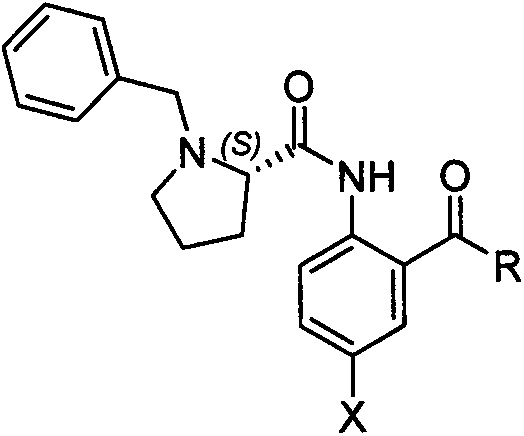
Preparation method of L-glufosinate-ammonium
A technology of glufosinate-ammonium and chiral prosthetic group, applied in the field of organic chemistry, can solve the problems of waste water generation, strict growth environment, and influence on enzyme activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
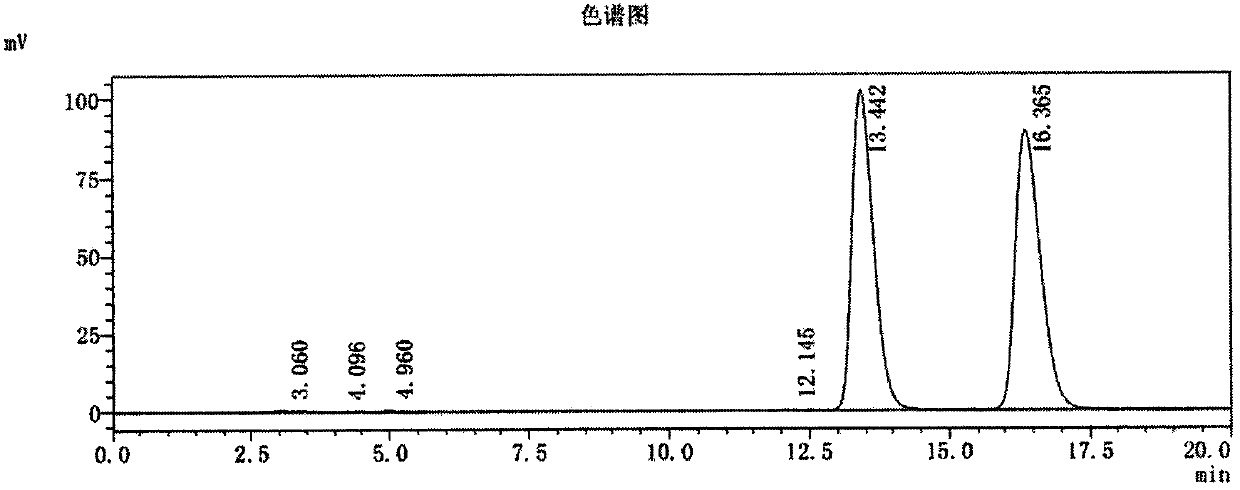
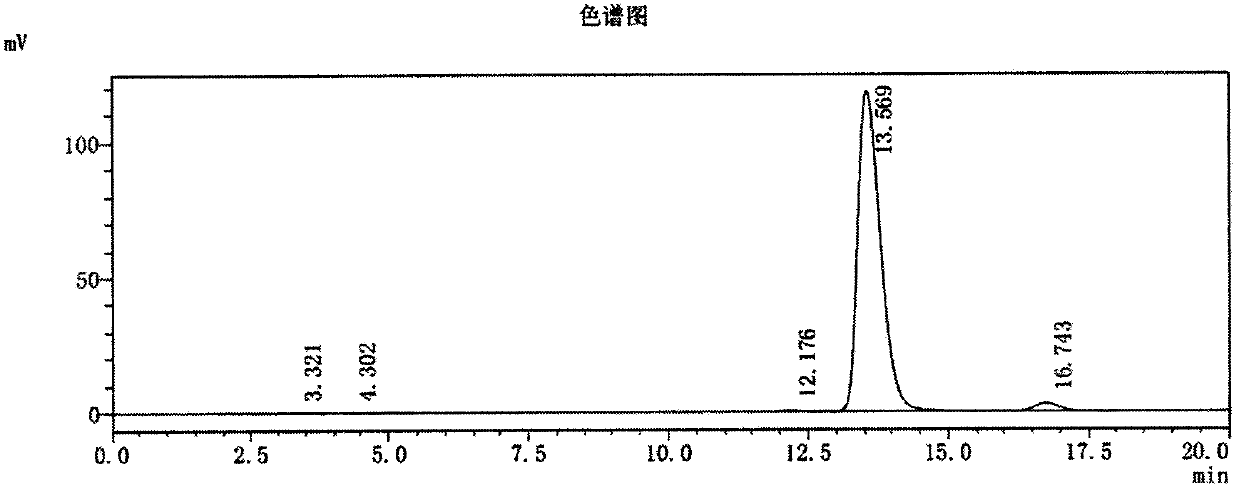
Image
Examples
Embodiment 1
[0017] Example 1: In a 250ml there-necked flask equipped with a thermometer and mechanical stirring, add 4.0 grams of sodium hydroxide, add 100 ml of methanol, 10.0 grams of DL-glufosinate-ammonium, 12.0 grams of nickel chloride hexahydrate, and 22.7 grams of chiral prosthetic groups, Slowly raise the temperature to 60°C for reaction. After the reaction, 60ml of methanol is distilled off under reduced pressure, the solid sodium hydroxide is filtered out, and the filtrate continues to concentrate to form a red solid. Add 30ml of 3M hydrochloric acid to the red solid, react at 100 degrees Celsius for 3 hours, the red color disappears, filter to obtain the chiral prosthetic group, dry and weigh 22.4 grams, dehydrate the filtrate, add 20ml of absolute ethanol, let it stand for 2 hours, and precipitate glufosinate-ammonium hydrochloride. The filtrate was concentrated to obtain 11.5 g of nickel chloride hexahydrate. Glufosinate-ammonium hydrochloride was dissolved in methanol, ammon...
Embodiment 2
[0018] Example 2: In a 250ml there-necked flask equipped with a thermometer and mechanical stirring, add 5.6 grams of potassium hydroxide, add 100 ml of methanol, 10.0 grams of DL-glufosinate-ammonium, 12.0 grams of nickel chloride hexahydrate, and 22.7 grams of chiral prosthetic groups, Slowly raise the temperature to 60°C for reaction. After the reaction is completed, 60ml of methanol is distilled off under reduced pressure, the potassium hydroxide solid is filtered out, and the filtrate continues to concentrate to form a red solid. Add 30ml of 3M hydrochloric acid to the red solid, react at 100 degrees Celsius for 3 hours, the red color disappears, filter to obtain the chiral prosthetic group, dry and weigh 22.2 grams, dehydrate the filtrate, add 20ml of absolute ethanol, let it stand for 2 hours, and precipitate glufosinate-ammonium hydrochloride. The filtrate was concentrated to obtain 11.6 g of nickel chloride hexahydrate. Dissolve glufosinate-ammonium hydrochloride in m...
Embodiment 3
[0019] Example 3: In a 250ml three-necked flask equipped with a thermometer and mechanical stirring, add 13.8 grams of potassium carbonate, 100 ml of acetonitrile, 10 grams of DL-glufosinate-ammonium, 13.1 grams of nickel sulfate hexahydrate, and 22.7 grams of chiral prosthetic groups, and slowly heat up Reaction at 60°C. After the reaction, 70ml of acetonitrile was distilled off under reduced pressure, potassium carbonate solid was filtered out, and the filtrate continued to concentrate to obtain a red solid. Add 20ml of 3M sulfuric acid to the red solid, react at 100 degrees Celsius for 3 hours, the red color disappears, filter to obtain the chiral prosthetic group, dry and weigh 22.2 grams, dehydrate the filtrate, add 20ml of absolute ethanol, let it stand for 2 hours, and precipitate glufosinate-ammonium hydrochloride. The filtrate was concentrated to obtain 12.8 g of nickel sulfate hexahydrate. Glufosinate-ammonium hydrochloride was dissolved in methanol, ammonia gas was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com