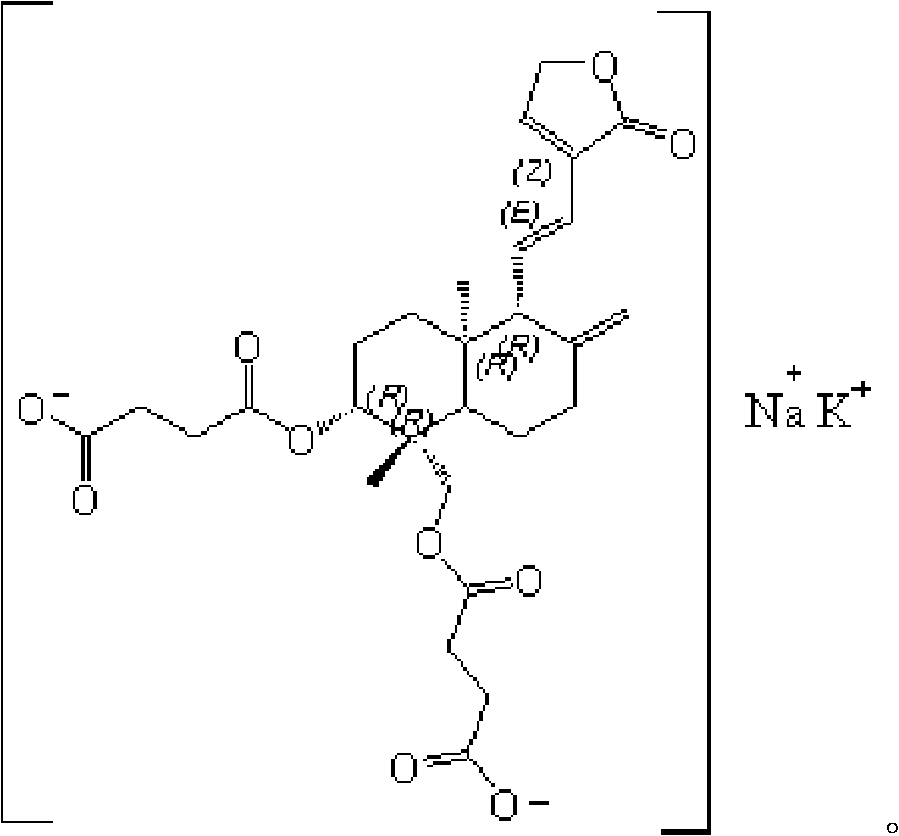
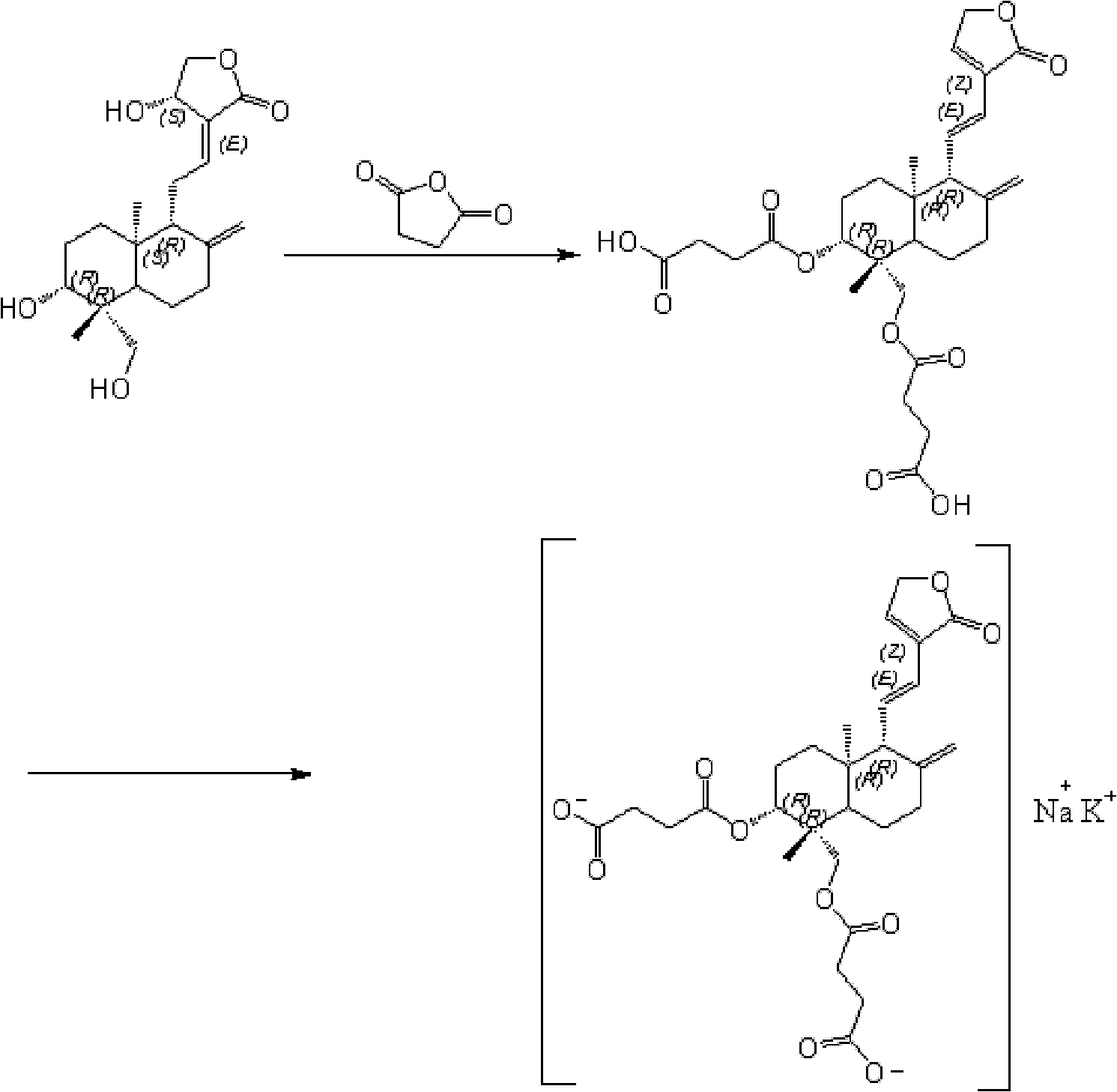
Preparation method of andrographolide
A technology of Yanshuning and succinic acid, applied in the field of preparation of Yanshuning, can solve the problems of high pyridine residue, low product purity, product hydrolysis, etc., and achieves the advantages of improving product purity, avoiding pyridine residue, and reducing reaction temperature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The embodiment of the invention discloses a preparation method of Yanhuning. Those skilled in the art can refer to the content of this article to appropriately improve the process parameters to achieve. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method of the present invention has been described through preferred embodiments, and relevant personnel can obviously make changes or appropriate changes and combinations to the method described herein without departing from the content, spirit and scope of the present invention to realize and apply the technology of the present invention .
[0035] For realizing the purpose of the present invention, the present invention adopts following technical scheme:
[0036] A preparation method of Yanhuning, comprising:
[0037] Step 1. Under the protection of inert gas, use an...
Embodiment 1
[0042] Add andrographolide (70g), succinic anhydride (105g), triethylamine (84g) and dioxane (350mL) into the reaction flask, seal it and start stirring. After vacuuming, argon gas was introduced to replace the air in the system, so that the system was under the protection of argon gas. Slowly heat up to 60±2°C with an oil bath, and after 7 hours of temperature-controlled reaction, cool down to 50±2°C, distill out the solvent dioxane and the catalyst triethylamine under reduced pressure. After completion, add 350mL 85% ethanol to dissolve and dilute. After adding activated carbon for decolorization and filtration, the filtrate was slowly added dropwise to 130mL potassium bicarbonate-sodium carbonate solution (sodium ion concentration: 2mol / L) using a constant pressure dropping funnel, and the temperature of the reaction was controlled at 30°C. After the addition, continue to stir 2h. 920 mL of acetone was added thereto for crystallization. Filter with suction, wash the filt...
Embodiment 2
[0044] Add andrographolide (100g), succinic anhydride (200g), triethylamine (150g) and ethyl acetate (1000mL) into the reaction flask, seal it and start stirring. After vacuuming, nitrogen gas was introduced to replace the air in the system, so that the system was under the protection of nitrogen gas. Slowly heat up to 80±2°C with an oil bath, and after a temperature-controlled reaction for 5 hours, lower the temperature to 40±2°C, distill off the solvent ethyl acetate and the catalyst triethylamine under reduced pressure. After completion, add 500mL 90% ethanol to dissolve and dilute. After adding activated carbon for decolorization and filtration, the filtrate was slowly added dropwise to 228mL potassium bicarbonate-sodium bicarbonate solution (sodium ion concentration is 1.5mol / L) with a constant pressure dropping funnel, and the temperature of the reaction was controlled to be 40°C. After the addition, Stirring was continued for 1 h. 1272 mL of acetone was added thereto ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com