Patents
Literature
220 results about "Bond formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
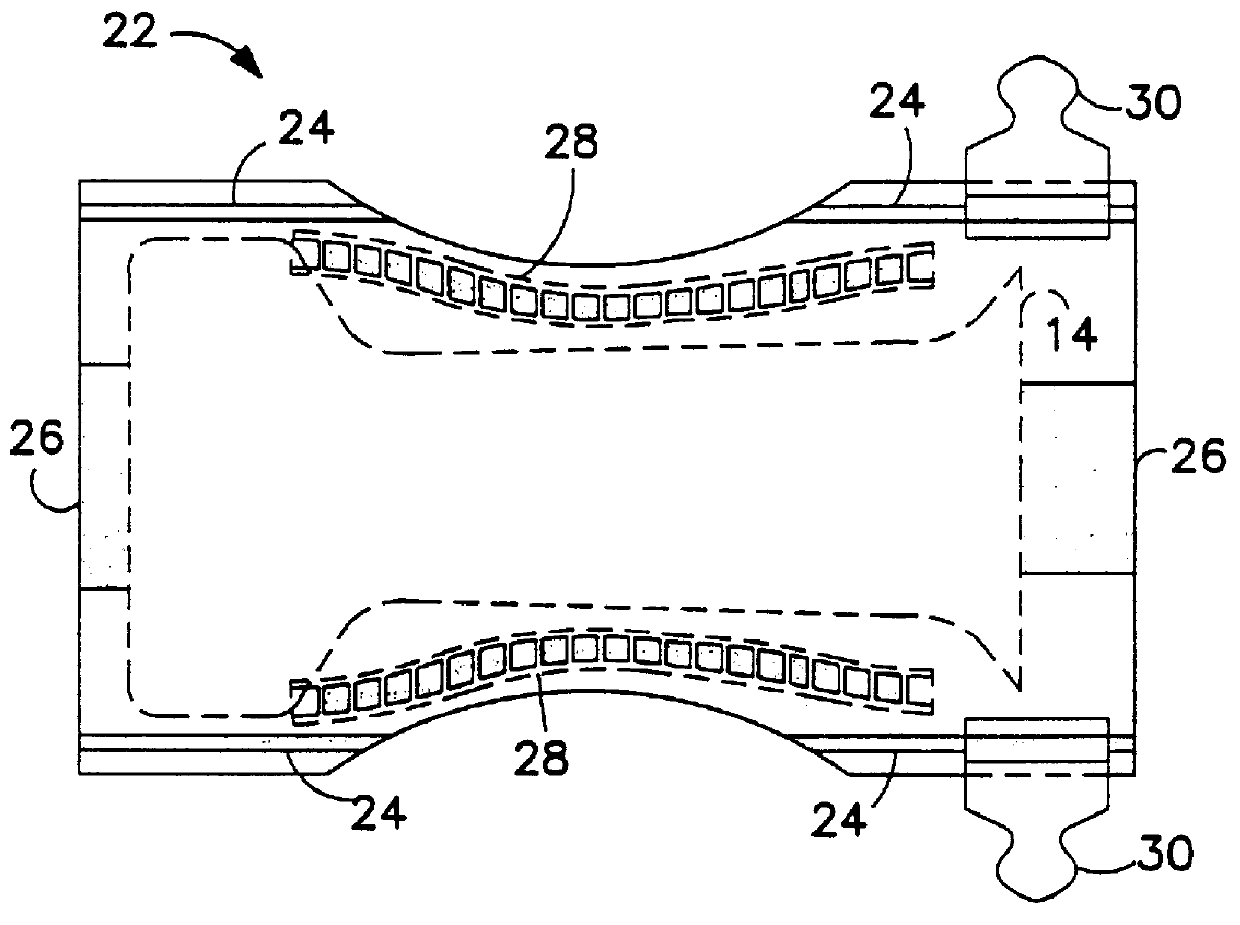
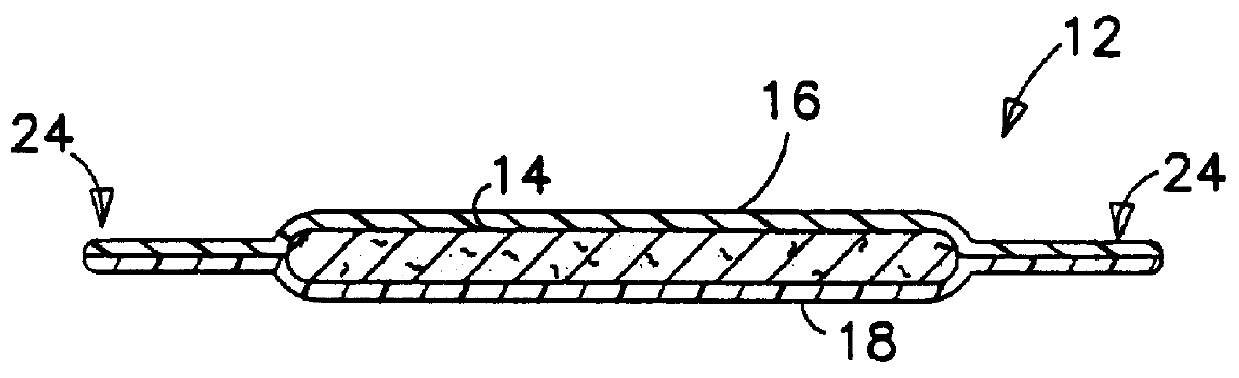
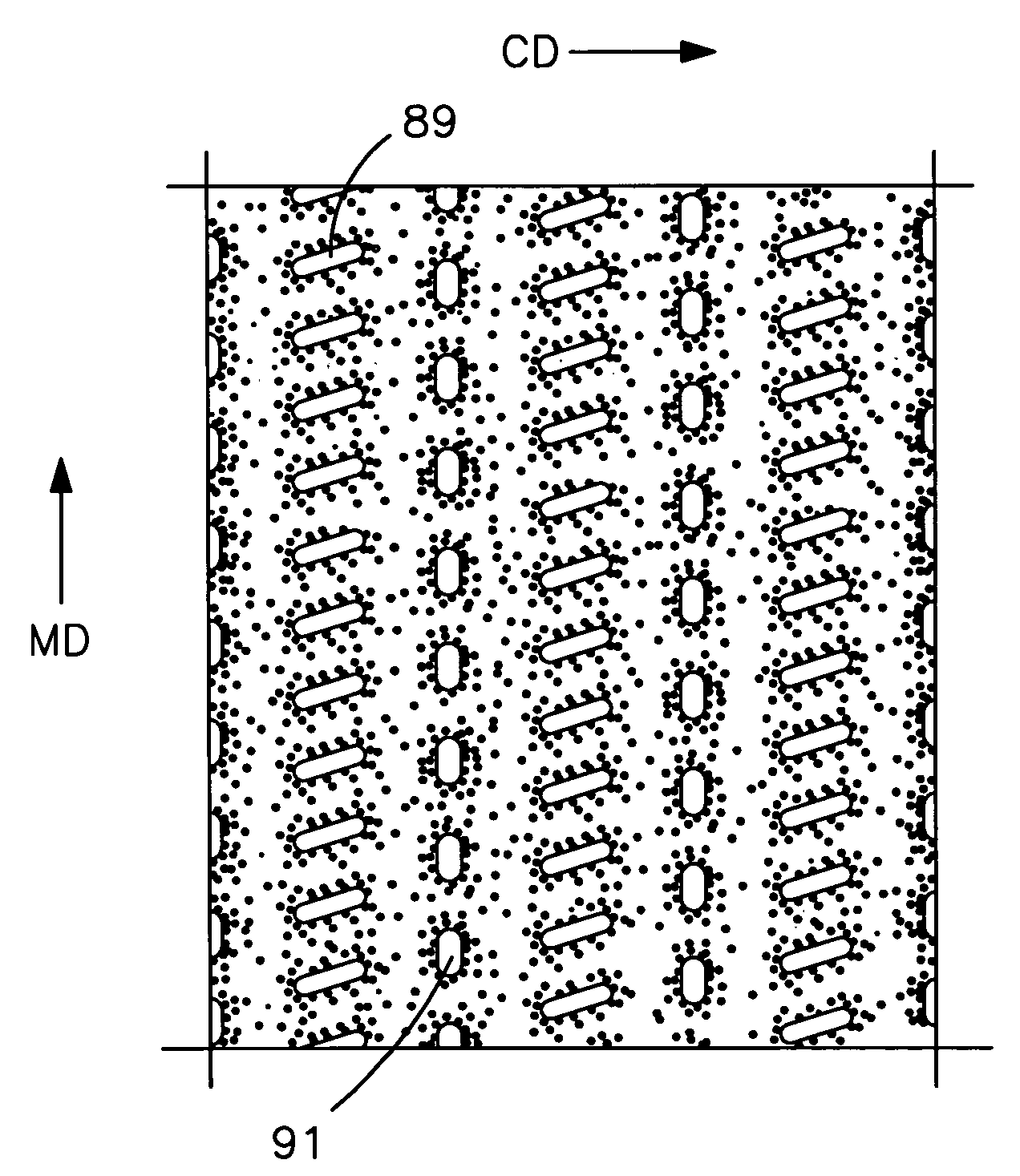
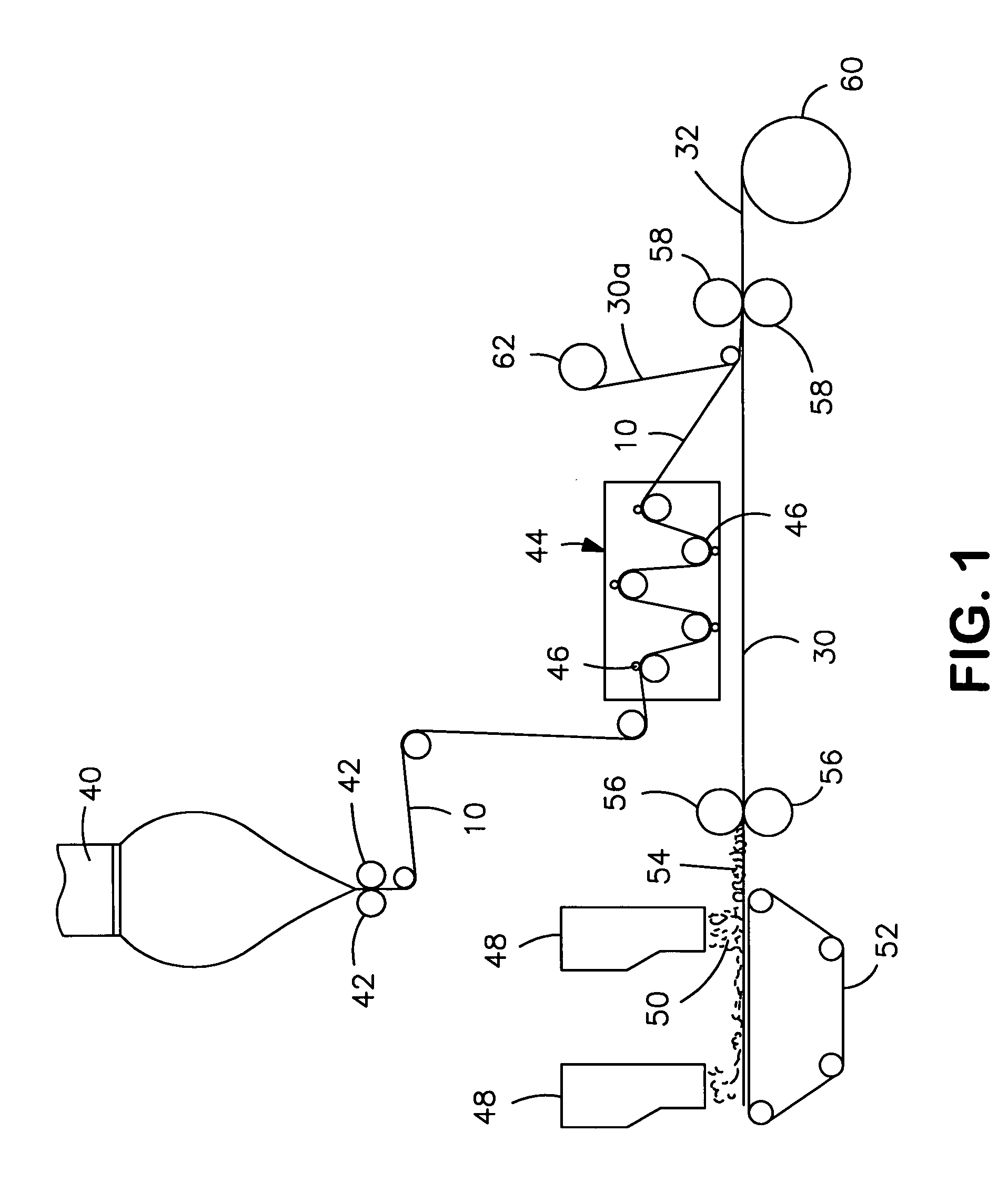
Nonwoven composite containing an apertured elastic film
ActiveUS7803244B2Lamination ancillary operationsSynthetic resin layered productsEngineeringBond formation
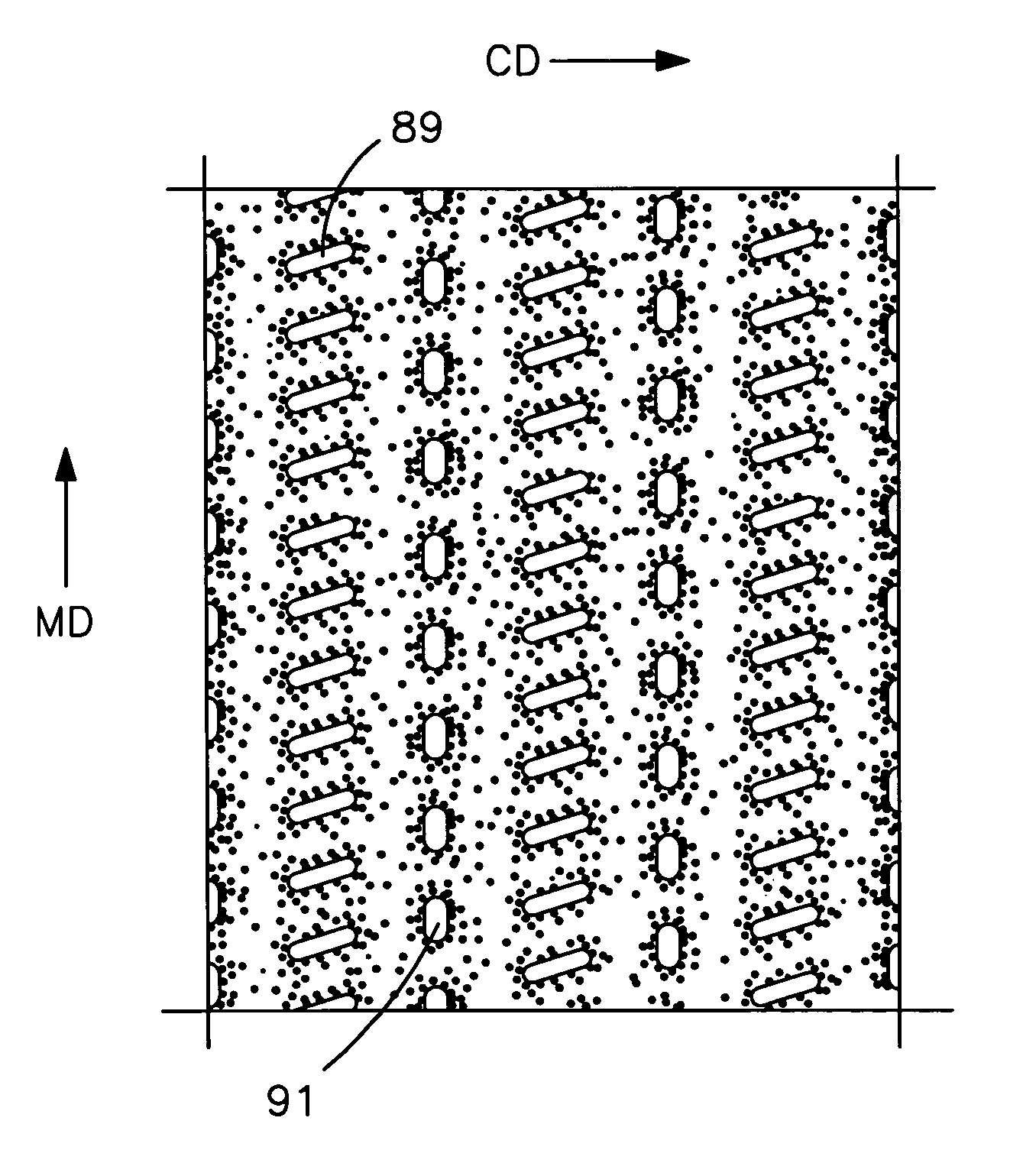
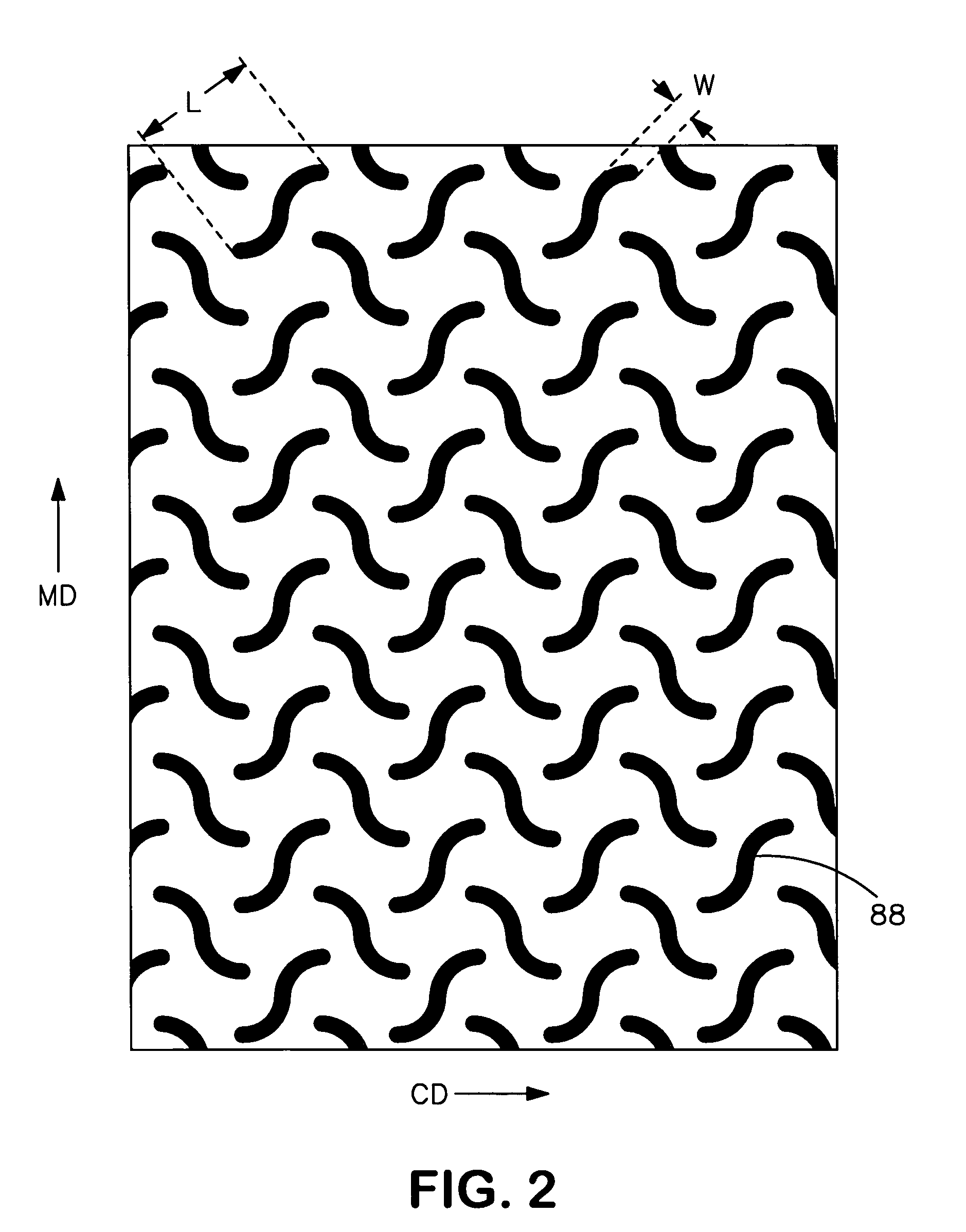
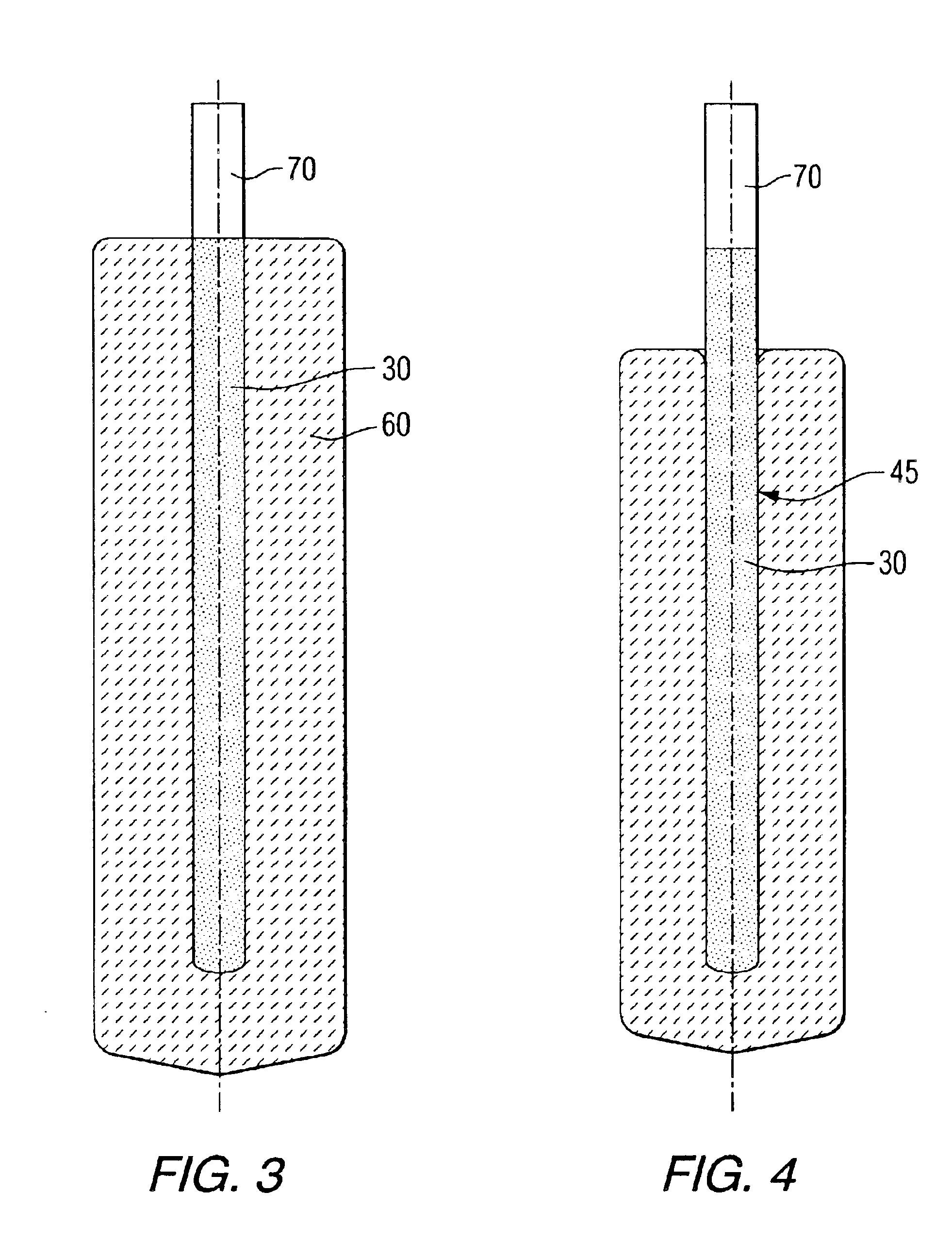
An elastic nonwoven composite that contains an elastic film laminated to one or more nonwoven web materials is provided. The composite is formed by passing the film through a nip to bond the film to the nonwoven web material(s). Concurrent with bond formation, apertures are also formed in the elastic film. The apertures are of a size sufficient to provide a desired level of texture, softness, hand feel, and / or aesthetic appeal to the composite without having a significant adverse effect on its elastic properties. Aperture and bond formation are accomplished in the present invention by selectively controlling certain parameters of the lamination process, such as film content, bonding pattern, degree of film tension, bonding conditions, etc.
Owner:KIMBERLY CLARK WORLDWIDE INC
Methods and apparatus for intermittent rotary ultrasonic bonding system
InactiveUS6123792AAvoids deleterious bounceExtended service lifeLaminationLamination apparatusFiberSurface velocity
This invention relates to apparatus and methods for intermittently bonding a substrate web in fabricating a blank subassembly for an absorbent article. The invention comprises ultrasonic bonding apparatus including an anvil roll, a substrate web thereon, and at least one rotary ultrasonic horn. The substrate web can comprise at least first and second layers of material. The rotary ultrasonic horn, in combination with the anvil roll, ultrasonically bonds intermittent segments of the first and second layers of the substrate web to each other. Such intermittent bonds can comprise end seals for the absorbent articles. The ultrasonic horn and anvil roll are periodically separated from each other to provide the intermittent bonding of the substrate web. An actuator apparatus periodically moves the anvil roll and ultrasonic horn from engaging contact with each other preventing bond formation. The actuator apparatus can include a cam mechanism moving one of the anvil roll and ultrasonic horn from engaging contact with the other during rotation of the rotary ultrasonic horn. The cam mechanism can create a physical gap between the ultrasonic horn and the anvil roll. The cam mechanism moves either the ultrasonic horn and the anvil roll toward the other of the ultrasonic horn and anvil roll at a velocity of no more than about 80 millimeters / second to prevent bounce or impact loading when the horn and anvil roll are in engaging contact. The anvil roll comprises a substrate-receiving surface generally moving the substrate web through the nip at a surface speed of at least 300 meters per minute. The ultrasonic system generally creates bonds between the first and second layers of the substrate web at least about 600 times per minute.
Owner:DUKANE IAS LLC
Nonwoven composite containing an apertured elastic film
ActiveUS20080095978A1Lamination ancillary operationsSynthetic resin layered productsEngineeringBond formation
An elastic nonwoven composite that contains an elastic film laminated to one or more nonwoven web materials is provided. The composite is formed by passing the film through a nip to bond the film to the nonwoven web material(s). Concurrent with bond formation, apertures are also formed in the elastic film. The apertures are of a size sufficient to provide a desired level of texture, softness, hand feel, and / or aesthetic appeal to the composite without having a significant adverse effect on its elastic properties. Aperture and bond formation are accomplished in the present invention by selectively controlling certain parameters of the lamination process, such as film content, bonding pattern, degree of film tension, bonding conditions, etc.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Curable composition
InactiveUS20090182091A1Less discolorationGood adhesivenessOther chemical processesEster polymer adhesivesSilylenePlasticizer
The present invention has its object to provide a curable composition which comprises a guanidine compound as a non-organotin type catalyst, is less discolored, has good surface curability, depth curability, strength rise and adhesiveness, and can retain the curability even after storage; the above object can be achieved by a curable composition which comprises: (A) an organic polymer containing a silyl group capable of crosslinking under siloxane bond formation, the silyl group being a group represented by the general formula (1): —SiX3 (1) (wherein X represents a hydroxyl group or a hydrolyzable group and the three X groups may be mutually the same or different), (B) a guanidine compound (B-1) as a silanol condensation catalyst, and (C) a plasticizer, wherein the content of the component (B-1) is not lower than 0.1 part by weight but lower than 8 parts by weight per 100 parts by weight of the component (A), and a non-phthalate ester plasticizer accounts for 80 to 100% by weight of the (C) component plasticizer.
Owner:KANEKA CORP
Coated and cured proppants
ActiveUS20130065800A1Improve crush resistanceGood fracture conductivityPretreated surfacesFluid removalAlcoholFirming agent
Solid proppants are coated with a coating that exhibits the handling characteristics of a pre-cured coating while also exhibiting the ability to form particle-to-particle bonds at the elevated temperatures and pressures within a wellbore. The coating includes a substantially homogeneous mixture of (i) at least one isocyanate component having at least 2 isocyanate groups, and (ii) a curing agent comprising a monofunctional alcohol, amine or amide. The coating process can be performed with short cycle times, e.g., less than about 4 minutes, and still produce a dry, free-flowing, coated proppant that exhibits low dust characteristics during pneumatic handling but also proppant consolidation downhole for reduced washout and good conductivity. Such proppants also form good unconfined compressive strength without use of an bond activator, are substantially unaffected in bond formation characteristics under downhole conditions despite prior heat exposure, and are resistant to leaching with hot water.
Owner:PREFERRED TECH
Negative active material for rechargeable lithium battery, and method of preparing the same
ActiveUS20090075173A1High initial capacityImprove initial efficiencyMaterial nanotechnologyElectrode thermal treatmentFree energiesEngineering
An negative active material for a rechargeable lithium battery includes a nano-composite including a Si phase, a SiO2 phase, and a metal oxide phase of formulation MyO, where M is a metal with an oxidation number x, a free energy of oxygen-bond formation ranging from −900 kJ / mol to −2000 kJ / mol, x, and x·y=2. The negative active material for a rechargeable lithium battery according to the present invention can improve initial capacity, initial efficiency, and cycle-life characteristics by suppressing its initial irreversible reaction.
Owner:SAMSUNG SDI CO LTD +1
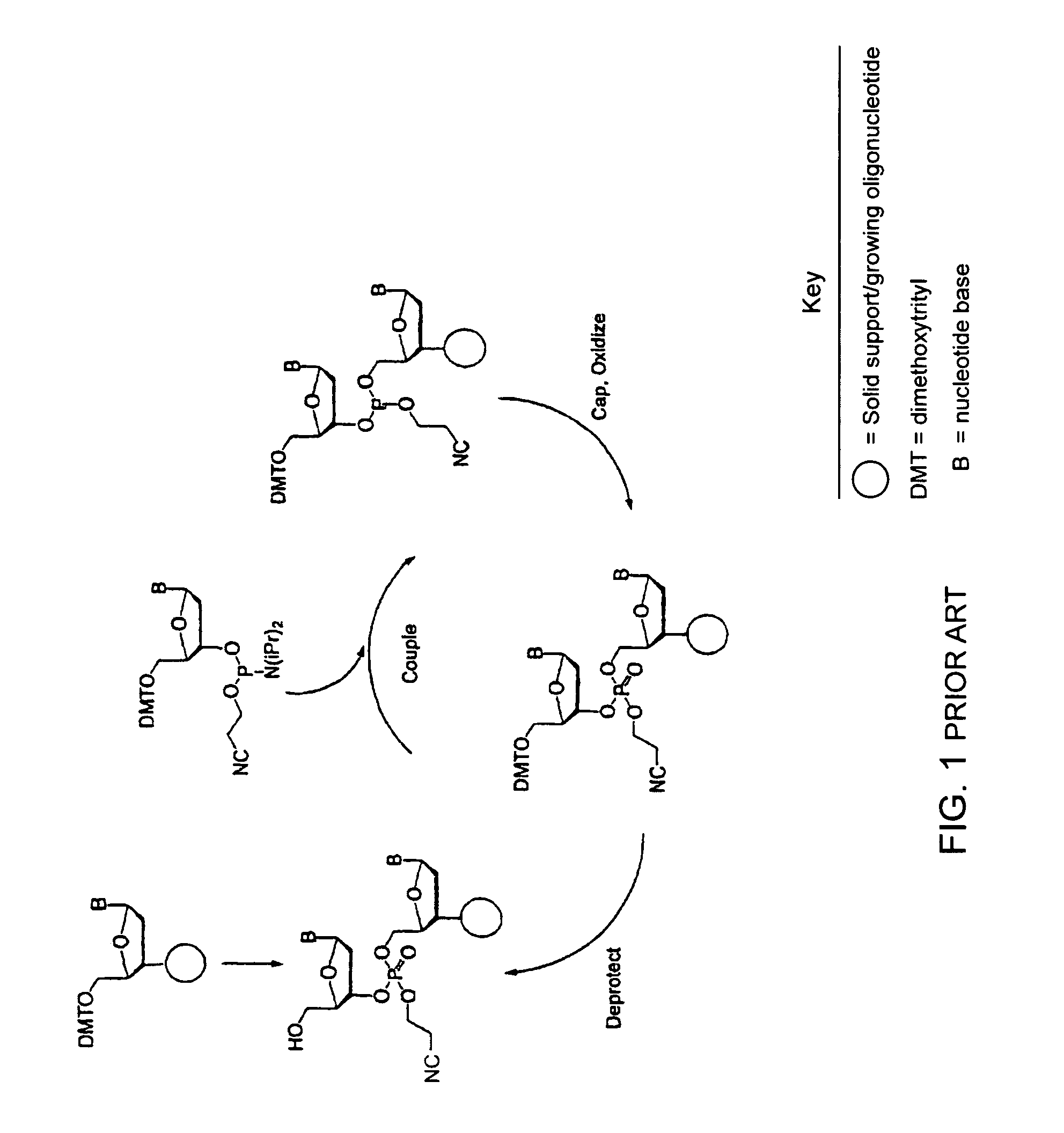
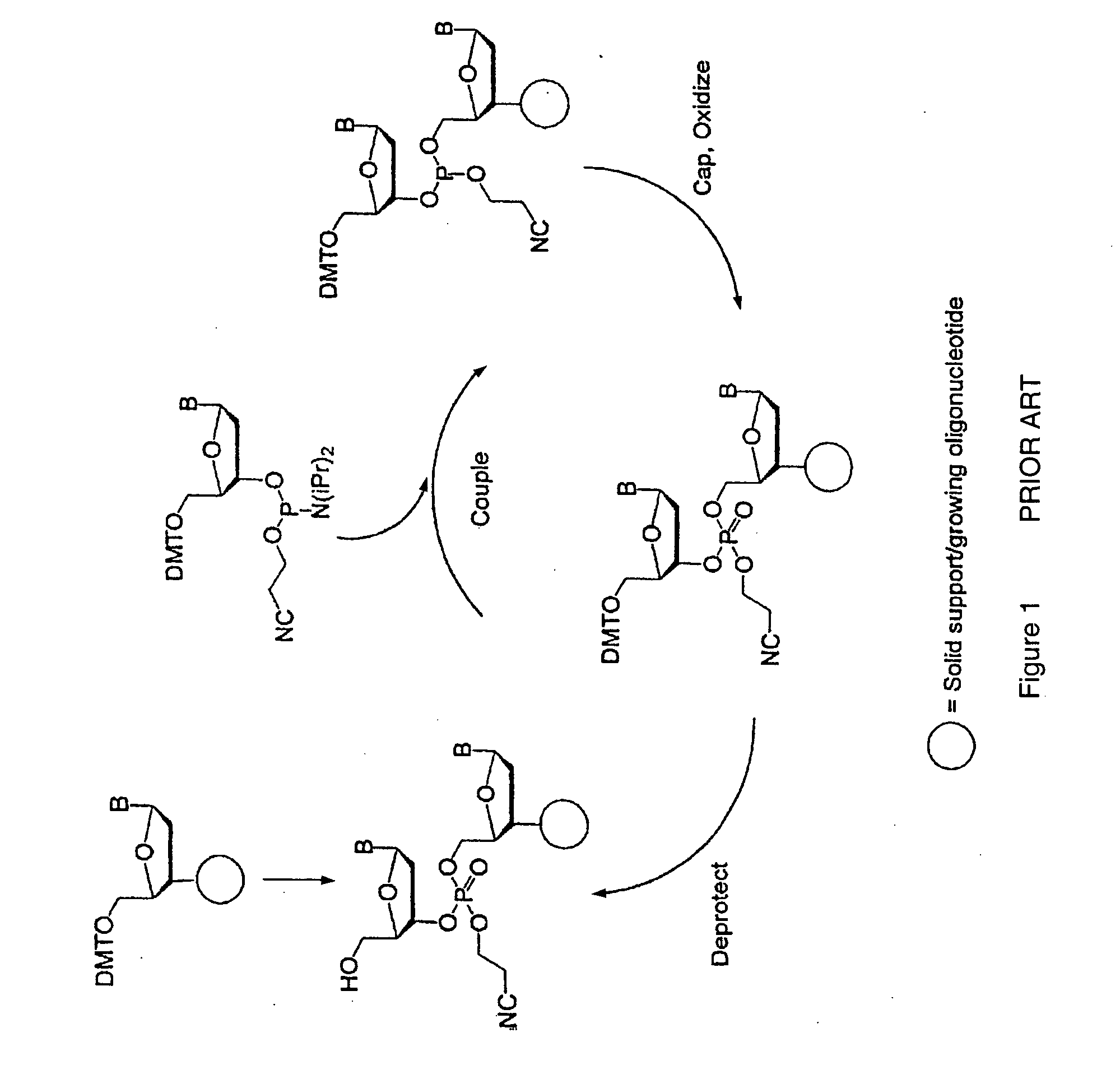
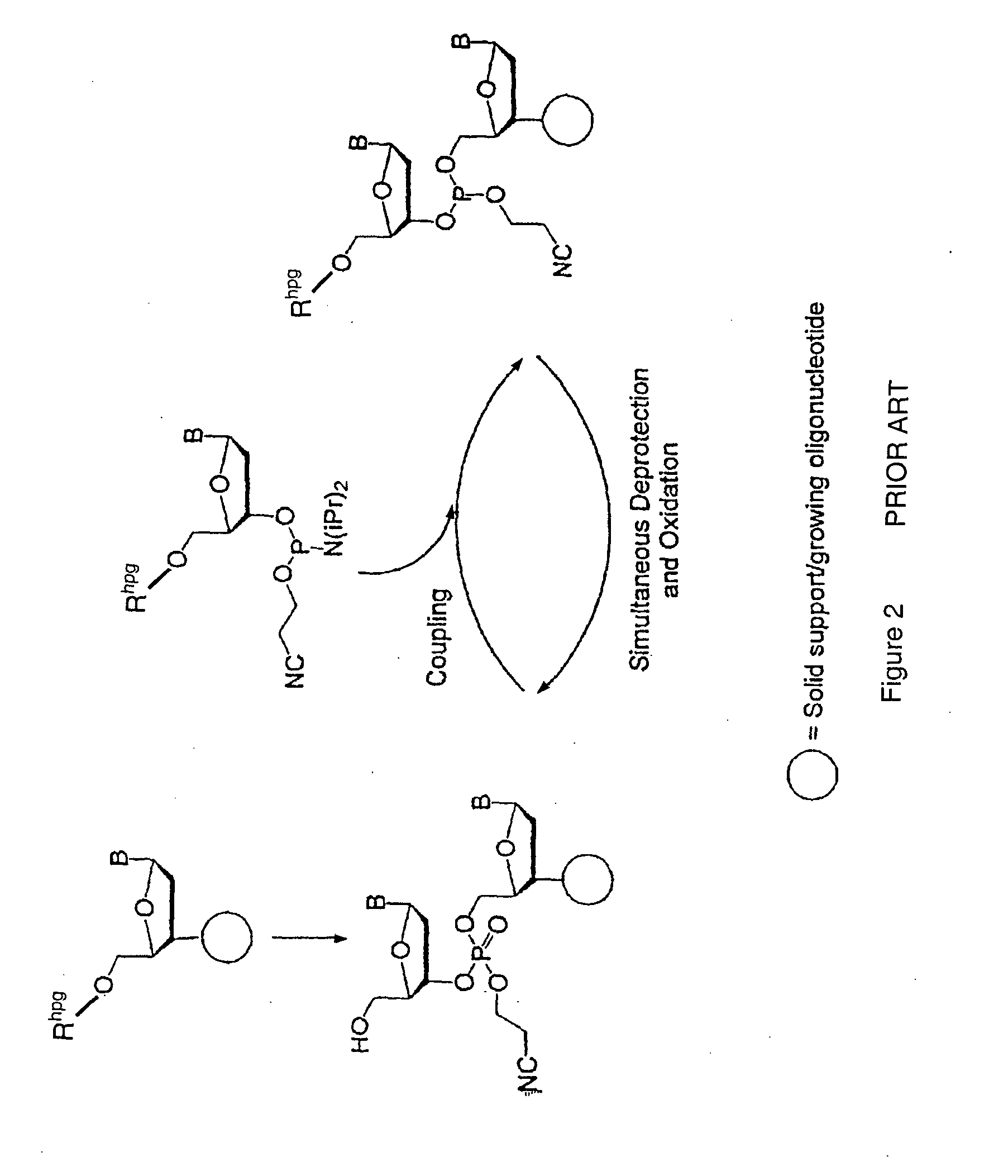
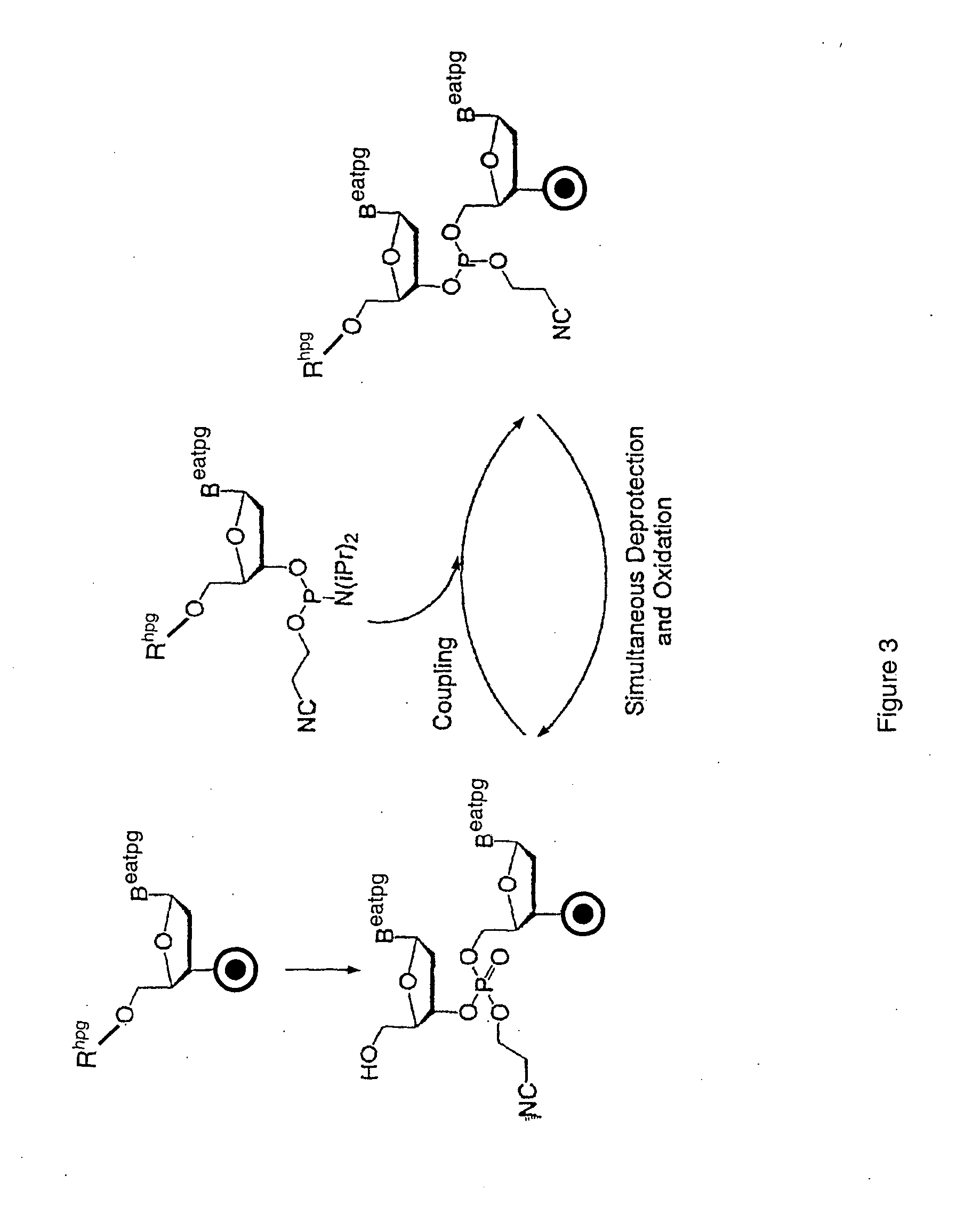
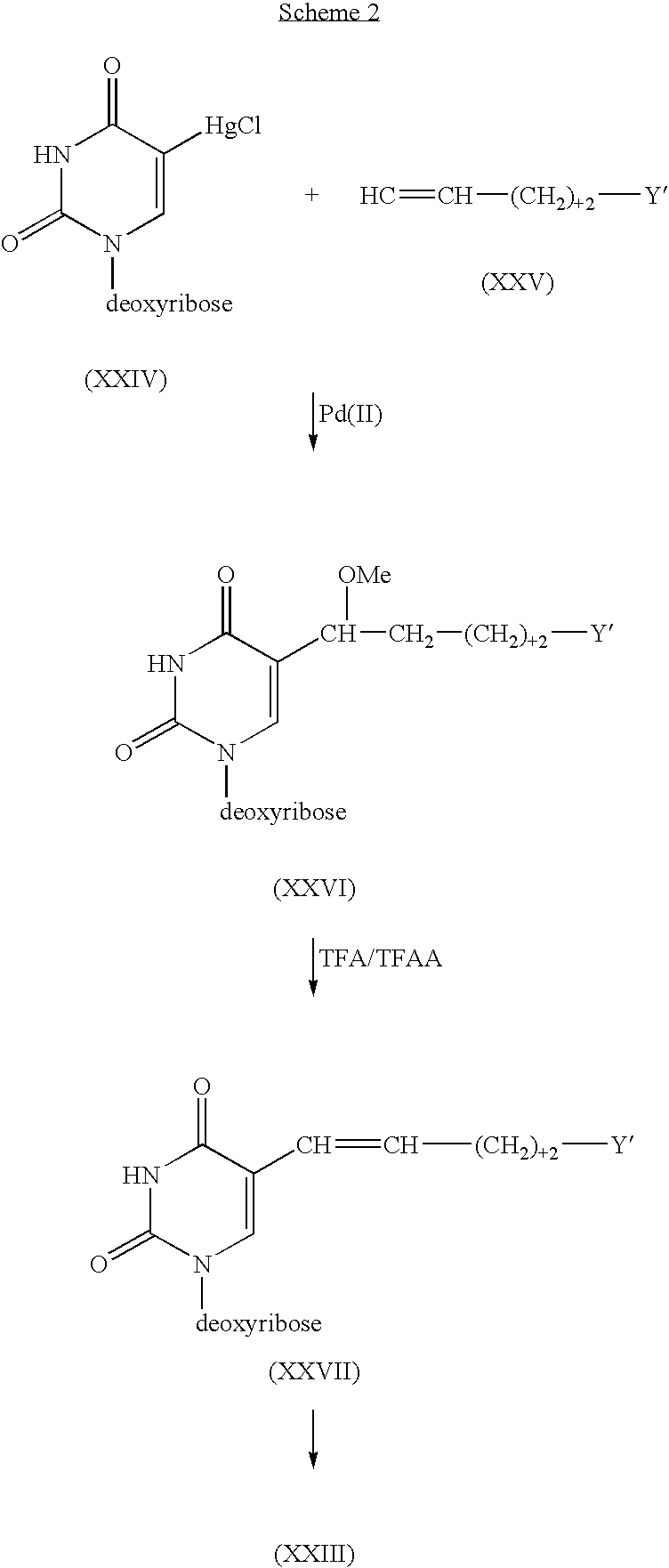
Method for polynucleotide synthesis
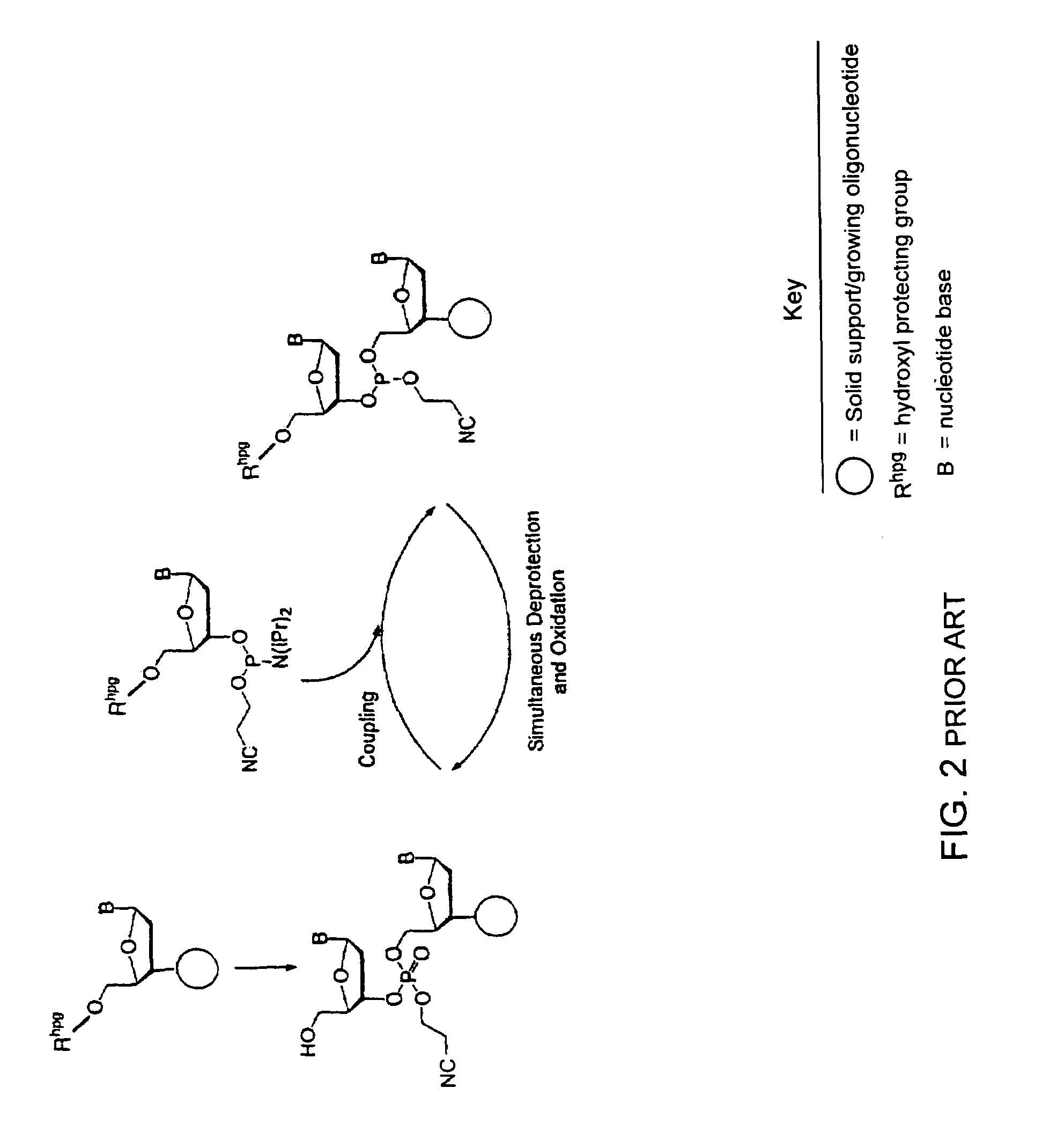
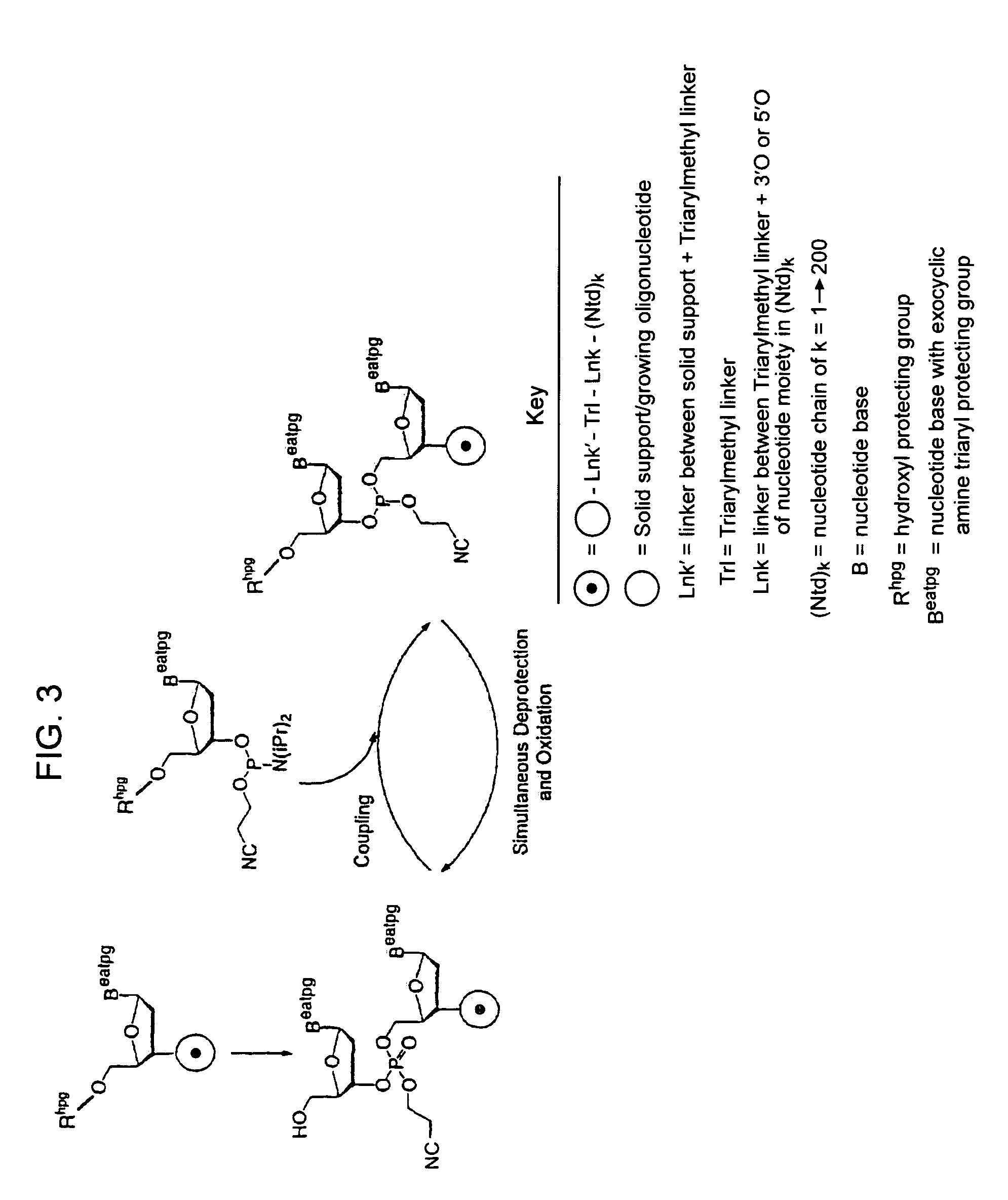
InactiveUS7417139B2Undesirable side reactionSugar derivativesPeptide/protein ingredientsHydrogenSynthesis methods
Methods of forming an internucleotide bond are disclosed. Such methods find use in synthesis of polynucleotides. The method involves contacting a functionalized support with a precursor having an exocyclic amine triaryl methyl protecting group under conditions and for a time sufficient to result in internucleotide bond formation. The functionalized support includes a solid support, a triaryl methyl linker group, and a nucleoside moiety having a reactive site hydroxyl, the nucleoside moiety attached to the solid support via the triaryl methyl linker group. In particular embodiments, the precursor has the structure:wherein:O and H represent oxygen and hydrogen, respectivelyR1 is hydrido, hydroxyl, protected hydroxyl, lower alkyl, modified lower alkyl, or alkoxy,one of R2 or R3 is a hydroxyl protecting group; and the other of R2 or R3 is a reactive group capable of reacting with the reactive site hydroxyl,Base is a heterocyclic base having an exocyclic amine group, andTram is the exocyclic amine triaryl methyl protecting group.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Ruthenium metathesis catalyst and method for producing olefin reaction product by metathesis reaction using the same
InactiveUS6175047B1Easy to prepareHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsCatalystsCarbon–carbon bondReducing agent
The invention has an object of safely and simply preparing a large amount of a ruthenium metathesis catalyst, which is used as a catalyst for a carbon-carbon bond formation using, particularly, a metathesis reaction. The metathesis catalyst has the following complex composition (A) or (B). The composition (A) includes RuX12(arene)(PR1R2R3) and R4CHX22, R5C=CH or R4CHX2 and a reducing agent, wherein X1 and X2 respectively are a halogen atom; arene is a hydrocarbon having a benzene ring; R1, R2 and R3, which may be the same or different, respectively are an alkyl group having 1-8 carbon atoms, a cycloalkyl group having 3-8 carbon atoms or an optionally substituted aryl group, wherein the substituent group is an alkyl group having 1-8 carbon atoms, an alkoxy group having 1-8 carbon atoms, an alkylamino group having 1-8 carbon atoms or a halogen atom; R4 is an alkyl group which has 1-8 carbon atoms and may have an ether bond or an ester bond, an optionally subsituted aryl group, wherein the substituent group is a halogen atom or a hydroxyl group; or cycloalkyl group having 3-8 carbon atoms; and R5 is an optionally substituted alkyl group which has 1-8 carbon atoms and may have an ether bond or an ester bond, wherein the substituent group is a halogen atom or a hydroxyl group, an aryl group or a cycloalkyl group having 3-8 carbon atoms. The composition B includes [RuX12(arene)]2, PR1R2R3, R5C=CH or R4CHX2 and a reducing agent, wherein X1, arene, R1, R2, R3, R4 and R5 are the same as defined above.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Sinter-bonded direct pin connections for inert anodes
InactiveUS6855234B2Reduce crackingStable electrical joint resistanceIsotope separationMetal working apparatusElectrical conductorMetallurgy
A sintered electrode assembly is made of an inert electrode (15) containing a sealed metal conductor (20) having a surface feature (30) such as a coating or wrapping which aids in bond formation with the inert electrode (15) at their interface (45), where the metal conductor (20) is directly contacted by and is substantially surrounded by the inert electrode (15) without the use of metal foam or metal powders.
Owner:ARCONIC INC
Composite form assembly with frangible bonded layers formed in-situ
The present invention relates to a non-impact printable, laminated form construction in which two or more layers are bonded together in-situ through use of a curable compound that creates a frangible bond between the layers which upon application of sufficient peeling pressure the form components or portions will separate from the composite form assembly. The in-situ bond formation occurs by passing select treatment energy through one or more layers of the laminate in order to cure the coating and thus secure the sheets or webs together. The present invention finds uses in a number of fields where it is generally desirable to create a tag, plate or other indicia containing device that is free of adhesive.
Owner:WARDKRAFT
Method for polynucleotide synthesis
InactiveUS20050048601A1Undesirable side reactionSugar derivativesPeptide/protein ingredientsNucleotideReactive site
Methods of forming an internucleotide bond are disclosed. Such methods find use in synthesis of polynucleotides. The method involves contacting a functionalized support with a precursor having an exocyclic amine triaryl methyl protecting group under conditions and for a time sufficient to result in internucleotide bond formation. The functionalized support includes a solid support, a triaryl methyl linker group, and a nucleoside moiety having a reactive site hydroxyl, the nucleoside moiety attached to the solid support via the triaryl methyl linker group.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Negative active material for rechargeable lithium battery, and method of preparing the same
ActiveUS8377592B2Improved initial capacity and initial efficiency and cycle-life characteristicInhibition of irreversible reactionsMaterial nanotechnologyElectrode thermal treatmentFree energiesEngineering
Owner:SAMSUNG SDI CO LTD +1
Curable composition
InactiveUS20100216925A1Excellent in surface curabilityImprove tensile propertiesTin organic compoundsOther chemical processesSilyleneBond formation
An object of the present invention is to provide a curable composition which has good curability and good tensile properties (high elongation), and contains a smaller amount of a dibutyltin compound which is regarded as toxic. The curable composition of the present invention comprises the following components (A) and (B) at a weight ratio of (A) / (B) of 25 / 75 to 75 / 25, (A) a reactive silyl group-containing organic polymer having, as a silicon-containing group cross-linkable by siloxane bond formation, a group represented by the formula (1): —SiX3, and (B) a reactive silyl group-containing organic polymer having, as a silicon-containing group cross-linkable by siloxane bond formation, a moiety represented by the formula (2): —O—R1—CH(CH3)—CH2—Si (R23-a)Xa wherein a is 1 or 2.
Owner:KANEKA CORP
Cross-linking oligonucleotides
Owner:DRUG ROYALTY TRUST 9
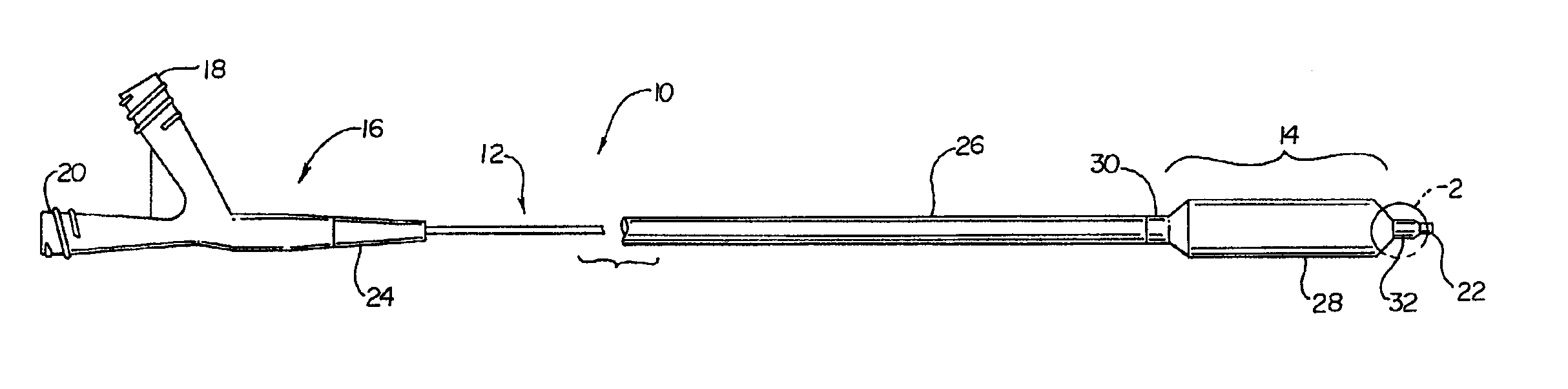
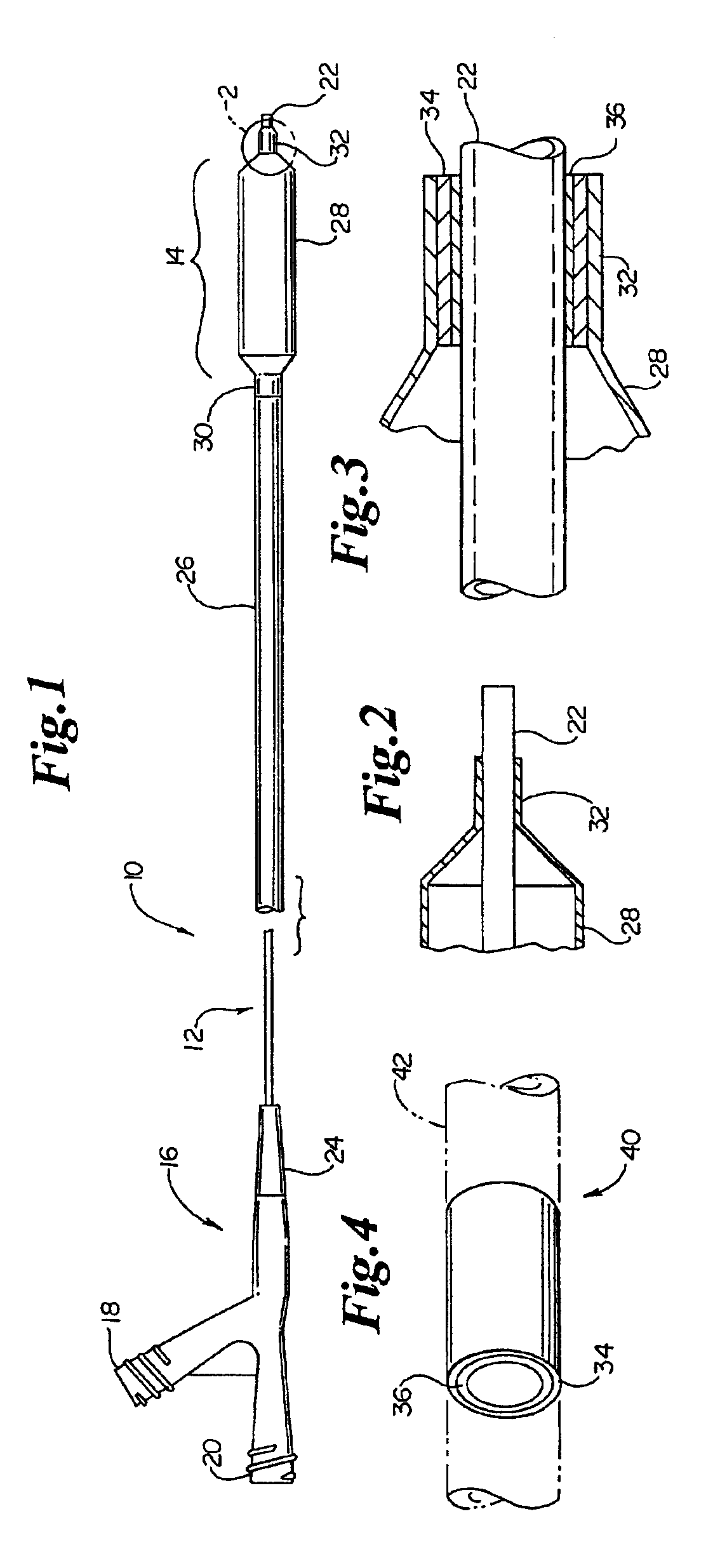
Catheter having an improved balloon-to-catheter bond
InactiveUS6923787B2Maximize the benefitsImprove bindingStentsBronchoscopesDistal portionBond formation
A tie layer insert is disclosed to aid in bond formation between an expandable balloon and a distal portion of a catheter shaft. The tie layer insert can be a single layer applied directly to the structural surfaces, or alternatively, the tie layer may be incorporated into a preformed polymeric insert. In the latter embodiment, the polymeric insert may include several layers of polymeric material. During the manufacturing process, the preformed polymeric insert is positioned between the distal portion of the expandable balloon and the catheter shaft. The entire distal region is then processed to form a sealably bonded expandable balloon to the distal end of the catheter shaft.
Owner:BOSTON SCI SCIMED INC
Curable composition
ActiveUS20100036049A1Excellent curabilityGood adhesivenessOther chemical processesCoatingsBond formationSilicon
The present invention has its object to provide a curable composition which allows slight bleedout of a liquid compound to occur to the cured product and shows good curability and adhesiveness using a non-organotin catalyst, an amine compound, as a silanol condensation catalyst.The present invention relates to a non-organotin-based curable composition which comprises:(A) an organic polymer having a silicon-containing group capable of crosslinking by siloxane bond formation, and(B) a specific amidine compound and / or a guanidine compound having a melting point of not lower than 23° C., as a silanol condensation catalyst.
Owner:KANEKA CORP
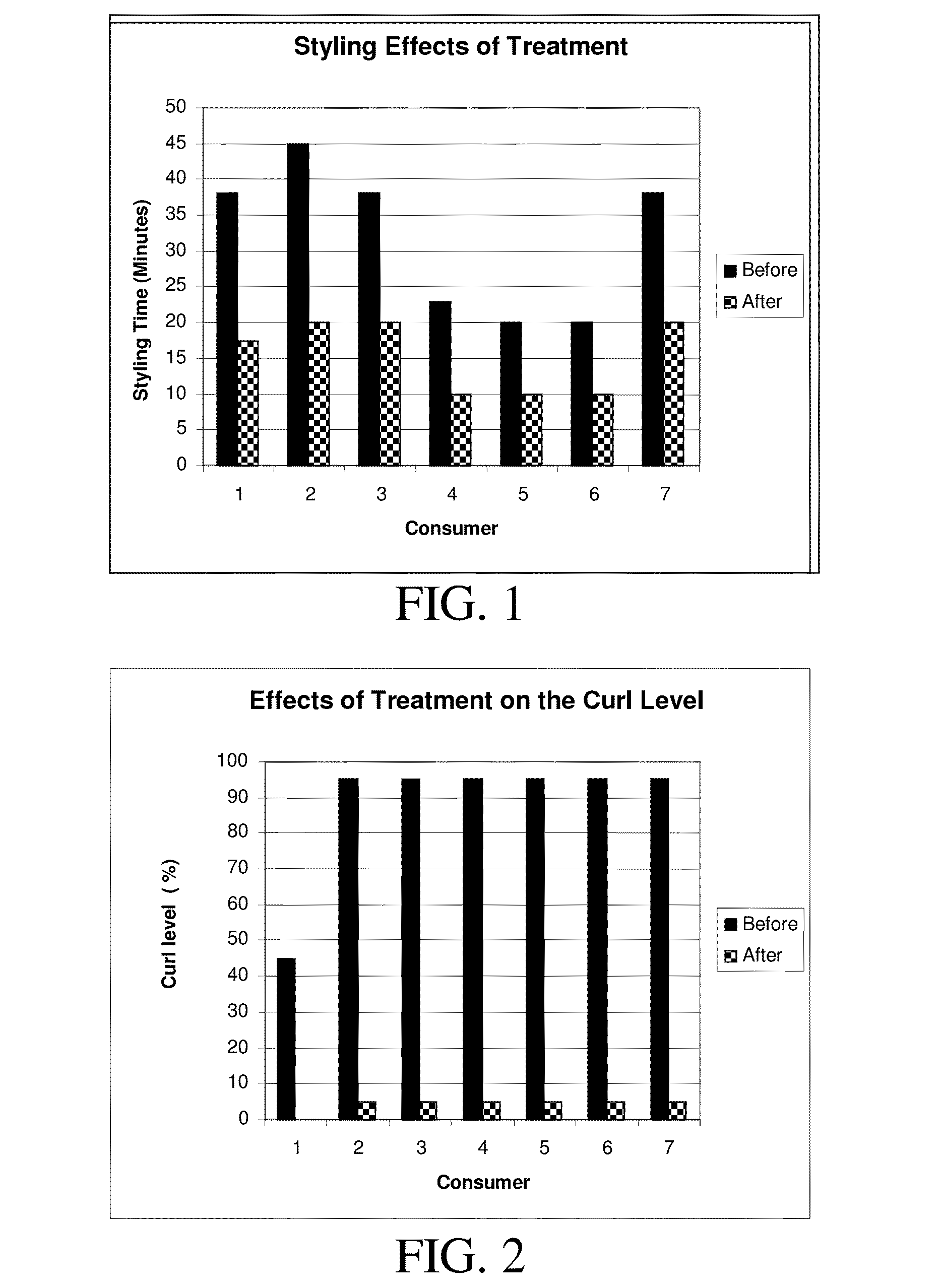
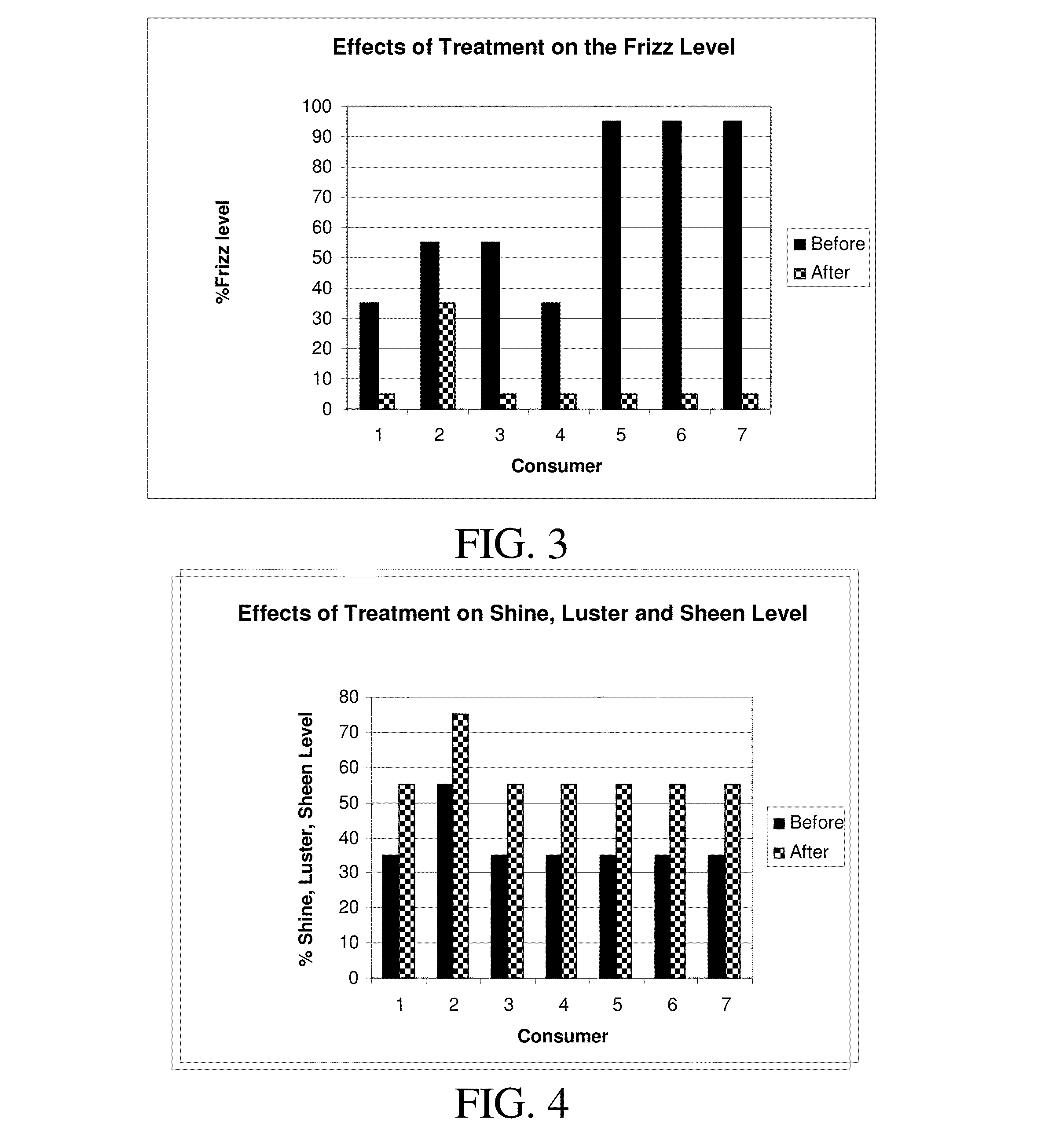
Formulations and methods for straightening and revitalizing hair
InactiveUS20140261518A1Long lasting curl reductionSafely be applied to hairCosmetic preparationsHair cosmeticsCross-linkFiber
The present invention is directed to methods and formulations of treating keratin fibers, in order to provide long lasting curl reduction and styling results for keratin fibers from a single application. The present invention may include forming sufficient active sites on the keratin fibers followed with fixation and / or cross-linking of active compounds via covalent and strong ionic bond formation to the active sites on the keratin fibers. The active sites may be produced by breaking a sufficient number of disulfide bonds in the keratin fibers by using one or more reducing agents. The active compounds may then be fixed and / or cross-linked to the active sites. The fixation and / or cross-linking reactions of the active compounds onto the keratin fibers is facilitated by allowing the formulations containing the active compounds to react on the keratin fibers at ambient conditions for a sufficient time, followed with heating the keratin fibers to approximately 400° F.
Owner:ZOTOS INT
Nonwoven Composite Containing an Apertured Elastic Film
An elastic nonwoven composite that contains an elastic film laminated to one or more nonwoven web materials is provided. The composite is formed by passing the film through a nip to bond the film to the nonwoven web material(s). Concurrent with bond formation, apertures are also formed in the elastic film. The apertures are of a size sufficient to provide a desired level of texture, softness, hand feel, and / or aesthetic appeal to the composite without having a significant adverse effect on its elastic properties. Aperture and bond formation are accomplished in the present invention by selectively controlling certain parameters of the lamination process, such as film content, bonding pattern, degree of film tension, bonding conditions, etc.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Apparatus and method for low pressure wirebond
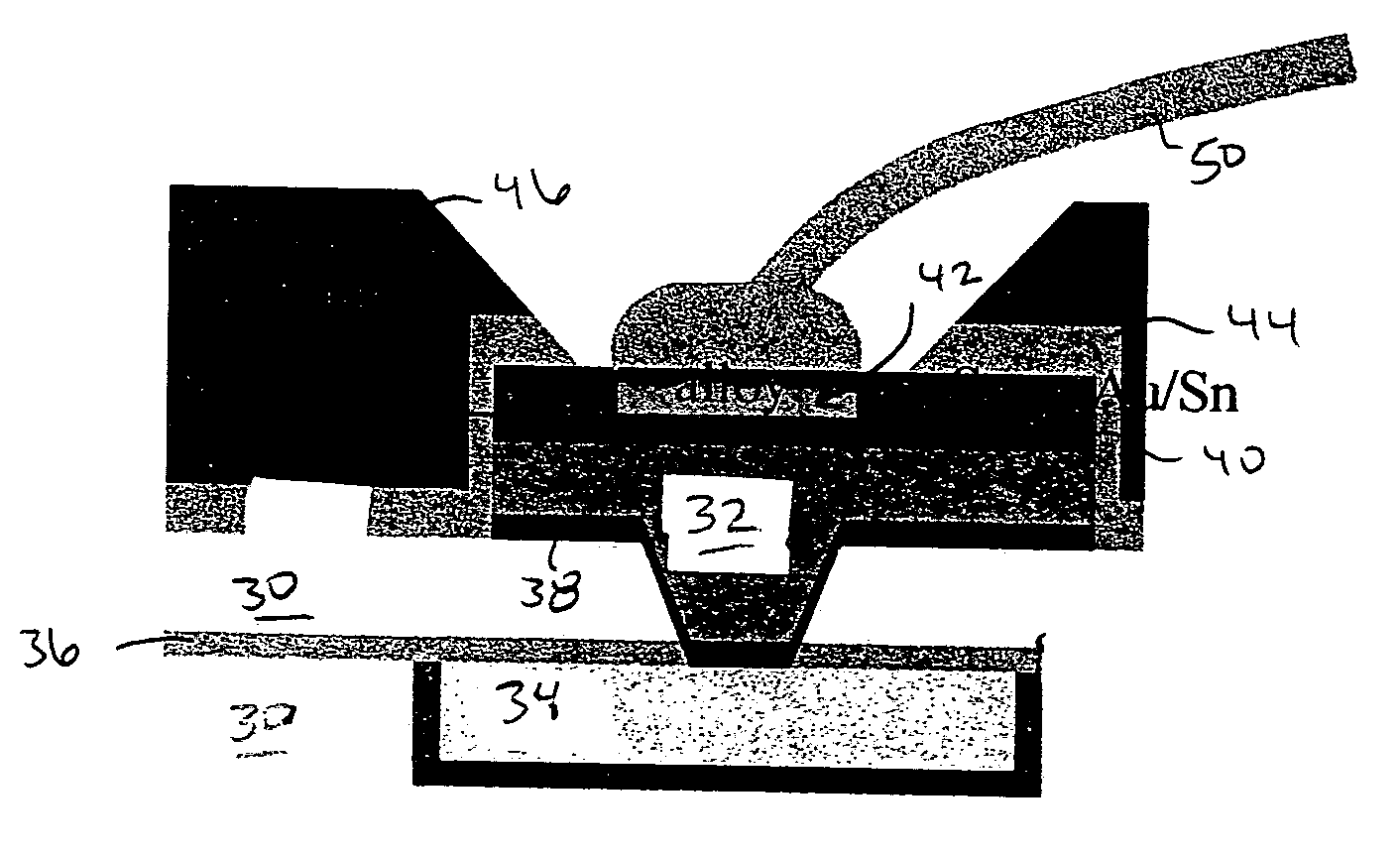
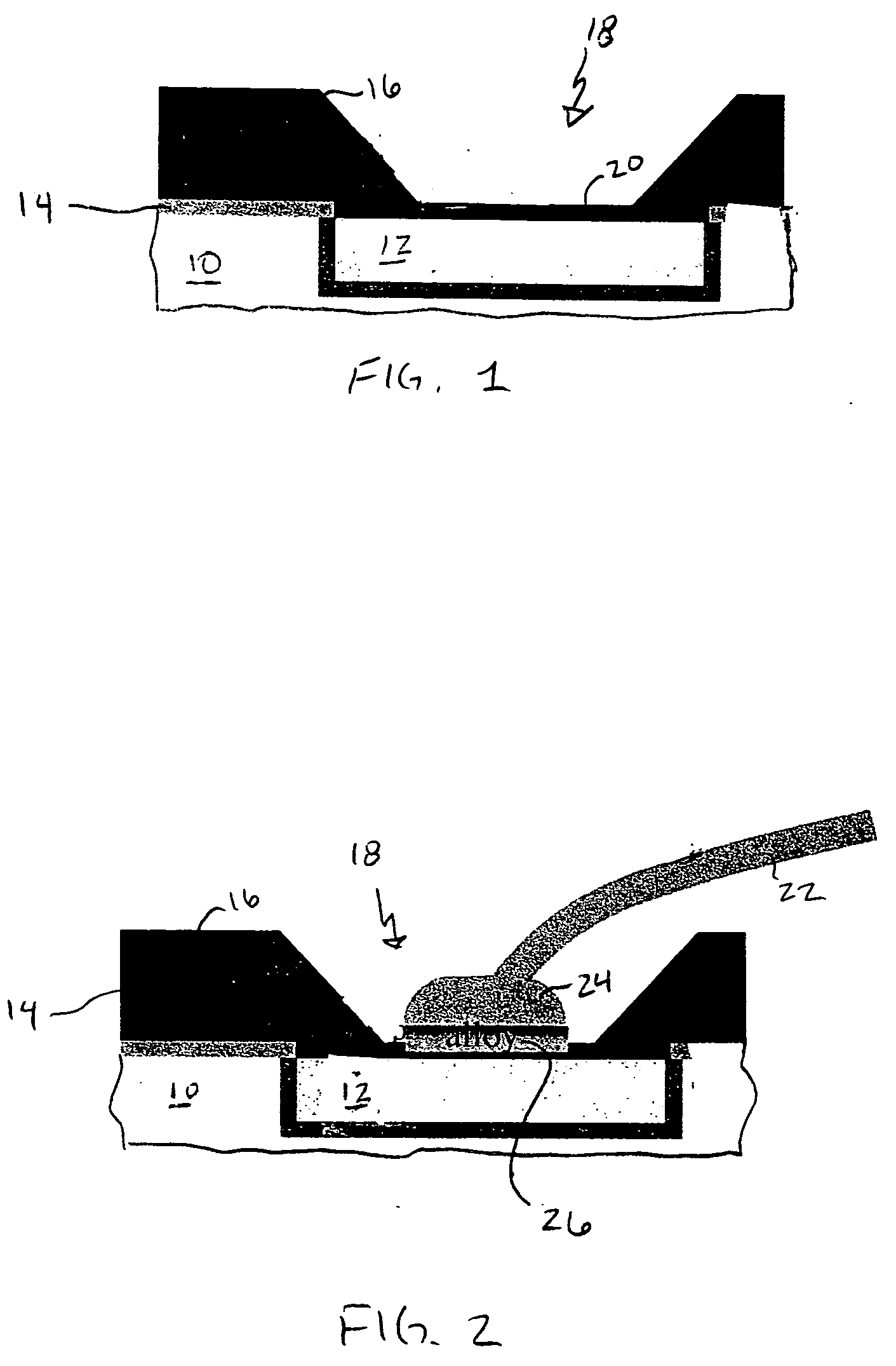
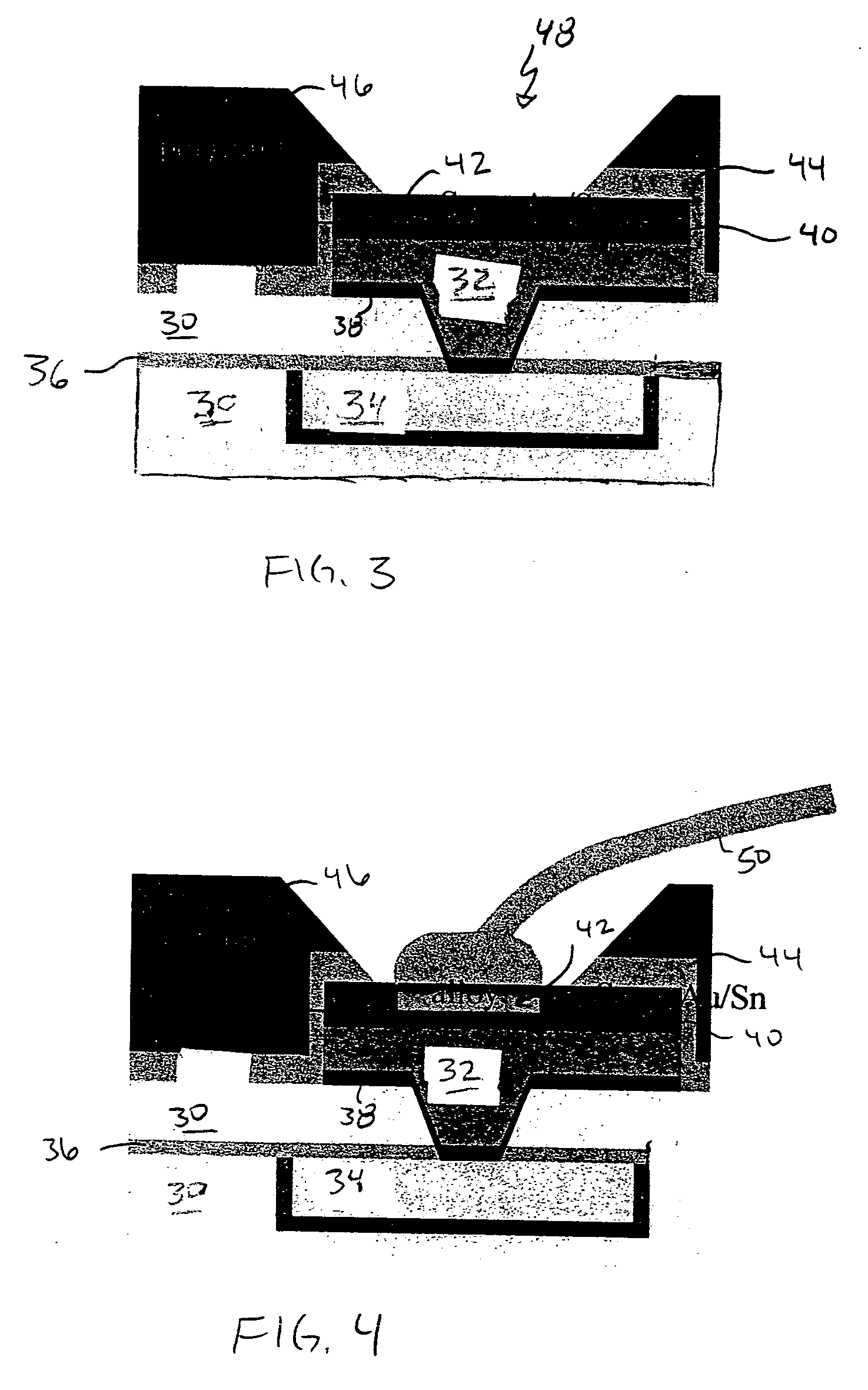
InactiveUS20050098605A1Reduction tendencySemiconductor/solid-state device detailsWelding/cutting auxillary devicesAlloyBond formation
A structure and method for low-pressure wirebonding, reducing the propensity of dielectric material to mechanical failure due to wirebond stress. A low temperature alloy on the surface of a bond pad allows alloy bond formation to occur between the wire and the bond pad at reduced bond pressures and reduced thermal and ultrasonic energies. Preferred alloys include Au—Sn and Au—In. The Au—Sn alloy may be formed over the Cu bond pad, incorporated in an aluminum bond pad stack, or deposited on a bond pad having Ni—Au capping of Cu or Al bond pads.
Owner:GLOBALFOUNDRIES INC
Additive for adjusting the glass transition temperature of visco-elastic polyurethane soft foams
The present invention is directed to the use of a disalt of malic acid in the production of a polyurethane foam to lower the glass transition temperature of the polyurethane foam obtained, wherein the disalt of malic acid is added to the reaction mixture comprising at least a polyol component, an isocyanate component, a catalyst to catalyse urethane or isocyanurate bond formation, an optional blowing agent and optionally further additives, and also to a polyurethane foam having a glass transition temperature of −20° C. to 15° C., which polyurethane foam is characterized in that it comprises disalts of malic acid or reaction products thereof with an isocyanate component, wherein the fraction accounted for by the disalts and the reaction products thereof with an isocyanate component is below 0.08 wt % based on the polyurethane foam.
Owner:EVONIK DEGUSSA GMBH
Curable composition
ActiveUS20090186993A1Excellent curabilityAdd depthOther chemical processesEster polymer adhesivesArylSilylene
Owner:KANEKA CORP
Carbon fiber bundle, process for producing the same, and thermoplastic resin composition and molded article thereof
A carbon fiber bundle is provided which can develop satisfactory interfacial adhesion to polyolefin-based resins, especially polypropylene resins. The carbon fiber bundle comprises a plurality of single fibers that was sized with a sizing agent comprising: a polymer having a main chain formed of carbon-carbon bonds, containing an acid group in at least part of side chains or at least a part of main chain ends, and representing an acid value of 23 to 120 mgKOH / g as measured in accordance with ASTM D1386; or a polymer having a main chain formed of carbon-carbon bonds and containing at least either of an epoxy group and an ester group in at least a part of side chains or at least a part of main chain ends.
Owner:MITSUBISHI CHEM CORP
Ligands for metals and improved metal-catalyzed processes based thereon
One aspect of the invention relates to ligands of transition metals. A second aspect of the invention relates to the use of catalysts comprising these ligands in transition metal catalyzed carbon-heteroatom and carbon-carbon bond forming reactions. The method offers many features to transition metal-catalyzed reactions, including suitable substrate scope, reaction conditions and improvements in efficiency.
Owner:MASSACHUSETTS INST OF TECH
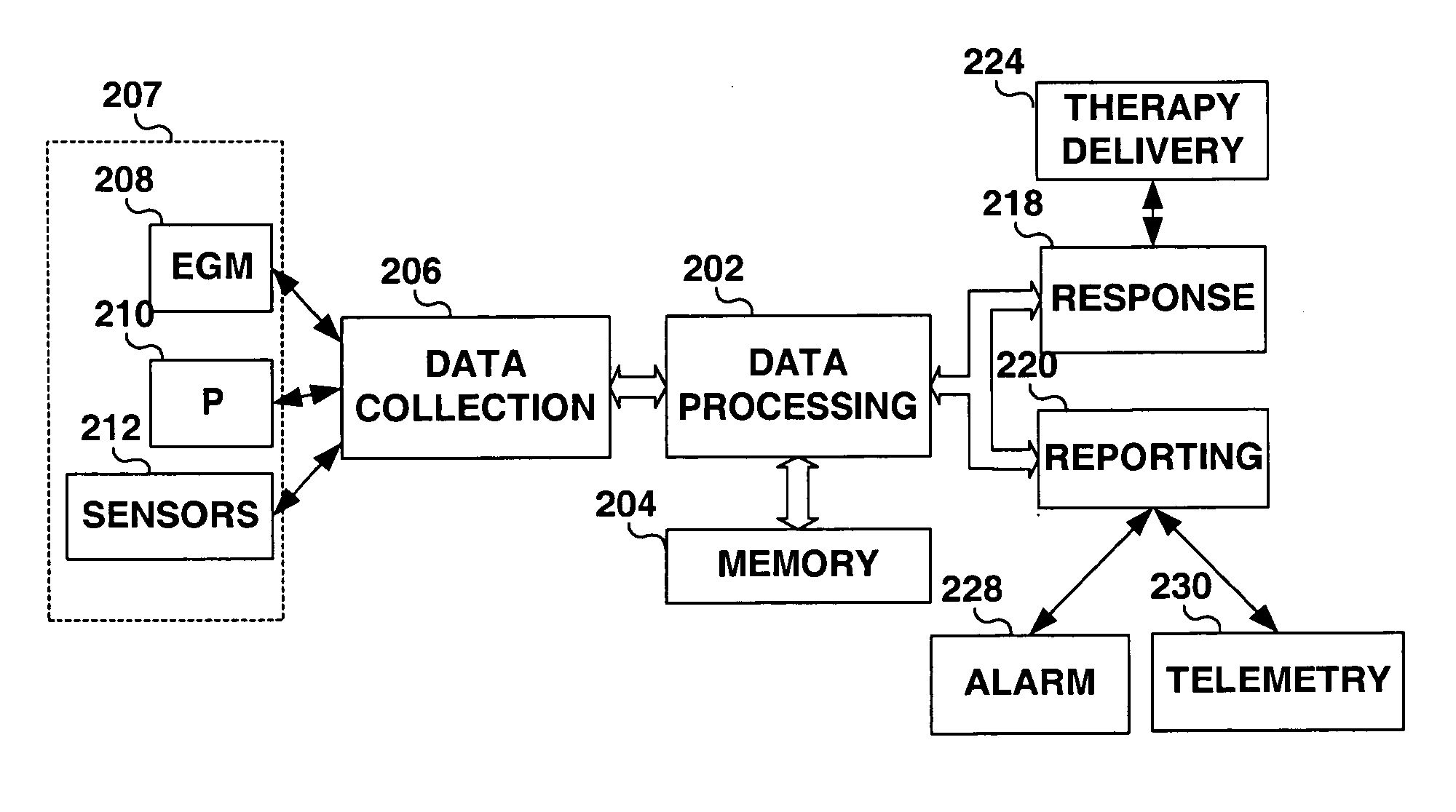
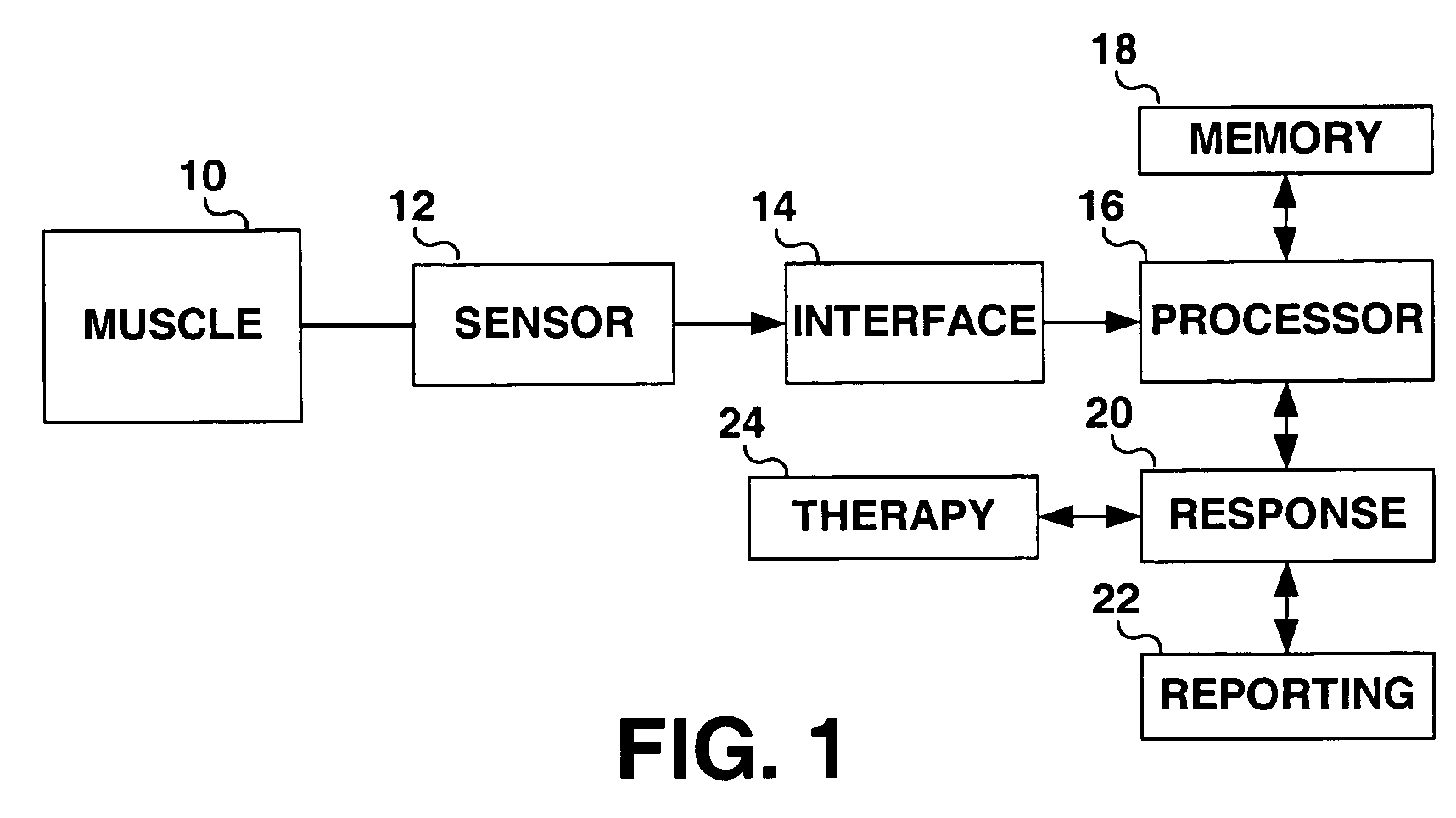
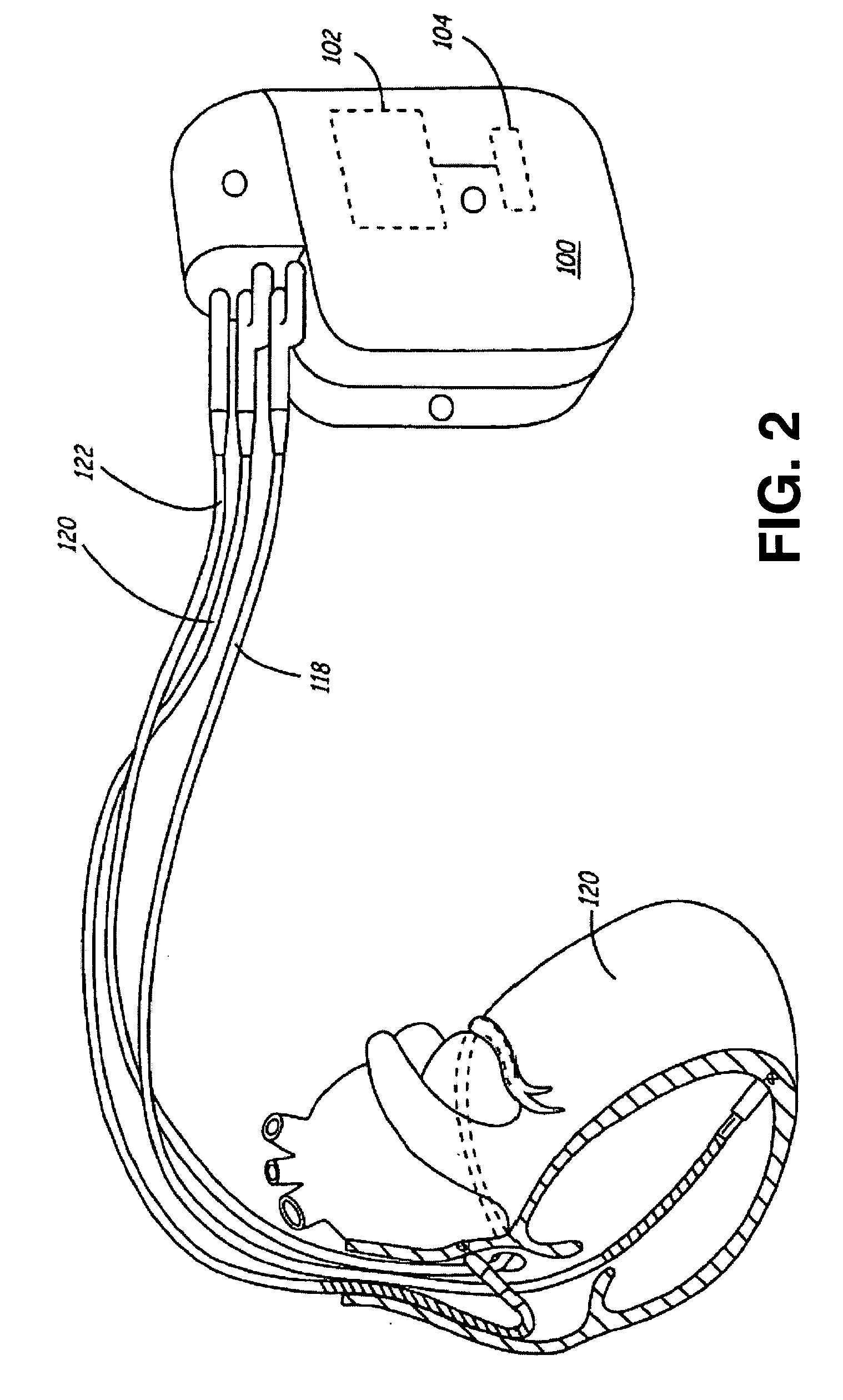
Method and apparatus for muscle function measurement
The present invention provides an apparatus and method for monitoring muscle function based on an index derived from a pressure or force signal. The muscle function index is derived from an instantaneous muscle stiffness ratio computed as the ratio of the first time derivative of the pressure or force waveform to the corresponding instantaneous pressure or force. The instantaneous stiffness ratio, {dot over (E)} / E(t), is in units of 1 / sec and relates to the rate of strong bond formation and will be influenced by calcium handling properties of the muscle fibers and the intracellular calcium concentration. As such, an index derived from {dot over (E)} / E(t) provides a measure of the inotropic status of the muscle.
Owner:MEDTRONIC INC
Surfactant-Enabled Transition Metal-Catalyzed Chemistry
ActiveUS20110130562A1Promote reaction efficiencyEliminating option for PEG-relatedUrea derivatives preparationCarboxylic acid nitrile preparationSolventBond formation
In one embodiment, the present application discloses mixtures comprising (a) water in an amount of at least 1% wt / wt of the mixture; (b) a transition metal catalyst; and (c) one or more solubilizing agents; and methods for using such mixtures for performing transition metal mediated bond formation reactions.
Owner:MYCELL TECH
Water-based metal surface treatment composition for forming lubricating film with excellent marring resistance
A water-based metal surface treatment composition for forming a lubricating film with excellent marring resistance, characterized in that it comprises (a) a water-compatible urethane resin which contains bisphenol skeletons and carboxyl groups in the resin backbone and has an average molecular weight of 3,000 or higher and which has been synthesized through reaction of an isocyanate in which the content of nitrogen atoms participating in the isocyanate reaction is 2 to 13 wt. % and the ratio of nitrogen atoms participating in urea bond formation to the nitrogen atoms participating in the isocyanate reaction is 10 / 100 to 90 / 100, (b) a hardener, (c) silica, and (d) a polyolefin wax, and that the sum of the ingredients (a) and (b) is 50 to 95 wt. % based on the whole composition (e) on a solid basis, the amount of functional groups contained in the ingredient (b) is 0.10 to 1.00 equivalent to the carboxyl groups contained in the backbone of the ingredient (a), the amount of the ingredient (c) is 3 to 40 wt. % based on the composition (e) on a solid basis, and the amount of the ingredient (d) is 2 to 30 wt. % based on the composition (e) on a solid basis.
Owner:HENKEL CORP
Nano-composite material and preparation method thereof
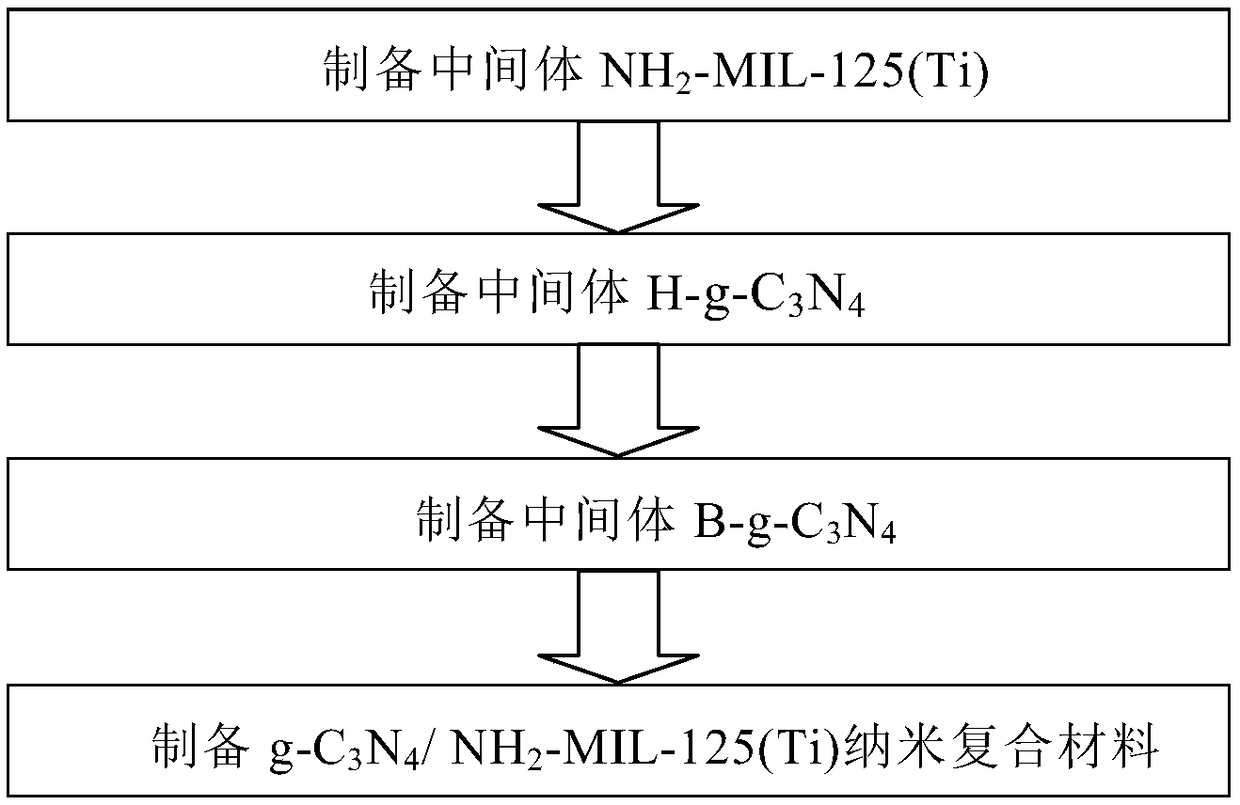
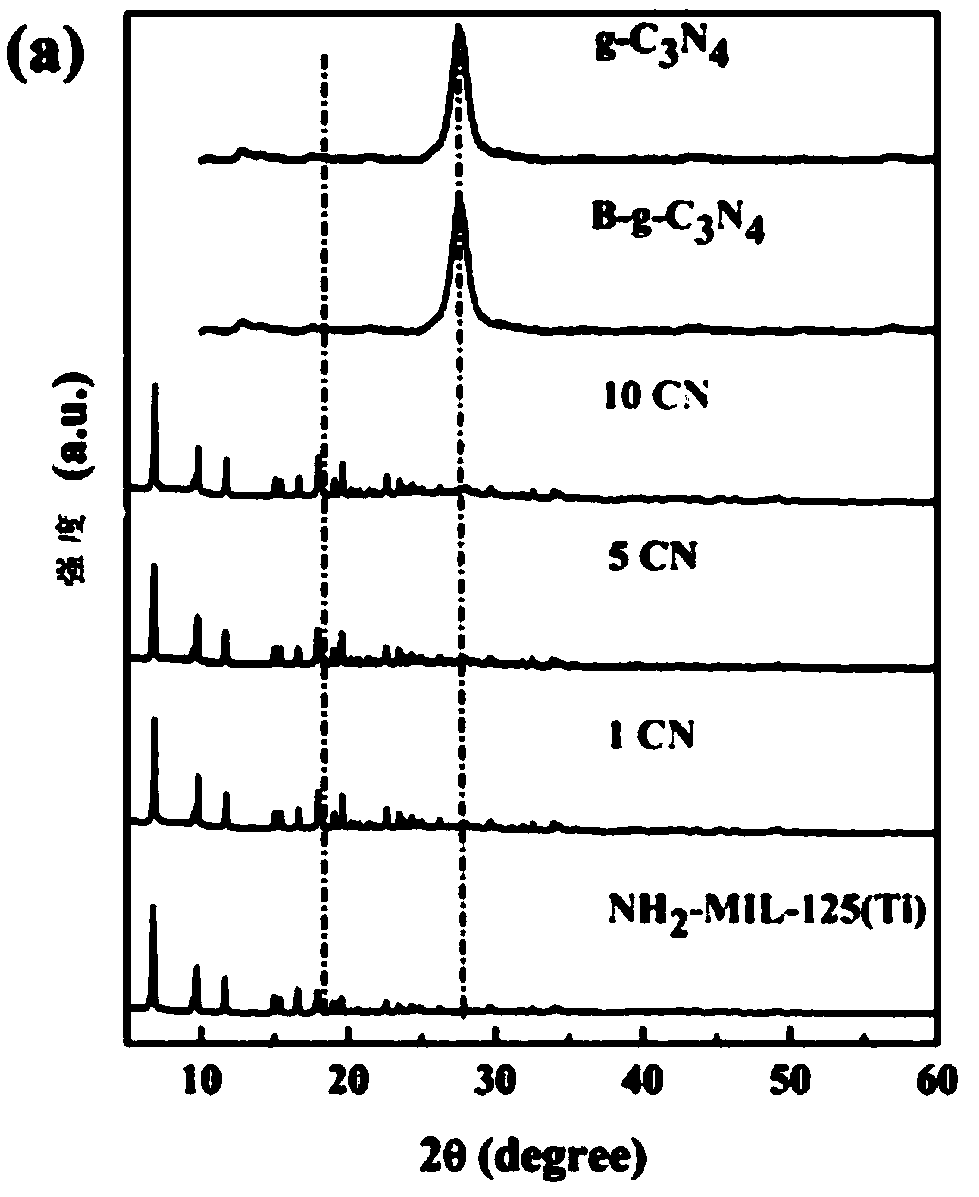
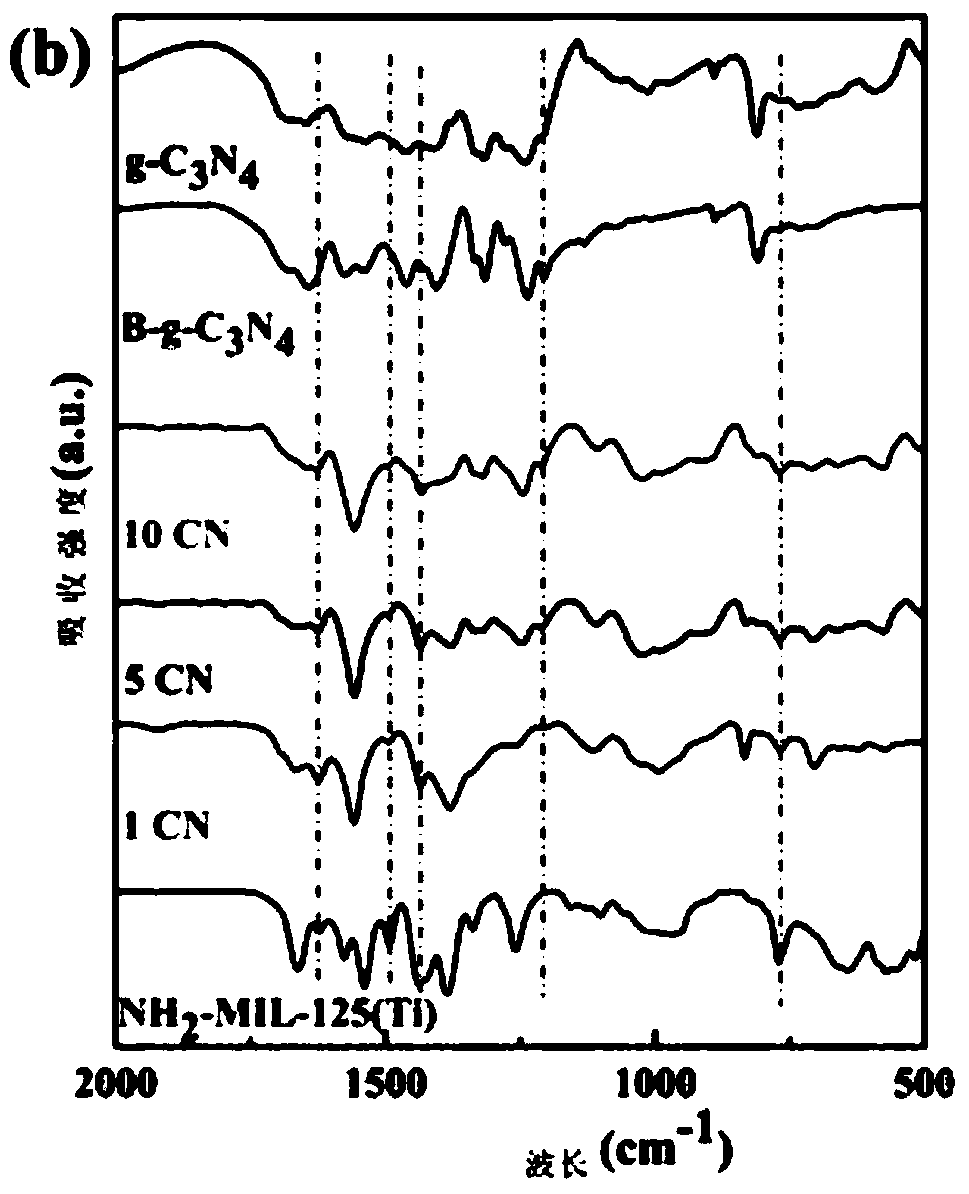
InactiveCN109012607AEfficient separationImprove photocatalytic reduction of U(VI) to U(IV) performanceOther chemical processesOrganic-compounds/hydrides/coordination-complexes catalystsHeterojunctionUranyl
The invention discloses a g-C3N4 / NH2-MIL-125(Ti) nano-composite material and a preparation method thereof, and relates to the field of preparation of uranyl ion removal materials. The preparation method includes the following steps: preparing an intermediate NH2-MIL-125(Ti), preparing an intermediate H-g-C3N4, preparing an intermediate B-g-C3N4, and finally preparing the g-C3N4 / NH2-MIL-125(Ti) nano-composite material. The B-g-C3N4 is used for the first time, and the B-g-C3N4 and an aminated NH2-MIL-125(Ti) material undergo amido bond formation to synthesizes the heterojunction photocatalytic composite material, and photo-induced electrons and holes are effectively separated, so the performance for catalytic reduction of U (VI) into U(IV) is improved; and when the mass proportion of the B-g-C3N4 is 1%, the composite material can effectively remove radioactive uranyl ions through the synergistic mechanism of adsorption and photocatalysis, so the U (VI) removal rate is improved.
Owner:南通寰宇博新化工环保科技有限公司
Device packages
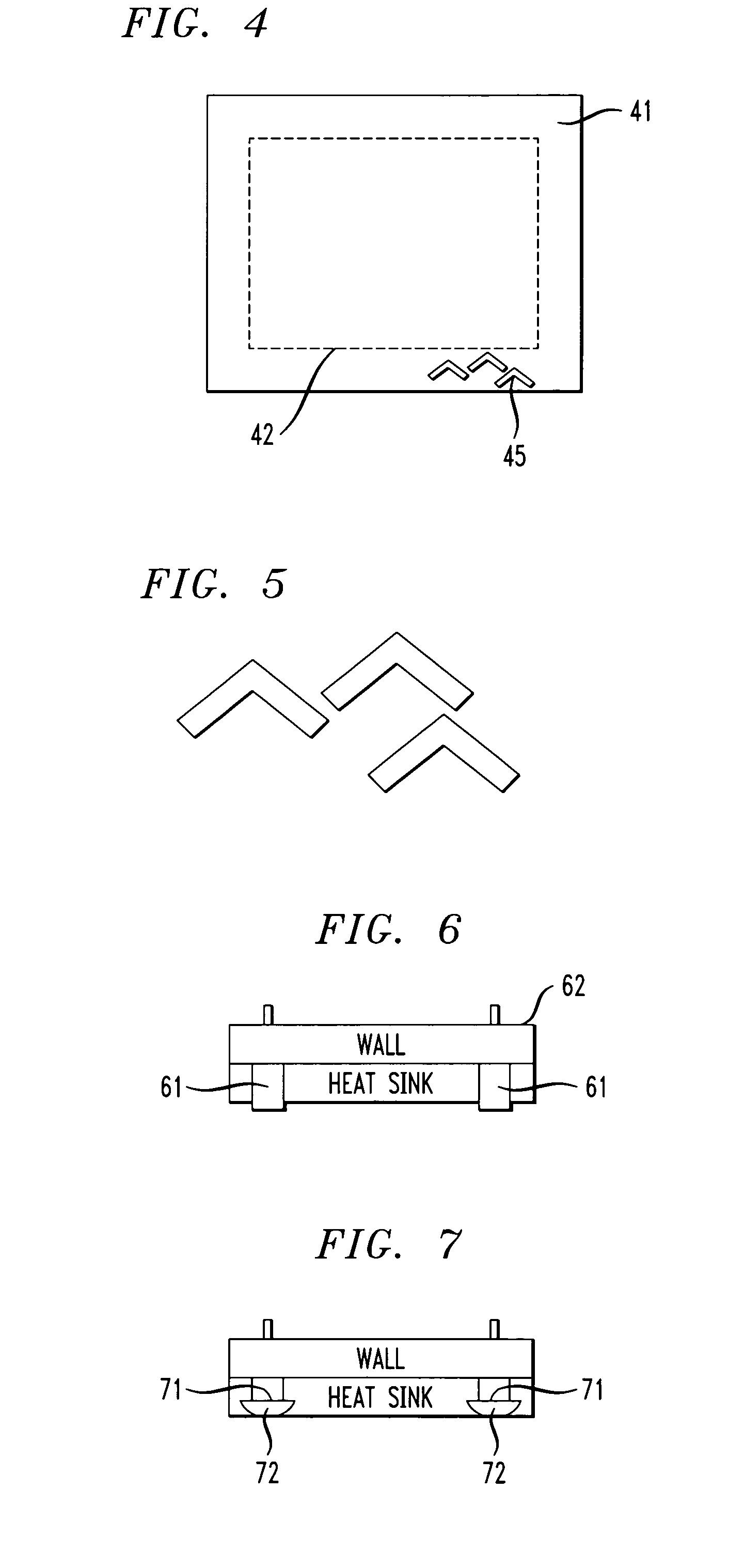
ActiveUS20060172465A1Undesirable interactionInhibiting undesirable interactionSemiconductor/solid-state device detailsSolid-state devicesFinal heightAudio power amplifier
Devices such as amplifiers are built on a heat sink having a perimeter wall surrounding active electronic devices. Surprisingly formation of wire bonds to such devices tends to be degraded if they have an aspect ratio greater than 2:1. This problem is overcome by forming wire bonds before such walls have a height of 30 mils and after bond formation extending the walls to their final height.
Owner:BELL SEMICON LLC
Amorphous inorganic ceramic material and method of producing same
InactiveUS20080057817A1Improve heat resistanceHigh strengthLayered productsWoven fabricsHydrogenHeat resistance
An amorphous inorganic ceramic material including silicon, carbon and oxygen is provided. In the ceramic material, the average elemental ratio between silicon, carbon and oxygen is represented by a compositional formula: SiCaOb wherein, a is a number that satisfies: 0.5≦a≦3.0, and b is a number that satisfies 0.5≦b≦4.0), the material has a siloxane skeleton formed of Si—O—Si bonds, and the hydrogen mass fraction is within a range from 0 to 1% by mass. The material exhibits excellent heat resistance, wettability with other materials, strength and elastic modulus, and is useful as a reinforcing material for composite materials, or as an exhaust gas filter material or the like.
Owner:SHIN ETSU CHEM IND CO LTD
Anticorrosion coatings with reactive polyelectrolyte complex system
InactiveUS20110244254A1Improve protectionEfficient use ofIon-exchanger regenerationAmphoteric ion-exchangersCationic polyelectrolytesIon release
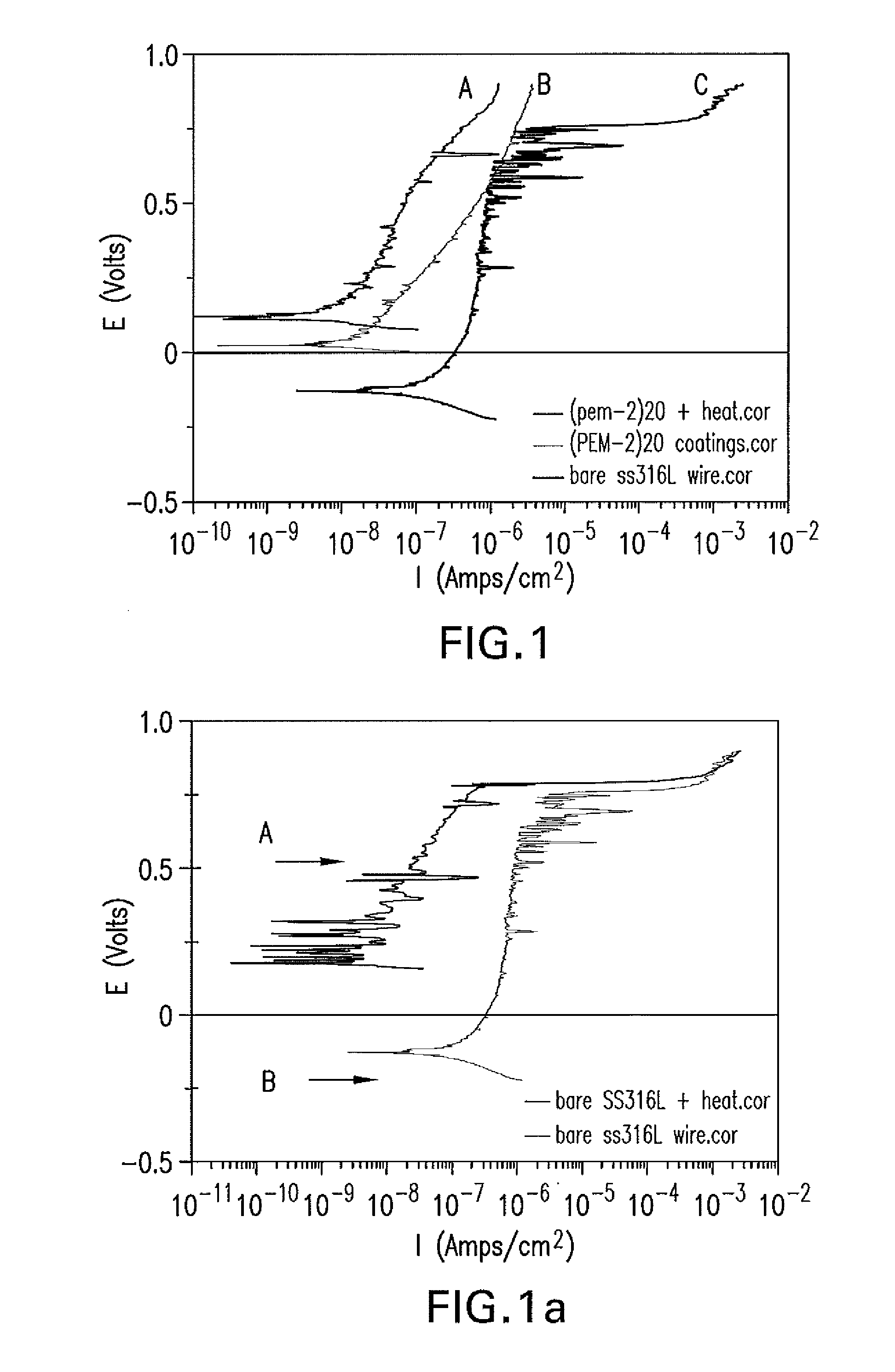
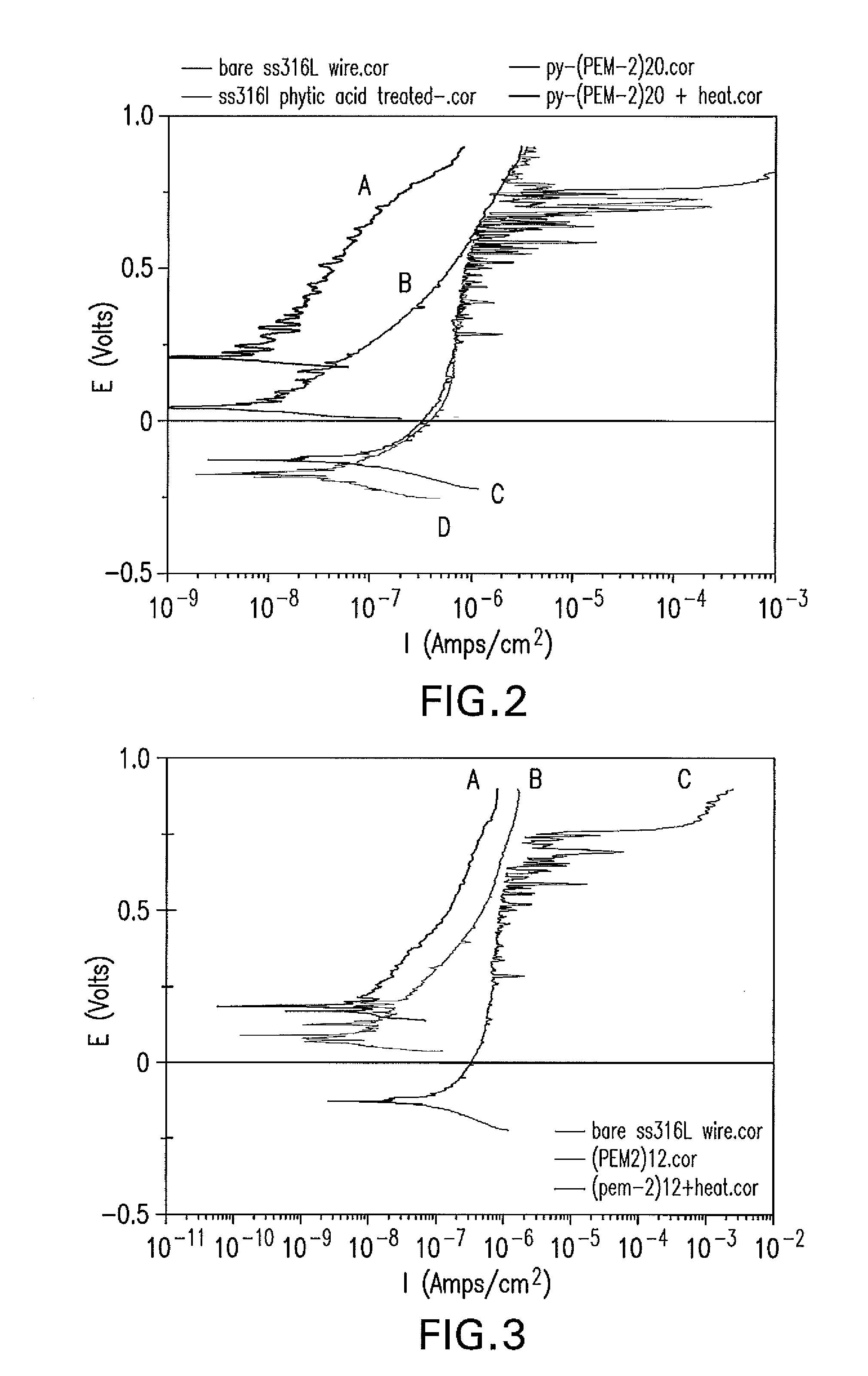
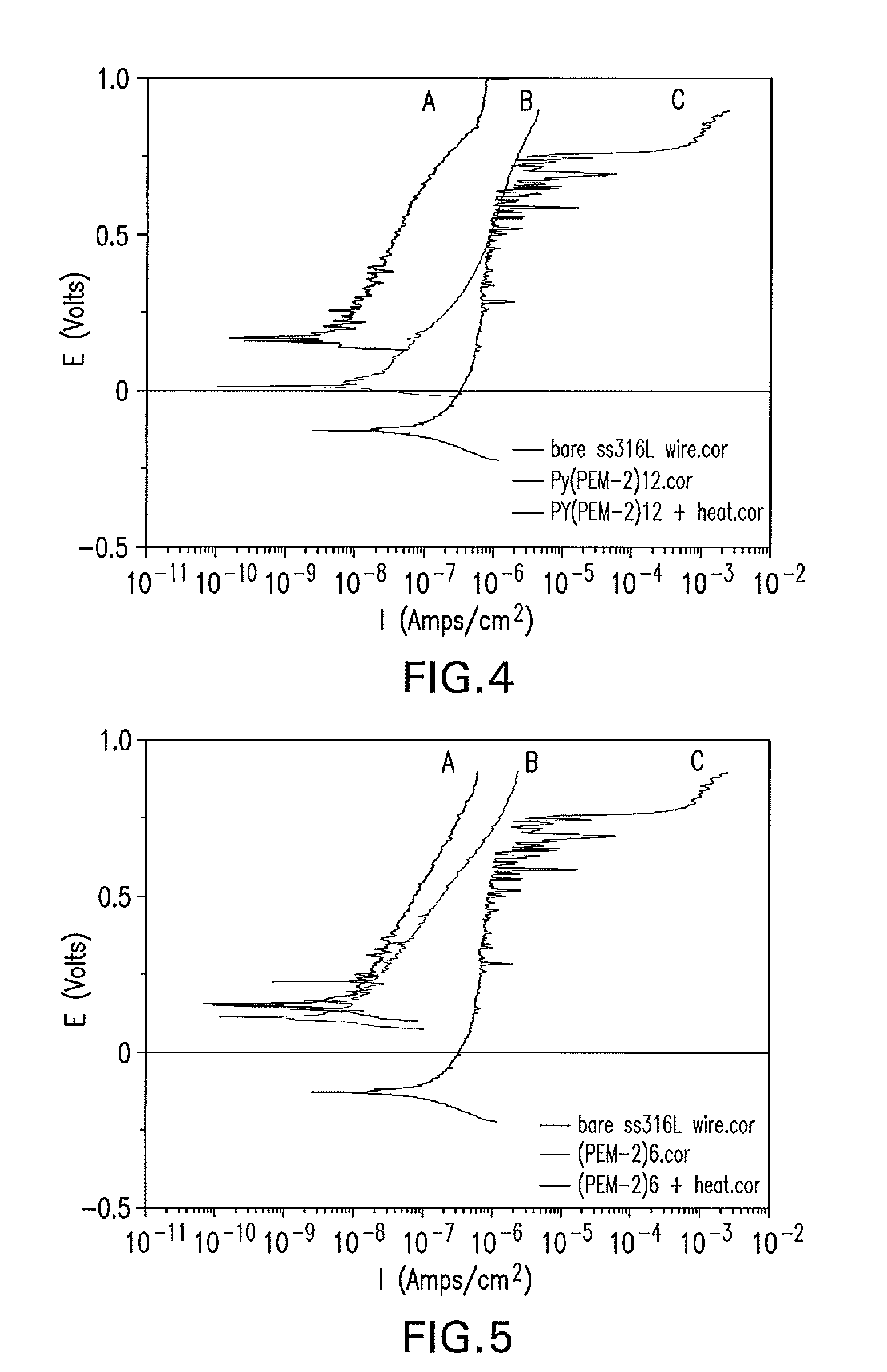
The present application is directed to anticorrosion coatings on metal substrates. In particular the coatings are especially suitable for metal containing medical devices and implants. The anticorrosion coatings comprise a combination of anionic and cationic polyelectrolytes which when applied to a metal substrate form a complex. In addition to cationic and anionic functionality, the polyelectrolytes also possess additional functionality which allows for further reacting to form covalent bonds between the anionic and cationic polyelectrolytes. The formed complex once applied to the metal substrate surface provides improved corrosion resistance, protection from metal ion release and improved mechanical properties.
Owner:SONG ZHIQIANG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com