Trimetazidine hydrochloride mono-layer osmotic pump controlled release tablet and preparation method thereof
A technology of trimetazidine hydrochloride and single-layer osmotic pump, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc. Response to large-scale problems, to achieve the effect of large-scale industrial production, cost saving, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
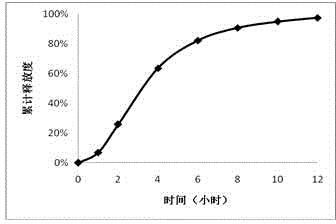
Embodiment 1
[0030] 1. Tablet core prescription
[0031] effect composition Dosage (mg) drug trimetazidine hydrochloride 35 osmotically active substances Sodium chloride 35 Suspension PEO N~750 70 Emulsifier Magnesium stearate 1
[0032] 2. Components of the coating solution (dosage for 100 tablets)
[0033] effect composition Dosage Film forming material Cellulose acetate 30g solvent acetone 950ml Water-soluble porogen, plasticizer PEG-4000 2g solvent water 50ml
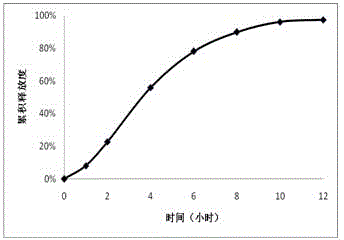
Embodiment 2
[0035] 1. Tablet core prescription
[0036] effect composition Dosage (mg) drug trimetazidine hydrochloride 35 osmotically active substances Sodium chloride 35 filler Microcrystalline Cellulose 101 60 Suspension PEO N~750 70 Emulsifier Magnesium stearate 2
[0037] 2. Components of the coating solution (dosage for 100 tablets)
[0038] effect composition Dosage Film forming material Cellulose acetate 30g solvent acetone 950ml Water-soluble porogen, plasticizer PEG-4000 2g solvent water 50ml
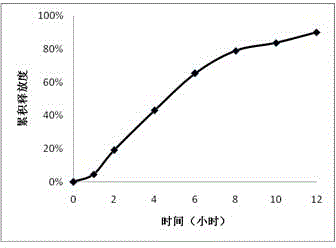
Embodiment 3
[0040] 1. Tablet core prescription
[0041] effect composition Dosage (mg) drug trimetazidine hydrochloride 35
[0042] osmotically active substances Sodium chloride 35 filler lactose 60 Suspension PEO N~750 70 Emulsifier Magnesium stearate 2
[0043] 2. Components of the coating solution (dosage for 100 tablets)
[0044] effect composition Dosage Film forming material Cellulose acetate 30g solvent acetone 950ml Water-soluble porogen, plasticizer PEG-4000 2g solvent water 50ml
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com