Andrographolide cyclodextrin inclusion compound, preparation method and application thereof
A technology of cyclodextrin inclusion compound and andrographolide, which is applied in the field of medicine, can solve the problems of limiting the application of andrographolide, instability, and inability to take it orally, so as to reduce the possibility of external interference, enhance stability, and improve solubility. Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of solid system
[0058] This example provides the preparation of a physical mixture of andrographolide and cyclodextrin and the preparation of a typical andrographolide-cyclodextrin inclusion compound of the present invention.
[0059] A physical mixture of andrographolide and cyclodextrin was prepared by rolling and mixing andrographolide and cyclodextrin for 15 minutes at a mixing molar ratio of andrographolide and cyclodextrin of 1:1 and 1:2.
[0060] The inclusion compound of andrographolide-cyclodextrin and the inclusion compound of andrographolide-cyclodextrin-polymer were prepared by spray drying. The andrographolide-cyclodextrin binary system was prepared as follows: the drug and the cyclodextrin in a molar ratio of 1:1 or 1:2 were dissolved in 50% ethanol (300ml of 50% ethanol solution contained 250mg of andrographolide), Sonicate the solution for 1h. Andrographolide-cyclodextrin-polymer ternary system was prepared as follows:...
experiment example 2
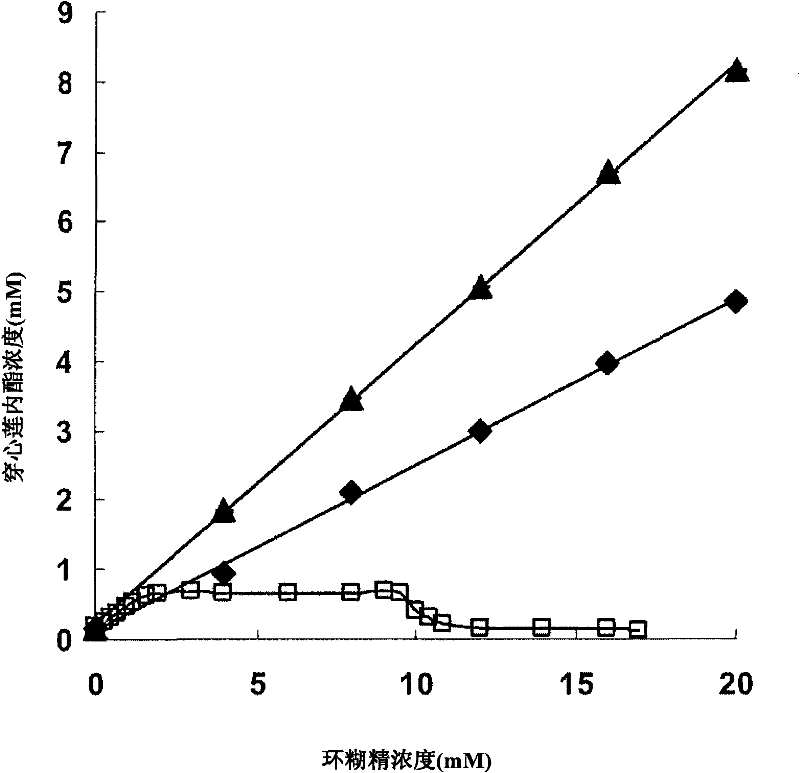
[0062] Experimental Example 2: Phase Solubility Study
[0063] Phase solubility studies were determined according to the method of Higuchi and Connors (T. Higuchi et al., Adv. Anal. Chem. Inst. 4 (1965) 117-121). Excess andrographolide was added to 10 ml of water or an aqueous solution containing various concentrations of the following cyclodextrins: β-cyclodextrin (0 to 16 mM), 2-hydroxypropyl β-cyclodextrin (0 to 20 mM) and Gamma cyclodextrin CD (0 to 20 mM). The suspension was shaken vigorously at 25 °C for 3 days. After reaching equilibrium, the samples were filtered through a 0.45 micron filter and diluted appropriately. The concentration of andrographolide was determined by ultraviolet spectrophotometry at a wavelength of UV 230nm (Hewlett Packard 8425A, Germany). Repeat the test 3 times. The apparent stability constant (K C ):
[0064]
[0065] where S 0 is the water solubility of andrographolide.
[0066] The phase solubility diagram of andrographolide in ...
experiment example 3
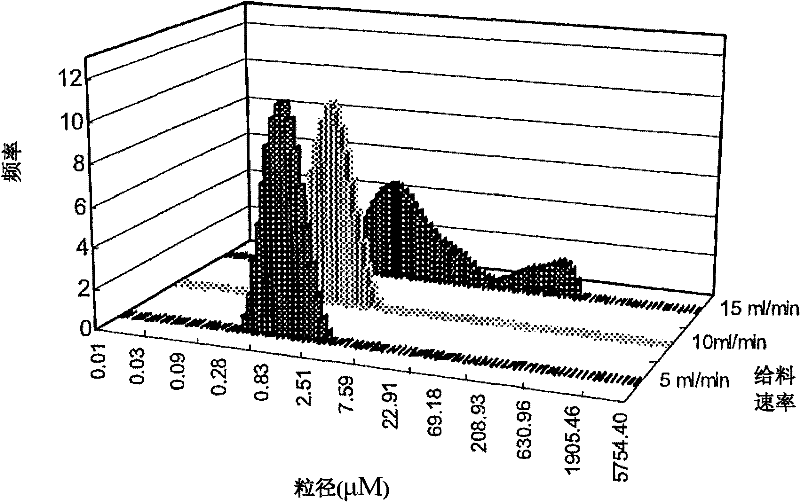
[0067] Experimental Example 3: Particle Size and Particle Size Distribution
[0068] The particle size of the sample powder was measured by a laser diffraction analyzer (MasterSizer 2000, Malvern Instrument, UK) using dry dispersion at a pressure of 3.5 bar. Repeat the measurement 3 times to get the average value. The results are expressed as the volume median diameter d(v; 0.5), where d refers to the diameter at 50% of the entire volume distribution.
[0069] The particle size of the spray-dried samples was determined by laser diffraction. The sample powder is dispersed by compressed air, which is then passed through a focused laser beam. Table 1 shows the particle size distribution of spray-dried granules produced with different andrographolide-CD ratios and under different operating conditions. Spray-dried particles with volume diameters in the range of 0.684-2.504 μΜ were successfully prepared. The results show that the drug-CD molar ratio does not affect the particl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com