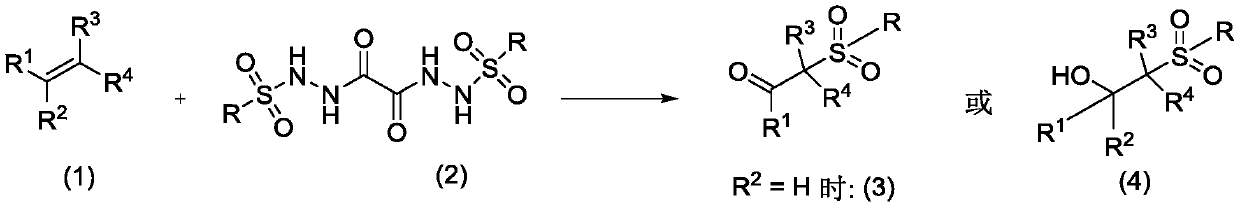
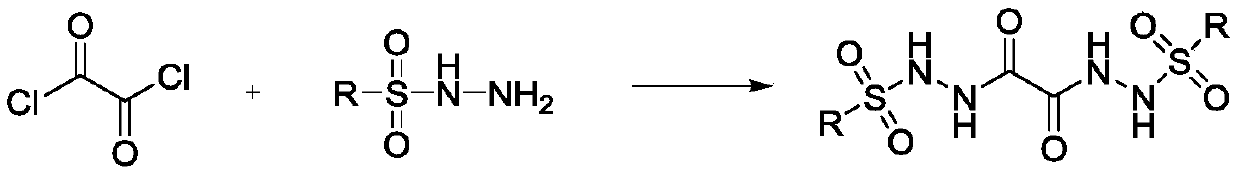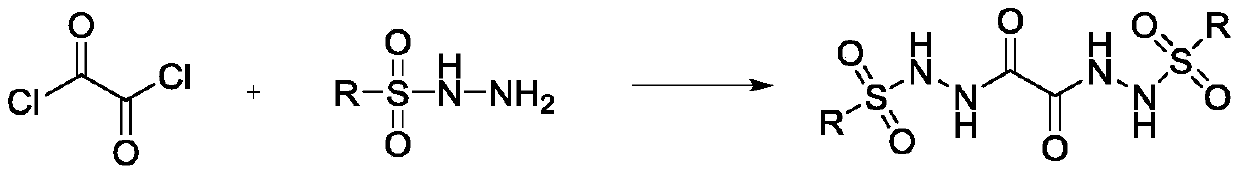Preparation method of oxalyl sulfonyl hydrazide and application of oxalyl sulfonyl hydrazide in olefin sulfonation reaction
A technology for the reaction of oxalylsulfonyl hydrazide and oxalyl chloride, which is applied to the preparation of sulfonic acid amide, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems that the preparation of oxalylsulfonyl hydrazide has not been reported, and achieve cheap raw materials , simple process and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment one (preparation of oxalyl p-toluenesulfonyl hydrazide):
[0022]
[0023] In a 100ml reaction flask, add 50ml of solvent methylene chloride and 3.2 grams (25mmol) of oxalyl chloride successively, cool in an ice bath to zero degree, then add 9.3 grams (50mmol) of p-toluenesulfonyl hydrazide and 5 grams (50mmol) of triethylamine successively dropwise. ), each dropping time was 10 minutes. After the dropwise addition, the reaction was continued at zero temperature for 5 hours, extracted three times with water and ethyl acetate. The water layer was removed, and the organic layer was dried with anhydrous sodium sulfate. The solvent was removed with a rotary evaporator, and then it was purified by silica gel chromatography (methanol / dichloromethane=1 / 10) to obtain 8.8 grams of pure oxalyl-p-toluenesulfonyl hydrazide, with a yield of 82%.
Embodiment 2
[0025] Add 2ml of solvent ethanol, 0.26g (0.6mmol) of oxalyl-p-toluenesulfonyl hydrazide, 0.052g (0.5mmol) of styrene, and 1.9mg (0.01mmol) of catalyst cuprous iodide in a 10ml reaction tube, open the Reaction at room temperature. Track the reaction until the complete disappearance of oxalyl-p-toluenesulfonyl hydrazide. After the reaction, it was extracted three times with water and ethyl acetate. The aqueous layer was removed, and the organic layer was dried over anhydrous sodium sulfate. The solvent was removed by a rotary evaporator, and then purified by silica gel chromatography (ethyl acetate / petroleum ether=1 / 10) to obtain 0.114 g of pure 2-p-toluenesulfonylacetophenone with a yield of 84%. 1 H NMR (400MHz, CDCl 3 )δ7.97(d, J=7.7Hz, 2H), 7.78(d, J=8.1Hz, 2H), 7.64(t, J=7.3Hz, 1H), 7.50(t, J=7.6Hz, 2H) ,7.35(d,J=8.0Hz,2H),4.74(s,2H),2.46(s,3H). 13 C NMR (101MHz, CDCl 3 ) δ 188.15, 145.36, 135.82, 134.31, 129.84, 129.34, 128.84, 128.62, 63.61, 21.70.
Embodiment 3
[0027] In Reaction Example 2, except that 0.052 g (0.5 mmol) of styrene was changed to 0.092 g (0.5 mmol) of 1-bromo-4-vinylbenzene, the reaction was carried out in the same manner as in Example 2. 2-p-toluenesulfonyl-4'-bromoacetophenone, yield 85%. 1 HNMR (400MHz, CDCl 3 )δ8.02(t, J=1.8Hz, 1H), 7.91(ddd, J=7.8, 1.6, 1.0Hz, 1H), 7.80–7.71(m, 3H), 7.38(dd, J=15.3, 7.8Hz ,3H),4.70(s,2H),2.47(s,3H). 13 C NMR (101MHz, CDCl 3 ) δ 186.69, 144.92, 137.41, 132.04, 130.34, 128.55, 127.98, 123.18, 63.98.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com