Patents
Literature
52 results about "Diisobutylaluminum hydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
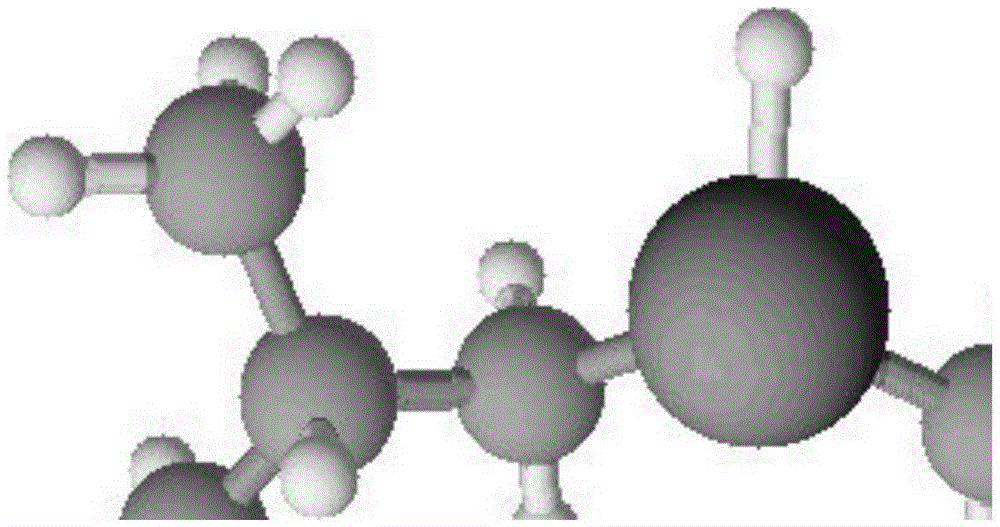
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH, /ˈdaɪbæl/ DY-bal) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound was investigated originally as a co-catalyst for the polymerization of alkenes.
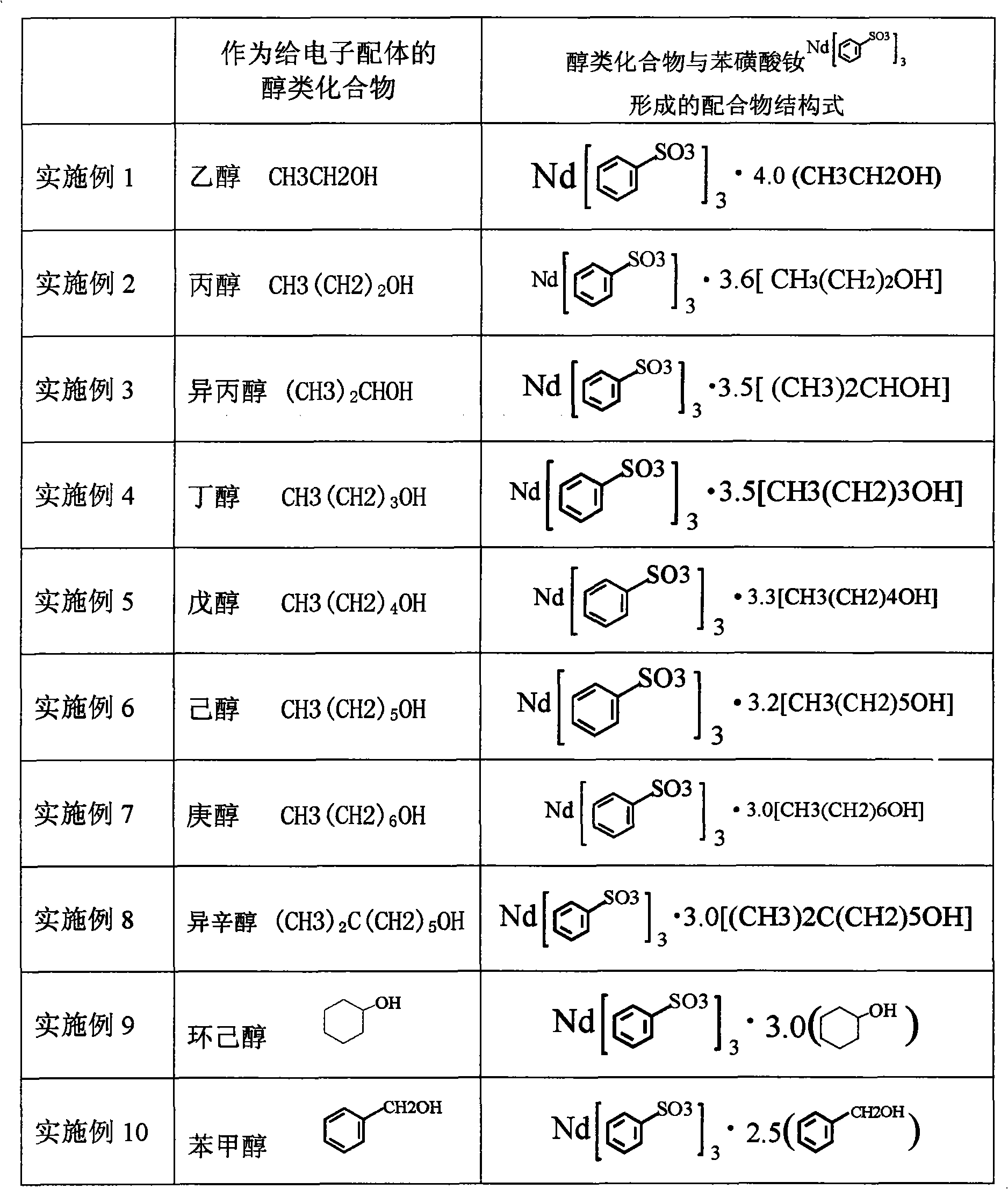
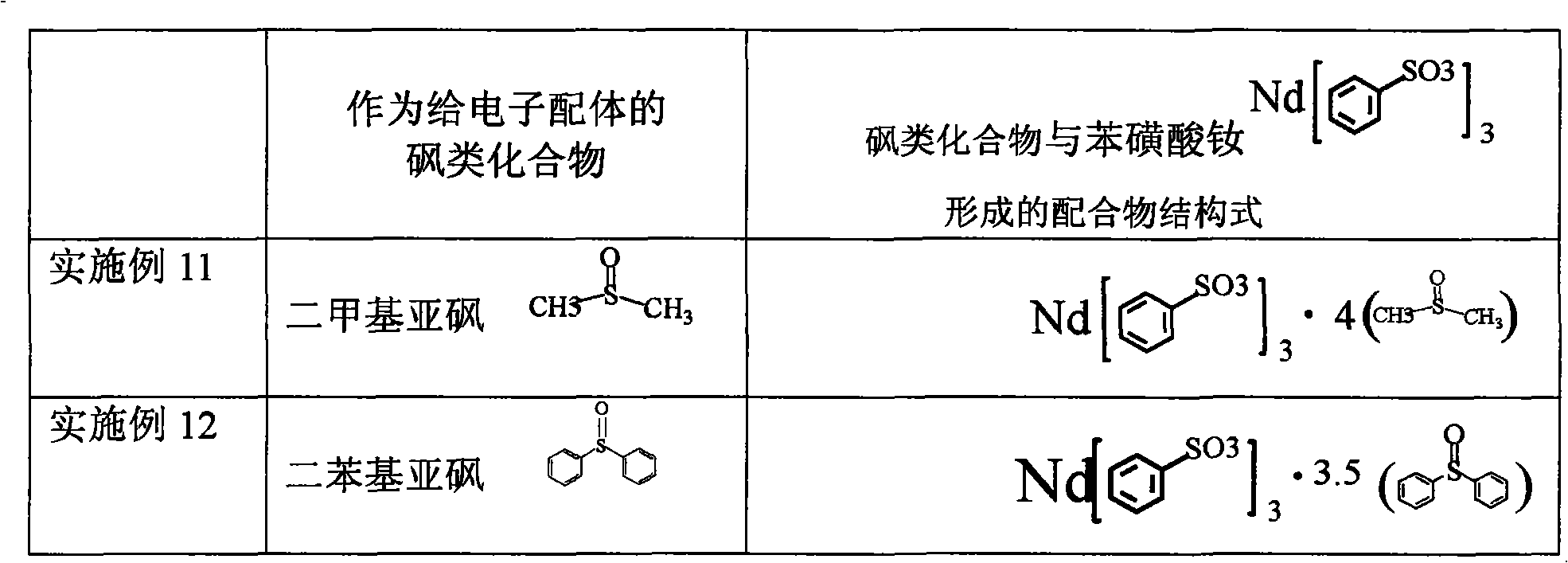
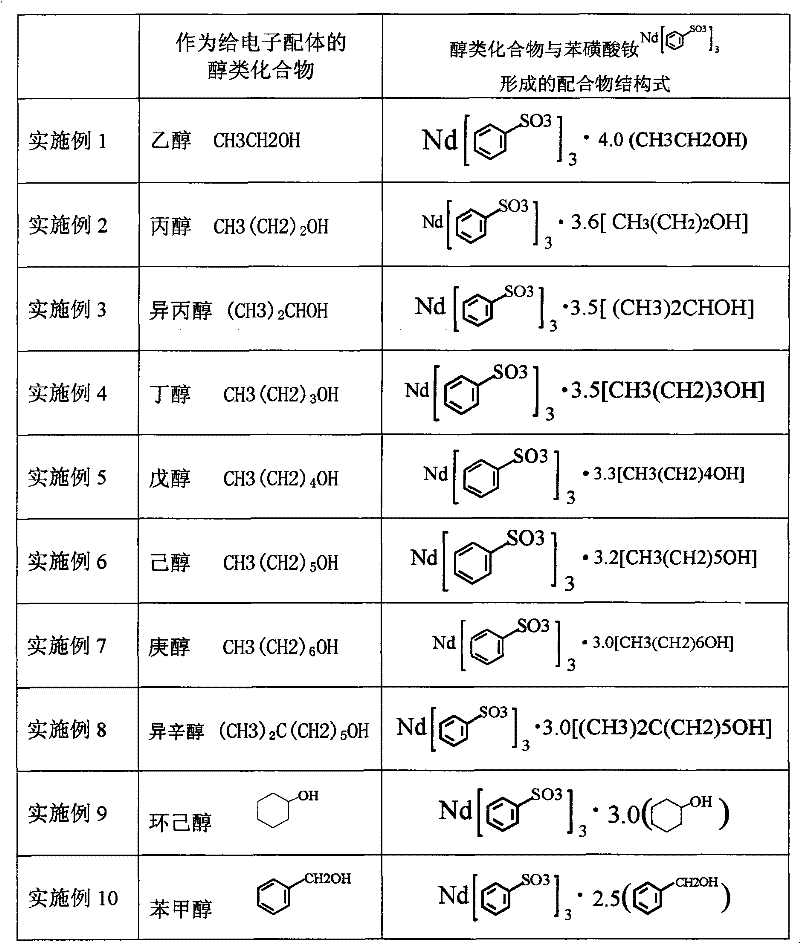
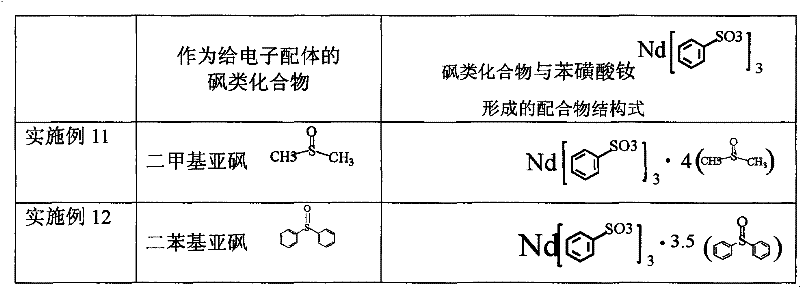
Sulfoacid rare earth catalyst for polymerizing high-cis-isoprene rubber and preparation method thereof
The invention relates to a sulfoacid rare earth catalyst for polymerizing high-cis-isoprene rubber and a preparation method thereof. The catalyst comprises benzenesulfonic acid neodymium rare earth complex and alkyl aluminum; the molar ratio of the alkyl aluminum to the rare earth neodymium in the benzenesulfonic acid neodymium rare earth complex is 15 to 40:1, wherein the benzenesulfonic acid neodymium rare earth complex comprises benzenesulfonic acid neodymium alcohol complex, benzenesulfonic acid neodymium sulphoxide complex, benzenesulfonic acid neodymium furan complex, benzenesulfonic acid neodymium amine complex, benzenesulfonic acid neodymium ether complex and benzenesulfonic acid neodymium ester complex; ligand comprises: alcohol compound, sulfoxide compound, furan compound, amine compound, ether compound and ester compound; and the alkyl aluminum comprises: 3-isobutyl aluminum, triethyl aluminum, diisobutyl aluminum hydride, or diethyl aluminum hydride. The sulfoacid rare earth complex catalyst is used for polymerizing isoprene, and can obtain the high-cis rare earth polyisoprene rubber with the cis 1,4 structure content more than 96.0 percent and the weight-average molecular weight more than 2.2 million.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Composition comprising neopentasilane and method of preparing same
ActiveUS8147789B2High purityReadily and efficiently removedSilicon organic compoundsOther chemical processesSilyleneSilanes
A composition comprising at least 93% (w / w) neopentasilane; and a method of preparing neopentasilane, the method comprising treating a tetrakis-(trihalosilyl)silane with diisobutylaluminum hydride.
Owner:NATA SEMICON MATERIALS CO LTD
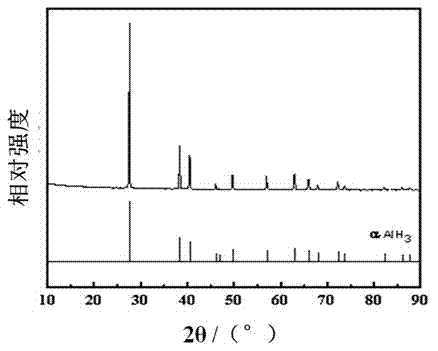
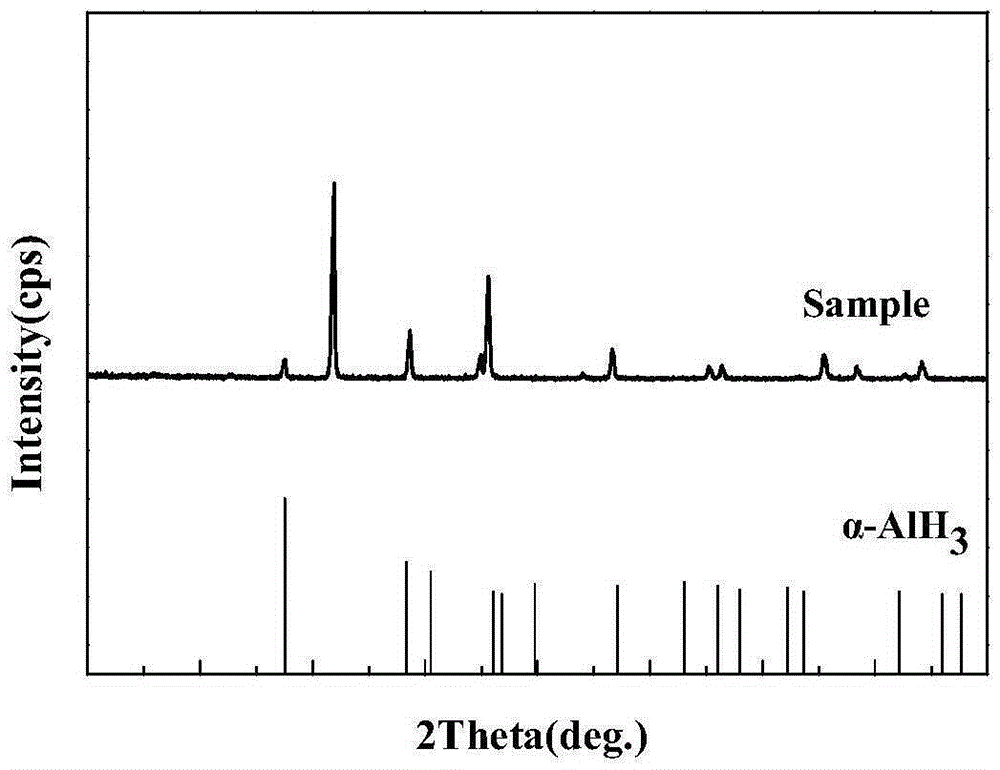
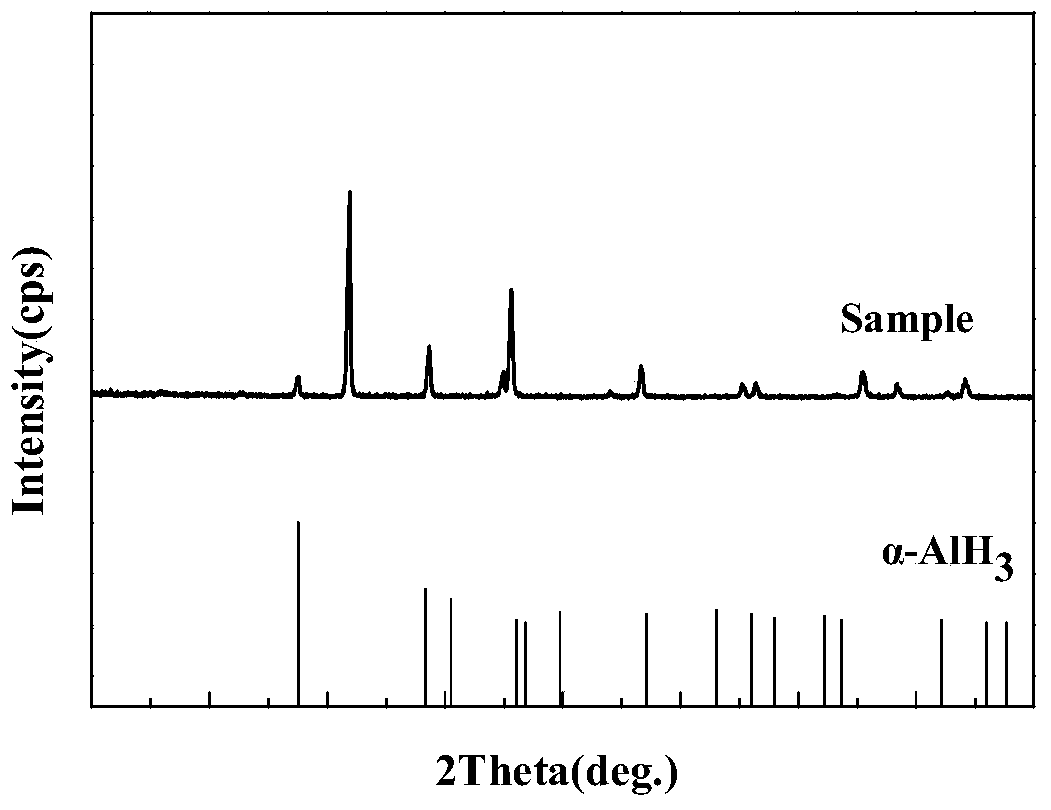
Method for preparing alpha-AlH3 under control of diisobutylaluminum hydride crystal transformation assistant
The invention discloses a method for preparing alpha-AlH3 under the control of a diisobutylaluminum hydride crystal transformation assistant. The method comprises the following steps: 1, dissolving AlH3 etherate and diisobutylaluminum hydride in anhydrous toluene or benzene, stirring at 92-94DEG C for 2-3h until a reaction ends, and standing until room temperature; and 2, filtering to obtain a white reaction product, washing by using ether 2-4 times, washing by using dilute hydrochloric acid 1-2 times, washing by using deionized water 2-3 times, and carrying out vacuum drying at 40-90DEG C for 5-8h under a degree of vacuum of 5-20Pa to obtain a white powder product. The method allows the above raw material to react under the action of the diisobutylaluminum hydride crystal transformation assistant in order to generate high yield alpha-AlH3, and realizes no side reactions.
Owner:HARBIN INST OF TECH
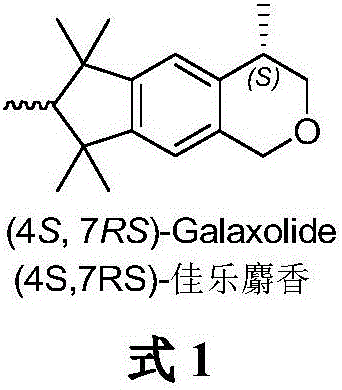
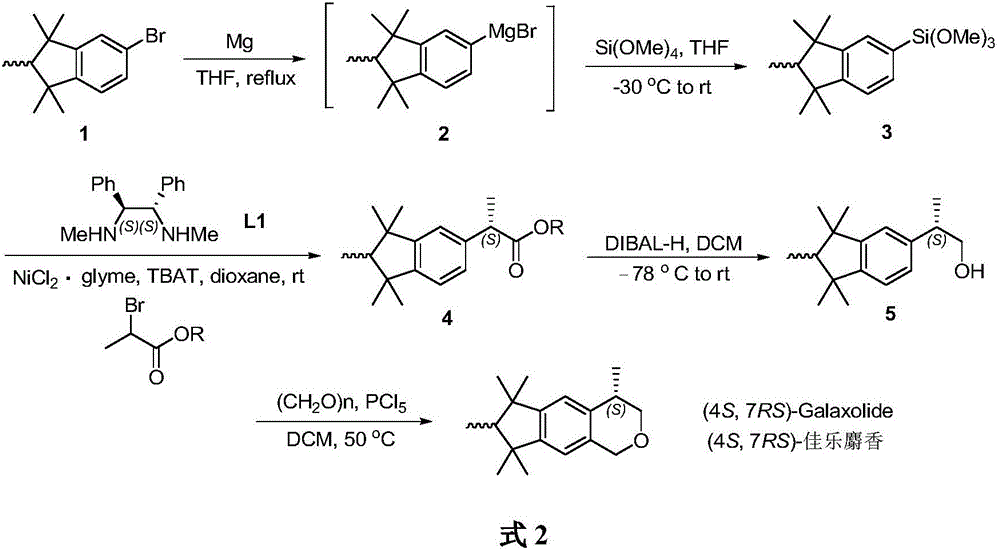
(4S, 7RS)-galaxolide synthesis method
The invention belongs to the technical field of chemical synthesis of essences and flavors and particularly relates to a (4S, 7RS)-galaxolide synthesis method. The method includes: subjecting bromo-pentamethyl indane and metallic magnesium to reaction to generate a Grignard reagent, and then subjecting to reaction with tetramethoxyl silane to obtain pentamethyl indane silane; subjecting the pentamethyl indane silane to asymmetric Hiyama cross coupling reaction with racemic 2-bromopropionate under catalysis of (1S, 2S)-N,N-dimethyl-1,2-diphenyl diaminoethane and nickel chloride to synthesize (S)-hexamethyl indane acid ester; reducing the (S)-hexamethyl indane acid ester by diisobutyl aluminum hydride (DIBAL-H) to obtain (S)-hexamethyl indanol, and finally subjecting to reaction with paraformaldehyde to obtain (4S, 7RS)-galaxolide. The (4S, 7RS)-galaxolide synthesis method is simple in synthetic route and mild in reaction condition, the overall yield reaches 40%, and product optical purity is 91%.
Owner:北京安胜瑞力科技有限公司
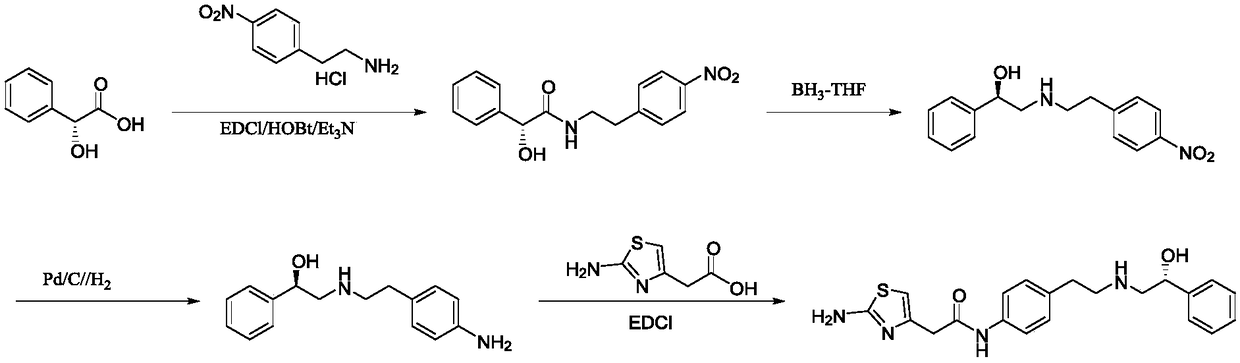
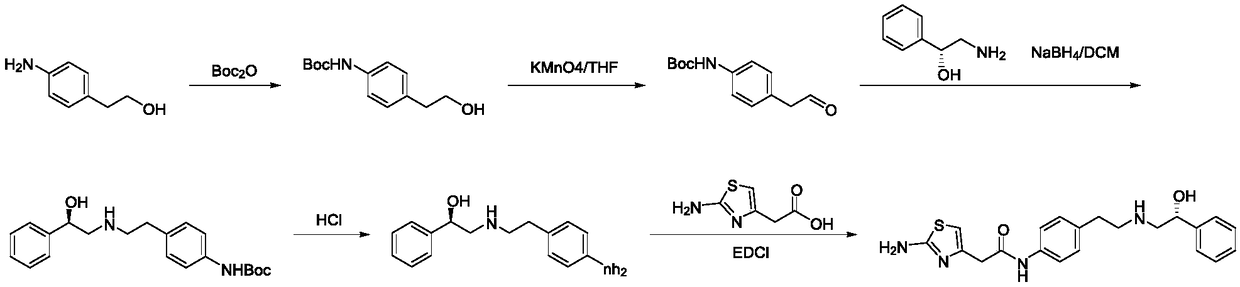
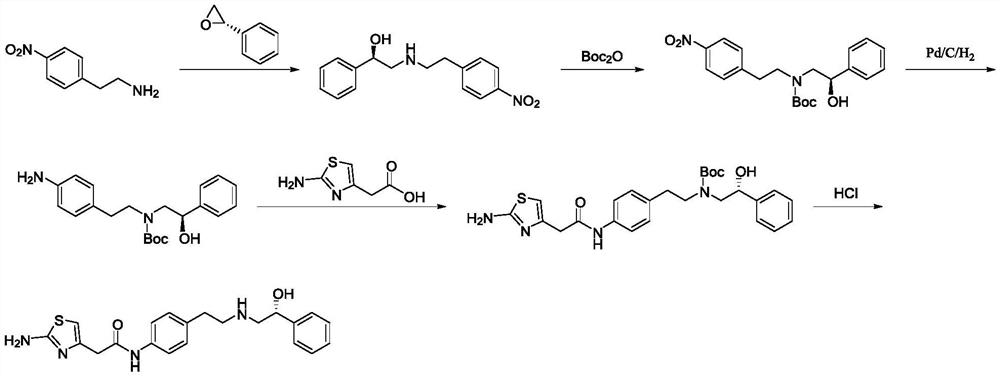
Preparation method of Mirabegron
The invention discloses a preparation method of Mirabegron and relates to the technical field of medicine preparation. The preparation method includes the steps of subjecting R-mandelic acid and 4-nitrophenylethylamine to amide condensation reaction to obtain an intermediate (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide, reducing amide carbonyl through diisobutyl aluminium hydride to obtainan intermediate (alphaR)-alpha-[[[2-(4-Nitrophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride, reducing nitryl through a ammonium formate-Pd / C reduction system to obtain an intermediate 2-[2-(4-amino-phenyl)-ethylamino]-1-phenyl-ethanol, and finally subjecting the intermediate and (2-aminothiazole-4-yl)acetic acid to condensation reaction to obtain Mirabegron. The Mirabegron prepared by themethod has high purity and high yield; the preparation method needs fewer steps of synthesis route and mild and controllable reaction conditions, is easy and convenient in operation, low in cost andadaptable to industrial production, and has broad prospect and high industrial application value.
Owner:ANHUI QINGYUN PHARMA & CHEM
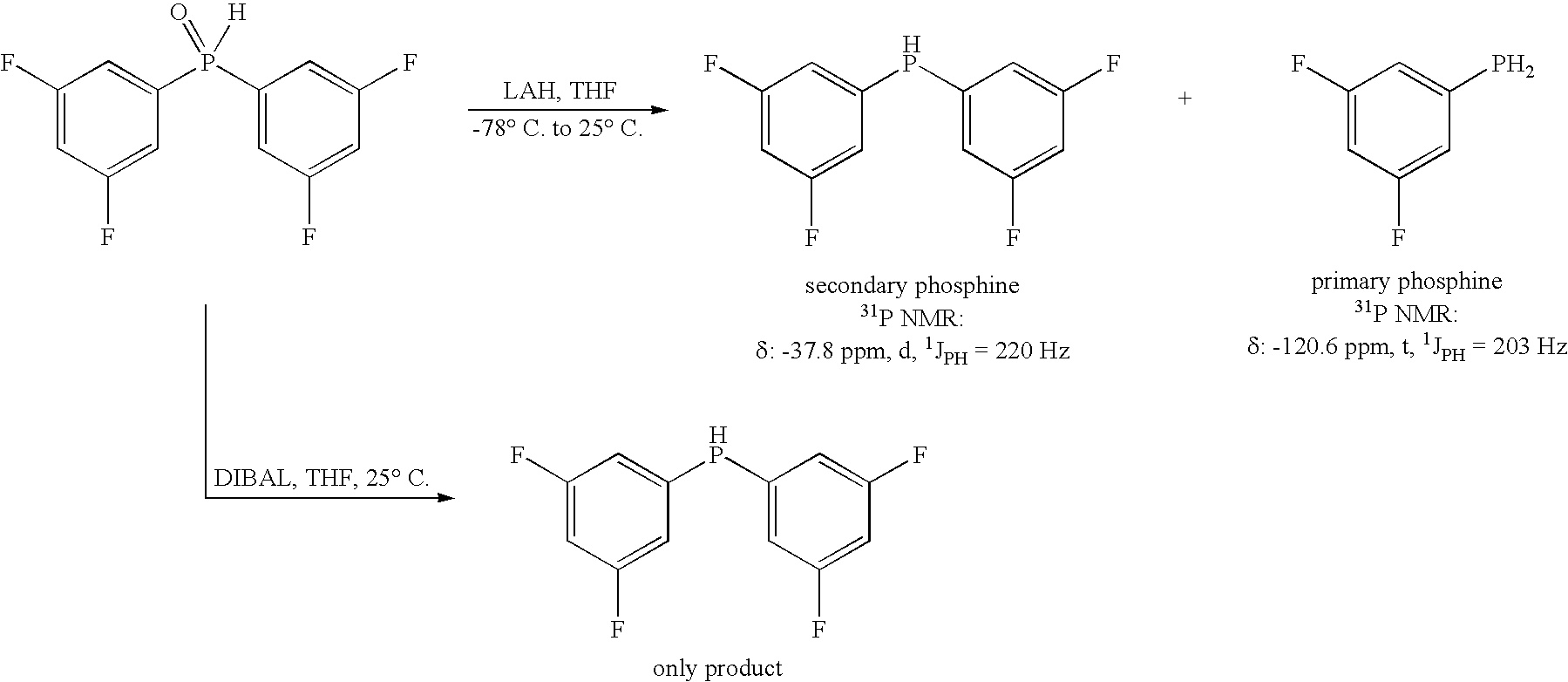
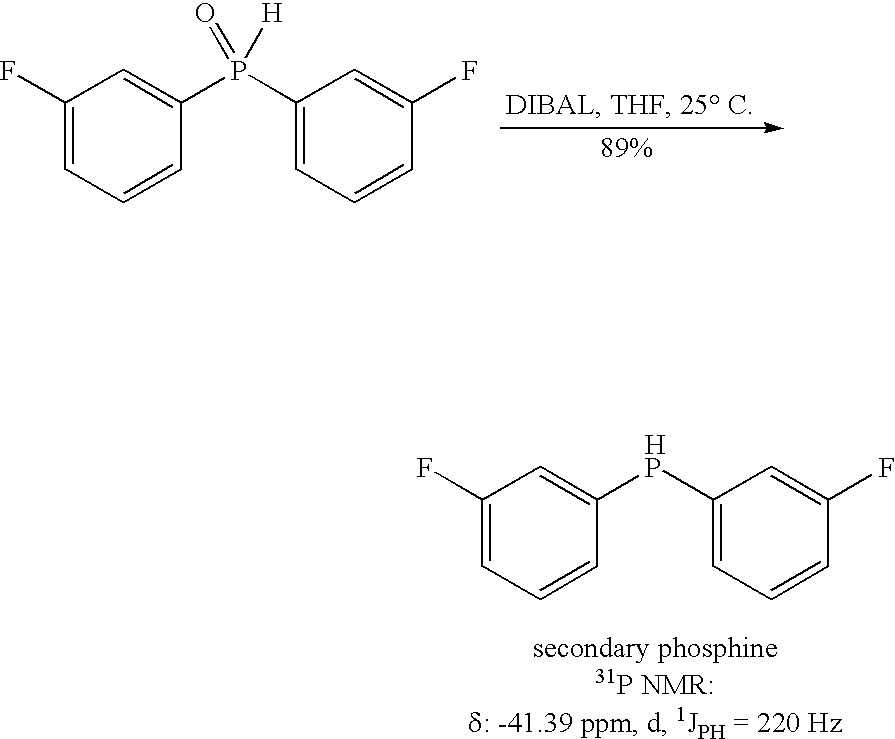
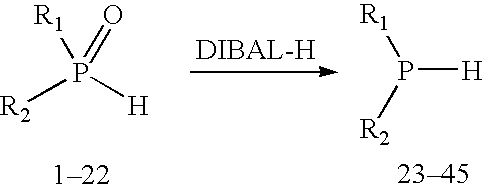
Method for generating secondary phosphines
ActiveUS20050203314A1Rapid responseReduced responsePhosphorus organic compoundsHydrogenTriisobutylaluminium
This invention provides a method for generating secondary phosphines from secondary phosphine oxides in the presence of a reducing agent, such as diisobutylaluminum hydride (DIBAL-H), triisobutyldialuminoxane, triisobutylaluminum, tetraisobutyldialuminoxane, or another reducing agent comprising: (i) an R1R2AlH moiety, wherein R1 and R2 are each an alkyl species or oxygen, and wherein at least one of R1 or R2 comprises at least 2 carbon atoms, or (ii) an R1R2R3Al moiety, wherein R1, R2, and R3 are not hydrogen, and wherein at least one of R1, R2, and R3 is an alkyl species comprising a β-hydrogen, not including triethylaluminum. Preferred reducing agents for the present invention include: diisobutylaluminum hydride, triisobutyldialiuminoxane, triisobutylaluminum, tetraisobutyldialuminoxane, and combinations thereof.
Owner:BOEHRINGER INGELHEIM PHARMA INC
Environmentally-friendly multi-effect antirust oil
InactiveCN103992850AMeet anti-rust requirementsDoes not affect lubricityLubricant compositionALUMINUM HYDRIDELubrication
The invention discloses an environmentally-friendly multi-effect antirust oil. The environmentally-friendly multi-effect antirust oil comprises, by weight, 61-70 parts of 75SN base oil, 10-18 parts of 150SN base oil, 5-7 parts of sodium petroleum sulfonate, 1-2 parts of sorbitan monostearate, 5-7 parts of calcium petroleum sulfonate, 0.1-0.2 parts of diisobutyl aluminum hydride, 1-2 parts of sodium hypophosphate, 2-3 parts of zinc dialkyl dithiophosphate, 0.5-1 part of tris(2,4-di-tert-butylpheny)phosphite, 2-3 parts of oleic acid and 10-14 parts of a film forming assistant. The antirust oil can be used for the rust prevention of various metal components, can meet antirust requirements of many metal materials comprising castings, steel, copper, aluminum and the like, is compatible with automobile oil, has no influences on the lubrication performance of lubrication oil, and has the advantages of thin oil film, easy cleaning, reasonable compounding of raw materials, safety and environmental protection.
Owner:TIANCHANG RUNDA METAL ANTIRUST AUX
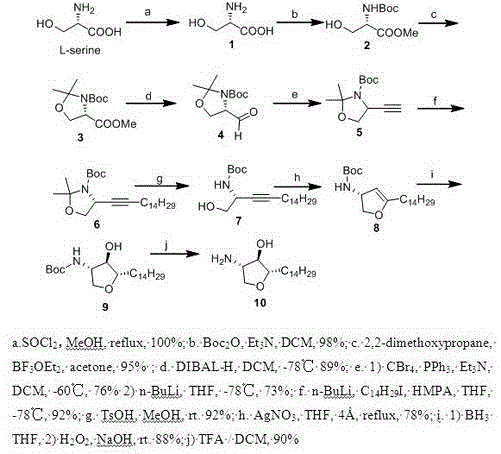
Method for synthesizing natural product Jaspine B isomer
InactiveCN103601706AInnovative designInnovative design ideasOrganic chemistryBulk chemical productionNatural productCombinatorial chemistry
The invention relates to a method for synthesizing natural product Jaspine B isomer, which comprises the following steps: by using cheap and accessible L-serine as an initial raw material, carrying out methyl ester protection, propylidene protection, diisobutylaluminum hydride reduction, Corey-Fuchs reaction, alkylation and the like to complete the synthesis of an intermediate disclosed as Formula 6; carrying out a metal catalysis process (silver nitrate catalysis) to complete the synthesis of a key intermediate disclosed as Formula 8; and completing the complete synthesis of the target molecule disclosed as Formula 10 at higher yield. The method has the advantages of novel and reasonable synthetic route design, cheap and accessible raw material, simple operating technique, mild reaction conditions and single product structure, efficiently completes the complete synthesis of the Jaspine B isomer with three chiral centers, and establishes firm foundation for subsequent complete synthesis of the natural product Jaspine B.
Owner:JIANGXI SCI & TECH NORMAL UNIV
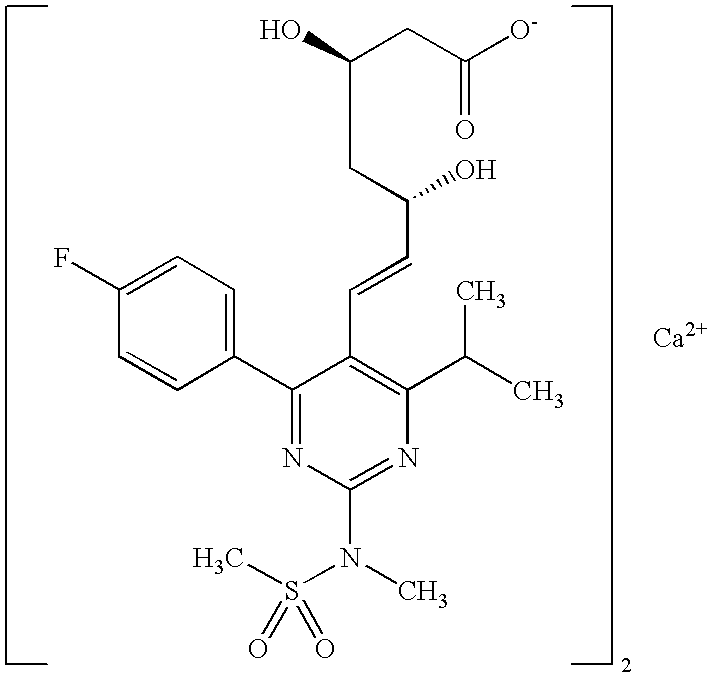
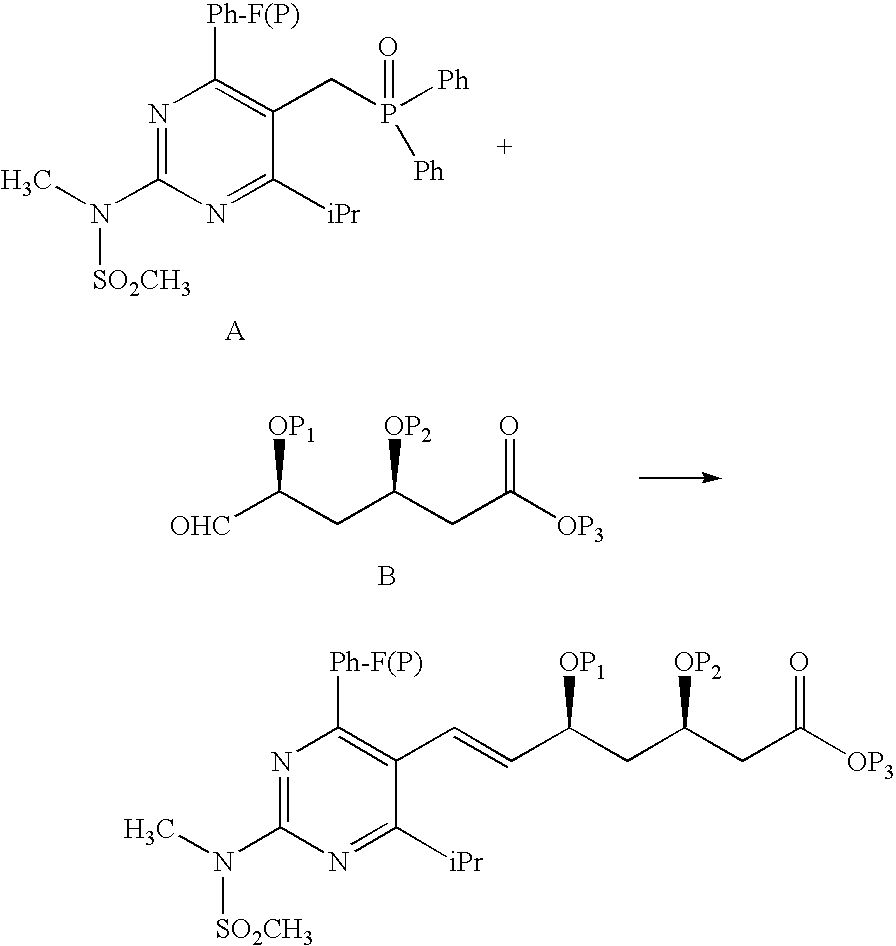
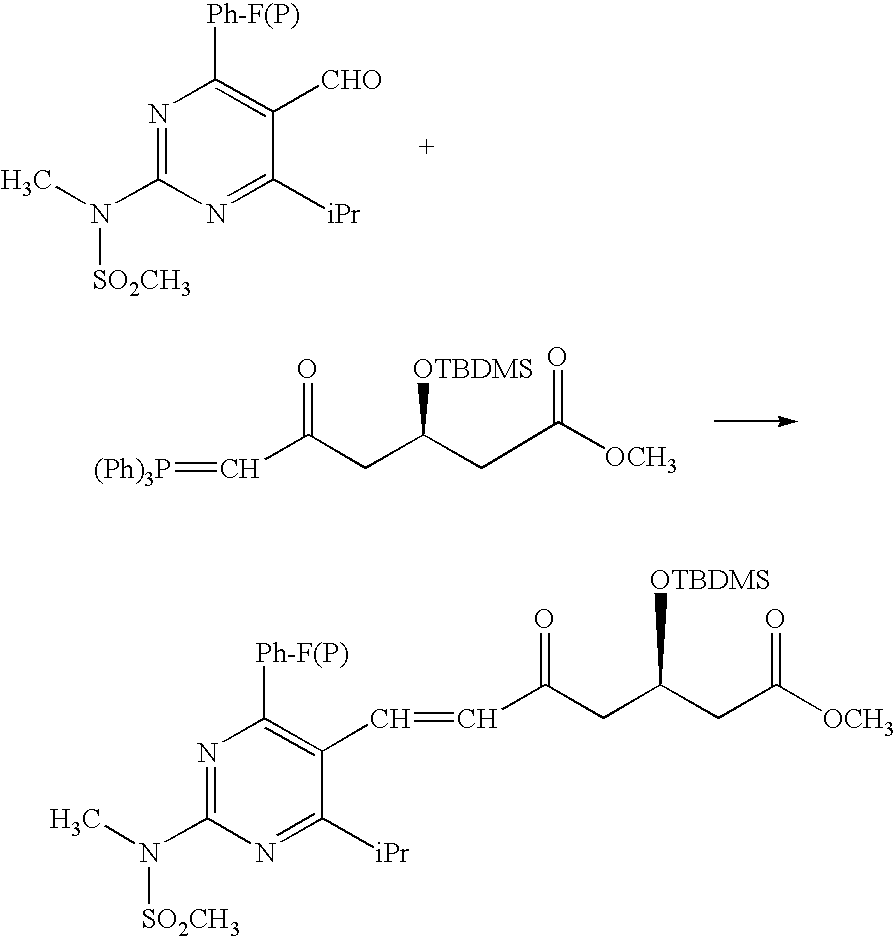
Synthetic method and intermediates of Rosuvastatin calcium and preparation methods of intermediates
ActiveUS8049010B2Increase not only reaction rate but also reaction stereoselectivityShort routeOrganic chemistryDiethyl phosphatePhosphate
The present invention publicly discloses a synthetic method and intermediates of rosuvastatin calcium and synthetic methods of the intermediates. The synthetic method uses 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-formaldehyde as the raw material, includes 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acrylonitrile (intermediate I) from a nitrilized reaction, and 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acraldehyde (intermediate II) from an aldehydized reaction of the intermediate I, and further goes through such unit processes as side-chain extension, ketone-group reduction, ethyl-group hydrolysis and neutralization reaction or decomposition reaction to obtain rosuvastatin calcium. The nitrilized reagent can be phosphate diethylacetonitrile, acetonitrile, etc.; the aldehyde reductant can be diisobutyl aluminum hydride, red aluminum, etc.; and the ketone-group reductant can be diethylmethoxyborane, NaBH4, KBH4, etc.
Owner:ANHUI QINGYUN PHARMA & CHEM
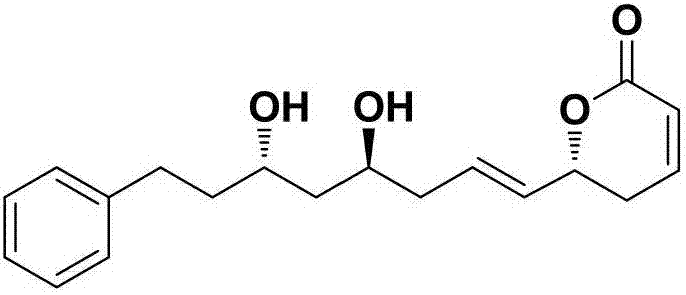
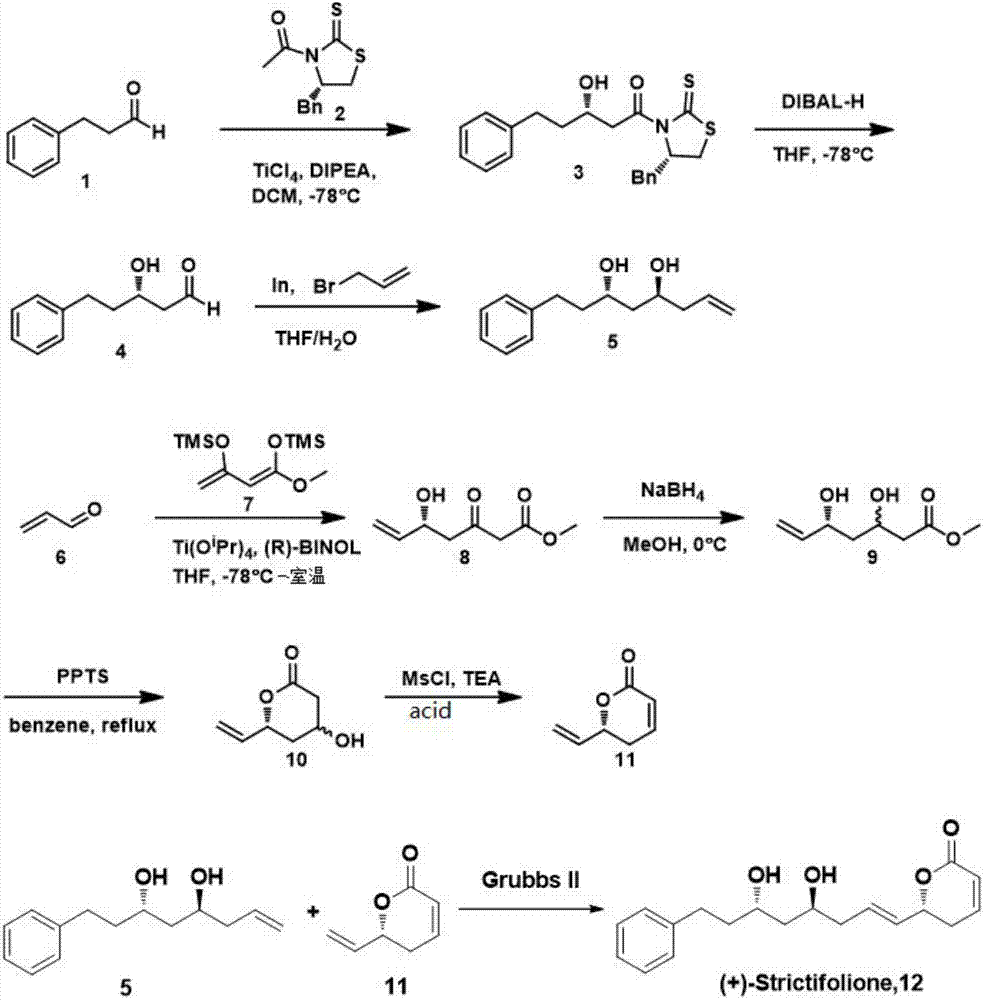
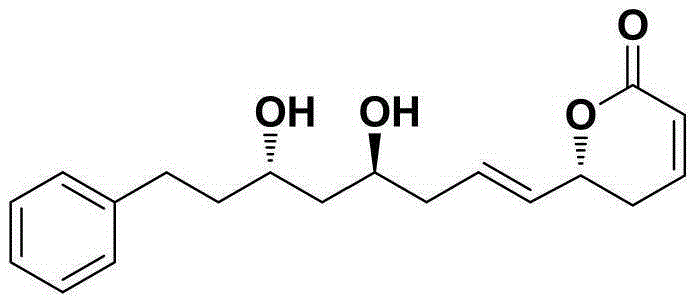
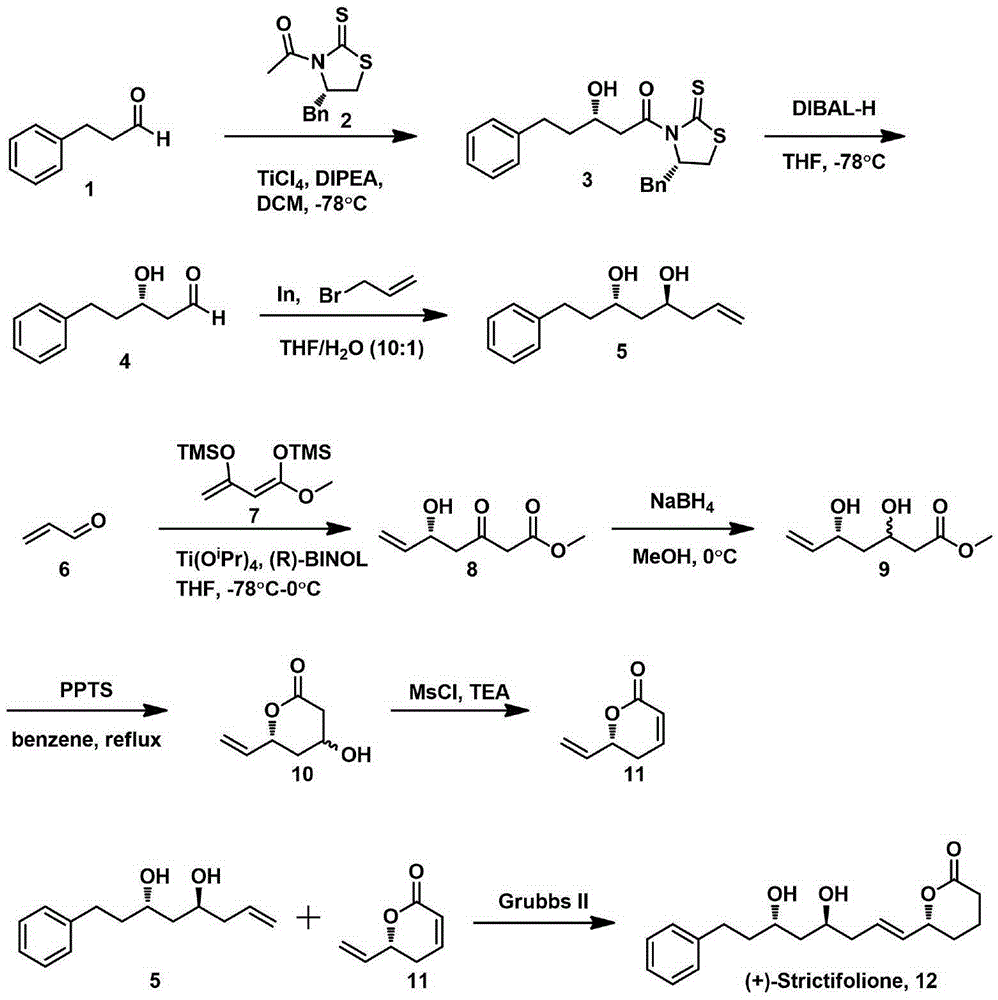
Natural product (+)-Strictifolione synthetic method
InactiveCN105254604ASimple and fast operationHigh yieldOrganic chemistryChemical recyclingIndiumStrictifolione
The invention discloses a natural product (+)-Strictifolione synthetic method. The method comprises the steps that 3-benzenepropanal and an Evans chiral auxiliary reagent are subjected to an aldol reaction for diisobutyl aluminum hydride reduction and then subjected to an addition reaction with metal indium activated 3-propylene bromine to obtain a compound shown in the formula 5; silyl enol ether reacts with acrolein under the action of titanium tetraisopropoxide and (R)-1,1-binaphthol, then carbonyl is reduced through sodium borohydride, p-toluenesulfonic acidpyridinium salt is used for cyclization, and acidification treatment is carried out after a methylsulfonyl group becomes a leaving group to obtain a compound shown in the formula 11; a Grubbs second-generation catalyst is used for carrying out a metal coupling reaction on the compounds shown in the formulas 5 and 11 to obtain (+)-Strictifolione. According to the bisection synthetic method, the design is simple and reasonable, operation is easy and convenient, the reaction condition is moderate, linear steps are simple, the product yield is high, and the production cost is greatly reduced.
Owner:JIANGXI SCI & TECH NORMAL UNIV
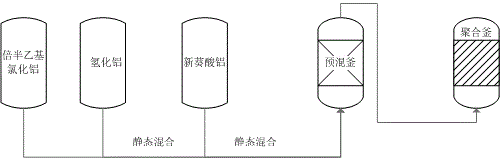
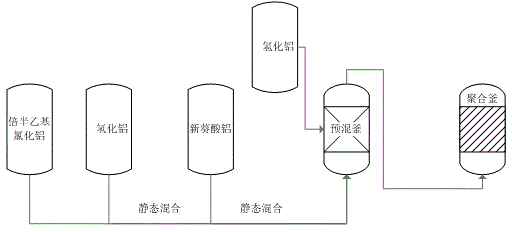
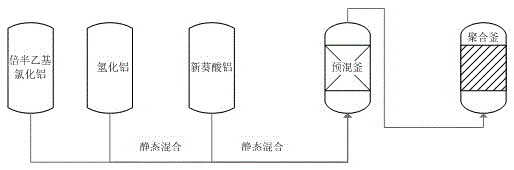
Catalyst charging method in neodymium polybutadiene rubber polymerization processes
The invention discloses a catalyst charging method in neodymium polybutadiene rubber polymerization processes. The catalyst charging method comprises following steps: (1) ethylaluminum sesquichloride, diisobutylaluminum hydride, and neodymium neodecanoate are mixed according to a certain ratio so as to obtain a mixed catalyst, the mixed catalyst is delivered into a static mixer for ternary aging, wherein aging time ranges from 25 to 35min, and aging temperature ranges from 25 to 35 DEG C; (2) the mixed catalyst obtained via aging is delivered into a premixing kettle with a mixture of butadiene and a solvent oil for buffer treatment, and then an obtained mixed material is delivered into a polymerization kettle for polymerization, wherein pressure of the polymerization kettle ranges from 0.35 to 0.45 MPa, a first polymerization kettle temperature ranges from 60 to 75 DEG C; and (3) a part of diisobutylaluminum hydride is directly added through the middle-lower part of the premixing kettle into the polymerization kettle without aging, and is reacted with the mixed material obtained via step (2) for polymerization so as to prepare polybutadiene rubber. Beneficial effects of the catalyst charging method are that: reaction is stable; activity is stable; temperature is stable; the catalyst charging method is beneficial for Mooney viscosity control; and polybutadiene rubber product quality is stable.
Owner:华宇橡胶有限责任公司
Synthetic method of beraprost
InactiveCN111116530AImprove protectionAvoid reactionOrganic chemistry methodsCombinatorial chemistryHydrolysis
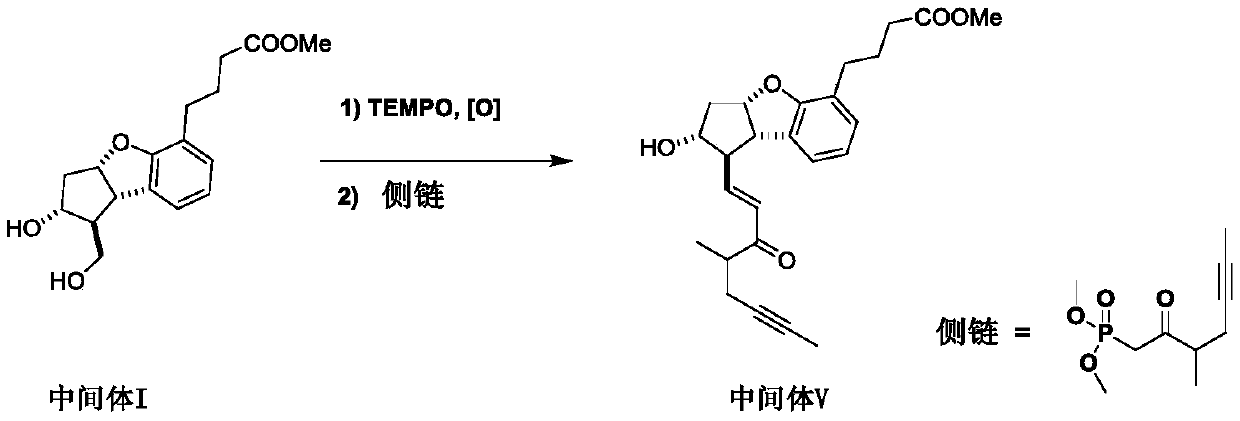
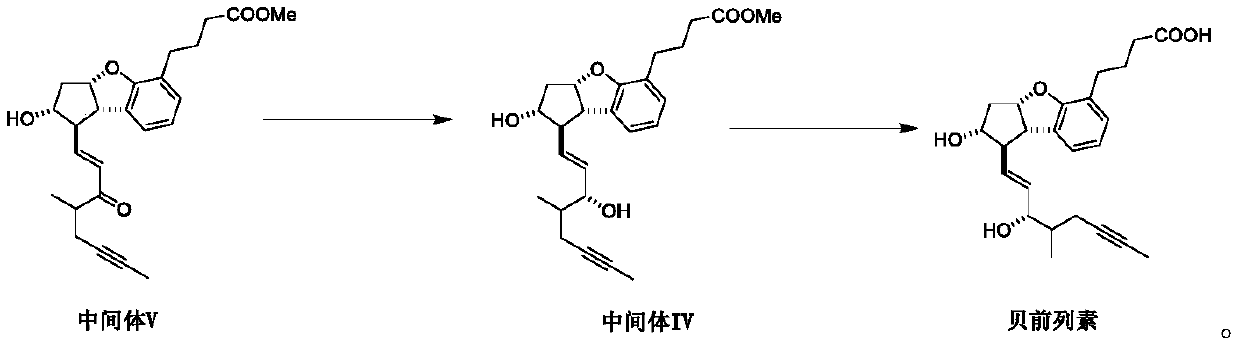
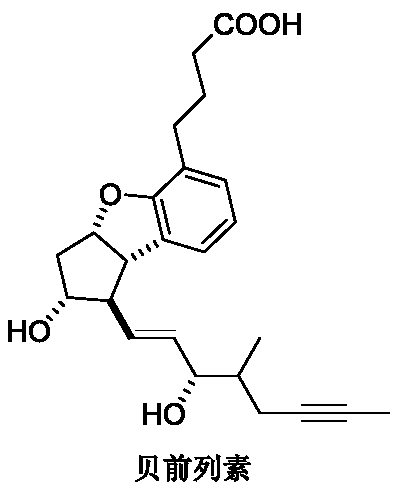
The invention relates to a synthetic method of beraprost. The synthetic method comprises the following steps: with an intermediate I as an initial raw material, carrying out selective primary alcoholoxidation and a Witting reaction to obtain an intermediate V; carrying out reduction and column chromatography purification on the intermediate V to obtain an intermediate IV; and hydrolyzing the intermediate IV to obtain beraprost. According to the synthetic method disclosed by the invention, two hydroxyl groups of the intermediate I are selectively oxidized, so a hydroxyl protection reagent is prevented from being used; in the oxidation step, ultralow-temperature (-60 to -80 DEG C) reactions and use of a reagent DCC with relatively high toxicity are avoided; in the reduction step, diisobutylaluminum hydride is prevented from being used; process operation units are greatly reduced, reaction steps are shortened, emission of three wastes is reduced, and the reactions are more efficient andenvironment-friendlier; and the main peak content of the prepared beraprost reaches 99.0% or above, and total process yield reaches 26% or above. The invention provides the synthetic method which ismore beneficial for industrial production.
Owner:JINAN KANGHE MEDICAL TECH
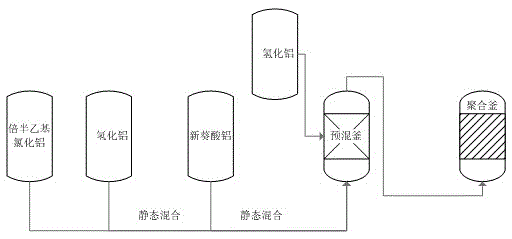
Catalyst feeding method in the polymerization process of neodymium-based polybutadiene rubber
The invention discloses a catalyst charging method in neodymium polybutadiene rubber polymerization processes. The catalyst charging method comprises following steps: (1) ethylaluminum sesquichloride, diisobutylaluminum hydride, and neodymium neodecanoate are mixed according to a certain ratio so as to obtain a mixed catalyst, the mixed catalyst is delivered into a static mixer for ternary aging, wherein aging time ranges from 25 to 35min, and aging temperature ranges from 25 to 35 DEG C; (2) the mixed catalyst obtained via aging is delivered into a premixing kettle with a mixture of butadiene and a solvent oil for buffer treatment, and then an obtained mixed material is delivered into a polymerization kettle for polymerization, wherein pressure of the polymerization kettle ranges from 0.35 to 0.45 MPa, a first polymerization kettle temperature ranges from 60 to 75 DEG C; and (3) a part of diisobutylaluminum hydride is directly added through the middle-lower part of the premixing kettle into the polymerization kettle without aging, and is reacted with the mixed material obtained via step (2) for polymerization so as to prepare polybutadiene rubber. Beneficial effects of the catalyst charging method are that: reaction is stable; activity is stable; temperature is stable; the catalyst charging method is beneficial for Mooney viscosity control; and polybutadiene rubber product quality is stable.
Owner:华宇橡胶有限责任公司
Method for synthesizing di-tert-butylphenylphosphonium tetrafluoroborate
ActiveCN109438511AReduce usageSimplify the experimental stepsGroup 5/15 element organic compoundsDichlorophenylphosphineTetrafluoroborate
The invention discloses a method for synthesizing di-tert-butylphenylphosphonium tetrafluoroborate, and belongs to the field of organic synthesis. The method comprises the following steps: in the anhydrous and anaerobic atmospheres, taking dichlorophenylphosphine as a raw material, after the reduction of diisobutyl aluminium hydride, reacting with tertiary butanol under the catalytic action of boron trifluoride, and then carrying out hydrolysis to generate the di-tert-butylphenylphosphonium tetrafluoroborate. Compared with the prior art, the method is high in yield and simple in aftertreatment, and is more applicable for industrial production.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Method for synthesizing N-Boc-3-pyrrolidine formaldehyde
The invention discloses a method for synthesizing N-Boc-3-pyrrolidine formaldehyde, which comprises the following steps of: (1) adding 1mol of glycine and 0.5 to 1.5mol of acrylonitrile into a methylbenzene solvent, refluxing the solution under the action of 1.5 to 2.5mol of paraformaldehyde, cooling and then performing suction filtering on the solution, concentrating the mother solution, distilling the mother solution under reduced pressure, and collecting the fraction of 102 to 105 degrees to obtain 3-cyanopyrrolidine; (2) adding 0.5mol of 3-cyanopyrrolidine obtained in the step (1) into dichloromethane, dripping 0.4 to 0.6mol of Boc acid anhydride into the solution at room temperature under the action of 0.5 to 0.75mol of triethylamine, performing room temperature reaction, washing the reaction solution by using hydrochloric acid solution, and separating, drying and concentrating the solution to obtain N-Boc-3-cyanopyrrolidine; and (3) dissolving 0.4mol of N-Boc-3-cyanopyrrolidine obtained in the step (2) into dichloromethane, cooling the solution, dripping 0.4 to 0.6mol of di-isobutyl aluminum hydride into the solution, dripping hydrochloric acid solution into the reaction solution after reaction to quench the reaction, separating the solution, concentrating the organic phase, distilling the concentrate under reduced pressure, and collecting the fraction of 110 to 114 degrees to obtain the N-Boc-3-pyrrolidine formaldehyde. The synthesizing method has a few steps, high yield and low cost, and is suitable for industrial large-scale production.
Owner:刘战朋
Method for preparing alpha-AlH3 by use of diisobutylaluminum hydride and LiBH4 as catalyst
The invention relates to a method for preparing alpha-AlH3 by use of diisobutylaluminum hydride and LiBH4 as the catalyst, relates to a preparation method for alpha-AlH3, and aims to solve the problem that conditions required by direct synthesis of alpha-AlH3 through aluminum and hydrogen are strict. The method comprises the following steps: adding aluminum powder, diisobutylaluminum hydride and LiBH4 in an autoclave; introducing hydrogen, reacting at the condition with the temperature of 100-150 DEG C, and drying to obtain the product. In the method, the reaction pressure is 10-15 MPa which is much lower than 10 GPa, the reaction temperature is 100-150 DEG C which is much lower than 650 DEG C, through XRD tests, the synthesized product contains alpha-AlH3, and the method is applied to preparation of alpha-AlH3.
Owner:HARBIN INST OF TECH
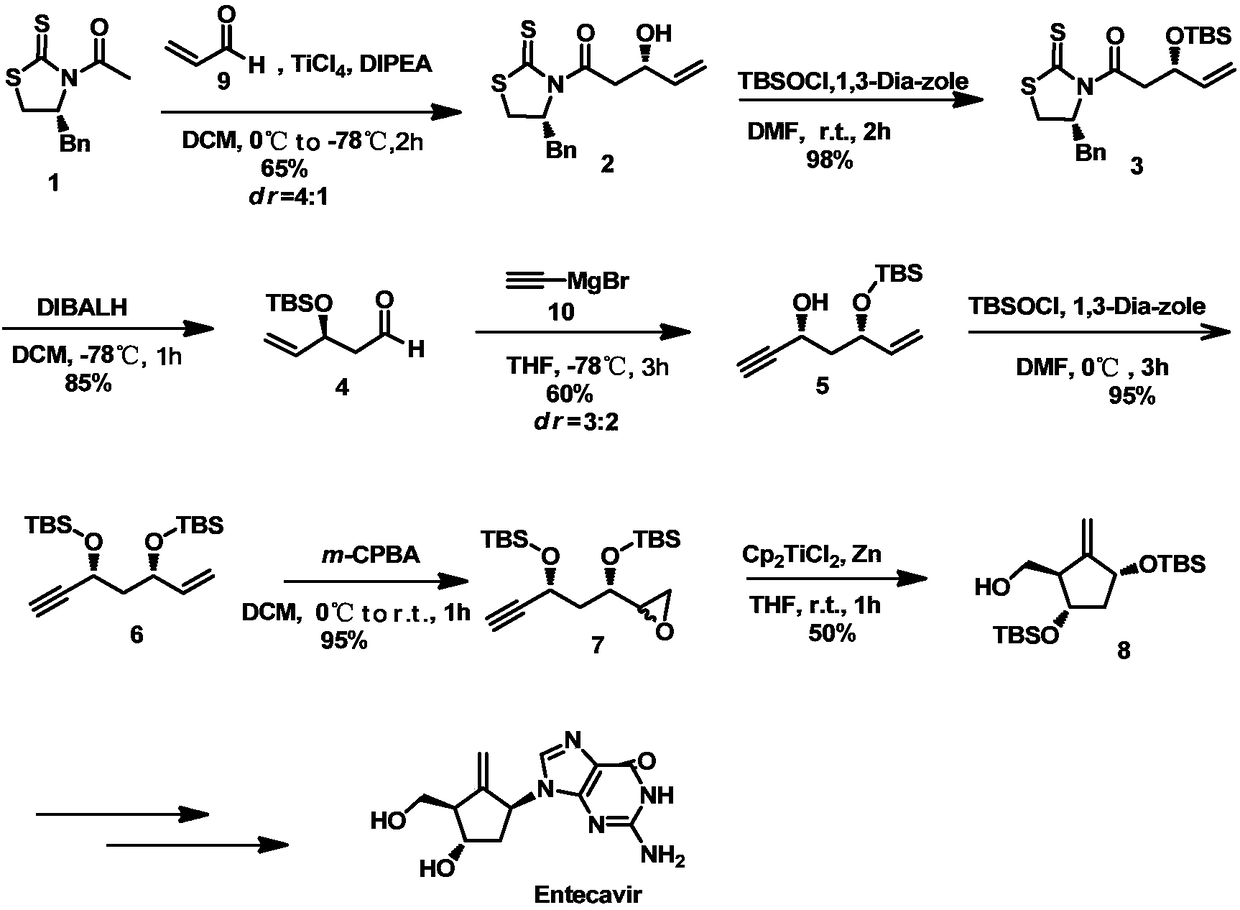
Novel synthesis method for key intermediate of anti-hepatitis B drug Entecavir
The invention discloses a novel synthesis method for a key intermediate of the anti-hepatitis B drug Entecavir. The method is characterized in that a known compound as shown by Formula 1 is adopted asa starting material, and synthesis of target molecules is achieved through a series of reaction steps such as Evans aldol reaction, hydroxyl protection through tert-butyldimethylsilyl chloride, reductive removal of a chiral auxiliary group through diisobutyl aluminum hydride (DIBAL-H) to obtain an aldehyde, addition of a Grignard reagent into the aldehyde, protection of a newly generated hydroxylthrough tert-butyldimethylsilyl, double-bond epoxidation reaction, free-radical ring closing reaction, hydroxyl protection through p-methoxybenzyl, etc. The method disclosed by the invention has theadvantages of unique and novel designing of the whole route, mild reaction conditions for the reaction steps, high rate, relatively fewer side effects, and high simplicity and convenience in operation; and common chemical reagents are adopted in the route, and raw materials are cheap and can be easily obtained, so that the synthesis cost can be greatly reduced.
Owner:DONGGUAN UNIV OF TECH
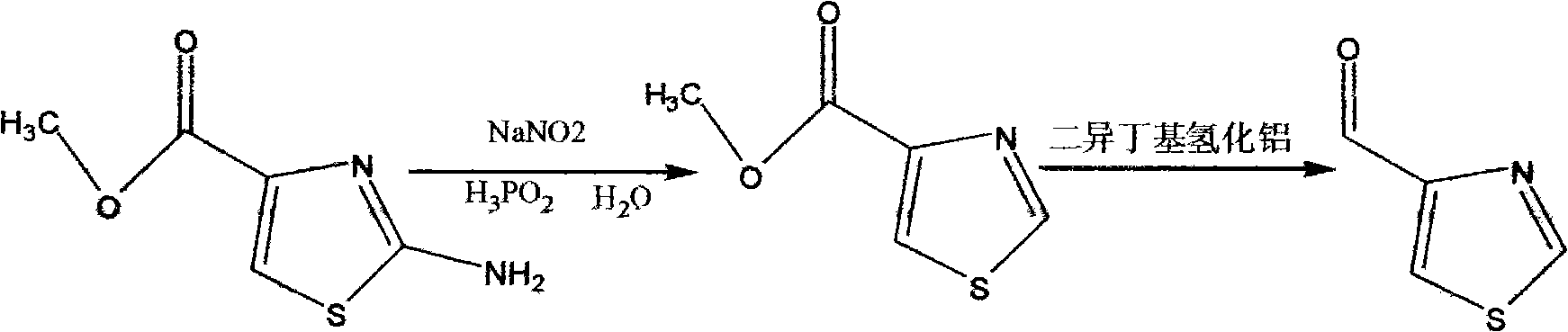
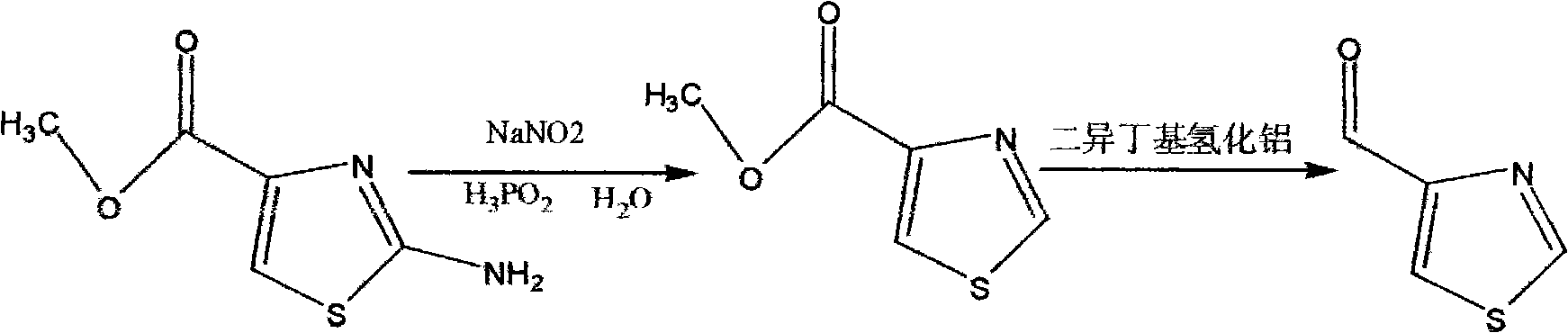
Synthesis method of thiazole-4-formaldehyde
The invention provides a synthesis method of thiazole-4-formaldehyde. The method comprises the following steps: using simple, common and cheap 2-amino-thiazole-4-methyl formate as raw material to perform diazotization and remove 2-amino, reducing with hypophosphrous acid to obtain thiazole-4-methyl formate, and reducing with dibal-H diisobutylaluminum hydride to obtain thiazole-4-formaldehyde. The method solves the defects that the mass production of thiazole-4-formaldehyde can not be realized; and the invention provides the industrial production method of thiazole-4-formaldehyde which is easy to operate and realize. The raw material of the synthesis method has simple preparation method and low price; and the preparation operation is simple and the industrial production is easy to realize.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
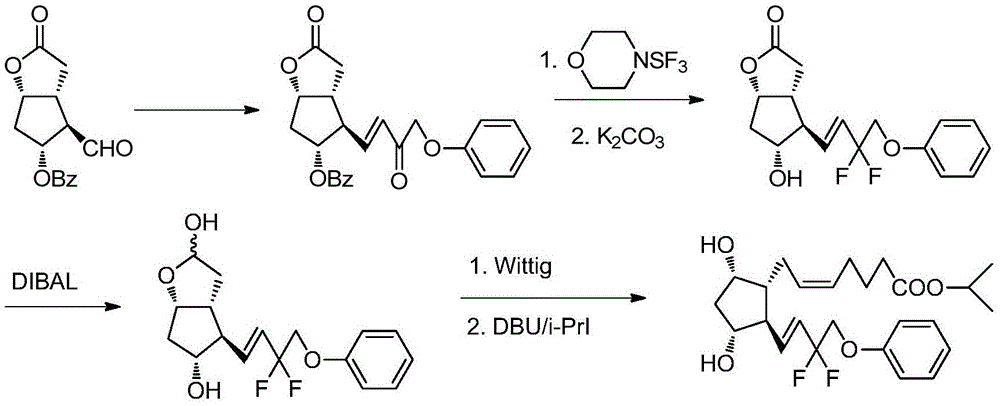
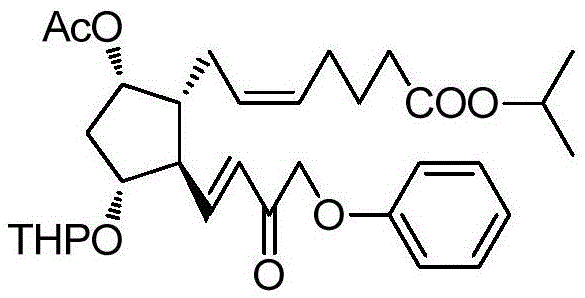
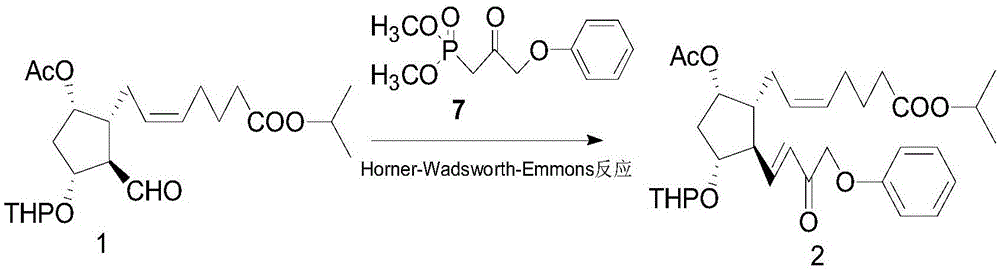
A kind of method of synthesizing tafluprost
ActiveCN103804195BOrganic compound preparationCarboxylic acid esters preparationWittig reactionTafluprost
Owner:TIEN TIANJIN PHARMA
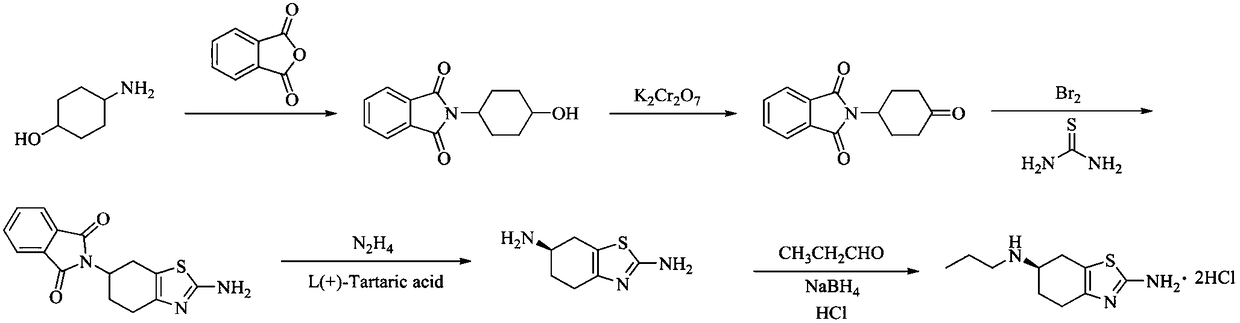
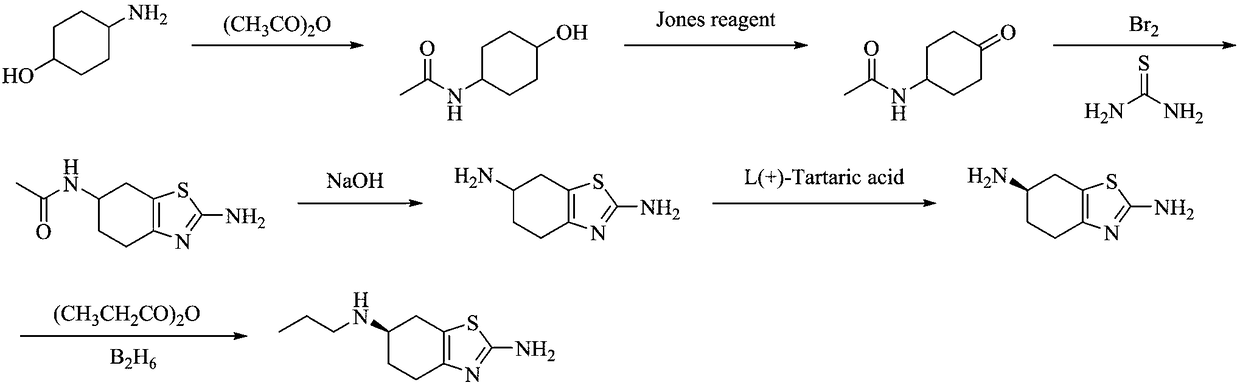
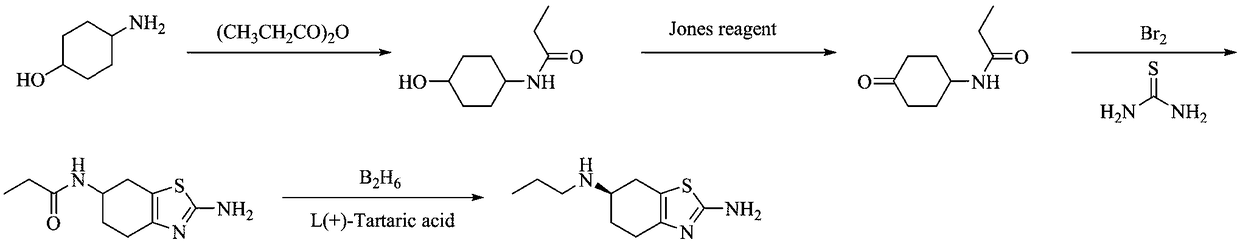
Preparation method of pramipexole hydrochloride
ActiveCN109232471AAvoid lostRaw materials are cheap and easy to getOrganic chemistryCyclohexanonePropionyl chloride
The invention discloses a preparation method of pramipexole hydrochloride. The method comprises the following steps: protecting an amino group of aminocyclohexanol by means of condensation of propionyl chloride to obtain p-propionyl cyclohexanol; then, carrying out catalytic oxidation to obtain p-propionyl cyclohexanone; carrying out oxidative bromination and Hantzsch condensation to obtain 2-amino-6-propionylamino-4, 5, 6, 7-tetrahydrobenzothiazole; then, reducing by using diisobutylaluminum hydride to obtain 2-amino-6-propylamino-4, 5, 6, 7-tetrahydrobenzothiazole; finally, carrying out L-(+)-tartaric acid separation and salification to obtain the pramipexole hydrochloride. The preparation method, provided by the invention, of pramipexole hydrochloride is low in price of raw materials, easy in obtaining of the raw materials, convenient and simple to operate, novel in route, environmentally-friendly and high in yield; the prepared pramipexole hydrochloride is good in purity.
Owner:ANHUI QINGYUN PHARMA & CHEM
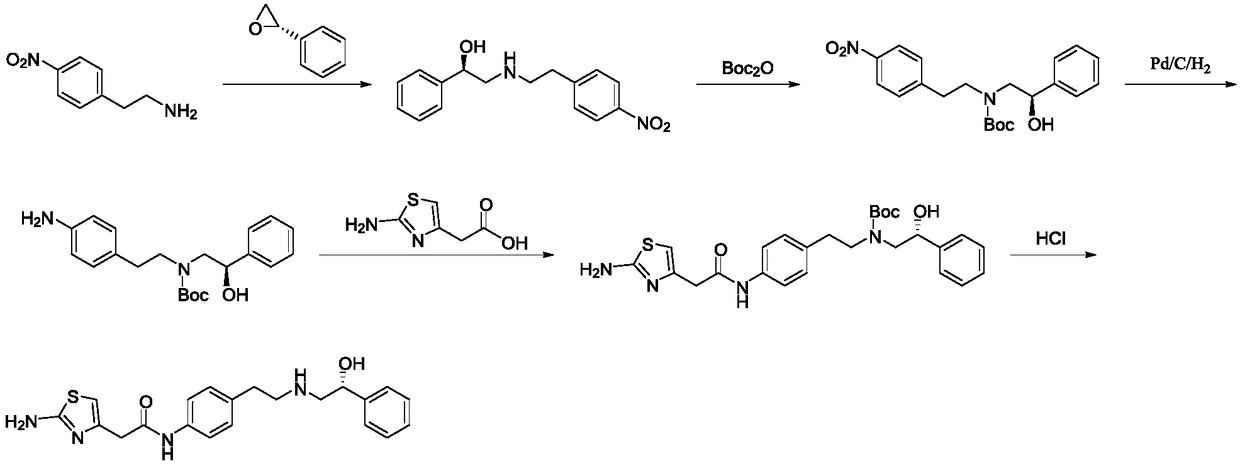
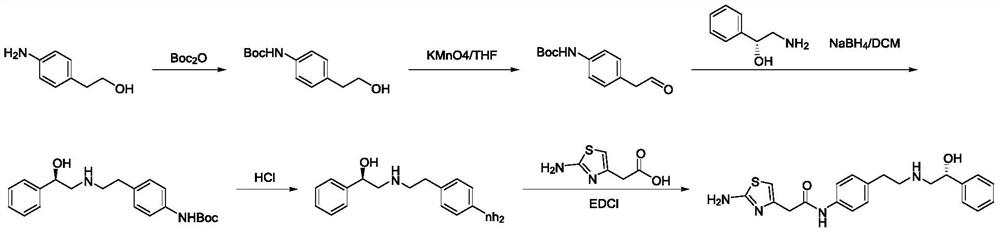
A kind of preparation method of Mirabegron
The invention discloses a preparation method of mirabegron, which relates to the technical field of medicine preparation, comprising the following steps: R-mandelic acid and p-nitrophenylethylamine undergo amide condensation reaction at high temperature to obtain intermediate (R)- 2‑Hydroxy‑N‑(4‑nitrophenethyl)‑2‑phenylacetamide; then the amide carbonyl is reduced by diisobutylaluminum hydride to obtain the intermediate (R)‑2‑((4‑nitro Phenylethyl)amino)-1-phenylethanol hydrochloride; then the nitro group is reduced by the ammonium formate-Pd / C reduction system to obtain the intermediate (R)-2-((4-aminophenylethyl)amino) ‑1‑phenylethanol; final condensation reaction with aminothiazoleacetic acid to obtain mirabegron. The mirabegron prepared by the present invention has good purity, high yield, few synthetic circuit steps, mild and controllable conditions, simple and convenient operation, low cost and is suitable for industrial production, and has broad prospects and industrial application value.
Owner:ANHUI QINGYUN PHARMA & CHEM
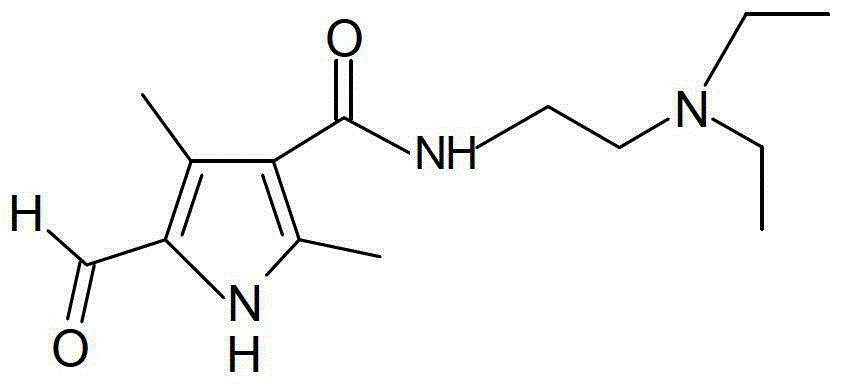
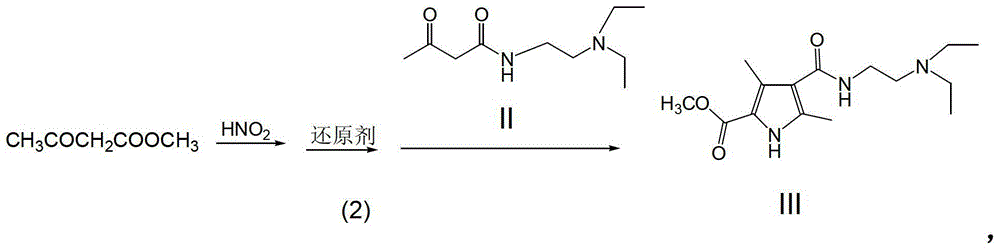
A kind of preparation method of sunitinib intermediate
The invention relates to a preparation method of a sunitinib intermediate 2, 4-dimethyl-5-formoxyl-1H-pyrrole-3-formyl (N, N-diethyl ethylenediamine). The method comprises the following steps: (1). carrying out a condensation reaction on ketene dimer and N, N-diethyl ethylenediamine to generate a compound II; (2). sequentially generating a nitrosation reaction and a reduction reaction on methyl acetoacetate to obtain a product, and reacting the product with the compound II obtained in the step (1) to generate a compound III; and (3). generating a reduction reaction on the compound III obtained in the step (2) in the presence of diisobutylaluminum hydride (DIBAL-H) to generate the sunitinib intermediate. Compared with an existing method, the method disclosed by the invention has the advantages of simplifying the reaction process, improving the yield of the intermediate and reducing the three-waste pollution, thereby having an important application value.
Owner:ZHANGJIAGANG HUACHANG PHARMA
Sulfoacid rare earth catalyst for polymerizing high-cis-isoprene rubber and preparation method thereof
The invention relates to a sulfoacid rare earth catalyst for polymerizing high-cis-isoprene rubber and a preparation method thereof. The catalyst comprises benzenesulfonic acid neodymium rare earth complex and alkyl aluminum; the molar ratio of the alkyl aluminum to the rare earth neodymium in the benzenesulfonic acid neodymium rare earth complex is 15 to 40:1, wherein the benzenesulfonic acid neodymium rare earth complex comprises benzenesulfonic acid neodymium alcohol complex, benzenesulfonic acid neodymium sulphoxide complex, benzenesulfonic acid neodymium furan complex, benzenesulfonic acid neodymium amine complex, benzenesulfonic acid neodymium ether complex and benzenesulfonic acid neodymium ester complex; ligand comprises: alcohol compound, sulfoxide compound, furan compound, aminecompound, ether compound and ester compound; and the alkyl aluminum comprises: 3-isobutyl aluminum, triethyl aluminum, diisobutyl aluminum hydride, or diethyl aluminum hydride. The sulfoacid rare earth complex catalyst is used for polymerizing isoprene, and can obtain the high-cis rare earth polyisoprene rubber with the cis 1,4 structure content more than 96.0 percent and the weight-average molecular weight more than 2.2 million.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
A kind of synthetic method of cephanolide C
ActiveCN108129432BRealized the first chemical synthesisSimple and fast operationOrganic chemistryChemical synthesisFormylation reaction
Owner:SHAANXI NORMAL UNIV
A kind of method using diisobutylaluminum hydride and libh4 as catalyst to prepare α-alh3
The invention relates to a method for preparing alpha-AlH3 by use of diisobutylaluminum hydride and LiBH4 as the catalyst, relates to a preparation method for alpha-AlH3, and aims to solve the problem that conditions required by direct synthesis of alpha-AlH3 through aluminum and hydrogen are strict. The method comprises the following steps: adding aluminum powder, diisobutylaluminum hydride and LiBH4 in an autoclave; introducing hydrogen, reacting at the condition with the temperature of 100-150 DEG C, and drying to obtain the product. In the method, the reaction pressure is 10-15 MPa which is much lower than 10 GPa, the reaction temperature is 100-150 DEG C which is much lower than 650 DEG C, through XRD tests, the synthesized product contains alpha-AlH3, and the method is applied to preparation of alpha-AlH3.
Owner:HARBIN INST OF TECH
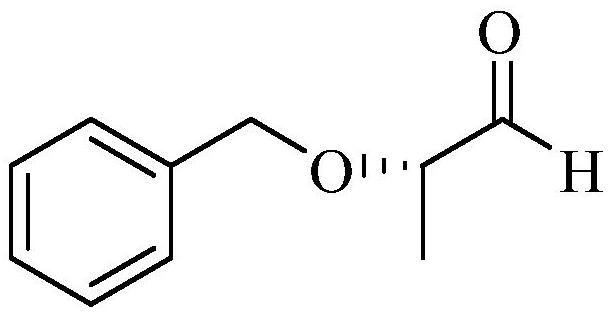
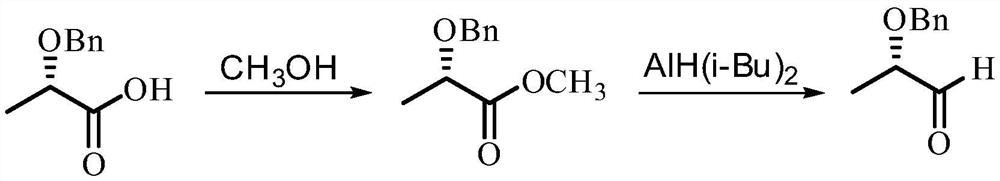
The method of synthesizing (s)-2-benzyloxypropanal
ActiveCN108101759BImprove operational safetyReduce manufacturing costOrganic compound preparationOrganic chemistry methodsOXALIC ACID DIHYDRATEPropanoic acid
The invention discloses a method for synthesizing (S)-2-benzyloxypropanal, the steps comprising: first reacting (S)-2-benzyloxypropionic acid with ethylenediamine to obtain (S)-2-( 1‑benzyloxyethyl)‑4,5‑dihydro‑1H‑imidazole; (S)‑2‑(1‑benzyloxyethyl)‑4,5‑dihydro‑1H‑imidazole was dissolved in Water and ethanol, and then add sodium metal under the protection of nitrogen to stir the reaction; after the reaction, remove the ethanol, and slowly add the residue to the saturated oxalic acid solution under the protection of nitrogen, stir evenly and then reflux for reaction. After the reaction is completed, extract, dry, filter, and remove The product (S)‑2‑benzyloxy propanal is obtained after solvent. The present invention prepares (S)-2-benzyloxypropanal in two steps; the preparation process does not use diisobutylaluminum hydride which is expensive and easy to catch fire during post-treatment, thereby fully improving the operational safety and reducing the Production cost; the preparation process is mild and easy to operate, without the anhydrous conditions required by traditional process reactions, and it is easy to realize industrial production.
Owner:东营睿港投资服务有限责任公司
A kind of synthetic method of crude torene terpenoid skeleton compound
ActiveCN108191803BSimple and fast operationLow costOrganic chemistryChemical synthesisFormylation reaction
The invention discloses a synthesis method of a cephalotaxus sinensis terpenoid framework compound. The method comprises the following steps: commercially available 2-methyl-5-bromoanisole as a synthetic raw material is subjected to Ei-ichi Negishi reaction for para-halogenation with a methoxyl group, then a product is subjected to formylation reaction with lithium diisopropylamide, and a 1,3-dicarbonyl compound is obtained; the 1,3-dicarbonyl compound is subjected to a Robinson cyclization reaction with 3-pentene-2-one, and a lactone compound is obtained through Luche reduction, hydrolysis and esterification reaction; acid treatment is performed after reduction by diisobutylaluminum hydride, a Heck reaction precursor is obtained and subjected to palladium-catalyzed carbonyl esterificationcoupling reaction, and chemical synthesis of the crude cephalotaxus sinensis terpenoid framework compound is achieved by esterification. The synthetic route has the advantages of being simple, efficient, convenient to operate, low in cost and the like, and the method is applicable to large-scale synthesis of the cephalotaxus sinensis terpenoid framework compound, and provides an important experimental basis for chemical synthesis of cephalotaxus sinensis terpenoid natural products.
Owner:SHAANXI NORMAL UNIV
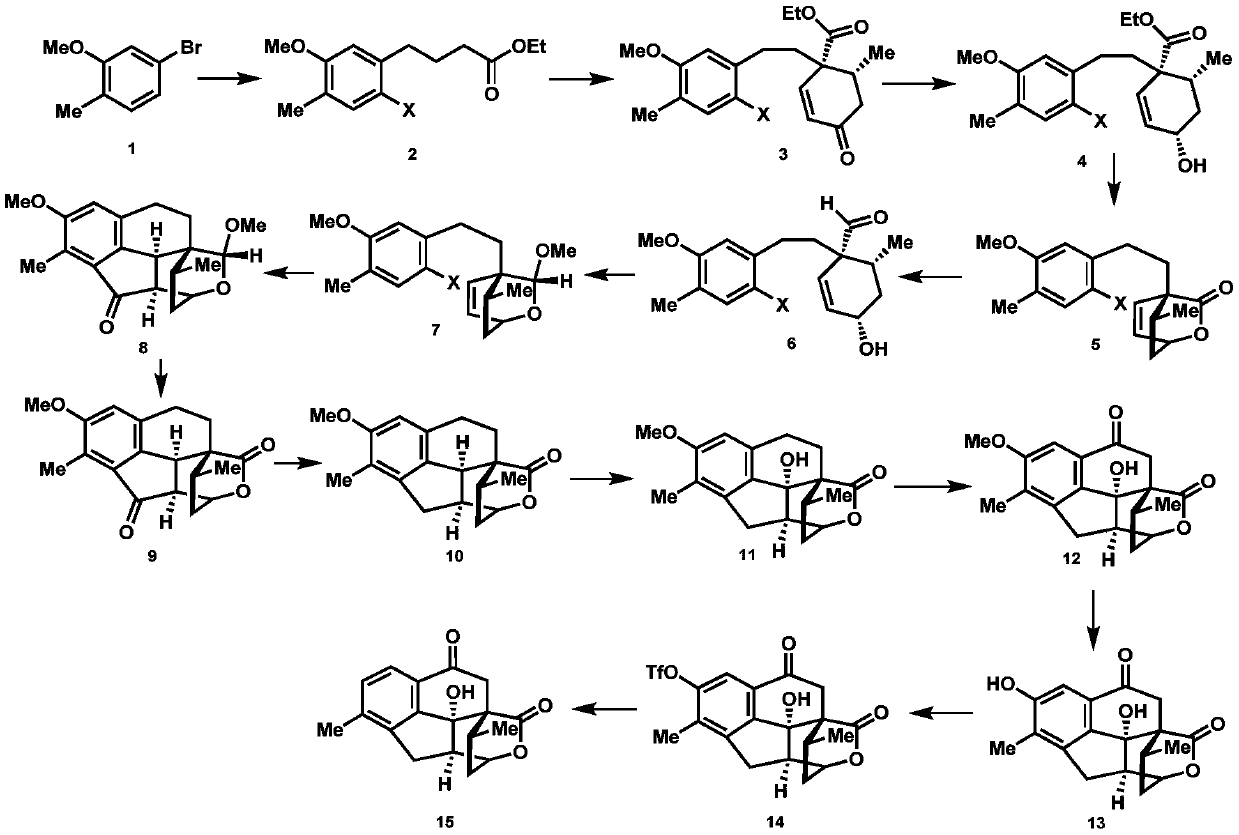
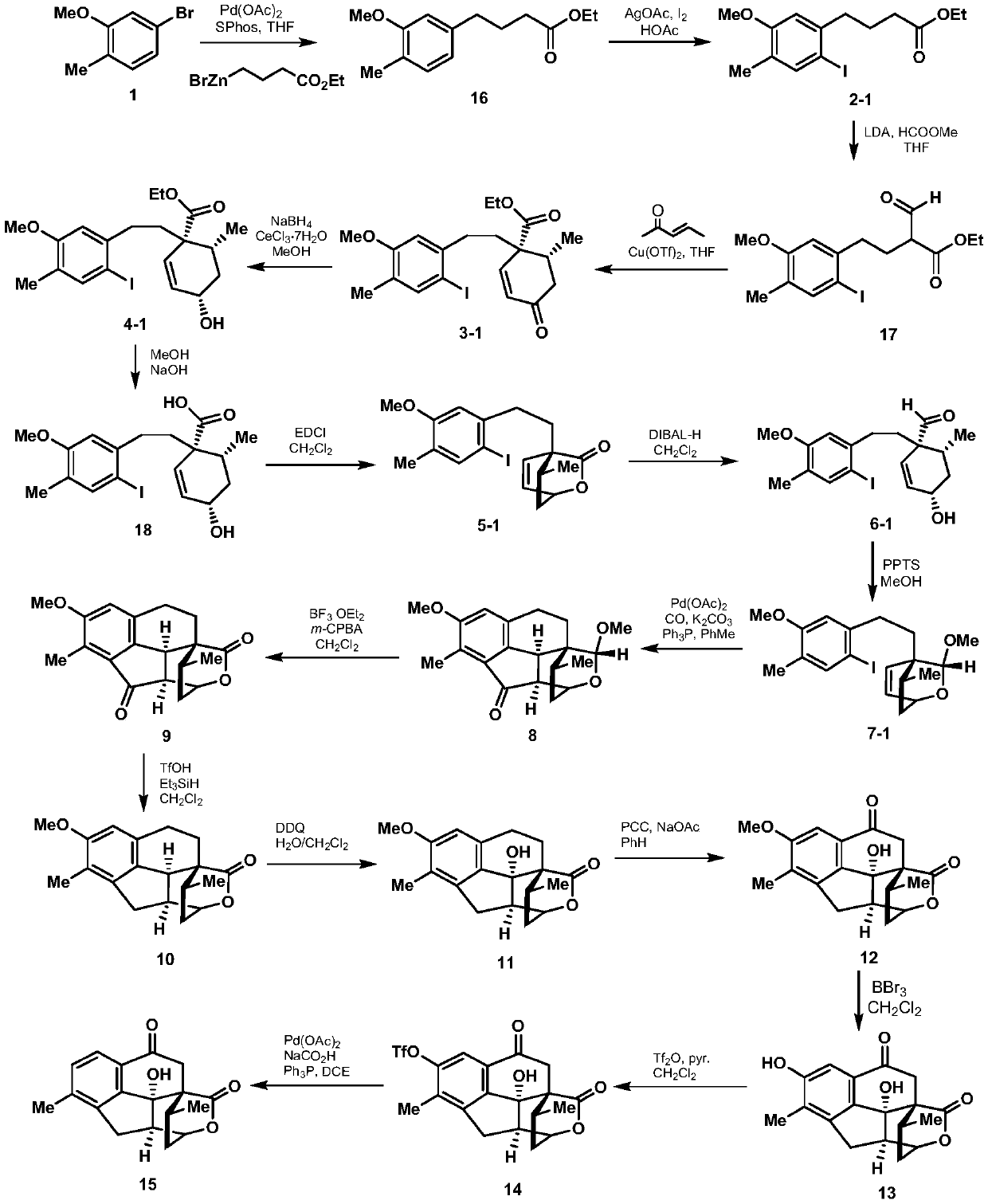
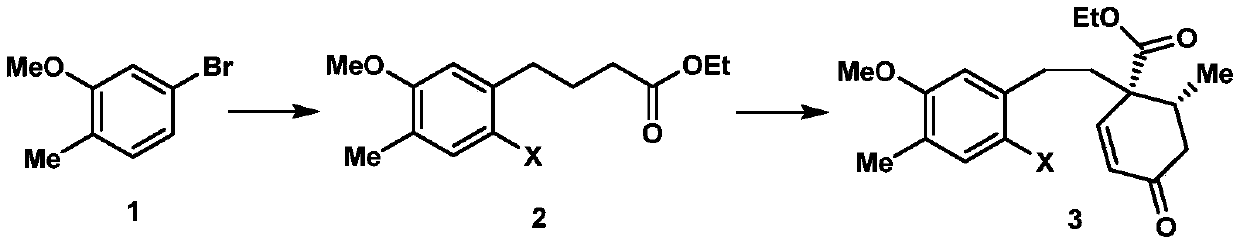
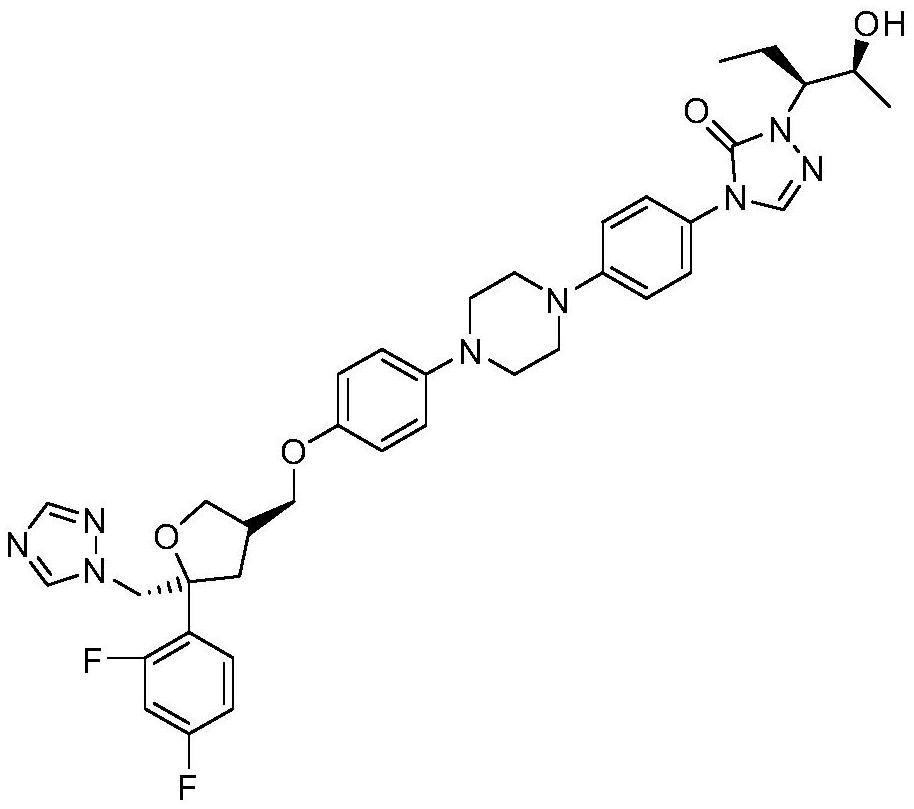
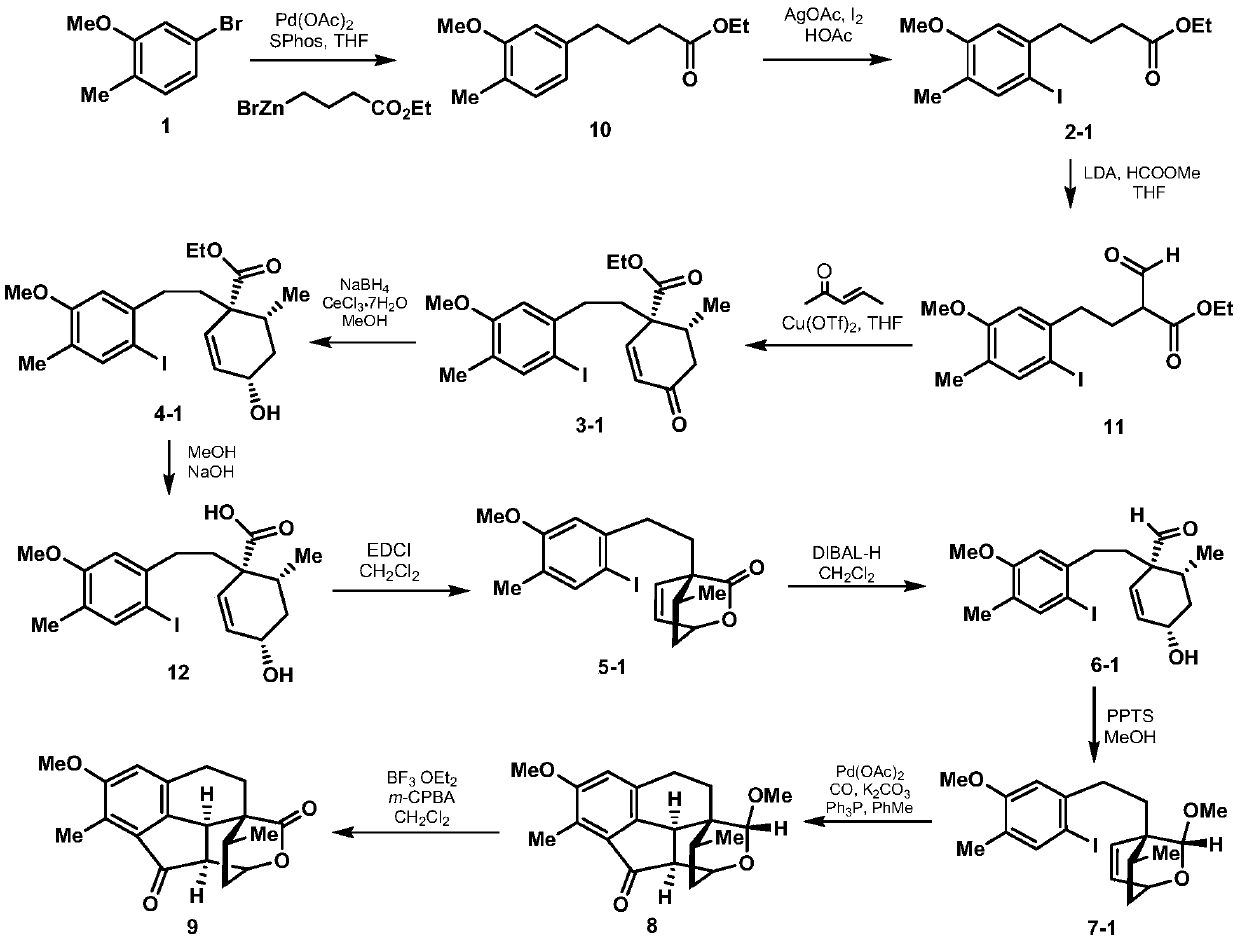
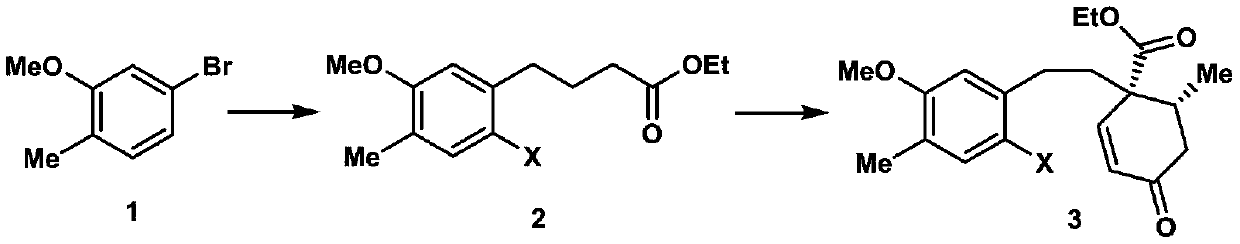
2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid
ActiveCN107383035BReasonable reaction process designThe method route is simpleOrganic chemistry methodsAcetic anhydrideLithium hydroxide
The invention relates to a preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid. The preparation method comprises 11 steps: carrying out esterification on a dicarboxylic acid piperidine compound 1 in thionyl chloride to obtain a diester compound 2; then reducing a pyridine ring by utilizing palladium carbon to obtain a compound 3; then taking the compound 3 to react with Boc acid anhydride to obtain a Boc protected compound 4; hydrolyzing the compound 4 by utilizing lithium hydroxide to obtain a dicarboxylic acid compound 5; carrying out ring closure in acetic anhydride to obtain a compound 6; carrying out reduction and ring opening by utilizing NaBH4 to obtain a mixture of position isomerism compounds 7A and 7B; carrying out the ring closure in the presence of iodomethane and potassium carbonate to generate position isomerism lactone 8A and 8B; step 8, reducing the 8A, obtained by column passing separation, by utilizing DIBAH (Diisobutylaluminum Hydride) to obtain a compound 9; carrying out Wittig reaction to obtain a compound 10; carrying out Michael addition under an alkaline condition to obtain a compound 11; finally, hydrolyzing the compound 11 under the alkaline condition to obtain a final compound.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +3
Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid
ActiveCN107383035AReasonable reaction process designThe method route is simpleOrganic chemistry methodsAcetic anhydrideTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid. The preparation method comprises 11 steps: carrying out esterification on a dicarboxylic acid piperidine compound 1 in thionyl chloride to obtain a diester compound 2; then reducing a pyridine ring by utilizing palladium carbon to obtain a compound 3; then taking the compound 3 to react with Boc acid anhydride to obtain a Boc protected compound 4; hydrolyzing the compound 4 by utilizing lithium hydroxide to obtain a dicarboxylic acid compound 5; carrying out ring closure in acetic anhydride to obtain a compound 6; carrying out reduction and ring opening by utilizing NaBH4 to obtain a mixture of position isomerism compounds 7A and 7B; carrying out the ring closure in the presence of iodomethane and potassium carbonate to generate position isomerism lactone 8A and 8B; step 8, reducing the 8A, obtained by column passing separation, by utilizing DIBAH (Diisobutylaluminum Hydride) to obtain a compound 9; carrying out Wittig reaction to obtain a compound 10; carrying out Michael addition under an alkaline condition to obtain a compound 11; finally, hydrolyzing the compound 11 under the alkaline condition to obtain a final compound.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +3
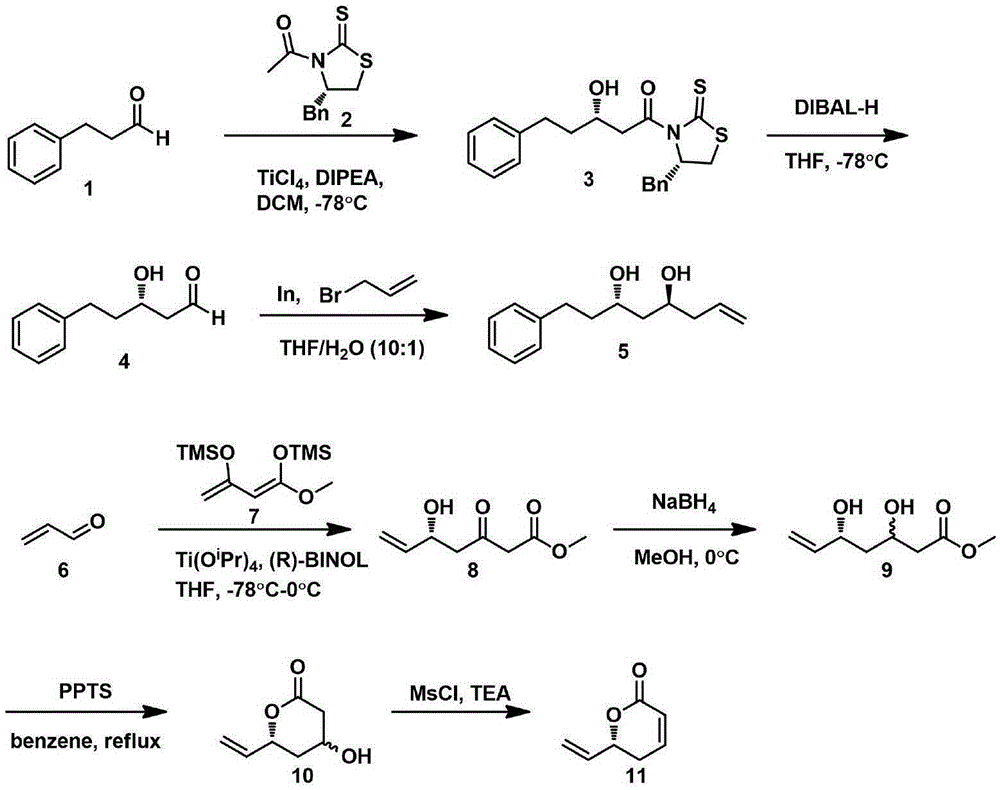
Synthetic method of natural product (+)-strictifolione
InactiveCN105254604BSimple and fast operationHigh yieldOrganic chemistryChemical recyclingStrictifolioneIndium
The invention discloses a method for synthesizing natural product (+)-Strictifolione. The present invention utilizes 3-phenylpropanal and Evans chiral auxiliary agent to carry out aldol condensation reaction, then reduces with diisobutyl aluminum hydride, and then adds 3-propylene bromide activated by metal indium to obtain the compound of formula 5; enol Silicone ether reacts with acrolein under the action of titanium tetraisopropoxide and (R)-1,1-binaphthol, then reduces the carbonyl group with sodium borohydride, and then uses pyridinium p-toluenesulfonate for ring closure, and then forms a After the sulfonyl group becomes a leaving group, the acid is added to obtain the compound of formula 11; the compound of formula 5 and the compound of formula 11 are subjected to metal coupling reaction using Grubbs second-generation catalyst to obtain (+)-Strictifolione. The "dichotomy" synthesis method of the present invention is simple and reasonable in design, easy to operate, mild in reaction conditions, less in linear steps, high in product yield, and greatly reduces production cost.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
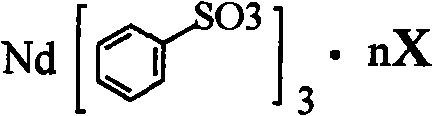
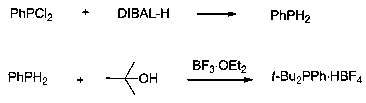

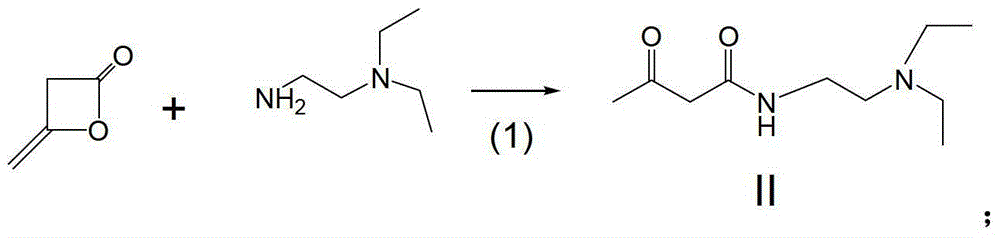
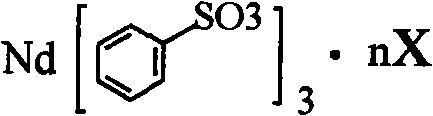
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/DEST_PATH_IMAGE003.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/505010DEST_PATH_IMAGE004.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/600639DEST_PATH_IMAGE002.png)
![Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/3100eb7b-3fd2-4cc9-8776-f6c91c9defda/505010DEST_PATH_IMAGE004.png)
![Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/3100eb7b-3fd2-4cc9-8776-f6c91c9defda/600639DEST_PATH_IMAGE002.png)
![Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/3100eb7b-3fd2-4cc9-8776-f6c91c9defda/DEST_PATH_IMAGE003.png)