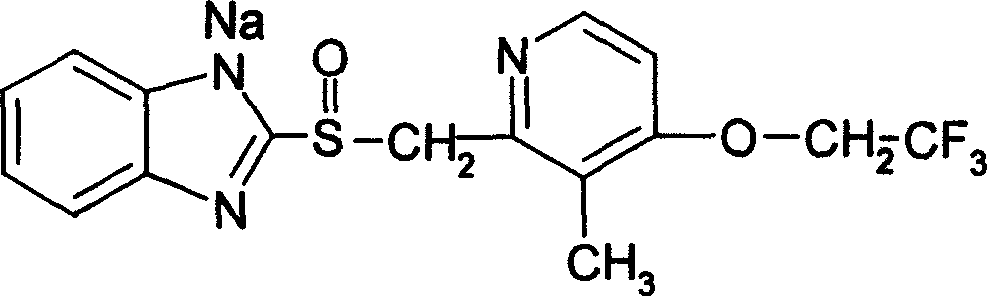
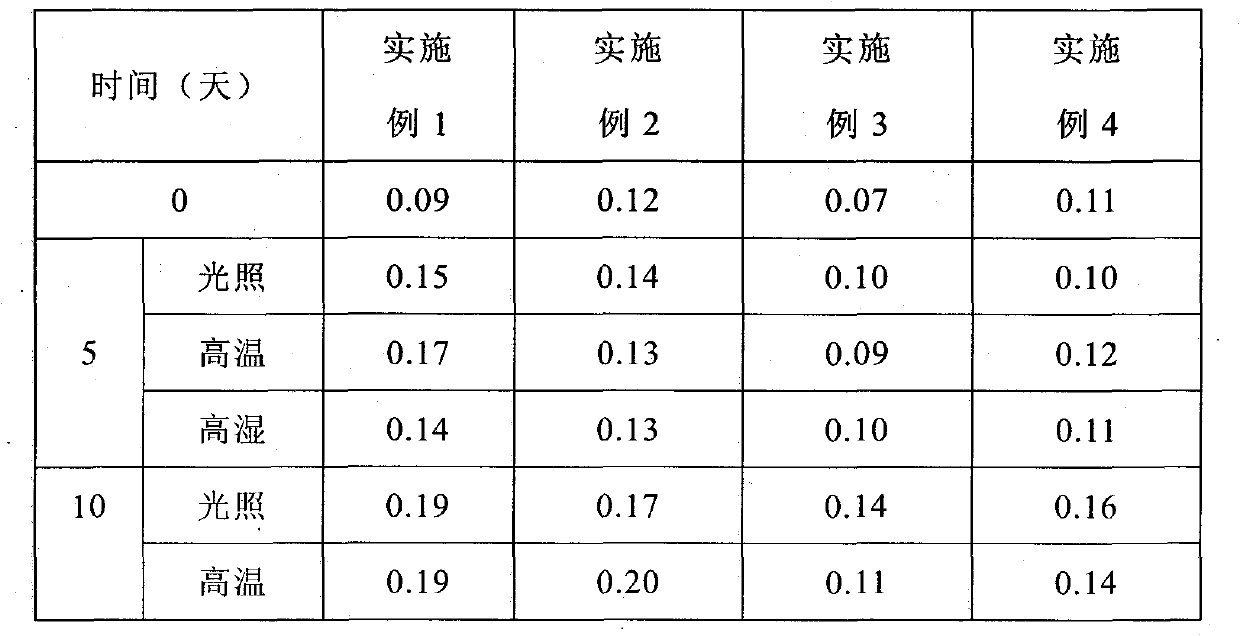
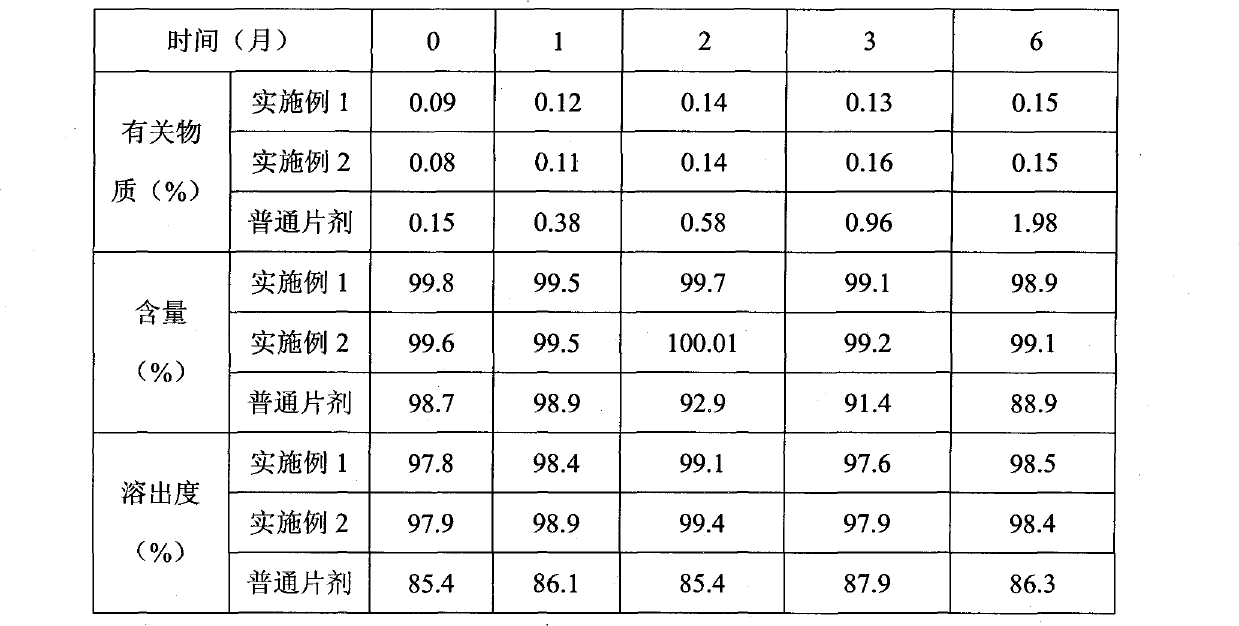
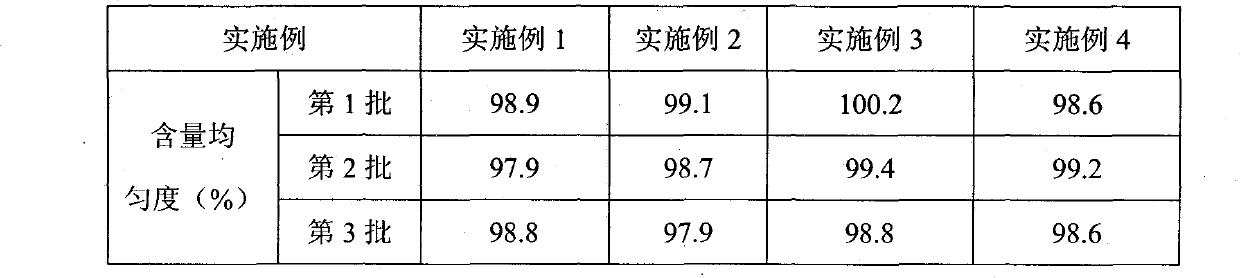
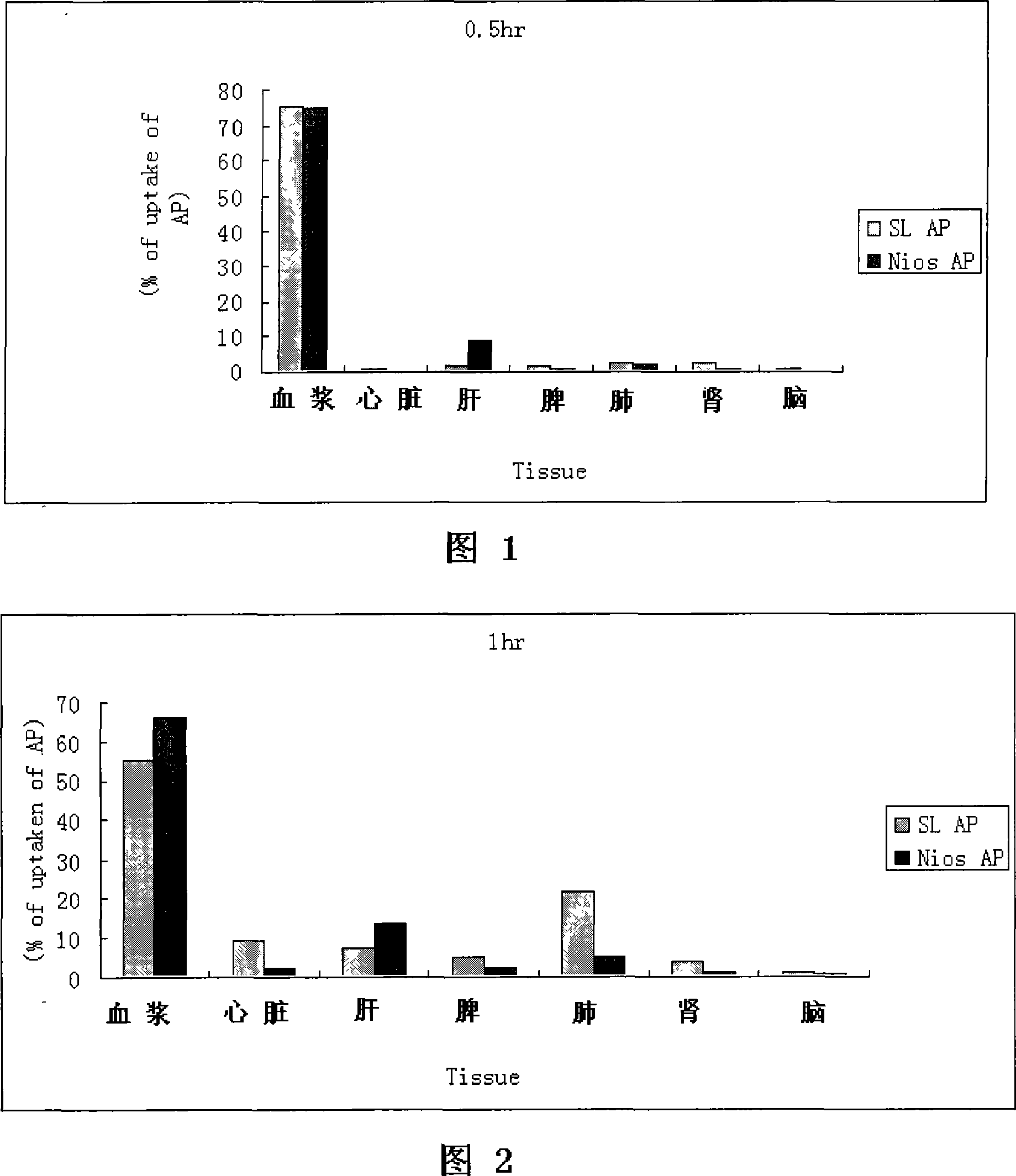
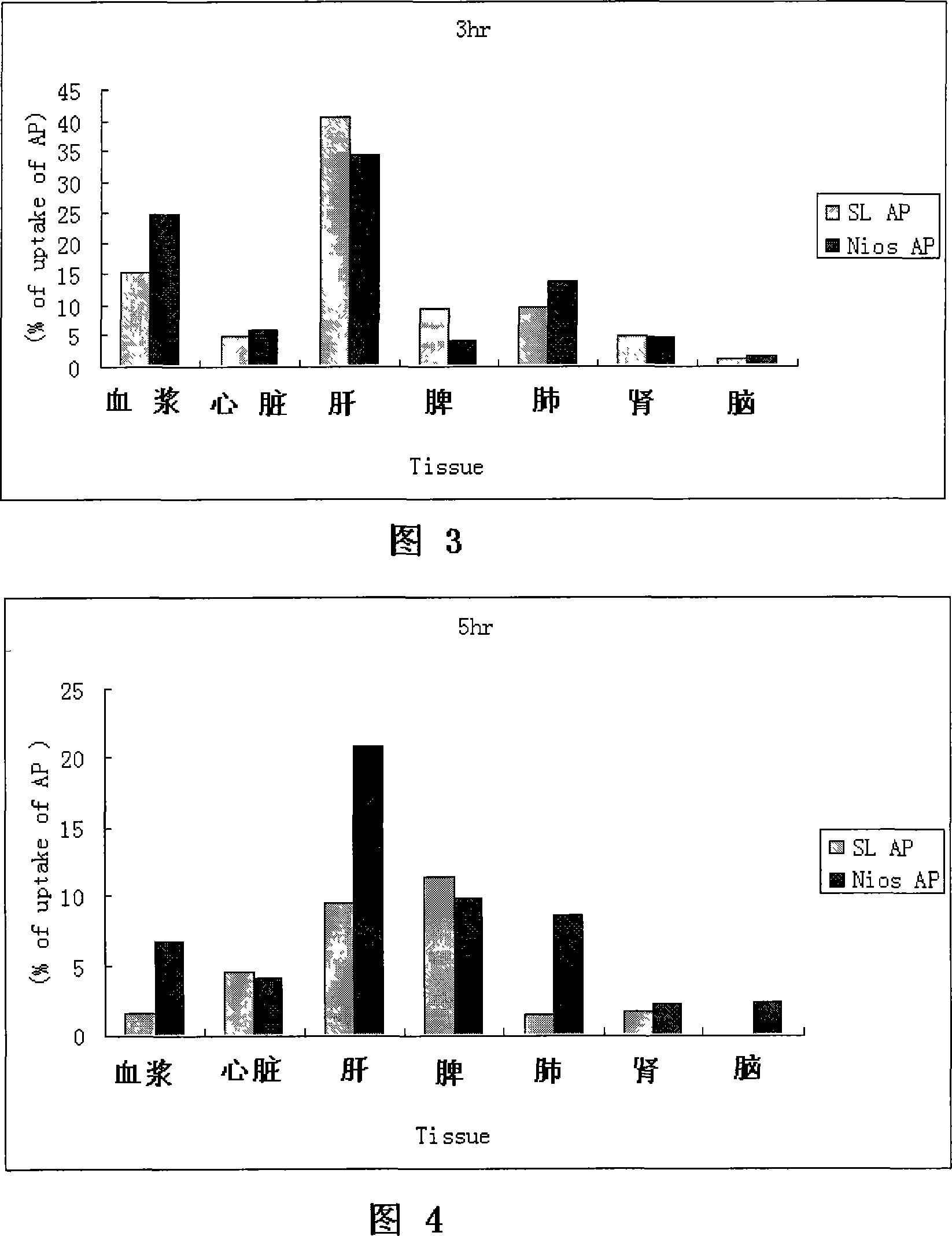
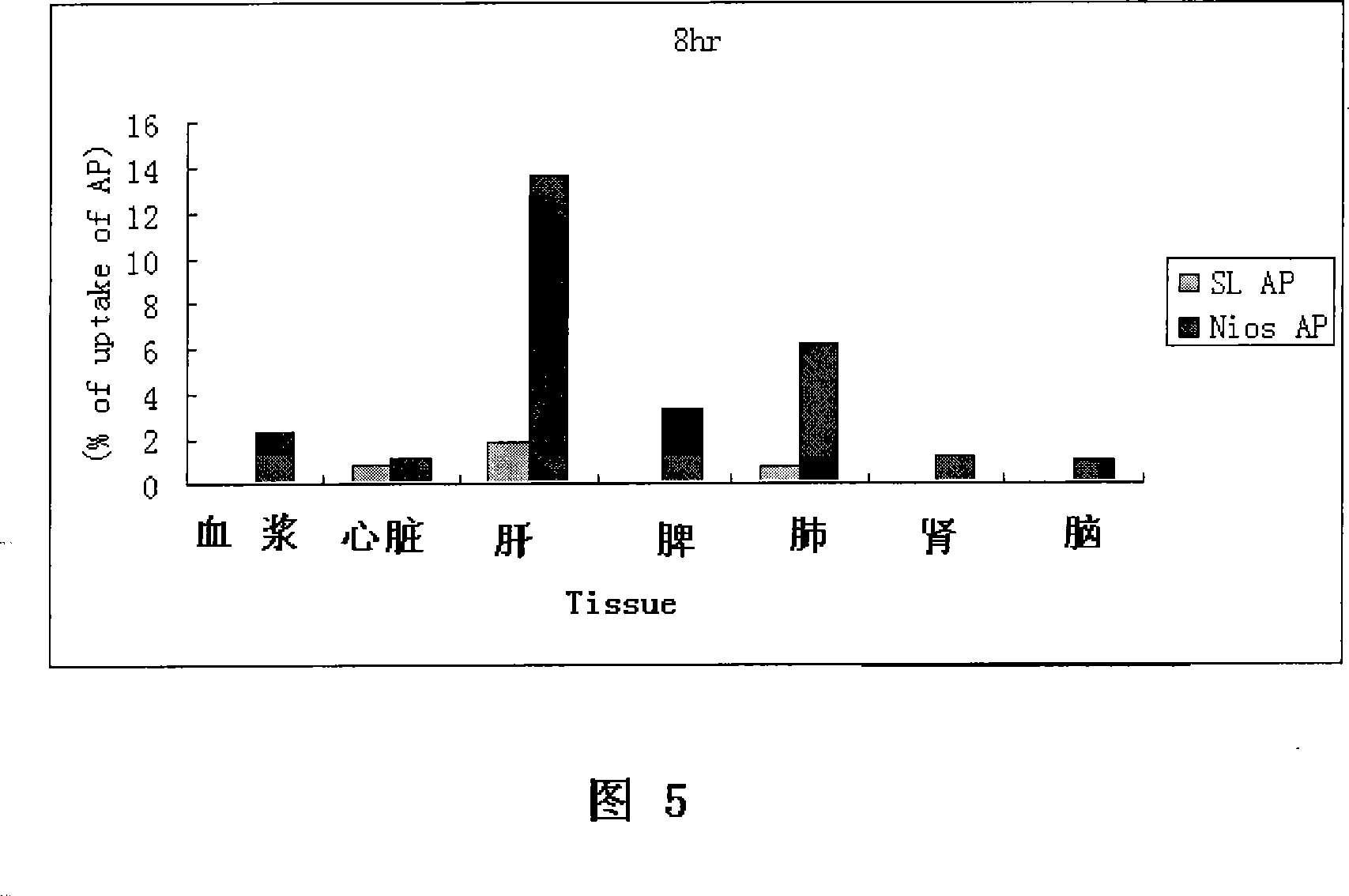
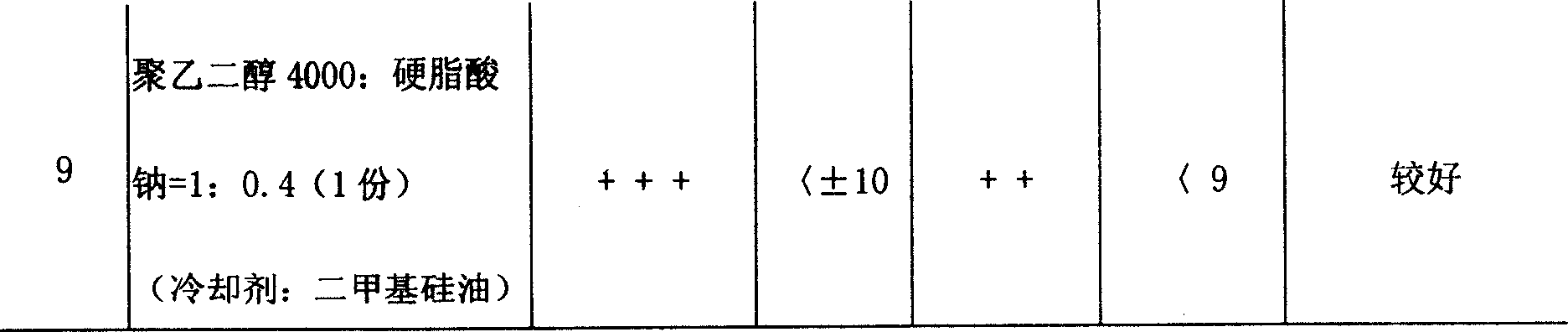Patents
Literature
874 results about "Drugs stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
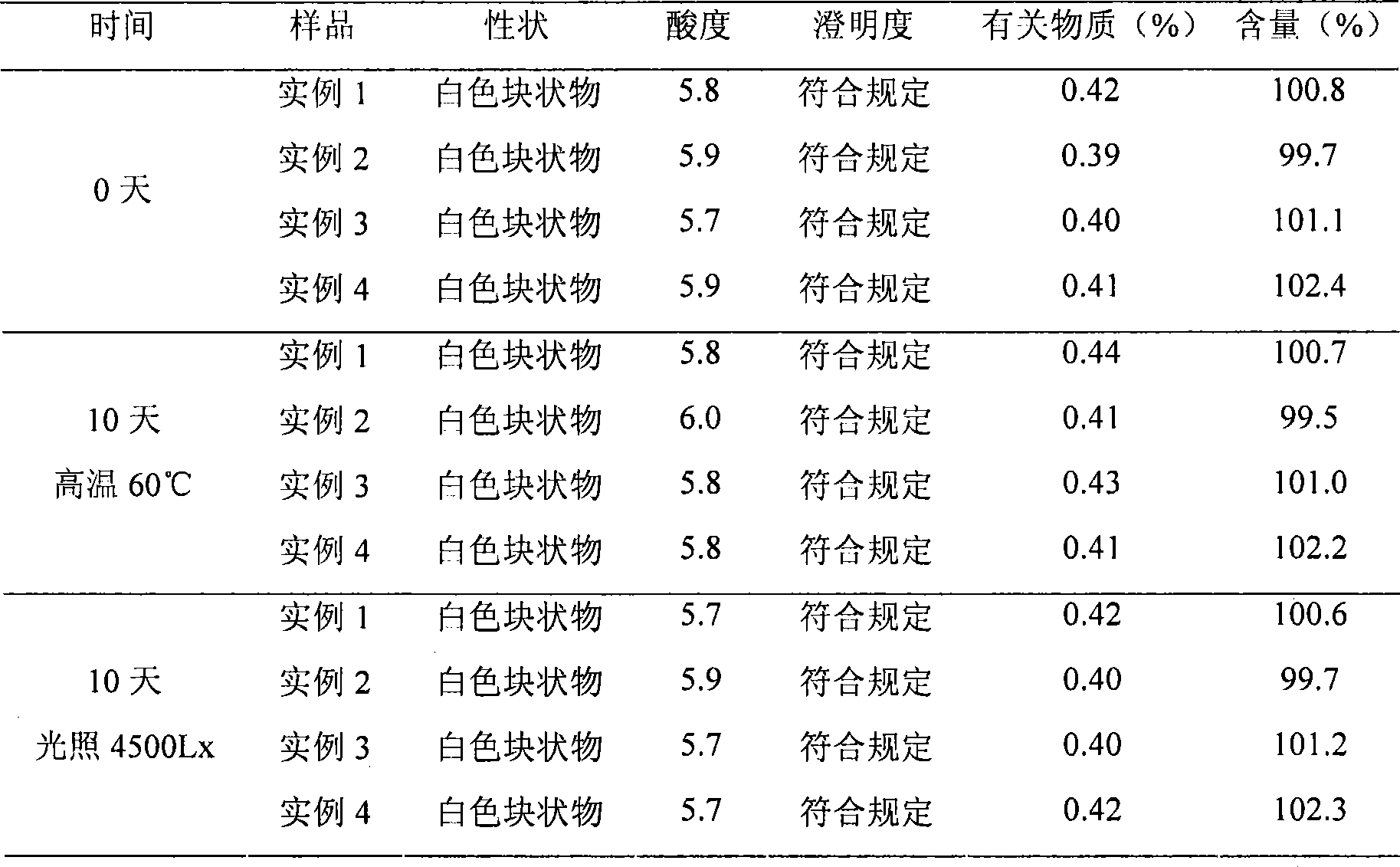
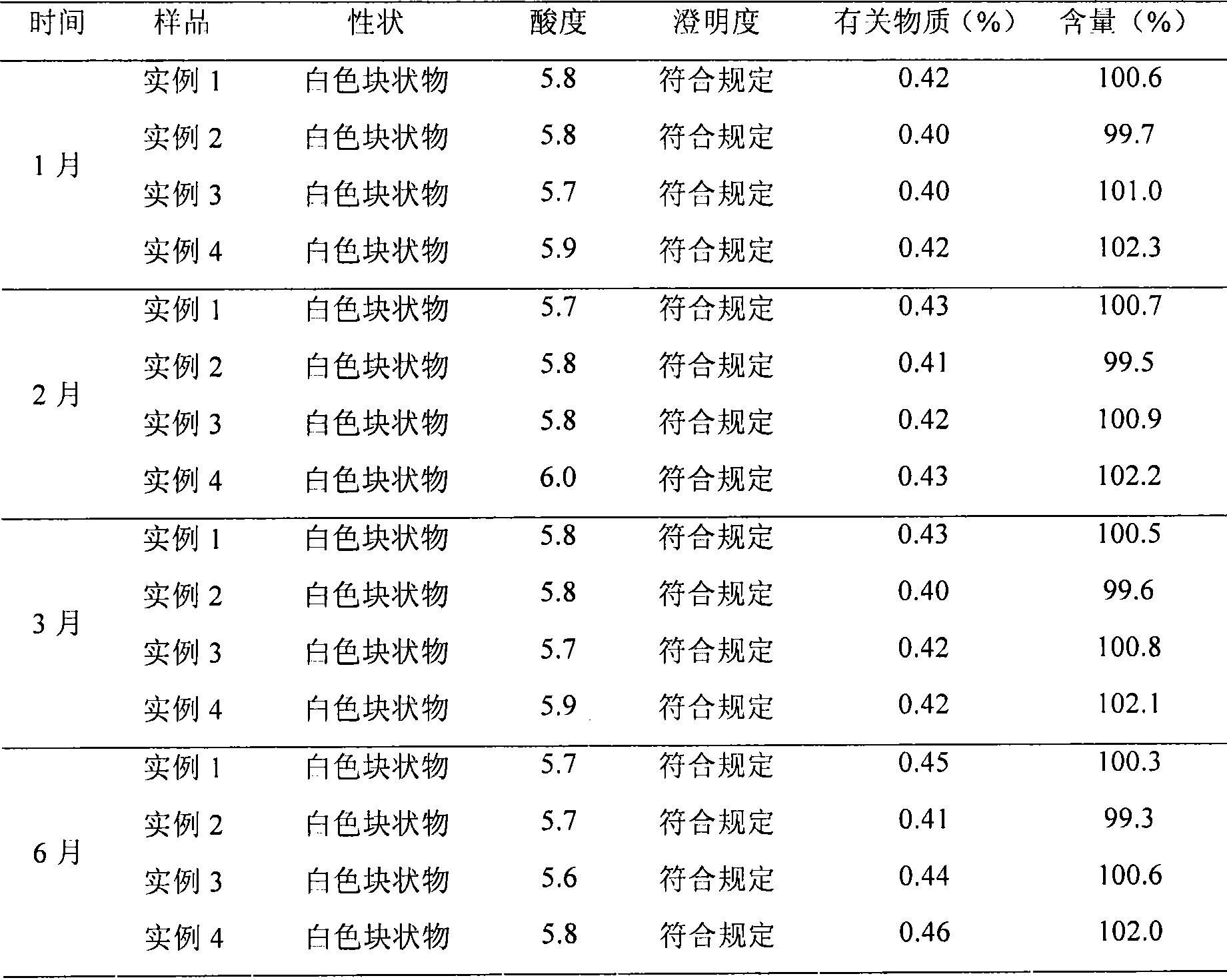
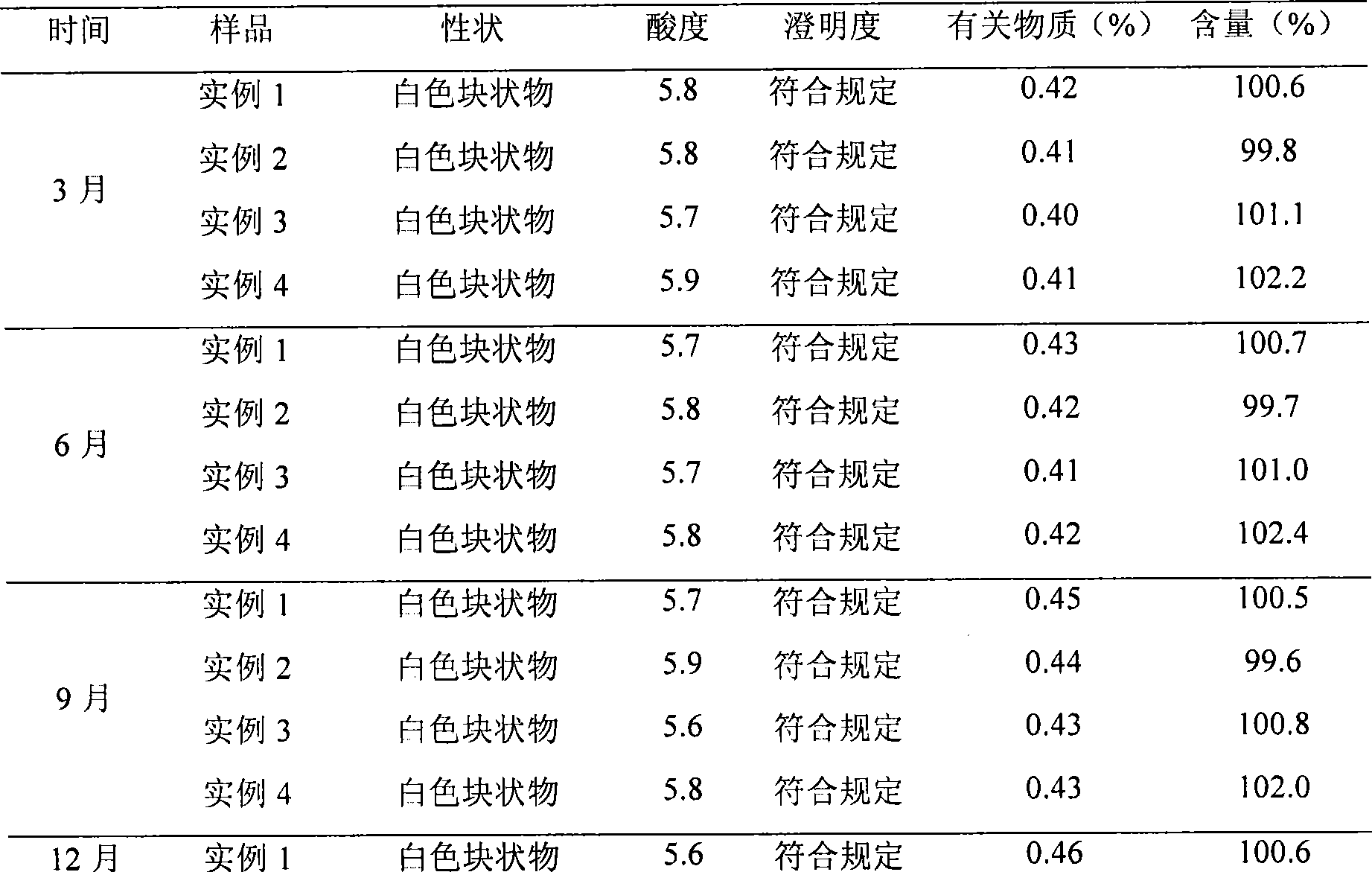
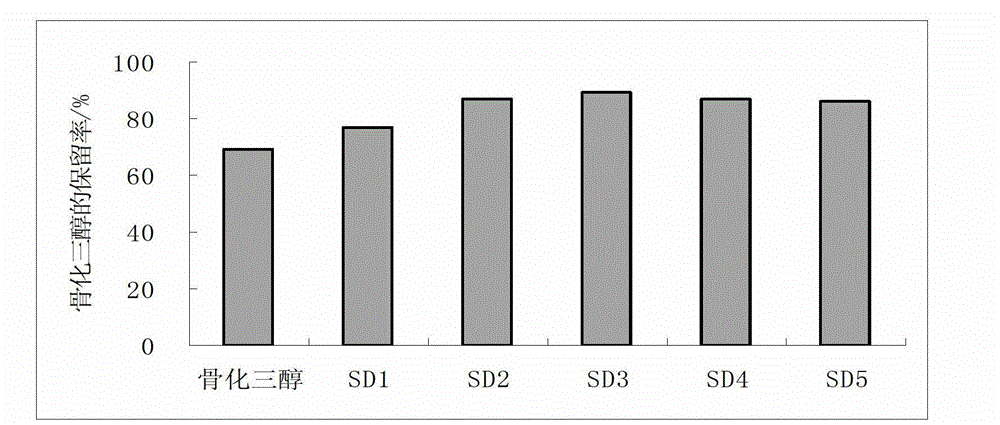
• Definition: Drug stability means the ability of the pharmaceutical dosage form to maintain the. physical, chemical, therapeutic and microbial properties during the time of storage and usage by the. patient.
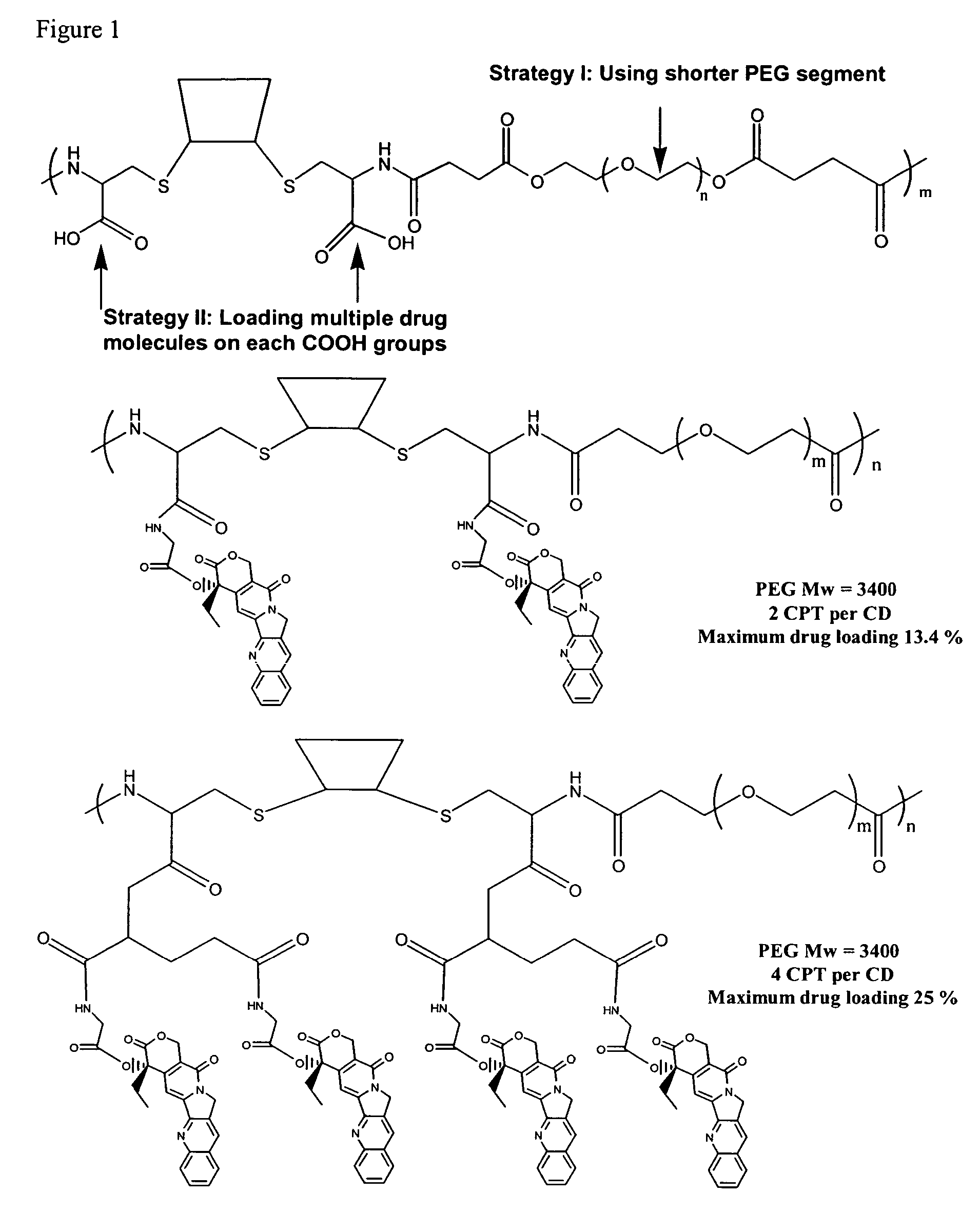
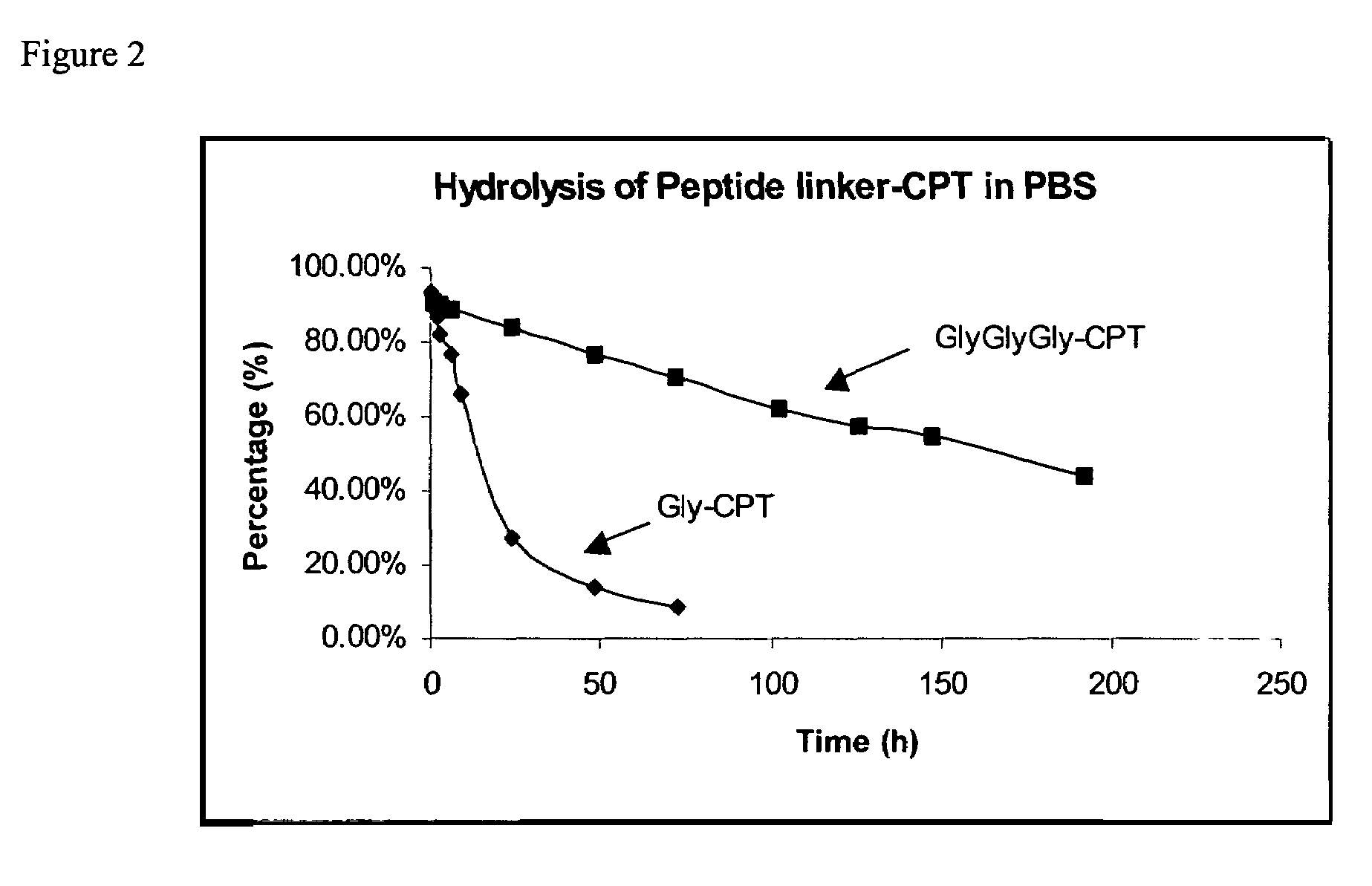
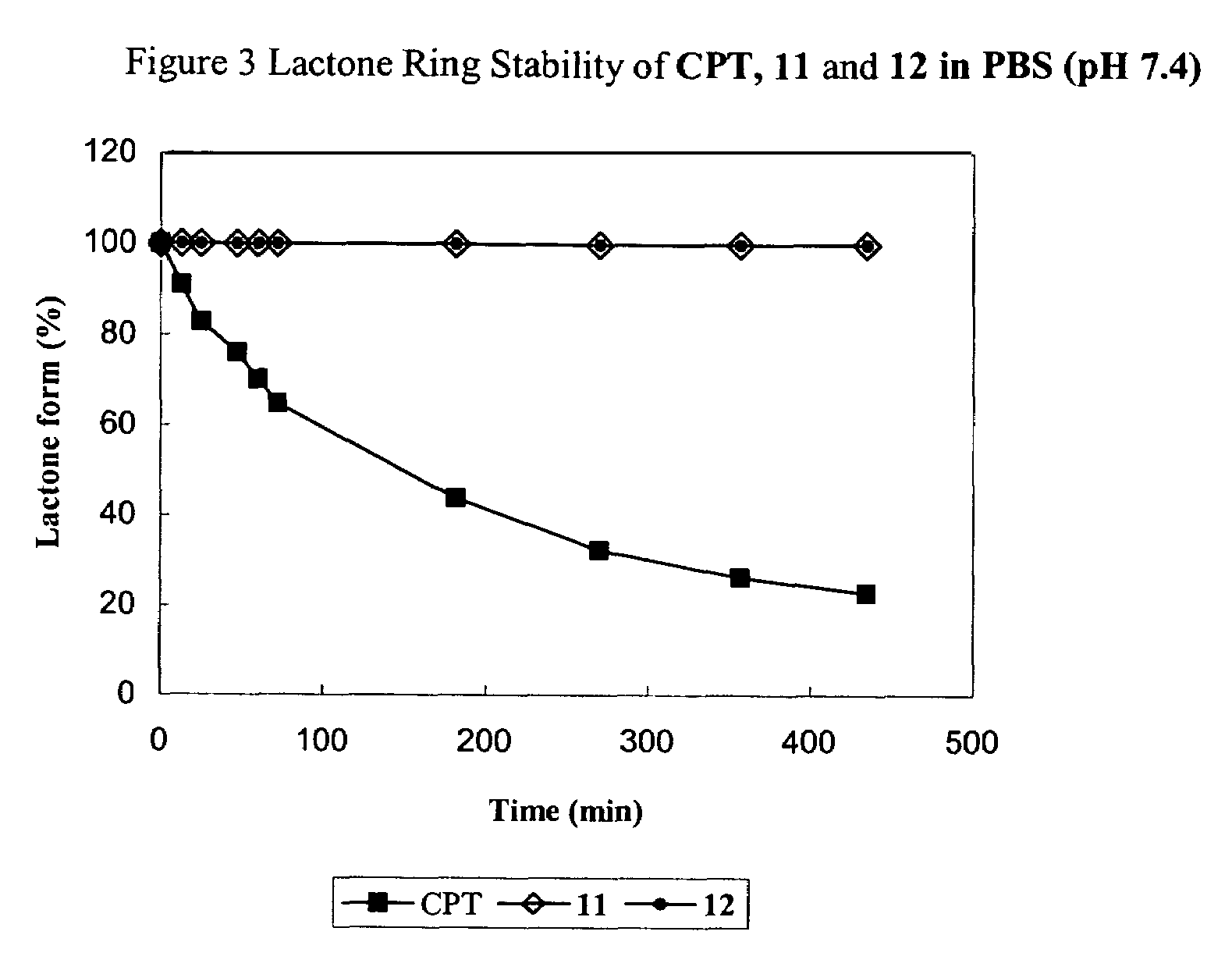
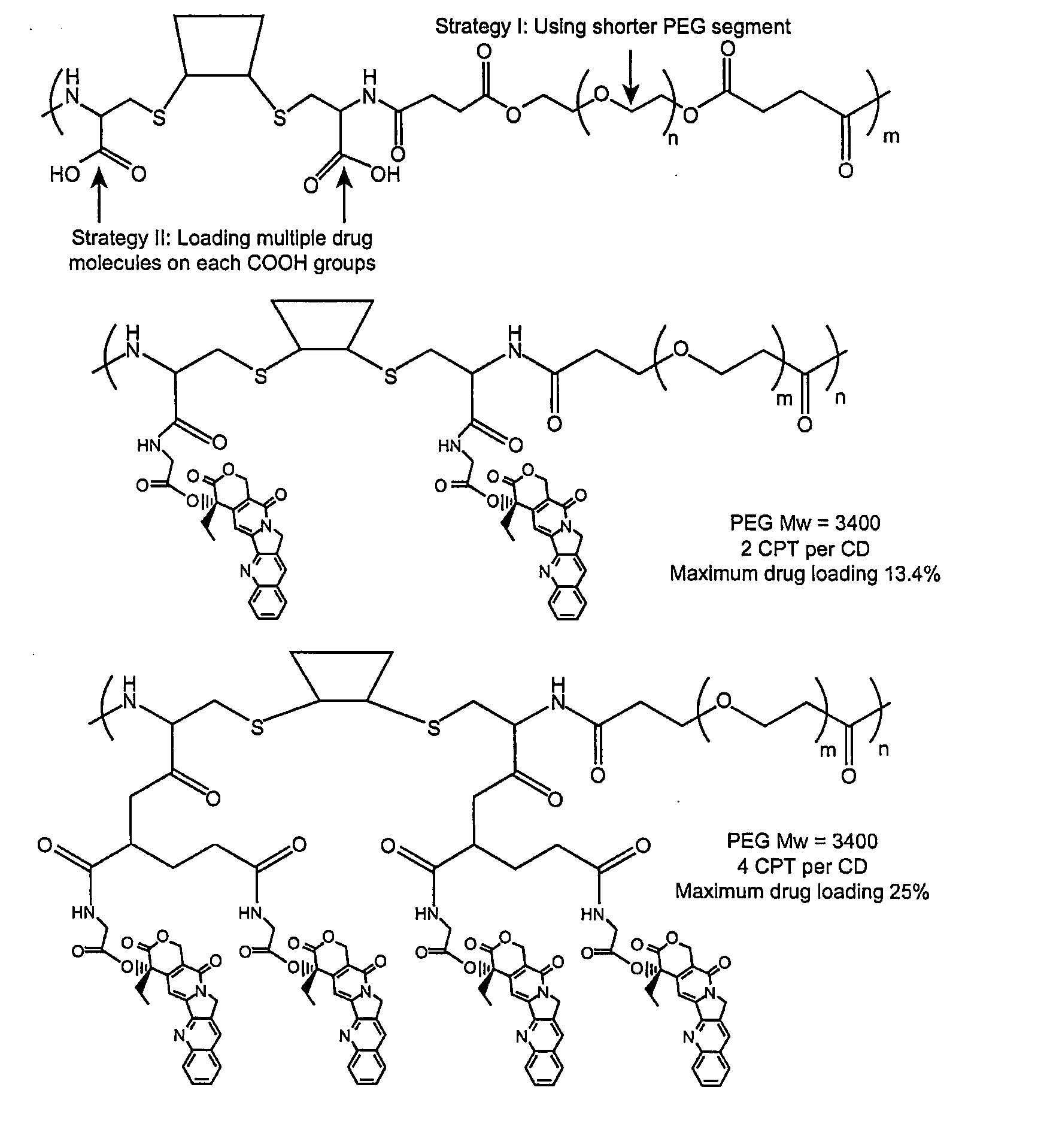
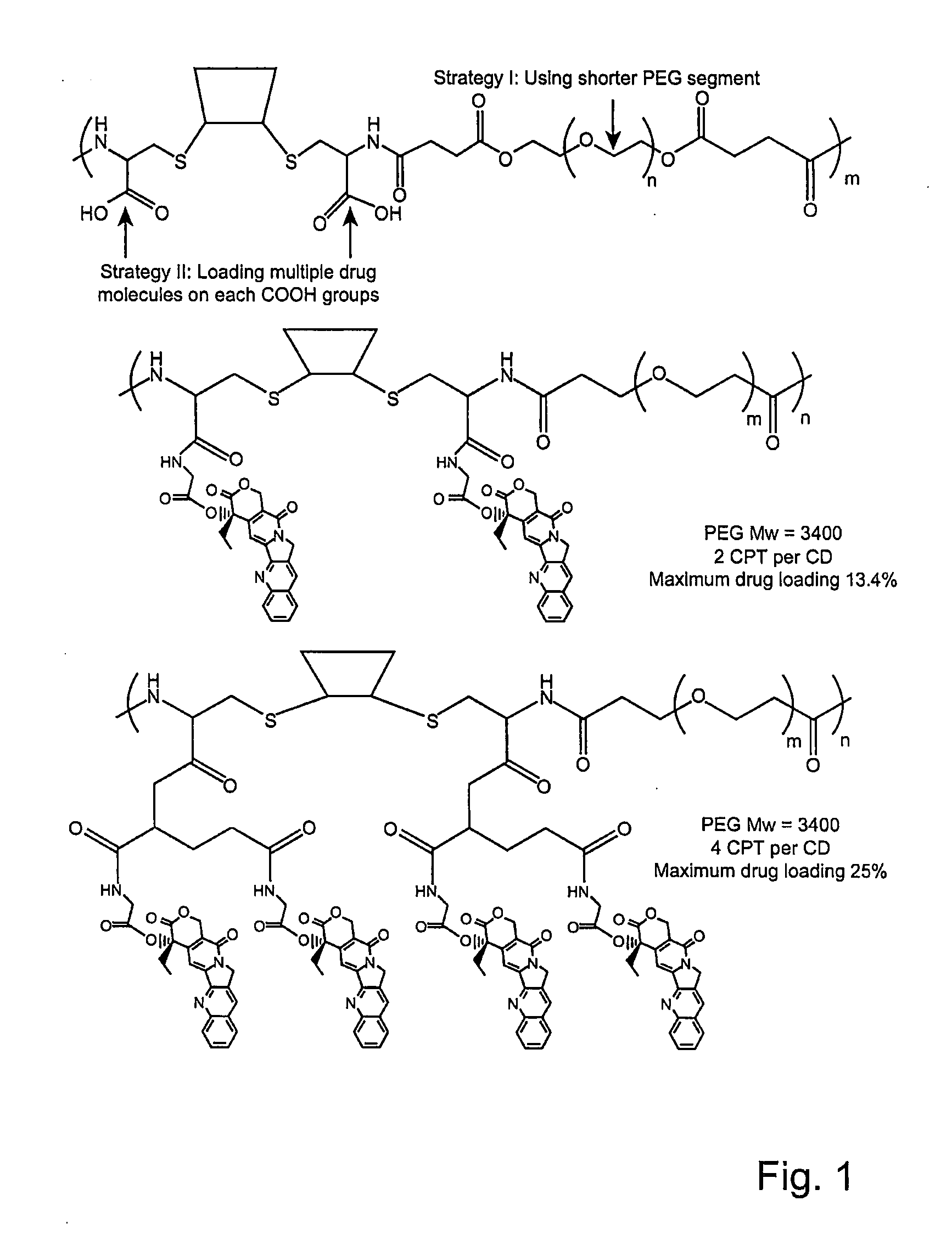
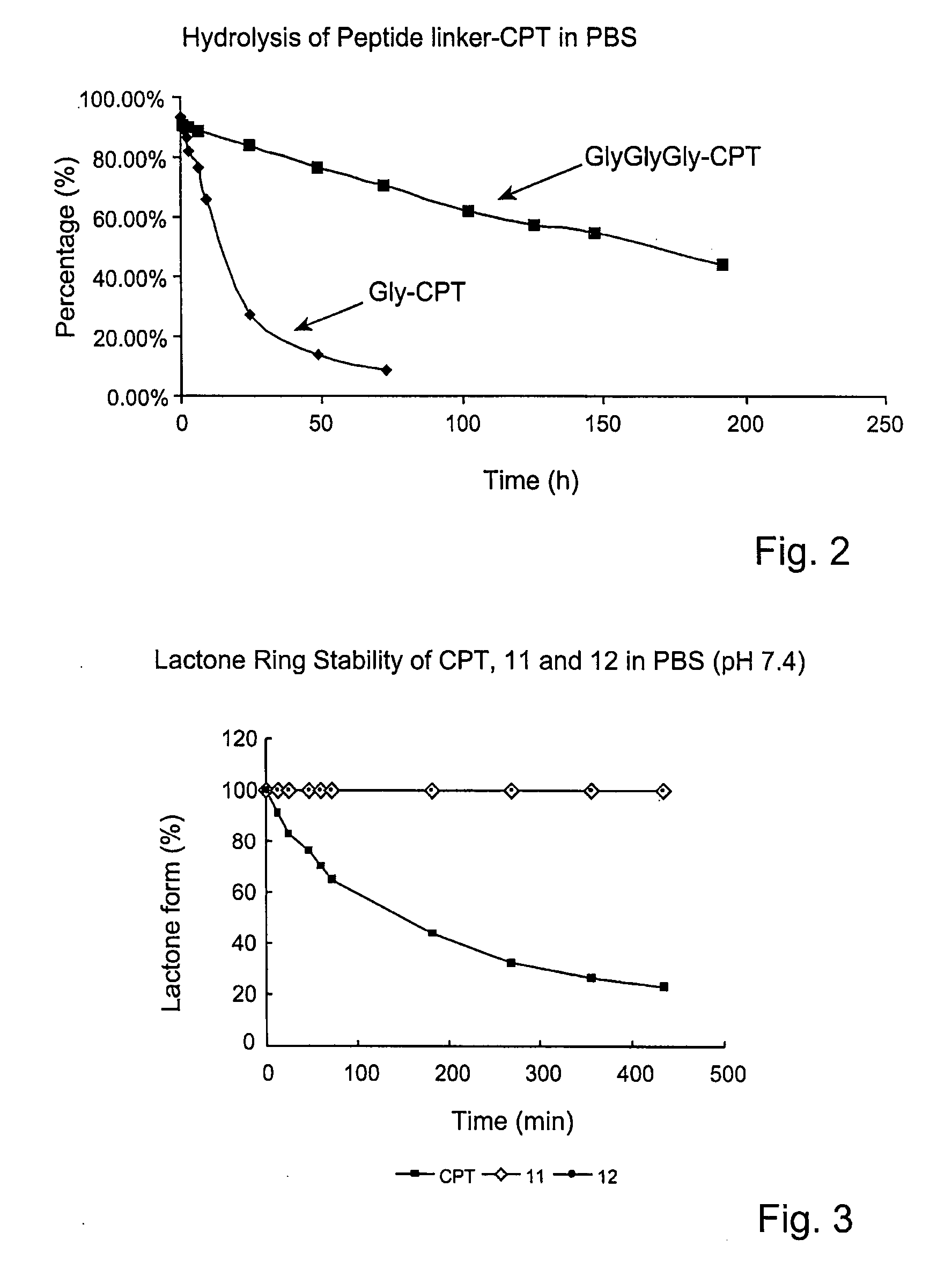
Cyclodextrin-based polymers for therapeutics delivery
ActiveUS7270808B2Improve drug stabilityImprove solubilityAntibacterial agentsBiocideSolubilityPresent method
The present invention relates to novel compositions of therapeutic cyclodextrin containing polymeric compounds designed as a carrier for small molecule therapeutics delivery and pharmaceutical compositions thereof. These cyclodextrin-containing polymers improve drug stability and solubility, and reduce toxicity of the small molecule therapeutic when used in vivo. Furthermore, by selecting from a variety of linker groups and targeting ligands the polymers present methods for controlled delivery of the therapeutic agents. The invention also relates to methods of treating subjects with the therapeutic compositions described herein. The invention further relates to methods for conducting pharmaceutical business comprising manufacturing, licensing, or distributing kits containing or relating to the polymeric compounds described herein.
Owner:CERULEAN PHARMA
Anti-inflammatory analgesic adhesive patch for external use
ActiveUS20120283671A1Promote absorptionLess irritatingAntipyreticAnalgesicsAdditive ingredientTackifier
An external patch containing diclofenac hydroxyethylpyrrolidine prepared by laminating an adhesive layer on a backing, wherein said adhesive layer is characterized by comprising 5-50% by weight of styrene•isoprene•styrene block copolymer, 20-50% by weight of a tackifier resin, 5-70% by weight of a softening agent, and 0.5-20% by weight of one or more solubilizers selected from N-methyl-2-pyrrolidone, propylene glycol and dimethyl sulfoxide as essential ingredients, and 0.5-20% by weight of diclofenac hydroxyethylpyrrolidine as an active ingredient. The patch has excellent transdermal absorption, less skin-irritation and excellent stability of the drug.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
Cyclodextrin-based polymers for therapeutics delivery
ActiveUS20080058427A1Efficient productionWell mixedAntibacterial agentsOrganic active ingredientsSolubilityPresent method
The present invention relates to novel compositions of therapeutic cyclodextrin containing polymeric compounds designed as a carrier for small molecule therapeutics delivery and pharmaceutical compositions thereof. These cyclodextrin-containing polymers improve drug stability and solubility, and reduce toxicity of the small molecule therapeutic when used in vivo. Furthermore, by selecting from a variety of linker groups and targeting ligands the polymers present methods for controlled delivery of the therapeutic agents. The invention also relates to methods of treating subjects with the therapeutic compositions described herein. The invention further relates to methods for conducting pharmaceutical business comprising manufacturing, licensing, or distributing kits containing or relating to the polymeric compounds described herein.
Owner:CERULEAN PHARMA
Daptomycin freeze-dried preparation for injection and preparing method
InactiveCN1616083AImprove solubilityEasy to storeAntibacterial agentsPowder deliveryFreeze-dryingAntibiotic Y
Active medicine daptomycin is mixed with certain amount of sodium hydroxide and pH regulator, the mixture is compounded with injection water to form solution in certain concentration, and the solution is filled into antibiotic bottle and freeze dried for 30-60 hr to prepare loose and water soluble freeze dried preparation. The freeze dried daptomycin preparation for injection has excellent medicine stability and convenience in carrying and clinical application. The preparation is used specially for intravenous injection to treat skin infection with resistance to other antibiotics.
Owner:魏雪纹 +2
Nano-emulsion medicine for treating foot rot and preparation method thereof
InactiveCN102552339AHigh thermodynamic stabilityEasy to operateAntibacterial agentsHydroxy compound active ingredientsPropanoic acidCutin
The invention discloses a nano-emulsion medicine for treating foot rot. The grain diameter of the nano-emulsion is in a range of 1-100 nanometers and the nano-emulsion is composed of the following raw materials: 0.1-10.0% of pine tar, 0.1-6.0% of cinnamyl aldehyde, 0.1-6.0% of carvacrol, 0.1-5.0% of camphor oil, 0.01-0.1% of clobetasol propionate, 18.0-35.0% of surfactant, 0-7.0% of co-surfactant and residual amount of distilled water; and the sum of the mass percentages of the components is 100%. The nano-emulsion disclosed by the invention has the functions of dissolving cutin, relieving itching, diminishing inflammation, restraining, partially disinfecting and resisting corrosion, accelerating absorption and the like, and is mainly used for treating animal foot rot. According to the nano-emulsion medicine for treating the foot rot, the solubility of the medicine is increased, the medicinal stability and the biological utilization rate are improved, the dosage of the medicine is reduced, the skin transmission rate is higher than that of common preparations including a medicinal extract and the like, and the cost is low, so that the nano-emulsion medicine has a wide market prospect in a veterinary medicine field.
Owner:NORTHWEST A & F UNIV
Instant lyophilized fibrinogen and fibrin ferment formulation composition, preparation method and use thereof
ActiveCN101371921ASolve the shortcoming of short storage timeImprove solubilityPeptide/protein ingredientsBlood disorderArginineIrritation
The invention provides a composition of instant freeze-dried fibrinogen and thrombin preparation, a preparation method and a use thereof, and the composition comprises composition 1 composed of 35-70mg / ml of fibrinogen, 9-15mg / ml of sodium citrate, 7-12mg / ml of sodium chloride, 0.3-0.6mg / ml of polysorbate-80, 10-15mg / ml of mannitol, 4 -8mg / ml of arginine and 3.5-5.5mg / ml of glutamic acid by mg / ml and composition 2 composed of 700-1,200 mg / ml of thrombin, 3-6mg / ml of dextran 20, 15-25mg / ml of glycine, 5-7mg / ml of sodium chloride and 4-7mg / ml of calcium chloride by mg / ml. A sealant avoids the risk of spreading of AIDS virus, and the like, increases the drug stability, reduces the degeneration during the virus inactivation process by dry-heat method, protects biological activity, avoids local liquid storage of the using part caused by high permeability and the irritation of a large amount of inorganic salts to tissues, is conductive to the healing of trauma sites and ensures product safety and independent package to the maximum extent, thereby increasing storage time, facilitating use and meeting the clinical and field first-aid needs.
Owner:HANBANG MEDICAL SCI & TECH HARBIN CITY
Enteric coated preparation of lansoprazole sodium and preparation method thereof
InactiveCN1883458AAvoid degradationImprove stabilityOrganic active ingredientsDigestive systemIsolation layerAqueous solution
Disclosed is a Lansoprazole sodium intestine-dissolving preparation, which is prepared from Lansoprazole sodium 15-30 parts, thinning agent 150-300 parts, moistening agent or binding agent 20-110 parts, crumbling agent 5-25 parts, lubricating agent 5-15 parts and coating agent 30-100 parts through the steps of disintegrating, sieving, mixing, tabletting, and dressing isolation layer.
Owner:JINZHOU JIUTAI PHARML CHINA
Cucurbitacin drop pill and preparing method thereof
The present invention utilizes ultramicropulverization and dripping pill preparation production process to make cucurbitacin dripping pills, and can attain the goal of raising disintegration and dissolution speed, quickly obtaining therapeutic effect, raising stability of medicine, reducing dose of auxiliary material, reducing production cost and convenient administration. Said pill not only can be sucked, but also can be swallowed.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Levamlodipine beaylate tablets and preparation method thereof
ActiveCN101766582AImprove stabilityRapid dissolutionOrganic active ingredientsPharmaceutical delivery mechanismActive componentLevamlodipine
The invention belongs to the technical field of medicament, and provides levamlodipine beaylate tablets and a preparation method thereof. The tablets consist of tablet cores using the levamlodipine beaylate as an active component and film coatings coated on the outer layer, wherein diluent in the tablet cores contains diatomite or aerosil, or a mixture of diatomite and aerosil, and contains other pharmaceutically acceptable supplementary materials; and the outer film coating accounts for 8 to 12 percent of the weight of the tablets, and can play a role in resisting humidity and avoiding light to ensure that the medicinal stability can be greatly improved, and related substances are obviously reduced. Furthermore, the tablets have small specification, so the tablets ensure uniform content, and improve dissolution; and the method is simple and controllable, and ensures that the hygroscopicity of the medicament is obviously reduced.
Owner:鲁南新时代生物技术有限公司
Niuhuangshangqing soft capsule and preparation method thereof
ActiveCN101444570AAvoid volatile lossPrevent oxidationHydroxy compound active ingredientsComponent separationVegetable oilMoisture absorption
The invention relates to a Niuhuangshangqing soft capsule and a preparation method thereof, as well as a quality control method of capsule contents. The Niuhuangshangqing soft capsule comprises contents and capsule shell. The contents include an extent of Chinese medicinal materials in Niuhuangshangqing prescription and a dispersion medium with a weight ratio of 1:(1-2), wherein, the dispersion medium is vegetable oil. The soft capsule is prepared by extracting effective components from Chinese medicinal materials in Niuhuangshangqing prescription in accordance with the properties of the effective components by using different modern extraction techniques and making into soft capsule. The soft capsule has embedding and masking effects, and can prevent volatilization loss of oil components and oxygenation or photodecomposition of effective components. The soft capsule also has the advantages of rapid disintegration, uniform dispersion, high bioavailability, good stability of effective components, low liability of moisture absorption, good air tightness, direct intestinal absorption after disintegration, masking of unpleasant odors of effective components, convenience for administration and carrying, and so on.
Owner:TAIJI GROUP
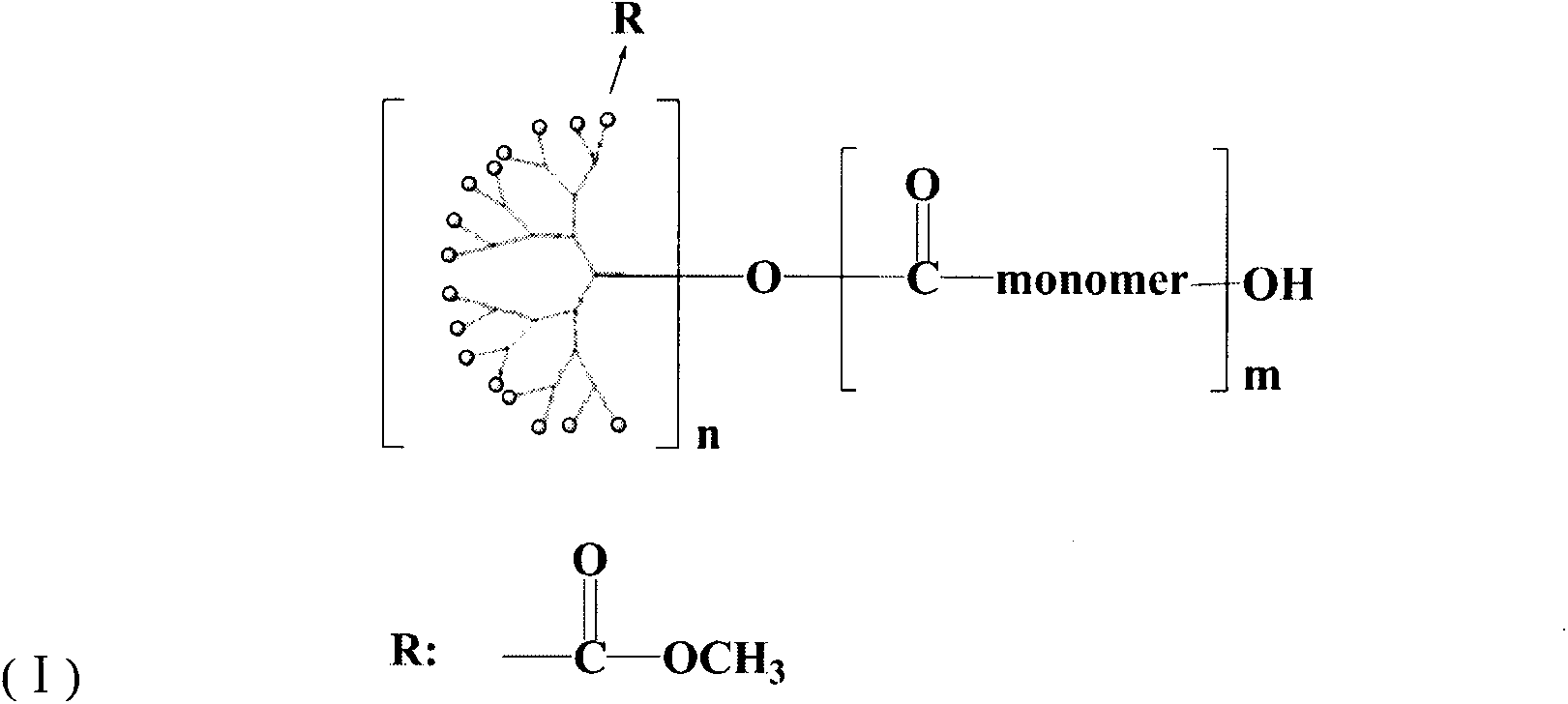
Preparation of multifunctional linear-dendritic segmented copolymer and application in pharmaceutics thereof
InactiveCN103242517ARapid drug releasePromote absorptionPowder deliveryPharmaceutical non-active ingredientsSide effectWater soluble drug
The invention relates to the fields of high polymer chemistry and pharmaceutical preparation and in particular relates to preparation of a multifunctional linear-dendritic segmented copolymer and application in pharmaceutics thereof. The multifunctional linear-dendritic segmented copolymer has the amphipathy, indissolvable drugs can be obviously dissolved, the drug stability and the in-vivo bioavailability are improved, particularly indissolvable or water-soluble drugs can be coated, and the drugs are self-assembled to form nano-micelle. The modified polyamidoamine structure has the charge inversion property of environmental response, so that the micelle carries the negative charge on the surface in body circulation, the charge responds to the faintly acid environment and is inversed into positive charge after reaching the tumor area, cellular uptake and intracellular drug delivery of the nano-micelle are promoted, the passive targeting property of the anti-tumor medicines is improved, the toxic or side effect of the medicine is reduced, and a new thought is provided for novel medicine targeting administration.
Owner:CHINA PHARM UNIV
Hemsleya amabilis drip pill and its preparation method
A dripping pill of hemsleyadine and its superfine pulverizing process for preparing it are disclosed. Its advantages are high dissolving and disintegrating speed, high stripping percentage, quickly taking its effect high stability and low cost.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Clonidine hydrochloride sustained release tablets and preparation method thereof
InactiveCN104352473AImprove stabilityDefinite curative effectOrganic active ingredientsPharmaceutical product form changeExtended release tabletsPlastic packaging
The invention provides clonidine hydrochloride sustained release tablets. The clonidine hydrochloride sustained release tablets are prepared from 0.2 part of clonidine hydrochloride, 70-90 parts of sustained release skeleton material and 10-20 parts of lubricating agent by weight. A preparation method of the clonidine hydrochloride sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The clonidine hydrochloride sustained release tablets and the preparation method have the beneficial effects that the clonidine hydrochloride sustained release tablets have good stability and definite curative effects and can be used for effectively treating hypertension; the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the clonidine hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Volatile oil solid self-emulsifying tablet and preparation method thereof
ActiveCN105395492AImprove solubilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsAdhesiveOil phase
Provided are a volatile oil solid self-emulsifying tablet and a preparation method thereof. The volatile oil solid self-emulsifying tablet is prepared from, by weight, 20-60 parts of volatile oil, 80-140 parts of emulsifier, 2-7 parts of oil phases, 20-60 parts of assistant emulsifier, 120-250 parts of inclusion materials, 400-800 parts of filler, 80-120 parts of disintegrating agent, 10-50 parts of adhesive and 0.5-10 parts of lubricating agent. According to the volatile oil solid self-emulsifying tablet and the preparation method thereof, a volatile oil solid self-emulsifying system of liquid and solid materials are combined to prepare solid preparations, the solid preparations have the advantages of the volatile oil solid self-emulsifying system and the solid materials, the solubility and the dissolution of a water-insoluble drug can be obviously improved, the drug stability is improved, the storage time of drugs is prolonged, irritation to gastrointestinal tracts is reduced, the drugs are convenient to take, the technology is simple, and large-scale production is facilitated.
Owner:HENAN UNIVERSITY
Negatively charged amphiphilic block copolymer as drug carrier
InactiveUS6890560B2Increase blood concentrationImprove drug stabilityPowder deliveryMaterial nanotechnologyBlood concentrationDrug carrier
The present invention provides an anionic group-containing amphiphilic block copolymer that is biocompatible and biodegradable and, when used as a drug carrier for a cationic drug, provides several advantages such as increased blood concentration and improved stability of the drug.
Owner:SAMYANG BIOPHARMLS CORP
Cyclosporine A micelle eye drop and preparing method thereof
ActiveCN103054796AImprove solubilityMicellar particle size is smallSenses disorderCyclic peptide ingredientsSolubilityReduced dose
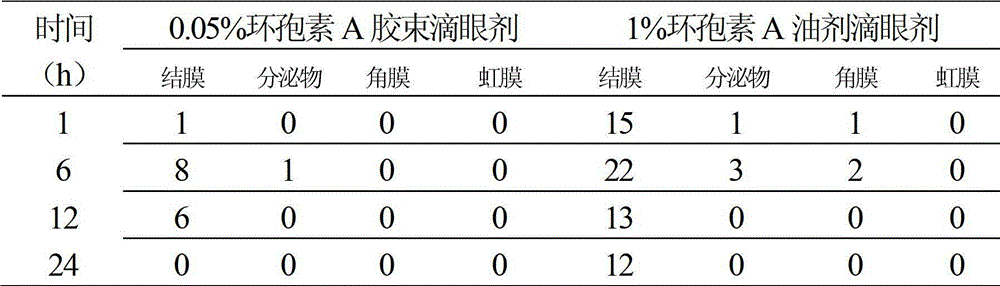
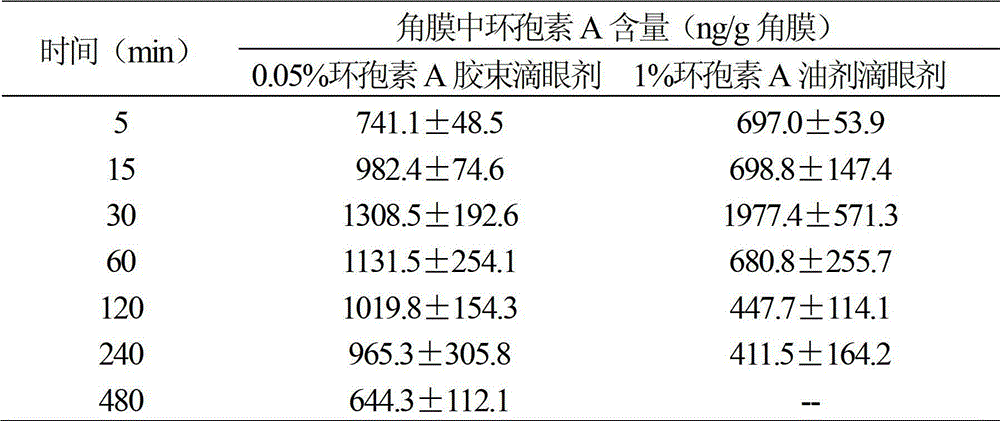
The invention relates to a cyclosporine A micelle eye drop and a preparing method thereof. The cyclosporine A micelle eye drop comprises cyclosporine A used as a main drug, aseptic and isoosmotic adjusting agents, and is characterized by further comprising a copolymer of polythene caprolactam, polyvinyl acetate and polyethylene glycol used as drug auxiliary materials. The preparing method includes: preparing a cyclosporine A micelle through a solvent rotary evaporation film forming method; obtaining cyclosporine A micelle powder after filtering, freezing and drying the cyclosporine A micelle; and dispersing and dissolving the powder into a buffer solution to obtain the cyclosporine A micelle eye drop. According to the cyclosporine A micelle eye drop, the cyclosporine A has good solubility in a water solution, the micelle is small in particle diameter and uniform in distribution range and drug stability is good. The cyclosporine A micelle eye drop not only reduces drug irritation, but also improves drug absorption of a cornea, reduces drug concentration, prolongs drug action time, reduces dosing frequency, improves patient compliance, and has good economical efficiency.
Owner:SHANDONG EYE INST
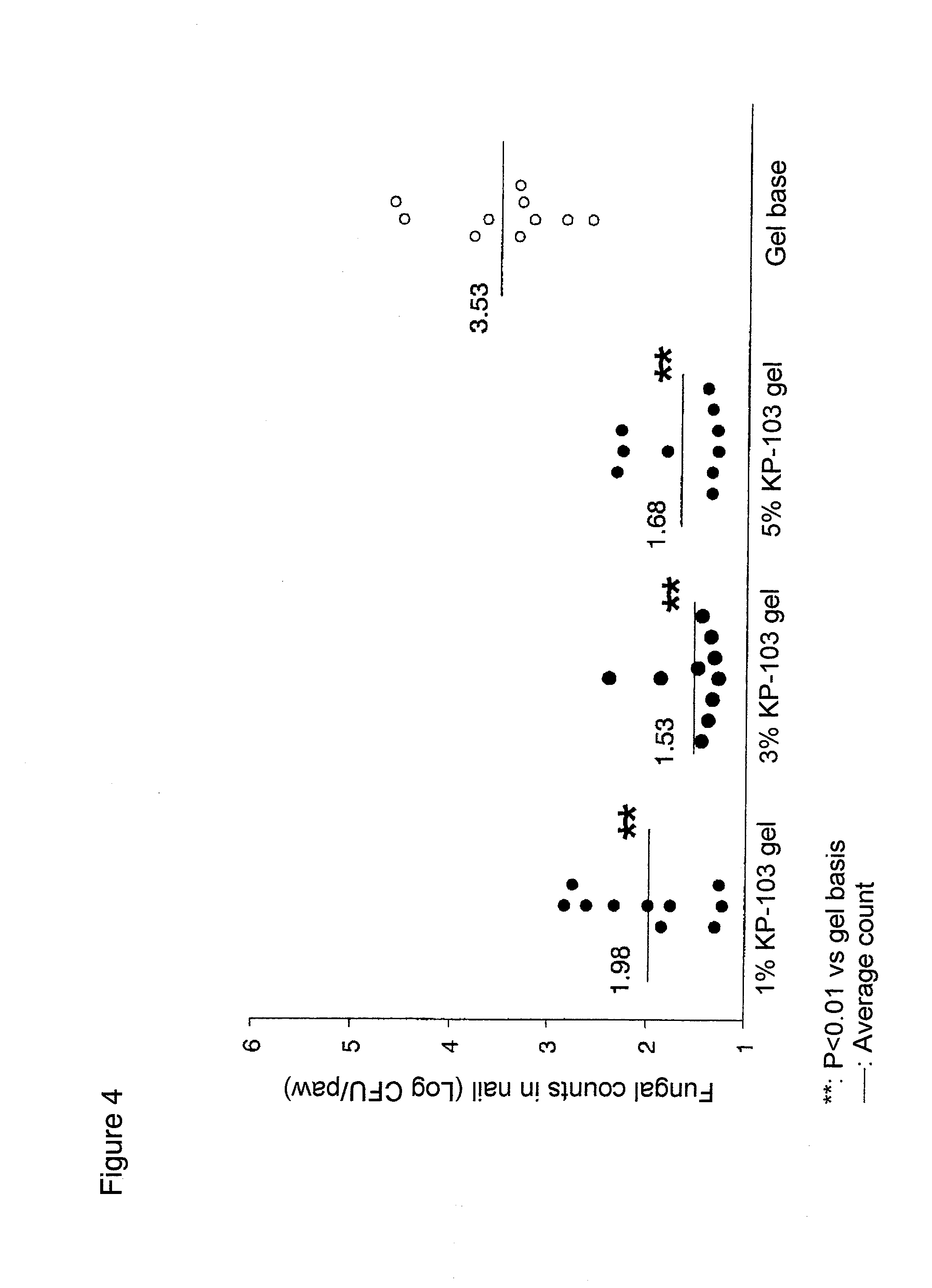
Gel composition for treating mycosis
ActiveUS20100317695A1Shorten the counting processUniform thicknessBiocideAntimycoticsFordyce's diseaseAlcohol
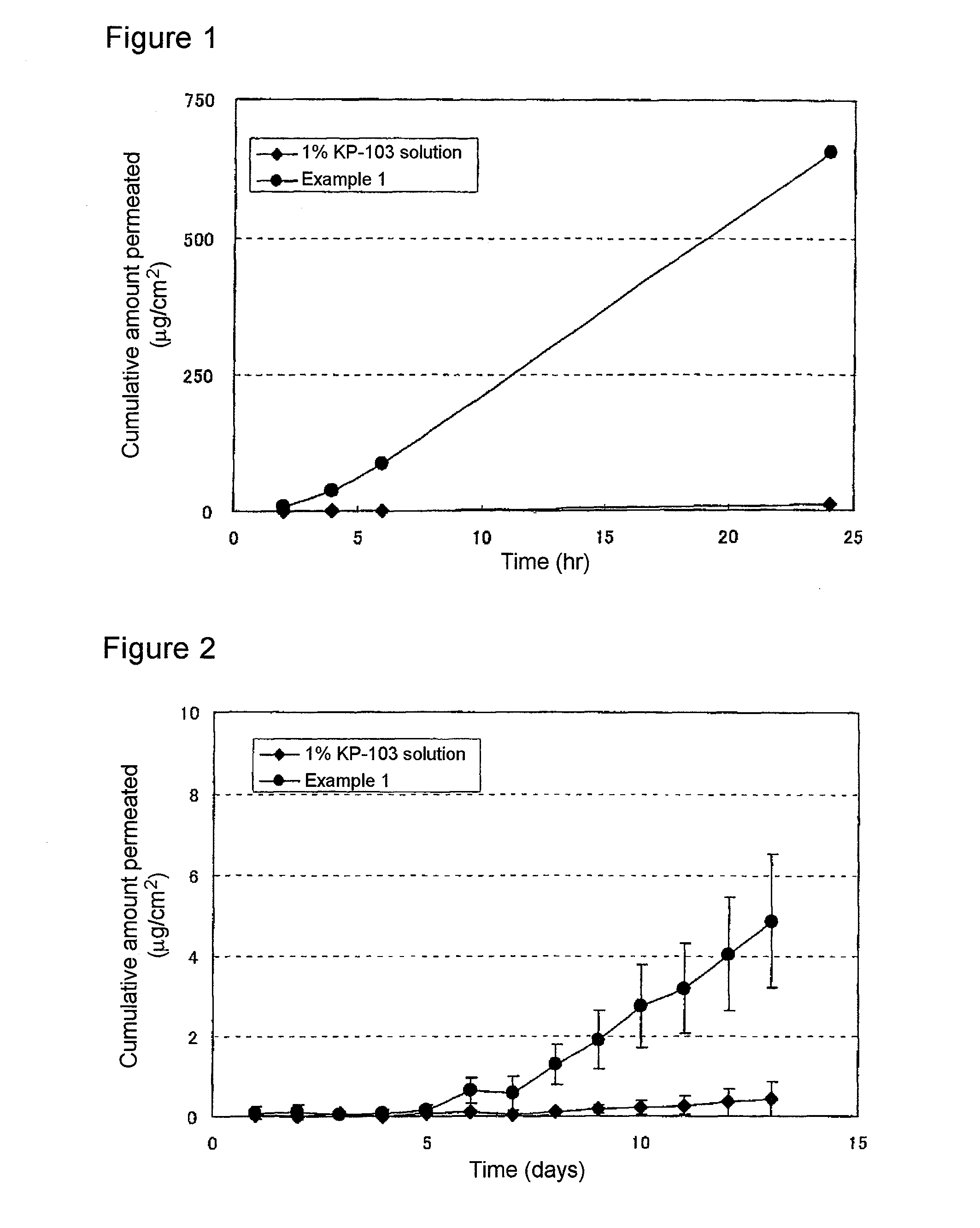
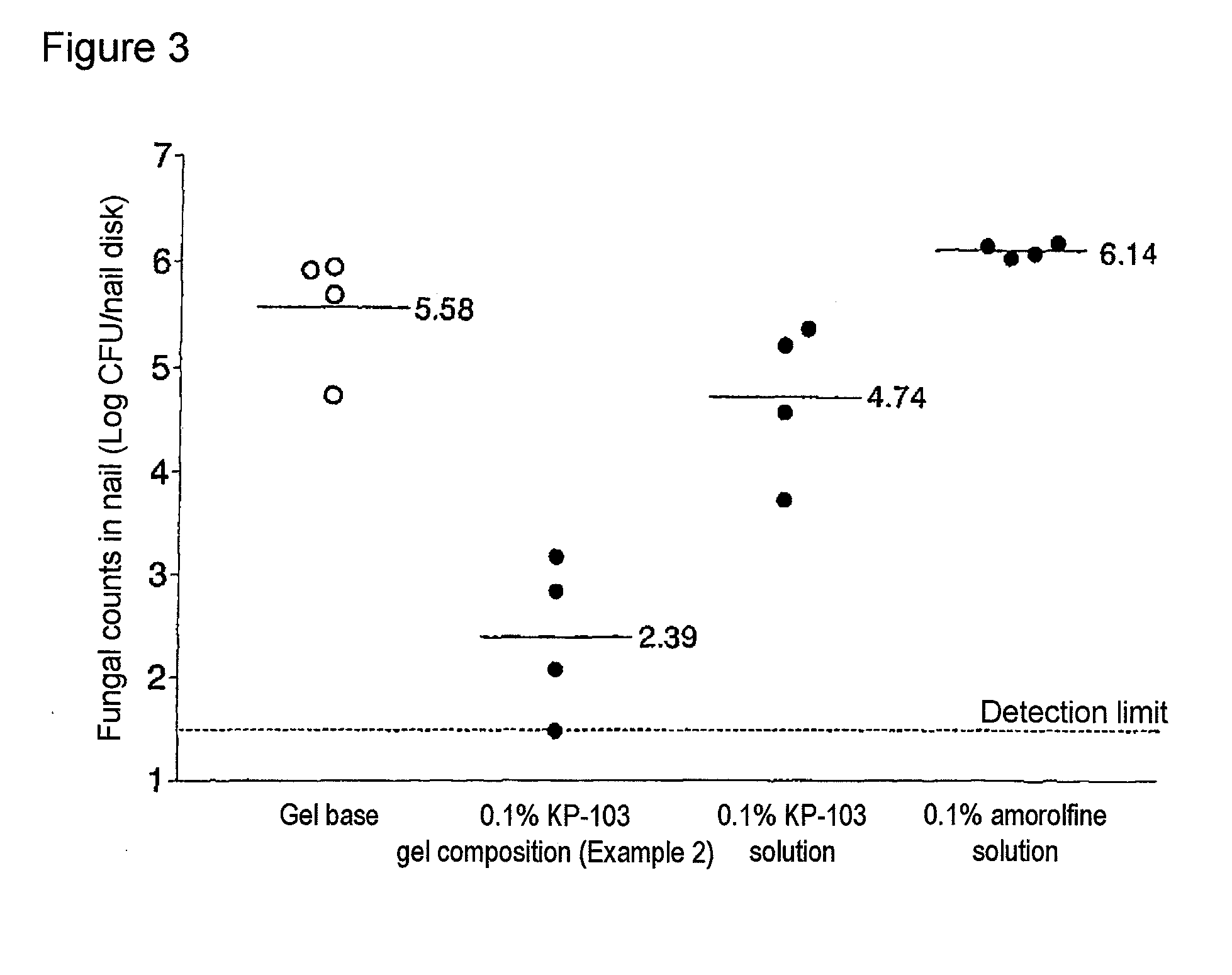
The present invention aims to provide a gel composition for mycosis treatment, which ensures good absorption and permeation of (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)-butan-2-ol into a target site (skin and nail). The present invention also aims to provide a gel composition for mycosis treatment, which is excellent in stability of this drug.It is a gel composition for mycosis treatment, which comprises (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)-butan-2-ol or an acid addition salt thereof, a lower alcohol, a polyhydric alcohol and a gel-forming polymer.The gel composition of the present invention is excellent in permeation of KP-103 into a target site and also excellent in permeation into the nail. Thus, the gel composition of the present invention allows the drug to be directly and rapidly absorbed and permeated into a target site in a constant manner, and thereby produces an excellent effect in mycosis treatment, particularly onychomycosis treatment.
Owner:KAKEN PHARMA CO LTD
Apigenin vesicle preparation and its preparation technology
InactiveCN101164536AProlong the action timeHigh encapsulation efficiencyOrganic active ingredientsNervous disorderApigeninCholesterol
The present invention provides an apigenin vesiculation preparation and its preparation process. Said preparation composition includes (by wt%) 0.25%-20% of apigenin, 40%-90% of non-ionic surface active agent, 8.7-48.8% of cholesterol and 2%-20% of lyophilization protection agent. The described non-ionic surface active agent can be Span-20, Span-40, Span-60, Span-80, Tween-20, Tween-60, Tween-80, benzozir, maizir, poloxamer or their mixture. Said invention can be made into preparations of injection, nose drops, ointment and oral liquor, etc, and can be prepared by adopting ethyl alcohol injection method, film dispersion method, inversed phase evaporation method, hydration method, extrusion method, mechanical method and pH gradient method.
Owner:SHENYANG PHARMA UNIVERSITY
Albumin binding type anti-tumor drug-maleimide molecule prodrug
ActiveCN108187063AExtended stayRapid drug releaseOrganic active ingredientsOrganic chemistryDocetaxelTumor cells
The invention belongs to the technical field of medicines, and relates to a redox sensitive albumin binding prodrug with a series of tumor tissue specific responses. The albumin binding prodrug is ananti-tumor drug-maleimide molecule prodrug particularly including three docetaxel-maleimide small molecule prodrugs; by utilizing a maleimide ring in a prodrug structure as a target head of plasma albumin 34-bit free sulfydryl in a binding body, so that after a drug enters into a body through intravenous injection, the drug is quickly and specifically bound with albumin to form an albumin-drug composition; therefore, the drug stability is enhanced, the circulation time of the drug in the boy is obviously prolonged, and accumulation of the drug on tumor tissues is realized by utilizing an EPR (Enhanced Permeability and Retention) effect. In addition, in the prodrug structure, by using a redox sensitive key as a binding key, quick release of the drug in tumor cells is promoted, a toxic effect of the drug on normal cells also can be alleviated, the prodrug has high selectivity, the aims of increasing an effect and reducing the toxicity of docetaxel are fulfilled, and a greater market application prospect is achieved.
Owner:SHENYANG PHARMA UNIVERSITY
Gynecologic rehabilitation capsules for treating gynecopathy
InactiveCN1679913ADisintegrate as soon as possibleImprove stabilityUnknown materialsCapsule deliveryDiseaseCurative effect
A Chinese medicine in the form of capsule for treating gynopathy, such as menoxenia, menorrhagia, menalgia, morbid leukorrhea, etc is prepared from 42 Chinese-medicinal materials including Chinese angelica root, white peony root, prepared rehmannia root, donkey-hide gelatin, astragalus root, etc.
Owner:贵州德昌祥医药股份有限公司
Self-assembly nano-particles of sulfhydrylation chitosan quaternary ammonium salt and preparation method and application thereof
InactiveCN101940551AExtended stayIncrease concentrationPowder deliveryGenetic material ingredientsPolyelectrolyteOrganic solvent
The invention belongs to the technical field of nano-particles, and relates to self-assembly nano-particles of a sulfhydrylation chitosan quaternary ammonium salt and a preparation method and application thereof. The sulfhydrylation chitosan quaternary ammonium salt is prepared from chitosan through quaterisation and sulfhydrylation modifications in turn. The nano-particles are generated from the positively charged sulfhydrylation chitosan quaternary ammonium salt and a negatively charged protein polypeptide medicament or a nucleic acid medicament through polyelectrolyte action. Compared with the prior art, the invention has the advantages that: the preparation process of the nano-particles is simple and easy to control, organic solvent is not needed, and the stability of medicaments can be effectively improved. Compared with the conventional chitosan quaternary ammonium salt nano particles, the nano-particles have better effects of promoting absorption of the protein medicament and promoting cell transfection of the nucleic acid medicament.
Owner:FUDAN UNIV
Enzalutamide soft capsule and preparation method thereof
ActiveCN104857517AHigh dissolution rateImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolBHA - Butylated hydroxyanisole
The invention discloses an enzalutamide soft capsule and a preparation method thereof. The enzalutamide soft capsule comprises capsule content and a capsule shell, wherein the capsule content comprises enzalutamide and pharmaceutical adjuvants; the capsule shell is formed by gelatin, glycerin, sorbitol, titanium dioxide, purified water and the like. The soft capsule can be prepared with the method comprising following steps: Labrasol, butylated hydroxyanisole, butylated hydroxytoluene and enzalutamide are mixed to obtain the capsule content material of the soft capsule; the gelatin, the glycerin, the sorbitol, titanium dioxide and the purified water are mixed to obtain the capsule shell material of the soft capsule; the capsule content material and the capsule shell material of the soft capsule are pelleted on a soft capsule making machine to obtain the enzalutamide soft capsule. The enzalutamide soft capsule and the preparation method have the following advantages: the soft capsule is convenient to administrate and carry, the drug stability is good, effective components are dissolved quickly, and the bioavailability is high.
Owner:NANJING HEALTHNICE MEDICAL TECH +1
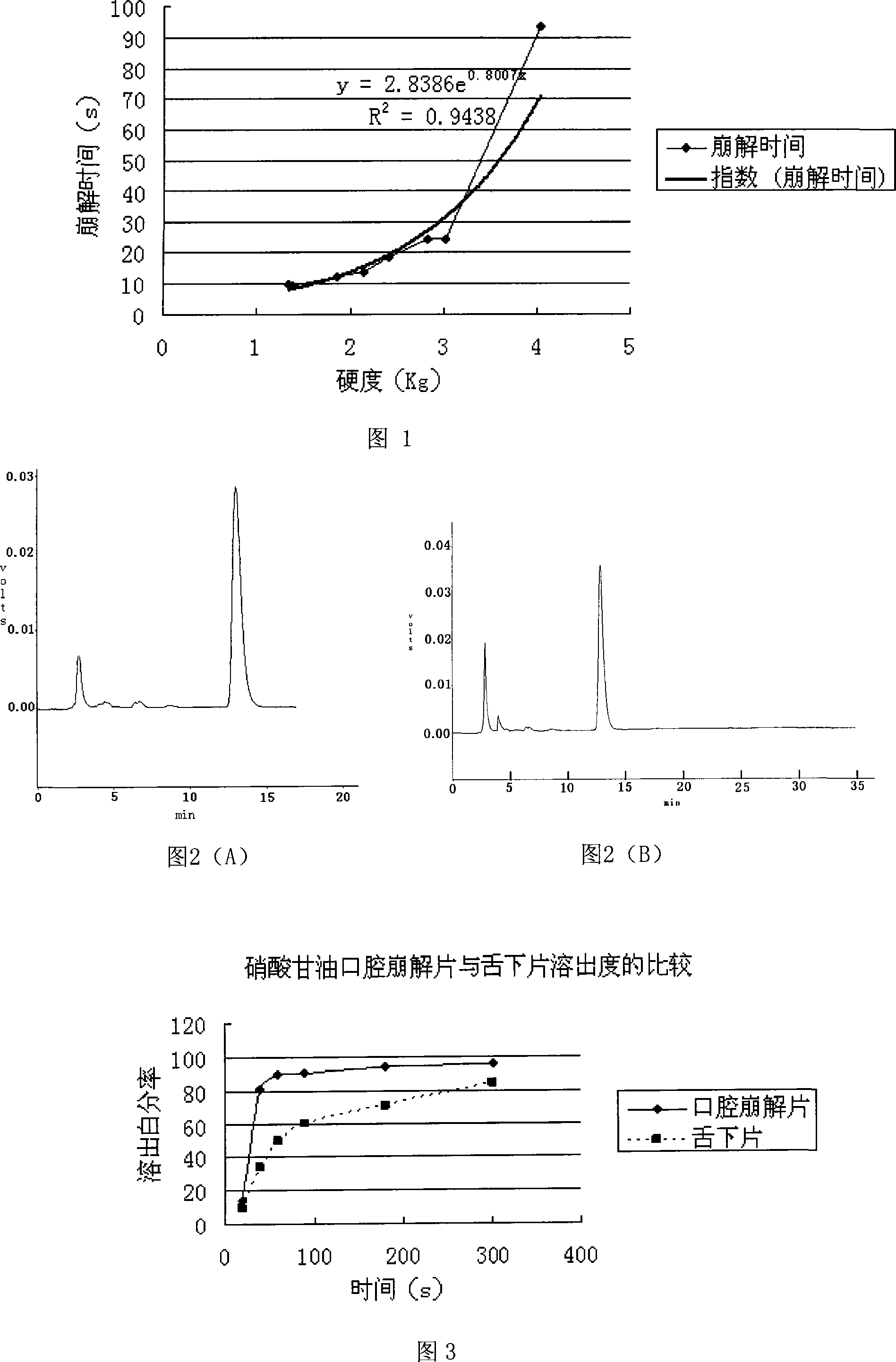
Glonoin Orally disintegrating tablets preparation of and preparing method thereof
InactiveCN101229148AImprove stabilityFast disintegration timePharmaceutical non-active ingredientsPill deliveryOrally disintegrating tabletLow-substituted hydroxypropylcellulose
The invention relates to a nitroglycerin cavity (hypoglossis) disintegrating tablet and a preparation method which mainly adopts water-soluble excipient. When coming across saliva, a disintegrating tablet can be disintegrated rapidly and most of the excipient is dissolved, which can be absorbed into body to recycle by hypoglossis mucosa. The main indigent of the invention is nitroglycerin ethanol solution occupying 10 percent in weight, and the containing weight uses the mixture of lactose and xylitol in every 10g is 0.8 to 0.9ml. The excipient comprises microcrystalline cellulose 101, low-substituted hydroxypropyl cellulose, disintegrant, lactose, xylitol, alcoholic solution of PVP30 and magnesium stearate. The invention adopts a wet method of preparing particle and tablet, combines with blank particle method to mix the blank particles and the particles containing medicine after being made into particles, which changes the traditional method of spraying medicine layer to blank particles and raises the stability of medicine. The pressure of a tabletting machine is adjusted to 1 to 3kg and the disintegration of the pressed tablets is speeded up.
Owner:TIANJIN MEDICAL UNIV
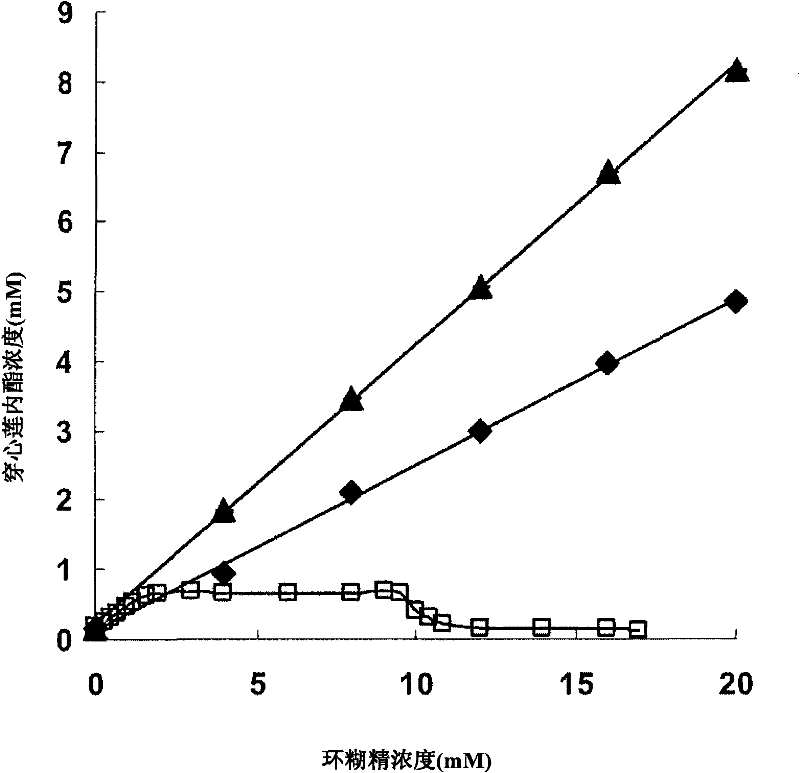
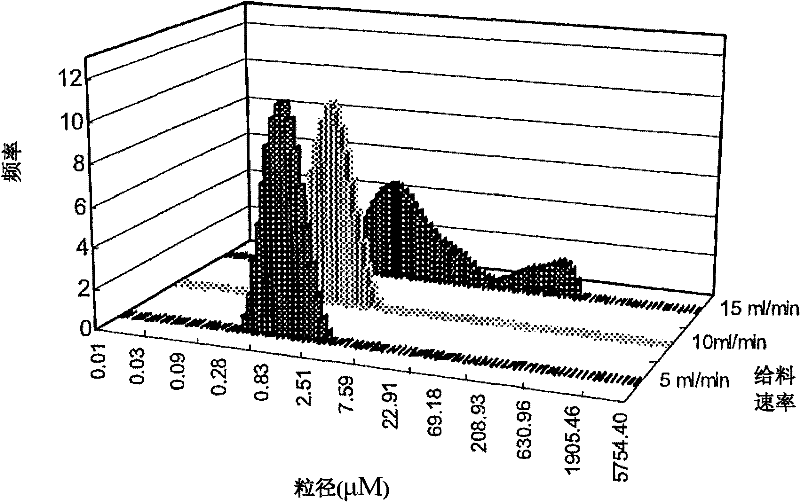
Andrographolide cyclodextrin inclusion compound, preparation method and application thereof
InactiveCN102343096AImprove solubilityImprove lipophilicityAntibacterial agentsOrganic active ingredientsSolubilityMicrometer
The present invention provides an andrographolide cyclodextrin inclusion compound. The basic components of the andrographolide cyclodextrin inclusion compound comprise: a) andrographolide, and b) pharmaceutically-acceptable cyclodextrin. The inclusion compound is prepared through carrying out spray drying for the CD and the andrographolide, wherein the entrance temperature is 100-200 DEG C, the material feeding speed is less than or equal to 20 ml / min, a molar ratio of the CD to the andrographolide is more than or equal to 1:1. The present invention further provides a preparation method for the inclusion compound, and an application of the inclusion compound. The inclusion compound provided by the present invention has excellent solubility, excellent drug stability and excellent bioavailability. With the method provided by the present invention, the inclusion compound of the andrographolide and the cyclodextrin or the inclusion compound of other plant compounds and the cyclodextrin can be prepared, wherein the inclusion compound has the particle size from micrometer to submicron, such that the bioavailability and the stability of the andrographolide are increased, a new delivery system for the diterpene plant compound is provided.
Owner:CITY UNIVERSITY OF HONG KONG
Thymalfasin-containing medicinal composition and preparation method thereof
The invention belongs to the field of medicaments, and particularly relates to a medicinal composition, in particular to a thymalfasin-containing medicinal composition and a preparation method thereof. The medicinal composition comprises the following components: thymalfasin, mannitol and sorbitol, wherein the weight ratio of the thymalfasin to the mannitol to the sorbitol is 16:(25-75):(25-75); and the thymalfasin, the mannitol and the sorbitol are processed and prepared into injection or freeze-dried powder injection. The medicinal composition has high medicinal stability.
Owner:HAINAN ZHONGHE PHARM CO LTD
Diacetyl rhein dropping pills and its preparing process
InactiveCN101015541ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsAntipyreticEngineeringDrugs stability
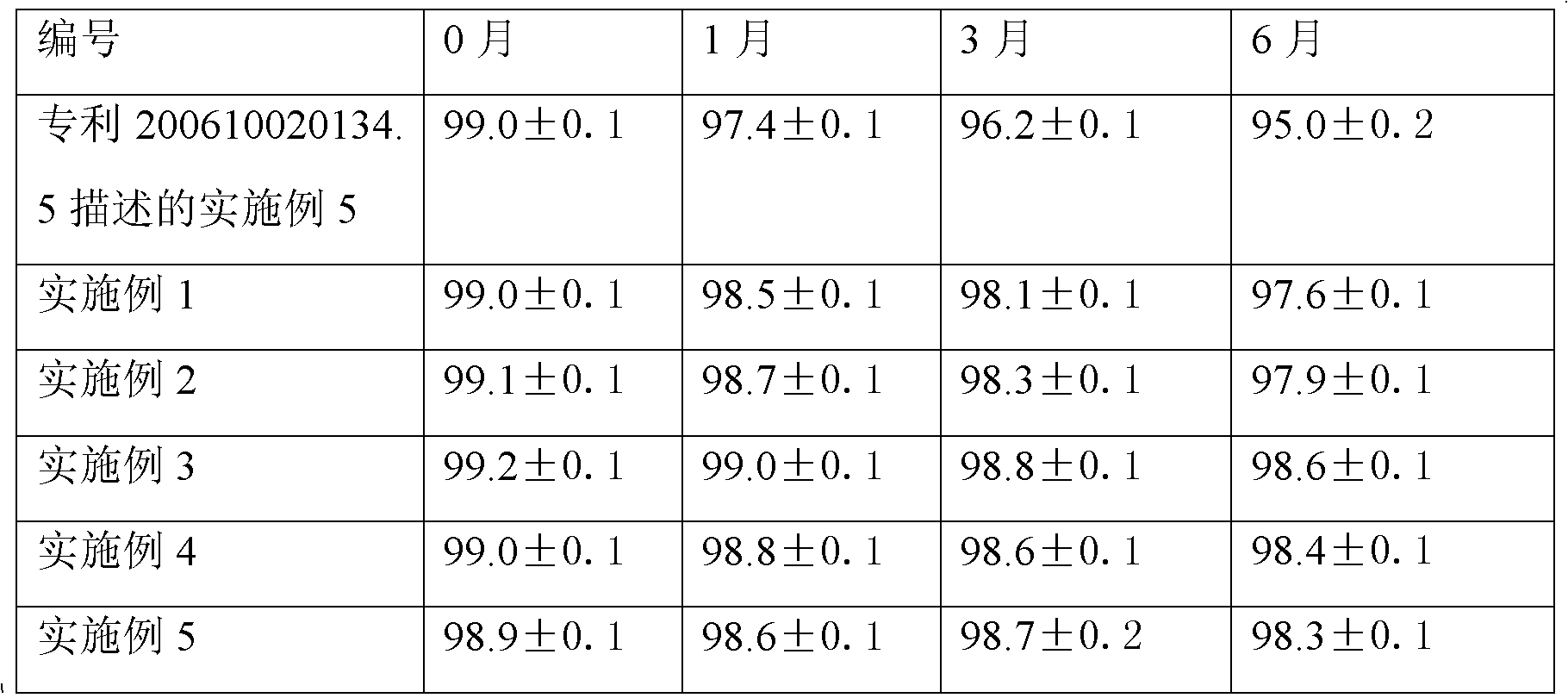
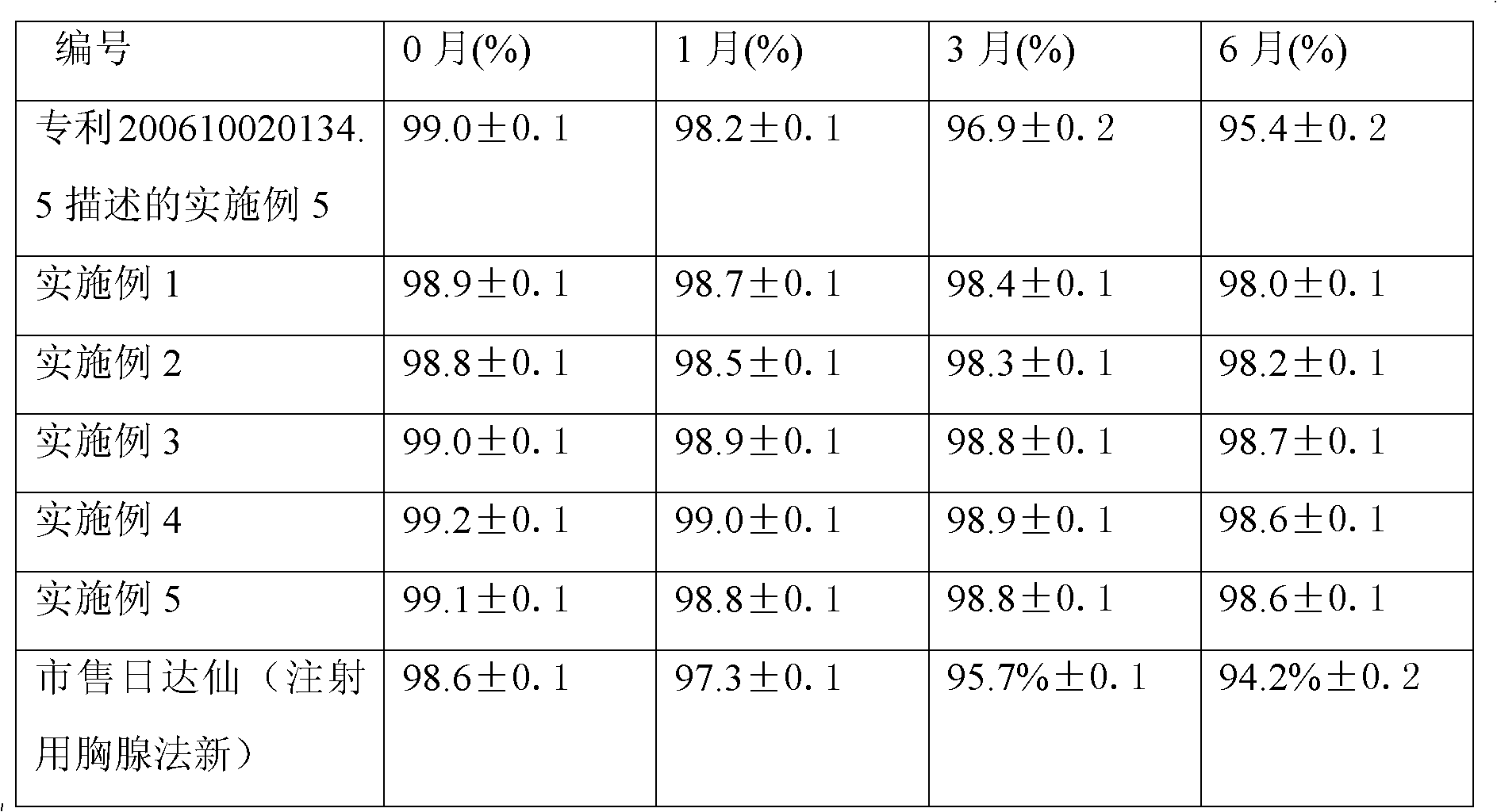
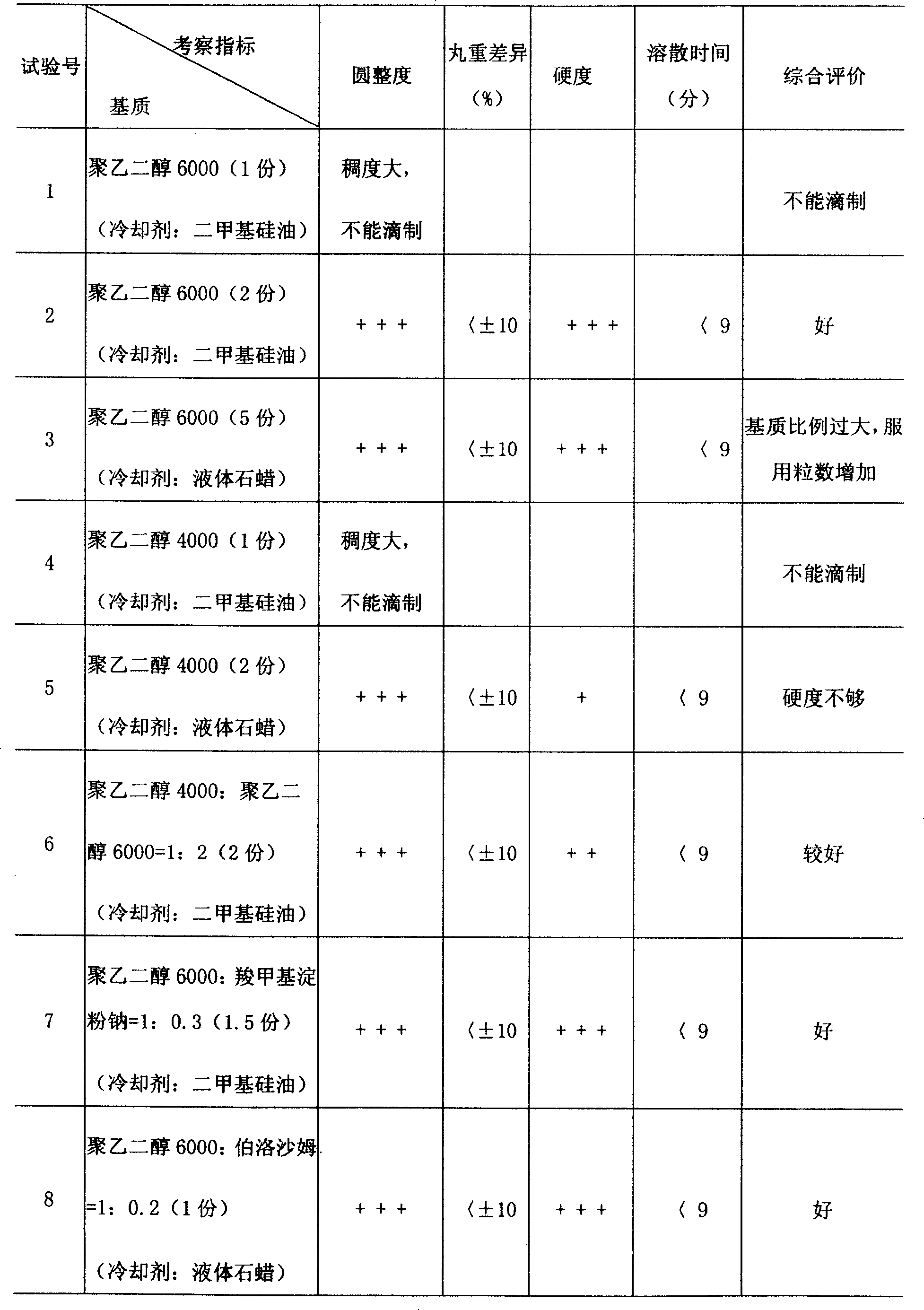
This invention relates to a dripping pill of diacetyl rhein. the pill is characterised in that it is prepared from diacetyl rhein and dripping pill substrate at a weight ratio of 1:1-5. the preparation method comprises pulverizing diacetyl rhein, adding substrate, heating, stirring to fusion, moving to a dripping pill engine with the temperature of materials and dripper at 75-85deg.C, stirring, dripping into coolant at 0-6deg.C and speed of 40+-10 pill / min, separating the dripping pill and coolant through a separating unit, and dripping to obtain the final product. The invention has the advantages of few air contact, uneasy oxidation, no hydrolysis, increase medicine stability, and improved shelf life.
Owner:上海慈瑞医药科技股份有限公司
Sub-microemulsion preparation for levocarnitine injection and preparation method thereof
InactiveCN101461782AHigh drug loadingImprove stabilityOrganic active ingredientsMetabolism disorderEmulsionCurative effect
The invention relates to a submicroemulsion preparation for injecting levocarnitine and a preparation method thereof. Proper auxiliary materials and preparation method are selected, so the emulsion has large drug loading, good stability and long storage period, and can prepare emulsions with different contents. The emulsion can be directly used for intravenous injection, has curative effect superior to that of the prior water injection preparation formulation, particularly has good drug stability at the same time, and is applicable to the mass preparation and industrialized production of the submicroemulsion preparation for injecting the levocarnitine.
Owner:HAINAN LINGKANG PHARMA CO LTD
Calcitriol solid lipidic dispersion and preparation method thereof
ActiveCN102973528AImprove stabilityImprove drug stabilityOrganic active ingredientsSkeletal disorderVitamin E AcetatePolyethylene glycol
The invention discloses a calcitriol solid lipidic dispersion and a preparation method thereof. Raw materials of the calcitriol solid lipidic dispersion comprise calcitriol, one or more lipidic carriers and solid carriers, wherein a mass ratio of calcitriol to the one or more lipidic carriers is 1: (90-200000); a mass ratio of the one or more lipidic carriers to the solid carriers is (1-2): 1; and the one or more lipidic carriers are selected from caprylic triglyceride, capric triglyceride, caprylic / capric triglyceride, laurin, caprylic dilaurin, capric dilaurin, vitamin E, vitamin E succinate, vitamin E polyethylene glycol succinate and vitamin E acetate. The calcitriol solid lipidic dispersion has high drug stability and can keep a constant treatment level. The preparation method does not adopt an inorganic solvent, is suitable for industrial production and is conducive to storage and taking. An experiment proves that the calcitriol solid lipidic dispersion has good stability and dispersion uniformity satisfying requirements.
Owner:SUN YAT SEN UNIV
Water-soluble silymarin and preparation method thereof
InactiveCN101810660ANo residueImprove qualityOrganic active ingredientsMetabolism disorderSolubilitySilybum Marianum Seed
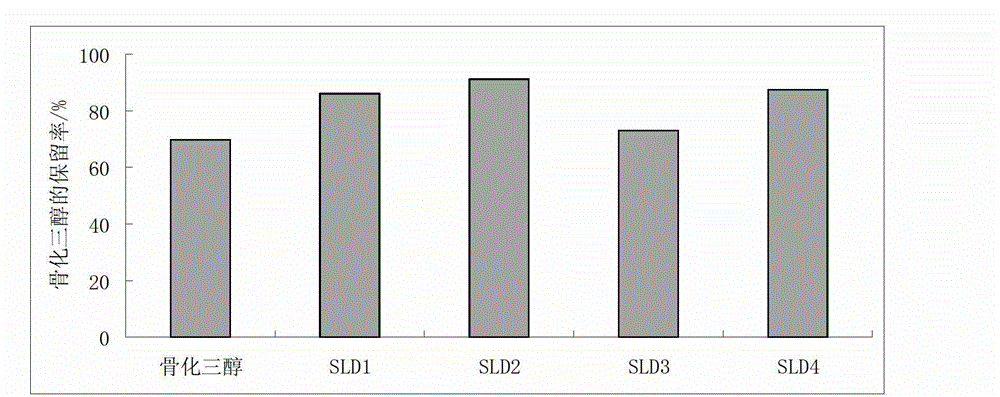
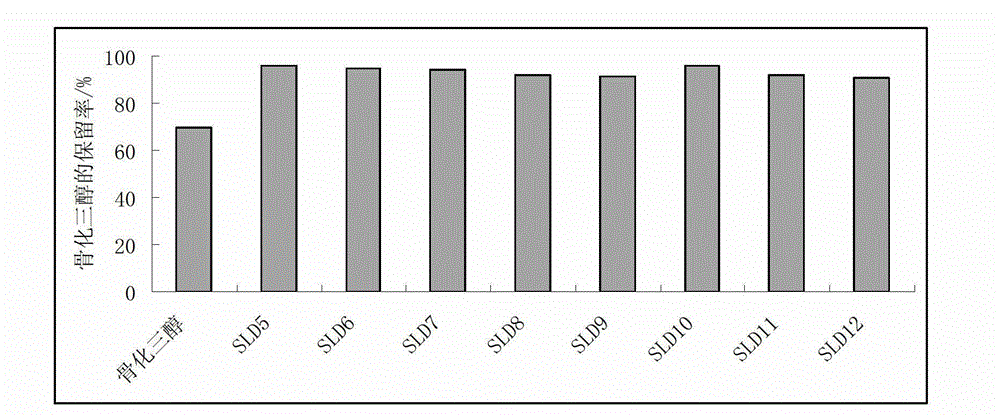
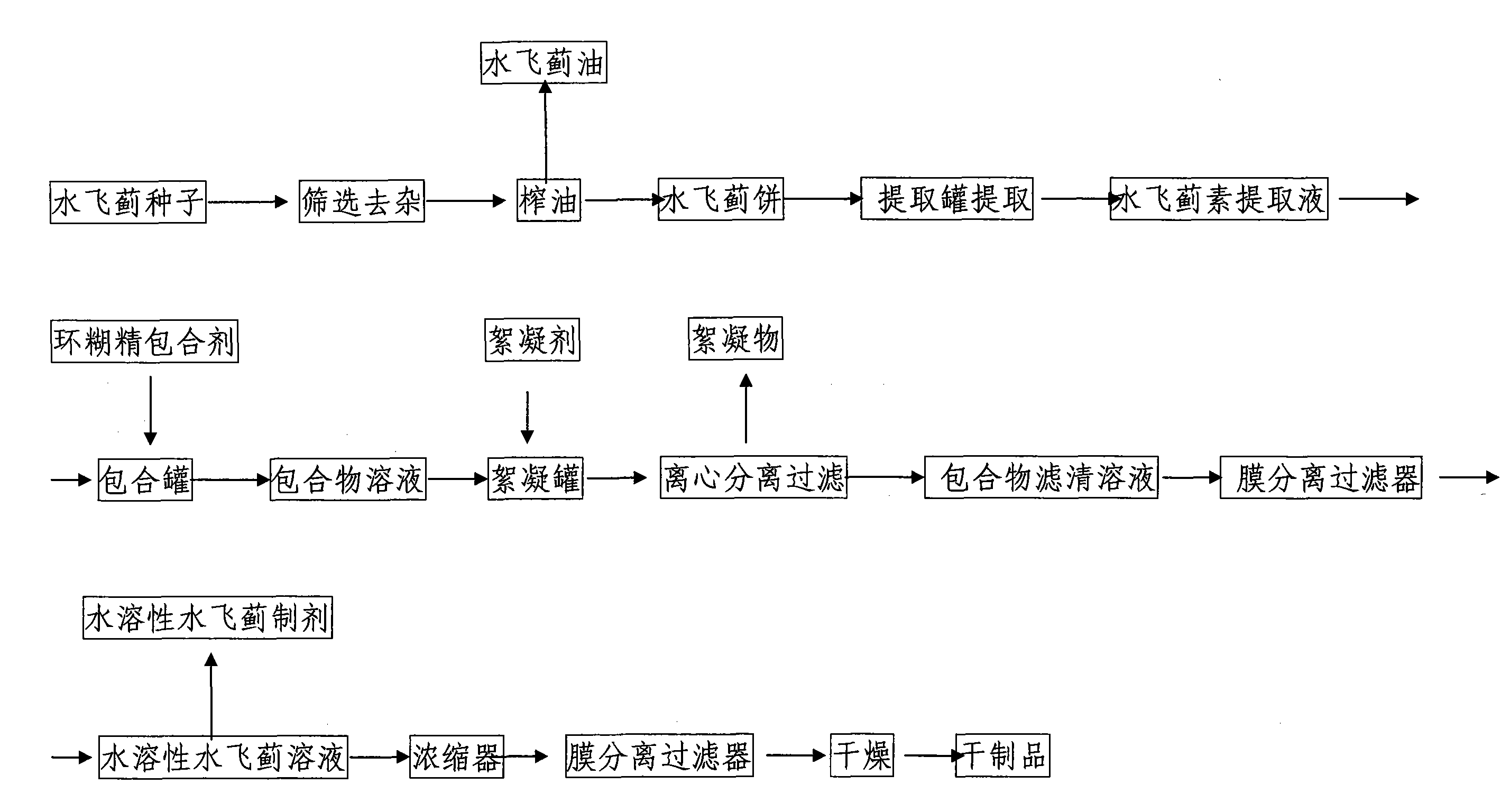
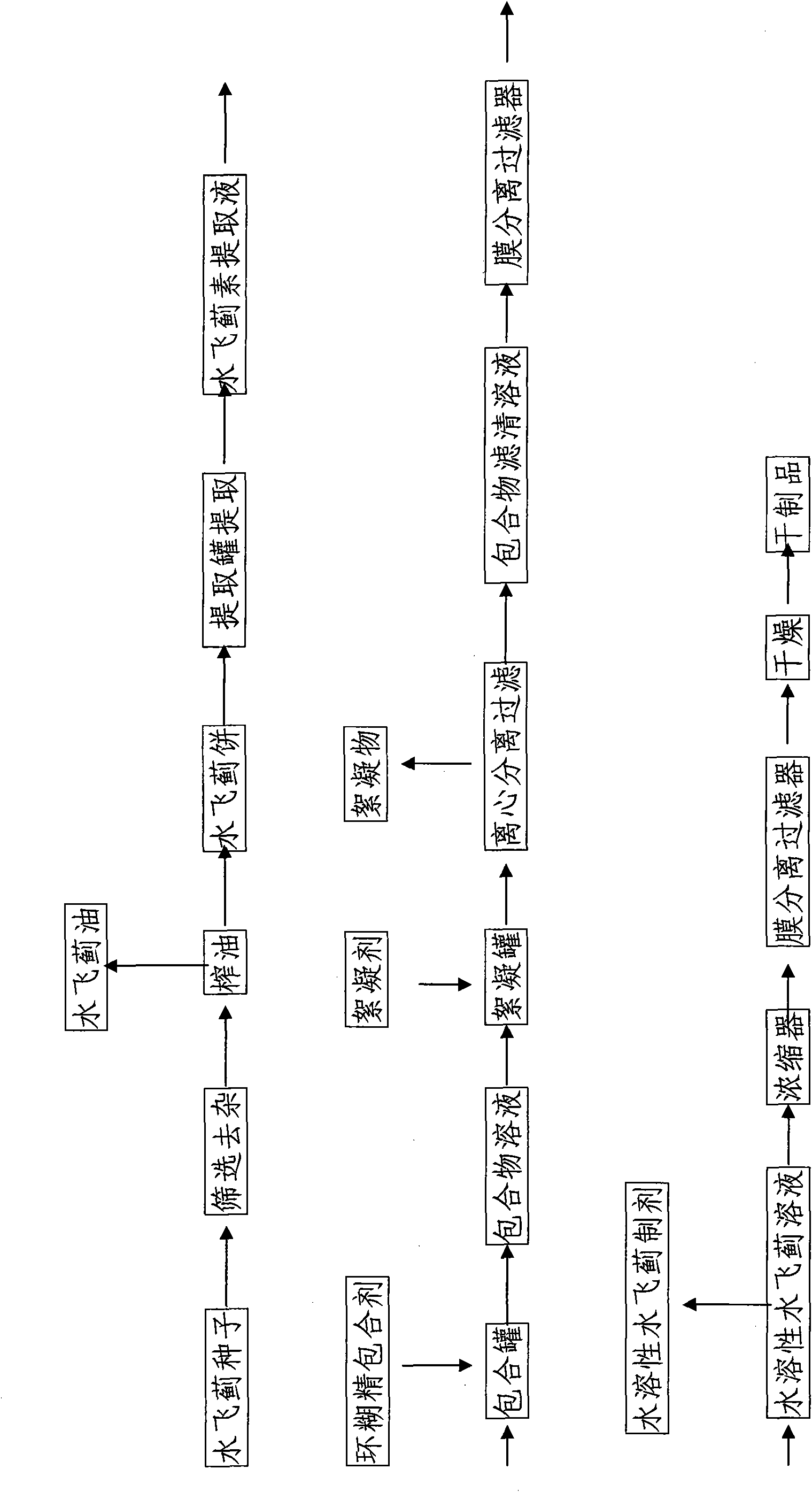
The invention discloses water-soluble silymarin, which is an inclusion compound that takes cyclodextrin as main molecules and silymarin as guest molecules; and the inclusion compound contains 12 to 20wt % of silybinin. The preparation method thereof comprises the following steps: silybum marianum seeds are squeezed into silybum marianum cakes; the silybum marianum cakes are added into alkali water ethanol solution and are heated to 75DEG C to 85DEG C, the constant temperature is kept for 1.5-2.5 hours, and silybum marianum extracting solution is prepared after 2-4 times of continuous extraction; the silybum marianum extracting solution is added with the cyclodextrin for inclusion, thus obtaining inclusion compound solution; flocculant is added, standing after being well stirred; after flocculate is filtered out, membrane separation process is carried out, thus obtaining water-soluble silymarin solution; and after the membrane separation process, the water-soluble silymarin solution is concentrated and dried, thus obtaining the dried product of the water-soluble silymarin. The water-soluble silymarin prepared by the method has the advantages of high solubility, good quality, good drug stability and high bioavailability.
Owner:江苏健佳药业有限公司
Inclusion method of medicament containing volatile component
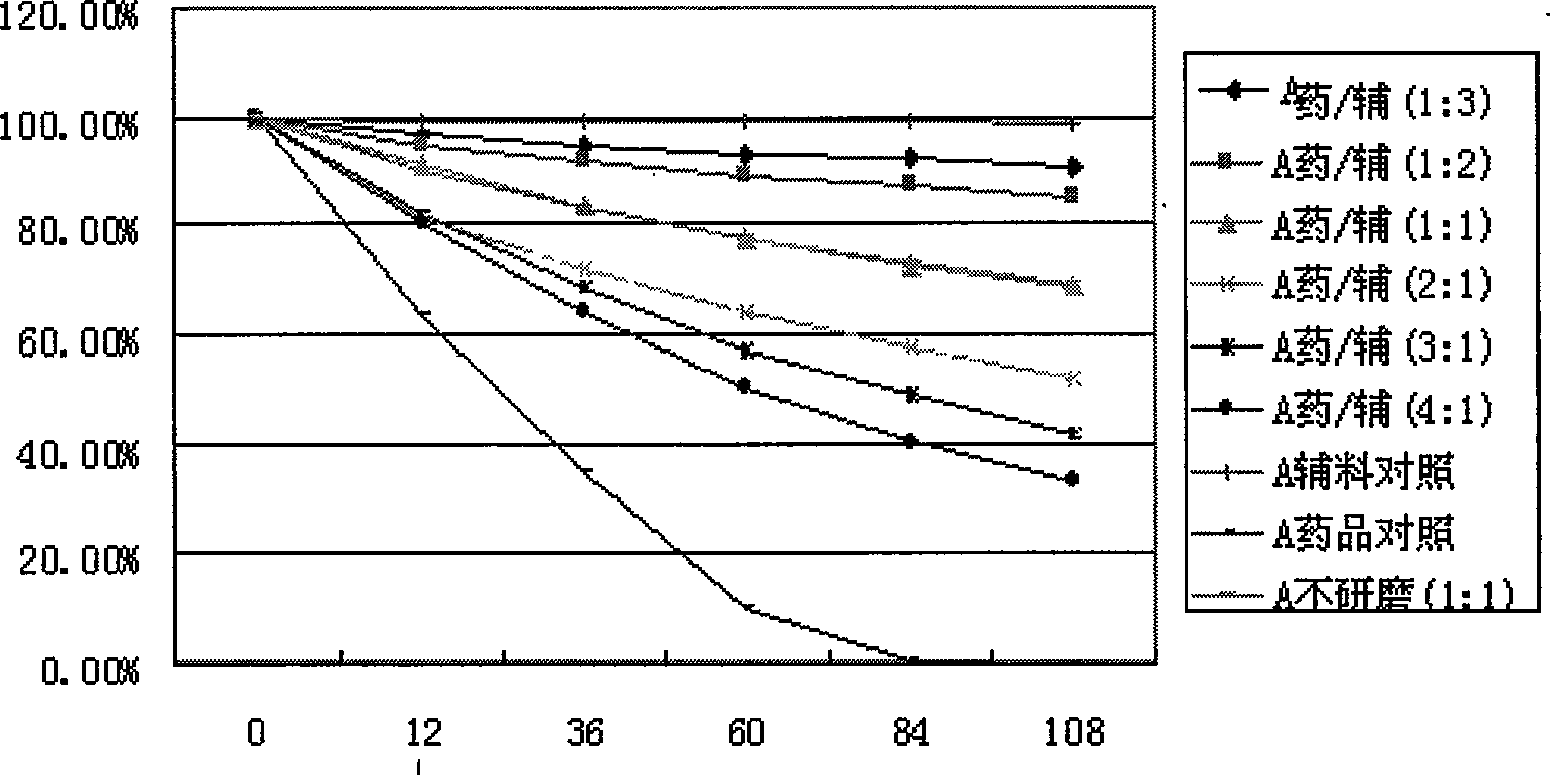
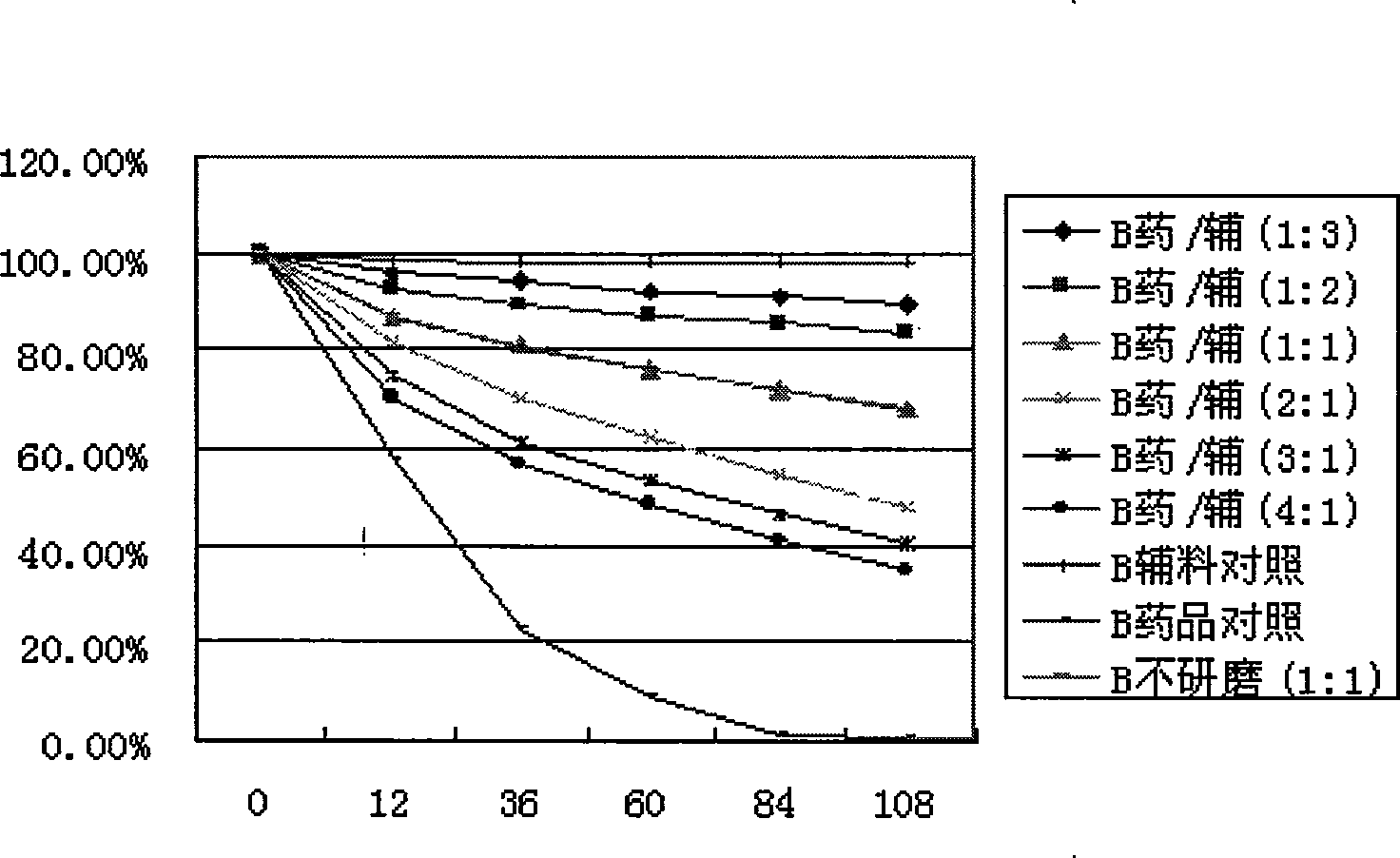
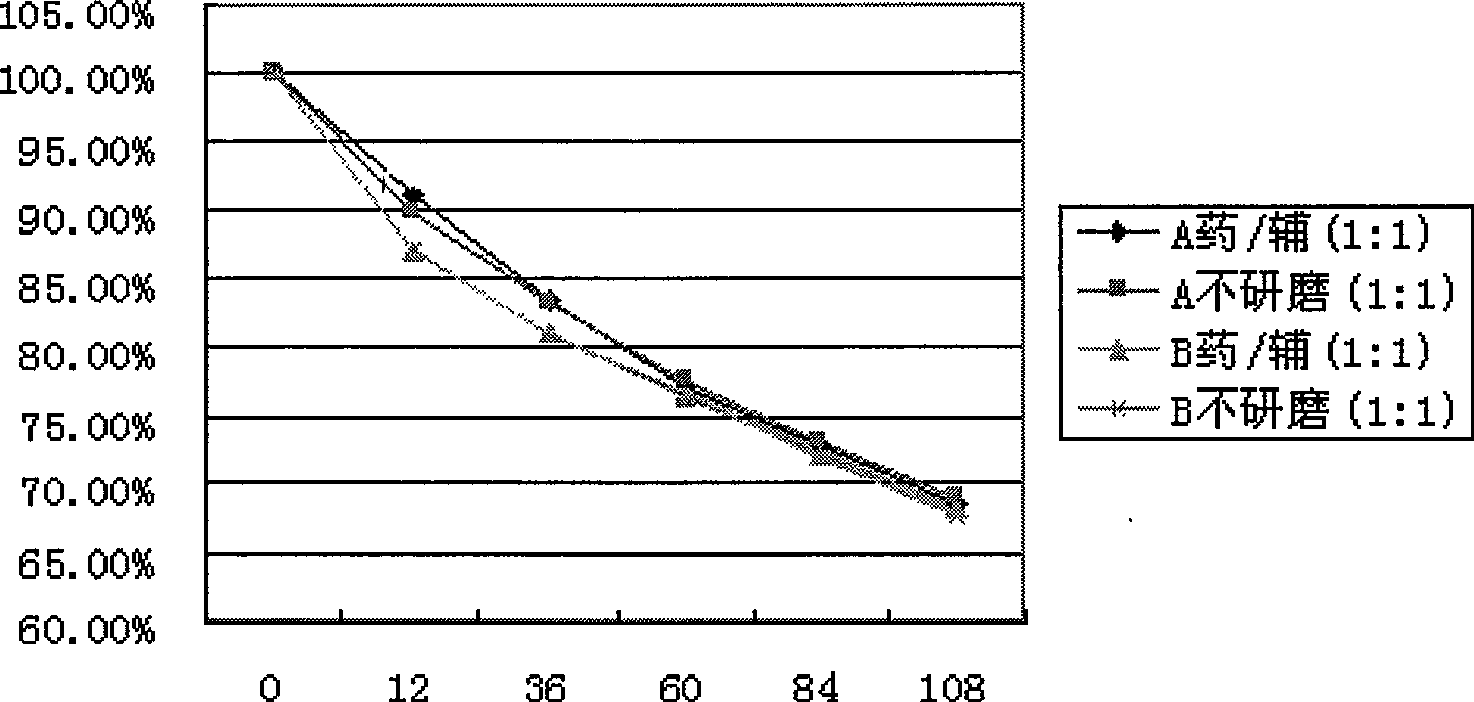
The invention discloses an inclusion method for enhancing the stability of drug containing volatile components. Crospovidone is added during the inclusion process of the drug containing the volatile component. The proportions of the drugs and the crospovidone are 4:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7 or 1:8. Materials can be ground during the inclusion process, and regular organic solvent such as ethanol, aether and the like can also be added. The proportions of the drug and the organic solvents are 2:1, 1:1 or 5:1, wherein the drugs mainly includes volatile traditional Chinese medicine in common use, such as borneol, menthol and the like. The invention, by experiments, proves that the crospovidone can reduce the volatile quantity of model drugs such as the borneol and the menthol, and the action can be promoted by certain mixing method and adding proper solvent.
Owner:PREMIER SPECIALTY CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com