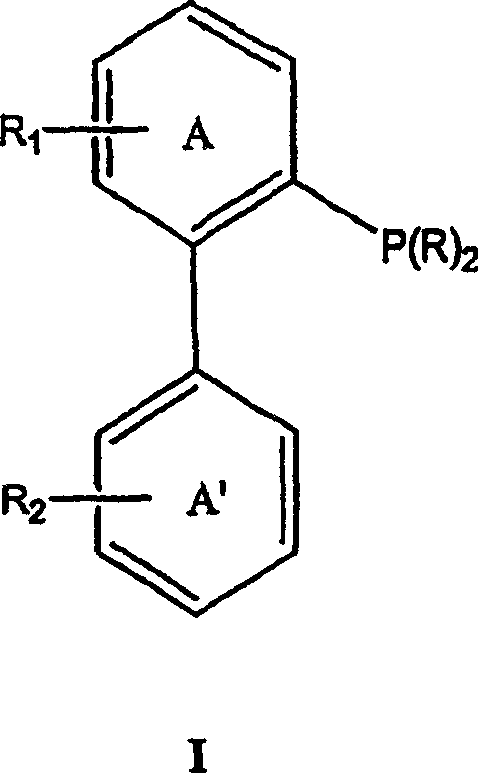
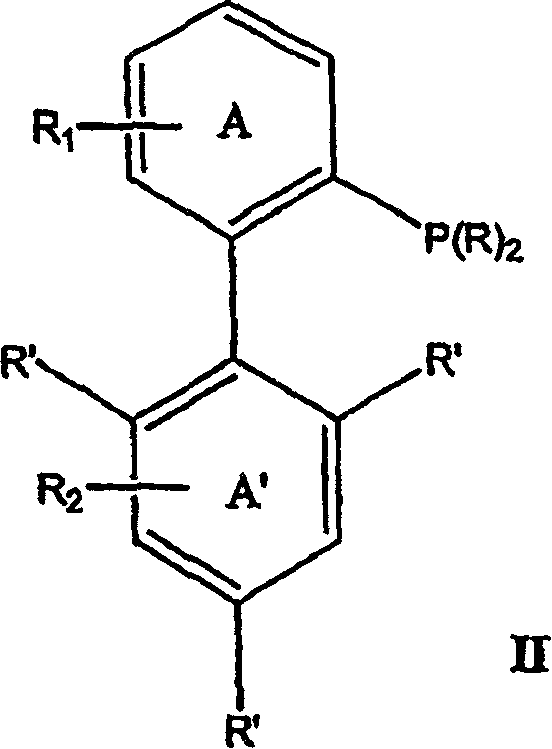
Ligands for metals and improved metal-catalyzed processes based thereon
A ligand and alkyl technology, applied in chemical instruments and methods, preparation of carbon-based compounds, compounds of Group 5/15 elements of the periodic table, etc., can solve problems such as high temperature, low product yield, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0655] Highly active catalysts for palladium-catalyzed cross-coupling reactions: Suzuki coupling and amination of inactive aryl chlorides at room temperature
[0656] A highly active palladium catalyst has been developed using the chelating aminophosphine ligand 1-(N,N-dimethylamino)-1'-(dicyclohexylphosphino)biphenyl (2). The catalyst is effective for the cross-coupling of aryl chlorides with amines, boronic acids and keto enolates. The ample reactivity of this system is suitable for room-temperature amination of aryl bromides and electron-deficient aryl chlorides, as well as facilitating room-temperature Suzuki coupling reactions of electron-rich and electron-deficient aryl chlorides. The coordination of the amine moiety is the key to enhanced reactivity and catalyst stability of the system.
[0657] Palladium-catalyzed C–N bond-forming reactions have been implicated in versatile and efficient synthetic transformations. The use of palladium catalysts supported by bidentate...
Embodiment 2
[0813] Synthesis of N-(2,5-dimethylphenyl)-N-methylaniline.
[0814]
[0815] The dried test tube was purged with argon and filled with Pd 2 (dba) 3 (4.6 mg, 0.005 mmol, 1.0 mol% Pd), Ligand 2 [Example 1] (6.0 mg, 0.015 mmol, 1.5 mol%) and NaOt-Bu (135 mg, 1.40 mmol). The test tube was fitted with a septum and toluene (2.0 mL), N-methylaniline (135 μL, 1.25 mmol) and 2-chloro-p-xylene (135 μL, 1.01 mmol) were added. The mixture was stirred at 80 °C for 13 hours, then cooled to room temperature, diluted with diethyl ether (20 mL), filtered and concentrated. The crude was purified by flash chromatography on silica gel to afford 202 mg (95%) of a colorless oil.
Embodiment 3
[0817] Synthesis of Di-n-Butyl-p-Toluidine
[0818]
[0819] The oven-dried resealable Schlenk tubes were purged with argon and loaded with Pd 2 (dba) 3 (2.3mg, 0.0025mmol, 0.05mol% Pd), Ligand 2 [Example 1] (2.9mg, 0.0075mmol, 0.075mol%) and NaOt-Bu (1.34g, 13.9mmol). Toluene (10 mL), di-n-butylamine (2.00 mL, 11.9 mmol) and 4-chlorotoluene (1.18 mL, 10.0 mmol) were added and the mixture was degassed with three freeze-pump-thaw cycles. The reaction vessel was placed under an argon atmosphere, sealed with a Teflon screw cap, and stirred at 100°C for 20 hours, after which time GC analysis showed complete consumption of the aryl halide. The reaction mixture was cooled to room temperature, diluted with ether (100 mL) and extracted with 1M HCl (3 x 100 mL). The combined aqueous acid phases were basified with 3M NaOH, then extracted with diethyl ether (3 x 150 mL). The ether extracts were dried over anhydrous sodium sulfate, filtered and concentrated to give 2.01 g (95%) of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com