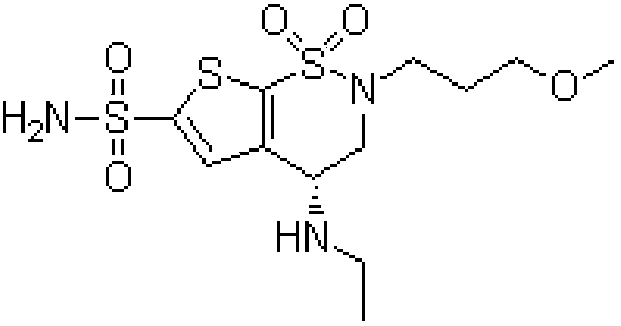
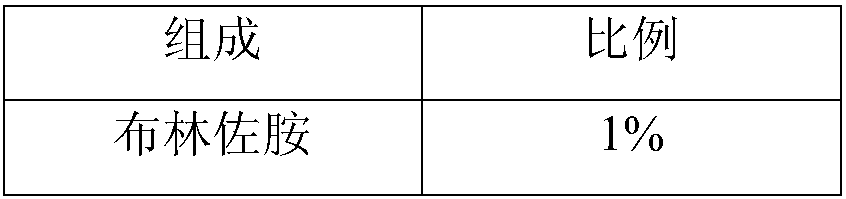
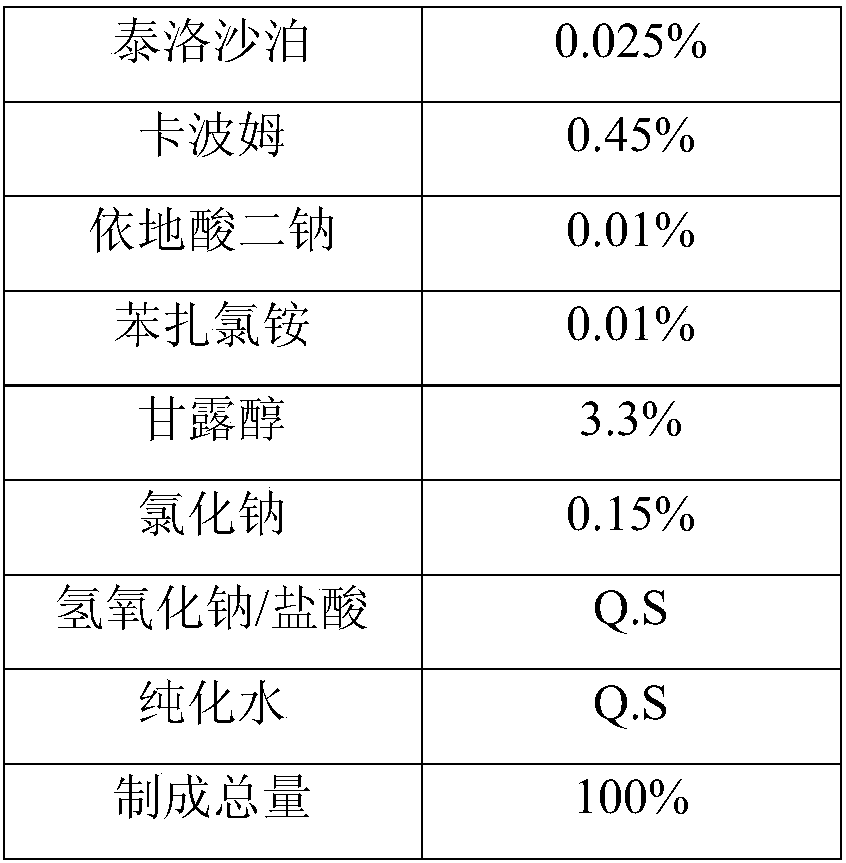
Brinzolamide eye drop preparation and preparation method thereof
A technology of brinzolamide and eye drops, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, oil/fat/wax non-active ingredients, etc., to achieve simple equipment, easy control of process parameters, and strong operability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028]
[0029] 【Preparation Process】
[0030] Put Tyloxapol and 5% of the total amount of purified water in a beaker according to the above table, dissolve, filter through a 0.22 μm filter membrane, add the raw material of brinzolamide to the filtrate, seal it, and prepare the suspension of the main drug; purify 30% of the total amount Put water in a beaker, sprinkle carbomer into it, stir while adding, heat to 60°C, after it swells completely, filter through a 10μm filter membrane, seal, add mannitol, sodium chloride, edetate disodium, benzalkonium chloride Ammonium and 30% of the total amount of purified water are placed in a beaker, heated to 60°C and stirred to dissolve, filtered through a 0.22 μm filter membrane, added to the above carbomer solution, stirred evenly, and adjusted to pH 7.5 with dilute hydrochloric acid or 10% sodium hydroxide solution ±0.2, stir evenly, add the above-mentioned main drug suspension, make up to volume with purified water, ful...
Embodiment 2
[0032]
[0033]
[0034] 【Preparation Process】
[0035] According to the above table, put poloxamer and 5% of the total amount of purified water in a beaker, dissolve, filter through a 0.22 μm filter membrane, add the raw material of brinzolamide to the filtrate, seal it, and prepare the main drug suspension; purify 30% of the total amount Put water in a beaker, sprinkle hydroxypropyl cellulose, stir while adding, heat to 60°C, after completely swelling, filter through a 10μm filter membrane, seal, add sorbitol, potassium chloride, disodium edetate, hydroxyl Benzyl ester and 30% of the total amount of purified water are placed in a beaker, heated to 60°C and stirred to dissolve, filtered through a 0.22 μm filter membrane, added to the above-mentioned hydroxypropyl cellulose solution, stirred evenly, adjusted with dilute hydrochloric acid or 10% sodium hydroxide solution When the pH value reaches 7.5±0.2, stir evenly, add the above main drug suspension, use purified water...
Embodiment 3
100%
[0038] 【Preparation Process】
[0039] Put polysorbate and 5% of the total amount of purified water in a beaker according to the above table, dissolve them, filter through a 0.22 μm filter membrane, add the raw material of brinzolamide to the filtrate, seal it, and prepare the suspension of the main drug; add 30% of the total amount of purified water Put it in a beaker, sprinkle sodium carboxymethyl cellulose, stir while adding, heat to 60°C, after it is completely swollen, filter through a 10μm filter membrane, seal it, add mannitol, boric acid, disodium edetate, ethyl hydroxybenzoate Put the ester and 30% purified water in a beaker, heat to 60°C and stir to dissolve, filter through a 0.22 μm filter membrane, add the above sodium carboxymethyl cellulose solution, stir well, adjust the pH with dilute hydrochloric acid or 10% sodium hydroxide solution value to 7.5±0.2, stir evenly, add the above main drug suspension, use purified water to make up the volume, fully h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com