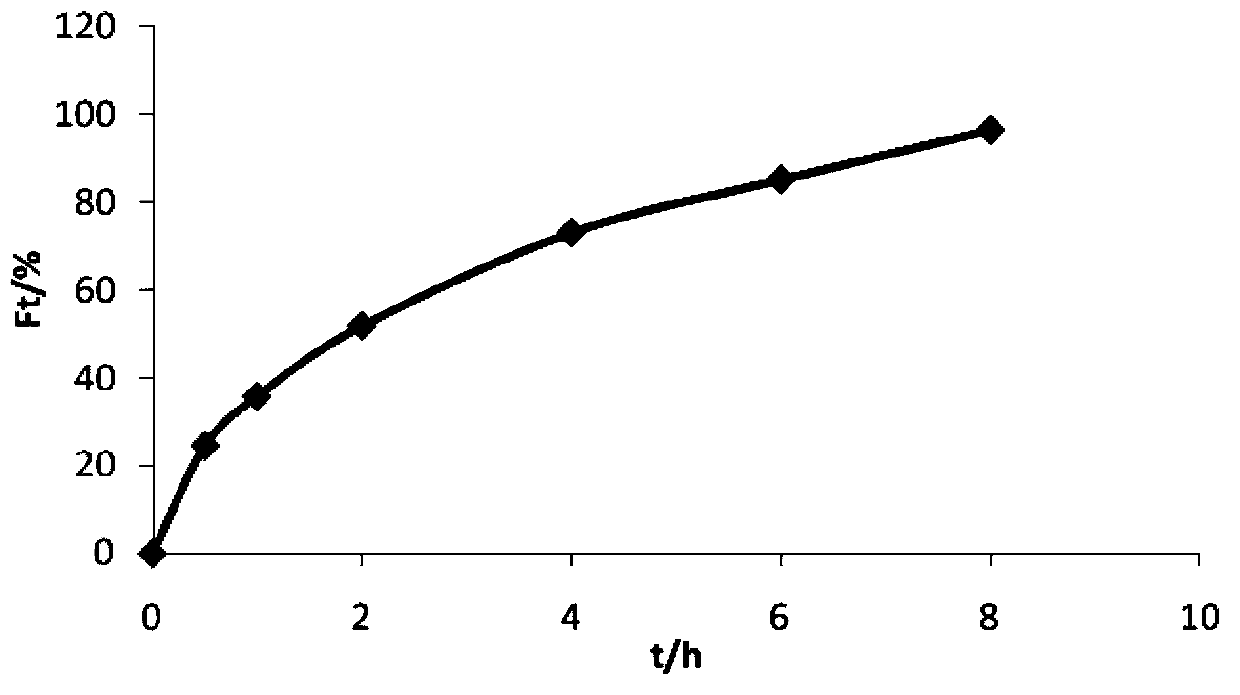
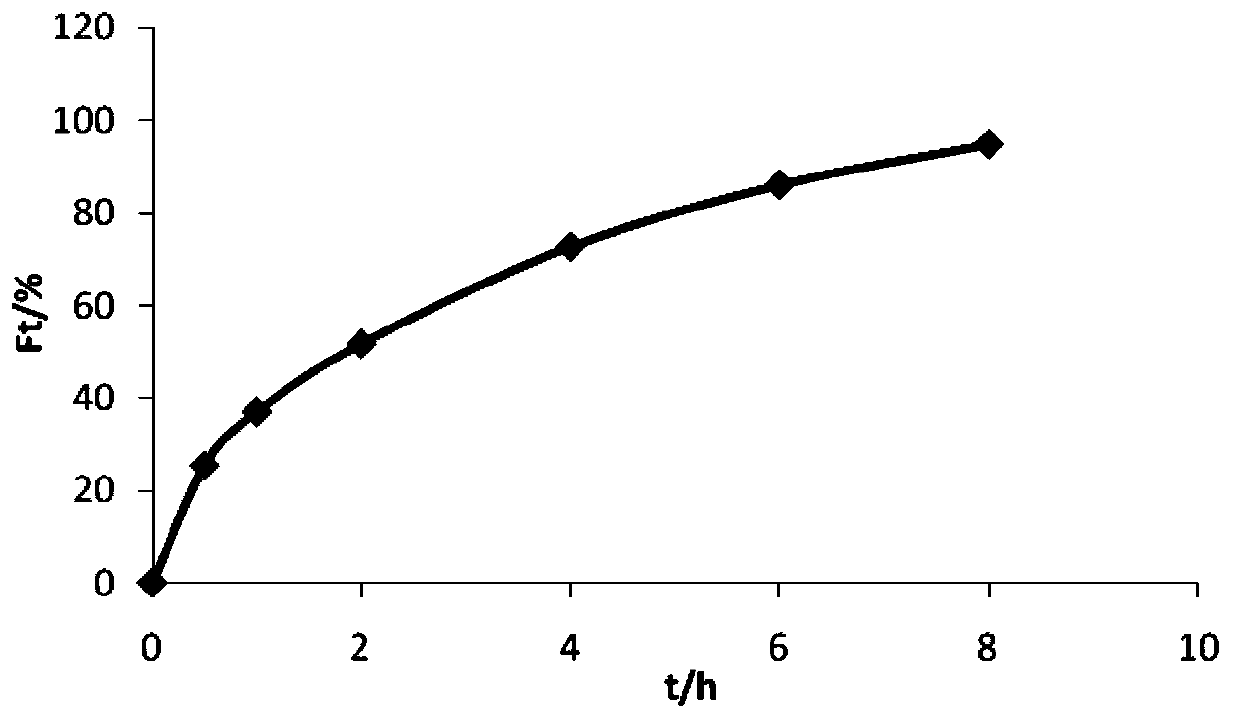
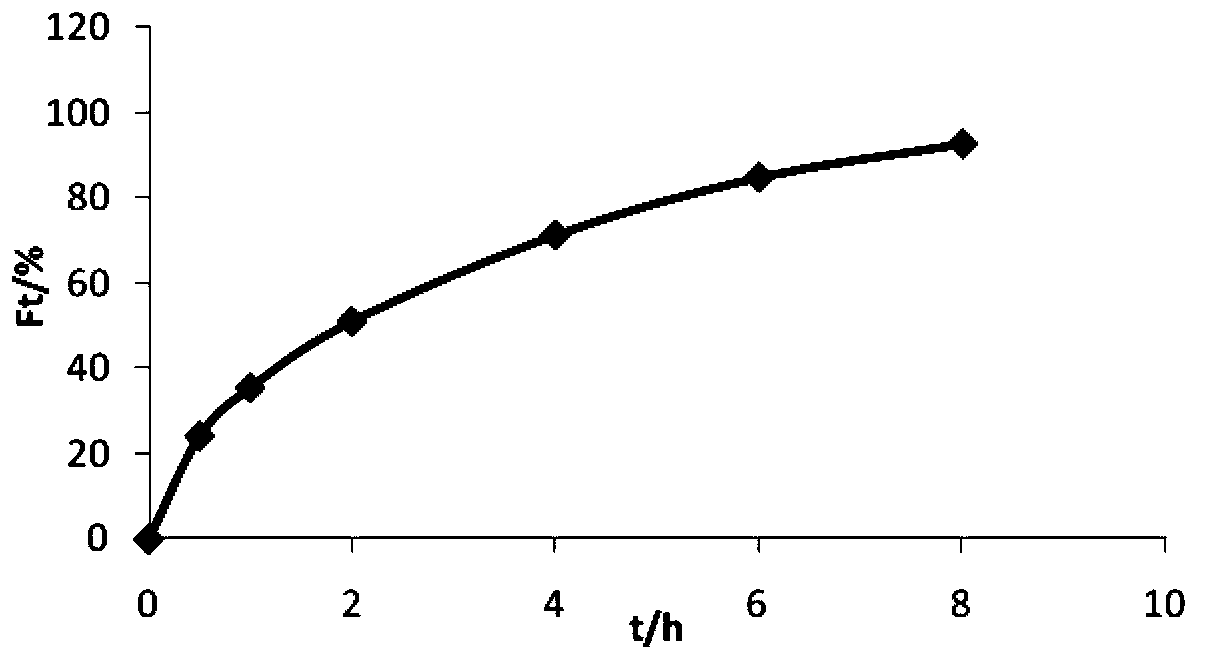
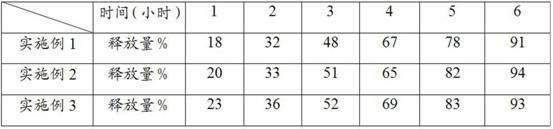
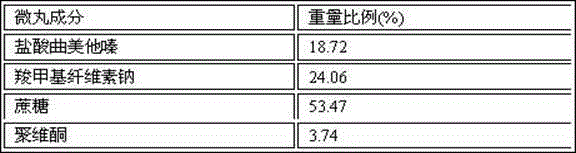
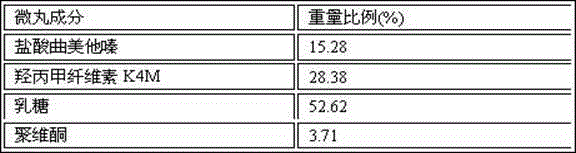
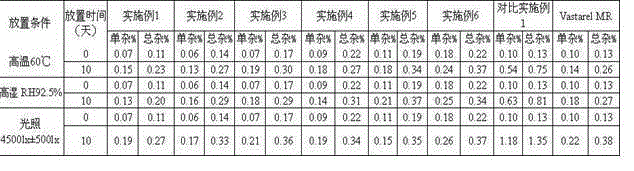
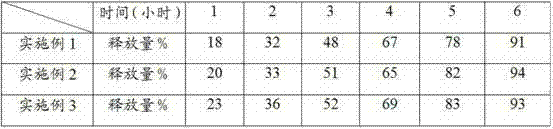
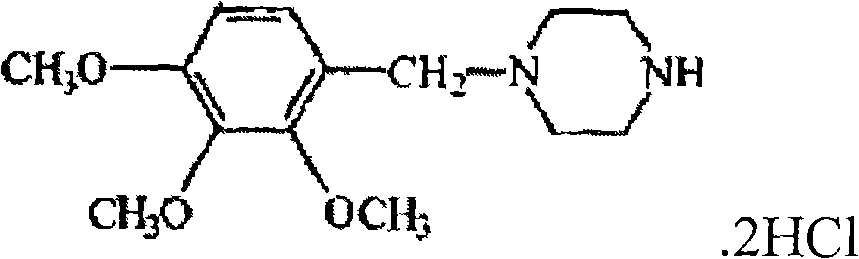
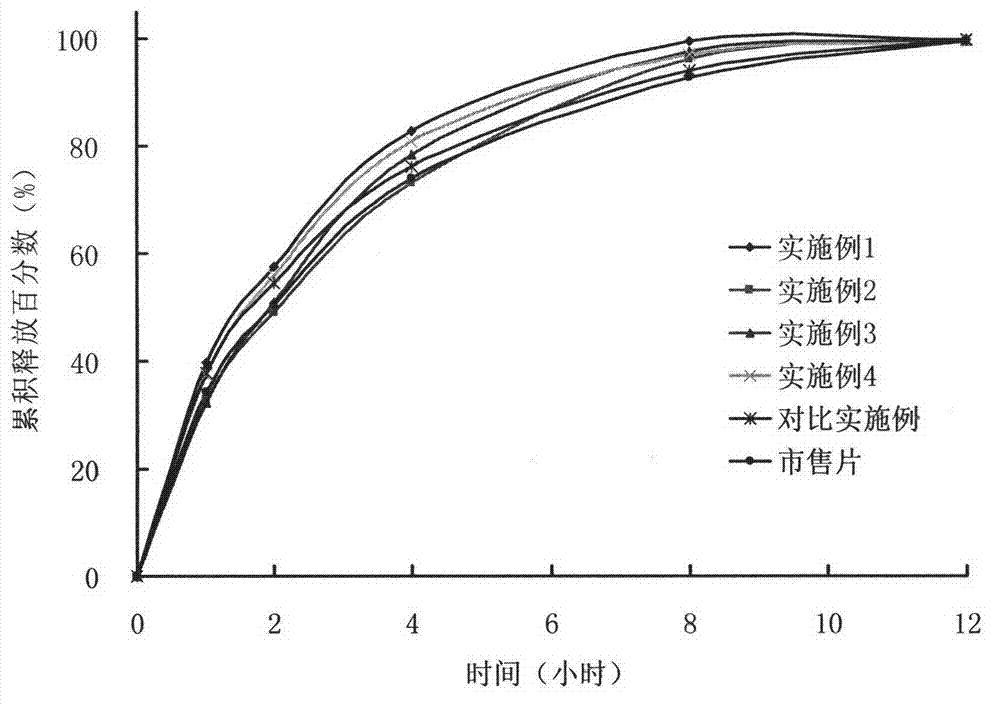
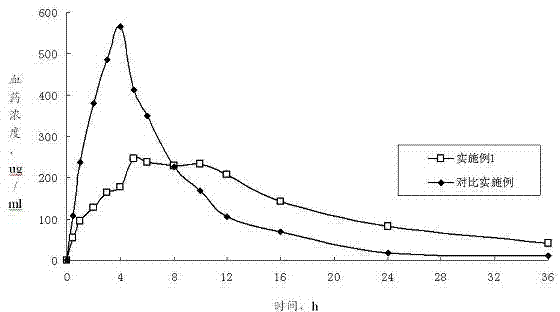
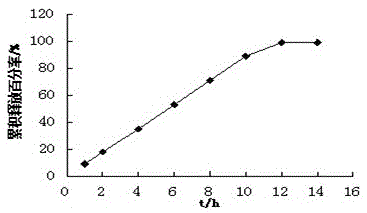
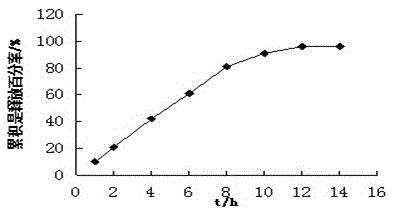
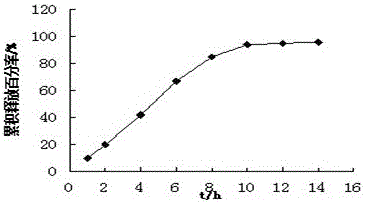
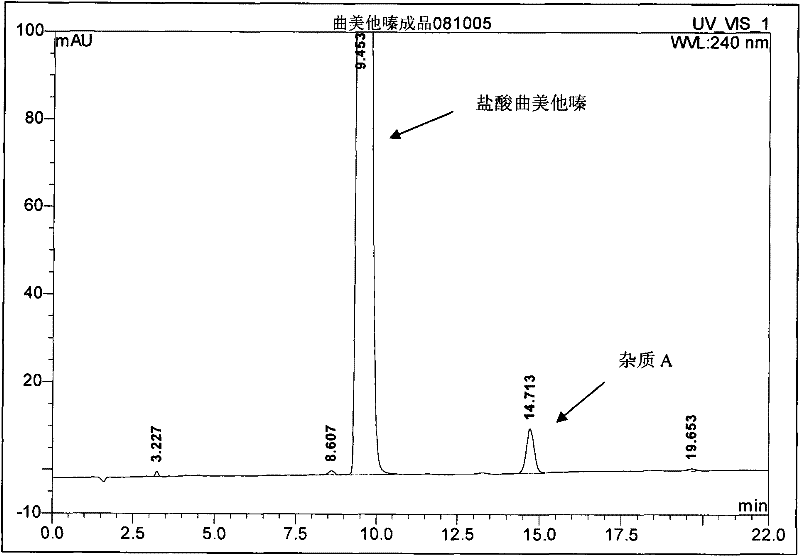
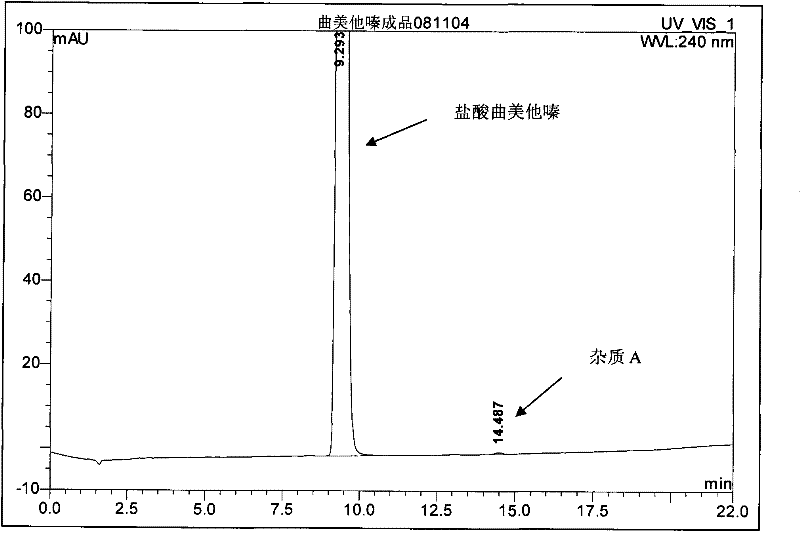
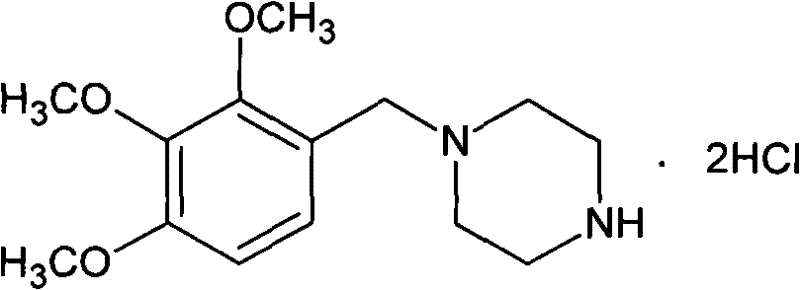
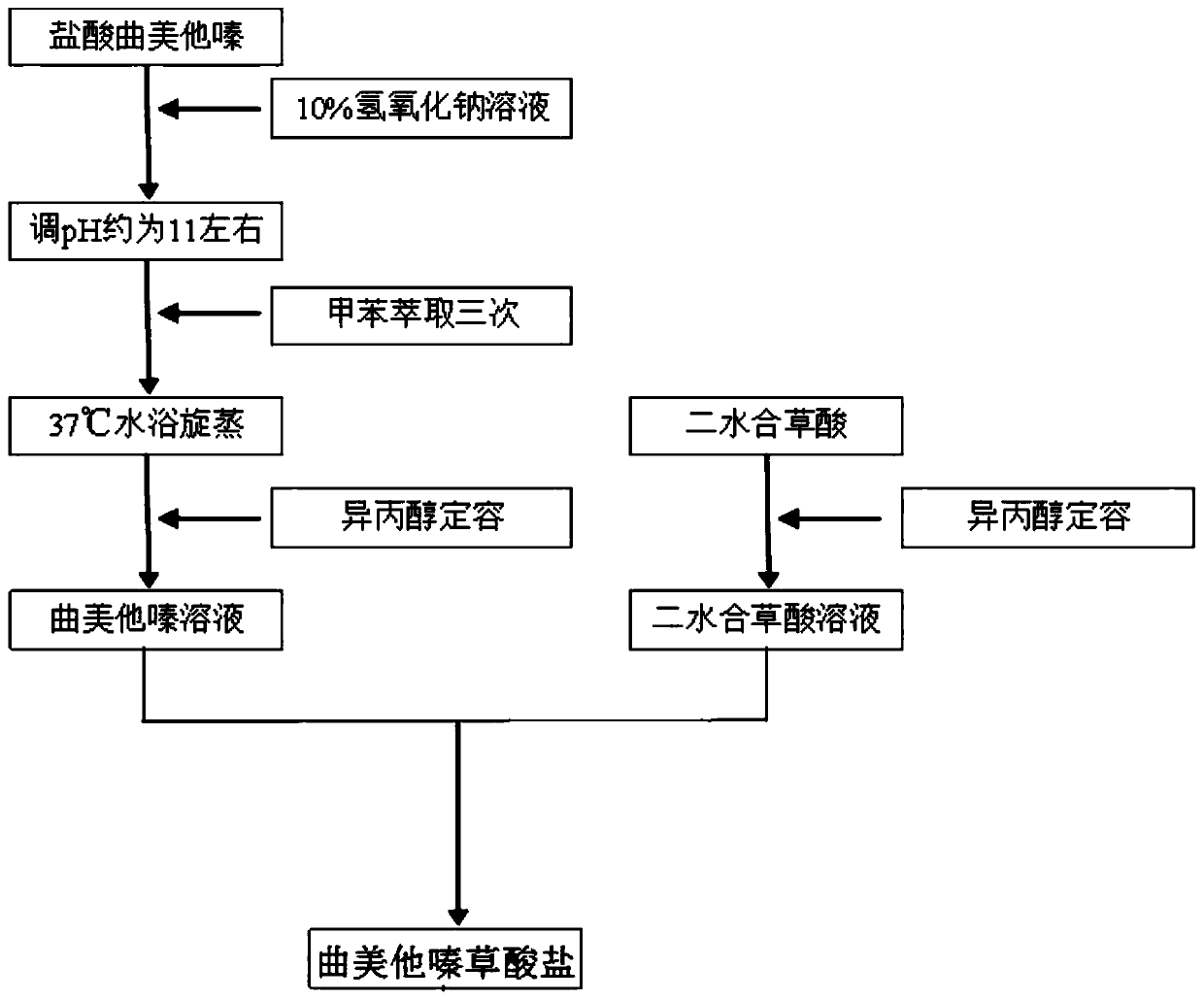
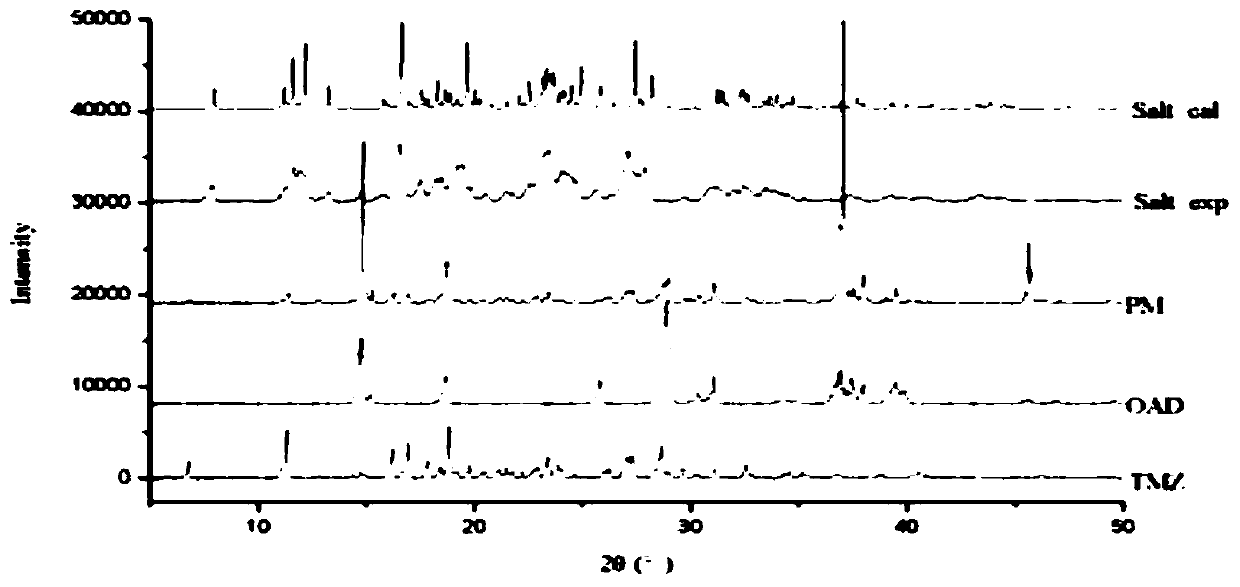
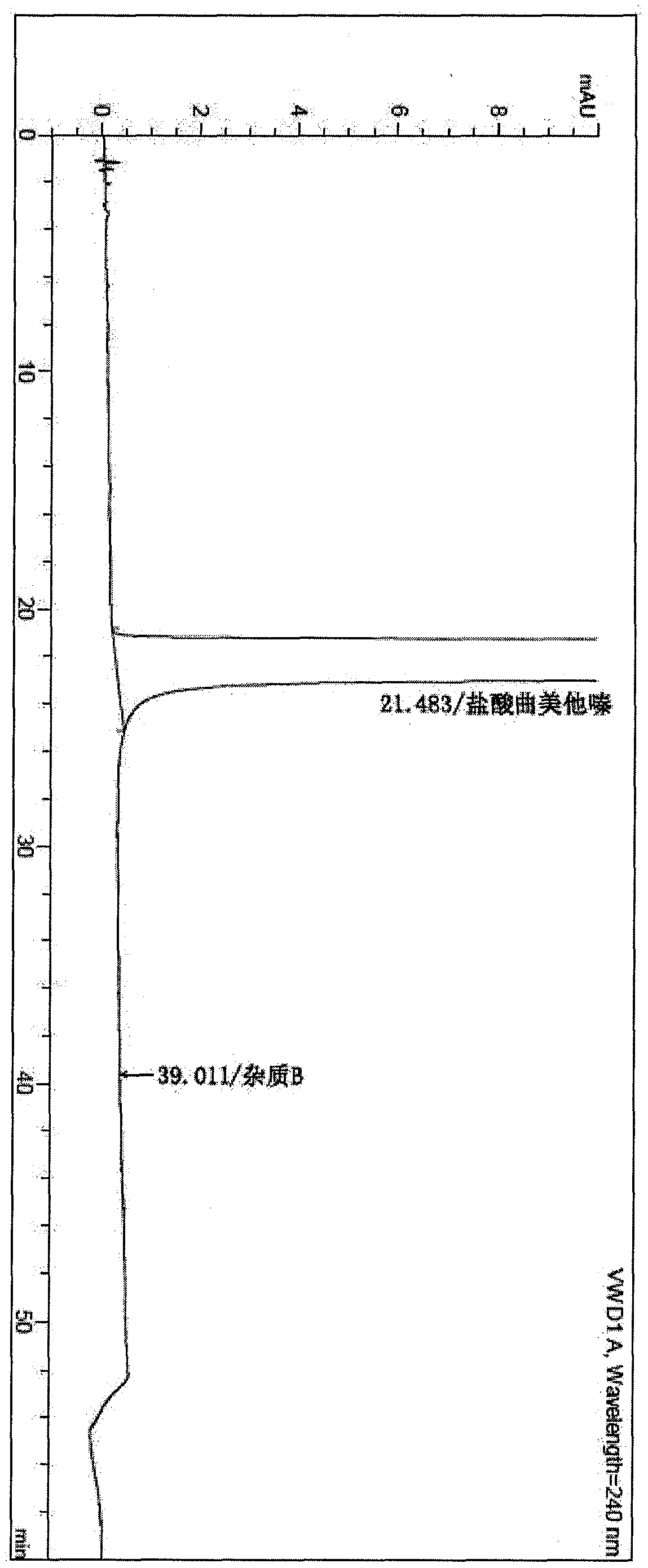
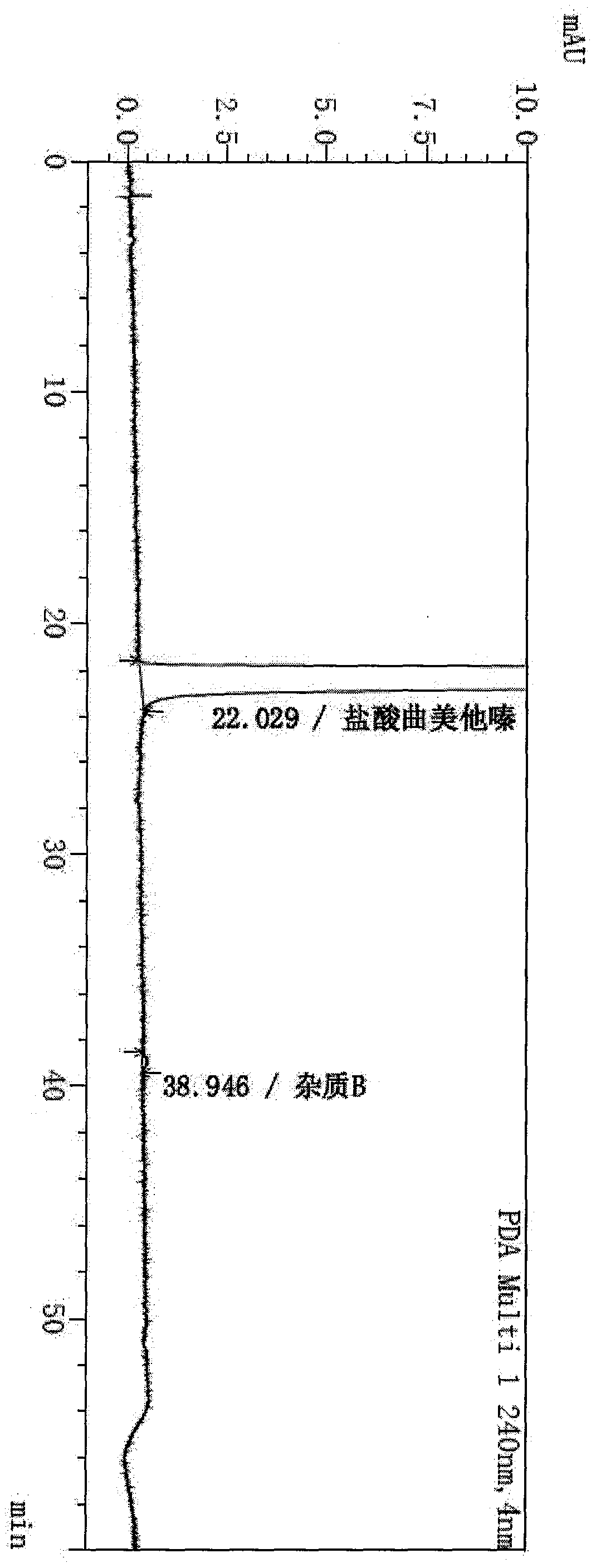
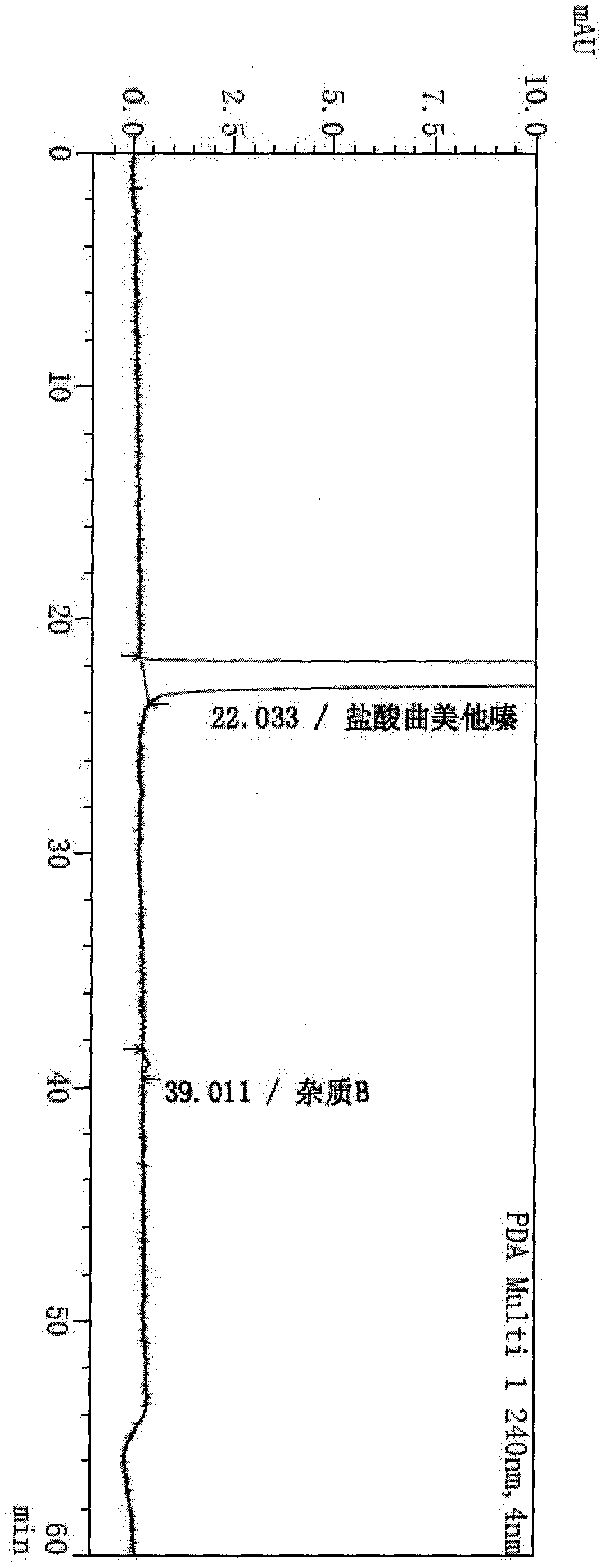
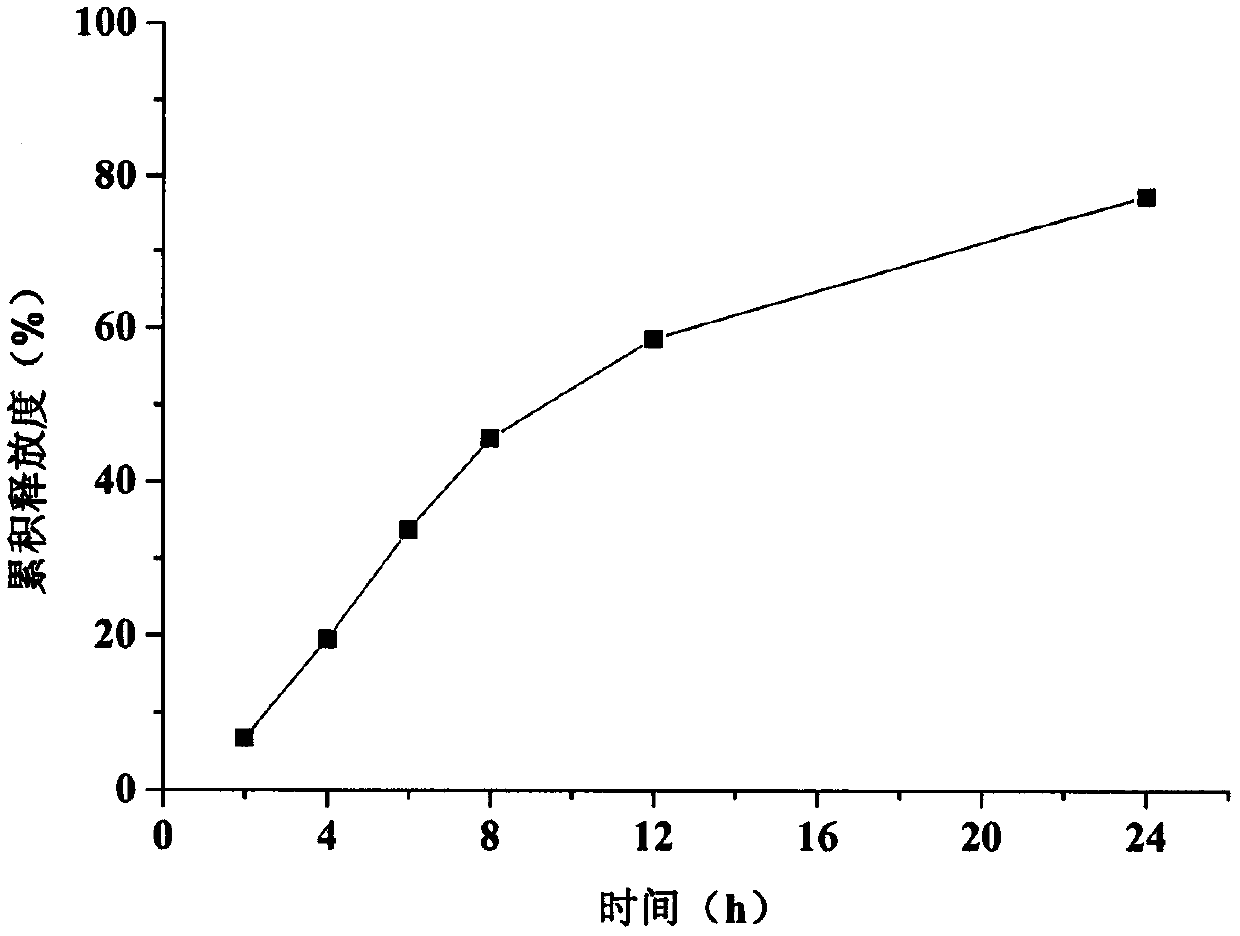
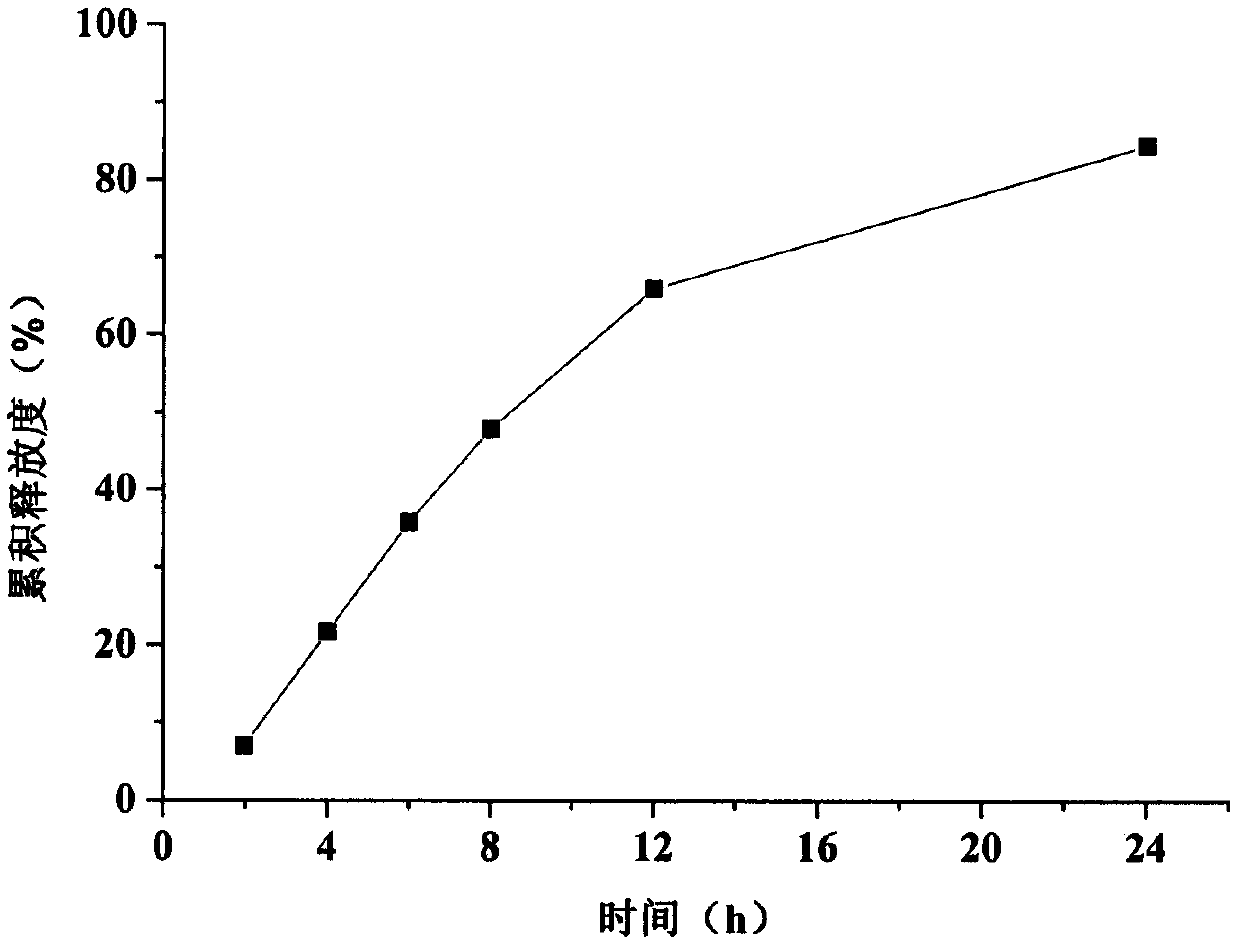
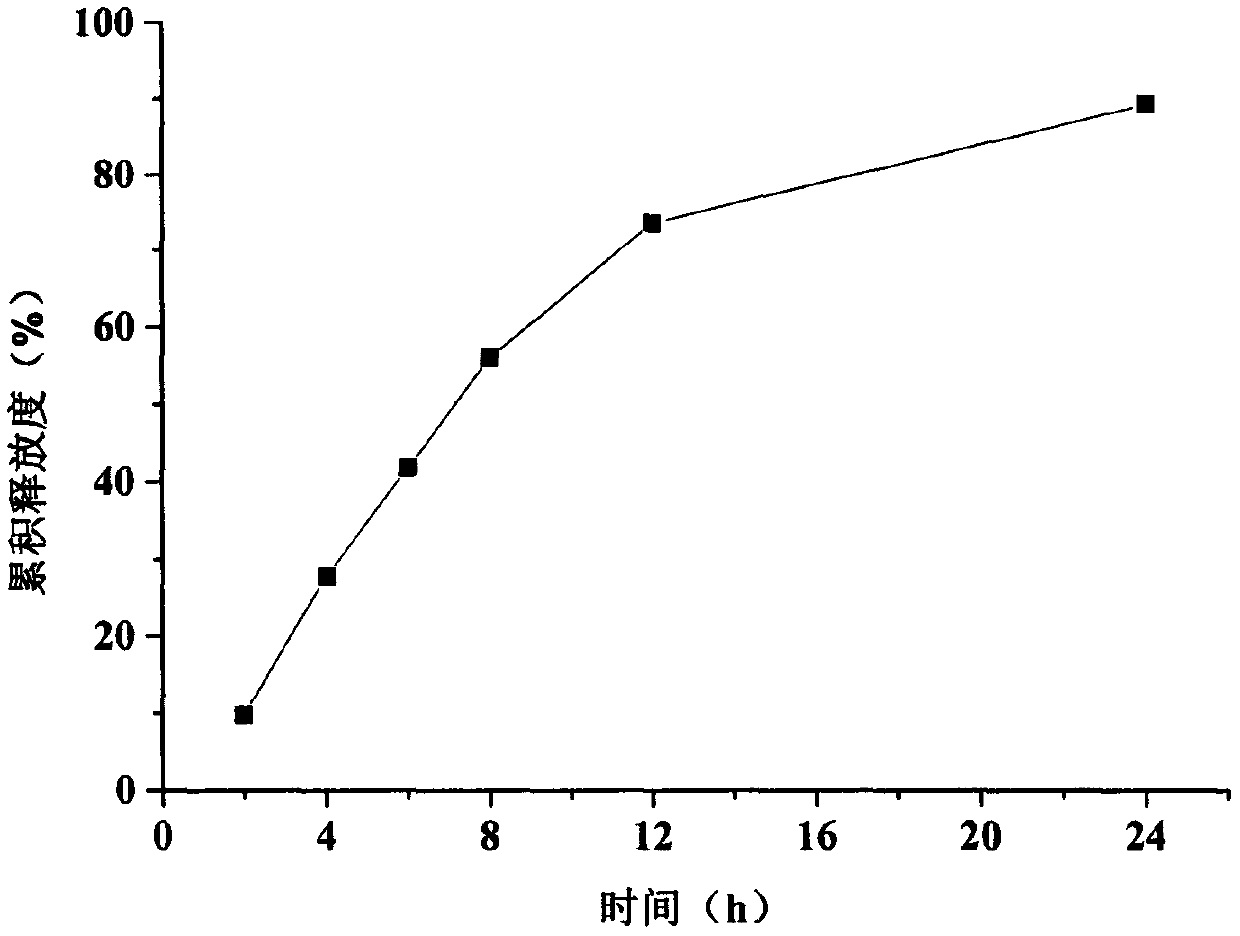
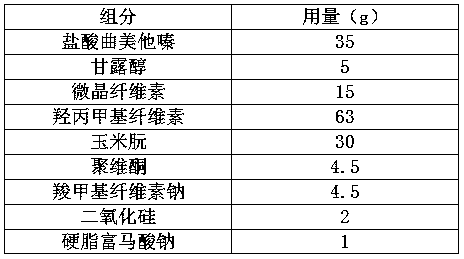
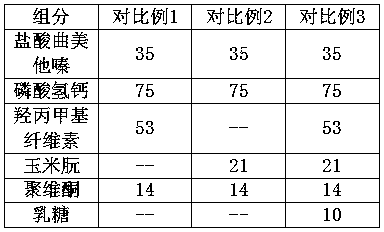
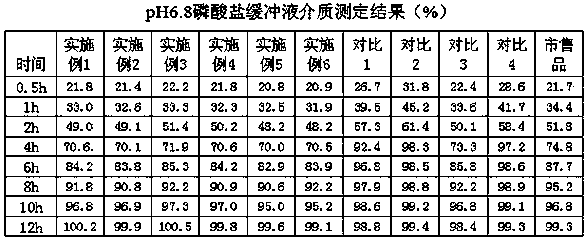
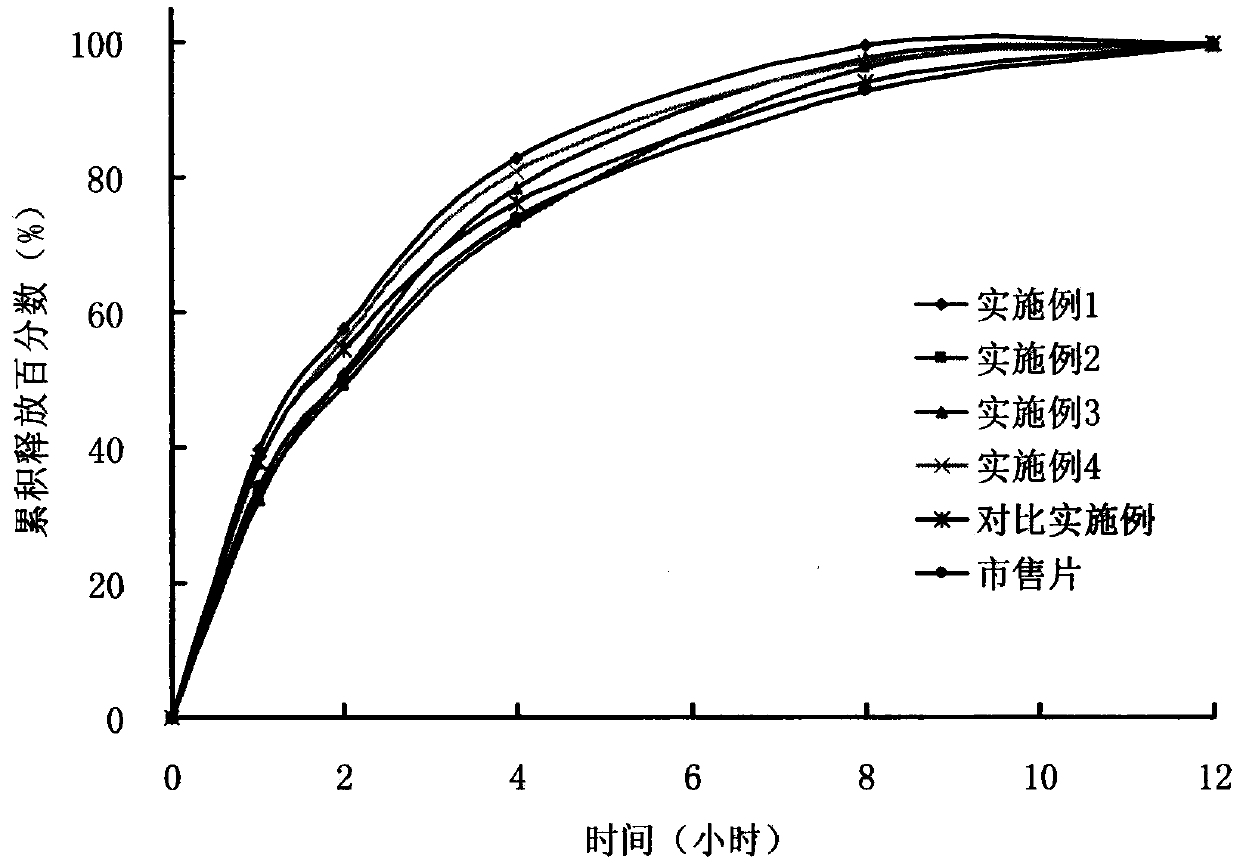
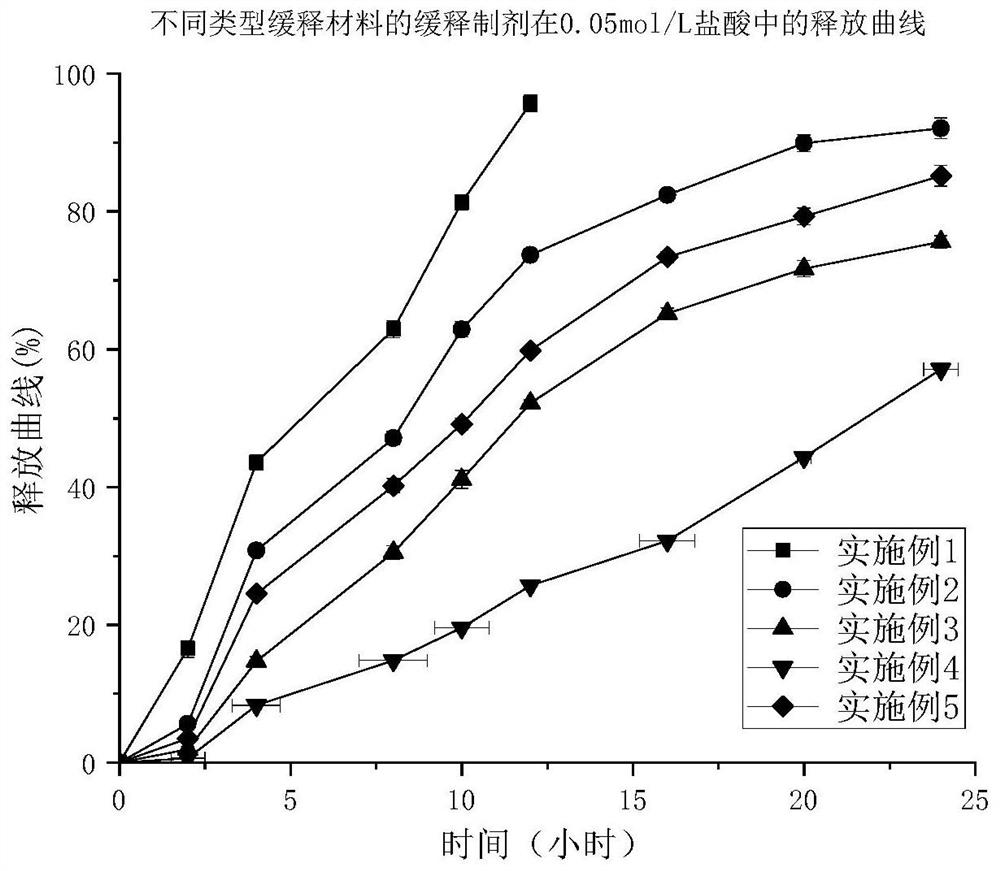
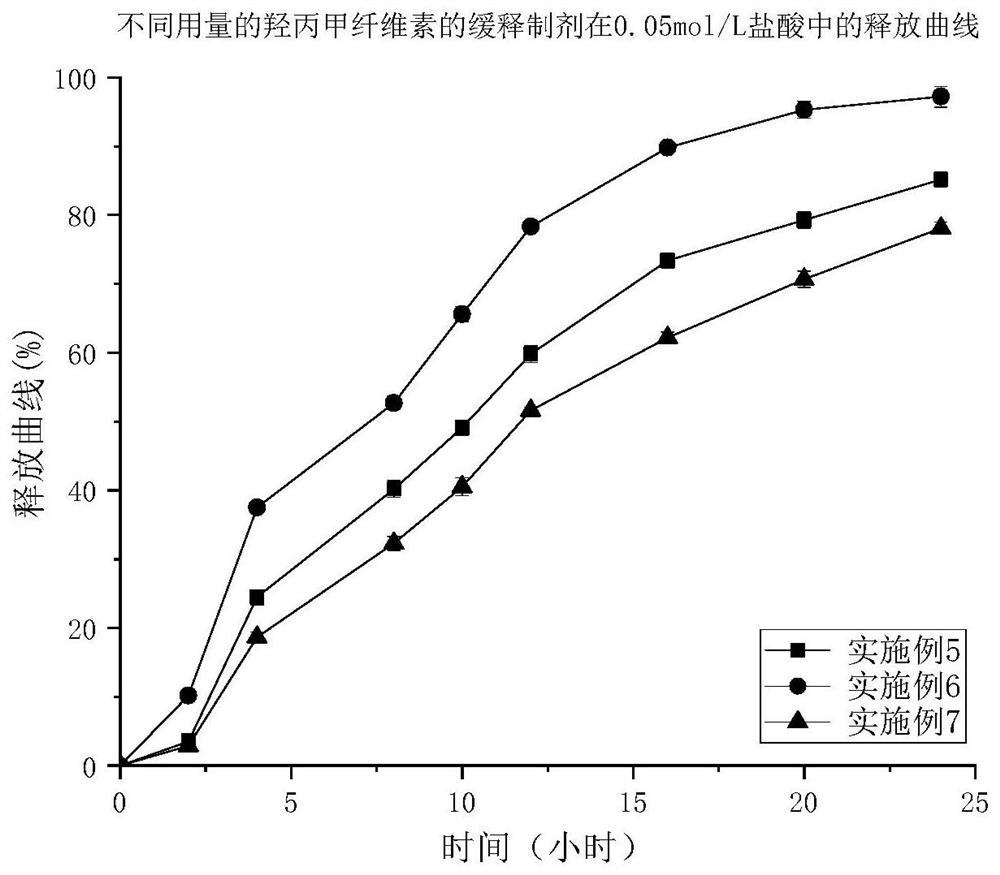
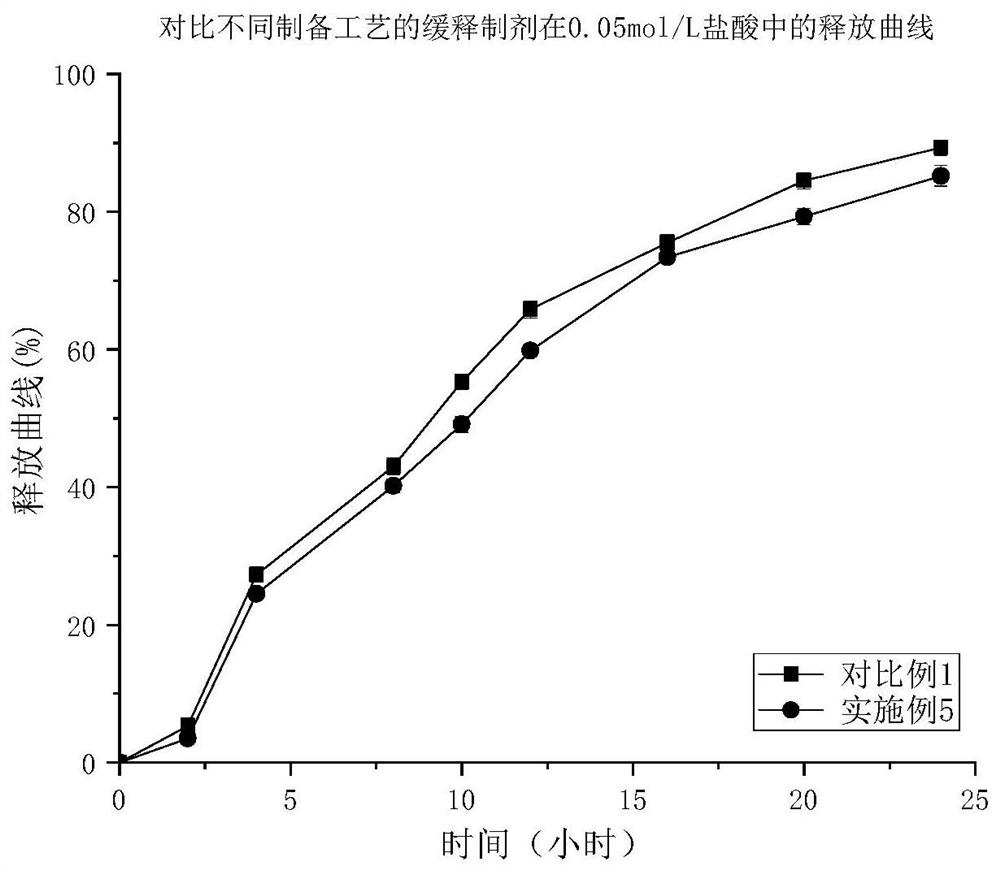
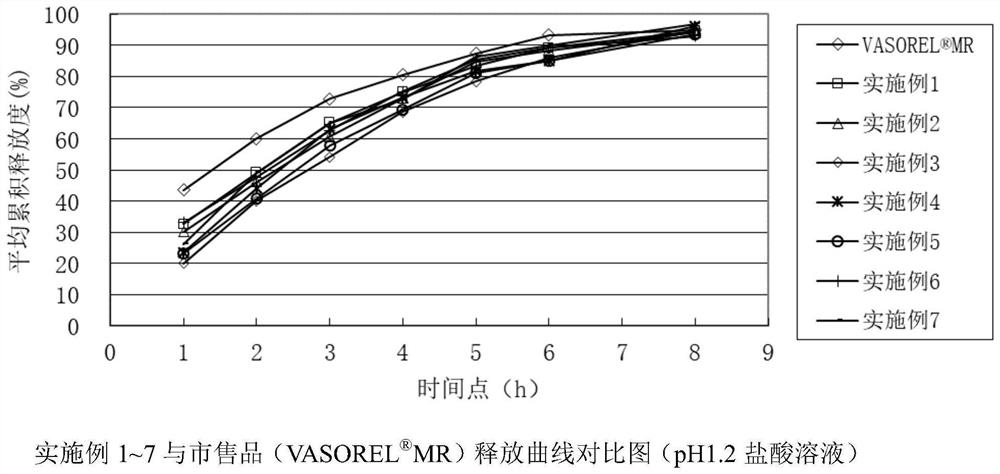
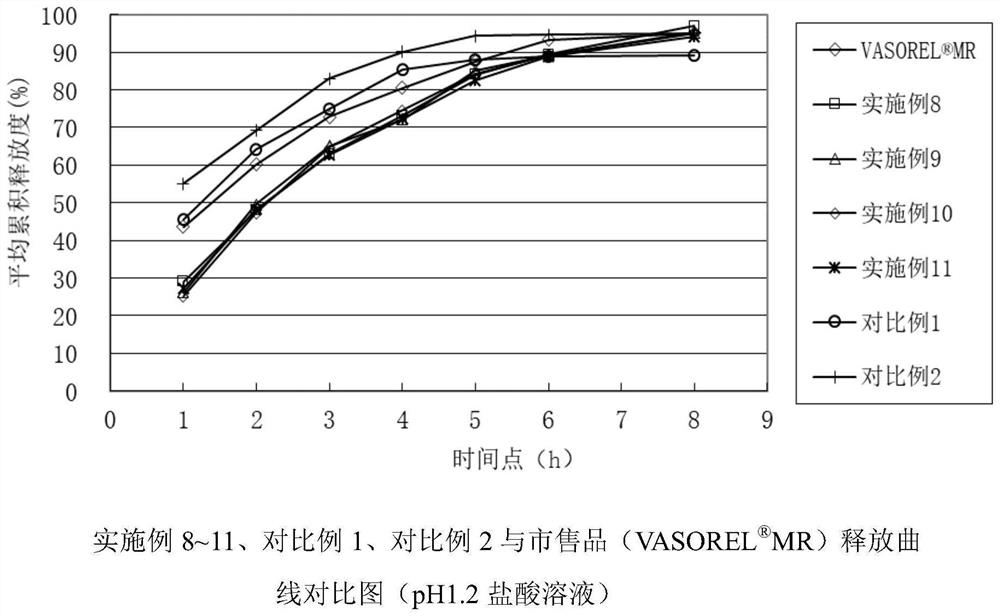
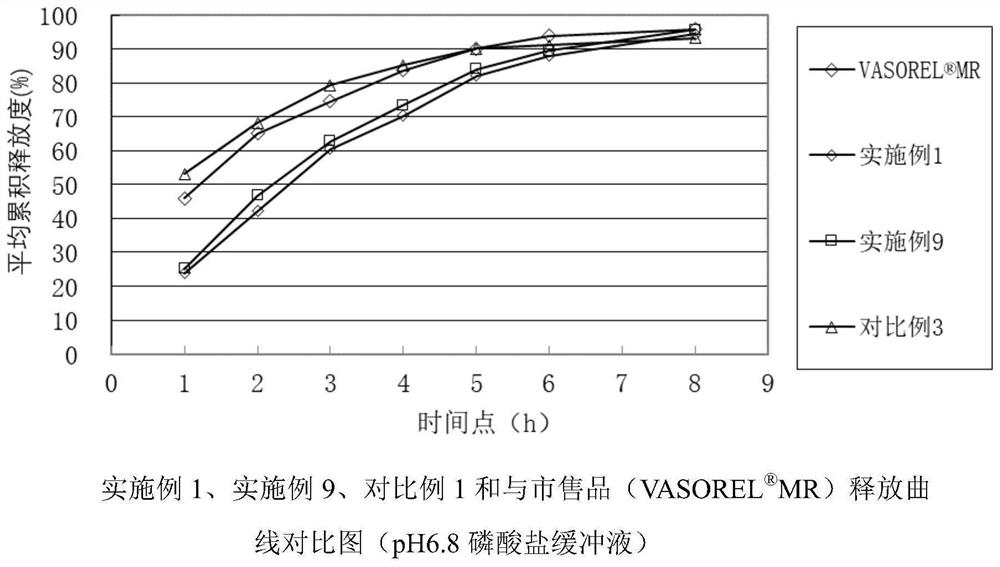
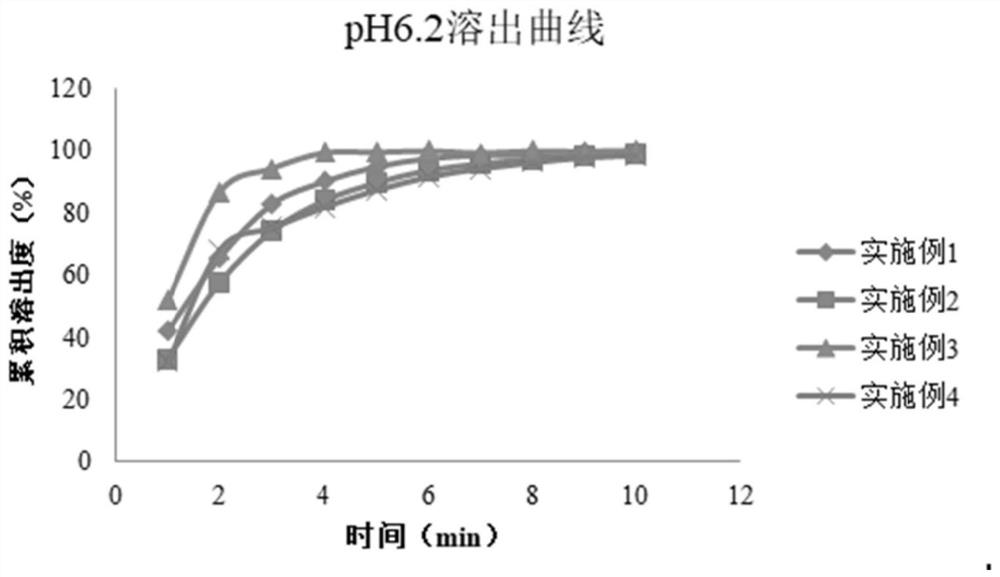
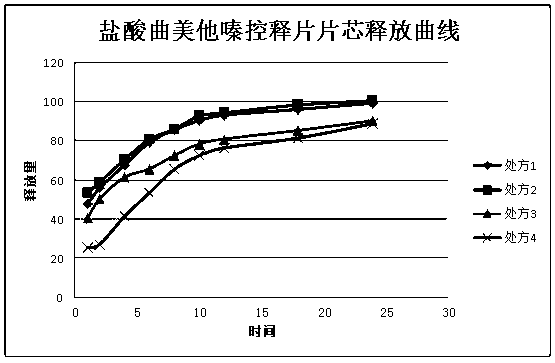
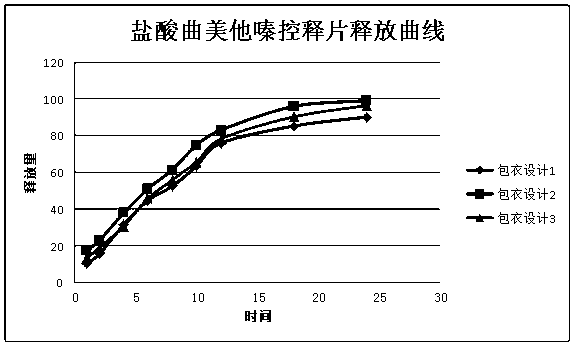
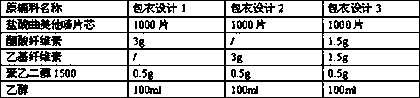
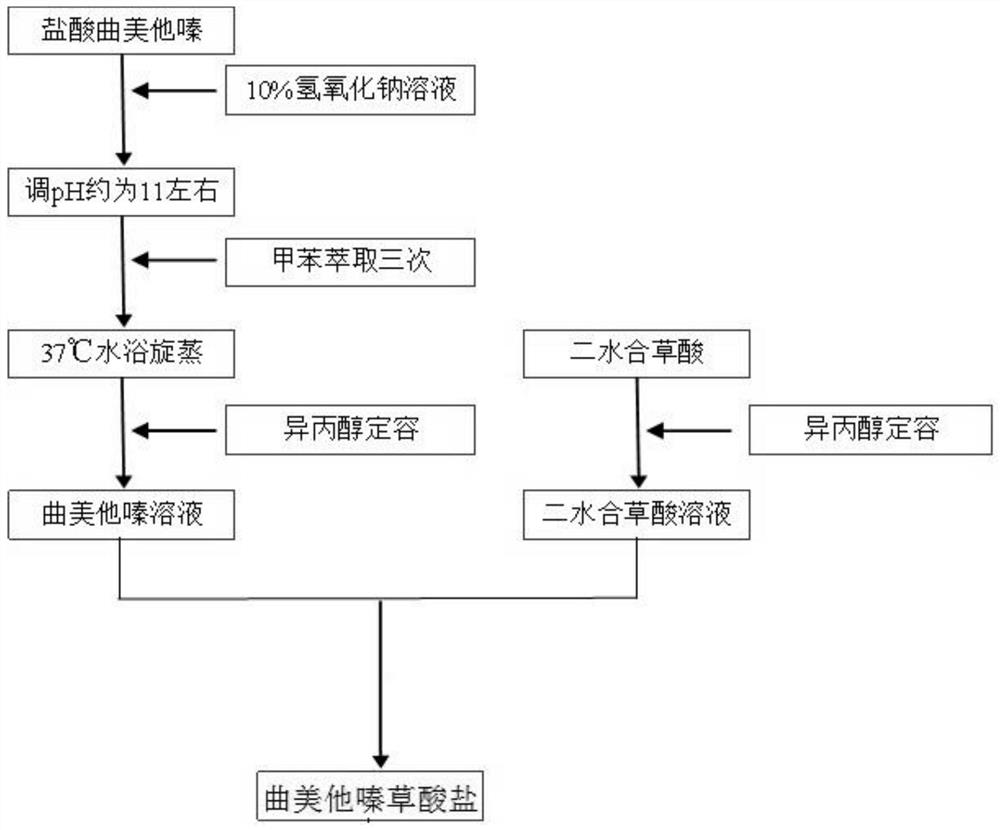
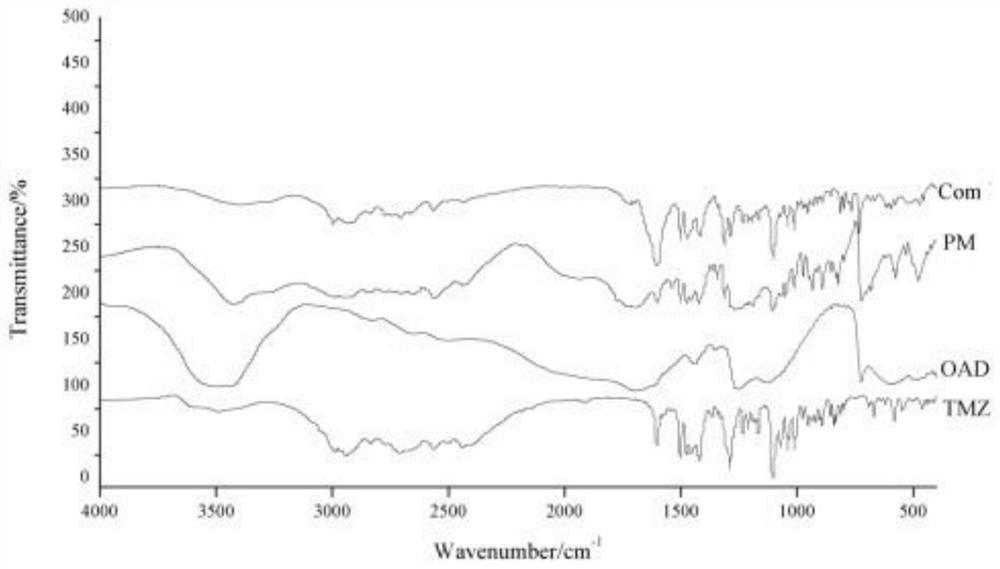
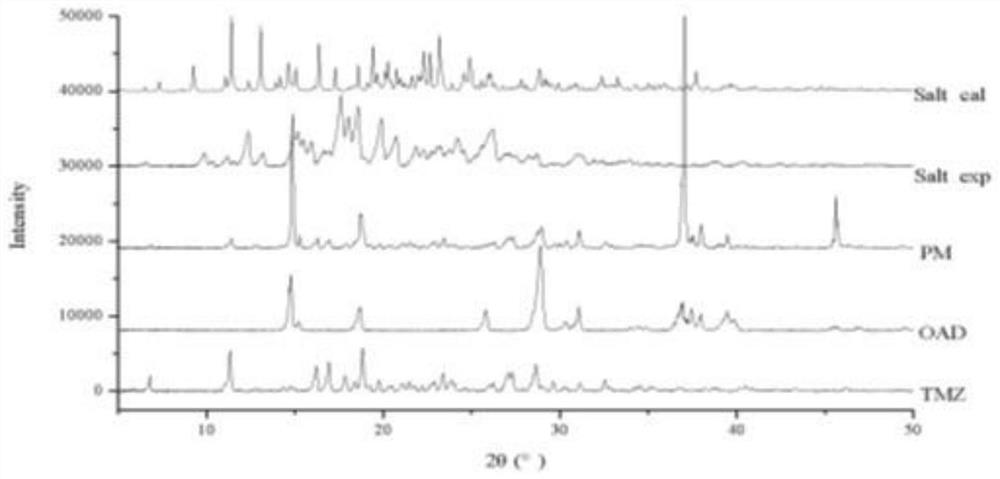
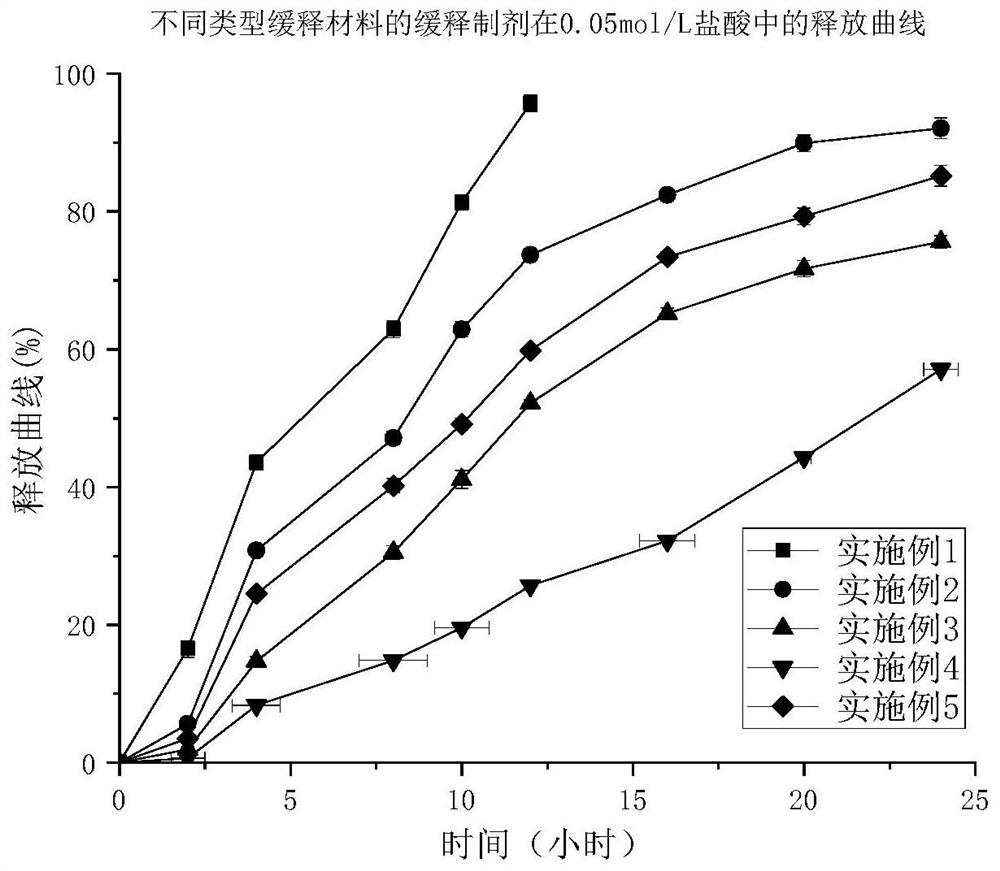
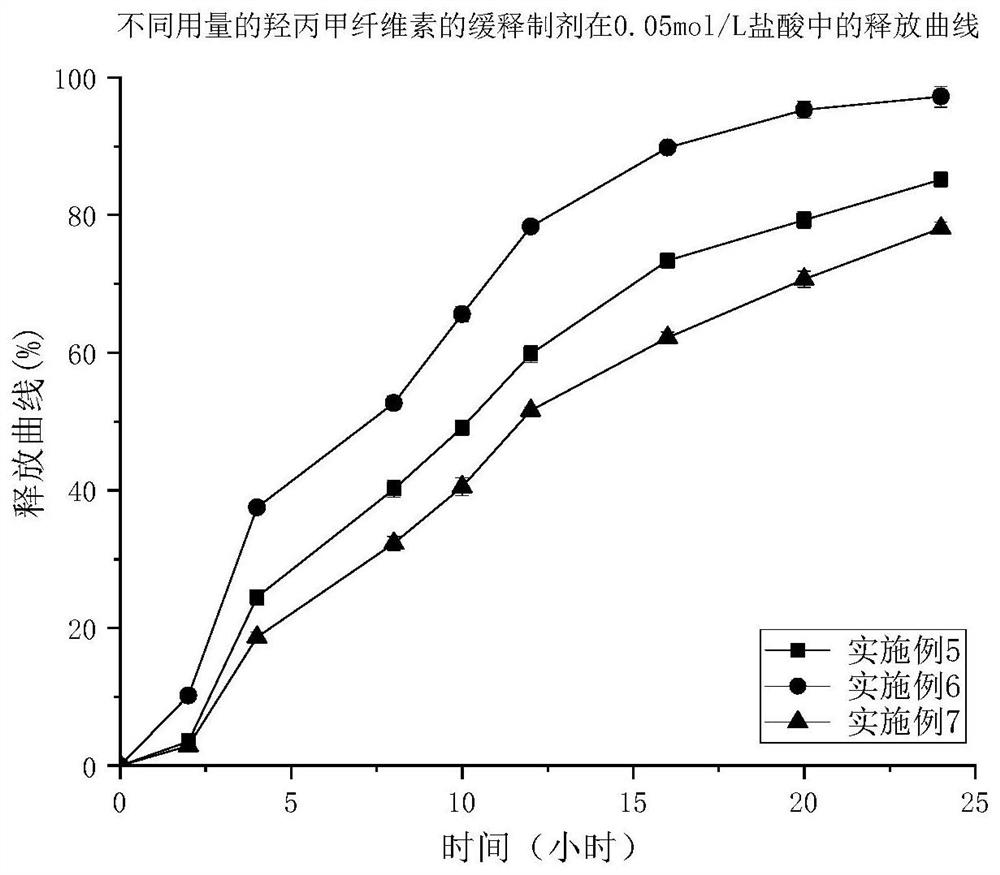
Patents
Literature
45 results about "Trimetazidine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
InactiveCN103385861AQuality improvementSmooth releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletAdhesive
The invention discloses a trimetazidine hydrochloride sustained release tablet prepared from the following ingredients in percentage by weight: 10-25% of trimetazidine hydrochloride, 20-60% of hydrophilic gel skeleton material, 1-20% of a hydrophobic blocker, 15-55% of filler, 1-10% of a lubricant, 0 or 1-10% of a coating material and a proper dosage of adhesive. A wet granulation method is adopted in the preparation process. After the trimetazidine hydrochloride sustained release tablet disclosed by the invention touches water, the hydrophilic gel skeleton material can expand rapidly to form a gel layer with a plurality of pores, a drug can be slowly released through the pores, and the hydrophobic blocker can partially block the pores to avoid the burst release of the drug. Proper mass and proper proportion of hydrophilic gel skeleton material and hydrophobic blocker are added to realize slow and uniform release of the drug under the cooperation of the two ingredients and well avoid the burst release phenomenon so as to beneficially ensure the safety and effectiveness of drug therapy.
Owner:SHANDONG UNIV
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
ActiveCN102319225ASmooth releaseImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention belongs to the field of sustained release medicament preparations, and particularly relates to a trimetazidine hydrochloride sustained release tablet and a preparation method thereof. The trimetazidine hydrochloride sustained release tablet is prepared from 40 to 45 parts of trimetazidine hydrochloride, 100 to 200 parts of polyoxyethylene, 100 to 200 parts of dextrin, 60 to 100 parts of 3-10 percent ethyl cellulose solution and 3 to 5 parts of magnesium stearate through material mixing, soft material preparing, drying, tabletting and other steps. In the trimetazidine hydrochloride sustained release tablet, the polyoxyethylene serves as an auxiliary material, and the sustained release tablet is prepared from the medicaments by a method of direct tabletting or tabletting aftergranulating. The drug dissolution of the trimetazidine hydrochloride sustained release tablet reaches about 90 percent 6 hours later, so the sustained release tablet is only required to be taken twice a day; therefore, the sustained release tablet has the advantages of releasing drug slowly and uniformly to reduce release rate and postpone peak time, reducing the number of administration times per day, improving the compliance of patients to the medicament and the like. Furthermore, the preparation method of the invention is simple and easy to operate.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Long-acting sustained-release pellet and preparation method thereof
InactiveCN103565751AEvenly wrappedReduced release rateOrganic active ingredientsGranular deliverySustained release pelletsBlood concentration
The invention discloses a long-acting sustained-release pellet and a preparation method thereof. The long-acting trimetazidine hydrochloride sustained-release pellet comprises a drug-containing core and a sustained-release coating layer from inside to outside sequentially, wherein an optional isolation coating layer is arranged between the drug-containing core and the sustained-release coating layer; the isolation coating layer accounts for 0-15 percent of the mass of the drug-containing core, and the sustained-release coating layer accounts for 5-30 percent of the mass of the drug-containing core; a gastrointestinal adhesive is contained in the sustained-release coating layer. According to the trimetazidine hydrochloride sustained-release pellet, the prescription is meticulously designed, the drug-containing core is uniformly coated by utilizing the sustained-release coating material, so that slow and uniform drug release of the drug-containing core can be maintained, the active component releasing speed can be reduced, the time for reaching the peak can be postponed, the pellet can be constantly and stably released in 24 hours, the blood concentration stability can be maintained, and the phenomenon of insufficient blood concentration in morning ischemia can be avoided. The administration frequency can be reduced to once a day, and the compliance of patients can be improved.
Owner:AC PHARMA CO LTD
Trimetazidine hydrochloride sustained-release capsule and preparation method thereof
InactiveCN104473905AOrganic active ingredientsSenses disorderControlled releaseSustained release pellets
The invention discloses a trimetazidine hydrochloride sustained-release capsule and a preparation method thereof, belonging to the technical field of medicines. The sustained-release capsule is prepared by filling sustained-release pellets containing trimetazidine in capsules, wherein the sustained-release pellets are a framework control preparation. The trimetazidine hydrochloride sustained-release capsule prepared by the preparation method is simple in process and low in cost. As the capsule is in a multiunit control release system of drug, the user does not need to worry about abrupt release of drug. Compared with preparations such as sustained release tablets, the trimetazidine hydrochloride sustained-release capsule is safe and reliable in medication and high in medication compliance.
Owner:万全万特制药(厦门)有限公司
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
ActiveCN102319225BSmooth releaseImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMagnesium stearate
The invention belongs to the field of sustained release medicament preparations, and particularly relates to a trimetazidine hydrochloride sustained release tablet and a preparation method thereof. The trimetazidine hydrochloride sustained release tablet is prepared from 40 to 45 parts of trimetazidine hydrochloride, 100 to 200 parts of polyoxyethylene, 100 to 200 parts of dextrin, 60 to 100 parts of 3-10 percent ethyl cellulose solution and 3 to 5 parts of magnesium stearate through material mixing, soft material preparing, drying, tabletting and other steps. In the trimetazidine hydrochloride sustained release tablet, the polyoxyethylene serves as an auxiliary material, and the sustained release tablet is prepared from the medicaments by a method of direct tabletting or tabletting aftergranulating. The drug dissolution of the trimetazidine hydrochloride sustained release tablet reaches about 90 percent 6 hours later, so the sustained release tablet is only required to be taken twice a day; therefore, the sustained release tablet has the advantages of releasing drug slowly and uniformly to reduce release rate and postpone peak time, reducing the number of administration times per day, improving the compliance of patients to the medicament and the like. Furthermore, the preparation method of the invention is simple and easy to operate.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Trimetazidine hydrochloride dispersible tablet and preparation method thereof
InactiveCN101283992AGood disintegrationDisintegrates quicklyOrganic active ingredientsPill deliveryTrimetazidine hydrochlorideDispersible tablet
The invention relates to trimetazidine hydrochloride dispersible tablets and a preparation method thereof. The dispersible tablets have quick disintegrating speed; can be administered by ingestion, chewing or dispersing into uniform suspension with good taste by using water; have good compliance; and are particularly suitable for the elderly people, people with ingestion difficulty and patients under special conditions. In addition, the peak time of the dispersible tablet is earlier than the conventional tablet, thus fully indicating quick action characteristic.
Owner:胡传良
High-stability sustained-release tablet prepared by using hydroxy propyl cellulose
ActiveCN102824644ASmooth releaseImprove stabilityOrganic active ingredientsSenses disorderCellulosePolymer science
The invention provides a high-stability sustained-release tablet prepared by using hydroxy propyl cellulose. The sustained-release tablet comprises the following components in parts by weight: 5-40 parts of trimetazidine hydrochloride and 10-85 parts of hydroxy propyl cellulose. A preparation method for the high-stability sustained-release tablet is a common wet granulation tableting process or a powder direct tableting process. According to the trimetazidine hydrochloride sustained-release tablet prepared by using the hydroxy propyl cellulose as a sustained-release material, the problem of low stability of a commercially available trimetazidine hydrochloride sustained-release tablet prepared by using the hydroxy propyl cellulose as a sustained-release material is solved.
Owner:ZHEJIANG CHENGYI PAHRMACEUTICAL +1
Trimetazidine hydrochloride tablets and preparation method thereof
InactiveCN105055352AImprove liquidityGood compressibilityOrganic active ingredientsSenses disorderLubricantTrimetazidine hydrochloride
The invention belongs to the technical field of medicine and particularly relates to trimetazidine hydrochloride tablets and a preparation method thereof. The trimetazidine hydrochloride tablets are prepared from raw materials as follows: trimetazidine hydrochloride, filler, a binder, a lubricant and a wetting agent. The trimetazidine hydrochloride tablets solve the problems of poor compressibility and difficulty in forming of the trimetazidine hydrochloride as the raw material. The invention further provides the preparation method of the trimetazidine hydrochloride tablets. The compressibility of the prepared trimetazidine hydrochloride tablets is improved, the trimetazidine hydrochloride tablets are formed easily, the use value of the trimetazidine hydrochloride tablets can be increased better, trimetazidine hydrochloride is prepared into tablets to facilitate taking of a patient, pain of the patient can be relieved timely, the process is simple, the quality is stable, a production process meets the requirement of GMP (Good Manufacture Practice of Drugs), and the trimetazidine hydrochloride tablets and the preparation method are applicable to industrial mass production.
Owner:REYOUNG PHARMA
Trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and preparation method thereof
ActiveCN103735528ARelease constant speedSmall toxicityOrganic active ingredientsPharmaceutical delivery mechanismSide effectMotility
The invention provides a trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and a preparation method thereof. The osmotic pump controlled release tablet is formed by a drug-containing layer, a booster layer and a coating film, wherein the drug-containing layer comprises the following components in percentage by weight: 10-50% of trimetazidine hydrochloride, 30-80% of suspension agent and the balance of other auxiliary materials; the booster layer comprises the following components in percentage by weight: 20-90% of swelling agent, 5-70% of osmotic active substance and 0.5-5% of lubricating agent; the semipermeable coating film comprises 10-20g of semipermeable high polymer material and 1-5g of water-soluble pore-forming agent every 100 tablets. The trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet can realizes constant release of drug in the body of a patient without being affected by pH value of a medium environment, enzyme, gastrointestinal motility and food, and is capable of maintaining the stability of plasma concentration of drug, reducing toxic and side effects of drug, decreasing dosing frequency and improving compliance of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
Trimetazidine hydrochloride mono-layer osmotic pump controlled release tablet and preparation method thereof
ActiveCN104887639AImprove securityLow toxicityOrganic active ingredientsPharmaceutical delivery mechanismSide effectFiller Excipient
The invention provides a trimetazidine hydrochloride mono-layer osmotic pump controlled release tablet and a preparation method thereof. The tablet is composed of a core, a coating membrane, and pores. The core comprises the following components, by weight percentage: 10-25% of trimetazidine hydrochloride, 8-35% of an osmotic pressure active substance, 16-50% of a suspension, 12-47% of a filler, and 0.5-1.5% of a lubricant. The coating membrane comprises, every 100 tablets: 1-10g of a pore forming agent, 1-10g of a plasticizer, 10-40g of a film forming material, and a proper amount of an solvent. The weight gain of the coating membrane is 4-10% of the weight of the core. The diameter of each pore is in a range of 0.6-1.2 mm. According to the trimetazidine hydrochloride mono-layer osmotic pump controlled release tablet, the auxiliary materials are high in security; the release accords with zero-level release; stability of a plasma concentration can be maintained; frequency of medicine administration for a patient is reduced; and the toxic and side effect of the medicine is minimized. The preparation method is simple, and the raw materials are less, so that the preparation method is suitable for industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
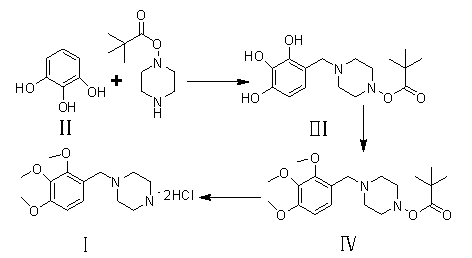
Novel synthesis path of trimetazidine hydrochloride
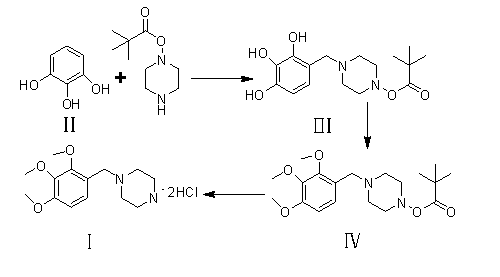
The invention provides a novel synthesis path of trimetazidine hydrochloride. The novel synthesis path comprises the steps of: reacting 1,2,3-phenol (II) react with Boc piperazine to generate a compound III, reacting the compound III react with dimethyl sulfate to generate a compound IV, and carrying out deprotection and salifying on the compound IV to obtain trimetazidine hydrochloride. The novel synthesis path has the advantages of simple process, high yield and low cost and is easy for industrial production.
Owner:WUHAN WUYAO PHARMA
Trimetazidine hydrochloride sustained release tablets and preparation method thereof
InactiveCN105878200AOrganic active ingredientsPharmaceutical delivery mechanismFiller ExcipientPharmaceutical formulation
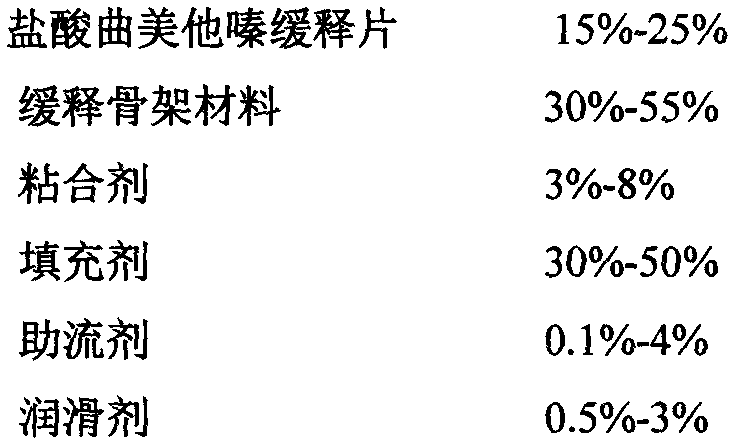
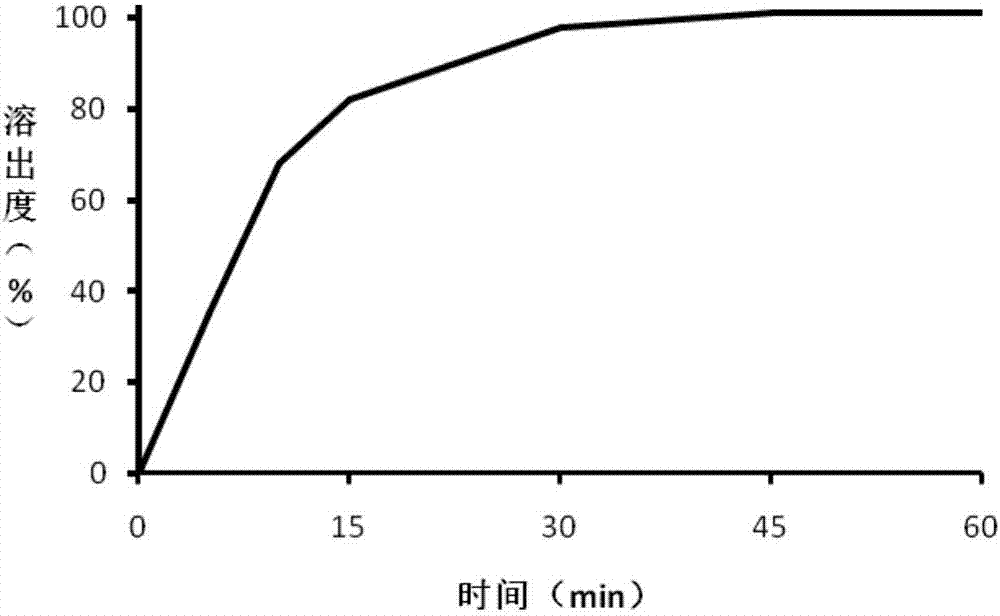
The invention belongs to the field of medicinal preparations, and in particular to trimetazidine hydrochloride sustained release tablets and a preparation method of the trimetazidine hydrochloride sustained release tablets. The trimetazidine hydrochloride sustained release tablets adopt hydrophilic gel as a sustained release framework material, and also contain a filling agent, a lubricating agent and other auxiliary materials. The prepared trimetazidine hydrochloride sustained release tablets are taken twice each day, 1 tablet each time, and the two rimetazidine hydrochloride sustained release tablets are taken at the breakfast time and the supper time respectively. Therefore, the trimetazidine hydrochloride sustained release tablets have the advantages that the drug can be slowly and uniformly released, thus the release rate is reduced, the peak time is delayed, the taking times each day is reduced, and the medicine-taking compliance of a patient is improved. In addition, the preparation method is simple in technology and easy to operate.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Trimetazidine hydrochloride tablet and preparation method thereof
ActiveCN107982231AImprove dissolution rateImprove securityOrganic active ingredientsPill deliveryFiller ExcipientAdhesive
The invention belongs to the field of medicine preparations, and particularly relates to a trimetazidine hydrochloride tablet and a preparation method thereof. The trimetazidine hydrochloride tablet is prepared from the following raw materials in parts by weight: 18 to 22 parts of trimetazidine hydrochloride, 58 to 67 parts of filling agent, 4 to 5 parts of adhesive, 1.7 to 2.7 parts of lubricant,and 8 to 13 parts of wetting agent. The preparation method comprises the following steps of screening the trimetazidine hydrochloride and the filling agent, so as to obtain the screened trimetazidinehydrochloride and the screened filling agent; respectively selecting the screened trimetazidine hydrochloride, the screened filling agent and the adhesive, and uniformly mixing in a wet way, so as toobtain a mixture B; adding the wetting agent into the mixture B, granulating, drying, and arranging, so as to obtain a granule; adding the lubricant into the granule, mixing, and tabletting, so as toobtain the trimetazidine hydrochloride tablet. The trimetazidine hydrochloride tablet has the advantages that the quality meets the standard; the trimetazidine hydrochloride tablet can be well absorbed, distributed and metabolized in a human body; the good clinical treatment effect and safety are realized.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
InactiveCN104706612AGuaranteed Del Sustained Release EffectEasy to carryOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletWestern medicine
The invention discloses a trimetazidine hydrochloride sustained release tablet and a preparation method thereof, and belongs to the field of western medicine preparation, the sustained-release tablet is prepared from the following components in parts by weight: 10-50 parts of trimetazidine hydrochloride, 100-200 parts of a gel skeleton material, 100-300 parts of filler, 40-80 parts of a glidant and 5-10 parts of a lubricant; the preparation method comprises the following steps: respectively crushing the trimetazidine hydrochloride, the gel skeleton material, the glidant and the filler into powder with the particle size controlled in 20-40 mesh, then mixing evenly, then adding the lubricant powder, mixing evenly to obtain a mixture; putting the mixture into a tabletting machine, tabletting, drying in an oven, and finally packaging. Using hydroxypropyl cellulose as the matrix skeleton material, the drug sustained release effect can be ensured, the preparation method is simple, easy to operate, and suitable for industrial mass production, and the tablet is convenient to carry, and easy for patients to carry and take.
Owner:ZIBO DEV ZONE YADA PHARMA
A kind of trimetazidine hydrochloride osmotic pump controlled-release tablet and preparation method thereof
ActiveCN103550183BPlay a protective effectNo effect on release characteristicsOrganic active ingredientsPharmaceutical delivery mechanismDrug release rateControl release
The invention discloses a trimetazidine hydrochloride osmotic pump controlled-release tablet and a preparation method thereof, which is a single-chamber osmotic pump controlled-release preparation, comprising a single-layer tablet core, an insoluble semipermeable film coat and small holes for drug release. The present invention can effectively improve drug hysteresis and drug release terminal residues by screening and optimizing the combination of fillers, adjust drug release rate and controlled release time, and reach a certain blood drug concentration in a relatively short period of time, forming a stable and constant rate release, and realizing rapid drug release. The purpose of drug controlled release design is to achieve effective, stable drug release and reduce side effects.
Owner:HEFEI LIFEON PHARMA
Trimetazidine hydrochloride double-layer osmotic pump controlled-release tablet and preparation method
ActiveCN103735528BSmall toxicityAvoid peaks and valleys in blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectTramadol Hydrochloride
The invention provides a trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and a preparation method thereof. The osmotic pump controlled release tablet is formed by a drug-containing layer, a booster layer and a coating film, wherein the drug-containing layer comprises the following components in percentage by weight: 10-50% of trimetazidine hydrochloride, 30-80% of suspension agent and the balance of other auxiliary materials; the booster layer comprises the following components in percentage by weight: 20-90% of swelling agent, 5-70% of osmotic active substance and 0.5-5% of lubricating agent; the semipermeable coating film comprises 10-20g of semipermeable high polymer material and 1-5g of water-soluble pore-forming agent every 100 tablets. The trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet can realizes constant release of drug in the body of a patient without being affected by pH value of a medium environment, enzyme, gastrointestinal motility and food, and is capable of maintaining the stability of plasma concentration of drug, reducing toxic and side effects of drug, decreasing dosing frequency and improving compliance of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
Method for preparing trimetazidine hydrochloride
ActiveCN102010386BSimple processOvercome the difficulty of low purityOrganic chemistrySolventPiperazine
The invention relates to a method for preparing trimetazidine hydrochloride. In the method, 2,3,4-trimethoxybenzaldehyde and piperazine are used as raw materials, palladium carbon is used as a catalyst for catalytic hydrogenation so as to salify to generate the trimetazidine hydrochloride, and a solvent used in the hydrogenation is mixed solution of water and ethanol. The solution ensures the complete reaction, cannot cause partial excess of the 2,3,4-trimethoxybenzaldehyde and can reduce impurities.
Owner:WUHAN WUYAO SCI & TECH
Trimetazidine oxalate and preparation method and application thereof
ActiveCN110183398AChange melting pointChange solubilityOrganic active ingredientsOrganic compound preparationOxalateOxalic acid
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Trimetazidine oxalate, preparation method thereof and application of oxalate
ActiveCN110054599AChange melting pointChange solubilityOrganic active ingredientsOrganic chemistry methodsOxalateMole ratio
The invention provides trimetazidine oxalate. A molecular formula of the trimetazidine oxalate is C18H26N2O11, and trimetazidine and oxalate react according to mole ratio of 1:2 to obtain the trimetazidine oxalate. A repose angle of the trimetazidine oxalate is smaller than that of trimetazidine hydrochloride, the surface appearance of the trimetazidine oxalate is flaky, flowability and hygroscopicity of a medicine are improved as compared with those of a prismatic crystal medicine prepared from trimetazidine hydrochloride raw materials, the maximum tensile strength of the trimetazidine oxalate is 4.66MPa in the pressure of 150MPa and remarkably higher than that of the trimetazidine hydrochloride at the same pressure, and tablet forming performance of the medicine is improved.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Synthesis method of trimetazidine hydrochloride
The invention provides a synthesis method of trimetazidine hydrochloride, which comprises the following steps: carrying out hydroamination reaction by using 2,3,4-trimethoxybenzaldehyde and piperazineanhydrous as raw materials, and catalyzing by using a Lindlar catalyst. The trimetazidine hydrochloride produced by the method does not contains trimetazidine hydrochloride impurity B and has high purity.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Trimetazidine hydrochloride single-layer osmotic pump controlled release tablet and preparation method thereof
ActiveCN110882229AQuick effectNo release time lagOrganic active ingredientsCoatingsBlood concentrationFoaming agent
The invention provides a trimetazidine hydrochloride single-layer osmotic pump controlled release tablet and a preparation method thereof. The osmotic pump is composed of a tablet core, a semipermeable membrane and small drug release holes. The tablet core contains the following components, by weight: 30% to 40% of trimetazidine hydrochloride, 20% to 55% of a penetration enhancer, 5% to 20% of a release regulator and 0.1% to 3% of a lubricant. The semipermeable membrane includes a semipermeable membrane forming material and a pore-foaming agent; the semipermeable membrane forming material accounts for 90% to 99%, the pore-foaming agent accounts for 1% to 10%; and the weight gain of the coating accounts for 5% to 15% of the mass of the tablet core; and the pore diameter of the drug releasepores on the surface of the semipermeable membrane is 0.4 mm to 1. 2mm. The trimetazidine hydrochloride single-layer osmotic pump controlled release tablet is taken once a day; the 12-hour cumulativerelease percentage of the medicine is 60 to 70%, release time lag is avoided; the stable blood concentration in the body is maintained while the medicine can take effect quickly; the toxic and side effects are reduced; and patient compliance is improved. The drug release behavior of the preparation is not influenced by physiological factors such as gastrointestinal tract peristalsis and pH.
Owner:CHINA PHARM UNIV
Trimetazidine hydrochloride intermediate preparation method
ActiveCN107382690ALow toxicityEmission reductionOrganic compound preparationCarbonyl compound preparationSolventNucleophilic substitution
The invention discloses a trimetazidine hydrochloride intermediate preparation method. The method takes 1,2,3-trichlorobenzene (II) as a starting raw material; a nucleophilic substitution reaction is performed under catalysis of a catalyst in a methanol solution of sodium methylate for obtaining an intermediate (III); and then a Duff reaction is performed on the intermediate (III), and finally an intermediate (I) is obtained. The solvent used in the method provided by the invention has low toxicity, and can be recycled and reused, so that the three-waste (waste gas, waste water and industrial residue) emission is reduced. The method simplifies the operation steps, reduces the production cost and is more beneficial to industrial reactions. The post-treatment process of the method is simpler, so that on the basis of increasing impurity removing efficiency, the complexity of the technological process is reduced further.
Owner:JIANGSU TOHOPE PHARMA
Trimetazidine hydrochloride sustained-release tablet
ActiveCN109316455AStable dissolution release propertiesNo burst phenomenonOrganic active ingredientsPill deliverySustained Release TabletHydrogen
The invention relates to a trimetazidine hydrochloride sustained-release tablet. The trimetazidine hydrochloride sustained-release tablet is specifically prepared from trimetazidine hydrochloride, a framework material, a thinner and an adhesive, wherein the framework material is a mixture of hydroxypropyl methyl cellulose and zein. The trimetazidine hydrochloride sustained-release tablet has the advantages that by using the hydroxypropyl methyl cellulose and the zein as the framework material, the burst release effect in the early period of trimetazidine hydrochloride is overcime; the in-vitrorelease of the obtained sustained-release tablet is free from the influence by the pH (potential of hydrogen) environment; the release degree in 8 hours is greater than 80%; the release curve is uniform with the release curve of a market product; the trimetazidine hydrochloride sustained-release tablet is suitable for industrialized large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
High-stability sustained-release tablet prepared by using hydroxy propyl cellulose
ActiveCN102824644BSmooth releaseImprove stabilityOrganic active ingredientsSenses disorderCelluloseSustained Release Tablet
The invention provides a high-stability sustained-release tablet prepared by using hydroxy propyl cellulose. The sustained-release tablet comprises the following components in parts by weight: 5-40 parts of trimetazidine hydrochloride and 10-85 parts of hydroxy propyl cellulose. A preparation method for the high-stability sustained-release tablet is a common wet granulation tableting process or a powder direct tableting process. According to the trimetazidine hydrochloride sustained-release tablet prepared by using the hydroxy propyl cellulose as a sustained-release material, the problem of low stability of a commercially available trimetazidine hydrochloride sustained-release tablet prepared by using the hydroxy propyl cellulose as a sustained-release material is solved.
Owner:ZHEJIANG CHENGYI PAHRMACEUTICAL +1
Trimetazidine hydrochloride sustained-release preparation and preparation method thereof
The invention provides a trimetazidine hydrochloride sustained-release preparation and a preparation method thereof. The trimetazidine hydrochloride sustained-release preparation is composed of sustained-release micro-tablets and capsule shells, and each sustained-release capsule is formed by filling the sustained-release micro-tablets with the diameter of 1-3mm. The trimetazidine hydrochloride micro-tablet is mainly composed of trimetazidine hydrochloride, an adhesive, a diluent, a sustained-release material and a lubricant. The trimetazidine hydrochloride sustained-release preparation comprises the following components in percentage by weight of 50 to 80 percent of trimetazidine hydrochloride, 1 to 20 percent of diluent, 1 to 20 percent of adhesive, 10 to 40 percent of sustained-releasematerial and 0.1 to 5 percent of lubricant. The invention also discloses a preparation method of the trimetazidine hydrochloride sustained-release preparation, which comprises the following steps of granulating the materials, tabletting, and filling into hollow capsules according to a certain quantity. The preparation process is good in stability, simple in process, high in production efficiency and suitable for industrial mass production.
Owner:南京康川济医药科技有限公司
A kind of trimetazidine hydrochloride sustained-release tablet and preparation method thereof
ActiveCN110623934BImprove complianceLittle side effectsOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMagnesium stearate
The invention relates to a trimetazidine hydrochloride sustained-release pharmaceutical composition and a preparation method thereof. The composition consists of an effective amount of trimetazidine hydrochloride and pharmaceutical auxiliary materials, the ratio of the main drug is 10%-20%, the slow-release material hypromellose is 10%-45%, and the slow-release material carbomer is 22%. ~40%, Sustained-release material Eudragit 0.2%~10%, diluent calcium hydrogen phosphate dihydrate 10%~48%, glidant colloidal silicon dioxide 0.3%~1.5%, magnesium stearate 0.5%~ 1.5%. The invention also provides a preparation method of the trimetazidine hydrochloride composition, that is, granulating through direct mixing process or fluidized bed, tableting and coating. The invention overcomes the defect of trimetazidine hydrochloride in the prior art that multiple administrations are required due to its short relative half-life, and can avoid side effects caused by excessive initial concentration of administration; the preparation process is simple and is suitable for large-scale industrial production.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Trimetazidine hydrochloride sublingual tablet pharmaceutical preparation
PendingCN114681415AQuality improvementEasy to makeOrganic active ingredientsPill deliveryPharmaceutical formulationPharmaceutical Substances
The invention relates to a trimetazidine hydrochloride tablet preparation, in particular to a sublingual tablet containing trimetazidine hydrochloride medicine, which contains trimetazidine hydrochloride, at least one diluent, at least two flavoring agents, at least one taste masking agent, at least one disintegrating agent and at least one lubricant, and the preparation contains 2mg of trimetazidine hydrochloride. The trimetazidine hydrochloride sublingual tablet in a specific proportion meets the requirements of a sublingual tablet preparation in quality, and has high disintegration and dissolution rates. Other advantages of the pharmaceutical preparation include simple process preparation. The invention further relates to a preparation method of the trimetazidine hydrochloride tablet, a sample prepared by the method is good in reproducibility, and the quality of the sample conforms to relevant regulations of sublingual tablets.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Trimetazidine hydrochloride controlled release tablet and preparation method therefor
InactiveCN111135151AOrganic active ingredientsPharmaceutical non-active ingredientsDrug utilisationControl release
Owner:HENAN TIANSHENG TAIFENG PHARM TECH CO LTD
A kind of trimetazidine oxalate and its preparation method and application
ActiveCN110183398BLarge specific surface areaImprove liquidityOrganic active ingredientsOrganic compound preparationOXALIC ACID DIHYDRATEOxalate
The invention provides a kind of trimetazidine oxalate, its molecular formula is C 16 h 24 N 2 o 7 , the trimetazidine oxalate is obtained by reacting trimetazidine and oxalic acid at a molar ratio of 1:1. The angle of repose of the trimetazidine oxalate is smaller than that of trimetazidine hydrochloride, and its surface morphology is flaky, which improves the fluidity and moisture absorption of the drug compared with the prismatic crystals of the trimetazidine hydrochloride bulk drug At the same time, the tensile strength of the trimetazidine oxalate reaches a maximum value of 3.99MPa at 150MPa, which is significantly higher than that of trimetazidine hydrochloride under the same pressure, which improves the tableting performance of the medicine.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
A kind of trimetazidine hydrochloride sustained-release preparation and preparation method thereof
The invention provides a trimetazidine hydrochloride sustained-release preparation and a preparation method thereof. The trimetazidine hydrochloride sustained-release preparation is composed of sustained-release microtablets and capsule shells, and each sustained-release capsule is composed of a Sustained release microtablets are filled. The microtablet is mainly composed of trimetazidine hydrochloride, a binder, a diluent, a sustained-release material and a lubricant. The trimetazidine hydrochloride sustained-release preparation comprises the following components in percentage by weight: trimetazidine hydrochloride 50-80%, diluent 1-20%, binder 1-20%, sustained-release material 10-40% %, lubricant 0.1 to 5%. The invention also discloses a preparation method of the trimetazidine hydrochloride sustained-release preparation: each material is granulated and then pressed into microtablets, and then filled into hollow capsules according to a certain amount. The preparation process has good stability, simple process and high production efficiency, and is suitable for industrialized mass production.
Owner:南京康川济医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com