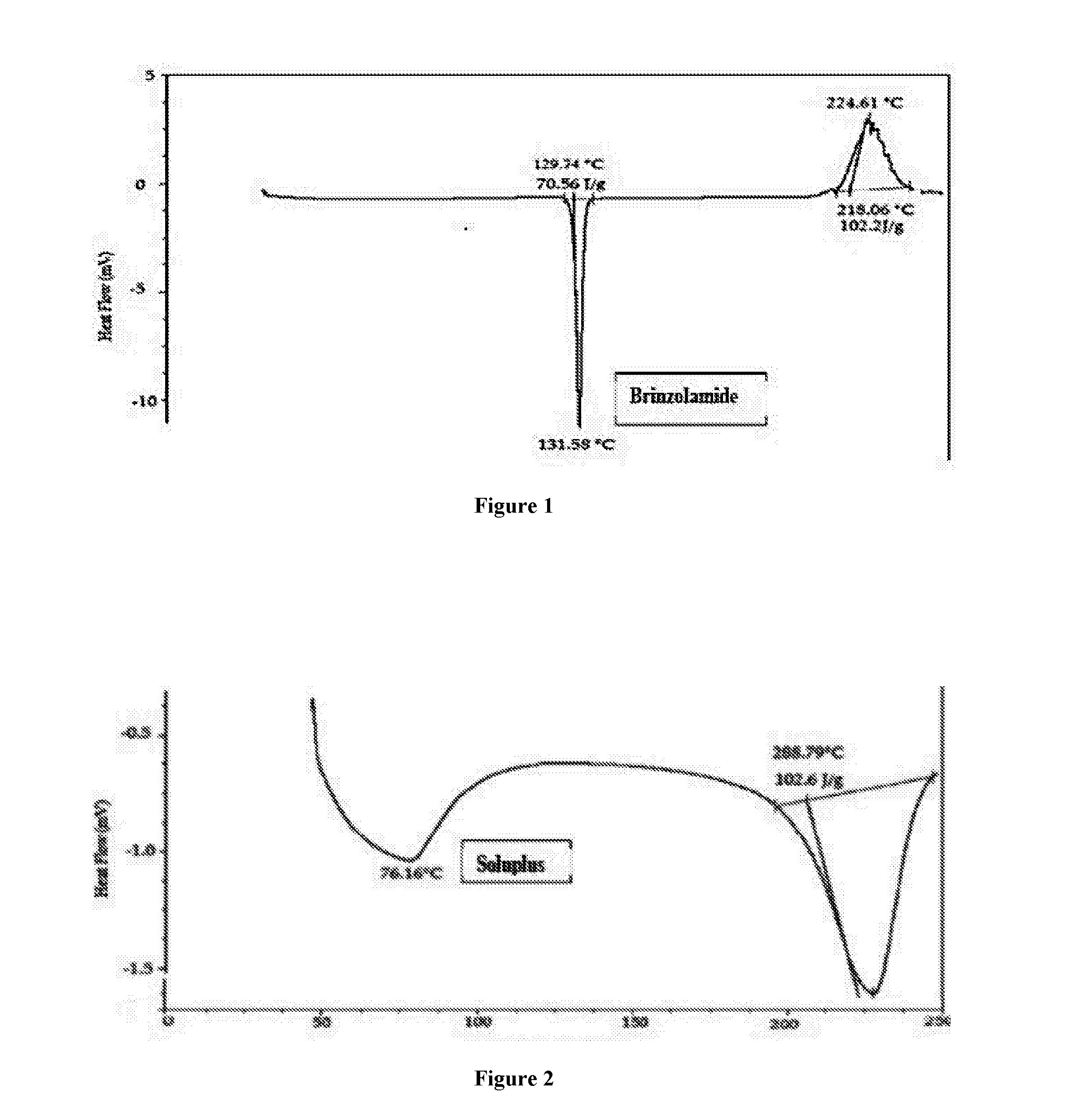
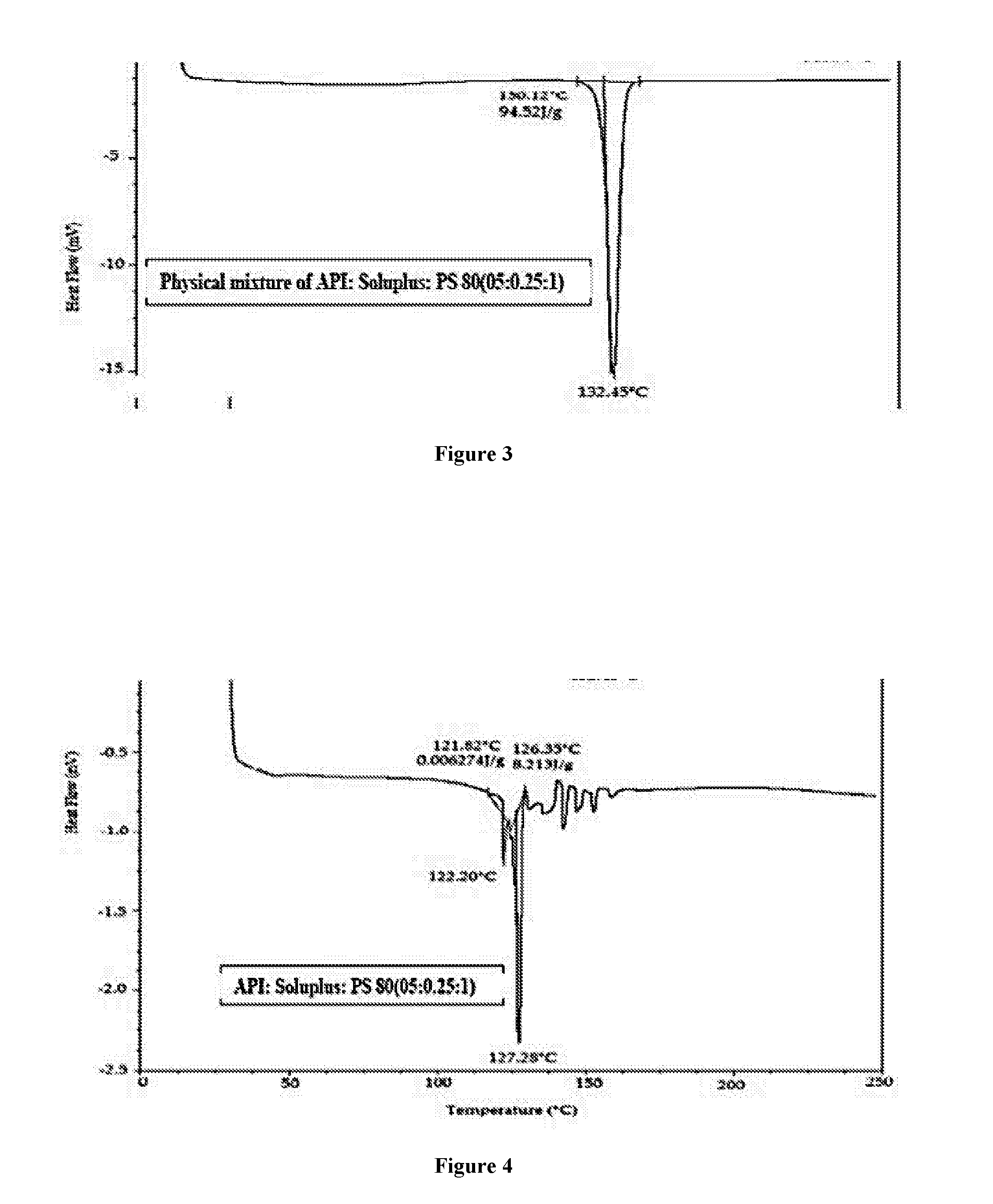
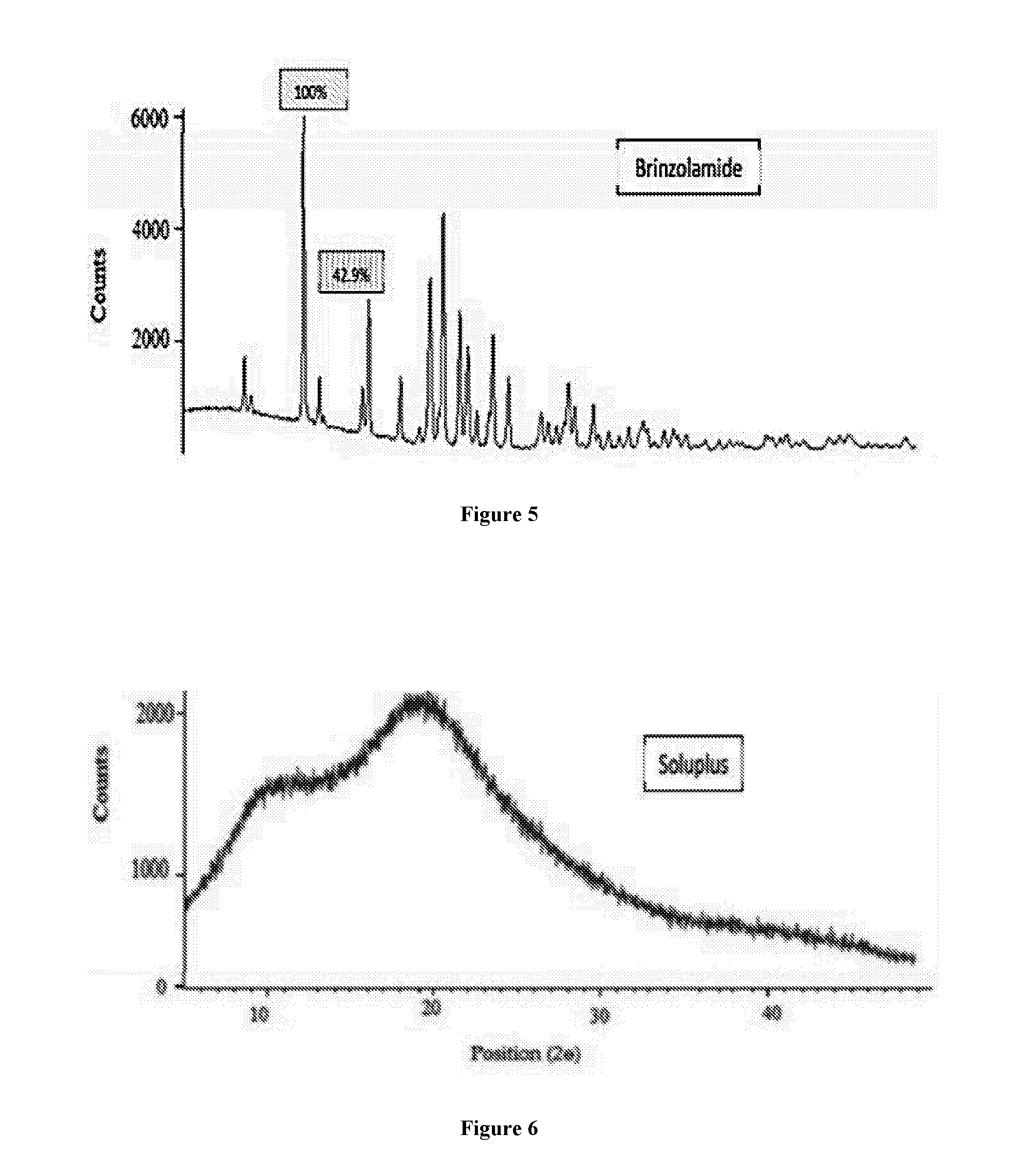
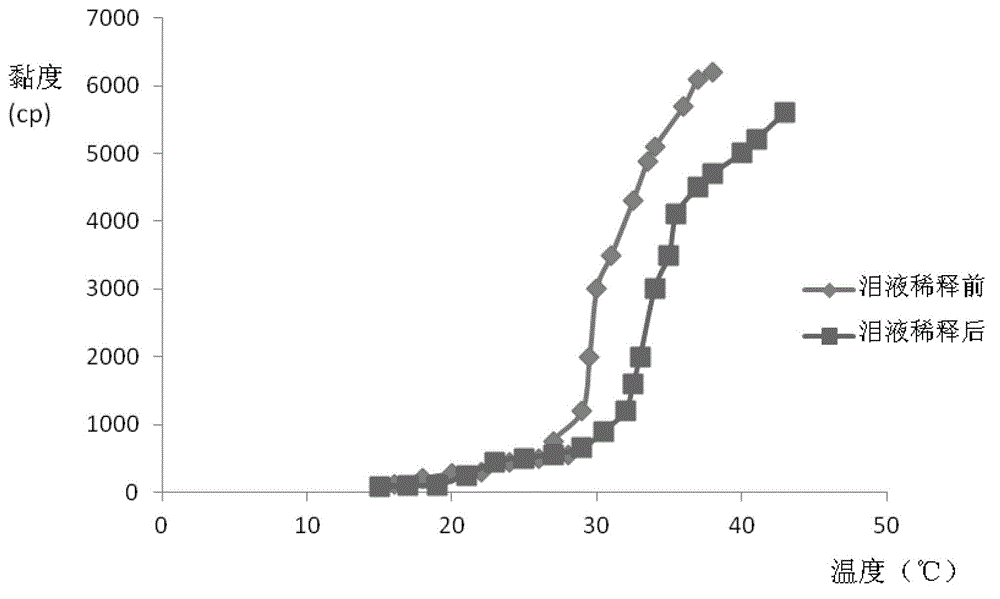
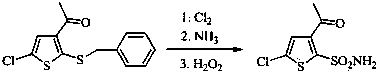Patents
Literature
34 results about "Brinzolamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
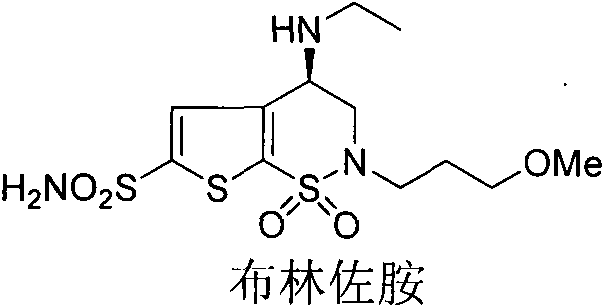
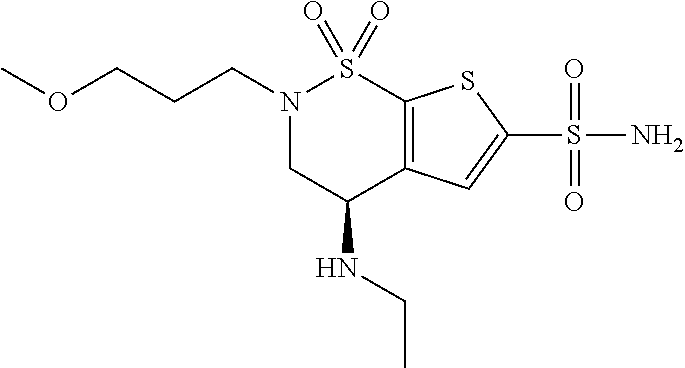
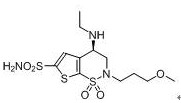
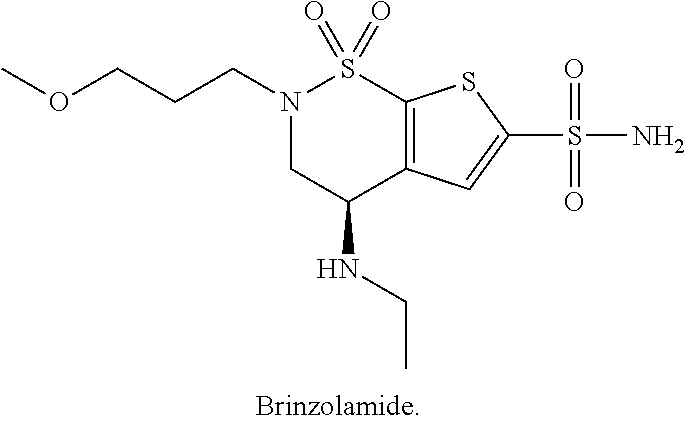
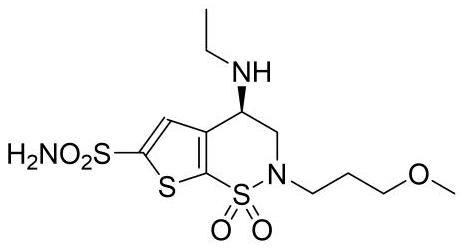
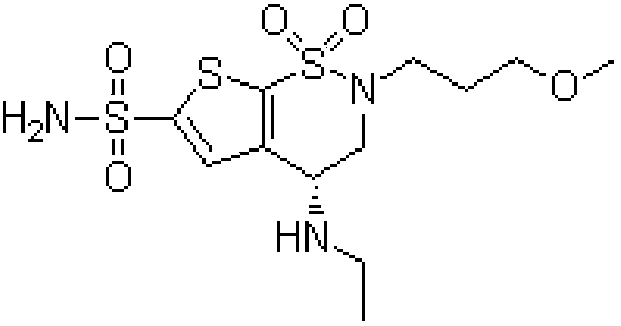
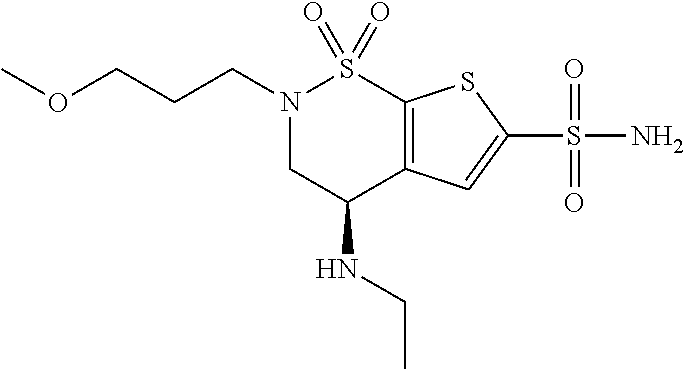
Brinzolamide is used to treat high pressure inside the eye due to glaucoma (open angle-type) or other eye diseases (e.g., ocular hypertension).
Pharmaceutical composition comprising brinzolamide
ActiveUS20160339105A1Improve bio-availabilityImprove manufacturabilityOrganic active ingredientsSenses disorderActive ingredientSURFACTANT BLEND
A sterile aqueous formulation of a carbonic anhydrase inhibitor such as brinzolamide in combination with polymers like Soluplus® and a surfactant like polysorbate 80, as well as methods of preparation thereof, is disclosed. The formulation relates to the highly solubilized or an amorphous form of poorly insoluble drugs / active ingredient(s) to improve its bio-availability and manufacturability.
Owner:SENTISS PHARMA
Brinzolamide eye preparations, and preparation method and use thereof
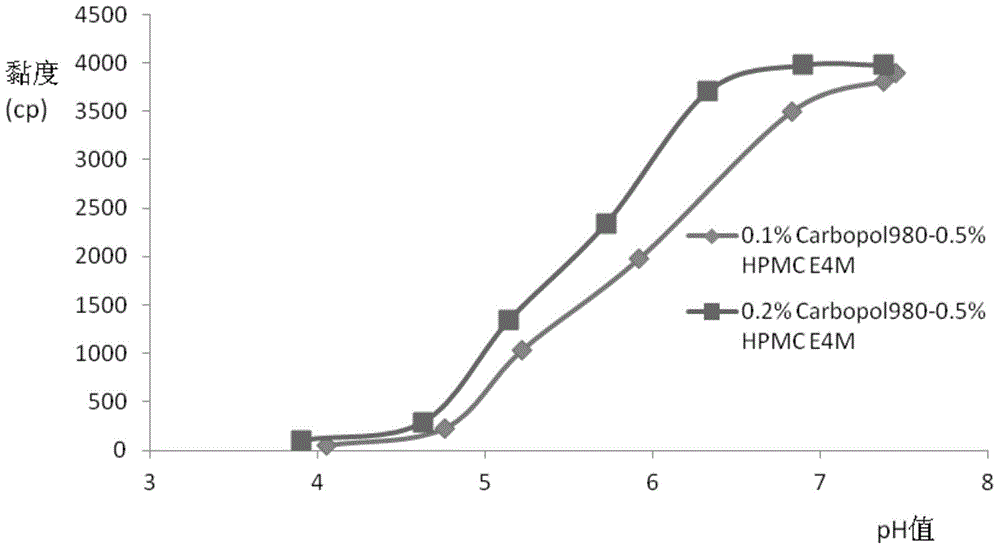
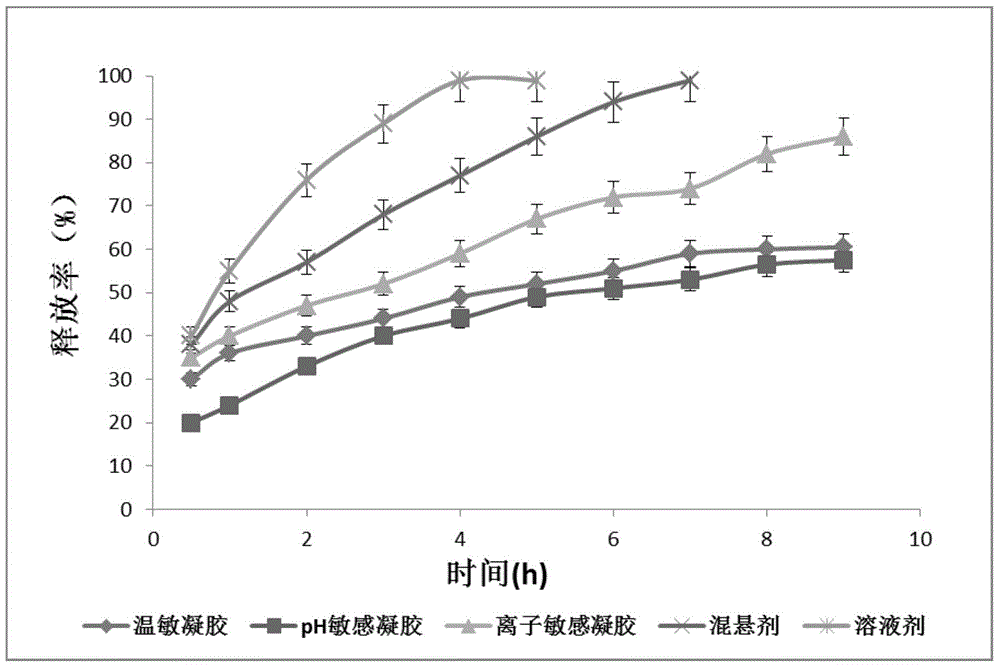
ActiveCN103142462AGood effectImprove solubilityOrganic active ingredientsSenses disorderGel preparationDisease
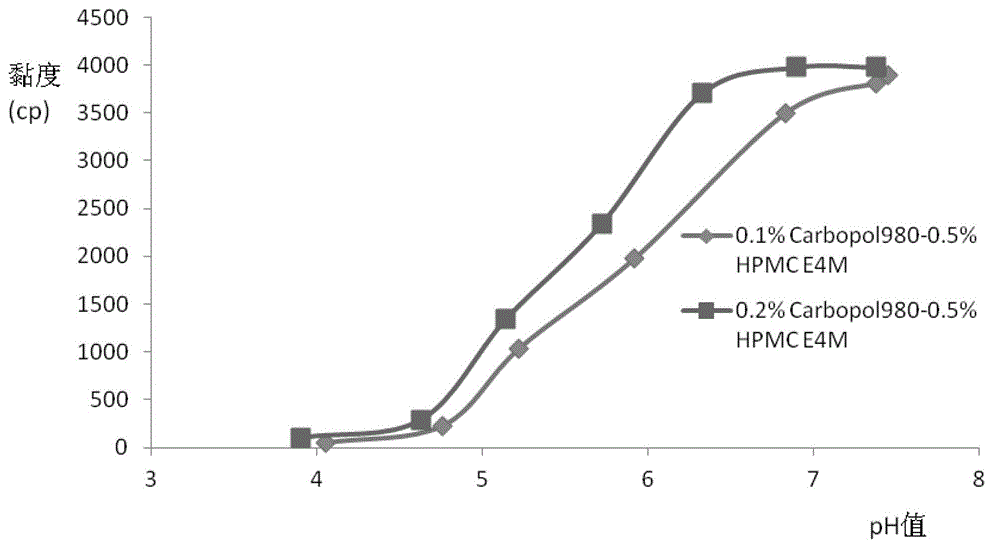
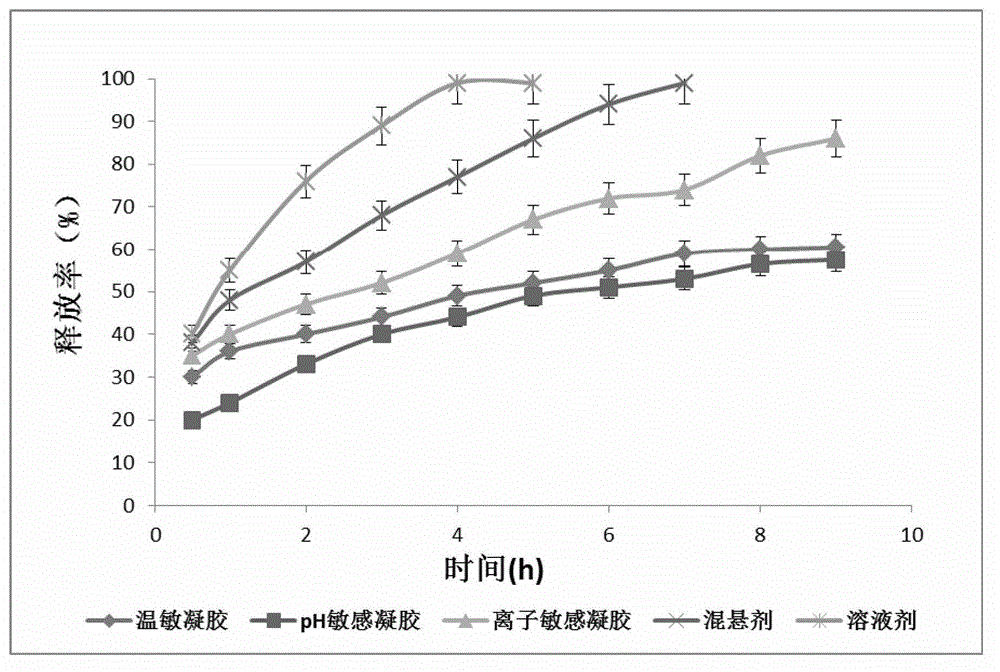
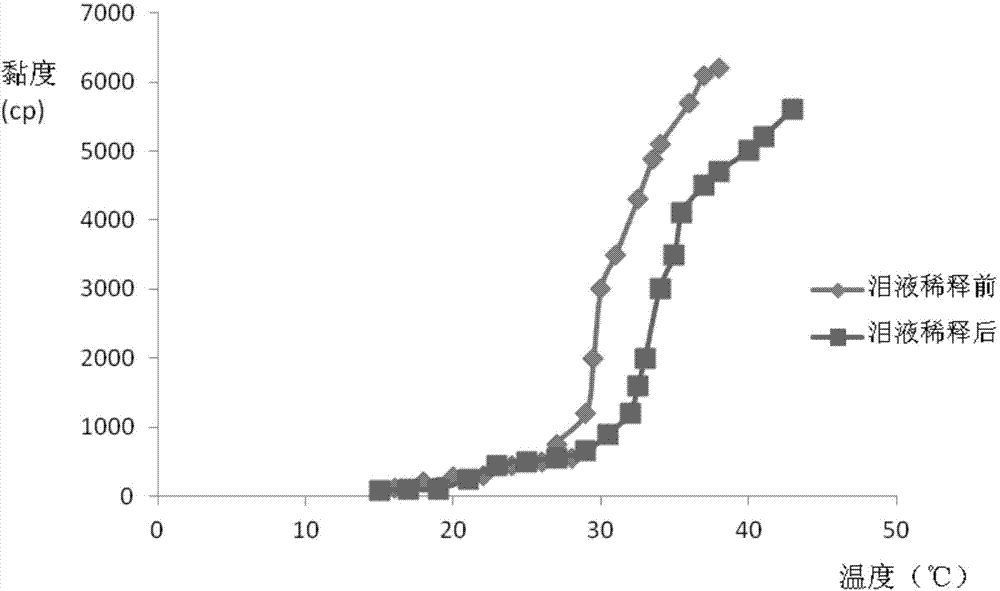
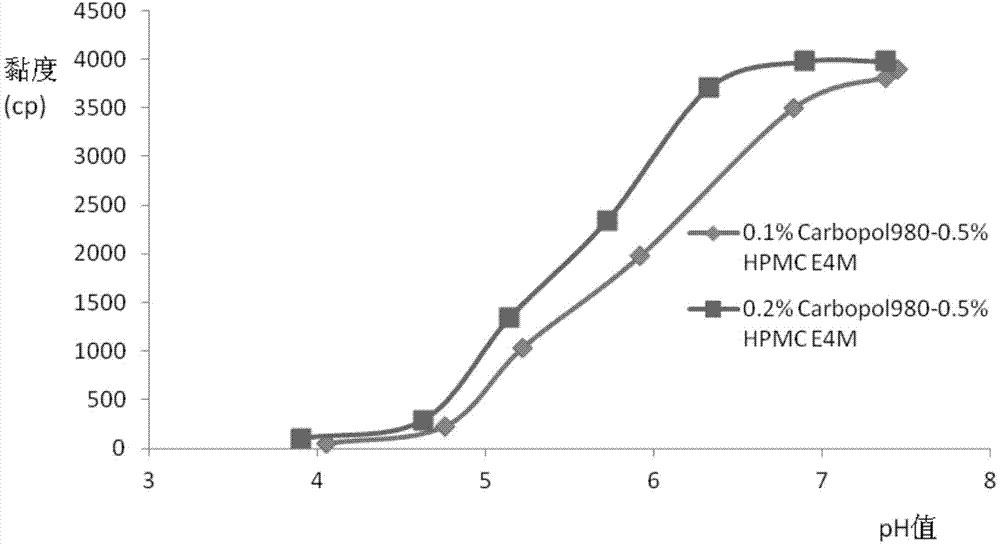
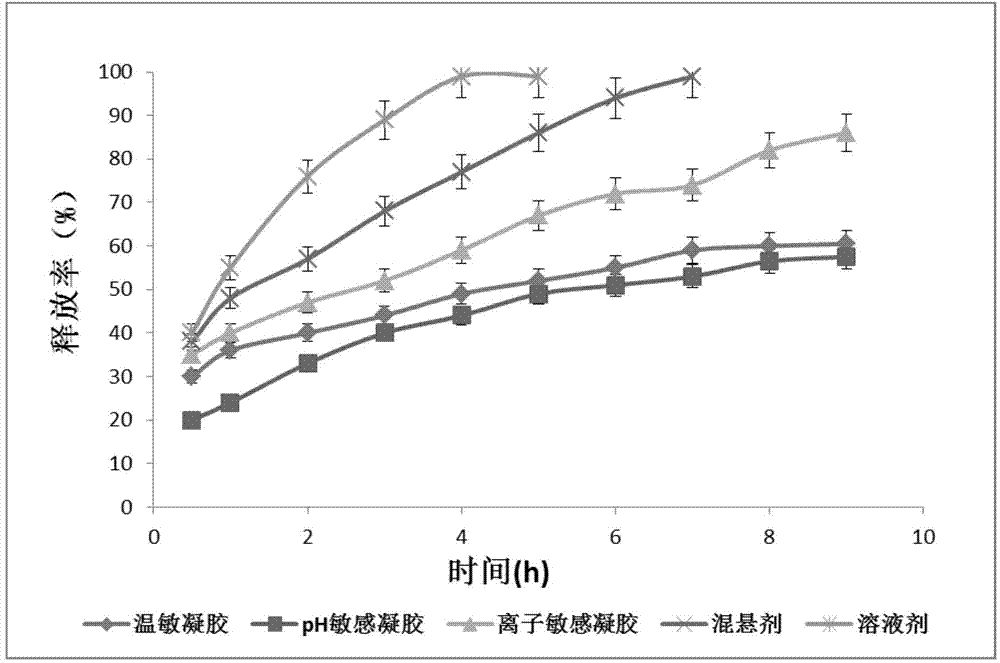
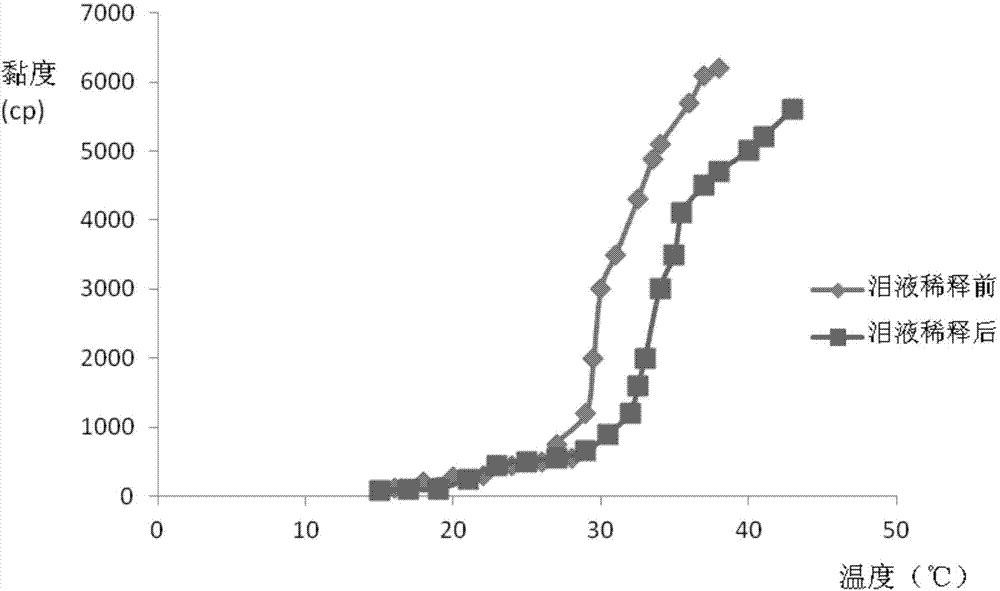
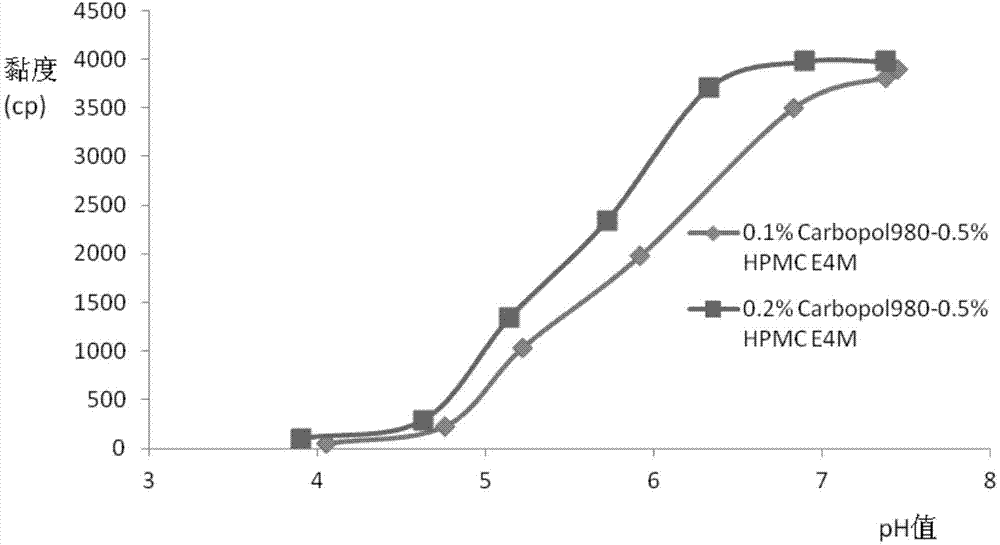
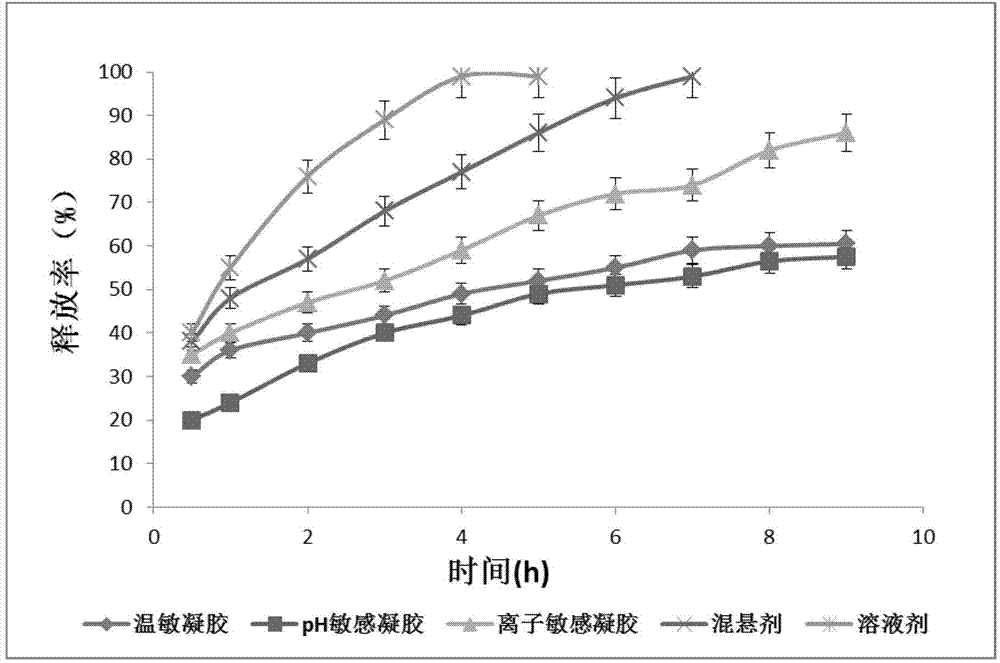
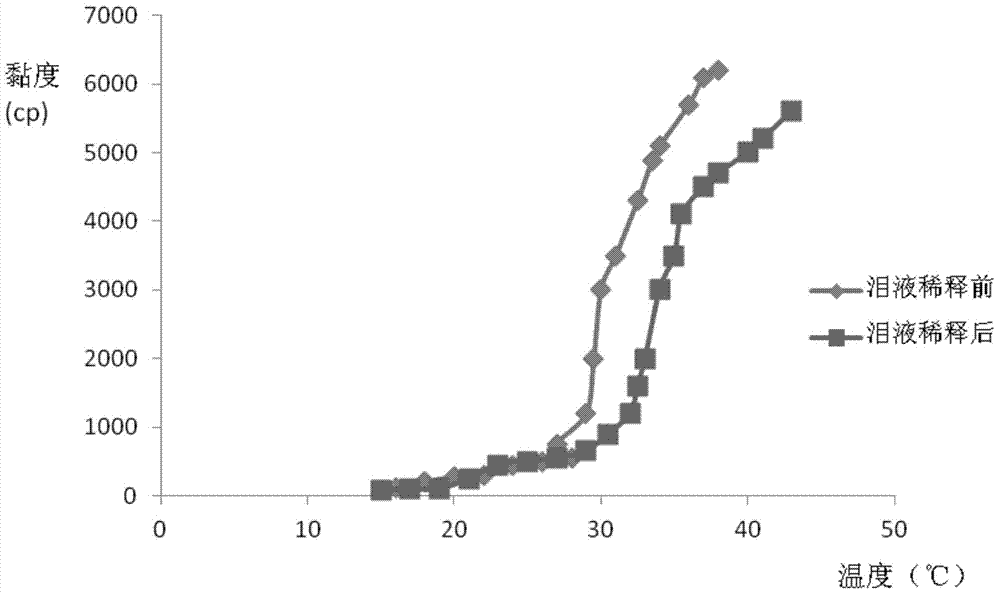
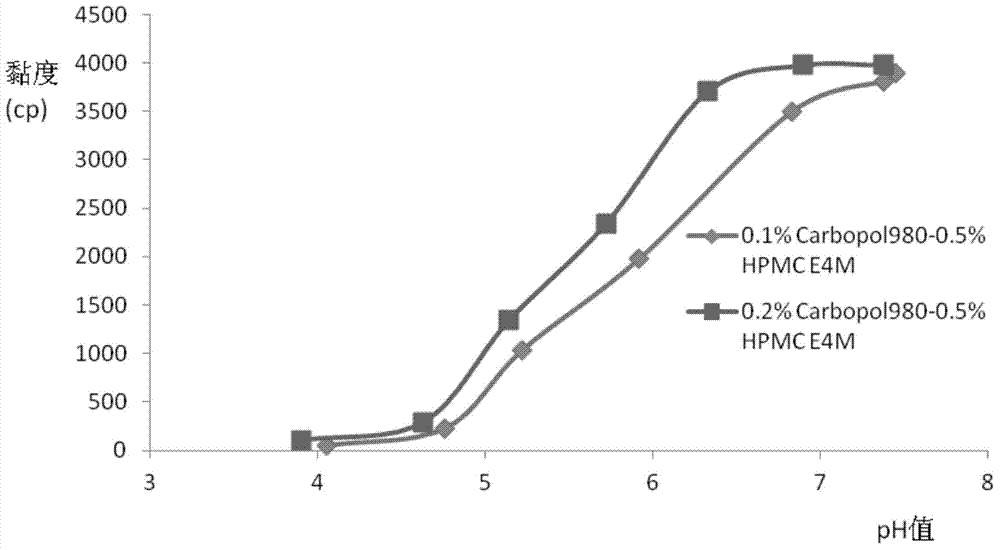
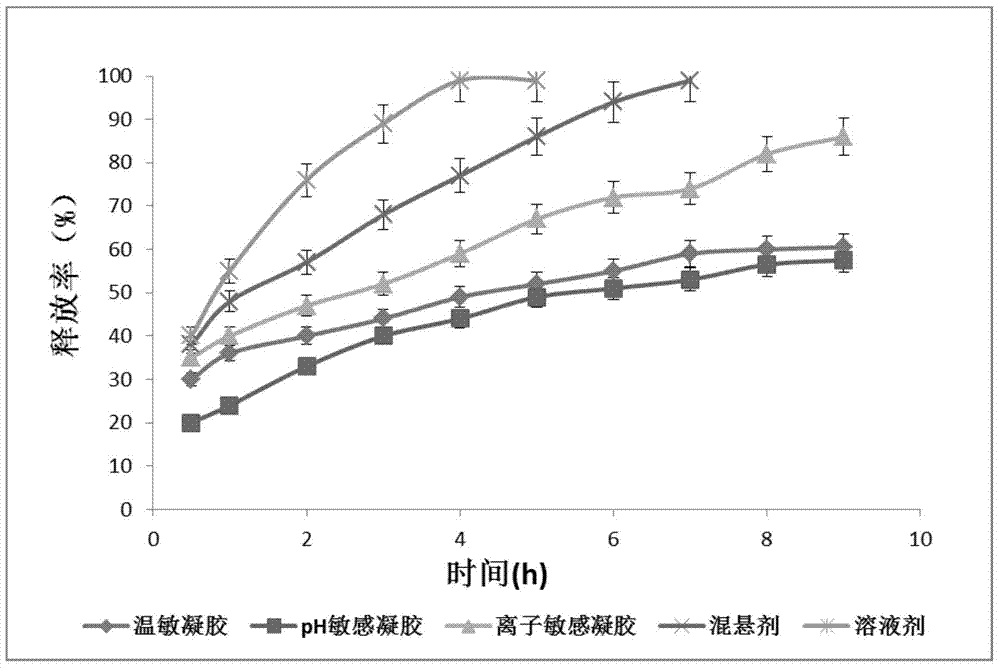
The invention relates to the medicine field, and concretely relates to brinzolamide eye preparations, and preparation methods and uses thereof. Several brinzolamide eye preparations are provided to prolong the eye stay time or improve the bioavailability, and provide a new dose form choice for the reduction of the intraocular tension or the treatment of glaucoma. The concrete dose forms of the preparations comprise a temperature-sensitive eye in-situ gel, a pH-sensitive eye in-situ gel, an ion-sensitive eye in-situ gel, a brinzolamide lipidosome, a brinzolamide nanoparticle, a brinzolamide nano-suspension, and a brinzolamide composite gel preparation, the preparations can be used for treating diseases comprising intraocular hypertension, glaucoma and the like, and the gels can prolong the stay time of medicines in eyes and reduce the administration frequency and the administration dosage, so the patient compliance is improved, and the side effects of medicines are reduced. The no addition of any antiseptics and the adoption of single-dosage packaging reduce the irradiation of the use of eye drops for a long term to eyes and substantially improve the medicine safety.
Owner:SICHUAN UNIV
Brinzolamide nanosuspension used for eyes and preparation method thereof
InactiveCN104721136AMedication convenienceImprove bioavailabilityPowder deliveryOrganic active ingredientsDiseaseSide effect
The invention relates to the field of medicines and in particular relates to a brinzolamide nanosuspension preparation used for eyes as well as a preparation method and application thereof, aiming to lengthen the residence time of the preparation on the eyes or improve the bioavailability of the preparation. The preparation can be used for treating the diseases, such as intraocular hypertension and glaucoma. Gels can conduce to lengthening the retention time of the medicine on the eyes and reducing the number of times of administration and the dosage of administration, so that not only is the patient compliance improved but also the side effects of the medicine can be reduced. Meanwhile, no preservative is added and single-dosage package is adopted, thus reducing the irritation of eye drops used for a long term to the eyes and greatly improving the medication safety.
Owner:SICHUAN UNIV
Brinzolamide nanoparticle preparation used for eyes and preparation method thereof
InactiveCN104721145AMedication convenienceImprove bioavailabilityPowder deliveryOrganic active ingredientsDiseaseSide effect
The invention relates to the field of medicines and in particular relates to a brinzolamide nanoparticle preparation used for eyes as well as a preparation method and application thereof, aiming to lengthen the residence time of the preparation on the eyes or improve the bioavailability of the preparation. The preparation can be used for treating the diseases, such as intraocular hypertension and glaucoma. Gels can conduce to lengthening the retention time of the medicine on the eyes and reducing the number of times of administration and the dosage of administration, so that not only is the patient compliance improved but also the side effects of the medicine can be reduced. Meanwhile, no preservative is added and single-dosage package is adopted, thus reducing the irritation of eye drops used for a long term to the eyes and greatly improving the medication safety.
Owner:SICHUAN UNIV
Brinzolamide clathrate compound preparation used for eyes and preparation method thereof
ActiveCN104721130ANo foreign body sensationImprove complianceOrganic active ingredientsSenses disorderDiseaseSide effect
The invention relates to the field of medicines and in particular relates to a brinzolamide clathrate compound preparation used for eyes as well as a preparation method and application thereof, aiming to lengthen the residence time of the preparation on the eyes or improve the bioavailability of the preparation. The preparation can be used for treating the diseases, such as intraocular hypertension and glaucoma. Gels can conduce to lengthening the retention time of the medicine on the eyes and reducing the number of times of administration and the dosage of administration, so that not only is the patient compliance improved but also the side effects of the medicine can be reduced. Meanwhile, no preservative is added and single-dosage package is adopted, thus reducing the irritation of eye drops used for a long term to the eyes and greatly improving the medication safety.
Owner:SICHUAN UNIV
Brinzolamide brimonidine eye drop and preparation method thereof
InactiveCN108261390ANo secondary damagePromote epithelial repairOrganic active ingredientsSenses disorderPreservative freePreservative
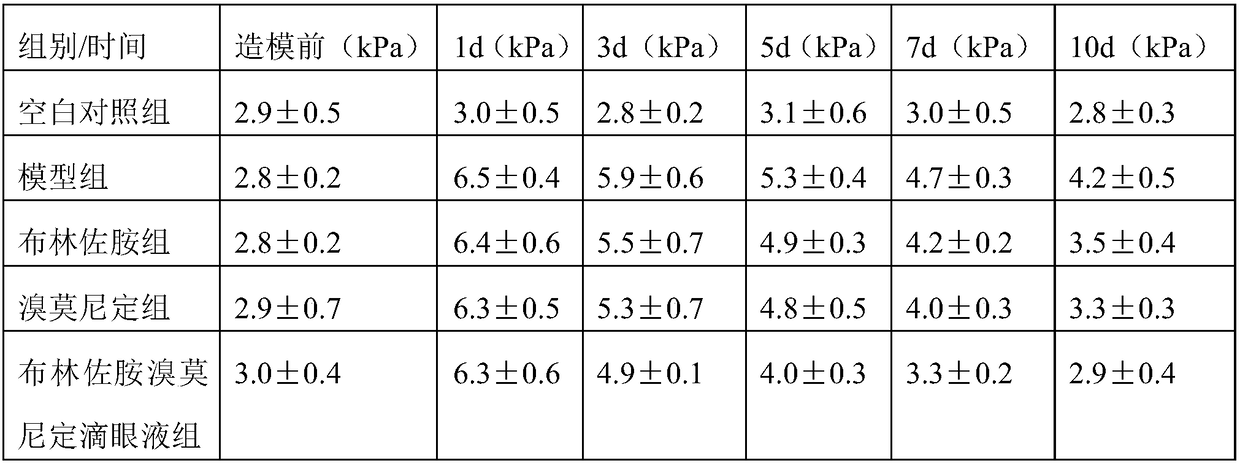
The invention belongs to the technical field of drugs, and particularly relates to a brinzolamide brimonidine eye drop and a preparation method thereof. The brinzolamide brimonidine eye drop is prepared from, by weight, 1-3% of brinzolamide, 1-5% of brimonidine, 0.3-0.8% of osmotic pressure conditioning agent, 0.1-1% of glycosaminoglycan, pH conditioning agent and balance water for injection, wherein the addition amount of the pH conditioning agent enables pH of the eye drop to be 5.5-7.5. The prepared eye drop containing the brinzolamide brimonidine and iso-osmia with tears have the advantages of not containing preservative, being capable of effectively reducing intraocular pressure and having no secondary damage to eyes caused by the preservative.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
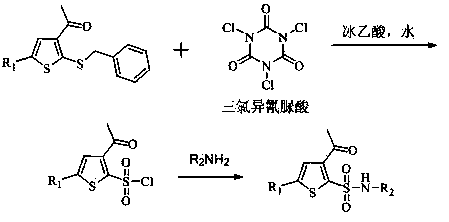
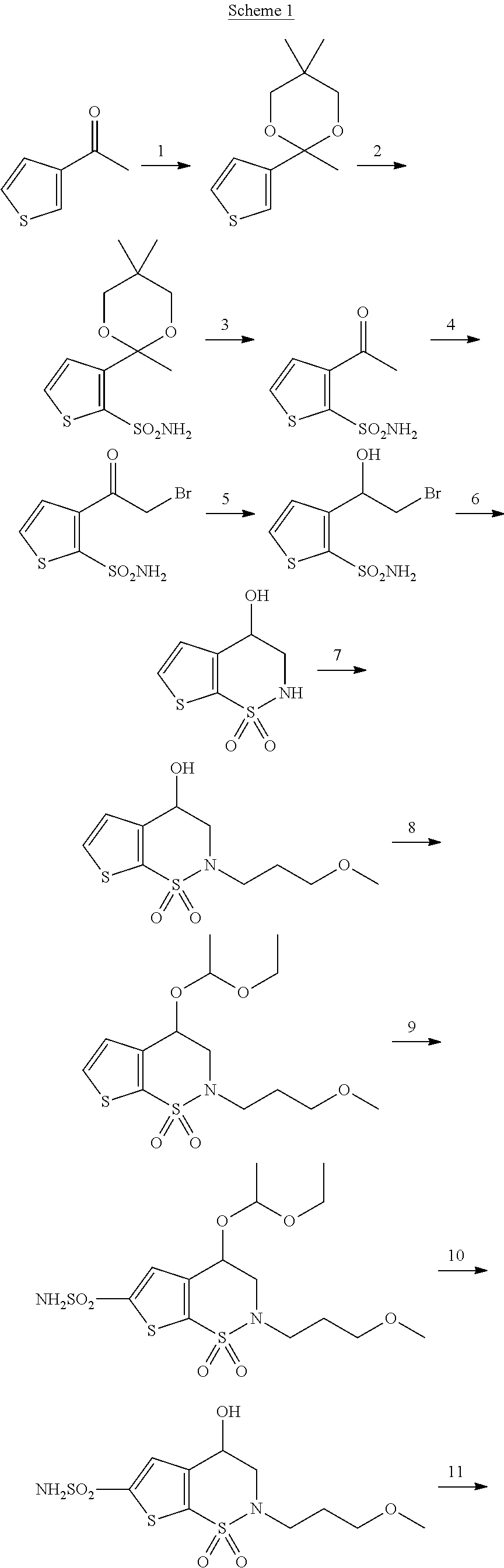
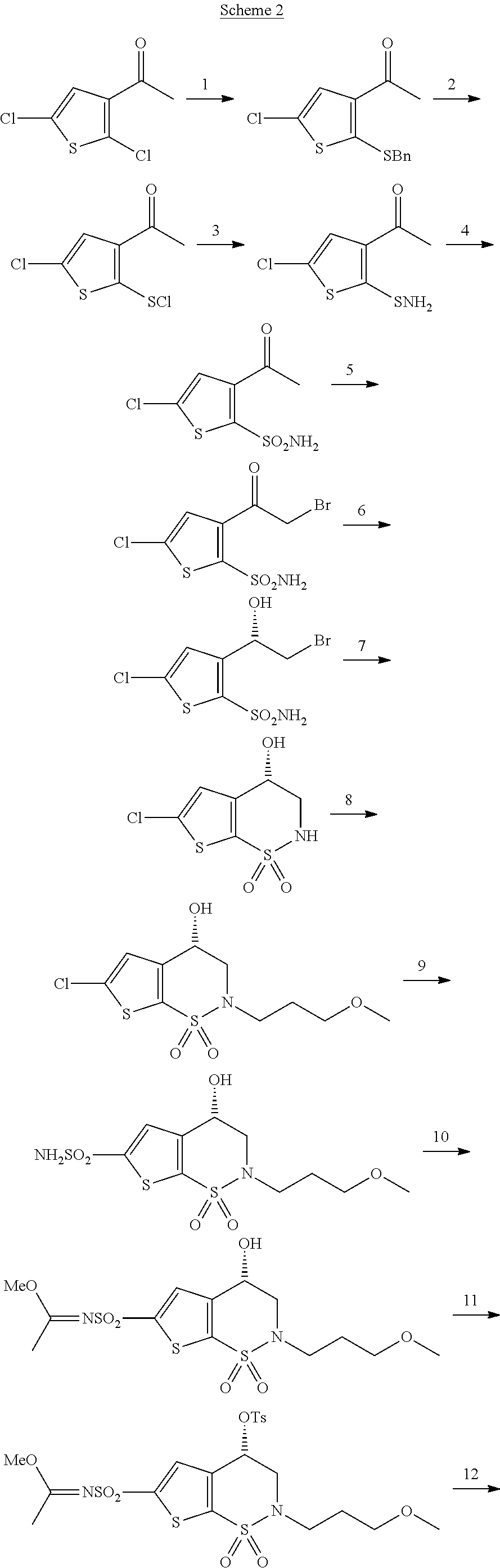
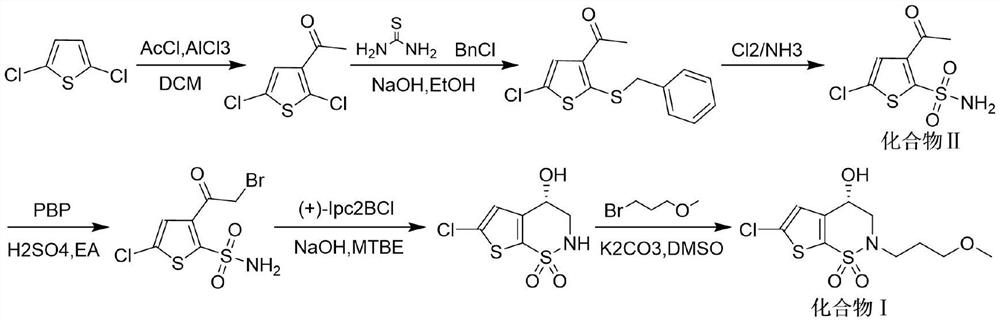
Method for preparing intermediate thiophene sulfonamide of brinzolamide
The invention belongs to the technical field of pharmaceutical chemicals, in particular relates to a method for preparing an intermediate thiophene sulfonamide of brinzolamide and is applicable to industrial production. The method comprises the following steps: dissolving thiophene benzyl sulfide in a proper solvent, adding glacial acetic acid and water, controlling the temperature to 0-15 DEG C, adding trichloroisocyanuric acid in batches in 20-40 minutes, reacting at the constant temperature until the raw materials disappear, performing vacuum concentration in a water bath at the temperature of 30-50 DEG C to remove the solvent, adding ethyl acetate petroleum ether solution with the mass concentration of ethyl acetate being 25%, stirring, filtering, evaporating the filtrate under reduced pressure, thereby obtaining thiophenesulfonyl chloride; controlling the temperature to 0-15 DEG C, diluting thiophenesulfonyl chloride by ethyl acetate, dropwise adding a dilution into ammonium hydroxide or a water solution of amine, stirring and reacting until the raw materials disappear, filtering, washing, drying the solids, and obtaining the thiophene sulfonamide. The product obtained by using the method is high in yield, simple and convenient in operation, low in cost and mild in reaction conditions and is suitable for large-scale production.
Owner:山东诚汇双达药业有限公司
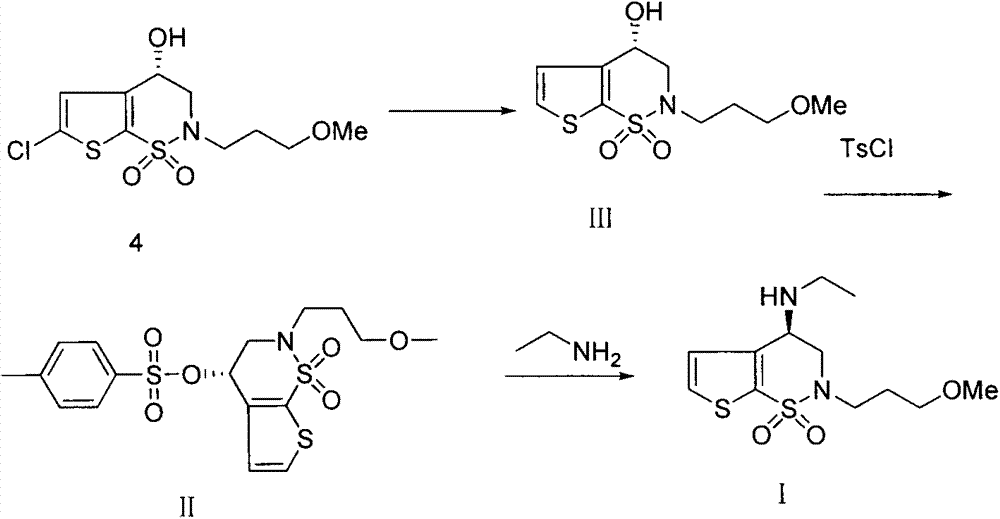
Preparation method of brinzolamide intermediate
ActiveCN103755727AMild reaction conditionsEasy to operateOrganic chemistryPhotochemistryBrinzolamide
The invention relates to a preparation method of a brinzolamide intermediate I and an intermediate compound III. The preparation method is simple to operate, mild in reaction condition, high in efficiency and low in cost, and is suitable for industrial production.
Owner:NANJING HUAWE MEDICINE TECH DEV
Preparation method of brinzolamide imprinted hydrogel contact lens for sustained and controlled release administration
ActiveCN111419851AGood flexibilityHigh oxygen permeabilityOrganic active ingredientsSenses disorder(Hydroxyethyl)methacrylateFunctional monomer
The invention discloses a preparation method of a molecularly imprinted hydrogel contact lens for sustained and controlled release administration. The method comprises the following steps: mixing choline chloride and methacrylic acid according to a certain proportion, and preparing a hydrophilic functional monomer deep eutectic solvent (DES) in an oil bath at 90 DEG C; taking brinzolamide as a template, hydroxyethyl methacrylate as a framework monomer, DES as a functional monomer, and polyethylene glycol dimethacrylate as a crosslinking agent to perform ultrasonic treatment under action of aninitiator AIBN to obtain a pre-polymerization solution; removing oxygen from the pre-polymerization solution, performing balancing in the dark, then injecting the pre-polymerization solution into a mold, performing ultraviolet polymerization, performing cutting by a round punch, performing eluting to remove unreacted substances, and performing drying to obtain the hydrogel contact lens. The hydrogel contact lens prepared by the preparation method has large drug loading amount, can realize slow release of drugs, and has the advantages of greatly improved bioavailability, good permeability, longretention time, better convenience and higher efficiency compared with a traditional ophthalmic preparation.
Owner:TIANJIN MEDICAL UNIV
Process for preparing pharmaceutical ophthalmic compositions of brinzolamide
InactiveUS20190209465A1Simple and cost-effective processPatient tolerability and bioavailabilityOrganic active ingredientsInorganic non-active ingredientsAlternative methodsAlternative process
The present invention relates to the field of drug delivery and, particularly, to alternative processes for preparing ophthalmic compositions of Brinzolamide or pharmaceutical acceptable salts thereof.
Owner:PHARMATHEN
Preparation methods of brinzolamide and an intermediate thereof
ActiveCN107759618AReduce dosageShort reaction timeOrganic chemistryTriethyl orthoacetateReaction temperature
The invention discloses preparation methods of brinzolamide and an intermediate thereof. The preparation method of the brinzolamide intermediate represented by a compound C comprises: carrying out a condensation reaction on a compound B and an orthoester-based compound at a temperature of 70-80 DEG C in an organic solvent in the presence of tertiary amine under an anhydrous condition, wherein theorthoester-based compound is one or a plurality of materials selected from trimethyl orthoacetate, triethyl orthoacetate and trimethyl orthobenzoate. According to the present invention, the condensation reaction is performed by using the tertiary amine as the catalyst, such that the reaction time for the amino protection is substantially shortened, the consumption of the protection reagent is reduced, the reaction temperature is reduced, the yield and the purity of the product are improved, the acid consumption and the alkali consumption during the post-treatment are reduced, the residue on ignition of the product is reduced, the operation is simplified, and the method is suitable for industrial production. The formulas B and C are defined in the specification.
Owner:SHANGHAI VIWIT PHARMA CO LTD
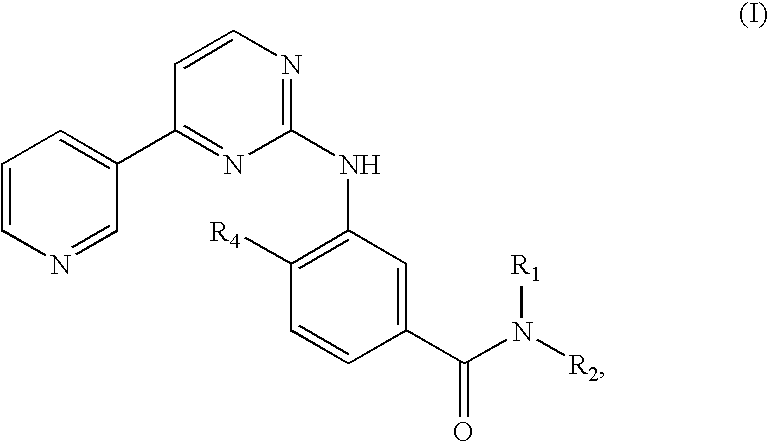
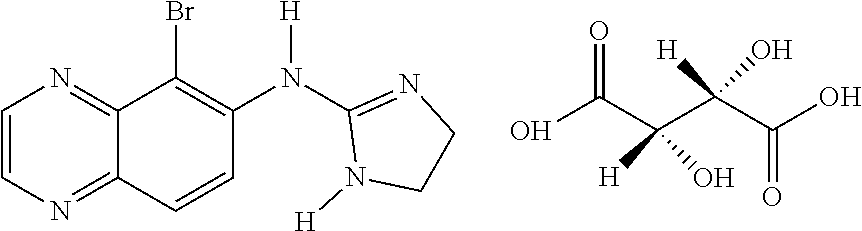
Pyrimidylaminobenzamide Derivatives For the Treatment of Neurofibromatosis
The present invention relates to the use of pyrimidylaminobenzamide derivatives for the preparation of a drug for the treatment of non-cancerous, benign brain tumors, especially for the curative and / or prophylactic treatment of NF, and to a method of treating non-cancerous, benign brain tumors, especially for the curative and / or prophylactic treatment of NF.
Owner:MANLEY PAUL W
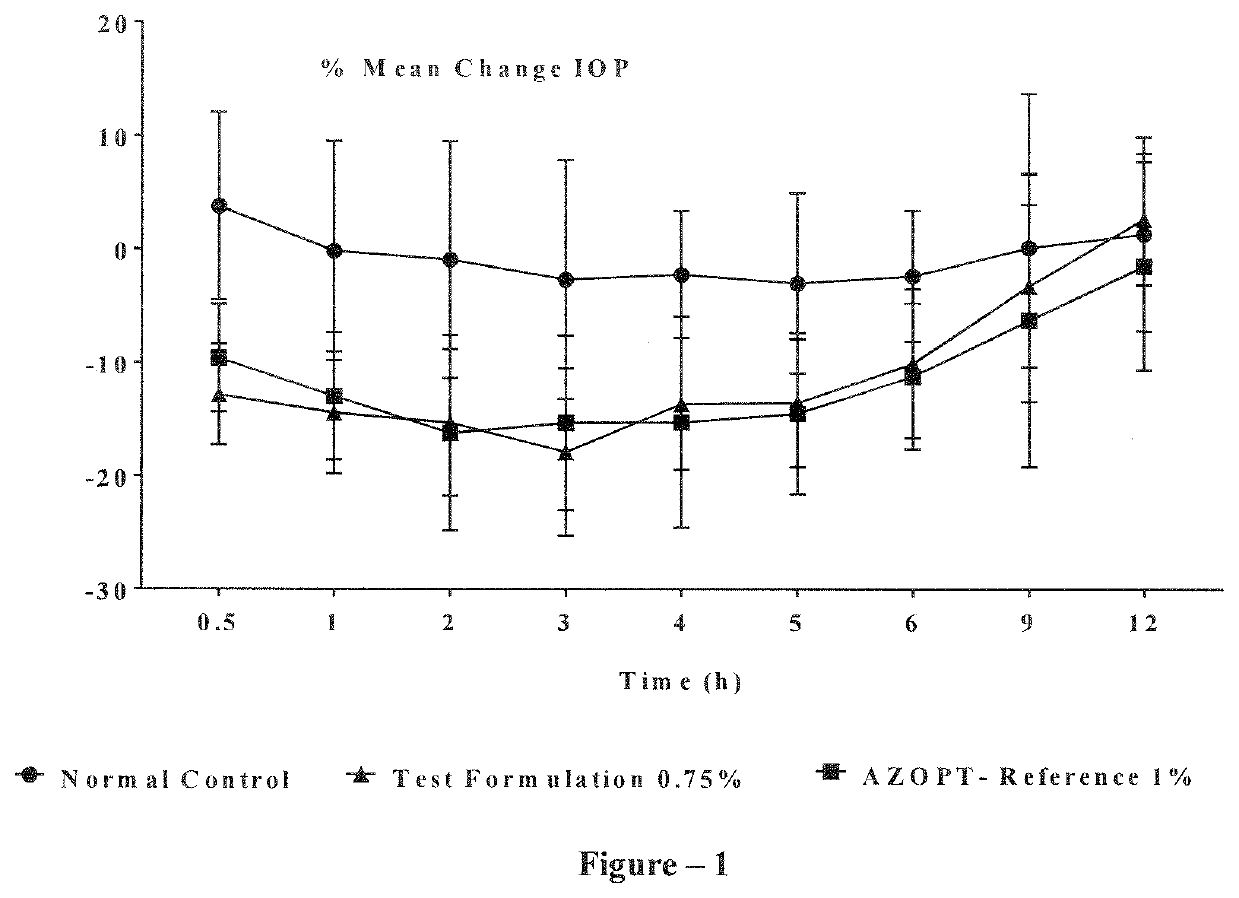
Ophthalmic compositions of brinzolamide
The present invention relates to an aqueous ophthalmic solution for treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma, the solution comprising at least 0.5 w / v % brinzolamide dissolved in the solution; hydroxy-propyl-β-cyclodextrin; polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer; and water and / or the process for preparation thereof.
Owner:SHILPA MEDICARE LTD
High-efficiency herbal eye drop
InactiveCN110870895AExtended stayAvoid rapid eliminationSenses disorderTetracycline active ingredientsDisodium EdetateHouttuynia
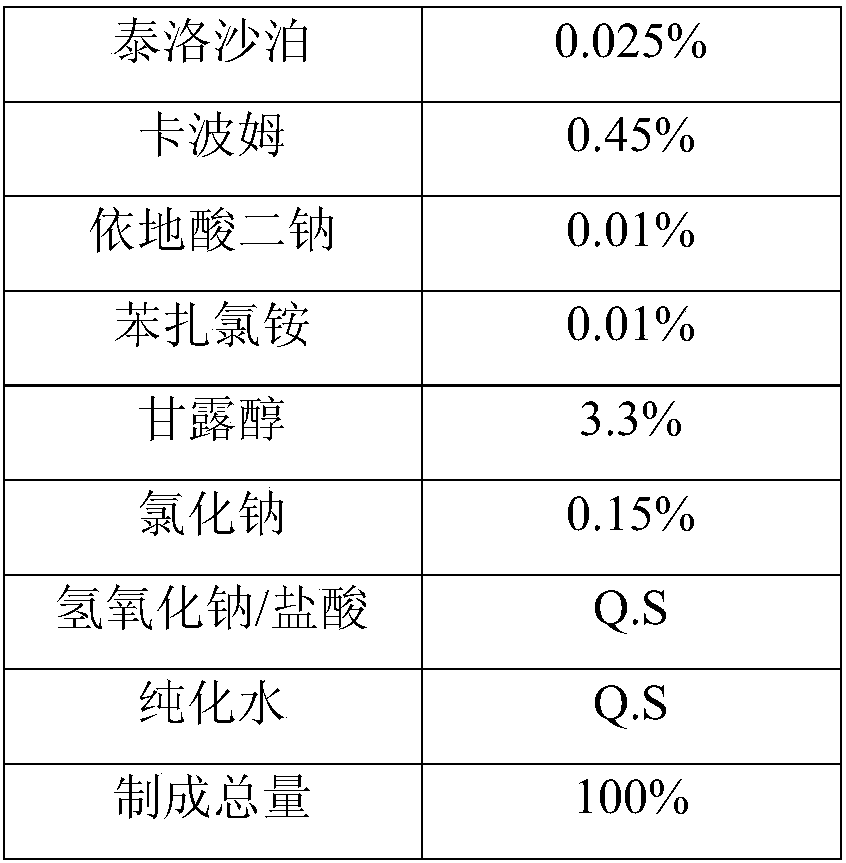
The invention discloses a high-efficiency herbal eye drop, and belongs to the field of pharmaceutical chemicals. The high-efficiency herbal eye drop contains the following components in parts by weight: 80-100 parts of water, 50-60 parts of polycarbophil AA, 30-40 parts of oxytetracycline, 25-30 parts of tafluprost, 20-25 parts of timclol maleate, 18-23 parts of herba houttuyniae extract, 16-21 parts of flos lonicerae extract, 13-18 parts of semen cassiae extract, 11-16 parts of rhizoma coptidis extract, 9-13 parts of sodium chloride, 6-11 parts of edetate disodium, 6-9 parts of poloxamer, 5-8parts of tyloxapol, 4-8 parts of timclol maleate, 4-6 parts of brinzolamide, 2-5 parts of mannitol, 2-5 parts of citric acid, 2-4 parts of sodium citrate, 1-3 parts of benzene, and 1-3 parts of a preservative. The high-efficiency herbal eye drop solves the problems that a traditional herbal eye drop has poor absorption and a poor effect, and a chemical preparation eye drop hurts eyes.
Owner:李达欣
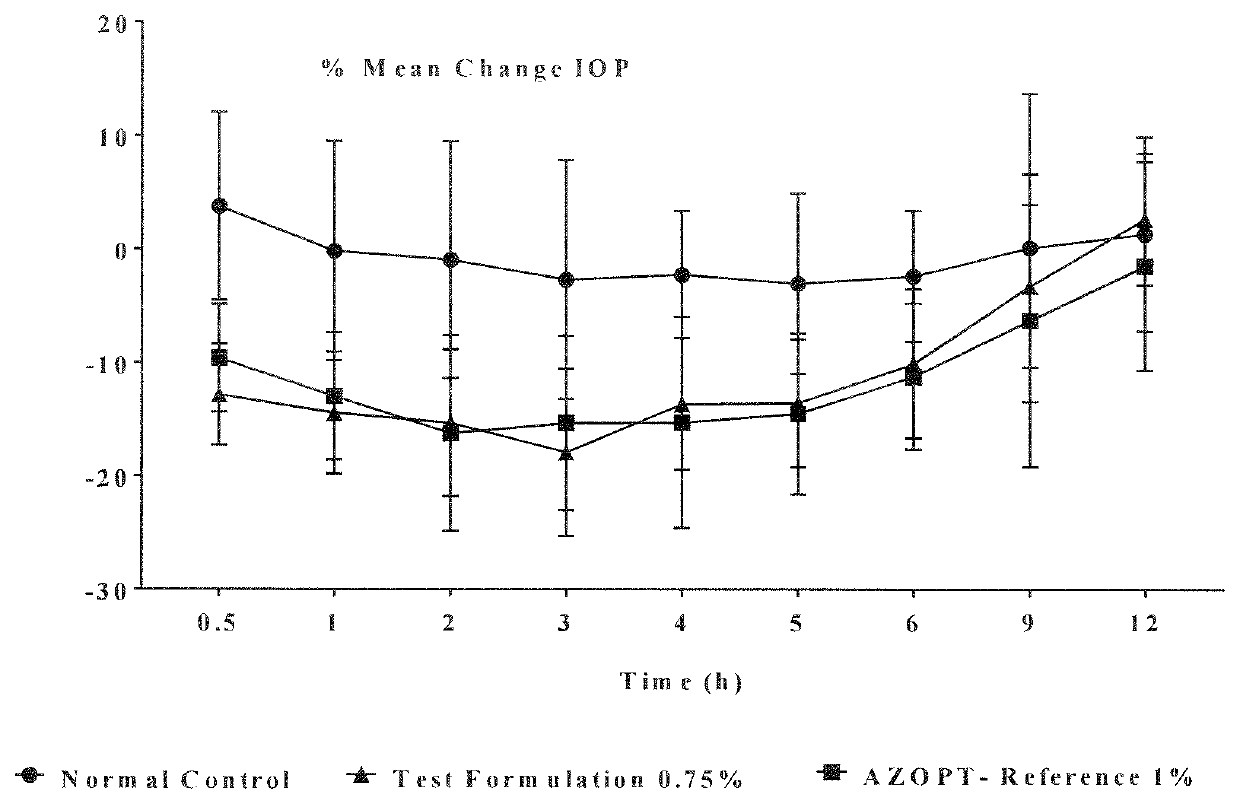
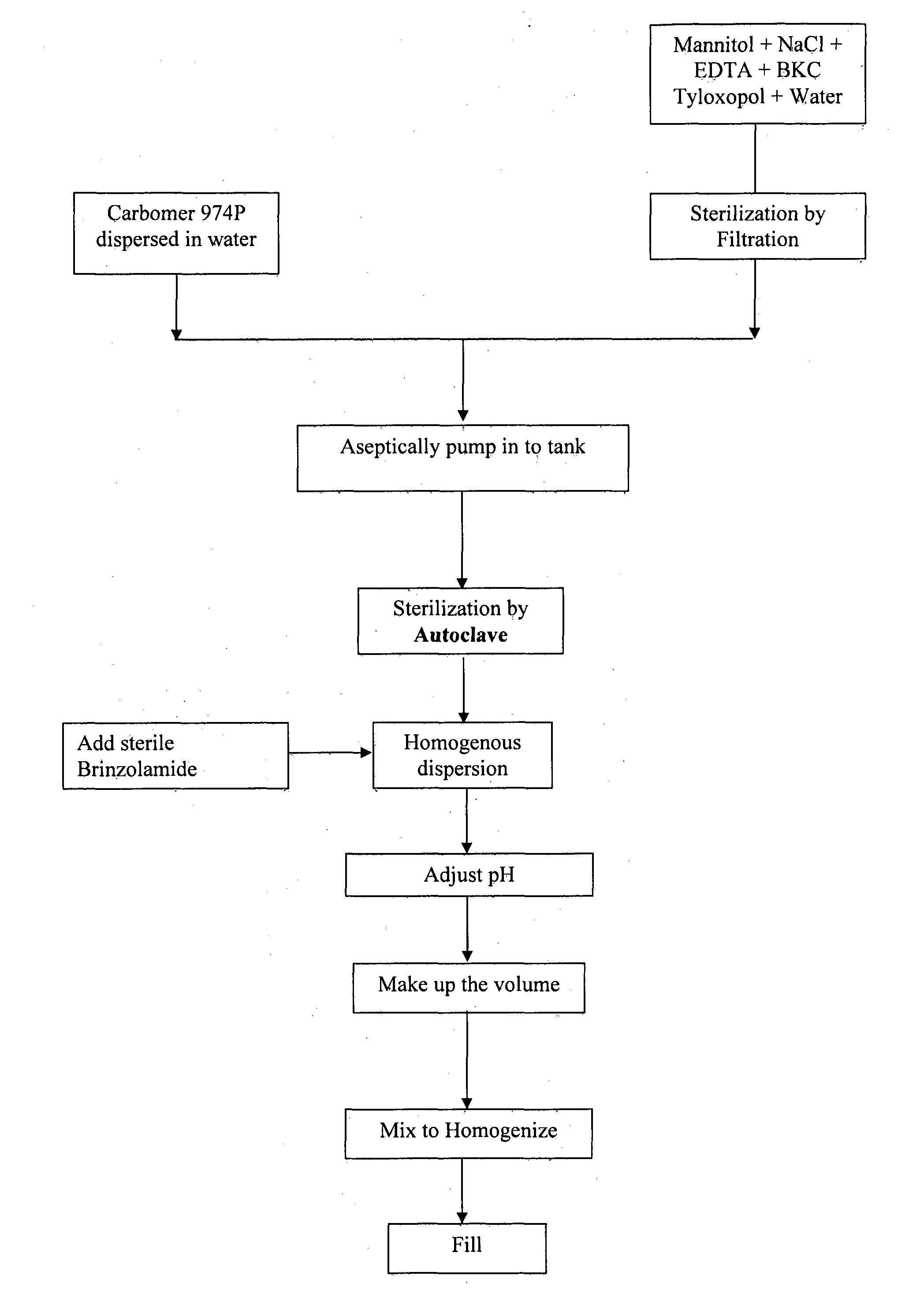
Process for preparing opthalmic suspension of brinzolamide
InactiveUS20150119386A1Simple and cost-effectiveInorganic non-active ingredientsPharmaceutical delivery mechanismOptometryBrinzolamide
The present invention relates to a process for preparing sterile ophthalmic suspension of carbonic anhydrase inhibitor. More particularly, the present invention relates to a process for preparing sterile ophthalmic suspension of brinzolamide.
Owner:AUROBINDO PHARMA LTD
Brinzolamide liposome eye preparation and preparation method thereof
InactiveCN104814924ANo foreign body sensationImprove complianceOrganic active ingredientsSenses disorderGel preparationDisease
The invention relates to the medicine field, and concretely relates to brinzolamide eye preparations, and preparation methods and uses thereof. Several brinzolamide eye preparations are provided to prolong the eye stay time or improve the bioavailability, and provide a new dose form choice for the reduction of the intraocular tension or the treatment of glaucoma. The concrete dose forms of the preparations comprise a temperature-sensitive eye in-situ gel, a pH-sensitive eye in-situ gel, an ion-sensitive eye in-situ gel, a brinzolamide lipidosome, a brinzolamide nanoparticle, a brinzolamide nano-suspension, and a brinzolamide composite gel preparation, the preparations can be used for treating diseases comprising intraocular hypertension, glaucoma and the like, and the gels can prolong the stay time of medicines in eyes and reduce the administration frequency and the administration dosage, so the patient compliance is improved, and the side effects of medicines are reduced. The no addition of any antiseptics and the adoption of single-dosage packaging reduce the irradiation of the use of eye drops for a long term to eyes and substantially improve the medicine safety.
Owner:SICHUAN UNIV
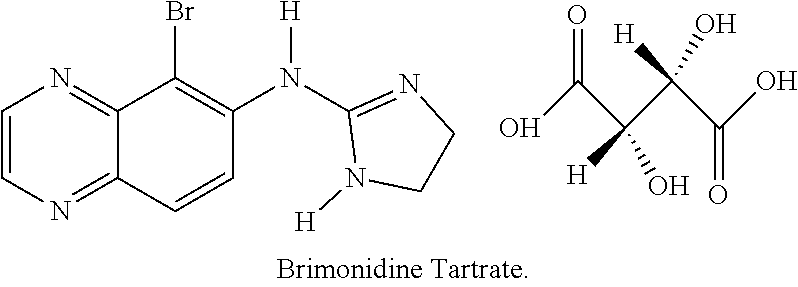
Safe use of brinzolamide and brimonidine compositions with enhanced benzalkonium chloride content
InactiveUS20220105103A1Organic active ingredientsInorganic non-active ingredientsDiseaseAdrenergic receptor agonists
Disclosed herein are safe pharmacologically acceptable and ophthalmologically suitable compositions and methods of their use in treating ophthalmic diseases or related conditions. Disclosed are compositions comprising effective amounts of carbonic anhydrase inhibitor(s) and alpha-2-adrenergic agonist(s) and an effective amount of a penetration enhancement component comprising one or more penetration enhancer compound(s) / molecule(s), e.g., benzalkonium chloride, which detectably or significantly increases the penetration of API(s) of the composition. In aspects, the invention provides compositions comprising a brinzolamide compound in an amount of about 0.1 wt. %-10 wt. % of the composition, a brimonidine compound, e.g., brimonidine tartrate, in an amount of about 0.01 wt. %-0.5 wt. % of the composition, one or more borate-polyol complexes in an amount of about 0.5 wt. %-6 wt. % of the composition, and a penetration enhancement component comprising benzalkonium chloride, in, for example, an amount of about 0.005-0.02 wt. % of the composition.
Owner:SOMERSET THERAPEUTICS LLC
Local-medicine-applying medicine composition including puerarin, brinzolamide and the like
PendingCN112972683AReduce adverse reactionsReduce pollutionOrganic active ingredientsSenses disorderDiseasePuerarin
The invention provides a medicine composition containing an effective dosage of puerarin and an effective dosage of dorzolamide or brinzolamide or prostaglandin, such as latanoprost, tafluprost and the like or / and an effective dosage of water-retaining agent or wetting agent or antixerophthalmic ingredients, and application in preparation of medicines, including eye-purpose medicines used for preventing or treating intraocular pressure or elevated intraocular pressure, glaucoma, fundi diseases, optic nerve protection, dry eyes or xerophthalmia or dry eye syndromes of the human being or mammals. The medicine disclosed by the invention has a better curative effect or a smaller side effect or better treatment compliance or a better treatment mode.
Owner:刘力
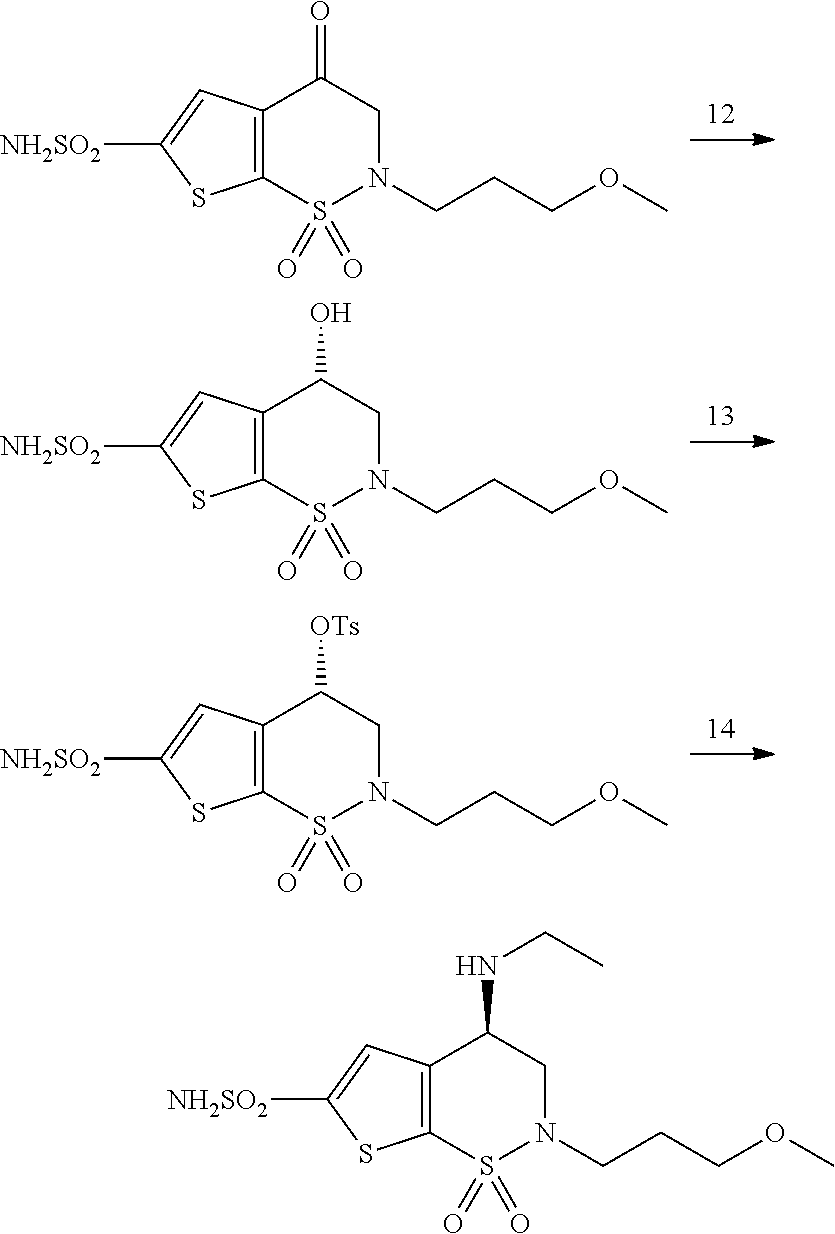
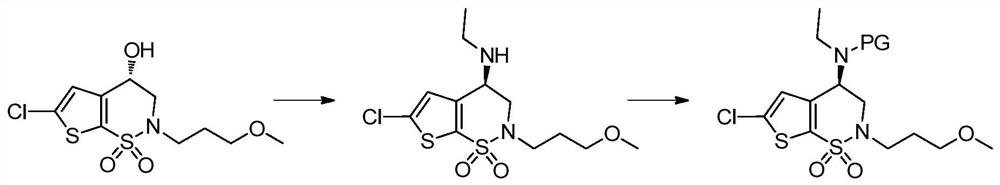
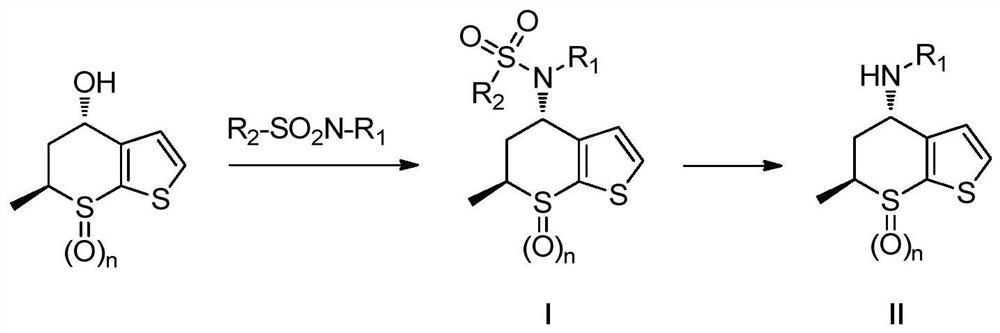
Process for the preparation of brinzolamide
The present invention relates to a process for the preparation of Brinzolamide, or 2H-thieno[3,2-e]-1, 2-thiazin-6-sulfonamide, 4-(ethyl amino)-3, 4-dihydro-2-(3-methoxypropyl)-, 1,1-dioxide,(4R)- via intermediates 2,3-dihydro-4H-thieno[3,2-e]-1, 2-thiazin-4-ones, 1,1-dioxide. Further objects of the present invention are the intermediates mentioned above and other intermediates of the synthesis.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Ophthalmic compositions of brinzolamide
ActiveUS20210353635A1Organic active ingredientsSenses disorderIntra ocular pressureOpen angle glaucoma
The present invention relates to an aqueous ophthalmic solution for treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma, the solution comprising at least 0.5 w / v % brinzolamide dissolved in the solution; hydroxy-propyl-β-cyclodextrin; polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer; and water and / or the process for preparation thereof.
Owner:SHILPA MEDICARE LTD
Compound nano eye drops and preparation method thereof
PendingCN114306226AReduce transshipmentReduce complexityOrganic active ingredientsSenses disorderAnterior corneaAntiseptic Agent
The invention provides compound nano eye drops and a preparation method thereof, and belongs to the technical field of eye drops. The compound nano eye drops provided by the invention simultaneously contain two medicines, namely the plinzolamide and the brimonidine tartrate, and the adhesive, the stabilizer and the osmotic pressure regulator are used in a matched manner, so that the complexity and the medication frequency of combined medication can be reduced; the particle size of solid particles in the eye drops is nanoscale, the specific surface area of the medicine is large, the residence time of the medicine in front of the cornea can improve the irritation effect of the large-particle medicine on eyes, and the compliance of a patient is improved. The adhesive increases the specific surface area of the brinzolamide medicine, increases the residence time of the hydrophobic medicine in front of cornea, reduces the loss of the medicine in eyes, reduces systemic side effects caused by loss through nasolacrimal ducts, improves the bioavailability, prolongs the medication cycle of the eye drops through slow release, and improves the antibacterial and anti-inflammatory effects; no preservative is contained, so that secondary injury to eyes of a patient is avoided.
Owner:SHANDONG INOMIC INST OF PHARM RES CO LTD
Safe brinzolamide and brimonidine compositions with enhanced benzalkonium chloride content
InactiveUS20220105100A1Organic active ingredientsInorganic non-active ingredientsDiseaseAdrenergic receptor agonists
Disclosed herein are safe pharmacologically acceptable and ophthalmologically suitable compositions and methods of their use in treating ophthalmic diseases or related conditions. Disclosed are compositions comprising effective amounts of carbonic anhydrase inhibitor(s) and alpha-2-adrenergic agonist(s) and an effective amount of a penetration enhancement component comprising one or more penetration enhancer compound(s) / molecule(s), e.g., benzalkonium chloride, which detectably or significantly increases the penetration of API(s) of the composition. In aspects, the invention provides compositions comprising a brinzolamide compound in an amount of about 0.1 wt. %-10 wt. % of the composition, a brimonidine compound, e.g., brimonidine tartrate, in an amount of about 0.01 wt. %-0.5 wt. % of the composition, one or more borate-polyol complexes in an amount of about 0.5 wt. %-6 wt. % of the composition, and a penetration enhancement component comprising benzalkonium chloride, in, for example, an amount of about 0.005-0.02 wt. % of the composition.
Owner:SOMERSET THERAPEUTICS LLC
Combination therapies using indazolylbenzamide derivatives for the treatment of cancer
InactiveUS20190134003A1Effective treatment of cancerAntineoplastic agentsHeavy metal compound active ingredientsCancer preventionCombination therapy
The invention relates to combination therapies useful in the treatment and / or prevention of cancer.
Owner:ESANEX
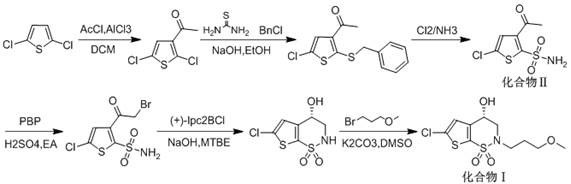
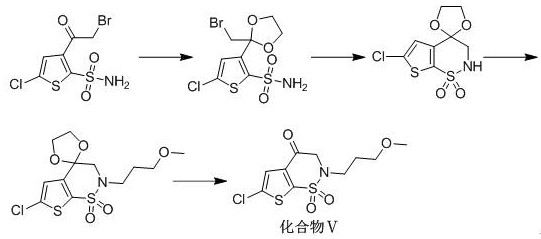
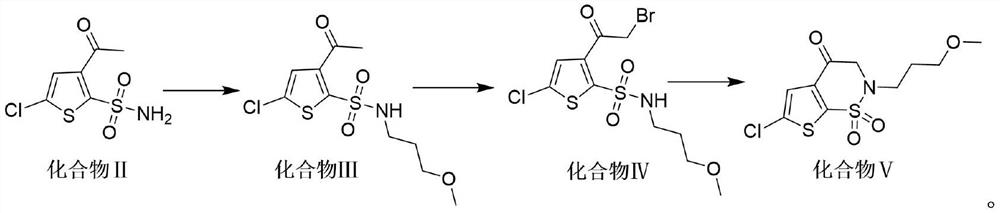
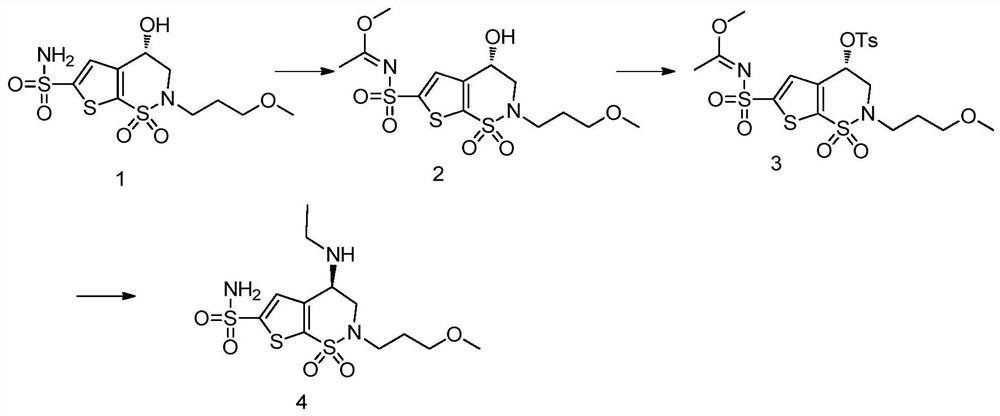
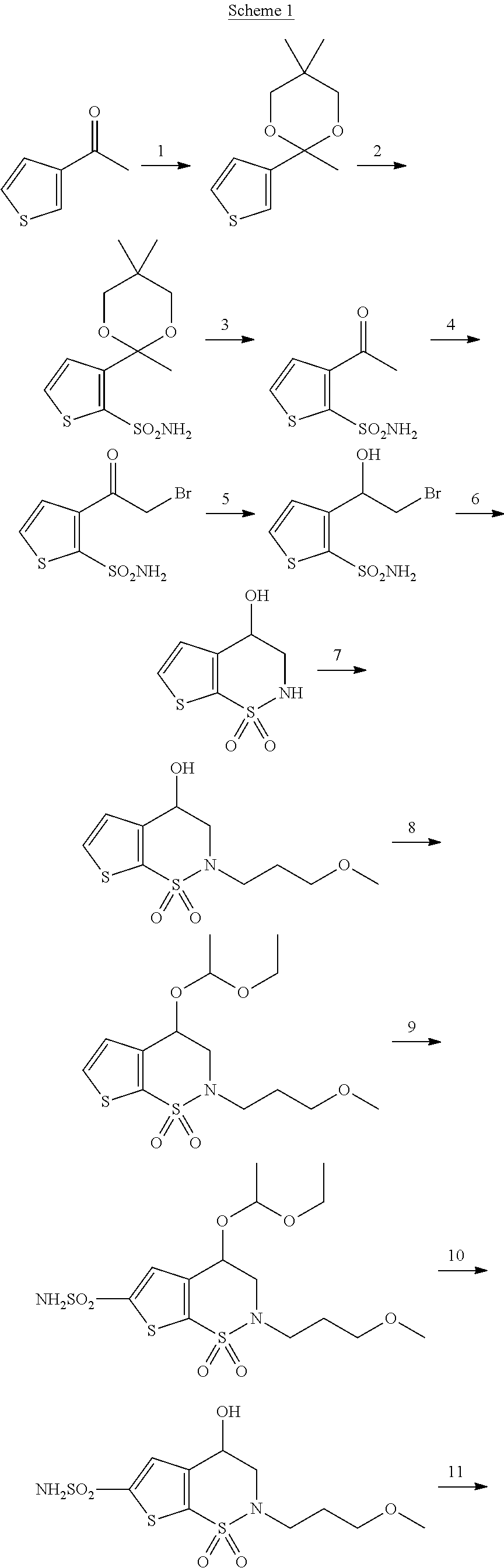
Synthesis method of brinzolamide key intermediate
ActiveCN113354665AEasy to separate and purifyHigh purityOrganic chemistryDrugs synthesisPharmaceutical Substances
The invention relates to the technical field of drug synthesis, in particular to a synthesis method of a brinzolamide key intermediate. A compound II as an initial raw material and 1-bromo-3-methoxypropane are subjected to an alkylation reaction to synthesize a compound III, the compound III is subjected to a bromination reaction to synthesize a compound IV, and the compound IV is subjected to an intramolecular ring closing reaction to finally synthesize a brinzolamide key intermediate compound V. The specific reaction is shown in the specification. The synthesis method provided by the invention is simple in synthesis route, the whole process is easy to operate, and the obtained product is easy to purify, high in purity, high in yield and suitable for large-scale production.
Owner:株洲壹诺生物技术有限公司
Methods of efficiently reducing intraocular pressure
PendingUS20220105102A1Components is relatively effectiveGood pharmacokinetic propertiesOrganic active ingredientsInorganic non-active ingredientsDiseaseAdrenergic receptor agonists
Disclosed herein are pharmacologically acceptable and ophthalmologically suitable compositions and methods of their use in treating ophthalmic diseases or related conditions. In aspects, the invention provides compositions comprising effective amounts of carbonic anhydrase inhibitor(s) and alpha-2-adrenergic agonist(s). In facets, compositions comprise a penetration enhancer component comprising one or more penetration enhancer compound(s) / molecule(s). In aspects, the invention provides compositions comprising effective amounts of brimonidine compound(s) and brinzolamide compound(s) capable of being administered once or twice daily for the treatment of elevated intraocular pressure, but which provide similar efficacy to a similar or substantially identical reference product requiring administration three times per day. In facets, the invention provides compositions capable of being administered at a frequency resulting in providing a lower total dose of both brinzolamide and brimonidine compound(s) to a recipient, but nonetheless are as or more effective in IOP control as such similar or substantially identical reference products.
Owner:SOMERSET THERAPEUTICS LLC
A kind of synthetic method of brinzolamide key intermediate
ActiveCN113354665BEasy to separate and purifyHigh purityOrganic chemistryDrugs synthesisPharmaceutical Substances
The invention relates to the technical field of drug synthesis, in particular to a method for synthesizing a key intermediate of brinzolamide, which uses compound II as a starting material, and compounds II and 1-bromo-3-methoxypropane are synthesized by alkylation reaction Compound Ⅲ, compound Ⅲ is synthesized into compound Ⅳ through bromination reaction, and compound Ⅳ is finally synthesized into brinzolamide key intermediate compound Ⅴ through intramolecular ring-closing reaction, the specific reaction is as follows: The synthesis method provided by the invention has a simple synthesis route, easy operation in the whole process, and the obtained product is easy to purify, has high purity and high yield, and is suitable for large-scale production.
Owner:株洲壹诺生物技术有限公司
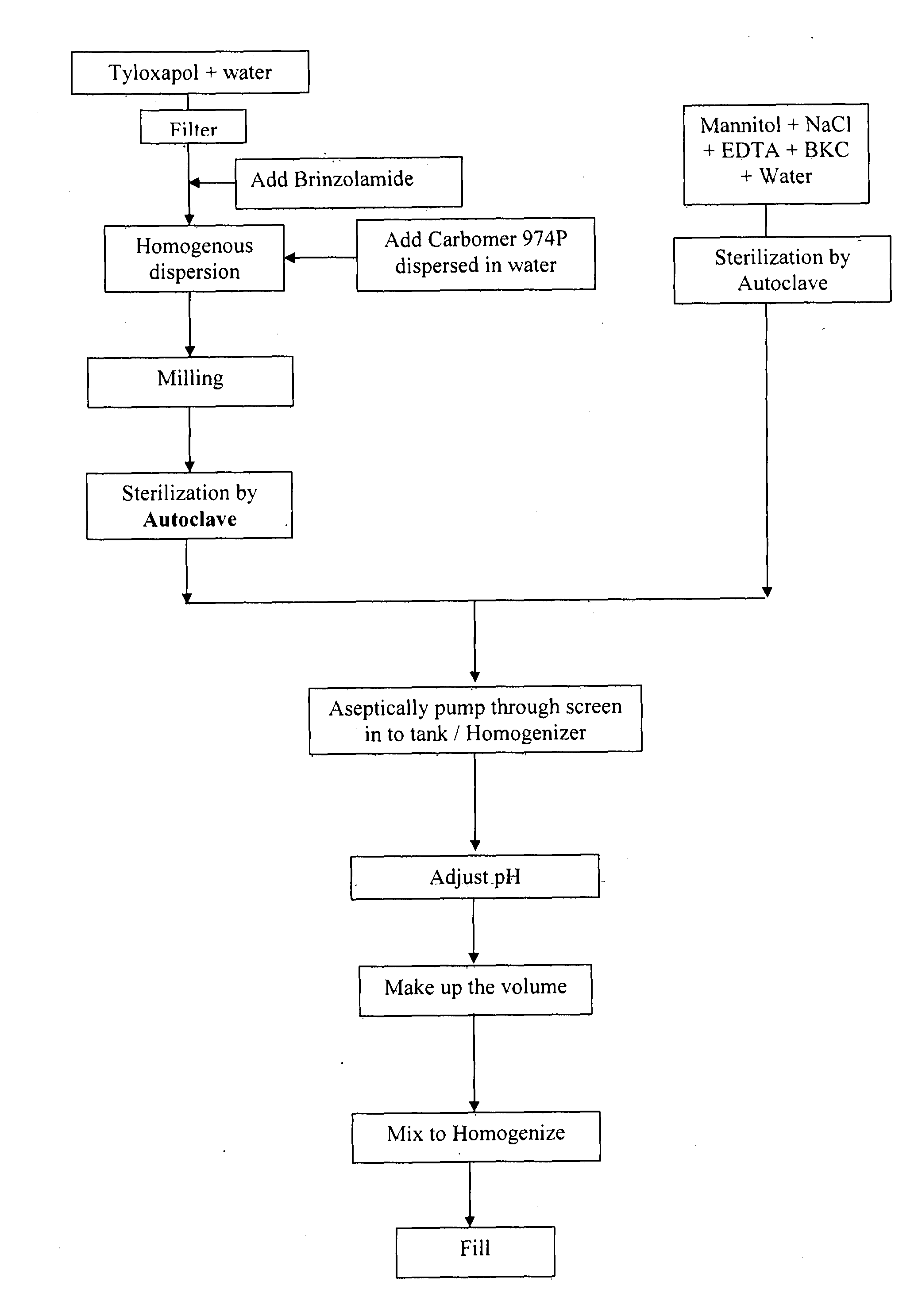
Brinzolamide eye drop preparation and preparation method thereof
InactiveCN107582519AThe prescription process is simpleStrong operability in industrial productionOrganic active ingredientsSenses disorderBULK ACTIVE INGREDIENTMedical prescription
A preparation method of a brinzolamide eye drop is disclosed. The method is as follows: dispersing active ingredient brinzolamide in a wetting agent, meanwhile completely swelling a suspending aidingagent at 60 DEG C, placing for two hours, dissolving an osmotic pressure regulator, a metal ion complexing agent and a preservative conditioner completely at 60 DEG C, adding a brinzolamide-containingwetting agent solution to a suspending aiding agent solution under homogeneous stirring conditions, continuing homogenizing stirring for 5min, adding other solutions, continuing homogenizing for 5min, adjusting pH, sterilizing and filling. The prescription of the brinzolamide eye drop is simple, the preparation method has strong industrialized production operationability, after direct micronization treatment of raw material medicines, a sample is prepared, a large number of manpower, material and financial resources can be saved, and the preparation method has the advantages of simple equipment required for the preparation project and easy process parameter control.
Owner:合肥远志医药科技开发有限公司
Efficient brinzolamide and brimonidine compositions
InactiveUS20220105101A1Components is relatively effectiveGood pharmacokinetic propertiesOrganic active ingredientsInorganic non-active ingredientsDiseaseAdrenergic receptor agonists
Disclosed herein are pharmacologically acceptable and ophthalmologically suitable compositions and methods of their use in treating ophthalmic diseases or related conditions. In aspects, the invention provides compositions comprising effective amounts of carbonic anhydrase inhibitor(s) and alpha-2-adrenergic agonist(s). In facets, compositions comprise a penetration enhancer component comprising one or more penetration enhancer compound(s) / molecule(s). In aspects, the invention provides compositions comprising effective amounts of brimonidine compound(s) and brinzolamide compound(s) capable of being administered once or twice daily for the treatment of elevated intraocular pressure, but which provide similar efficacy to a similar or substantially identical reference product requiring administration three times per day. In facets, the invention provides compositions capable of being administered at a frequency resulting in providing a lower total dose of both brinzolamide and brimonidine compound(s) to a recipient, but nonetheless are as or more effective in IOP control as such similar or substantially identical reference products.
Owner:SOMERSET THERAPEUTICS LLC
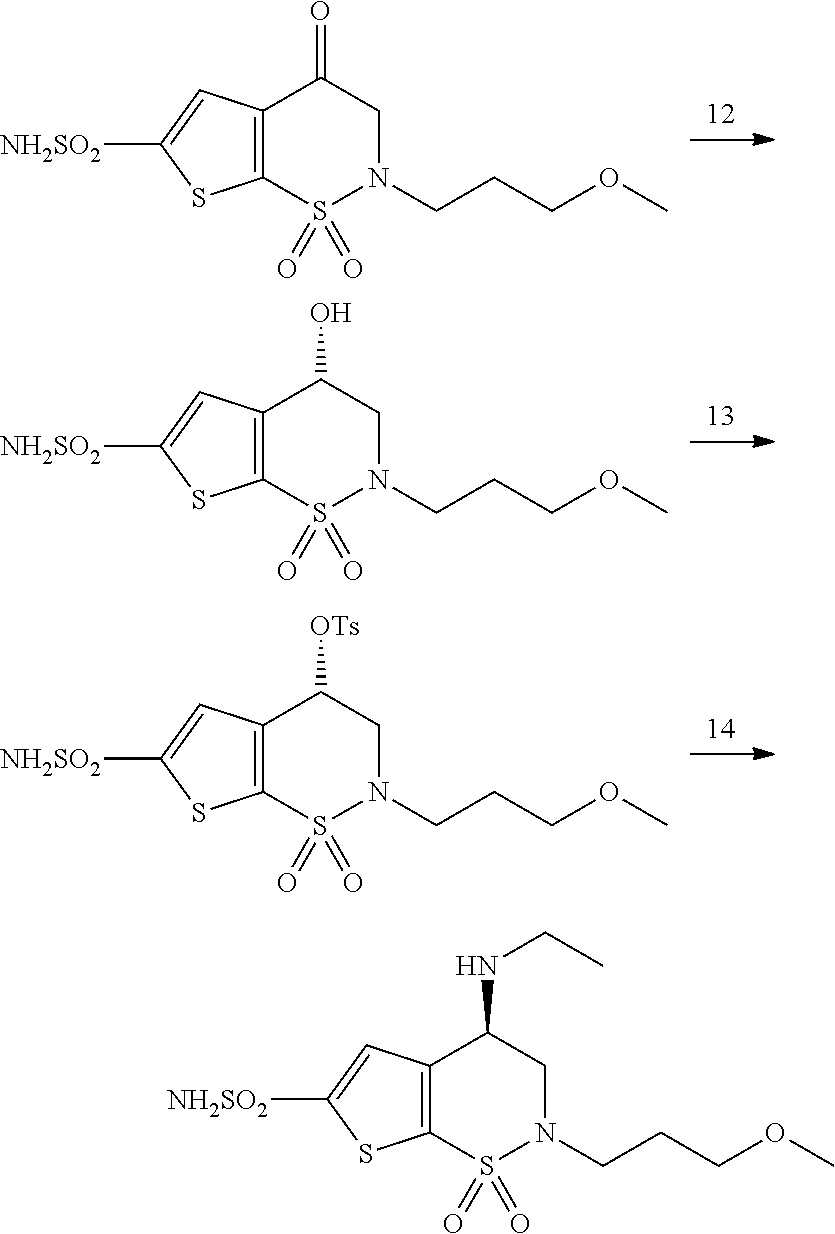
A kind of preparation method of chiral amino compound and its intermediate
ActiveCN107722043BSimple and fast operationHigh yieldOrganic chemistry methodsBulk chemical productionThiolCombinatorial chemistry
The invention discloses a preparation method of brinzolamide and its intermediate. The preparation method of the present invention comprises the following steps: in a solvent, carry out the following deprotection reaction on the compound of formula D and thiolate; wherein, in the compound of formula D, X is selected from O or NMe; X is When O, Y is Me, when X is NMe, Y is H; n is selected from 0, 1, 2, 3, 4 or 5; each R is independently NO 2 or CN; the thiol salt is the salt formed by thiol and metal, expressed as RS ‑ m + ; among them, M + Is an alkali metal ion, specifically can be selected from Li + 、Na + 、K + 、Ru + and Cs + One or more of; R is C 14‑24 alkyl. The preparation method of the invention has the advantages of simple and convenient operation, mild reaction conditions, high yield and good product quality, and is suitable for industrialized production.
Owner:CHEMVON BIOTECH CO LTD
Process for the preparation of brinzolamide
The present invention relates to a process for the preparation of Brinzolamide, or 2H-thieno[3,2-e]-1,2-thiazin-6-sulfonamide, 4-(ethyl amino)-3,4-dihydro-2-(3-methoxypropyl)-, 1,1-dioxide, (4R)-via intermediates 2,3-dihydro-4H-thieno[3,2-e]-1,2-thiazin-4-ones, 1,1-dioxide. Further objects of the present invention are the intermediates mentioned above and other intermediates of the synthesis.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com