Method for large-scale preparation of tafluprost
A tafluprost and fluorination technology, which is applied in the field of large-scale preparation of tafluprost, can solve the problems of many side effects, etc., and achieve the effect of reducing the generation of impurities and timely and reasonable quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
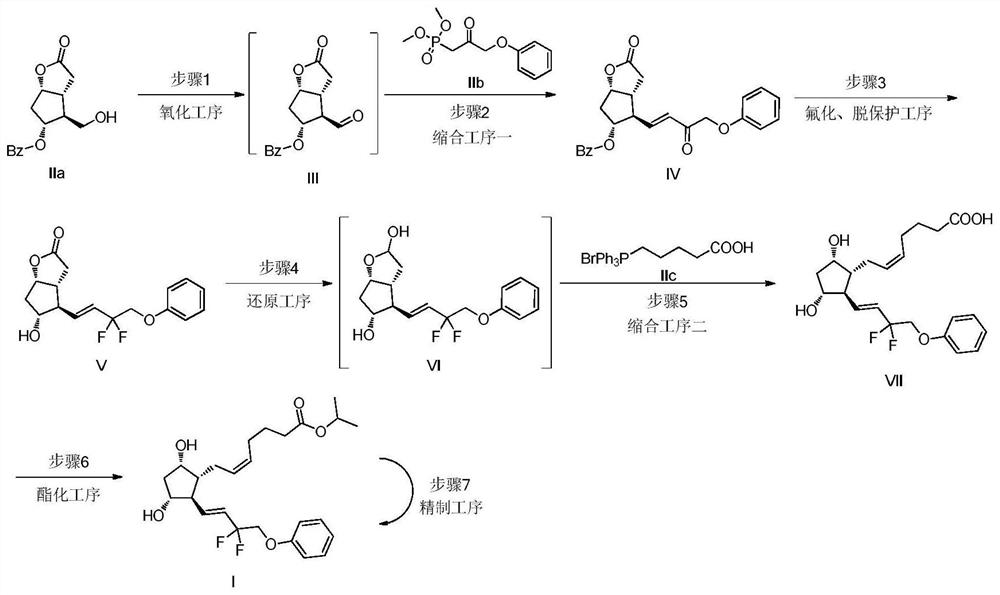
[0020] 1. Oxidation reaction: Add 109.4g of trichloroacetonitrileuric acid (TCCA), 1.3L of dimethyl carbonate and 325mL of ethyl acetate into the reaction flask, stir and cool down to between 0°C and 5°C. Add tetramethylpiperidine nitrogen oxide (TEMPO0.74g) into the system, and then add corylactone. After reacting for 1.5 hours, the reaction solution was added to the suspension of sodium thiosulfate pentahydrate, dipotassium hydrogen phosphate trihydrate and water to quench the reaction, filter with suction, wash the filter cake with dimethyl carbonate, and let the filtrate stand The layers were separated, and the organic phase was dried by adding anhydrous sodium sulfate and stirring. The filtrate was concentrated to dryness under reduced pressure to obtain 117.3 g of intermediate III.
[0021] 2. Condensation reaction 1: Add 17.57g of sodium hydride and 1.76L of tetrahydrofuran into the reaction bottle, start stirring, cool down to -5°C to 0°C, and drop 103.1g of 2-carbony...
example 2
[0032] Refined Purification:
[0033] Reverse-phase column chromatography: use a dynamic axial compression column to The pore diameter of carbon octadeca is used as filler, and the mobile phase is acetonitrile-ammonium acetate-water solution (10:1:10). Dissolve 42 grams of tafluprost crude product, pass through the column, collect relevant effluents, and concentrate to dryness under reduced pressure.
[0034] Normal-phase column chromatography: The mobile phase was dissolved in n-hexane-isopropanol (8:2), and passed through a COSMOSILCHIRAL 5C 20ID×250mm column. The relevant effluent was collected and concentrated to dryness under reduced pressure. 35.28 g was obtained, and the content of tafluprost detected by HPLC was 99.9%. Impurity A: 0.021%, impurities B, C, D, F, G not detected, unknown maximum single impurity 0.065%, total impurity: 0.086%
example 3
[0036] Refined Purification:
[0037] Reverse-phase column chromatography: use a dynamic axial compression column to The pore diameter of carbon octadeca is used as a filler, and the mobile phase is acetonitrile-ammonium acetate-water solution (10:1:10). Dissolve 35 grams of tafluprost crude product, pass through the column, collect relevant effluents, and concentrate to dryness under reduced pressure.
[0038] Normal-phase column chromatography: The mobile phase was dissolved in n-hexane-isopropanol (8:2), and passed through a COSMOSILCHIRAL 5C 20ID×250mm column. The relevant effluent was collected and concentrated to dryness under reduced pressure. 24.71 g was obtained, and the content of tafluprost detected by HPLC was 99.9%. Impurity A: 0.020%, impurities B, C, D, F, G not detected, unknown maximum single impurity 0.062%, total impurity: 0.082%
[0039] Chemical name of each impurity:
[0040] Impurity A: (5E)-7-[(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-phenoxy-1-buten-1-y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com