Continuous flow synthesis method of (3aS, 6aR)-lactone
A flow synthesis, lactone technology, applied in chemical instruments and methods, organic chemistry, chemical/physical/physical chemical processes, etc., can solve the problems of low enantiomeric selectivity, difficult industrial scale-up, long reaction time, etc. To achieve the effect of shortened reaction time, convenient synthesis and high degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
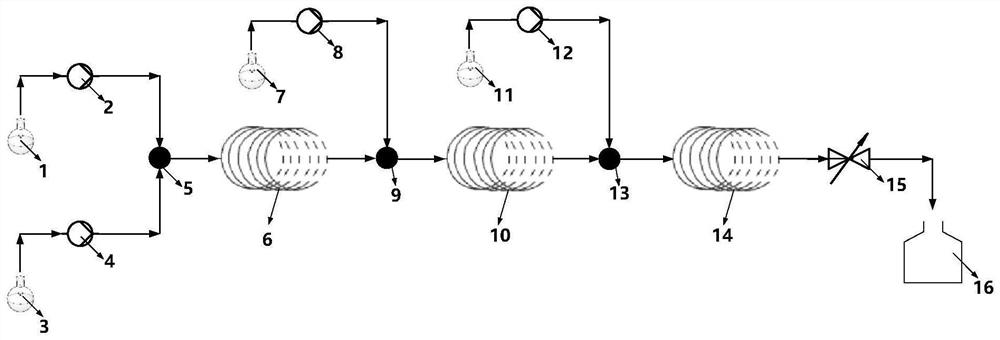
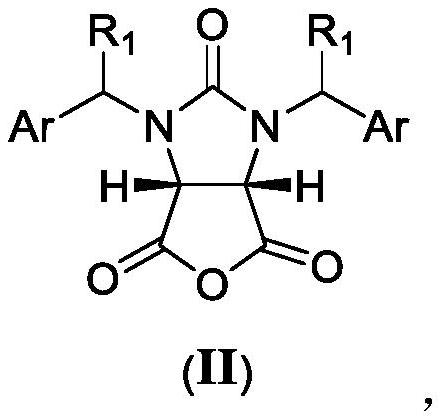
[0073] The substrate cyclic anhydride (II) (R in formula II 1 is hydrogen, Ar is phenyl, and its full name is cis-1,3-dibenzylimidazolin-2-one-2H-furo[3,4-d]imidazole-2,4,6-trione)( 33.6g, 0.10mol) was dissolved in 200ml of anhydrous toluene and placed in the substrate liquid storage tank 1, and the chiral auxiliary agent (S)-1,1-bis([1,1'-biphenyl]-4-yl )-1,2-propanediol (III) (R in formula III 2 is hydrogen, R 3 Hydrogen) (39.9g, 0.105mol) and newly distilled n-butylamine (23.90ml, 0.10mol) were dissolved in 200ml of anhydrous toluene and placed in the chiral auxiliary agent solution storage tank 3 to prepare a tetrahydrofuran solution of lithium borohydride (1mol / L) is placed in the borohydride solution storage tank 7, and an aqueous hydrochloric acid solution (2mol / L) is prepared and placed in the inorganic mineral acid solution storage tank 11.
[0074] With feed pump 2 and feed pump 4, substrate liquid and chiral auxiliary agent solution are transported respectively i...
Embodiment 2
[0077] This embodiment is the same as Embodiment 1, the only difference is that the micro-mixer 5, the micro-mixer 9 and the micro-mixer 13 in this embodiment are all T-shaped micro-mixers. In this example, the substrate cyclic anhydride (II) was completely converted, the yield of the target product (I) was 92.1%, and the enantioselectivity was 98%.
Embodiment 3
[0079] This embodiment is the same as Embodiment 1, the only difference is that the micro-mixer 5, the micro-mixer 9 and the micro-mixer 13 in this embodiment are all Y-shaped micro-mixers. In this example, the substrate cyclic anhydride (II) was completely converted, the yield of the target product (I) was 87.6%, and the enantioselectivity was 97.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com