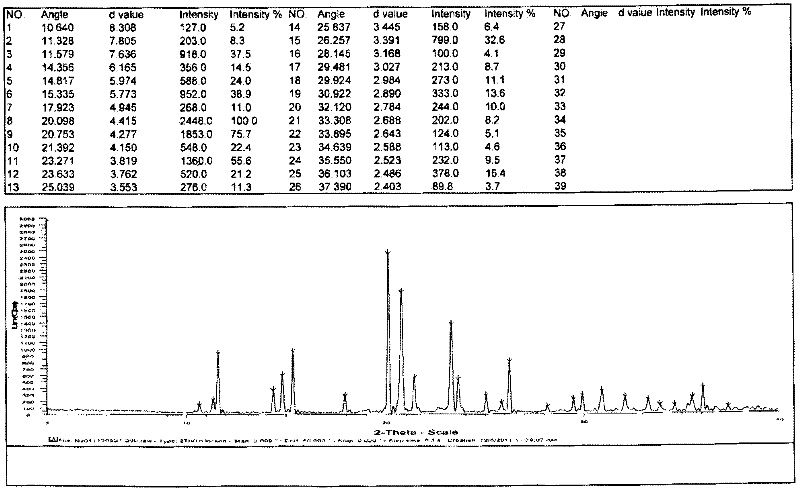
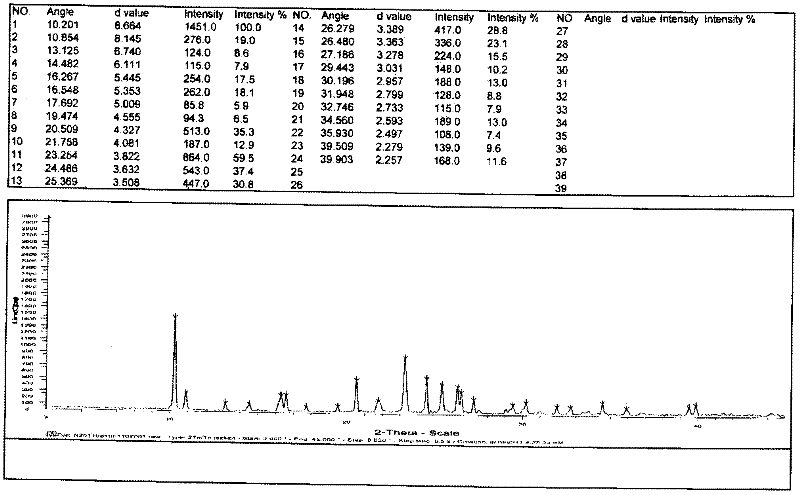
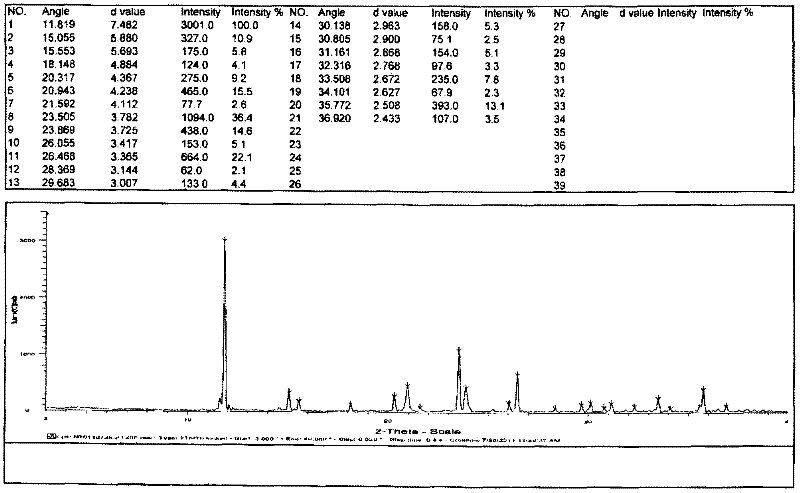
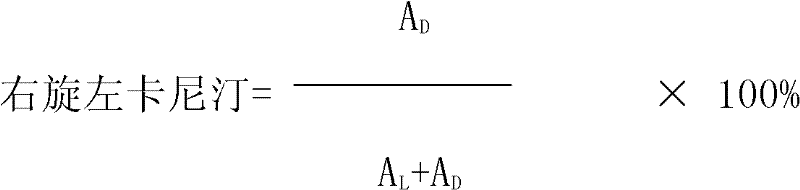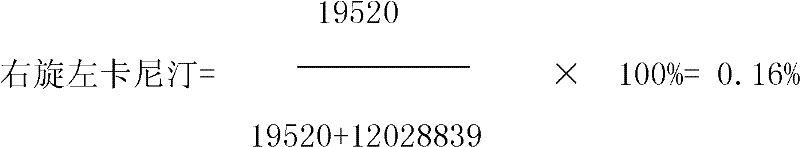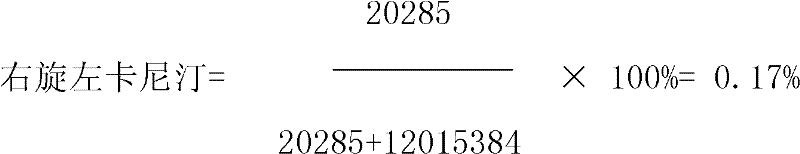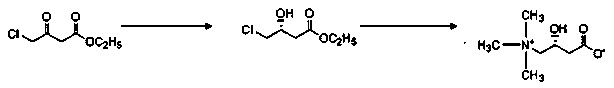Patents
Literature
115 results about "Levocarnitine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
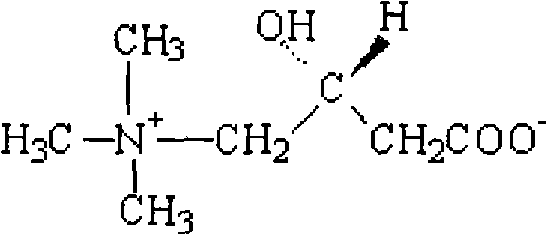
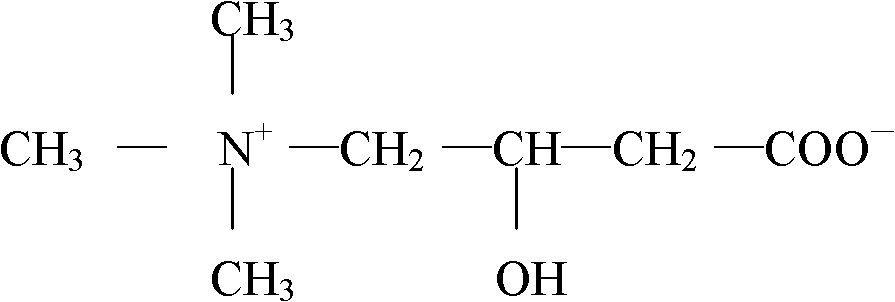
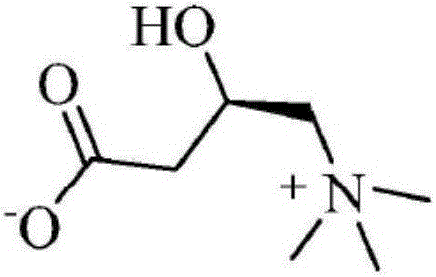
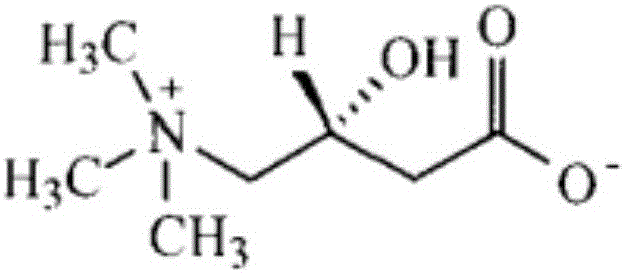
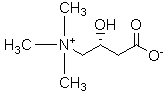
An amino acid derivative. Levocarnitine facilitates long-chain fatty acid entry into mitochondria, delivering substrate for oxidation and subsequent energy production. Fatty acids are utilized as an energy substrate in all tissues except the brain. Check for http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=301896&idtype=1 active clinical trials or http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=301896&idtype=1&closed=1 closed clinical trials using this agent. (http://nciterms.nci.nih.gov:80/NCIBrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&code=C26657 NCI Thesaurus)
Levocarnitine liposomes injection
InactiveCN101637450AUnexpected effectImprove stabilityOrganic active ingredientsMetabolism disorderAcute toxicity testingCholesterol
The invention discloses levocarnitine liposomes injection, which is characterized by comprising the following active ingredients in parts by weight: 1 part of levocarnitine, 3-15 parts of soybean lecithin, 0.4-7.5 parts of cholesterol and 0.02-1 part of antioxidant, and a pharmaceutically acceptable carrier, wherein, the antioxidant is one or more of L-cysteine, thiourea, vitamin E and butylated hydroxyanisole and is most preferably the vitamin E. The invention also discloses a preferable preparation method of the levocarnitine liposomes injection, namely ammonium sulphate pH gradient method.The invention provides levocarnitine liposomes injection which has excellent stability, high entrapment rate and low leakage rate in the process of long-term storage. The acute toxicity tests, unusualtoxicity tests and heat source tests adopting the levocarnitine liposomes injection all conform to the specifications and are applicable to industrialized production.
Owner:HAINAN YONGTIAN PHARMA INST
Medicine combination capable of reducing myocardial infarction area and use thereof
The invention relates to a pharmaceutical composition for reducing myocardial infarction area, and a pharmaceutical preparation containing the same and an application thereof. The pharmaceutical composition comprises levocarnitine or a derivative thereof and a drug for improving hemodynamics. The dosages of levocarnitine or the derivative thereof and the drug for improving hemodynamics are dosages capable of effectively reducing myocardial infarction area.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Levocarnitine composition for injection and preparation method of levocarnitine composition
ActiveCN102327238AReduce dosageReduce skeletonOrganic active ingredientsPowder deliveryPorosityFreeze-drying
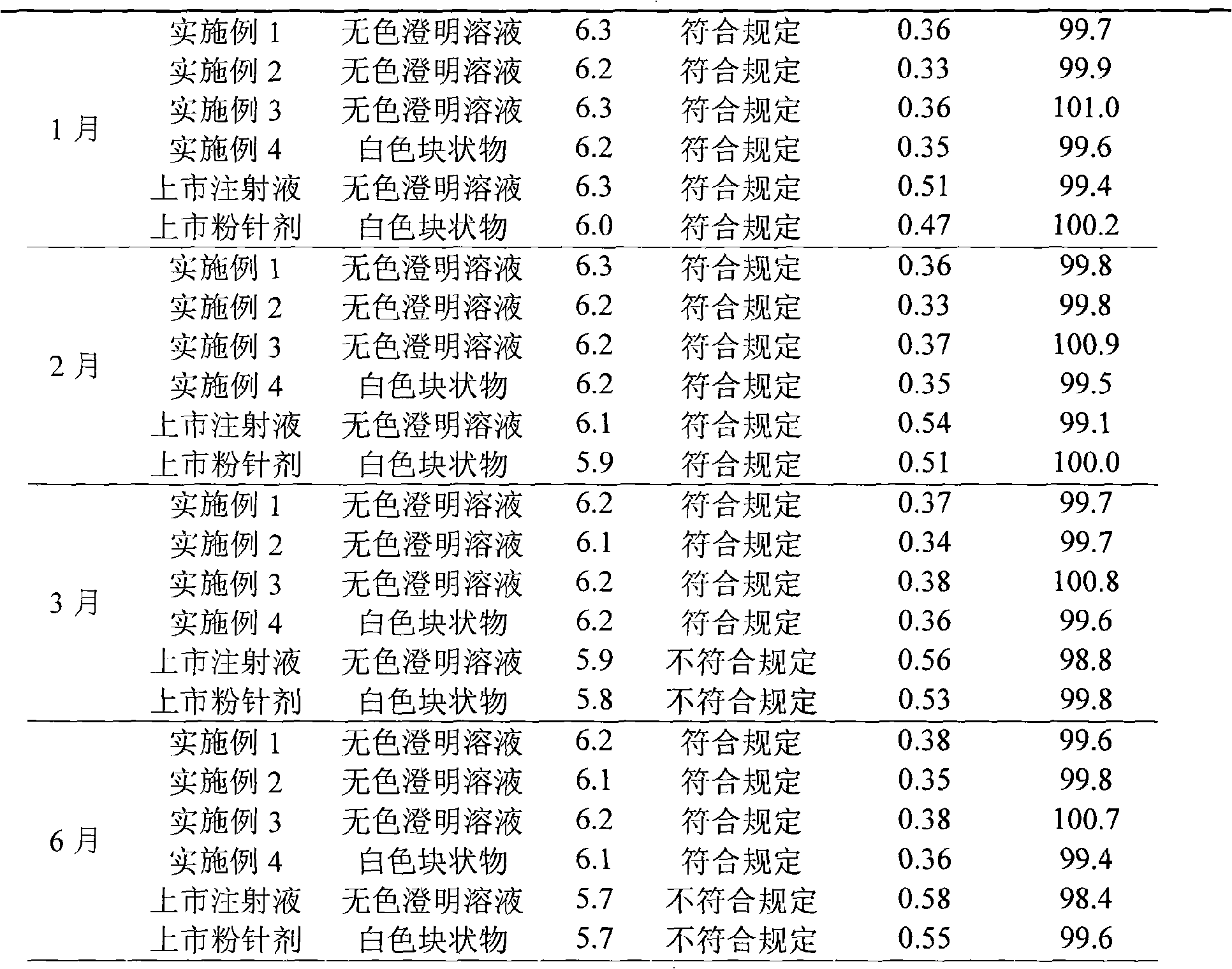
The invention relates to a levocarnitine composition for injection and a preparation method of the levocarnitine composition; the levocarnitine composition is lyophilized powder containing levocarnitine and mannitol, wherein the weight ratio of the levocarnitine to the mannitol is 1:(0.75-1.25); the average particle diameter of the lyophilized powder is 90-130 nm, and the porosity is 94-98%. The preparation method comprises the steps of: 1) preparing: weighing levocarnitine and mannitol, putting the levocarnitine and the mannitol in a preparing tank, adding injection water, agitating to enable the levocarnitine and the mannitol to be completely dissolved and uniformly mixing, regulating the pH to 5.7-6.3 through a 0.1mol / L hydrochloric acid solution; 2) decarburizing and sterile filtering; 3) sterile packaging; 4) vacuum freeze drying to obtain the levocarnitine composition . The levocarnitine composition has the advantages of simple preparation, advanced technique, uniform quality, excellent stability, better redissolution capability and clinic medicine application safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
A kind of preparation method and quality control method of compound injection
The invention relates to a preparation method and a quality control method of a compound injection, belonging to the fields of preparation methods and quality control methods of injections. The compound injection is prepared from levocarnitine or derivatives / pharmaceutical salts thereof and trimetazidine or derivatives / pharmaceutical salts thereof as active pharmaceutical ingredients, and a pharmaceutically acceptable vector. By adopting a separate active pharmaceutical ingredient preparation process and a thick mixing / thin mixing technique, the preparation method maximally reduces the loss of the active pharmaceutical ingredients, ensures the product quality and implements the practicability of the technique; and in the quality control method, the contents of the two active pharmaceutical ingredients are simultaneously detected by high efficiency liquid chromatography, and dextrocarnitine is used as a related substance to carry out the detection. Thus, the quality control method is scientific and reasonable, and can be used for instructing production.
Owner:CHANGZHOU SIMM DRUG RES & DEV CENT
Levocarnitine for injection and preparation method thereof
ActiveCN102309475ANon-allergenicEfficient processOrganic active ingredientsMetabolism disorderActivated carbonPenicillin
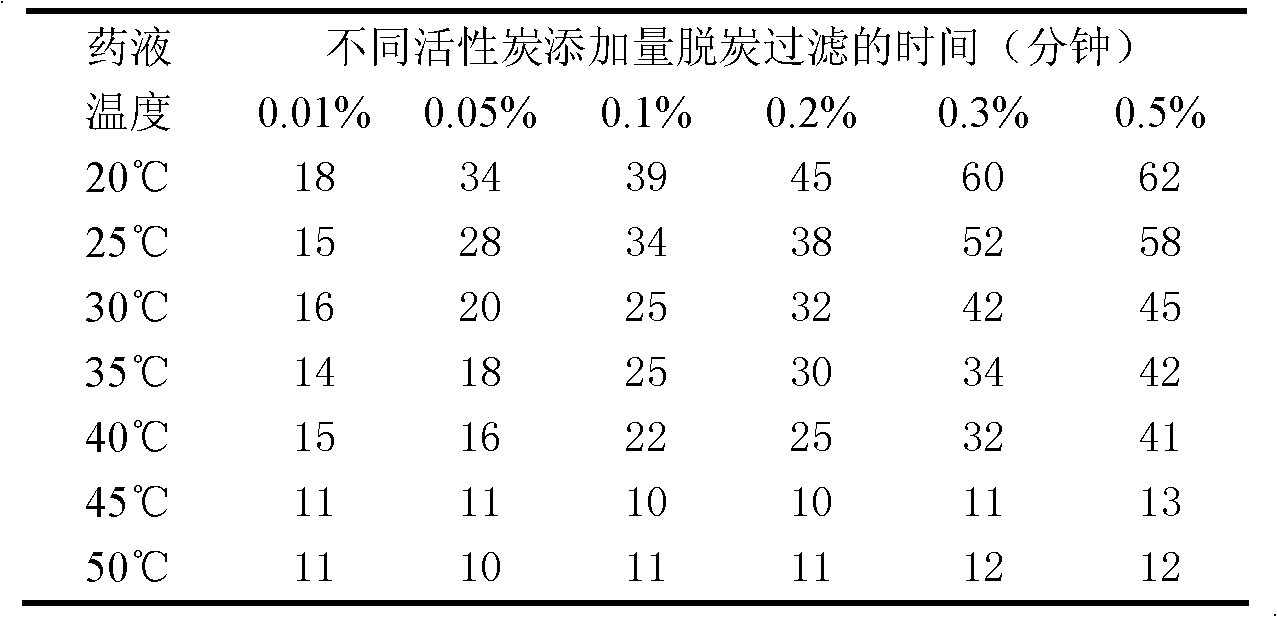
The invention discloses a levocarnitine for injection and a preparation method thereof. The preparation method comprises the following steps: weighing sodium dihydrogen phosphate and sodium hydroxide, using injection water to dissolve the weighed sodium dihydrogen phosphate and sodium hydroxide and diluting and uniformly mixing, dissolving levocarnitine and mannitol in the buffer solution, addingneedle active carbon and stirring uniformly, stirring for adsorption, and using filtering membrane for filtering and removing carbon; carrying out fine filtration through 0.22 mum microporous membrane, after the intermediate passed the inspection, filling the filtrate with the amount of 5 ml in 15 ml penicillin bottle (using up the liquor in 6 h after degerming), and carrying out half-plug pressing, freeze-drying, capping, visual inspection, labeling, and packaging. According to the invention, buffer salts (the buffer salt ion pair consisting of sodium dihydrogen phosphate and sodium hydroxide) with buffer effect are used in stead of traditional hydrochloric acid to regulate the pH value of the levocarnitine solution, so that the main drug is always in a stable pH environment in the wholepreparation process, and the API degradation caused by violent change of pH value of levocarnitine is avoided. Compared with the similar kind products, the levocarnitine disclosed in the invention has higher effectiveness and higher safety.
Owner:长春海悦药业股份有限公司
Pharmaceutical composition for reducing the area of myocardial infarction and its use
This invention relates to a pharmaceutical composition for preventing and curing myocardium ischemia and reducing area of myocardial infarction, its pharmaceutical preparation and applications. The composition includes (a) levocarnitine or its derivatives, and (b) trimetazidine or its medicative salts. The quantity of levocarnitine or its derivatives, and trimetazidine or its medicative salts in the composition is effective amount for treating myocardial ischemia and reducing the area of myocardial infarction.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Health-care food with anti-oxidation and lowering weight functions
InactiveCN100998411AImprove stabilityObvious effectOrganic active ingredientsPowder deliverySoftgelGrape seed
A health-care food in the form of softgel, capsule, particle, tablet, or oral liquid for losing weight and antioxidizing is proportionally prepared from extract of grape seed, extract of oolong tea, levocarnitine, and wheat embryo bud oil.
Owner:吉林云尚保健食品有限公司
Medicament composition and application thereof
The invention relates to a medicament composition, a medicament preparation containing the medicament composition and application of the medicament composition. The medicament composition comprises levocarnitine or derivative thereof and rhein or pharmaceutically acceptable salt thereof. The medicament composition shows an excellent effect of preventing and treating mucosal injury caused by various reasons or diseases caused or characterized by the injury.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Desalinisation process for salt-containing water solution of levocarnitine
InactiveCN101337902AReduce consumptionLess investmentOrganic compound preparationAmino-carboxyl compound preparationDesalinationWastewater
A method for desalting a salt-containing L-carnitine solution comprises the following steps: selecting the salt-containing L-carnitine solution with a weight concentration of 6.1% 2000 Kg, adjusting the pH value to 5 to 10, separating impurities, pre-desalting by an electrodialysis method to achieve the desalination rate of 60 to 90%, separating inorganic ammonium salt to obtain the pre-desalted salt-containing L-carnitine solution, desalting the pre-desalted salt-containing L-carnitine solution by resin exchange method to obtain a salt-containing L-carnitine solution, evaporating, concentrating, removing ammonia, and crystallizing to obtain 113 Kg L-carnitine product with a content of 99.74%, wherein the yield of L-carnitine is 92.6%. By adopting electrodialysis and resin exchange for desalting the salt-containing L-carnitine solution, the method can greatly reduce the use amount of resin and achieve less equipment investment, lower consumption of auxiliary materials, lower energy consumption, less amount of wastewater, higher yield, higher quality of the L-carnitine product, and greatly reduced desalination cost of the salt-containing L-carnitine solution. Additionally, useful inorganic ammonium byproducts can be obtained.
Owner:开原亨泰精细化工厂
Levocarnitine thin film coated tablets and preparation method thereof
ActiveCN102349881AReduce crushReduce the sieving processOrganic active ingredientsMetabolism disorderFiller ExcipientPolyethylene glycol
The invention relates to levocarnitine thin film coated tablets applied in medicine and sanitation industries. The levocarnitine thin film coated tablets are prepared from a levocarnitine tablet core and a coating layer, wherein the levocarnitine tablet core is composed of 45-55wt% of levocarnitine, 40-50wt% of filling agent, 0.5-5wt% of glidant and 0.5-3wt% of lubricant; the coating layer comprises an isolation coating layer and a moisture-proof coating layer; the filling agent is one or more of microcrystalline cellulose, lactose, calcium hydrogen phosphate, hydroxypropyl cellulose and pregelatinized starch; the glidant is one or more of silicon dioxide and talc powder; the lubricant is one or more of magnesium stearate, polyethylene glycol and leucine; and the isolation coating layer of the coating layer is one or more film forming materials of hydroxypropyl methyl cellulose, ethyl cellulose and polyvinyl pyrrolidone or a premixed material containing the film forming materials. Thelevocarnitine thin film coated tablets and the preparation method thereof have the advantages that: the levocarnitine tablet core is prepared by direct tabletting through prescription screening; the operation is easy to control; the raw material pulverizing and screening processes are omitted; moisture absorption of the raw materials after pulverization is avoided; the process is simplified; the production cost is lowered; and the production efficiency is improved.
Owner:NORTHEAST PHARMA SHENYANG SCI & TECH DEV +1
Wrapping agent in levo-carnitine wrapping technology and composition of ten series products and preparation method
InactiveCN102125692AIncrease content concentrationIncreased sensitivityOrganic active ingredientsAntipyreticMoistureHard Capsule Dosage Form
The name of the invention is: wrapping agent in levo-carnitine wrapping technology and composition of ten series products and preparation method. The wrapping agent in levo-carnitine (also known a L-carnitine, levocarnitine, vitamin BT, carnitine) wrapping technology is applicable to the application and manufacture in medicine, health food, both edible and medicinal food, and food fields. Polyacrylic resin No. II is used as a wrapping material, and 95% anhydrous alcohol is used as a diluent; the materials are mixed and soaked in proportion to prepare the wrapping agent; with the wrapping agent, air isolation of products prepared by levo-carnitine is achieved, and the problems are solved that products prepared by levo-carnitine are quite easy to absorb moisture and become denatured, and is not applicable to solid preparations. The ten series products are series products with levo-carnitine as a main raw material and used for treating and regulating different symptoms with different compositions; based on the granulation process in medicine manufacturing process, traditional Chinese medicines of different compositions with levo-carnitine as a main raw material are mixed with the wrapping agent to prepare granules, and the granules are canned into a hard capsule dosage form or a granule dosage form.
Owner:王华明
Preparation method of levocarnitine
ActiveCN106748843AHigh optical purityHigh yieldOrganic compound preparationCarboxylic acid esters preparationAlkaline hydrolysisMedicinal chemistry
The invention provides a preparation method of levocarnitine. The preparation method comprises the following steps: taking epoxy chloropropane as a starting material, then carrying out amination, cyaniding and carrying out ester exchange under the action of lipase CALB to obtain corresponding chiral ester, then carrying out alkaline hydrolysis and acidification, and then removing chlorine ions under the action of strongly alkaline resin, so that the levocarnitine finished product is obtained. In the invention, acid resin is used in an ester exchange process, and recemization can be realized, so that yield and optical purity of the levocarnitine are improved.
Owner:WUXI FORTUNE PHARMA
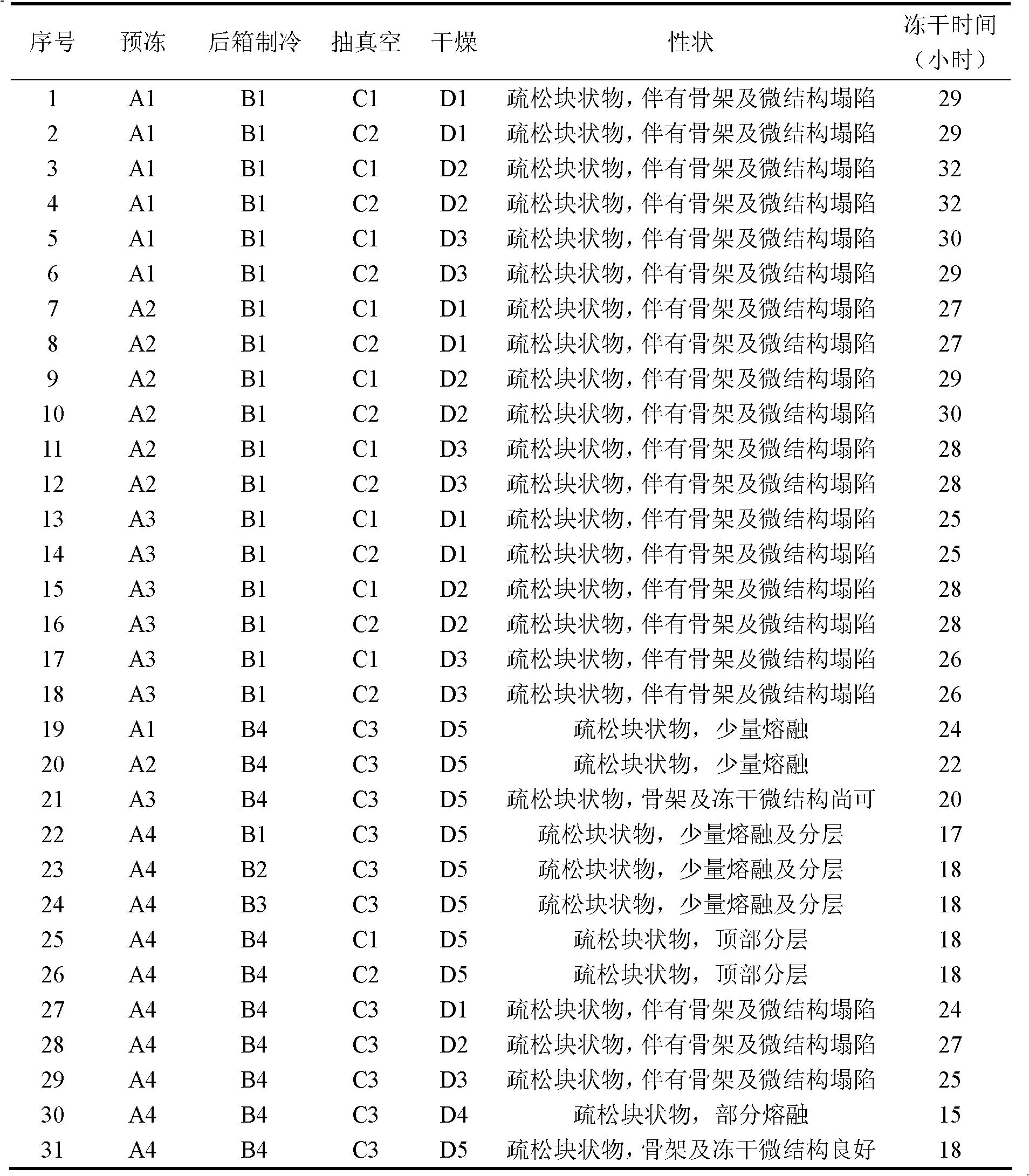
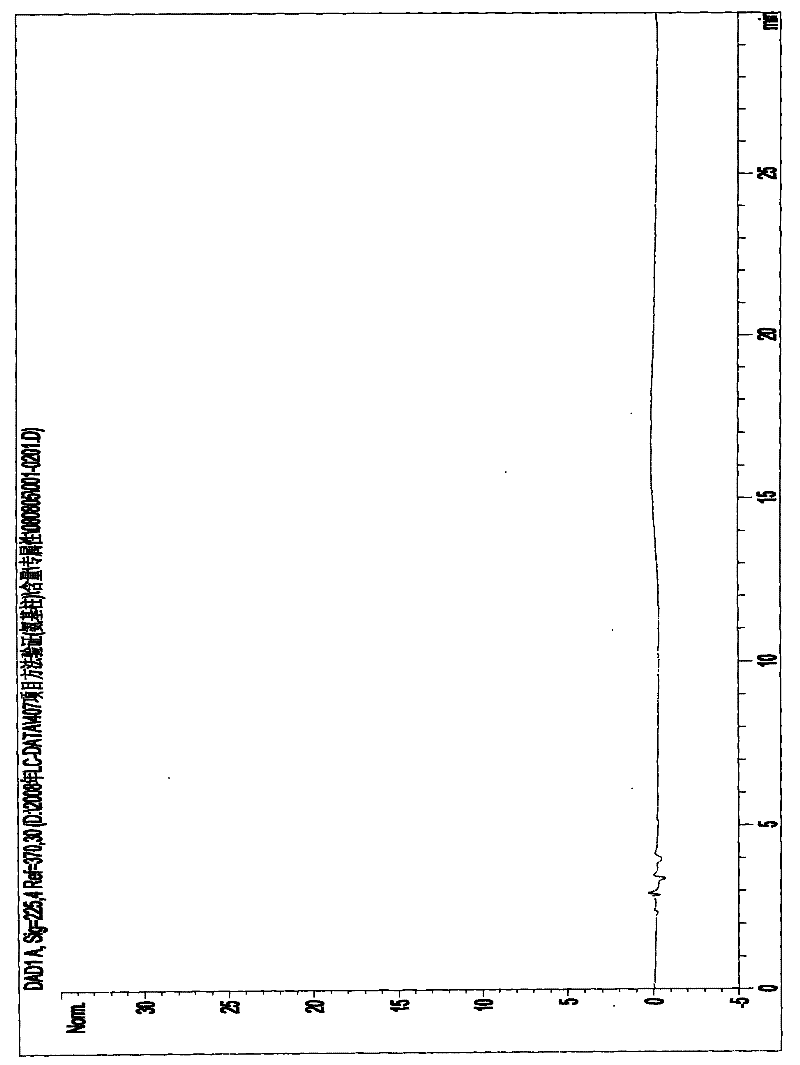
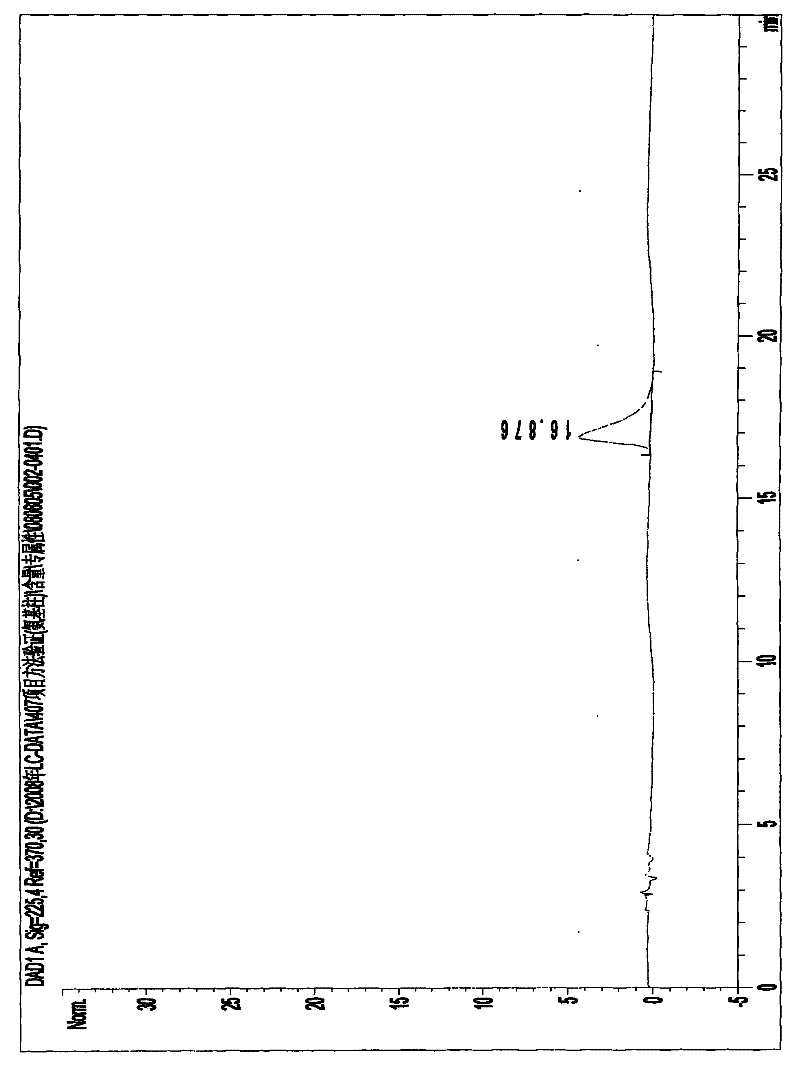
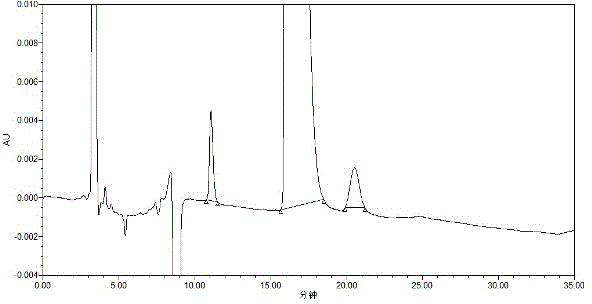
High performance liquid detection method for content of D-Carnitine in levocarnitine and levocarnitine salt product
ActiveCN104698101AEfficient separationHigh sensitivityComponent separationRetention timeCyclodextrin
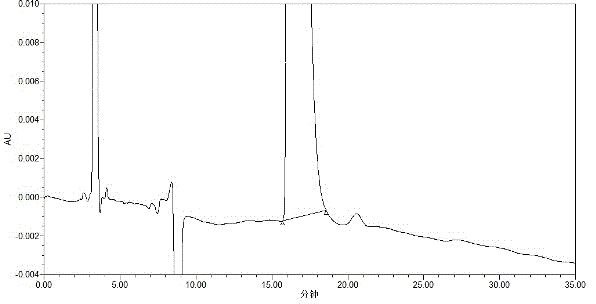
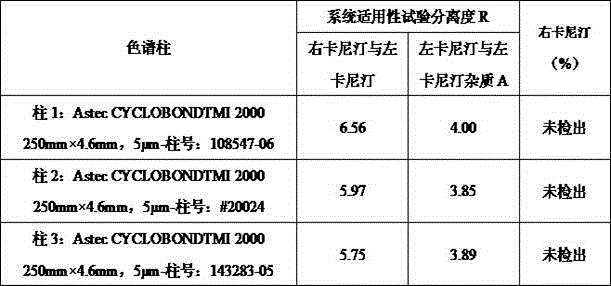
The invention discloses a high performance liquid detection method for the contents of D-Carnitine in levocarnitine and a levocarnitine product, and belongs to the field of medicament detection. According to the method, a chiral stationary phase high performance liquid chromatography method is adopted; a chiral stationary phase is formed by bonding cyclodextrin and a derivative of cyclodextrin with a silica gel filler, so that the separation effect of three components such as levocarnitine, D-Carnitine and levocarnitine impurities A is good, and the method is high in repetitiveness and durability; a flowing phase adopts a triethylamine-acetic acid aqueous solution and an acetonitrile system; the flowing phase is basically lossless relative to a chiral chromatographic column; the chromatographic peak retention time is high in repetitiveness, and the peak type is symmetric.
Owner:NORTHEAST PHARMA GRP
A pharmaceutical composition treating severe high-altitude diseases
InactiveCN104138377AEffective treatmentOrganic active ingredientsAntinoxious agentsDiseaseTrimetazidine
The present invention provides a pharmaceutical composition used for treating severe altitude sickness, characterized by a composition consisting of trimetazidine or pharmaceutically acceptable salt thereof and L-carnitine or derivative thereof or pharmaceutically acceptable salt thereof, and the weight ratio of the above being 1:200.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
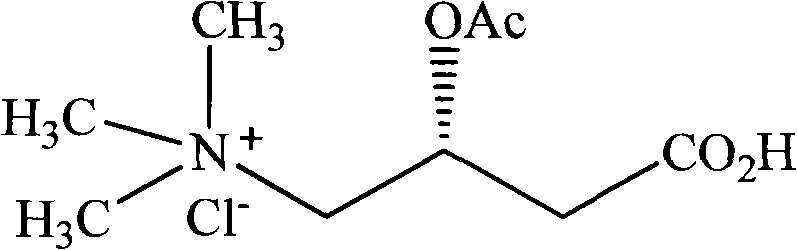
Polycrystal substance of acetyl chloride levocarnitine
The invention provides new crystal solid forms of acetyl chloride levocarnitine, namely acetyl chloride levocarnitine of type II and type III, and a composition comprising the crystal solid forms and a method for preparing the crystal solid forms.
Owner:南京海辰药业股份有限公司
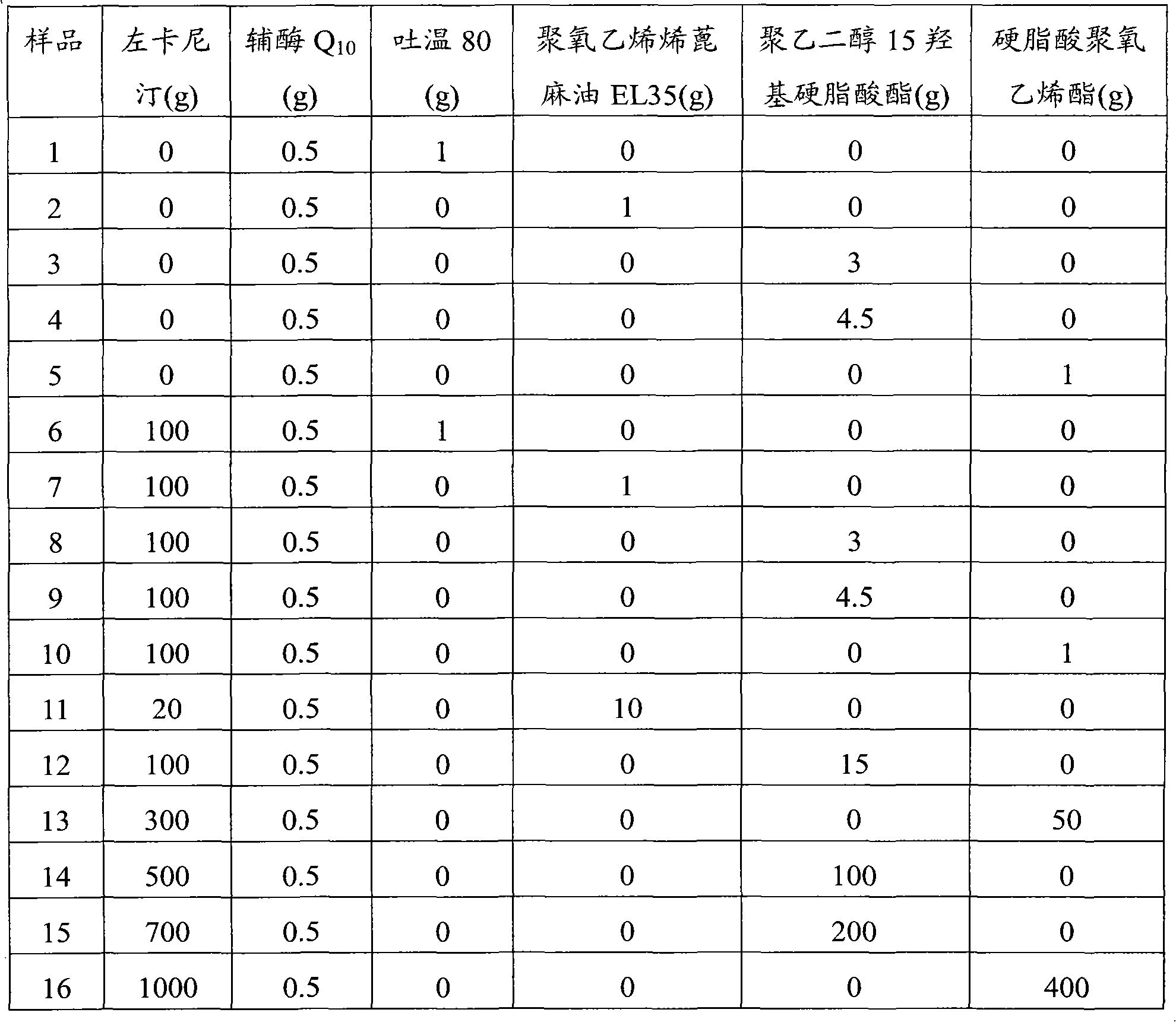
Pharmaceutical composition containing L-carnitine and coenzyme Q10 as well as its preparation method
InactiveCN102552923AImprove stabilityImprove securityOrganic active ingredientsMetabolism disorderPolyoxyethylene castor oilPolyethylene glycol
The present invention provides a pharmaceutical composition containing L-carnitine and coenzyme Q10 as well as its preparation method. The pharmaceutical composition comprises L-carnitine or its pharmaceutically acceptable salt or ester, coenzyme Q10 or its pharmaceutically acceptable salt or ester, and a solubilizing agent, wherein the solubilizing agent comprises one or more selected from polyoxyethylene castor oil, polyoxyethylene hydrogenated castor oil, stearic acid polyoxyethylene ester and polyethylene glycol 15 hydroxy stearate . The prepared pharmaceutical composition which takes L-carnitine and coenzyme Q10 and / or their pharmaceutically acceptable salts as an active ingredient has the advantages of good stability, high security, stable preservation for long-term and safe usage; and the pharmaceutical composition can fully perform the synergy of two ingredients, has the merits of convenient usage, economical and practical effects, and possesses a good prospect.
Owner:LIAONING THINKING DECKER MEDICAL TECH +4
A sustained release agent improving anoxia endurance
InactiveCN104138376AIncrease blood oxygen saturationOrganic active ingredientsAntinoxious agentsTrimetazidinePerylene derivatives
The present invention discloses a slow-release agent for increasing hypoxia tolerance; the slow-release agent is a composition formed by combining trimetazidine or pharmaceutically acceptable salt thereof and L-carnitine or derivative thereof or pharmaceutically acceptable salt thereof, according to the ratio 1:1.6-6400.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Drug combination containing levocarnitine derivatives and preparation method of drug combination
InactiveCN102579370ASubstance increaseReduce contentOrganic active ingredientsPowder deliveryLiquid stateMedicine
The invention relates to a drug combination containing levocarnitine derivatives and a preparation method of the drug combination, wherein, the drug combination is stable under both a solid state and a liquid state. The drug combination comprises ethanoyl chloride levocarnitine with crystal form II and / or crystal form III, proper lyophilized injectable powder excipients and alkali or buffer agents.
Owner:南京海辰药业股份有限公司
Composition for treating diabetic nephropathy
InactiveCN103751450AOrganic active ingredientsAnthropod material medical ingredientsRhizomeDiabetic nephropathy
The invention discloses a composition for treating diabetic nephropathy, wherein the composition is prepared from the following raw medicines: 0.5-1.5g of levocarnitine, 20-40g of astragalus membranaceus, 20-40g of bighead atractylodes rhizome, 10-30g of the root of red-rooted salvia, 10-30g of cortex cinnamomi, 5-25g of parasitic loranthus, 5-25g of ootheca mantidis, 5-25g of flatstem milkvetch seed, 5-25g of poria cocos, 5-25g of forged fossil fragments, 5-25g of forged concha ostreae, 2-15g of red flower and 2-10g of rhizoma alismatis. In use, the composition mainly comprises qi-tonifying medicine, interior-warming medicine, blood-activating blood and stasis-dissolving medicine, heat-clearing medicine, water-disinhibiting and damp-percolating medicine and is supported by western medicine components, so that therapeutic principle and method for tonifying deficiency and tonifying qi, activating blood and dissolving stasis and clearing heat and promoting dieresis are determined; finally, the unexpected technical effect is achieved.
Owner:青岛山大齐鲁医院(山东大学齐鲁医院(青岛))
Detection method for dextroisomer in levocarnitine
The invention provides a detection method for dextroisomer in levocarnitine, which belongs to the field of pharmaceutical analysis. The detection method comprises the following steps: preparing chromatographic conditions, that is, using a C18 column with a filling agent of octadecylsilane chemically bonded silica and a particle size of 5 mu m as a chromatographic column, setting detection wavelength of excitation wavelength lambda ex and fluorescence wavelength lambda em to be 260 nm and 315 nm respectively, and using tetra-n-butylammonium hydroxide-methanol and acetonitrile-water as mobile phases; preparing a solvent of tetra-n-butylammonium hydroxide; preparing a reagent of fluorenylmethyl chloroformate; preparing a system suitability solution; preparing a solution of a test sample; carrying out a derivative reaction; carrying out detection by using HPLC (using a fluorescence detector and the method of gradient elution). The method provided in the invention has reproducibility, sensitivity and accuracy according with requirements when used for detection of dextroisomer in levocarnitine.
Owner:NORTHEAST PHARMA GRP
Levocarnitine composition for injection and preparation method thereof
ActiveCN106265544AImprove freeze-drying efficiencyReduce adverse effectsOrganic active ingredientsPowder deliveryPorosityGlycine
The invention belongs to the technical field of medicine and particularly relates to a levocarnitine composition for injection and a preparation method thereof. The levocarnitine composition for injection comprises levocarnitine, tranexamic acid, mannitol and glycine. The levocarnitine composition for injection has the remarkably-excellent freeze-drying structure, average grain diameter and porosity, the redissolution performance of the levocarnitine composition for injection can be effectively improved, and the stability and consistency of the performance within the period of validity of a preparation are guaranteed.
Owner:REYOUNG PHARMA
Pharmaceutical composition for reducing the area of myocardial infarction and its use
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
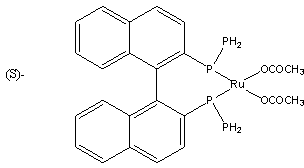
Preparation method of L-carnitine compound
ActiveCN104030934AHigh optical purityHigh yieldOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisTwo step
The invention discloses a preparation method of a levocarnitine compound, and the preparation method is applied to the technical field of pharmaceutical chemical synthesis. The preparation method comprises the following steps that (1), 4-chloroacetoacetic acid ethyl ester serves as the initial raw material, under the condition that a solvent exists, an asymmetric catalyst phosphine ligand ruthenium complex is applied for hydrogenation reduction, and (R)-4-chlorine-3-hydroxybutyrate ethyl ester is obtained by vacuum concentration and high vacuum distillation; (2), a trimethylamine squeous solution and the (R)-4-chlorine-3-hydroxybutyrate ethyl ester are added dropwise and slowly in the solvent comprising inorganic base, the dropping speed is controlled, low temperature reaction is carried out, then indoor temperature reaction is carried out, the pH of the mixture is adjusted to be 6 through concentrated hydrochloric acid after reaction is finished, and the levocarnitine compound is obtained by resin column purification. According to the method, the asymmetric catalytic reaction is applied, the two-step reaction that an optical active intermediate with high optical purity and high yield can be obtained and the optical active intermediate is converted to be the levocarnitine compound is the one-pot reaction, the water serves as the reaction solvent, the inorganic base is used for catalysis, the unique process of indoor temperature reaction is adopted, the product quality is good, the purity is high, the yield can reach up to 80%, the reaction steps of the preparation method are short, operation is easy, pollution to environment is small, and green resources are protected.
Owner:NORTHEAST PHARMA GRP
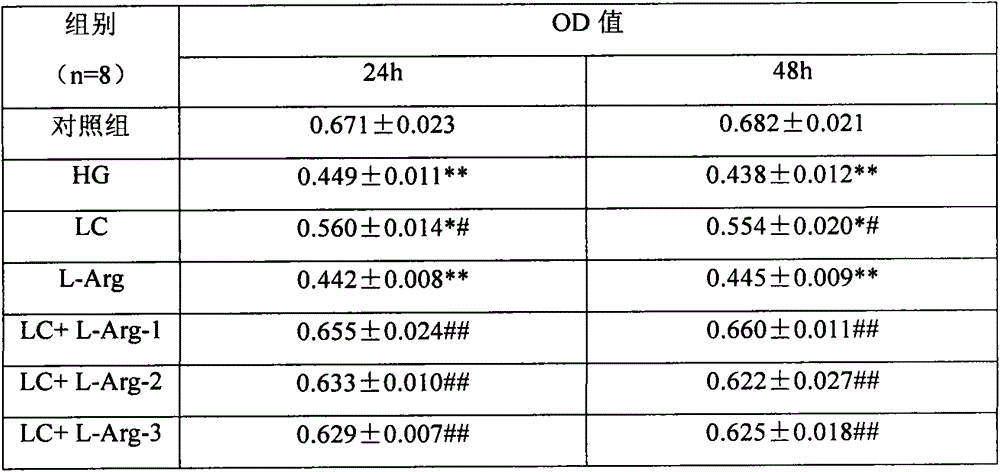
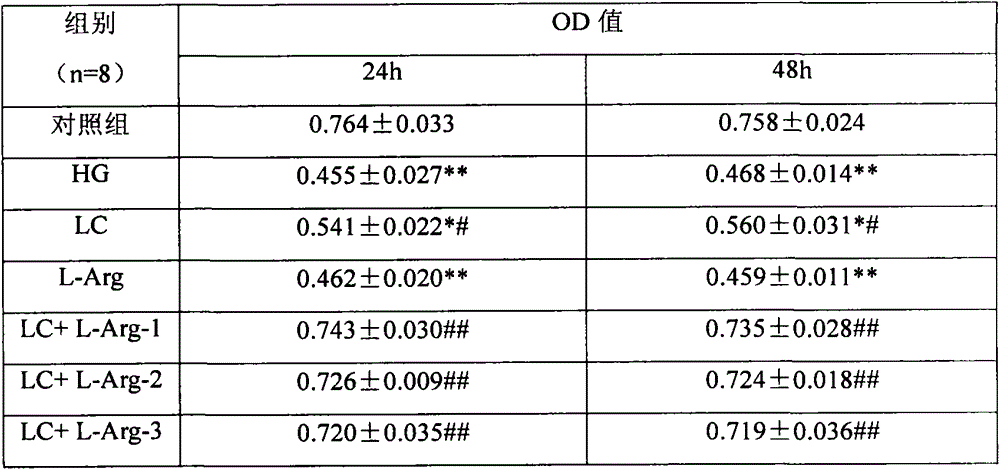
Application of combination of levocarnitine and L-arginine in preparation of drugs for treatment of diabetic retinopathy nerve damage
InactiveCN103948581AProtects against nerve damageOrganic active ingredientsSenses disorderDiabetes retinopathyArginine
The invention discloses application of a pharmaceutical composition containing L-arginine and levocarnitine in combined preparation of drugs for treatment of diabetic retinopathy nerve damage, and particularly relates to application of the pharmaceutical composition in preparation of the drugs for treatment of retinal nerve damage and Muller cell injury of diabetic retinopathy nerve damage. Cell and animal experiments show that compared with the single use of the levocarnitine, the diabetic retinopathy nerve damage and particularly the retinal nerve damage and Muller cell injury of diabetic retinopathy nerve damage can be more effectively protected by combination of the levocarnitine and the L-arginine, and the technical effect of synergistic interaction can be achieved.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Levocarnitine composition and preparation method thereof
InactiveCN105853347AImprove thermal stabilityReduce contentOrganic active ingredientsMetabolism disorderThermal stabilityImpurity
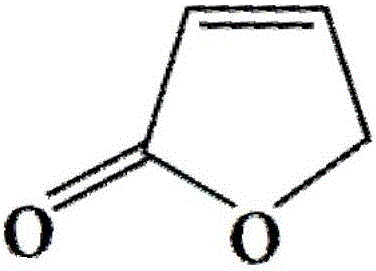
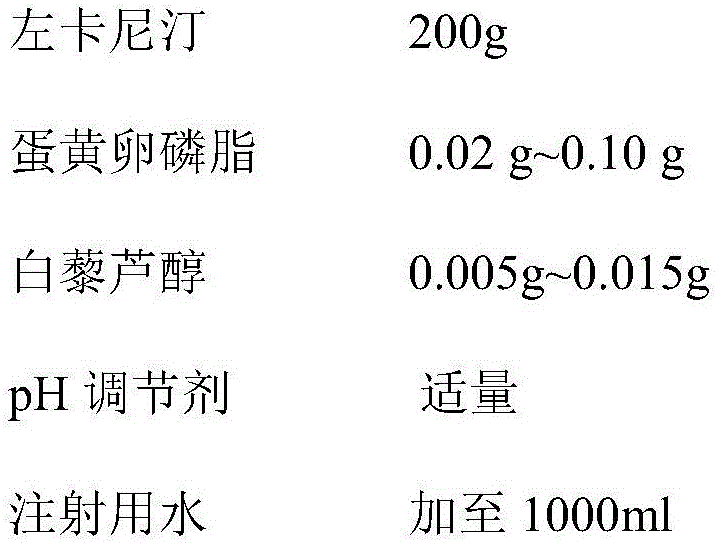
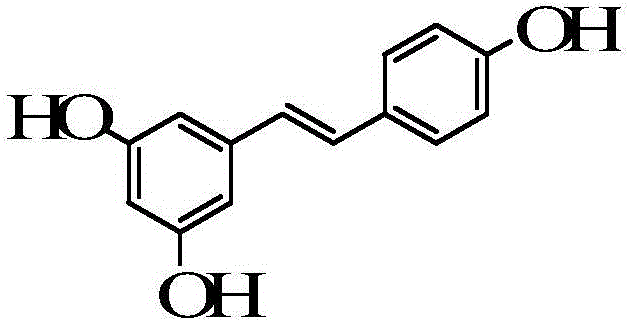
The invention belongs to the technical field of medicines, and particularly relates to a levocarnitine composition and a preparation method thereof. The composition is prepared from the following components: 200g of levocarnitine, 0.02-0.10g of egg yolk lecithin, 0.005-0.015g of resveratrol, a proper amount of pH regulator and water for injection which is added until the total volume is 1000ml. The composition has excellent thermal stability, and the content of pyrolyzed impurity 2(5H)-furanone generated in a high-temperature sterilization process can be greatly reduced.
Owner:HAINAN HERUI PHARMA
Method for synthesizing levocarnitine by taking D-(-)-tartaric acid as raw material
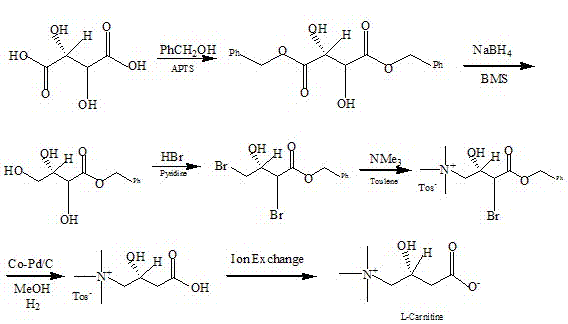
ActiveCN103044278ASimple production technologyMild reaction conditionsOrganic compound preparationAmino-carboxyl compound preparationIon exchangeBENZYL ALCOHOL/WATER
The invention discloses a method for synthesizing levocarnitine by taking D-(-)-tartaric acid as raw material, and relates to a method for synthesizing levocarnitine. The method takes D-(-)-tartaric acid as raw material and comprises the following steps of: (I) synthesizing D-(-)-dibenzyl tartrate with benzyl alcohol by taking boric acid as a catalyst; (2) reducing the D-(-)-dibenzyl tartrate to obtain D-(-)-2,3,4-methyl trihydroxy benzene by taking sodium borohydride as a catalyst; (III) bromizing in a hydrogen bromide acetate solution to obtain D-(-)-3-hydroxy-2,4-methyl dibromo-benzene; (IV) introducing hydrogen and performing selective debromination hydrogenation to obtain D-(-)-3-hydroxyl-4-methyl bromobenzene by using a Pd / C catalyst; and (V) performing trimethylamine amination, hydrolysis and ion exchange to obtain levocarnitine. The process flow does not adopt a resolving agent and cyanide, the reaction conditions are mild, pollution is prevented, and the cost is low.
Owner:开原亨泰营养科技有限公司
Antiaging composition
InactiveCN103800735APromote growthImprove the environmentOrganic active ingredientsAntinoxious agentsWestern medicineTraditional medicine
The invention discloses an antiaging composition, which is characterized in that the antiaging composition is prepared from the following raw materials: levocarnitine 0.5-1.5 g, Astragalus membranaceus 25-45 g, Acanthopanax senticosus 25-45 g, Ziziphus jujuba 10-30 g, parched Coix lacryma-jobi seed 10-20 g, and Radix Glycyrrhizae 10-30 g. In the formula, Astragalus membranaceus has effects of invigorating qi and consolidating superficial resistance; Acanthopanax senticosus has effects of supplementing qi and tranquilizing mind; Ziziphus jujuba has effects of tranquilizing mind and supplementing qi; parched Coix lacryma-jobi seed has effects of strengthening the spleen and eliminating dampness; Radix Glycyrrhizae has effect of regulating various medical materials. The antiaging composition can generate synergistic antiaging effect with Western medicinal components.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Fatty liver therapeutic drug
InactiveCN1520810AEasy to carryEasy to useOrganic active ingredientsDigestive systemVascular diseaseWestern medicine
The present invention relates to fatty liver treating medicine, and is especially one kind of Western medicine preparation with levocarnitine as main component. The medicine preparation consists of levocarnitine in 20-100 wt% and mannitol in 0-80 wt% as matrix, and is prepared through crushing levocarnitine, mixing levocarnitine with the matrix, pelletizing and preparing into tablet or capsule. The medicine preparation is easy to absorb, fast in acting and obvious in treating effect, and is used in treating fatty liver, slimming, cardiac and cerebral vascular diseases, etc.
Owner:张永 +1
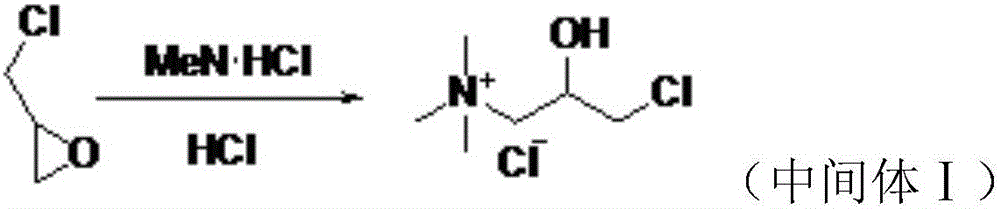
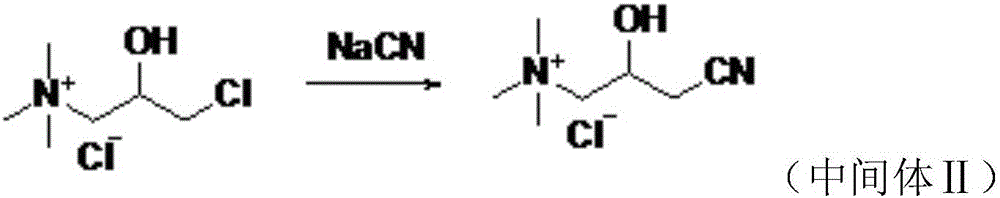
Preparation method of levocarnitine intermediate
PendingCN108727221AGood colorLow impurity contentOrganic compound preparationPreparation by cyanide reactionPhytic acidSodium cyanide
The invention discloses a preparation method of a levocarnitine intermediate. The preparation method comprises the following steps: taking S-epichlorohydrin as a starting raw material; firstly, takingS-epichlorohydrin and trimethylamine hydrochloride to be subjected to amination reaction, so as to obtain L-3-chloro-2-hydroxypropyltrimethyl ammonium chloride; then taking L-3-chloro-2-hydroxypropyltrimethyl ammonium chloride and sodium cyanide to be subjected to cyanation reaction to obtain the levocarnitine intermediate, i.e., L-3-cyano-2-hydroxypropyltrimethyl ammonium chloride. After the cyanation reaction is finished, a complexing agent is used for treating a reaction system; preferably, the complexing agent is phytic acid; after the cyanation reaction is finished, an adsorbent is usedfor treating the reaction system; preferably, the adsorbent is activated carbon. According to the method disclosed by the invention, the complexing agent is added to treat after the amination reaction, so that the color of an L-quaternary ammonium salt solution can be remarkably improved, and furthermore, the color of L-nitrile can be remarkably improved and the impurity content of L-nitrile is reduced. According to the method disclosed by the invention, the adsorbent is added to treat after the cyanation reaction, so that the color of L-nitrile can be remarkably improved, and furthermore, theimpurity content of L-nitrile is reduced.
Owner:CHANGZHOU LANLING PHARMA
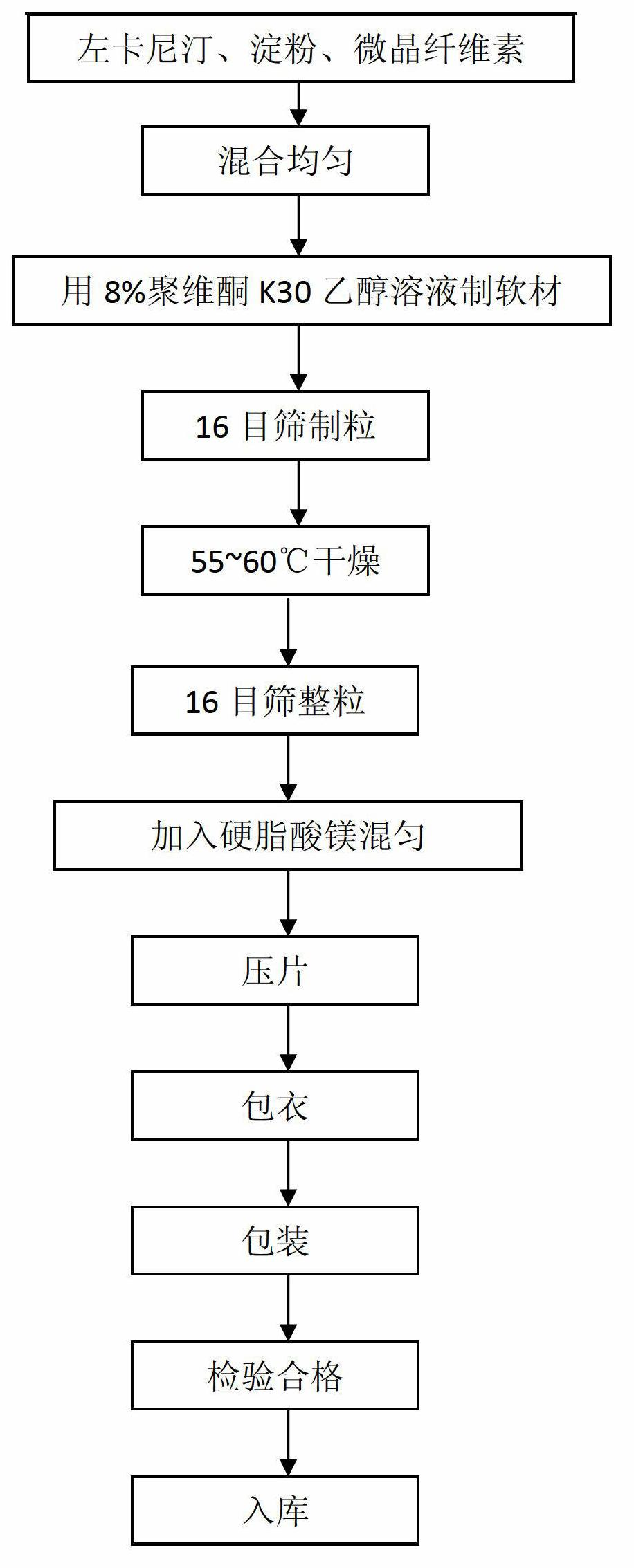
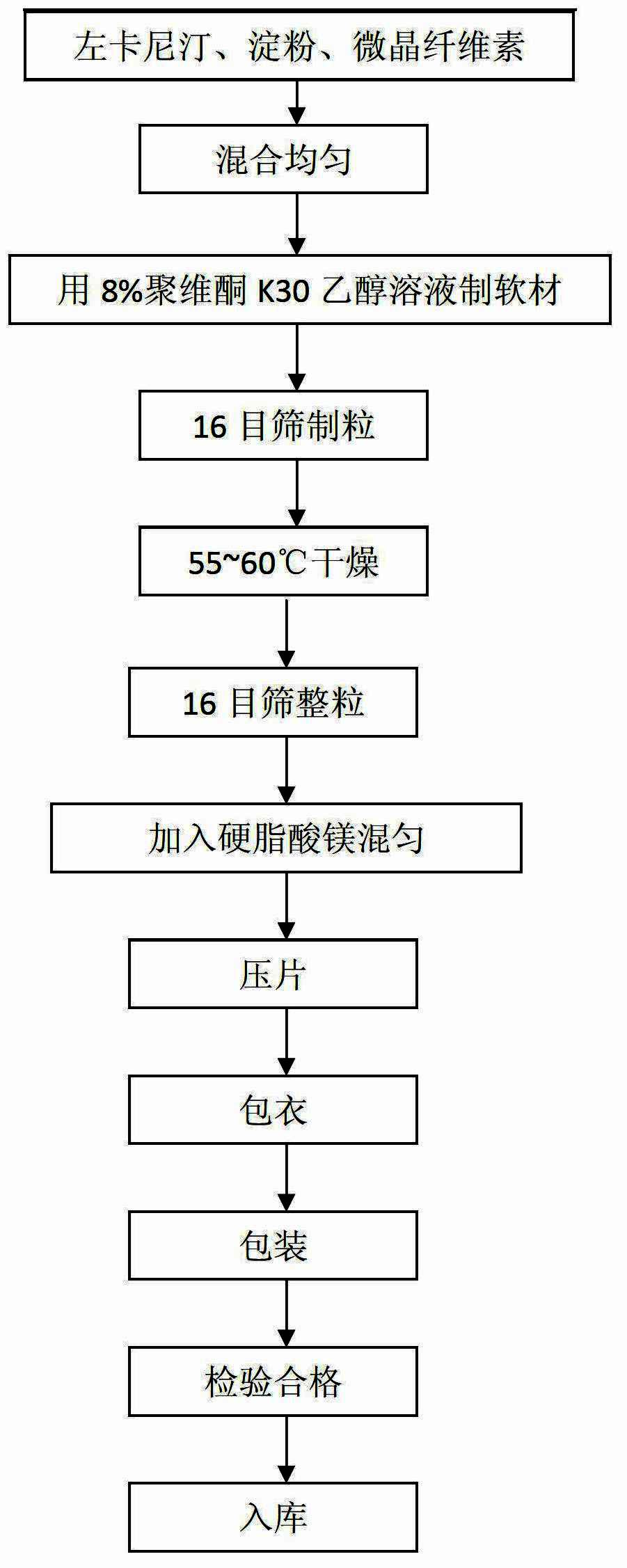
Levocarnitine coating tablet and preparation method thereof
InactiveCN102657632ASolve the moisture problemLong shelf lifeOrganic active ingredientsMetabolism disorderMagnesium stearateSoft materials
The invention discloses a levocarnitine coating tablet and a preparation method thereof, and belongs to the technical field of the preparation of medicines. The levocarnitine coating tablet comprises levocarnitine, starch, microcrystalline cellulose, 8 mass percent ethanol solution of povidone K30, magnesium stearate, an ethanol solution of an Opadry 21K film coating premix and an aqueous solution of Opadry 85 G film coating premix. The preparation method comprises the following steps of: sieving the levocarnitine, the starch, the microcrystalline cellulose and the magnesium stearate with an 80-mesh sieve for later use; mixing the levocarnitine, the starch and the microcrystalline cellulose uniformly to prepare mixed powder, adding the ethanol solution of the povidone K30 into the mixed powder to prepare soft materials, and sieving with a 16-mesh sieve to prepare granules and drying; adding the dried granules into the magnesium stearate, sieving with a 16-mesh sieve, and mixing uniformly; and tabletting, and spraying a film coat on the surface of the tablet. The levocarnitine coating tablet can be stored easily for a long term and does not absorb moisture, and is low in production cost; and the preparation method is easy to operate and high in efficiency.
Owner:QINGDAO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com