New isoandrographolidume sulfonate, pharmaceutical composition containing sulfonate, preparation method and applications thereof
A technology of andrographolide and composition is applied to the new isoandrographolide sulfonated compound, the pharmaceutical composition containing the sulfonated compound and the fields of preparation and use thereof, which can solve the problem of affecting the clinical curative effect of the product, difficult to formulate product quality standards, etc. problem, to achieve the effect of simple composition, controllable quality and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
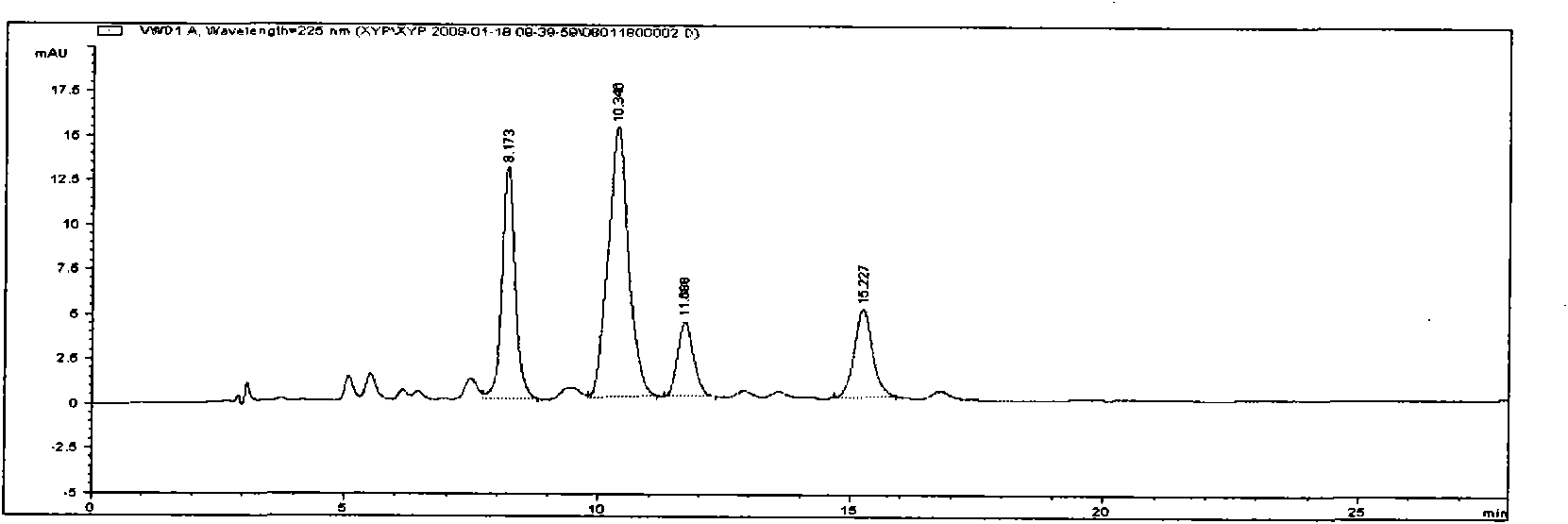
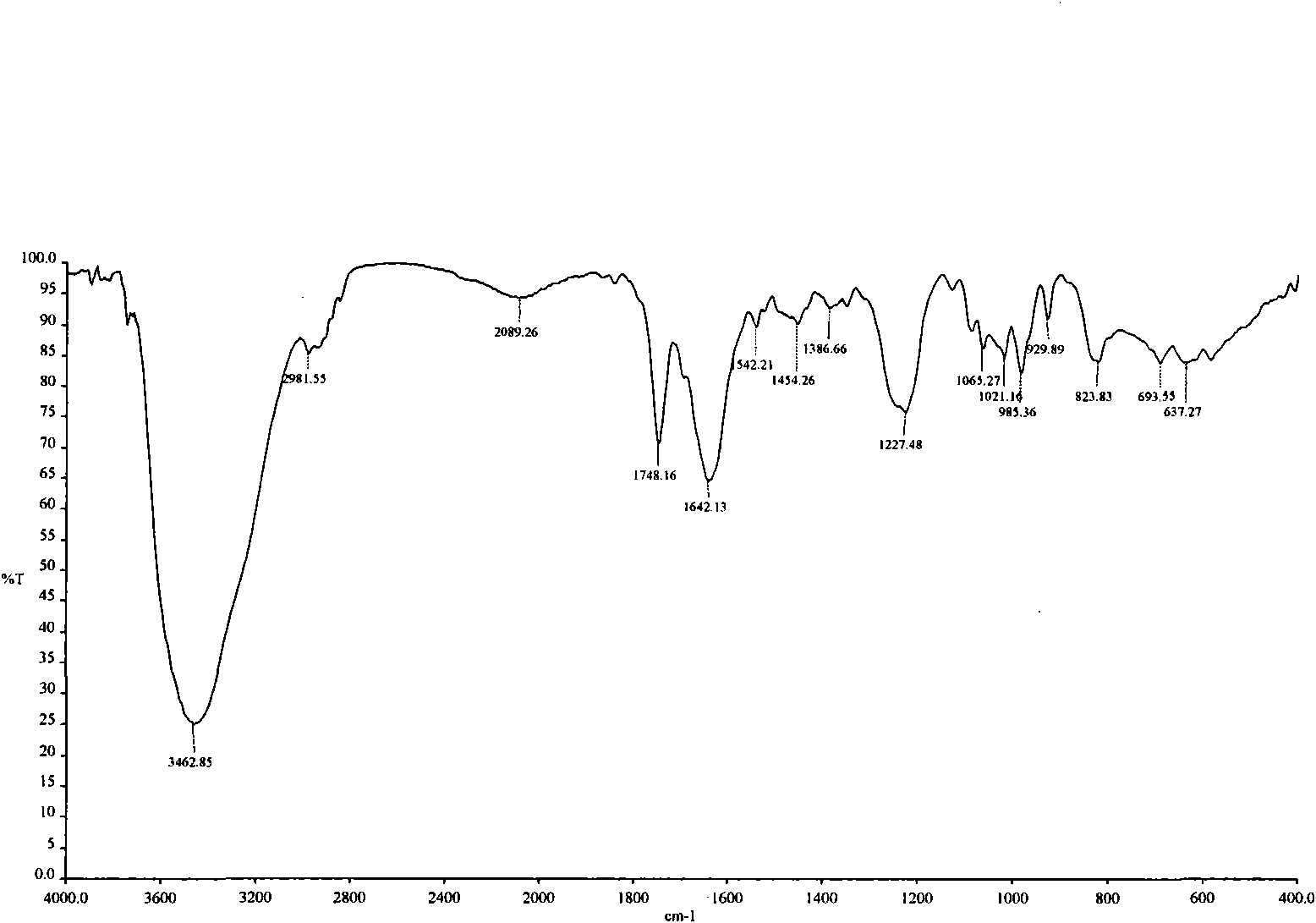
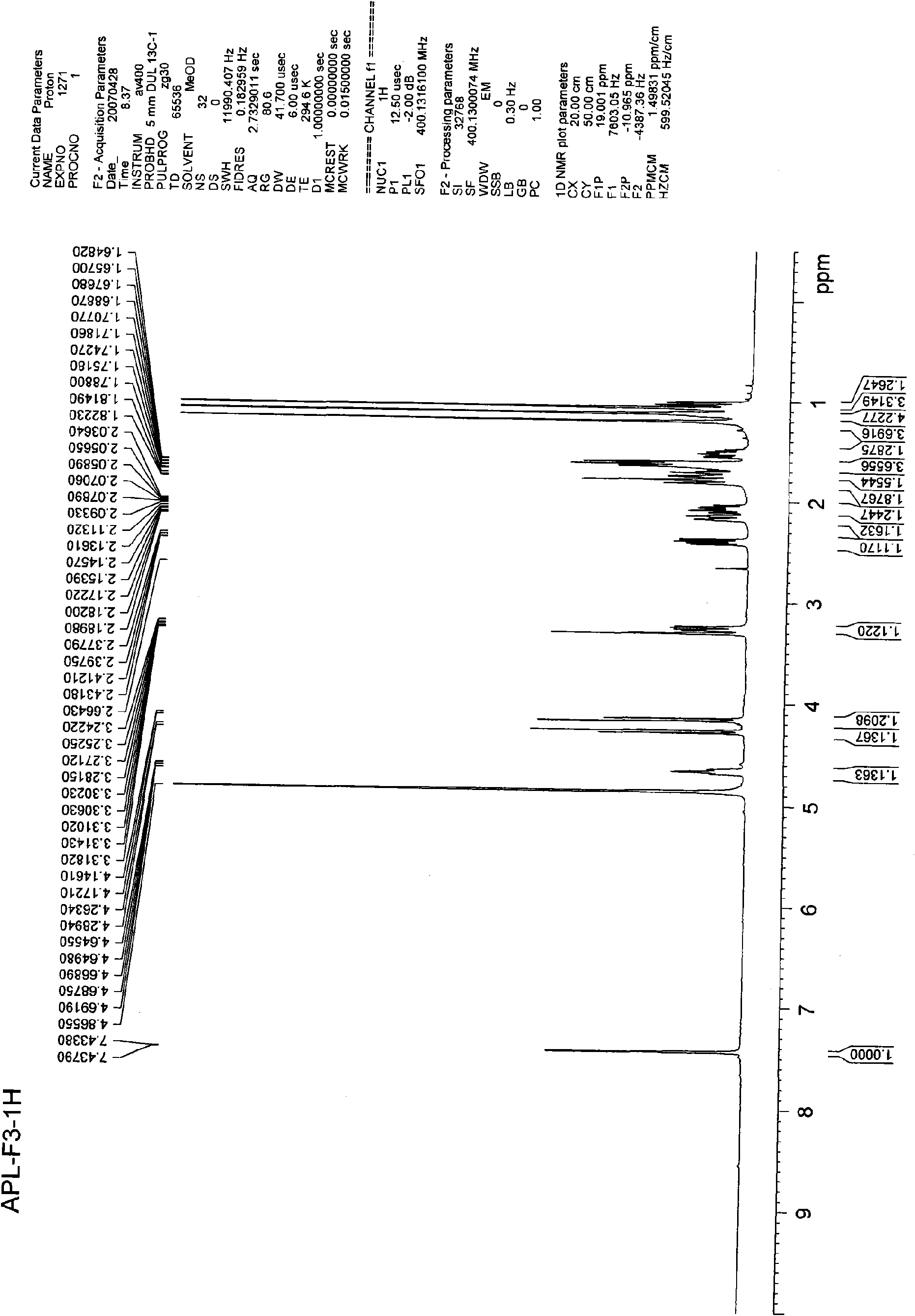
[0037] This example discloses the preparation method of isoandrographolide and a composition containing isoandrographolide-19-sulfate, andrographolide sulfonate E, andrographolide, and andrographolide
[0038] (1) Preparation of total sulfonated andrographolide (refer to Chinese patent, patent authorization number CN1162420C):
[0039] Take 1000mL of absolute ethanol and place it in a reactor, slowly add 1000mL of concentrated sulfuric acid dropwise, stir well, add 1000g of andrographolide, stir well, and place it at room temperature for 72h. Add 1000mL of ethanol in a water bath, stir well, adjust the pH to 7.0, add ethanol until the alcohol content reaches 80%, let it stand for 24 hours, add a small amount of activated carbon, filter, recover ethanol from the filtrate, condense into a thick paste, and vacuum dry to obtain Andrographis paniculata Total sulfonates of lactones.
[0040] (2) The total sulfonates of andrographolide are separated by column chromatography to prepa...
Embodiment 2
[0066] Isoandrographolide-19-sulfate was prepared according to the method in Example 1, weighed 1g, dissolved in about 800mL of glucose isotonic solution, added 0.1% activated carbon and stirred for 10 minutes, filtered, added glucose isotonic solution to 1000mL, filtered , use 10% sodium hydroxide solution to adjust the pH value to 4.5-6.5, potting (injection solution with large capacity, specifications are 50mL / bottle, 100mL / bottle, 250mL / bottle, 500mL / bottle), sterilized at 100°C for 30 minutes , after the clarity inspection, the package is ready.
Embodiment 3
[0068] The composition is prepared according to Example 1. Weigh 25g of the composition, add 600mL of water for injection, stir to dissolve, add 25g of mannitol, stir to dissolve, add water for injection to 1000mL, mix well, and filter with a 0.22μm microporous membrane to obtain The solution was divided into 7mL vials, 2mL per vial, capped, put into a lyophilizer, and lyophilized according to the designed lyophilization curve to obtain a composition lyophilized powder injection (50 mg each).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com