Enantioselective phosphoramidite compounds and catalysts
A phosphoramidite and compound technology, applied in the field of phosphoramidite compounds and catalyst complexes, can solve the problems such as not being clear, and achieve the effect of being easily obtained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0295] The following examples serve to illustrate the invention.
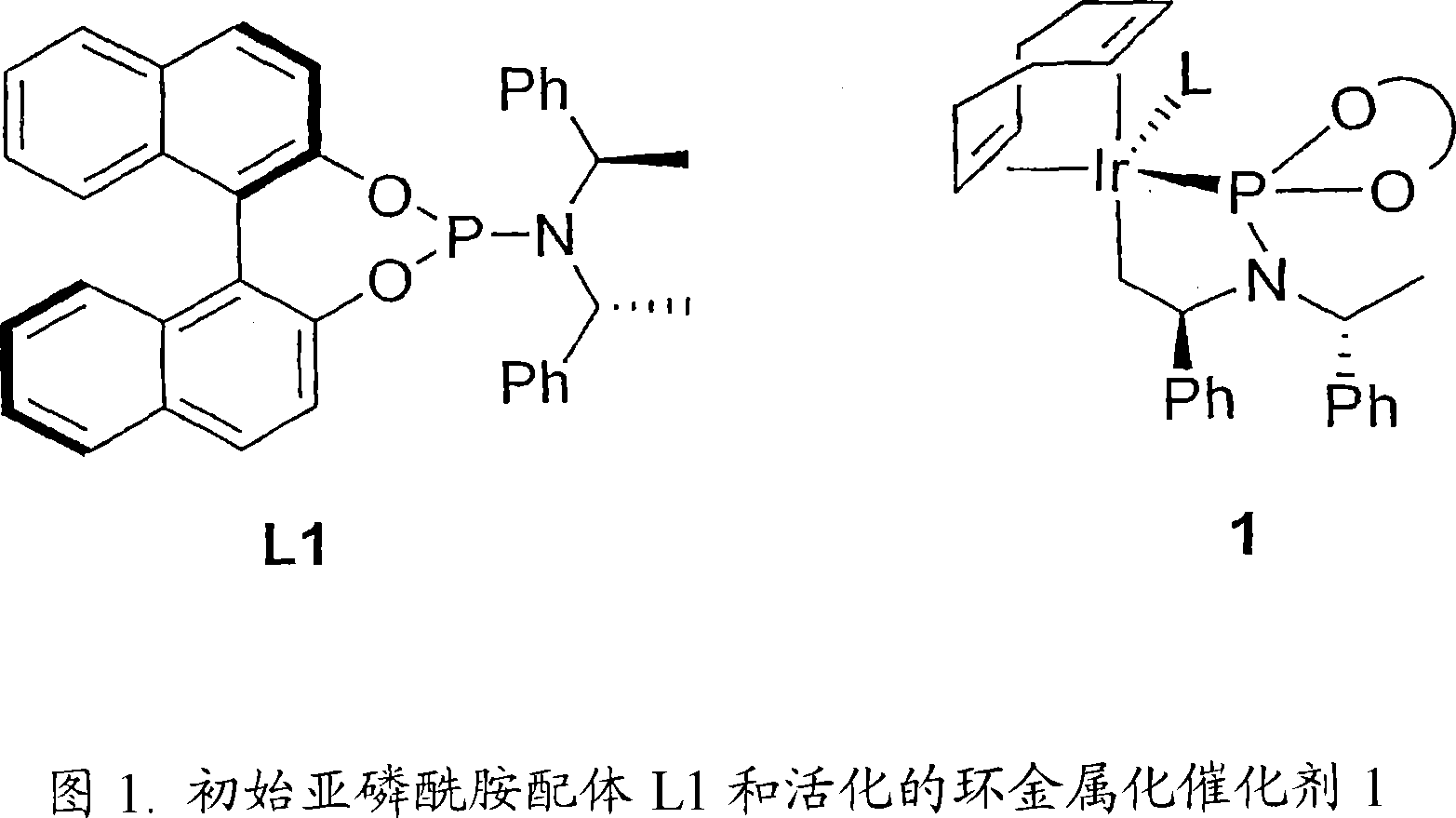
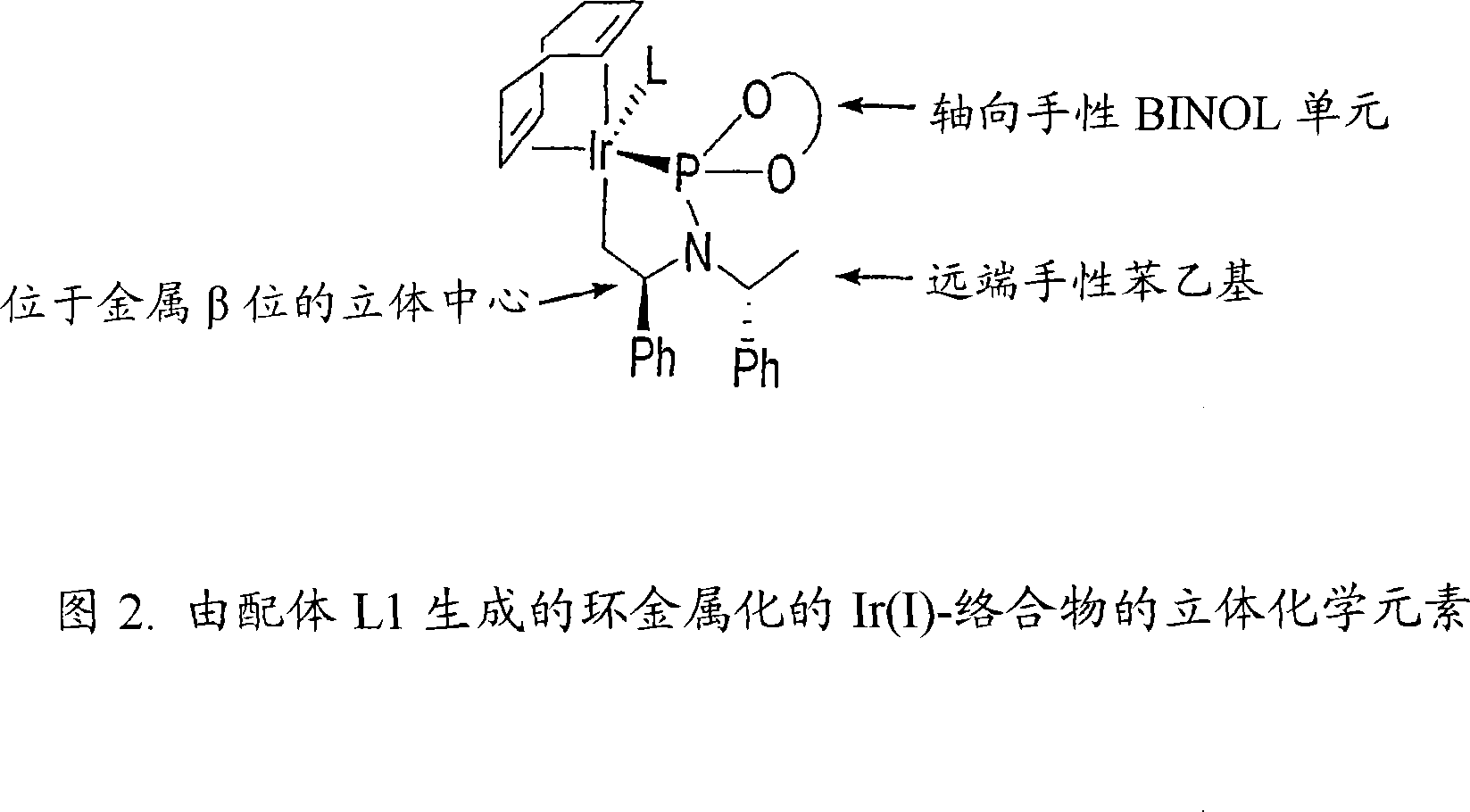
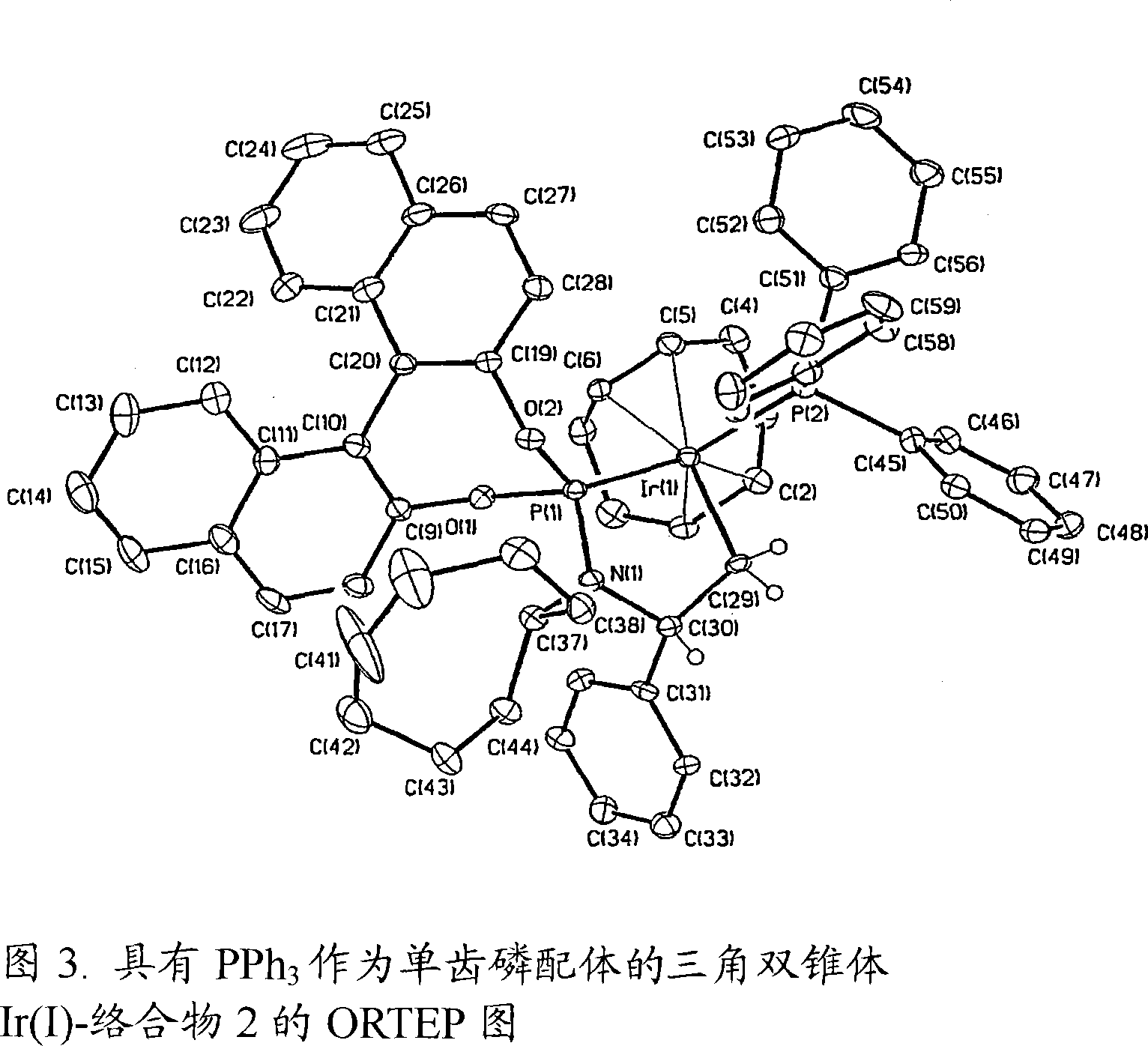
[0296] We first carried out the etherification reaction of highly enantioselective amination using allyl carbonate and a phosphoramidite ligand L1 containing a binaphtholate unit and a bis-phenethylamino group. We subsequently found that the active catalyst in the above reaction was generated by cyclometallation on one methyl group of the phenethylamino group. Cyclometallation destroys the C of the ligand 2 symmetry, generated with only C 1 product of symmetry. Utilizing the information that active catalysts are generated by inducing cyclometallation with basic reagents, we have extended the scope of the method of the present invention to employ weakly basic nucleophiles such as aromatic amines. To this end, we performed the reaction using catalytic amounts of aliphatic amines to induce cyclometallation or performed a catalytic step after precatalytic activation with volatile aliphatic amines.
[0297] In p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com