All-trans retinoic acid-camptothecin anticancer drug conjugate as well as preparation method and application thereof
A technology of all-trans retinoic acid and anti-cancer drugs, which is applied in drug combinations, anti-tumor drugs, and pharmaceutical formulations. effect of nature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of ATRA-SN38 conjugate (C10 hydroxyl)
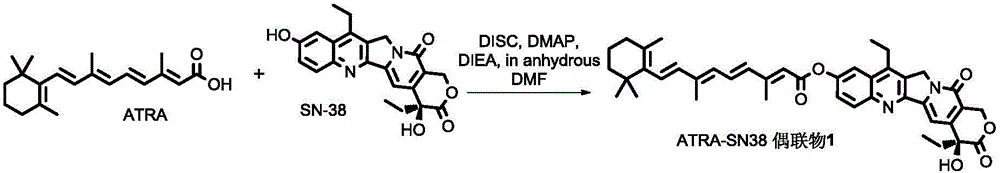
[0055] The preparation method of a kind of ATRA-SN-38 conjugate (C-10 hydroxyl) of this embodiment (synthetic route sees figure 1 ),include:
[0056] SN-38 (500mg, 1.27mmol), DMAP (171.4mg, 1.40mmol), ATRA (383.2mg, 1.27mmol), EDC·HCl (268mg, 1.40mmol) were dissolved in 15mL DMF, and DIEA (180.9mg, 1.40mmol) was added ), reacted overnight, after removing the reaction solvent, the solid was dissolved in dichloromethane, washed 7 times with water, washed 1 time with saturated aqueous sodium bicarbonate solution, 5% citric acid and saturated saline successively, dried over anhydrous sodium sulfate, filtered, After collecting the filtrate, the solvent was removed under reduced pressure; the solid was separated and purified by column chromatography (ethyl acetate: n-hexane = 1:1) to obtain the product ATRA-SN38 conjugate 1 (hereinafter referred to as conjugate 1).
[0057] NMR data of conjugate 1:
[0058] 1 H...
Embodiment 2
[0059] Example 2 The first preparation method of the conjugate 1 nanoparticle
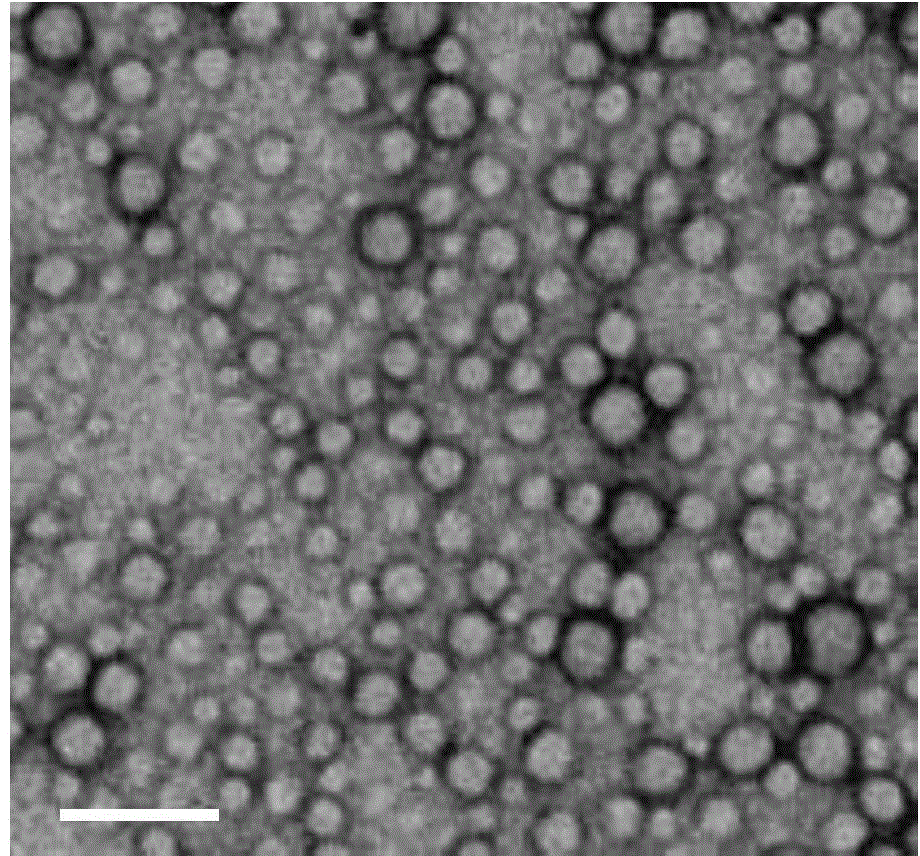
[0060] The conjugate 1 (20 mg) of Example 1 was dissolved in 1 mL of Tween 80 solution, and then slowly injected into water (final concentration 2 mg / mL), and gently shaken and vibrated to form nanoparticles, which were designated as RSN38. The nanoparticle solution can be directly used for clinical oral administration or injection.
Embodiment 3
[0061] Example 3 The second preparation method of conjugate 1 nanoparticles
[0062] The conjugate 1 (20 mg) obtained in Example 1 was dissolved in 1 mL of dimethyl sulfoxide (DMSO), and then rapidly injected into water (final concentration 2 mg / mL). The solution was dialyzed to remove DMSO and form nanoparticles. The nanoparticle solution can be directly used for clinical oral administration or injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com