Method for synthesizing amidine compounds through oxidative amidation of aryl methyl ketone under catalysis of copper (II)
A technology for oxidizing amides and aryl methyl ketones with aryl methyl ketones, which is applied in the field of synthesis of amidine compounds, can solve the problems of unfavorable large-scale production, poor stability, and unseen problems, and achieve low cost and good stability , the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~19
[0046] Embodiment 1~19 carries out according to the following method:
[0047] Add ketones (0.5 mmol), aniline (1.1 mmol), CuCl to the sealed tube 2 (13.4mg, 0.1mmol), PhCOONa (36mg, 0.25mmol) and DMSO (1.0mL), and the reaction mixture was stirred at 80°C under 1atm oxygen atmosphere for 30 hours, the organic layers were mixed, washed with Na 2 SO 4 Drying, filtration and concentration in vacuo and purification by silica gel column color classification (eluent: petroleum ether / ethyl acetate) gave amidines.
[0048] Concrete reaction process is as follows:
[0049]
Embodiment 1
[0051] Ketone compound raw material:
[0052] Target product:
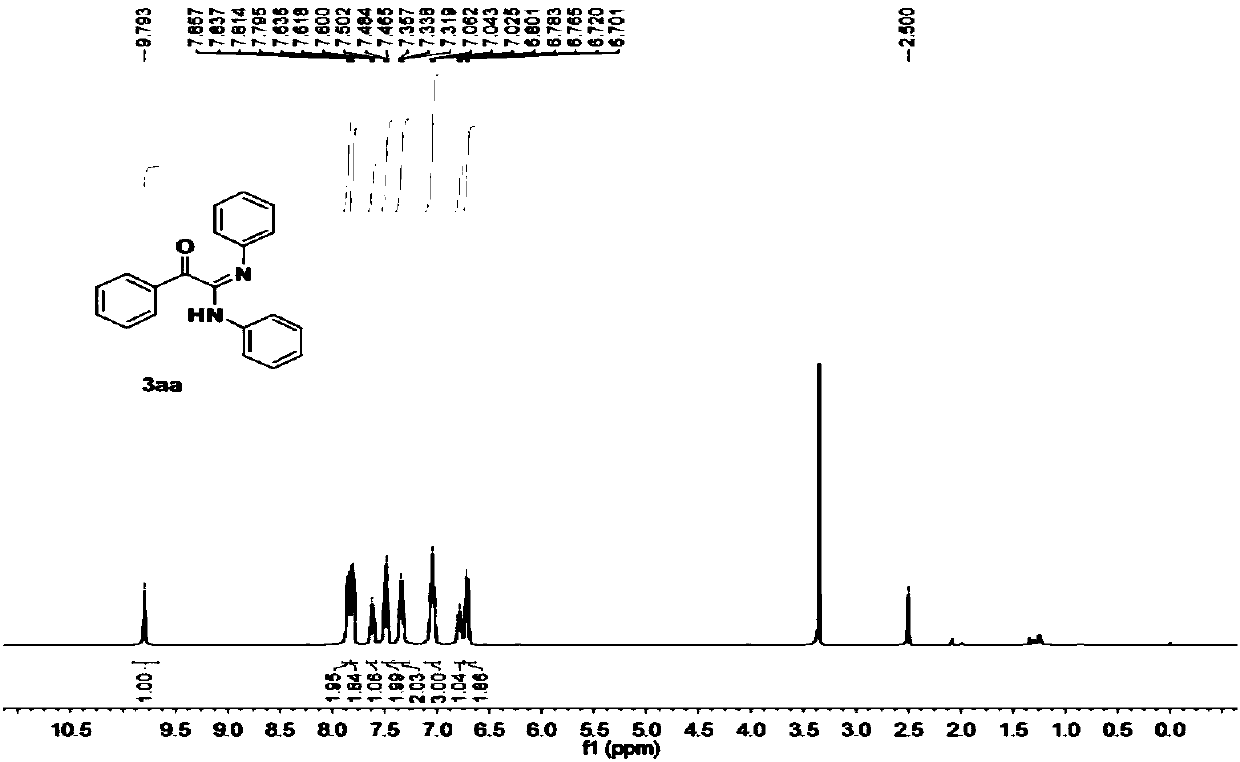
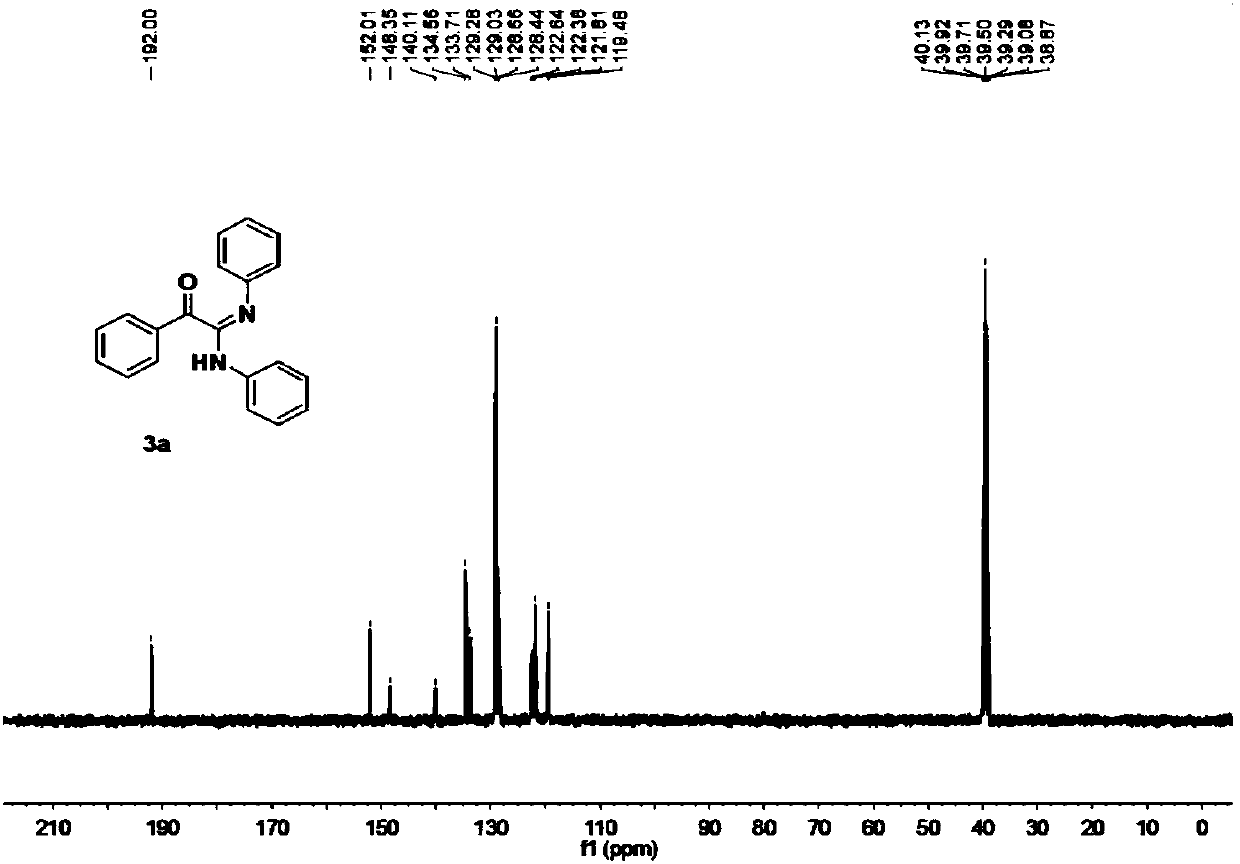
[0053] Obtained 128.4 mg of the target product with a yield of 86%; a yellow solid; 1 H NMR (400MHz, DMSO) δ9.79(s, 1H), 7.85(d, J=7.9Hz, 2H), 7.80(d, J=7.7Hz, 2H), 7.62(t, J=7.3Hz, 1H ),7.48(t,J=7.4Hz,2H),7.34(t,J=7.5Hz,2H),7.04(t,J=7.4Hz,3H),6.78(t,J=7.2Hz,1H), 6.71(d,J=7.6Hz,2H). 13 C NMR (101 MHz, DMSO) δ 192.00, 152.01, 148.35, 140.11, 134.56, 133.71, 129.28, 129.03, 128.66, 128.44, 122.64, 122.38, 121.81, 119.48.
Embodiment 2
[0055] Ketone compound raw material:
[0056] Target product:
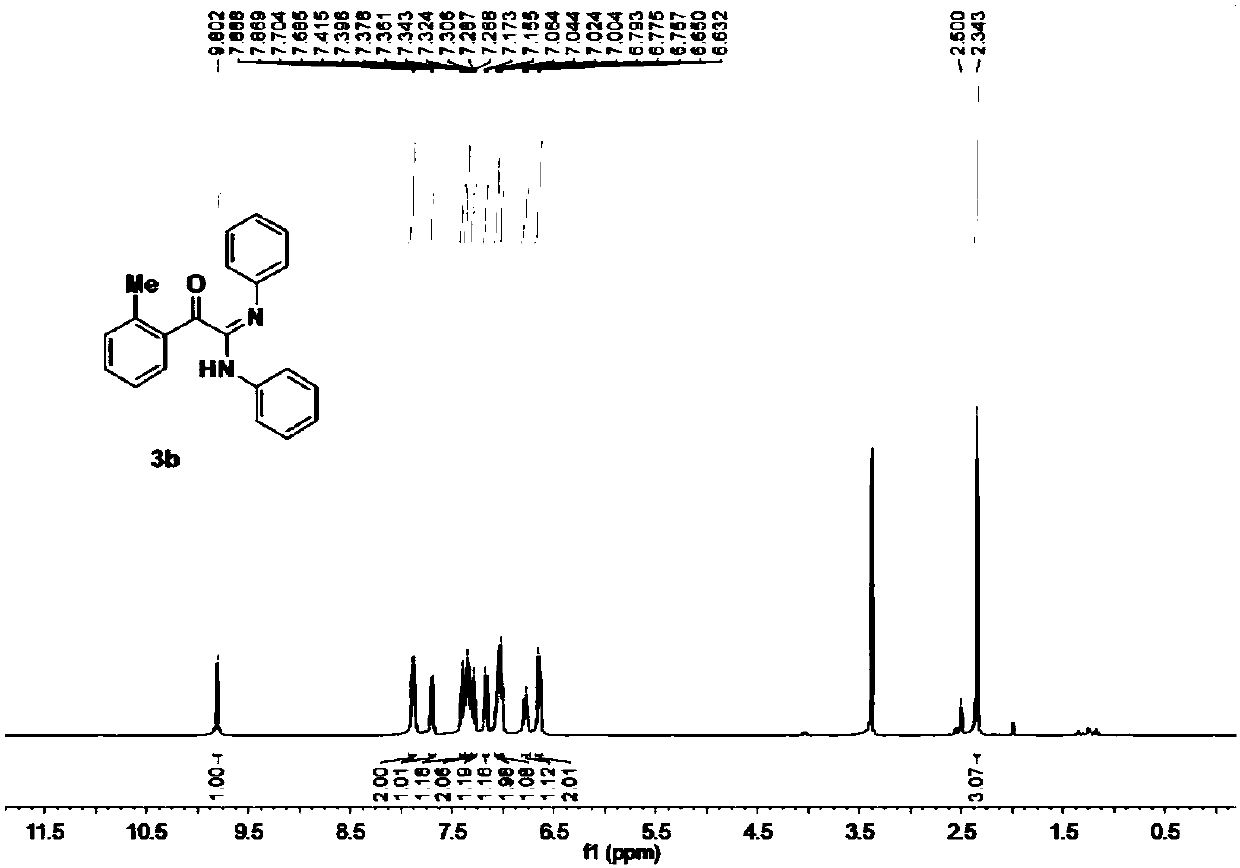
[0057] 105 mg of the target product was obtained; the yield was 67%; a yellow solid; 1 H NMR (400MHz, DMSO) δ9.80(s, 1H), 7.88(d, J=7.7Hz, 2H), 7.69(d, J=7.7Hz, 1H), 7.40(t, J=7.4Hz, 1H ),7.34(t,J=7.5Hz,2H),7.29(t,J=7.6Hz,1H),7.16(d,J=7.5Hz,1H),7.05(d,J=7.9Hz,2H), 7.00(s,1H),6.77(t,J=7.1Hz,1H),6.64(d,J=7.5Hz,2H),2.34(s,3H). 13 C NMR (101MHz, DMSO) δ194.22, 153.04, 148.38, 140.22, 139.36, 133.20, 133.17, 131.84, 131.81, 128.64, 128.29, 126.10, 122.56, 122.27, 121.62, 119.49.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com