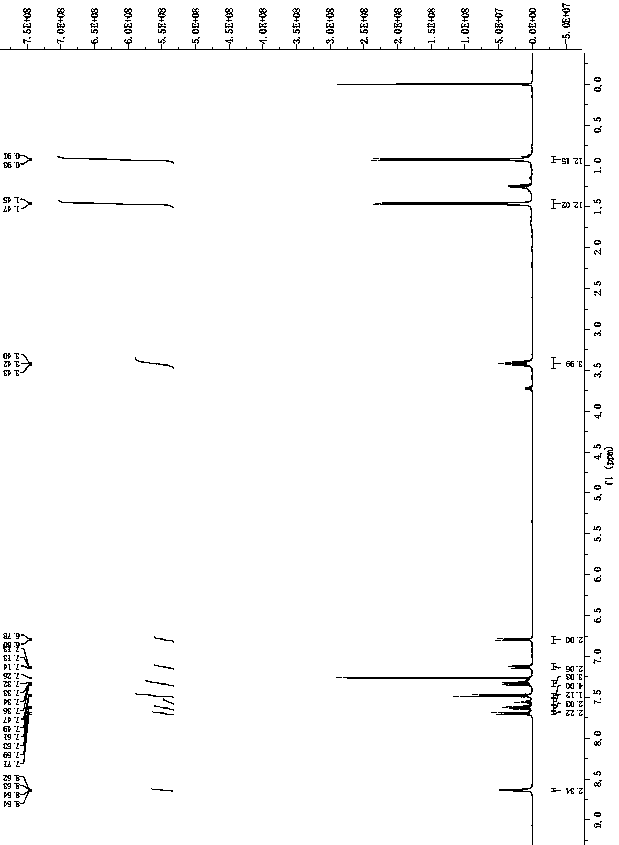
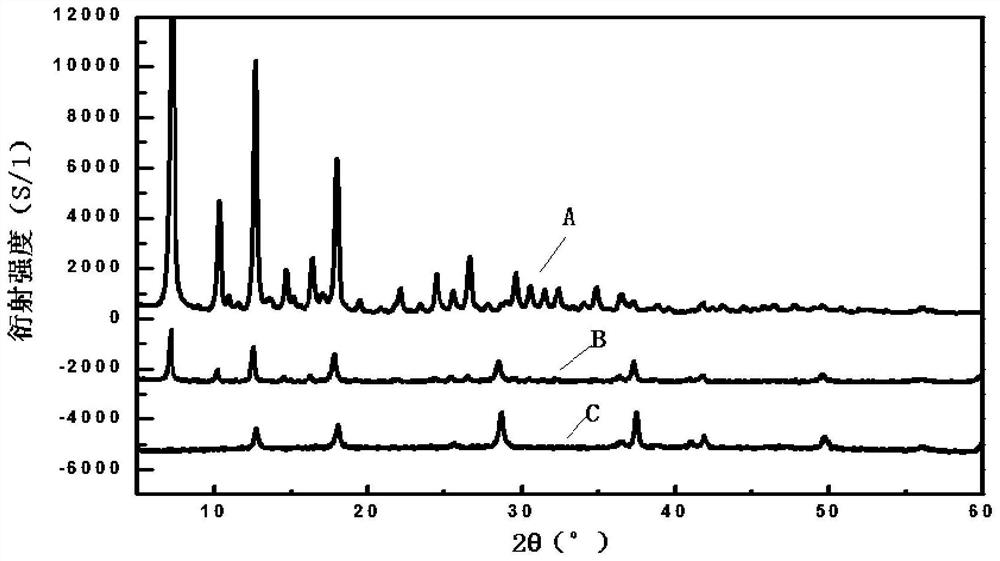
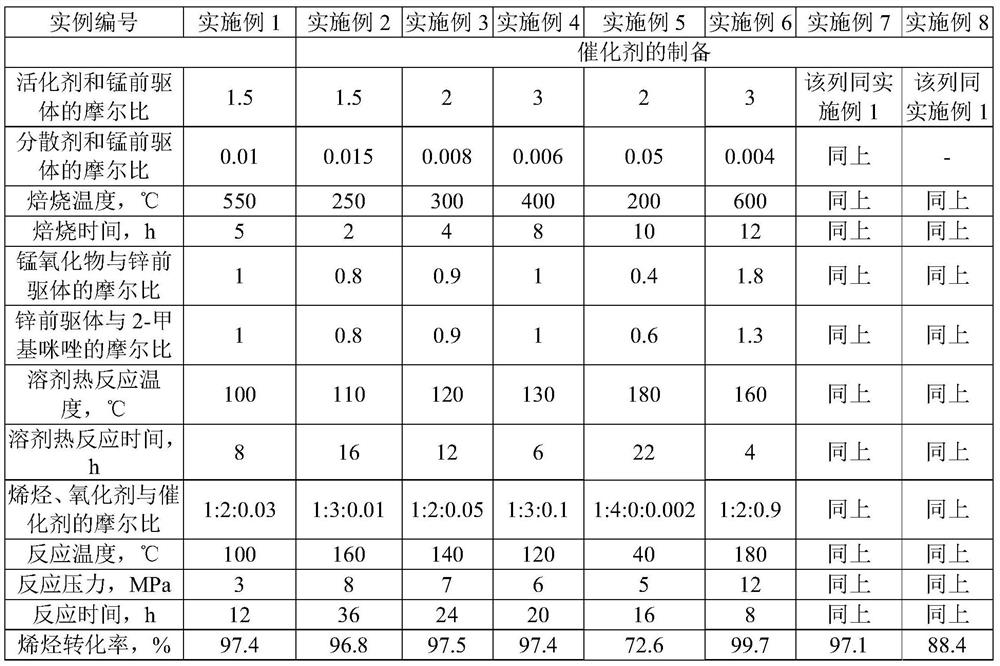
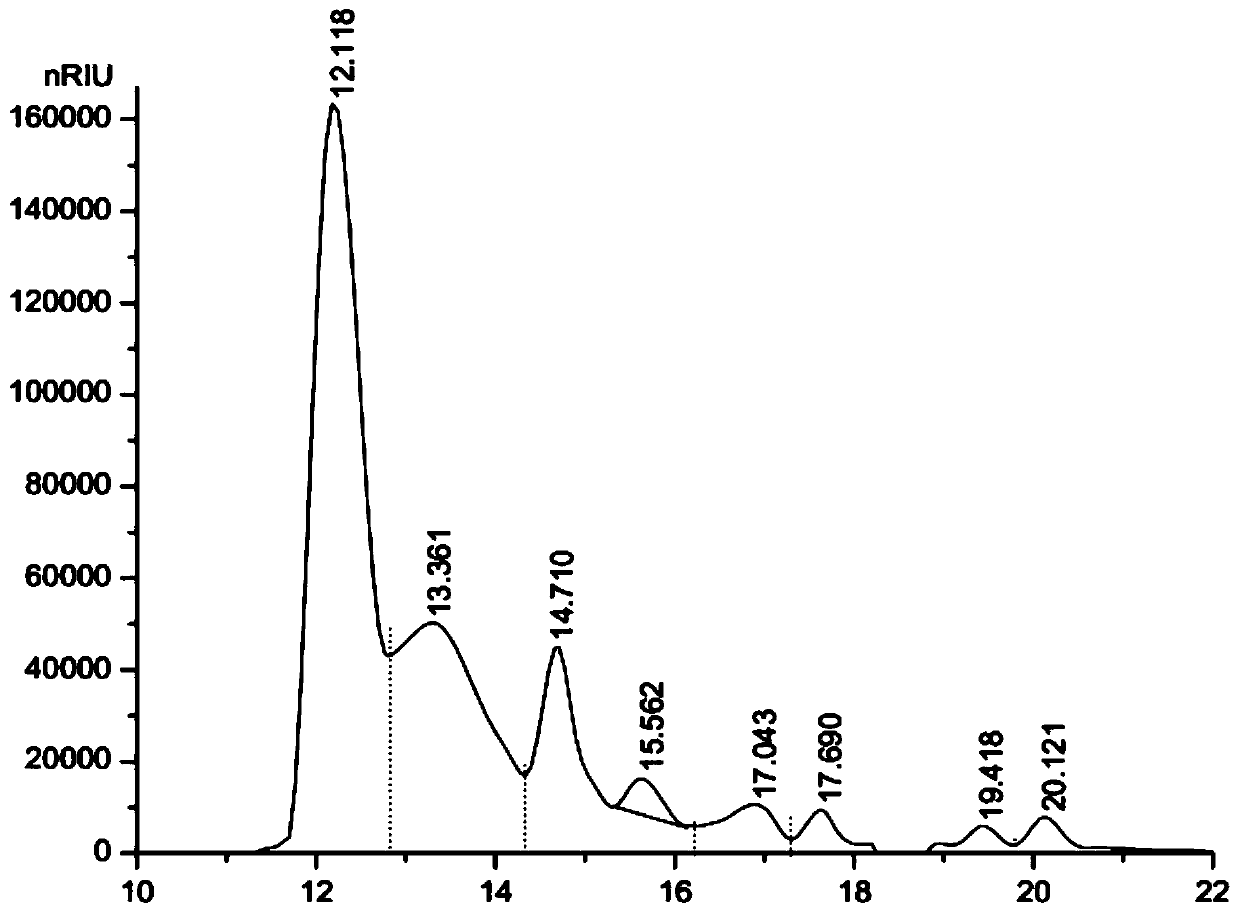
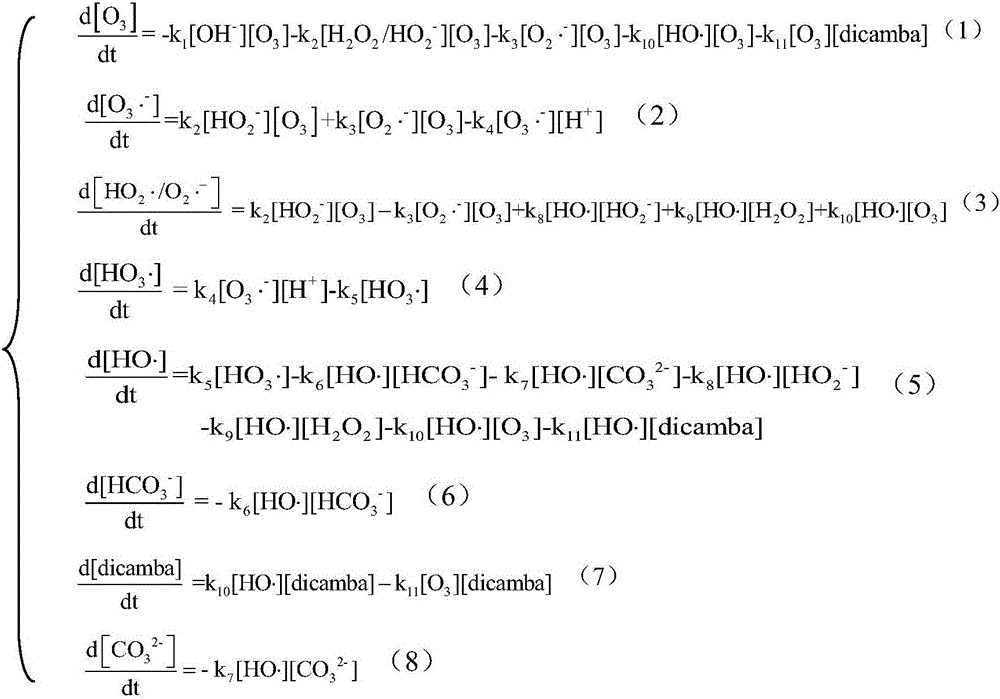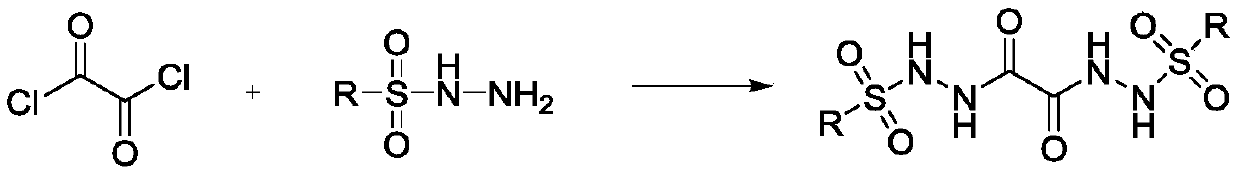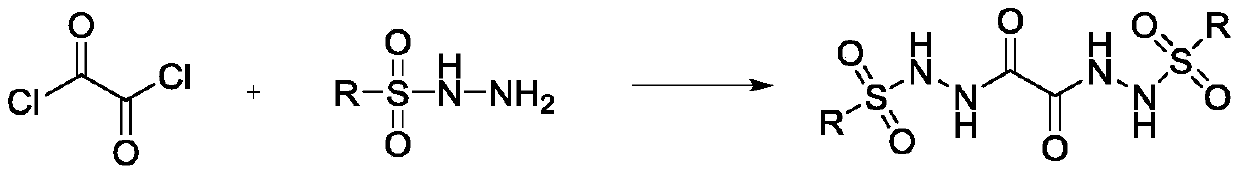Patents
Literature
44 results about "Oxidative addition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination.
Oxidative reactions using membranes that selectively conduct oxygen
InactiveUS6730808B2Eliminate the problemNot possibleAlkali metal oxides/hydroxidesFunctional group formation/introductionLanthanideOxygen
Reactor membranes for used in oxidation reactions of hydrocarbons involving oxygen comprising a selective oxidation catalyst on a mixed conducting, oxide ion selective ceramic membrane of the composition (Sr1-xCax)1-yAyMn1-zBzO3-delta, whereA is Ba, Pb, Na, K, Y, an element of the lanthanide group or a combination thereof,B is Mg, Al, Ga, In, Sn, an element of the 3d or 4d period or a combination thereof,x is from 0.2 to 0.8,y is from 0 to 0.4,z is from 0 to 0.6, anddelta is a number, dependent on x, y and z, that renders the composition charge neutral.
Owner:BASF AG
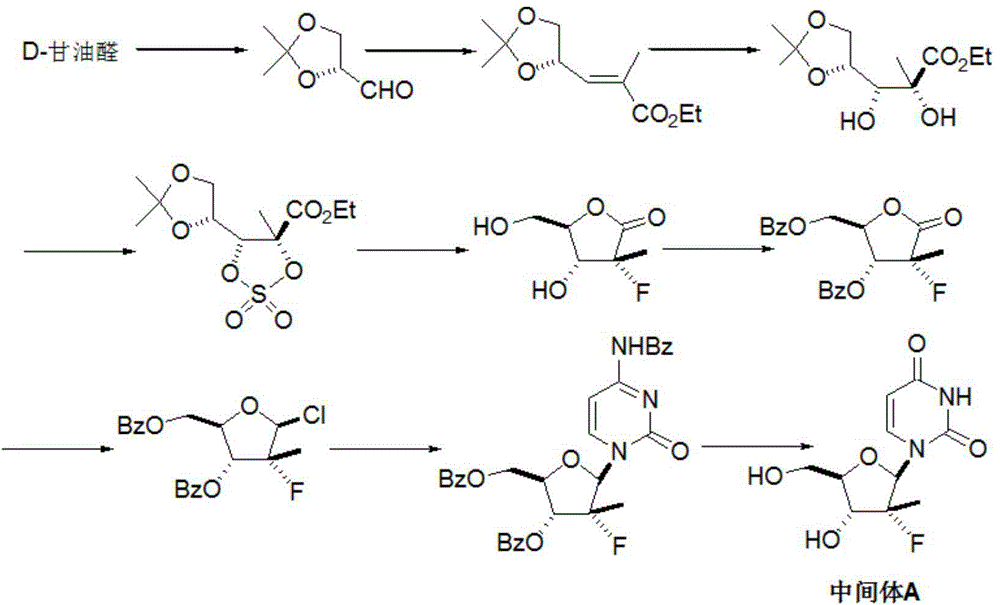
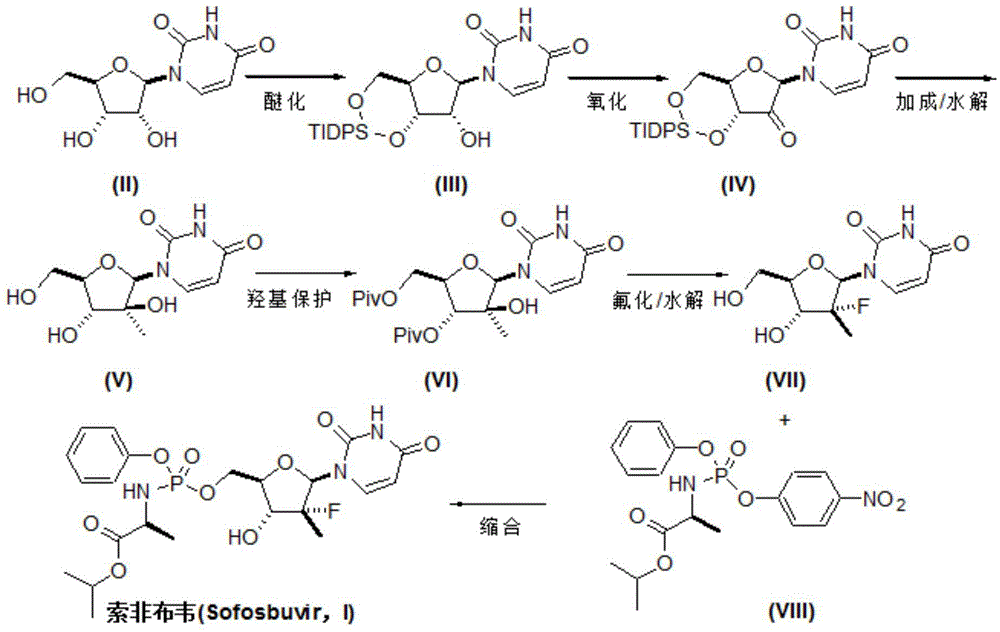
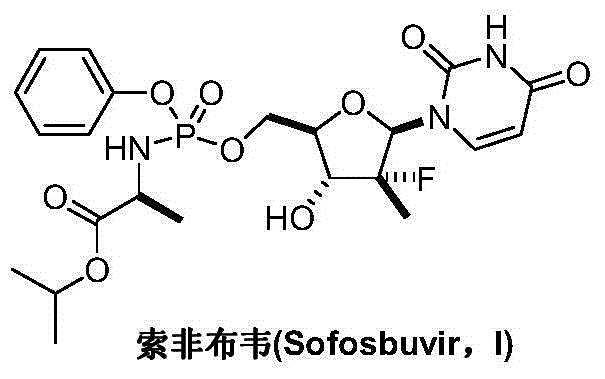
Preparation method for sofosbuvir
InactiveCN104478976AEase of industrial productionRaw materials are easy to getSugar derivativesSugar derivatives preparationUridine NucleotidesSofosbuvir
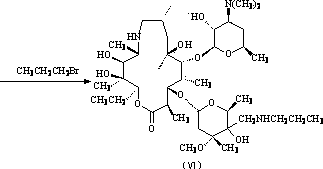
The invention discloses a preparation method for preparing sofosbuvir (Sofosbuvir,I) with uridine as a raw material and through etherification, oxidation, addition, condensation and other steps; the preparation method has the advantages of easily obtained raw materials, concise process, economy, and environmental protection, and is suitable for industrialized production.
Owner:SUZHOU MIRACPHARMA TECH
A synthetic method of high-purity tulathromycin
InactiveCN103864865ASugar derivativesSugar derivatives preparationAzithromycinBiochemical engineering
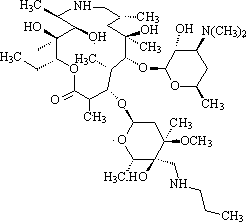
The invention provides a synthetic method of high-purity tulathromycin. The method includes subjecting demethylated azithromycin adopted as a raw material to substitution, oxidation, addition, reduction, condensation, and the like, so as to obtain a target compound. According to the synthetic method, the cheaper raw material is adopted, and a traditional process employing low-temperature reaction is changed. The method has the advantages of short process route, easy-to-control reaction, high yield, and the like. The method is suitable for large-scale industrialized production.
Owner:QINGDAO VLAND BIOTECH INC
Preparation method of high-yield and low-cost peptizer DBD
ActiveCN105061272ALow costThe path is simple and convenientHydropoly/poly sulfide preparationBenzoyl chlorideAlkaline hydrolysis
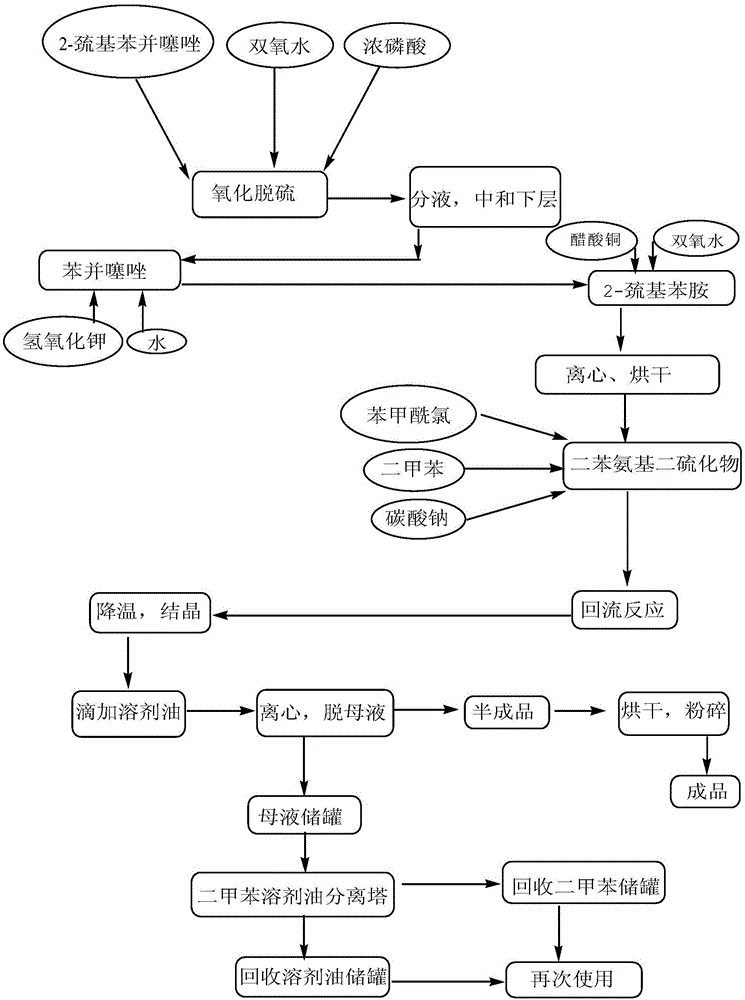
The invention relates to a preparation method of a high-yield and low-cost peptizer DBD. The method comprises steps as follows: (1) desulfurizing 2-mercaptobenzothiazole to obtain benzothiazole; (2) performing alkaline hydrolysis and decarbonization on the benzothiazole to obtain 2-aminothiophnol; (3) oxidizing the 2-aminothiophnol to produce diphenyl amino disulfide; (4) performing acylation on the diphenyl amino disulfide and benzoyl chloride to produce the DBD. According to the method, the 2-mercaptobenzothiazole is used as a raw material, so that the production cost is greatly reduced; the path for synthesizing the 2-aminothiophnol is simple and convenient, workshop operation is easy, and the yield is high.
Owner:SHANDONG YANGGU HUATAI CHEM
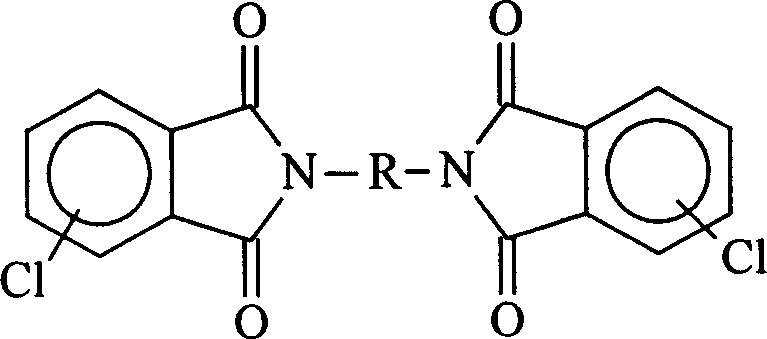
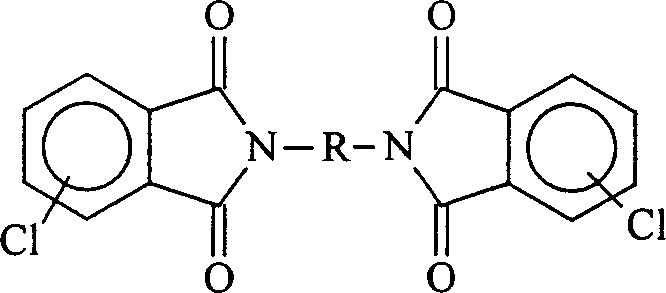
Process for preparing biphenyl polyimide
A process for preparing biphenyl polyimide includes such steps as dissolving bichlorodiphthalimide monomer, triphenylphosphino nickel as catalyst and zinc powder as reducer in non-proton solvent, reaction at 60-120 deg.C to generate Ni mating substance, oxydizing addition reaction on bichlorodiphthalimide to generate intermediate, and dereducing reaction to generate its condensate and zinc chloride while reducing Ni to react on chloroimide to generate polyimide. Its advantages are simple process and low cost.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
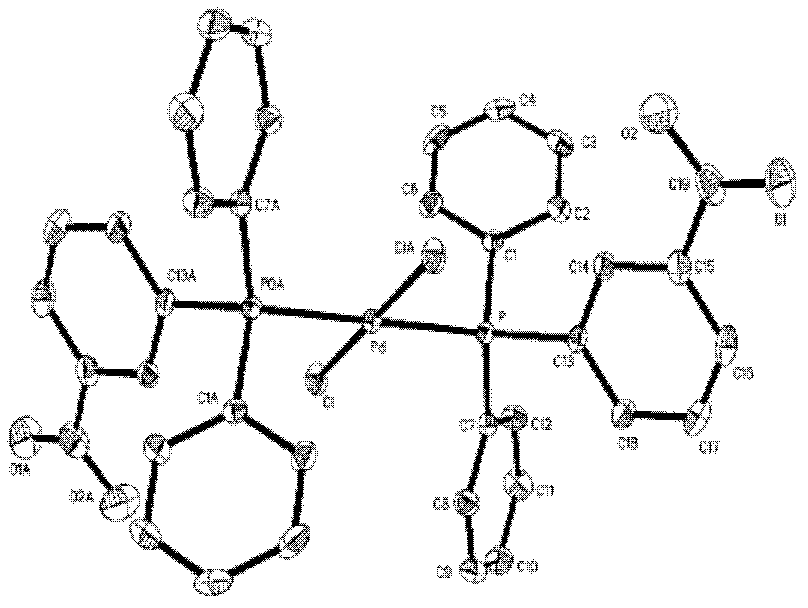
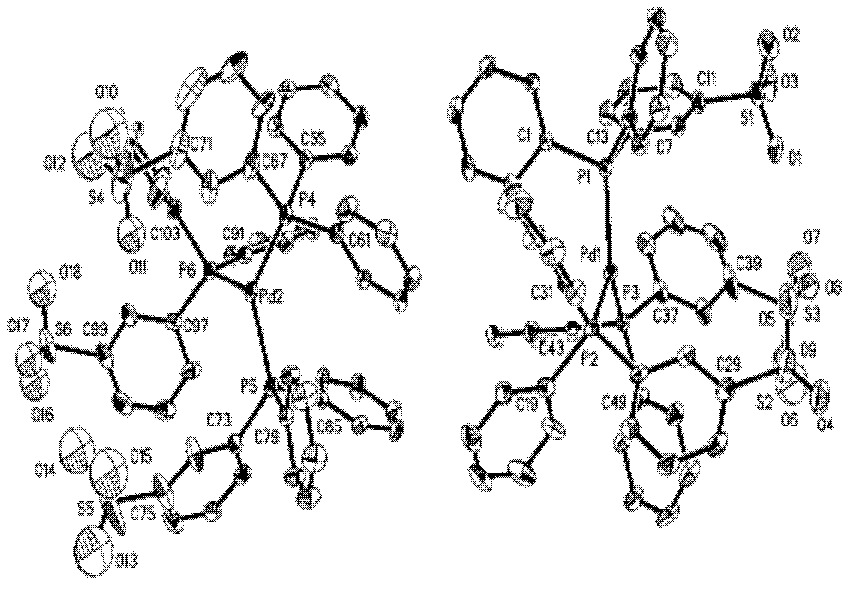
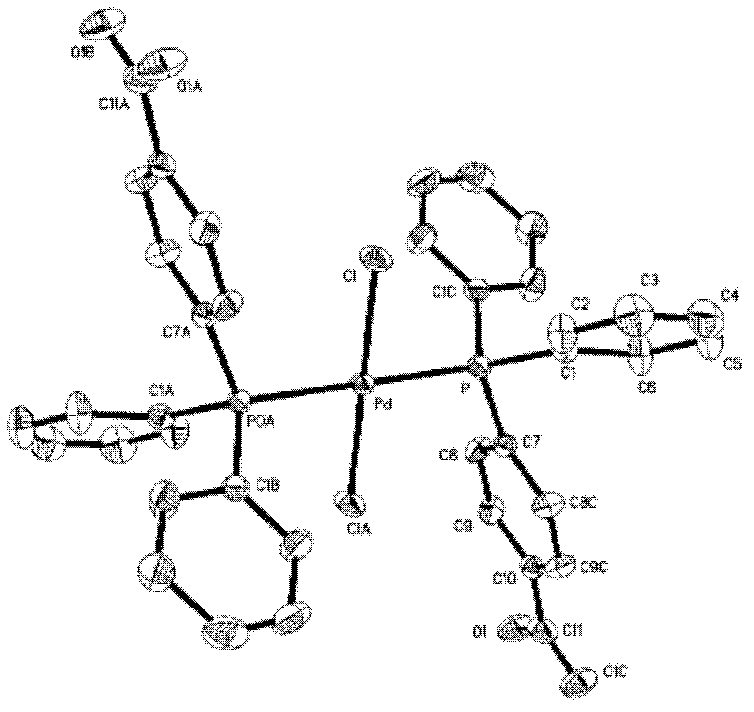
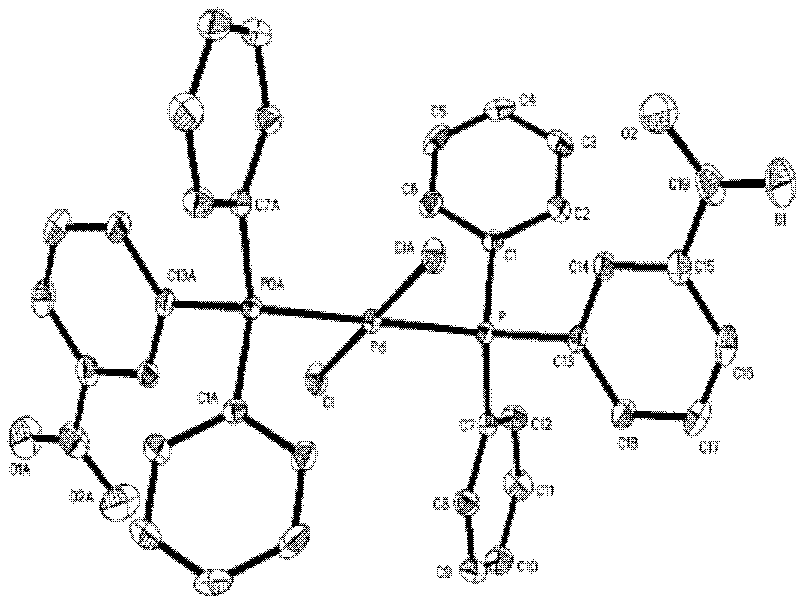
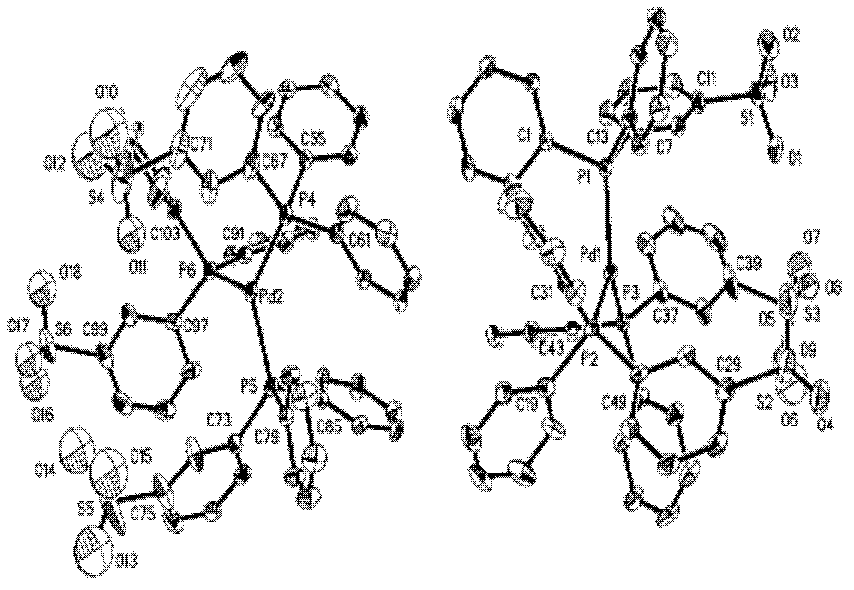
Preparation method of palladium complex and conjugated aromatic polymer
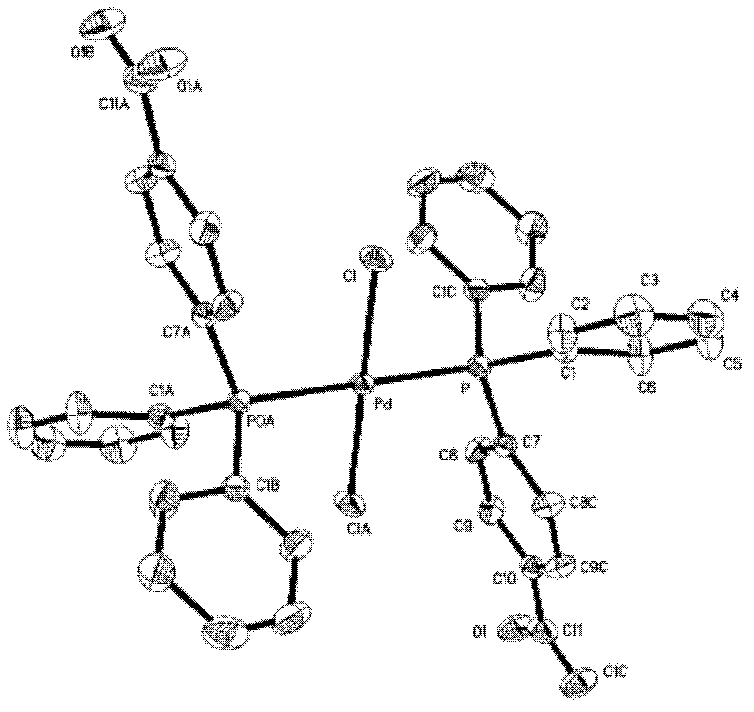
The invention provides a palladium complex of general formula (I) or general formula (II), wherein, X represents halogen, L represents phosphorus ligand or nitrogenous ligand, G represents a sulfonic group, a carboxylic group, a phosphonate group, a hydroxy group or a polyether chain, G is positioned at para-position or meta-position of L, n represents a positive integer, and m represents 2, 3 or 4. The invention further provides a preparation method of the palladium complex, and a method for preparing a conjugated aromatic polymer by using a palladium complex catalyst. According to the invention, because a neutral hydrophilic substituent group is introduced to the palladium complex catalyst ligand, the palladium complex catalyst is amphiphilic and can simultaneously dissolved in water phase and organic phase; the catalyst undergoes oxidative addition with a polymer monomer easily, and can promote the transmetallation step to let the coupling polymerization reaction carry out continuously and rapidly, thus the conjugated aromatic polymer having high molecular weight is obtained. The conjugated aromatic polymer is represented as PdX2(L-Gn)2 (I) or Pd(L-Gn)m (II).
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
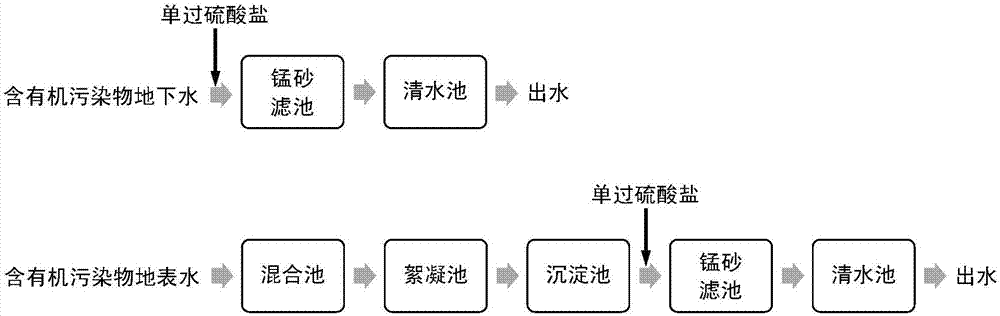
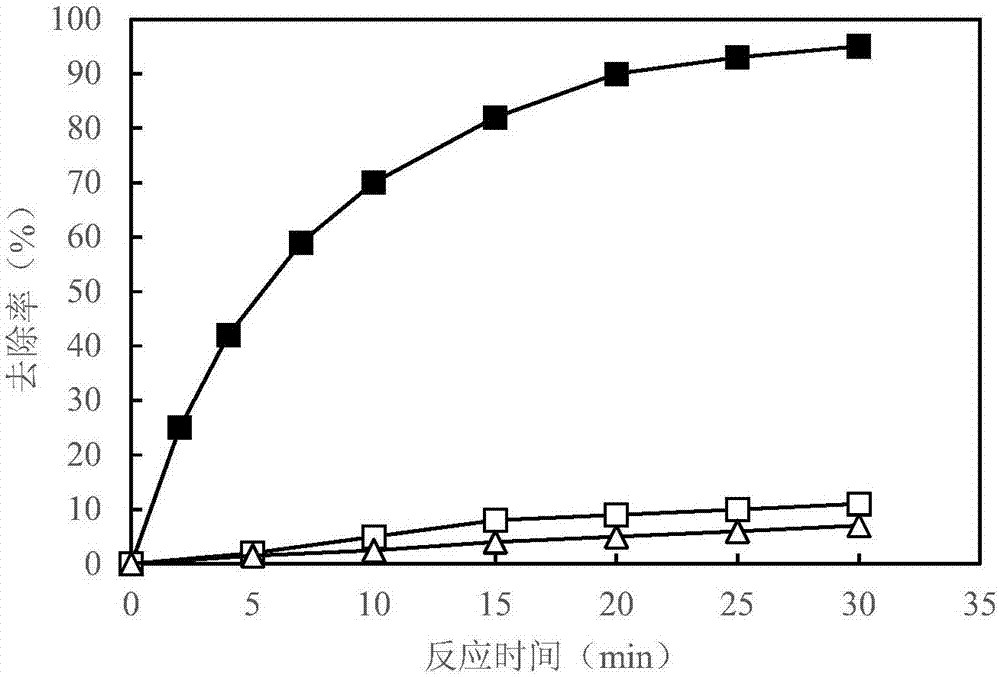
Water treatment method for oxidative degradation of organic pollutants by catalyzing monopersulfate through manganese sand
InactiveCN108002511AChemically stableEasy to transportSpecific water treatment objectivesWater contaminantsSulfate radicalsPersulfate
The invention discloses a water treatment method for oxidative degradation of organic pollutants by catalyzing monopersulfate through manganese sand, and relates to a water treatment method which solves the problems that an existing pre-oxidizing agent is poor in oxidizing ability, poisonous and harmful byproducts are liable to generate, the existing pre-oxidizing agent is inconvenient to use andoperate, and operation investment cost is high. The water treatment method comprises the following steps: adding the monopersulfate into to-be-treated water, introducing water into a filter bed with manganese sand to filter, catalyzing the monopersulfate by manganese sand to generate a sulfate radical free group to oxidize and degrade organic pollutants in water, thereby completing oxidative removal on organic substances. The monopersulfate is stable in chemical property, is convenient to transport and store, and is normally listed as a drinking water disinfector product summary in China; andthe water treatment method is simple to operate, does not need to additionally arrange equipment, does not change the original treatment process of the water feeding plant, can be applied on a large scale; and the monopersulfate is catalyzed by the manganese sand, so that the organic substance removal efficiency is high; and moreover, residual monopersulfate further can be used for sterilizing filtered water as a disinfector, so that poisonous and harmful byproducts are not generated.
Owner:JILIN JIANZHU UNIVERSITY
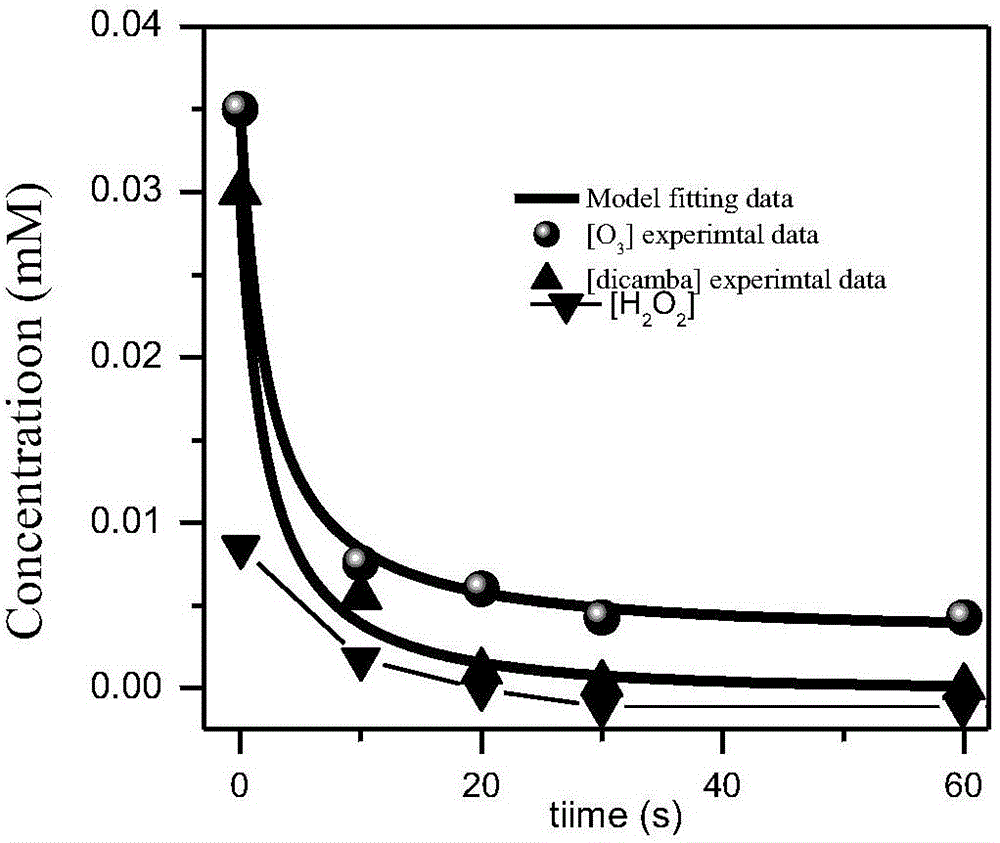
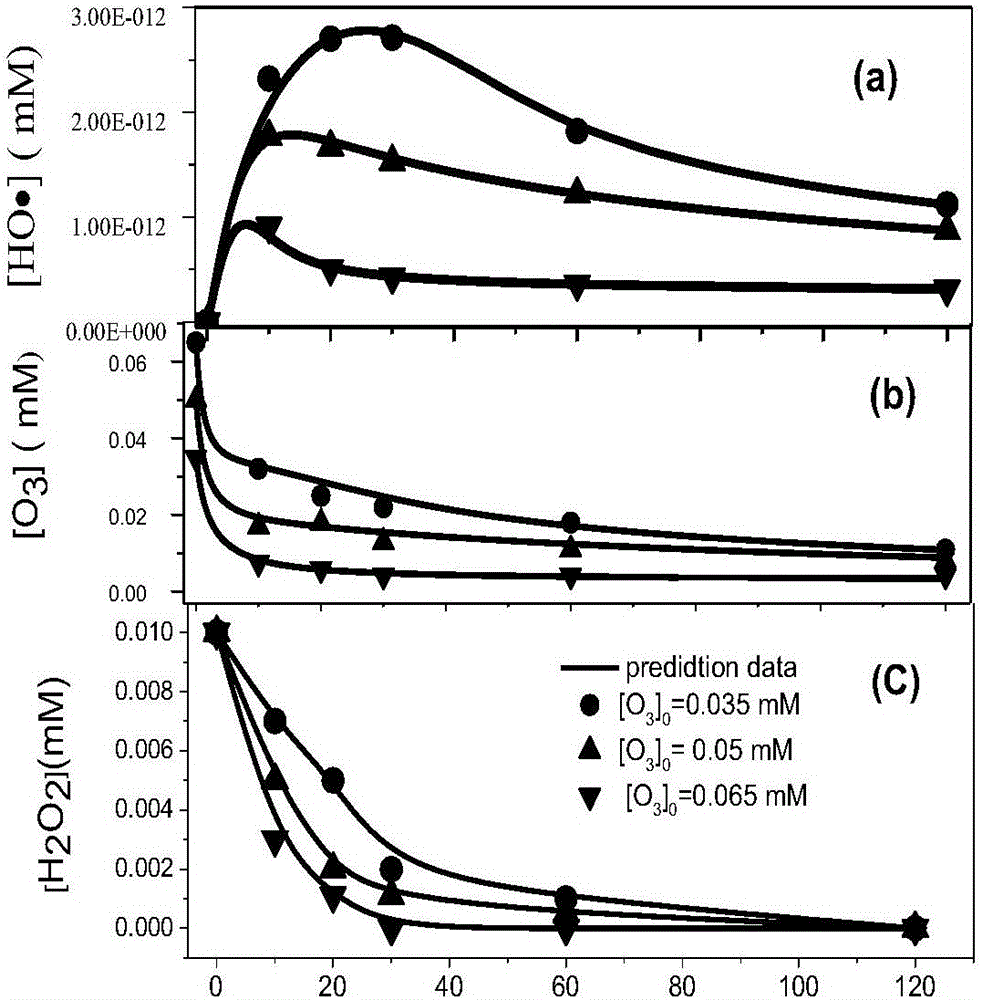
Method for predicting oxidative degradation for removing dicamba in organic wastewaterofozone/hydrogen peroxide
ActiveCN106202899ALow costComputational theoretical chemistrySpecial data processing applicationsRoom temperatureWastewater
The invention discloses a methodfor predicting oxidative degradation for removing dicamba in organic wastewaterofozone / hydrogen peroxide.The method includes the following steps that step 1, adynamical modelfor oxidative degradation is established, and the dynamical model is established according to free radicalchain reaction mechanism of ozone and hydrogen peroxide and dicamba containing organic wastewater; step 2, the efficiency of degrading the dicamba in the organic wastewater of the ozone / hydrogen peroxide is predicted according to the model in the step 1; step 3, degradation is executed according to results in the steps 1 and 2, and the degradation is achieved by mixing the ozone and hydrogen peroxide and the dicamba containing organic wastewater and performing degradation reaction at room temperature, wherein the ozone concentration in the mixed liquid is 0.001-1 mM, and the hydrogen peroxide concentration is 0.001-1 mM. The method can simulate the dicamba removal situation by establishing the model, and the cost is saved.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of deuterated benzene compound
PendingCN113979822AThe synthesis method is simpleEasy to getCarbonyl compound separation/purificationBulk chemical productionDeuterated benzenePtru catalyst
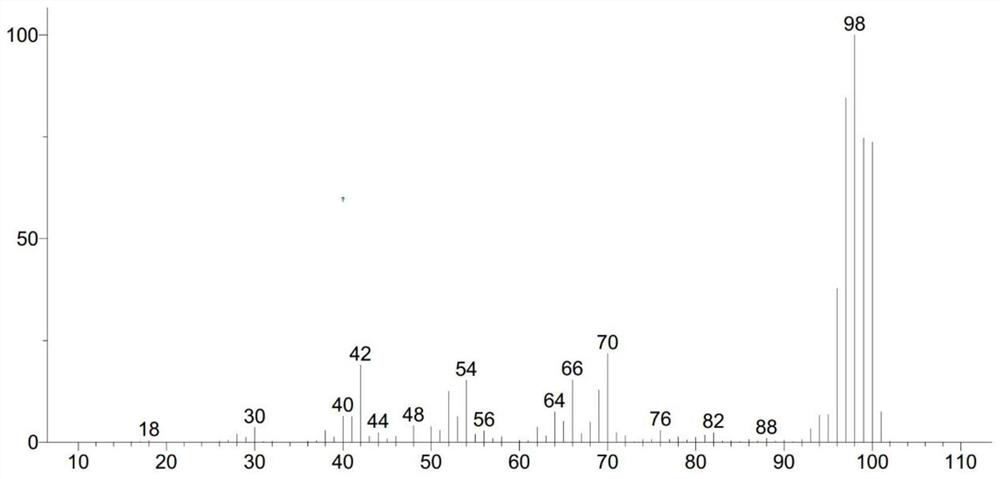
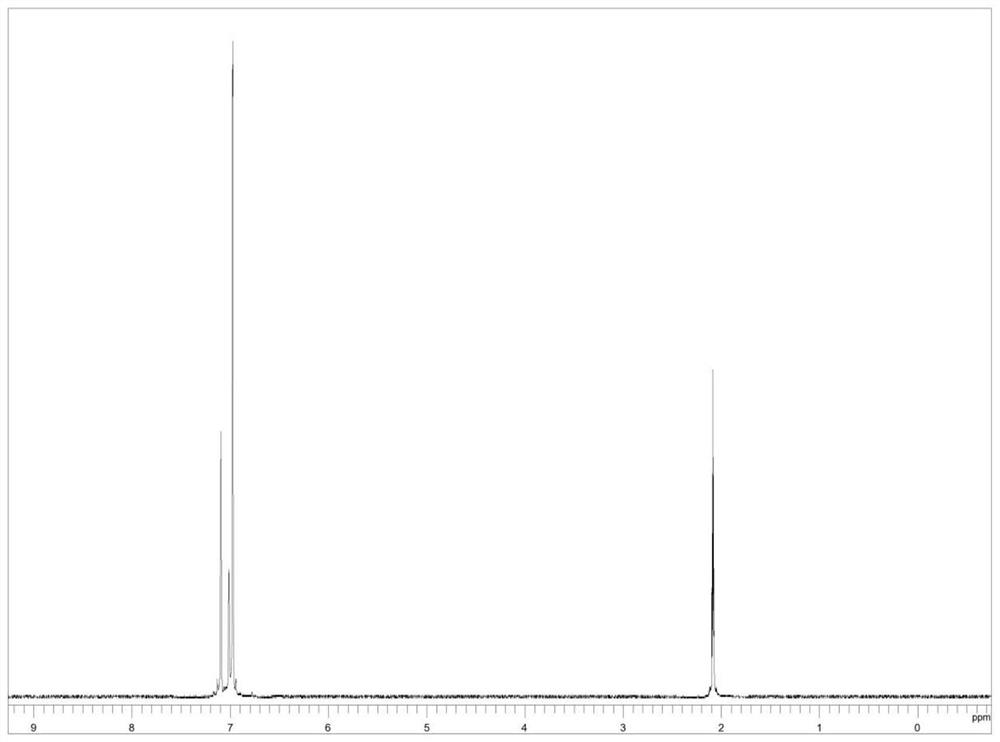
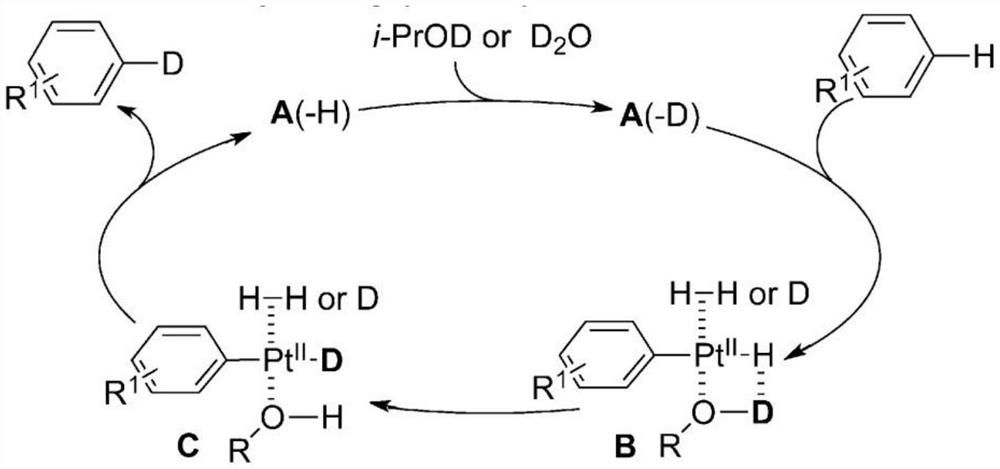
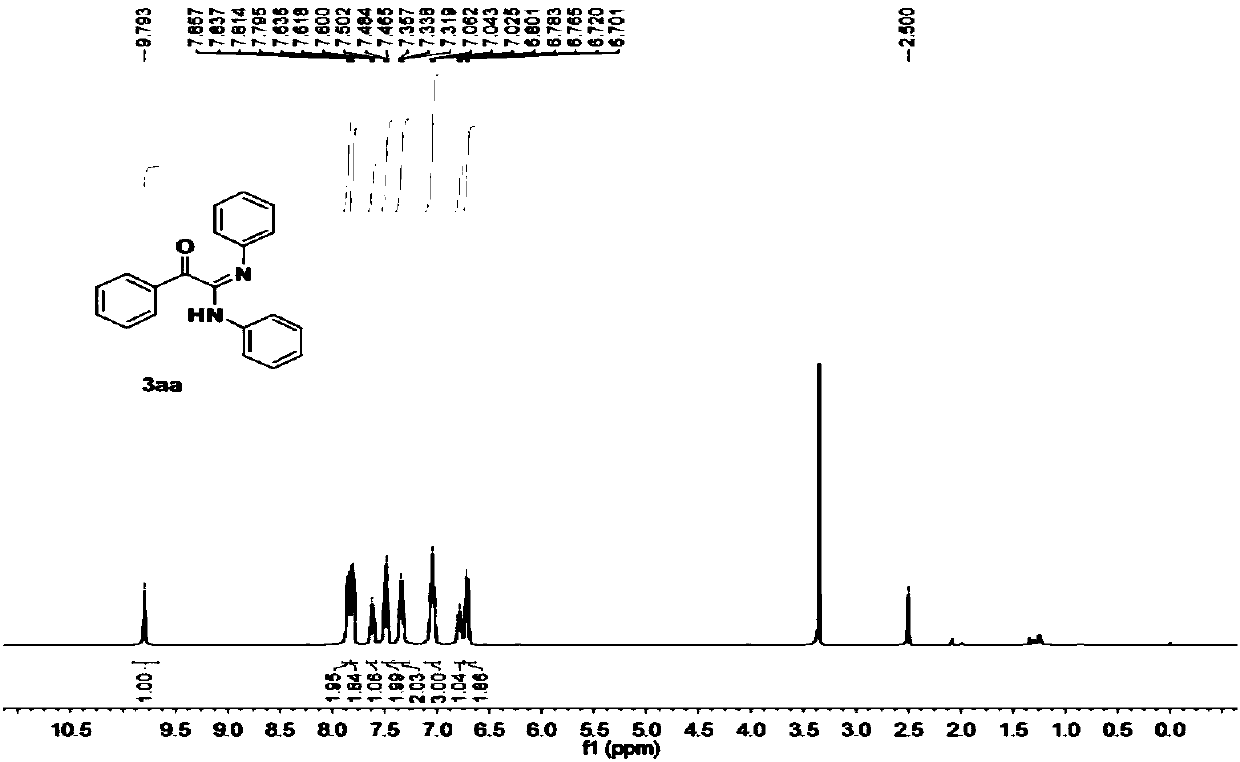
The invention relates to the field of organic chemistry, and discloses a preparation method of a deuterated benzene compound, in the method, active carbon supported noble metal is mainly used as a catalyst, deuterium water is used as a deuterium source, and the deuterated benzene compound is prepared from a benzene compound. Wherein in a reaction system, isopropanol is activated on the surface of a catalyst, trace active hydrogen is generated in situ, oxidative addition and reduction elimination reactions are carried out, and finally deuterium atoms in deuterium water and hydrogen atoms in a benzene ring compound are exchanged to generate the deuterated benzene compound. Compared with a traditional deuteration reaction, the method has the following advantages that conditions are mild, reaction raw materials are easy to obtain, and the deuteration reaction of benzene substances can be completed under the hydrogen-free condition; and in the reaction process, deuterium gas is prevented from being used as a deuterium source, so that the occurrence of excessive deuterium addition reaction and the safety problem are avoided, and the producing cost is reduced. And the reaction product is clean and has high yield.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing amidine compounds through oxidative amidation of aryl methyl ketone under catalysis of copper (II)
The invention discloses a method for synthesizing amidine compounds through oxidative amidation of aryl methyl ketone under the catalysis of copper (II). The method comprises the following steps: in an oxygen-containing atmosphere and in an organic carboxylate / DMSO mixed system, performing a reaction on the aryl methyl ketone and aryl primary amine under the catalysis of divalent copper salt, or performing a reaction on the aryl methyl ketone and the aryl primary amine and secondary amine under the catalysis of the divalent copper salt to obtain the amidine compounds. By the method, the aryl methyl ketone and amine compounds are used as raw materials for producing the amidine compounds through the one-step reaction; the method has the characteristics of a simple step, low cost and the like, and is conducive to industrial production.
Owner:YUANJIANG HUALONG CATALYST TECH
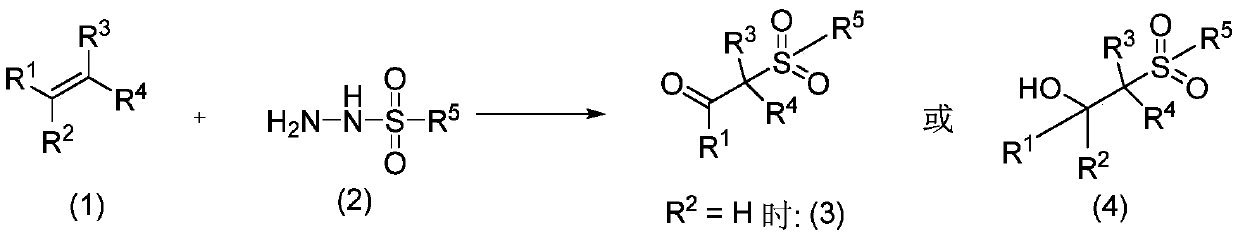
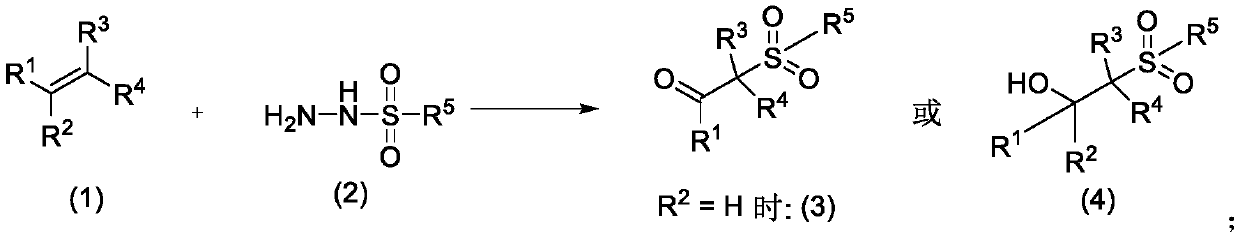
Preparation method of oxalyl sulfonyl hydrazide and application of oxalyl sulfonyl hydrazide in olefin sulfonation reaction
ActiveCN109748829ASave raw materialsSimple processOrganic compound preparationSulfonic acid amide preparationChemical synthesisOxygen
The invention belongs to the technical field of organic chemical synthesis and particularly relates to a preparation method of oxalyl sulfonyl hydrazide and application of the oxalyl sulfonyl hydrazide in olefin sulfonation reaction. The preparation method allows aryl sulfonyl hydrazide to have reaction with oxalyl chloride in the presence of alkali to prepare the oxalyl sulfonyl hydrazide. The invention further provides a novel method using the oxalyl sulfonyl hydrazide to have reaction with substituted olefin to prepare beta-keto-sulfone or beta-hydroxyl sulfone. The novel method uses the substituted olefin and the oxalyl sulfonyl hydrazide as the raw materials and oxygen in air as the oxidizing agent to perform oxidative addition reaction in the presence of a catalyst to generate the beta-keto-sulfone or beta-hydroxyl sulfone.
Owner:SHANGHAI MAIPU NEW MATERIAL TECH CO LTD
O-aminoalcohol compounds, preparation method thereof and application thereof
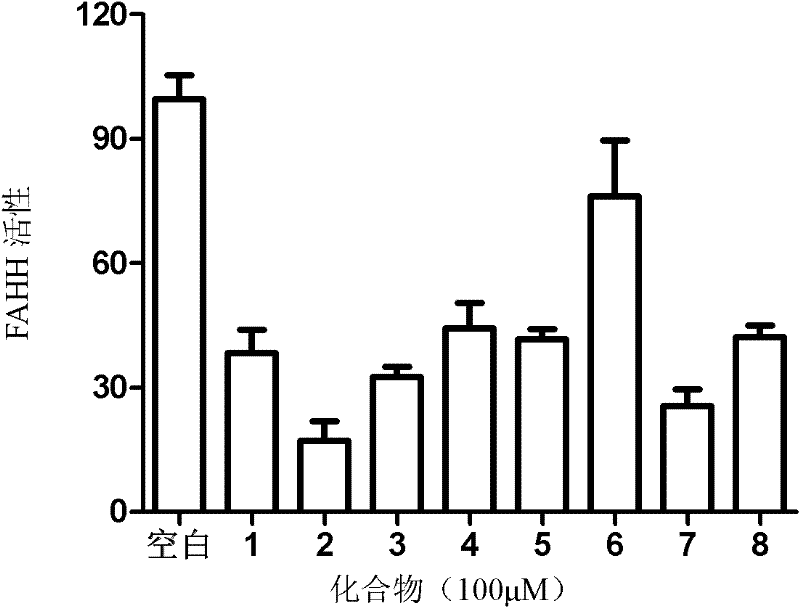
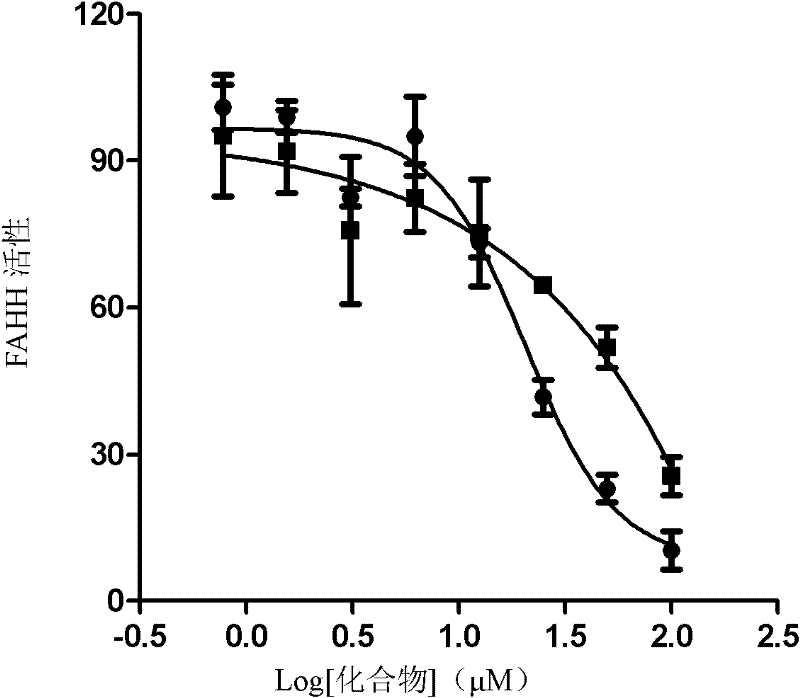
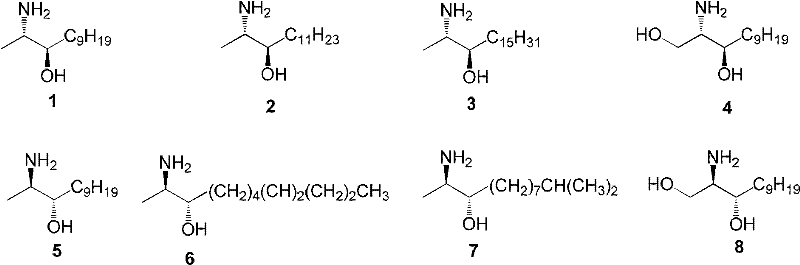
InactiveCN102241596ARaw materials are cheap and easy to getStereoselective single realizationOrganic active ingredientsNervous disorderBottleTwo step
The invention which relates to alcohol compounds provides o-aminoalcohol compounds, a preparation method thereof and an application thereof. Eight natural products of o-aminoalcohols having important physiological activities and derivatives thereof are prepared through carrying out a single stereoselective synthesis and a four-step process on an amino acid which is cheap and easily available and is treated as a raw material. A one-bottle / two-step process which is adopted in oxidative addition allows a target of single stereoselectivity to be realized and C-2 racemization not to be generated in asymmetric synthesis. The activity of the compounds as an FAAH enzyme inhibitor is analyzed through active experiments, so results provide reliable and valuable basis for medicinal development. The synthetic process which has the advantages of generality, concision, high efficiency, single selectivity, cheap and easily available raw materials, and simplicity of operation and separation of each step is suitable for industrial large-scale production.
Owner:XIAMEN UNIV
Synthetic method of cis-4-methoxycyclohexyl-1-carbamic acid
InactiveCN109796373AReduce the feed ratioLow costCarbamic acid derivatives preparationOrganic compound preparationMicroreactorWastewater
The invention provides a synthetic method of cis-4-methoxycyclohexyl-1-carbamic acid. The synthetic method includes the steps of catalytic hydrogenation, oxidative reaction, replacement reaction and hydrolytic reaction; the hydrolytic reaction is carried out in a microreactor, with a Jones reagent acting as an oxidant; barium hydroxide octahydrate is used as an alkaline material in the hydrolyticreaction. The synthetic method has few steps and shorter reaction time; the salt content in reaction wastewater is low, the content of heavy metals is low, and discharged waste is little; the finishedproduct has the content of 98% and above; the total reaction yield is 45% and above.
Owner:NANTONG JIANGSHAN AGROCHEM & CHEM LIMITED LIABILITY
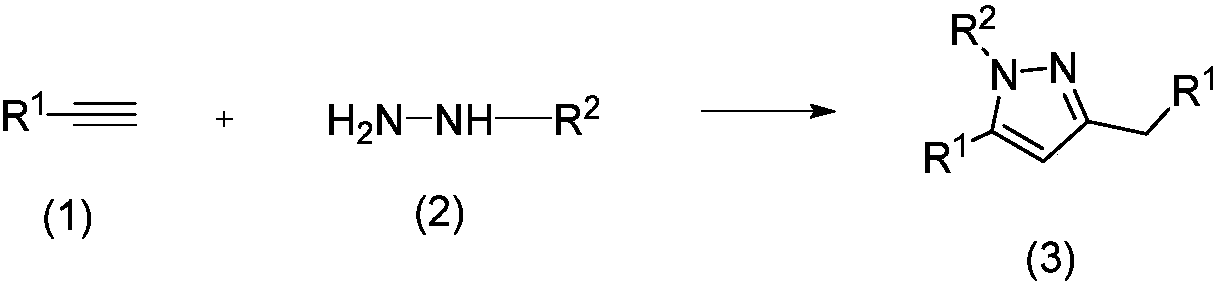
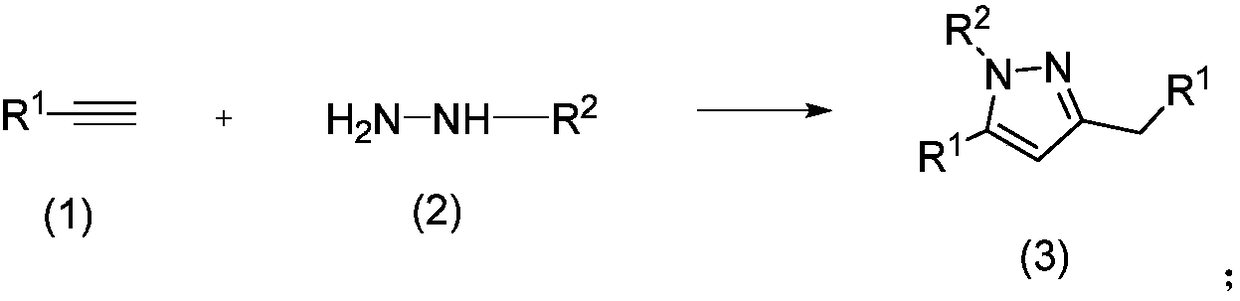
Method for preparing polysubstituted pyrazole through one-pot reaction of substituted alkyne and hydrazine or hydrazine substitute
The invention belongs to the technical field of drug intermediate preparation and specifically relates to a method for preparing polysubstituted pyrazole through one-pot reaction of substituted alkyneand hydrazine or hydrazine substitute. The polysubstituted pyrazole is generated from oxidation addition reaction of substituted alkyne and hydrazine or hydrazine substitute served as raw materials and air served as oxidizing agent under the existence of photo-sensitizer and copper salt and under the irradiation of visible light. The method provided by the invention has the advantages of low costof raw materials, simple and convenient process and easiness in industrial preparation.
Owner:陕西君境迈德生物医药科技有限公司
Adenaphtho-imidazolyl nitrogen heterocyclic carbene metal palladium complex catalyst and preparation and application thereof
InactiveCN109794295AHigh charge densityHigh selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCarbeneNitrogen gas
The invention discloses an acenaphtho-imidazolyl nitrogen heterocyclic carbene metal palladium complex catalyst, which is prepared by the following steps: 3-chloropyridine or pyridine is taken as an axial ligand, acenaphtho-imidazole salt is taken as a framework, and coordination is carried out on the 3-chloropyridine or pyridine and a metal ligand PdCl2 in the presence of potassium carbonate under the protection of nitrogen so as to obtain the acenaphtho-imidazole nitrogen heterocyclic carbene metal palladium complex catalyst. According to the acenaphtho-imidazole nitrogen heterocyclic carbene metal palladium complex, due to the introduction of the anthraquinone skeleton, the coordination of carbon 2 as atoms and metals is affected, the charge density of the metal center is increased, thesigma electron-donating capacity is enhanced, the oxidation addition reaction is facilitated, and the catalytic circulation is further promoted. The experimental results show that the selectivity ofthe carbonylation cyclization reaction is high (the selectivity is more than 99%) and the conversion rate is high (more than 85%) by using the acenaphtho-imidazole nitrogen heterocyclic carbene metalpalladium complex catalyst. In addition, the dosage of the catalyst is small, the reaction conditions are mild, the use of toxic phosphine ligands is avoided, and the method is safe and environment-friendly.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Decoloration method for producing edible vegetable oil with low content of trans fatty acid at CO2 supercritical state
The invention relates to a decoloration method for producing edible vegetable oil with low content of trans fatty acid at a supercritical CO2 state. The decoloration method of the invention controls the decoloration pressure of the supercritical CO2, the temperature, the carclazyte adding amount, the time and the stirring speed, takes the content of the trans fatty acid as an indicator, and regulates and controls each main parameter to obtain the edible vegetable oil with low content of trans fatty acid finally. In the method of the invention, the decolored carclazyte is utilized to decolor the oil at a supercritical liquid CO2 state; as the active decolored carclazyte has stronger Van der Waals' force at the supercritical liquid CO2 state, the triglyceride polarity is extremely small, and the pigment is larger relative to the polarity, the decolored carclazyt has stronger acting force on the pigment matters in the oil; and at the supercritical liquid state, the liquidity of the pigment is better, reactions, such as oxidation and addition reaction and the like are not easy to occur the oil; and the trans fatty acid content is low.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Catalyst and preparation method and application thereof, and method for preparing unsaturated carbonate
ActiveCN113877634ASimple processReduce lossOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCarbonate ester
The invention relates to the field of unsaturated carbonic ester preparation, and discloses a catalyst and a preparation method and application thereof, and a method for preparing unsaturated carbonic ester. The catalyst comprises manganese oxide and a zinc metal organic framework compound, wherein a molar ratio of manganese element to zinc element in the catalyst is 0.3-2. The preparation method of the catalyst comprises the following steps: (1) mixing a manganese precursor with an activating agent and a first solvent, then sequentially carrying out separating, optional washing and optional drying, and then conducting roasting; and (2) mixing the obtained manganese oxide with a zinc precursor, 2-methylimidazole and a second solvent for a solvothermal reaction, and then carrying out separating, wherein a molar ratio of the usage amount of the manganese oxide in terms of elemental manganese to the usage amount of the zinc precursor in terms of elemental zinc is 0.3-2. According to the catalyst, unsaturated carbonic ester can be prepared only through one-step oxidation addition, a process is simple, and the comprehensive effect of excellent conversion rate and unsaturated carbonic ester selectivity can be obtained.
Owner:CHINA PETROLEUM & CHEM CORP +1
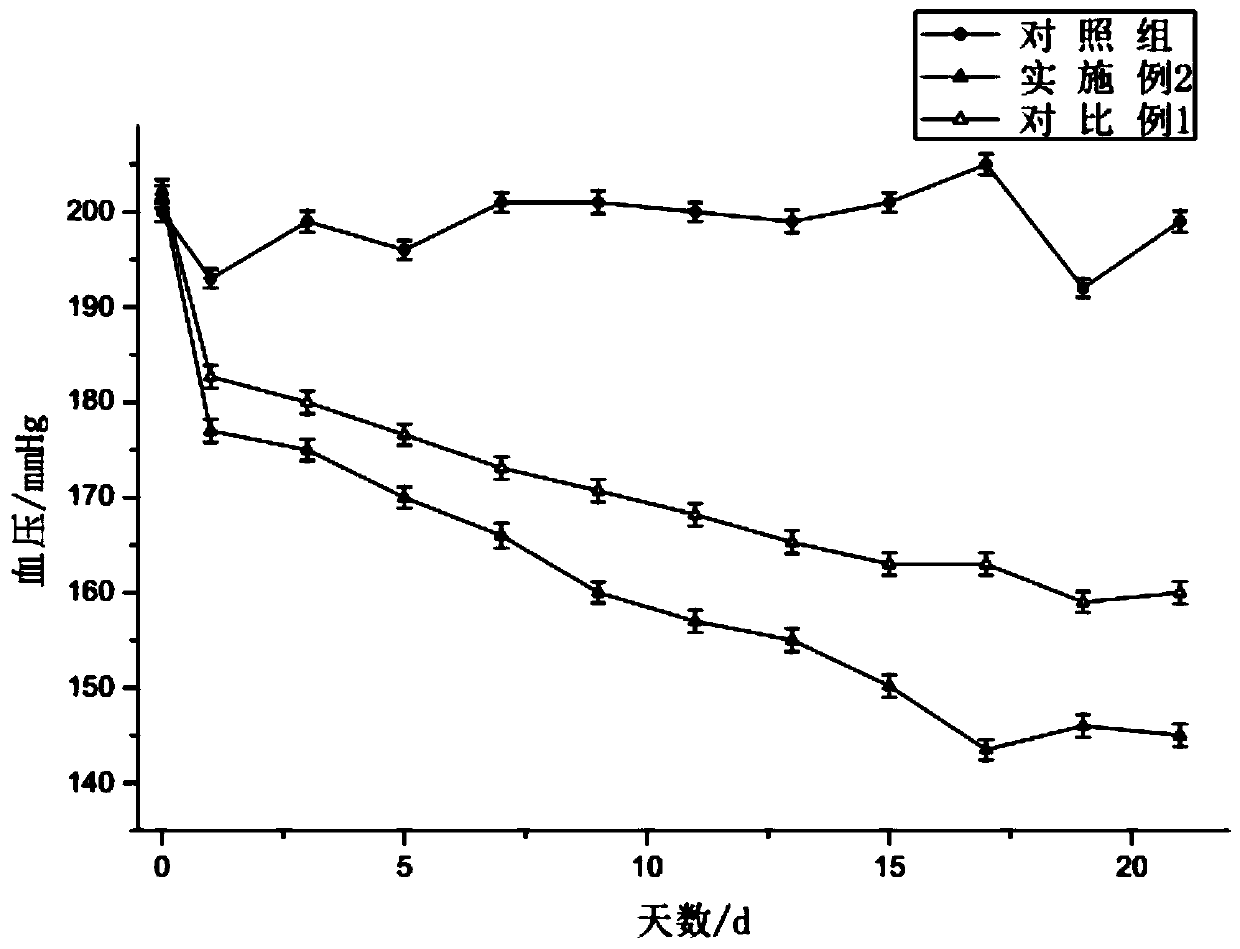
Oral liquid capable of lowering blood pressure and resisting thrombus
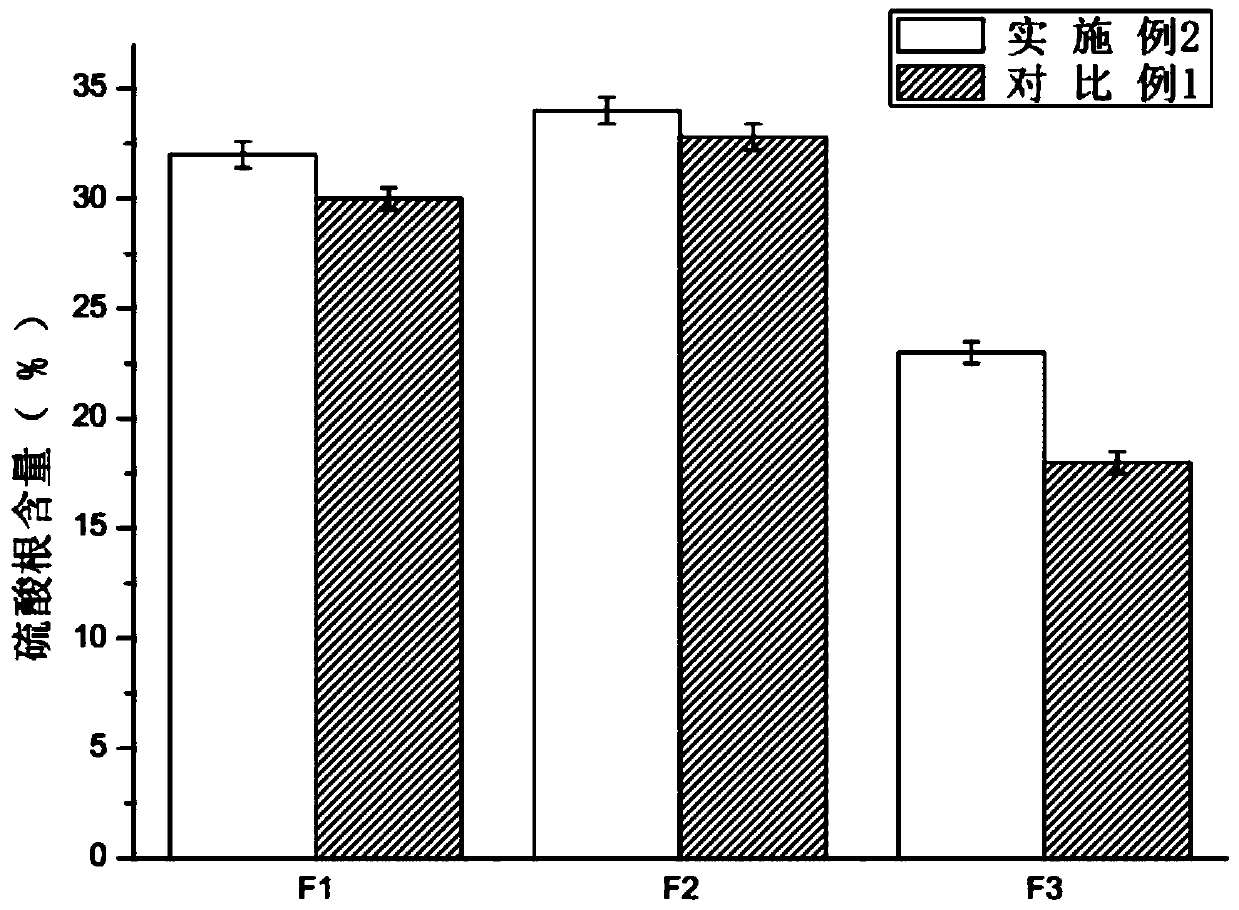
ActiveCN110251462AShorten the covalent bond lengthReduce sheddingOrganic active ingredientsDispersion deliverySulfate radicalsFiltration
The invention provides oral liquid capable of lowering blood pressure and resisting thrombus and belongs to the technical field of biological preparations. A preparation method of the oral liquid includes: extracting laminarin; separating and purifying fucoidan; using free radicals to degrade the fucoidan in an oxidative manner, wherein choline chloride is added during the oxidative degradation of the fucoidan; dissolving; performing rough filtration; flavoring; sterilizing; filling; sealing. The oral liquid is small in molecular weight, easy to absorb, high in sulfate radical content, high in biological activity and capable of further increasing the anti-thrombus effect of the fucoidin by improving hemorheology.
Owner:安徽草珊瑚生物科技有限公司
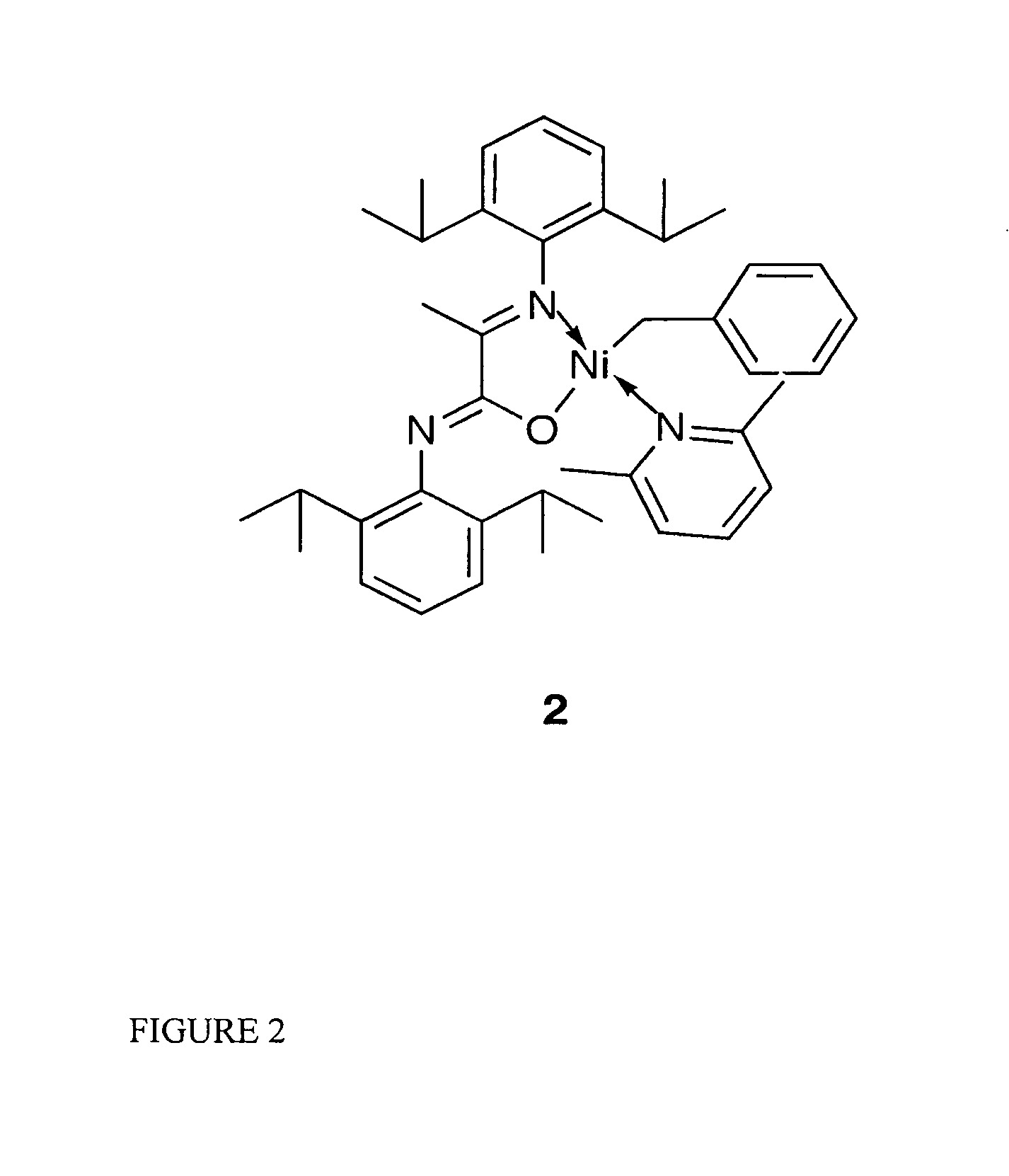
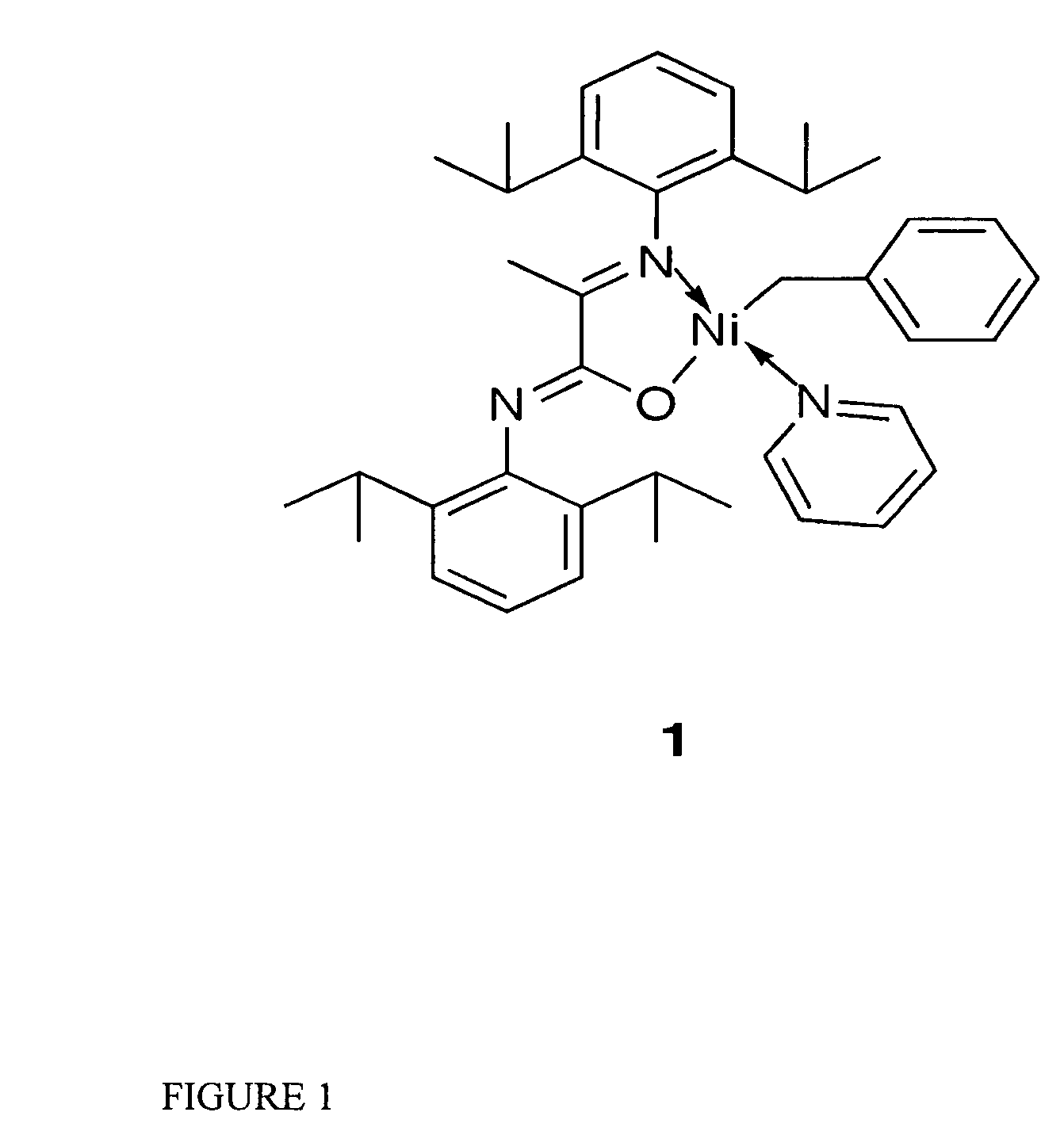
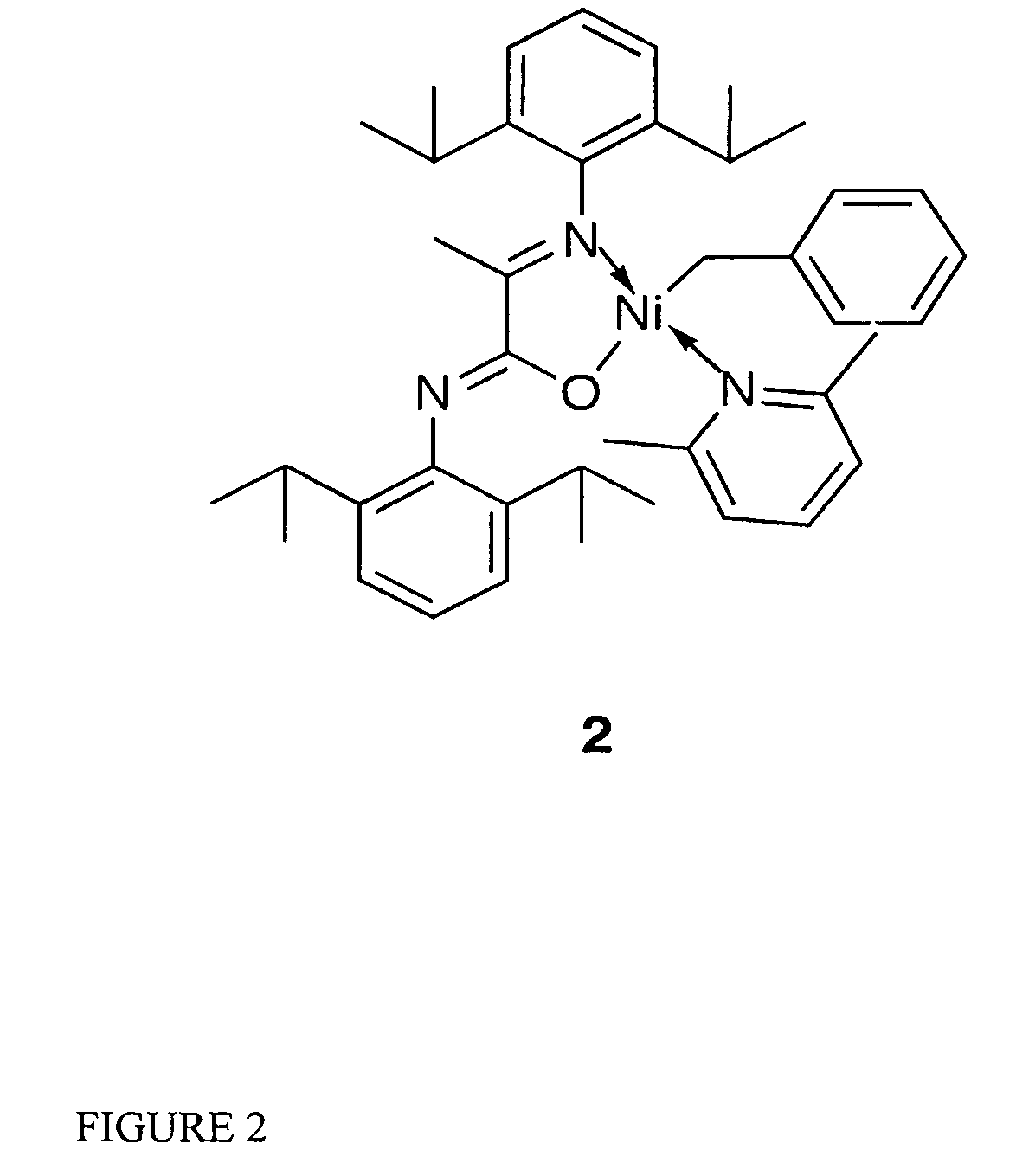
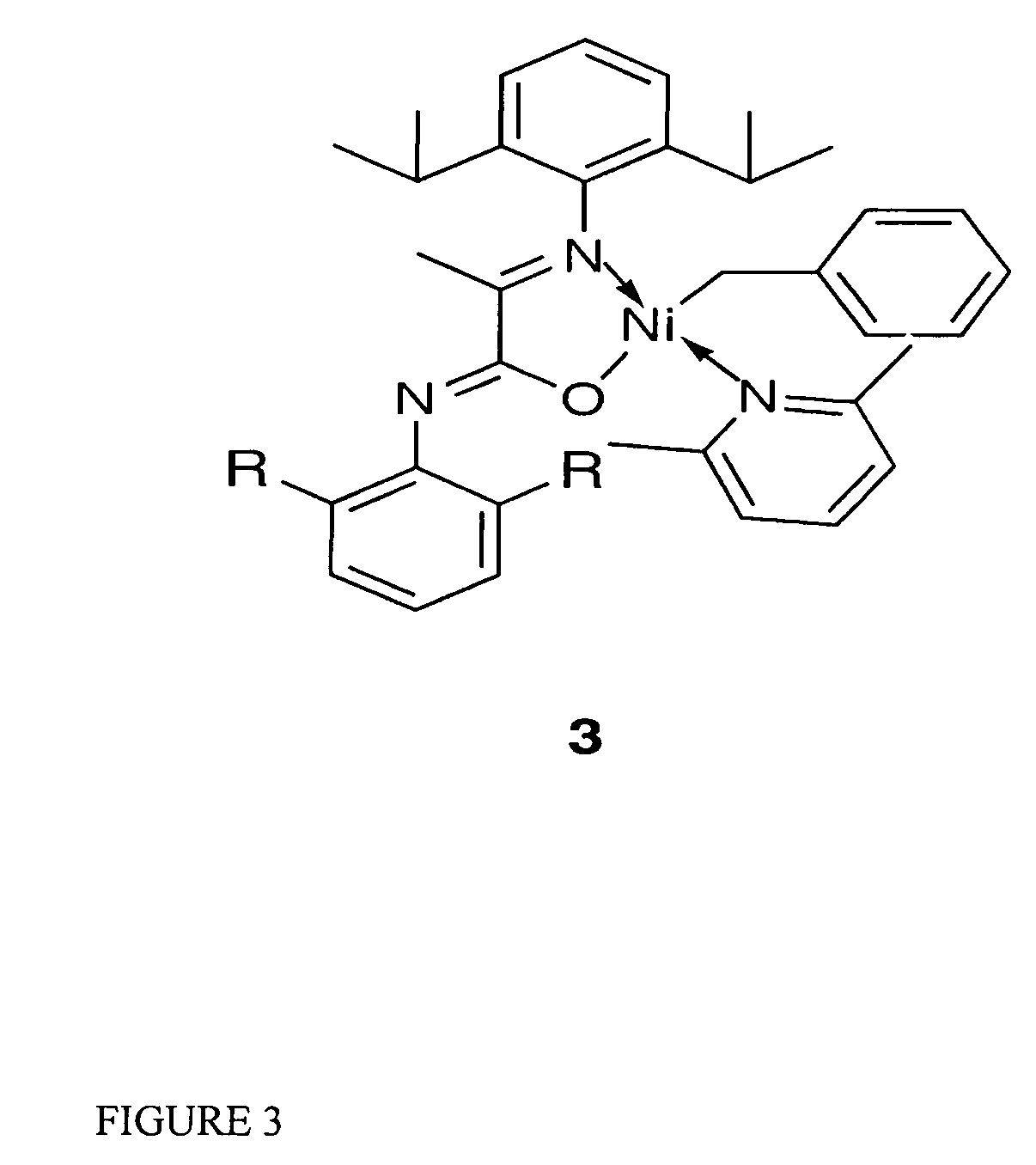
Single component, phosphine-free, initiators for ethylene homopolymerization and copolymerization with functionalized co-monomers
InactiveUS20070225456A1High material utilizationImprove propertiesOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsProtonationElectron donor
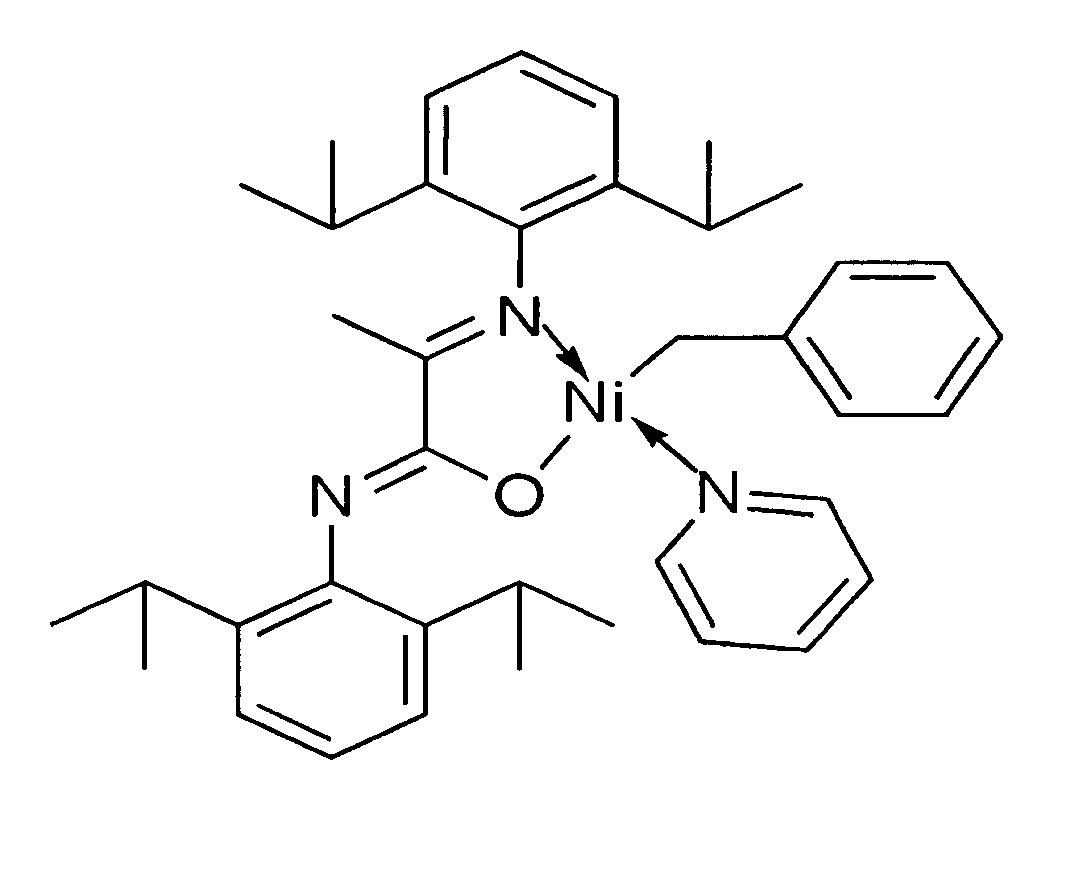
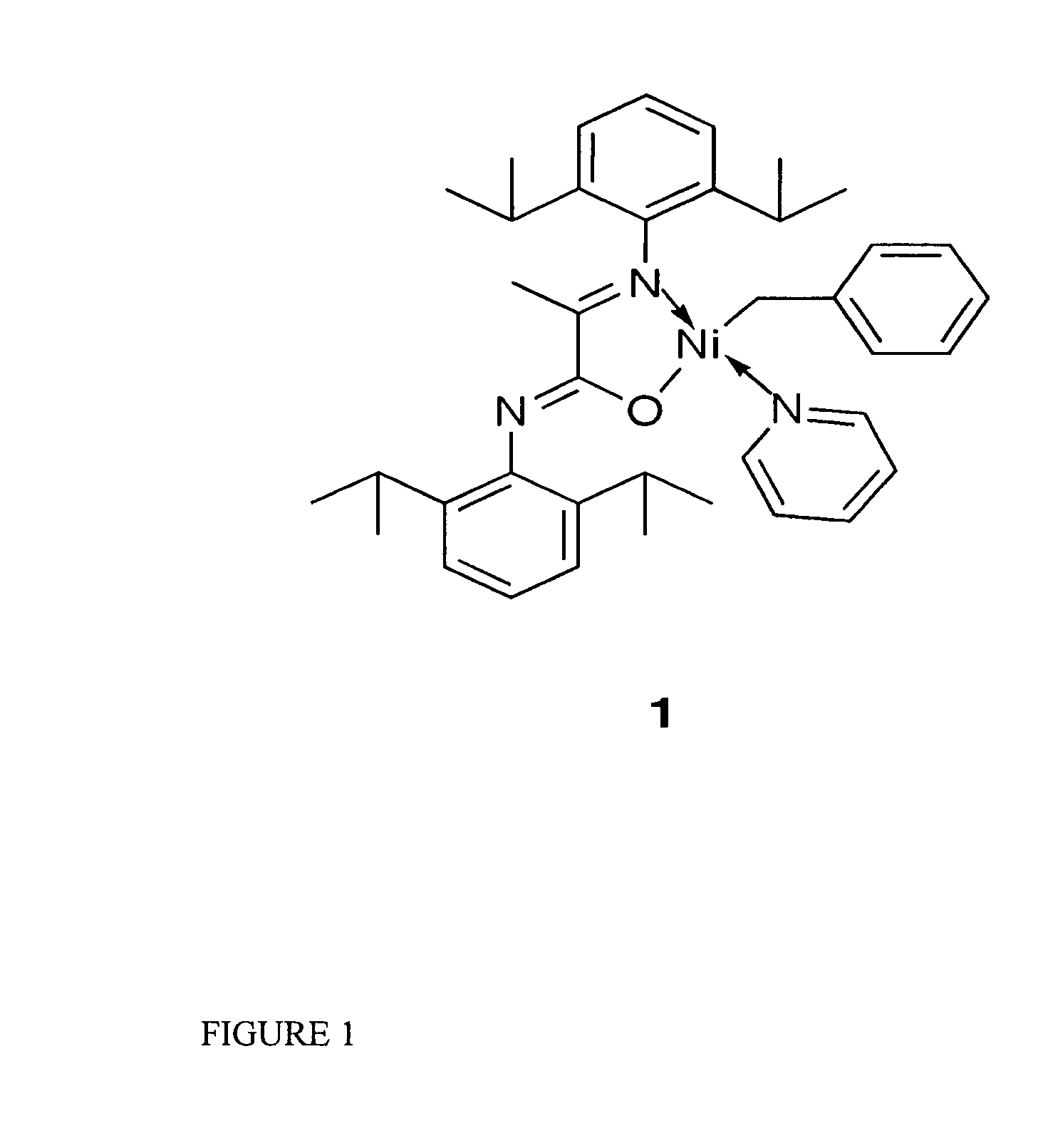
Novel phosphine-free non-ionic single catalysts, and method for making such catalysts, for the homo-polymerization and copolymerization of olefins such as ethylene, a-olefins and functionalized olefins without the use of additional co-activators, are disclosed. These phosphine-free non-ionic single catalysts are also active for co-polymerization of olefins with monomers with polar functionalities. The catalyst of this invention comprise of a late transition metal with a chelating monoanionic ligand, an R group and a neutral 2 electron donor ligand. Catalysts are prepared by the oxidative addition of benzylhalide (halide=Cl, Br or I) to an appropriate metal source in the presence of a stabilizing agent, such as nitrogen based ligands, followed by the addition of the deprotonated form of the chelating ligand.
Owner:RGT UNIV OF CALIFORNIA
An immobilized monoamine oxidase and its application in the synthesis of chiral azabicyclic compounds
ActiveCN105624128BStable separationEasy to separateImmobilised enzymesFermentationAzabicyclo CompoundsOxidative enzyme
Owner:弈柯莱生物科技(集团)股份有限公司
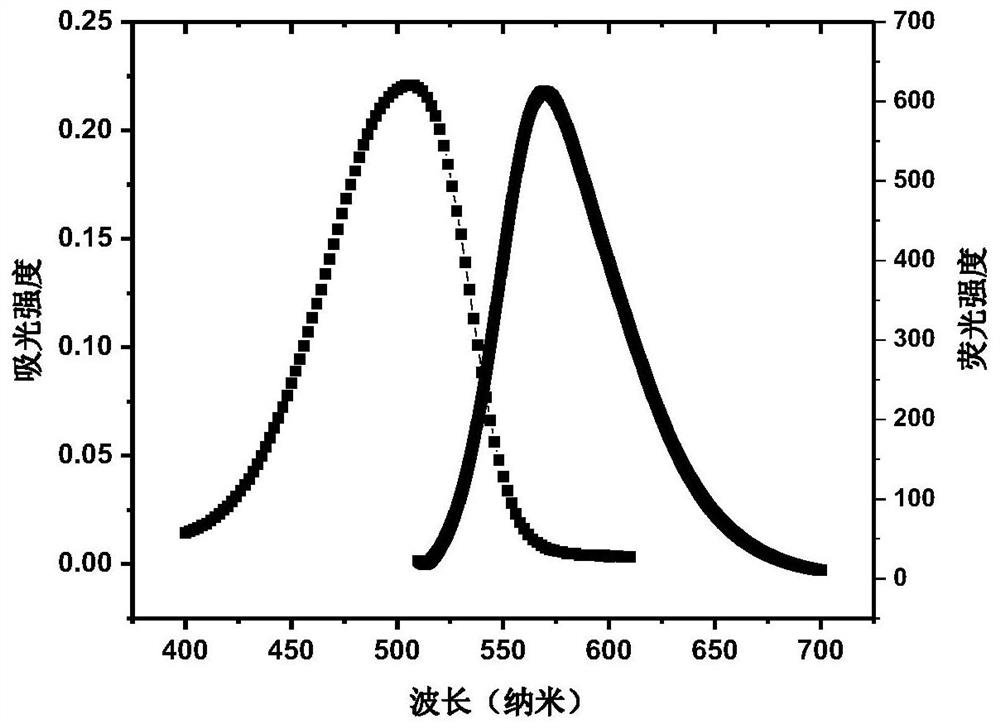
A photosensitizer with high singlet oxygen yield and preparation method thereof
ActiveCN112375089BHigh quantum yieldStrong absorption capacityOrganic chemistryPhotodynamic therapyQuantum yieldSinglet oxygen
The invention discloses a photosensitizer with a high yield rate of singlet oxygen and a preparation method thereof. The Suzuki coupling reaction is adopted to synthesize 4,8-dibromo-6-(2-ethylhexyl)-[1,2, 5] Thiadiazo[3,4-F]benzotriazole and 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolin-2-yl)pyridine , under the catalysis of [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride, the photosensitizer is obtained through oxidative addition and reductive elimination. The photosensitizer disclosed by the invention has a strong absorption ability in visible light, has a stable structure under slightly acidic to weakly alkaline conditions, and has a high quantum yield and high singlet oxygen generation ability, and can be used as a new photosensitizer. Fluorescence imaging or photodynamic therapy of tumors.
Owner:SHANDONG UNIV
Preparation method of isotope labeled N-(1, 3-dimethylbutyl)-N'-phenyl p-benzoquinone
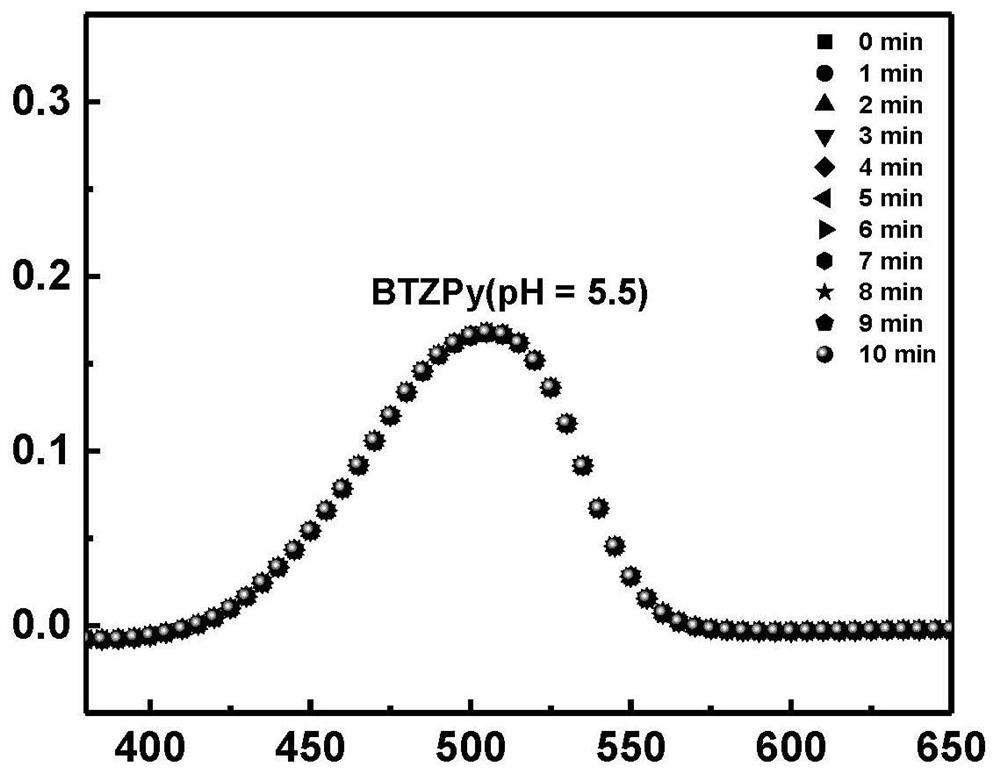
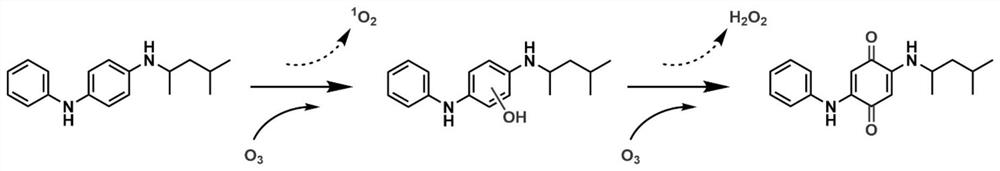
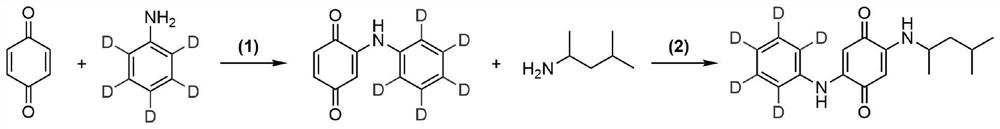
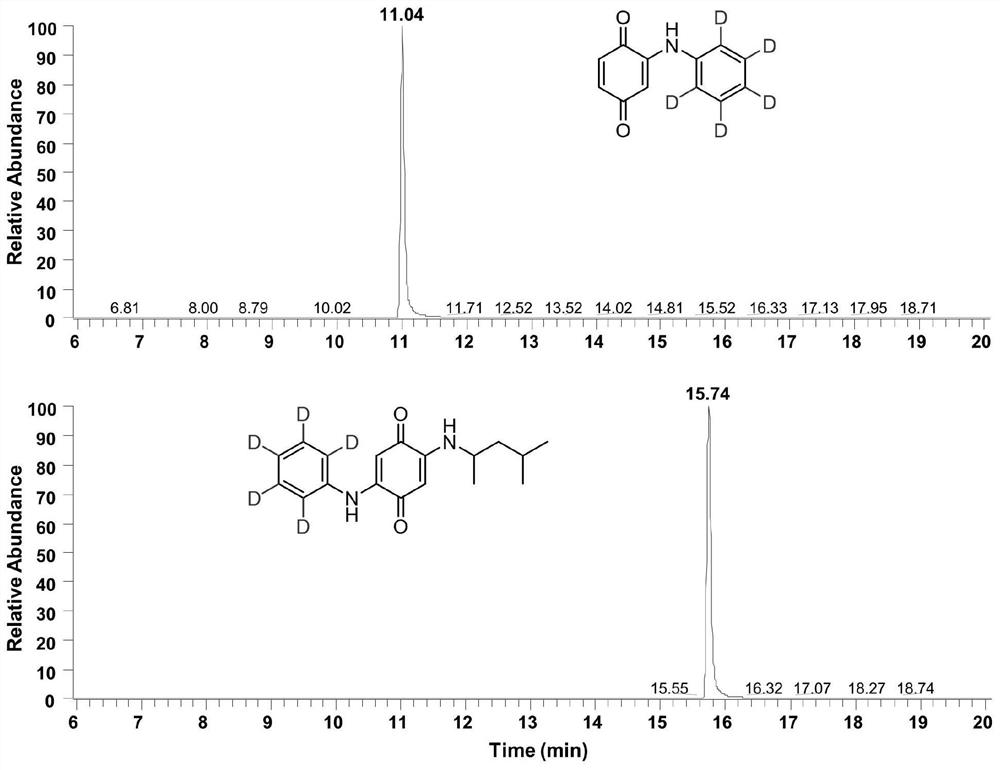
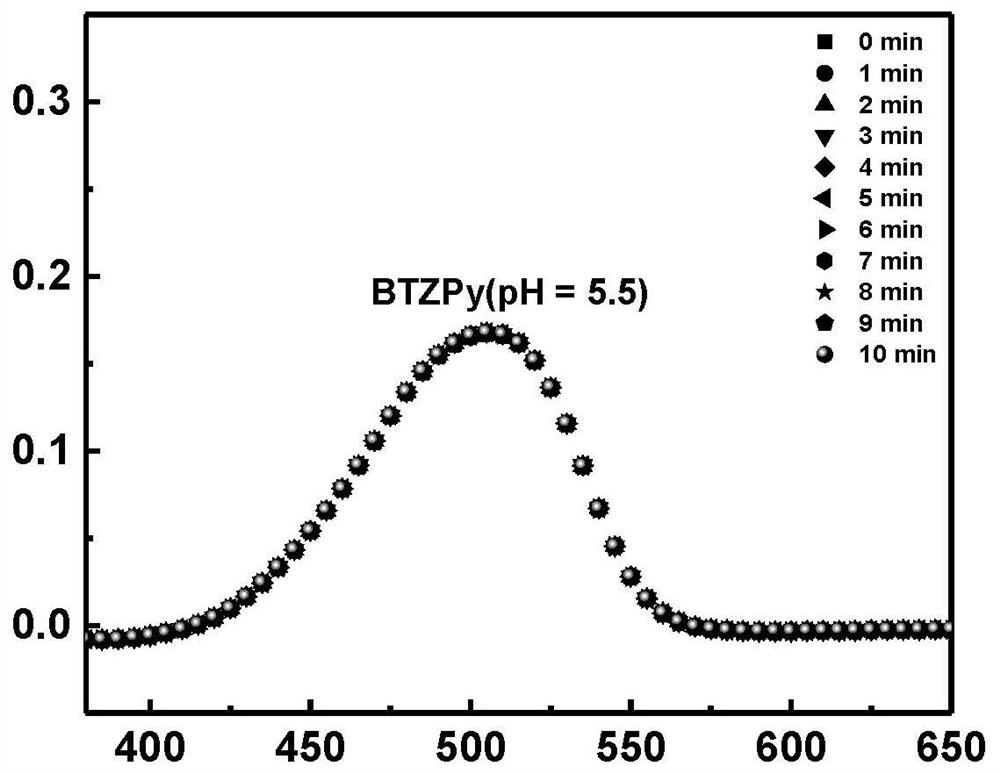
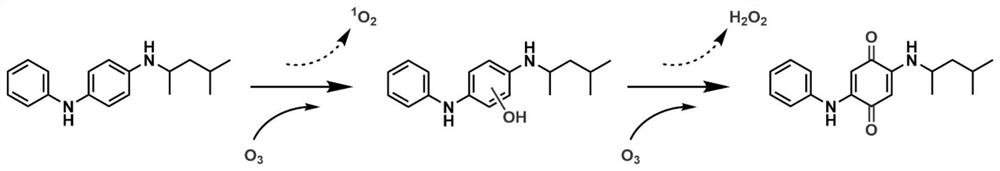
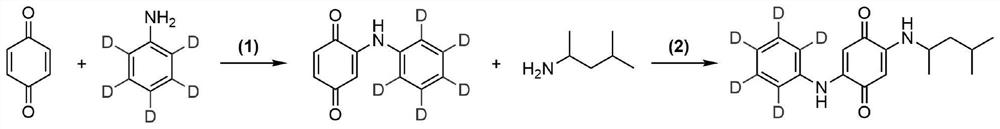
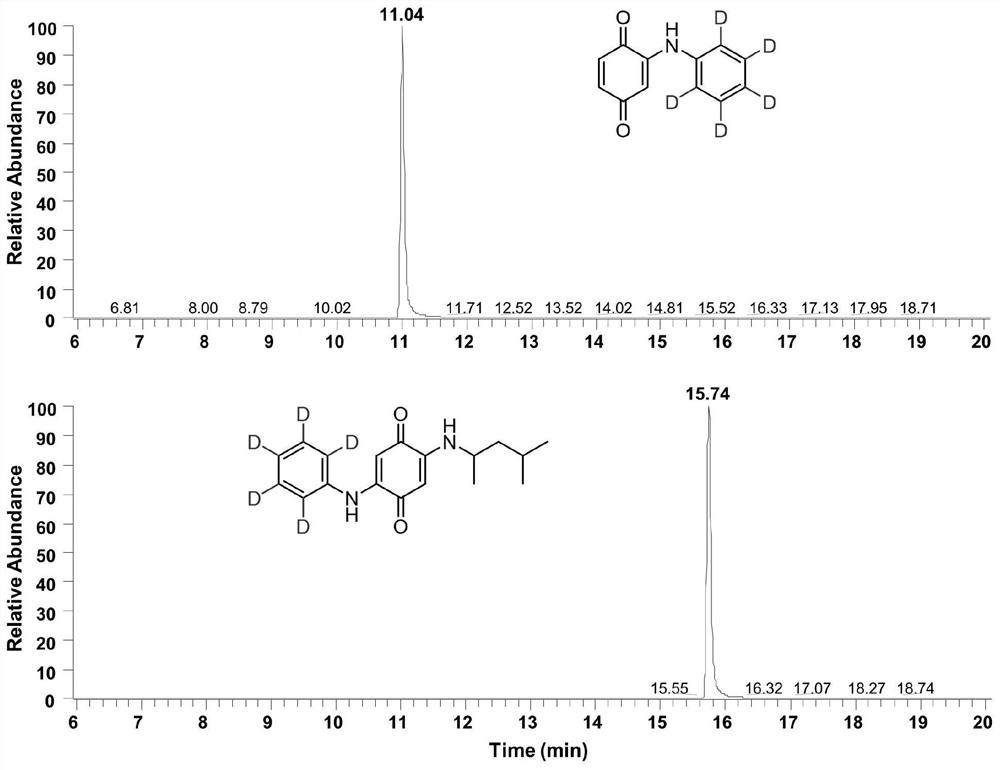
ActiveCN113683524AImprove conversion efficiencyGood economical applicabilityOrganic compound preparationOrganic chemistry methodsQuinoneDeuterated benzene
The invention discloses a preparation method of isotope labeled N-(1, 3-dimethylbutyl)-N'-phenyl p-benzoquinone. The method comprises the following steps: reacting p-benzoquinone with aniline with all deuterated benzene ring hydrogen protons to obtain deuterated p-benzoquinone-2-aniline; and enabling reaction between the deuterated p-benzoquinone-2-aniline and 1, 3-dimethyl butylamine to obtain the N-(1, 3-dimethyl butyl)-N'-phenyl p-benzoquinone labeled by the isotope D. According to the invention, the continuous oxidative addition reaction of p-benzoquinone, aniline with all deuterated benzene ring hydrogen protons and 1, 3-dimethyl butylamine is adopted, so that the isotope D-labeled 6ppd-quinone can be simply and efficiently prepared under relatively mild conditions. The isotope D labeled 6ppd-quinone prepared by the method can be widely applied to content detection of environmental samples, cell and animal toxicological experiments and other related researches as a standard substance.
Owner:BEI JING NORMAL UNIV HONG KONG BAPTIST UNIV UNITED INT COLLEGE
A kind of preparation method of resveratrol compound
ActiveCN109970517BAvoid generatingHigh yieldOrganic compound preparationOrganic chemistry methodsPtru catalystOrganic synthesis
The invention provides a preparation method of resveratrol compounds, belonging to the technical field of organic synthesis. In the present invention, first, alkoxy-substituted benzyl halide, alkoxy-substituted benzaldehyde and metal catalyst undergo oxidative addition and reduction elimination reactions to obtain alkoxy-substituted diacetophenone; then the alkoxy-substituted diphenylethyl Reduction, trans-elimination and selective debenzylation of ketones and metal catalysts in a hydrogen atmosphere give resveratrol compounds. In the preparation method of the present invention, hydrogenation reduction, trans elimination and selective debenzylation reactions can be realized through a one-pot method, and the reaction directly obtains trans olefins, avoiding the generation of isomers; the reaction selectively catalyzes debenzylation Benzyl removes Lewis acid from the source, and has the advantage of high yield, which is a green and environmentally friendly process. Experimental results show that the products obtained by the preparation method provided by the present invention are all trans-olefins, the purity can reach more than 99.5%, and the yields are all above 80%.
Owner:HANGZHOU NORMAL UNIVERSITY +1
Organic transition metal compound, preparation method and method for forming transition metal-containing thin film
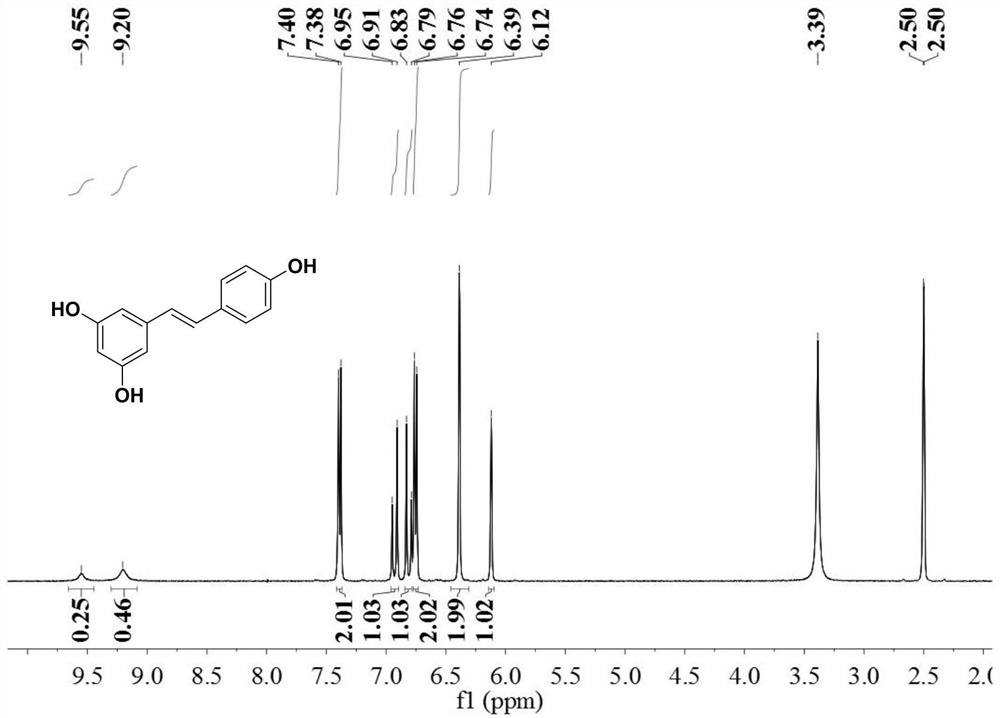
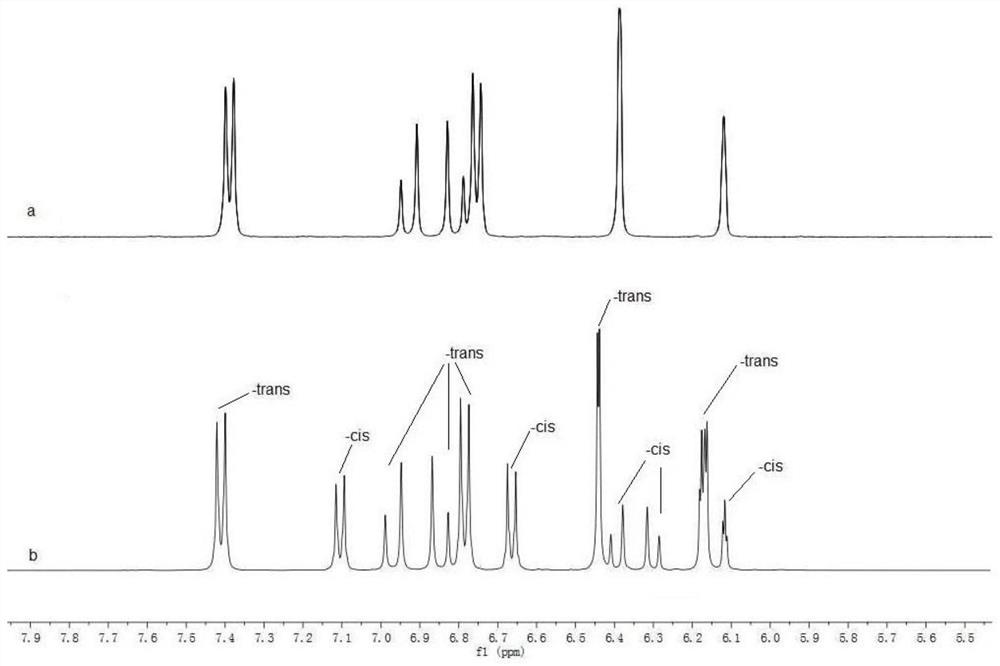
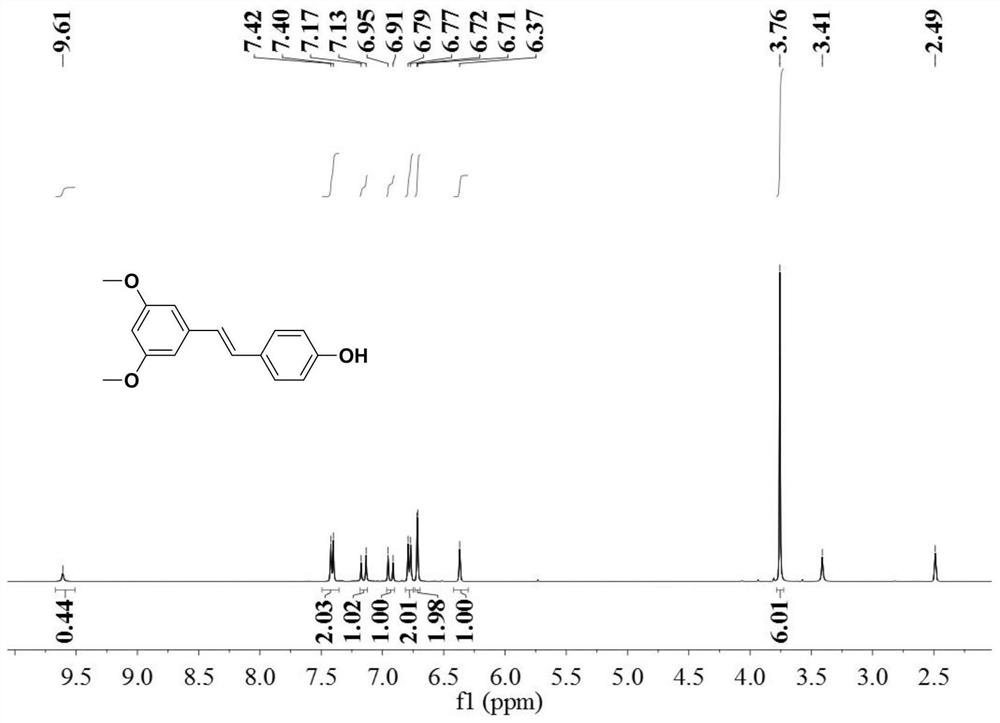
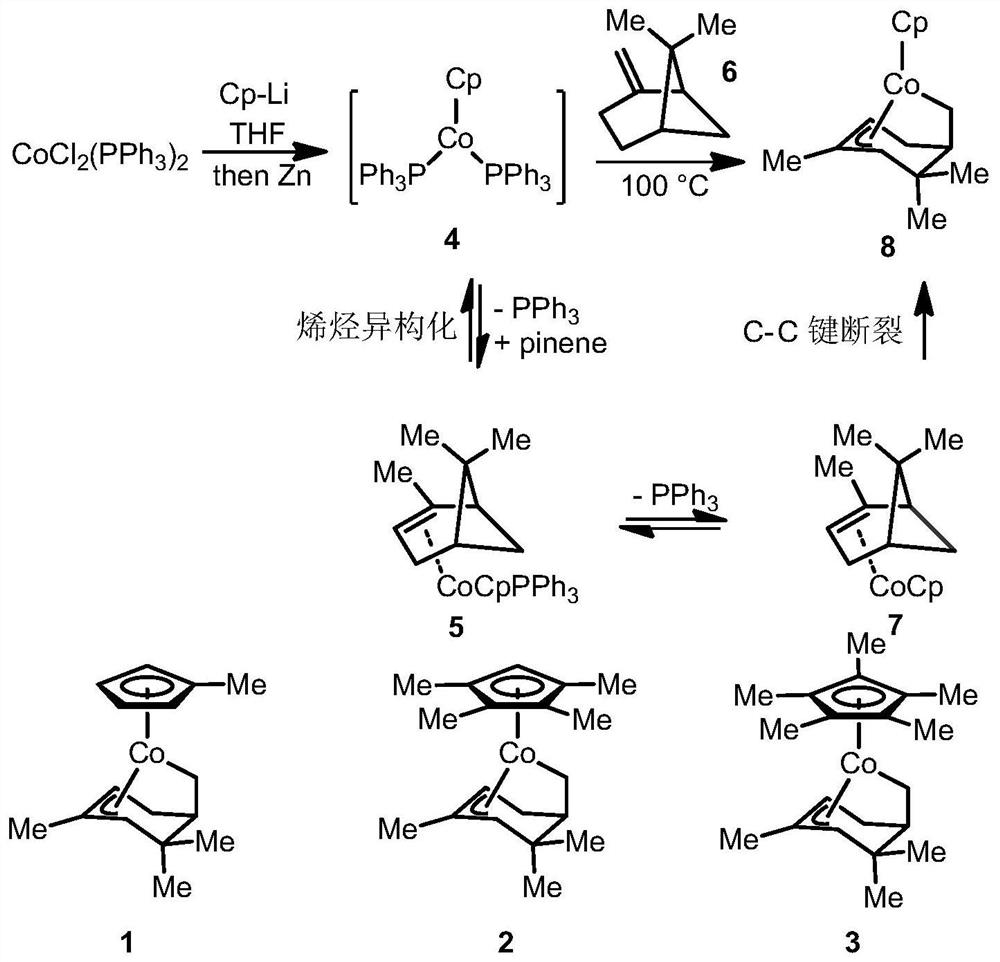
ActiveCN113201029AThe synthesis steps are simpleGood volatilization effectCobalt organic compoundsChemical vapor deposition coatingPhysical chemistryThin membrane
The invention discloses an organic transition metal compound, a preparation method and a method for forming a transition metal-containing thin film. The structural formula of the organic transition metal compound is selected from one of formulas shown in the specification. A low-valence transition metal and pinene are subjected to carbon-carbon bond oxidative addition to obtain a chelated organic transition metal compound. An organic transition metal metal complex is used as a metal precursor, and research on the atomic deposition process of the metal complex proves that the metal complex well follows ideal atomic layer deposition growth so as to deposit a transition metal-containing film with high purity and smooth surface, and further proves that in the atomic layer deposition process, the transition metal-containing film can be deposited in a deep and wide groove in a shape-preserving manner. It is indicated that the method is very suitable for a complex or porous three-dimensional nano stereo structure substrate, and the transition metal-containing film is uniformly deposited in a shape-preserving manner to obtain the transition metal-containing film.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Photosensitizer with high singlet oxygen yield and preparation method thereof
ActiveCN112375089AHigh quantum yieldStrong absorption capacityOrganic chemistryPhotodynamic therapyQuantum yieldSinglet oxygen
The invention discloses a photosensitizer with high singlet oxygen yield and a preparation method thereof. 4, 8-dibromo-6-(2-ethylhexyl)-[1, 2, 5] thiadiazole [3, 4-F] benzotriazole and 4- (4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborane-2-yl) pyridine are subjected to a Suzuki coupling reaction under the catalysis of a [1, 1'-bis (diphenylphosphino) ferrocene] palladium dichloride, and oxidation addition and reduction elimination are performed to obtain the photosensitizer. The photosensitizer disclosed by the invention has relatively strong absorption capability in visible light, is stable in structure under the condition of slight acid to weak base, has very high quantum yield and high singlet oxygen generation capability, and can be applied to fluorescence imaging or photodynamic therapy oftumors as a novel photosensitizer.
Owner:SHANDONG UNIV
A kind of preparation method of isotope-labeled n-(1,3-dimethylbutyl)-n'-phenyl-p-benzoquinone
ActiveCN113683524BImprove conversion efficiencyGood economical applicabilityOrganic compound preparationOrganic chemistry methodsQuinoneIsotopic labeling
The invention discloses a preparation method of isotope-labeled N-(1,3-dimethylbutyl)-N'-phenyl-p-benzoquinone. The method comprises the steps of: reacting p-benzoquinone with all deuterated aniline of benzene ring hydrogen protons to obtain deuterated p-benzoquinone-2-aniline; said deuterated p-benzoquinone-2-aniline and 1,3-di Methylbutylamine is reacted to obtain isotope D-labeled N-(1,3-dimethylbutyl)-N'-phenyl-p-benzoquinone. The present invention adopts the continuous oxidative addition reaction of p-benzoquinone and aniline with all deuterated hydrogen protons of benzene ring and 1,3-dimethylbutylamine, and realizes simple, high-efficiency, and can prepare isotope D under relatively mild conditions. Labeled 6ppd‑quinone. The isotope D-labeled 6ppd-quinone prepared by the method can be widely used as a standard in the content detection of environmental samples and in related researches such as cell and animal toxicity experiments.
Owner:BEI JING NORMAL UNIV HONG KONG BAPTIST UNIV UNITED INT COLLEGE
Process for preparing biphenyl polyimide
A process for preparing biphenyl polyimide includes such steps as dissolving bichlorodiphthalimide monomer, triphenylphosphino nickel as catalyst and zinc powder as reducer in non-proton solvent, reaction at 60-120 deg.C to generate Ni mating substance, oxydizing addition reaction on bichlorodiphthalimide to generate intermediate, and dereducing reaction to generate its condensate and zinc chloride while reducing Ni to react on chloroimide to generate polyimide. Its advantages are simple process and low cost.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of palladium complex and conjugated aromatic polymer
ActiveCN102424694BWith oxidative additionFacilitate transfer stepsOrganic chemistryHalogenHydroxy group
The present invention provides a palladium complex with a structure such as formula (I) or formula (II), wherein X is a halogen; L is a phosphine ligand or a nitrogen-containing ligand; G is a sulfonic acid group, a carboxylic acid group, a phosphonic acid group , a hydroxyl group or a polyether chain; the position of the G is at the para or meta position of the L; n is a positive integer; m is 2, 3 or 4. The invention also provides a preparation method of the palladium complex and a method for preparing the conjugated arene polymer by using the palladium complex catalyst. Due to the introduction of neutral hydrophilic substituents in the palladium complex catalyst ligand, the palladium complex catalyst has amphiphilicity and can be dissolved in both the water phase and the organic phase, and the catalyst is easy to carry out oxidative addition with the polymerized monomer, and The metal transfer step can be promoted, and the coupling polymerization reaction can be carried out continuously and rapidly, so high molecular weight conjugated arene polymers can be obtained, PdX2(L-Gn)2 (I); Pd(L-Gn)m (II).
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
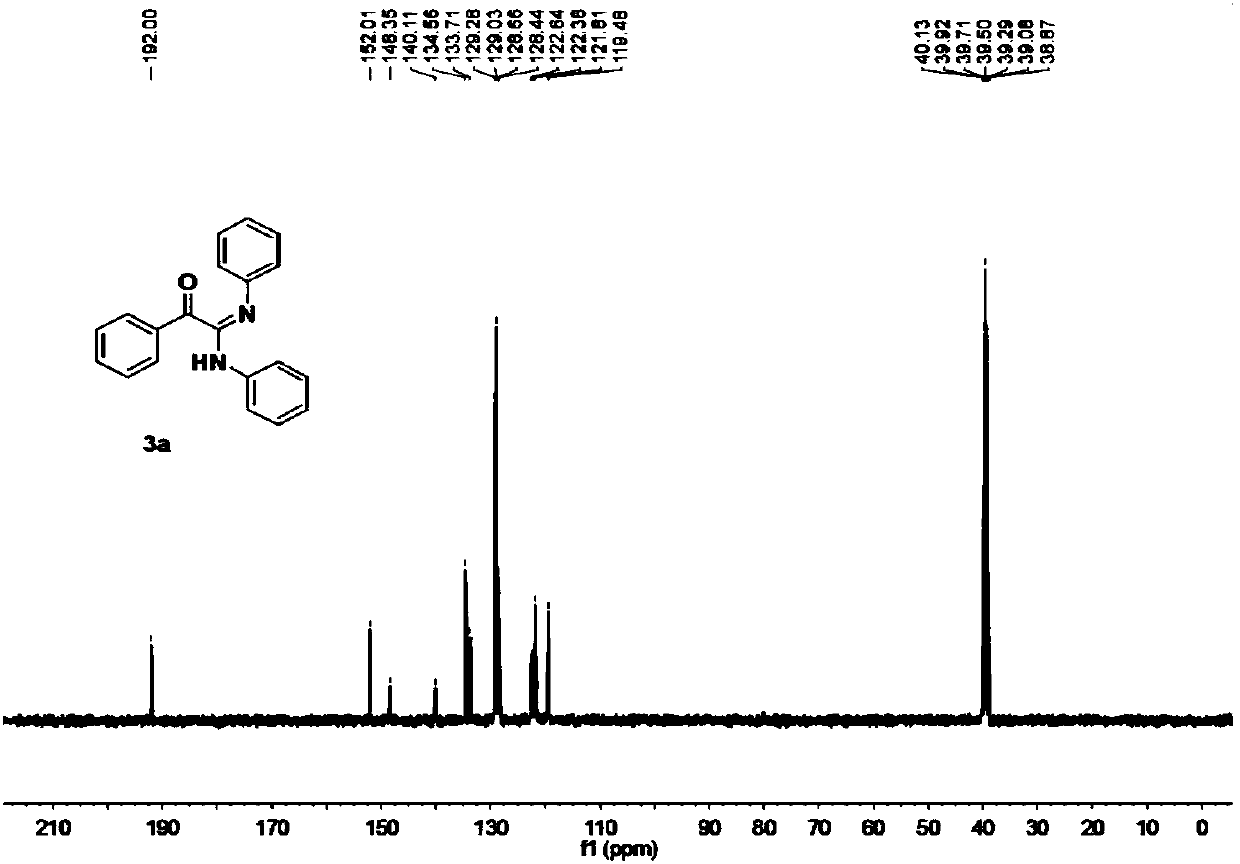
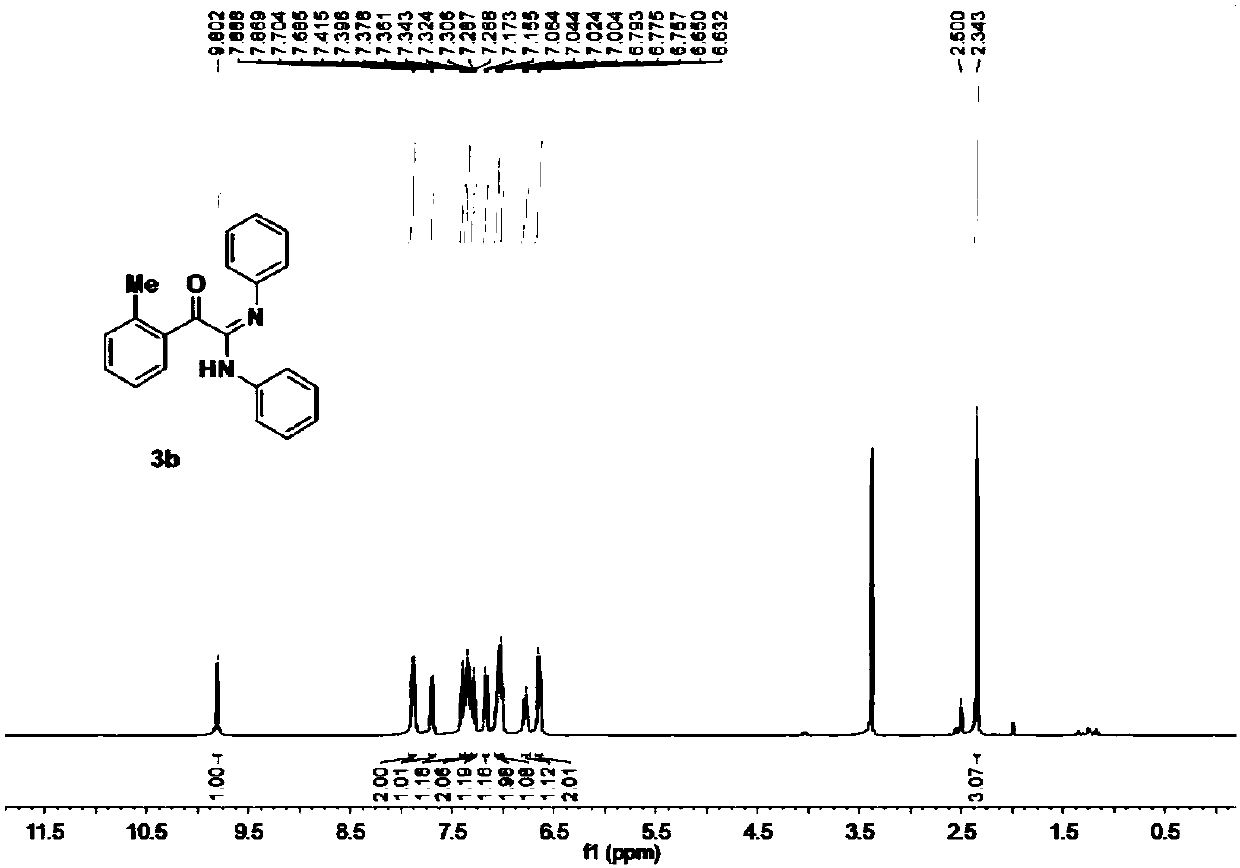
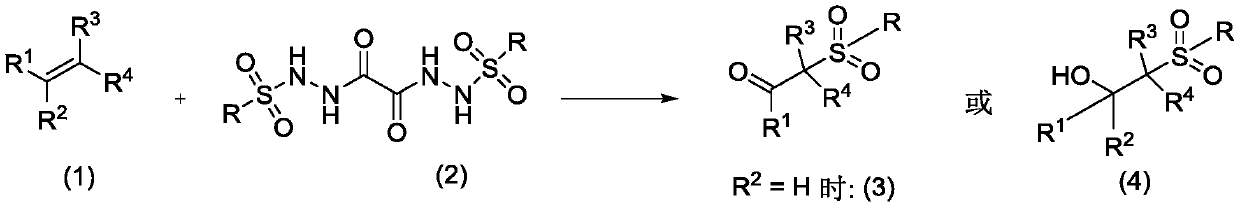
Method for preparing β-ketosulfone or β-hydroxysulfone by reaction of substituted alkenes and sulfonyl hydrazide derivatives
ActiveCN109232331BSave raw materialsSimple processOrganic chemistryOrganic compound preparationChemical synthesisSulfohydrazide
Owner:JIANGXI MENHOVER CHEM TECH CO LTD
Single component, phosphine-free, initiators for ethylene homopolymerization and copolymerization with functionalized co-monomers
InactiveUS7968487B2High material utilizationImprove propertiesOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsProtonationElectron donor
Novel phosphine-free non-ionic single catalysts, and method for making such catalysts, for the homo-polymerization and copolymerization of olefins such as ethylene, α-olefins and functionalized olefins without the use of additional co-activators, are disclosed. These phosphine-free non-ionic single catalysts are also active for co-polymerization of olefins with monomers with polar functionalities. The catalyst of this invention comprise of a late transition metal with a chelating monoanionic ligand, an R group and a neutral 2 electron donor ligand. Catalysts are prepared by the oxidative addition of benzylhalide (halide=Cl, Br or I) to an appropriate metal source in the presence of a stabilizing agent, such as nitrogen based ligands, followed by the addition of the deprotonated form of the chelating ligand.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com