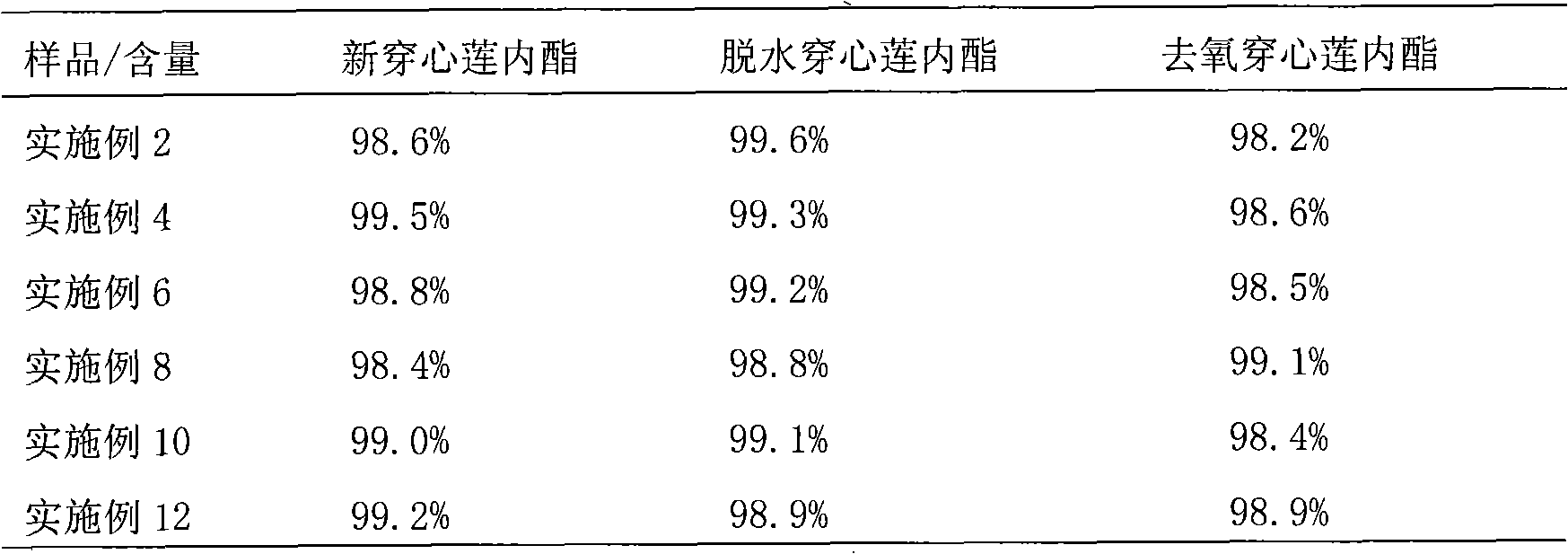
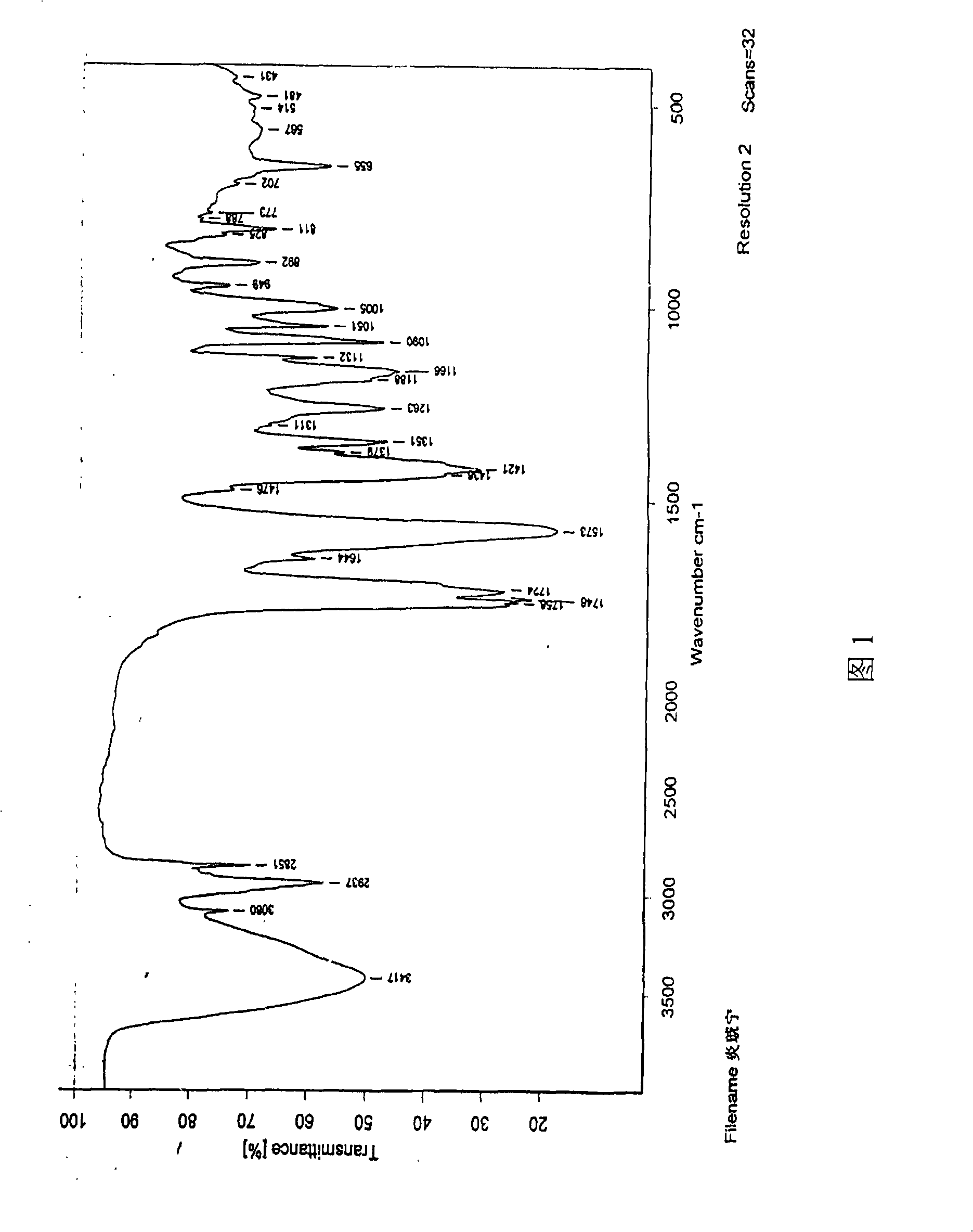
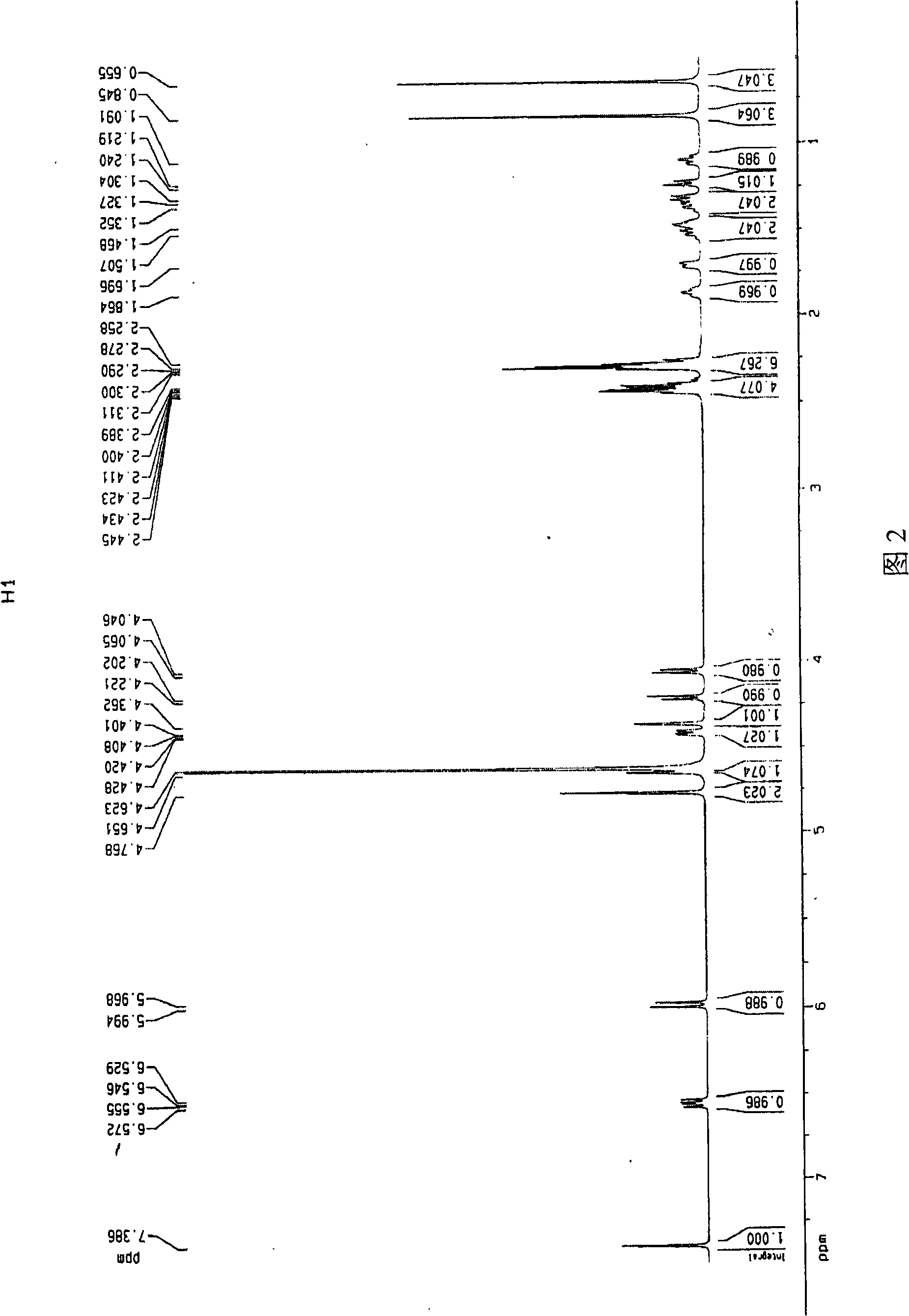
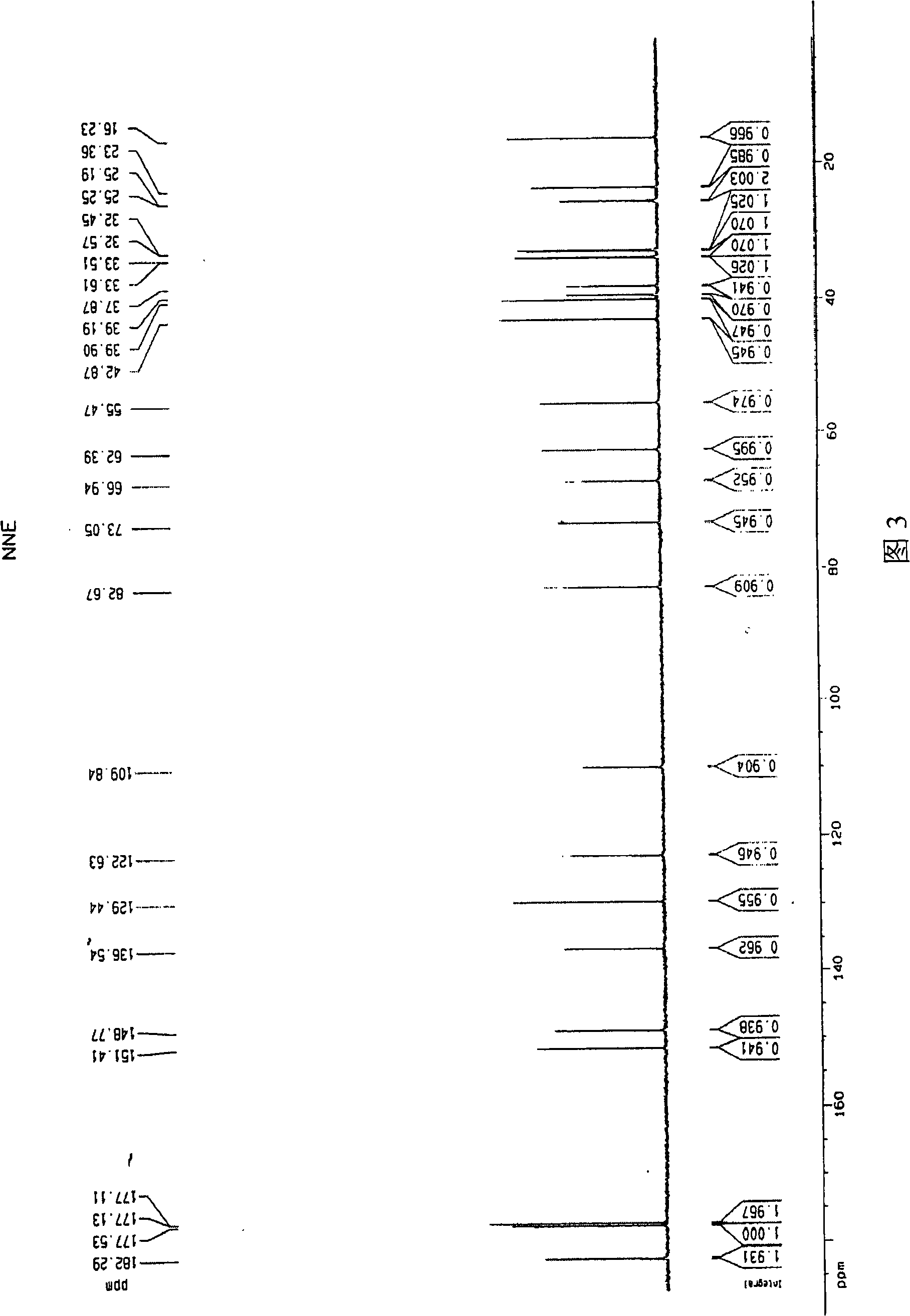
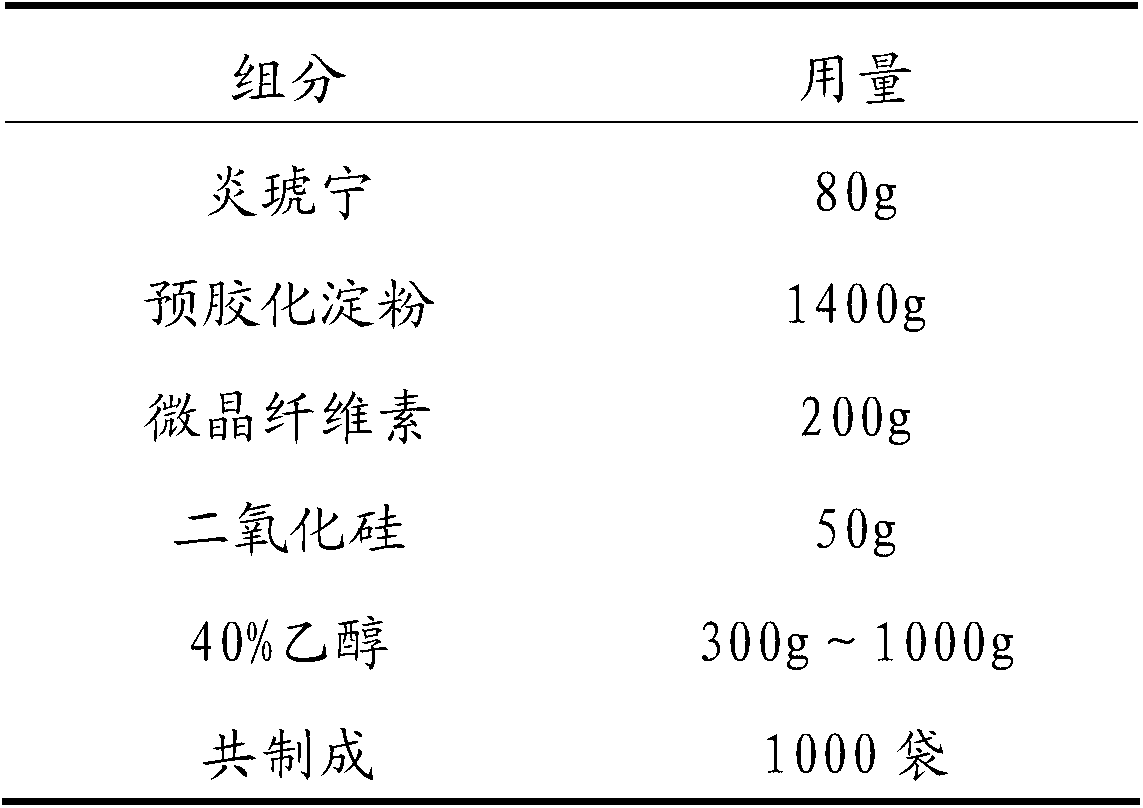
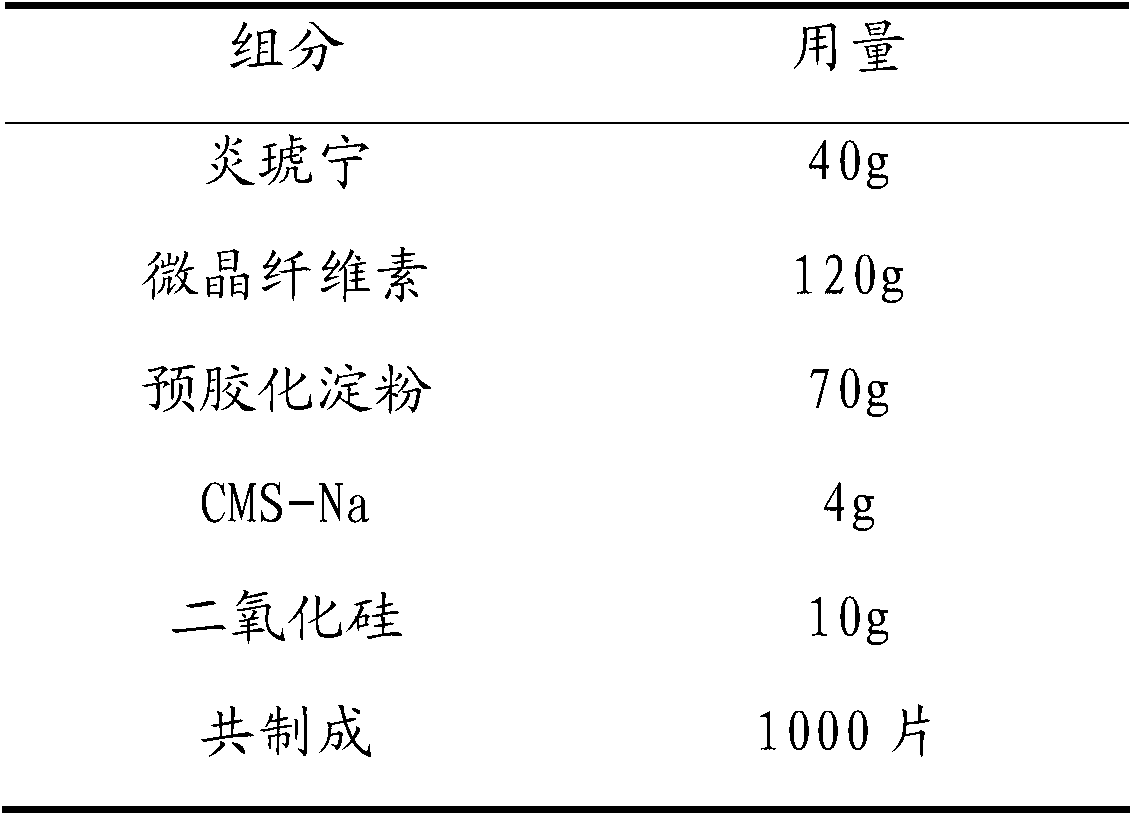
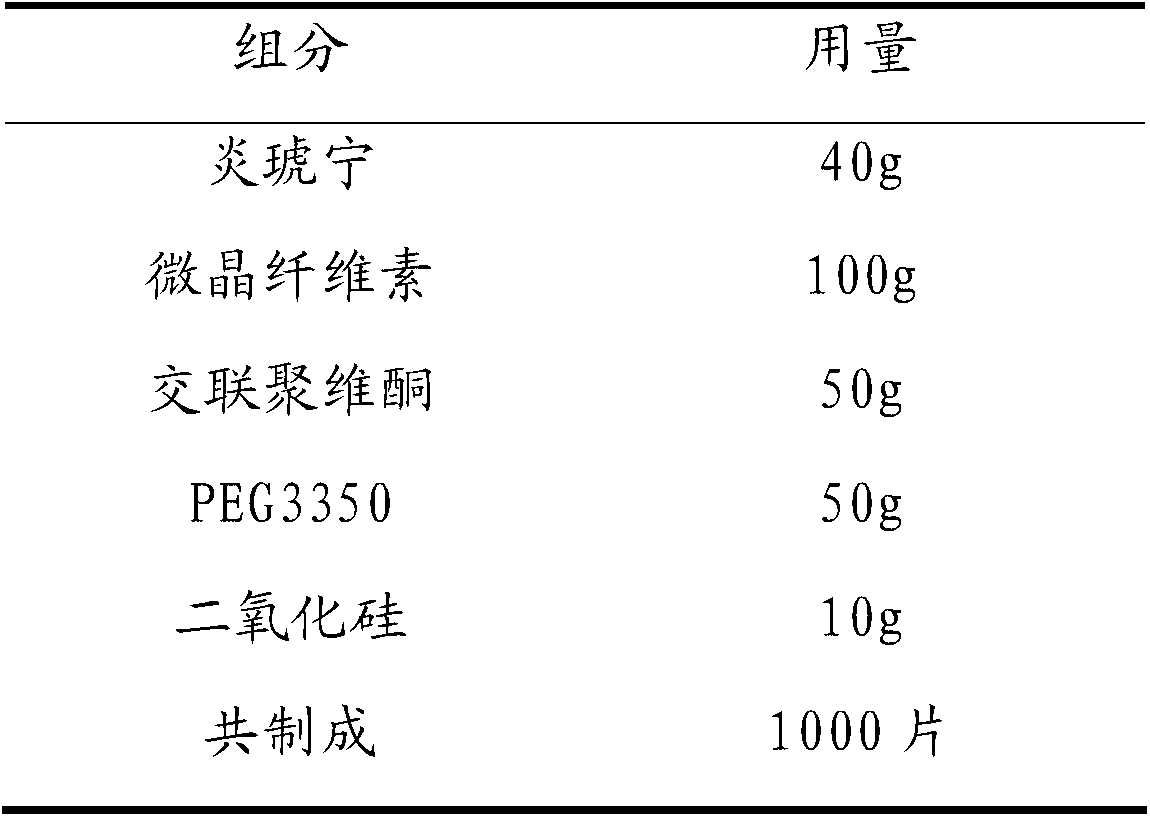
Patents
Literature
470 results about "Andrographolide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
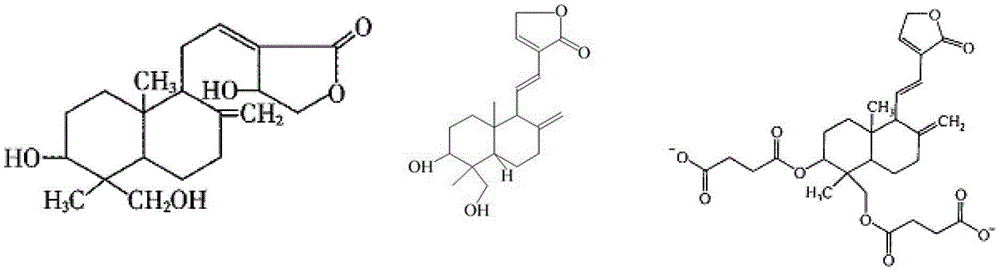
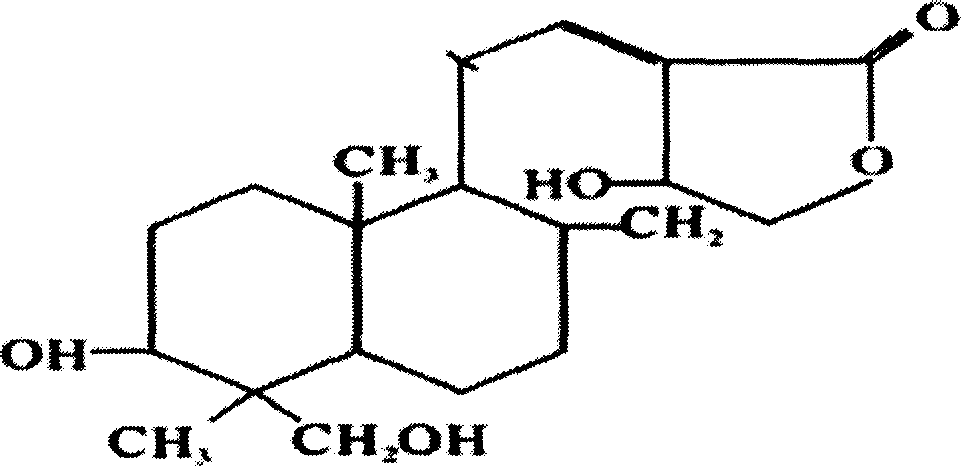
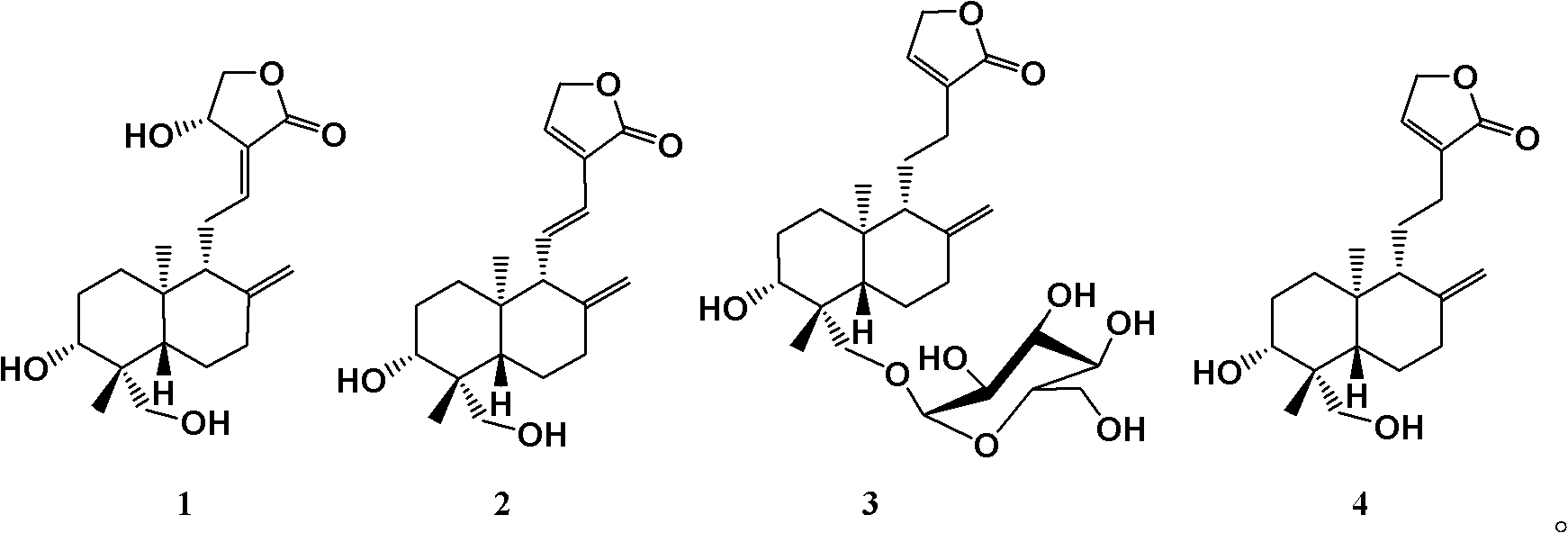
Andrographolide is a labdane diterpenoid that has been isolated from the stem and leaves of Andrographis paniculata. Andrographolide is an extremely bitter substance. Andrographolide has been studied for its effects on cell signaling, immunomodulation, and stroke. Study has shown that andrographlide may bind to a spectrum of protein targets including NF-κB and actin by covalent modification.
Sulfonated derivative of andrographolide and combination of medication
ActiveCN1687049AClear structureGood antibacterialAntibacterial agentsPowder deliveryDiseaseTonsillitis
The present invention discloses II kinds of andrographolide sulfonated derivatives with the actions of resisting bacteria, relieving inflammation and reducing fever and medicine composition containing them. They can be used for preparing freeze-dried powder, injection or oral preparation, and can be used for curing the diseases of pneumonia, bronchitis, tonsillitis and bacillary dysentery, etc.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
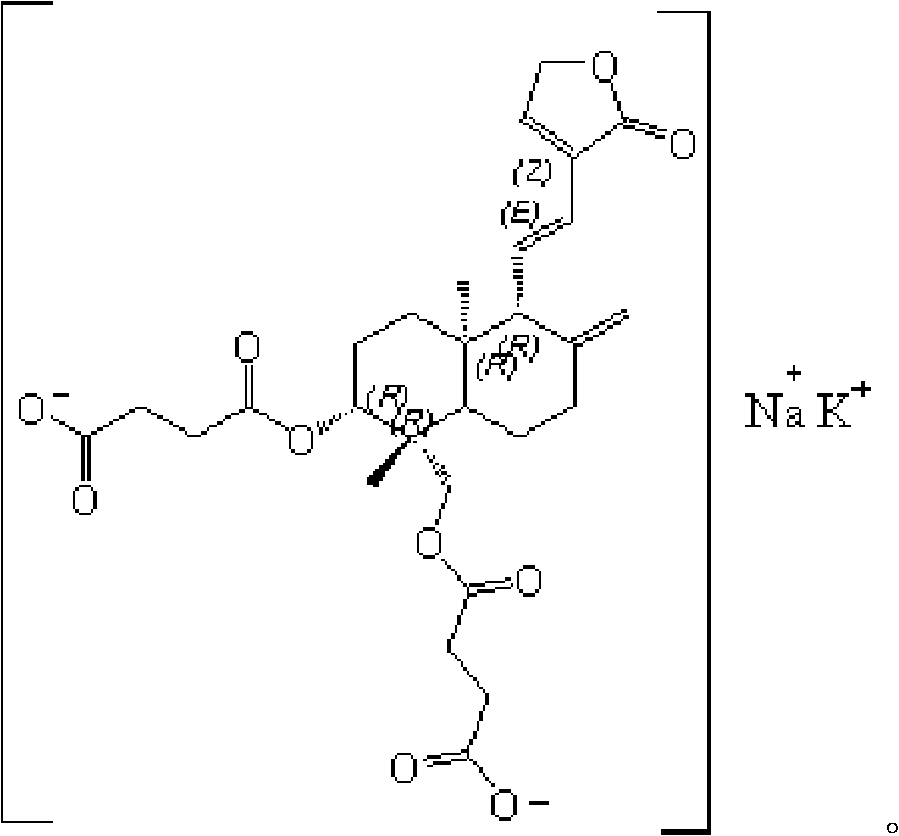
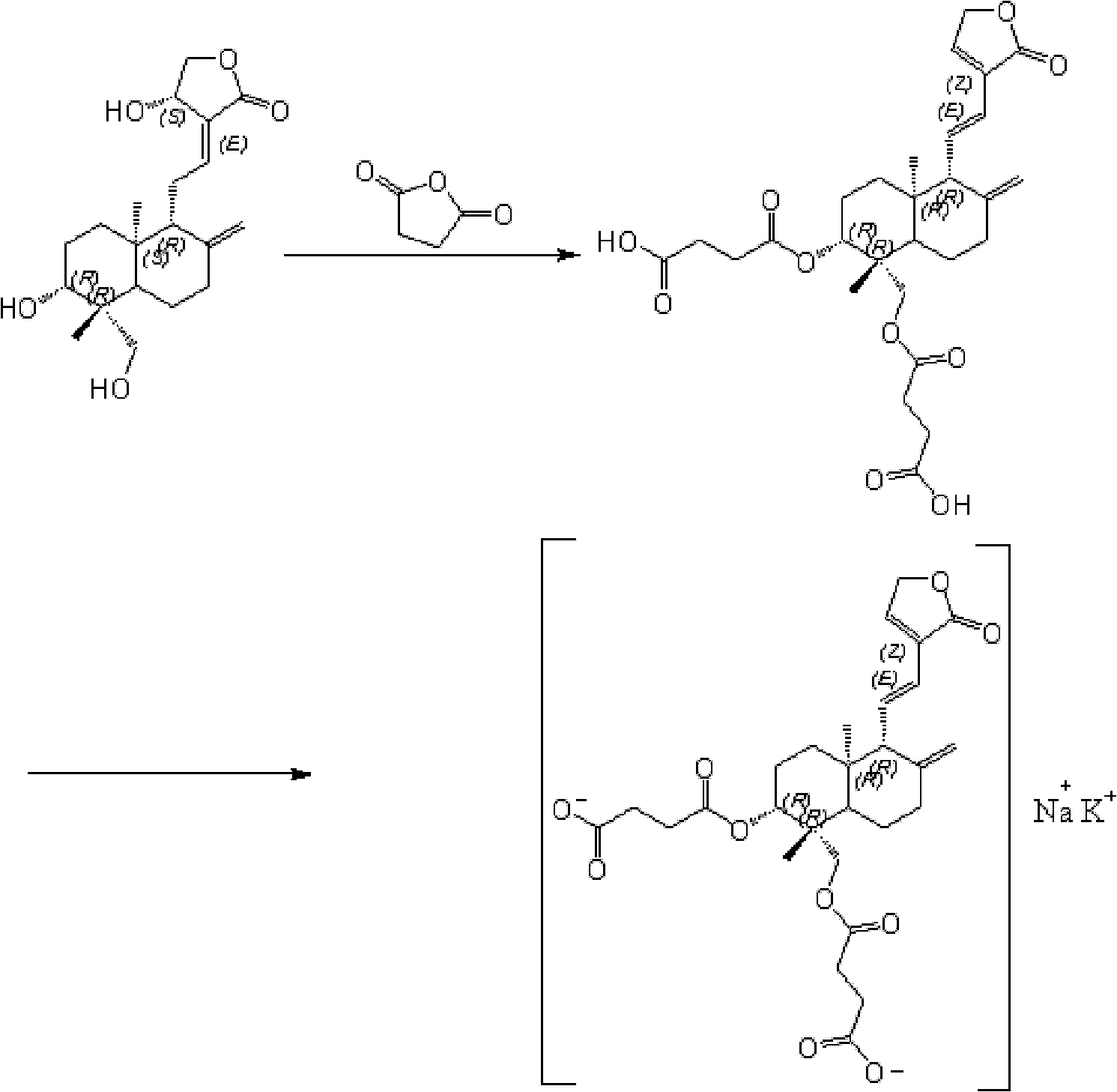
Preparation method of potassium sodium dehydroandroan drographolide succinate, potassium sodium dehydroandroan drographolide succinate preparation and preparation method thereof
ActiveCN1927854ADifficult to purifyImprove responsePowder deliveryOrganic active ingredientsNitrogen gasAndrographolide
The present invention relates to the preparation process and preparation of Yanuning. Yanuning is prepared through an esterification reaction and a salt-forming reaction. During the esterification reaction, andrographolide and succinic anhydride react in pyridine solution and the resultant is post-treated to obtain dehydroandrographolide semi-succinate. During the salt-forming reaction, dehydroandrographolide semi-succinate and KOH, KHCO3 or K2CO3 react in water to form monopotassium salt of dehydroandrographolide semi-succinate, and through post-treatment, Yanuning is prepared. The present invention has mild reaction condition and nitrogen protection to avoid oxidation and degradation, excessive pyridine added into the reaction for dewatering without need of vacuumizing, and heating reflux in the later reaction stage for complete reaction.
Owner:珠海晨安医药有限公司
Production technique of andrographolide and neoandrographolide, dehydroanddrographolide, oxyandrographolide
ActiveCN101559088ARaise the ratioReduce usageAntibacterial agentsOrganic chemistryChemical industryImpurity
The invention discloses a production technique of andrographolide and neoandrographolide, dehydroanddrographolide and oxyandrographolide; the technique comprises the following steps of: firstly preparing stem and leaf extract of andrographis paniculata, removing fat-soluble impurities such as chlorophyll and the like with petroleum ether, and then hot-melting the extract in lower alcohol or aqueous lower alcohol, conducting reflux and decolorization with active carbon, separating out a majority of andrographolide crystals, then removing flavonoid through an alumina column or alkali cleaning, obtaining the andrographolide, cold-melting the andrographolide with trichloromethane or dichloromethane for 2 to 4 times, filtering and obtaining two parts of filtrate and insoluble substances, concentrating the filtrate, then conducting solvent crystallization and recrystallization or column chromatography for separation, and finally, respectively obtaining dehydroanddrographolide and pure product of andrographolide; the insoluble substances go through solvent crystallization and recrystallization or column chromatography for separation to obtain the neoandrographolide and pure product of andrographolide. The technique has simple production equipment, simplified routes and easy operation, can realize industrialized batch production; and the proportion of neoandrographolide, dehydroanddrographolide and oxyandrographolide in the obtained andrographolide is high, while the content of impurities is low. The obtained neoandrographolide, dehydroanddrographolide and oxyandrographolide all have monomer purity of higher than 98 percent, thus being capable of being used as chemical reference substance of the traditional Chinese medicine, or being applied as raw materials of medicine and chemical industry.
Owner:雷允上药业集团有限公司
Method for preparing potassium dehydroandrographolide succinate or potassium sodium dehydroandroan drographolide succinate
The invention discloses a method for preparing potassium dehydroandrographolide succinate or potassium sodium dehydroandroan drographolide succinate, which comprises that firstly, andrographolide serves as a raw material, the andrographolide reacts with succinic anhydride to produce dehydroandroan drographolide disuccinate in a specific non-pyridine solvent under the heating reflux in the presence of a catalyst, and the usage amount ratio (W / W) of the andrographolide and the catalyst is (1:10)-(1:1); and secondly, the purified dehydroandroan drographolide disuccinate reacts with potassium hydroxide, sodium hydroxide, alkalinity sylvite or alkalinity sodium salt to produce the potassium dehydroandrographolide succinate or the potassium sodium dehydroandroan drographolide succinate in methanol, ethanol or hydrosolvent. The method is practical, convenient to operate, low in cost and nontoxic. The potassium dehydroandrographolide succinate or the potassium sodium dehydroandroan drographolide succinate is prepared easily under the normal atmosphere through usage of the solvent, the product quality is guaranteed, and the method is suitable for industrial production particularly.
Owner:HUBEI HOPE PHARMA
Technique for preparing potassium sodium dehydroandroandrographolide succinic by using potassium dehydroandrographolide succinate
The invention discloses a process of preparing Dehydroandrographolide Succinate Sodium and Potassium salts with Potassium Dehydroandrograpolide Succinate. The process comprises the steps of: weighing right amount of Potassium Dehydroandrograpolide Succinate, adding absolute ethyl alcohol about 3 to 6 times to make suspension; weighting mole Sodium bicarbonate in equal weight, adding water to dissolve, dripping slowly Sodium bicarbonate solution in potassium dehydroandrograpolide succinate suspension in a water bath at a temperature of between 40 and 60 DEG C, mixing into liquid medicine and defecating, dissolving impurity and filtering, putting on a 0.22 mu m filter membrane, adding absolute ethyl alcohol about 7 to 12 times while mixing, after 10 to 30 minutes of mixing, natural crystallization seeds out after 13 to 18 hours standing in room temperature; filtering and washing 2 to 3 times with right amount of absolute ethyl alcohol; filtering and drying with less pressure. The invention provides a process route of preparing potassium sodium dehydroandroan drographolide succinate for injection with high purity, which is high in process yields, high in product purity, good in solubility, less in impurity and complete in combination of Potassium and Sodium; no obvious toxic and side effect in clinic application, the preparation is more stable compared with the similar and can be prepared into various preparations.
Owner:CHANGCHUN MAILING BIOLOGICAL ENG CO LTD
Preparation method of andrographolide
ActiveCN102382083AImprove transfer rateResidue reductionOrganic chemistryActivated carbonOrganic solvent
The invention provides a preparation method of andrographolide. The preparation method includes steps of extracting ethanol of common andrographis herb crude medicine, filtering, decolorizing by the aid of activated carbon, filtering again, concentrating cooling of a concentrated liquor, removing supernatant fluid, collecting deposit, washing the deposit by ethanol to obtain macro-crystal, dissolving the macro-crystal by ethanol, decolorizing the macro-crystal by the aid of activated carbon, filtering the macro-crystal, concentrating filtrate, recrystallizing and drying so that andrographolide is obtained. The transfer rate of andrographolide of the method is high, remained organic solvent is less, and a product is safer and effective.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
New isoandrographolidume sulfonate, pharmaceutical composition containing sulfonate, preparation method and applications thereof
InactiveCN101560198AClear structureGood water solubilityOrganic active ingredientsOrganic chemistryInflammatory factorsSulfonate
The invention discloses a compound as a formula (1), a kalium salt, a sodium salt or an ammonium salt thereof and also discloses a pharmaceutical composition containing the compound as the formula (1), an isoandrographolidume sulfonate E, an andrographolic acid and andrographolidume and a preparation method thereof. The compound and the composition obtained by the method have favorable effects, such as an inflammatory factor resistant effect, an antibacterial effect, an analgesic effect and an antineoplastic effect. The invention also discloses a peroral preparation or an injection preparation prepared by using the compound and the composition.
Owner:YANTAI TARGET DRUG RES
Method for simultaneously preparing andrographolide and dehydrated andrographolide
InactiveCN101168537ASimple processEasy to operateOrganic chemistryMagnoliophyta medical ingredientsAlcoholGradient elution
The invention relates to a method for simultaneously preparing andrographolide and didehydroandrographolide. The method includes that common andrographis herbs are smashed and then added into alcohol solvent with certain concentration to be abstracted, extracting solution is filtered, solvent is recycled to lead the concentration of the alcohol solvent in filtrate to be lower than 20 percent; after being filtered, the filtrate is taken to be exchanged through macroporous resin and to be absorbed, the alcohol solvent with certain concentration is used to operate the gradient elution, so as to lead the andrographolide and the didehydroandrographolide to be desorbed in elutriant with different concentration; after being concentrated, the elutriant is recrystallized and refined to obtain andrographolide crystal and didehydroandrographolide crystal. The purity degree of the two crystals obtained through the method reaches above 97 percent, and the method has the advantages that the process is simple, the operation is easy, the cost is low, the industrializing production can be operated, etc.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI
Andrographolide ground suspending liquid, preparation method thereof, and application of pharmaceutical preparation
InactiveCN102614133AImprove stabilityDissolution rate is fastAntibacterial agentsOrganic active ingredientsImmediate releaseDrugs preparations
The invention relates to andrographolide ground suspending liquid, a preparation method thereof, and the application of pharmaceutical preparation, which belongs to the field of pharmaceutical preparation. The preparation method comprises the steps of adding andrographolide into hydrophilic accessory solution with certain concentration, and grinding the andrographolide in a basket grinder to prepare suspending liquid with particle sizes smaller than 3000 nm. Liquid layers of pharmaceutical suspending liquid are laminated onto blank pellet cores with certain particle size range, to prepare andrographolide immediate-release pellets. After grinding, by reducing pharmaceutical particle sizes, increasing particle surface areas and improving the wettability of pharmaceutical particles, the dissolution in vitro of the drug is improved, and hydrophilic carriers are adopted to effectively prevent the aggregation of pharmaceutical particles so as to improve the stability of the pharmaceutical preparation. The preparation method is simple and easy for industrialized production, and the dissolving-out speed of the prepared andrographolide immediate-release pellets is high, so that the bioavailability is obviously improved.
Owner:SHENYANG PHARMA UNIVERSITY
Potassium sodium dehydroandroandrographolide succinates and their preparations
InactiveCN1557812AHigh purityImprove solubilityAntibacterial agentsOrganic active ingredientsSide effectSuccinates
The present invention relates to one kind of sodium-potassium andrographolide-semisuccinate and its preparations. On the basis of potassium andrographolide-semisuccinate, which is not enough stable and is likely to produce precipitate and negative effect, sodium-potassium andrographolide-semisuccinate is developed and has relatively high stability and less side effect. The sodium-potassium andrographolide-semisuccinate is prepared into injection, powder for injection and other forms.
Owner:YAOPHARMA CO LTD
Preparation method of andrographolide bulk pharmaceutical
ActiveCN102584752ASimple preparation processHigh yieldOrganic chemistrySuccinatesMedicinal chemistry
The invention discloses a novel preparation method of an andrographolide bulk pharmaceutical, belonging to the field of pharmaceutical chemistry. The method comprises the following steps of: reacting andrographolidume serving as a raw material with succinyl oxide under the action of a catalyst to obtain a 14-deoxy-11,12-didehydroandrographolidume-3,19-disuccinate (dehydroandrographolide succinate) intermediate at high yield and high quality; reacting the intermediate with a mixed salt; and crystalizing to directly obtain andrographolide which is consistent with an injection standard. The andrographolide bulk pharmaceutical has the advantages of mild reaction conditions, total reaction yield of over 70 percent and purity of over 98 percent, and is suitable for industrial production.
Owner:KAIFENG PHARMA GRP +1
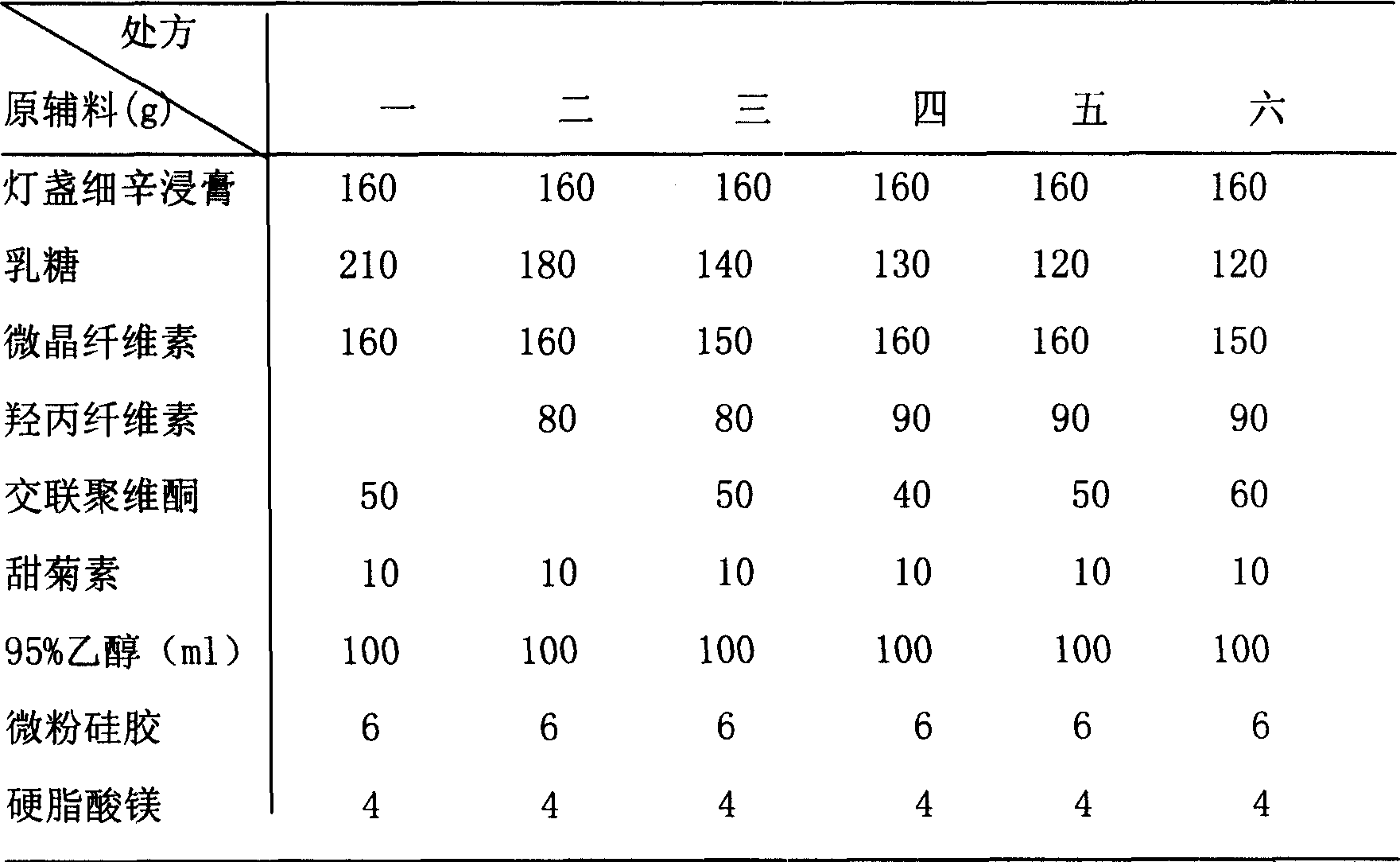
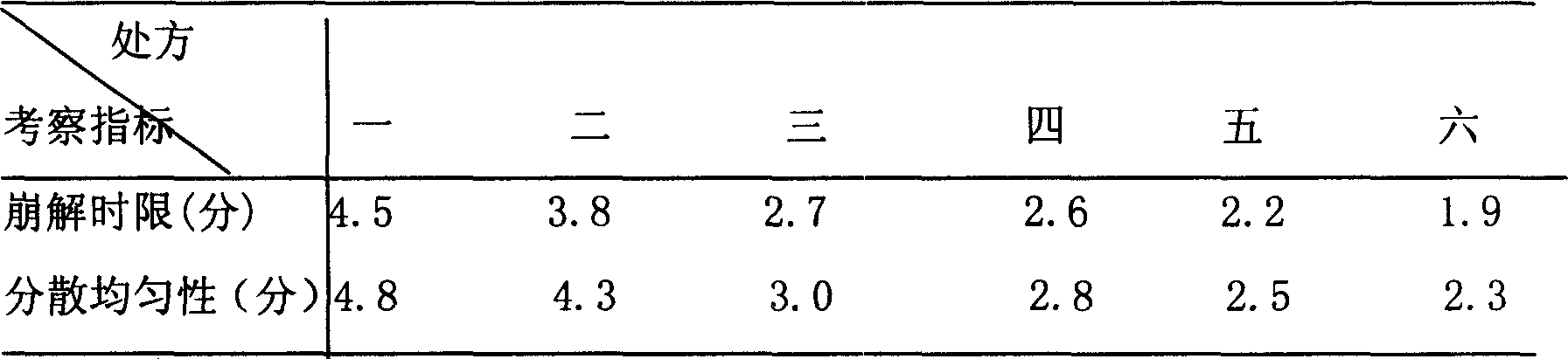
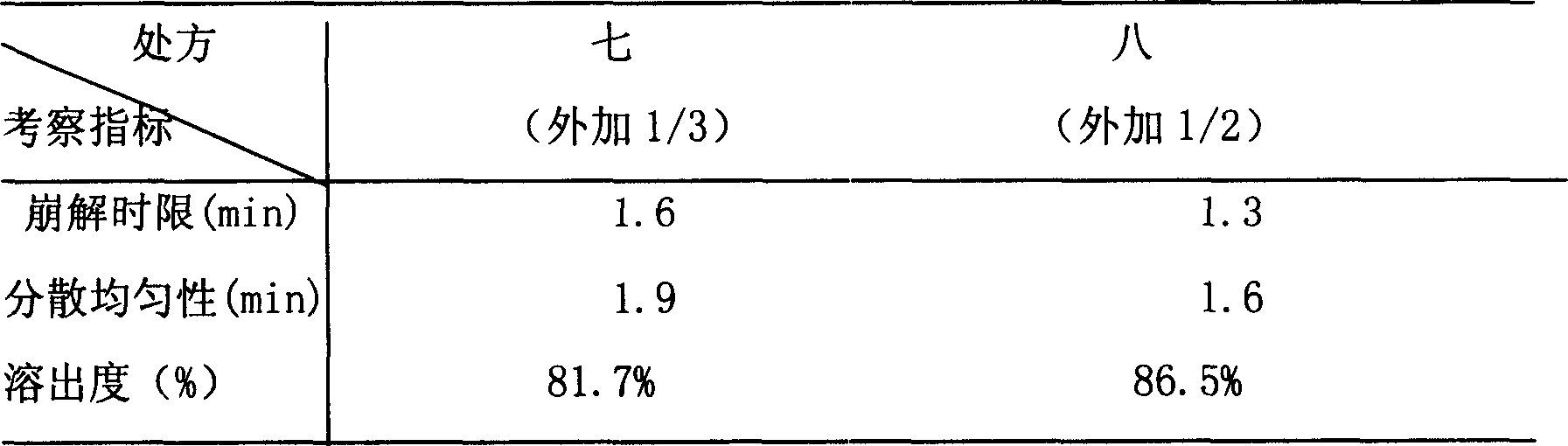
Yimaikang dispersion tablet and its preparing method
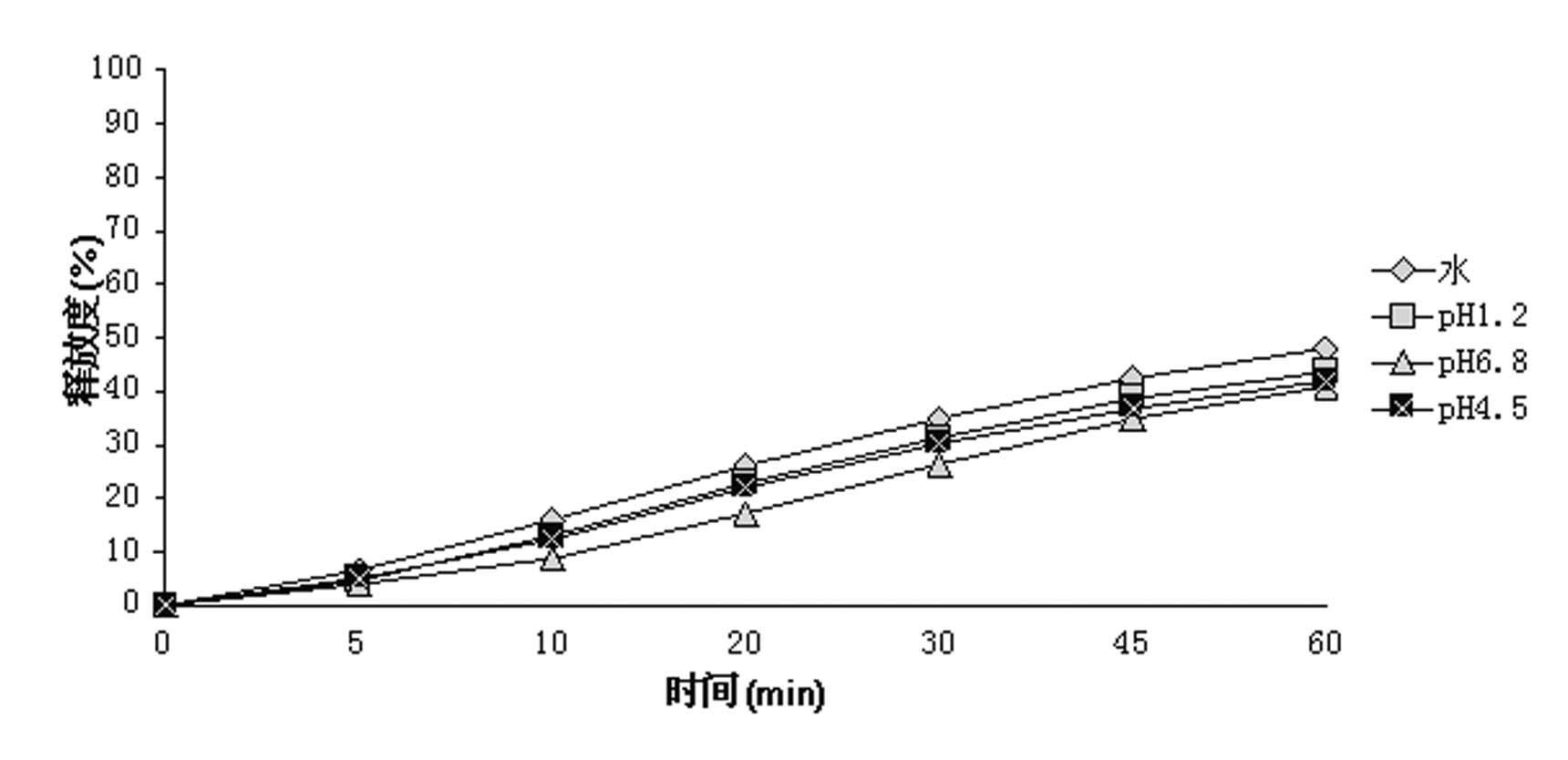
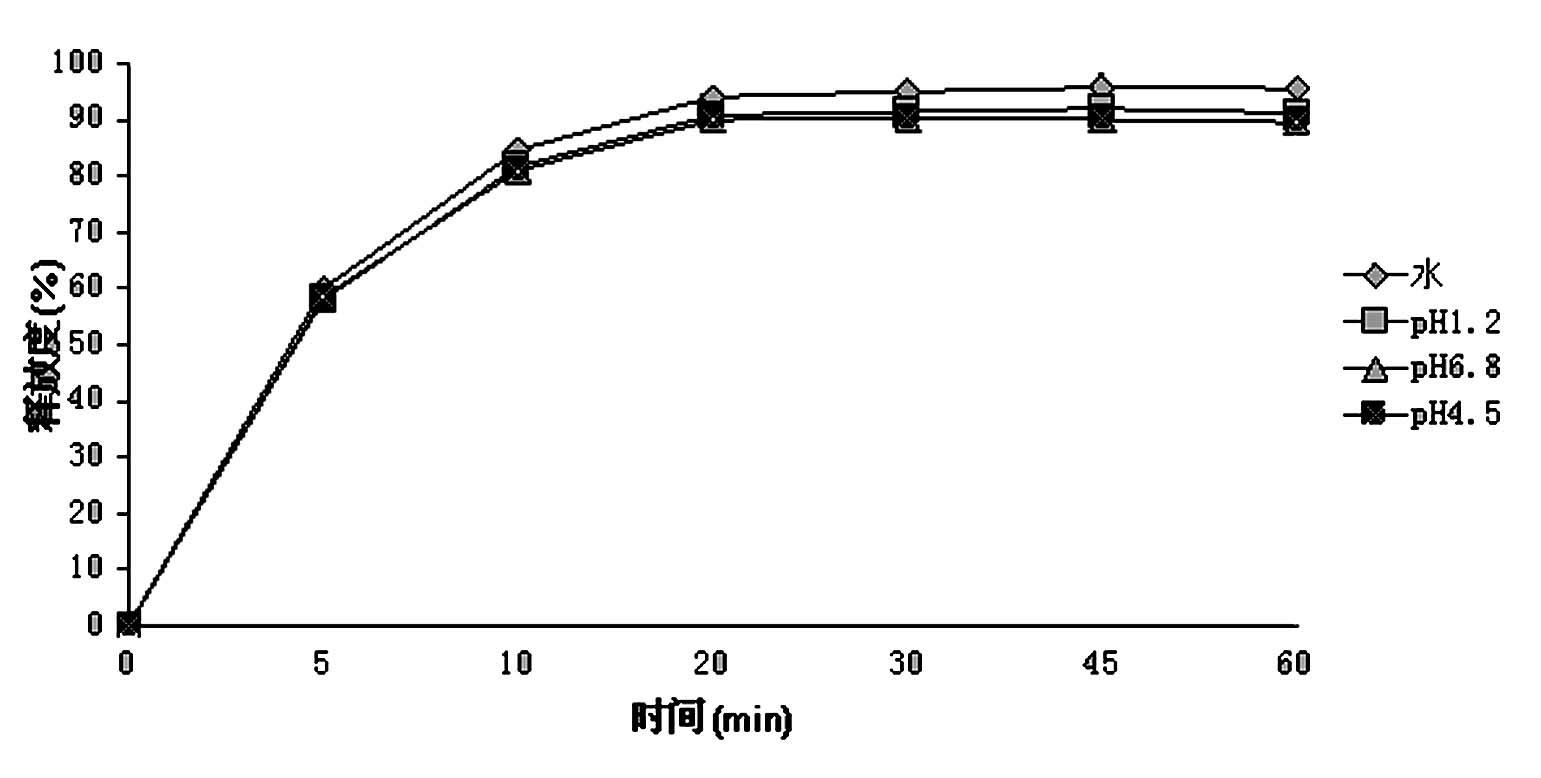
The Yimaikang dispersion tablet consists of fleabane extractum and medicine carrier. Its recipe includes fleabane extractum, lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked polyvidon, steviosin, alcohol, fine silica gel powder and magnesium stearate. The dispersion tablet can disintegrate completely in 3 min to reach homogeneous dispersive state and its effective component andrographolide has dissolving rate reaching 60 % in 10 min. It is used in treating ischemic cerebral vascular diseases, cerebral hemorrhage sequela, vein obstruction, etc. and has fast acting, good taste and convenient taking.
Owner:云南白药集团大理药业有限责任公司
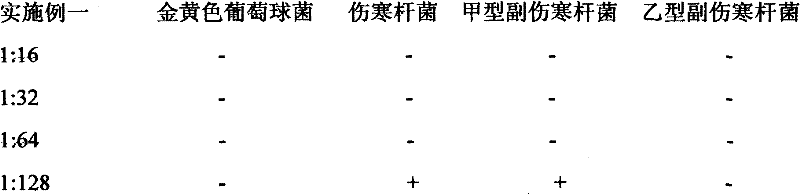
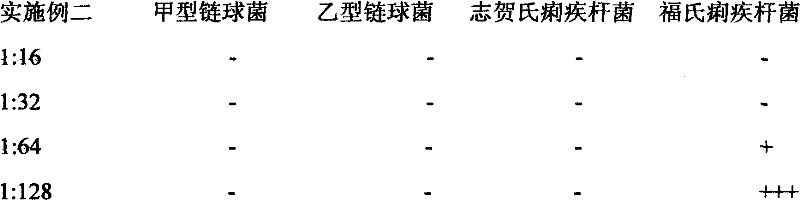
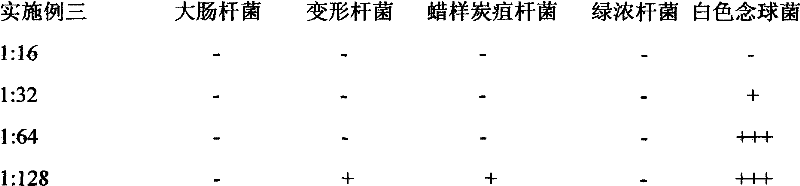
Chinese herb biological disinfectant
InactiveCN102578169AEffective disinfectionAntibacterial agentsBiocideDisinfectantVolumetric Mass Density
The invention relates to a Chinese herb biological disinfectant to solve the public nuisance problem of the traditional disinfectant. The Chinese herb biological disinfectant is prepared by adopting the steps of preparing primary liquid medicine by ethanol precipitation of the following bulk drugs in weight ratio: common andrographis herb 40-120, santonin 40-120, celastrus angulatus 35-105, camphor tree 35-105, stephania tetrandra 35-105, rhododendron molle 35-105, macleaya cordata 30-90, pomegranate rind 30-90, clematis root 30-90, radix stemonae 30-90, eucalyptus robusta 30-90, sophora flavescens 30-90, cortex dictamni 25-75, Chinaberry bark and root-bark 25-75, rhizoma corydalis 25-75 and liquorice root 25-75; and adding sodium usnic acid with weight ratio of 0.01-0.03% in the primary liquid medicine, wherein the sodium usnic acid is dissolved with a solvent before the sodium usnic acid is added in the primary liquid medicine. The Chinese herb biological disinfectant contains 0.2% andrographolide, with color being brownish red, ph being 7-8 and density being 1.10g / cm<3>. The Chinese herb biological disinfectant has the advantages of effective disinfection and no harm to human.
Owner:蔡葵荣 +1
Oral preparation containing andrographolide and preparation method thereof
InactiveCN103070843AImprove toleranceAvoid direct accessOrganic active ingredientsAntiviralsEnteric-coated granulesSide effect
The invention relates to an oral preparation containing andrographolide and a preparation method thereof, belonging to the field of medicines. The oral preparation containing andrographolide is in dosage forms of enteric-coated granules, enteric-coated tablets, enteric-coated capsules and enteric-coated dispersible tablets which are prepared by mixing andrographolide and pharmaceutically acceptable auxiliary materials. The prepared oral preparation can reduce incidence of adverse reactions and side effects thereof and improve the medication safety while ensuring the therapeutic effect, and uses the andrographolide more widely.
Owner:司鹏 +1
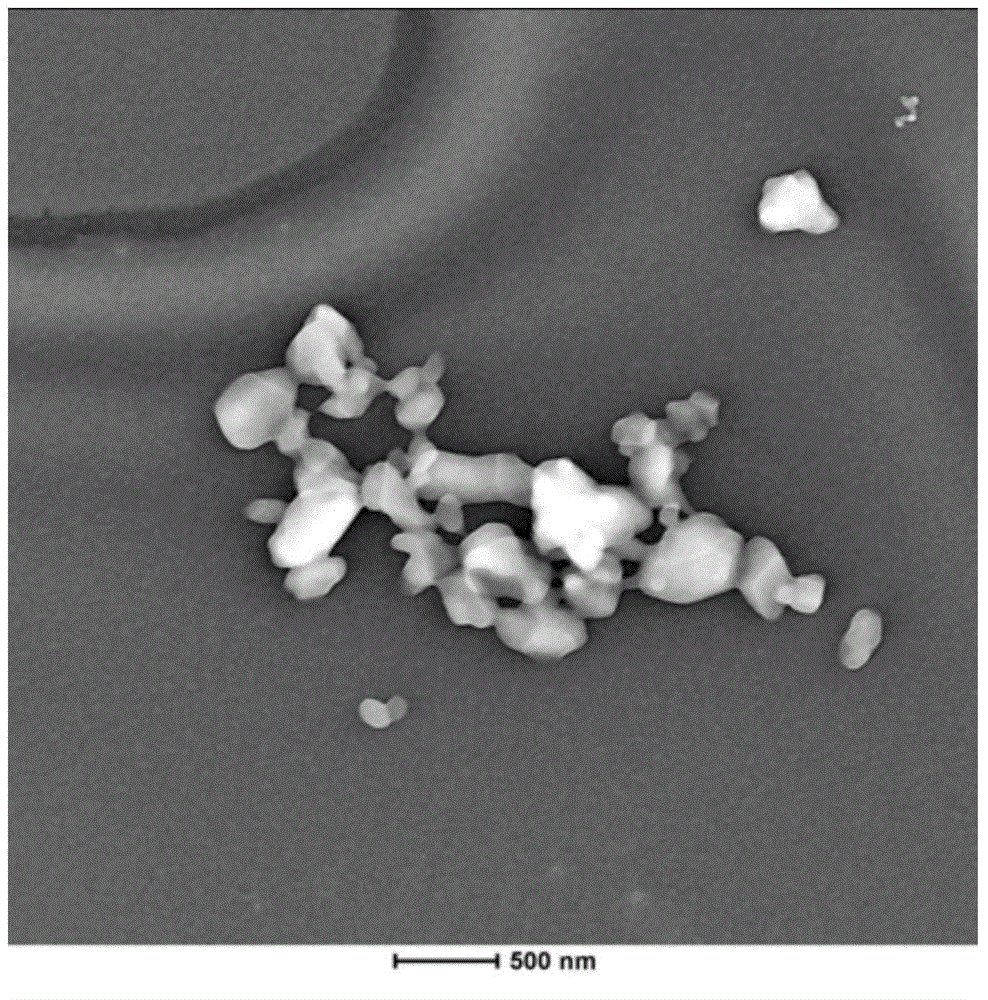
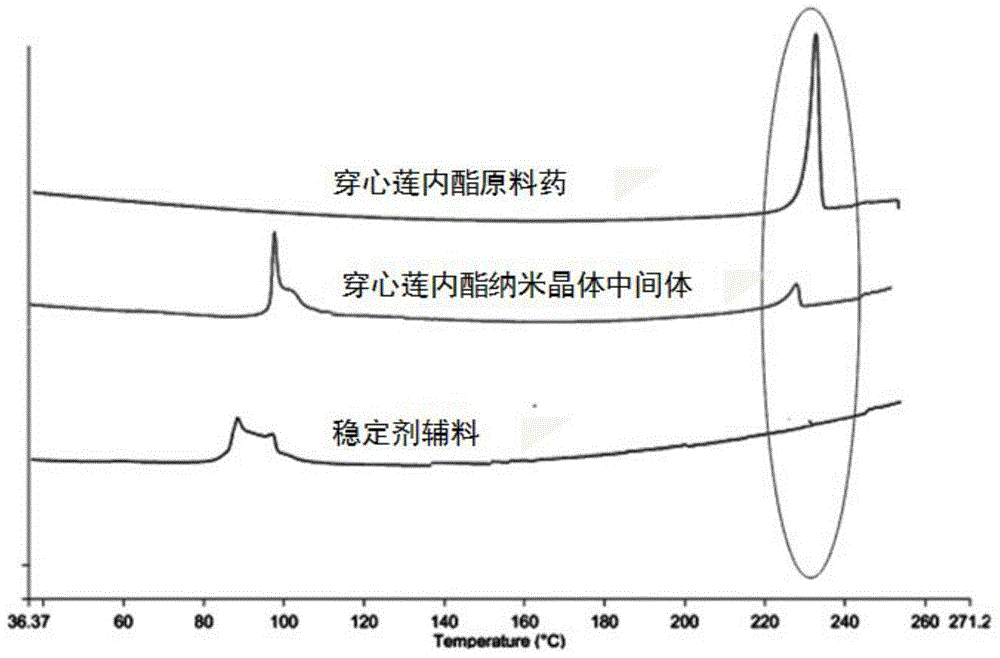
Andrographolide nano crystal intermediate, preparation method and applications thereof
InactiveCN104983688AImprove bioavailabilityImprove wettabilityAntibacterial agentsPowder deliverySolubilitySolvent
The invention belongs to the field of medicinal preparation, and relates to an andrographolide nano crystal intermediate and a preparation method thereof. The provided andrographolide nano crystal intermediate can be immediately dispersed into a nano state after being contacted with water; the solubility, dissolving-out speed, and bioavailability of andrographolide are prominently improved; the intermediate can be made into different dosage forms containing the nano crystal intermediate; the intermediate can be disintegrated into nano crystals after being contacted with water; the drug stability is enhanced, and the drug function is reinforced. The preparation technology is simple, no toxic solvent is used, the cost is low, and the technology can be easily industrialized, and thus has a good application prospect.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
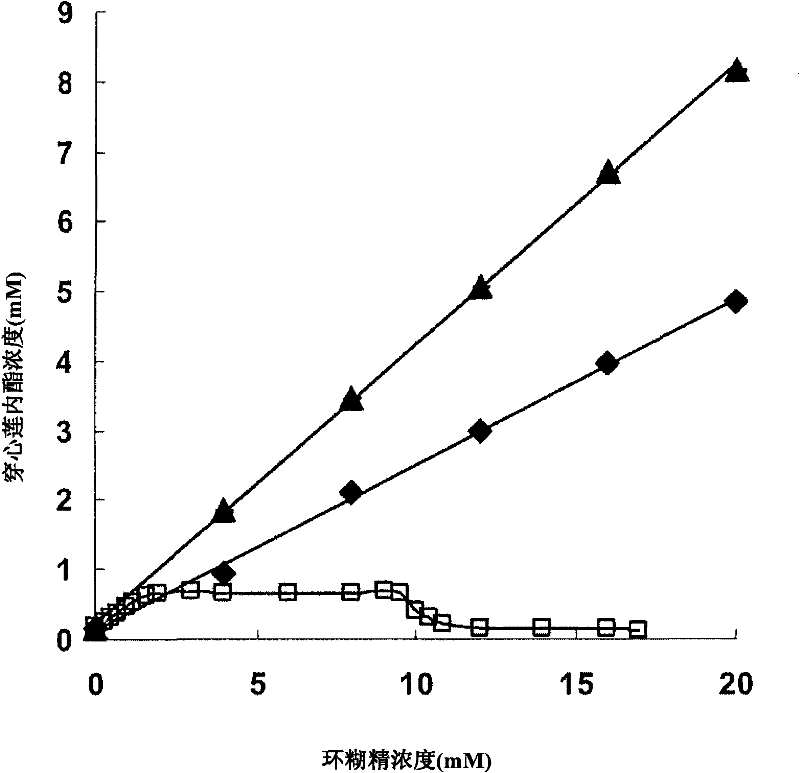
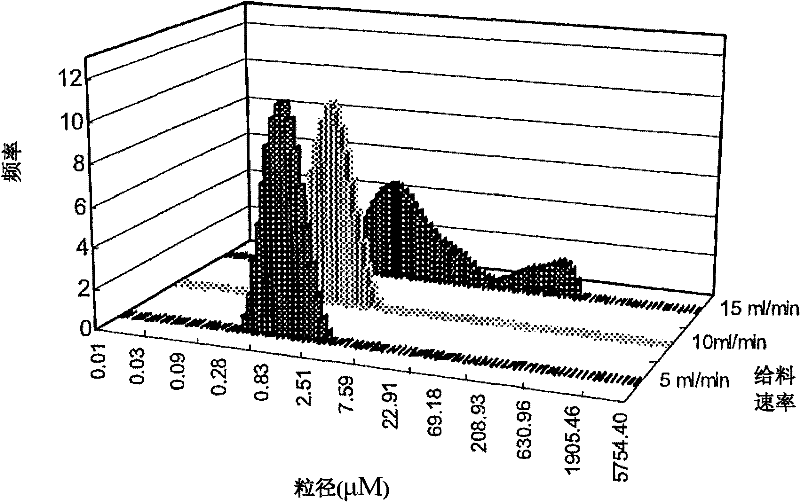
Andrographolide cyclodextrin inclusion compound, preparation method and application thereof
InactiveCN102343096AImprove solubilityImprove lipophilicityAntibacterial agentsOrganic active ingredientsSolubilityMicrometer
The present invention provides an andrographolide cyclodextrin inclusion compound. The basic components of the andrographolide cyclodextrin inclusion compound comprise: a) andrographolide, and b) pharmaceutically-acceptable cyclodextrin. The inclusion compound is prepared through carrying out spray drying for the CD and the andrographolide, wherein the entrance temperature is 100-200 DEG C, the material feeding speed is less than or equal to 20 ml / min, a molar ratio of the CD to the andrographolide is more than or equal to 1:1. The present invention further provides a preparation method for the inclusion compound, and an application of the inclusion compound. The inclusion compound provided by the present invention has excellent solubility, excellent drug stability and excellent bioavailability. With the method provided by the present invention, the inclusion compound of the andrographolide and the cyclodextrin or the inclusion compound of other plant compounds and the cyclodextrin can be prepared, wherein the inclusion compound has the particle size from micrometer to submicron, such that the bioavailability and the stability of the andrographolide are increased, a new delivery system for the diterpene plant compound is provided.
Owner:CITY UNIVERSITY OF HONG KONG
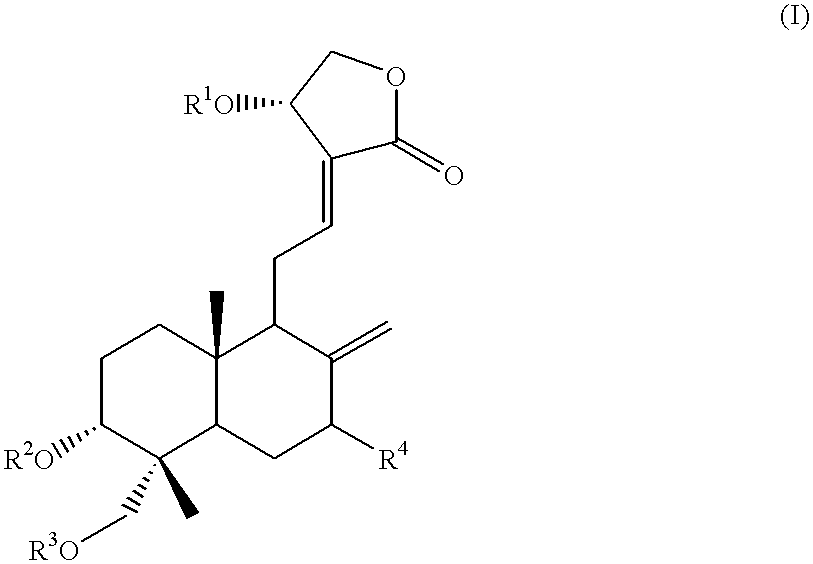
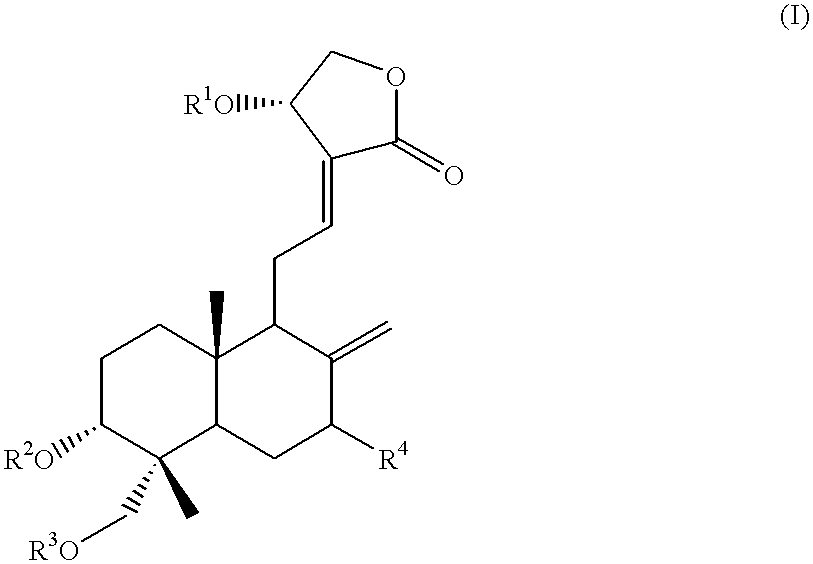
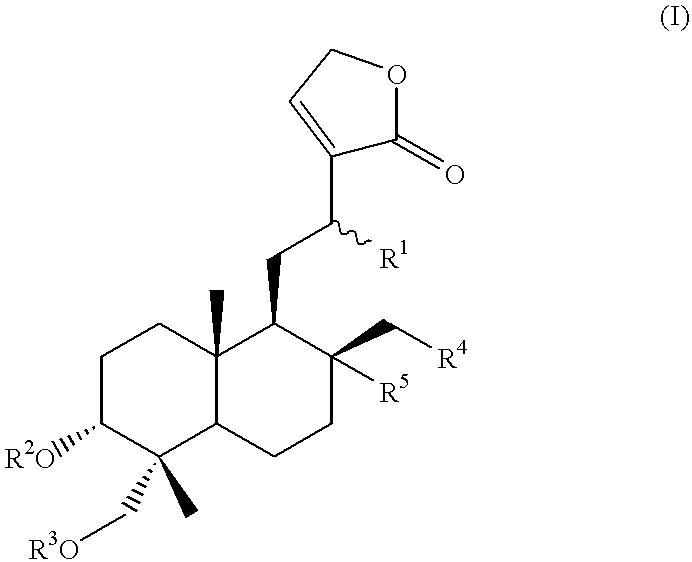
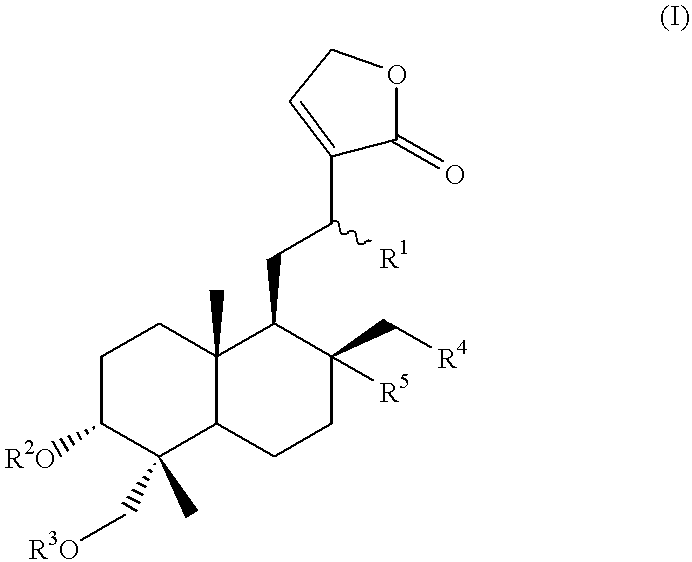
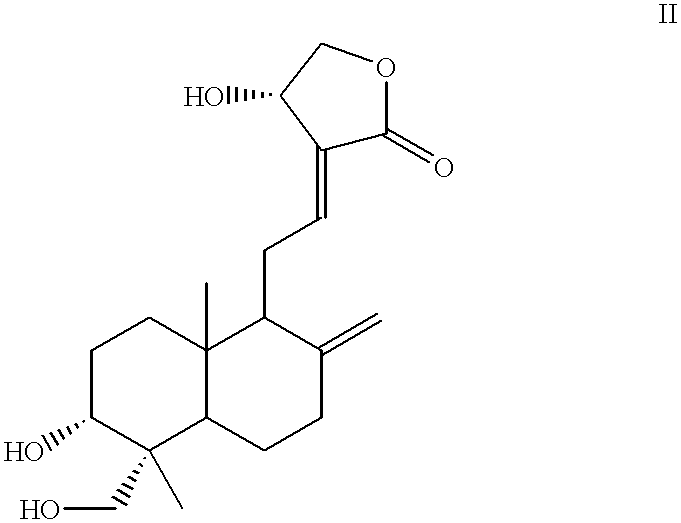
Novel anticancer compounds : process for their preparation and pharmaceutical compositions containing them
InactiveUS20020016363A1High activityLow toxicityBiocideSilicon organic compoundsCombinatorial chemistryStereoisomerism
The present invention relates to novel anticancer agents, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, and their pharmaceutically acceptable solvates. The present invention more particularly relates to novel derivatives of andrographolide, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, and their pharmaceutically acceptable solvates. The novel derivatives of andrographolide have the general formula (I).
Owner:DR REDDYS LAB LTD
Method for extracting andrographolide from andrographis paniculata
InactiveCN103435578AHigh yieldIncreased diffusion areaOrganic chemistryPlant tissueBiochemical engineering
The invention relates to a method for extracting andrographolide from andrographis paniculata and in particular relates to a method for extracting andrographolide by employing the combination of a plant tissue crushing method and enzymolysis. The processing steps comprises: crushing the whole andrographis paniculata by the plant tissue crushing extraction method, carrying out enzymolysis on the crushed andrographis paniculata, extracting by the assistance of ultrasonic wave and microwave, filtering, extracting by petroleum ether, purifying by macroporous resin, concentrating under reduced pressure and re-crystallizing, thus obtaining the andrographolide with high purity. The method for extracting andrographolide from andrographis paniculata carries out crushing at room temperature, and is free from damage to ingredient, saves time, has high efficiency and saves energy, is simple and convenient to operate, beneficial to environment protection, high in extraction efficiency and purity, and applicable to production in large scale.
Owner:NANJING TONGZE AGRI SCI & TECH
Andrographolide and preparation method thereof
The invention relates to andrographolide and a preparation method thereof. Specifically, the andrographolide provided by the invention contains lower than 2% of dehydrated andrographolide and higher than 95% of andrographolide. In addition, the method for preparing andrographolide comprises the following steps of: (1) extracting a herba andrographitis medicinal material by ethanol, and filtering to reserve an extracting solution; (2) decoloring the filtrate through active carbon, and filtering; (3) concentrating, cooling the concentrate, discarding supernatant liquid, and collecting educt; (4) washing the educt through ethanol to obtain a crude crystal; (5) dissolving the crude crystal through ethanol, decoloring through active carbon and filtering; and (6) concentrating and recrystallizing filtrate, and drying to obtain a product. The method disclosed by the invention is high in extracting efficiency and low in impurity content, and is specifically applicable to industrial production.
Owner:成都天台山制药股份有限公司
Compounds having anticancer activity : process for their preparation and pharmaceutical compositions containing them
InactiveUS6576662B2High activityNo toxicityBiocideOrganic chemistryMedicinal chemistryStereoisomerism
Owner:DR REDDYS LAB LTD
Method for preparing and rographalide
The invention relates to an andrographolide preparing method, especially relating to a method for separating and purifying andrographolide with macroporous resin.
Owner:TIANJIN TASLY PHARMA CO LTD
Method for planting dendrobium officinale on tree
InactiveCN106613829AMeet growth needsImprove survival rateMagnesium fertilisersGrowth substratesCambiumNutrient solution
The invention provides a method for planting dendrobium officinale on a tree, and belongs to the technical field of dendrobium officinale planting. The method comprises the steps of selecting and treating an adnascent tree, pretreating seedlings, treating moss, binding the tree and fixing planting, performing water and fertilizer management after fixing planting, and the like. During treatment on the adnascent tree, a notch which does not damage a stem cambium is formed in a tree trunk, the tree trunk is coated with a pulpous nutrition medium, and the dendrobium officinale is planted after the tree is planted for 3 to 5 days, wherein the used pulpous medium is prepared from dimethomorph, manganese zinc myclobutanil, ivy extracts, gibberellins, andrographolide, polyvinyl acetate emulsion, sorbitol, potassium nitrate, ammonium nitro-humate and monocalcium phosphate. The tree is treated in advance, so that the varieties of the selectable trees can be increased; the dendrobium officinale domesticated seedlings are soaked with planting fixing nutritional liquid before planting fixing and then is irrigated after planting fixing, so that the root system of the domesticated seedlings is developed quickly, plant diseases and insect pests are few, and the survival rate is above 98 percent.
Owner:陈勇
Use of andrographolides
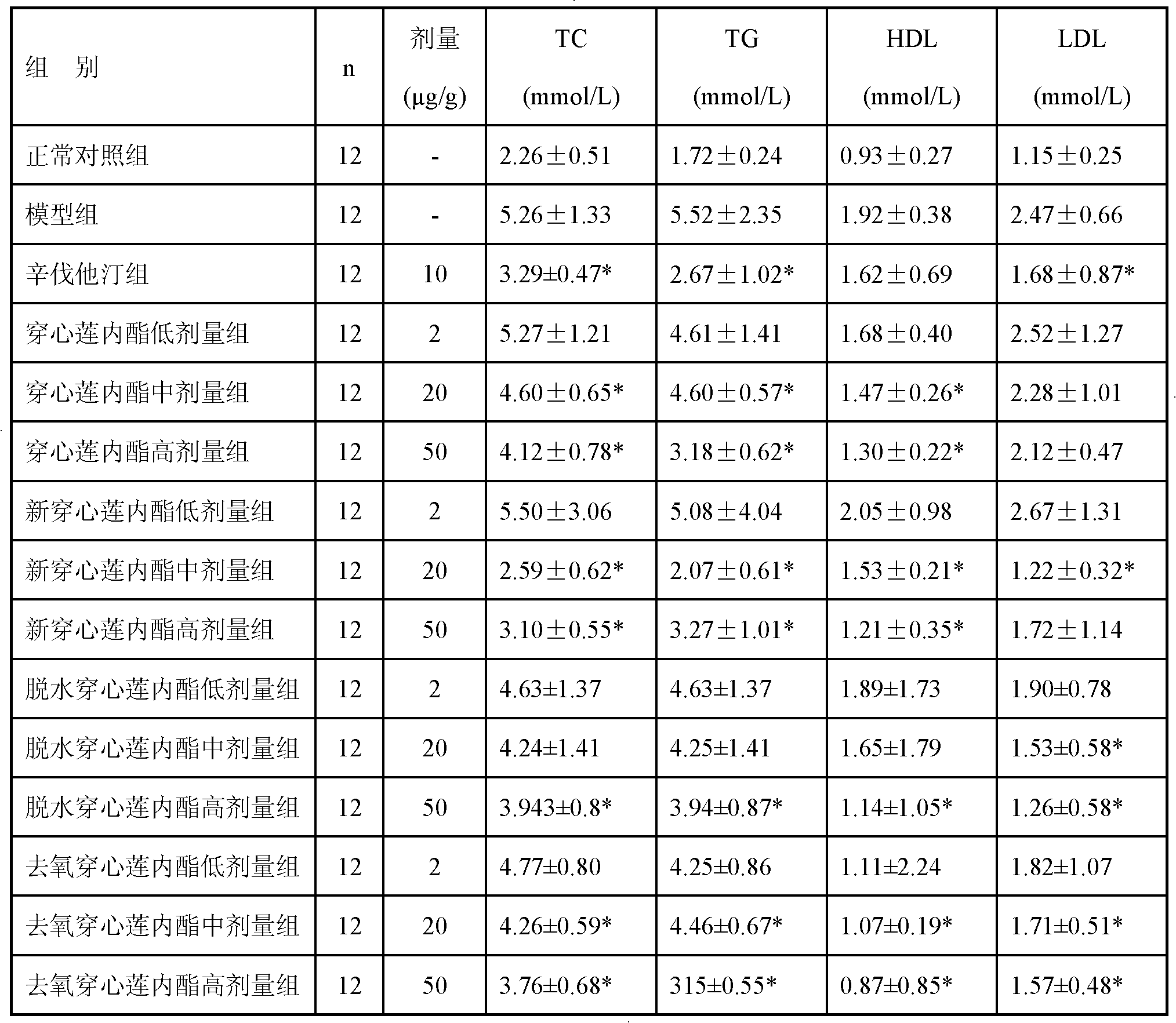
The invention discloses the use of andrographolides, dehydrated andrographolides, new andrographolides and deoxidized andrographolides, which refers to the use of at least one of the andrographolides, the dehydrated andrographolides, the new andrographolides and the deoxidized andrographolides as a unique active component in preparation of medicinal preparations for preventing and treating hyperlipidaemia. In the invention, a new medical value of andrographolide compounds is developed.
Owner:SHANGHAI UNIV OF T C M
Andrographis paniculata extract and method for preparing same
InactiveCN103006724AHigh yieldAvoid side effectsAntibacterial agentsMagnoliophyta medical ingredientsMedicinal herbsActivated carbon
The invention discloses an andrographis paniculata extract and a method for preparing the same. The andrographis paniculata extract comprises 32.3- 40.5% by mass of andrographolide and 2.23-5.52% by mass of dehydroandrographolide. The method comprises the following steps: grinding the andrographis paniculata by adopting the ball milling technology, extracting by ethanol, filtrating the obtained extract by a micro-filtration memebrane, purifying by activated carbon fiber, concentrating by a nanofiltration membrane, eluting by a macroporous resin, spraying and drying to obtain the andrographis paniculata extract. The active components such as the andrographolide in the andrographis paniculata which is a medicinal herb are kept to the maximum extent, the impurities such as the pigment in the andrographis paniculata are removed, and the strong anti-bacterial and anti-inflammatory activities of the andrographis paniculata are retained. The method of preparing the andrographis paniculata extract is easy to operate, omits the step of heating, consumes the least energy for preparation and is suitable for industrial mass production.
Owner:GUANGDONG PHARMA UNIV
Nanometer emulsion oral liquid of andrographolide and its prepn process
InactiveCN1931130AHeat-clearing and detoxifyingWith cooling blood and detumescenceAntibacterial agentsOrganic active ingredientsDistilled waterOil phase
The nanometer emulsion oral liquid of andrographolide consists of surfactant, oil, andrographolide and distilled water. Its preparation process includes the following steps:weighing surfactant with or without co-surfactant; calculating HLB value and selecting oil for reaching emulsifying HLB value near that of the surfactant phase; changing the ratio between the surfactant phase and the oil phase regularly to dissolve andrographolide in the surfactant; adding distilled water slowly at 20-25 deg.c to form clear and flowing O / W type stable nanometer emulsion oral liquid. The nanometer emulsion oral liquid has high dissolubility of andrographolide, raised bioavailability of andrographolide, less stimulation to gastrointestinal tract and raised patient's compliance, and may be also taken easily by children and persons with dysphagia.
Owner:NORTHWEST A & F UNIV
Dripping pills of andrographolide and its preparing method
The invention discloses dripping pills of andrographolide and its preparing method, wherein the andrographolide is filed by right amount of findings to obtain drop pills. The invention also discloses the pharmaceutical actions of the traditional Chinese medicine.
Owner:TIANJIN TASLY PHARMA CO LTD
Andrographolide synthetic method
The invention belongs to the field of medicaments, in particular relates to an andrographolide synthetic method and aims at providing a synthetic method for improving the synthesis yield of the andrographolide. The andrographolide synthetic method comprises the two following steps of: esterification reaction and satifying reaction, wherein in the esterification reaction, dehydration paniculta lactone succinate half ester is prepared; and in the satifying reaction, dehydration paniculta lactone succinate half ester potassium sodium salt, namely andrographolide, is prepared. The andrographolide synthetic method comprises a first step of esterification reaction as follows: raising the temperature of andrographolide, succinic anhydride, pyridine and anhydrous sodium sulfite to react at a pressure reduction vacuum state; dissolving in a warm heat water; and crystallizing at a low temperature to obtain the dehydration paniculta lactone succinate half ester; and a step of satifying reaction as follows: dissolving the dehydration paniculta lactone succinate half ester into an ethanol solution with the concentration of more than or equal to 95 percent by volume; adding a kali salt solution to react to obtain the dehydration paniculta lactone succinate half ester potassium sodium salt; dissolving the dehydration paniculta lactone succinate half ester potassium sodium salt into absolute ethyl alcohol and adding a sodium salt solution to react, filter and wash to obtain the andrographolide.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Cleaning agent for oral cavity and preparation method thereof
InactiveCN102058502AEnhance phagocytosisEnhance immune functionCosmetic preparationsToilet preparationsMenthyl anthranilateCleansing Agents
The invention relates to a cleaning agent for an oral cavity, mainly comprising the following ingredients in percentage by weight: 0.1-30 percent of green tea, 0.1-50 percent of turmeric, 0.01-10 percent of menthyl anthranilate, and 0.01-10 percent of sodium cyclamate and acesulfame. According to different conditions of patients, 0.1-50 percent of scutellaria, 0.1-50 percent of camellia, 0.1-30 percent of andrographolide and 0.01-10 percent of borneol can also be added. Due to adoption of the cleaning agent for the oral cavity, effective ingredients or / and extracts are taken into the oral cavity in a manner of directly gargling in the mouth without using a manner of gargling again with clean water after gargling, and the purposes of inhibiting bacteria, diminishing inflammation, cleaning the oral cavity and preventing decayed teeth are achieved.
Owner:TIANJIN BIJIA PHARMA CO LTD
Method for induding andrographolide by cyclodextrin and medicinal preparation
InactiveCN1437939ADoes not change chemical propertiesGood water solubilityOrganic active ingredientsAntipyreticCyclodextrinUltrasonic generator
The present invention discloses a method for including andrographolide by using cyclodextrin and its medicinal preparation. The andrographolide is an importent active extract of medicinal plant andrographis. Its inclusion method includes: adding 10-60g of cyclodextrin or its derivative into distilled water and dissolving it; adding 6g of andrographolide extract into 5-10 ml of medicinal methyl alcohol, heating and dissolving it; pouring the cyclodextrin aqueous solution into andrographolide ethyl alcohol solution; ultrasonic vibrating mixed solution in ultrasonic generator for 20-30 min., adopting thin-layer chromatography to identify the cyclodextrin-andrographolide inclusion quality. It does not change chemical property and effective activity of the andrographolide, can be prepared intovarious dosage forms.
Owner:侯文阁
Preparation method of andrographolide
The invention relates to the field of pharmaceutical chemistry, in particular to a preparation method of andrographolide. Andrographolidume and succinic anhydride serve as raw materials under protection of inert gas, in an aprotic solvent, aliphatic series micromolecule amine serves as a catalytic agent, an ester forming reaction is carried out to prepare dehydroandrograpolide succinate, dehydroandrograpolide succinate is subjected to salt forming reaction with an alkaline compound of sodium and an alkaline compound of potassium to prepare andrographolide, and the aprotic solvent is selected from halogenate hydrocarbon, esters, ethers and cyclic ether or a ketone or nitrile compound containing 1-4 carbon atoms. According to the invention, the low-toxicity aprotic solvent is used for replacing high-toxicity bad-smell pyridine to serve as a solvent, and simultaneously, the low-boiling-point low-toxicity aliphatic series micromolecule amine is used to replace pyridine to serve as the reacted catalyst, so that pyridine residues of finished products can be avoided, product purity and productivity are improved, and simultaneously, the pressure of environmental protection is lightened.
Owner:CHONGQING LUMMY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com