield by using acetal in the existing methods. Different from the existing methods for preparing glufosinate-ammonium, the method disclosed by the invention comprises the following steps: firstly reacting acetal with acetylchloride to obtain an enol ether intermediate, reacting the enol ether intermediate with sodium cyanide to obtain amino-nitrile, and finally hydrolyzing the amino-nitrile to obtain the glufosinate-ammonium. The method has the advantages of higher reaction yield and capacity of remarkably reducing the production cost of the glufosinate-ammonium.
Owner:GUANGAN LIER CHEM CO LTD
Acyclic enol ethers, isomers thereof, organoleptic uses thereof and processes for preparing same
InactiveUS7175871B2Sustainable heat and light and base stabilityChewing gumEssential-oils/perfumesEnol etherHair preparations
Described are synthetically produced substantially pure enol ether compositions which are cis and / or trans isomers of enol ethers having the structures:wherein R1 is C4–C10 straight chain alkyl or C9 8-alkenyl; and wherein R2 is C1–C4 alkyl, C3–C4 2-alkenyl, C4 3-alkenyl or C10 non-allenic alkadienyl and uses thereof in imparting, augmenting or enhancing the aroma and / or taste of a consumable material such as a perfume composition, a cologne, a perfumed article such as a soap, cosmetic, hair preparation or detergent, a foodstuff, a chewing gum or an alcoholic or non-alcoholic beverage such as a carbonated beverage or fruit liquer. Also described is a process for synthesis of such enol ethers by means of (i) first forming an acetal having the structure:R1—CH2—CH(OR2)2 (ii) then carrying out a thermal decomposition reaction at pH<7 and then (iii) fractionally distilling the resulting reaction product to provide the enol ether.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
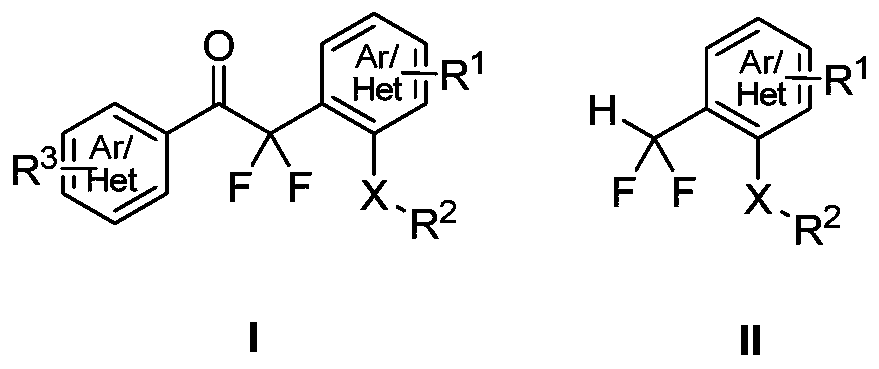
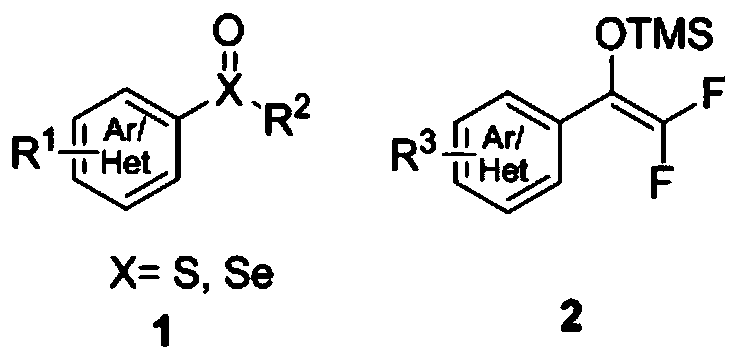
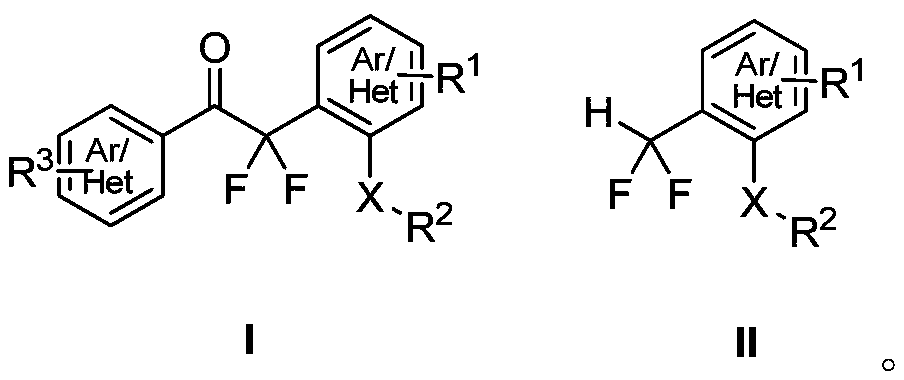
Method for synthesizing difluoroalkyl or difluoromethyl sulfur-containing or selenium-containing compounds
ActiveCN110156646ARaw materials are easy to getSimple and fast operationSulfide preparationArylEnol ether
The invention discloses a method for preparing difluoroalkyl or difluoromethyl substituted sulfur-containing or selenium-containing compounds. According to the method provided by the invention, one aryl or heteroaryl sulfoxide and selenoxide and one aryl or heteroaryl difluorosilyl enol ether are used as raw materials, and under the action of an activator, [3,3]-sigma rearrangement is performed tosynthesize one corresponding difluoroalkyl substituted sulfur-containing or selenium-containing compound; and the difluoroalkyl substituted sulfur-containing or selenium-containing compounds are prepared by the [3,3]-sigma rearrangement and a Haller-Bauer reaction in one pot, the synthetic method is simple, and has a high yield and mild conditions, thereby having good application prospects.
Owner:TAIZHOU UNIV +1
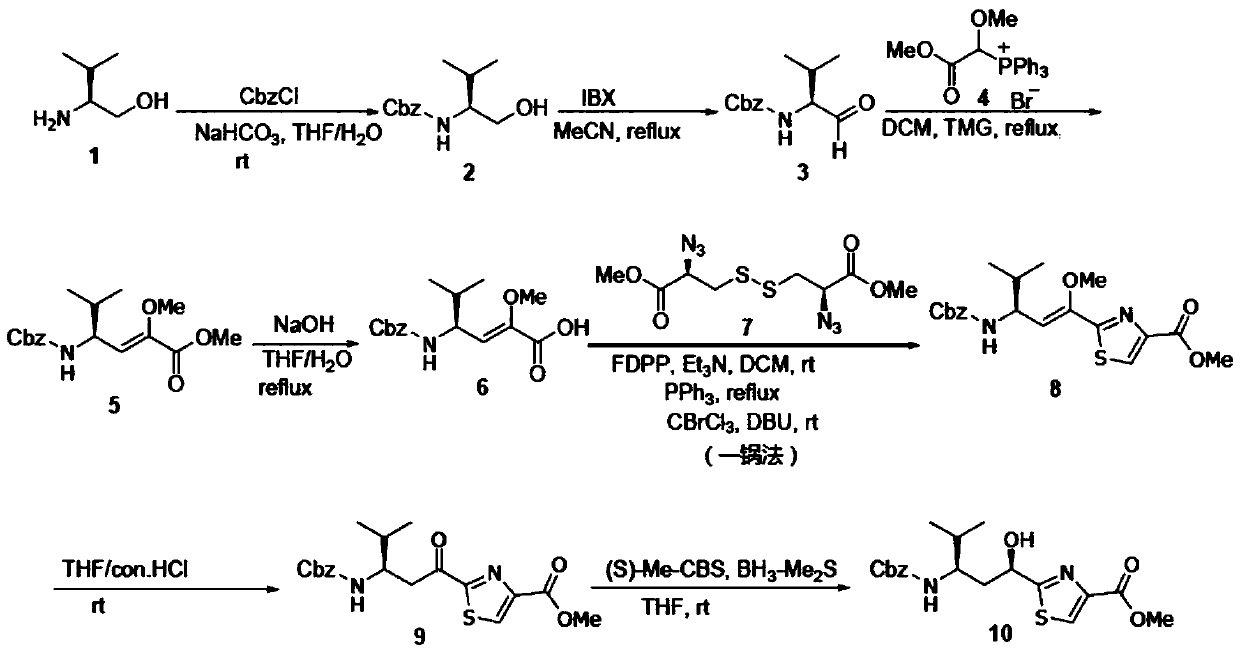
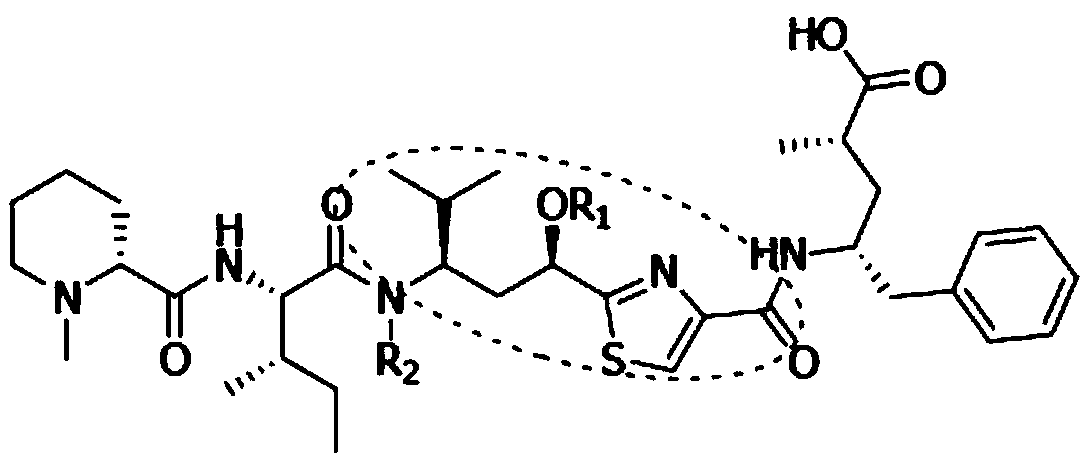
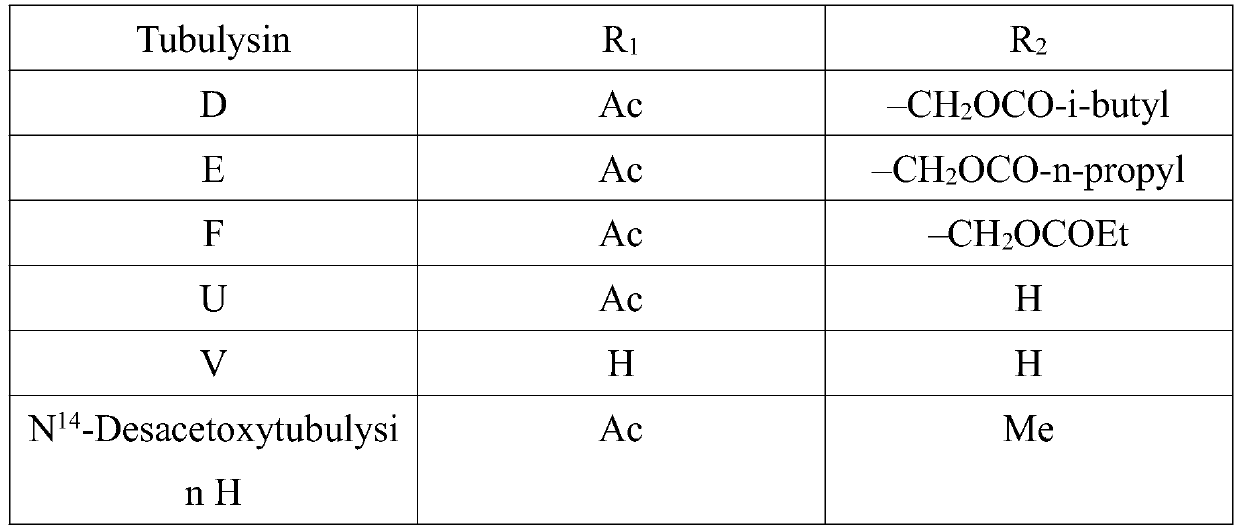
Synthetic method of key intermediate Tuv of natural anti-cancer drug Tubulysins
The invention belongs to the technical field of chemical synthesis, and particularly relates to a synthesis method of a key intermediate Tuv of a natural anti-cancer drug Tubulysins. With L-valinol (1) which is cheap and easy to obtain as a raw material, the synthesis method includes steps of: firstly, protecting amino groups with CbzCl; then carrying out an oxidation reaction and a Wittig reaction; hydrolyzing methyl ester to obtain carboxylic acid, and carrying out a reaction with carboxylic acid serving as a substrate with beta-azido disulfide under mild reaction conditions under the combined action of a coupling reagent and an organic phosphine reagent to prepare a thiazoline intermediate product;, then reacting the and further efficiently synthesizing the 2,4-disubstituted thiazole compound by adding an oxidizing reagent through a one-pot method. The preparation method comprises the following steps: by taking (S)-2-methyl-CBS-oxazolyl borane as a raw material, hydrolyzing under anacidic condition, converting methyl enol ether into a ketone compound, and finally performing asymmetric reduction reaction by taking (S)-2-methyl-CBS-oxazolyl borane as a catalyst, thereby obtainingthe target compound.
Owner:SHENZHEN ELDERLY MEDICAL RES INST +1
LED substrate dewaxing cleaning agent
ActiveCN105623895AImprove wax removal abilityProduce synergyNon-ionic surface-active compoundsOrganic detergent compounding agentsEnol etherCarboxylic salt
The invention discloses an LED substrate dewaxing cleaning agent. The LED substrate dewaxing cleaning agent comprises a dispersing agent, isomeric alcohol ether carboxylate, triethanolamine oleate, an organic solvent, 1H-benzotriazole, isopropylate triphenyl phosphate, 2-methyl-4-isothiazolin-3-one, isomeric alcohol ethoxylates, enol ether, a penetrant and ethyl acetate. The LED substrate dewaxing cleaning agent can be applied to various LED substrates, is high in cleaning efficiency, dewaxing capability and safety, free from environmental pollution, energy saving, low in washing cost and free from damage to the substrates during washing.
Owner:TAICANG HE S CIRCUIT BOARD CO LTD
Alpha - (1,3- or 1, 2- dicarbonylenol ether methyl ketones as cysteine protease inhibitors
Cysteine protease inhibitors which deactivate the protease by covalently bonding to the cysteine protease and releasing the enolate of a 1,3-dicarbonyl (or its enolic form). The cysteine protease inhibitors of the present invention accordingly comprise a first portion which targets a desired cysteine protease and positions the inhibitor near the thiolate anion portion of the active site of the protease, and a second portion which covalently bonds to the cysteine protease and irreversibly deactivates that protease by providing a carbonyl or carbonyl-equivalent which is attacked by the thiolate anion of the active site of the cysteine protease to sequentially cleave a beta -dicarbonyl enol ether leaving group.
Owner:PROTOTEK
1,3-cyclodiketone enol ether compound, 1-asymmetirc donor-receptor cyclopropane as well as synthesis methods thereof
The invention provides a 1,3-cyclodiketone enol ether compound, a 1-asymmetirc donor-receptor cyclopropane as well as synthesis methods thereof. By use of a reaction method for selectively using oxygen atoms of 1,3-cyclodiketone as nucleophilic sits to perform nucleophilic attack on 1-symmetric donor-receptor cyclopropane, a 1,3-cyclodiketone enol ether compound is obtained; a novel 1-asymmetirc donor-receptor cyclopropane 5 is obtained through intracellular conversion of the compound 3. The 1-asymmetirc donor-receptor cyclopropane 5 is obtained efficiently through desymmetrization of 1-asymmetirc donor-receptor cyclopropane through the simple two-step reaction. Meanwhile, the 1-asymmetirc donor-receptor cyclopropane 5 contains a cycloketene structure which is a very good intermediate module for constructing complex compounds in Domino and multi-component reactions.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Method for application of enamine in palladium catalytic asymmetry allyl group alkylated reaction
InactiveCN101139270ASimple and safe operationHigh reactivityOrganic compound preparationCarbonyl compound preparationAllyl acetateEnamine
The present invention relates to a method in the chemical technological field wherein the enamine is used in the palladium-catalyzed asymmetric allyl alkylation; the chiral ligand and the palladium catalyst are dissolved in the solvent first and react at the room temperature; the substituted allyl acetate is added; the mixture is stirred; then the enamine category compounds are added in the reaction; the cold saturated ammonium chloride solution is used for hydrolysis after the reaction is finished; thus, the product can be made. The enamine is a quite convenient and quite promising pro-nuclear reagent and overcomes the trouble of the unstable enol ether caused by the replacement of the alkali with the simple ketone; the operation is simple and safe; and the catalytic reaction has the high activity and selectivity of the reaction.
Owner:SHANGHAI JIAO TONG UNIV
Alpha-(1,3-dicarbonylenol ether) methyl ketones as cysteine protease inhibitors
InactiveUS20020128434A1Improve solubilityImproved toxicity profileTripeptide ingredientsImmunoglobulinsLeaving groupProteinase activity
Cysteine protease inhibitors which deactivate the protease by covalently bonding to the cysteine protease and releasing the enolate of a 1,3-dicarbonyl (or its enolic form). The cysteine protease inhibitors of the present invention accordingly comprise a first portion which targets a desired cysteine protease and positions the inhibitor near the thiolate anion portion of the active site of the protease, and a second portion which covalently bonds to the cysteine protease and irreversibly deactivates that protease by providing a carbonyl or carbonyl-equivalent which is attacked by the thiolate anion of the active site of the cysteine protease to sequentially cleave a .beta.-dicarbonyl enol ether leaving group.
Owner:ZIMMERMAN MARY P +2
Method for synthesizing ultra-pure o-cresol formaldehyde epoxy resin
The invention discloses a method for synthesizing an ultra-pure o-cresol formaldehyde epoxy resin. The method comprises the following steps: preparing an enol ether monomer by taking o-cresol and allyl chloride as raw materials; adding formaldehyde and acid catalysts for reaction to obtain a solution of o-cresol formaldehyde allyl ether resins; finally, oxidizing by using peroxyacetic acid to obtain the o-cresol formaldehyde epoxy resin. According to the invention, a process of synthesizing the o-cresol formaldehyde epoxy resin is optimized, the product purity is high, the content of hydrolyzable chlorides is low, and the method has the obvious advantages that residual chlorine groups are avoided in the synthetic process, chlorine exists in an inorganic chlorine form, and the content of the hydrolyzable chlorides in the o-cresol formaldehyde epoxy resin is greatly reduced and is generally 20ppm or lower.
Owner:JIANGSU SANJILI CHEM
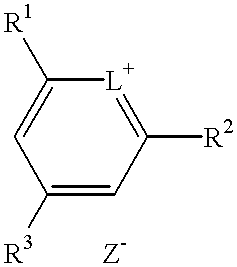
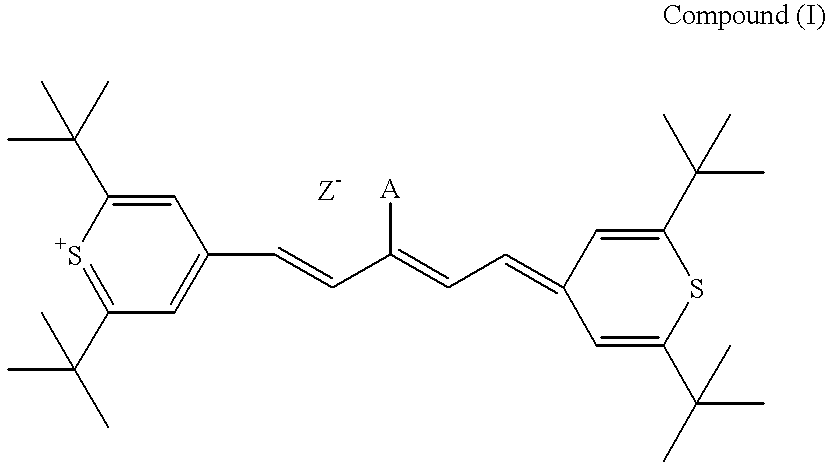
Method for preparation of an intermediate dye product
The present invention provides a novel method for the synthesis of an intermediate dye product having the following formula:whereinL is S, Te, or Se;R1 and R2 are either the same or different aryl or alkyl compounds;R3 is hydrogen or a short chain alkyl group; andZ is an anion.The process to formulate this intermediate compound entails reacting an R1-acetylene compound with an R2-acetylene compound (compounds A) into an enol ether compound with the R1 and / or R2 constituents (compound D). And from compound D, it forms into an intermediate dye compound having an L-based cyclic ring with the R1 and / or R2 constituents (compound F). With compound F the desired dye can be made with a greater overall yield for mass production.
Owner:RES FOUND OF STATE UNIV OF NEW YORK THE STATE UNIV OF NEW YORK AT BUFFALO
Proportion research of novel drilling fluid lubricant
InactiveCN104673254AReduce the impactImprove the lubrication effectDrilling compositionEnol etherResidual oil
The invention aims to provide a novel drilling fluid lubricant. The novel drilling fluid lubricant comprises the following components in percentage by weight: polyoxy enol ether, an emulsifier, fatty acid aluminum and residual oil according to the ratio of (5-20): (0.1-5): (1-10): (0.1-0.5). The novel drilling fluid lubricant has the beneficial effects of attractive lubricating property; by utilizing the lubricant in the field, the torque of a drill steam and the resistance of a summer drill can be reduced effectively, the accident of hamming of a drilling tool is reduced, the drilling fluid is not affected, the thickening and bubbling are avoided, the filter loss is reduced a little, and the environmental influence is small.
Owner:QINGDAO HUICHENG PETROCHEM TECH
Synthesis method of alkyl acid testosterone
ActiveCN111995650AReduce generationLow yieldSteroidsBulk chemical productionOrganic solventEnol ether
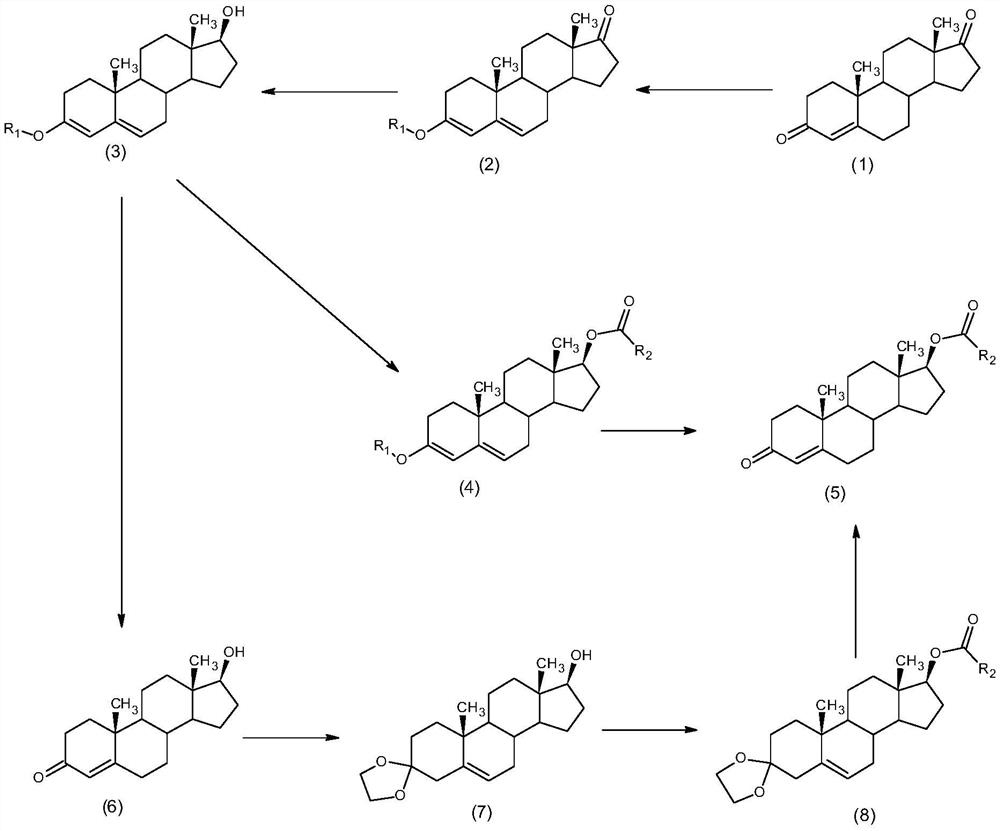
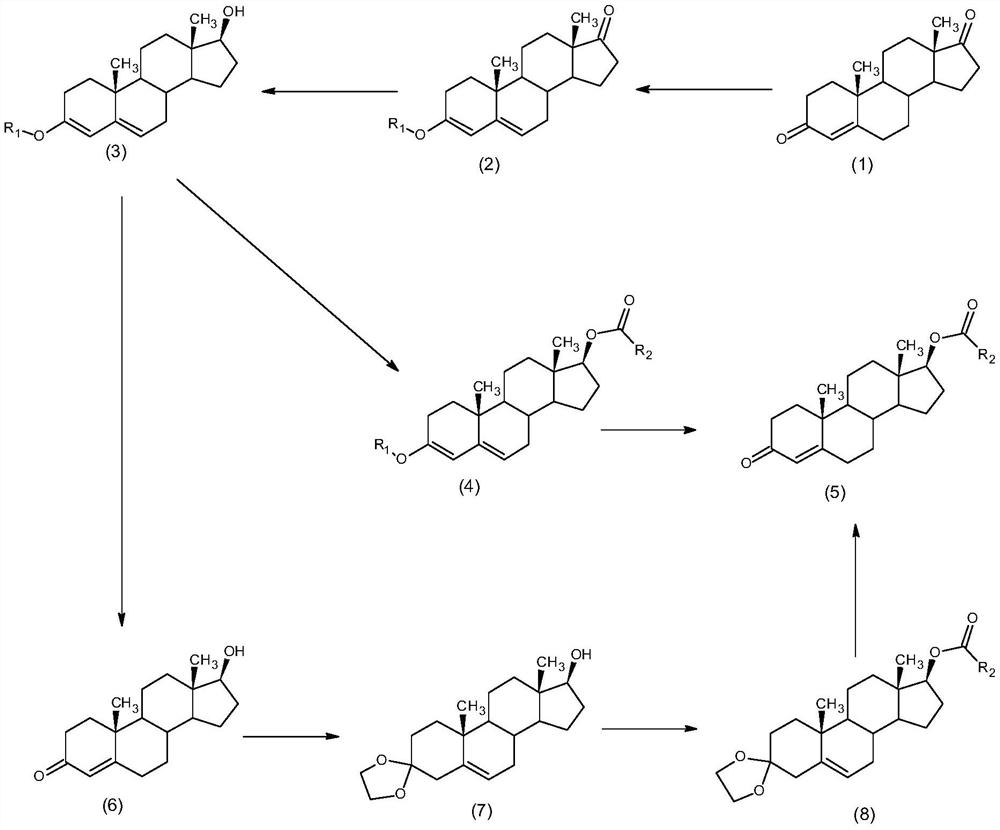
The invention discloses a synthesis method of alkyl acid testosterone, and belongs to the technical field of synthesis and processing of medicines. The method comprises the following steps of: taking4-androstenedione (4AD) as an initial raw material, firstly, carrying out enol ether protection on the keto group at the site 3, and reducing carbonyl at the site 17 into hydroxyl; or taking 4-androstenedione (4AD) as an initial raw material, firstly carrying out enol ether protection on the keto group at the site 3, then reducing carbonyl at the site 17 into hydroxyl, then carrying out hydrolysison the site 3 to obtain testosterone, and carrying out esterification and third-site hydrolysis to obtain the testosterone ester after testosterone third-site ketal protection. According to the method disclosed by the invention, the third site is protected during esterification reaction, the generation of impurities can be reduced, and an esterification reaction solvent is a water-insoluble organic solvent, so that after the reaction is completed, products can be directly extracted in a layered manner, a large amount of water does not need to be added to separate out the products, the amountof wastewater is reduced, the solvent can be recycled, and the process is more suitable for industrial production.
Owner:ZHEJIANG SHENZHOU PHARMA
Process for preparing vinyl chloroformate
ActiveUS8273914B1High yieldSimple and cost efficient processPreparation by transesterificationHalogenSilylene
Disclosed is a process for making vinyl chloroformate which includes reacting (a) a carbonyl compound of formula I:wherein R is a halogen or an alkyl group of 1 to about 25 carbon atoms; with (b) a silyl-containing enol ether and in the presence of an effective amount of a Group VIII-containing catalyst.
Owner:BAUSCH & LOMB INC
Garland chrysanthemum extract compounds, and synthesis method and application thereof
InactiveCN101851245AThe synthesis method is simpleMild reaction conditionsBiocideOrganic chemistryUnsaturated hydrocarbonSynthesis methods
The invention relates to garland chrysanthemum extract compounds, and a synthesis method and application thereof. The compounds have enol ether-spiro ketal-acetals and unsaturated side chains. The garland chrysanthemum extract compounds have the following structural formula, wherein the exocyclic double bond can be cis or trans exocyclic double bond; Ar is a C2-14 unsaturated alkyl group; and R1 is a methyl group or C2-14 unsaturated alkyl group. The method for synthesizing the compounds is simple and suitable for industrial production; and the compounds have obvious activity of insect antifeedant.
Owner:SOUTH CHINA UNIV OF TECH
Enol-ether capped polyethers and surfactants produced therefrom
The use of enol ether capped polyether-polysiloxane copolymers as surfactants in polyurethane foam applications is taught herein. These enol ether capped surfactants exhibit a high capping efficiency and yield good performance. Moreover, they are stable in water / amine premixes.
Owner:GENERAL ELECTRIC CO
Catalyst compositions and their use for hydrogenation of nitrile rubber
InactiveUS20150126683A1Organic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsNitrile rubberEnol ether
This invention relates to novel catalyst compositions based on ruthenium or osmium carbene-complex catalysts, pref. of the Grubbs-I, -II or -III type or fluorenylidene analogues thereof, and terminal olefins, pref. enol ethers such as ethyl vinyl ether (EVE or VEE) as co-catalysts and to a process for selectively hydrogenating nitrile rubbers in the presence of such catalyst compositions, pref. with a preceding metathesis step using the same complex catalyst as in the hydrogenation step.
Owner:ARLANXEO DEUT GMBH
Process for preparing graft polyols using enol ethers as reaction moderators
The present invention provides a process for preparing a stable graft polyol dispersion. The graft polyol dispersion has low viscosity and a high solids content. An ethylenically unsaturated monomer is polymerized in a polyol mixture including a macromer polyol and a carrier polyol. A free radical initiator and a reaction moderator are also present during polymerization. The reaction moderator is of the formula: wherein R is a phenyl group, R1 is selected from the group of an aliphatic hydrocarbon radical having 1 to 18 carbon atoms, and R2 is selected from the group of a phenyl group, a nitrile group, a methoxycarbonyl group, a carbamoyl group, and combinations thereof.
Owner:BASF CORP
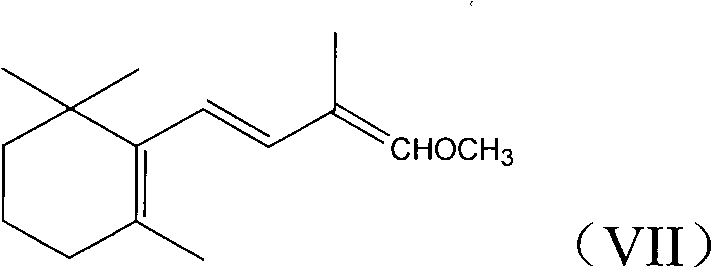
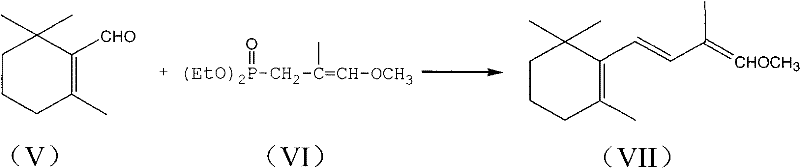
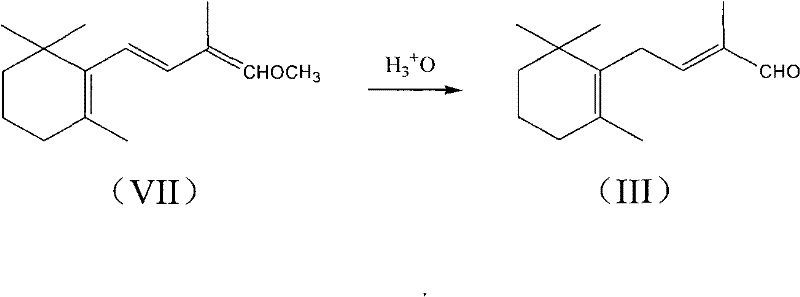
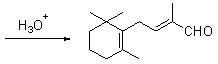
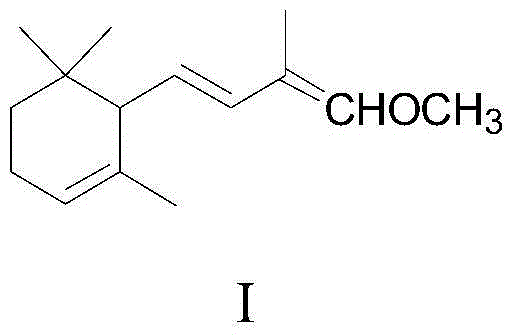
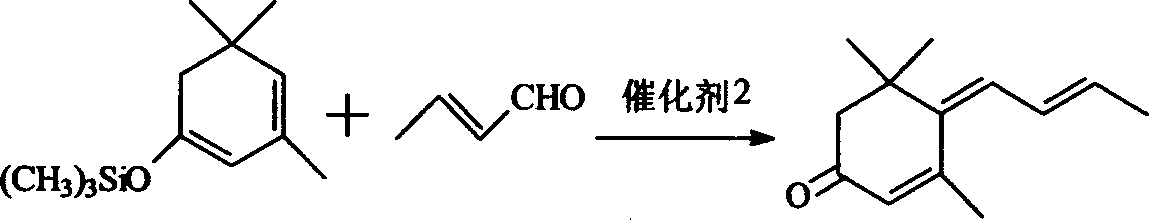
1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-1,3-butadiene and preparation method thereof
InactiveCN104418713ASimple process routeRaw materials are easy to getOrganic compound preparationCarbonyl compound preparation by hydrolysisCyclohexeneEnol ether
The invention provides an enol ether 1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-1,3-butadiene shown in a formula I and a preparation method thereof. In the presence of an acid catalyst, the 1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-1,3-butadiene is subjected to hydrolysis reaction to prepare 2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-2-butene-1-aldehyde. The process line is simple, easily available in raw materials and low in cost, and has industrial value.
Owner:SHAOXING UNIVERSITY +1
Preparation technology of alpha, alpha-dimethyl-ethide phenylpropyl aldehyde
InactiveCN101525280AReduce pollutionEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcid waterGas phase
The invention discloses a preparation technology of alpha, alpha-dimethyl-ethide phenylpropyl aldehyde. The preparation technology is as follows: ethide benzyl chloride and isobutylaldehyde are taken as major raw materials, aromatic hydrocarbon is a solvent under the condition of alkalescence and is added with a catalytic agent, the aromatic hydrocarbon is stirred and heated under the temperature of 60-95 DEG C to be reacted, and then the aromatic hydrocarbon is washed by protonic acid water under the temperature of 50-95 DEG C to eliminate enol ether so as to obtain the alpha, alpha-dimethyl-ethide phenylpropyl aldehyde. The preparation technology not only has simple operation and reduces the environment pollution, but also has high yield coefficient of products; in addition, the content of the gas phase chromatography of obtained products is 80-95 percent.
Owner:孙玉明
Aromatic enol ether paint additives
Owner:EASTMAN CHEM CO
Synthetic method for beta-isophorone trimethyl silane enol ether and its use in synthetic large column trienic ketone
The synthesis process of beta-isophorone trimethyl silane enol ether includes the reaction between beta-isophorone and trimethyl chlorosilane under the catalysis of sodium iodide to obtain beta-isophorone trimethyl silane enol ether. The said beta-isophorone trimethyl silane enol ether is made to produce Aldol react with crotonaldehyde, and the product is heated to dewater to produce large column trienic ketone. The present invention has the advantages of simple operation and high yield, and is suitable for industrial production. The synthesized large column trienic ketone is used in cigarette essence and has excellent effect.
Owner:APPLE FLAVOR & FRAGRANCE GRP +1
Enol ethers
Disclosed are enol ethers compounds. The enol ethers exhibit low volatile organic content and are useful in a variety of chemical applications. The enol ethers can be used in applications as diluents, wetting agents, coalescing aids, paint additives and as intermediates in chemical processes. The enol ethers also have particular utility as film-hardening additives in coating formulations.
Owner:EASTMAN CHEM CO
Process for Production of Polysubstituted Cyclobutanes and Polysubstituted Cyclobutenes
InactiveUS20080051598A1High chemical yieldHigh stereoselectivityGroup 4/14 element organic compoundsOrganic compound preparationCyclobutaneEnol ether
The prior art required specialized substrates or reaction conditions to be used to manufacture polysubstituted cyclobutane compounds and polysubstituted cyclobutene compounds, and the method had poor generality. The type or quantity of the catalyst or solvent used was also problematic in the industrial manufacture of polysubstituted cyclobutane compounds. The present invention provides a method for manufacturing a polysubstituted cyclobutane compound with high stereoselectivity that has low environmental load (is ecologically advantageous) and is applicable to industrial manufacturing from the standpoint of operation, substrate generality, catalyst, solvent, and efficiency. A polysubstituted cyclobutane, a cyclobutene, and a bicyclo[4.2.0]octane compound can be manufactured efficiently, stereoselectively, and in an ecologically advantageous manner by causing a Brønsted acid to act on a mixture of an enol ether compound or 2-siloxydiene compound with an alkene or alkyne compound in which a carbonyl group is substituted at the 1-position in a non-aqueous solvent or without a solvent.
Owner:TOHOKU UNIV
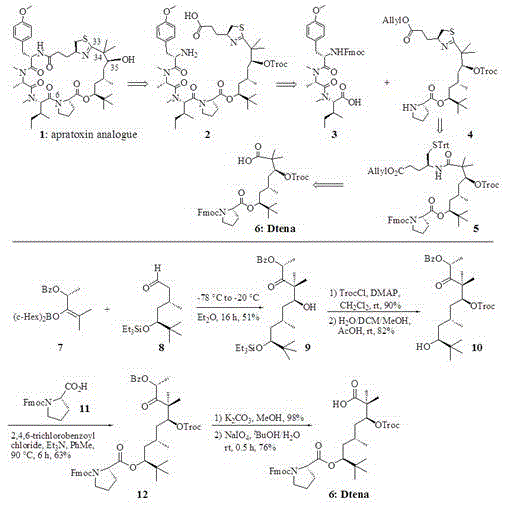
Synthetic method for Dtena modified fragment of apratoxin marine natural product
InactiveCN105481746AFew reaction stepsSuitable for industrial productionOrganic chemistryChemical synthesisAlcohol
The invention relates to a synthetic method for a Dtena modified fragment of an apratoxin marine natural product, which belongs to the field of chemical synthesis. The synthetic method comprises the following steps: subjecting boryl enol ether and aldehyde to adol reaction so as to prepare beta-hydroxyl ketone; preparing O-Troc alcohol through the Troc protection reaction of the hydroxyl group of beta-hydroxyl ketone and silyl ether deprotection reaction; subjecting the O-Troc alcohol and N-Fmoc proline to a coupling reaction to prepare o-O-Bz-yl ketone containing a proline constitutional unit; and carrying out O-Bz deprotection reaction of the o-O-Bz-yl ketone and oxidation reaction of newly generated o-hydroxyl ketone so as to prepare the Dtena modified fragment (as in a figure in the specification) of the apratoxin marine natural product. The synthetic method has the characteristics of a few reaction steps, high overall yield, good product selectivity, suitability for industrial production, etc.
Owner:HARBIN INST OF TECH AT WEIHAI
Aromatic enol ethers
Disclosed are aromatic enol ethers that have utility as film-hardening additives for coating formulations. The aromatic enol ethers have particular utility as film-hardening additives for water-based coating formulations. The aromatic enol ethers provide improvements in hardness and hardness related properties such as block resistance without contributing to the volatile organic content of the composition.
Owner:EASTMAN CHEM CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com