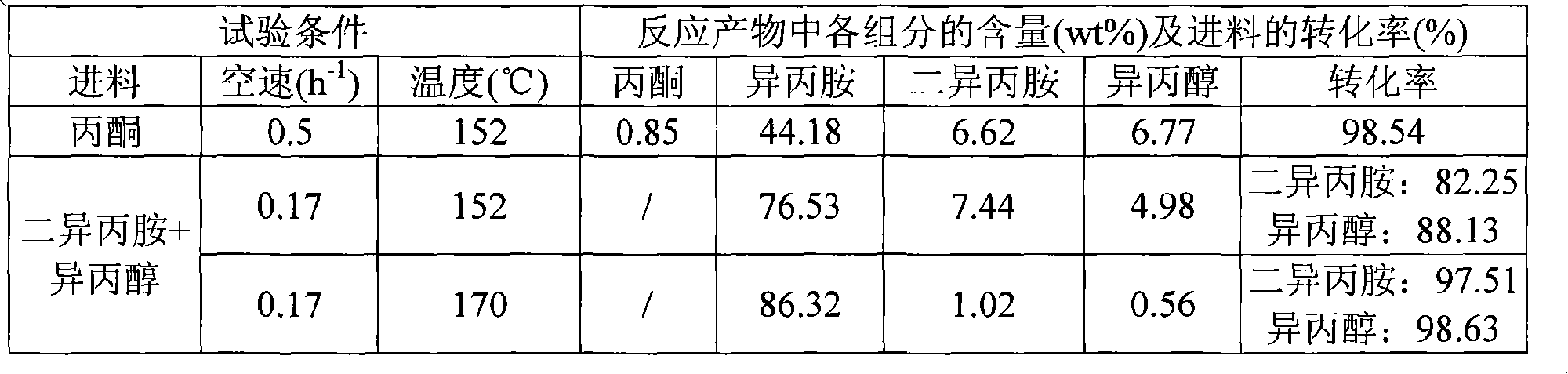
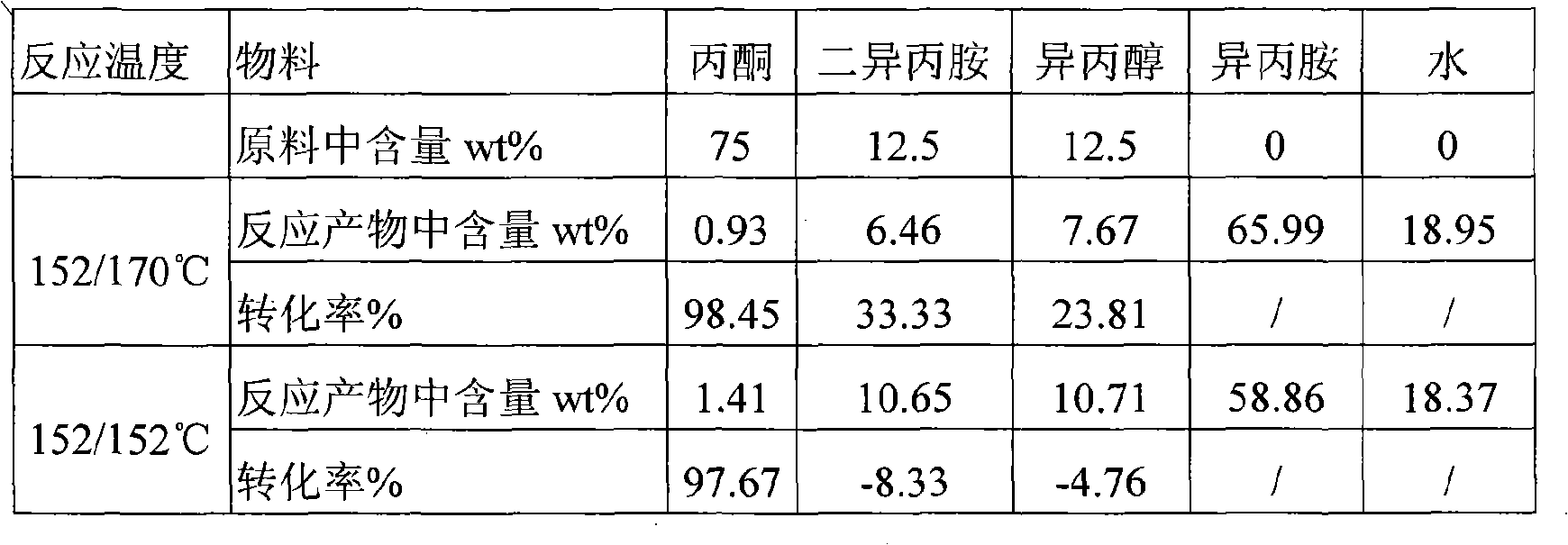
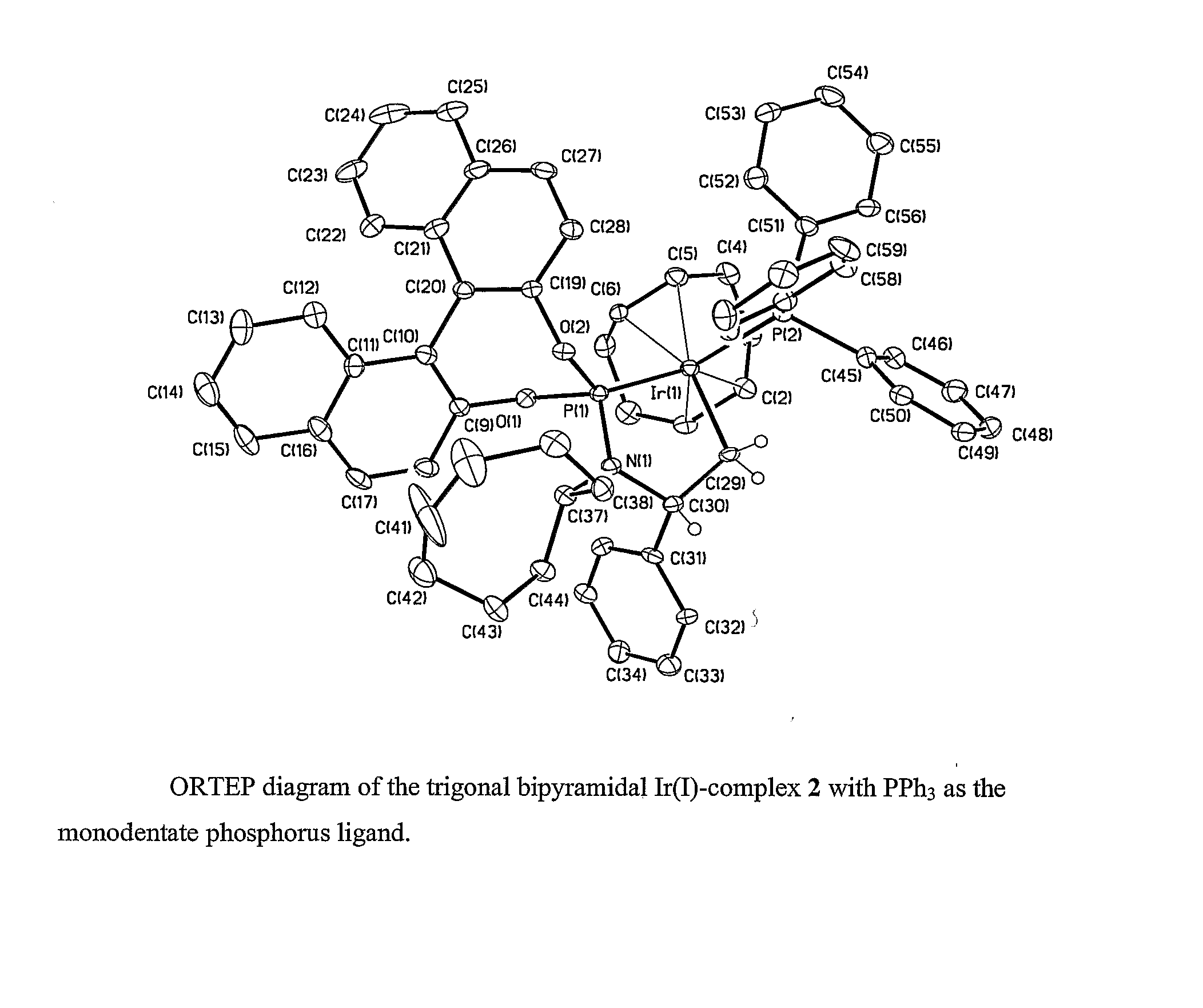
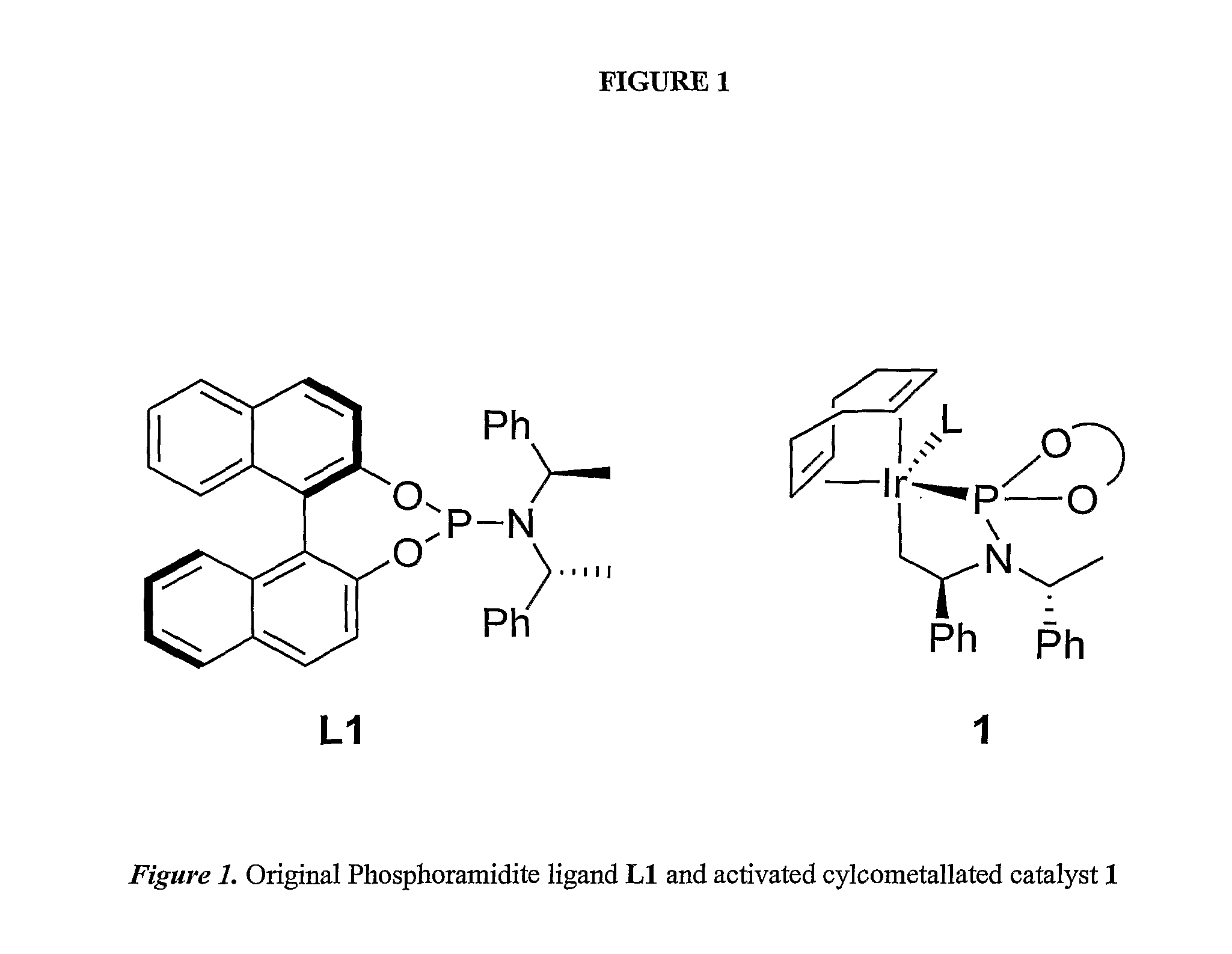
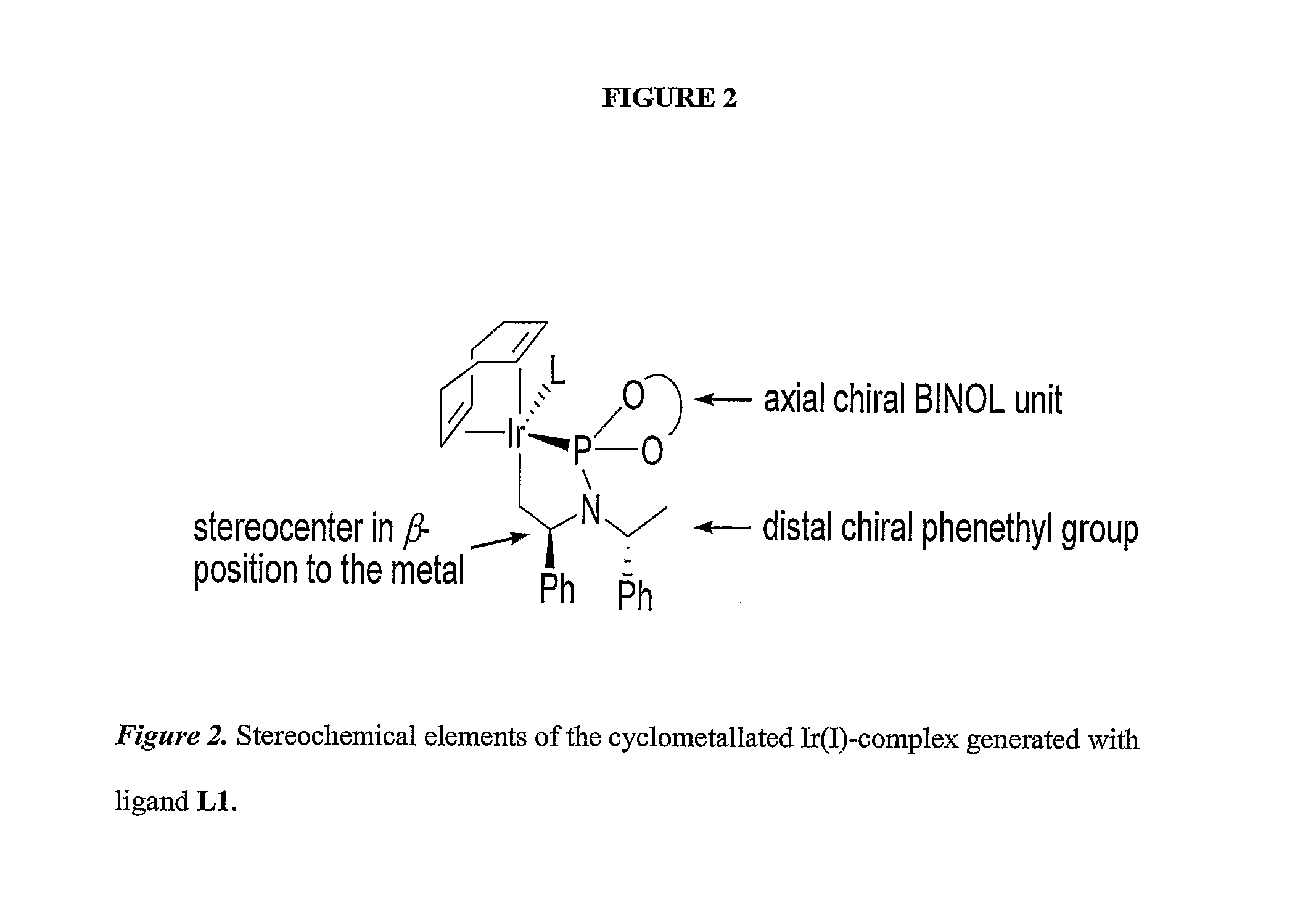
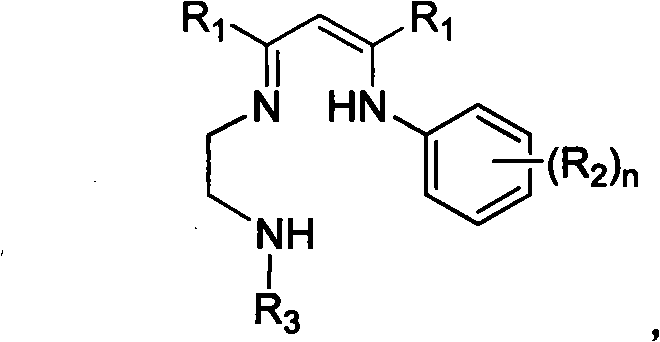
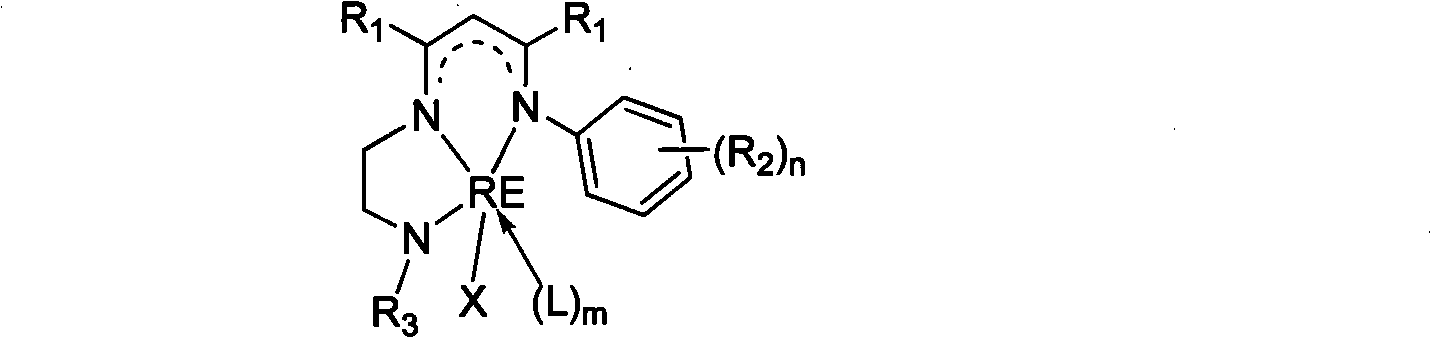
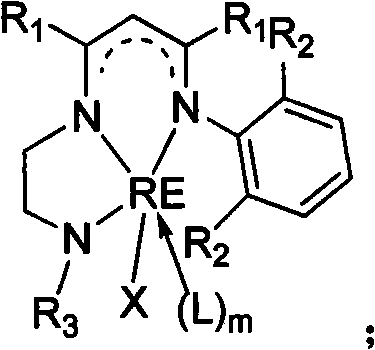
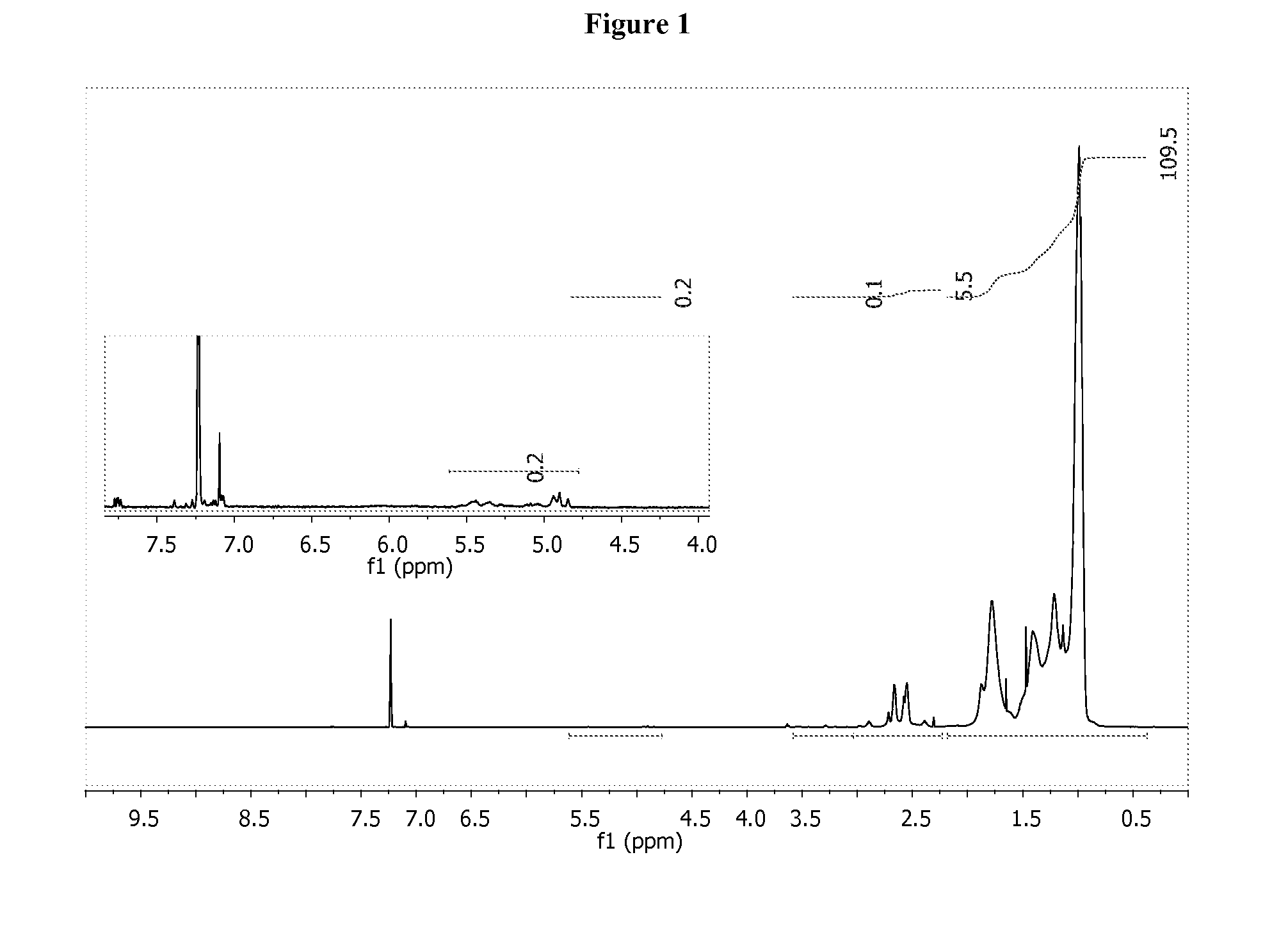
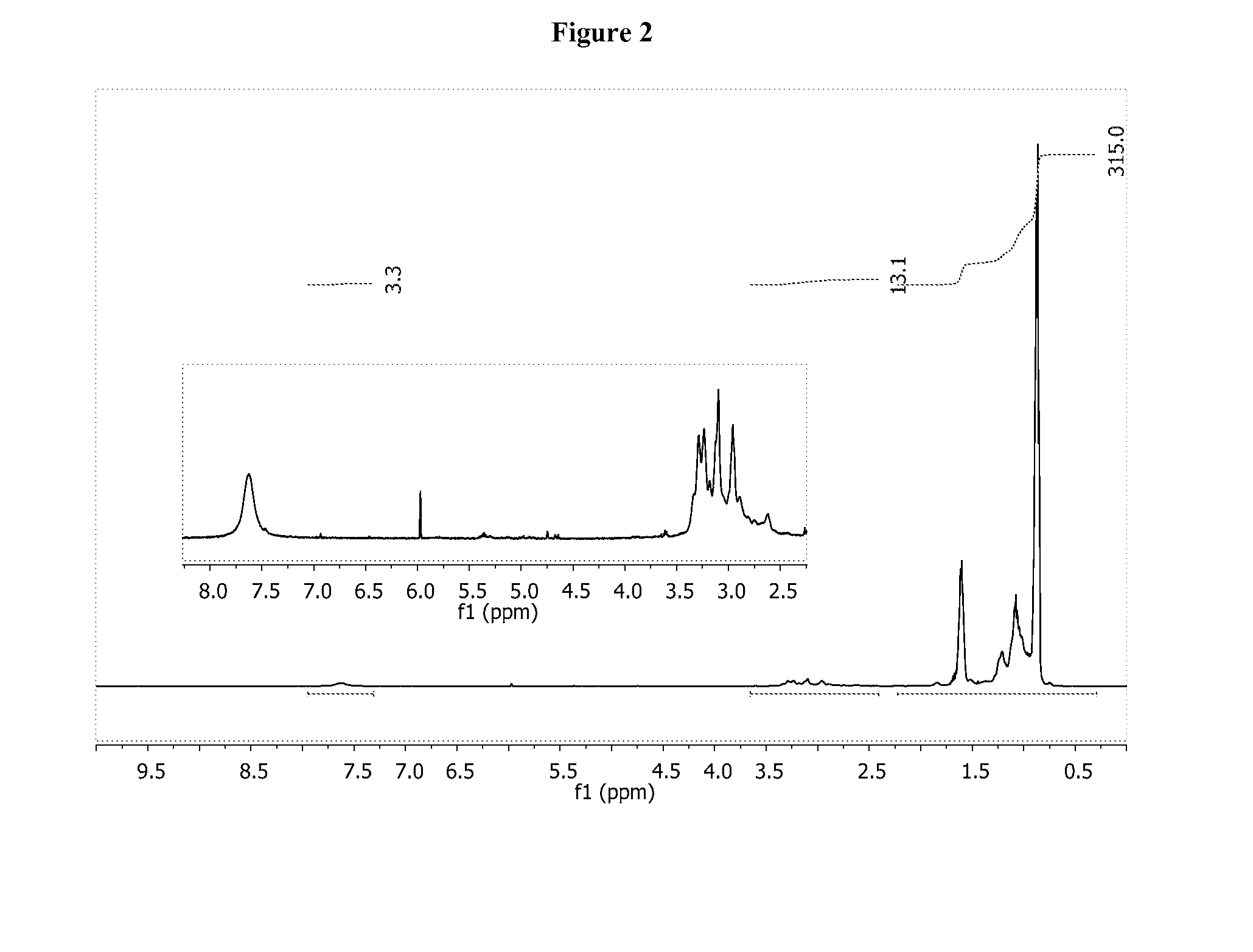
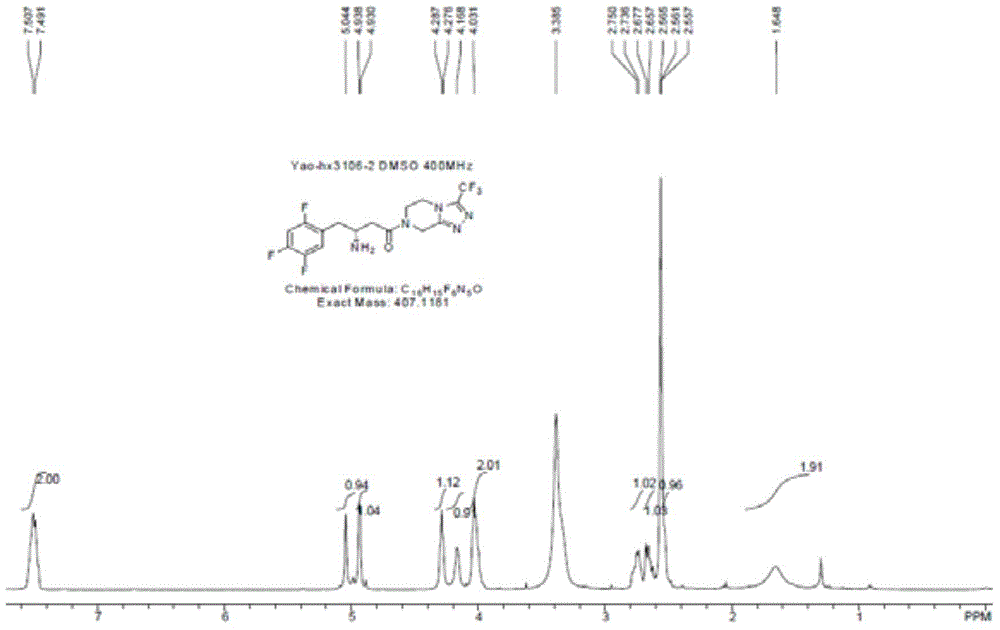
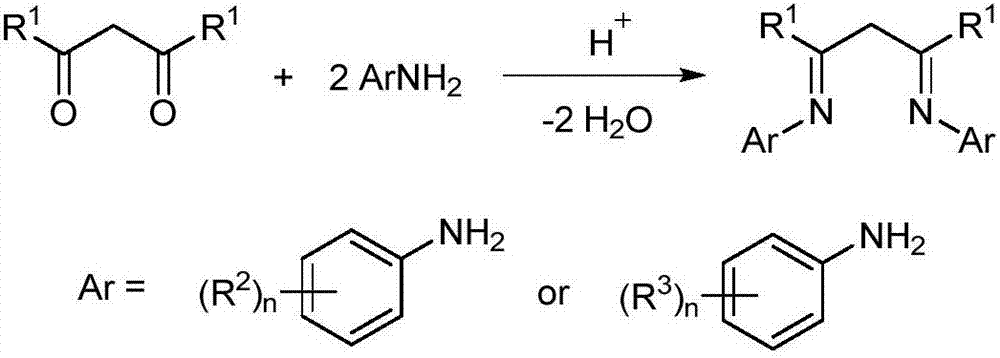
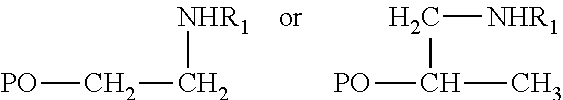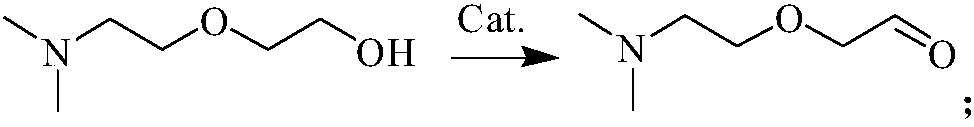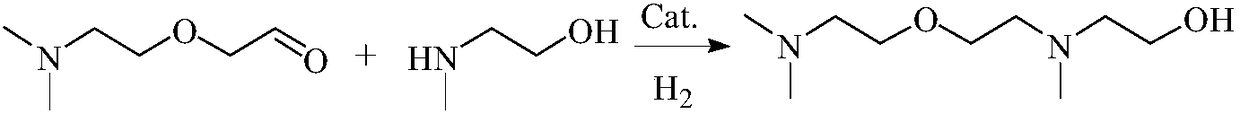Patents
Literature
82 results about "Hydroamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
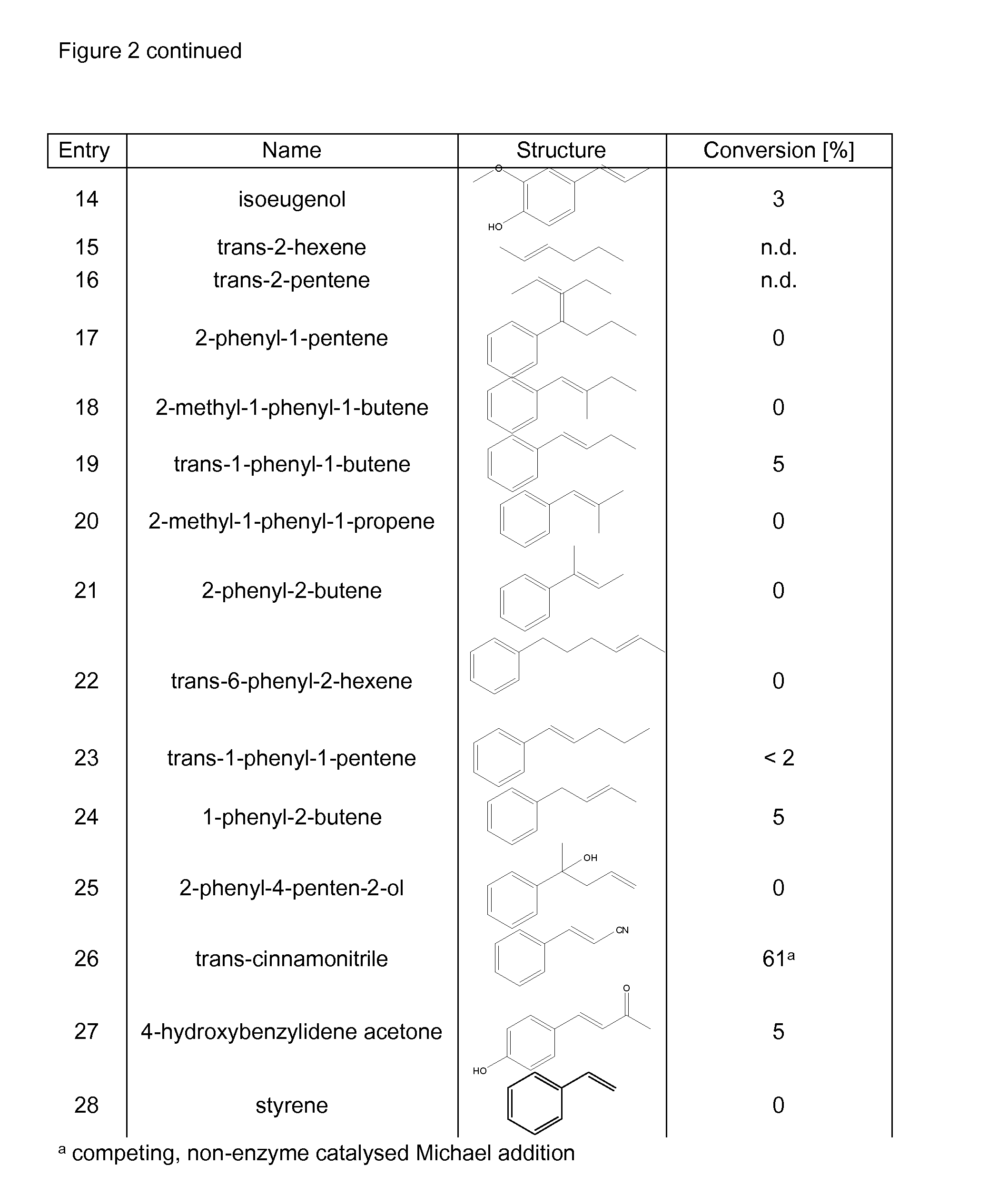
Hydroamination is the addition of an N-H bond of an amine across a carbon-carbon multiple bond of an alkene, alkyne, diene, or allene. In the ideal case, hydroamination is atom economical and green. Amines are common in fine-chemical, pharmaceutical, and agricultural industries.
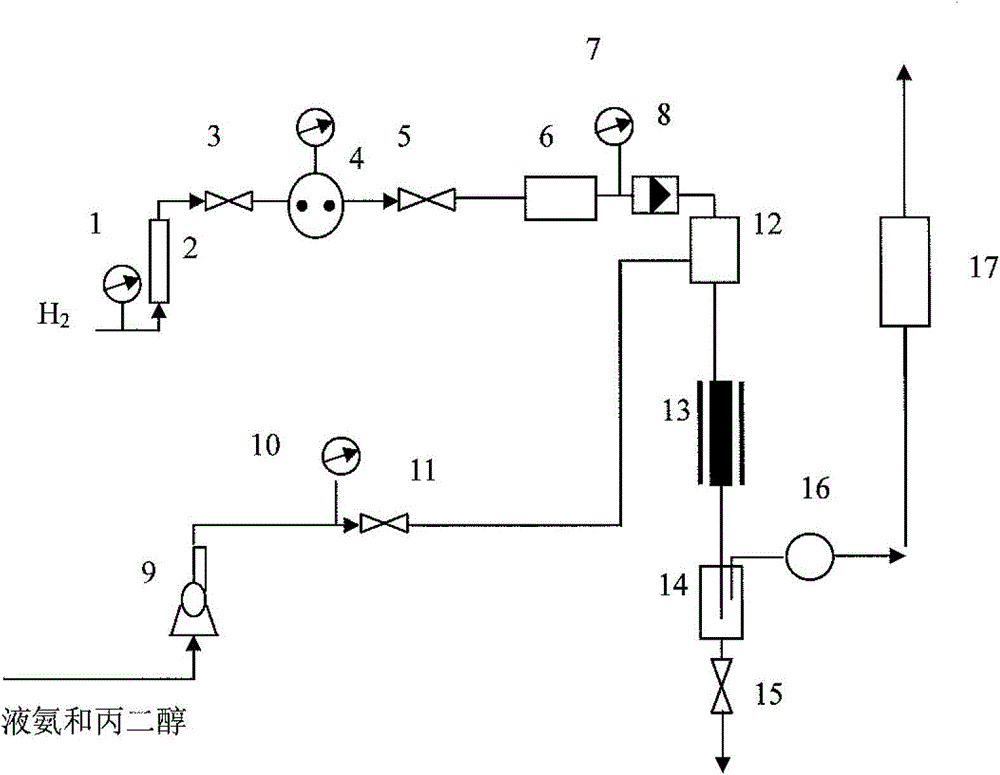
Method and device for preparing propane diamine by taking propylene glycol and liquid ammonia as raw materials
ActiveCN104693038AHigh selectivityLow one-time investment costOrganic compound preparationAmino compound preparationHydrogenPropylene glycol
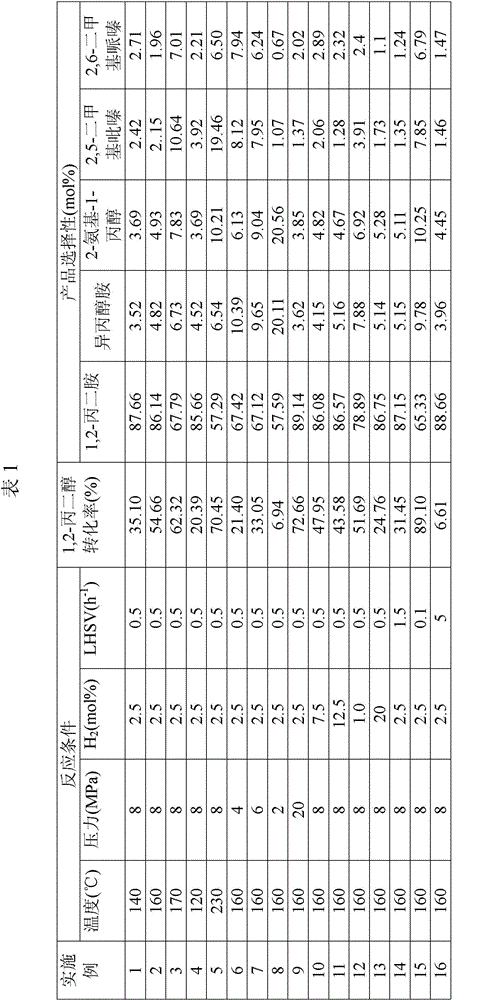
The invention relates to a method for preparing propane diamine by taking propylene glycol and liquid ammonia as raw materials. Propylene glycol and liquid ammonia are mixed in a certain ratio and are pumped into a reactor by virtue of a pump, and reaction is carried out in presence of a catalyst and hydrogen. The method for preparing the propane diamine by taking the propylene glycol and liquid ammonia as the raw materials has the advantages that a novel catalyst is adopted, catalytic performance is excellent, and long-time operation can be easily carried out; propylene glycol is subjected to hydroamination for producing a propane diamine product at lower reaction pressure, and reaction conditions are adjusted and changed, so that composition of the product can be flexibly adjusted and changed, selectivity of a target product is improved, a reaction process is simple, one-time investment of a production unit and production cost are reduced, a reaction product and a catalyst can be simply separated, and large-scale continuous industrial production can be easily realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
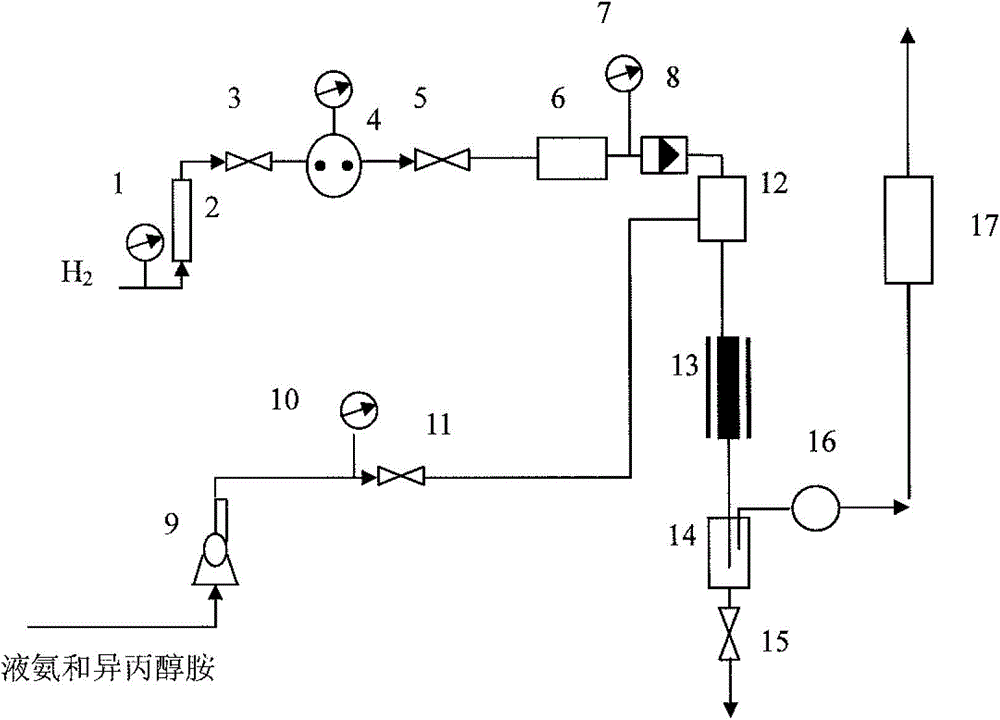
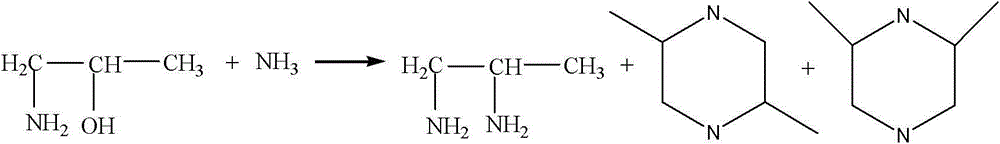
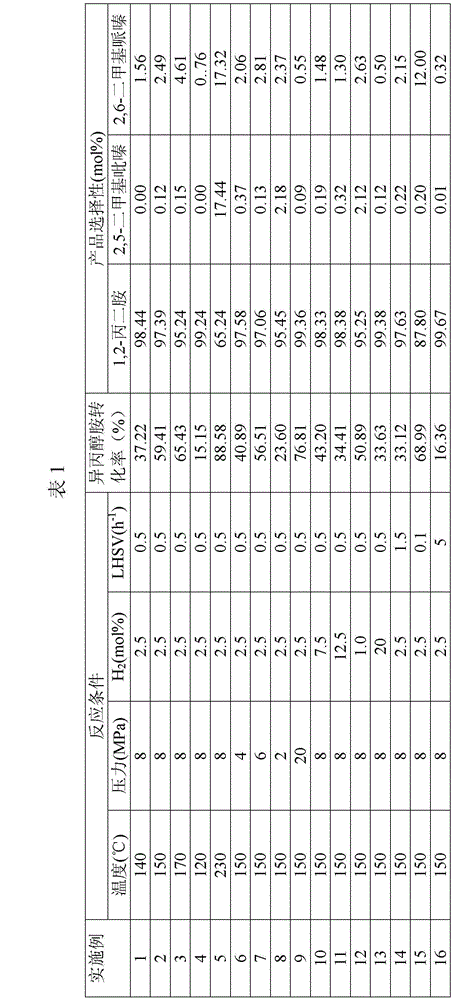
A method of preparing 1,2-diaminopropane from isopropanolamine and liquid ammonia and a device thereof
ActiveCN104693037AHigh selectivityLow one-time investment costOrganic compound preparationAmino compound preparationHydrogenPropylenimine
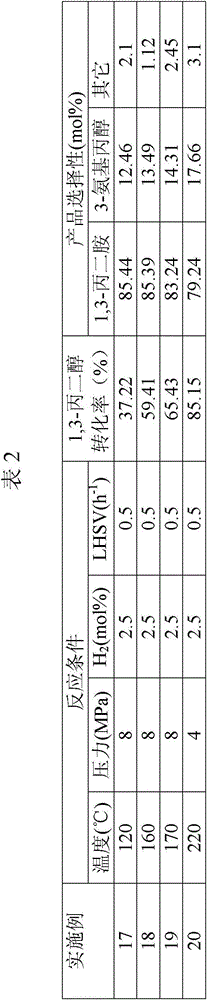
The invention relates to a method of preparing 1,2-diaminopropane from isopropanolamine and liquid ammonia. The method includes mixing the isopropanolamine and the liquid ammonia according to a certain ratio, pumping the mixture into a reactor and reacting under existence of a catalyst and hydrogen. The method adopts the novel catalyst which is excellent in catalytic performance and prone to long-term operation. The method allows production of a diaminopropane product by hydroamination of the isopropanolamine to be achieved under a low reaction pressure. By adjustment of reaction conditions, composition of products can be regulated flexibly and selectivity of a target product is improved. A reaction process is simple. One-time investment of production devices and a production cost are reduced. Separation of a reaction product from the catalyst is simple. The method is prone to large-scale continuous industrial production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
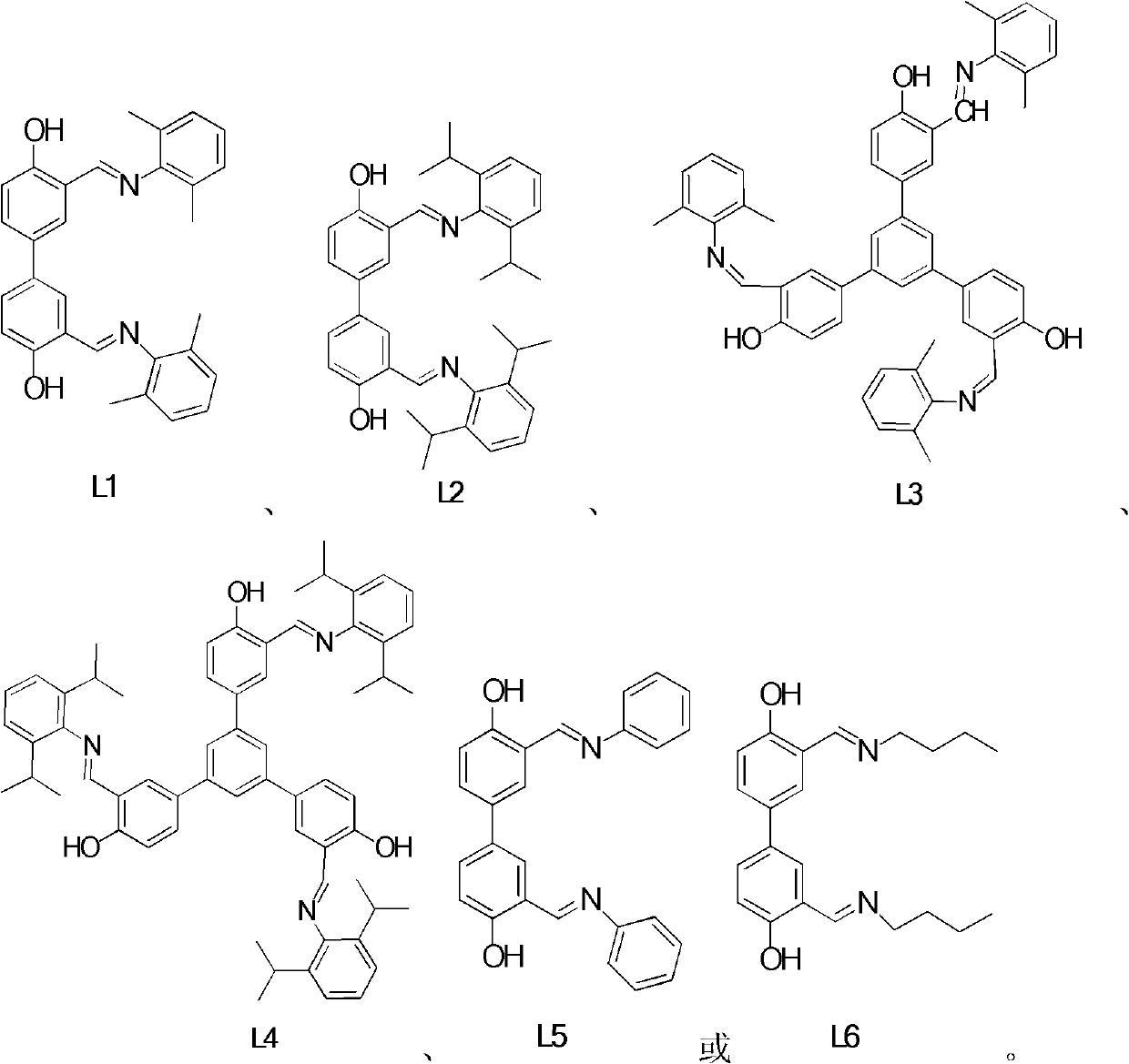
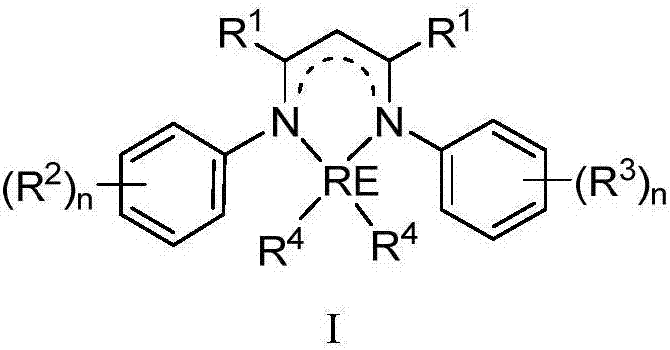
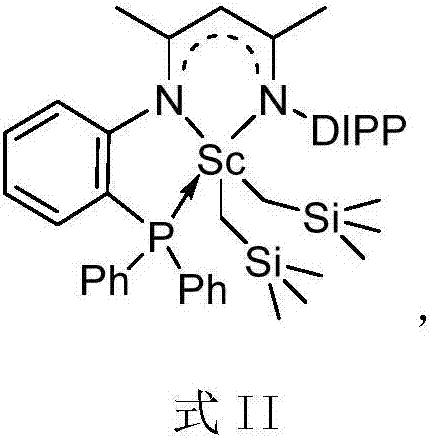
Schiff-base ligand-based rare-earth metal complex, preparation method and applications
InactiveCN102503966AOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsAlkaneHydrogen
The invention relates to a Schiff-base ligand-based rare-earth metal complex, a preparation method and applications. The complex is prepared by reacting a Schiff-base ligand with a rare-earth metal amino compound. When the complex is used as a catalyst, hydrogen-alkane oxidation reaction and hydroamination can be directly and efficiently catalyzed without the assistance of any activating agents or cocatalysts, and moreover, the complex can be recycled in catalytic reaction. The structural general formula of the rare-earth supported catalyst is shown as follows in the specification.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
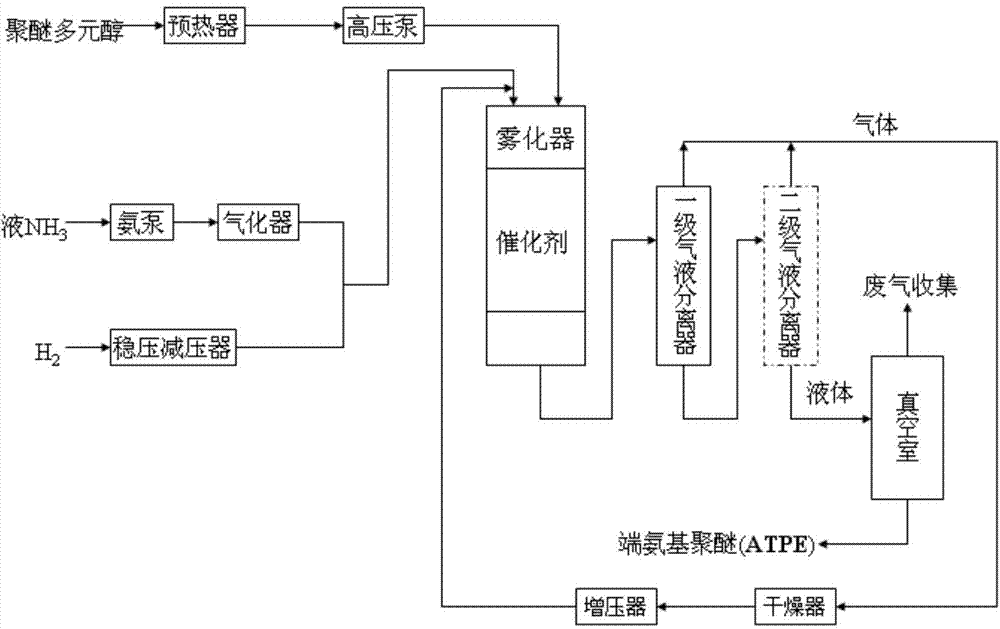
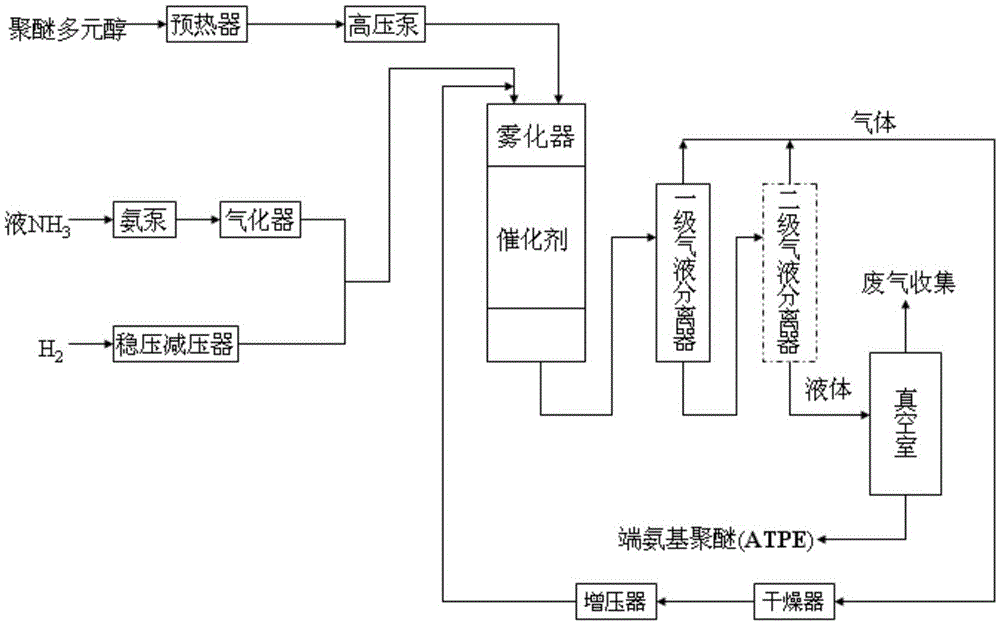
Continuous preparation method of amine-terminated polyether
The invention discloses a continuous preparation method of amine-terminated polyether, and the method comprises the following steps of: taking polyether polyol, H2 and NH3 as raw materials, executing critical hydroamination in a fixed bed reactor under a critical hydroamination catalyst, executing gas-liquid separation in a gas-liquid separator to feed liquid from the fixed bed reactor, reusing NH3 and H2 without being reacted after being dried and pressurized; accessing the liquid material into a vacuum chamber, removing H2O and other small-molecule materials in vacuum, continuously discharging to prepare amine-terminated polyether. The method is continuous in operating processes and more steady in a product quality batch method; in preparation, the gas can be reused to prevent environmental pollution; the method can effectively solve the problems of decreased conversion and weak selectivity caused by poorly contacting the gas and the catalyst during large-molecular polyether reaction; the method has the advantages of higher reaction conversion ratio and primary amine selectivity.
Owner:HONGBAOLI GRP CO LTD +1
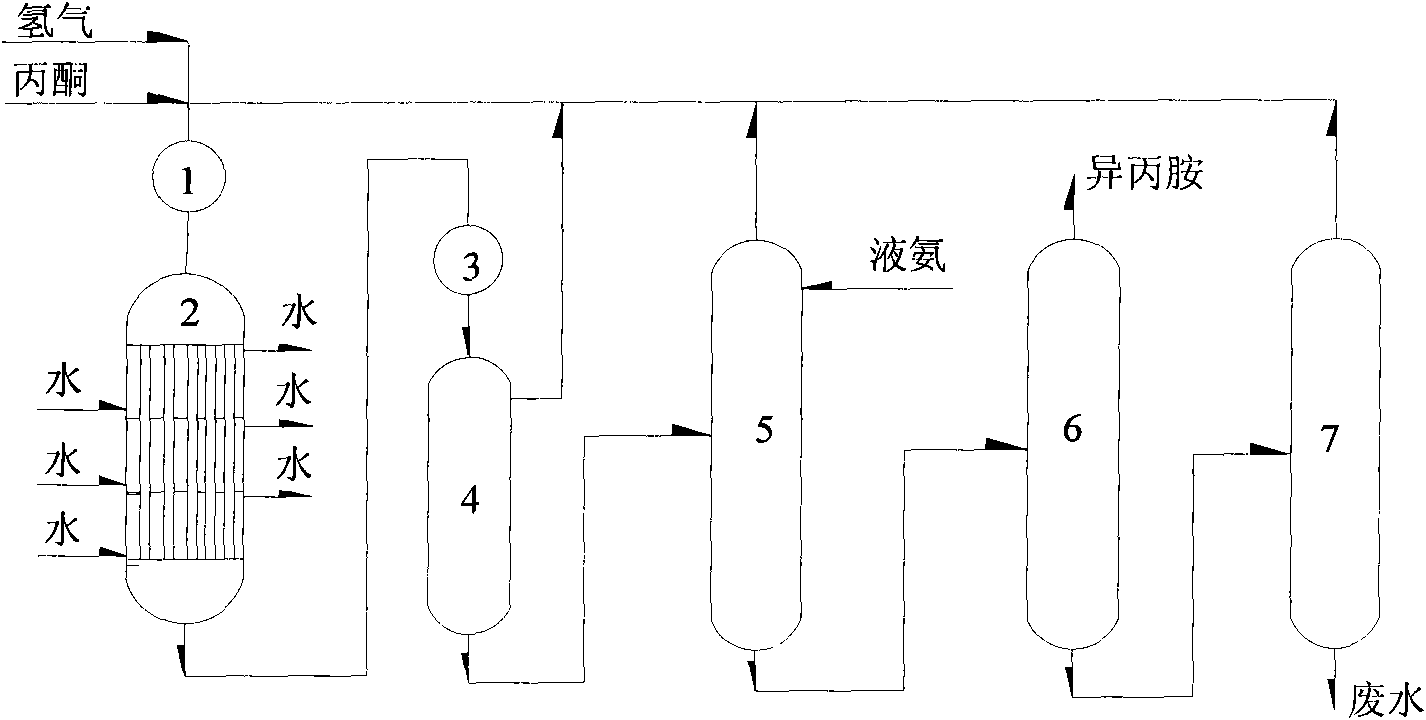
Method for producing isopropylamine
ActiveCN101684073AReduce heatReduce energy costsAmino compound purification/separationPreparation by reductive alkylationReaction temperatureEconomic benefits
Owner:CHINA PETROLEUM & CHEM CORP +1
Enantioselective Phosphoramidite Compounds and Catalysts
InactiveUS20070259774A1Easy to getWithout compromising activity and degree of chemical selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystEnantio selectivity
This invention relates to phosphoramidite compounds and catalyst complexes which can be used to provide enantioselective reactions including hydroamination reactions, etherification reactions and conjugate addition reactions and allylic substitution reactions, among others. In a first aspect, the present invention is directed to phosphoramidite and related compounds according to general structure (I), where Z is absent or is a group containing O, N or S, preferably O; R1 and R2 are independently an optionally substituted C1-12 alkyl group, an optionally substituted (CH2)n-aromatic group or (CH2)n-heteroaromatic group, or are linked together to form an optionally substituted aliphatic or (CH2)n-aromatic dianion of a diol, diamine, dithiol, aminoalcohol, aminohiolate or a alcoholthiol group; R3′ and R3 are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group with the proviso that R3′ and R3 are not both H, or together R3′ and R3 form an optionally substituted C5-C15 saturated or unsaturated carbocyclic ring; R4 is H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group; R5 is absent, H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic or (CH2)n-heteroaromatic group; Ra and Ra′ are each independently H or a C1-C3 alkyl group, or Ra and Ra′ together with the carbon to which they are attached form a optionally substituted C5-C15 saturated or unsaturated carbocyclic or heterocyclic ring, or an aromatic or heteroaromatic ring; R6 and R7 are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group, with the proviso that R5, R6 and R7 cannot simultaneously be H, and when Ra and Ra′, together with the carbon to which they are attached, form a carbocyclic ring, heterocyclic ring or an aromatic or heteroaromatic ring, R5 is absent or is preferably H; R6 and R7 are preferably H or CH3; and each n is independently 0, 1, 2, 3, 4, 5 or 6 and wherein at least one of the carbon atoms attached to the nitrogen of the phosphoramidite group is a chiral center.
Owner:YALE UNIV
Novel three-tooth nitrogen ligand and rare earth metal complex
InactiveCN101492390ASilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsDiketoneAlkene
The invention relates to a novel tridentate nitrogen ligand, rare earth metal complexes thereof and the application of the rare earth metal complexes in the olefin hydroamination reaction and the polyester synthesis. The ligand of the kind can be obtained from corresponding beta diketone and amine by two-step condensation reaction; the rare earth complexes can be obtained by the reaction between the ligand and rare earth metal alkyl and amidine compounds. The rare earth complexes can effectively catalyze the olefin hydroamination reaction and the ring-opening polymerization of lactones. The structural general formulas of the tridentate nitrogen and the corresponding rare earth complexes are shown as above.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Hydroamination Of Aldehyde-Containing Macromonomers
This invention relates to a polyolefin composition comprising one or more of the following formulae:wherein the PO is the residual portion of a vinyl terminated macromonomer (VTM) having had a terminal unsaturated carbon of an allylic chain and a vinyl carbon adjacent to the terminal unsaturated carbon; R1 is a hydrogen atom, an aryl group, or an alkyl group, wherein the aryl group or alkyl group can include one or more heteroatoms, an alkyl or aryl amino group, a polyalkylamino group; wherein the VTM is a vinyl terminated macromonomer.
Owner:EXXONMOBIL CHEM PAT INC
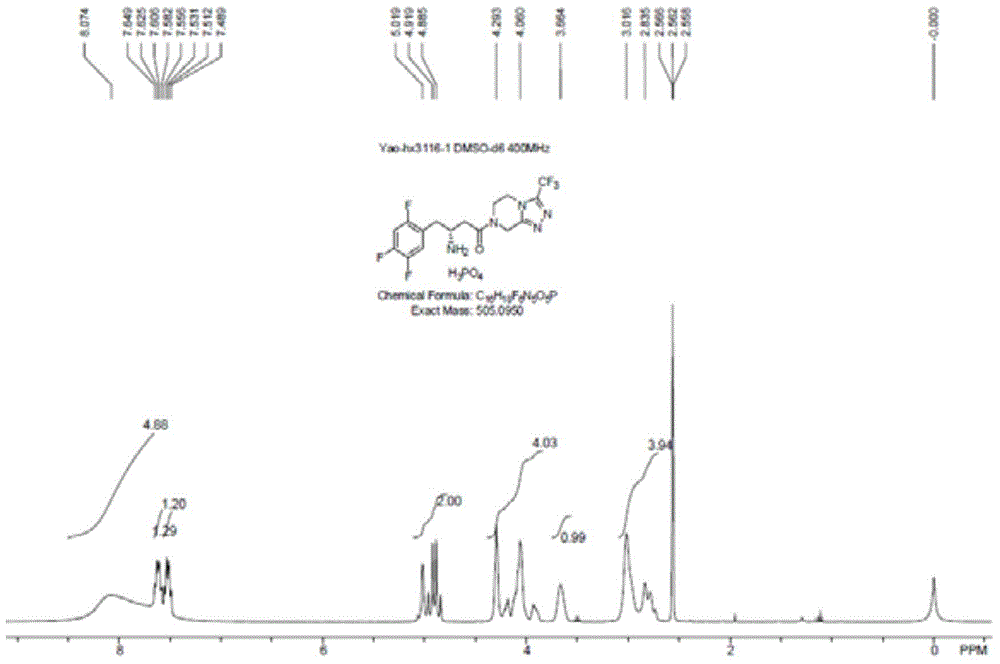
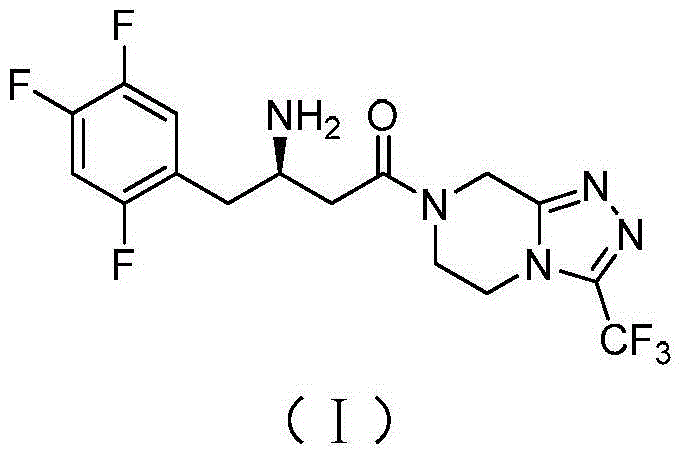
Synthetic method of sitagliptin and salt thereof
The invention discloses a synthetic method of sitagliptin and a salt thereof. The synthetic method comprises the steps: carrying out an esterification reaction, a reduction reaction, an oxidizing reaction and a witting reaction on 2,4,5-trifluorophenylacetic acid as a starting raw material to obtain 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate; then carrying out a hydroamination reaction on 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate and chiral amine in the presence of butyl lithium or hexamethyldisilazane sodium to form a chiral hydroamination product; carrying out an esterolysis reaction, a condensation reaction and a hydrogenation reaction to obtain sitagliptin. The raw materials used in the synthetic method of sitagliptin are low in price and easy to obtain; the synthetic method of sitagliptin is less in step, easy to operate and capable of effectively reducing cost. By the use of the method, high-purity sitagliptin can be obtained, a sitgliptin phosphate obtained through salifying has an HPLC (High Performance Liquid Chromatography) and an ee (enantiomeric excess) value of more than 99% and can be applied to the field of medicine.
Owner:ZHEJIANG NHU CO LTD +1
Preparation method of alkylamine and method of increasing hydroamination activity of calicining zeolite catalyst
InactiveCN1534014AHigh activityMolecular sieve catalystsOrganic compound preparationNitrogenNitrogen gas
Alkylamine production by reaction of olefins with ammonia or primary or secondary amines under hydroamination conditions with a calcined zeolitic catalyst is such that at most 24 hours before the reaction the catalyst is heat- activated at 100-550degreesC in a gas stream of air, nitrogen and / or other inert gases.
Owner:BASF AG
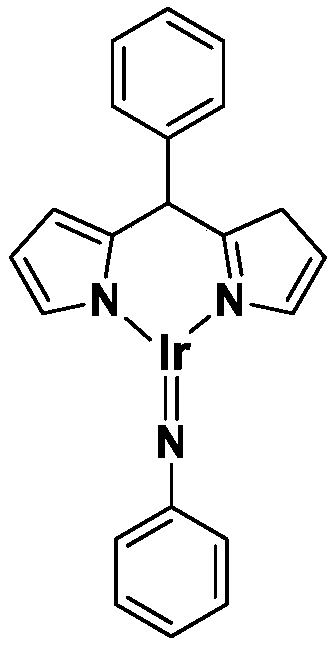
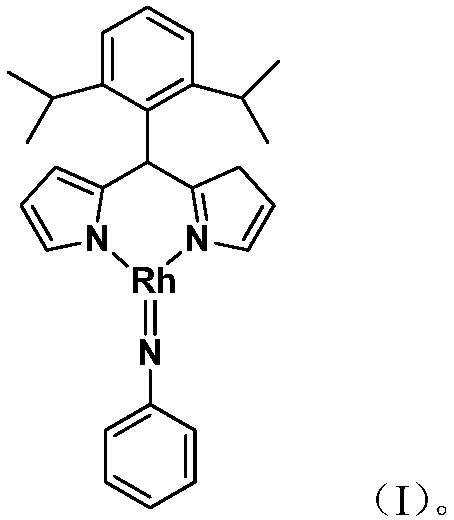
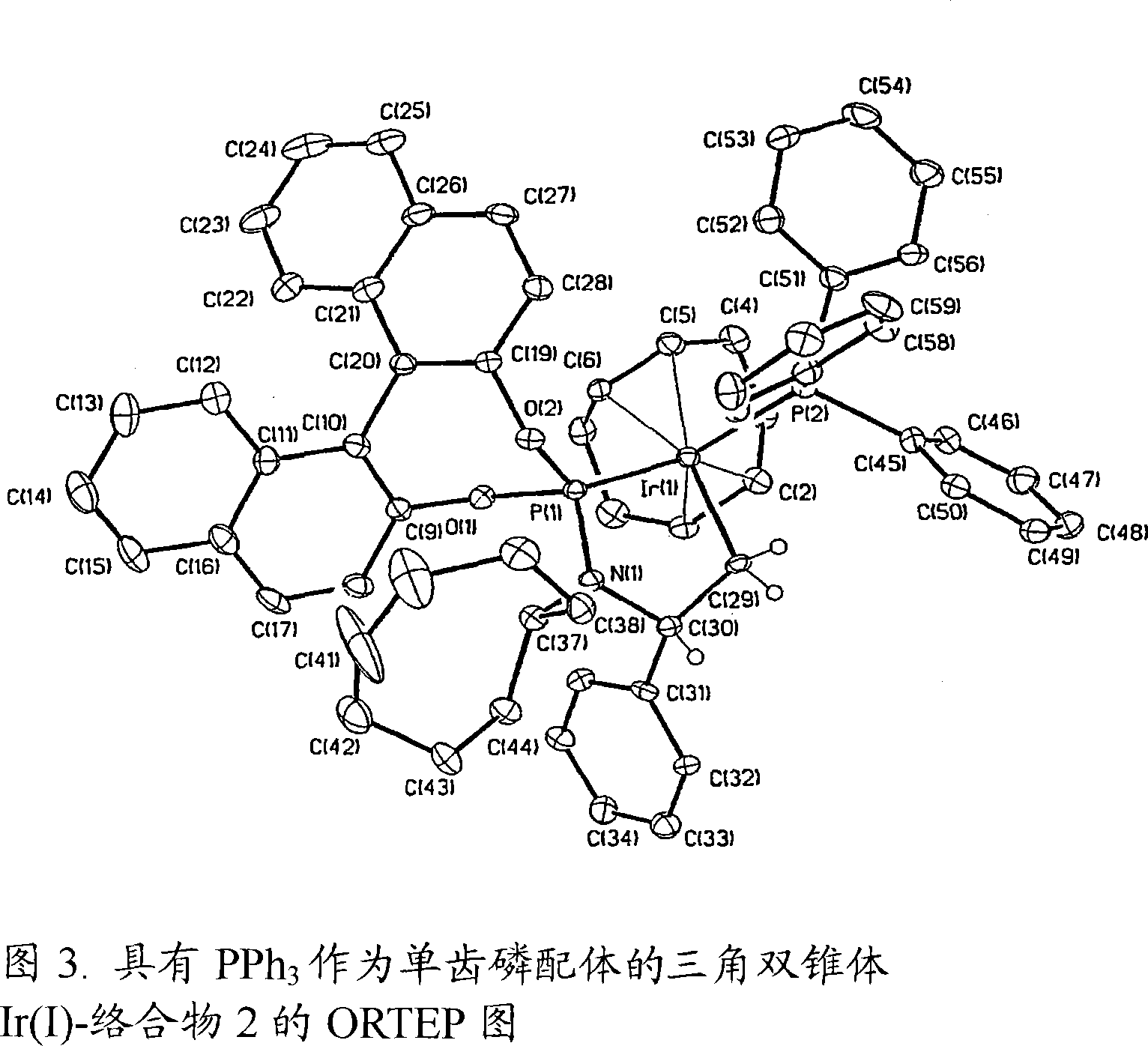
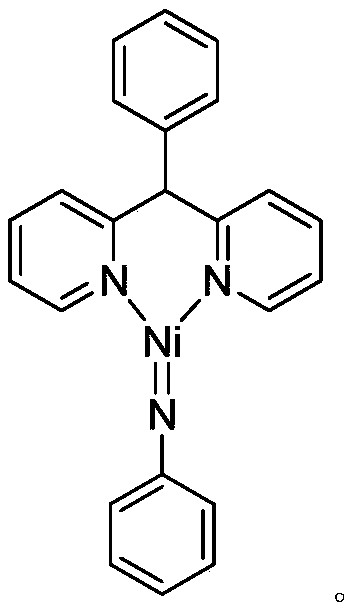
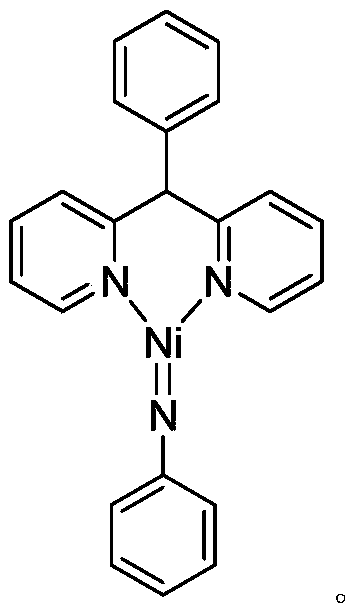
Trivalent iridium imine complex containing iridium nitrogen double bonds, preparation method and application of trivalent iridium imine complex
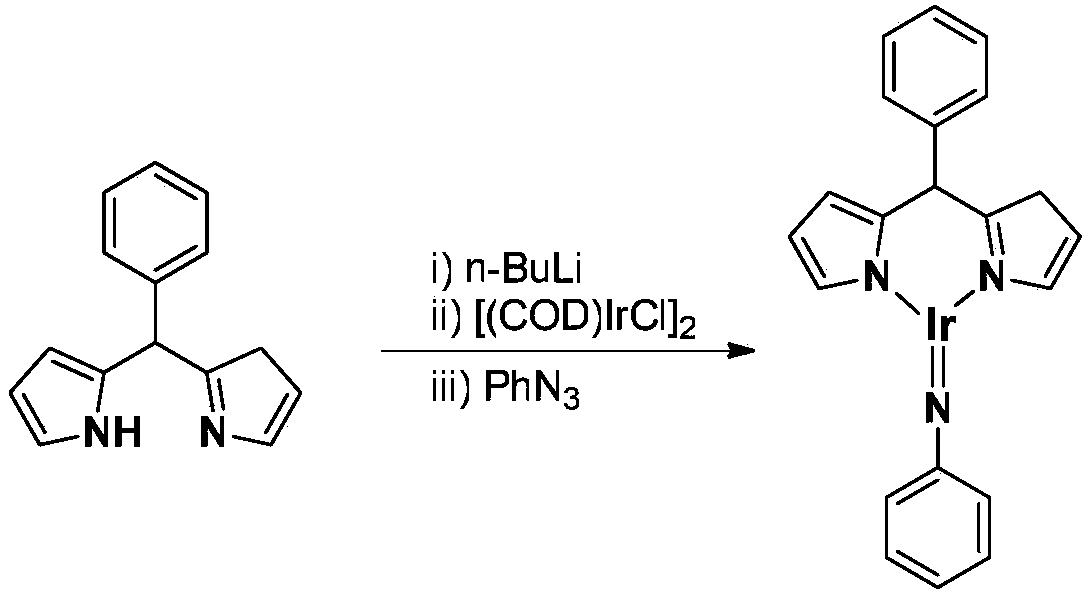
ActiveCN109293706AEasy to prepareHigh yieldIndium organic compoundsOrganic compound preparationMarkovnikov's ruleDouble bond
The invention belongs to the technical field of synthetic chemistry, and in particular relates to a trivalent iridium imine complex containing iridium nitrogen double bonds, a preparation method and an application of the trivalent iridium imine complex. A binuclear iridium compound cyclooctadiene iridium chloride dimer [ (COD) IrCl ]2 is taken as a raw material, and the binuclear iridium compoundiridium(I) cyclooctadiene chloride, dimer [ (COD) IrCl ]2 reacts with phenyl dipyrrole compounds under an alkaline condition to obtain a precursor containing a monovalent iridium complex, and the precursor is oxidized into the trivalent iridium imine compound containing the iridium nitrogen double bonds by using an azide oxidation method. The synthesis process is simple and green, and has excellent selectivity and higher yield. The trivalent iridium imine complex has the characteristics of stable physical and chemical properties, thermal stability and the like, and shows excellent activity andregioselectivity (anti-Markovnikov's rule) in hydroamination of olefins.
Owner:SHANGHAI INST OF TECH
Environmental-friendly and efficient preparation method of quinolone compound
InactiveCN104262249ASimple processLow costOrganic compound preparationAmino-hyroxy compound preparationIce waterPtru catalyst
The invention discloses an environmental-friendly and efficient preparation method of a quinolone compound. According to the adopted technical scheme, the environmental-friendly and efficient preparation method comprises the following steps: putting an intermediate 1 (I) in a reactor, dropwise adding an intermediate 2 (II) at a room temperature, and mixing and stirring uniformly under the condition without solvent to carry out solvent-free hydroamination; after a detection reaction is completed, carrying out chromatography on reaction liquid through a silicagel column, concentrating through reduced pressure distillation to obtain a yellow oily intermediate 3 (III); then putting the intermediate 3 (III) in the reactor, directly adding a catalyst, heating for dissolution while stirring to carry out an intramolecular cyclization reaction; after the detection reaction is completed, quenching the reaction by using ice water, extracting, concentrating and recrystallizing to obtain a target compound 4 (IV). The preparation method of the quinolone compound disclosed by the invention is environmental-friendly, efficient, simple, convenient and safe to operate, and the produced quinolone compound is low in cost.
Owner:YUNNAN MINZU UNIV
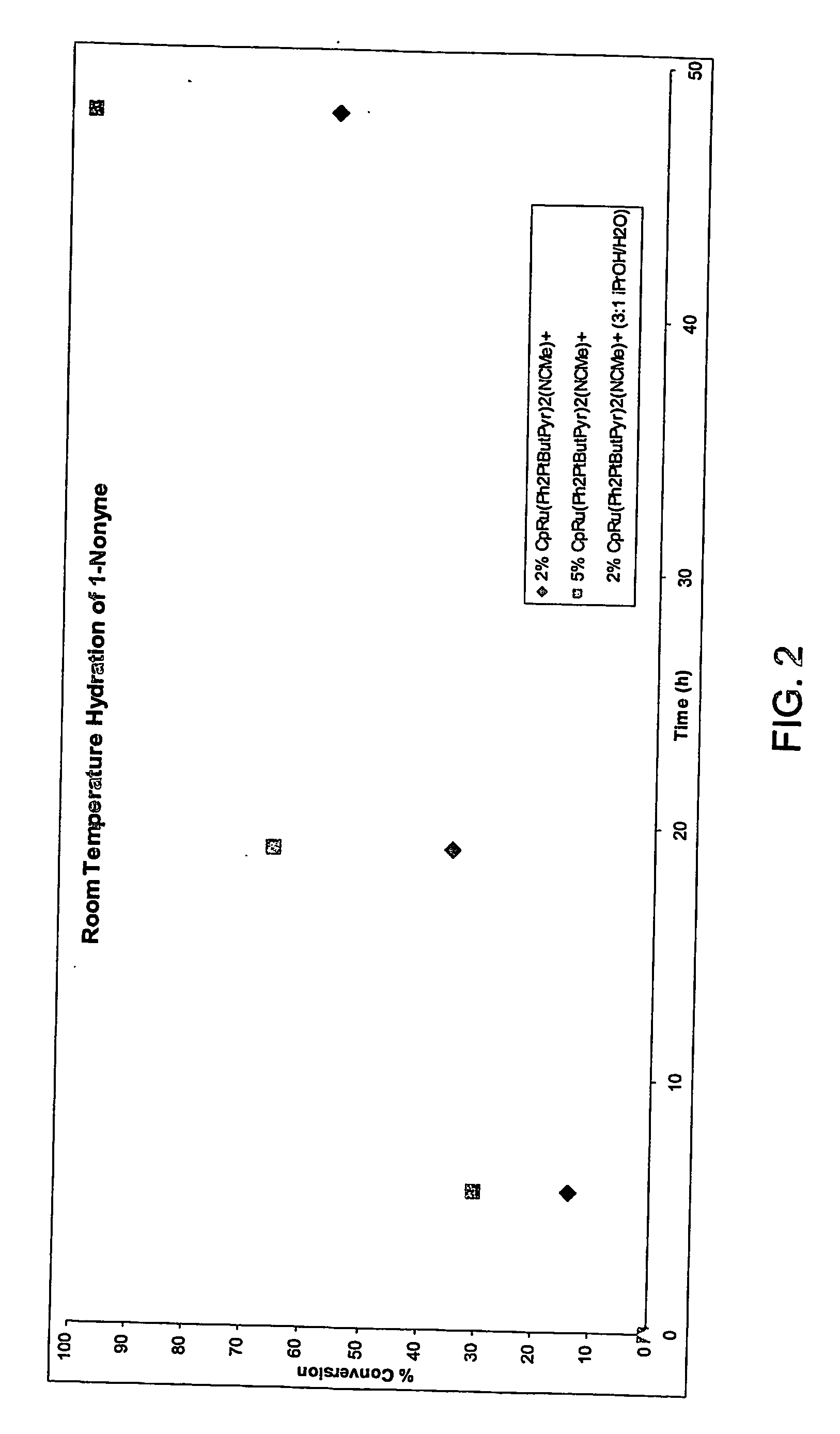
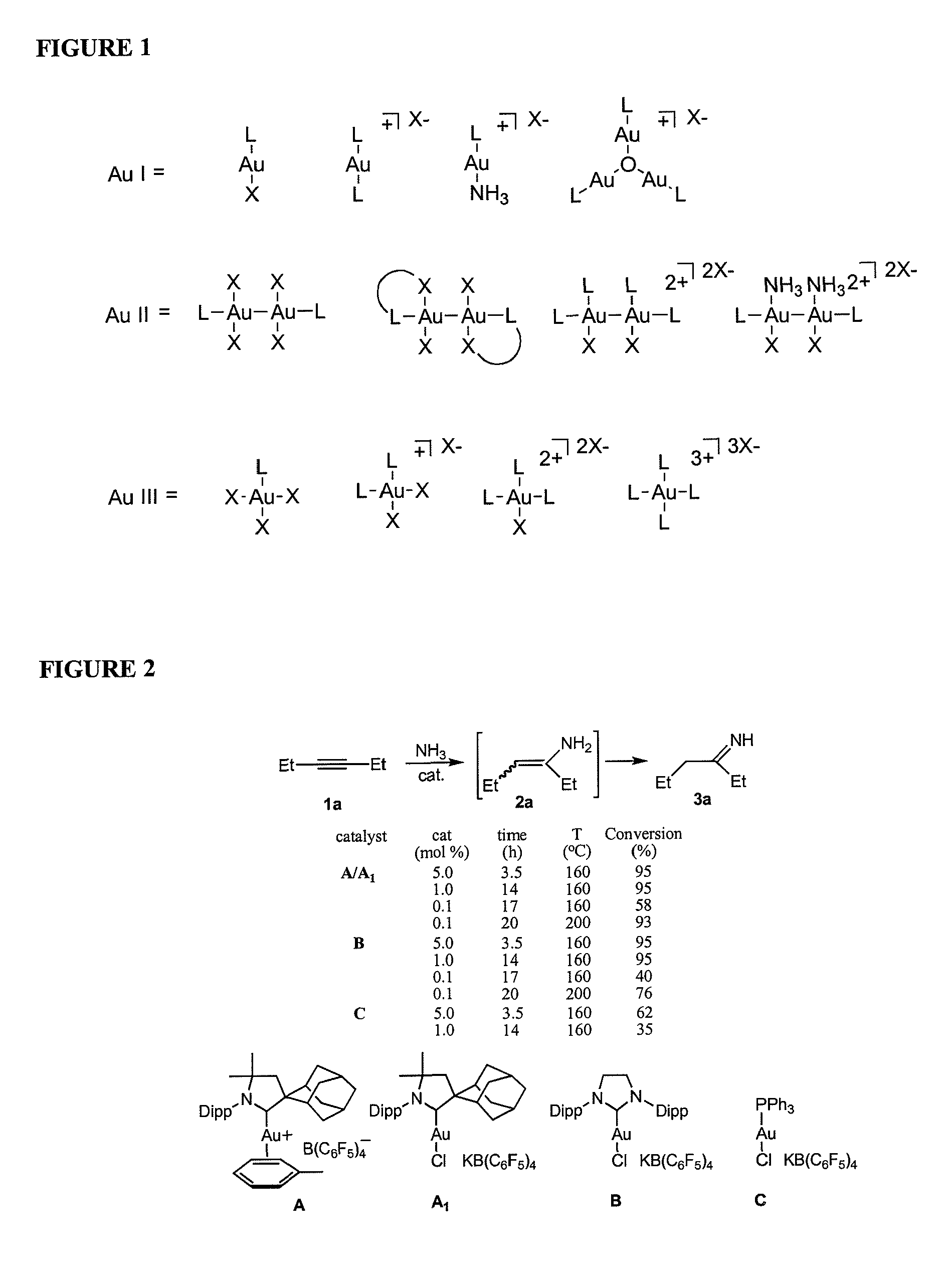
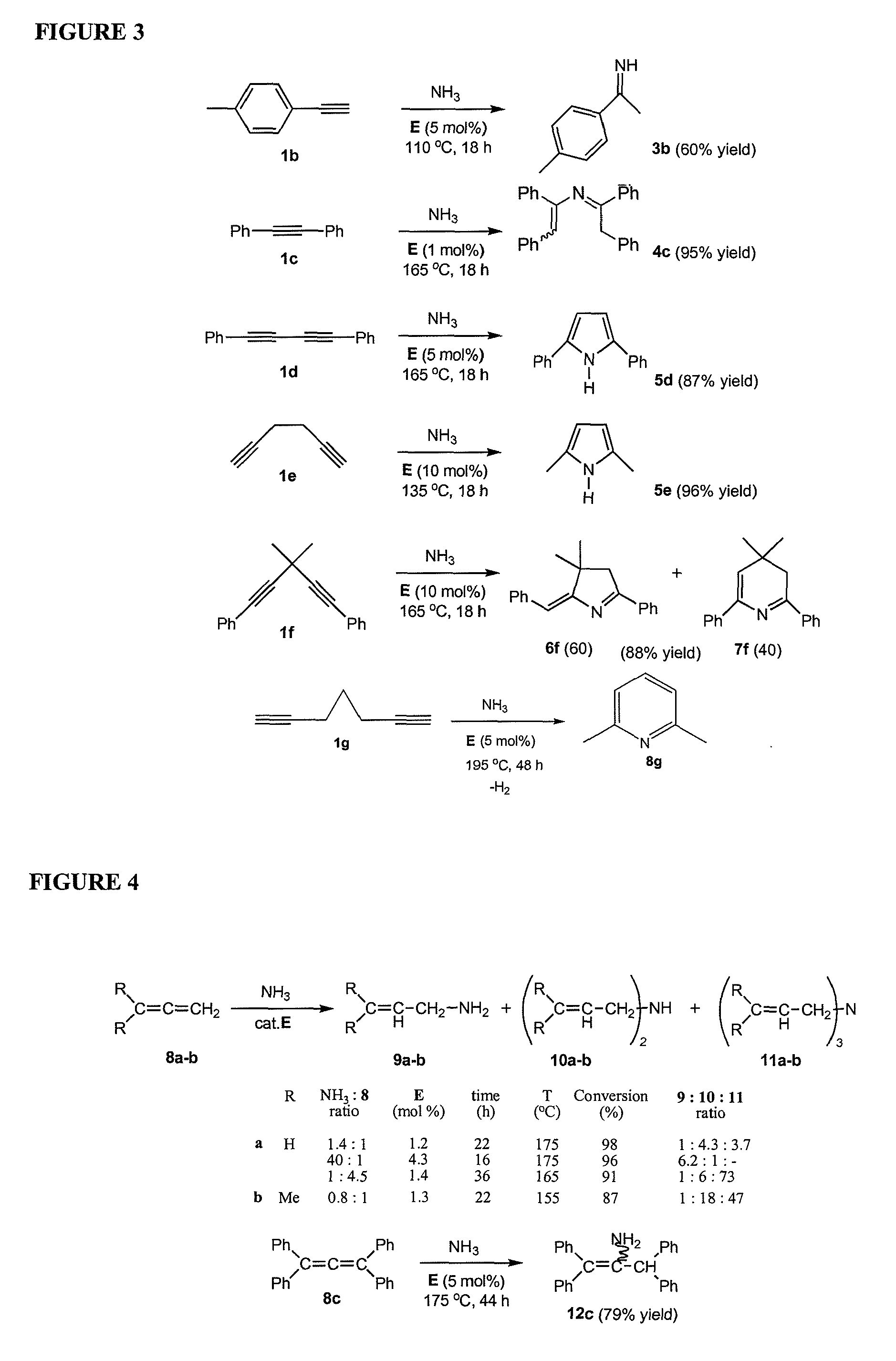
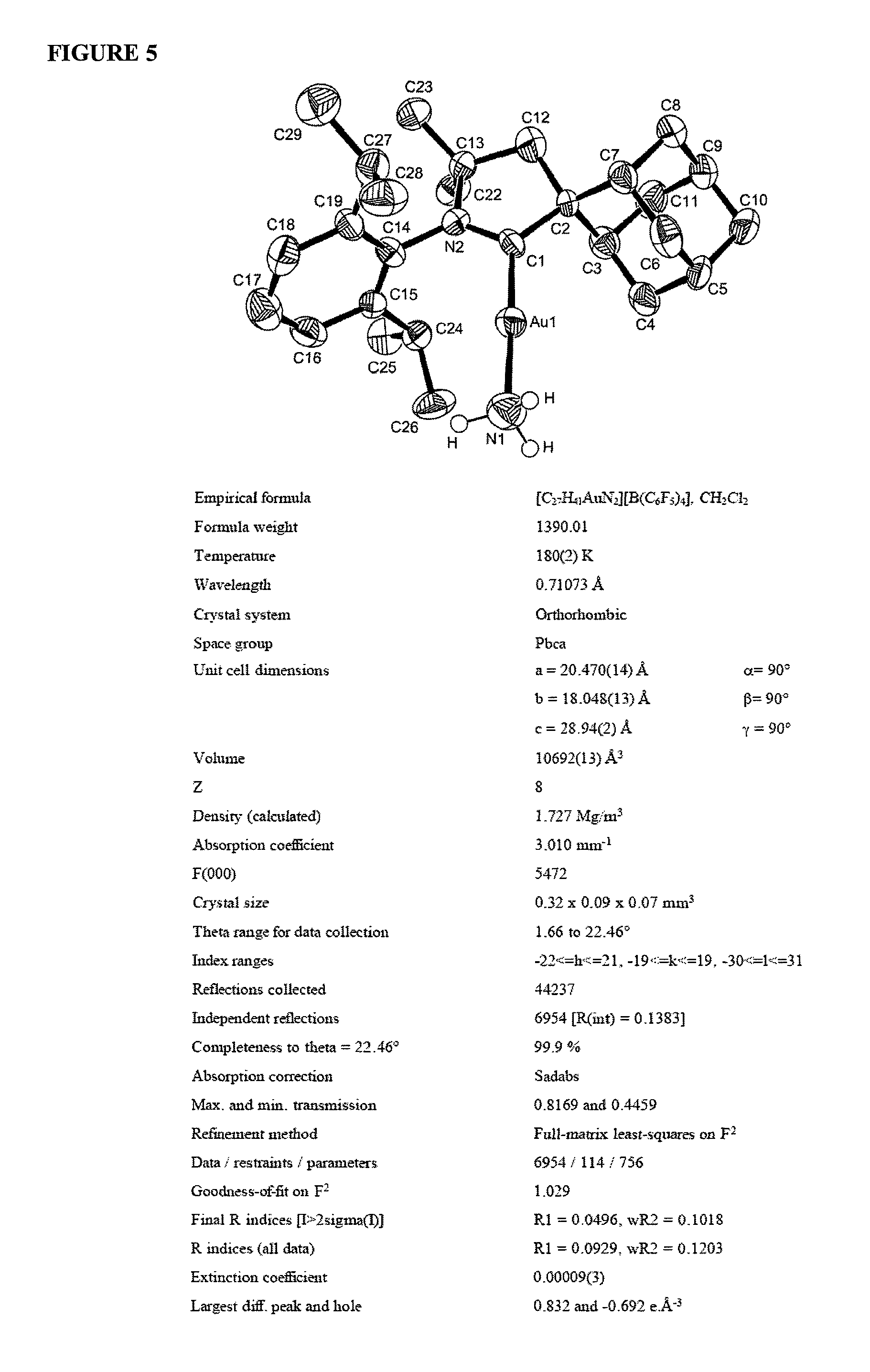
Gold catalyzed hydroamination of alkynes and allenes
InactiveUS20110166349A1Isocyanic acid derivatives preparationOrganic compound preparationAlkyneAllene
Methods are provided for the catalytic hydroamination of compounds having an alkyne or allene functional group, in which the compound is contacted with ammonia or an amine in the presence of a catalytic amount of a gold complex under conditions sufficient for hydroamination to occur.
Owner:RGT UNIV OF CALIFORNIA
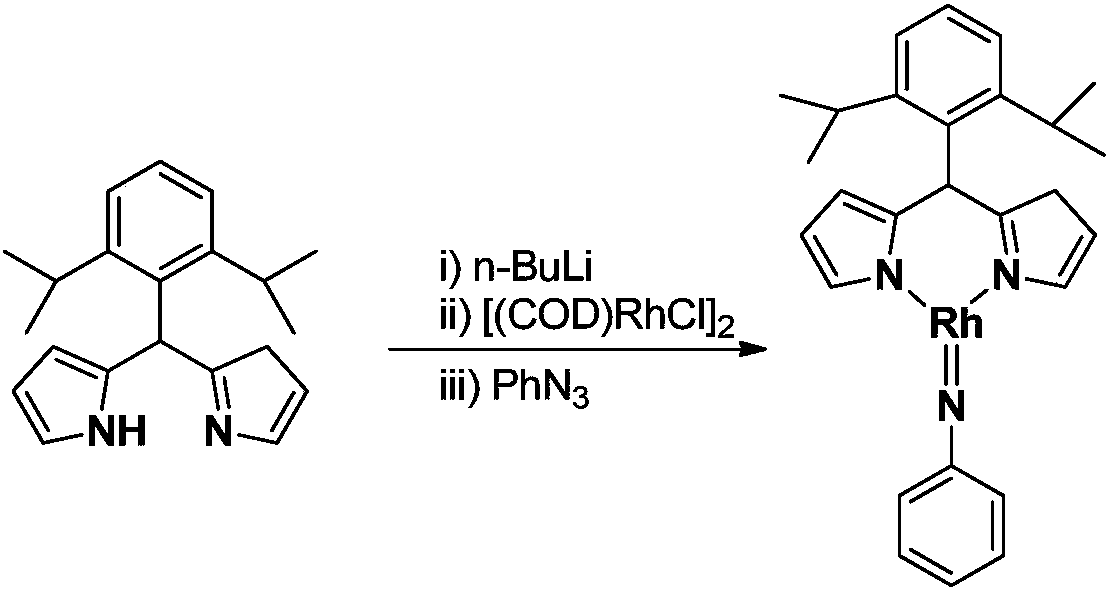
Preparation of large-steric-hindrance trivalent rhodium imide complex and application thereof
ActiveCN109651445AEasy to prepareHigh yieldRhodium organic compoundsOrganic compound preparationImideRegioselectivity
The invention provides a large-steric-hindrance trivalent rhodium imide complex containing a rhodium-nitrogen dual-key structure. The large-steric-hindrance trivalent rhodium imide complex is characterized by including the following structure shown in the description. A synthesis technology is simple and green, and has excellent selectivity and a high yield. The large-steric-hindrance trivalent rhodium imide complex has the features of stable physical and chemical properties and thermal stability, and has excellent activity and regional selectivity in an anti-Markovnikov hydroamination reaction of olefins.
Owner:SHANGHAI INST OF TECH
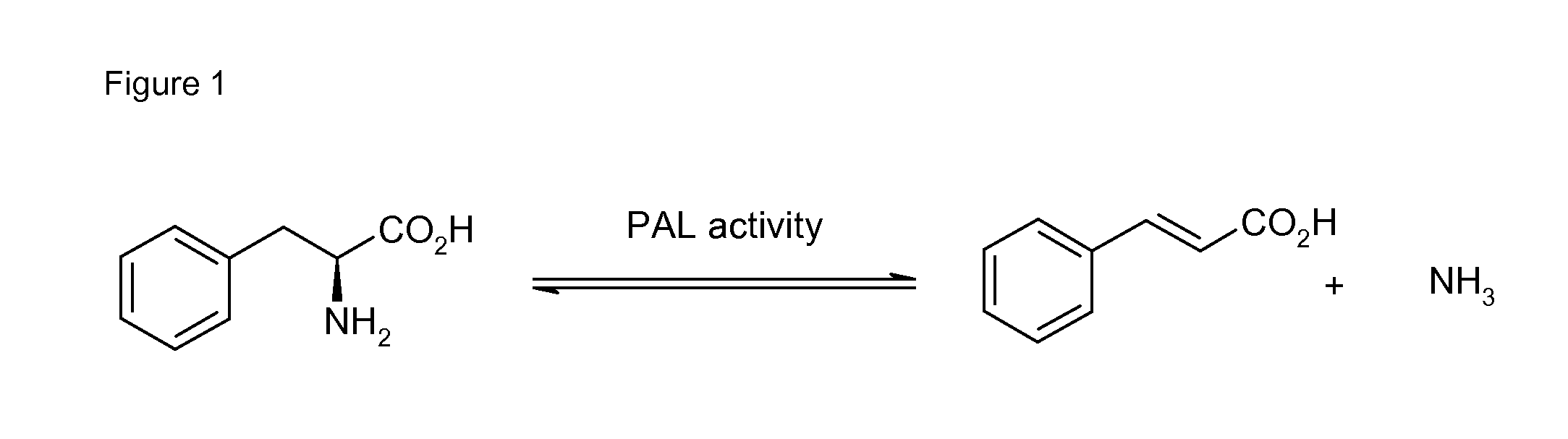
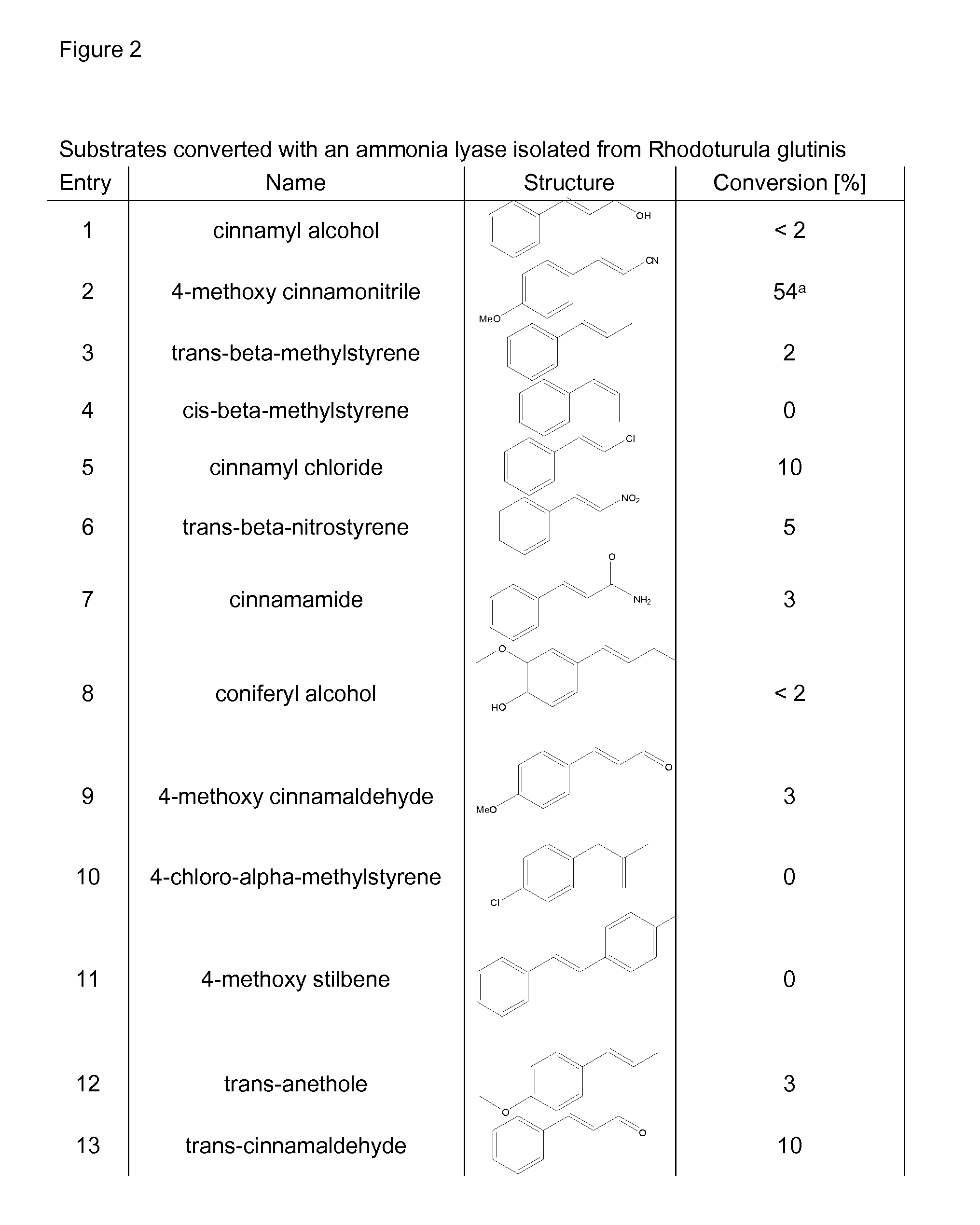
Biocatalyst for catalytic hydroamination
The present invention relates to a method for the enzymatic hydroamination of C—C double bonds catalyzed by enzymes structurally and / or functionally related to phenylalanine ammonia lyase (PAL) isolated from microorganisms of Petroselinum crispum, Rhodoturula glutinis and / or functional active derivatives thereof.
Owner:BASF AG
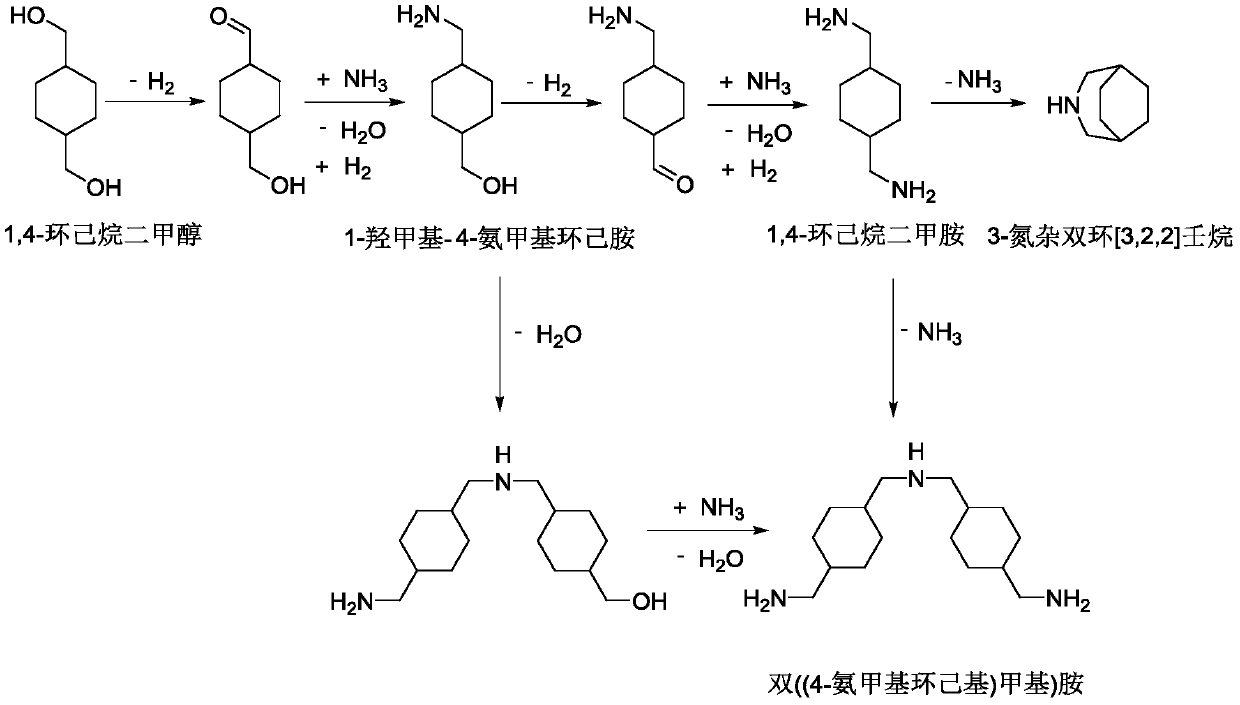
Method for preparing cyclohexane dimethylamine
ActiveCN110981705AOrganic compound preparationPreparation by dehydrogenationPtru catalystDehydrogenation
The invention discloses a method for preparing cyclohexane dimethylamine. The method comprises the following steps: (1) carrying out a dehydrogenation reaction on cyclohexane dimethanol under the catalysis of a dehydrogenation catalyst to obtain cyclohexanediformaldehyde; and (2) carrying out a hydroamination reaction on the cyclohexane dicarboxaldehyde obtained in the step (1), liquid ammonia andhydrogen under the action of an amination catalyst to obtain cyclohexane dimethylamine. According to the method, by adopting different types of catalysts and different reaction control conditions insections, the reaction processes of dehydrogenation of cyclohexane dimethanol, imidization and hydrogenation are controlled, and the generation of secondary amine and high polymer byproducts in the amination process is inhibited, so that the selectivity and the yield of the cyclohexane dimethylamine product are greatly improved.
Owner:WANHUA CHEM GRP CO LTD
A preparing method of 2,2,6,6-tetramethyl-4-piperidylamine by a catalytic amination method
A preparing method of 2,2,6,6-tetramethyl-4-piperidylamine by a catalytic amination method is disclosed. The preparing method includes following steps of: (1) adding 2,2,6,6-tetramethyl-4-piperidone, sodium hydroxide, ammonia water and a skeleton nickel catalyst into a high-pressure kettle having a condenser, a pressure gage, a stirrer and a thermocouple, feeding nitrogen to replace air in the kettle, feeding hydrogen until the pressure is 2.5 MPa, stirring, heating to 110 DEG C, and reacting for 30 min; and (2) after the reaction is completed, cooling to room temperature, stopping stirring, venting, taking reaction materials out, filtering, subjecting filtrate to distillation under atmospheric pressure to distill ammonia gas and water, subjecting the residue in the kettle to vacuum distillation, and collecting a fraction that is the 2,2,6,6-tetramethyl-4-piperidylamine. The preparing method adopts the skeleton nickel as the catalyst, synthesizes the 2,2,6,6-tetramethyl-4-piperidylamine by hydrogenation and amination, and is high in yield and purity of products.
Owner:青岛欧美亚橡胶工业有限公司
Enantioselective phosphoramidite compounds and catalysts
InactiveCN101090904AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystEnantio selectivity
This invention relates to phosphoramidite compounds and catalyst complexes which can be used to provide enantioselective reactions including hydroamination reactions, etherification reactions and conjugate addition reactions and allylic substitution reactions, among others. In a first aspect, the present invention is directed to phosphoramidite and related compounds according to general structure (I), where Z is absent or is a group containing O, N or S, preferably O; R<1> and R<2> are independently an optionally substituted C1-12 alkyl group, an optionally substituted (CH2)n-aromatic group or (CH2)n-heteroaromatic group, or are linked together to form an optionally substituted aliphatic or (CH2)n-aromatic dianion of a diol, diamine, dithiol, aminoalcohol, aminohiolate or a alcoholthiol group; R<3'> and R<3> are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group with the proviso that R<3'> and R<3> are not both H, or together R<3'> and R<3> form an optionally substituted C5-C15 saturated or unsaturated carbocyclic ring; R<4> is H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group; R<5> is absent, H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic or (CH2)n-heteroaromatic group; R and R are each independently H or a C1-C3 alkyl group, or R and R together with the carbon to which they are attached form a optionally substituted C5-C15 saturated or unsaturated carbocyclic or heterocyclic ring, or an aromatic or heteroaromatic ring; R<6> and R<7> are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group, with the proviso that R<5>, R<6> and R<7> cannot simultaneously be H, and when R and R, together with the carbon to which they are attached, form a carbocyclic ring.
Owner:YALE UNIV
Sequence-controllable linear/hyperbranched polymer prepared through metal-free catalyzed multi-component polymerization, and preparation method and application thereof
ActiveCN106243351ASimple methodMild conditionsFluorescence/phosphorescencePolymer scienceFluorescence
The invention belongs to the technical field of organic chemistry, and discloses a sequence-controllable linear / hyperbranched polymer prepared through metal-free catalyzed multi-component polymerization, and a preparation method and an application thereof. The method comprises the following steps: 1, adding alkyne and amine into a reaction container, and carrying out a hydroamination reaction with a polar solvent as a reaction medium to obtain an alkenyl ester intermediate, wherein the alkyne is a butyldiacetyl ester compound; 2, adding amine, aldehyde and a catalyst into the reaction container provided with the alkenyl ester intermediate, and carrying out a polymerization to obtain a polymer solution, wherein the catalyst is acid; and 3, adding the polymer solution to a settling agent in a dropwise manner under a stirring condition, allowing the obtained solution to stand, filtering the solution, washing the obtained material, and drying the washed material to obtain the sequence-controllable linear / hyperbranched polymer. The method has the advantages of simplicity, mild conditions, no metal catalysis, high yield of the polymer, controllable unit sequence of the polymer structure, realization of the preparation of linear and hyperbranched polymers, and potential application values in the field of biological and chemical luminescence detection.
Owner:SOUTH CHINA UNIV OF TECH
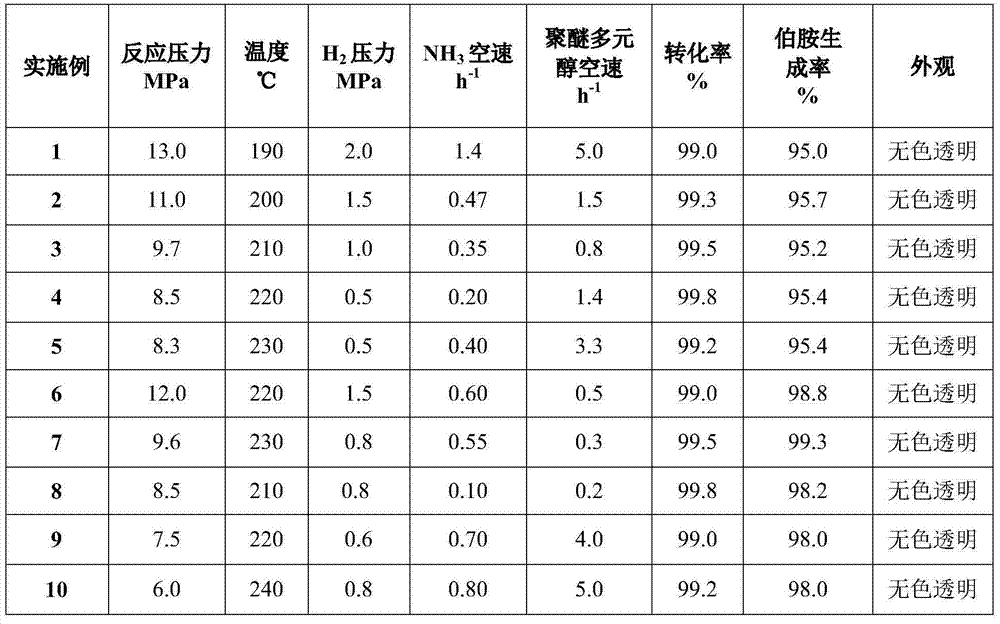
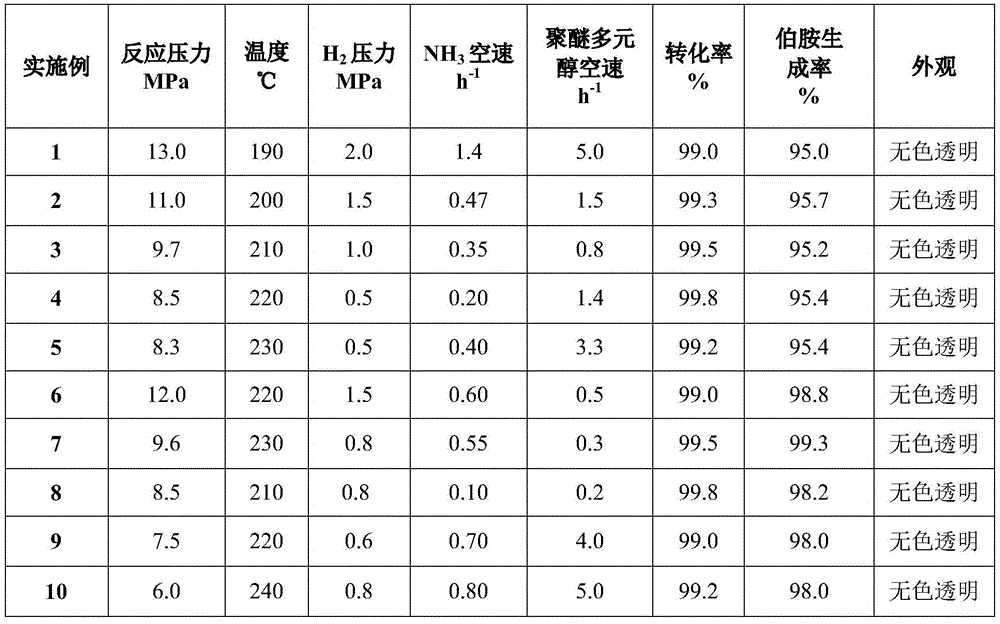
Method for preparing polyether amine by hydroamination of polyether polyol
ActiveCN112898558AImprove conversion rateHigh selectivityOrganic compound preparationMetal/metal-oxides/metal-hydroxide catalystsPolymer sciencePolyol
The invention discloses a method for preparing polyether amine by hydroamination of polyether polyol, a catalyst in the method is mainly composed of a multi-metal active component, an auxiliary agent and a carrier, the multi-metal active component comprises M1-M2 (M1 is one or more than two of transition metals Ni, Co and Cu, and M2 is one or two of transition metals Re and Ru). The catalyst may also comprise an auxiliary agent, and the auxiliary agent is one or a combination of Fe, Cr, Mn, B, Mg, Ba, Pt and other metals or oxides. The catalyst disclosed by the invention is characterized in that in the preparation process of the catalyst, a complexing agent or a stabilizing agent needs to be added into the prepared precursor liquid of the multi-element metal active component and the auxiliary agent so as to improve the catalytic performance. When the catalyst is used for preparing polyether amine through polyether polyol hydroamination, the conversion rate is high, the catalytic performance is stable, the target product selectivity is high, the technological process is simple, the catalyst is suitable for a continuous or batch reactor, and the market prospect is wide.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for catalyzing intermolecular hydroamination reaction of alkyne and amine
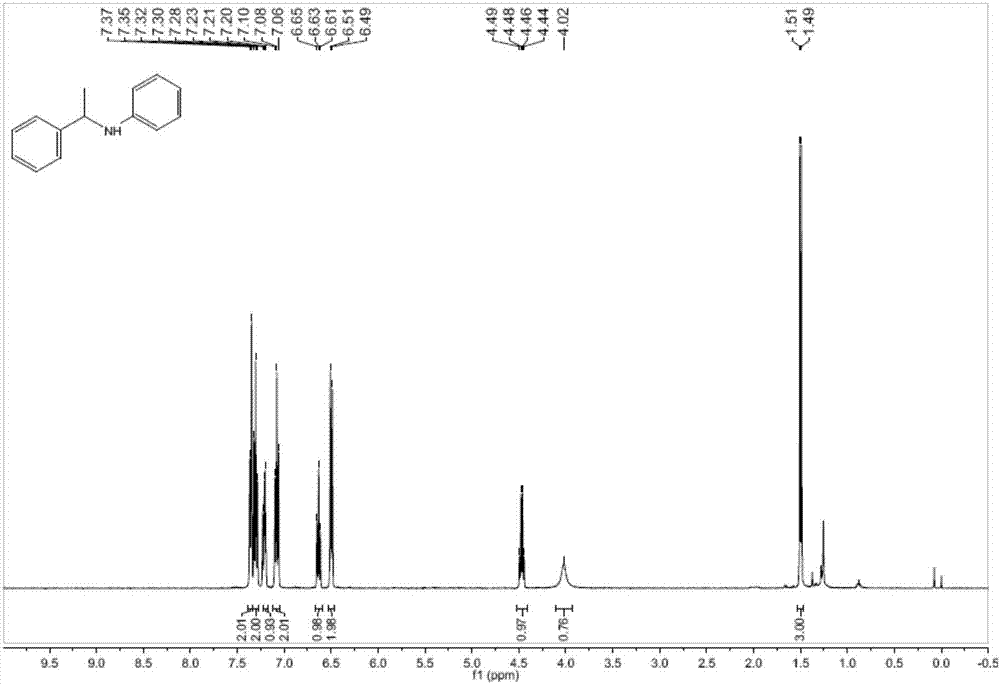
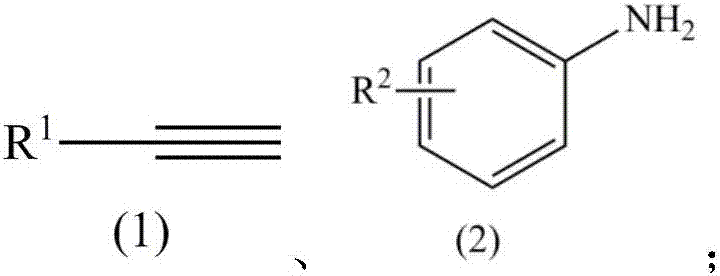
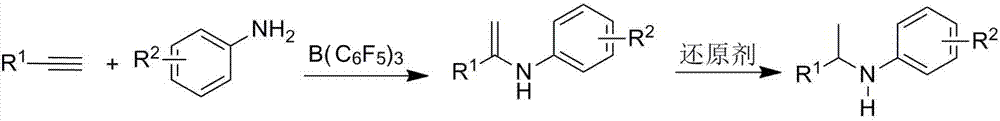
ActiveCN107382741ASimple and fast operationMild reaction conditionsOrganic compound preparationAmino compound preparation by condensation/addition reactionsAlkyneSolvent
The invention relates to a method for catalyzing intermolecular hydroamination reaction of alkyne and amine. The method comprises the following steps: reacting a terminal alkyne as shown in a formula (1) and a primary aromatic amine as shown in a formula (2) in an aprotic polar solvent at 25-100 DEG C for 6-24 hours under catalysis of tris(pentafluorophenyl)borane and under airtight conditions containing protective atmosphere, wherein structural formulae of the formula (1) and the formula (2) are as shown in the specification, wherein R1 is selected from C1-10 alkyl and aryl; and R2 is selected from hydrogen, C1-10 alkyl, alkoxy, cyano, trifluoromethyl, nitro, halogen and amino. The method is simple and available in raw material, simple in operation, relatively mild in reaction condition, relatively high in yield and relatively wide in application range of a substrate.
Owner:SUZHOU UNIV
Method for preparing enamine compound by catalyzing phenylacetylene and utilizing hydroamination reaction
ActiveCN109651160AImprove stabilityImprove thermal stabilityAmino compound preparation by condensation/addition reactionsEnamineSolvent
The invention provides a method for preparing an enamine compound by catalyzing phenylacetylene and utilizing the hydroamination reaction. The method is characterized by comprising the steps that thephenylacetylene and secondary amine are taken as raw materials, under the effect of a nickelous imine complex catalyst, the hydroamination reaction is conducted in a solvent, after finishing of the reaction and after-treatment, the enamine product is obtained, wherein the molecular formula of the nickelous imine complex catalyst is [R1R2C (C5H4N) 2] Ni=NPh, R1 and R2 are independently selected from H, CH3 and Ph, and arene is the solvent. The catalyst has extremely high catalytic activity and regioselectivity, the synthesizing technology is simple and green, good selectivity and high yield areachieved, the atom economy is good, and production of waste gas, waste water and industrial residues is reduced; the method protects the environment, and is simple in operation and suitable for industrial production.
Owner:SHANGHAI INST OF TECH
Compositions and methods for facilitating reaction at room temperature
InactiveUS20070029528A1Various chemical reactionEfficient CatalysisOrganic-compounds/hydrides/coordination-complexes catalystsConductive materialHydration reactionHeteroatom
Compositions and methods useful in facilitating or conducting a reaction at effective conditions, such as room temperature (e.g. about 70 degrees F.), utilize a compound including at least two different heteroatoms, and optionally a heterocycle, and a transition metal. The compound is effective in facilitating a variety of reactions including hydrolysis reactions, alcoholysis reactions, aminolysis reactions, carbon dioxide conversion reactions, hydroamination reactions, hydration reactions, and the like.
Owner:SAN DIEGO STATE UNIVERSITY
Gold catalyzed hydroamination of alkynes and allenes
Methods are provided for the catalytic hydroamination of compounds having an alkyne or allene functional group, in which the compound is contacted with ammonia or an amine in the presence of a catalytic amount of a gold complex under conditions sufficient for hydroamination to occur.
Owner:RGT UNIV OF CALIFORNIA
A kind of continuous preparation method of amino-terminated polyether
The invention discloses a continuous preparation method of amine-terminated polyether, and the method comprises the following steps of: taking polyether polyol, H2 and NH3 as raw materials, executing critical hydroamination in a fixed bed reactor under a critical hydroamination catalyst, executing gas-liquid separation in a gas-liquid separator to feed liquid from the fixed bed reactor, reusing NH3 and H2 without being reacted after being dried and pressurized; accessing the liquid material into a vacuum chamber, removing H2O and other small-molecule materials in vacuum, continuously discharging to prepare amine-terminated polyether. The method is continuous in operating processes and more steady in a product quality batch method; in preparation, the gas can be reused to prevent environmental pollution; the method can effectively solve the problems of decreased conversion and weak selectivity caused by poorly contacting the gas and the catalyst during large-molecular polyether reaction; the method has the advantages of higher reaction conversion ratio and primary amine selectivity.
Owner:HONGBAOLI GRP CO LTD +1
Rare earth complex based on diimine ligands and application of rare earth complex
ActiveCN107312034ASilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAlkyl transferDiimine
The invention belongs to the technical field of organic synthesis. A catalyst is a rare earth complex based on diimine ligands. The invention further provides application of the rare earth catalyst in aromatic amine hydroamination and alkylation reaction.
Owner:SUZHOU UNIV
Nickelous imine complex with nickel nitrogen double-bond structure and preparation and application of nickelous imine complex
ActiveCN110204580AStable physical and chemical propertiesImprove thermal stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDouble bondCoordination complex
The invention relates to a nickelous imine complex with a nickel nitrogen double-bond structure and preparation and application of the nickelous imine complex. The preparation method of the nickelousimine complex comprises the following steps that 1, a phenyldipyridine ligand solution is added into a zero-valent nickel precursor solution for a reaction for 3-5 hours at room temperature; 2, azidobenzene is added for a reaction for 2-5 hours at room temperature, and through aftertreatment, the nickelous imine complex is obtained. The nickelous imine complex is used for catalyzing an anti-Markovnikov hydroamination reaction of styrene to prepare a linear-chain amine compound. Compared with the prior art, the synthesizing technology is simple and green, the selectivity and the yield are high,the nickelous imine complex prepared by using the method has the advantages of stable physicochemical properties, thermal stability and the like, and the nickelous imine complex can show excellent activity and regioselectivity in the reaction of catalyzing the anti-Markovnikov hydroamination reaction of the styrene.
Owner:SHANGHAI INST OF TECH
Synthesis method of N, N, N'-trimethyl-N'-hydroxyethyl diaminoethyl ether
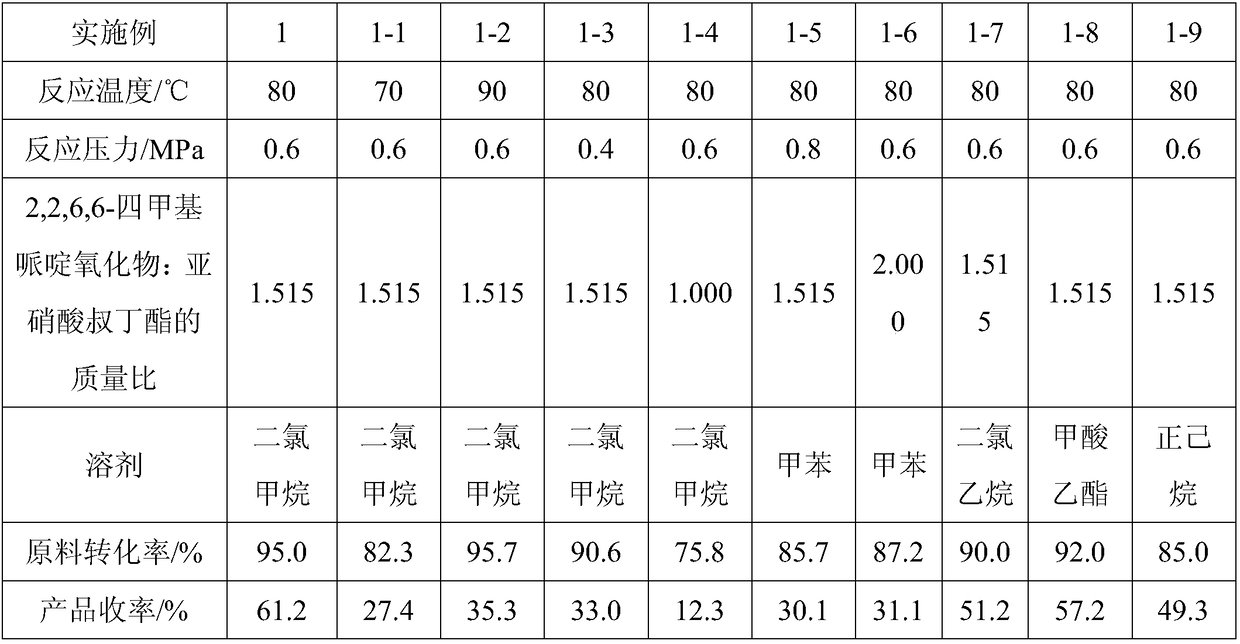
InactiveCN108084040AReduce pollutionNo pollution in the processOrganic compound preparationAmino-hyroxy compound preparationNitriteSynthesis methods
The invention discloses a synthesis method of N, N, N'-trimethyl-N'-hydroxyethyl diaminoethyl ether. The synthesis method comprises the following steps of: in a solvent I, adopting dimethylaminoethoxyethanol as a raw material, and under a catalytic system of 2, 2, 6, 6-tetramethyl piperidinoxide-FeCl3-tertbutyl nitrite, utilizing oxygen gas to oxidize the dimethylaminoethoxyethanol into 2-[2-(dimethylamino)ethoxy]acetaldehyde; in a solvent II, mixing N-methylethanolamine with the 2-[2-(dimethylamino) ethoxy]acetaldehyde, adopting Raney Ni as a catalyst, adopting the above mixture as a reactionsystem to carry out hydrogenation and amination, and preparing the N, N, N'-trimethyl-N'-hydroxyethyl diaminoethyl ether. The N, N, N'-trimethyl-N'-hydroxyethyl diaminoethyl ether synthesized by adopting the synthesis method has the characteristics of simple process, low cost, high yield and less pollution.
Owner:ZHEJIANG UNIV
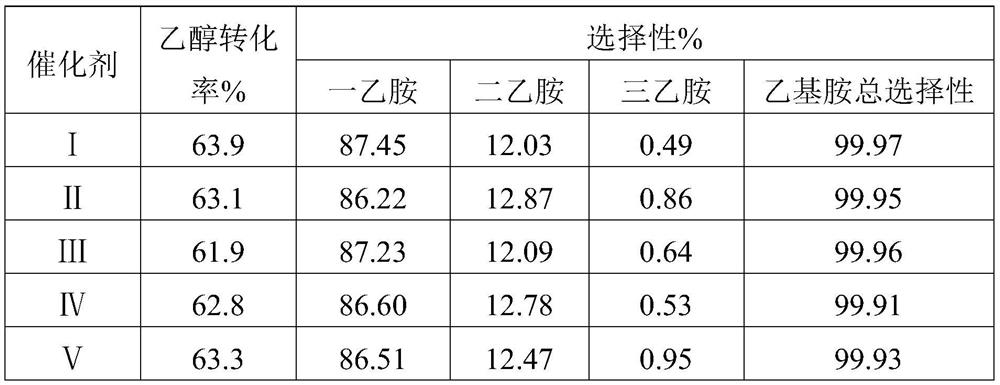
Catalyst for synthesizing monoethylamine, preparation method and application
ActiveCN112044447AObvious advantagesHigh industrial application valueOrganic compound preparationCatalyst activation/preparationPtru catalystEthyl group
The invention discloses a catalyst for synthesizing monoethylamine as well as a preparation method and application thereof. The catalyst comprises aluminum oxide particles and cobalt, palladium and rhenium deposited on the surfaces of the aluminum oxide particles. Cobalt accounts for 10%-40% of the total mass of the catalyst, palladium accounts for 0.5%-5% of the total mass of the catalyst, and rhenium accounts for 0.1%-1% of the total mass of the catalyst. The catalyst is prepared by adopting a spraying method. By adopting the catalyst provided by the invention, when monoethylamine is prepared through hydroamination of ethanol, the monoethylamine selectivity is greater than or equal to 86%(the conversion rate is greater than or equal to 61%, and the ethylamine selectivity is greater thanor equal to 99.9%), the advantages are obvious, and the catalyst has relatively high industrial application value.
Owner:XIAN MODERN CHEM RES INST
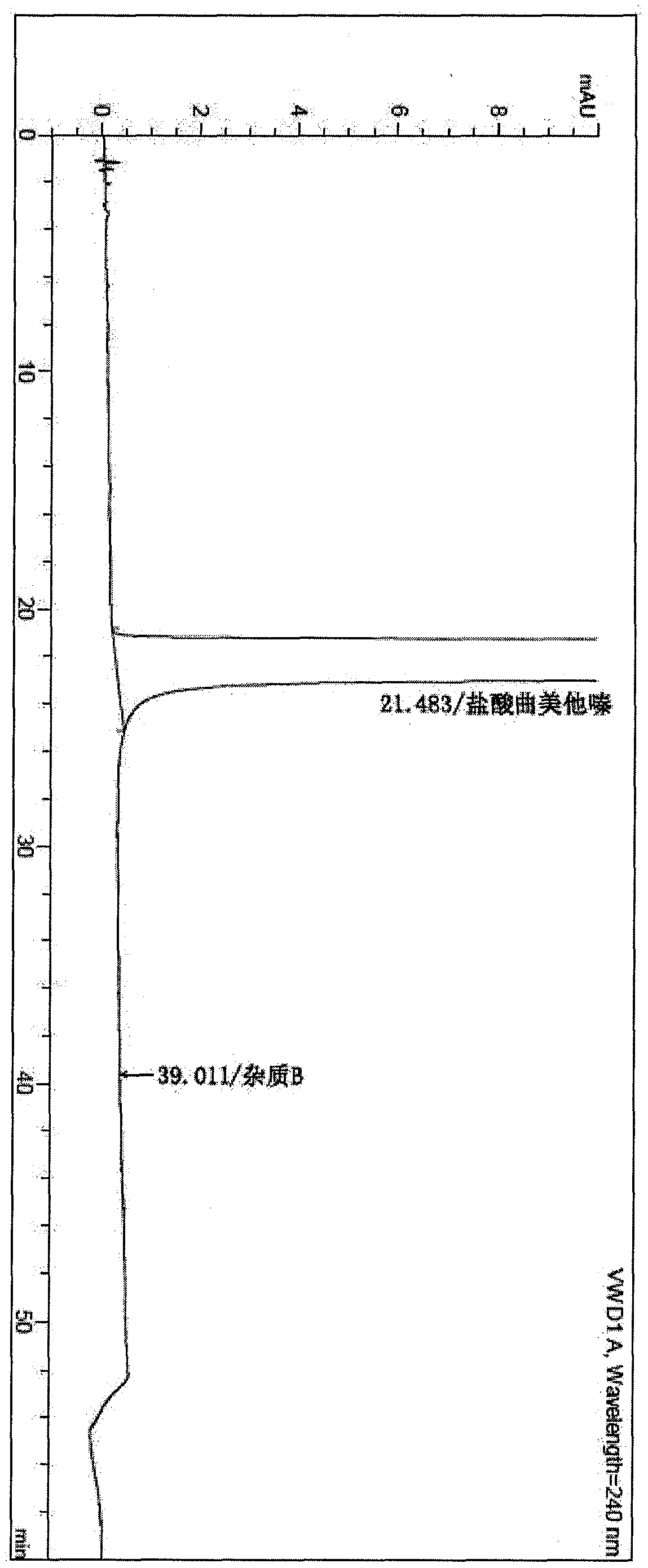
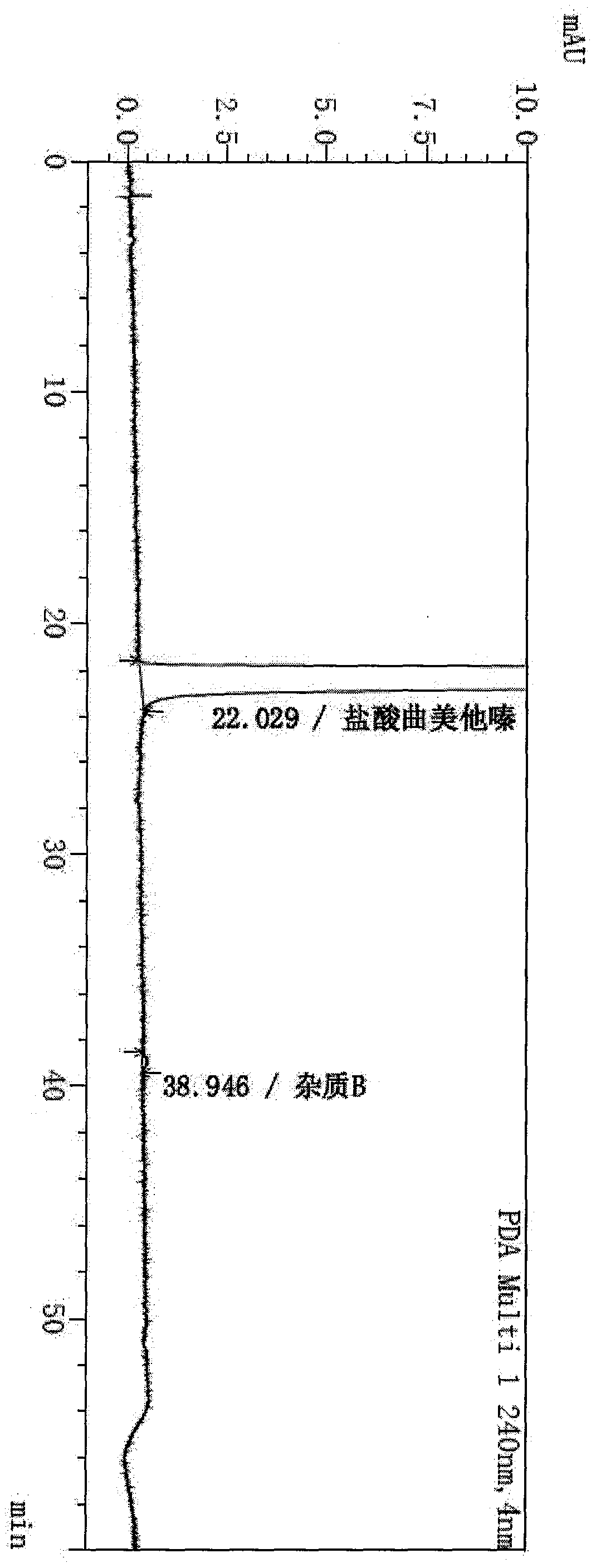
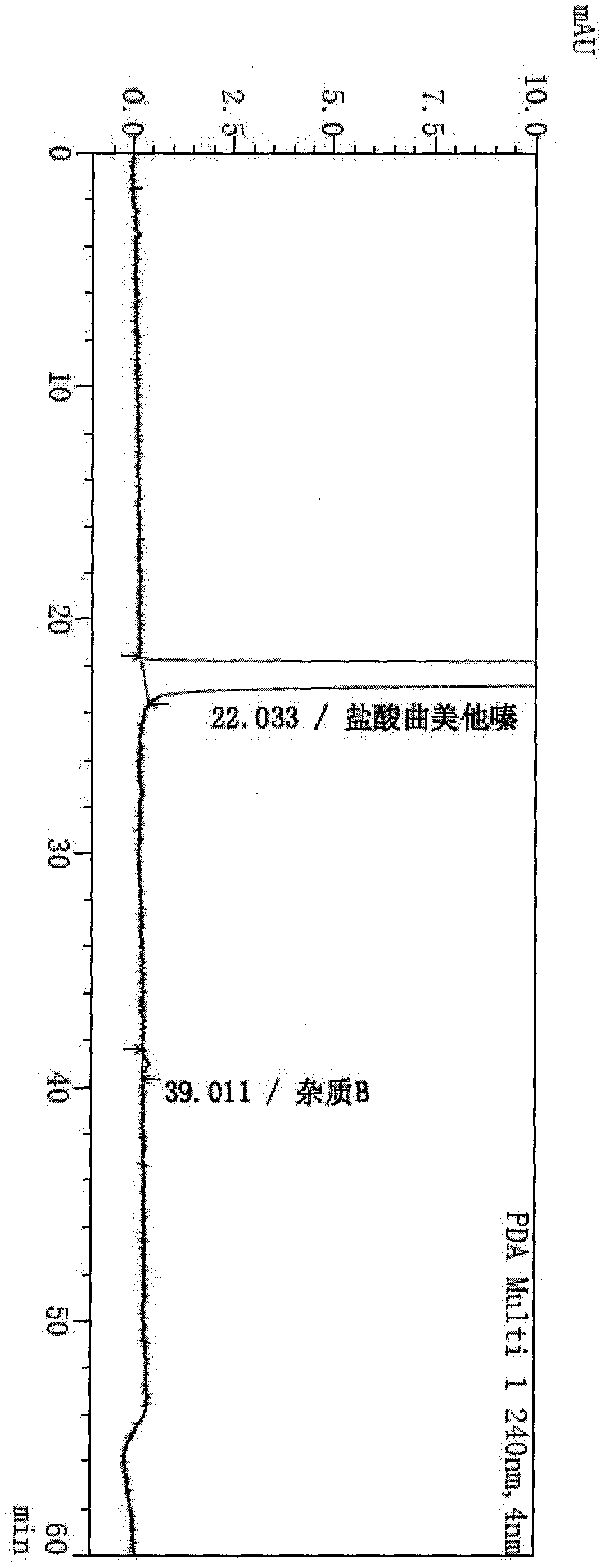
Synthesis method of trimetazidine hydrochloride
The invention provides a synthesis method of trimetazidine hydrochloride, which comprises the following steps: carrying out hydroamination reaction by using 2,3,4-trimethoxybenzaldehyde and piperazineanhydrous as raw materials, and catalyzing by using a Lindlar catalyst. The trimetazidine hydrochloride produced by the method does not contains trimetazidine hydrochloride impurity B and has high purity.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com