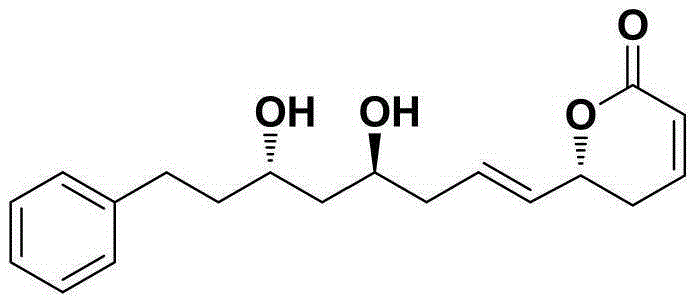
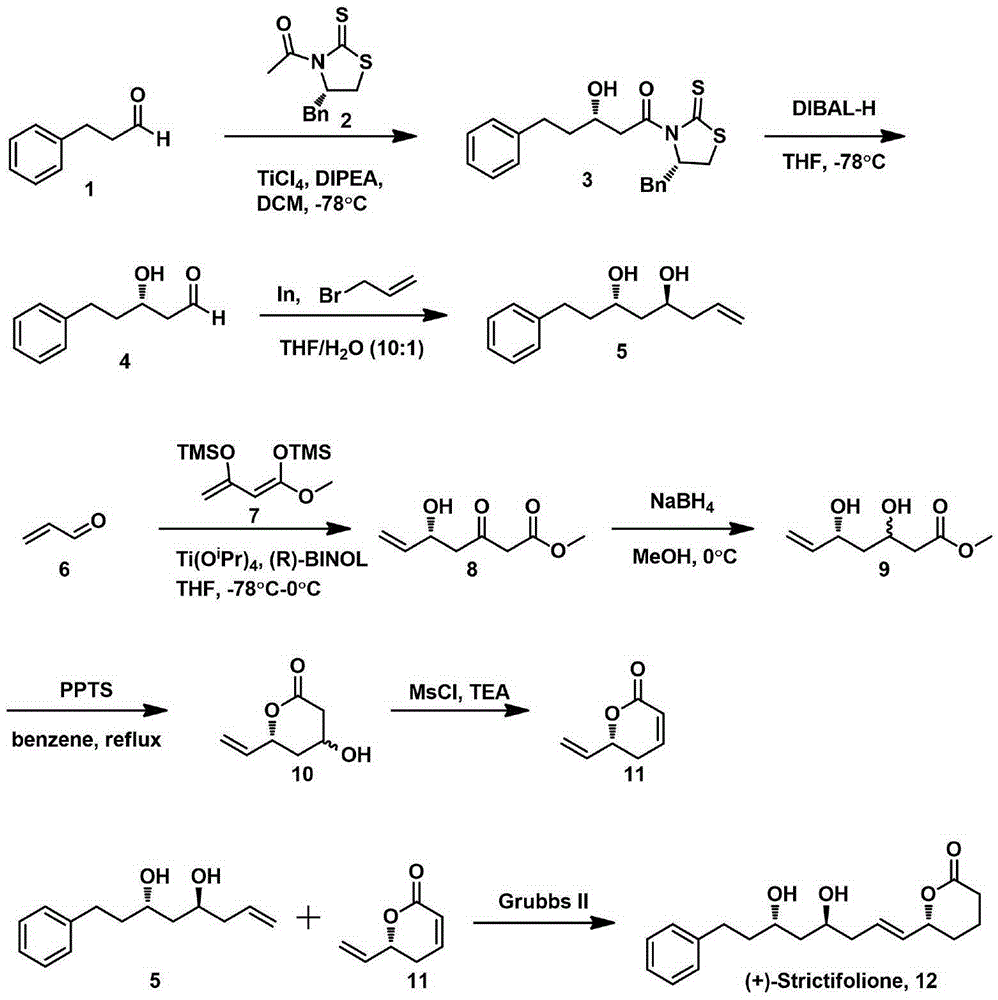
Natural product (+)-Strictifolione synthetic method
A technology of natural products and synthetic methods, applied in chemical recovery, organic chemistry and other directions, can solve the problems of high synthesis cost and long route, and achieve the effects of simple operation, good reaction selectivity and lower production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Under the protection of nitrogen, dissolve the Evans chiral auxiliary (0.47g, 1.87mmol) of formula 2 in dichloromethane (20mL), cool down to -78°C and slowly add titanium tetrachloride (TiCl 4 ) (0.21mL, 1.91mmol); 5 minutes later, N, N-diisopropylethylamine (DIPEA) (0.364mL, 2.09mmol) was added and reacted for 30 minutes; then 3-phenylpropanal (0.285mL, 2.06 mmol) was dissolved in 10 mL of dichloromethane and added dropwise; after 30 minutes, it was quenched with saturated ammonium chloride, extracted with dichloromethane, spin-dried and passed through the column, and concentrated to obtain a yellow solid, that is, the compound of formula 3, yield: 60 %;
[0030] Dissolve the prepared compound 3 (0.3g, 0.78mmol) in tetrahydrofuran (8mL), cool down to -78°C and slowly add diisobutylaluminum hydride (DIBAL-H) (2.33mL, 1MinHexane) and react for 20 minutes Diluted with a small amount of ethyl acetate, quenched by adding 4 mL of saturated potassium sodium tartrate, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com