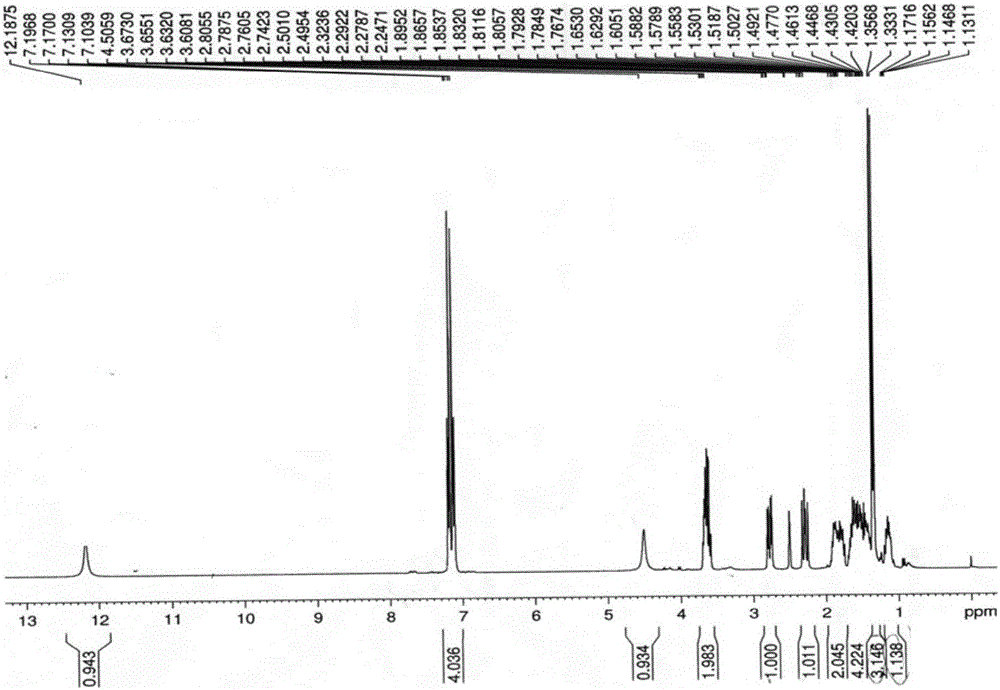
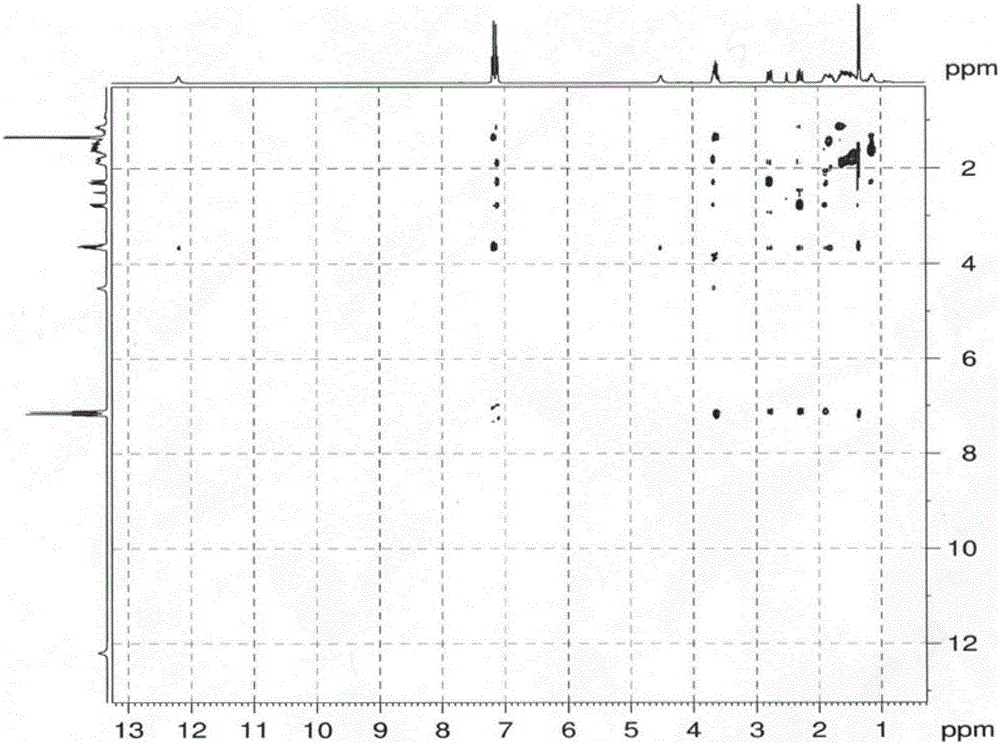
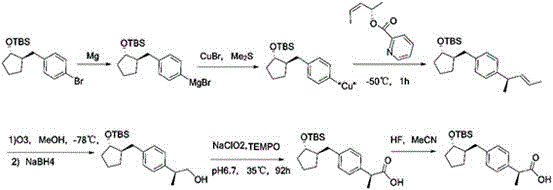
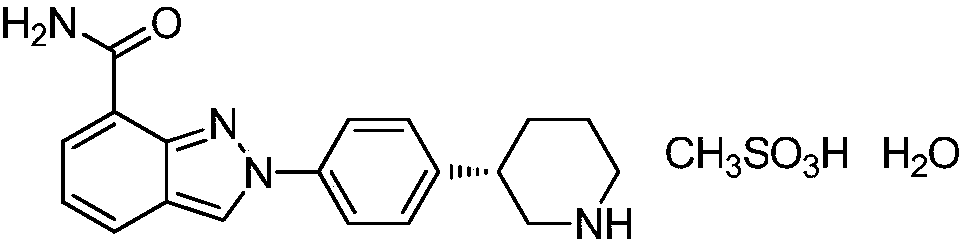
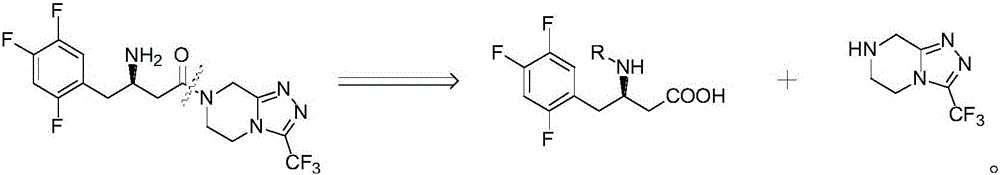
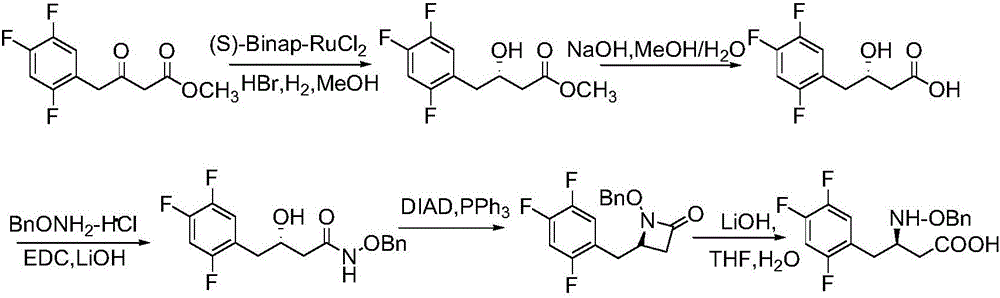
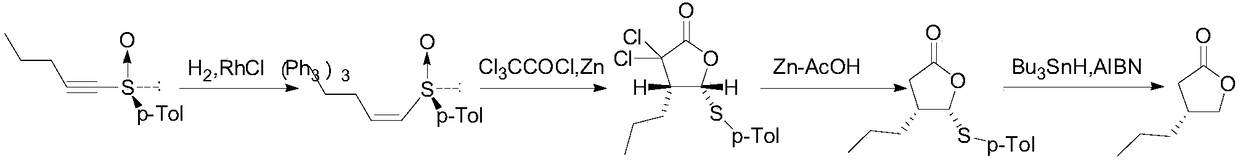
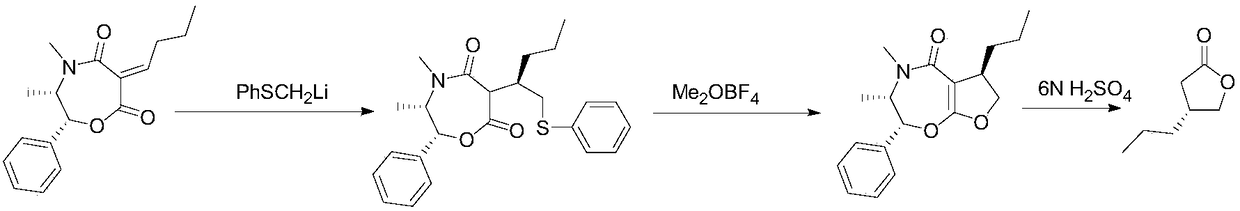
Patents
Literature
168 results about "Chiral auxiliary" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.
Chiral auxiliaries
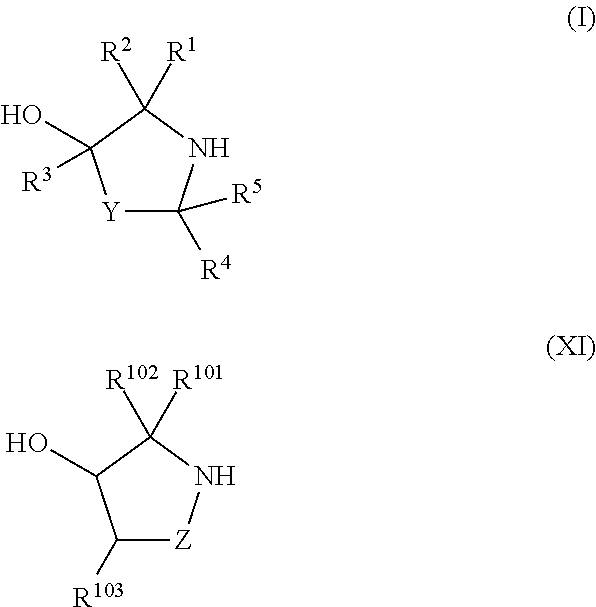
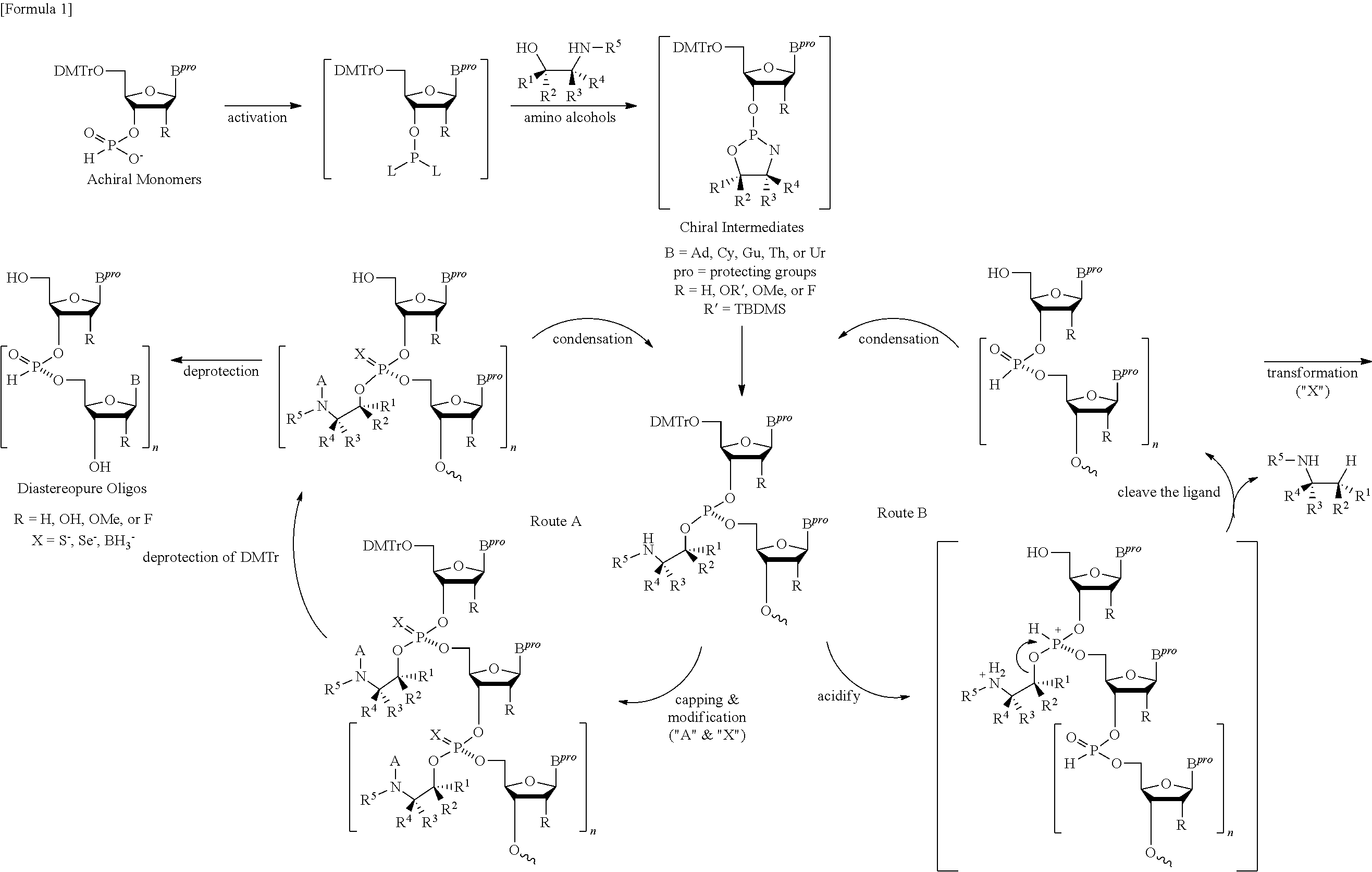
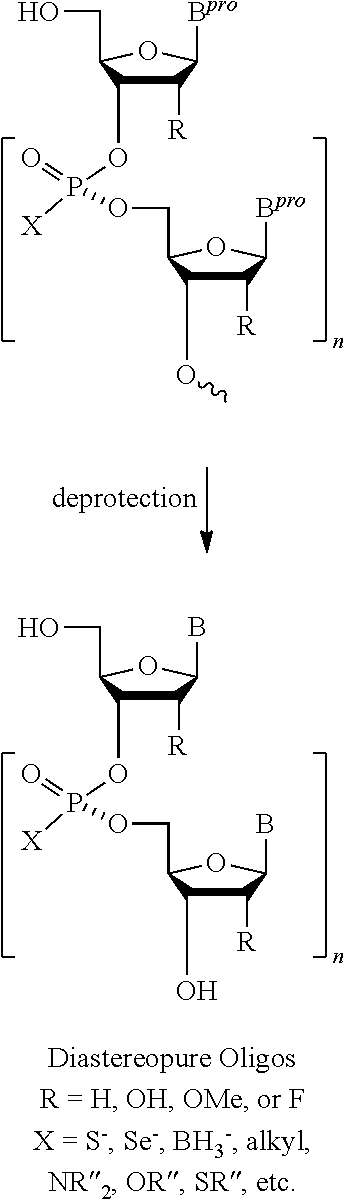
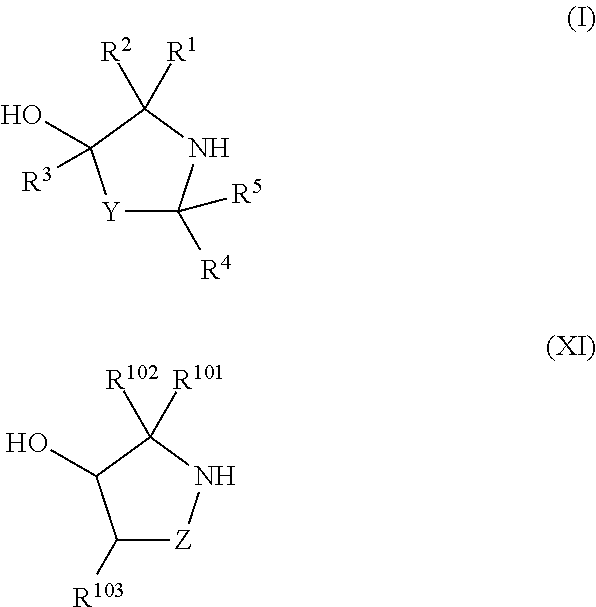
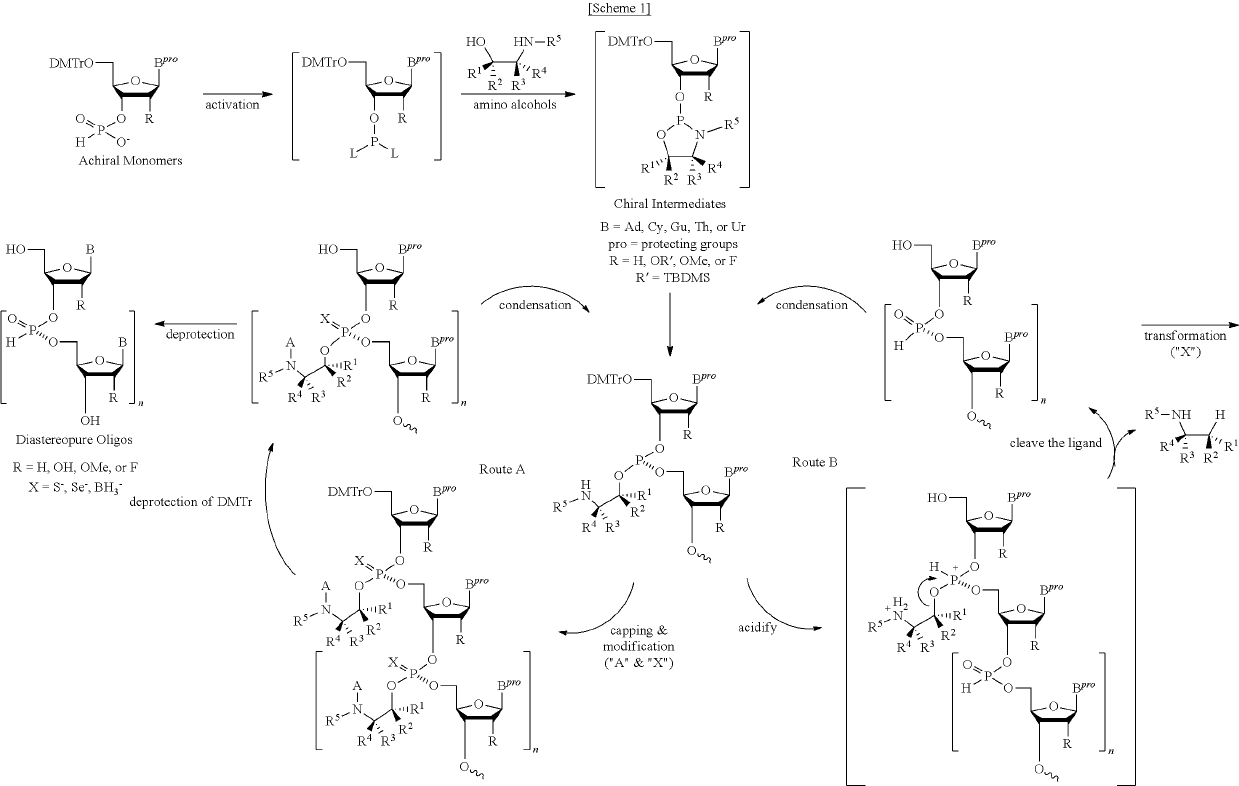
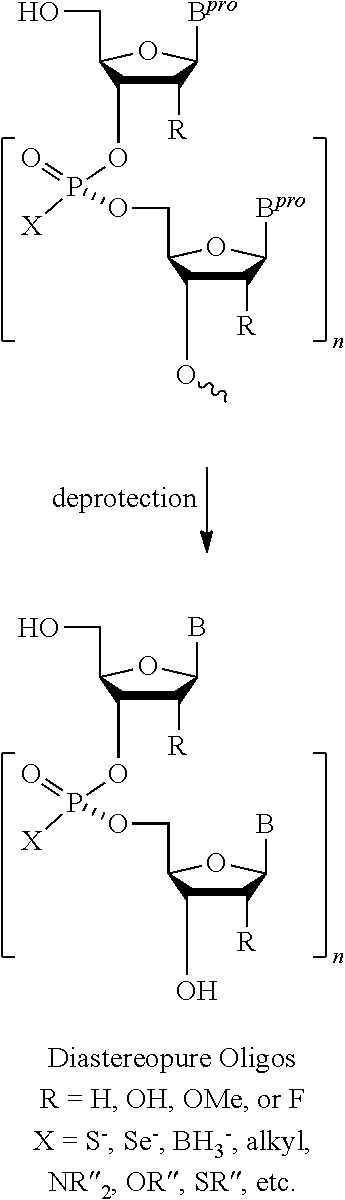
ActiveUS20130178612A1High asymmetric yieldHigh stereoregularitySugar derivativesSugar derivatives preparationPhosphorus atomStereochemistry
Chiral auxiliaries useful for efficiently producing a phosphorus atom-modified nucleic acid derivative with high stereoregularity, and compounds represented by the following the general formula (I) or the general formula (XI) for introducing the chiral auxiliaries.
Owner:WAVE LIFE SCI LTD
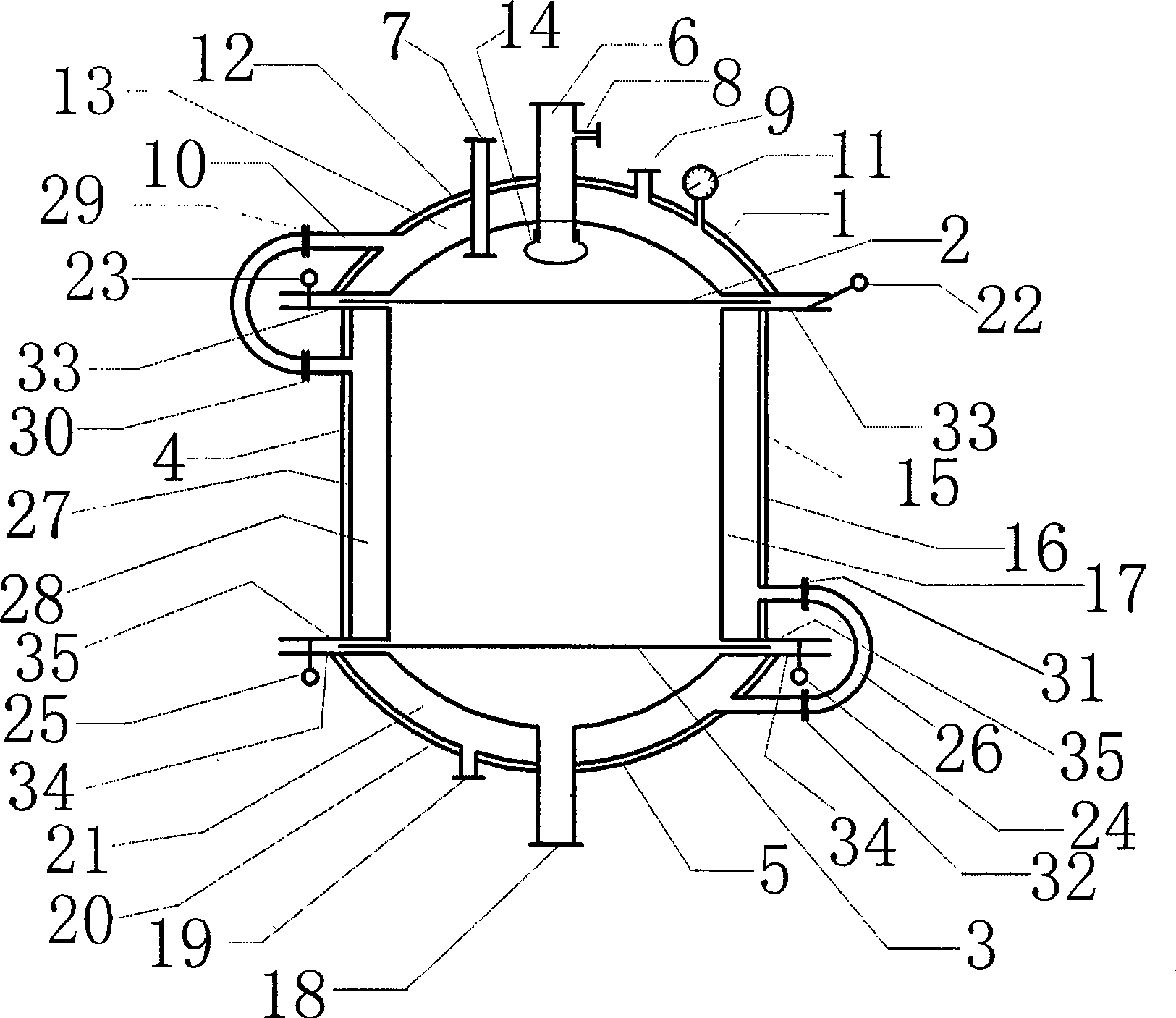
Production method of levamlodipine besylate
The invention takes N, N-dimethylformamide as a chiral auxiliary and separates amlodipine with tartaric acid resolution reagent to prepare l-amlodipine. In addition, benzene sulfonic acid and l-amlodipine alkali are directly salified and refined, filtered and dried via a special filter to produce levoamlodipine besylate. The upper part and the lower part of the special filter adopted by the invention are respectively provided with a hemispherical top cap and a hemispherical bottom cap, the middle part is provided with a lauter tank and ring groove filter plates are respectively arranged between the top cap and the lauter tank or the bottom cap and the lauter tank. A feed pipe, inlet and outlet of inert gases, an outlet of cooling fluid and a temperature meter are installed on the top cap of the filter; a discharge pipe and the outlet of cooling fluid are installed on the bottom cap. The filter is provided with an insulating layer and an interlayer, thus can control the temperature, avoid light, be filled with inert gases and protect the feed liquid and filtrate from oxidation, illumination and high temperature damage. The filtration efficiency is high, the effect is good and the structure is simple, the filter operation, disassembly, assembly and cleaning are convenient and the levoamlodipine besylate enjoys high synthesis and production yield and stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
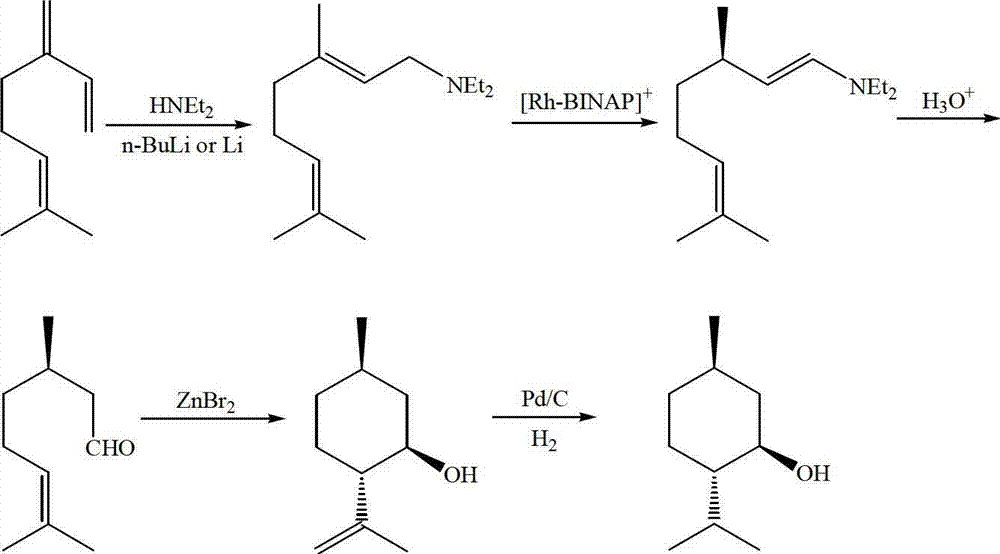
Method for asymmetric synthesis of levorotation menthol
InactiveCN103044204AEasy to makeMild reaction conditionsPreparation by hydrogenationLewis acid catalysisDihydropyridine derivatives
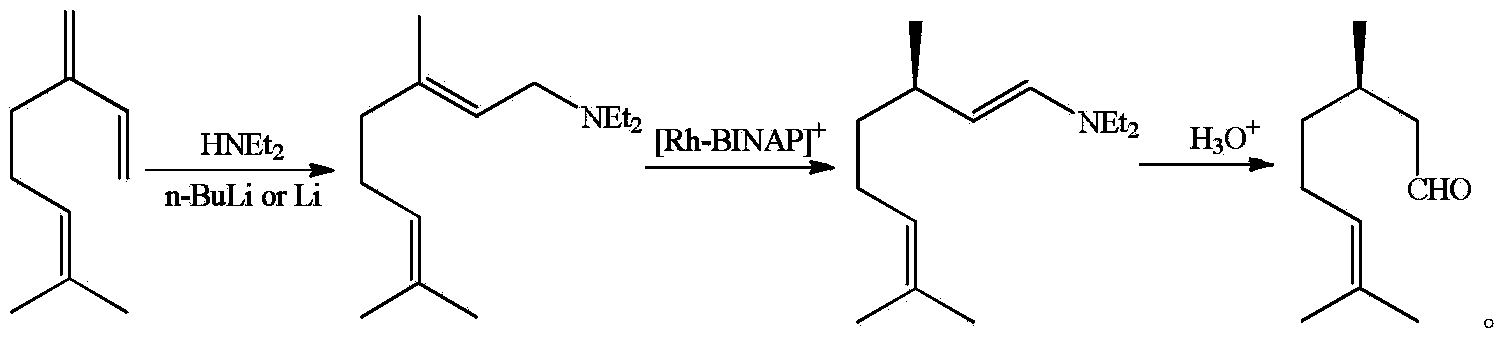
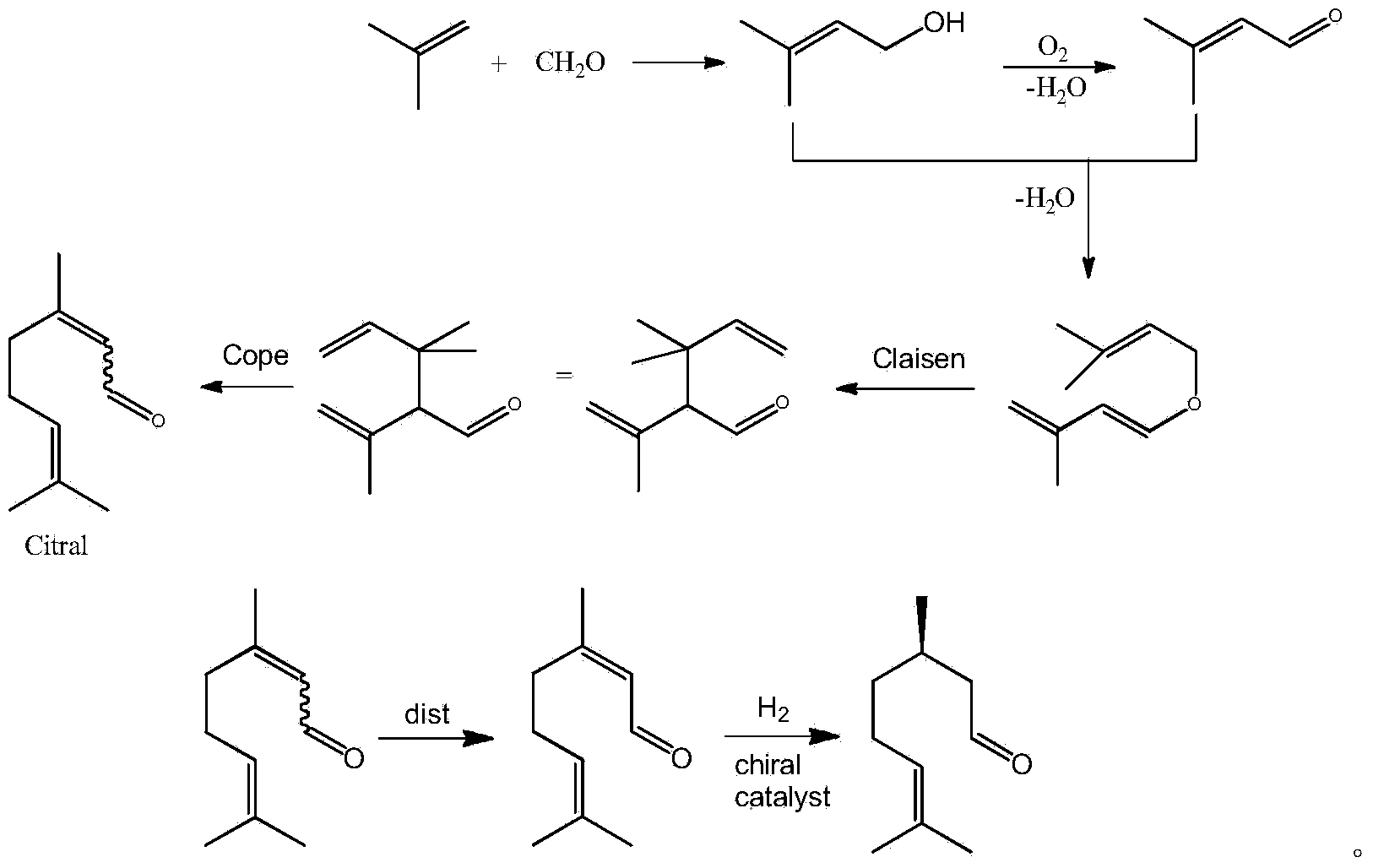
The invention discloses a method for asymmetric synthesis of levorotation menthol. The method comprises the following steps: citral is taken as an initial raw material, a dihydropyridine derivant is taken as a negative hydrogen source, chiral amine serves as a chiral auxiliary agent for catalytic and asymmetric hydrogenization synthesis of dextrorotation citronellal, the dextrorotation citronellal is catalyzed by Lewis acid for ring-closing synthesis of levorotation isopulegol, and the levorotation isopulegol is subject to catalytic hydrogenation to finally produce the levorotation menthol. The total yield of the levorotation menthol produced by adopting the method is larger than 60 percent, and the ee (enantiomeric excess) value is larger than 90 percent. The method has the characteristics of mild reaction conditions, simple synthetic process, simplicity in catalyst preparation, convenience in catalyst recovery and the like and is suitable for large-scale industrial production of levorotation menthol.
Owner:GUANGDONG FOOD IND INST +1
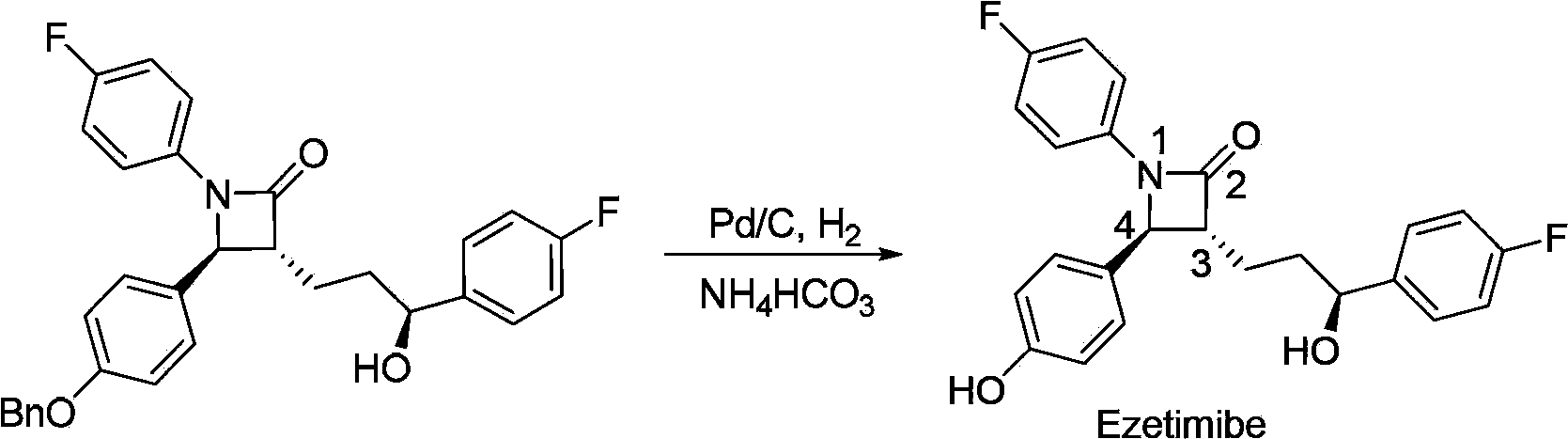
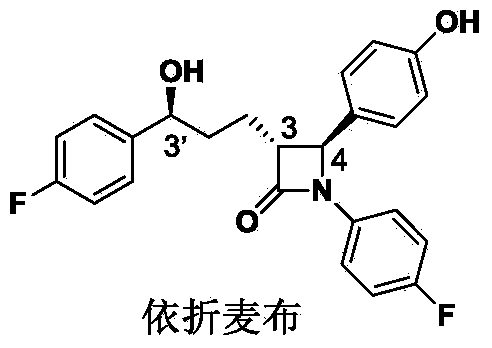
Stereselective synthesis method for lipid-lowering drug ezetimibe
ActiveCN103965089AHigh chemical purityHigh optical purityOrganic chemistryBulk chemical productionLipid lowering drugSynthesis methods
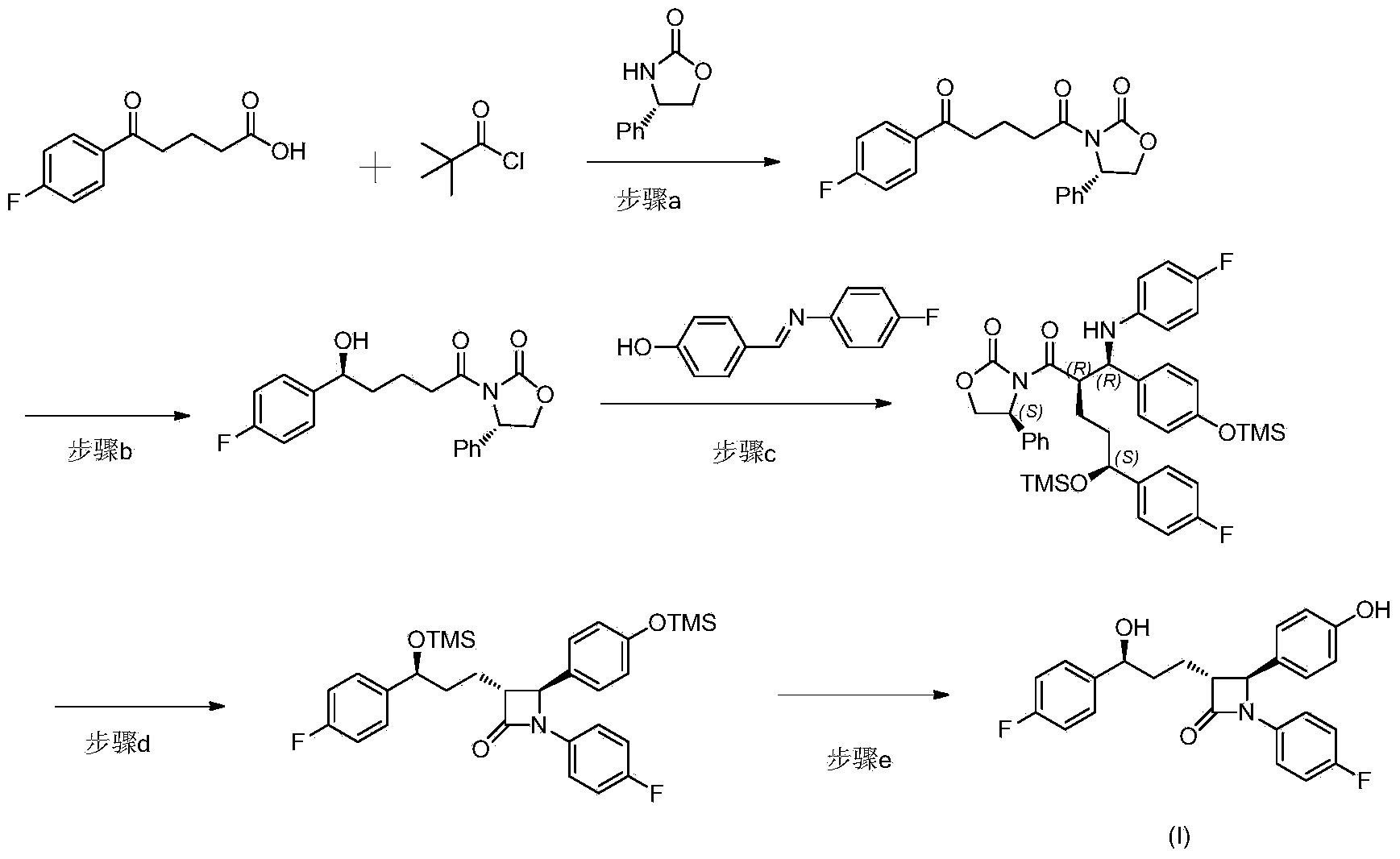
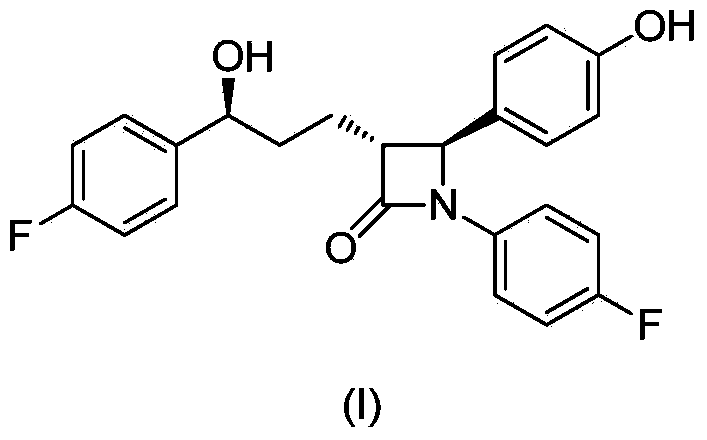
The invention provides a stereselective synthesis method for a lipid-lowering drug ezetimibe shown in formula I. The method comprises the following steps: a, P-fluorobenzoyl butyric acid shown in formula II reacts with a chiral auxiliary shown in formula III to obtain ketone shown in formula IV; b, under the existence of a chiral catalyst, the ketone shown in formula IV is reduced to chiral alcohol shown in formula V; c, chiral alcohol shown in formula V reacts with a silicyl protective agent to obtain a protected compound shown in formula VI, and then the compound shown in formula VI and imine shown in formula VII are subjected to addition and protecting groups are removed, so that a compound shown in formula VIII and a diastereomer thereof shown in formula IX are obtained, and through recrystallization with an appropriate solvent, an optically pure compound shown in formula VIII is obtained; d, the compound shown in formula VIII is protected with an acylation reagent, so that a compound shown in formula X is obtained, and amide shown in formula X is cyclized with a fluorinion catalyst, so that protected lactam shown in formula XI is obtained; then protecting groups are removed, and the ezetimibe shown in formula I is obtained.
Owner:SHANGHAI FANGNAN PHARMA
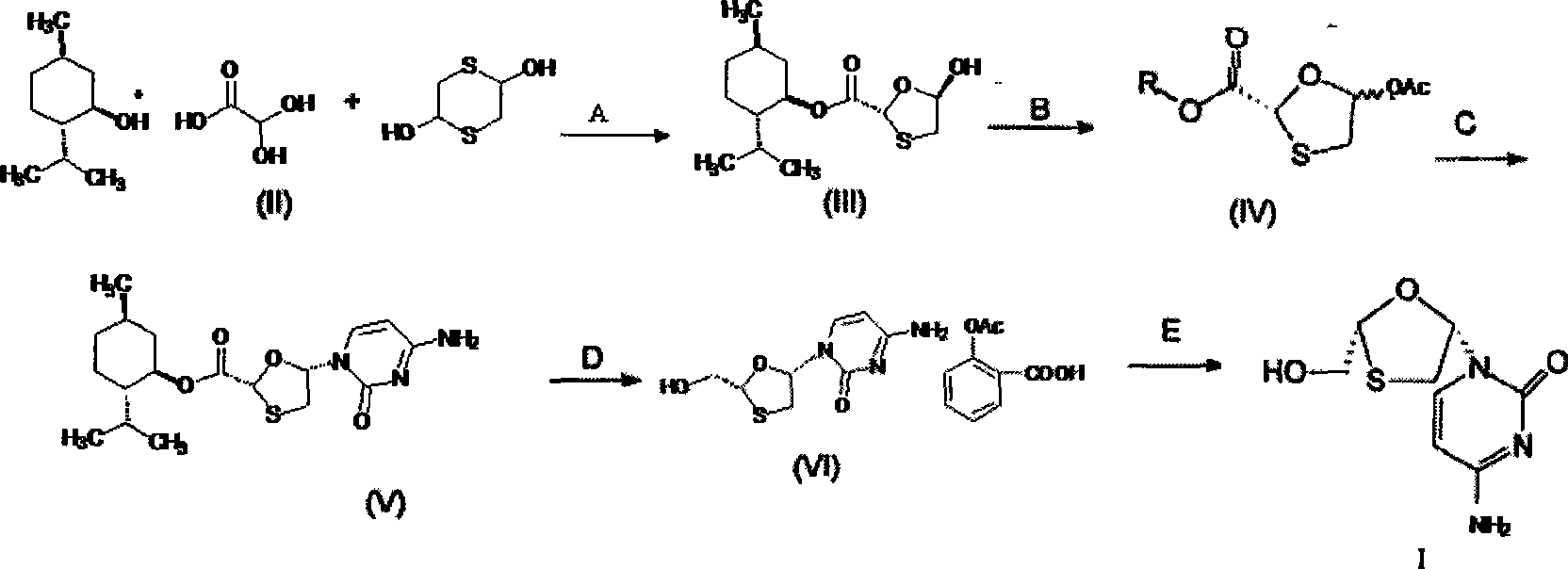
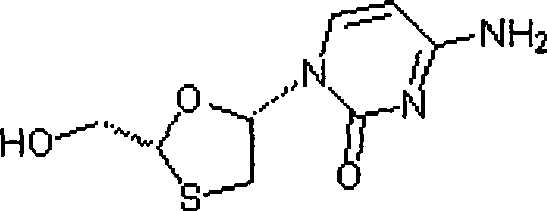
Lamivudine diastereoselective synthesis method
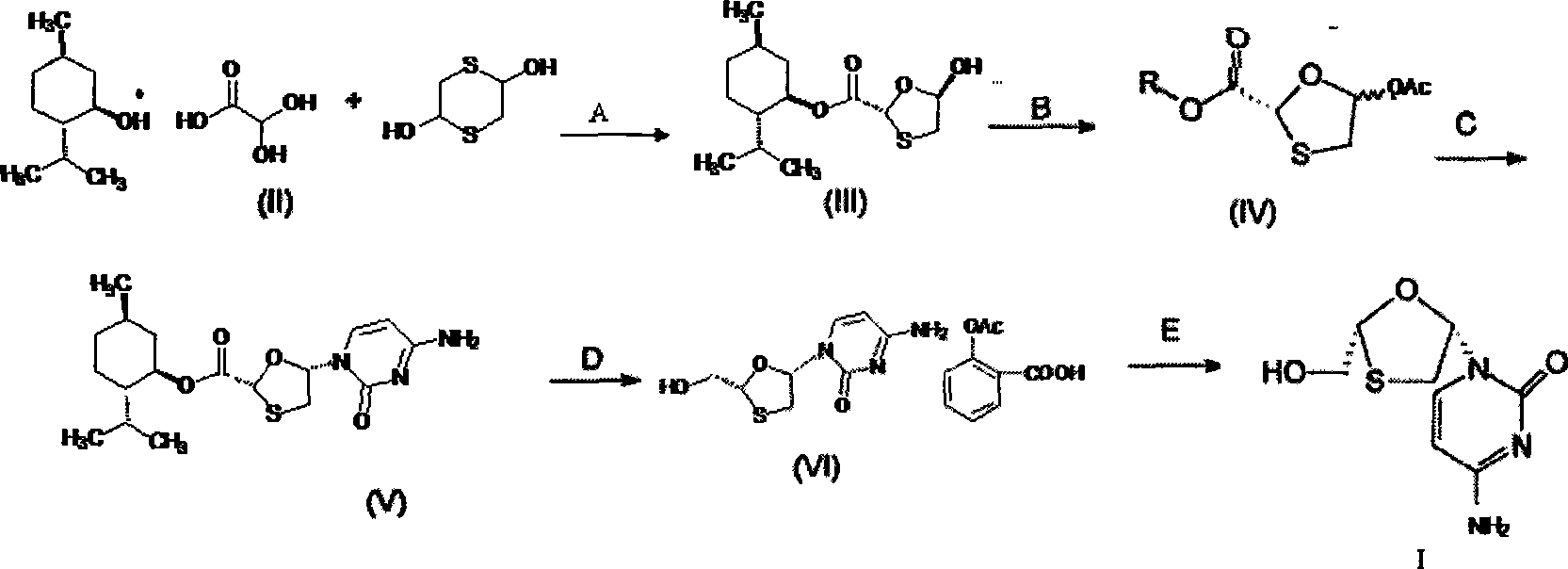
A lamivudine diastereoselective synthesis method, which takes chiral auxiliary agent L-menthol as the initial material, synthesizes trans-5-hydroxyl-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthol ester under the action of concentrated sulfuric acid, choose triethanolamine to obtain trans-isomer trans-5-hydroxyl-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthol ester, and let the trans-isomer to react with acylating agent to obtain trans-5-acetoxy-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthol ester, glycosidate with cytosine under the action of alkali to obtain 5S-cytosine-1'-radical-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthyl ester, and then deoxidize with a deoxidizer, and salifying with aspirin, to ionize and liberate lamivudine. Since triethanolamine is added as in the course of reaction interconverting agent, the yield of lamivudine is increased greatly. Aspirin is added in the course of reaction, so that the lamivudine forms an aspirin salt that has poor water solubility, and therefore can effectively separate and liberate lamivudine from the medium.
Owner:湖南千金湘江药业股份有限公司
Chiral auxiliaries
ActiveUS10428019B2High yieldSugar derivativesSugar derivatives preparationPhosphorus atomStereochemistry
The disclosed and claimed compounds are chiral auxiliaries useful for efficiently producing a phosphorus atom-modified nucleic acid derivative with high stereoregularity. The disclosed and claimed compounds include those represented by the following formula (I) or formula (XI) for introducing the chiral auxiliaries.
Owner:WAVE LIFE SCI LTD
Preparation method of tamsulosin
InactiveCN101284807ALow costRaw materials are easy to getSulfonic acid amide preparationChemical productsSubstituted phenethylamine
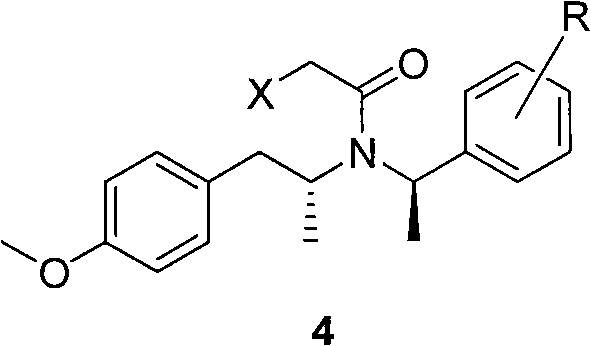
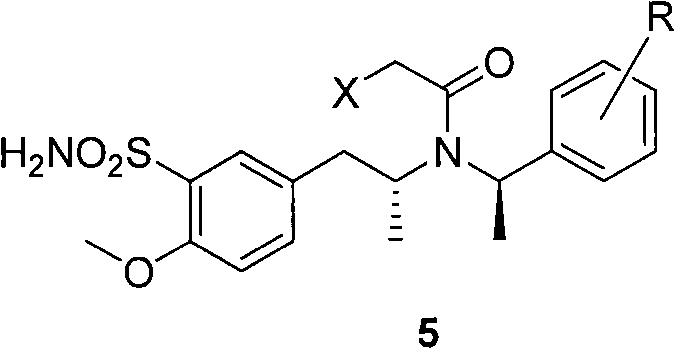
The invention discloses a method for making tamsulosin, which takes methoxyphenylacetone as the initial raw material and (R)-1-phenethylamine or substituted phenethylamine as chiral auxiliary reagent, and obtains the final raw material drug, namely tamsulosin through diastereoselective reductive amination, salifying, haloacetylization, halosulfonation, amination, alkylation, acylamide reduction and debenzylation. The method for making the tamsulosin has the advantages that: the method has low cost and easy-obtaining raw materials, each reaction is suitable for industrial production, and the obtained chemical product has high purity.
Owner:2Y CHEM
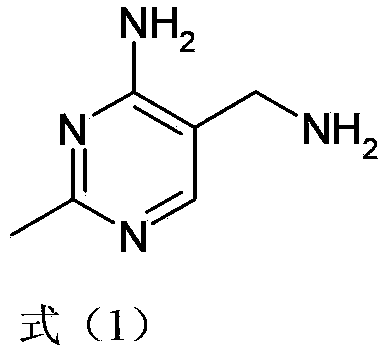
Simple and convenient preparation method of key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for vitamin B1
The invention relates to a simple and convenient preparation method of a key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for the vitamin B1. According to the method, lewis acid is used for catalyzing addition condensation reaction of acetamidine and 3-formyl amino ethyl cyanide and then catalyzing reaction of the condensation product and triethyl orthoformate to introduce a formyl chiral auxiliary, an imdo group and the formyl chiral auxiliary are subjected to ring formation to form 2- methyl-4-formyl amino-5- amino methyl pyrimidine, and finally basic hydrolysis is performed, so that 2-methyl-4-amino-5-amino methyl pyrimidine is obtained. The reaction procedures are carried out sequentially by adopting the one-pot method, the products in each step require no separation and purification, and the operation is simple and convenient. According to the method, highly toxic o-chloroaniline and other phenylamine compounds are not used, so that residue of o-chloroaniline compounds in the vitamin B1 product can be completely avoided. Meanwhile, the production wastewater is less and the yield is high.
Owner:XINFA PHARMA
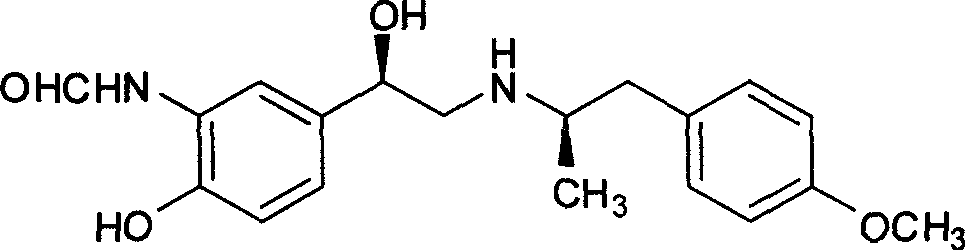
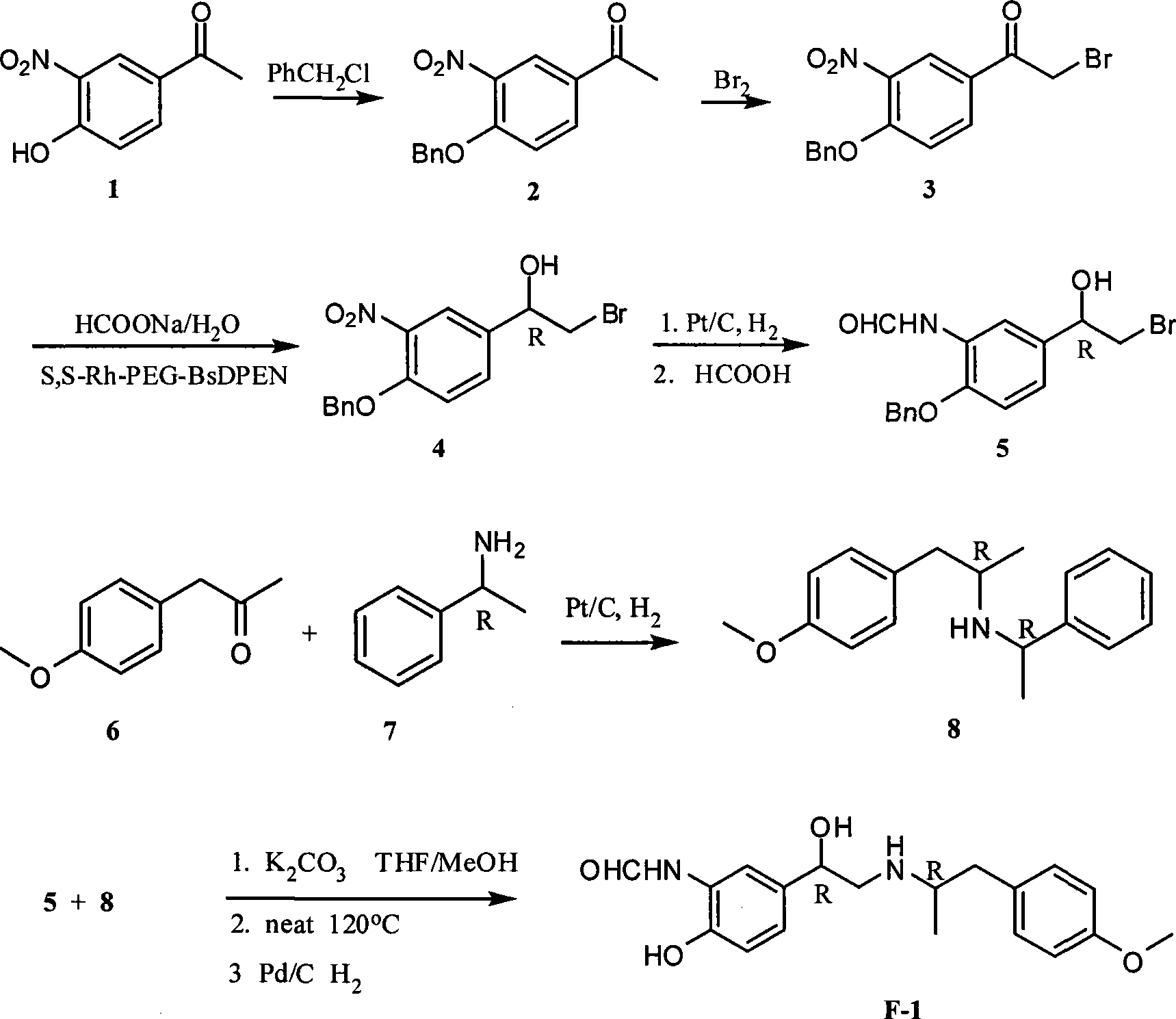
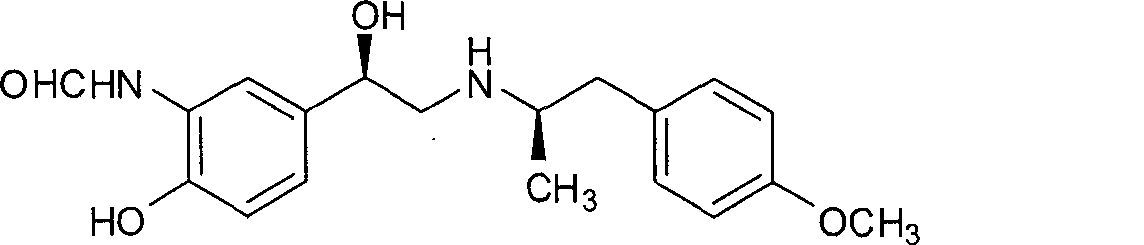
Unsymmetrical hydrogen migration synthesizing method for (R, R)-formoterol
InactiveCN101468954AIn line with the concept of green chemistryLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
The invention relates to an asymmetric hydrogen transfer synthesis method for (R,R)-formoterol, and relates to a novel method for synthesizing an optical pure beta 2-adrenoreceptor excitant, namely formoterol. The method comprises: firstly, taking 4-hydroxyl-3 nitroacetophenone as a raw material, using benzyl groups to protect phenolic hydroxyl groups, and obtaining alpha-bromo keto after bromination; secondly, taking (S,S)-Rh-PEG-BsDPEN as a catalyst and formic acid and derivatives of the formic acid as hydrogen sources, and synthesizing chiral alcohol intermediate by an asymmetric hydrogen transfer method; thirdly, using (R)-alpha-methyl phenylethylamine and methoxyl phenylacetone to generate imine compounds, and obtaining chiral amine intermediate through hydrogenation reduction under the catalysis of Pt / C; and fourthly, reacting and coupling the chiral alcohol intermediate and the chiral amine intermediate, removing protective groups, and obtaining the (R,R)-formoterol. The invention uses the asymmetric hydrogen transfer method and a chiral auxiliary reagent to synthesize the (R,R)-formoterol, and has high yield and good ee value. Compared with a method for synthesizing chiral formoterol through chemical splitting, the method has the advantages of high total yield, mild reaction conditions, low cost and so on, and is favorable for industrial production.
Owner:SUN YAT SEN UNIV
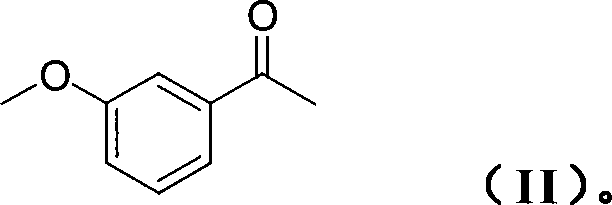
Technique for producing rivastigmine hydrogen tartrate
InactiveCN101239934AAvoid huge lossesLow production costCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsRivastigmine
The invention discloses a production process of rivastigmine, comprising using m-methoxy hypnone as raw material, adding chiral auxiliary, reducing and aminating through an asymmetry synthesis method to obtain chiral amine, then demethylatig and acylating to prepare rivastigmine, and finally reacting rivastigmine with tartaric acid to obtain rivastigmine. The invention has advantages of simple operation, low cost, high yield, small pollution, and is suitable for industrialisation production.
Owner:苏州凯达生物医药技术有限公司
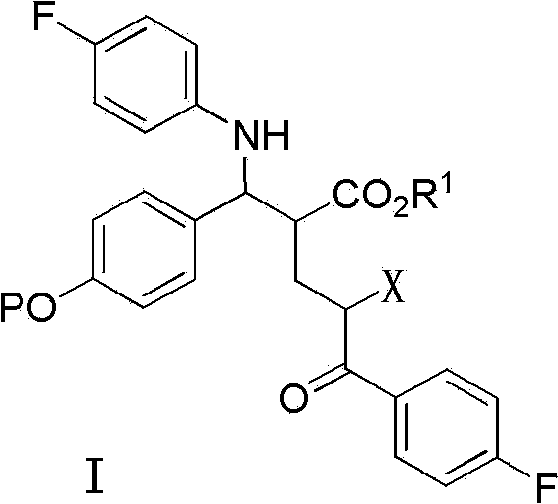
Preparation midbody for Ezetimibe and preparation method of preparation midbody
ActiveCN104230978AEasy to manufactureGroup 4/14 element organic compoundsBulk chemical productionChlorobenzeneAcyl group
The invention provides a preparation midbody for Ezetimibe which is shown in a general formula I, wherein PG is an acetyl group, a T-butyloxycarbonyl group, a benzyl group, a benzyloxycarbonyl group, a trityl group, a trimethylsilyl group or a diphenylmethyl group silicon group. The invention further provides a preparation method of the midbody I, and the application for preparing the medicine Ezetimibe. The method for preparing the Ezetimibe by adopting the compounds shown in the general formula I is different from the method in the conventional document, the newer chirality assistant (S)-4-(2-chlorphenyl)-2-oxazolone is adopted, the productive rate reaches 91%, and the optical purity reaches 100%, so that the productive rate and the optical purity are higher than those of the previously applied (S)-4-phenyl-2-oxazolone. Besides, the selected chirality assistant (S)-4-(2-chlorphenyl)-2- oxazolone can be conveniently prepared by the original commercialized ((S)-2-chlorobenzene glycine potassium).
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
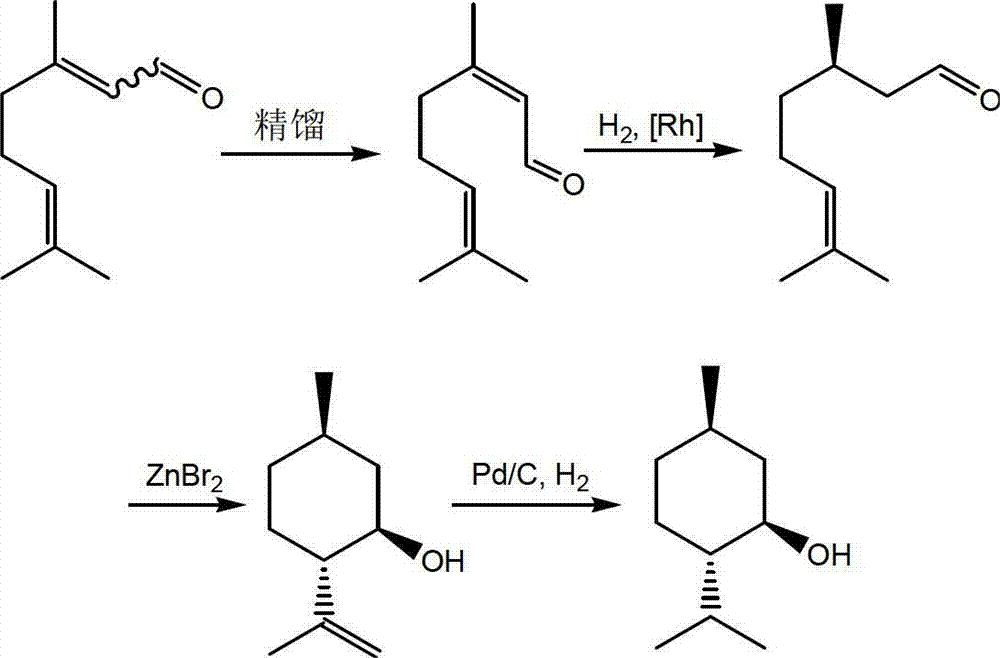
Asymmetric synthesis method of dextral citronellal
InactiveCN103724170AEasy to makeMild reaction conditionsOrganic compound preparationCarbonyl compound preparationHydrogenDihydropyridine
The invention discloses an asymmetric synthesis method of dextral citronellal. The method takes citral as a starting material, dihydropyridine derivative as a negative hydrogen source, and chiral amine salt as a chiral auxiliary agent, and asymmetric hydrogenation reaction is generated in a bi-catalytic system to synthesize dextral citronellal. By adopting the synthesis method disclosed by the invention for the synthesis of dextral citronellal, the total yield is more than 85%, and the ee (enantiomeric excess) value is greater than 80%; the synthesis method has the characteristics of mild reaction conditions, simple synthesis process, simple catalyst preparation, convenience in recycling and the like, and is suitable for industrial production of dextral citronellal.
Owner:GUANGDONG FOOD IND INST +1
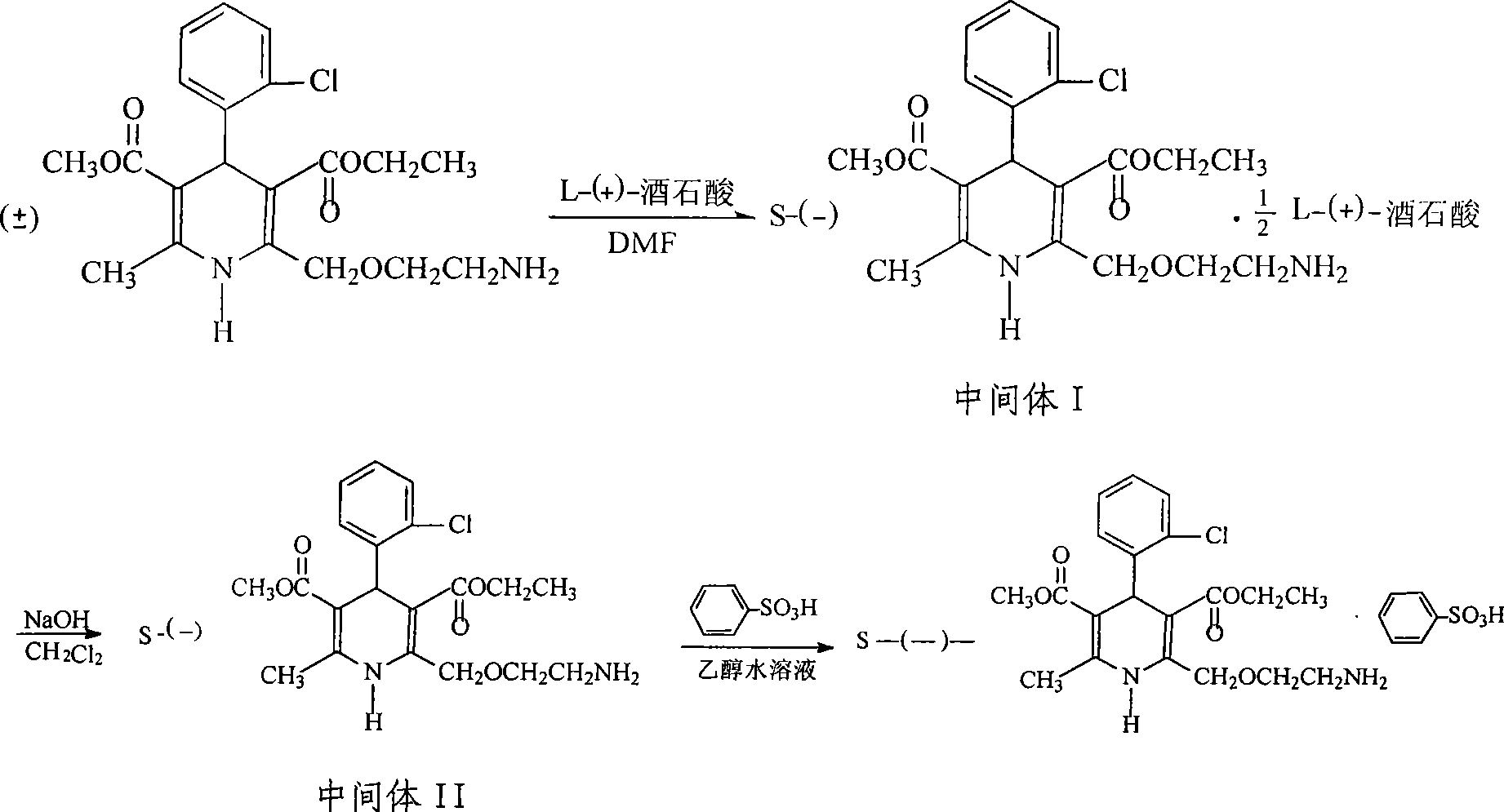
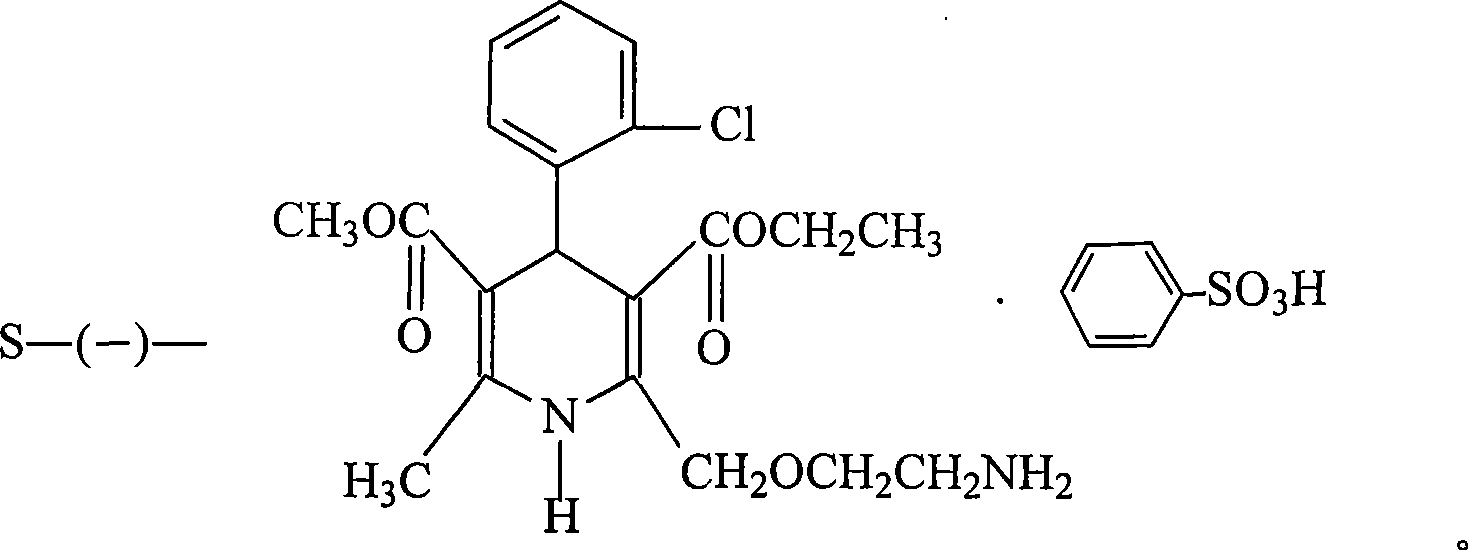
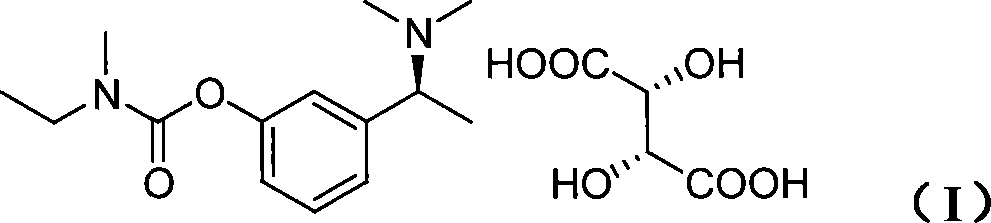
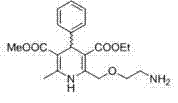
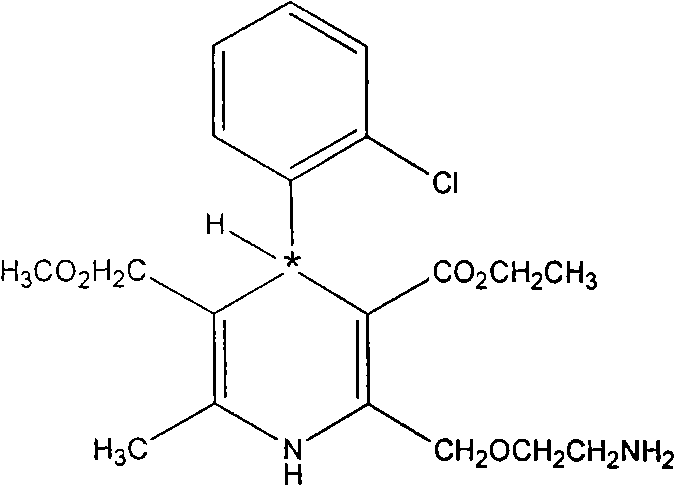
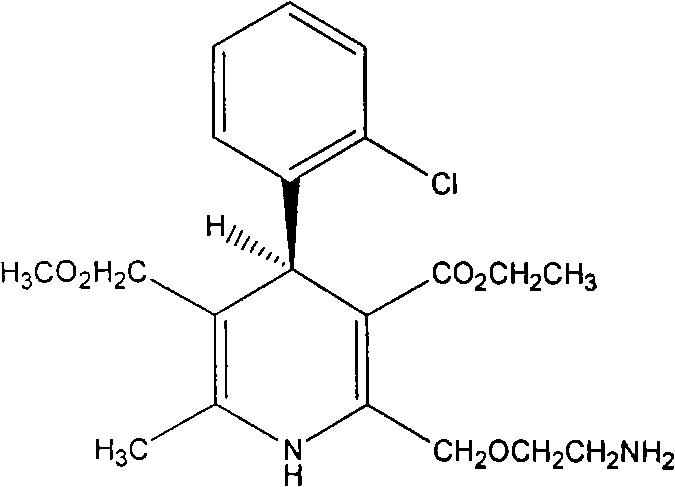
Method for producing S-(-)-amlodipine besylate
The invention provides a method for producing S-(-)-amlodipine besylate. The method comprises the following steps: 1, reacting R,S-amlodipine with the structural formula represented by formula I and a resolving agent which are treated as raw materials in a mixed solution of water and an organic solvent to obtain an intermediate 1; and 2, directly reacting the intermediate 1 with benzenesulfonic acid in the mixed solution of water and the organic solvent to obtain the S-(-)-amlodipine besylate, wherein the resolving agent in step 1 is selected from L-tartrate or D-tartrate. The method of the invention, which adopts cheap tartrate as the resolving agent and certain proportions of water and the organic solvent as chiral assistants, allows the optical purity of the obtained S-(-)-amlodipine besylate to reach 99.9%, the S-(-)-amlodipine besylate to be obtained by directly reacting the intermediate 1 with benzenesulfonic acid without hydrolysis, and the yield and the crystal form to be verygood, so the method has a good industrial application prospect.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
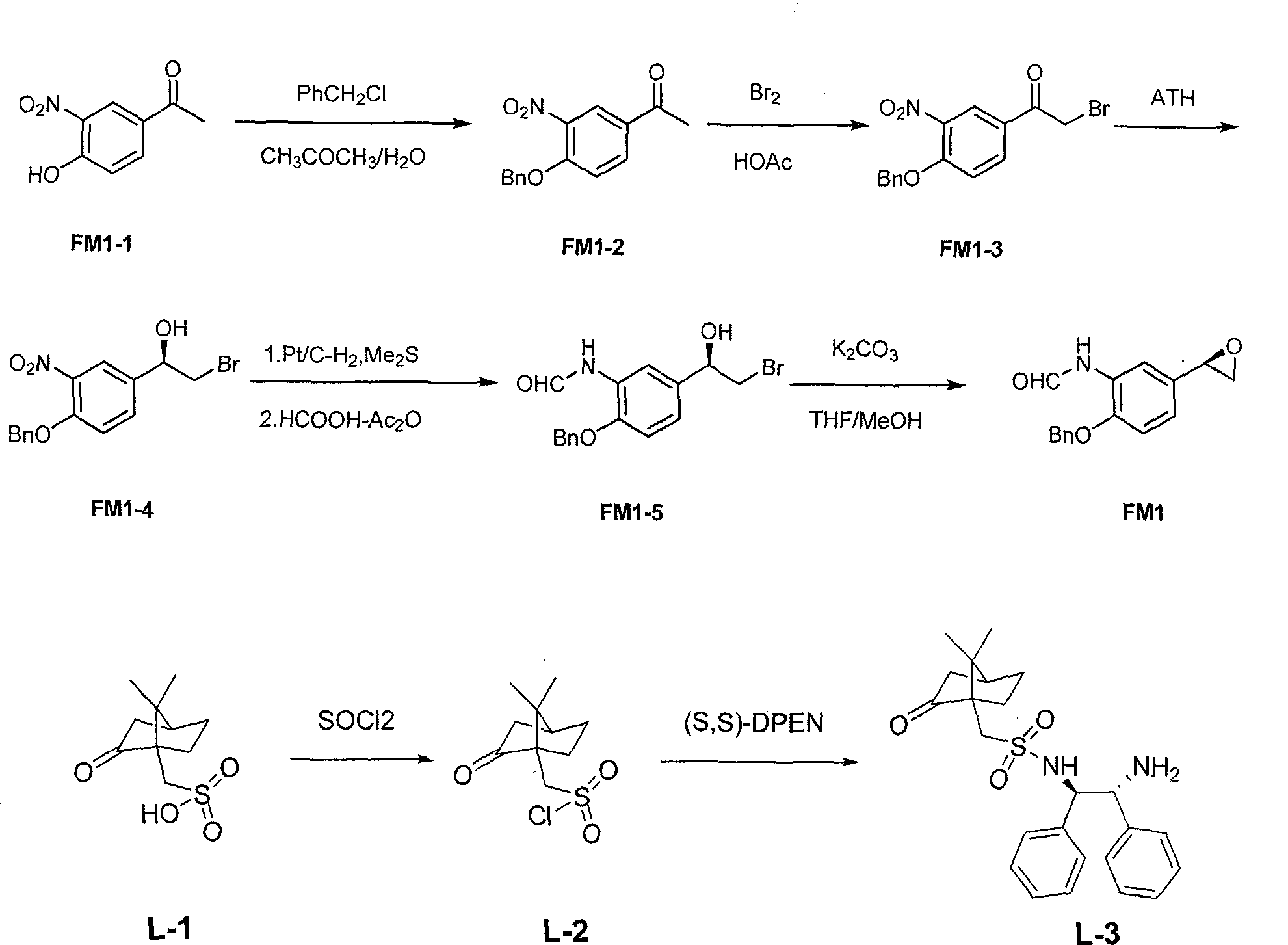
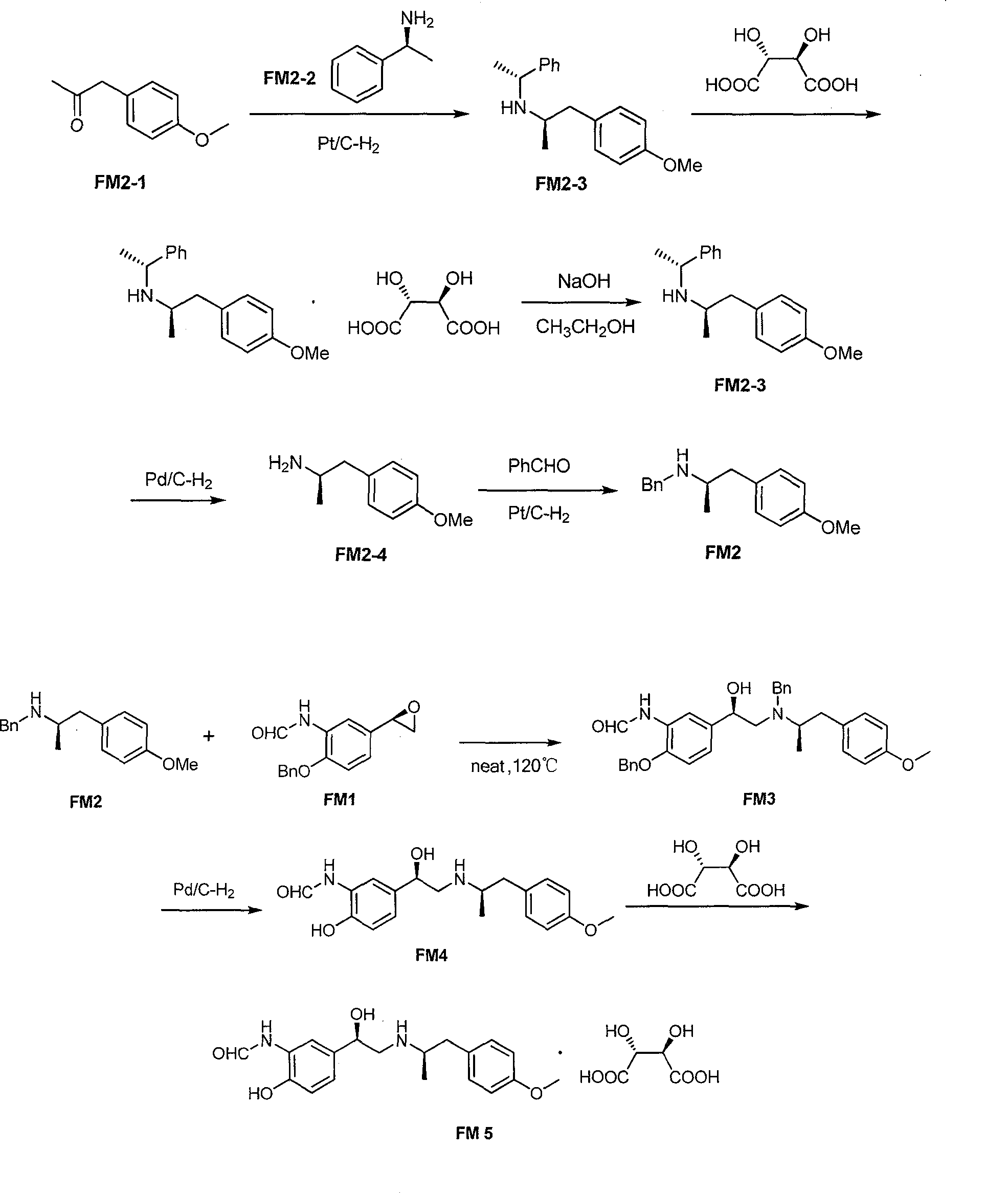
Asymmetric synthesis method of (R,R)-formoterol tartrate
InactiveCN103664677AHigh yieldHigh enantioselectivityCarboxylic acid amides optical isomer preparationCarboxylic acid salt preparationSynthesis methodsBenzaldehyde
The invention relates to an asymmetric synthesis method of (R,R)-formoterol tartrate, which comprises the following steps: by taking (S,S)-CsDPEN and transition metal complex as a catalyst, performing asymmetric hydrogen transfer reaction on alpha-bromoketone used as a raw material, thus obtaining a chiral alcohol intermediate compound; performing reaction steps of nitro-reduction, formylation, cyclization and the like, thus obtaining a key intermediate compound FM 1; by taking Pt / C as a catalyst and alpha-methylphenylethylamine as a chiral assistant, synthesizing an intermediate compound FM 2-3; performing tartaric acid salification, ionization and alpha-methylphenethyl removal, and reacting with benzaldehyde, thus preparing a chiral amine intermediate compound FM 2; reacting and coupling the two key intermediate compounds, and performing protective group removal to obtain (R,R)-formoterol FM 4; and performing tartaric acid salification on the FM 4, thus preparing the target product (R,R)-formoterol tartrate FM 5. According to the invention, the (R,R)-formoterol is synthesized through an asymmetric hydrogen transfer method by means of the chiral assistant, and high yield and favorable ee value are achieved. Compared with a chemical resolution method for synthesizing chiral formoterol, the method provided by the invention has the advantages of high overall yield, mild reaction conditions, low cost and the like, thereby being beneficial to industrial production.
Owner:SUN YAT SEN UNIV +1
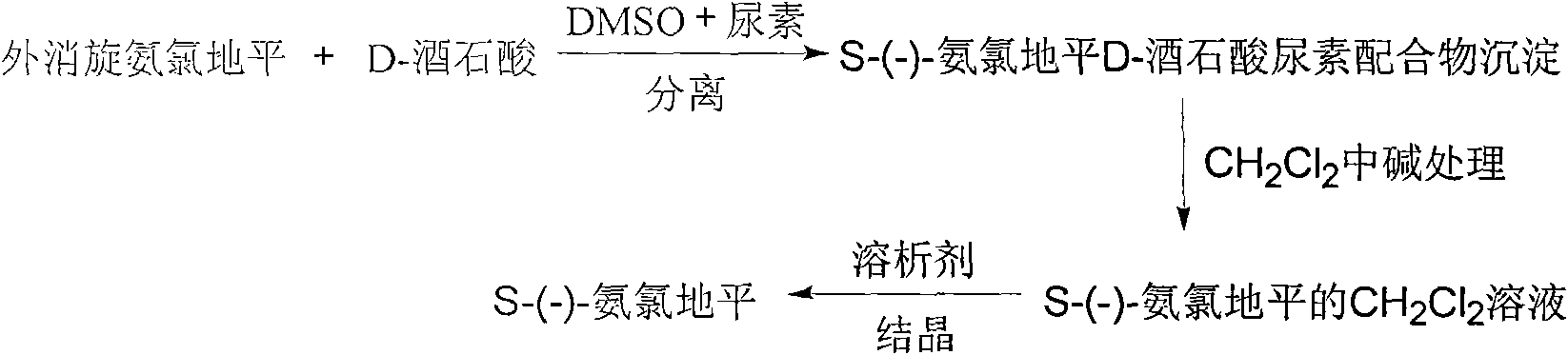
Method for obtaining S-(-)-amlodipine in splitting manner
ActiveCN101654429AMoisture content no special requirementsShort reaction timeOptically-active compound separationOrganic racemisationEnantiomerLevamlodipine
The invention relates to a method for obtaining S-(-)-amlodipine in a splitting manner. The method comprises the following steps: dissolving racemic amlodipine and D-tartaric acid into a mixed cosolvent containg dimethylsulfoxide and urea to carry out a complex reaction, carrying out alkaline treatment, solvent-out crystallization and the like on the S-(-)-amlodipine, the D-tartaric acid and the urea complexes solid sedimentation obtained after the complex reaction is finished, and then obtaining S-(-)-amlodipine pure crystal. The method introduces the other chiral auxiliary reagent-urea in the prior dimethylsulfoxide solution, thus the racemic amlodipine can better react with a splitting agent D-tartaric acid in the cosolvent containing the dimethylsulfoxide and urea, the reaction time islargely shortened, and the reaction does not have special requirements on the water content of the used solvent; the enantiomeric purity of the obtained levamlodipine is over than 99% and the rate ofrecovery is over than 80%. The method can be applied to preparing the intermediate of other chiral medicaments basically meeting the standard of the medical industry, is simple and provides a betterprospect for the preparation of various agents by the amlodipine single enantiomer.
Owner:JIANGXI SHIMEI PHARM CO LTD
Medetomidine industrial splitting method
The invention discloses a medetomidine industrial splitting method which takes L-(-) camphorsulfonic acid as a chiral reagent and C1-C5 alcohol as a chiral auxiliary. According to the method disclosed by the invention, the splitting yield reaches up to over 40% and the splitting optical purity reaches over 99.5%. The method is simple in industrialized operation and can be used for industrialized and commercialized production.
Owner:安庆生命科技园发展有限公司
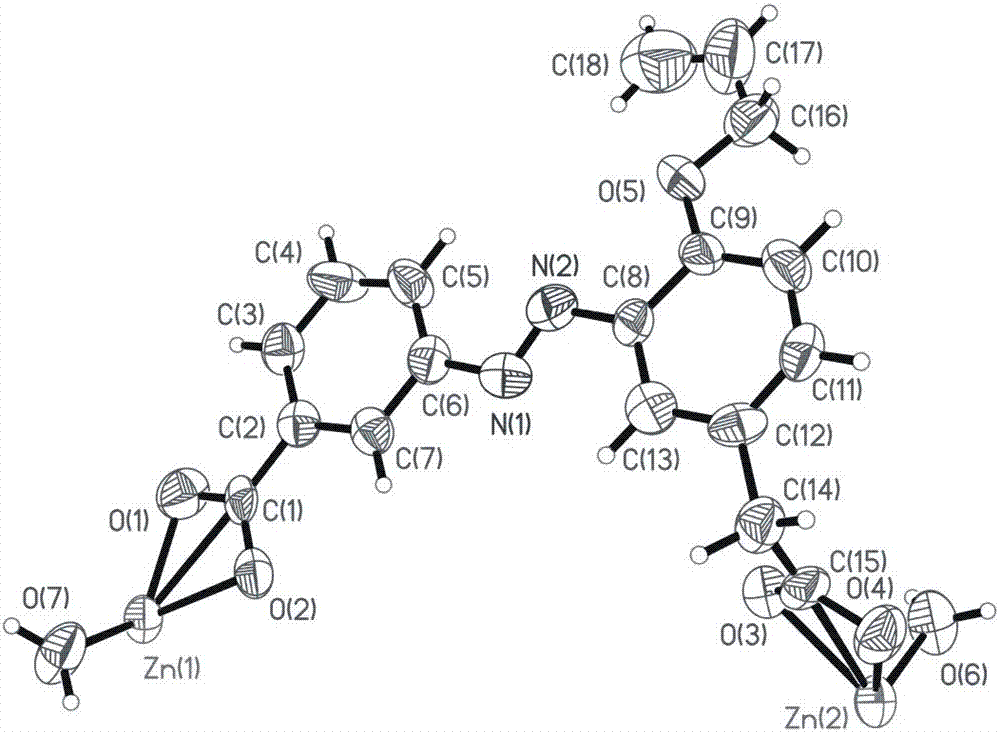
Chiral coordination polymer crystal and preparation method thereof
The present invention discloses a chiral coordination polymer crystal and a preparation method thereof, (1) the chiral coordination polymer crystal has the characteristics of simple preparation, and single chiral structure; (2) ligand 3-(3-carboxylic)-4-allyloxy-azo phenylacetic acid exists in general in a trans stable configuration, and has a special structure, a specific spiral mode is used for coordination reaction in the coordination reaction, a coordination polymer can be obtained by the specific spiral mode under normal temperature liquid phase conditions from the ligand and a metal ion, and preferably, when the metal ion is Zn<2+>, a single chiral crystal structure can be obtained; (3) chiral auxiliaries and chiral ligands are not needed in the preparation method of the chiral coordination polymer crystal, a single chiral spiral can be realized by lattice orientation special selectivity, and the single chiral crystal can be formed, and has potential application in efficient chiral separation and chiral recognition and other aspects.
Owner:SHANDONG UNIV OF SCI & TECH
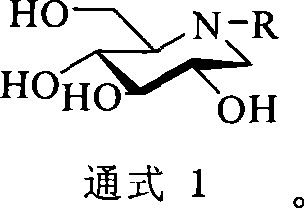
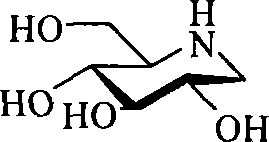
1-deoxidization nojiri toxin derivant, production method and uses thereof
The invention discloses a 1-deoxidized nojiri toxin derivative and a relative preparation method, which uses natural cheap D-glucose as material to synthesize dicarbonyl-D-glucose, and processes enamine double-reduction reaction on the dicarbonyl-D-glucose and amine, to obtain 1-deoxidized nojiri toxin derivative with high yield and spatial selectivity. The derivative can be used as intermediate and chiral auxiliary of organic synthesis or drug synthesis, to chirally synthesize organic or drug. The invention further discloses a full synthesis process of 1-deoxidized nojiri toxin derivative, with spatial control, simple operation, mild condition, high yield and industrialization suitability.
Owner:ZHENGZHOU UNIV
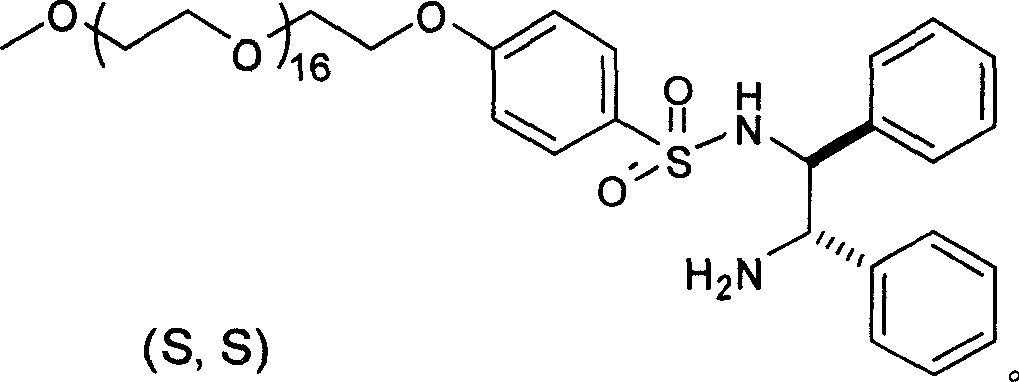
Method for synthesizing vilanterol intermediate and salt thereof
The invention provides a method for preparing a vilanterol intermediate (formula V) and a salt (formula VII) thereof, belonging to the field of chemical drug synthesis. The method comprises the steps of carrying out ring opening reaction on an epoxy compound 2,2-dimethyl-6-ethylene oxide-4H-benzo[d][1,3]dioxane and an amine chiral auxiliary to prepare a chiral compound V; then, separating the compound V from a mixture in a way of forming a crystal salt with an acid. A reagent used in the method provided by the invention is cheap and easily available, and hypertoxic chiral oxazaborolidine is prevented from being used, so that the cost is reduced, and the environment pollution is reduced.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
Linear polystyrene-supported (4S)-oxazolidine-2-benzimine as well as preparation method and application thereof
InactiveCN101914174ARealize recyclingHigh optical purityOrganic compound preparationCarboxylic acid amides preparationPolystyreneOxazolidine
The invention relates to linear polystyrene-supported (4S)-oxazolidine-2-benzimine which has the following structural formula, wherein m:n=1:1-4, and Mw=8300-14000. In the invention, (4S)-oxazolidine-2-benzimine is supported on linear polystyrene to prepare the linear polystyrene-supported (4S)-oxazolidine-2-benzimine as a chiral auxiliary reagent to induce asymmetric reaction into homogeneous reaction, and the reaction is rapid and convenient for on-line detection. The chiral auxiliary reagent not only reserves the high yield and the high stereoselectivity of the asymmetric reaction induced by the oxazolidine-2-benzimine as the chiral auxiliary reagent to obtain a chiral compound with high optical purity, bus also realize the recycling of the (4S)-oxazolidine-2-benzimine. The synthesized chiral compound with high optical purity is used as the precursor, the intermediate and the final product of medicines, agricultural chemicals, spices and functional materials.
Owner:HUBEI UNIV
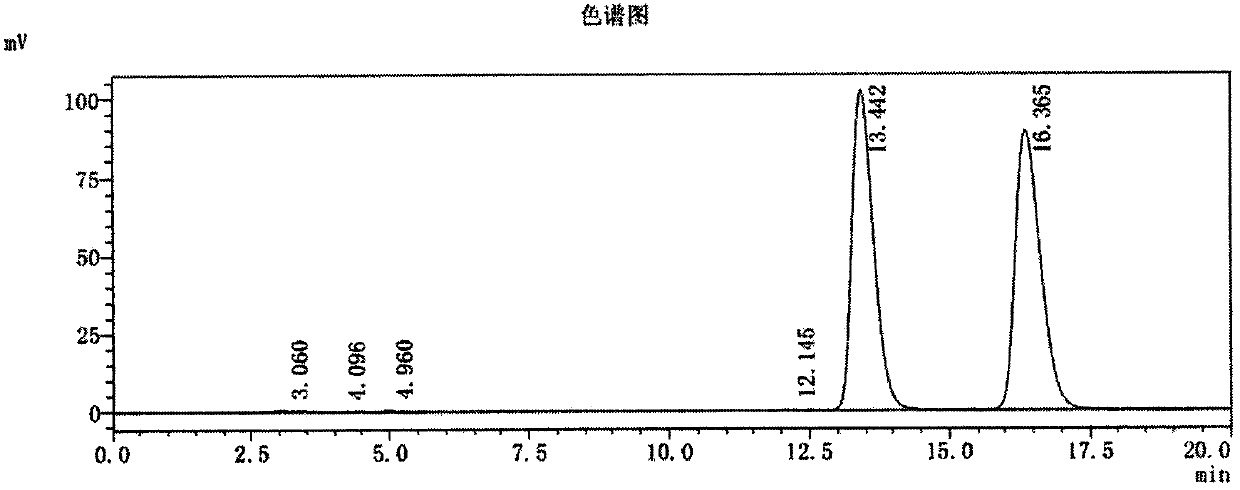
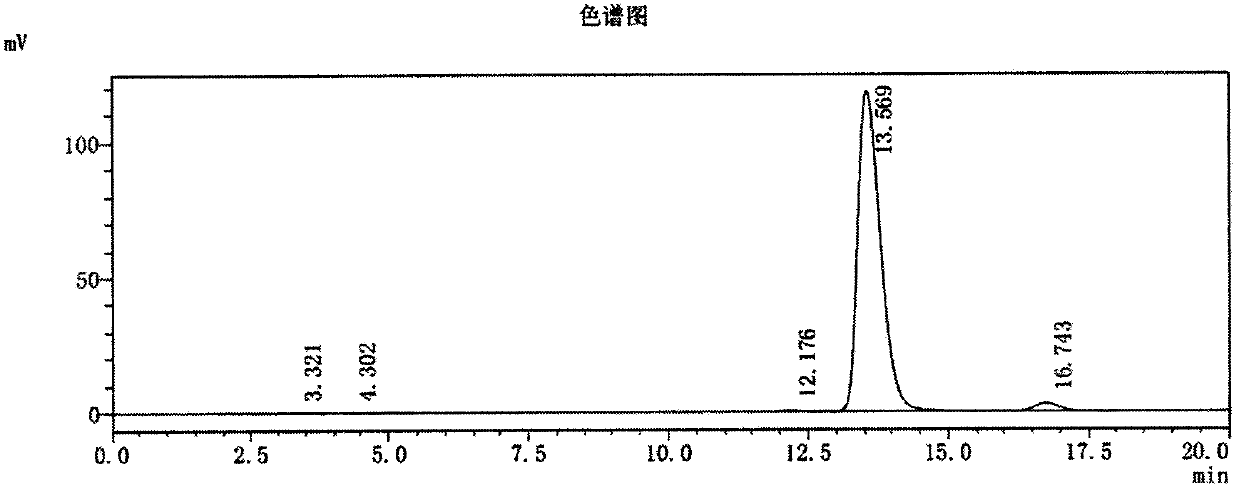
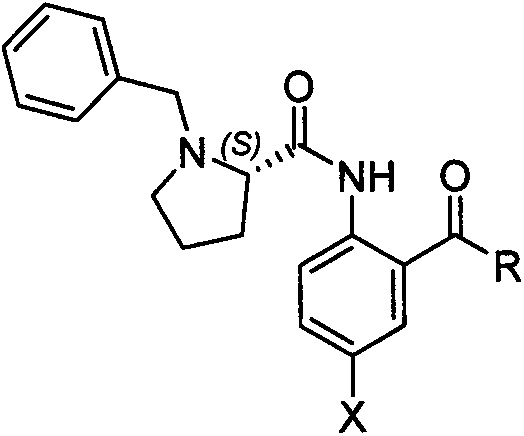
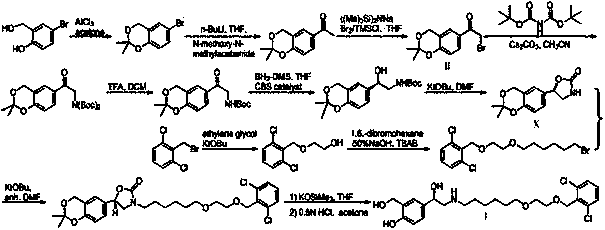
Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane
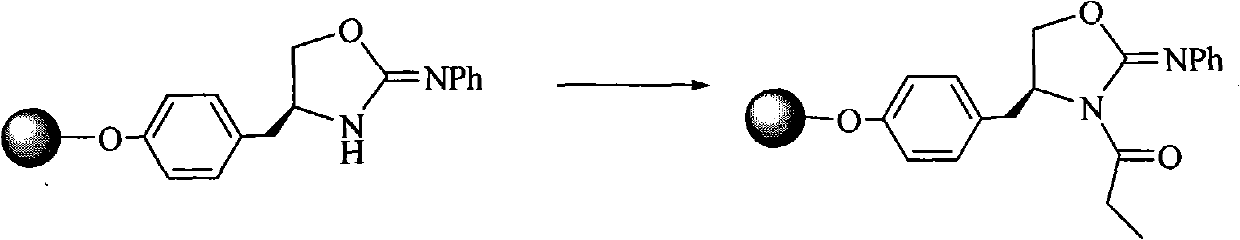
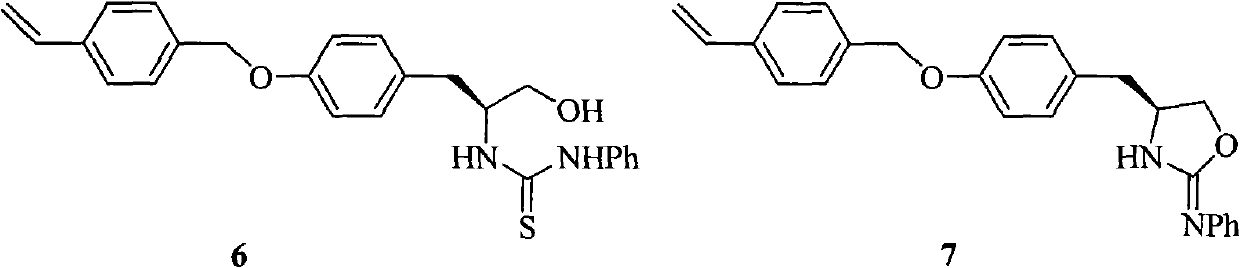
ActiveCN107793414ASimple process routeHigh yieldOrganic chemistryBulk chemical productionHydrogen atomNitrogen
The invention discloses a method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane. The method comprises the following steps of: (a) adopting a compound which is shown in a formula V and comprisesa chiral auxiliary group and an amino protecting group as a raw material, and performing an intramolecular cyclization reaction to obtain a compound shown in a formula VI; (b) removing the chiral auxiliary group and amino protecting group from the compound shown in the formula V so as to obtain a compound shown in a formula VII, wherein when X is a hydrogen atom, the compound shown in the formulaVII is (S,S)-2,8-diazabicyclo[4.3.0]nonane; and (c) when X is an oxygen atom, performing a reduction reaction on the compound shown in the formula VII by using amide so as to obtain (S,S)-2 ,8-diazabicyclo[4.3.0]nonane.
Owner:SHANGYU JINGXIN PHARMA +1
Preparation method of L-glufosinate-ammonium
The invention discloses a preparation method of L-glufosinate-ammonium, and the method comprises the following steps: by using DL-glufosinate-ammonium as a starting raw material, complexing the DL-glufosinate-ammonium with a chiral auxiliary group and metal ions under the action of an inorganic alkali to form a coordination compound, and inversing the configuration of D-glufosinate-ammonium in theprocess to generate the L-glufosinate-ammonium. And by hydrolyzing the coordination compound, the L-glufosinate-ammonium is obtained, and meanwhile the chiral auxiliary group and the metal salt are recovered. The method is simple in process route, high in yield, low in cost and suitable for industrial production of the L-glufosinate-ammonium.
Owner:CHINA PHARM UNIV
Synthetic method of ezetimibe, and intermediate used in synthetic method
ActiveCN103570574AReduce usageHigh activityGroup 4/14 element organic compoundsIndium organic compoundsEzetimibeCarboxylic acid
The invention discloses a synthetic method of ezetimibe, and an intermediate used in the synthetic method. According to the synthetic method, allyl amination of Morita-Baylis-Hillman adducts is performed so as to realized production of a chiral beta-aromatic amido alpha-methylene carboxylic acid derivative with high activity and selectivity, and the chiral ezetimibe synthetic intermediate is obtained via simple conversion reaction; and chiral medicament ezetimibe can be synthesized further. According to the synthetic method, chirality of ezetimibe is realized via chiral catalysis, so that the use of chiral auxiliary base oxazolidinone is avoided, cost is low, and the synthetic method is friendly to the environment.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
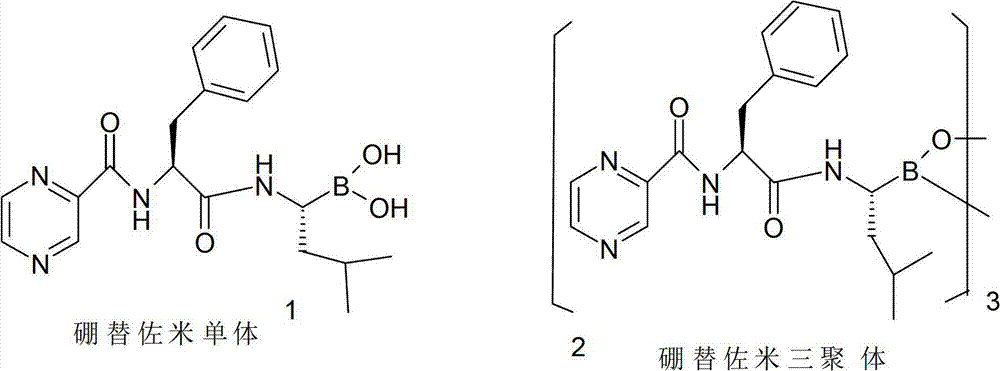
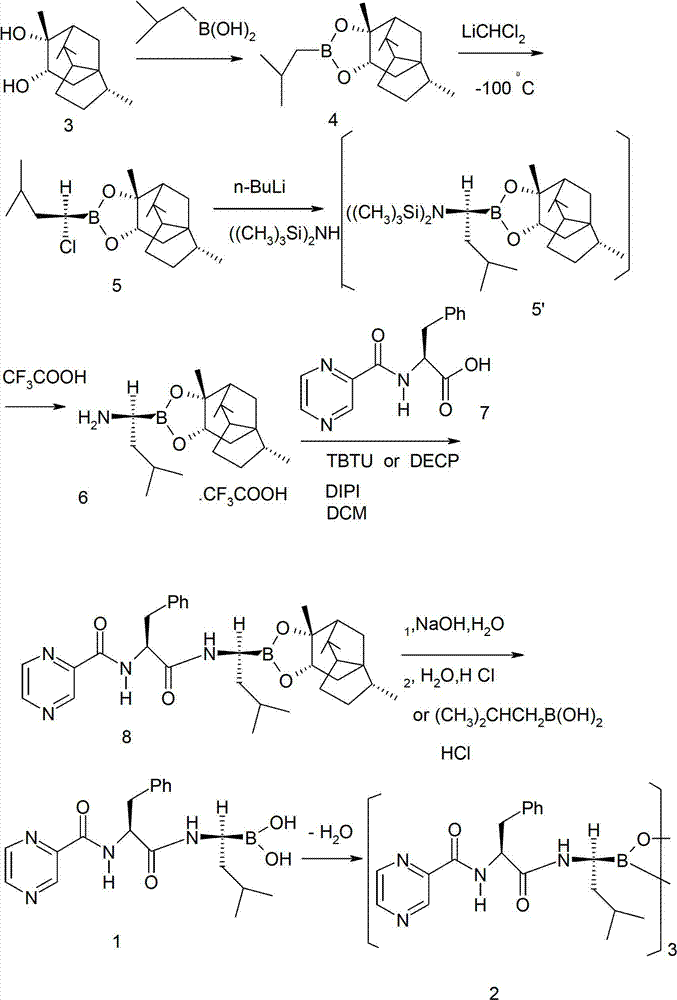
Method for preparing bortezomib with (one)-cypress camphor serving as chiral auxiliary reagent
InactiveCN102898501AEasy to recycleReduce manufacturing costPeptidesBulk chemical productionBoronic acidPhenylalanine
The invention discloses a method for preparing bortezomib with (one)-cypress camphor serving as a chiral auxiliary reagent. The technological process includes that the (one)-cypress camphor and boronic acids in an organic solvent form boronic acid-(one)-cypress camphor esters; the boronic acid-(one)-cypress camphor esters are condensed and rearranged at a low temperature to obtain 2-chloro isoamyl boronic acid-(one)-cypress camphor esters; the 2-chloro isoamyl boronic acid-(one)-cypress camphor esters are aminated by lithium bis (trimethylsilyl) amide and removed protecting groups to obtain (one)-cypress camphor-(R)-1-amino-3-isobutyl-borate trifluoroacetic acid salt; the (one)-cypress camphor-(R)-1-amino-3-isobutyl-borate trifluoroacetic acid salt and pyrazine formyl-L-phenylalanine react under the existence of a condensing agent to generate bortezomib-(one)-cypress camphor esters; and the bortezomib-(one)-cypress camphor esters are hydrolyzed to generate bortezomib monomers and further generate bortezomib tripolymers.
Owner:朱锦桃 +3
Method for preparing loxoprofen active metabolite
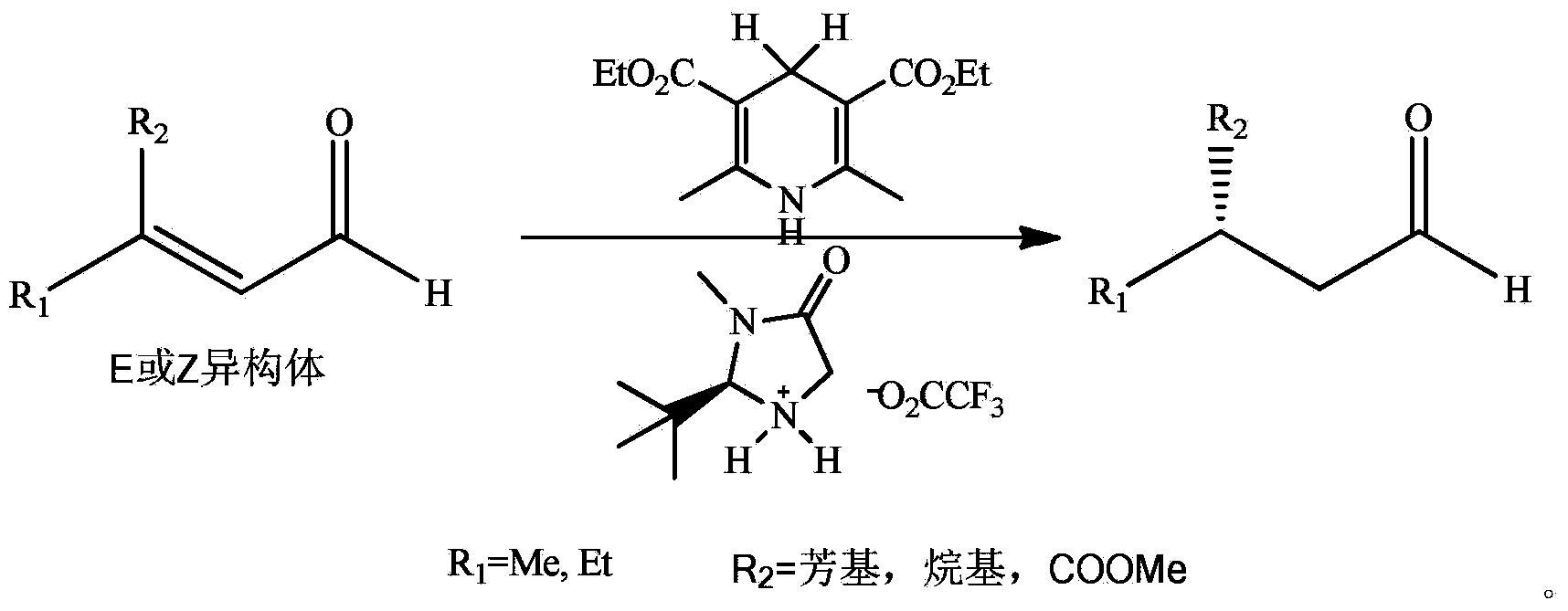
ActiveCN106045842ABeneficial technical effectBeneficial progressCarbamic acid derivatives preparationOrganic compound preparationCompound aPropanoic acid
The invention discloses a method for compounding a trans-hydroxyl active metabolite of loxoprofen. The method comprises the following steps: taking 2-[p(bromomethyl)phenyl]propionic acid as a raw material and carrying out resolution and methyl esterification, thus obtaining an intermediate, namely, a compound as shown in a formula 3; preparing a chiral assistant, namely, a compound as shown in a formula 7, by starting from L-phenylalaninol; firstly forming Schiff base as shown in a formula 9 by cyclopentanone and the chiral assistant, namely, the compound as shown in the formula 7, and then condensing the Schiff base as shown in the formula 9 and the intermediate, namely, the compound as shown in the formula 3, into an intermediate, namely, a compound as shown in a formula 11; carrying out acidic hydrolysis on the intermediate, namely, the compound as shown in the formula 11, and perfroming stereoselective reduction on cyclopentanone carbonyl groups, thus obtaining the trans-hydroxyl active metabolite, namely, a compound as shown in a formula TM. In a compounding path, raw materials can be easily obtained, the operation is convenient, environmental friendliness is realized, a separation means of column chromatography is prevented from being used, and the technical requirements of industrial large-scale production can be completely met.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD
Key intermediate of PARP inhibitor and preparation method thereof
InactiveCN108997313AEasy to operateMild conditionsOrganic chemistry methodsBulk chemical productionAlkyl transferPARP inhibitor
The invention discloses a preparation method of Niraprib of a PARP inhibitor and two key intermediates used in the preparation method. The preparation method comprises the following steps: taking 4-methyl alpha-bromophenylacetate and (S)-tert-butanesulfinyl amide as raw materials, and performing condensation, asymmetry alkylation, reduction and coupling on the raw materials, so as to obtain a keyintermediate compound 5; condensing and coupling the compound 5, so as to obtain a compound 6; hydrolyzing the compound 6 under the action of p-toluenesulfonic acid to remove a protecting group, so asto obtain a target product Niraprib. The preparation method has the beneficial effects that the conventional resolution and chiral source methods are overcome by introducing the asymmetry alkylationinduced chirality of a chiral auxiliary, so that the preparation method is simple in operation, mild in conditions, good in yield and relatively high in chemical purity and optical purity; in addition, key intermediates 5 and SM3 are condensed to form a target compound 1, so that the original patent is broken through, and the preparation method is suitable for industrial production.
Owner:CHANGZHOU PHARMA FACTORY
Process for preparing intermediates for synthesis of antifungal agents
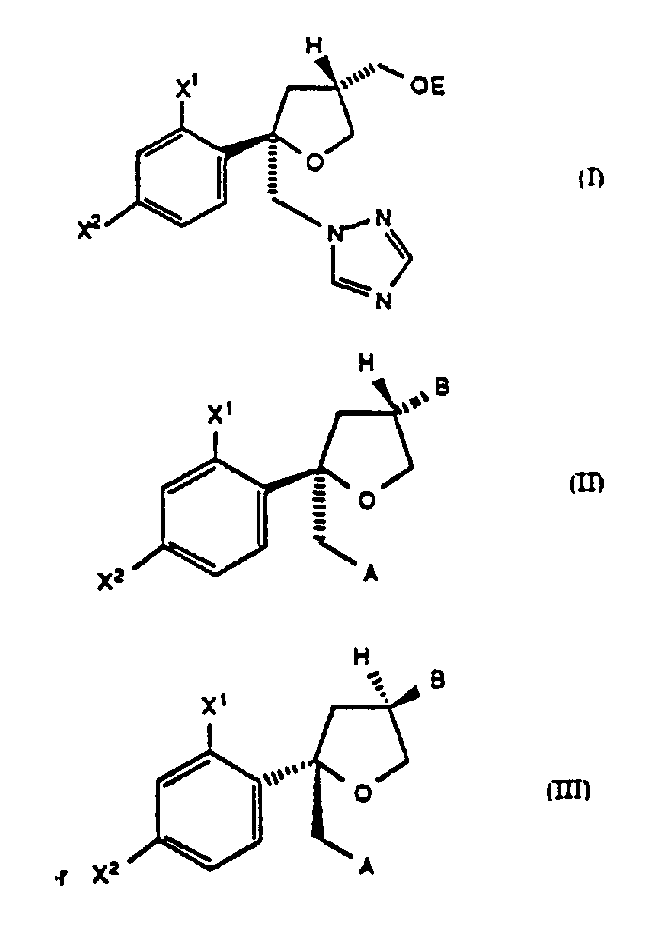
Disclosed is a process for preparing chiral compounds of formula (I) wherein: X<1> and X<2> are independently F or Cl; and E is -SO2R<2>, wherein R<2> is C1-C6 alkyl, -C6H4CH3 or -CF3; its enantiomer and racemates thereof, useful in the synthesis of tetrahydrofuran azole antifungals. Novel compounds of formula (II) or (III) wherein: X<1> and X<2> are independently F or Cl; B represents -C(O)Q* or -CH2OR'; Q* represents a chiral auxiliary group; R'' represents a hydroxy protecting group; and A represents Cl, Br, I, triazolyl or imidazolyl; are also disclosed.
Owner:SCHERING AG
Preparation method of Sitagliptin midbody of beta-amino acid
InactiveCN105968030AChiral raw materials are cheap and readily availableShort reaction pathCarbamic acid derivatives preparationOrganic compound preparationAlkaneAlcohol
The invention discloses a preparation method of a Sitagliptin midbody of beta-amino acid. The method comprises the following steps of 2,4,5-fluorobenzene acetaldehyde 2, L-benzedrine alcohol and tributyl allyl tin alkanes perform asymmetrical allylation reaction under the effect of a catalyst A to obtain a compound 3; the compound 3 is oxidized to remove chiral auxiliary radials to obtain a compound 4; the compound 4 is subjected to amino radial protection to obtain a compound 5; the compound 5 is subjected to double-bond oxidation to obtain beta-aminobutyric acid 1. The method provided by the invention has the advantages that the raw materials are cheap and can be easily obtained; the reaction route is short; the operation is simple and convenient; the reaction conditions are mild; no special requirements exist on the equipment; the yield is high; the selectivity is high; good industrial application and economic values are realized.
Owner:ZHEJIANG UNIV OF TECH
Optically pure (R)-4-n-propyl-dihydrofuran-2(3H)-one preparation method
The present invention relates to an optically pure (R)-4-n-propyl-dihydrofuran-2(3H)-one preparation method, wherein optically pure (S)-3-n-pentanoyl-4-substituted oxazole-2-one is used as a raw material, alkylation reaction with an olefin or alkyne reagent, reducing removal of a chiral auxiliary group, oxidation of double bond or triple bond and other steps are performed to prepare the opticallypure (R)-4-n-propyl-dihydrofuran-2(3H)-one. According to the present invention, the preparation method has characteristics of easily available raw material, low cost, high total yield, high optical purity of the obtained product, simple reaction conditions and simple operation process.
Owner:BEIJING ABLEPHARMTECH CO LTD
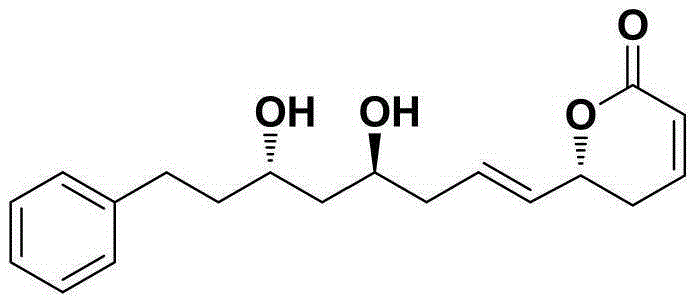
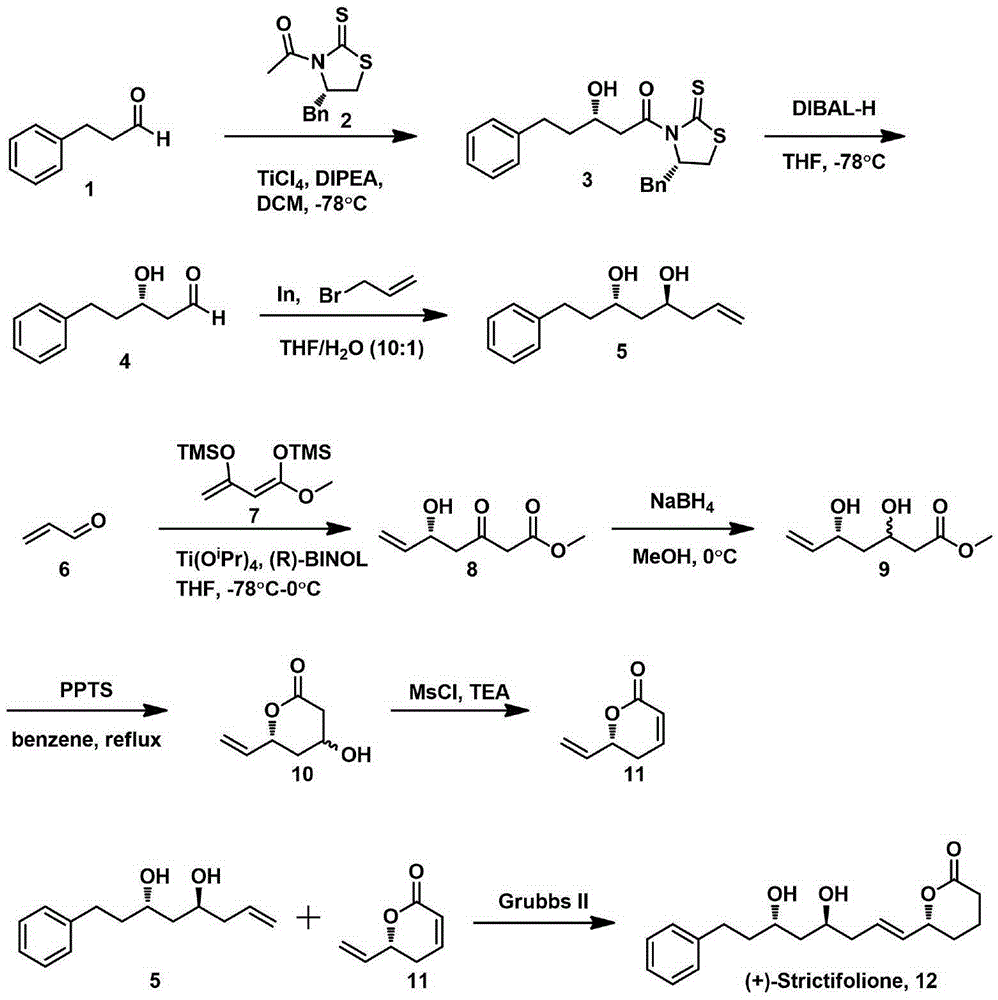
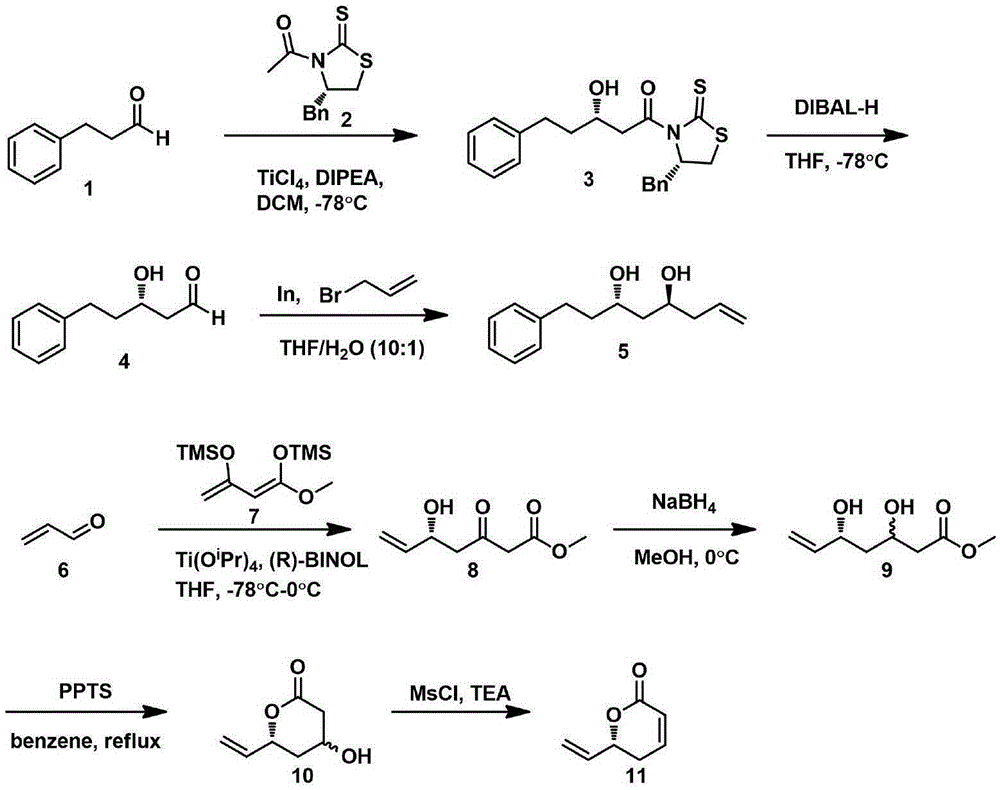
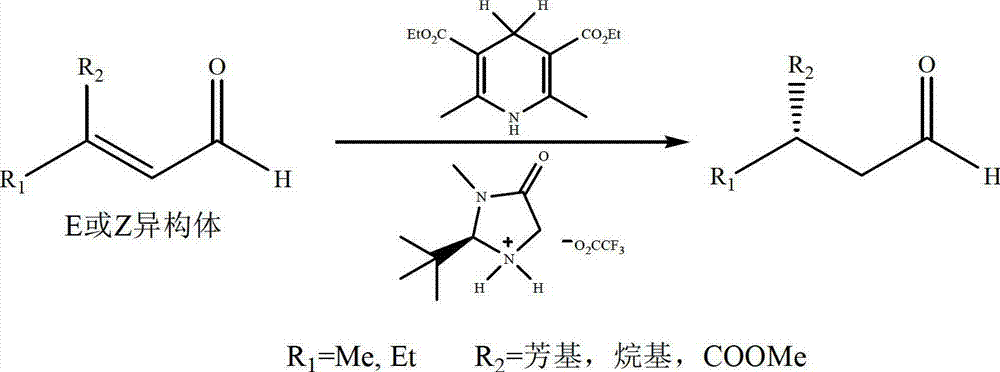
Natural product (+)-Strictifolione synthetic method
InactiveCN105254604ASimple and fast operationHigh yieldOrganic chemistryChemical recyclingIndiumStrictifolione
The invention discloses a natural product (+)-Strictifolione synthetic method. The method comprises the steps that 3-benzenepropanal and an Evans chiral auxiliary reagent are subjected to an aldol reaction for diisobutyl aluminum hydride reduction and then subjected to an addition reaction with metal indium activated 3-propylene bromine to obtain a compound shown in the formula 5; silyl enol ether reacts with acrolein under the action of titanium tetraisopropoxide and (R)-1,1-binaphthol, then carbonyl is reduced through sodium borohydride, p-toluenesulfonic acidpyridinium salt is used for cyclization, and acidification treatment is carried out after a methylsulfonyl group becomes a leaving group to obtain a compound shown in the formula 11; a Grubbs second-generation catalyst is used for carrying out a metal coupling reaction on the compounds shown in the formulas 5 and 11 to obtain (+)-Strictifolione. According to the bisection synthetic method, the design is simple and reasonable, operation is easy and convenient, the reaction condition is moderate, linear steps are simple, the product yield is high, and the production cost is greatly reduced.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
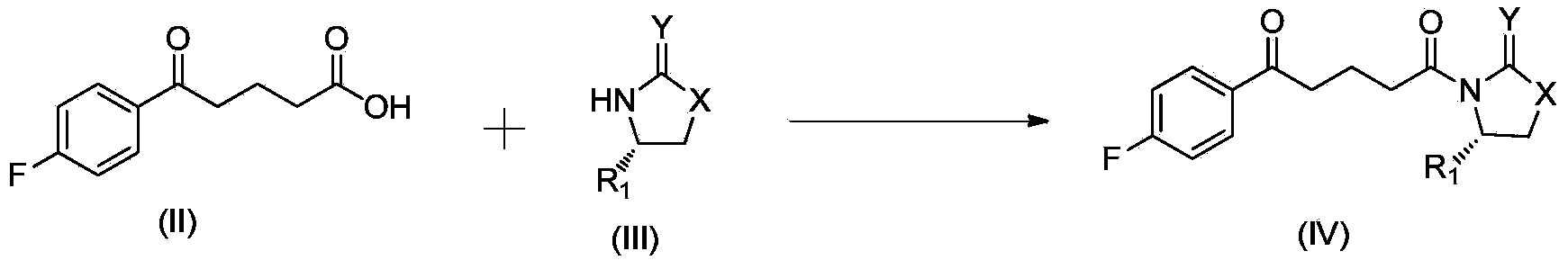
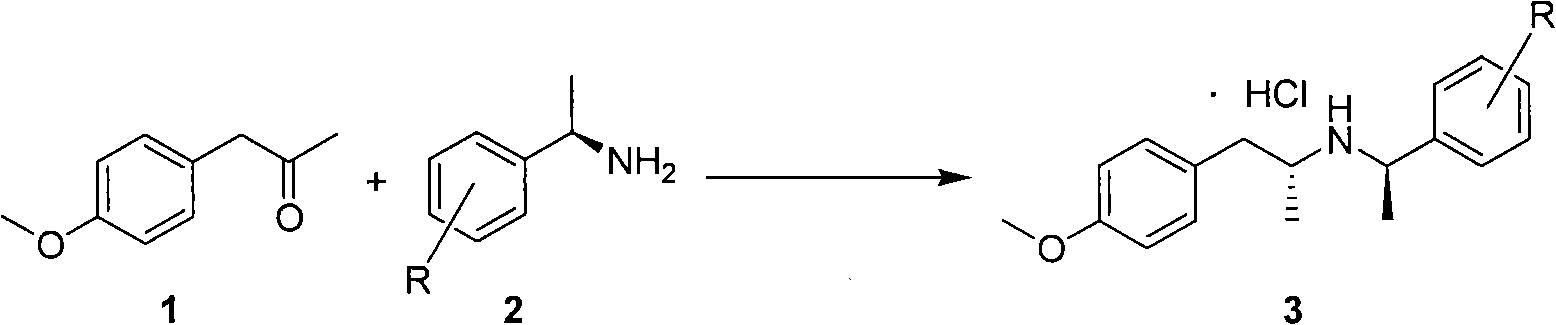
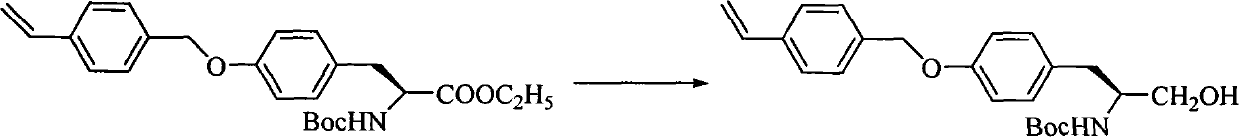
![Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane](https://images-eureka.patsnap.com/patent_img/274c98d1-31ba-4a2a-b4fc-e857a6b8911c/FDA0001110043610000011.png)
![Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane](https://images-eureka.patsnap.com/patent_img/274c98d1-31ba-4a2a-b4fc-e857a6b8911c/FDA0001110043610000012.png)
![Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane](https://images-eureka.patsnap.com/patent_img/274c98d1-31ba-4a2a-b4fc-e857a6b8911c/FDA0001110043610000021.png)