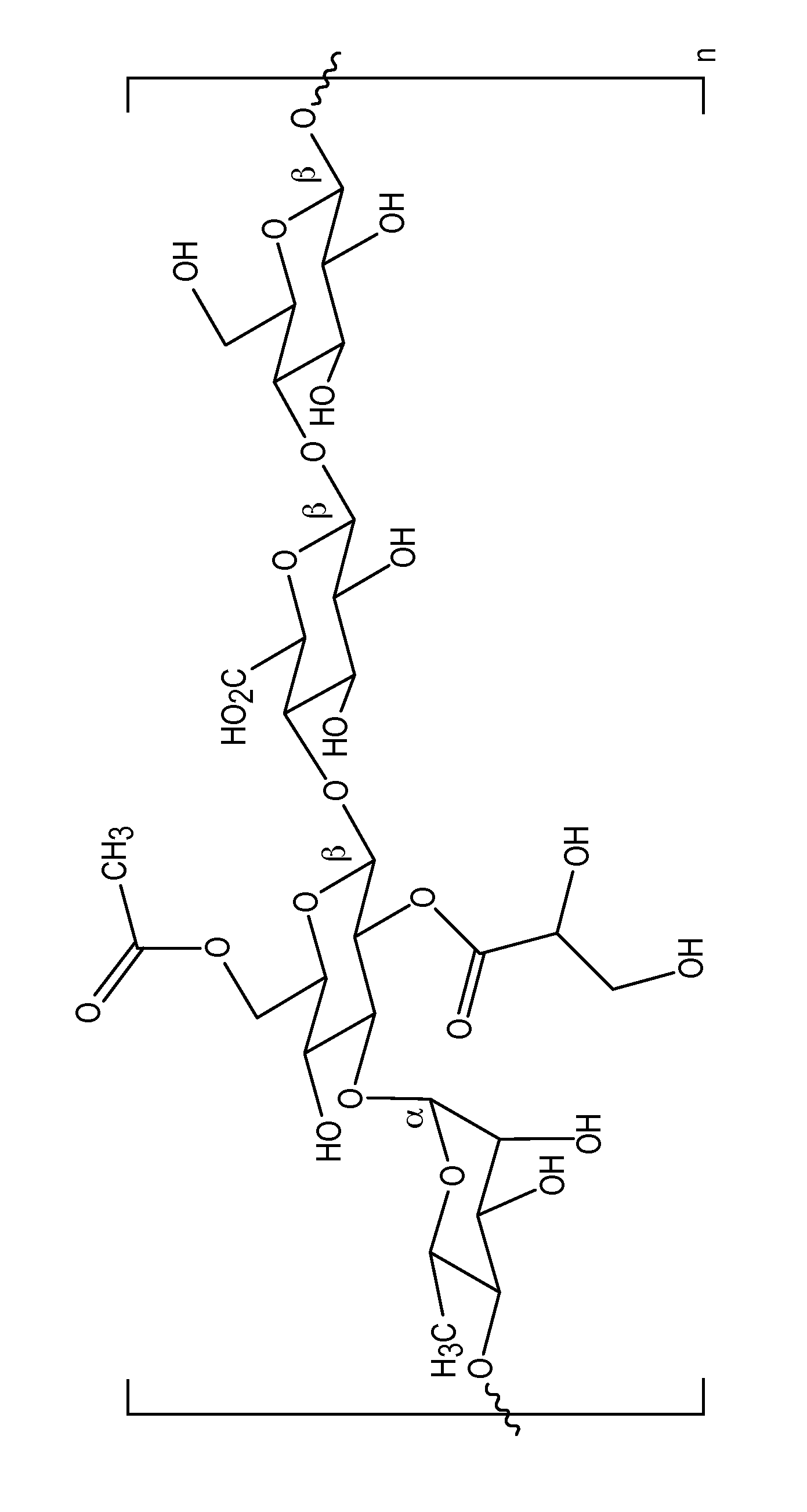
Patents
Literature
88 results about "Drug suspension" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In technical terms, an oral suspension is a liquid medium in which coarse, insoluble drug particles have been dispersed. Oral suspensions make the administration of insoluble drugs in liquid format feasible. Administering Oral Suspension Medicines.
Suspending vehicles and pharmaceutical suspensions for drug dosage forms
InactiveUS20060216242A1Process stabilityPeptide/protein ingredientsAerosol deliverySUSPENDING VEHICLESolvent
Suspending vehicles and pharmaceutical suspensions that include a biocompatible polymer that can be combined with a hydrophobic solvent and a hydrophilic solvent to provide vehicles and suspensions that are substantially free of stiff gels upon contact with an aqueous medium are provided. Vehicles and suspensions remain flowable out of a pump-driven dosage form over the life of the dosage form. Such vehicles and suspensions are also biocompatible, suitable for creating and maintaining drug suspensions, and capable of providing stable drug formulations.
Owner:DURECT CORP
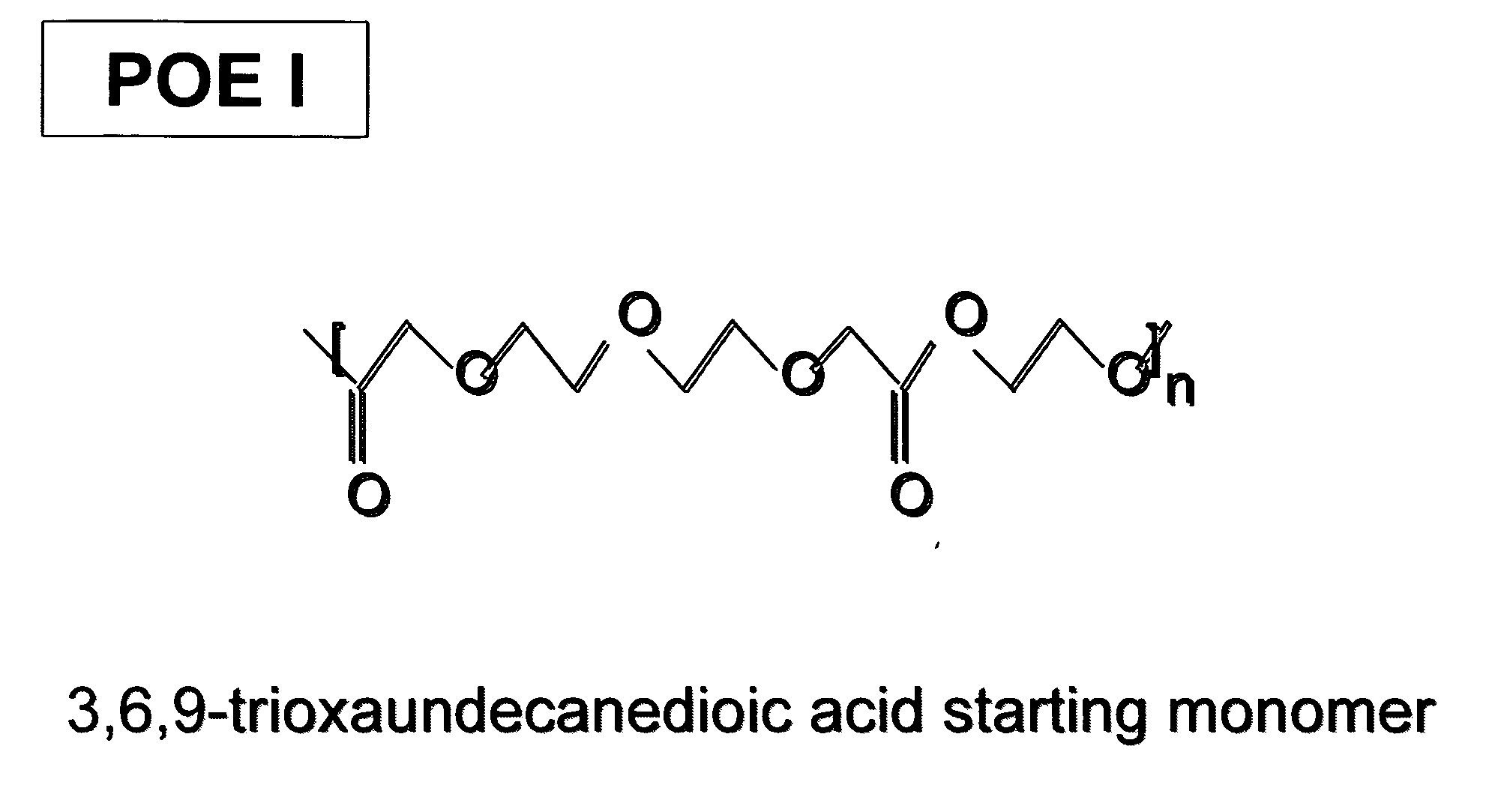
Polyoxaester suspending vehicles for use with implantable delivery systems
ActiveUS20060246138A1LimitMaintain stabilityPowder deliveryOrganic active ingredientsSUSPENDING VEHICLEActive agent
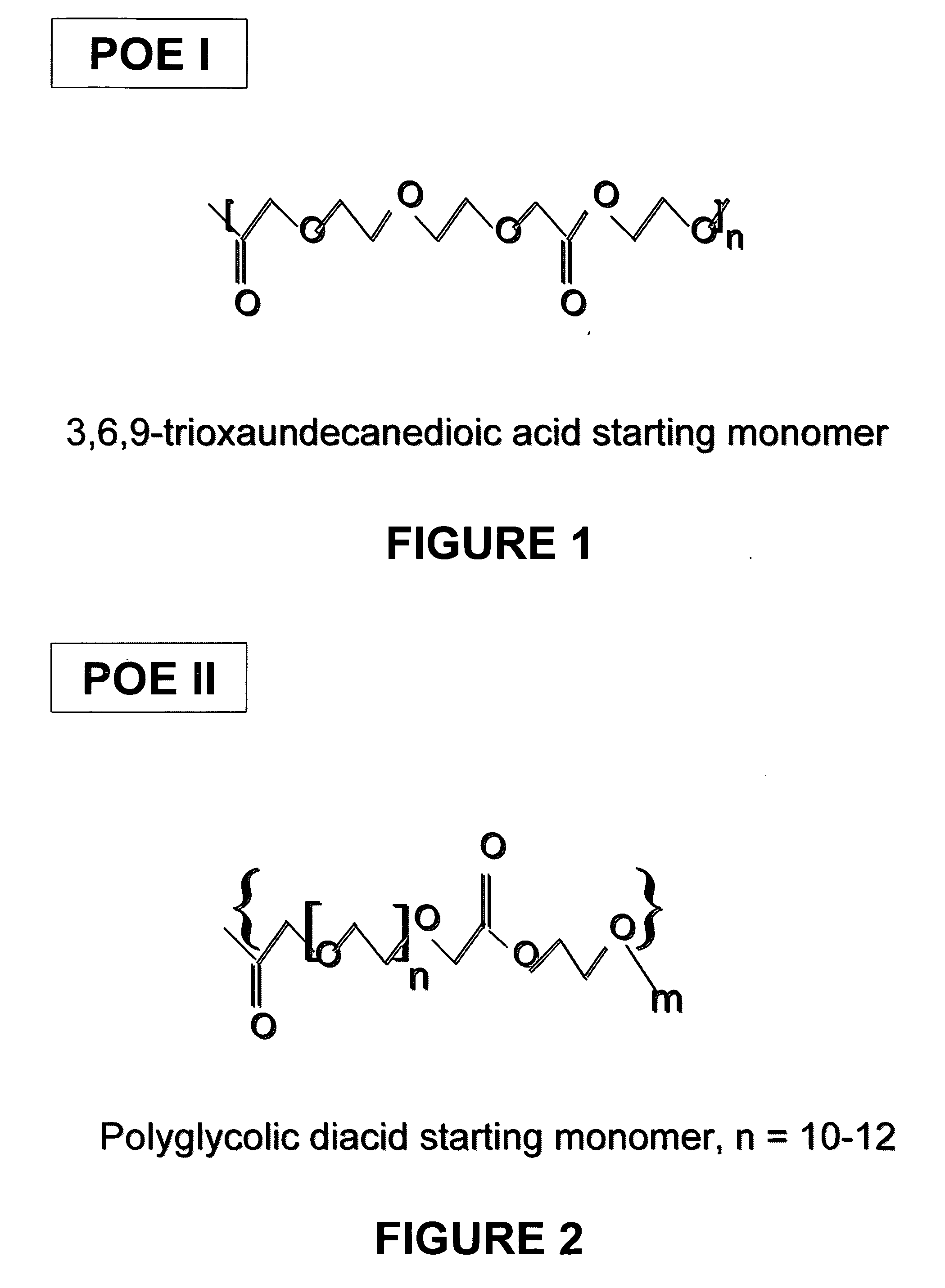
Liquid polyoxaester polymer materials are provided as suspending vehicles suitable for dispensing of pharmaceutically active agents, such as proteins, from delivery devices, for example, pump-driven dosage forms. Polyoxaesters are made from at least one diacid and at least one diol. Through the use of polyoxaesters virtually solvent-free pharmaceutical suspensions can be created.
Owner:INTARCIA THERAPEUTICS INC
Pharmaceutical suspension composition
An aqueous oral liquid pharmaceutical composition system with reduced propensity for agglomeration and phase separation which is particularly amendable to the suspension of one or more pharmaceutical actives that are substantially insoluble in water. The oral liquid pharmaceutical composition may further comprise pharmaceutical actives that are soluble in water and dissolve in the aqueous medium. In the composition of the invention both suspended and any dissolved active agents are distributed homogeneously.
Owner:WYETH LLC
Pharmaceutical suspension compositions lacking a polymeric suspending agent
InactiveUS20020037877A1Improve physical stabilityLow viscosityBiocideOrganic chemistrySuspending AgentsDrug suspension
Stable aqueous pharmaceutical suspension compositions containing lecithin as a stabilizing additive and lacking a polymeric suspending agent are disclosed.
Owner:ALCON INC
Stable nanoparticulate drug suspension
InactiveUS20100323020A1Convenient route of administrationEasy to doOrganic active ingredientsPowder deliverySodium bicarbonateDisease
A liquid pharmaceutical composition comprises an aqueous medium having suspended therein a solid particulate Bc1-2 family protein inhibitory compound such as ABT-263, having a D90 particle size not greater than about 3 μm; wherein the aqueous medium further comprises at least one pharmaceutically acceptable surfactant and at least one pharmaceutically acceptable basifying agent such as sodium bicarbonate in amounts that are effective together to inhibit particle size increase. The composition is suitable for oral or parenteral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bc1-2 family proteins, for example cancer.
Owner:ABBVIE INC
Polyoxaester suspending vehicles for use with implantable delivery systems
ActiveUS7959938B2LimitMaintain stabilityOrganic active ingredientsPowder deliverySUSPENDING VEHICLEActive agent
Liquid polyoxaester polymer materials are provided as suspending vehicles suitable for dispensing of pharmaceutically active agents, such as proteins, from delivery devices, for example, pump-driven dosage forms. Polyoxaesters are made from at least one diacid and at least one diol. Through the use of polyoxaesters virtually solvent-free pharmaceutical suspensions can be created.
Owner:INTARCIA THERAPEUTICS INC
Pharmaceutical suspension composition
An aqueous oral liquid pharmaceutical composition system with reduced propensity for agglomeration and phase separation which is particularly amendable to the suspension of one or more pharmaceutical actives that are substantially insoluble in water. The oral liquid pharmaceutical composition may further comprise pharmaceutical actives that are soluble in water and dissolve in the aqueous medium. In the composition of the invention both suspended and any dissolved active agents are distributed homogeneously.
Owner:WYETH LLC
Iontophoretic transdermal delivery device
A conducting silicone matrix incorporating a suspension of a drug in ionized and non-ionized phases in an emulsion of a hydrophobic polymer. In one version, the drug is prepared as a concentrated aqueous suspension incorporated in a silicone matrix with a silicone surfactant. An electrolyte may be incorporated into the silicone matrix for increasing its conductivity. When a current is applied, the drug in individual globules in the drug suspension migrates away from the electrode and becomes concentrated at the distal side of the globules eventually resulting in an increase in the drug concentration distal to the electrode and adjacent to the skin and thereby resulting in transfer of the active drug through the skin. This system provides a matrix with minimal electroendosmotic flow that is current efficient and provides a drug reservoir that can last for several days during application of the drug.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
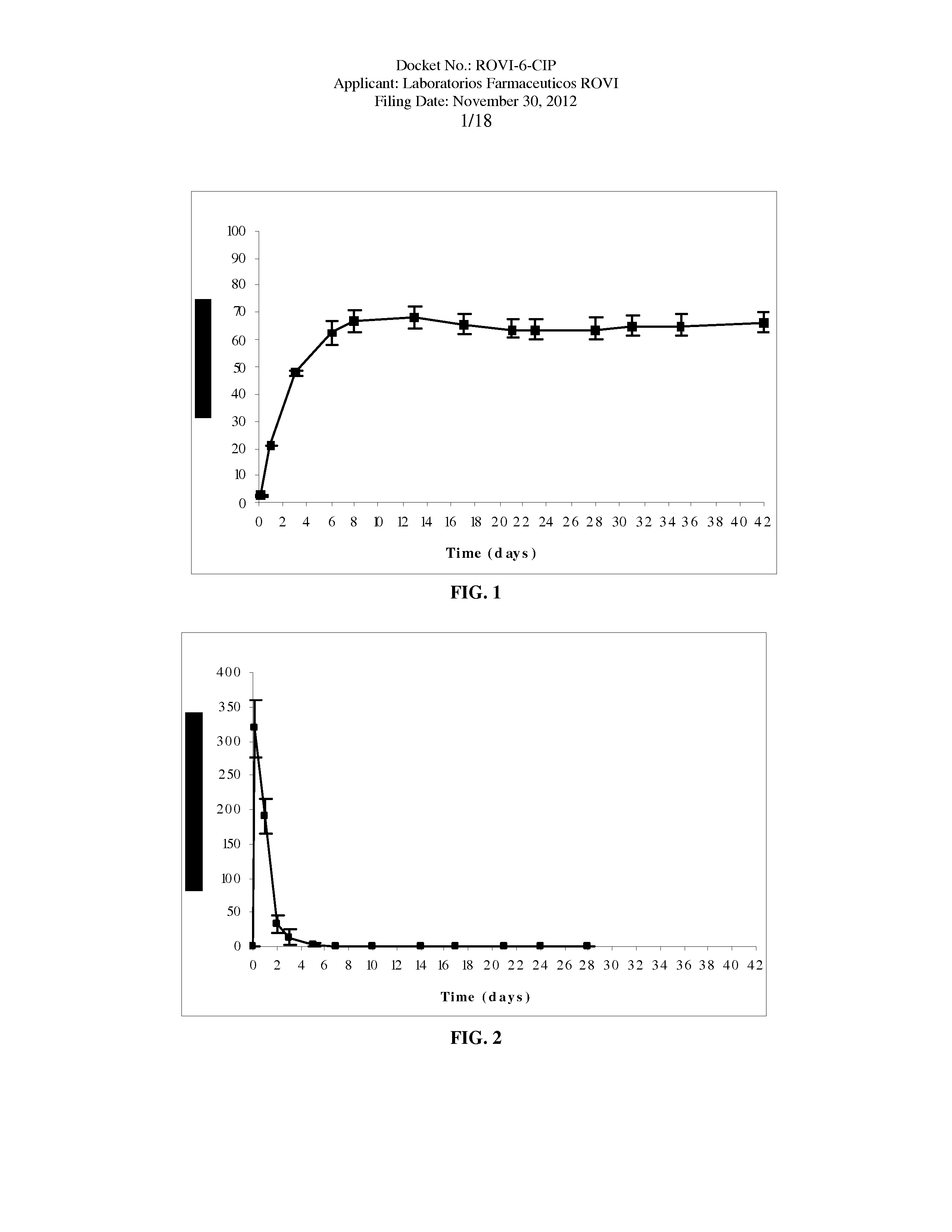
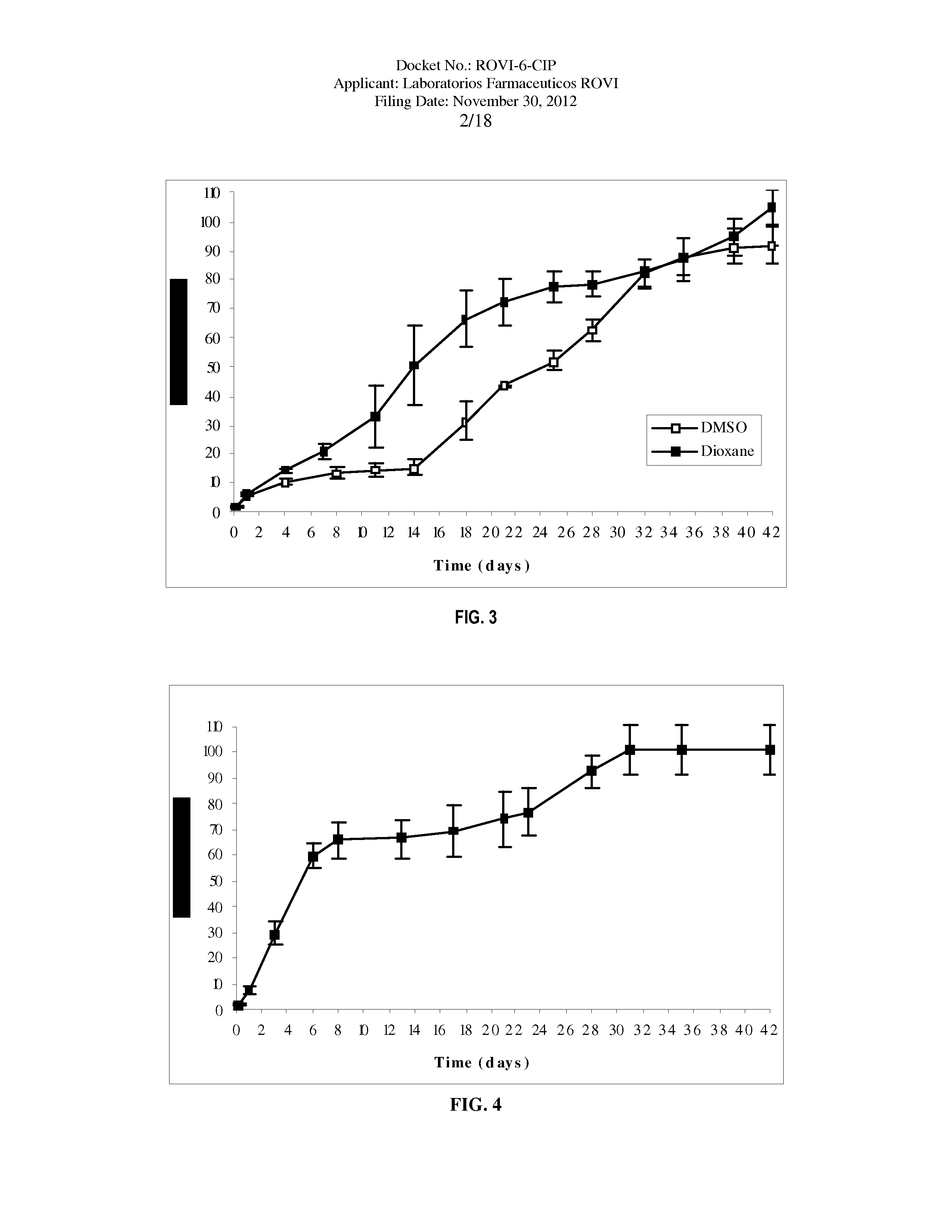
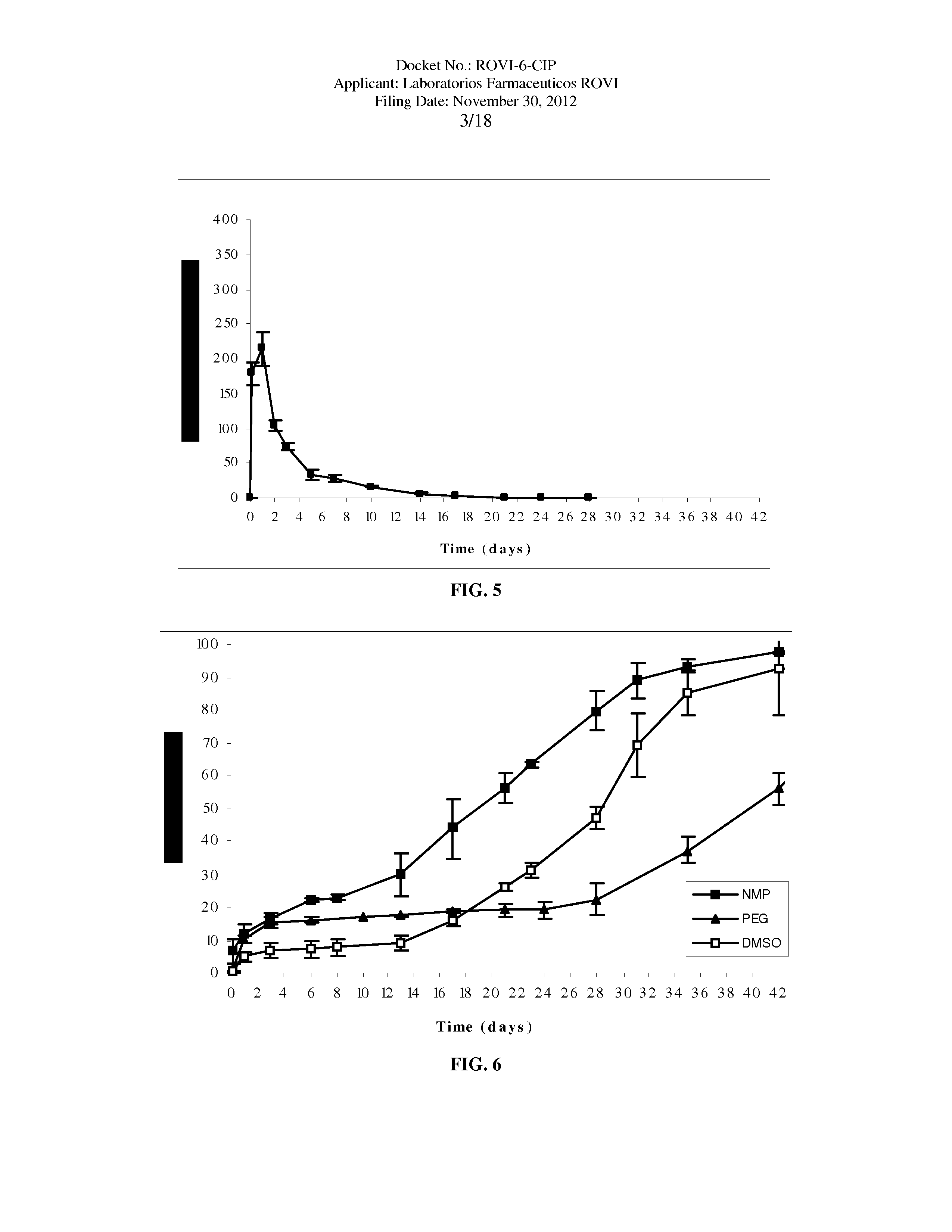
Antipsychotic Injectable Depot Composition
The present invention is directed to a composition that can be used to deliver an antipsychotic drug such as risperidone as an injectable in-situ forming biodegradable implant for extended release providing therapeutic plasma levels from the first day. The composition is in the form of drug suspension on a biodegradable and biocompatible copolymer or copolymers solution using water miscible solvents that is administered in liquid form. Once the composition contacts the body fluids, the polymer matrix hardens retaining the drug, forming a solid or semisolid implant that releases the drug in a continuous manner. Therapeutic plasma levels of the drug can be achieved since the first day up to at least 14 days or more even up to at least four weeks.
Owner:LAB FARM ROVI SA
Andrographolide ground suspending liquid, preparation method thereof, and application of pharmaceutical preparation
InactiveCN102614133AImprove stabilityDissolution rate is fastAntibacterial agentsOrganic active ingredientsImmediate releaseDrugs preparations
The invention relates to andrographolide ground suspending liquid, a preparation method thereof, and the application of pharmaceutical preparation, which belongs to the field of pharmaceutical preparation. The preparation method comprises the steps of adding andrographolide into hydrophilic accessory solution with certain concentration, and grinding the andrographolide in a basket grinder to prepare suspending liquid with particle sizes smaller than 3000 nm. Liquid layers of pharmaceutical suspending liquid are laminated onto blank pellet cores with certain particle size range, to prepare andrographolide immediate-release pellets. After grinding, by reducing pharmaceutical particle sizes, increasing particle surface areas and improving the wettability of pharmaceutical particles, the dissolution in vitro of the drug is improved, and hydrophilic carriers are adopted to effectively prevent the aggregation of pharmaceutical particles so as to improve the stability of the pharmaceutical preparation. The preparation method is simple and easy for industrialized production, and the dissolving-out speed of the prepared andrographolide immediate-release pellets is high, so that the bioavailability is obviously improved.
Owner:SHENYANG PHARMA UNIVERSITY
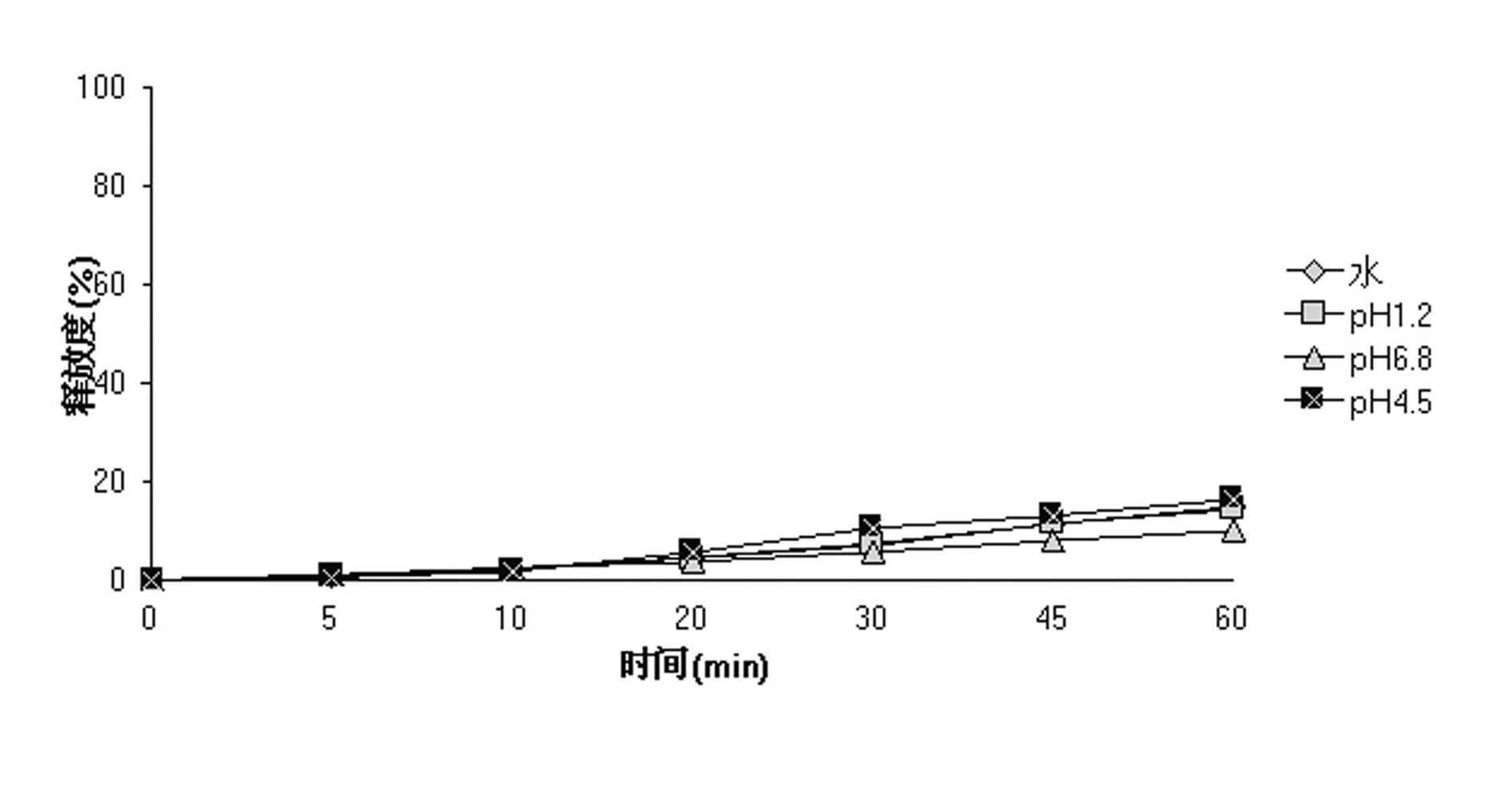
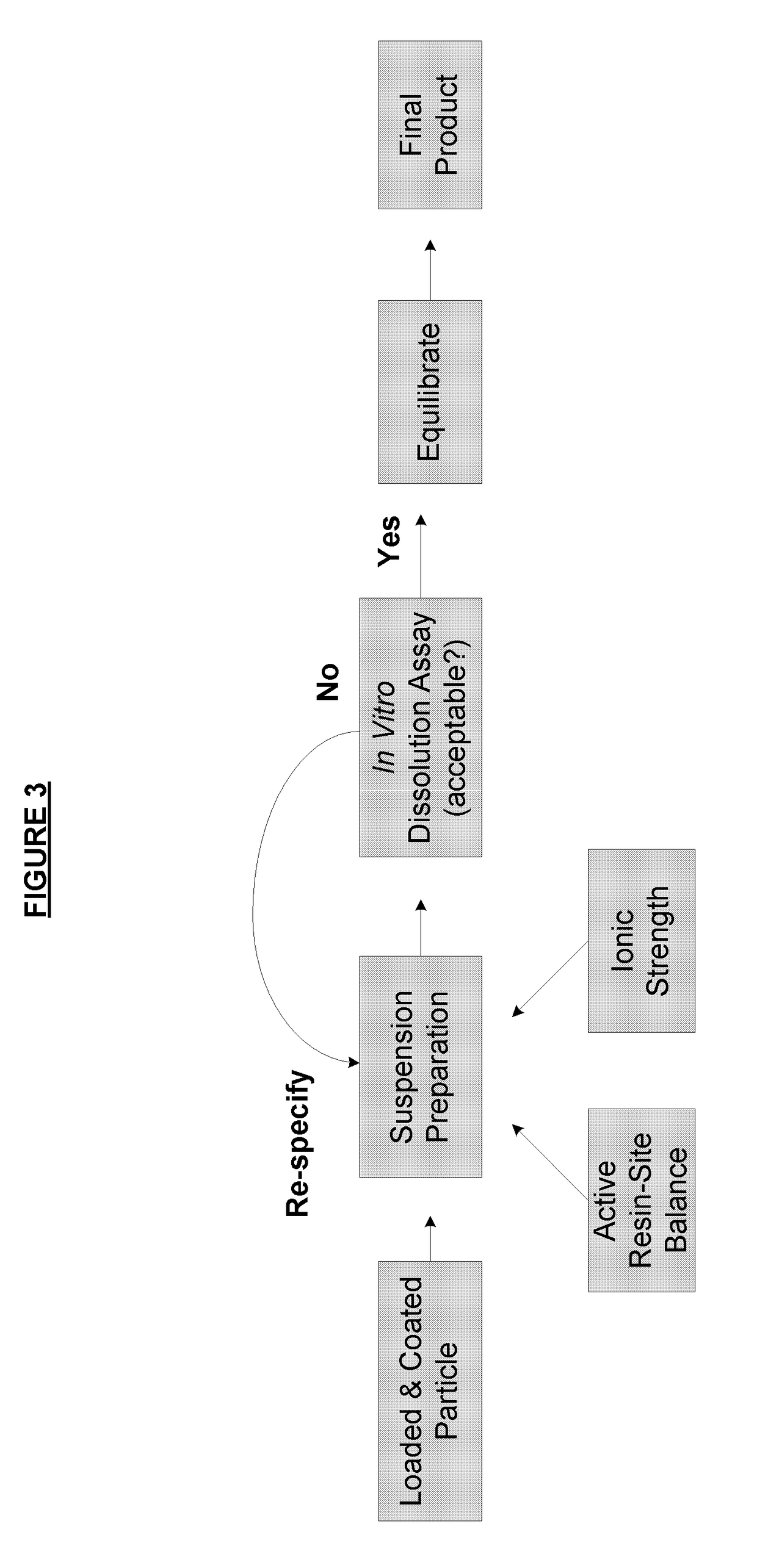
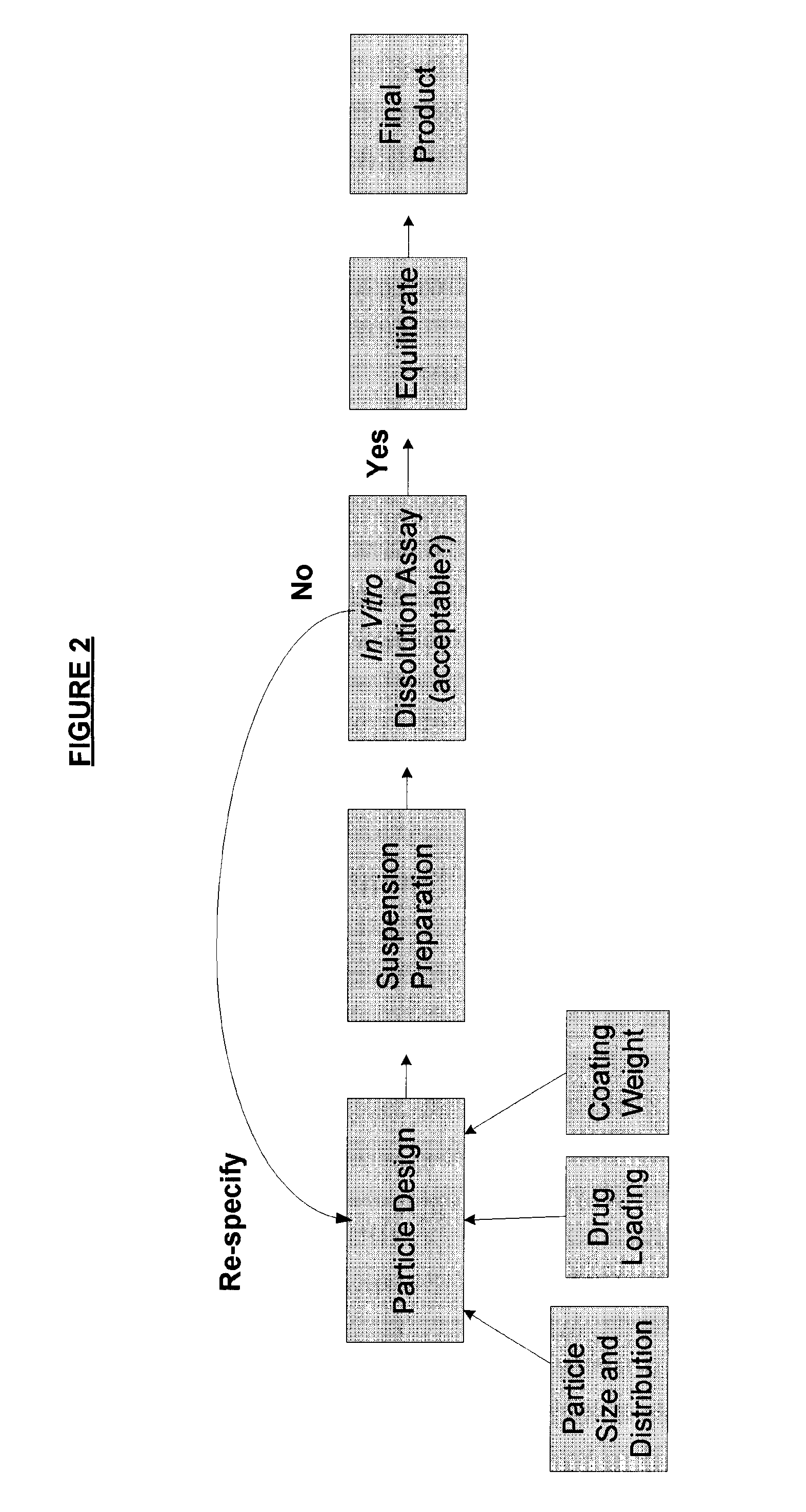
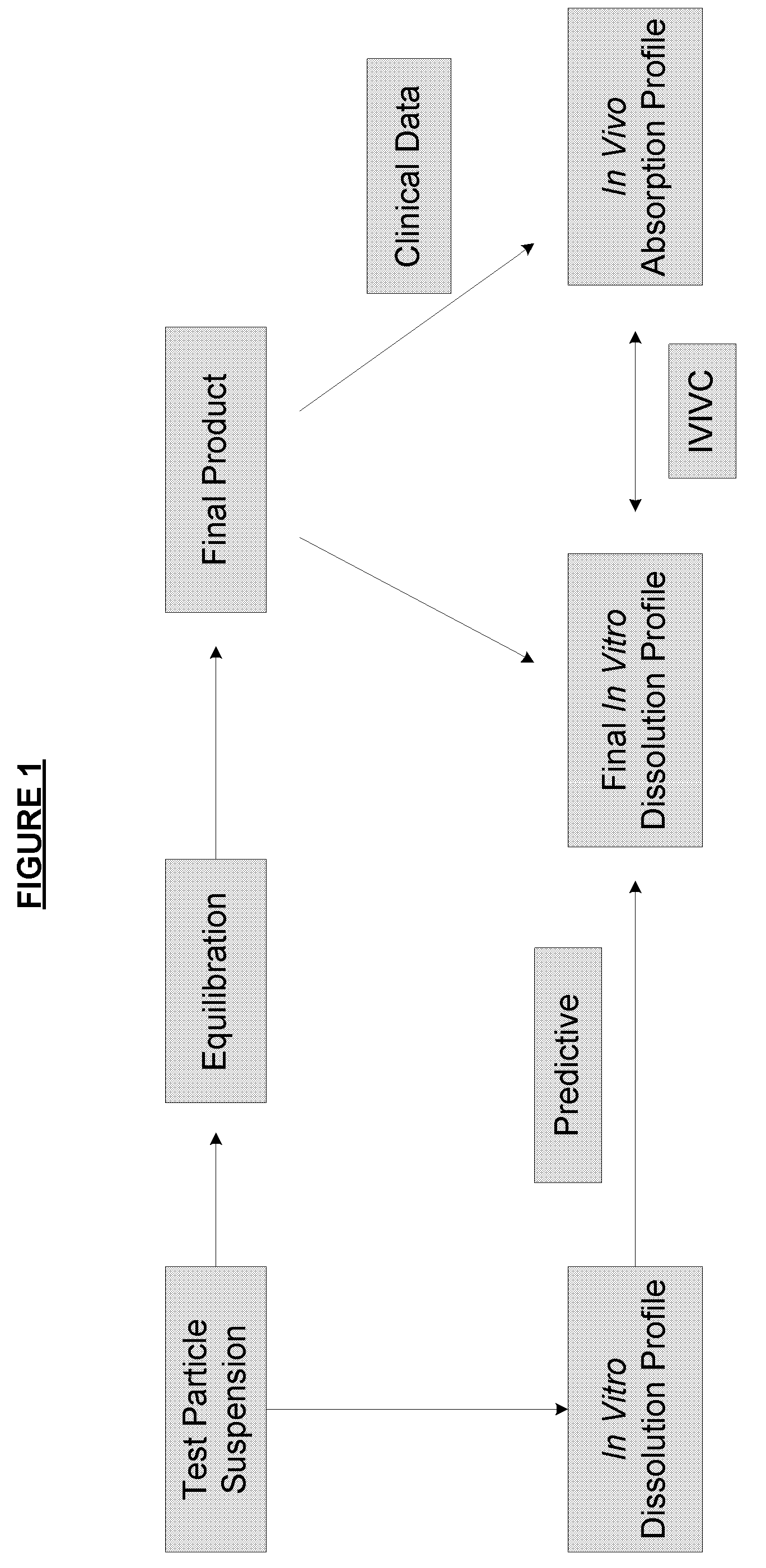
Method of formulating and designing liquid drug suspensions containing ion exchange resin particles
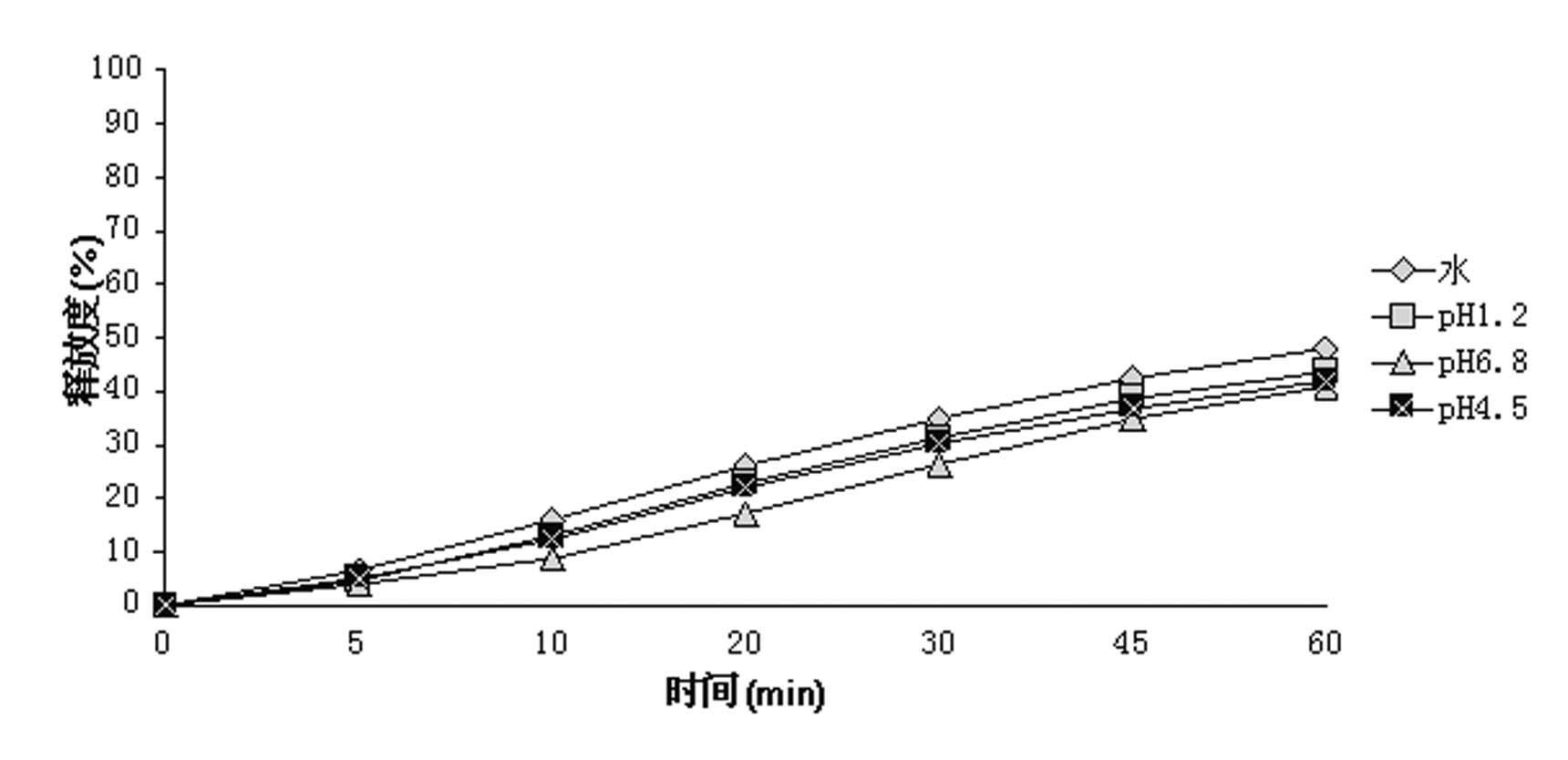
ActiveUS8470375B1Maintaining bioequivalenceMaintaining bioavailabilityDispersion deliverySolution deliveryQuality controlDissolution
The invention relates to the formulation and quality control of liquid drug suspensions. In particular, the invention relates to methods of formulating liquid suspensions comprising drug-containing resin particles. The invention also relates to methods of confirming the acceptability of drug-containing resin particles for use in formulating liquid drug suspensions. The invention further relates to methods of formulating liquid suspensions in which drug-containing resin particles, the liquid suspension, or both are modified to achieve a desired in vitro dissolution profile. The invention also relates to a novel dissolution method and methods of predicting in vivo bioequivalence based on in vitro dissolution methods.
Owner:NEOS THERAPEUTICS LP
Pharmaceutical suspensions containing drug particles, devices for their administration, and methods of their use
ActiveUS20170172961A1Convenient treatmentDisperse fastPowder deliveryOrganic active ingredientsDrug suspensionDrug delivery
Owner:SYNAGILE CORP
Drug delivery device for drug suspensions
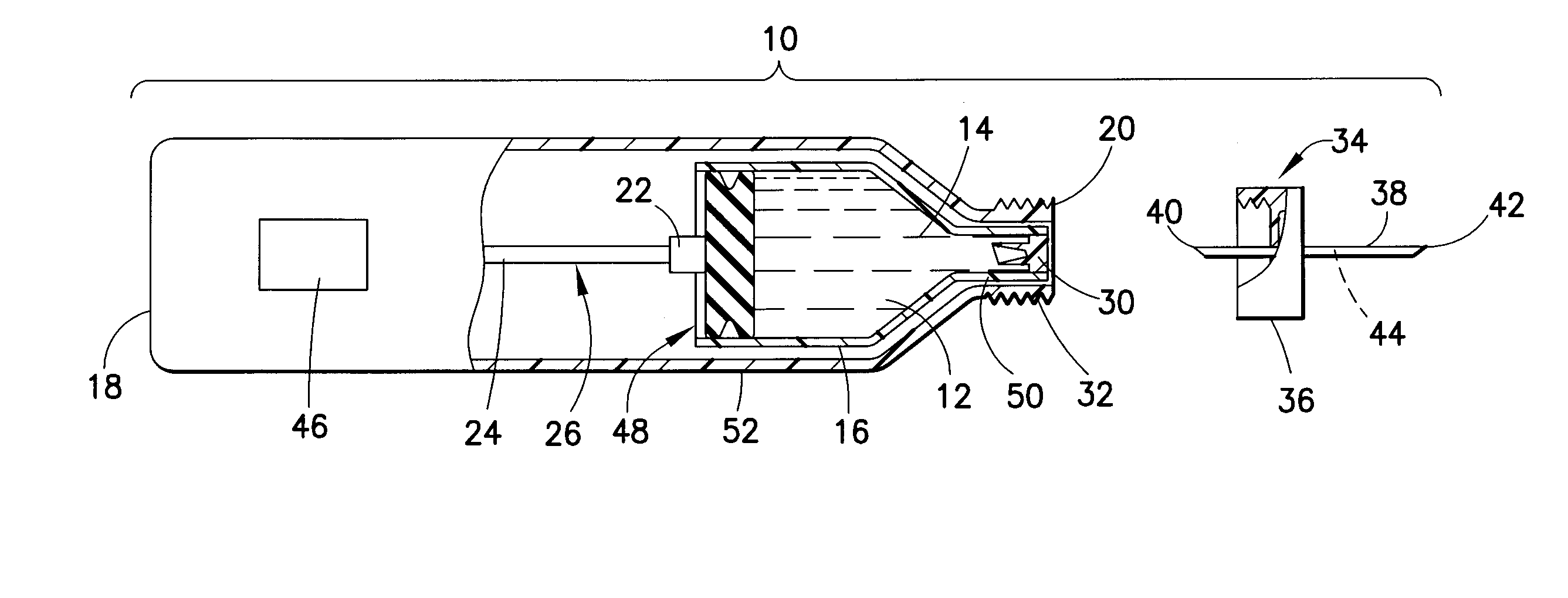
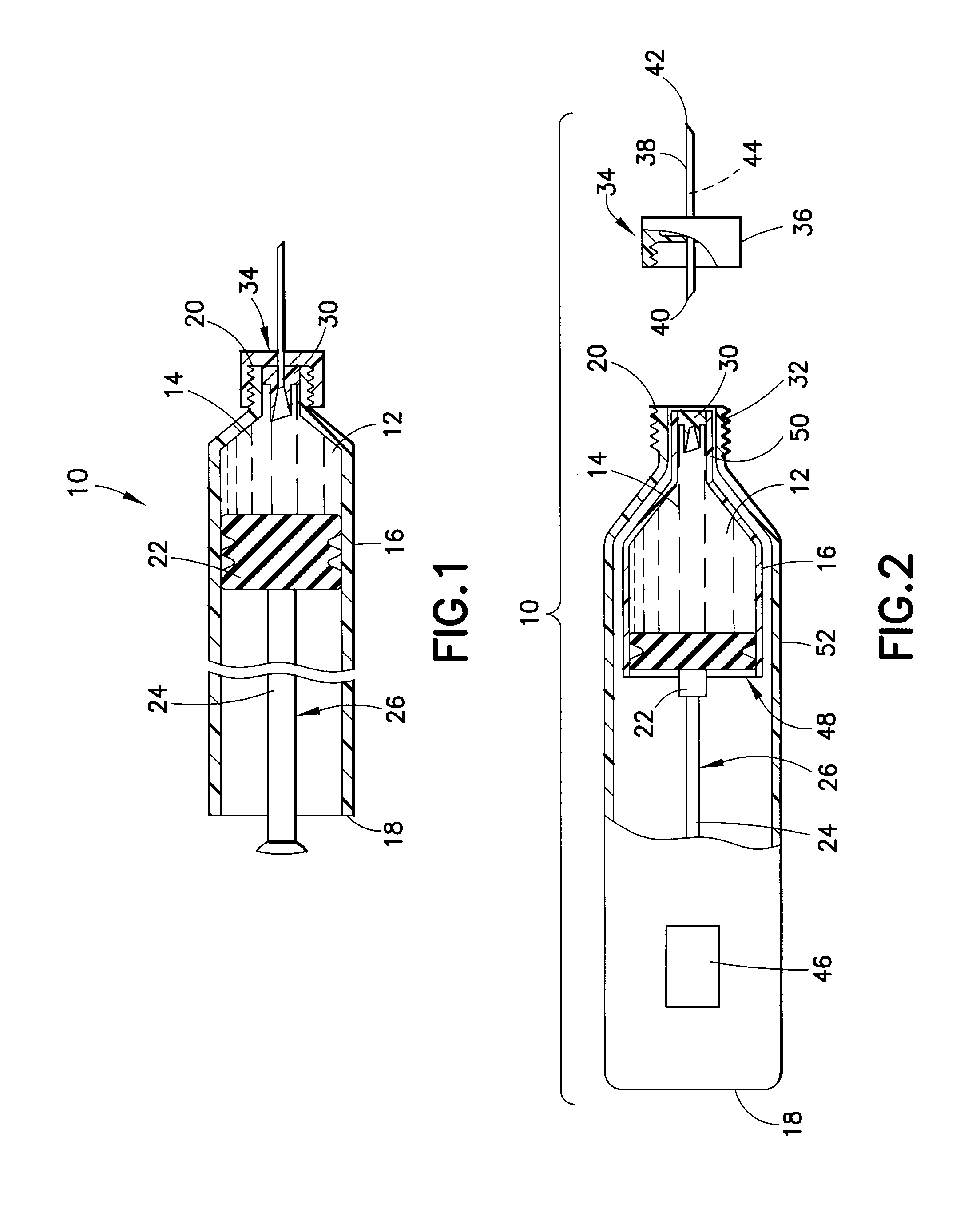
InactiveUS20100292656A1Easy maintenanceLow inner diameter requirementAmpoule syringesInfusion needlesMedicineSolid particle
A drug delivery device is provided herein, the device including a reservoir for containing a medicament. The medicament includes a suspension of solids in a liquid carrier. The device also includes a needle having a distal end for injection into a patient, a proximal end in communication with the reservoir, and a lumen extending between the distal and proximal ends. A path is defined from the reservoir to the distal end of the needle through the lumen, the path having an inner diameter that decreases in the proximal to distal direction along at least a portion thereof. Advantageously, with the subject invention, a flow path may be defined which provides a more gradual transition in diameter from the reservoir to the distal tip of a needle. In this manner, changes in velocity of the suspension may be less abrupt than in the prior art, thus better maintaining solid particles in the suspension.
Owner:BECTON DICKINSON & CO
Antipsychotic Injectable Depot Composition
ActiveUS10463607B2Simple methodAvoiding irregular initial burst release of drugOrganic active ingredientsPharmaceutical delivery mechanismMedicineSolvent
The present invention is directed to a composition that can be used to deliver an antipsychotic drug such as risperidone, paliperidone or a combination thereof, as an injectable in-situ forming biodegradable implant for extended release providing therapeutic plasma levels from the first day. The composition is in the form of drug suspension on a biodegradable and biocompatible copolymer or copolymers solution using water miscible solvents that is administered in liquid form. Once the composition contacts the body fluids, the polymer matrix hardens retaining the drug, forming a solid or semisolid implant that releases the drug in a continuous manner. Therapeutic plasma levels of the drug can be achieved from the first day up to at least 14 days or more even up to at least four weeks.
Owner:LAB FARM ROVI SA
Methods of Formulating and Designing Liquid Drug Suspensions Containing Ion Exchange Resin Particles
The invention relates to the formulation and quality control of liquid drug suspensions. In particular, the invention relates to methods of formulating liquid suspensions comprising drug-containing resin particles. The invention also relates to methods of confirming the acceptability of drug-containing resin particles for use in formulating liquid drug suspensions. The invention further relates to methods of formulating liquid suspensions in which drug-containing resin particles, the liquid suspension, or both are modified to achieve a desired in vitro dissolution profile. The invention also relates to a novel dissolution method and methods of predicting in vivo bioequivalence based on in vitro dissolution methods.
Owner:NEOS THERAPEUTICS LP
Methodc for preparing sub-micron gemfibrozil medicament powder
The present invention is process of preparing gemfibrozil medicine powder, and belongs to the field of insoluble medicine micronizing technology. Through dissolving gemfibrozil medicine in water solution of sodium hydroxide, neutralizing and replacing with added water solution of hydrochloric acid containing surfactant to obtain suspension of gemfibrozil medicine, filtering, washing and drying, spheroid gemfibrozil medicine powder in submicron size is produced. The process is simple, low in cost and easy in industrial application, and the obtained gemfibrozil medicine powder has regular shape, narrow size distribution and high dispersivity. The present invention lays foundation for the industrial production and the new preparation form development of gemfibrozil medicine.
Owner:BEIJING UNIV OF CHEM TECH
Methods of formulating and designing liquid drug suspensions containing ion exchange resin particles
The invention relates to the formulation and quality control of liquid drug suspensions. In particular, the invention relates to methods of formulating liquid suspensions comprising drug-containing resin particles. The invention also relates to methods of confirming the acceptability of drug-containing resin particles for use in formulating liquid drug suspensions. The invention further relates to methods of formulating liquid suspensions in which drug-containing resin particles, the liquid suspension, or both are modified to achieve a desired in vitro dissolution profile. The invention also relates to a novel dissolution method and methods of predicting in vivo bioequivalence based on in vitro dissolution methods.
Owner:NEOS THERAPEUTICS LP
Pharmaceutical suspensions containing drug particles, devices for their administration, and methods of their use
ActiveUS9901561B2Disperse fastDissolve rapidly in salivaOrganic active ingredientsPowder deliveryPharmaceutical SubstancesMedicinal chemistry
Owner:SYNAGILE CORP
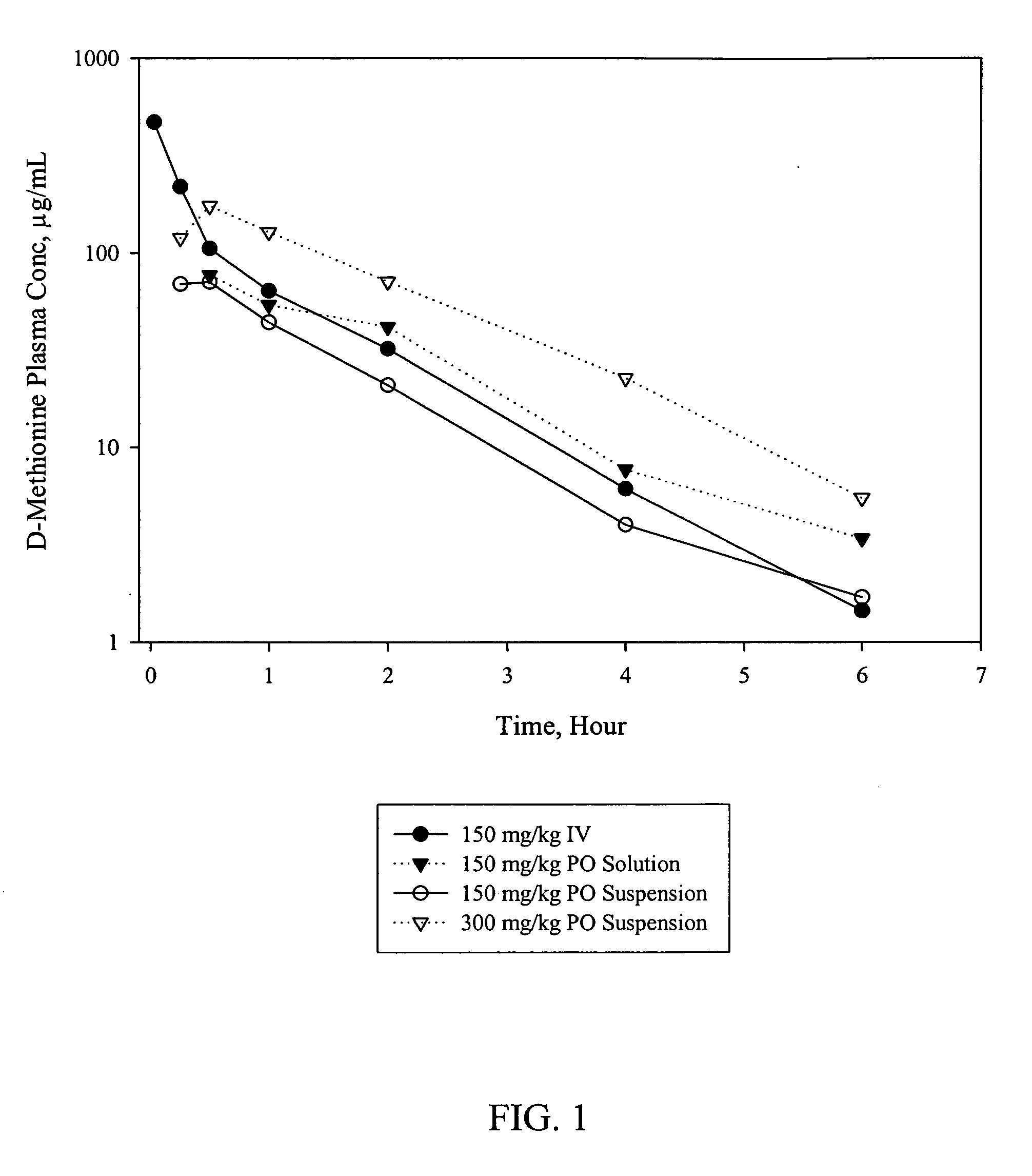
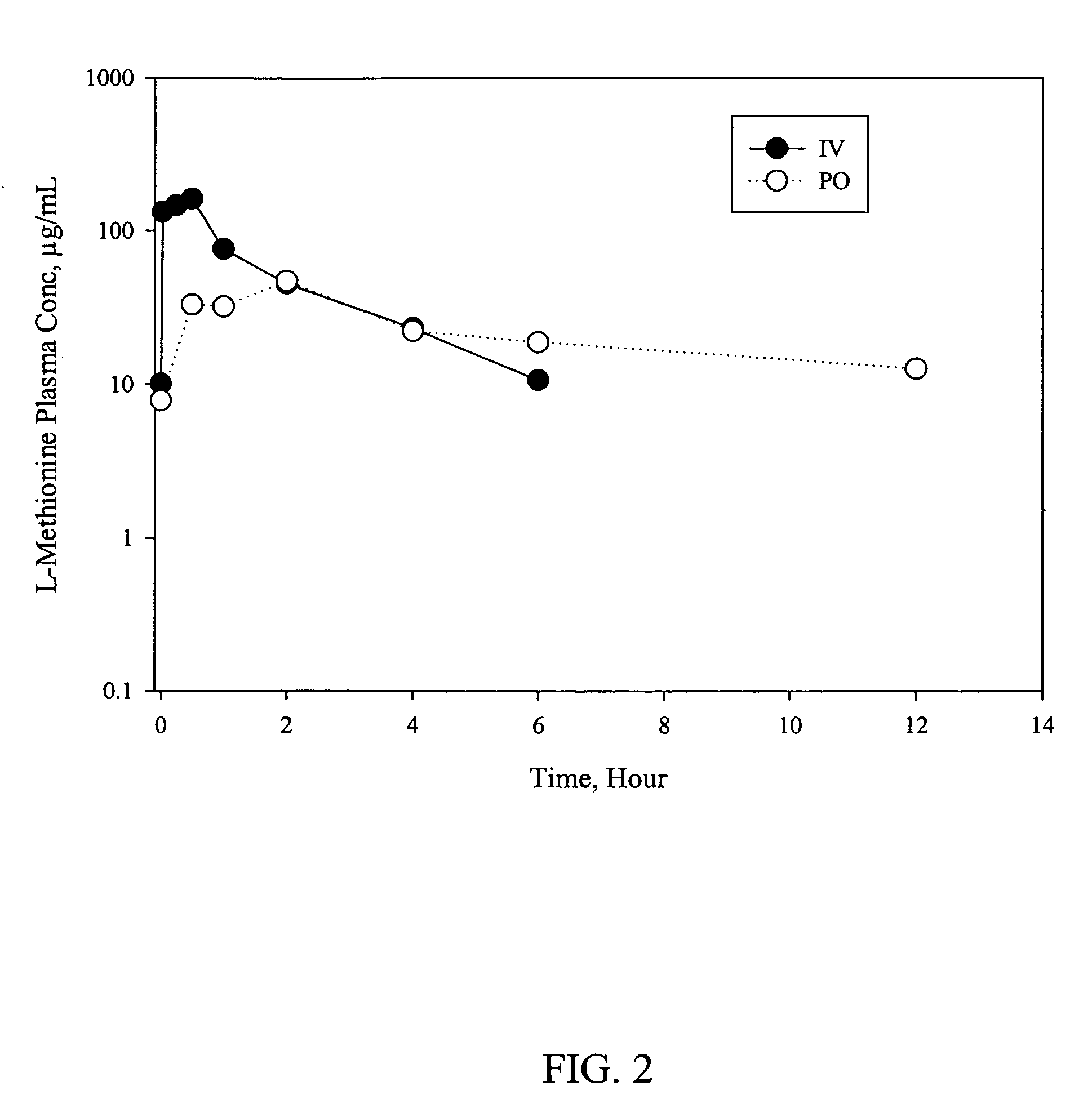
D-methionine formulation with improved biopharmaceutical properties
InactiveUS20060058390A1Treat and prevent and ameliorate neuronal damageOrganic active ingredientsBiocideDiseaseHigh doses
The present invention provides pharmaceutical suspensions of D-methionine in which the aqueous solubility of D-methionine is exceeded, thereby allowing oral administration of higher doses. The present invention also provides processes for preparing these suspensions. The present invention further provides methods for preventing, treating, or ameliorating oral mucositis, hearing loss due to chemotherapy, antibiotics and noise, neuronal damage due to various CNS disorders and injuries, and anthracycline toxicity.
Owner:MOLECULAR THERAPEUTICS INC
Pharmaceutical Suspensions and Related Methods
A pharmaceutical suspension having a therapeutically effective amount of phenylephrine and a therapeutically effective amount of a first active agent consisting essentially of a first substantially water insoluble active agent having an average particle size of between about 10 and about 100 microns, an effective amount of non-reducing sweetener; an effective amount of water; and an effective amount of a suspending system; wherein the pharmaceutical suspension has a pH of from about 4 to about 6 and is substantially free of a reducing sugar and related methods.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Antipsychotic Injectable Depot Composition
ActiveUS20180221272A1Simple methodConstant effectiveNervous disorderPharmaceutical delivery mechanismSolventBlood plasma
Owner:LAB FARM ROVI SA
Pharmaceutical nanosuspension
The present invention in general relates to a pharmaceutical suspension comprising nano-sized cocrystals of at least one active ingredient and at least one dicarboxylic acid. It in particular relates to a pharmaceutical suspension comprising nano-sized cocrystals of at least one anthelmintic drug and at least one dicarboxylic acid. The invention further relates to uses, methods for use and methods for manufacturing the pharmaceutical suspension according to this invention.
Owner:UNIV GENT
Density-matched suspension vehicles and pharmaceutical suspensions
InactiveUS20060045891A1Inorganic non-active ingredientsSolution deliverySUSPENDING VEHICLEActive agent
Density-matching is used to provide suspending vehicles, pharmaceutical suspensions, dosage forms, and kits as well as methods of making and using the vehicles, suspensions, and dosage forms. Pharmaceutical suspensions comprising a pharmaceutically active agent having an active agent density, ρA, and a suspending vehicle having a suspending vehicle density, ρSV; wherein the suspending vehicle density, ρSV is substantially equal to the active agent density, ρA, are provided. Suspending vehicles comprise at least one suspending agent. The suspending vehicles can further comprise at least one density-modifying solid in such a combination with the suspending agent as to create a suspending vehicle that has a density that substantially matches the density of a desired drug particle or combination of drug particles. Pharmaceutical suspensions that remain homogenous during prolonged storage can be obtained.
Owner:ALZA CORP
Preparation method of poorly soluble medicine liposome
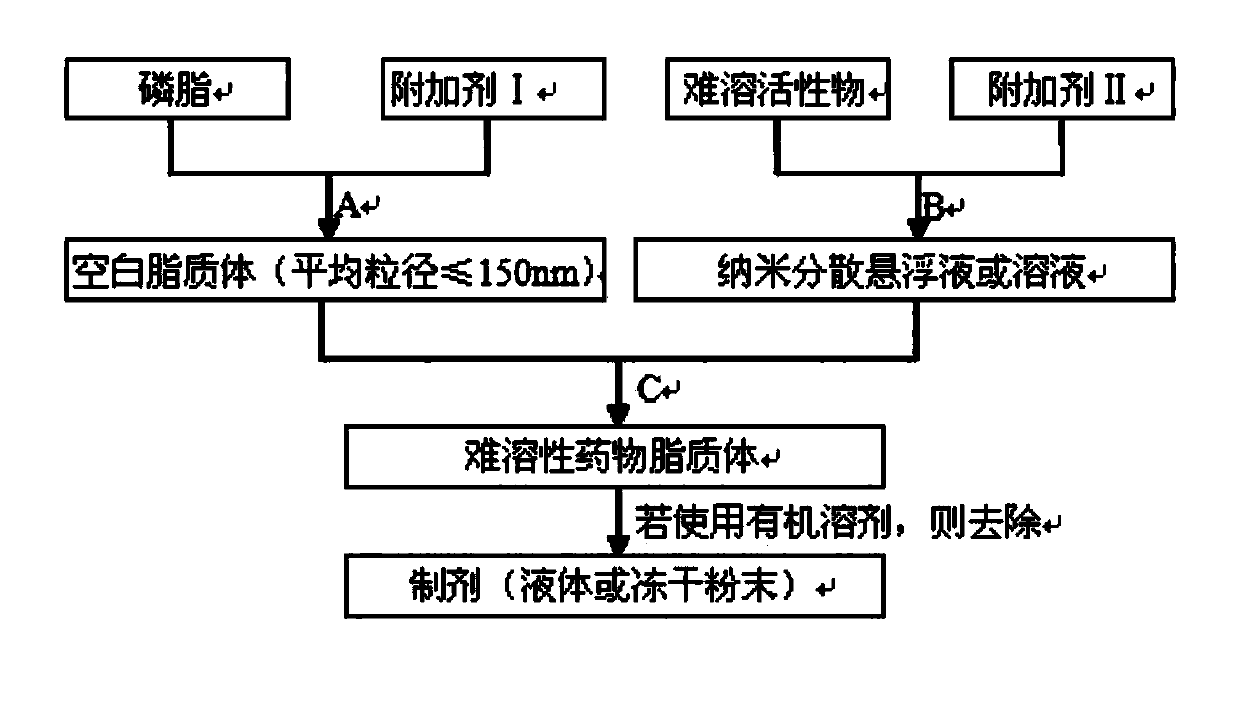
ActiveCN103622911ARealize industrial productionReduce usageOrganic active ingredientsLiposomal deliveryDrugs solutionProcess equipment
The invention belongs to the field of medicine preparation, and particularly relates to a preparation method of a poorly soluble medicine liposome. A blank liposome free of a medicine and a medicine suspension or a medicine solution are separately prepared, and then the blank liposome and the medicine are mixed under the conditions of a certain temperature and a certain rotating speed. According to the poorly soluble medicine liposome prepared by the method, the encapsulation efficiency is greater than or equal to 90%, and the mean grain size is smaller than or equal to 150nm. According to the preparation method, conventional process equipment is adopted, the product is stable in quality, the method is simple in technology, and low in cost, and the technical problem that the poorly soluble medicine liposome is difficultly industrialized is solved.
Owner:常州金远药业制造有限公司
Dye-free pharmaceutical suspensions comprising sorbitol as a non-reducing sweetener and at least one active agent
A dye-free pharmaceutical suspension having a therapeutically effective amount of a first active agent consisting essentially of a first substantially water insoluble active agent having an average particle size of between 10 and 100 microns, an effective amount of non-reducing sweetener; an effective amount of water; and an effective amount of a suspending system; wherein the dye-free pharmaceutical suspension has a pH of from about 5 to about 6 and is substantially free of a reducing sugar and related methods.
Owner:MCNEIL PPC INC
Method for Administering Metformin
A device and method for administering a consistent and steady dosage of Metformin to patients which includes an implantation component with a composition of Metformin adapted to be inserted either subdermally or subcutaneously to administer a steady incremental dosage of Metformin to the patient throughout a predetermined period of time. A Metformin drug suspension is dispersed in a plurality of microreservoirs and the mixture of microreservoirs and placed in a silicone polymer tube for in situ polymerization and molding, which would allow Metformin molecules to initially diffuse through the microreservoir membrane and then through the silicone polymer containing membrane. In a further embodiment, Metformin implants may be formed by dispersing the drug in a copolymer matrix, which is then coated with a copolymer that serves as a rate-controlling membrane. The drug permeation through the polymer membrane occurs at a rate that is slower than that through the polymer matrix.
Owner:GOPINATHAN SAJI
Bismuth containing liquid pharmaceutical suspensions
A liquid pharmaceutical suspension for oral administration containing a bismuth-containing pharmaceutical agent, a suspension system, and water. The suspension system can contain from about 0.001% to about 0.2% gellan gum and from about 0.001% to about 0.75% magnesium aluminum silicate.
Owner:PROCTER & GAMBLE CO
Method for rapidly dissolving/swelling water-soluble high polymer suspending agent in drug suspension
InactiveCN108938563AReduce preparation timeFully contactedOrganic active ingredientsSolution deliveryPolymer scienceOrganic solvent
The invention discloses a method for rapidly dissolving / swelling a water-soluble high polymer suspending agent in drug suspension. The method comprises the following steps: adding the suspending agentinto an organic solvent mixable with water, uniformly stirring and mixing until no granule exists, and adding the organic solvent with the suspending agent dispersed therein into a dispersion mediumcontaining drugs and excipients. When the suspended solution is prepared by the traditional method, the high polymer suspending agent easily floats on the water surface or block mass that is wet in exterior and dry in interior is formed, the suspending agent needs to be added into water to be uniformly stirred and mixed in the small-amount multiple manner, and the agent needs to be completely dissolved or swelled in water within 10-20 hours even longer time. Moreover, when the high polymer suspending agent is dispersed in the organic solvent, the suspending agent can be directly added into theorganic solvent, uniformly stirred and mixed and then mixed with the aqueous solution, the dispersed suspending agent can be rapidly and fully contacted with water and can be fully dissolved or swelled within 5-30 minutes, the drug preparation time can be greatly saved, and the production efficiency is improved.
Owner:CHONGQING BULL ANIMAL PHARMA
Pharmaceutical suspension
The present invention is directed to the provision of a pharmaceutical suspension. The suspension includes high molecular weight polyethylene glycol as a suspending agent. The suspension also typically includes an antimicrobial agent (e.g., polymeric quaternary ammonium compound), an antimicrobial system (e.g., borate / polyol complex system) or both. The suspension has been found particularly useful as an ophthalmic suspension, but can be used in other instances as well.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com