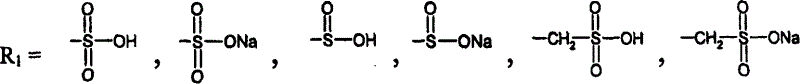Patents
Literature
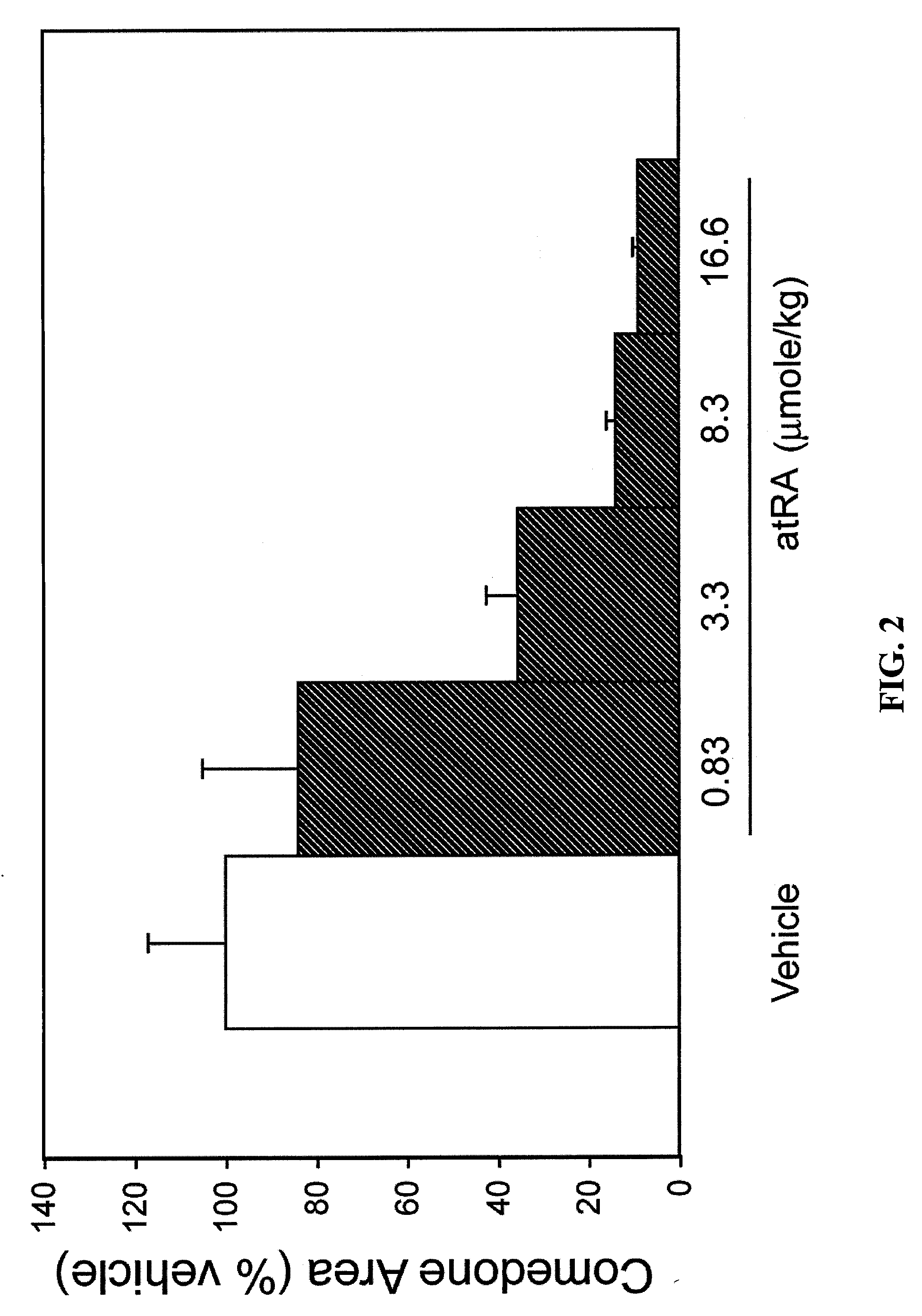
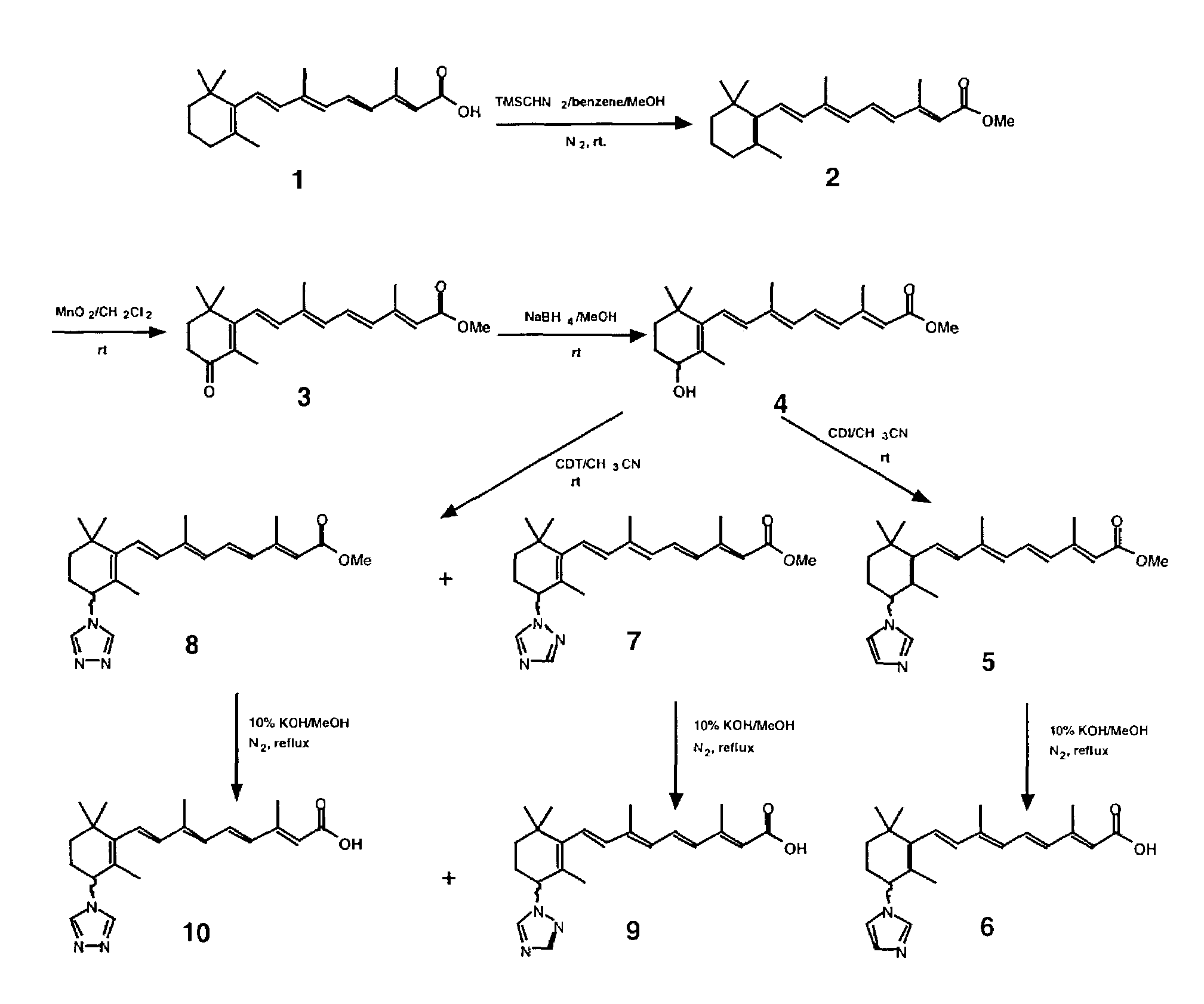
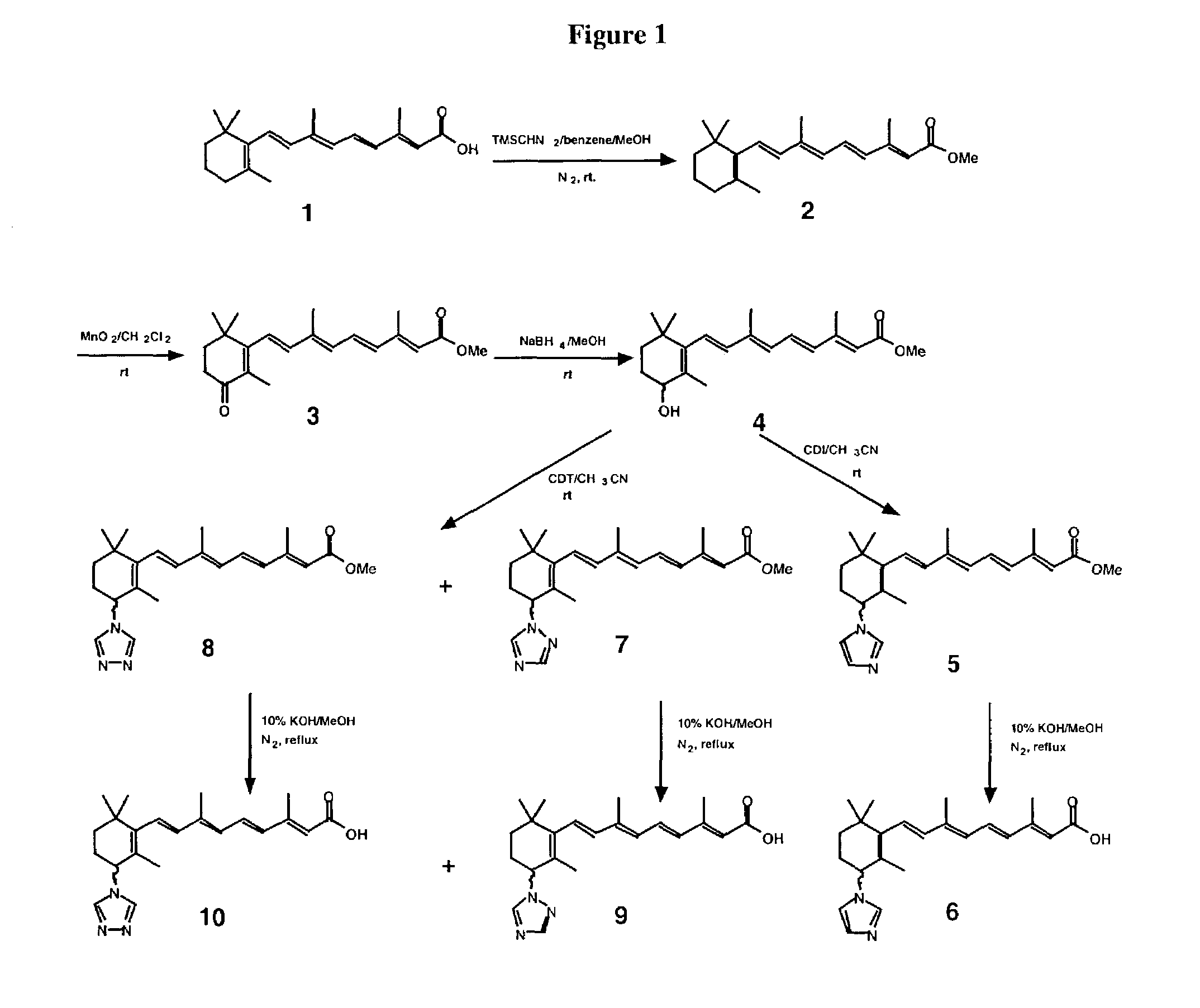
168 results about "All trans" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
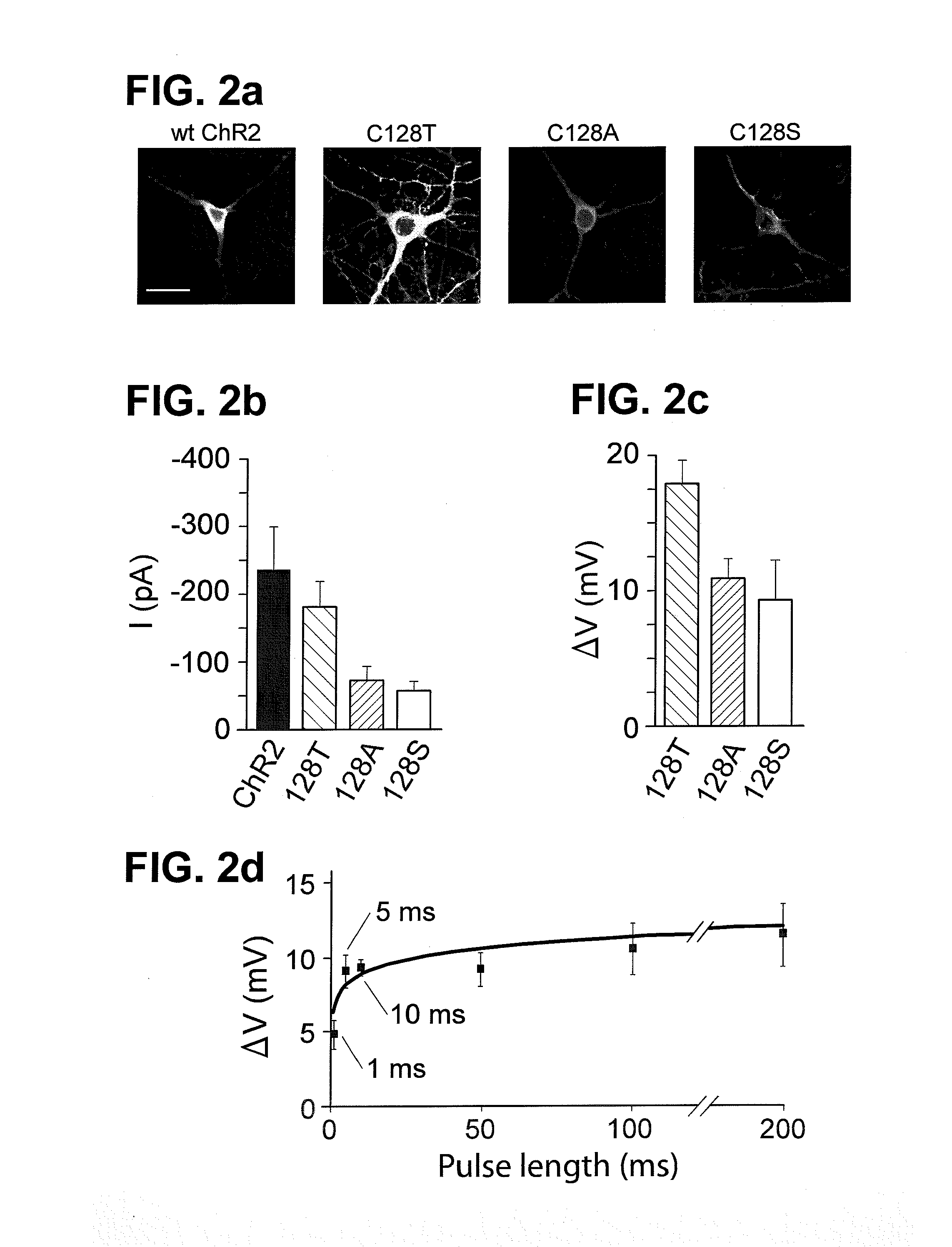
Optically-based stimulation of target cells and modifications thereto
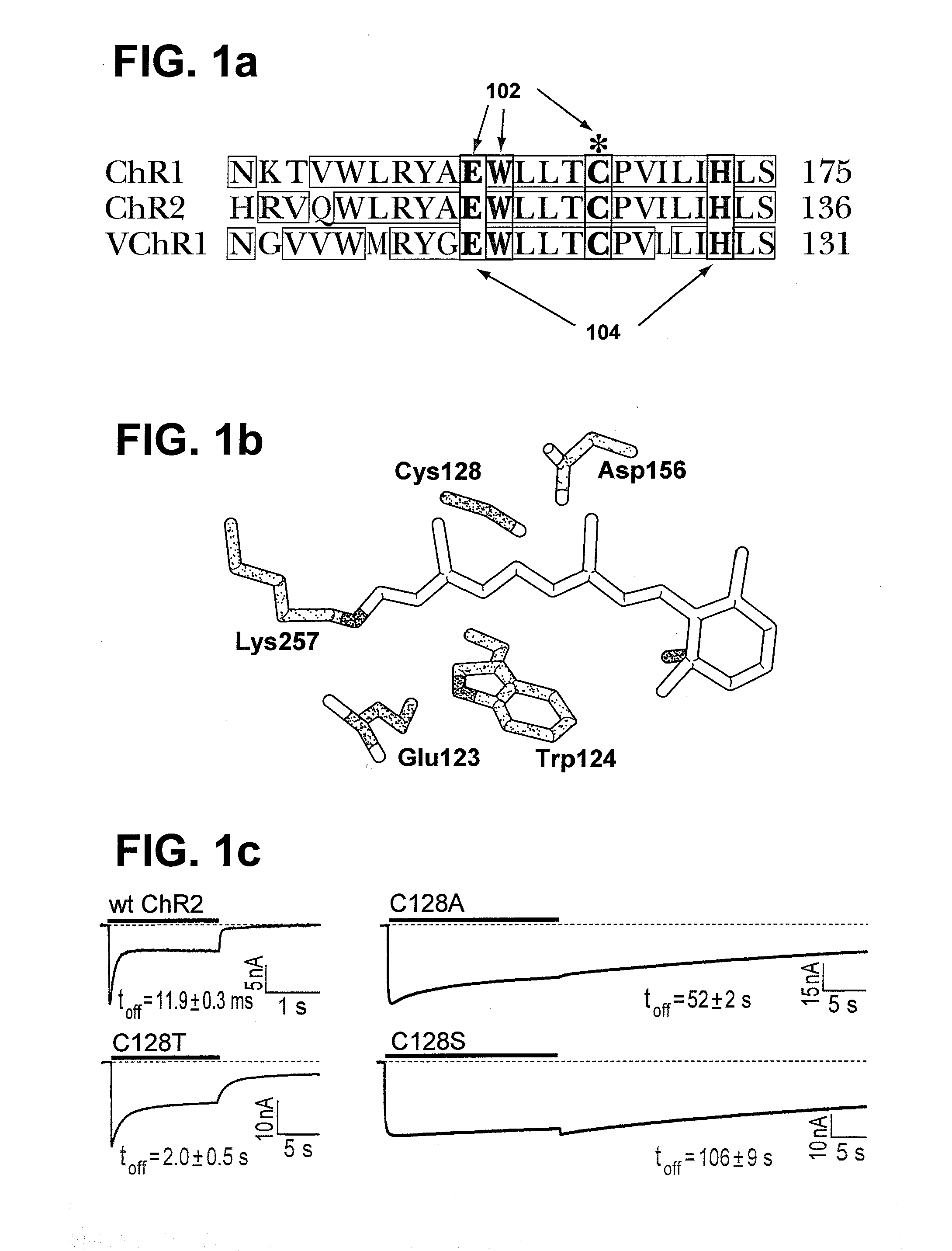
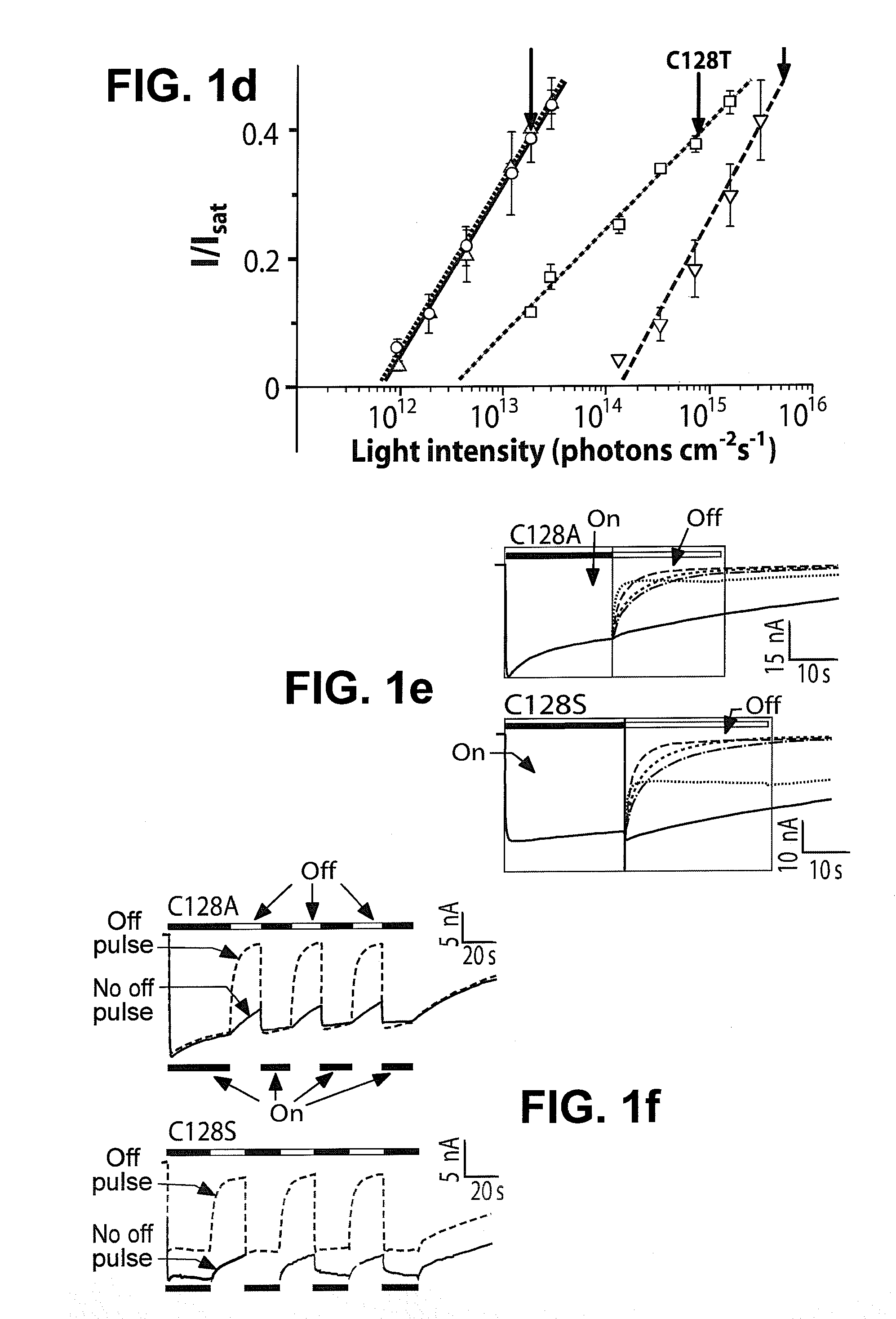
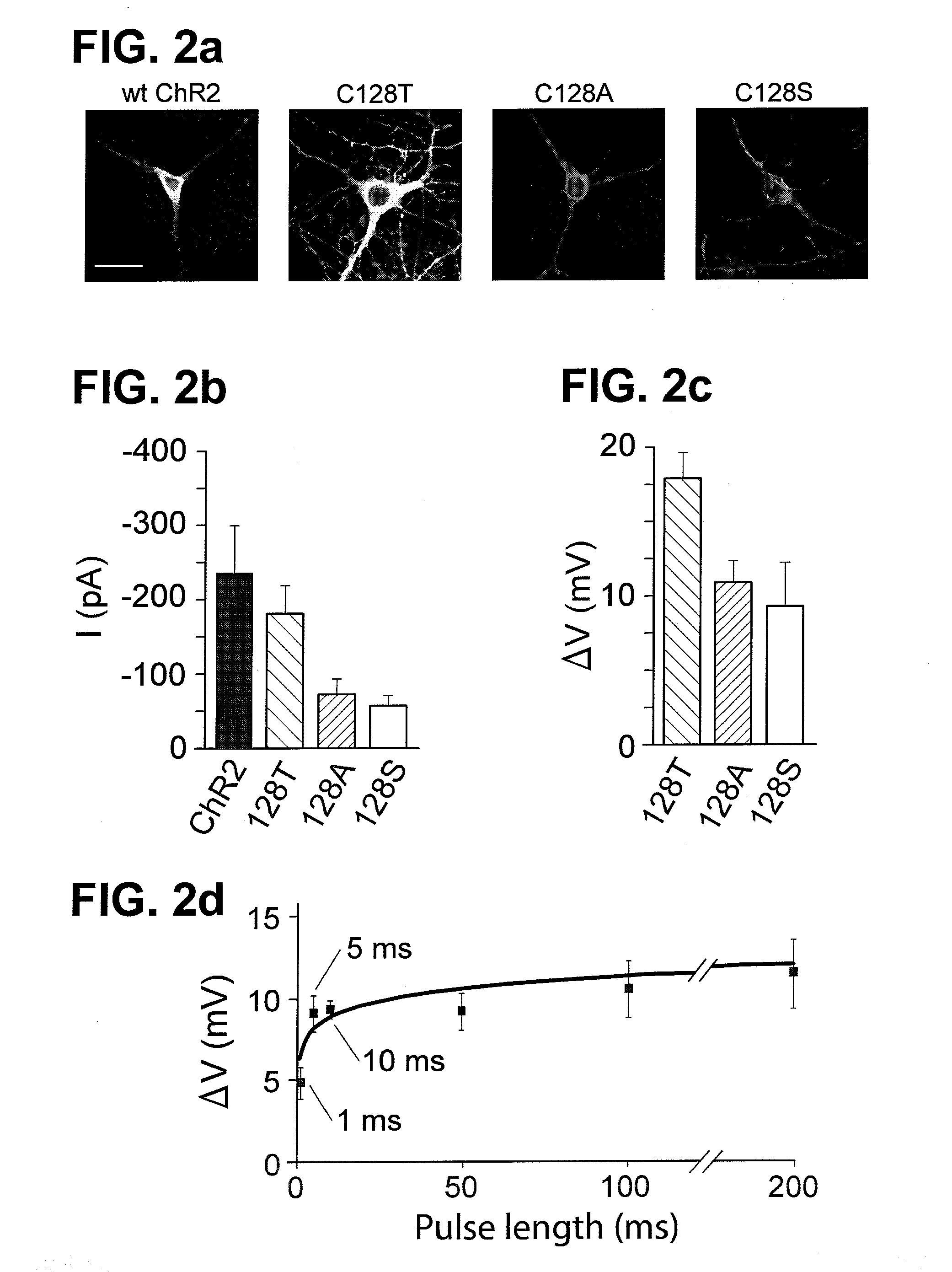
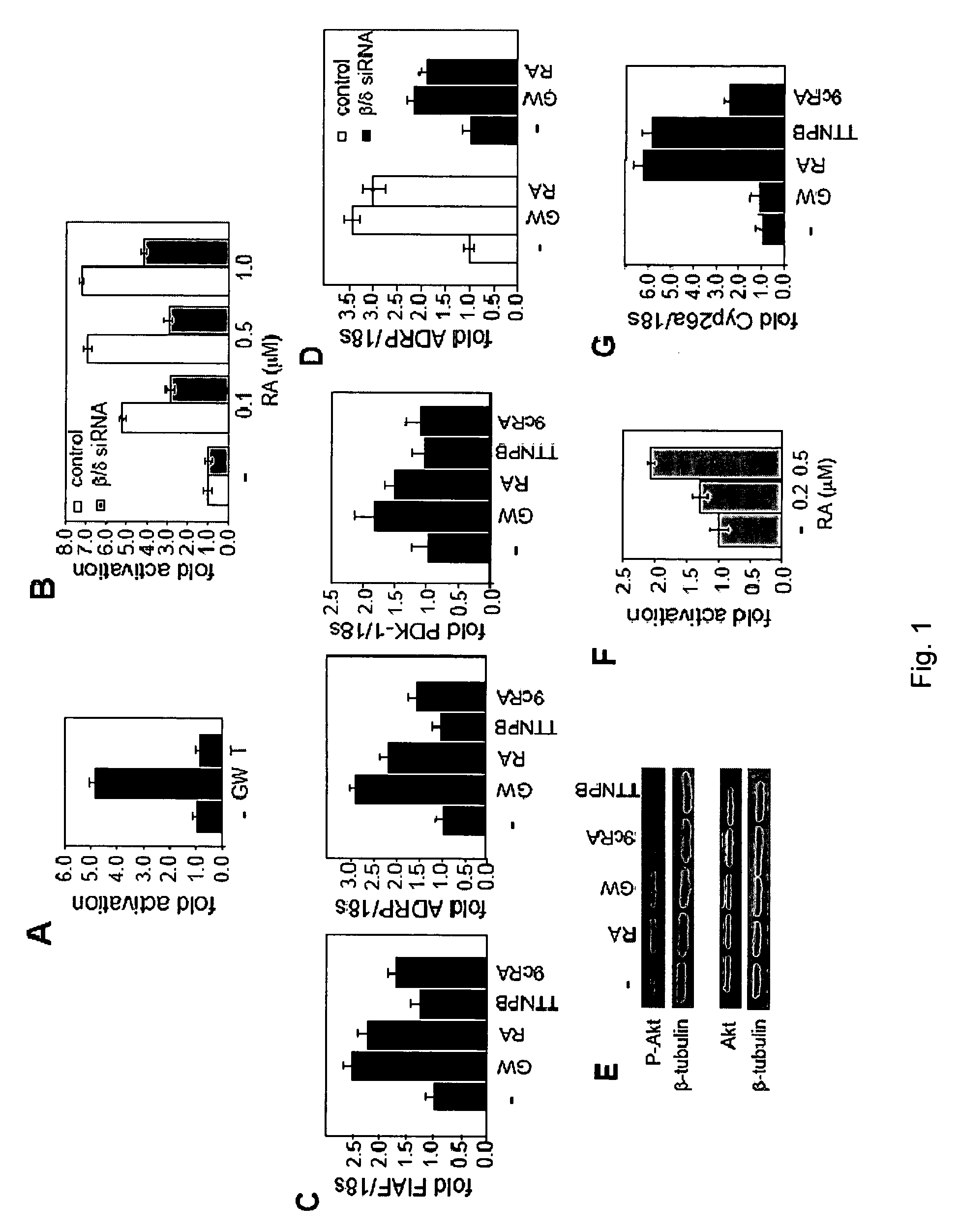
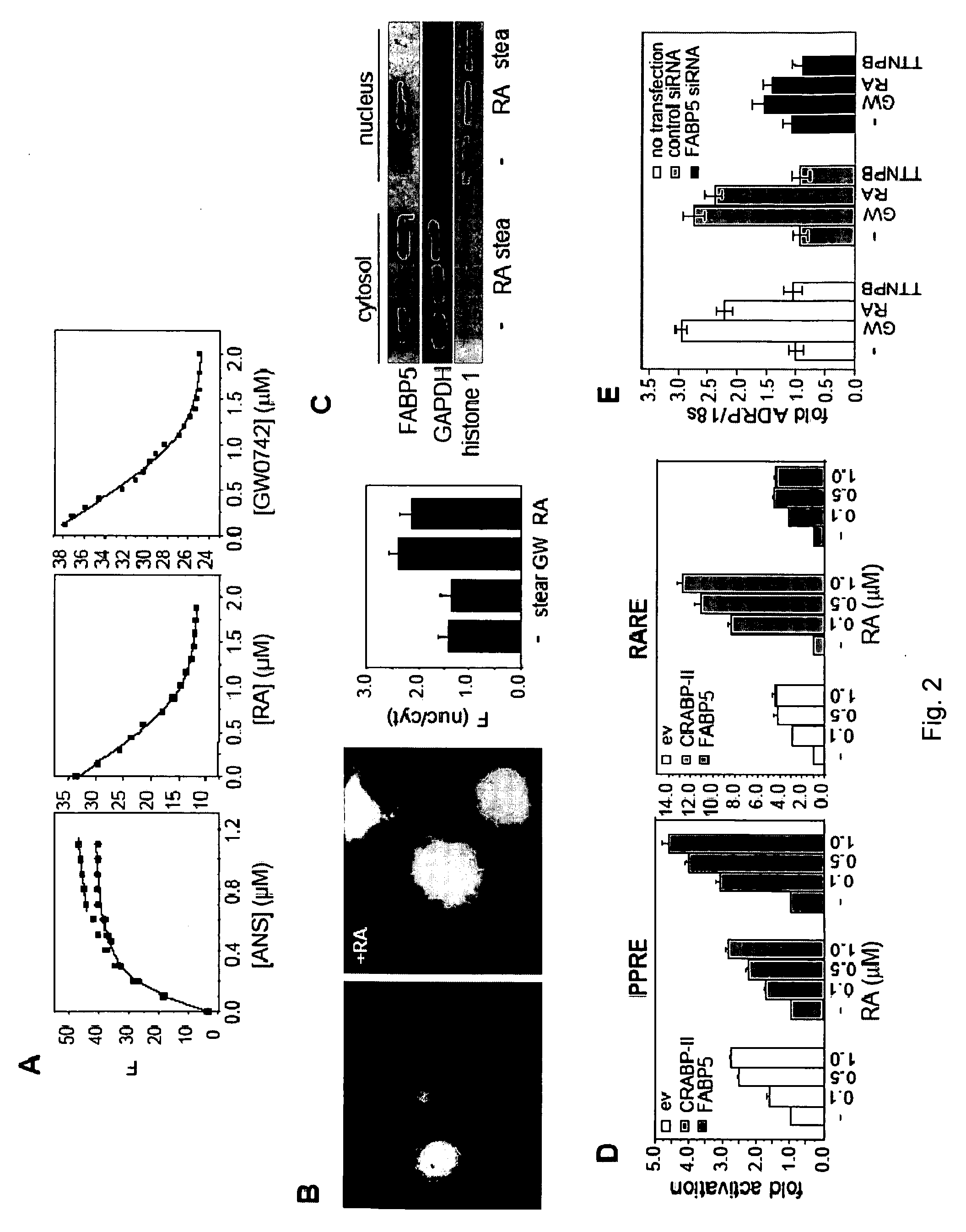
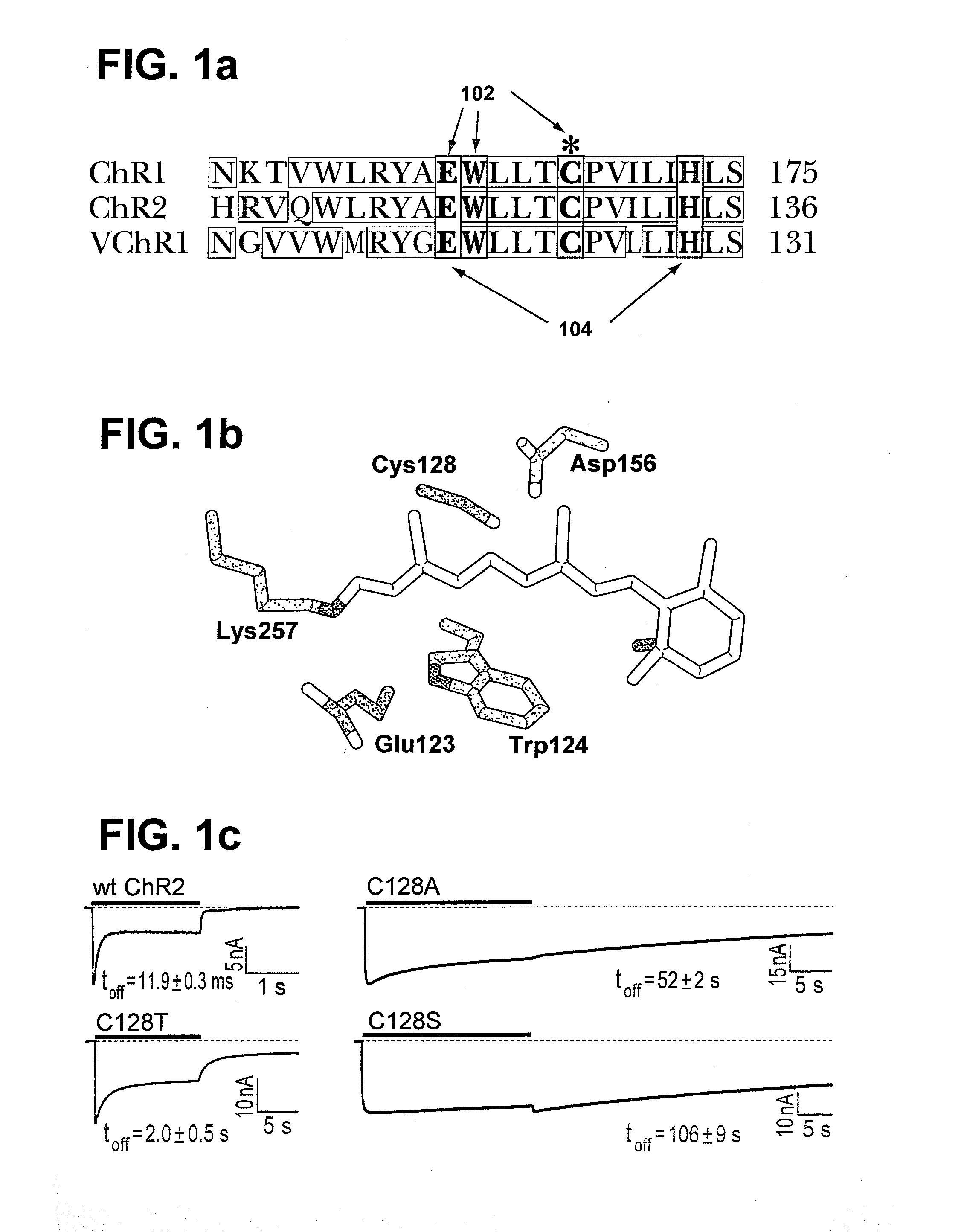
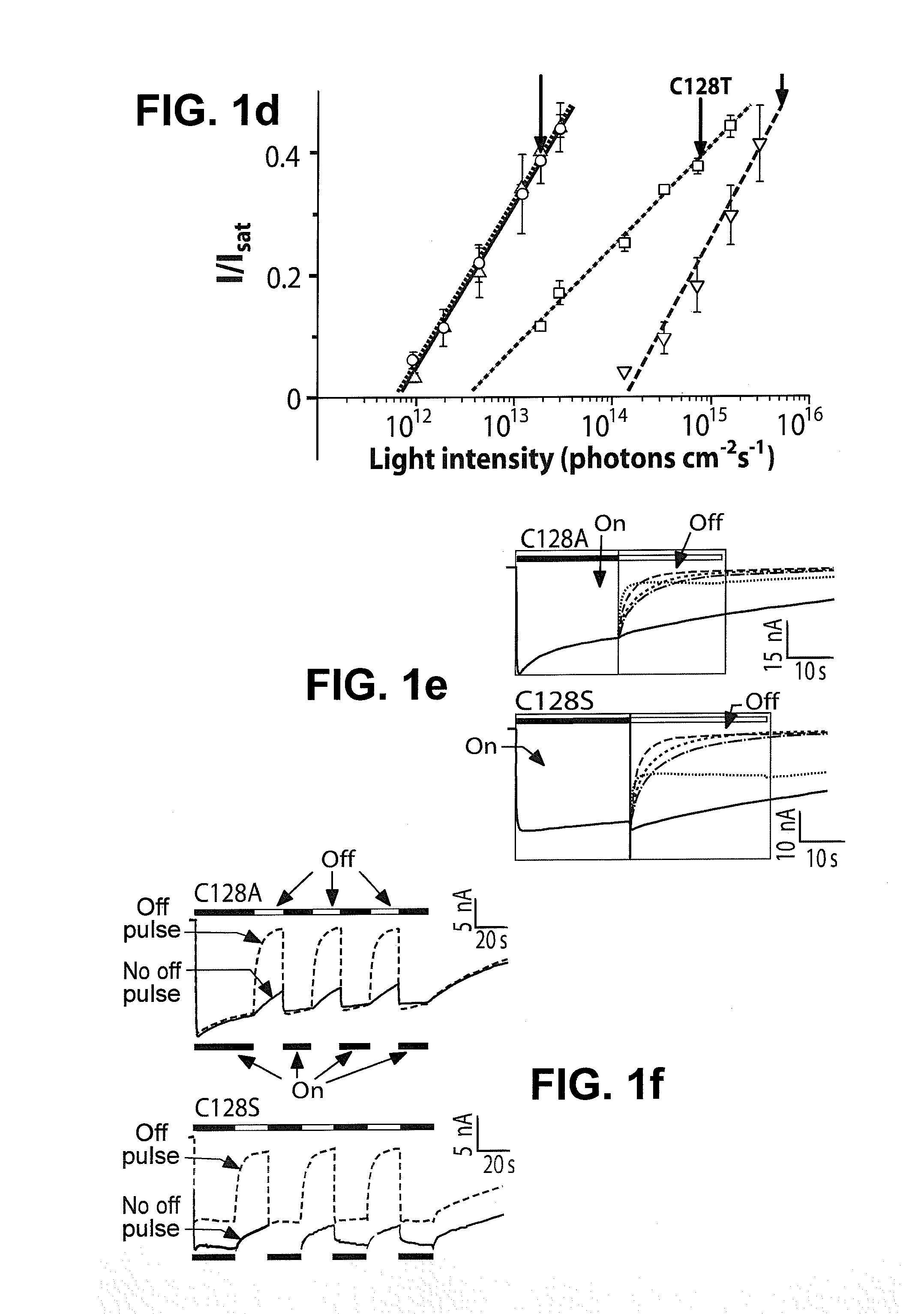
Stimulation of target cells using light, e.g., in vivo or in vitro, is implemented using a variety of methods and devices. One example involves a vector for delivering a light-activated molecule comprising a nucleic acid sequence that codes for light-activated molecule. The light-activated molecule includes a modification to a location near the all-trans retinal Schiff base, e.g., to extends the duration time of the open state. Other aspects and embodiments are directed to systems, methods, kits, compositions of matter and molecules for ion channels or pumps or for controlling currents in a cell (e.g., in in vivo and in vitro environments).
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
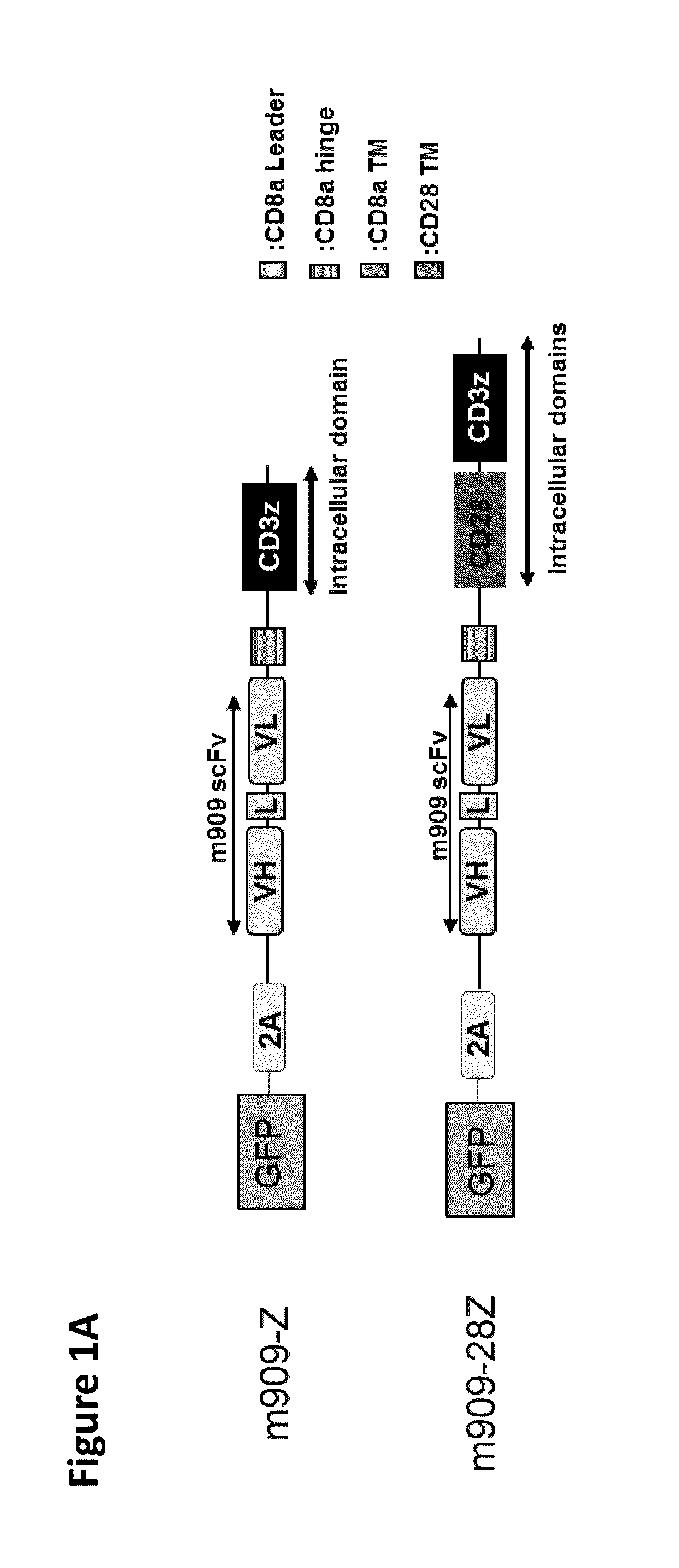
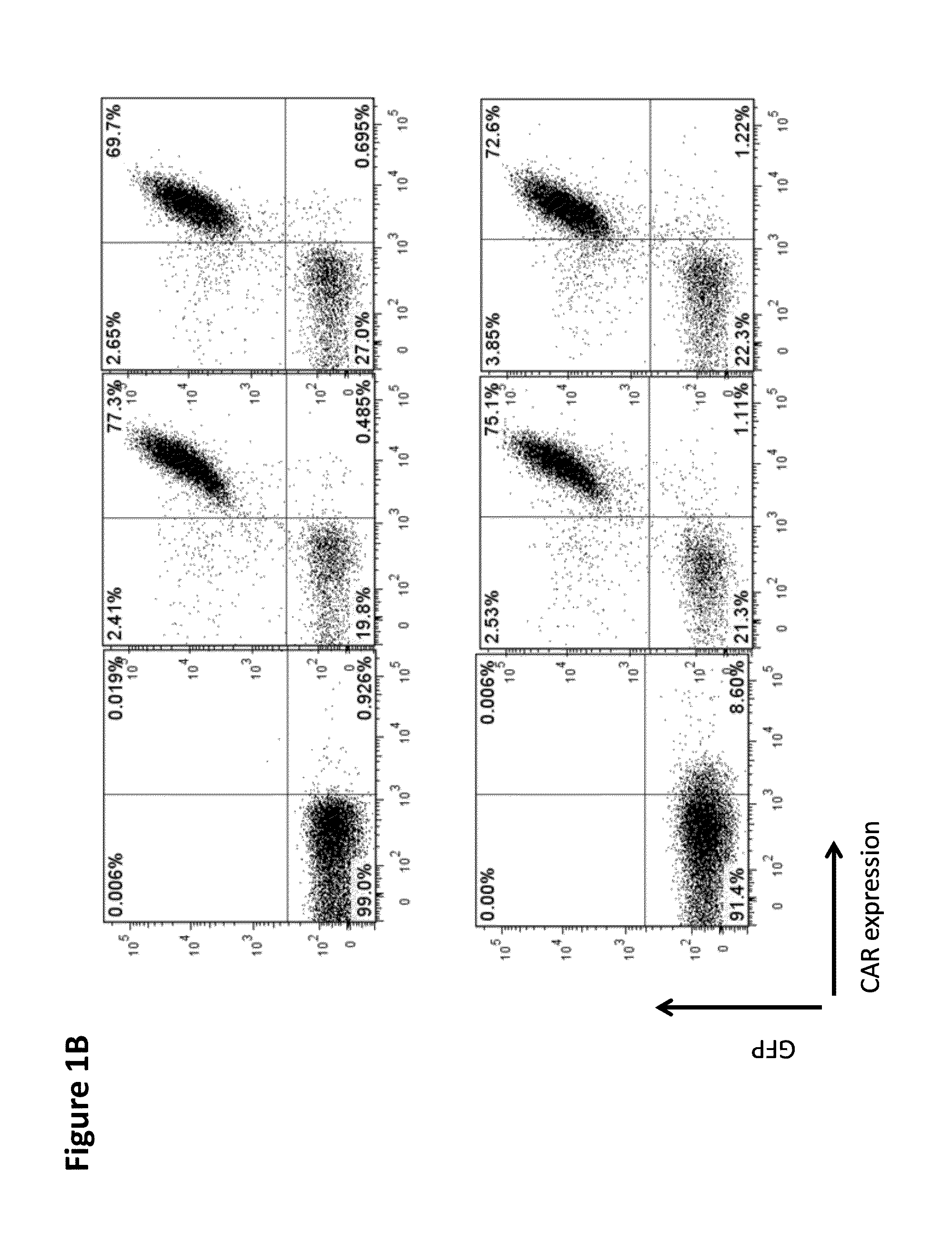
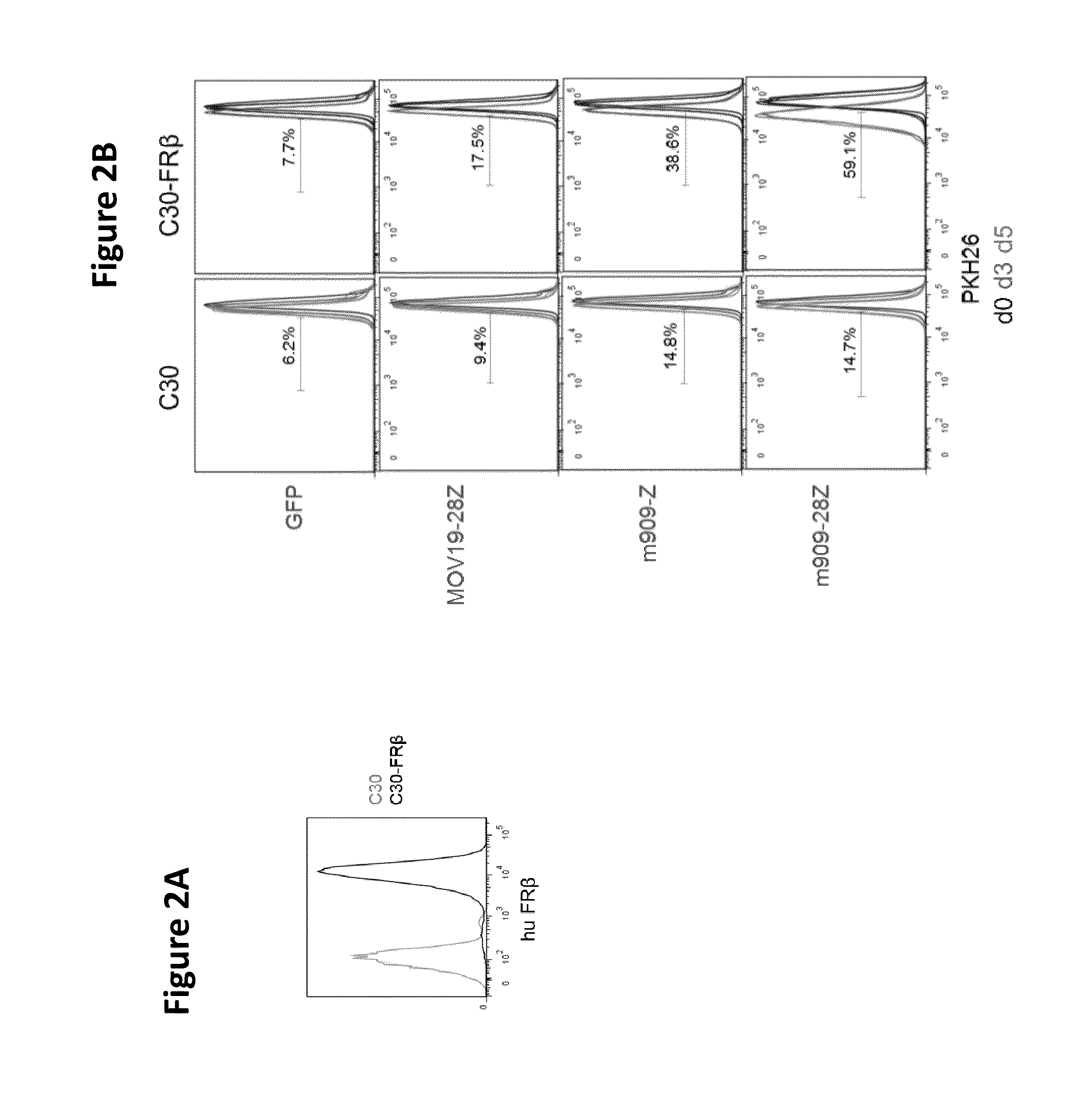
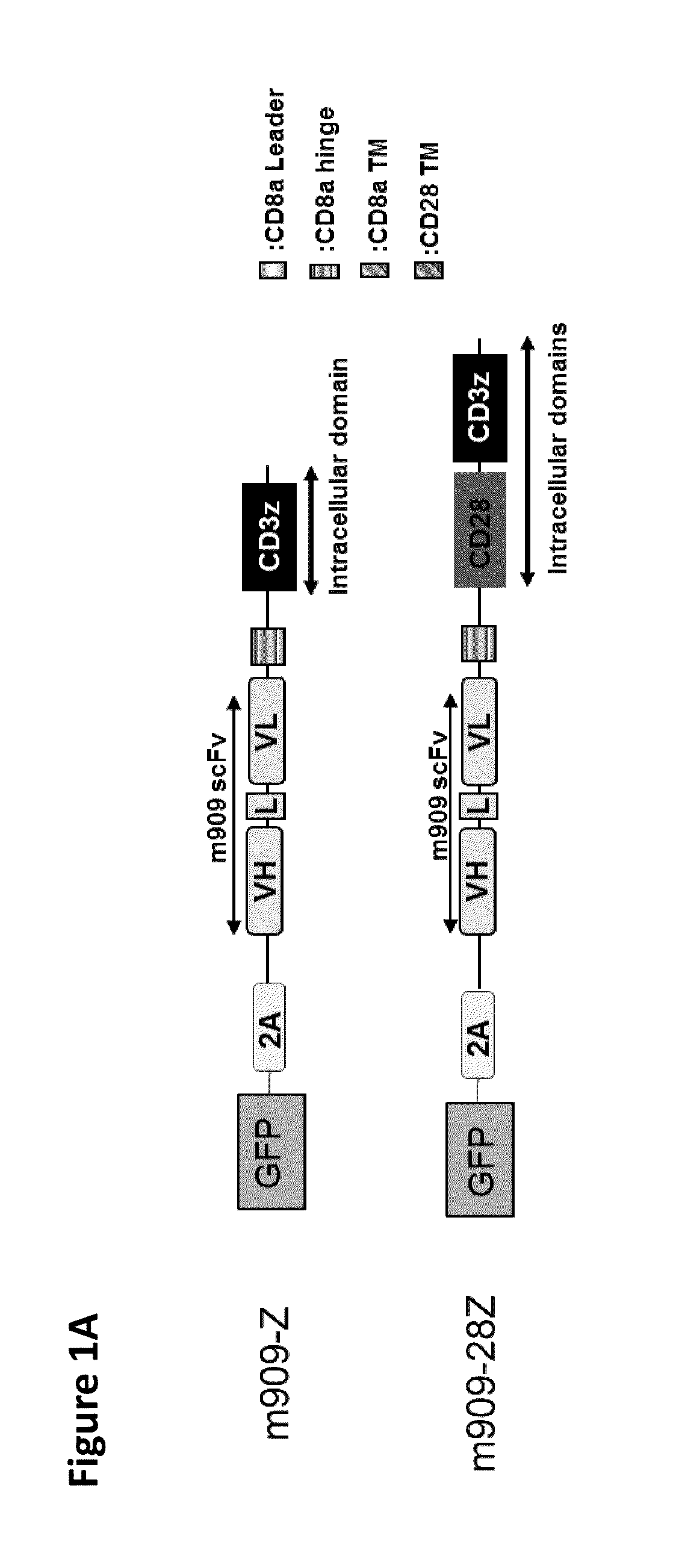
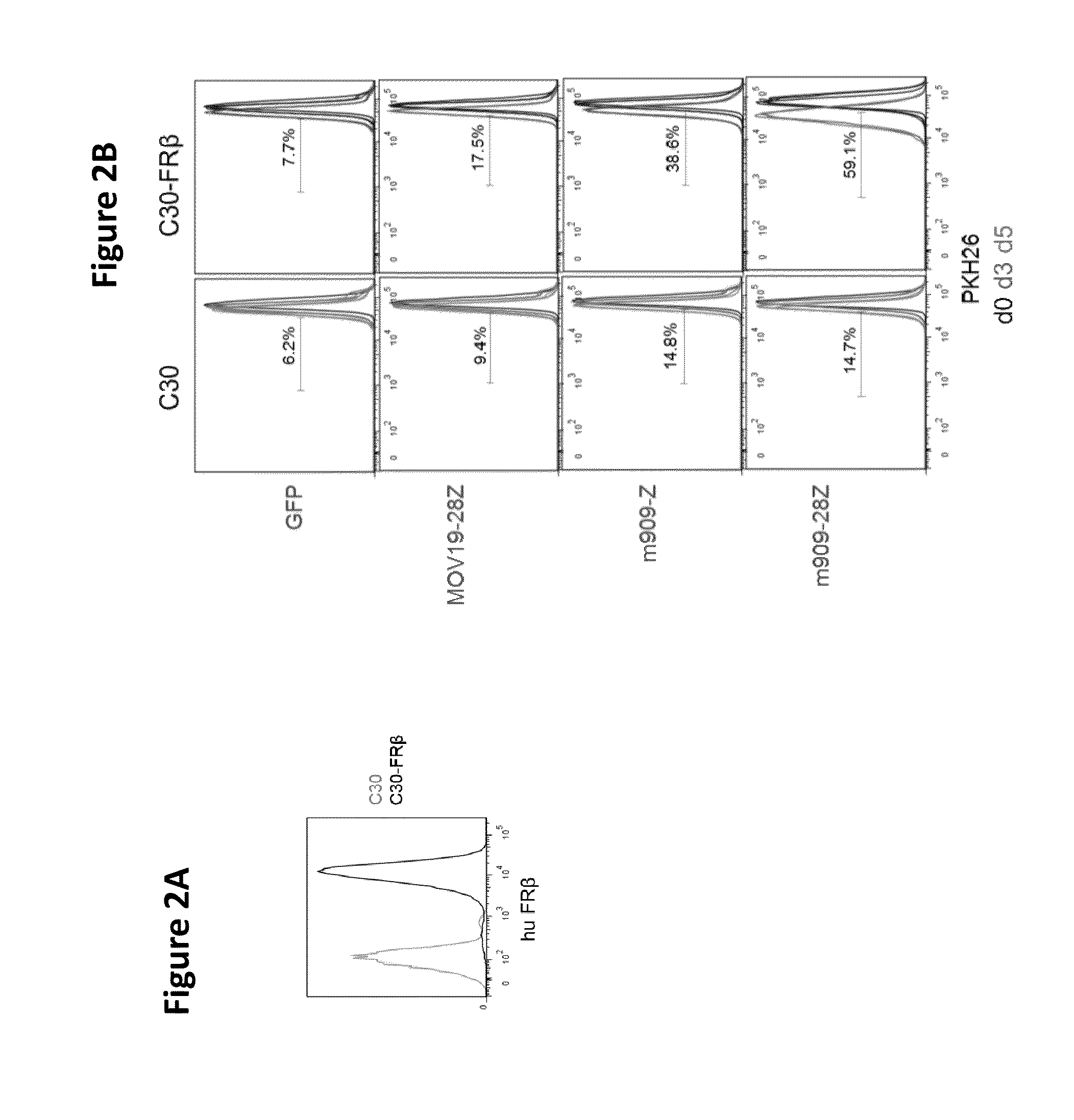
Chimeric antigen receptor specific for folate receptor beta
ActiveUS20140286973A1High affinityLow toxicityBiocideAntibody mimetics/scaffoldsAgonistBinding domain
The invention provides compositions and methods for treating leukemia, for example, acute myeloid leukemia (AML). The invention also relates to at least one chimeric antigen receptor (CAR) specific to folate receptor beta (FRβ), vectors comprising the same, and recombinant T cells comprising the FRβ CAR. The invention also includes methods of administering a genetically modified T cell expressing a CAR that comprises a FRβ binding domain in combination with a RXR agonist, such as all-trans retinoic acid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
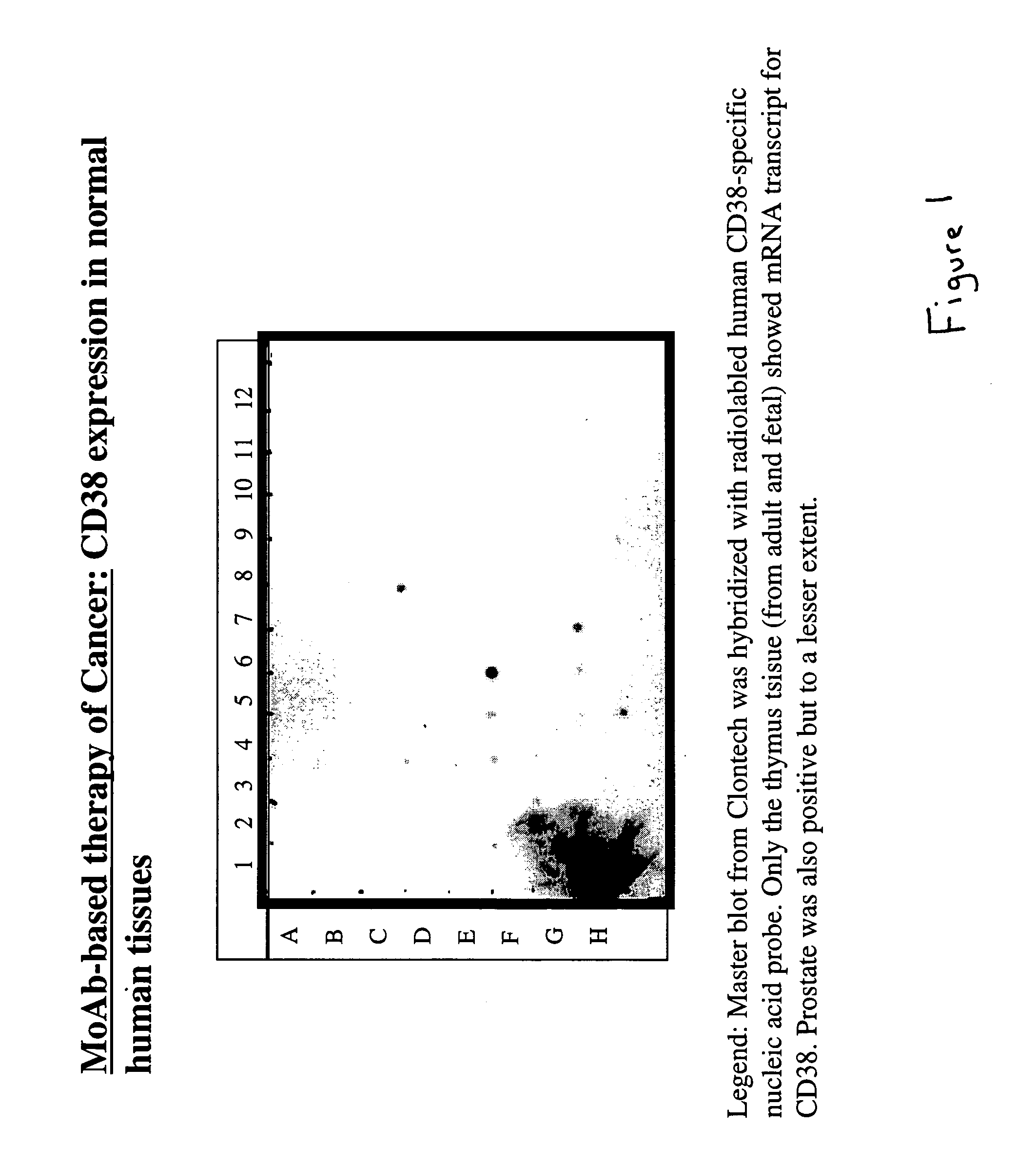
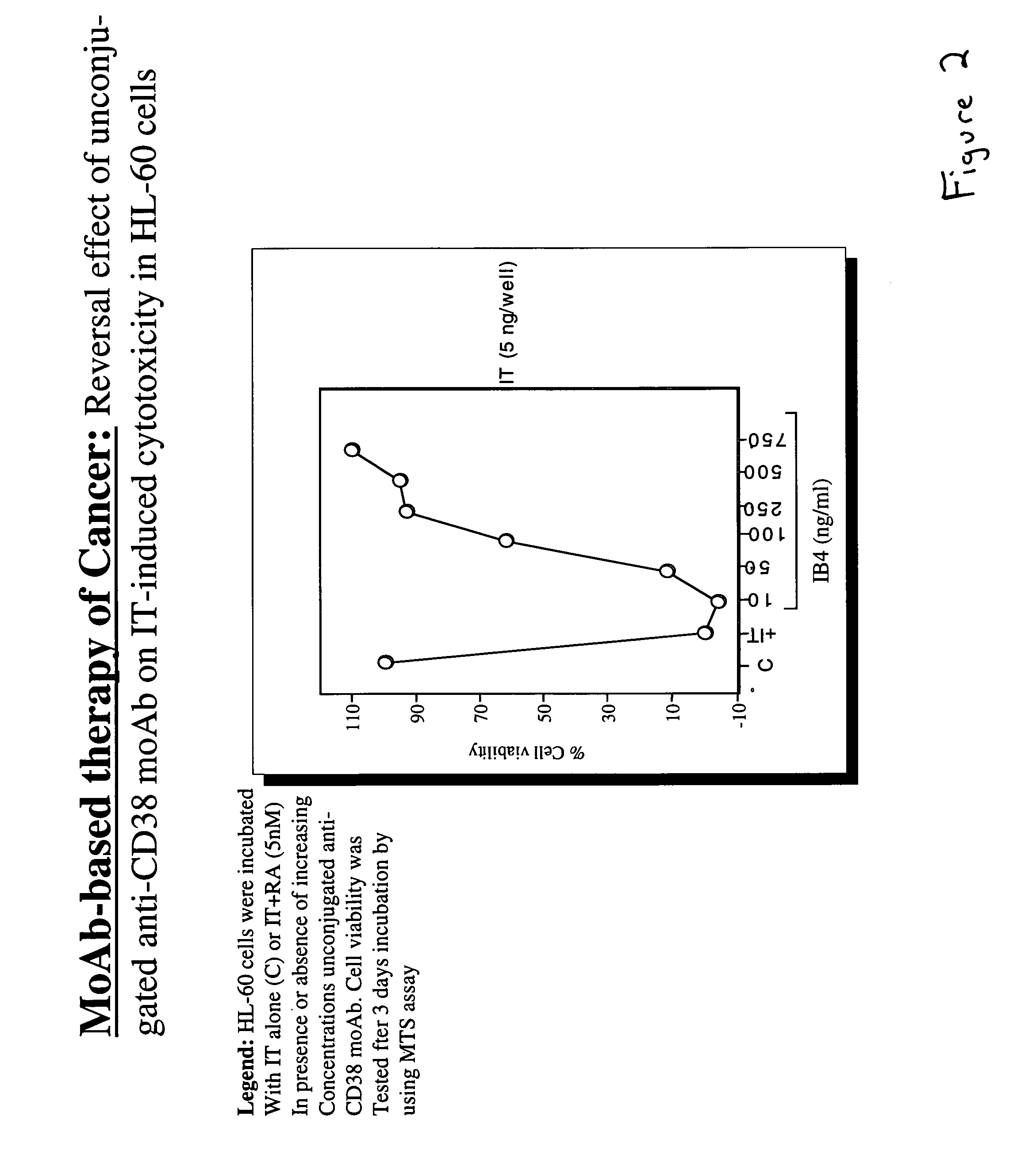
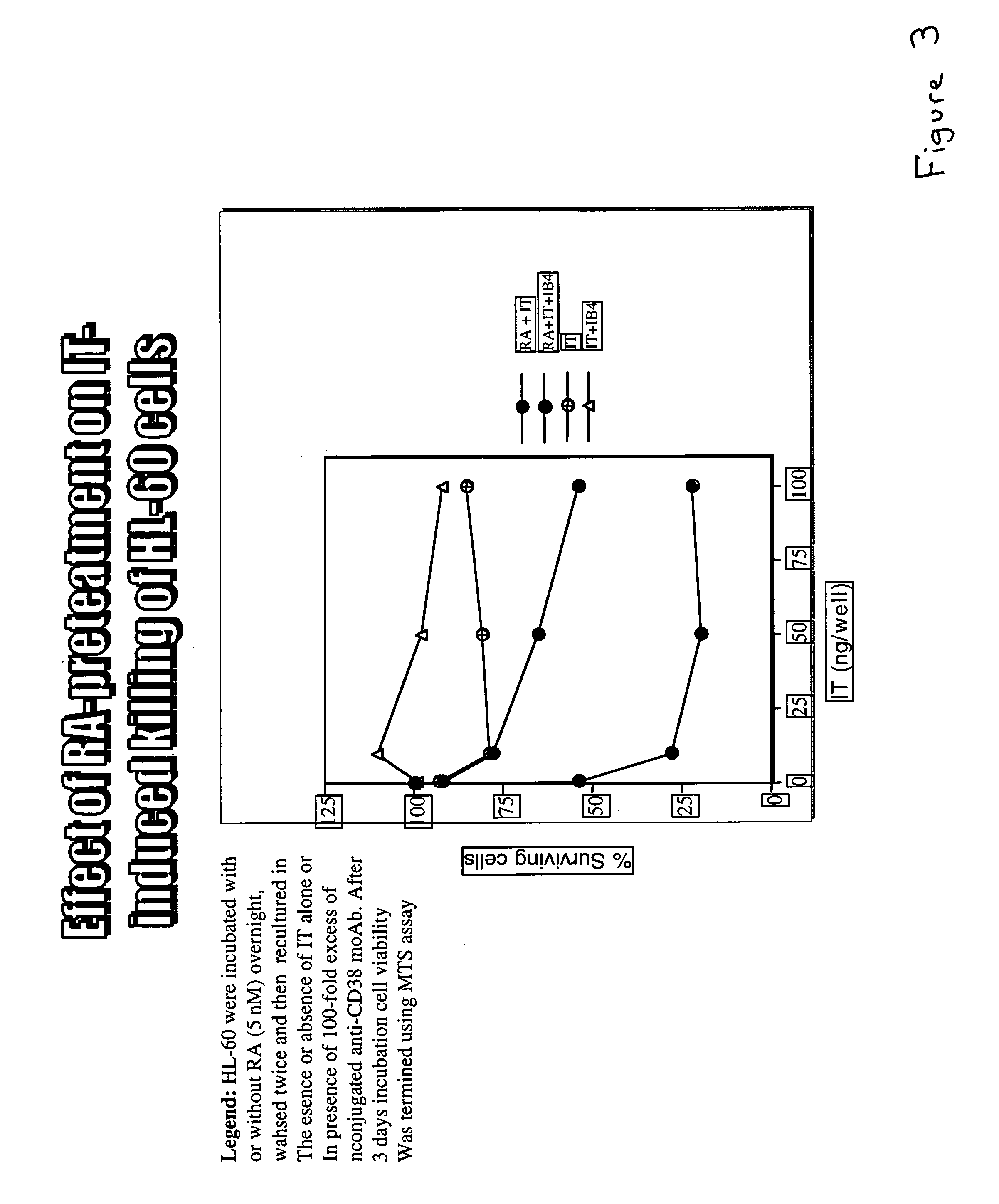
Potentiation of anti-CD38-Immunotoxin cytotoxicity
The present invention is directed to the use of agents that induce high levels of cell surface molecules to provide targets for immunotoxins directed against the same cell surface molecules. A specific example is given in which all-trans-retinoic acid (RA) is used to induce high levels of CD38 cell surface antigen expression in several myeloid and lymphoid leukemia cells. CD38 was then used as target for delivering plant toxin (gelonin) to leukemia cells. Treatment of leukemia cells with RA induced high levels of CD38 in those cells that otherwise had low CD38 expression. The RA-induced leukemia cells then became exquisitely sensitive to an immunotoxin constructed from an anti-CD38 monoclonal antibody conjugated to the plant toxin gelonin.
Owner:BOARD OF REGENTS
Therapeutic compounds
InactiveUS20040048923A1Improve pharmacological activityGood curative effectBiocideOrganic compound preparationDocetaxel-PNPTreatment effect
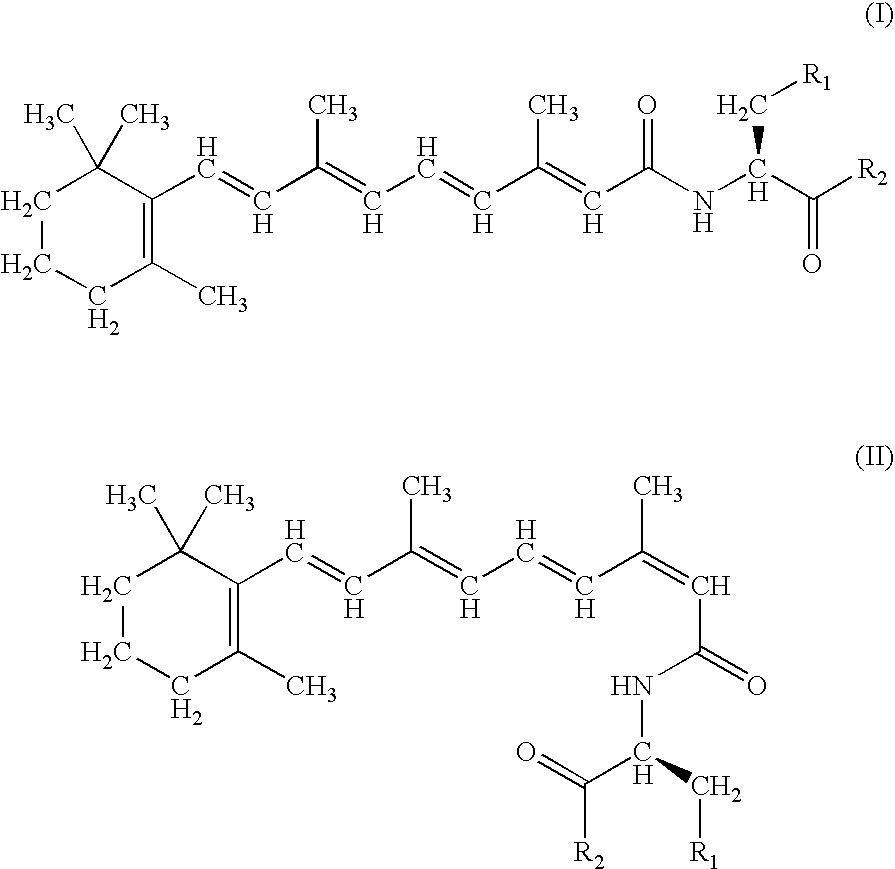
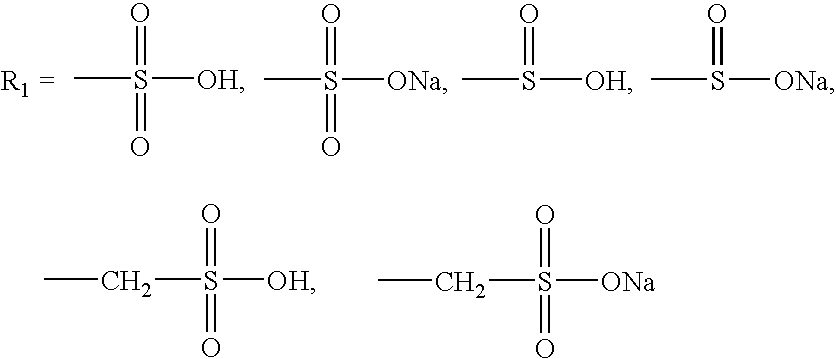
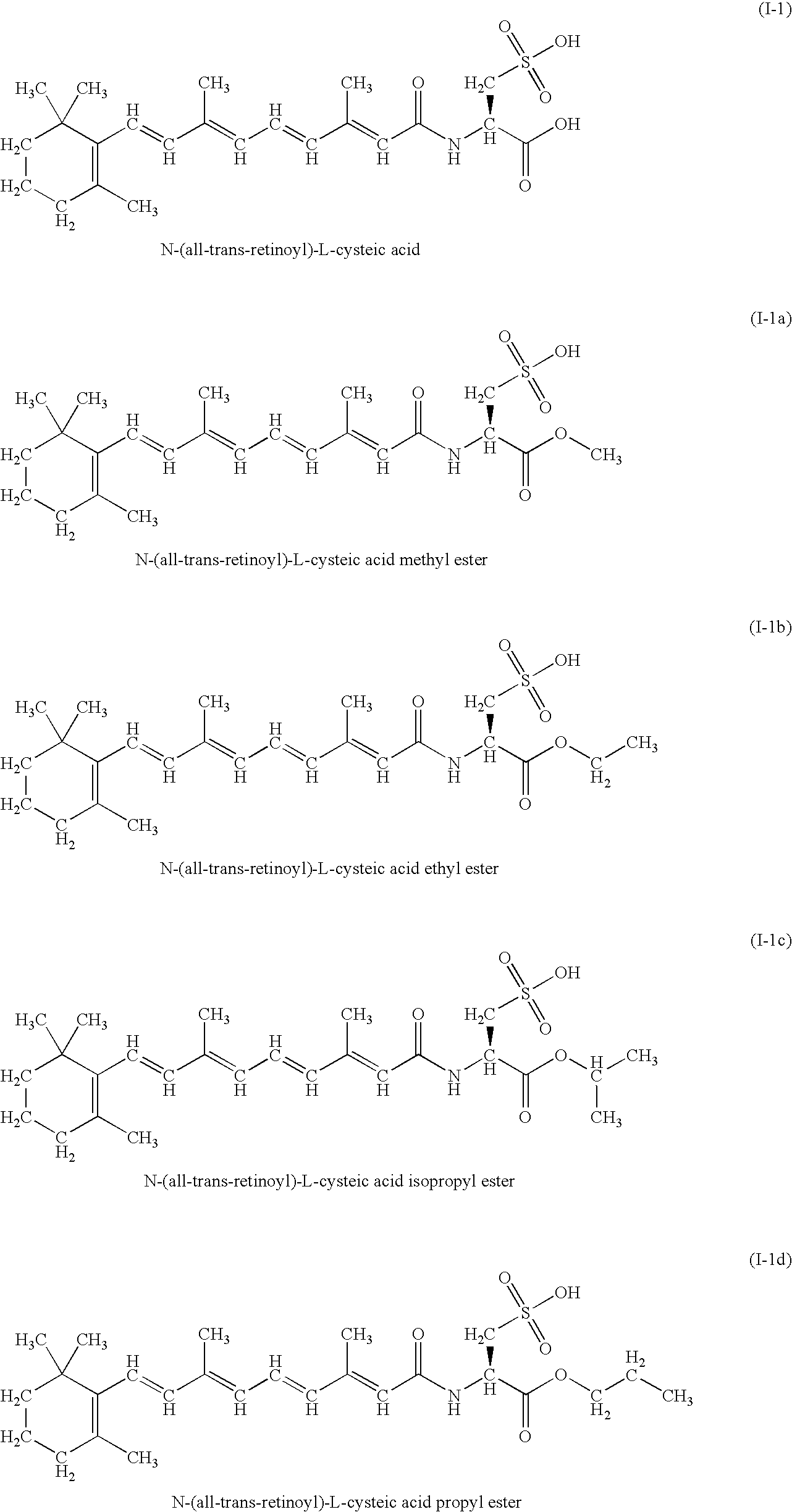
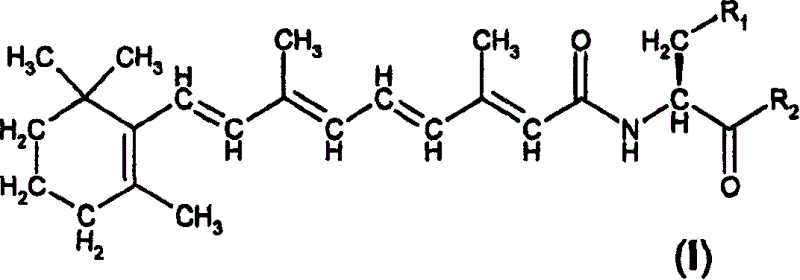
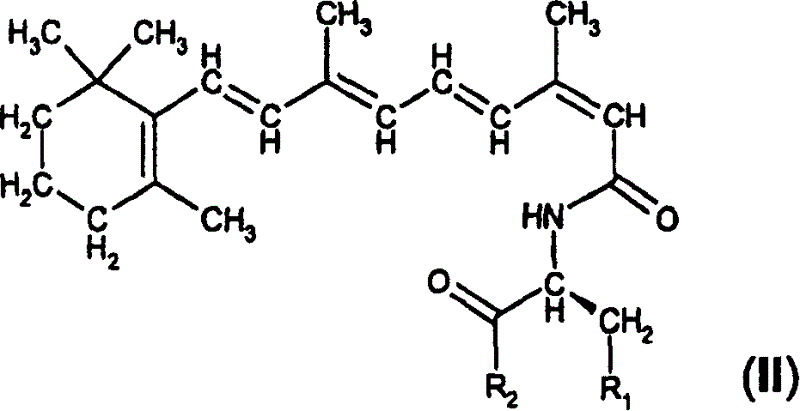
A group of new compounds, N-(all-trans-Retinoyl)-L-cysteic acid, N-(13-cis-Retinoyl)-L-cysteic acid, N-(all-trans-Retinoyl)-L-cysteinesulfinic acid, N-(13-cis-Retinoyl)-L-cysteinesulfinic acid, N-(all-trans-Retinoyl)-L-homocysteic acid, N-(13-cis-Retinoyl)-L-homocysteic acid, and sodium salts of these compounds, including sodium salts of their esters and amides, is shown to exhibit therapeutic effects per se, and which compounds in combination with cytotoxic compounds, such as docetaxel, paclitaxel, doxorubicin and mitoxantrone, exhibit a synergistic effect. These compounds make it possible to manufacture new formulations of poorly soluble pharmaceutical compounds, and the present invention discloses a process of manufacturing water-soluble formulations of such compounds, exemplified by docetaxel, and paclitaxel, exhibiting enhanced pharmacological activity, and formulations of water-soluble pharmaceuticals exemplified by doxorubicin and mitoxantrone, exhibiting improved therapeutic efficacy.
Owner:VIVESTO AB
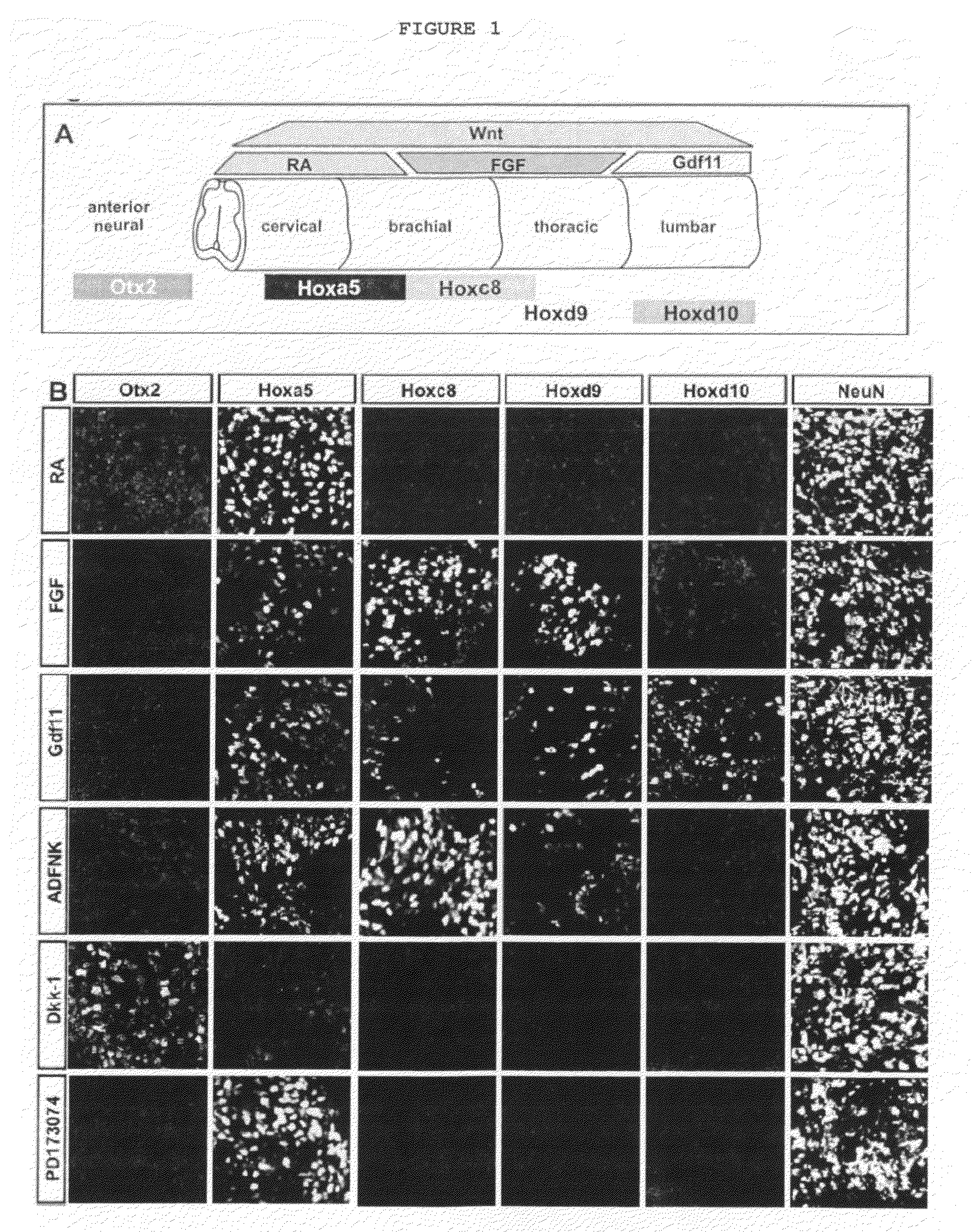
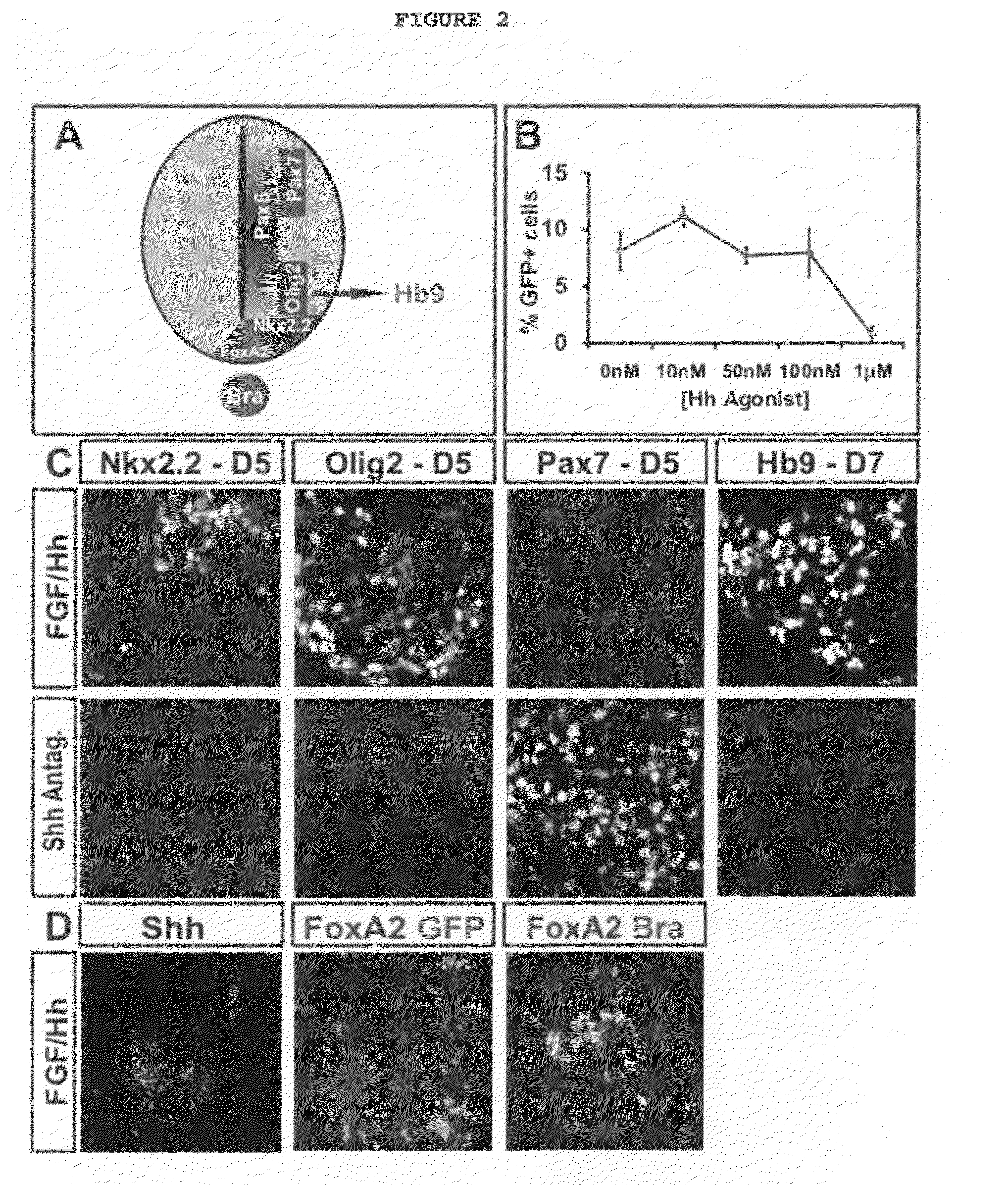
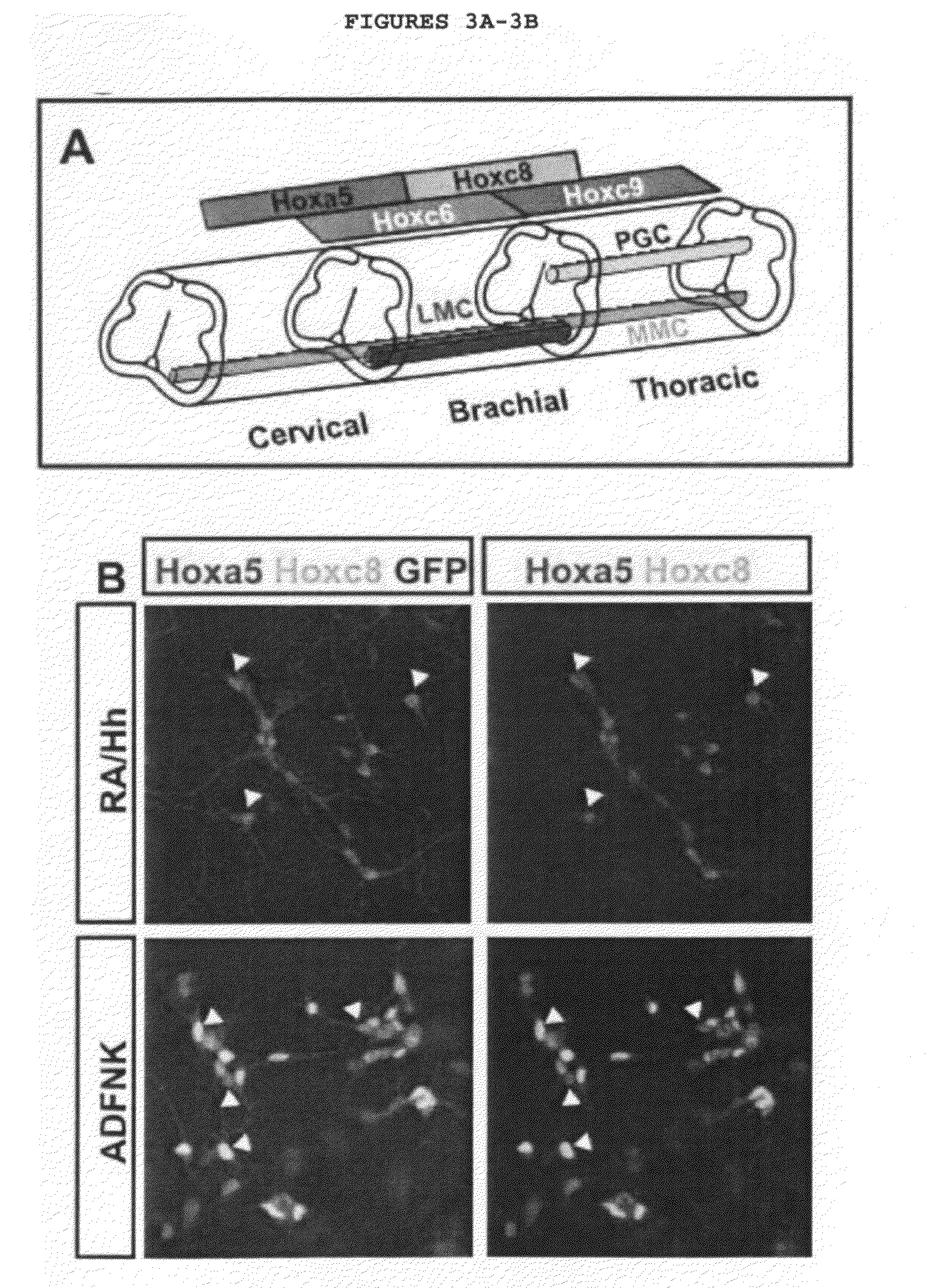
Generation of brachial, thoracic and lumbar spinal motor neurons from embryonic stem cells in the absence of all-trans retinoic acid supplement
InactiveUS20100196332A1Increase the number ofAvoid signalingBiocidePeptide/protein ingredientsFOXP1Retinoid
Disclosed are methods for generating a neuron expressing Hoxc8 transcription factor or a caudal motor neuron comprising culturing an embryonic stem cell in a composition which is essentially free of retinoids and comprises an isotonic salt solution, so as to generate the neuron which expresses Hoxc8 transcription factor or the caudal motor neuron. Disclosed are also methods for generating a caudal brachial motor neuron, a thoracic motor neuron, or a lumbar motor neuron from an embryonic stem cell in a composition essentially free of retinoids and comprising ADFNK medium, an amount of FGF-2, or Gdf11 respectively. Disclosed are also methods of transplanting a motor neuron into a subject comprising generating the motor neuron and transplanting the motor neuron into the subject. Disclosed is also a population of motor neuron cells enriched for motor neuron cells expressing Foxp1 and expressing a gene associated with Spinal Muscular Atrophy (SMA) or Amyotrophic Lateral Sclerosis (ALS).
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
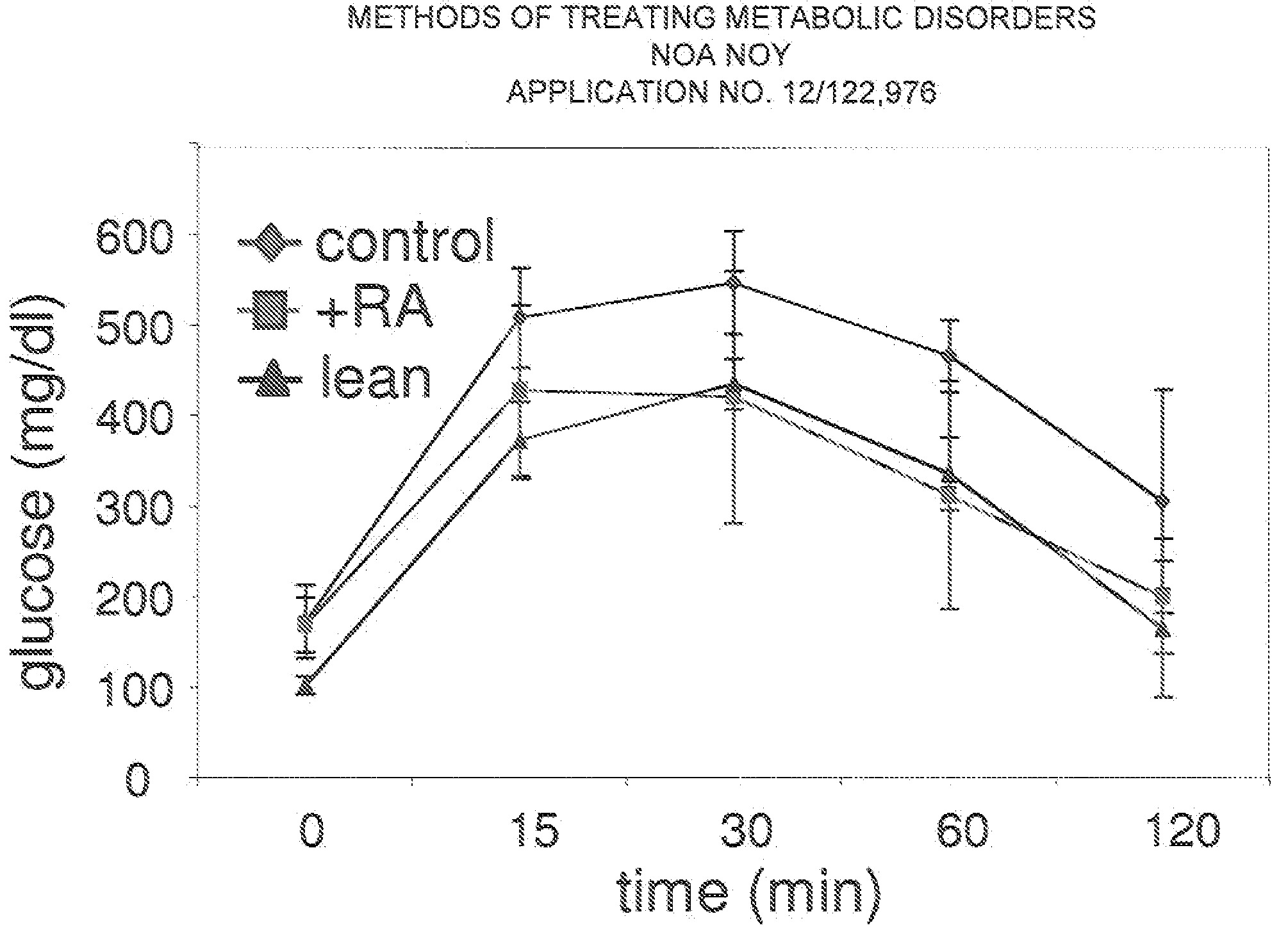
Methods of treating metabolic disorders
InactiveUS20090137671A1High sensitivityBiocideMetabolism disorderInsulin sensitivityActivating receptors
A method of increasing the insulin sensitivity of insulin resistant cells includes administering to the cells an amount of all-trans-retinoic acid effective to activate transcription factor perosixome proliferator-activated receptor (PPAR) β / δ of the cells.
Owner:CASE WESTERN RESERVE UNIV
Optically-Based Stimulation of Target Cells and Modifications Thereto
Stimulation of target cells using light, e.g., in vivo or in vitro, is implemented using a variety of methods and devices. One example involves a vector for delivering a light-activated molecule comprising a nucleic acid sequence that codes for light-activated molecule. The light-activated molecule includes a modification to a location near the all-trans retinal Schiff base, e.g., to extends the duration time of the open state. Other aspects and embodiments are directed to systems, methods, kits, compositions of matter and molecules for ion channels or pumps or for controlling currents in a cell (e.g., in vivo or in vitro environments).
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Combination Therapies with Anti-CD38 Antibodies
InactiveUS20160067205A1Hydroxy compound active ingredientsAntibody ingredientsCombination therapyCancer research
The present invention relates to combination therapies with anti-CD38 antibodies and all-trans retinoic acid.
Owner:JANSSEN BIOTECH INC
Method and application for inducing human umbilical cord mesenchyme stem cells to be differentiated into testicular interstitial cells
InactiveCN102174468AHigh differentiation efficiencyGood secretion effectSkeletal/connective tissue cellsViruses/bacteriophagesCorpus luteum graviditatisTesticular Interstitial Cells
The invention discloses a method and application for inducing human umbilical cord mesenchyme stem cells to be differentiated into testicular interstitial cells. The method comprises the following step of culturing human umbilical cord mesenchyme stem cells of patients suffering from adenovirus and carrying mice steroidogenic factor-1 genes in a DMEM-F12 culture solution containing 0.3-3ng / ml of luteinizing hormone, 200-800mu M of dibutyryl cyclic adenosine monophosphate, 5*10<-6>-5*10<-4>M of all-trans retinoic acid (ATRA), 10mU / ml of human chorionic gonadotropin and 2.4uM of adrenocorticotrophic hormone for a week. Induced by the method in the invention, the human umbilical cord mesenchyme stem cells can be differentiated into testicular interstitial cells in vitro and provides important sources of cells for treating testosterone shortage by the cell replacing method or the genetic method.
Owner:JINAN UNIVERSITY
Method for preparing lutein water-soluble dry powder
ActiveCN101177540AMultiple smallImprove solubilityNatural dyesFood preparationSolubilityFluidized bed drying
The invention discloses a preparation method of food-grade lutein water-soluble dry powder. The content of the all-trans isomer of the active ingredient in the product obtained by the existing method is low, and the structure is amorphous, which affects the coloring effect or bioavailability of the product. The steps of the present invention are as follows: after mixing lutein crystals with a low-boiling, volatile organic solvent with high solubility to lutein crystals, heating and dissolving to obtain an oil phase; mixing denatured starch with water, heating and dissolving, and cooling to obtain an aqueous phase ; The oil phase is slowly added to the water phase under stirring to obtain an emulsified mixed solution; the emulsified mixed solution is uniformed through a high-pressure homogenizer, so that the particle size of the emulsion reaches the nanometer level; the organic matter in the emulsion system is removed by conventional separation methods Solvent; use spray drying method or spray-starch fluidized bed drying method to remove water in the emulsion to obtain dry powder. The invention greatly increases the all-trans content of the active ingredients in the final product, is all amorphous, and has good coloring and nutritional strengthening effects.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Method for preparing lutein water-soluble dry powder
ActiveCN101177540BMultiple smallImprove solubilityNatural dyesFood preparationSolubilityFluidized bed drying
The invention discloses a preparation method of a food grade phylloxanthin water-soluble dry powder. The content of the all-trans isomer of the active ingredient of the product obtained by means of the prior method is low with an amorphous structure, so that the coloring effect or bioavailability of the product is affected. The procedures of the invention are as follows: when a phylloxanthin crystal is mixed with a low boiling point and volatile organic solvent which has a big solubility to the phylloxanthin crystal, and then the mixer is heated and dissolved so as to get an oil phase; a modified starch is mixed with water, and the mixer is warmed up and dissolved, then is cooled so as to get a water phase; the oil phase is slowly added into the water phase after mixed around, so that an emulsive mixed liquor is gotten; the emulsive mixed liquor is uniform through a high-pressure uniform machine, thereby the latex grain diameter can reach the nanometer level; the organic solvent in thelatex system can be removed by a conventional separation method; the normal water in the latex can be removed to get the dry powder by a spray drying process or spray starch fluidized bed drying process. The invention can greatly improve the all-trans content of the active ingredient of the termination product in amorphous form, thereby the invention has the advantages of good coloring and nutrition strengthening effects.
Owner:ZHE JIANG MEDICINE CO LTD XINCHANG PHARMA FAB
Chimeric antigen receptor specific for folate receptor β
The invention provides compositions and methods for treating leukemia, for example, acute myeloid leukemia (AML). The invention also relates to at least one chimeric antigen receptor (CAR) specific to folate receptor beta (FRβ), vectors comprising the same, and recombinant T cells comprising the FRβ CAR. The invention also includes methods of administering a genetically modified T cell expressing a CAR that comprises a FRβ binding domain in combination with a RXR agonist, such as all-trans retinoic acid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for preparing all-trans tretinoin
ActiveCN101774954AEasy to separateThorough responseOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsWittig reactionTretinoin
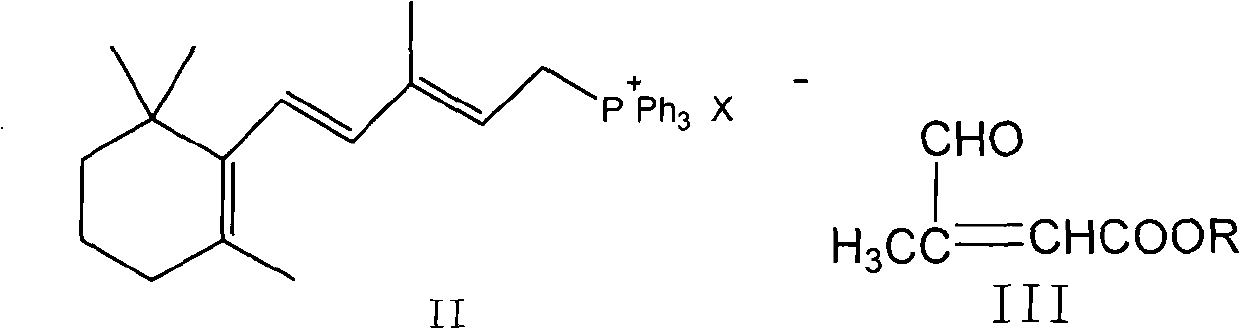
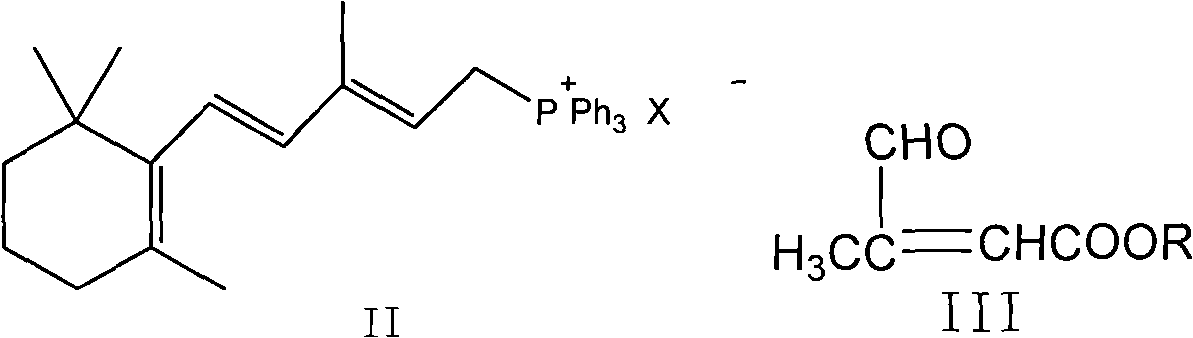
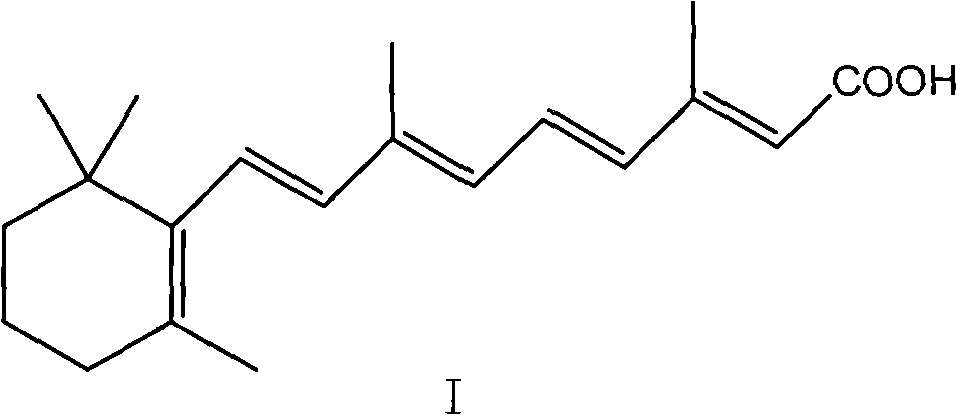
The invention provides a method for transforming 11-cis-tretinoin into all-trans tretinoin, which is characterized by using a palladium nitrate composite catalyst. The invention also provides a method for synthesizing the all-trans tretinoin, which comprises the following steps of: using compounds (II) and (III) as raw materials to perform WITTIG reaction under the action of alkali so as to obtain a mixture of all-trans retinoic acid ester and 11-cis-retinoic acid ester; performing hydrolysis and acidification to obtain a mixture of the all-trans tretinoin and an isomer 11-cis-tretinoin; and transforming the isomer to obtain the target product namely the all-trans tretinoin. The method can be finished by two steps and has the advantages of complete reaction, high yield, less isomer content in the finished product and low cost, and is suitable for industrial production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Method of preparing nano-dispersed high-all-trans-carotenoid microcapsules
ActiveUS20120018912A1Easy to operateEasy to controlCosmetic preparationsPowder deliveryAntioxidantDesolvation
A method of preparing nano-dispersed high-all-trans-carotenoid microcapsules is provided, comprising: preparing 10-20% carotenoid suspension by milling the high-all trans-carotenoid crystals with dichloromethane until the particle size thereof is in the range of 2-5 μm, then supplying the suspension together with preheated dichloromethane of another pass into a dissolving tank to obtain a solution of 0.5-2%; delivering the solution together with ethanol or isopropanol into a crystallization device having high gravity rotating packed bed simultaneously and continuously, and then into a wiped-film evaporator for desolvation until the solid content is 10-20%, then a transparent alcohol dispersion of carotenoid is obtained; mashing the alcohol dispersion together with an aqueous solution containing an antioxidant and protective colloid and spray drying to obtain nano-dispersed high-all-trans-carotenoid microcapsules. As the crystals are nano-dispersed and the content of trans-isomer is more than 90%, the carotenoid microcapsules of present inventions exhibit high bioavailability.
Owner:ZHEJIANG NHU CO LTD +2
Composite of all-trans-retinoic acid and liposome and application thereof
InactiveCN101843584AInhibition of reproductionPrevent recurrenceHydroxy compound active ingredientsPharmaceutical non-active ingredientsLipid formationVeratric acid
The invention discloses a composite of all-trans-retinoic acid and liposome and application thereof. The composite of all-trans-retinoic acid and liposome consists of the liposome and the all-trans-retinoic acid encapsulated in the liposome. The composite is prepared by a method comprising: 1) dissolving lipid materials and the all-trans-retinoic acid to prepare a mixture of a lipid membrane and the all-trans-retinoic acid; 2) hydrating the mixture of the liquid membrane and the all-trans-retinoic acid to prepare a hydration product; and 3) performing ultrasonic on the hydration product to prepare the composite of the all-trans-retinoic acid and the liposome. Experiments of the invention prove that the composite of the all-trans-retinoic acid and the liposome can be used for inhibiting the reproduction of tumor cells, and the combined application of the composite of the all-trans-retinoic acid and the liposome and Vinorelbine invisibile liposome produces the strongest inhibitory effect on the recrudesce of tumors.
Owner:PEKING UNIV
Formulations useful against hepatitis C virus infections
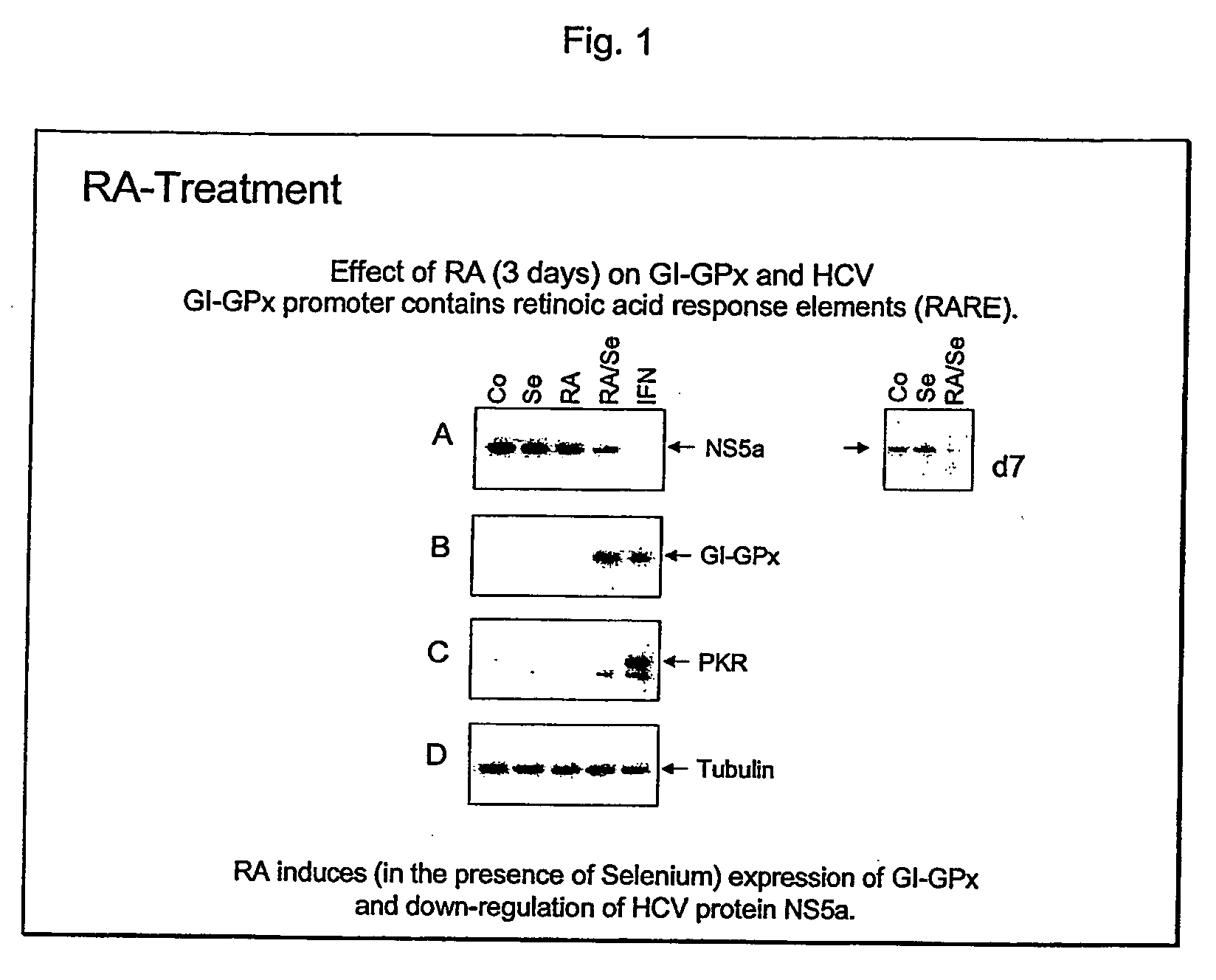
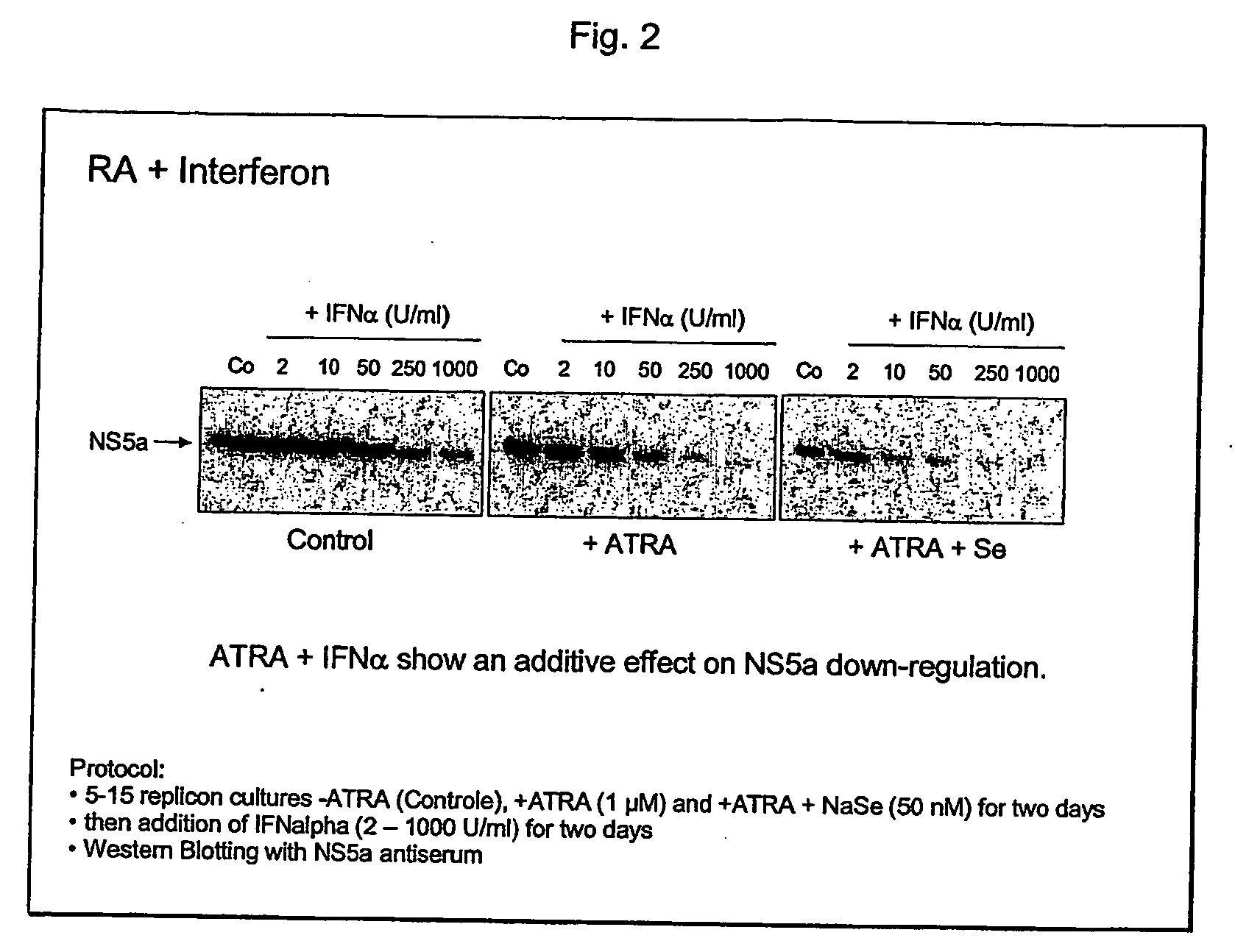
The present invention relates generally to chemical compounds and substances which are effective against Hepatitis C virus (HCV) infections. Moreover, the present invention relates to compositions comprising said compounds and / or substances, to methods for preventing HCV infections as well use of the compounds and / or substances for the preparation of compositions useful for the prophylaxis and / or treatment of HCV infections. Useful compounds and substances according to the invention are selenium, selenium salts, Vitamin D3 and retinoids, like all trans retinoic acid and salts thereof, C1-C alkyl amides of all trans retinoic acid and salts thereof, C1-C10 alkyl esters of all trans retinoic acid and salts thereof, 9-cis retinoic acid and salts thereof, C1-C10 alkyl amides of 9-cis retinoic acid and salts thereof, C1-C10 alkyl esters of 9-cis retinoic acid and salts thereof, (E)-4-[2(5,6,7,8-tetrahydro-5,5,8,8-tetra methyl-2-naphthalenyl-1-propenyl)benzoic acid (TTNPB), (4-[5,6,7,8-tetrahydro5,5,8,8-tetramethyl-2-naphthalenyl)carboxamido]benzoic acid (AM-580), N-(4-hydroxyphenyl)retinamide (4-HPR), and 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (AHPN).
Owner:GPC BIOTECH AG
Pharmaceutical for prevention and treatment of ophthalmic disease induced by in-crease in vasopermeability
InactiveUS20090281184A1Improve efficiencyImprove permeabilityBiocideSenses disorderDiseaseBenzoic acid
A medicament for preventive and / or therapeutic treatment of an ocular disease resulting from vascular hyperpermeability, for example diabetic retinopathy or age-related macular degeneration, which comprises as an active ingredient a retinoid such as all trans retinoic acid or 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl]benzoic acid.
Owner:SAPPORO MEDICAL UNIVERSITY
Preparation method of hemopoietic progenitor cells and special medium for the same
InactiveCN102206610APromote productionInhibition of developmentMicrobiological testing/measurementEmbryonic cellsVascular endothelial growth factorPhases of clinical research
Owner:PEKING UNIV +1
Method for differentiation of embryonic stem cells into nerve cells through in vitro induction
InactiveCN102899285APromote partial recoveryPromote regenerationNervous disorderMammal material medical ingredientsOLIG2Conceptus
The invention belongs to the field of biomedicine, and relates to a method for differentiation of embryonic stem cells into nerve cells through in vitro induction, especially to a method for induction of Olig2<+>-GFP<+>-mES nerve cell differentiation through a purine derivative Purmorphamine, and uses thereof. According to the present invention, an embryoid body mediated nerve induction method is adopted, Olig2-GFP<+>-mES is adopted as a cell model, a purine derivative Purmorphamine and all-trans retinoic acid are combined to carry out directed induction on the Olig2-GFP<+>-mES cells to obtain spinal motor neurons and oligodendrocyte progenitor cells through differentiation; experiment results show that the Purmorphamine can be used as a substitute of SHH, can effectively induce Olig2<+>-GFP<+>-mES differentiation to obtain high purity and function spinal motor neurons and oligodendroglial cells, and can cause expression changes of related genes; and transplant experiment results show that the induced nerve cells can promote partial function and morphology restoration after rat spinal cord injury, and have effects of spinal cord injury regeneration promotion and function reconstruction.
Owner:FUDAN UNIV
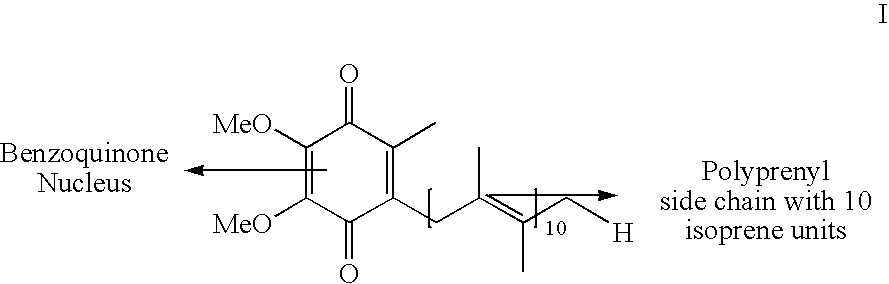
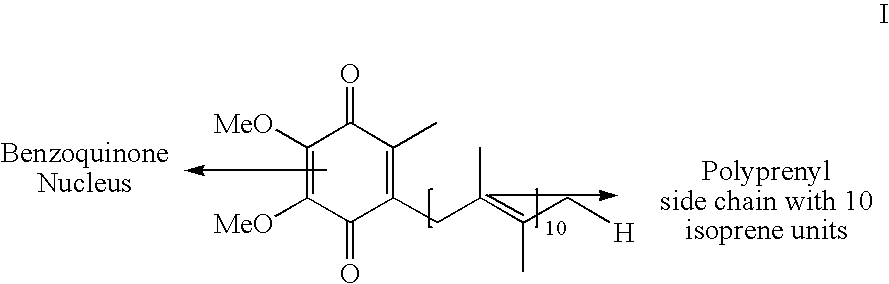
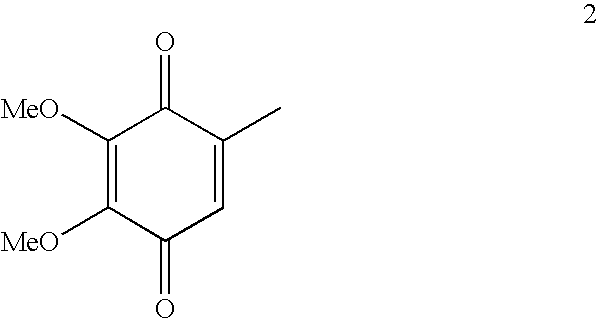
Novel Intermediates, Process for Their Preparation and Process for the Preparation of Coq10 Employing the Said Novel Intermediates
InactiveUS20080200702A1Speed up the processSimple and cost-effective and commercially applicableOrganic compound preparationQuinone preparation by oxidation1,4-BenzoquinoneMethyl group
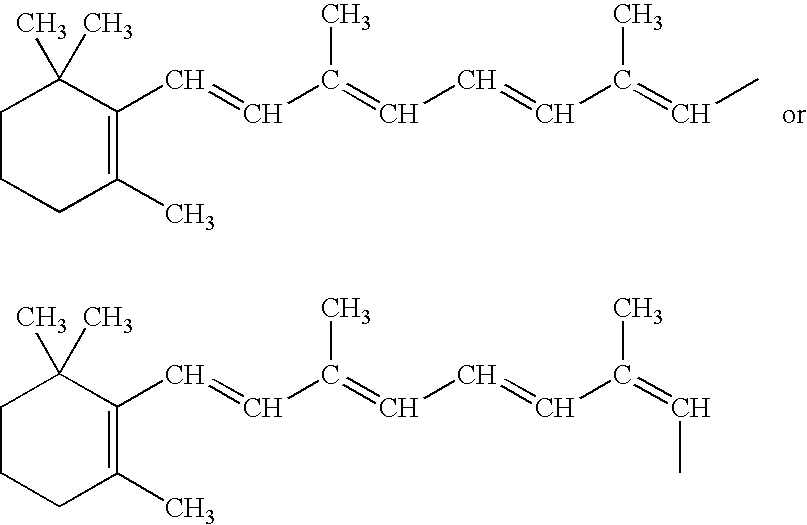
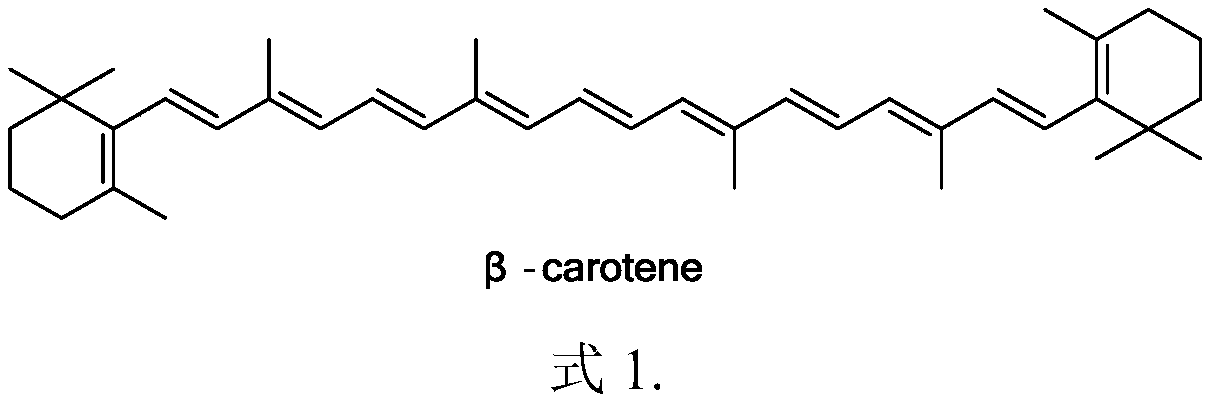
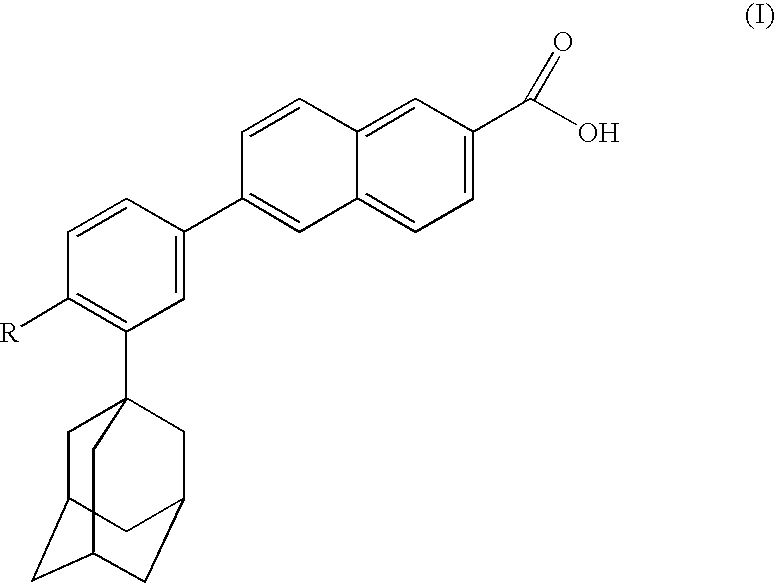
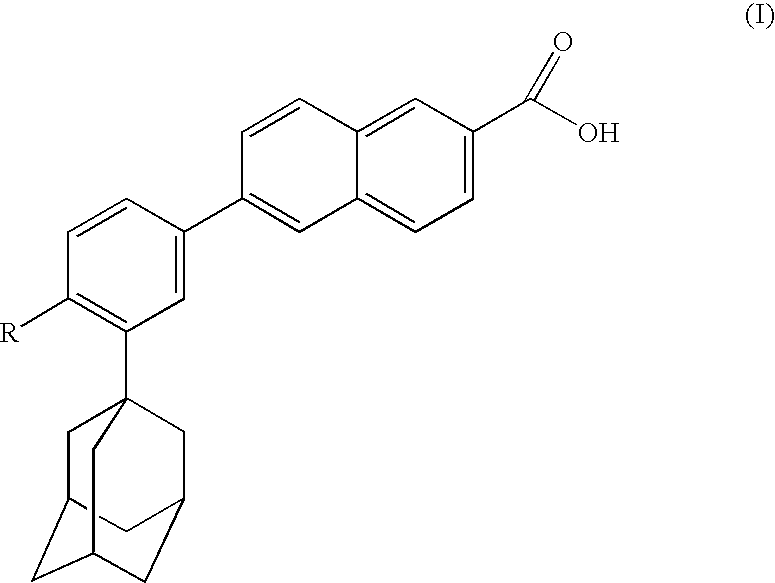
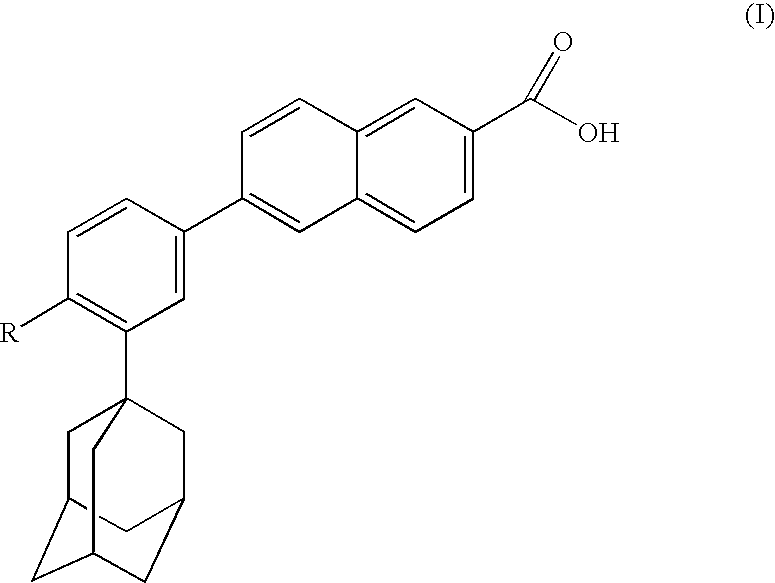
The present invention relates to an improved process for the preparation of Coenzyme Q. Coenzyme Q10 or CoQ10 has the chemical name 2-[(all-trans)-3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl]-5,6-dimethoxy-3-methyl-1,4-benzoquinone and has the formula I.The invention also provides new intermediates useful for the preparation of CoQ10 and processes for their preparation.
Owner:NICHOLAS PIRAMAL INDIA LTD
All-trans retinoic acid injection and application
InactiveCN107753473AImprove solubilityInduced differentiationPowder deliveryHydroxy compound active ingredientsSolubilityCell mass
Owner:HIGHFIELD BIOPHARM CORP
HPLC-MS detection method of xanthophylls cis-trans-isomers in xanthophylls products
InactiveCN103018351AMeasurement precisionAssay stabilityComponent separationInjection volumeIsomerization
The invention belongs to the field of analysis technology, and relates to an HPLC-MS detection method of xanthophylls cis-trans-isomers in xanthophylls products. The method is characterized by performing a light iodine isomerization reaction for the all-trans xanthophylls to obtain the cis-isomeride of the xanthophylls; using a YMC Carotenoid C30 chromatographic column to substantially separate the xanthophylls isomer, wherein a mobile phase is methanol / water = 98 / 2, a time is 70 minutes, a flow velocity rate is 1.0 mL / min, a DAD detector is used, a column temperature is 25 DEG C, an injection volume is 20 [mu]l and a detection wavelength is 450 nm; and using a positive ion mass spectrometry (APCI / MS), wherein a flow rate of the components from the chromatographic column into the mass spectrometer is 10 [mu]L / min, a scanning range m / z is 200 to 800, a capillary temperature is 150 DEG C, a vaporization temperature is 450 DEG C, a capillary voltage is 10 V, and a flow rate of dry gases is 8 mL / min. According to information of the mass spectrum and the spectrum, the xanthophylls isomers are respectively determined as all trans, 9-cis, 9'-cis, 13-cis and 13'-cis xanthophylls. The analysis method is rapid and effective, good in reproducibility and high in recovery rate, and can quantitatively analyze content of the xanthophylls cis-trans-isomers in the xanthophylls products.
Owner:NORTHEAST FORESTRY UNIVERSITY
Method for preparing all-trans-beta-carotene
ActiveCN108752251AIncrease alkalinityReduce solubilityOrganic chemistry methodsGroup 5/15 element organic compoundsBeta-CaroteneZinc
The invention provides a method for preparing all-trans-beta-carotene. According to the method for preparing the all-trans-beta-carotene, an alkaline ionic liquid provides a strong alkaline environment, so that 2,4-pentadiene pentadecarbonate and 2,7-dimethyl-2,4,6-zinc triene-1,8-dialdehyde react under the weak base action to obtain the all-trans-beta-carotene directly. The method for preparing the all-trans-beta-carotene has the advantages that a strong alkaline ionic liquid serves as an alkaline assistant, the selectivity and yield of beta-carotene both reach 94% or above, and the purity oftrans-beta-carotene in a product reaches 96%; the reaction operation is simple, the product is separated quickly, and the method is environmentally friendly and suitable for industrialized production.
Owner:WANHUA CHEM GRP CO LTD
Retinol derivatives, their use in the treatment of cancer and for potentiating the efficacy of other cytotoxic agents
A group of new compounds, N-(all-trans-Retinoyl)-L-cysteic acid, N-(13-cis-Retinoyl)-L-cysteic acid, N-(all-trans-Retinoyl)-L-cysteinesulfinic acid, N-(13-cis-Retinoyl)-L-cysteinesulfinic acid, N-(all-trans-Retinoyl)-L-homocysteic acid, N-(13-cis-Retinoyl)-L-homocysteic acid, and sodium salts of these compounds, including sodium salts of their esters and amides, is shown to exhibit therapeutic effects per se, and which compounds in combination with cytotoxic compounds, such as docetaxel, paclitaxel, doxorubicin and mitoxantrone, exhibit a synergistic effect. These compounds make it possible to manufacture new formulations of poorly soluble pharmaceutical compounds, and the present invention discloses a process of manufacturing water-soluble formulations of such compounds, exemplified by docetaxel, and paclitaxel, exhibiting enhanced pharmacological activity, and formulations of water-soluble pharmaceuticals exemplified by doxorubicin and mitoxantrone, exhibiting improved therapeutic efficacy.
Owner:OASMIA PHARMA AB
Dermatological compositions comprising at least one retinoid compound, an Anti-irritant compound and benzoyl peroxide
InactiveUS20100160439A1Improve toleranceOvercome problemsCosmetic preparationsBiocideRetinoidIsotretinoin
Dermatological compositions contain, formulated into a physiologically acceptable medium, at least one retinoid compound selected from among all-trans retinoic acid, isotretinoin, motretinide, and naphthoic acid compounds of formula (I), and salts and esters thereof:wherein R is a hydrogen atom, a hydroxyl radical, a branched or unbranched alkyl radical having from 1 to 4 carbon atoms, an alkoxy radical having from 1 to 10 carbon atoms, or a cycloaliphatic radical which is substituted or unsubstituted, and benzoyl peroxide, and also at least one anti-irritant compound selected from among 18β-glycyrrhetinic acid, and its salts and derivatives thereof.
Owner:GALDERMA RES & DEV SNC
Non-spherical drug-loaded particles and controlled release preparation of lactyl polymer and preparation methods thereof
InactiveCN101953776AStable structureNo hemolyticPowder deliveryHydroxy compound active ingredientsSolventSustained-Release Preparations
The invention relates to non-spherical drug-loaded particles and a controlled release preparation of a lactyl polymer and preparation methods thereof. The non-spherical particles of polylactic-co-glycolic acid (PLGA) are prepared by using an emulsion-solvent volatilization method assisted by small molecule materials. The PLGA is used as raw material coated with at least one of the following hydrophobic drugs: all-trans retinoic acid, paclitaxel, epirubicin, camptothecin or roxithromycin, wherein the mass ratio of the hydrophobic drug to a lactyl polymer high polymer material is 1:4-40. The drug-loaded particles of the all-trans retinoic acid are prepared and subjected to in vitro drug release evaluation. The results show that the preparation method has the advantages of simple preparation process, good reproducibility, significantly increased drug loading amount and encapsulation efficiency relative to spherical particles, very good controlled-release effect, no hemolysis initiation and safety use. The novel carrier and preparation have a potential industrial production value in the field of long-circulating controlled release of the hydrophobic drugs.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Compounds, compositions, kits and methods of use to orally and topically treat acne and other skin conditions by administering a 19-nor containing vitamin d analog with or without a retinoid
Oral and topical pharmaceutical compositions, kits and methods of treatment thereof for treating various skin disorder including acne, psoriasis, ichthyosis, photoaging, photodamaged skin, and, skin cancer. Exemplary vitamin D analogs as active pharmaceutical ingredients include 2-methylene-19-nor-20(S)-1α-hydroxy-bishomopregnacalciferol, 19-nor-26,27-dimethylene-20(S)-2-methylene-1α,25-dihydroxyvitamin D3, 2-methylene-1α,25-dihydroxy-(17E)-17(20)-dehydro-19-nor-vitamin D3, 2-methylene-19-nor-(24R)-1α,25-dihydroxyvitamin D2, 2-methylene-(20R,25S)-19,26-dinor-1α,25-dihydroxyvitamin D3, 2-methylene-19-nor-1α-hydroxy-pregnacalciferol, 1α-hydroxy-2-methylene-19-nor-homopregnacalciferol, (20R)-1α-hydroxy-2-methylene-19-nor-bishomopregnacalciferol, 2-methylene-19-nor-(20S)-1α-hydroxy-trishomopregnacalciferol, 2-methylene-23,23-difluoro-1α-hydroxy-19-nor-bishomopregnacalciferol, 2-methylene-(20S)-23,23-difluoro-1α-hydroxy-19-nor-bishomopregnancalciferol, (2-(3′hydroxypropyl-1′,2′-idene)-19,23,24-trinor-(20S)-1α-hydroxyvitamin D3, 2-methylene-18,19-dinor-(20S)-1α,25-dihydroxyvitamin D3, a stereoisomer thereof, a prodrug thereof in oral compositions, a salt thereof, and / or a solute thereof. Compounds that activate retinoic acid receptors, such as retinoyls and retinoyl esters, include 13-cis-retinoic acid, all-trans-retinoic acid, (2E,4E,6Z,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexeneyl)nona-2,4,6,8-tetraenoic acid, 9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-tetraenoic acid, 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-napthoic acid, 4-[1-(3,5,5,8,8-pentamethyl-tetralin-2-yl)ethenyl]benzoic acid, retinobenzoic acid, ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl]pyridine-3-carboxylate, retinoyl t-butyrate, retinoyl pinacol, retinoyl cholesterol, an isomer thereof, a prodrug thereof for oral compositions, an ester thereof, a salt thereof, and / or, a solute thereof. Combinations of such active ingredients demonstrate synergistic efficacy.
Owner:WISCONSIN ALUMNI RES FOUND
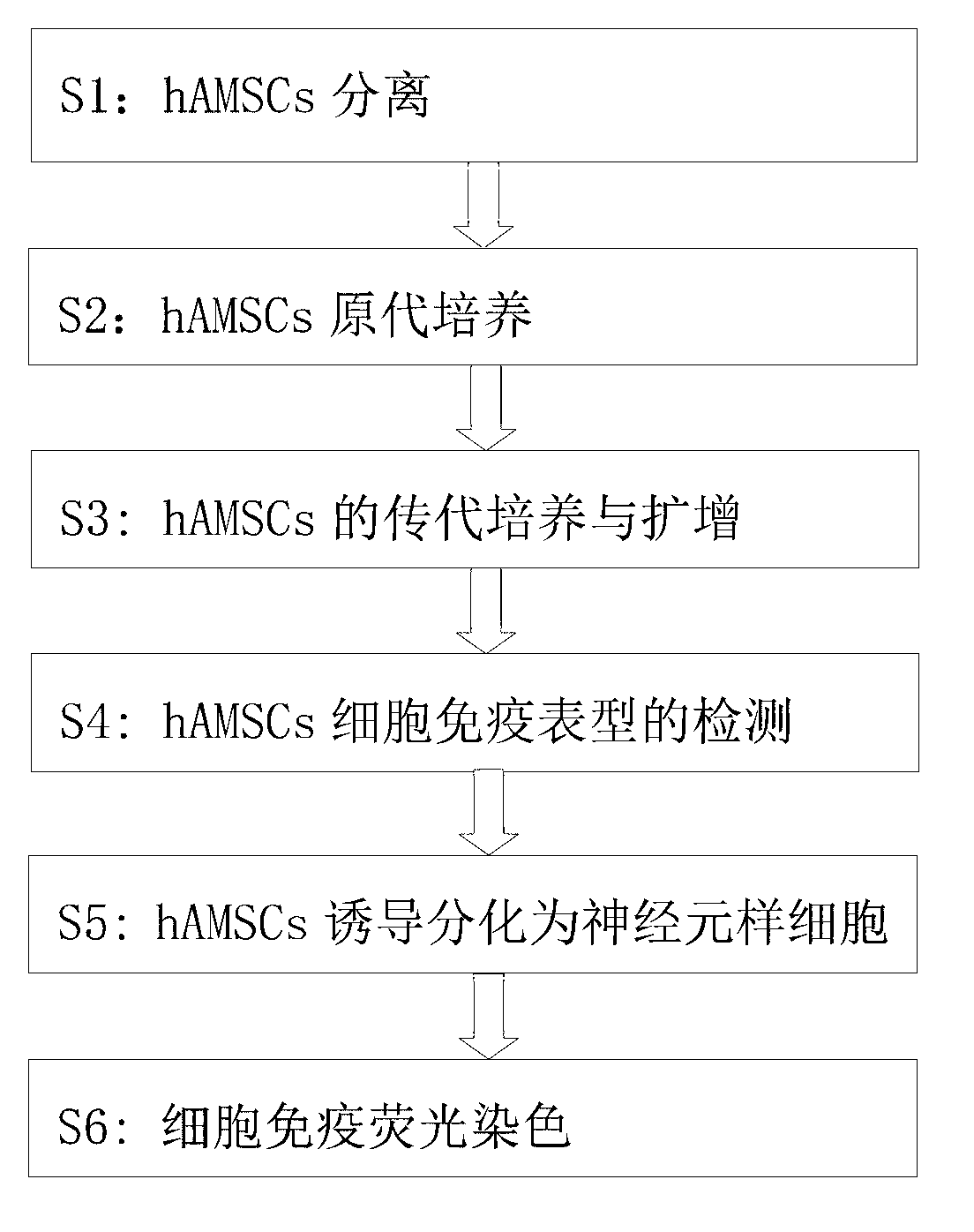
Method for inducing human amniotic mesenchymal stem cells to differentiate into neuron-like cells
InactiveCN103013917ANervous system cellsSkeletal/connective tissue cellsGerm layerGlial fibrillary acidic protein
The invention provides a method for inducing differentiating human amniotic mesenchymal stem cells (hAMSCs) to differentiate into neuron-like cells by adopting all-trans retinoic acids, a basic fibroblast growth factor (bFGF) and an epidermal growth factor (EGF). The method comprises the following steps of: separating the hAMSCs, carrying out primary culture of the hAMSCs, subculturing and amplifying the hAMSCs, detecting hAMSCs immunophenotyping, inducing the hAMSCs to differentiate into the neuron-like cells and carrying out cellular immunity fluorescence staining. According to the inducing method provided by the invention, umbilical cord mesenchymal stem cells are induced to differentiate into neutral stem cells by using the all-trans retinoic acids in combination of the bFGF and the EGF; the neural stem cells not only have the typical morphology of nerve cells, but also express neuron marker antigen neuron-specific emolase and astrocyte marker antigen glial fibrillary acidic proteins; and the capability of a mesenchymal cell trans-germinal layer differentiating into non mesenchymal cells is realized so that the mesenchymal cells are likely to turn into more ideal seed cells for clinical application in further.
Owner:陆华
C-4 substituted retinoids
InactiveUS7265143B2Improved pharmacokinetic (PK) parameterStrong inhibitory activityBiocideHydroxy compound active ingredientsRetinoidCis-Retinoic Acid
C-4 substituted retinoic acid analogs, synthesis methods of C-4 substituted retinoic acid analogs and methods of using C-4 substituted retinoic acid analogs to treat various cancers and dermatological diseases and conditions. The C-4 substituted retinoic acid analogs include C-4 all-trans retinoic acid (ATRA) and 13-cis retinoic acid (13-CRA) analogs. The C-4 substituted retinoic acid analogs inhibit all-trans retinoic acid (ATRA) 4-hydroxylase activity, thereby inhibiting the catabolism of ATRA. The C-4 substituted retinoic acid analogs also have ATRA-mimetic activity. The preferred substitutions at C-4 are an azole group, a sulfur, oxygen, or nitrogen containing group, a pyridyl group, an ethinyl group, a cyclopropyl-amine group, an ester group, or a cyano group, or forms, together with the C-4 carbon atom, an oxime, an oxirane or aziridine group.
Owner:MARYLAND UNIV OF
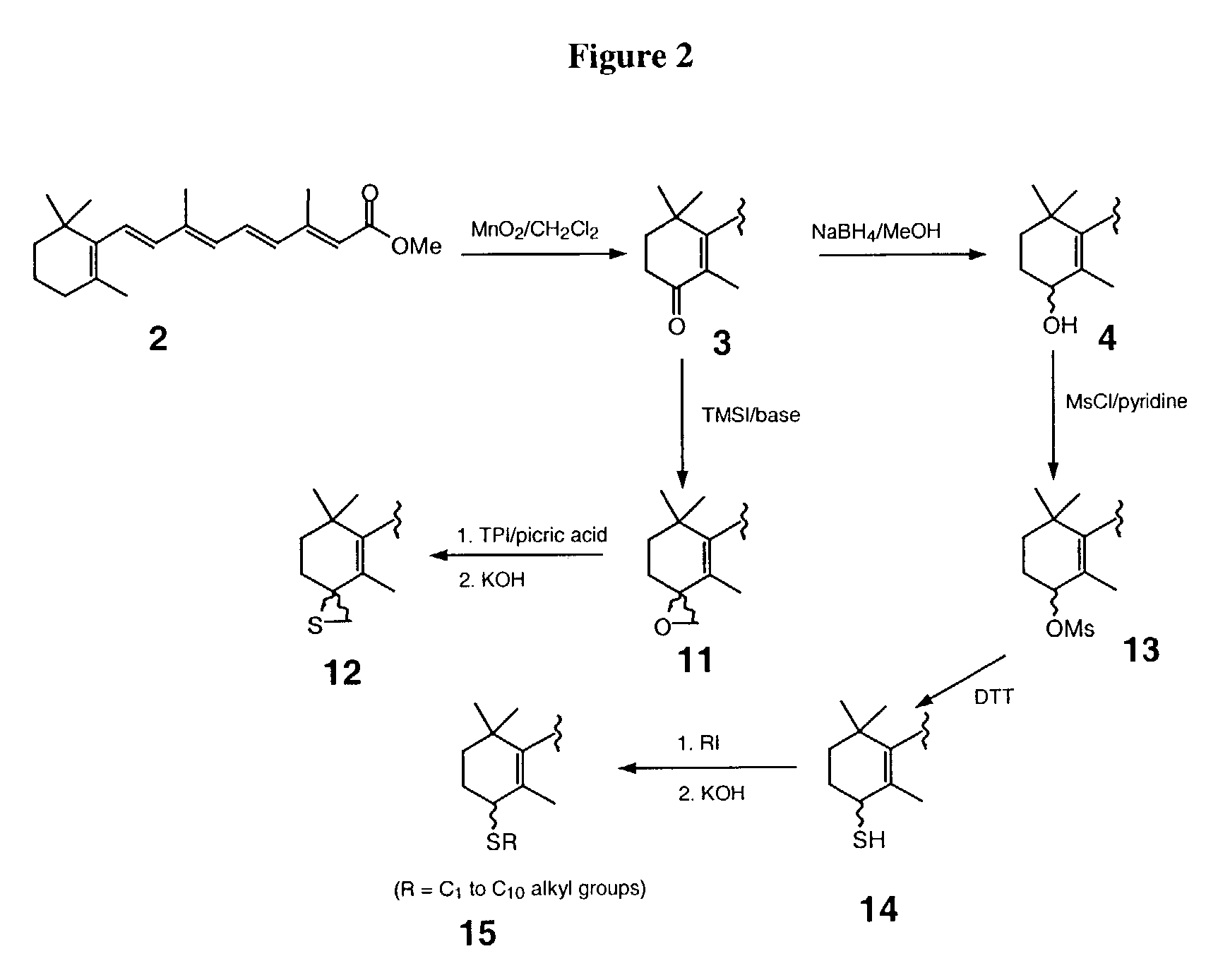
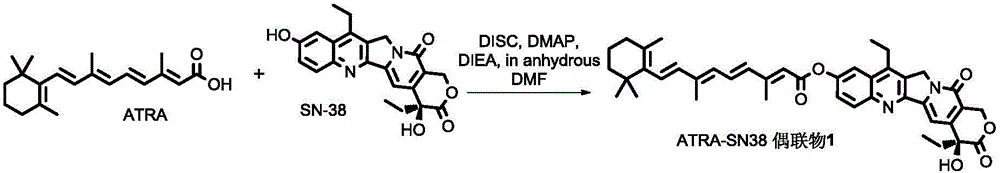
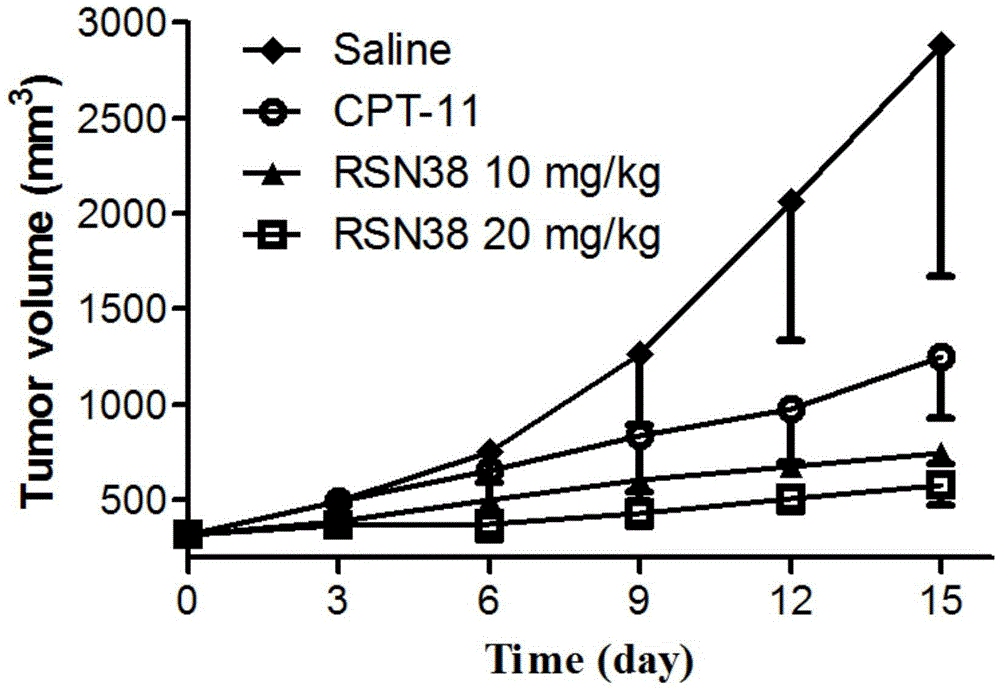
All-trans retinoic acid-camptothecin anticancer drug conjugate as well as preparation method and application thereof
ActiveCN104478890AImprove solubilityExpand the scope of clinical applicationOrganic active ingredientsOrganic chemistryPolyoxyethylene castor oilSolubility
The invention discloses an all-trans retinoic acid-camptothecin anticancer drug conjugate as well as a preparation method and application thereof. The structural formula of the all-trans retinoic acid-camptothecin anticancer drug conjugate is shown in a formula (I), (II), (III), (IV), (V) or (VI). The all-trans retinoic acid-camptothecin anticancer drug conjugate has good solubility in Tween, polyoxyethylene castor oil, a Poly(ethylene adipate)-polylactic acid copolymer and a Poly(ethylene adipate)-poly (lactic acid-glycollic acid) copolymer, can be self assembled into nanometer grains in water, can be directly injected or taken orally or processed into other dosage forms. According to the all-trans retinoic acid-camptothecin anticancer drug conjugate disclosed by the invention, as all-trans retinoic acid and SN-38 or camptothecin take synergistic effect, compared with a conjugate only containing one of irinotecan, SN-38 and all-trans retinoic acid, the all-trans retinoic acid-camptothecin anticancer drug conjugate has good tumor suppression effect.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com