Patents
Literature
35 results about "Tafluprost" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
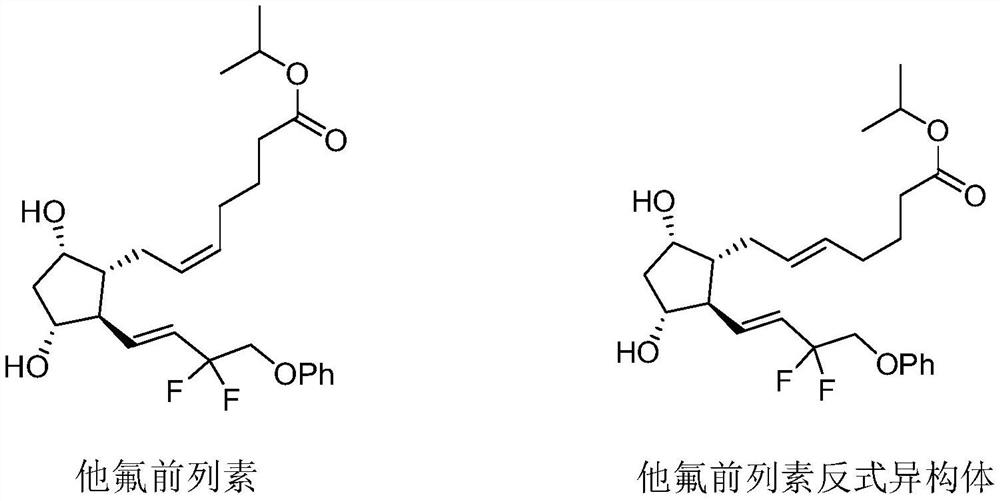
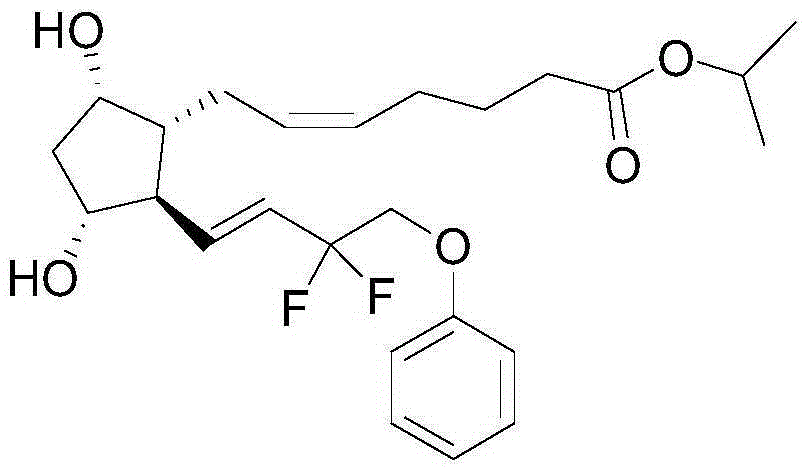
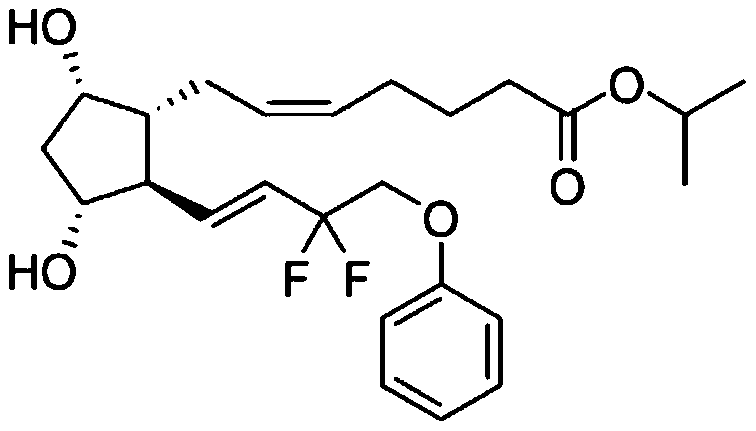
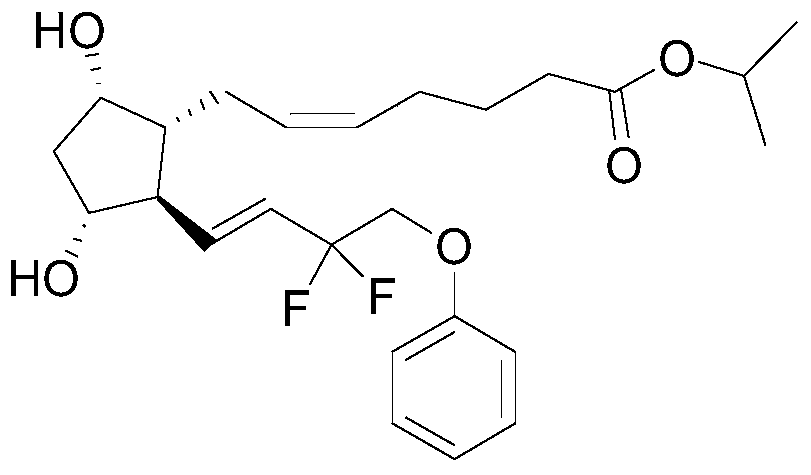
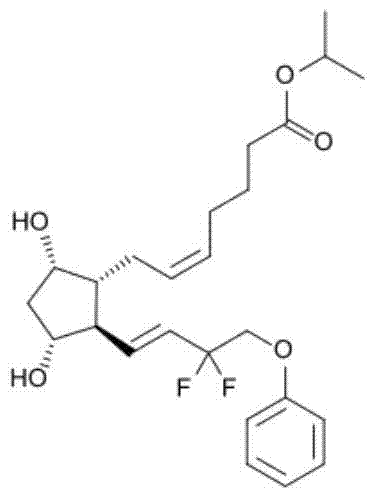
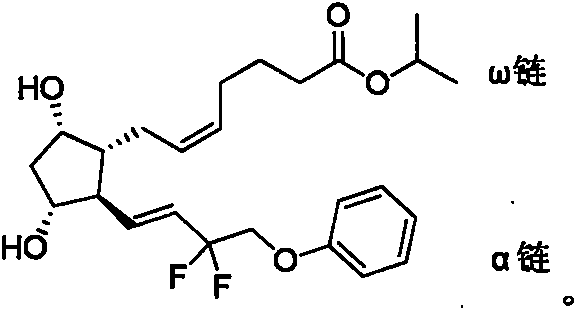
Tafluprost is used to treat high pressure inside the eye due to glaucoma (open-angle type) or other eye diseases (such as ocular hypertension).
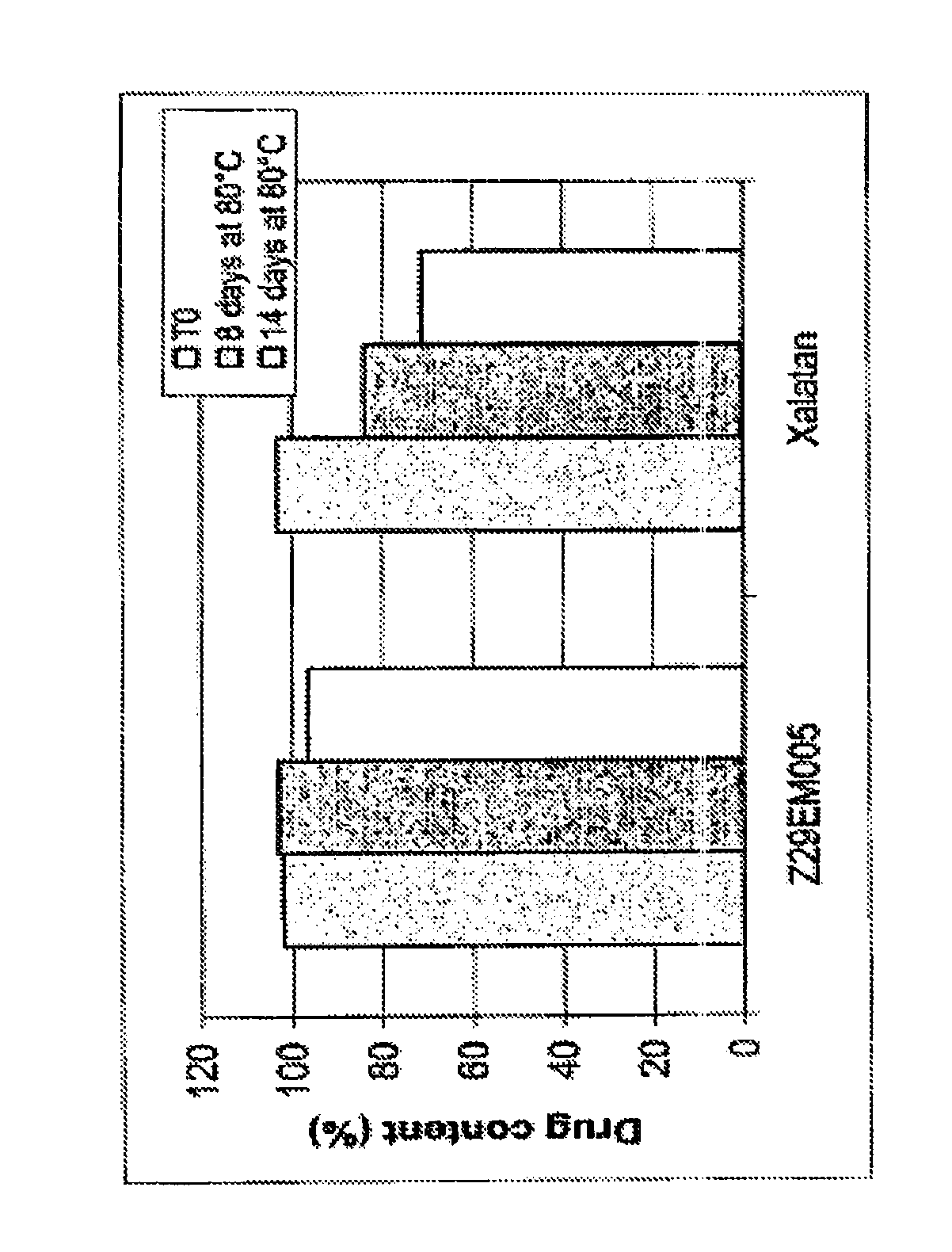
Ophthalmic emulsions containing prostaglandins
ActiveUS8273362B2Good chemical stabilityToxic reductionAntibacterial agentsPowder deliveryNon ionicChemical stability
Cationic ophthalmic oil-in-water type emulsions, include colloid particles having an oily core surrounded by an interfacial film, the emulsion including at least one cationic agent and at least one non ionic surfactant, the oily core including a prostaglandin selected from the group comprising in particular latanoprost, unoprostone isopropyl, travoprost, bimatoprost, tafluprost, 8-isoprostaglandinE2, or a mixture thereof, for treating ocular hypertension and / or glaucoma. These emulsions have the property to increase the chemical stability of prostaglandins.
Owner:SANTEN SAS
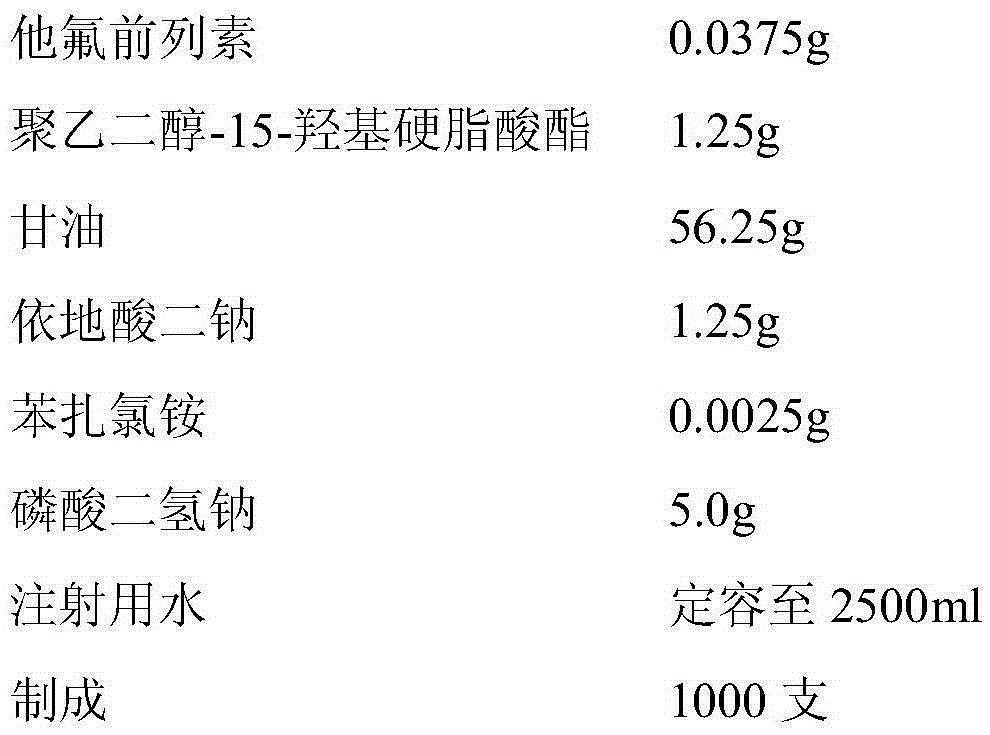
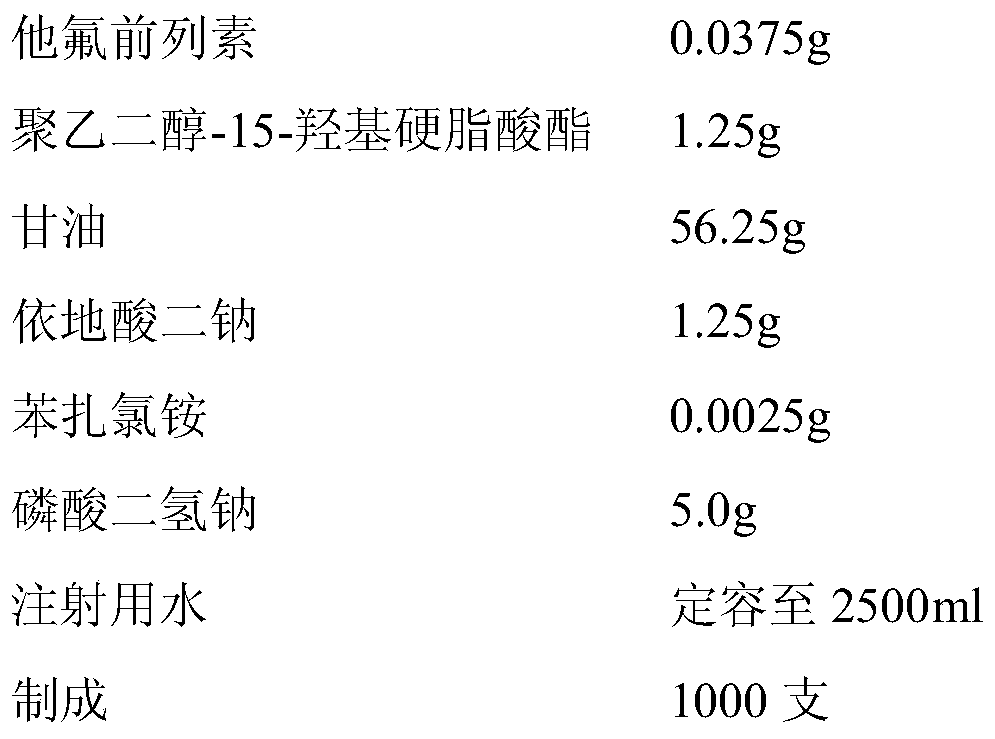
Tafluprost eye drop and preparation method thereof
ActiveCN105380901AReasonable preparation processEasy to operateOrganic active ingredientsSenses disorderSolubilityPolyethylene glycol
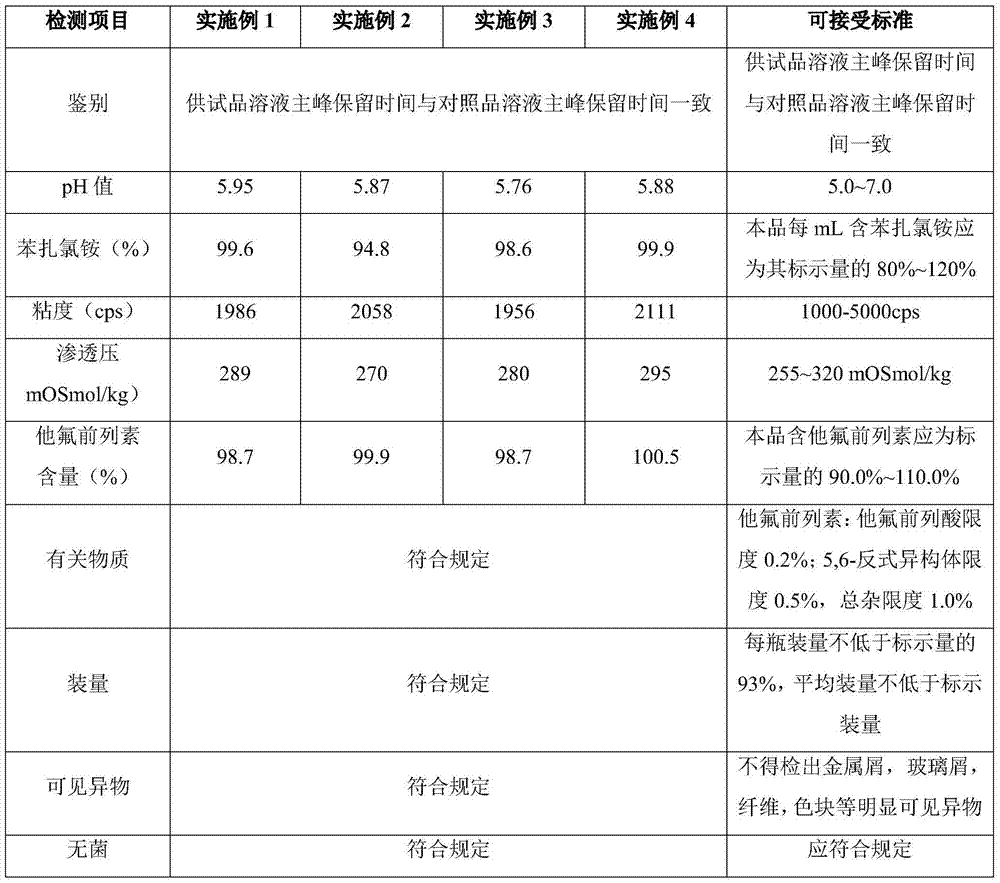
The invention relates to a tafluprost eye drop and a preparation method thereof. The eye drop takes tafluprost as an active ingredient and also contains a non-ionic solubilizer and a pharmaceutically acceptable carrier, wherein the non-ionic solubilizer is polyethylene glycol-15-hydroxyl stearate. According to the invention, the problem of low solubility of tafluprost in the preparation process can be solved, the stability of tafluprost in an aqueous solution can be increased, and formation of related substances is reduced.
Owner:苏州工业园区天龙制药有限公司
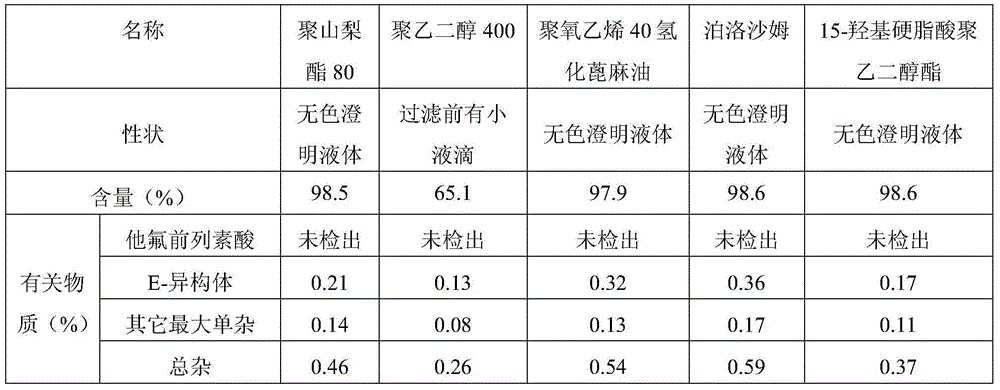
Good-stability eye-drop preparation containing PGF2alpha derivative and preparation method thereof
InactiveCN105012231AImprove stabilityDecrease increaseOrganic active ingredientsSenses disorderHydroxystearic AcidSolvent
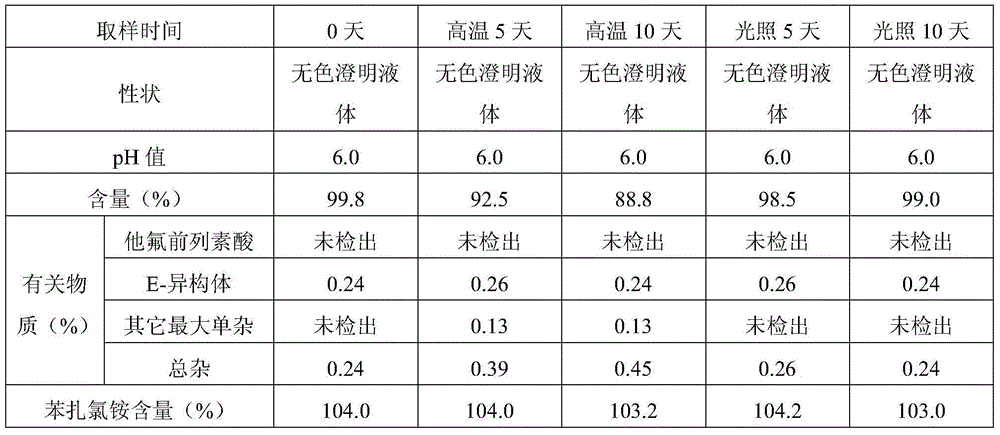
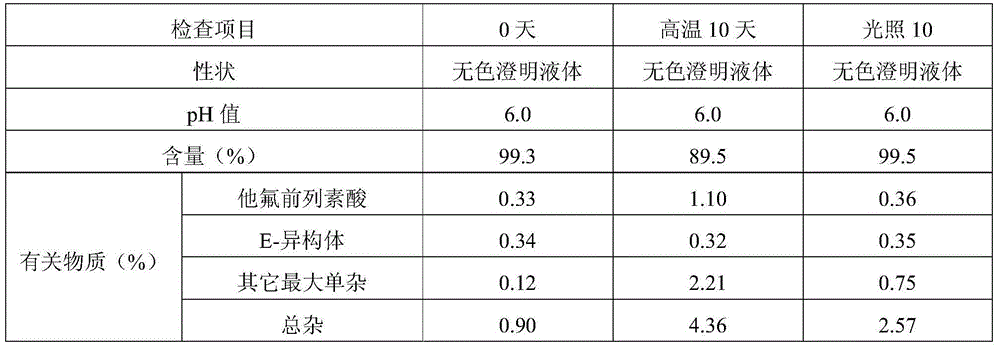
The invention provides a good-stability eye-drop preparation containing a PGF2alpha derivative. The eye-drop preparation comprises the PGF2alpha derivative serving as an active constituent and also comprises 15-hydroxy stearic acid polyglycol ester. It is surprised to find that after the 15-hydroxy stearic acid polyglycol ester serves as a solutizer of the PGF2alpha derivative (such as tafluprost) eye-drop preparation, the stability of active medicine is obviously improved; after the product is subjected to tests of the high temperature, the hard light, the acceleration stability and the long-term stability, compared with existing eye drops, the increase amount of related substances is obviously smaller, and the pharmacological activity and safety of the product are further guaranteed.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Eye drops containing tafluprost and preparation method thereof
ActiveCN104622798AGood bioadhesionImprove complianceOrganic active ingredientsSenses disorderTafluprostEnhanced bioavailability
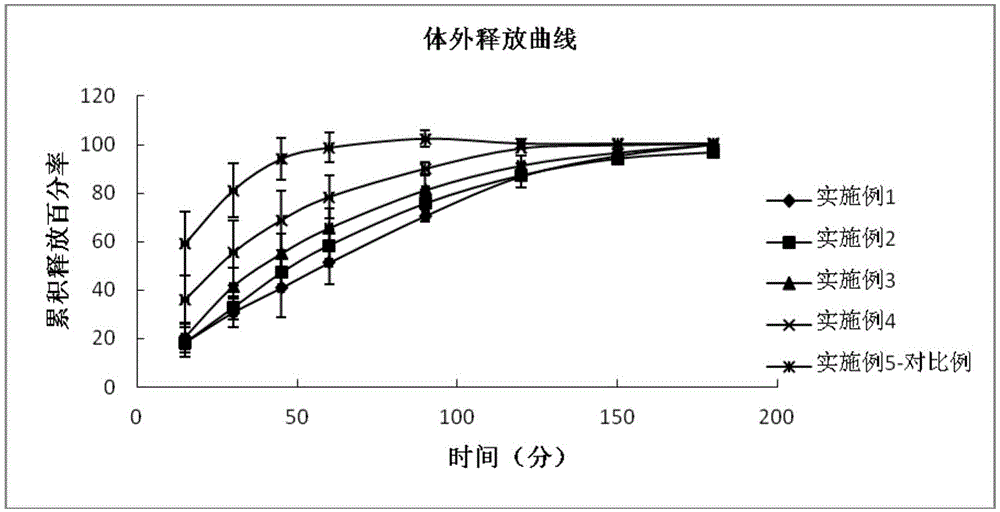
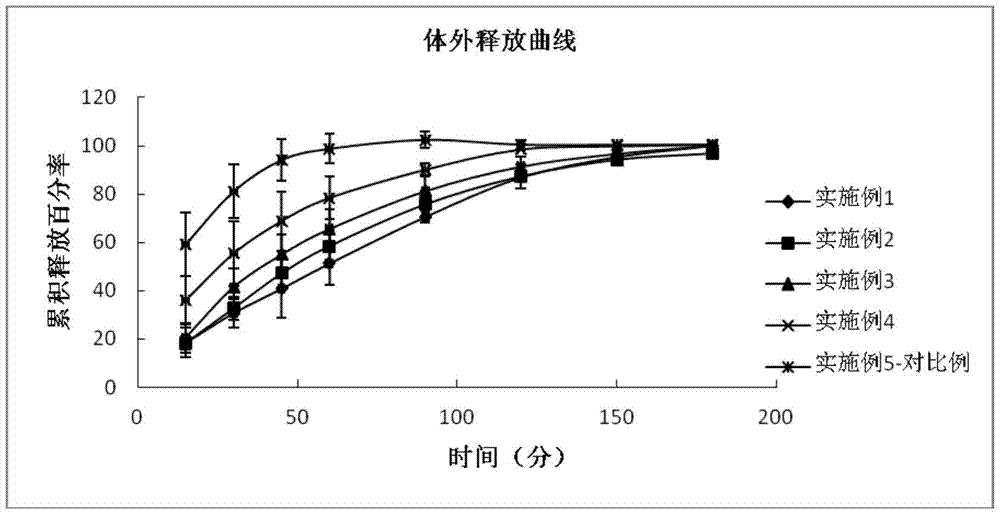
The invention provides eye drops containing tafluprost. The eye drops comprise tafluprost and a hydrophilic polymer, wherein the hydrophilic polymer is selected from polycarbophil or carbomer and the content of the hydrophilic polymer is 0.4-1.0%. Medicament storage formed by the eye drops plays a role of slowly releasing medicaments, prevents medicaments from being rapidly eliminated, and improves the bioavailability. The preparation method of the eye drops is simple and feasible and suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Method of purification of prostaglandins including fluorine atoms by preparative high performance liquid chromatography
InactiveCN103588692AOvercoming Purification ProblemsOrganic compound preparationCarboxylic acid esters preparationPreparative hplcChemical structure
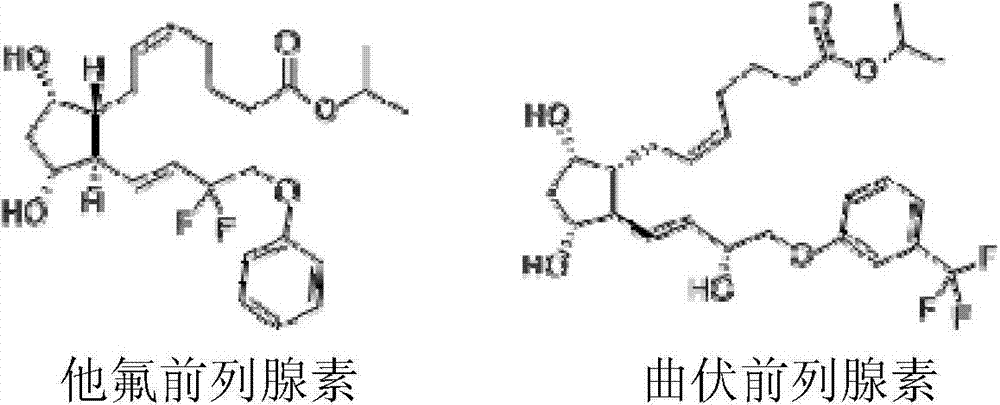
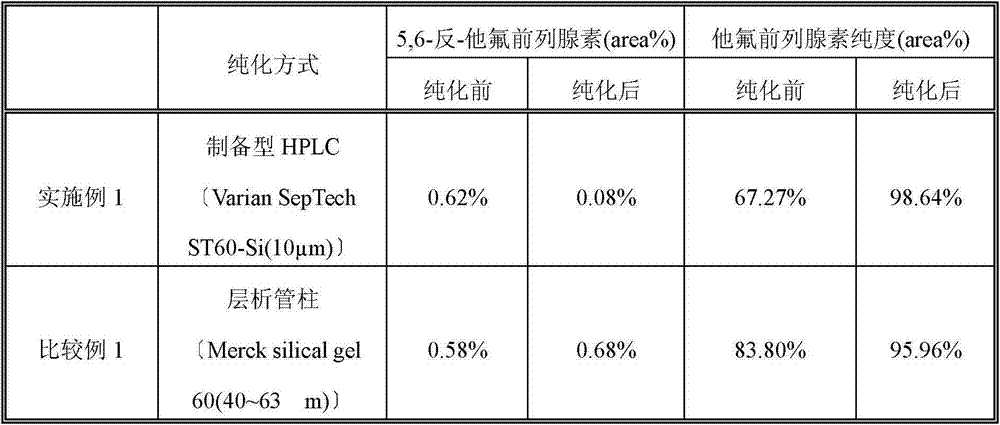
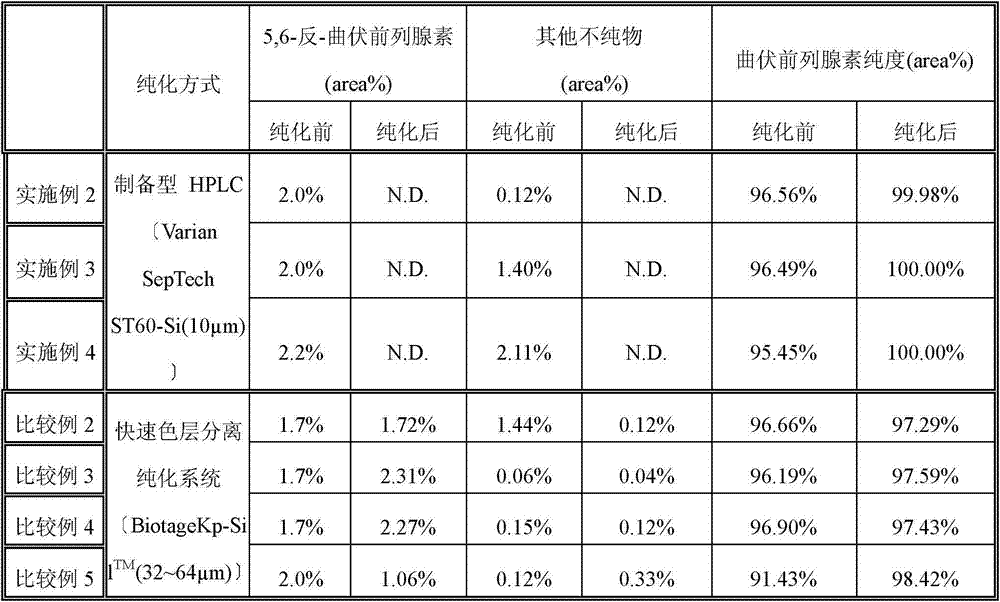
The present invention discloses a method of purification of prostaglandins including fluorine atoms by using preparative HPLC. Tafluprost and Travoprost are prostaglandins including fluorine. The chemical structure of the impurities in crude Tafluprost and crude Travoprost also contain fluorine, therefore, the removal of the impurities is difficult. Purification by using preparative high performance liquid chromatography (HPLC) can achieve high-quality liquid bulk drugs.
Owner:EVERLIGHT CHEMICAL INDUSTRIAL CORPORATION
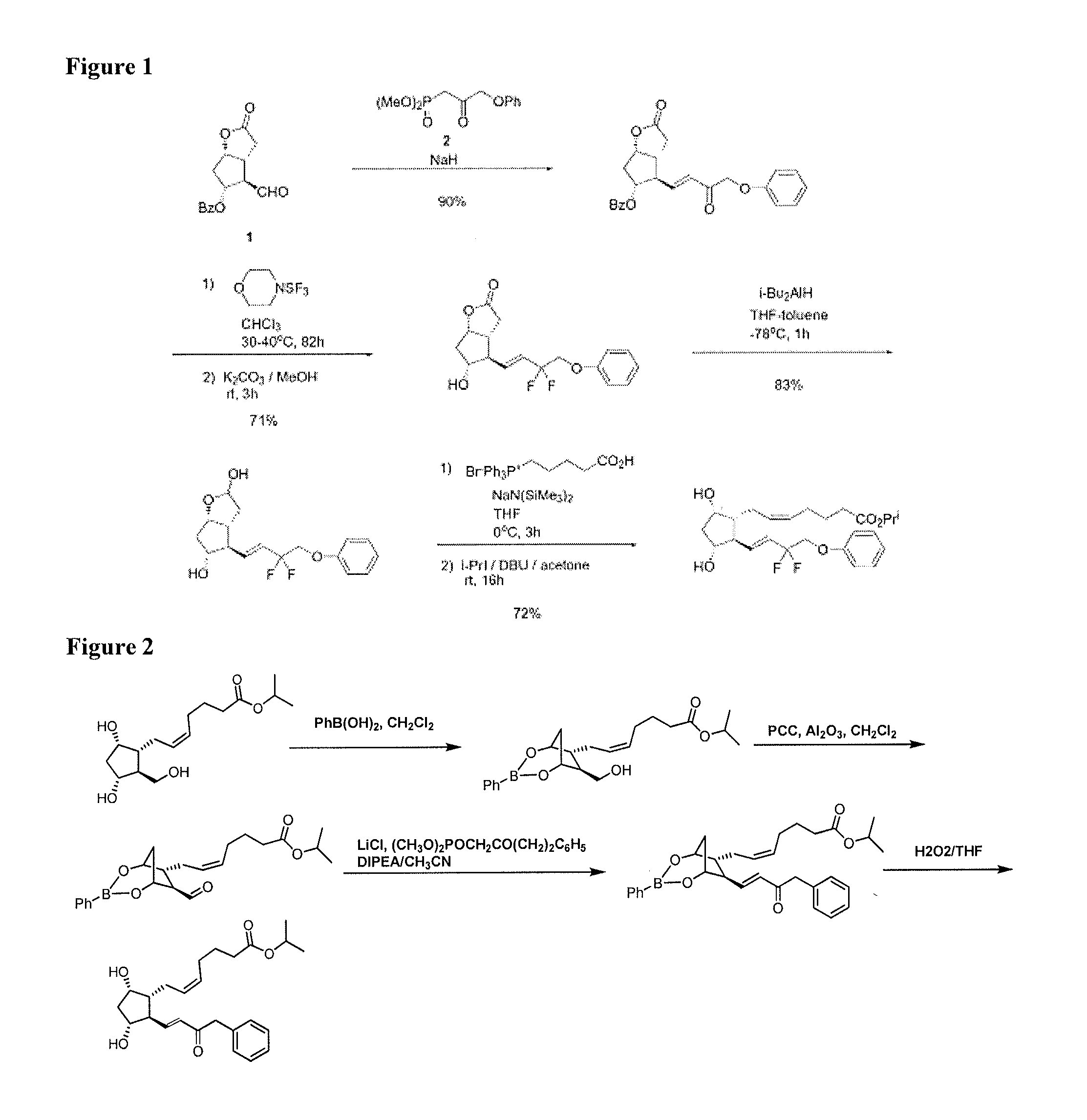
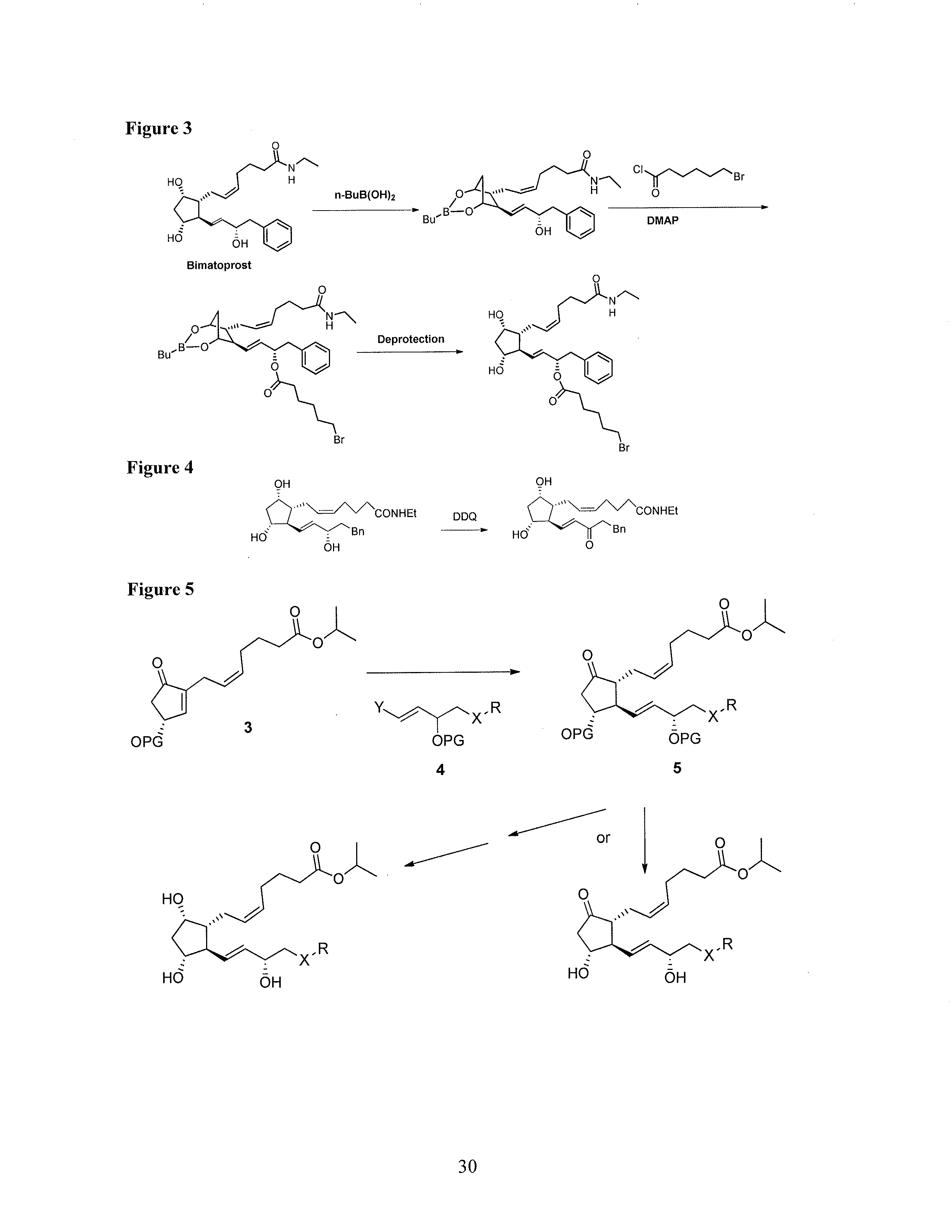
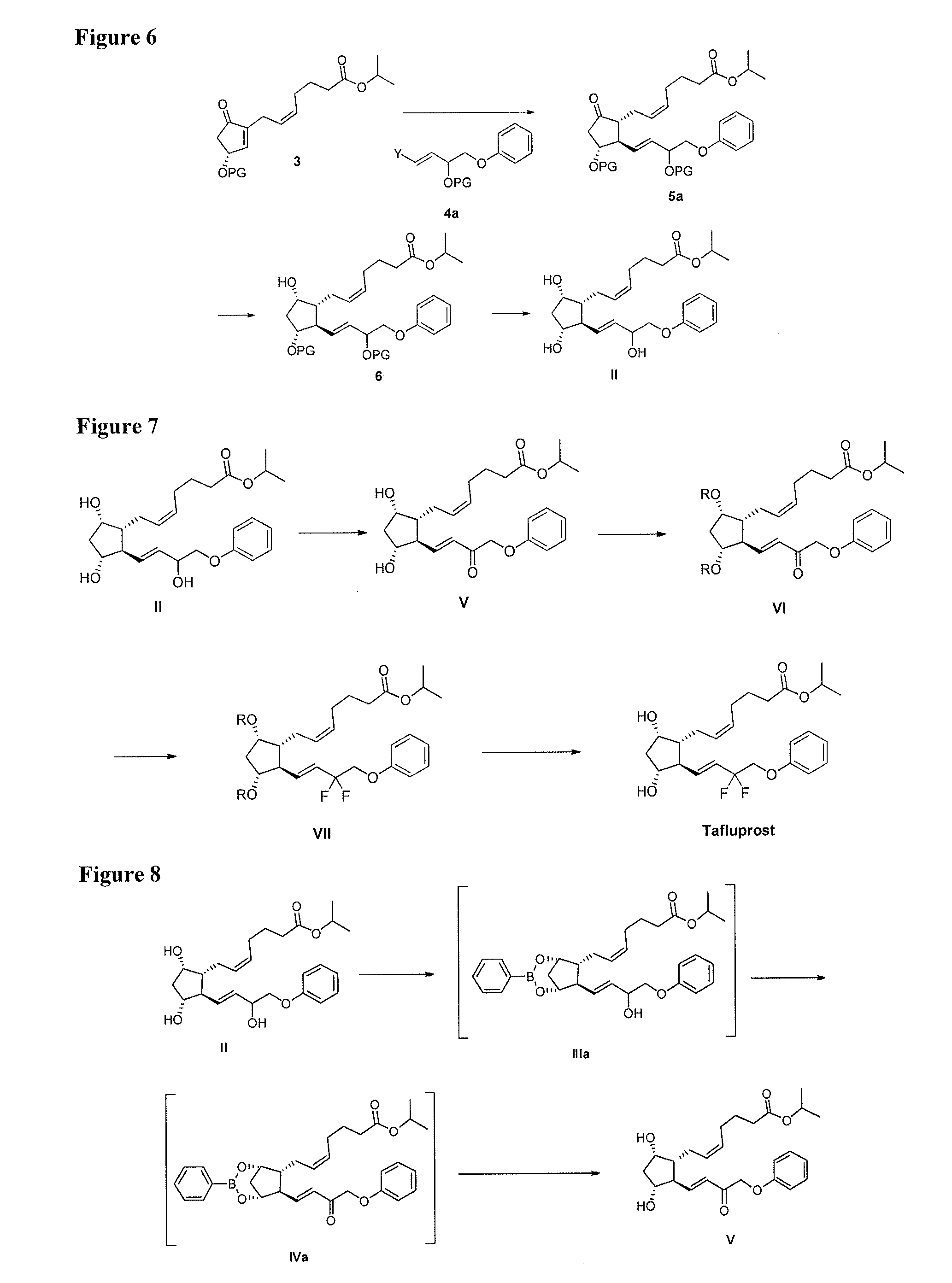
Process for the preparation of tafluprost and intermediates thereof
InactiveUS20140046086A1Senses disorderPreparation from carboxylic acid halidesSynthetic ProstaglandinsProstaglandin analog
The present invention provides efficient, economical and environmental friendly methods for synthesis of prostaglandin analogs including tafluprost and intermediates thereof. The invention involves a selective oxidation using in situ boronate ester protection and a unique crystallization method to remove the undesired isomers of fluorinated intermediates.
Owner:SCINOPHARM CHANGSHU PHARMA
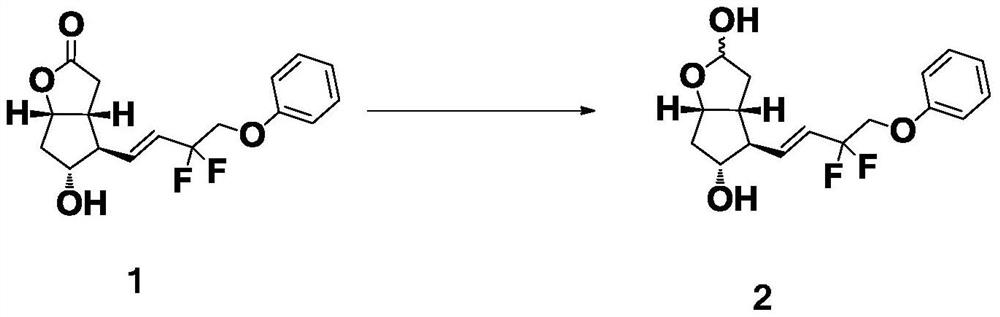
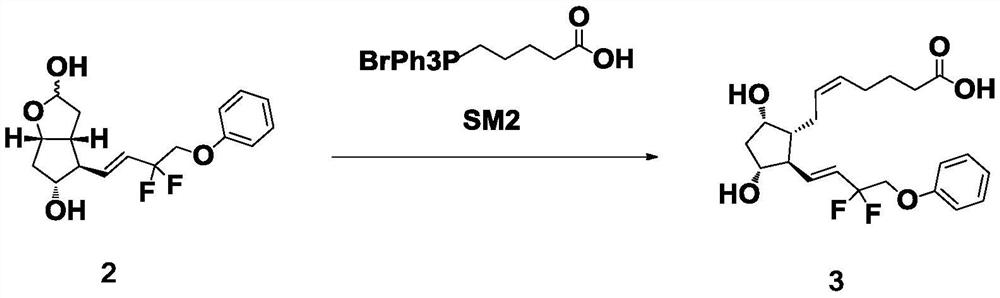
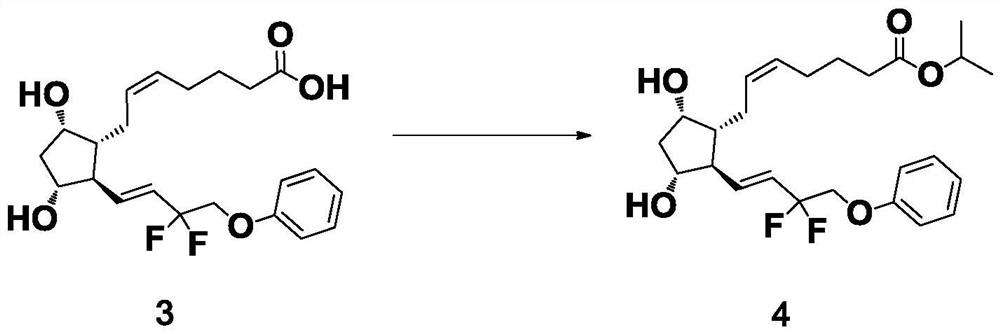
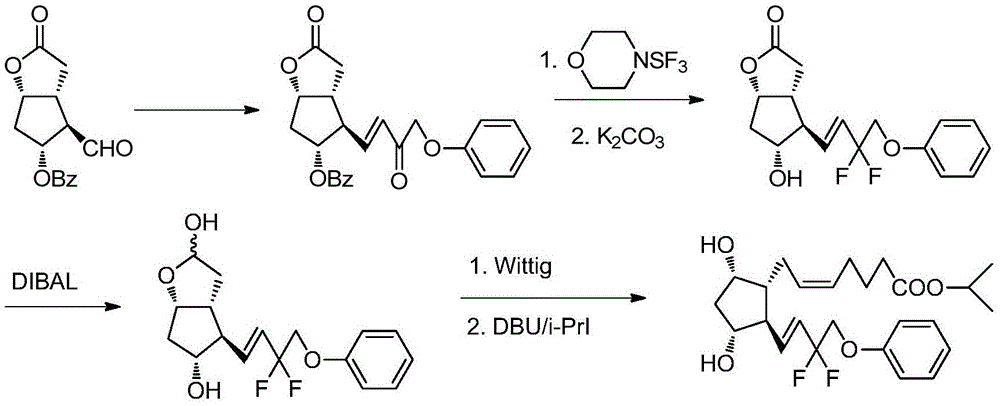
Preparation method of Tafluprost
ActiveCN106986766AHigh purityQuality is easy to controlOrganic compound preparationCarboxylic acid esters preparationWittig reactionEsterification reaction
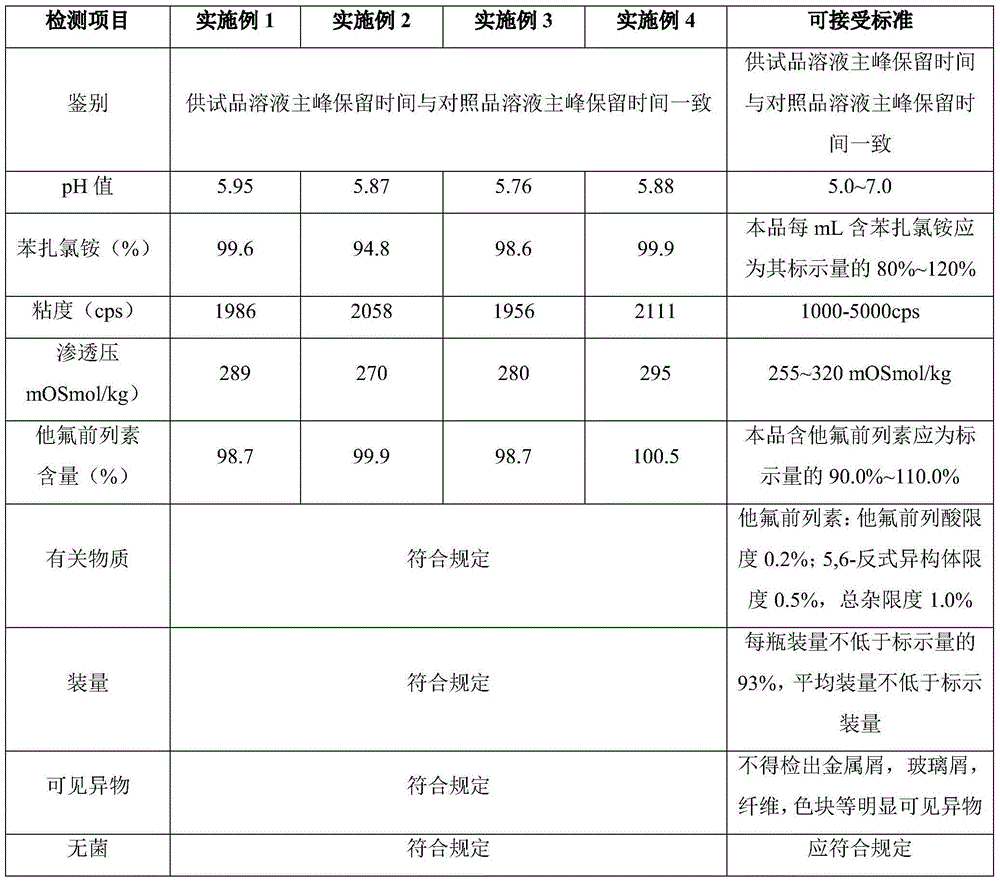
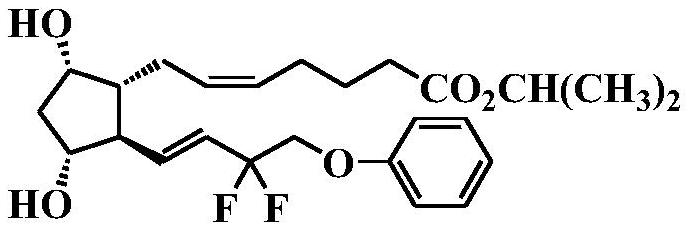
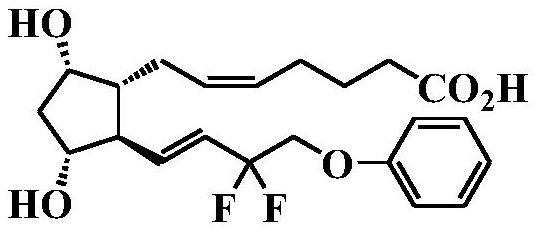
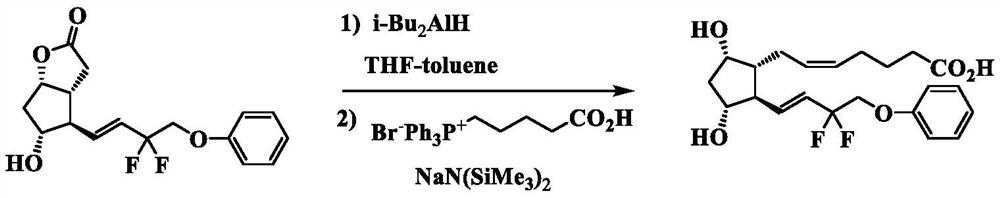
The invention discloses a preparation method of Tafluprost. The preparation method comprises the following steps: adopting a compound of formula 1 as a raw material, and carrying out the steps of DIBAL-H reduction, a Wittig reaction and an esterification reaction, wherein alkaline amino acids are adopted for refining after the Wittig reaction. According to the preparation method disclosed by the invention, the total mass yield of the three-step reaction can reach 70%, the technology is stable, a prepared Tafluprost product is colorless or faint yellow thick oily liquid, and the purity reaches up to more than 99%.
Owner:YANGTZE RIVER PHARM GRP CO LTD
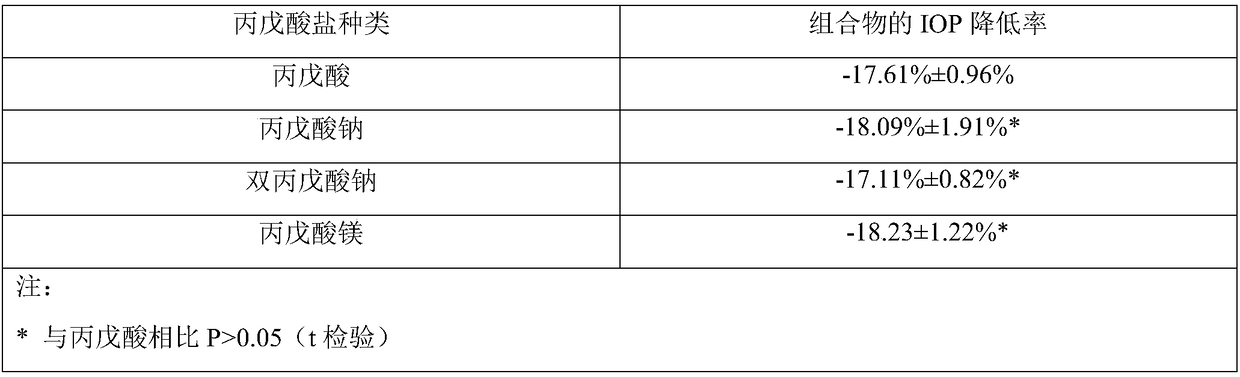
Composition comprising prostaglandin derivatives and ophthalmic liquid formulation comprising the composition
ActiveCN109172580AReduce concentrationReduce usageSenses disorderPharmaceutical delivery mechanismSodium divalproexMagnesium Valproate
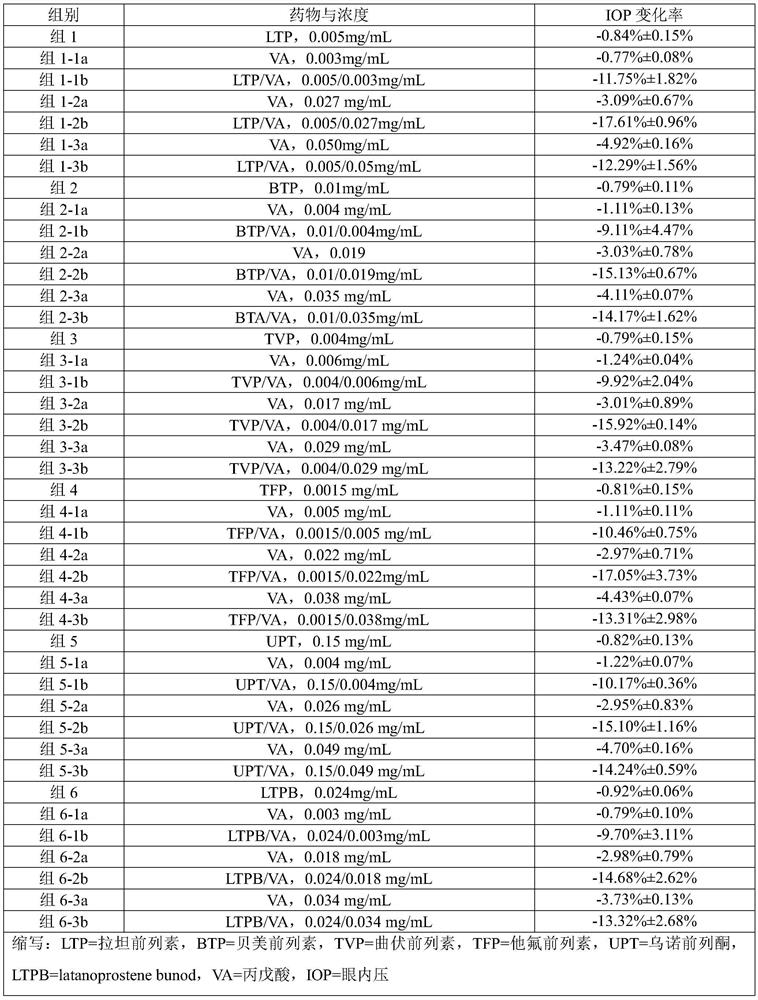
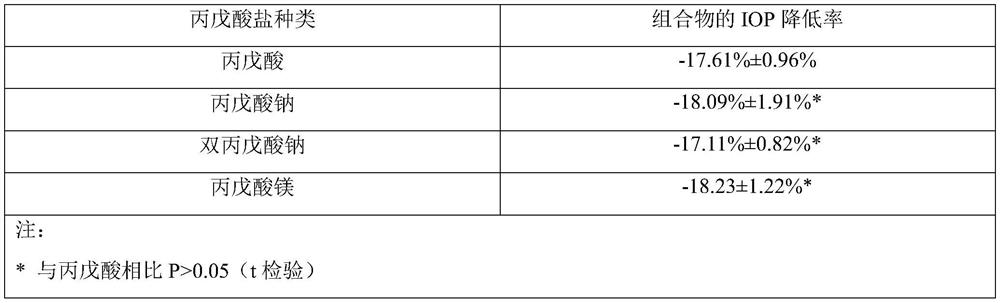
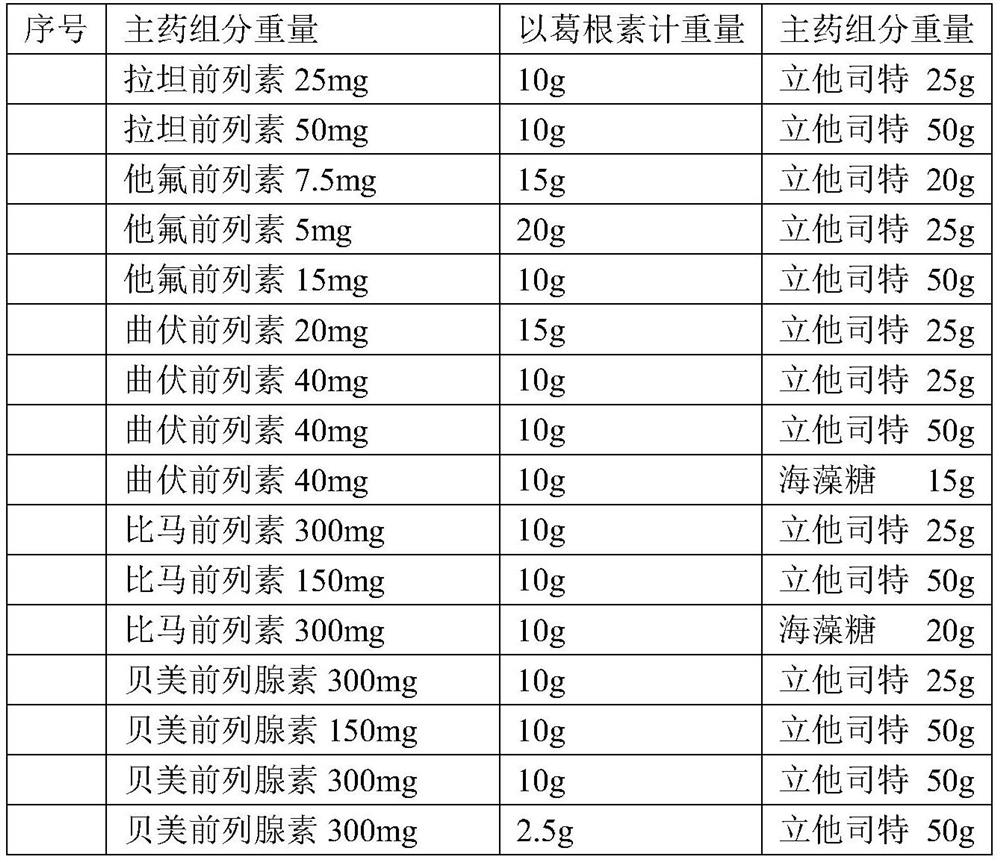
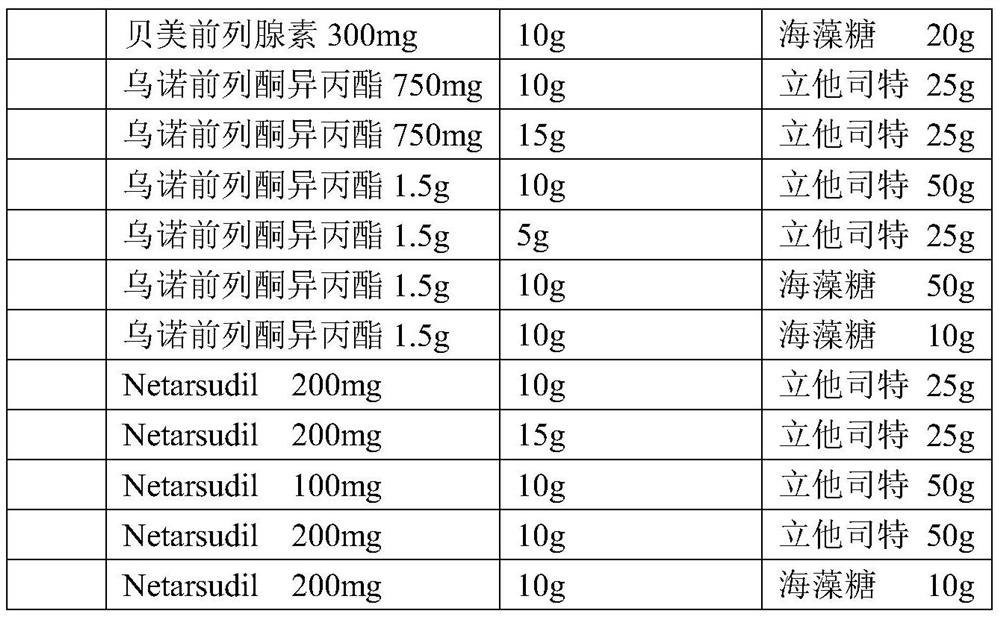
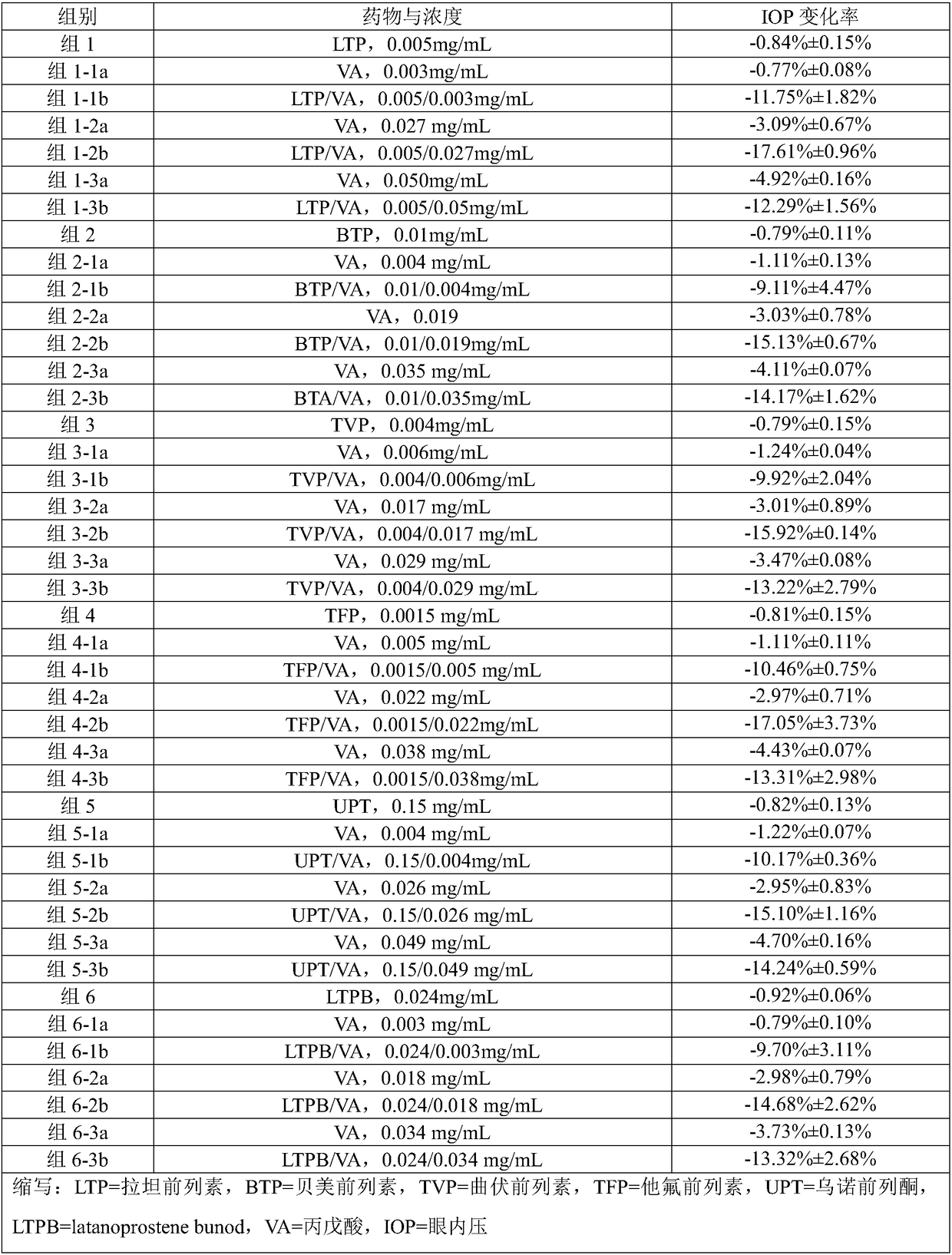
The invention provides a composition comprising prostaglandin derivatives and valproic acid or a pharmaceutically acceptable salt thereof and an ophthalmic liquid formulation comprising the composition, wherein the prostaglandin derivative is preferably one of latanoprost, bemeprost, travoprost, taflurane, unoprost and latanoprostene, and valproic acid or a pharmaceutically acceptable salt thereofis preferably one of valproic acid, sodium valproate, sodium divalproate and magnesium valproate. The composition provided by the invention can synergistically reduce intraocular pressure, avoid theuse of cosolvent, and improve the safety of medicament.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Method for large-scale preparation of tafluprost
PendingCN112209863AReduce generationGood effectOrganic chemistryTrichloroacetonitrileCombinatorial chemistry
The invention discloses a method for industrially preparing tafluprost. The method comprises the following steps: taking Corey lactone as an initial raw material, oxidizing, condensing, fluorinating,deprotecting, reducing, re-condensing, esterifying and refining to obtain the tafluprost, according to the method for large-scale preparation of the tafluprost provided by the invention, a reversed-phase and normal-phase preparative column purification technology is used, a molecular group evacuation technology is adopted, and a molecular group'evacuation agent 'is added into a mobile phase, so that a relatively good effect is achieved, in addition, a trichloroacetonitrile uric acid(TCCA)reagent is adopted in an oxidation method, and the yield of the tafluprost is improved. The use of variousreaction quenching agents is also the characteristic of the method, and the timely and reasonable quenching of the reaction has certain significance for reducing the generation of impurities.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Amine salts of prostaglandin analogs
InactiveUS20150011755A1Organic compound preparationCarboxylic acid esters preparationProstaglandin analogTafluprost
The present application relates to amine salts of prostaglandin analogs and their uses for the preparation of substantially pure prostaglandin analogs. Specific embodiments relate to amine salts of tafluprost and their uses for the preparation of substantially pure tafluprost.
Owner:DR REDDYS LAB LTD
Tafluprost eye drop agent and preparation method thereof
InactiveCN110711175AIncrease contentLess impuritiesOrganic active ingredientsSenses disorderAntioxidantBULK ACTIVE INGREDIENT
The invention belongs to the field of bio-medicine, and discloses a tafluprost eye drop agent and a preparation method thereof. The eye drop agent take tafluprost as an active ingredient, a hydrophilic gel skeleton material is used as a skeleton and a solubilizer, the tafluprost eye drop agent further includes an antioxidant and a stabilizer, and the antioxidant and the stabilizer are macromolecular compounds. A process of the tafluprost eye drop agent improves the asepsis level of a product, the characteristics of simple preparation composition, easy, convenient and quick operation, high compatibility for medicinal packaging materials, low cost, stable properties of liquid medicine and high quality standard are achieved, and the tafluprost eye drop agent is suitable for industrial production.
Owner:南京华盖制药有限公司
Preparation method of Tafluprost crude drug
ActiveCN109053452ALow impurity contentProcess stabilityOrganic compound preparationOrganic chemistry methodsAlkaneTafluprost
The invention discloses a preparation method of a tafluprost crude drug. The preparation method comprises the following steps: preparing and separating by adopting a high-performance preparation liquid phase, and pulping C6 to C8 alkane. The preparation method of the invention is stable in process, and high in operability. The total purity of the prepared Tafluprost is greater than 99.5 percent, the content of trans isomer impurities is less than 0.1 percent, and the content of other monomer impurities is less than 0.1 percent.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of tafluprost eye drop and preparation method thereof
ActiveCN105380901BReasonable preparation processEasy to operateOrganic active ingredientsSenses disorderSolubilityPolyethylene glycol
The invention relates to a tafluprost eye drop and a preparation method thereof. The eye drop takes tafluprost as an active ingredient and also contains a non-ionic solubilizer and a pharmaceutically acceptable carrier, wherein the non-ionic solubilizer is polyethylene glycol-15-hydroxyl stearate. According to the invention, the problem of low solubility of tafluprost in the preparation process can be solved, the stability of tafluprost in an aqueous solution can be increased, and formation of related substances is reduced.
Owner:苏州工业园区天龙制药有限公司
Hair growth promoting agent containing 15,15-difluoroprostaglandin F2α derivative as active ingredient
InactiveUS9089720B2Promote growthCosmetic preparationsOrganic active ingredientsDiseaseOpen angle glaucoma
Owner:AGC INC +1
A kind of method of synthesizing tafluprost
ActiveCN103804195BOrganic compound preparationCarboxylic acid esters preparationWittig reactionTafluprost
Owner:TIEN TIANJIN PHARMA
Hair growth promoting agent containing 15,15-difluoroprostaglandin f2alpha derivative as active ingredient
ActiveUS20110152373A1Promotes hair growthNot be made thinBiocideCosmetic preparationsDiseaseOpen angle glaucoma
The present invention provides a new pharmaceutical application of a 15,15-difluoroprostaglandin F2α derivative. As a result of intensive studies in order to find a new pharmaceutical application of a 15,15-difluoroprostaglandin F2α derivative, it was found that, in a European Phase III clinical trial for tafluprost, one of the 15,15-difluoroprostaglandin F2α derivatives, with patients with open-angle glaucoma or ocular hypertension, tafluprost has actions of growing eyelashes, making eyelashes thicker, and changing the color thereof, that is, has an effect of promoting the growth of hair (eyelashes). Therefore, a 15,15-difluoroprostaglandin F2α derivative is useful as a hair growth promoting agent, and is expected to be useful as an active ingredient of a preventive or therapeutic agent for a disease associated with hair such as alopecia and a hair care product or a hair cosmetic product for regrowing hair, growing hair, increasing hair density, nourishing hair, or the like.
Owner:ASAHI GLASS CO LTD +1
Method for purifying tafluprost
ActiveCN112851510AAvoid decompositionEasily and efficiently provideOrganic active ingredientsSenses disorderBiochemical engineeringSilica gel
The purpose of the present invention is to provide a simple and efficient method for purifying tafluprost that can be scaled up in proportion. The present invention relates to the method for purifying tafluprost, which comprises a step for purifying a crude product of tafluprost by silica gel column chromatography and collecting a component containing tafluprost by HPLC analysis. In addition, the present invention also relates to a method for producing tafluprost, which comprises the aforementioned method for purifying tafluprost.
Owner:ASAHI GLASS CO LTD
A kind of eye drop containing tafluprost and preparation method thereof
ActiveCN104622798BGood bioadhesionImprove complianceOrganic active ingredientsSenses disorderMedicineTafluprost
The invention provides an eye drop containing tafluprost, comprising tafluprost and a hydrophilic polymer, wherein the hydrophilic polymer is selected from polycarbophil or carbomer, and the hydrophilic polymer The content of the substance is 0.4 to 1.0%. The drug depot that can be formed by the eye drops of the present invention has the effect of slowly releasing drugs, avoids rapid elimination of drugs, and improves bioavailability; the preparation method of the eye drops is simple and easy, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Cocrystal composed of resveratrol and prostaglandin analog and use thereof in preparation of antitumor drugs
InactiveCN110437043AHydroxy compound active ingredientsOrganic compound preparationCancer cellProstaglandin analog
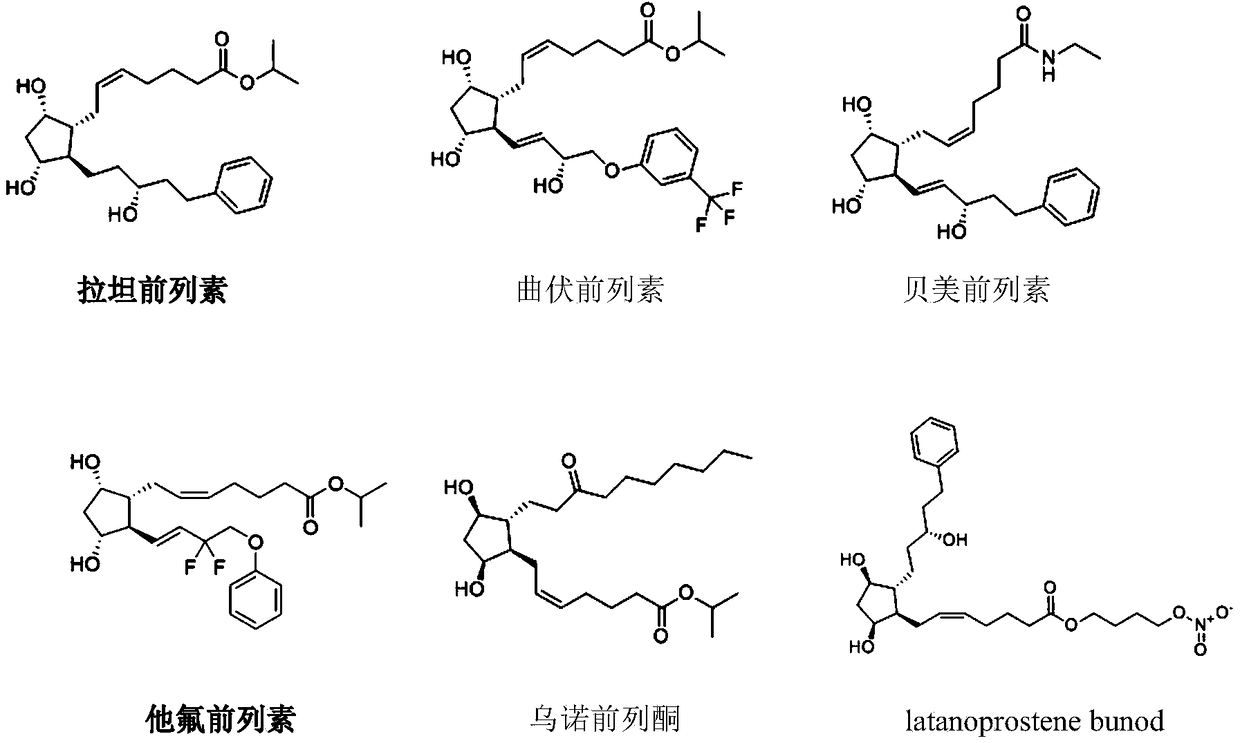
The present invention provides a cocrystal composite composed of resveratrol and a prostaglandin analog and a pharmaceutical composition comprising the same. The cocrystal composite and the composition are characterized in that the prostaglandin analog is one selected from the latanoprost, travoprost, bimatoprost, tafluprost and Latanoprostene Bunod. The cocrystal composite can generate synergistic inhibit effects (with CI being less than 1) on cell proliferation of various cells of cancer such as lung cancer and gastric cancer.
Owner:黄泳华
High-efficiency herbal eye drop
InactiveCN110870895AExtended stayAvoid rapid eliminationSenses disorderTetracycline active ingredientsDisodium EdetateHouttuynia
The invention discloses a high-efficiency herbal eye drop, and belongs to the field of pharmaceutical chemicals. The high-efficiency herbal eye drop contains the following components in parts by weight: 80-100 parts of water, 50-60 parts of polycarbophil AA, 30-40 parts of oxytetracycline, 25-30 parts of tafluprost, 20-25 parts of timclol maleate, 18-23 parts of herba houttuyniae extract, 16-21 parts of flos lonicerae extract, 13-18 parts of semen cassiae extract, 11-16 parts of rhizoma coptidis extract, 9-13 parts of sodium chloride, 6-11 parts of edetate disodium, 6-9 parts of poloxamer, 5-8parts of tyloxapol, 4-8 parts of timclol maleate, 4-6 parts of brinzolamide, 2-5 parts of mannitol, 2-5 parts of citric acid, 2-4 parts of sodium citrate, 1-3 parts of benzene, and 1-3 parts of a preservative. The high-efficiency herbal eye drop solves the problems that a traditional herbal eye drop has poor absorption and a poor effect, and a chemical preparation eye drop hurts eyes.
Owner:李达欣
Effective glaucoma therapeutic agent prepared from prostaglandin composition
InactiveCN108815169AAvoid adverse reactionsImprove solubilityOrganic active ingredientsSenses disorderGlucocorticoidIntraocular pressure
The invention discloses an effective glaucoma therapeutic agent prepared from a prostaglandin composition. The glaucoma therapeutic agent is prepared by compounding prostaglandins and inhibitors, andfurther comprises glucocorticoid, an adjuvant drug capable of acting on eyes, and isotope calibration objects for tracing, wherein the glucocorticoid specifically refers to one or several of hydrocortisone, prednisone, prednisolone and medicinal salts or compound esters thereof; the adjuvant drug specifically refers to any one or a combination of several of unoprostone, tafluprost and AL-6589. Theeffective glaucoma therapeutic agent can achieve the effect of persistently and efficiently reducing the intraocular pressure to treat glaucoma, overcomes eye disorders caused by prostaglandins, obviously reduces the incidence rate of the disorders while achieving the same intraocular pressure reducing effect, particularly can promote dissolution of active ingredients, has an antibacterial effect, and provides a basis of observation for accurate treatment by displaying the targeted area and the drug flow direction by virtue of the isotope calibration objects for tracing.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
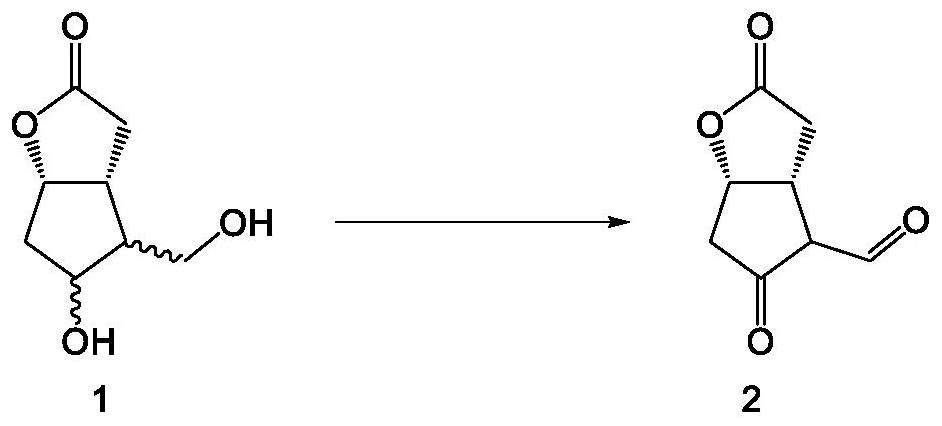
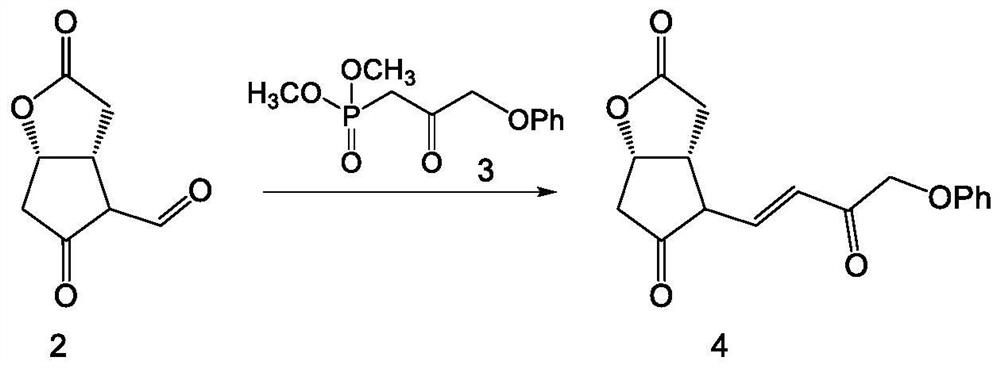
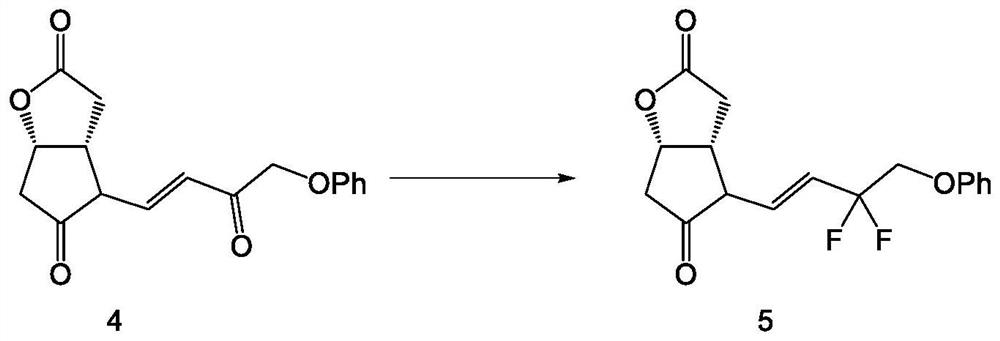
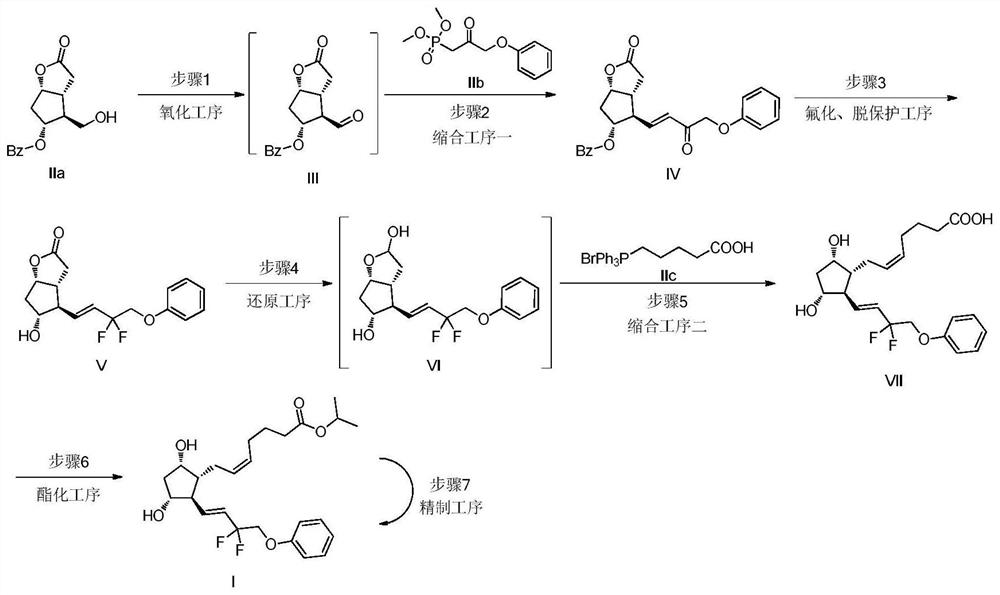
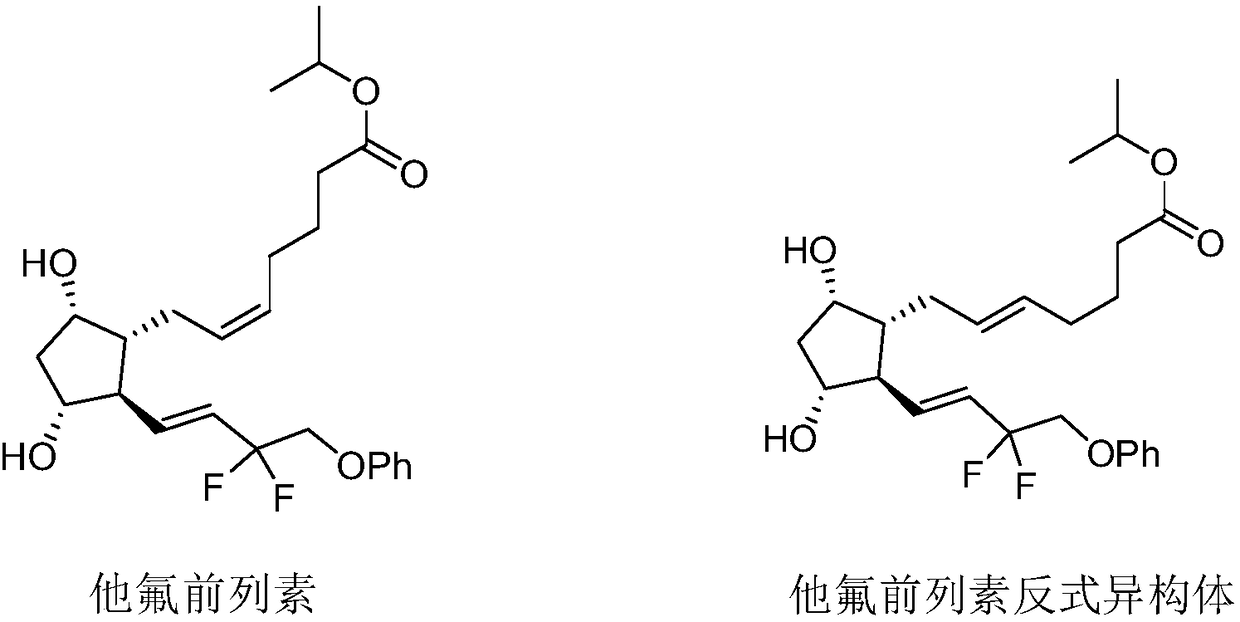
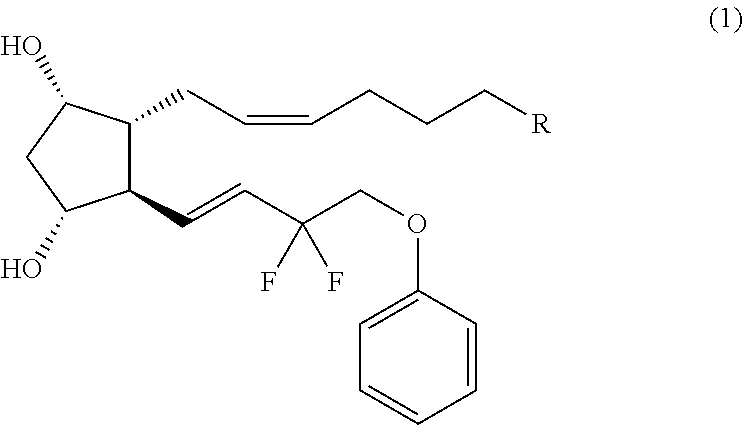
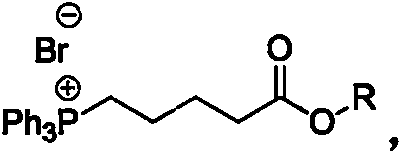
Preparation method of high-purity Tafluprost and analogs thereof and intermediate compound
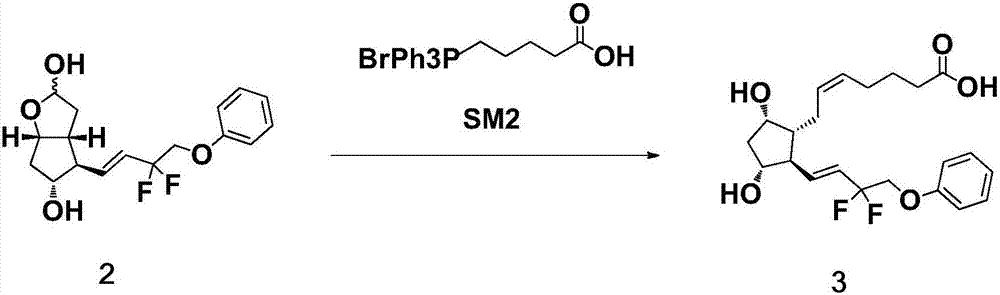
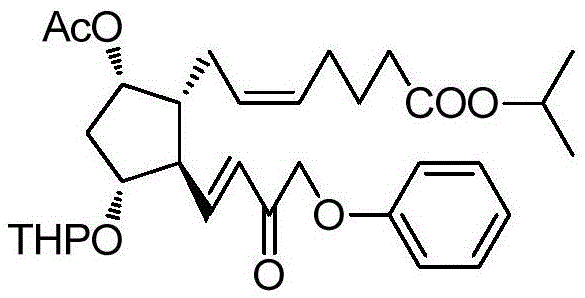
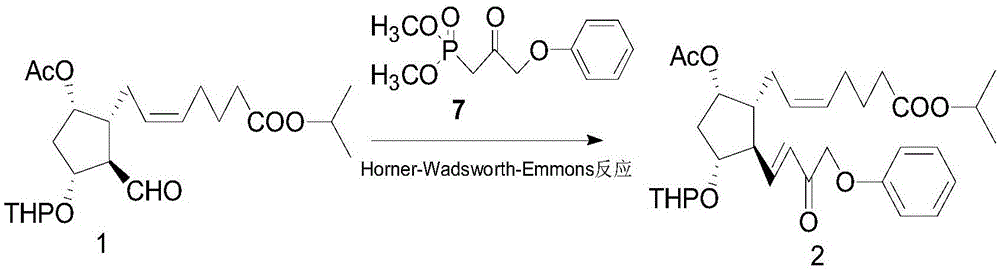
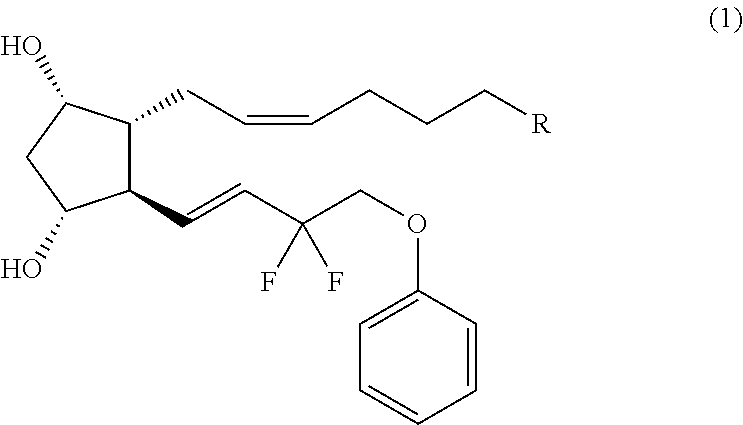
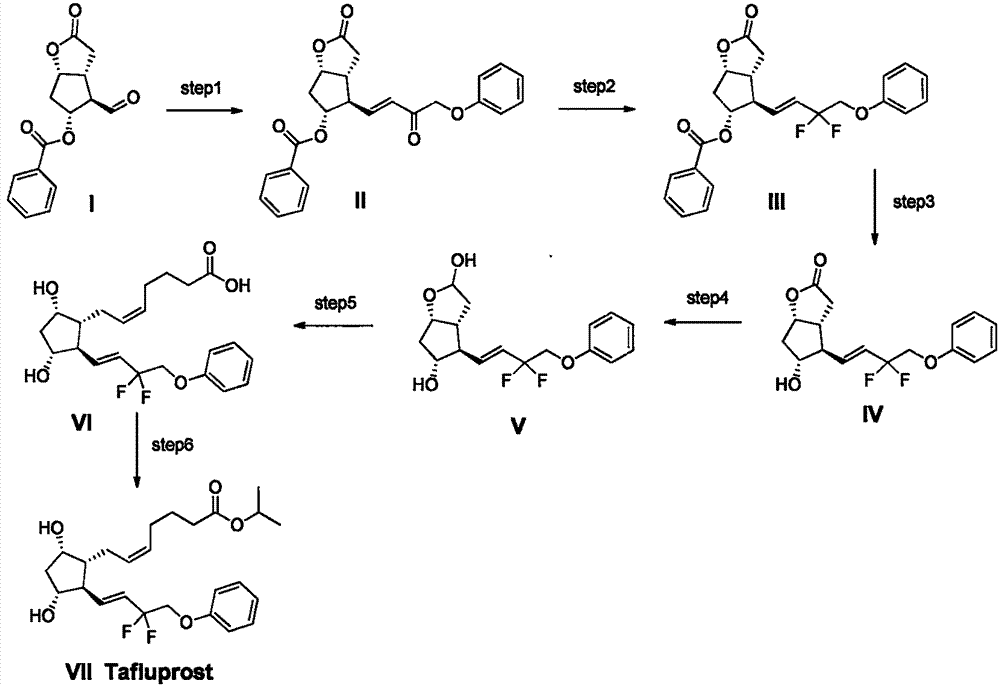
The invention belongs to the technical field of medicine, and relates to a preparation method of Tafluprost and analogs thereof and a related intermediate compound. The preparation method is characterized in that (4-carboxybutyl)triphenylphosphonium bromide having a structure shown below is used as a witting reaction reagent, wherein R is alkyl or aryl. The method is simple, feasible and convenient for industrial production; and the Tafluprost and analogs thereof which are basically free of omega chain trans-isomers can be prepared.
Owner:SUZHOU LANXITE BIOTECH
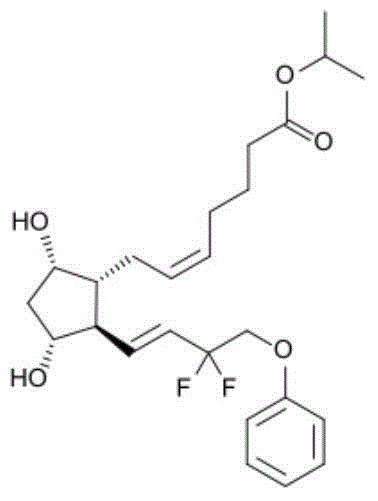
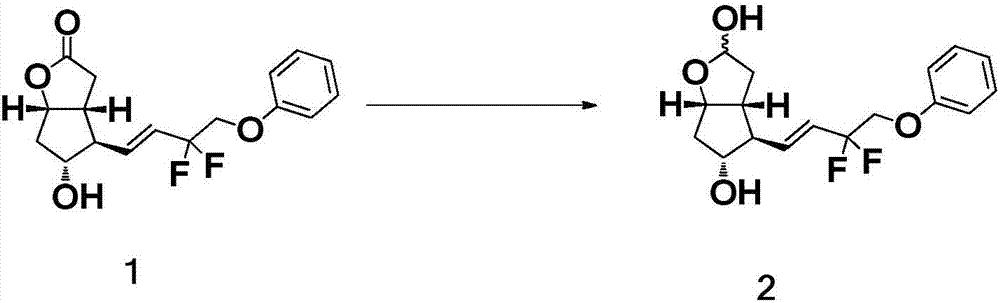
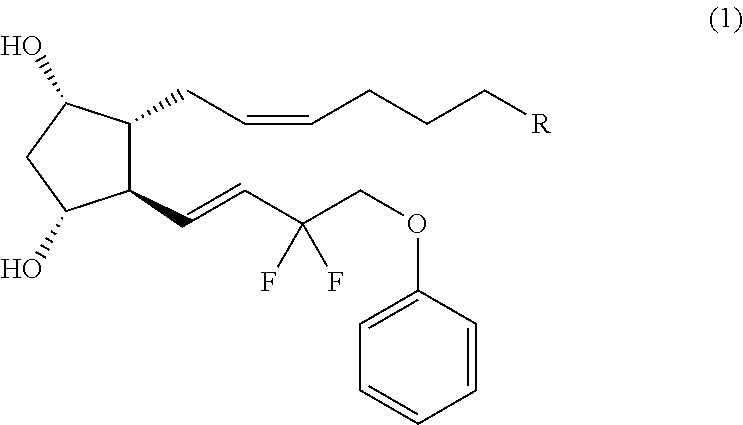
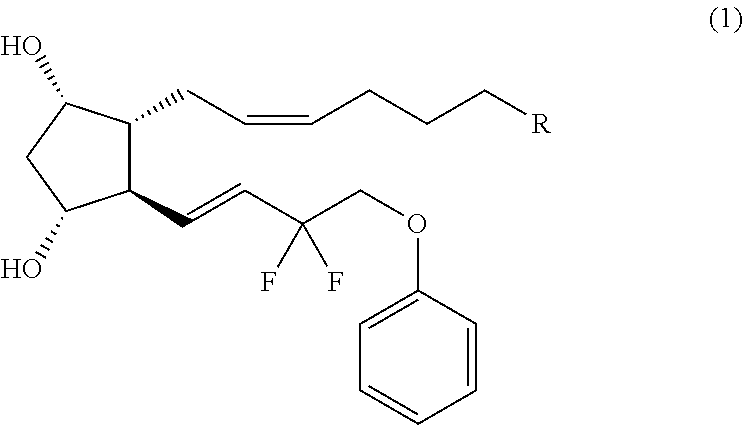
Crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate, and preparation method of corresponding crystals thereof
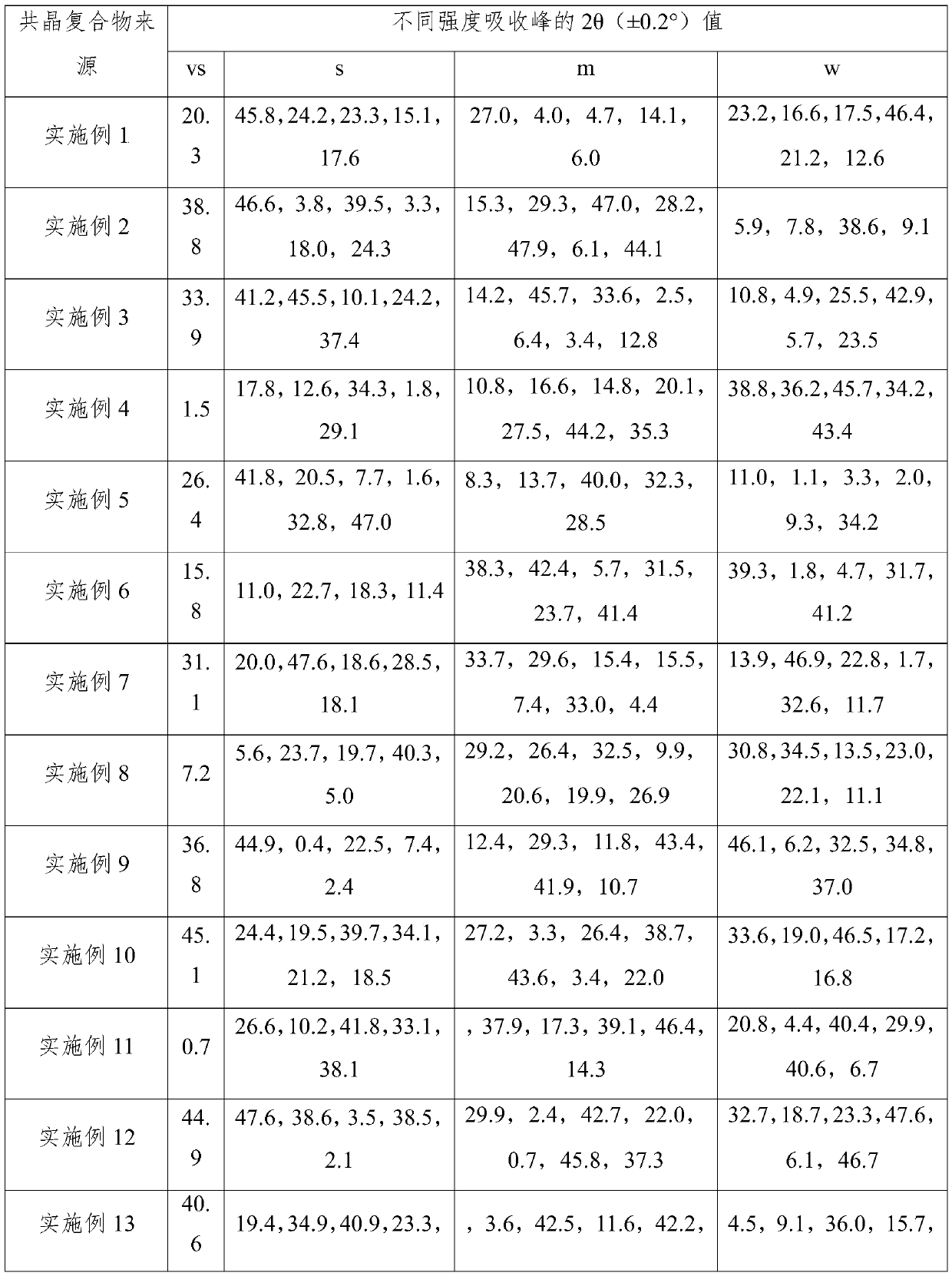
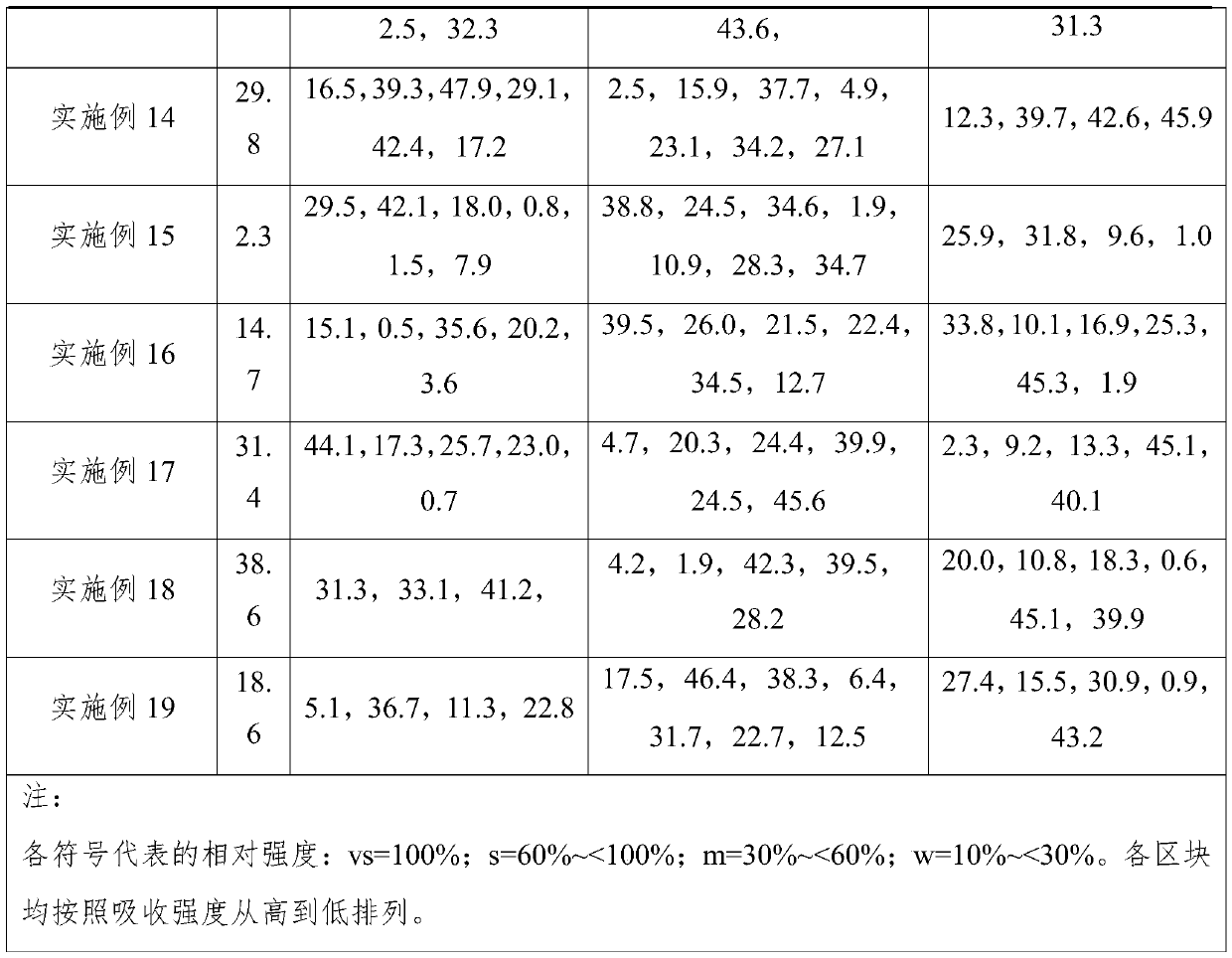
The invention provides a crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate (a tafluprost intermediate, a difluorination product 2), and a preparation method of corresponding crystals of the crystal form. Crystal form parameters comprise characteristic peak values (2theta) of the XRPD spectrum of the crystals, and DSC and IR characteristic atlas parameters. White crystal powder of the product 2 is obtained through single solvent or mixed solvent recrystallization by adopting a simple crystallization method, the primary crystallization purity is greater than 90% generally, the highest purity after secondary crystallization reaches 99.8%, and the proportion of each of all unimodal impurities is lower than 0.1%, so the purification process of tafluprost synthesized by using the product 2 as a raw material is substantially simplified.
Owner:TIEN TIANJIN PHARMA
Ophthalmic compositions comprising tafluprost for the treatment of glaucoma
PendingCN112153970AHalogenated hydrocarbon active ingredientsSenses disorderIntra ocular pressureAlkane
Owner:NOVALIQ GMBH
Composition comprising prostaglandin derivative and ophthalmic liquid preparation comprising same
ActiveCN109172580BReduce concentrationReduce usageSenses disorderPharmaceutical delivery mechanismKetonePharmaceutical medicine
The present invention provides a composition comprising a prostaglandin derivative and valproic acid or a pharmaceutically acceptable salt thereof and an ophthalmic liquid preparation comprising the composition, wherein the prostaglandin derivative is preferably latanoprost , bimatoprost, travoprost, tafluprost, unoprostone and latanoprostene, valproic acid or its pharmaceutically acceptable salts are preferably valproic acid, sodium valproate, diprostene One of sodium valproate and magnesium valproate. The composition provided by the invention can produce a synergistic effect of lowering ocular pressure, avoids the use of solubilizers, and improves the safety of medicines.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Local-medicine-applying medicine composition including puerarin, brinzolamide and the like
PendingCN112972683AReduce adverse reactionsReduce pollutionOrganic active ingredientsSenses disorderDiseasePuerarin
The invention provides a medicine composition containing an effective dosage of puerarin and an effective dosage of dorzolamide or brinzolamide or prostaglandin, such as latanoprost, tafluprost and the like or / and an effective dosage of water-retaining agent or wetting agent or antixerophthalmic ingredients, and application in preparation of medicines, including eye-purpose medicines used for preventing or treating intraocular pressure or elevated intraocular pressure, glaucoma, fundi diseases, optic nerve protection, dry eyes or xerophthalmia or dry eye syndromes of the human being or mammals. The medicine disclosed by the invention has a better curative effect or a smaller side effect or better treatment compliance or a better treatment mode.
Owner:刘力
Method for synthesizing tafluprost
PendingCN113816856AHigh purityImprove economyOrganic compound preparationCarboxylic acid esters preparationCombinatorial chemistryEsterification reaction
The invention provides a method for synthesizing tafluprost, and relates to the technical field of tafluprost synthesis. The method for synthesizing the tafluprost comprises the following specific preparation steps: S1, primary reaction, S2, secondary reaction, S3, third reaction, S4, fourth reaction, S5, fifth reaction and S6, final reaction. The invention provides the method for synthesizing tafluprost. According to the method for synthesizing tafluprost, racemic Corey lactone diol is used as a raw material, the tafluprost is obtained through oxidation, condensation, fluorination, reduction, recondensation and esterification reaction, the total mass yield of six-step reaction of the preparation method can reach 22%, the process is stable, the prepared tafluprost product is colorless viscous oily liquid, and the purity of the tafluprost product is up to 99% or above.
Owner:上海京河医药科技有限公司
Composition for treating or preventing glaucoma comprising a sulfonamide compound and another drug
InactiveUS20160228421A1Good effectOrganic active ingredientsOrganic chemistryIntra ocular pressureOphthalmology
Owner:SANTEN PHARMA CO LTD
The preparation method of tafluprost
ActiveCN106986766BHigh purityQuality is easy to controlOrganic compound preparationCarboxylic acid esters preparationWittig reactionBiochemical engineering
The invention discloses a preparation method of Tafluprost. The preparation method comprises the following steps: adopting a compound of formula 1 as a raw material, and carrying out the steps of DIBAL-H reduction, a Wittig reaction and an esterification reaction, wherein alkaline amino acids are adopted for refining after the Wittig reaction. According to the preparation method disclosed by the invention, the total mass yield of the three-step reaction can reach 70%, the technology is stable, a prepared Tafluprost product is colorless or faint yellow thick oily liquid, and the purity reaches up to more than 99%.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of preparation method of tafluprost crude drug
ActiveCN109053452BHigh purityHigh yieldOrganic compound preparationOrganic chemistry methodsAlkaneFluid phase
The invention discloses a preparation method of a tafluprost raw material drug, which comprises the preparation and separation of a high-efficiency preparation liquid phase, and the beating of C6-C8 alkane; the preparation method of the invention is stable in process and strong in operability, and can obtain other The total purity of the fluprostin is greater than 99.5%, wherein the impurity content of the trans isomer is less than 0.1%, and the content of other simple impurities is less than 0.1%.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate, and preparation method of corresponding crystals thereof Crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate, and preparation method of corresponding crystals thereof](https://images-eureka.patsnap.com/patent_img/22d7128e-c9f7-408a-a992-ab807fd41762/2014100598570100003DEST_PATH_IMAGE001.PNG)
![Crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate, and preparation method of corresponding crystals thereof Crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate, and preparation method of corresponding crystals thereof](https://images-eureka.patsnap.com/patent_img/22d7128e-c9f7-408a-a992-ab807fd41762/2014100598570100003DEST_PATH_IMAGE002.PNG)
![Crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate, and preparation method of corresponding crystals thereof Crystal form of (3aR,4R,5R,6aS)-4-((E)-3,3-difluoro-4-phenoxylbutyl-1-alkyl-1-yl)-2-oxohexahydro-2H-cyclopentane[b]furan-5-benzoate, and preparation method of corresponding crystals thereof](https://images-eureka.patsnap.com/patent_img/22d7128e-c9f7-408a-a992-ab807fd41762/2014100598570100003DEST_PATH_IMAGE003.PNG)