1,3-cyclodiketone enol ether compound, 1-asymmetirc donor-receptor cyclopropane as well as synthesis methods thereof
A technology for cyclic diketone enol ethers and synthetic methods, which is applied in the field of 1-asymmetric donor acceptor cyclopropane and 1,3-cyclic diketone enol ether compounds, and can solve the problems of limited types and synthetic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The synthesis method includes the following steps: in an organic solvent, using scandium trifluoromethanesulfonate as a catalyst, reacting a 1,3-cyclic diketone compound 1 with a 1-symmetric donor acceptor cyclopropane 2 to obtain a 1,3-cyclic Diketone Enol Ether Compound 3.
[0042] The molar ratio of 1,3-cyclic diketone compound 1 to 1-symmetric donor acceptor cyclopropane 2 is 1.2:1. The molar ratio of 1,3-cyclic diketone compound 1 to scandium trifluoromethanesulfonate is 24 :1 or 12:1. The reaction temperature is 25°C or 45°C; the reaction time is 24 hours.
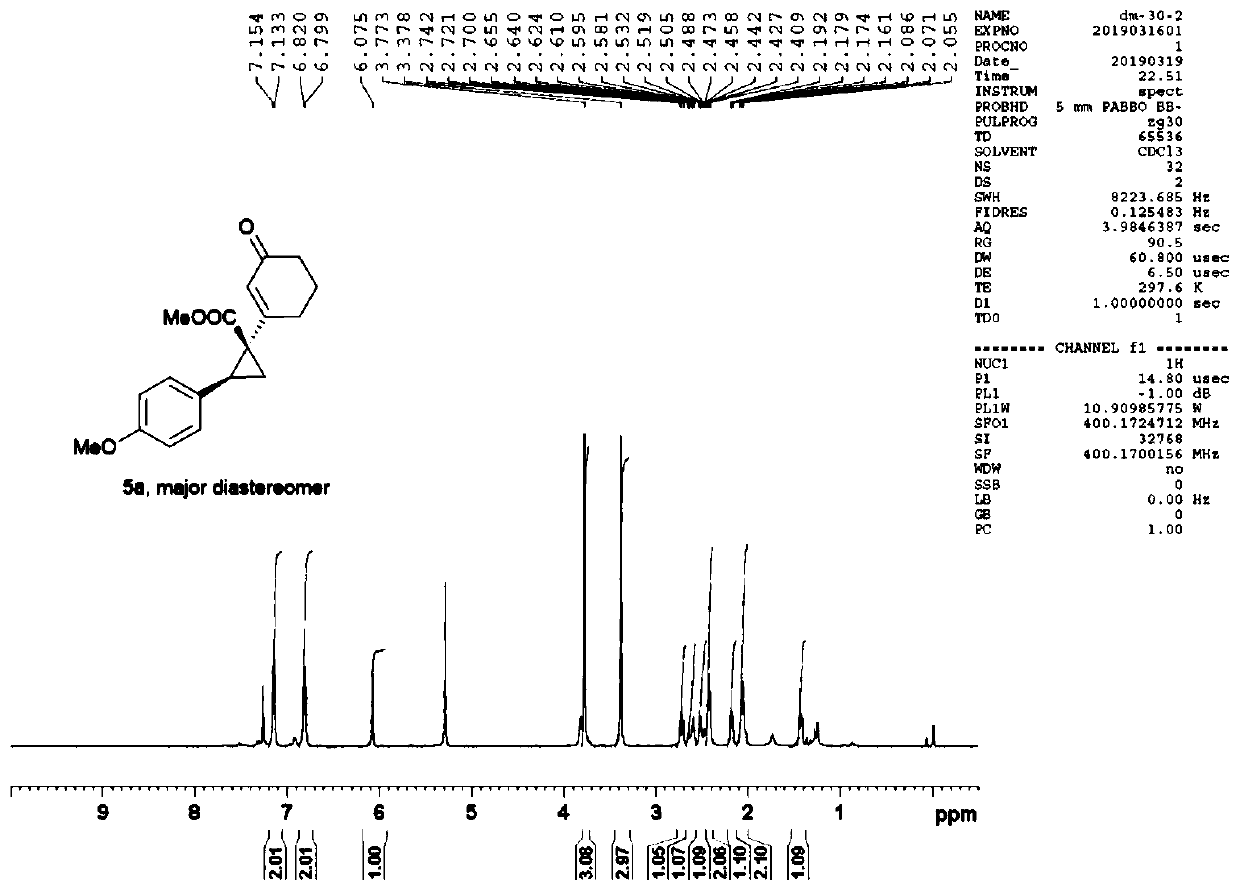
[0043] The present invention also provides a 1-asymmetric donor acceptor cyclopropane, the structure of the 1-asymmetric donor acceptor cyclopropane 5 is as follows:
[0044]
[0045] Wherein, R is H or methyl; Ar is substituted phenyl, 2-thienyl or 3-(1-tert-butoxycarbonyl)indolyl; n is 0 or 1.
[0046] 1-The synthetic method of asymmetric donor acceptor cyclopropane 5, the synthetic route is:
[0047]...
Embodiment 1
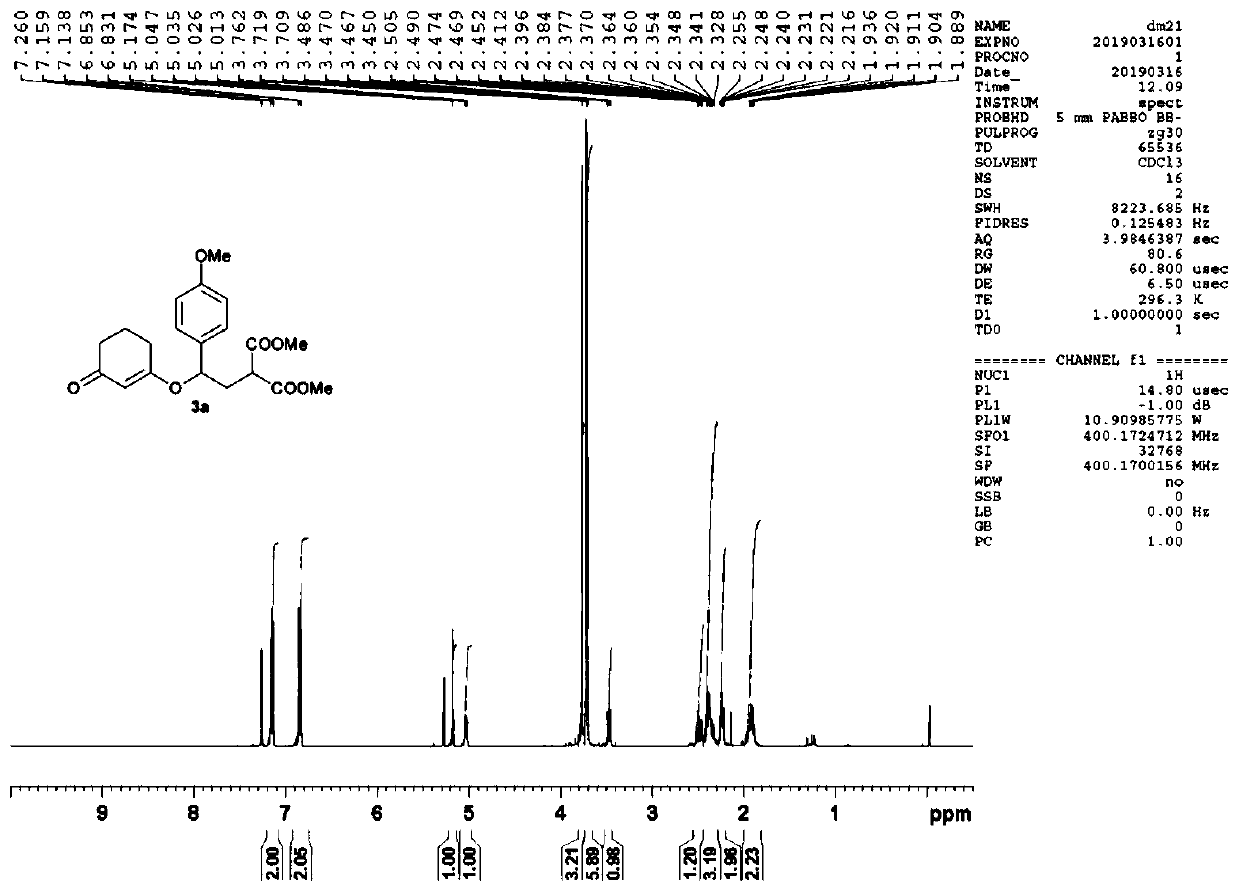
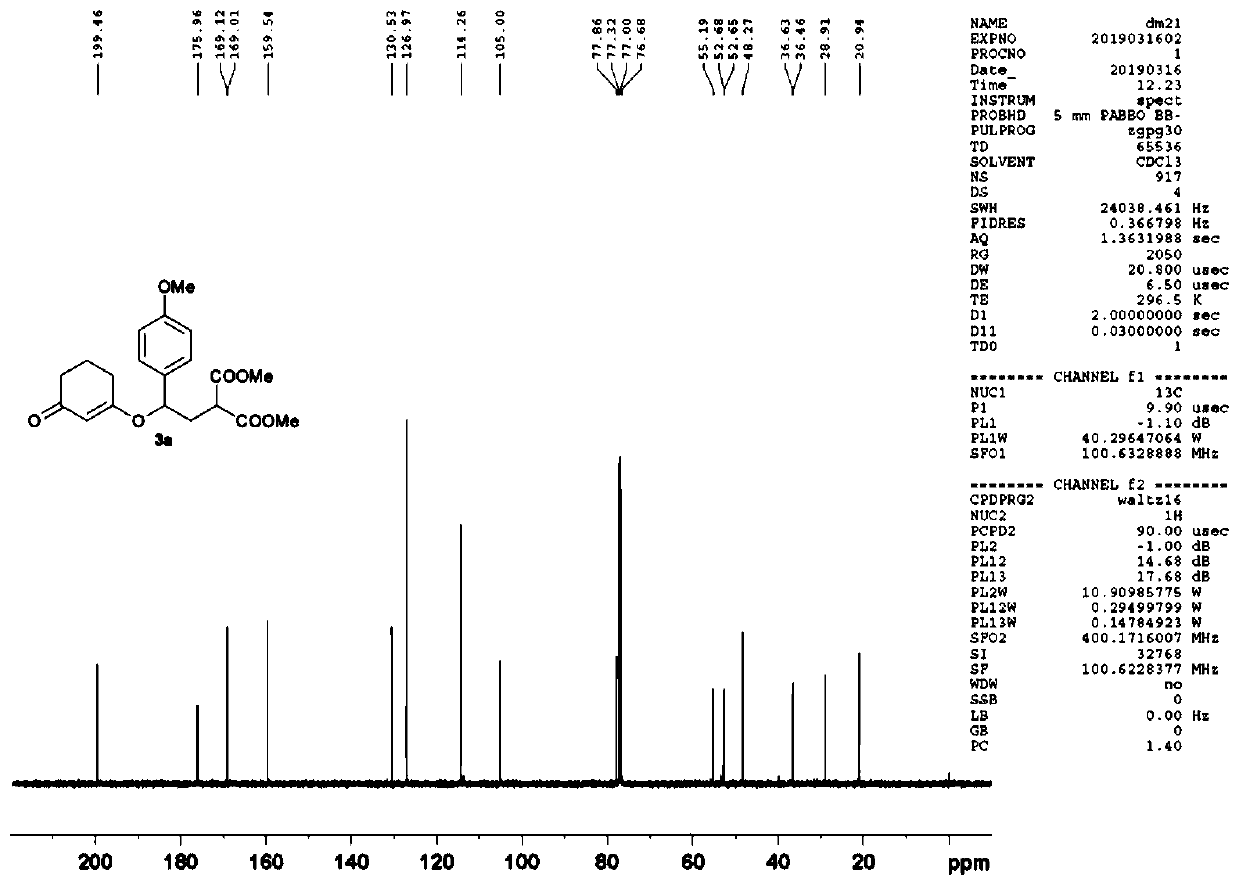
[0050] Embodiment 1: the synthesis of compound 3a (gram level)
[0051]
[0052] Add scandium triflate (0.25mmol ), and the reaction was stirred at room temperature (25° C.) for 24 hours. Then the reaction solution was diluted with dichloromethane and washed with saturated NaHCO 3 solution, the aqueous phase was extracted three times with dichloromethane, all organic phases were combined, washed with saturated brine, NaSO 4Drying, filtration, spin-drying, column chromatography (petroleum ether: ethyl acetate = 2:1-1:1), and rotary evaporation gave 1.74 g of a colorless viscous liquid 3a with a yield of 93%. 1 H NMR (400MHz, CDCl 3 ):δ=1.87-1.97(m,2H,CH 2 ),2.22-2.25(m,2H,CH 2 ),2.30-2.53(m,4H,CH 2 ×2), 3.47(dd, J=6.8Hz, 7.8Hz, 1H, CH), 3.71(s, 3H, CH 3 ),3.72(s,3H,CH 3 ),3.76(s,3H,CH 3 ), 5.03(dd, J=4.9Hz, 8.5Hz, 1H, CH), 5.17(s, 1H, CH), 6.84(d, J=8.7Hz, ArH), 7.15(d, J=8.7Hz, 2H ,ArH)ppm; 13 C NMR (100MHz, CDCl 3 ): δ=20.9, 28.9, 36.5, 36.6, 48.3, 52.6, 52.7, ...
Embodiment 2
[0053] Embodiment 2: the synthesis of compound 3a
[0054] Add scandium triflate (0.0075mmol ), and the reaction was stirred at room temperature (25° C.) for 24 hours. Then the solvent was spin-dried, column chromatography (petroleum ether: ethyl acetate = 2:1-1:1), and rotary evaporation to obtain 51.3 mg of colorless viscous liquid compound 3a with a yield of 91%.
[0055] Compounds 3b, 3c, 3f, 3g, 3i, 3j, 3k, 3l, and 3m similar to 3a in the following examples are all passed through the corresponding 1,3-ring diketone compound 1 and the corresponding compound 1 by the method in Example 2. 1-symmetrical donor-acceptor cyclopropane 2 reaction synthesis. Specifically: in the mixed solution of 1,3-cyclohexanedione compound 1 (0.18mmol) and 1-donor acceptor cyclopropane 2 (0.15mmol) in dichloromethane (1.0mL), add trifluoromethanesulfonic acid scandium (0.0075 mmol), and the reaction was stirred at room temperature (25° C.) for 24 hours. Then spin dry the solvent, perform col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com