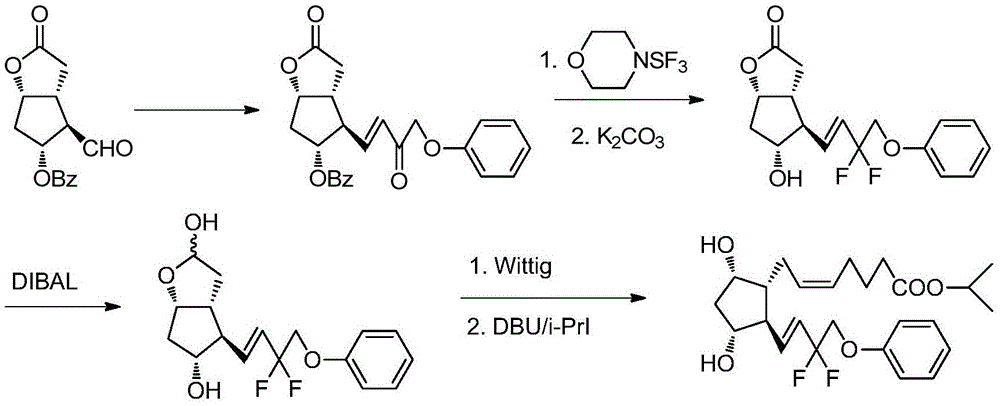
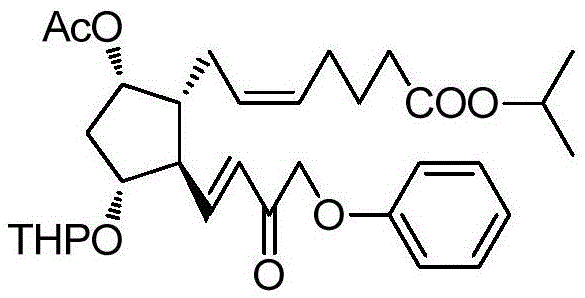
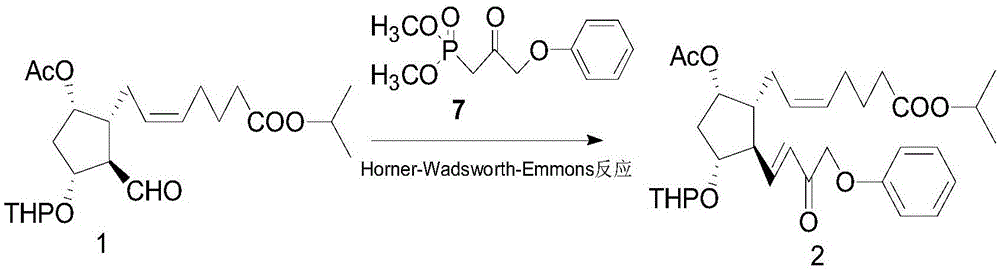
A kind of method of synthesizing tafluprost
A compound, selected technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (Z)-7-((1R,2R,3R,5S)-5-Acetoxy-2-((E)-3-oxo-4-phenoxybut-1-en-1-yl)- Isopropyl 3-((tetrahydro-2H-pyran-2-yl)oxy)cyclopentyl)hept-5-enoate 2.
[0049] 2.6 g of dimethyl 2-oxo-3-phenoxypropylphosphonate 7 was dissolved in 30 ml of toluene, and 0.42 g of LiOH.H 2 O, 0.05 g of tetrabutylammonium bromide, stirred at -20°C for 1 hour. Added 5 g of (Z)-7-((1R,2R,3R,5S)-5-acetoxy-2-formyl-3 -((Tetrahydro-2H-pyran-2-yl)oxy)cyclopentyl)hept-5-enoic acid isopropyl ester 1 and 60 ml of toluene mixed solution, continue to stir for 24 hours, TLC detects that raw material 7 remains , the reaction solution was washed twice with brine, dried over anhydrous sodium sulfate, and concentrated to obtain 4.4 g of a light yellow liquid of 2. The yield was 78.5%. It was directly used in subsequent reactions. A small sample was purified by silica gel, ethyl acetate / n-hexane (1:2) was eluted to obtain 2 as a yellow liquid. 1 H-NMR (CDCl 3 ,300MHz)δ(ppm):1.23(d,6H),1.45-2.05(m,13H),2.07(s,3H),...
Embodiment 2
[0051] (Z)-7-((1R,2R,3R,5S)-5-Acetoxy-2-((E)-3-oxo-4-phenoxybut-1-en-1-yl)- Isopropyl 3-((tetrahydro-2H-pyran-2-yl)oxy)cyclopentyl)hept-5-enoate 2.
[0052]Dissolve 3.6 g of dimethyl 2-oxo-3-phenoxypropylphosphonate 7 in 30 ml of dichloromethane, add 0.58 g of butyllithium, 0.05 g of tetrabutylammonium bromide, room temperature (20-30°C ) and stirred for 1 hour. Added 5 g of (Z)-7-((1R,2R,3R,5S)-5-acetoxy-2-formyl-3-((tetrahydro-2H-pyran-2- base)oxy)cyclopentyl)hept-5-enoic acid isopropyl ester 1 and 60 ml of dichloromethane mixed solution, continue to stir at room temperature for 15 hours, TLC detects that there is no residue of raw material 1, and the reaction solution is washed twice with brine , dried over anhydrous sodium sulfate, and concentrated to obtain 5.6 g of a light yellow liquid of 2. The yield was 85.4%. It was directly used in subsequent reactions.
Embodiment 3
[0054] (Z)-7-((1R,2R,3R,5S)-5-Acetoxy-2-((E)-3-oxo-4-phenoxybut-1-en-1-yl)- Isopropyl 3-((tetrahydro-2H-pyran-2-yl)oxy)cyclopentyl)hept-5-enoate 2.
[0055] 6.1 g of 2-oxo-3-phenoxypropyl phosphonic acid dimethyl ester 7 was dissolved in 45 ml of tetrahydrofuran, added 1.0 g of t-BuOK, 0.05 g of tetrabutylammonium bromide, and stirred at 80°C for 1 hour. Added 5 Gram (Z)-7-((1R,2R,3R,5S)-5-acetoxy-2-formyl-3-((tetrahydro-2H-pyran-2-yl)oxy)cyclopentene base) Hept-5-enoic acid isopropyl ester 1 and 60 ml of tetrahydrofuran mixed solution, continue to stir for 1 hour, TLC detects that there is no raw material 1 residue, the reaction solution is poured into 200 ml of brine, extracted with 50ml×4 ethyl acetate, combined The organic phase was washed twice with brine, dried over anhydrous sodium sulfate, and concentrated to obtain 4.9 g of a light yellow liquid of 2. The yield was 74.9%. It was directly used in subsequent reactions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com