A kind of preparation method and quality control method of compound injection
A quality control method and injection technology, which are used in medical preparations containing active ingredients, pharmaceutical formulas, measuring devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
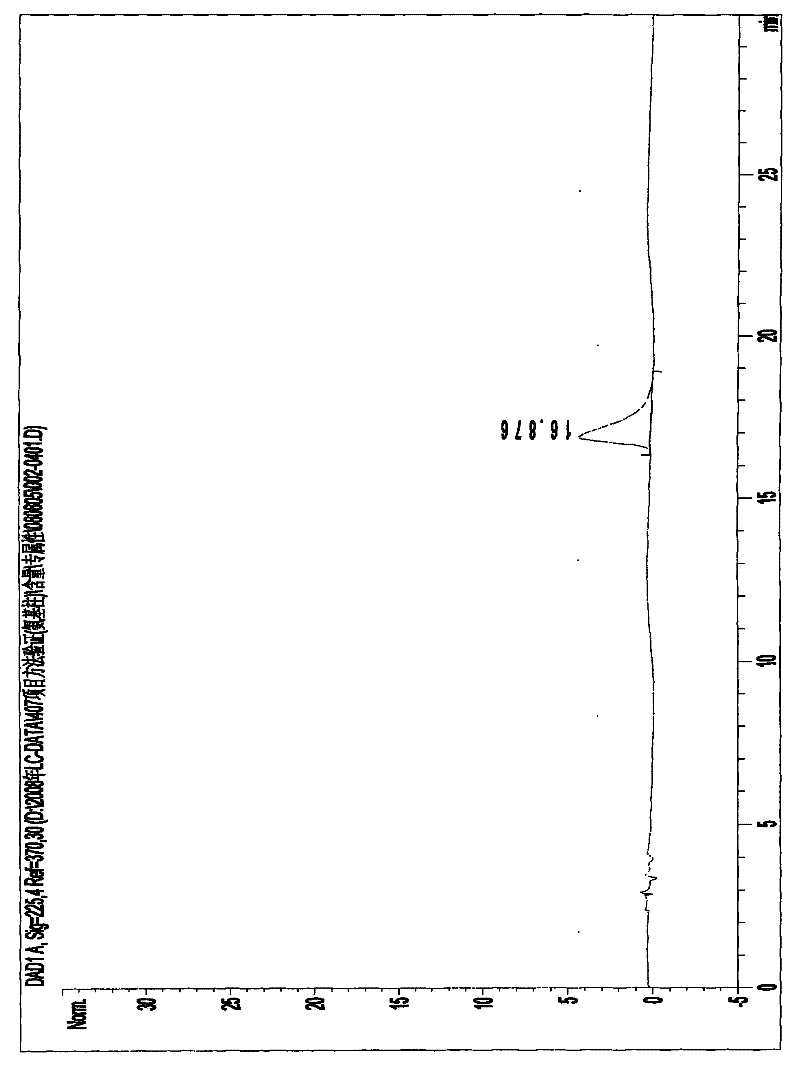
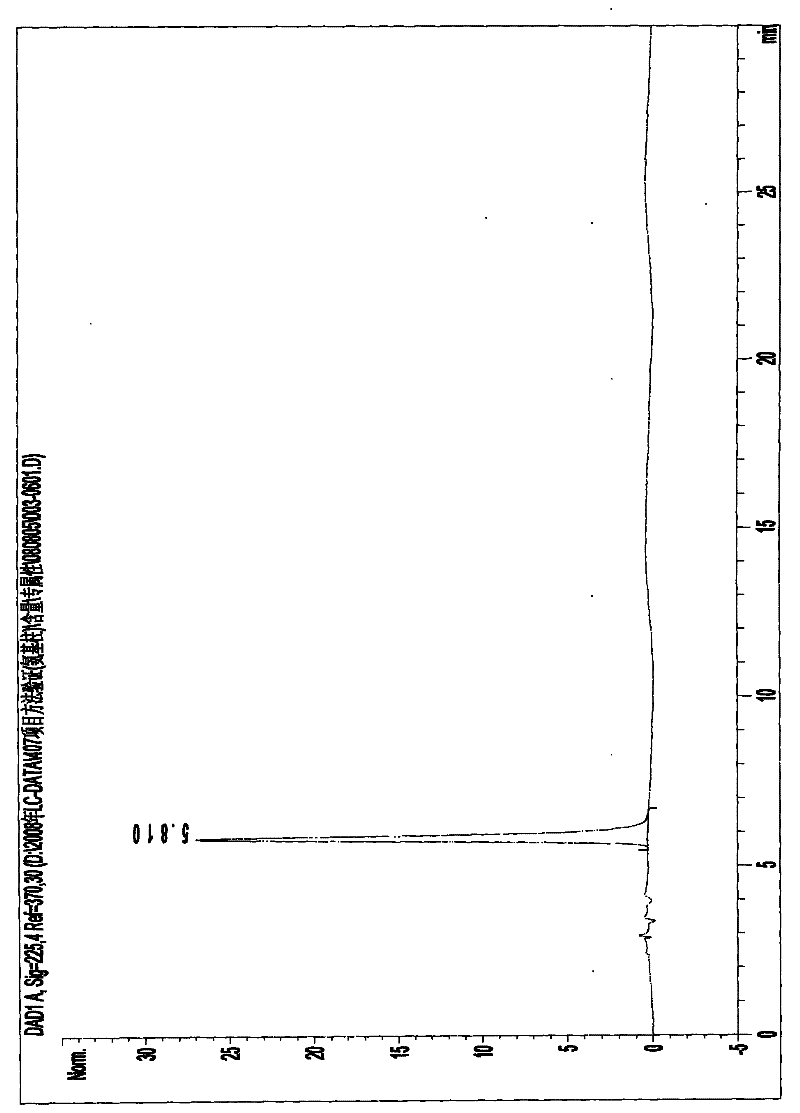
Image
Examples
Embodiment 1
[0068] Compound levocarnitine injection (5mL: levocarnitine 1000mg, trimetazidine hydrochloride 5mg) preparation prescription
[0069]
[0070] Experimental operation
[0071] 1.20% levocarnitine solution dilute
[0072] (1) Feeding (for preparing compound levocarnitine injection 100000mL)
[0073]
[0074] (2) Preparation: Weigh 20.8 kg of levocarnitine raw material, stir and dissolve in 60,000 mL of water for injection at 60°C, after dissolving, add water for injection at 60°C to about 90,000 mL.
[0075] (3) pH adjustment: stir and add hydrochloric acid to the above-mentioned levocarnitine solution, and adjust the pH to 6.02.
[0076] (4) Decolorization: Weigh 401.21g of activated carbon (0.4% cast, w / v), add it into the pH-adjusted levocarnitine solution while it is hot, stir in a water bath at 60°C for 30 minutes, and the stirring speed is about 200r / min.
[0077] (5) Filtration: After the decolorization is completed, when the temperature of the solution drops be...
Embodiment 2
[0100] Compound acetyl-levocarnitine injection (5mL: acetyl-levocarnitine 1000mg, trimetazidine 5mg) prescription
[0101]
[0102] Experimental operation
[0103] 1. Dilute 20% acetyl-levocarnitine solution
[0104] (1) Feeding (for the preparation of compound acetyl-levocarnitine injection 5000mL)
[0105]
[0106] (2) Preparation: Weigh 1.06kg of acetyl-levocarnitine raw material, stir and dissolve it in 3000mL of water for injection at 60°C and add 1.50g of EDTA. After dissolving, add to 4900mL with 60°C of water for injection.
[0107] (3) pH adjustment: Stir and drop hydrochloric acid into the above-mentioned acetyl-levocarnitine solution to adjust the pH to 5.6.
[0108] (4) Decolorization: Weigh 10.02g of activated carbon (0.2%, W / V), add it to the pH-adjusted acetyl-levocarnitine solution while it is hot, decolorize in a water bath at 60°C for 20min, and stir at a speed of about 250r / min.
[0109] (5) Filtration: After the decolorization is completed, when th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com