Andrographolide nano crystal intermediate, preparation method and applications thereof
A technology of andrographolide and nanocrystals, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of adding a large amount of carrier materials or toxic solvents, poor stability of liquid preparations, The problem of low bioavailability of the preparation can be solved, and the effect of prolonging the adhesion time and residence time, enhancing the adhesion and improving the bioavailability of the drug can be achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of different average particle size ranges (500nm, 600nm, 700nm, 800nm, 900nm) andrographolide nanocrystal suspension
[0037] Weigh 0.15g of polyvinylpyrrolidone-K30 (stabilizer Ⅰ) and add 100ml of water to dissolve, mix well, then add 0.15g of D-alpha-tocopheryl succinate polyethylene glycol (stabilizer Ⅱ) and mix well, Obtain stabilizer solution; Then, the andrographolide of 0.5g is dispersed in stabilizer solution, shear through high-speed shear emulsification method, and rotating speed is: 13000rpm, and shearing time is 5min; The primary particle diameter that makes is 45 μ m Suspension; then the above primary suspension is subjected to high-pressure homogenization, the homogenization temperature is controlled at 10°C, and nanocrystal suspensions of different particle size ranges are prepared according to the following homogenization process:
[0038] Homogenization process ①: First, homogenize 5 times at 200bar, 400bar, 600bar, 800bar,...
Embodiment 2
[0043] Example 2: Determination of Saturation Solubility of Andrographolide Nanocrystal Suspensions with Different Particle Sizes
[0044] Dissolution was determined by the paddle method, using 900 mL of pH 7.4 phosphate buffer solution as the dissolution medium, and the speed was 100 r min -1 , the temperature of the water bath is 37 ° C, and the average particle diameter prepared in the above example 1 is 10ml each of 500nm, 600nm, 700nm, 800nm, and 900nm, and the suspension is placed in the dissolution cup for 0.5min and 1min. 1.5min, 4min, 6min, 10min, 20min, 1mL of samples were taken after 30min, filtered through a 0.22μm microporous membrane, and determined by HPLC. According to the standard curve of andrographolide, A=29593C(R 2 =0.9994), linear range 0.0456~0.0912mg / ml, calculate its dissolution rate, its content is shown in Table 1, and the dissolution rate and time relationship curve of andrographolide nanocrystal suspension in different particle size ranges are show...
Embodiment 3
[0049] Embodiment 3: the preparation of andrographolide nano crystal intermediate powder
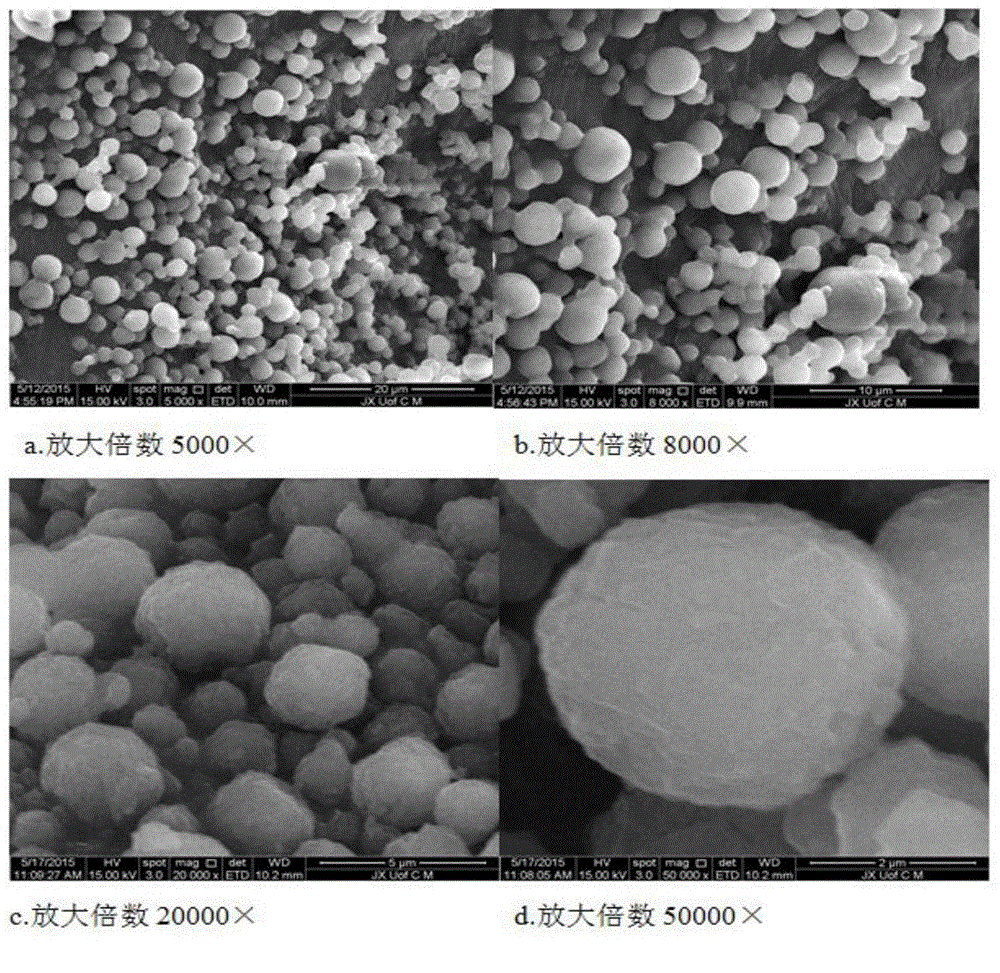
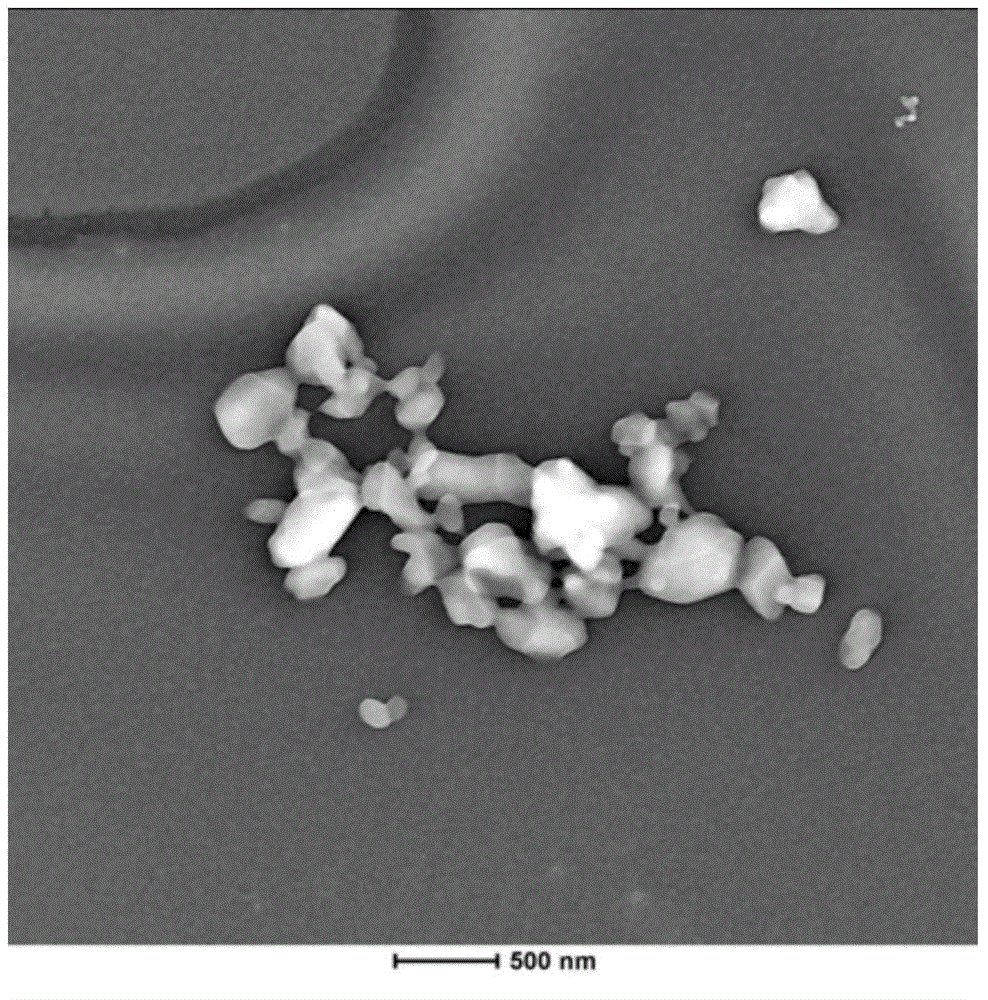
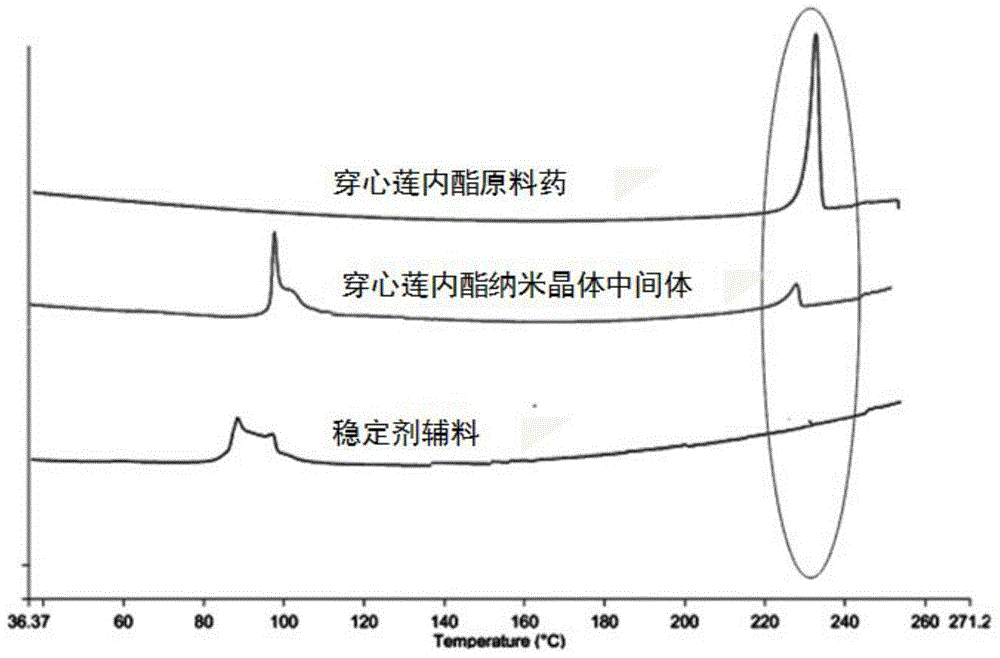
[0050] Experimental method: Get the nanocrystal suspension sample that the average particle diameter that makes according to the experimental method of embodiment 1 is 500nm, add 1.0g lactose (stabilizer III) to disperse and dissolve, then adopt spray drying rapid solidification technology, spray drying hot air temperature The temperature is 120° C., the air outlet temperature is 60° C., and the peristaltic speed of the pump is 5 ml / min. After drying and solidification, the andrographolide nanocrystal intermediate powder is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com