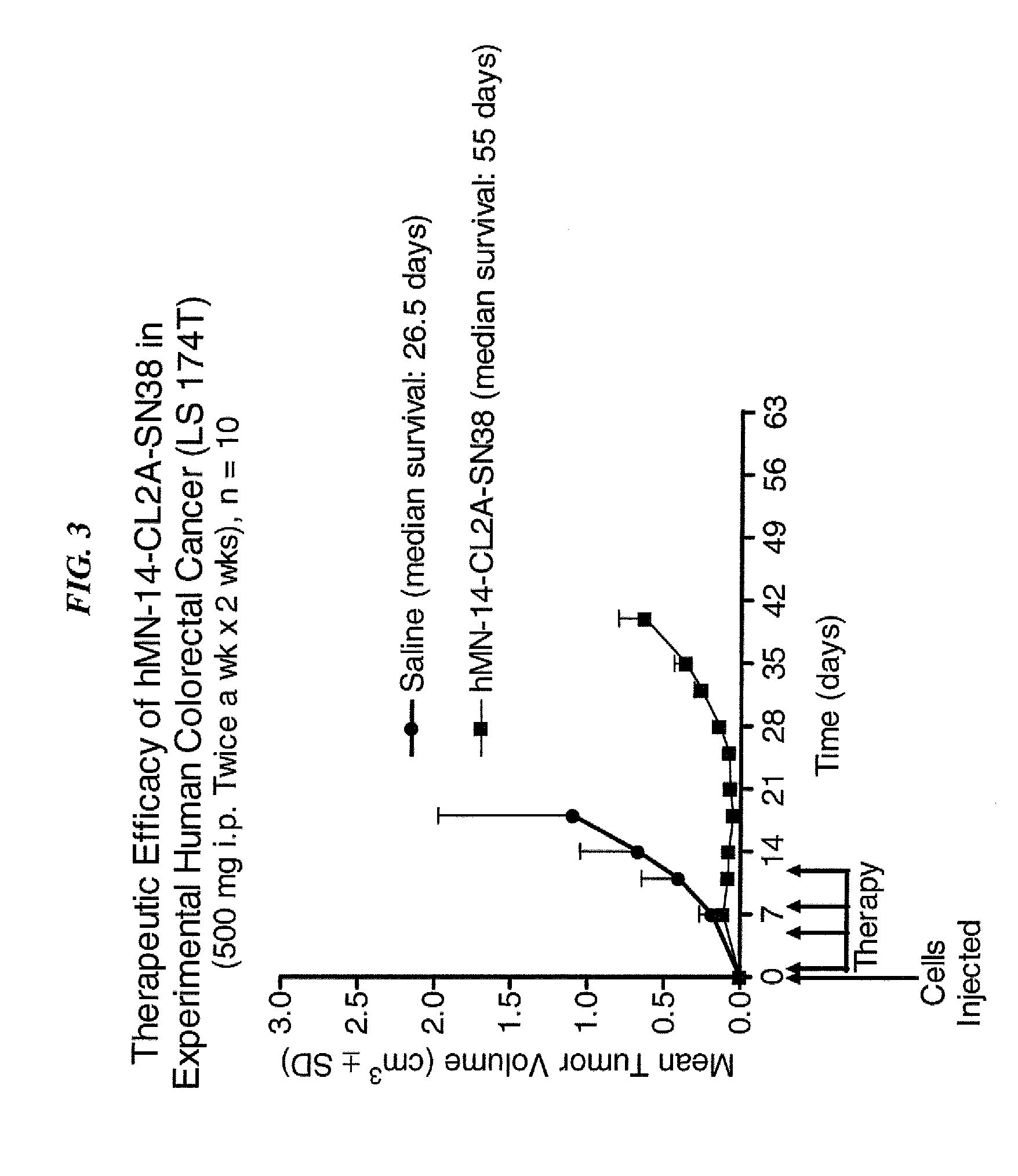
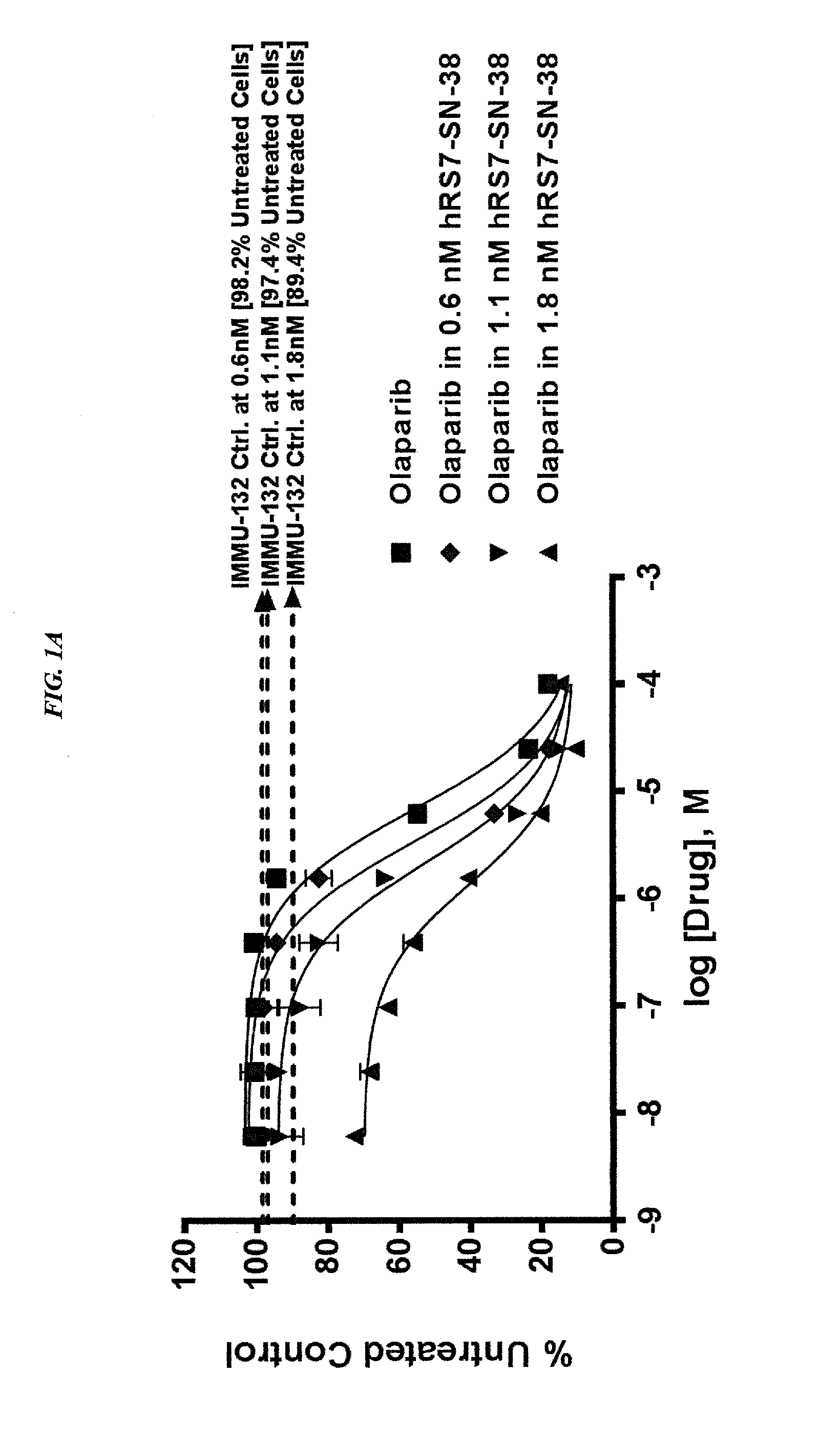
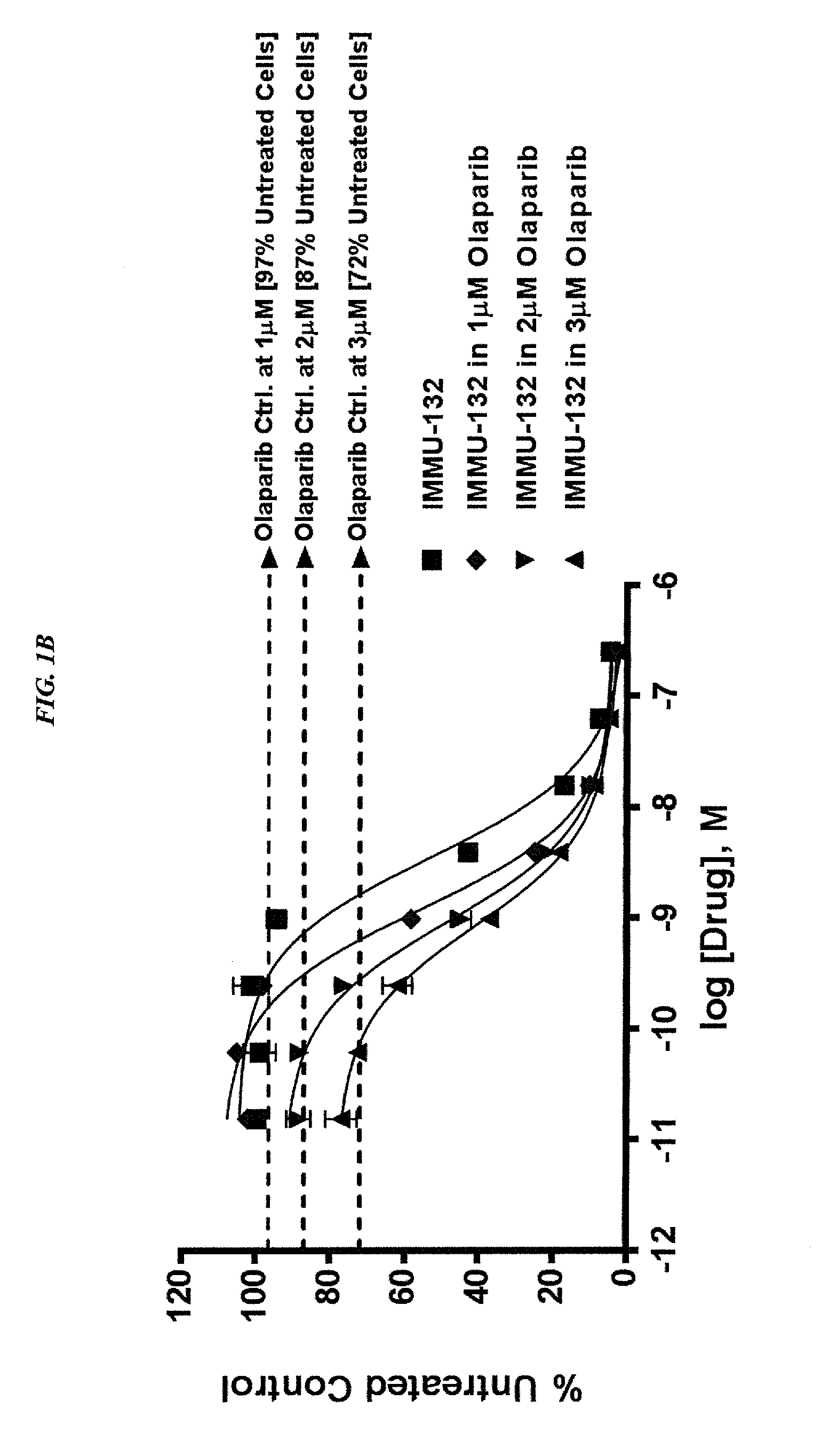
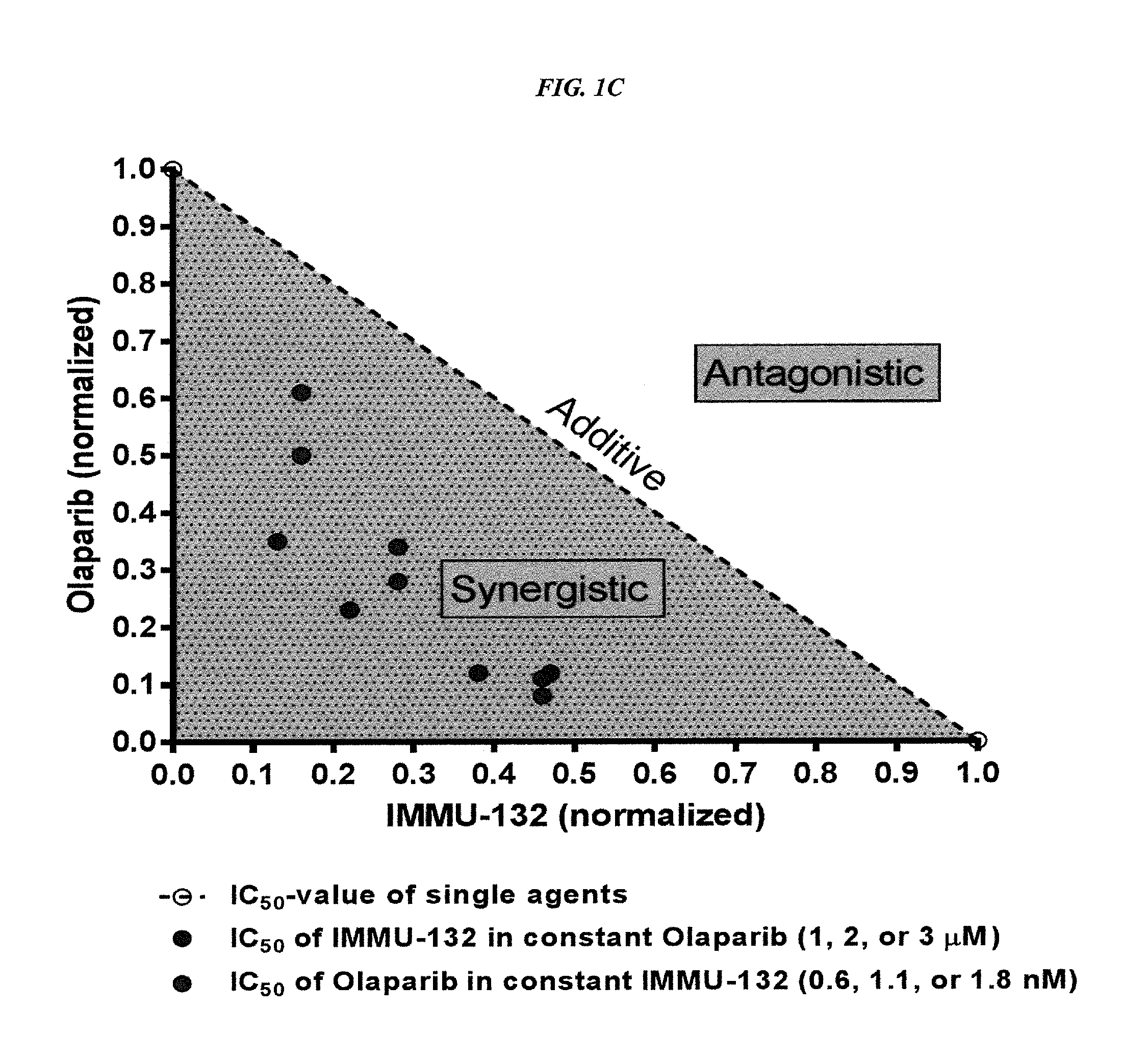
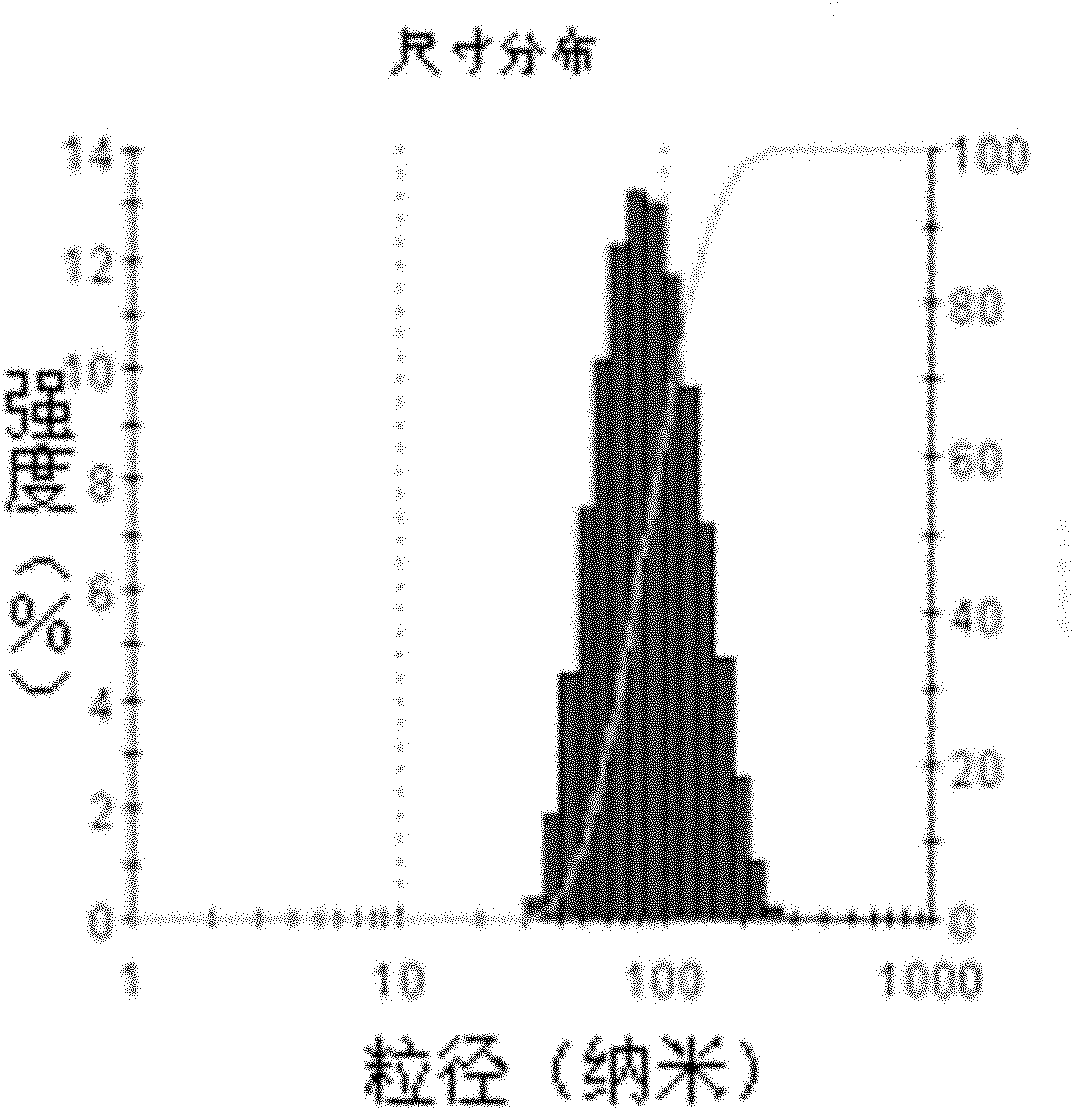
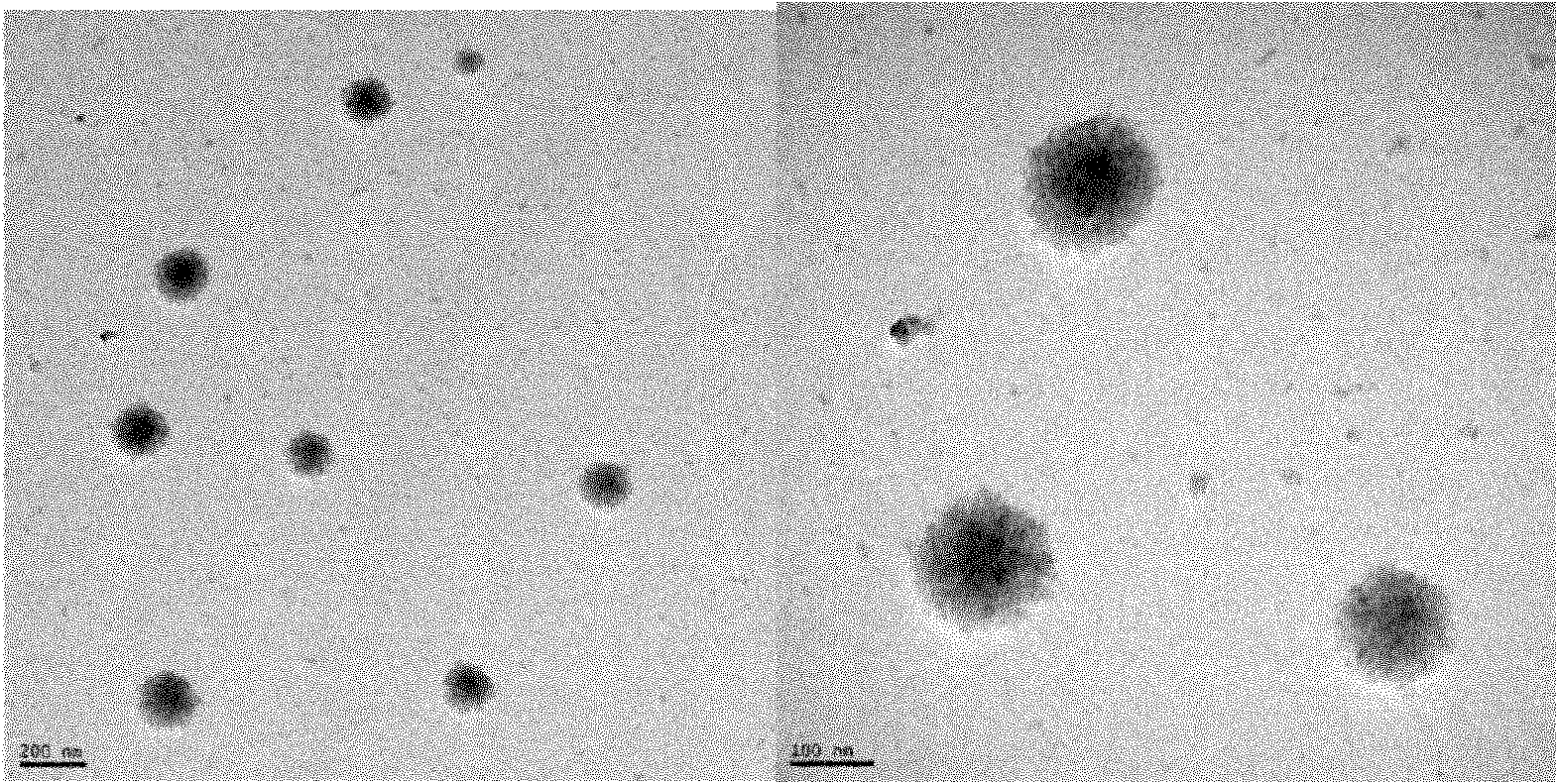
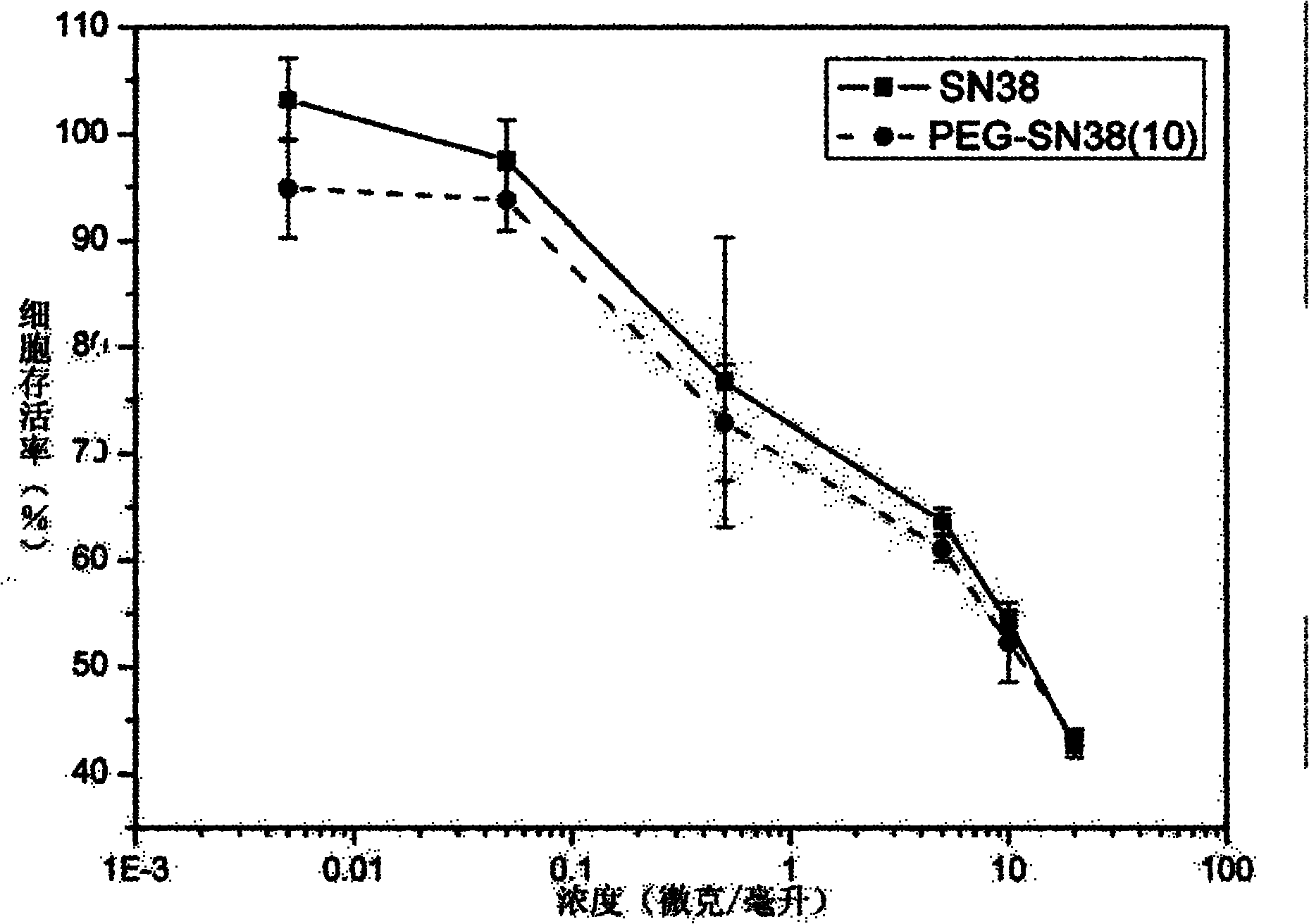
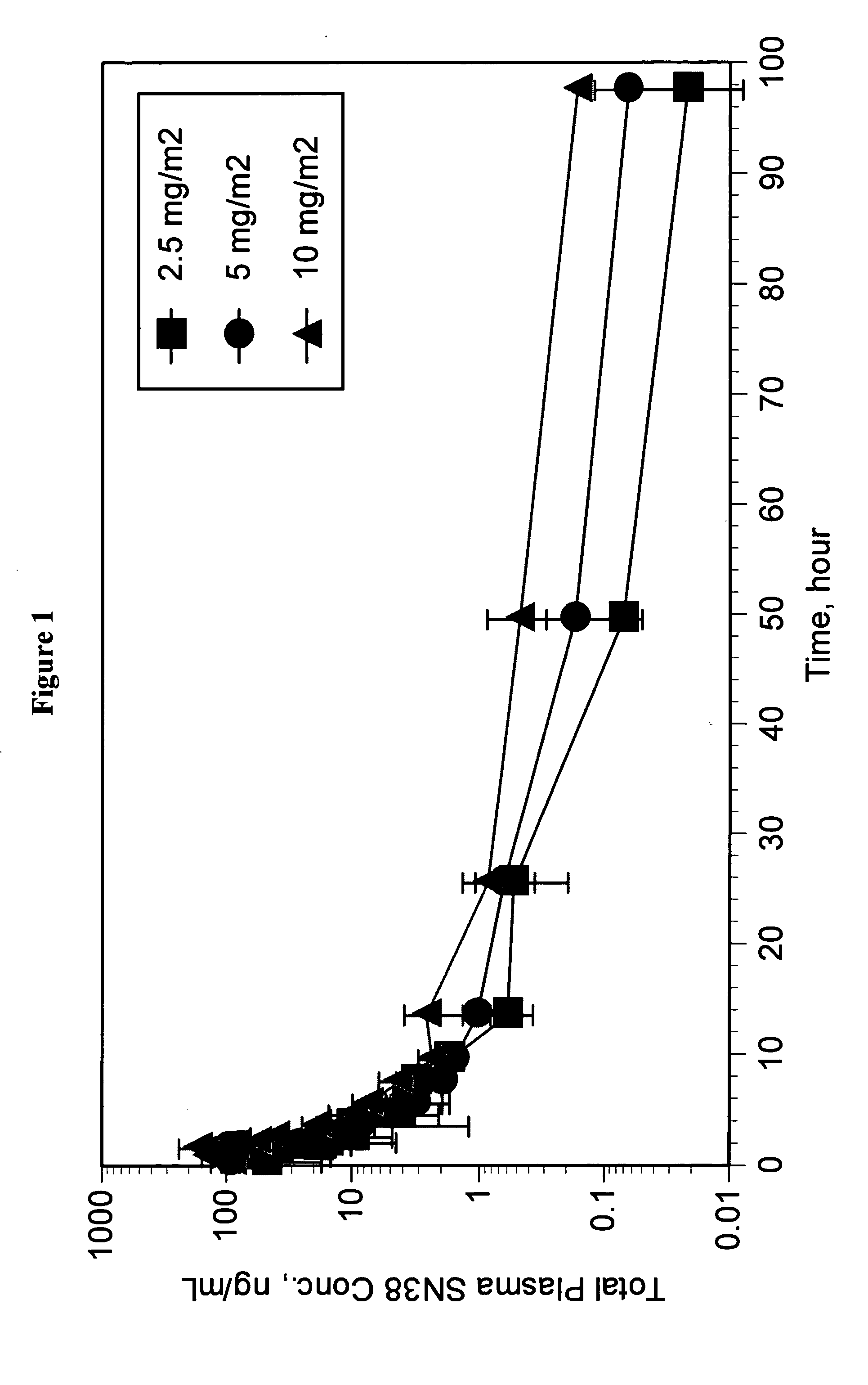
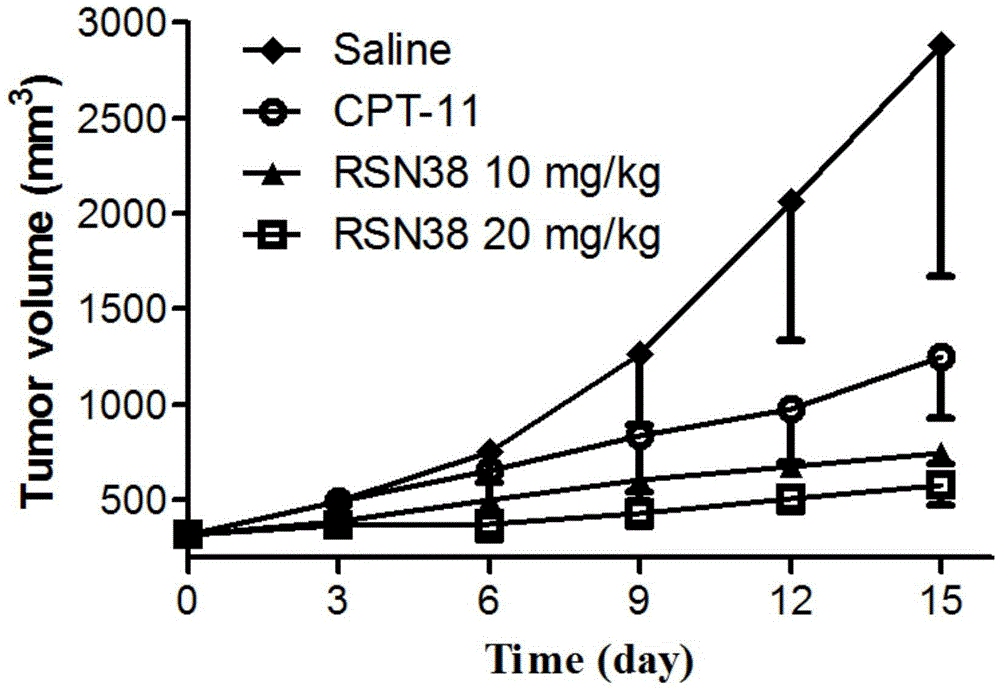
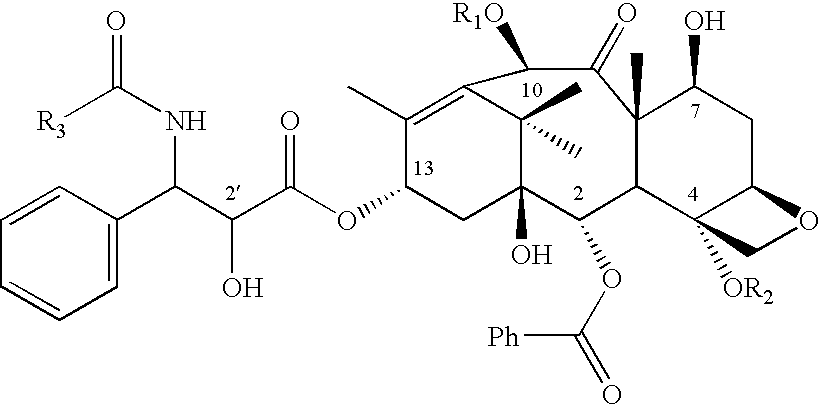
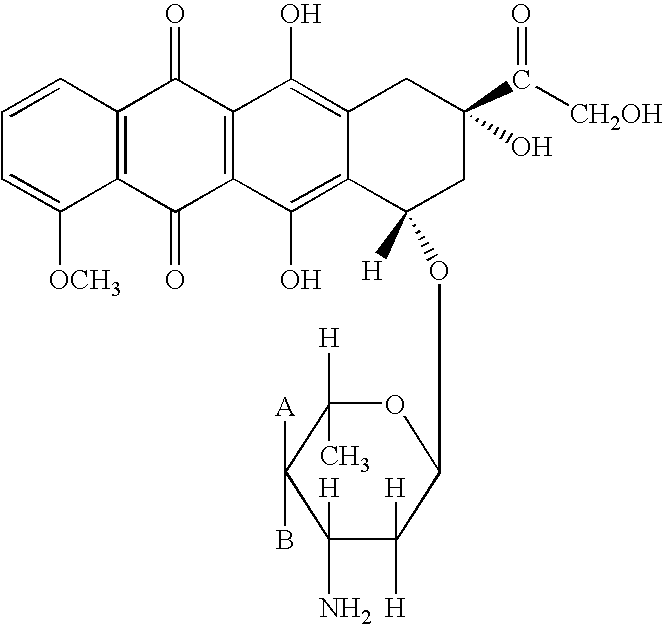
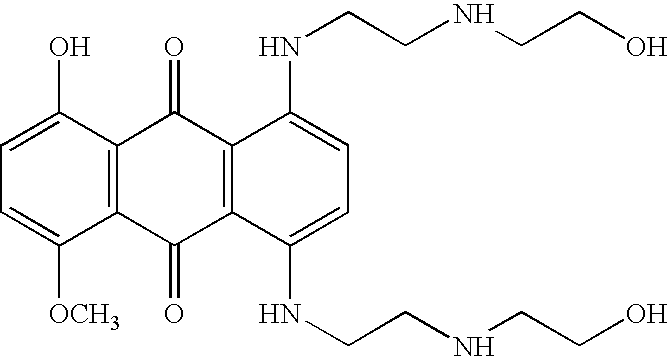
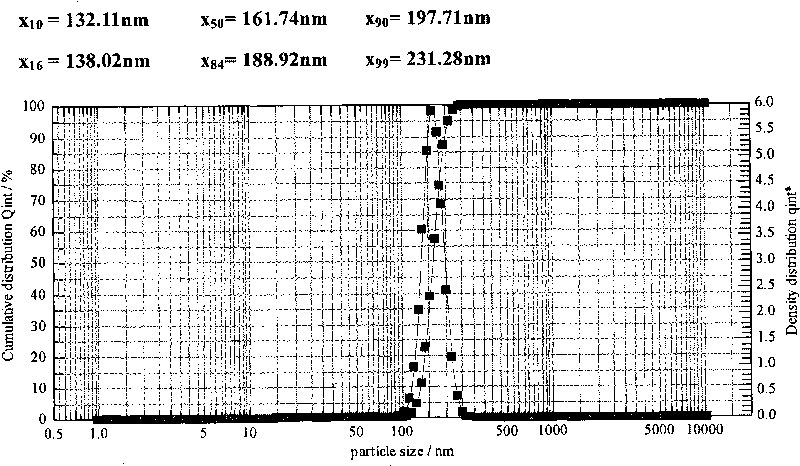
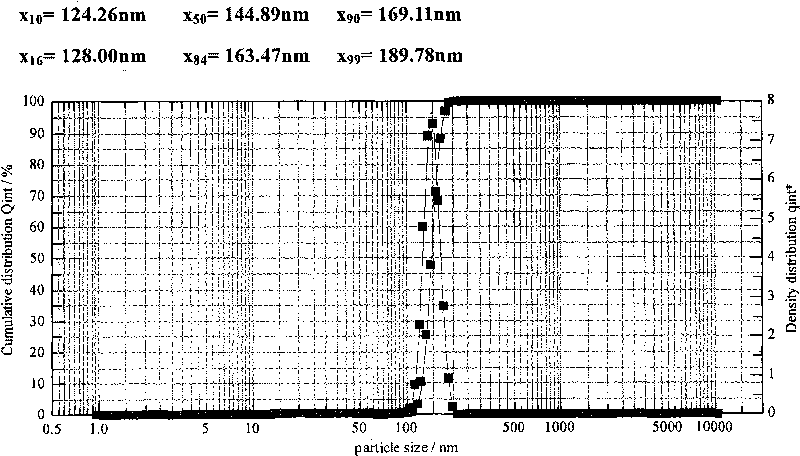
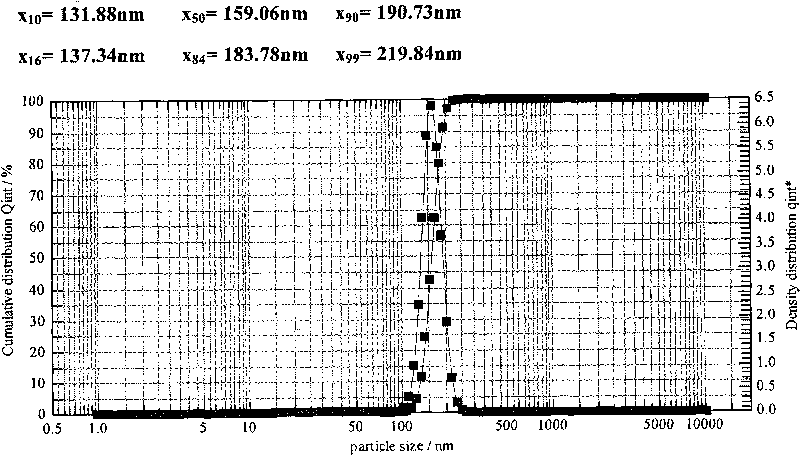
Patents
Literature
56 results about "SN-38" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
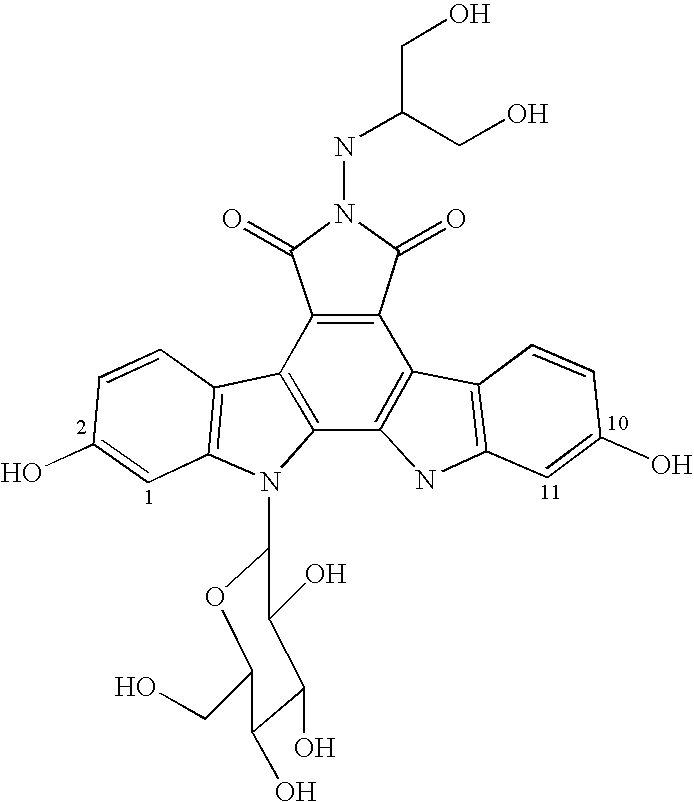
SN-38 is an antineoplastic drug. It is the active metabolite of irinotecan (an analog of camptothecin - a topoisomerase I inhibitor) but has 1000 times more activity than irinotecan itself. In vitro cytotoxicity assays show that the potency of SN-38 relative to irinotecan varies from 2- to 2000-fold.
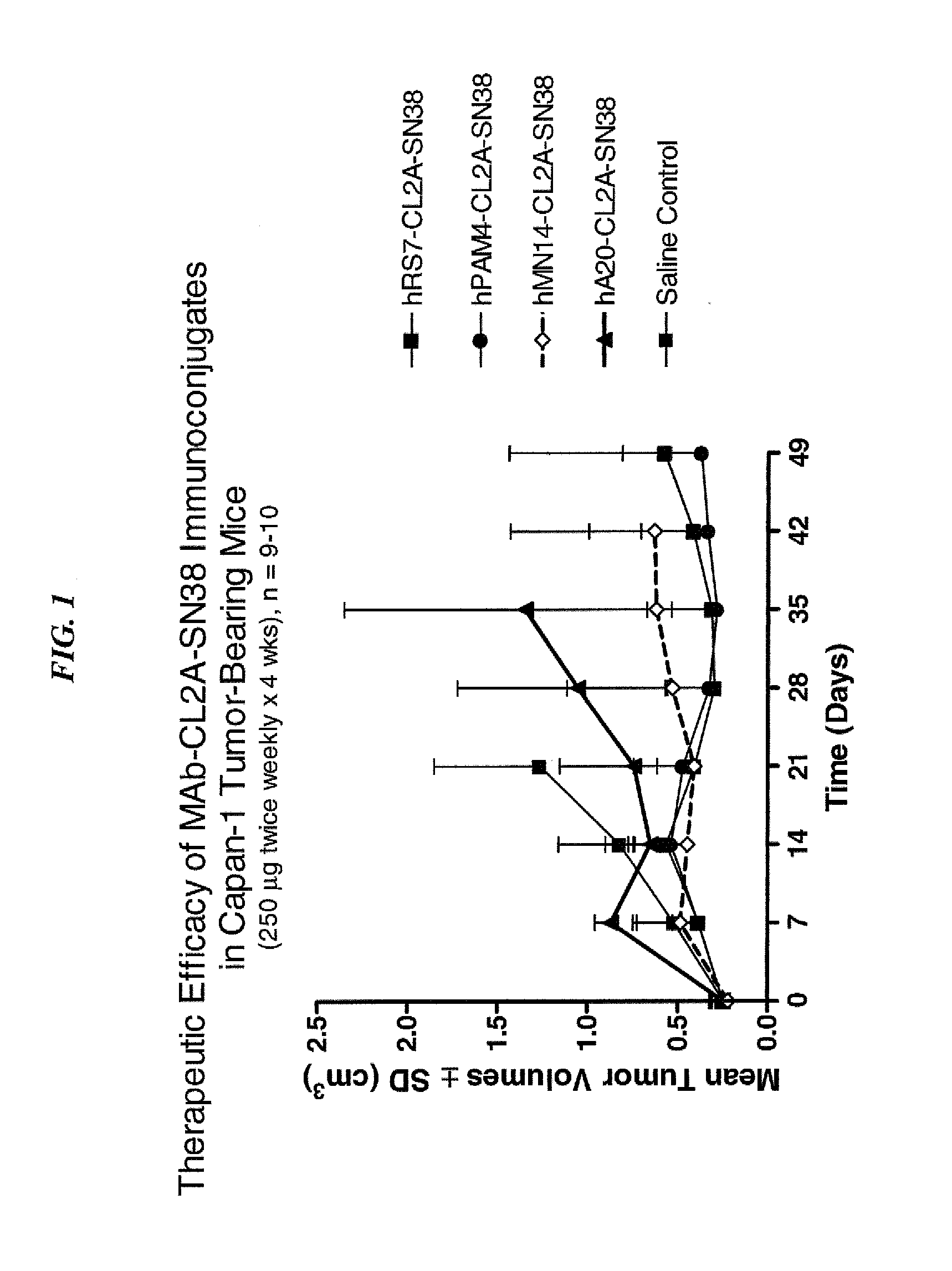
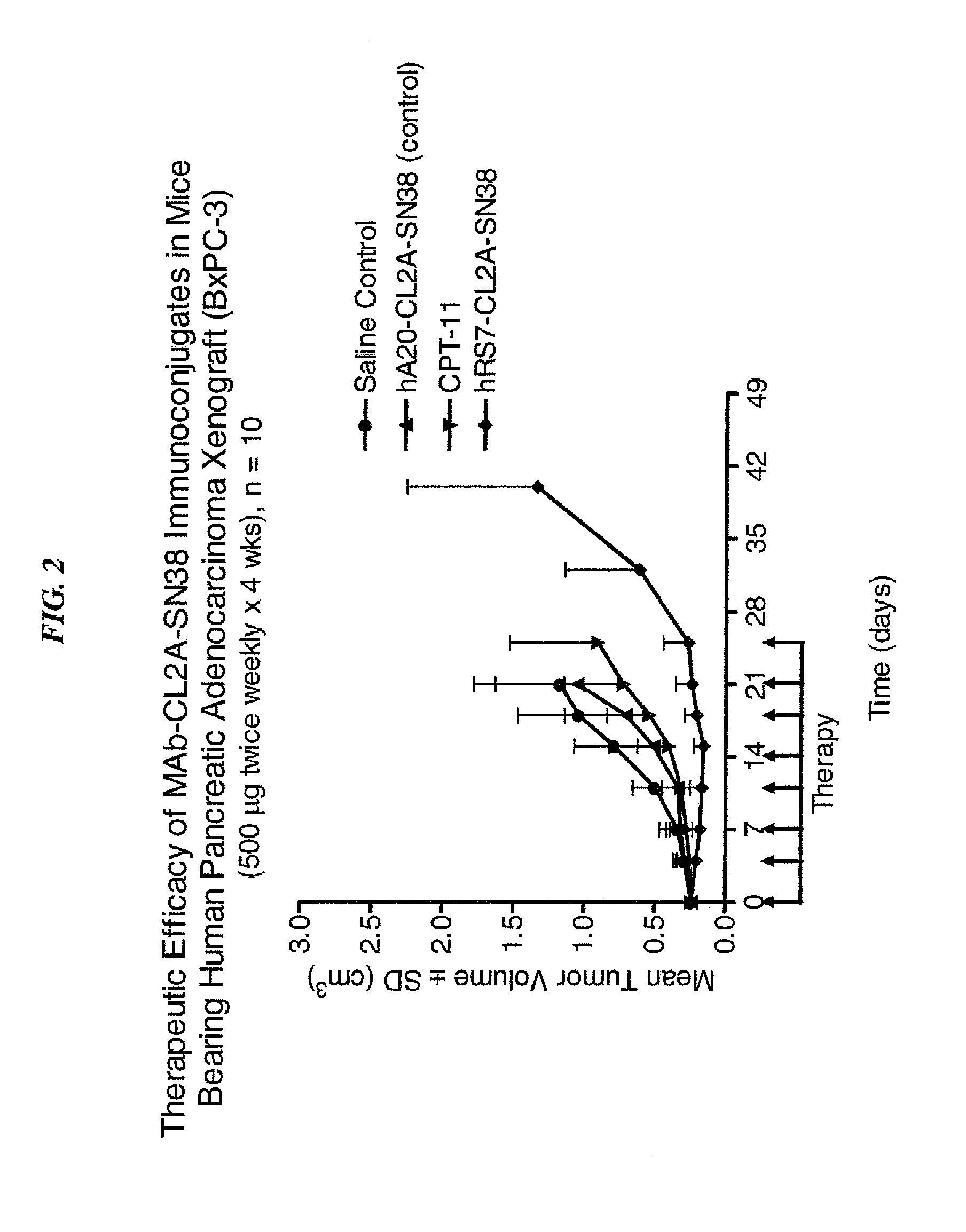
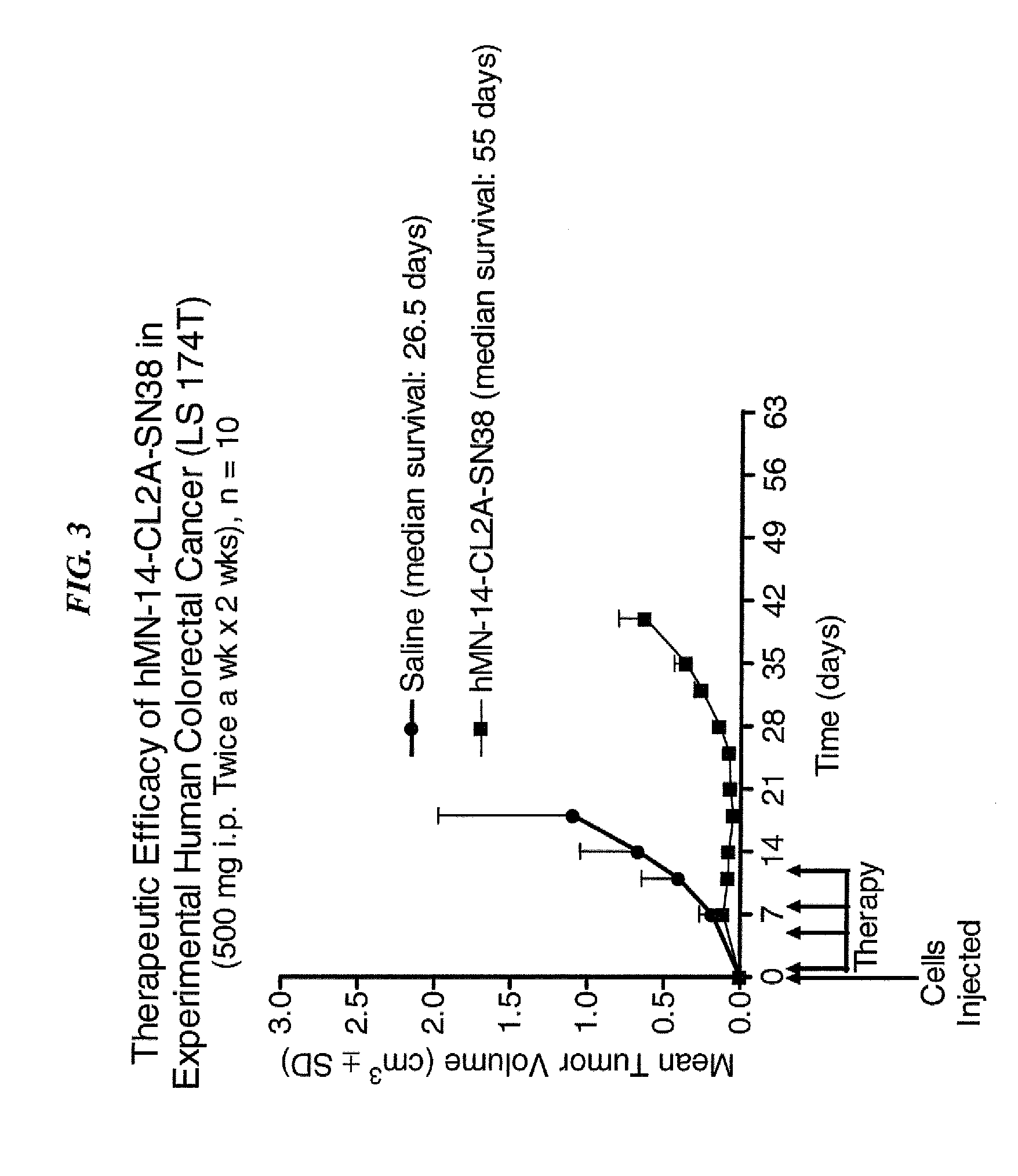
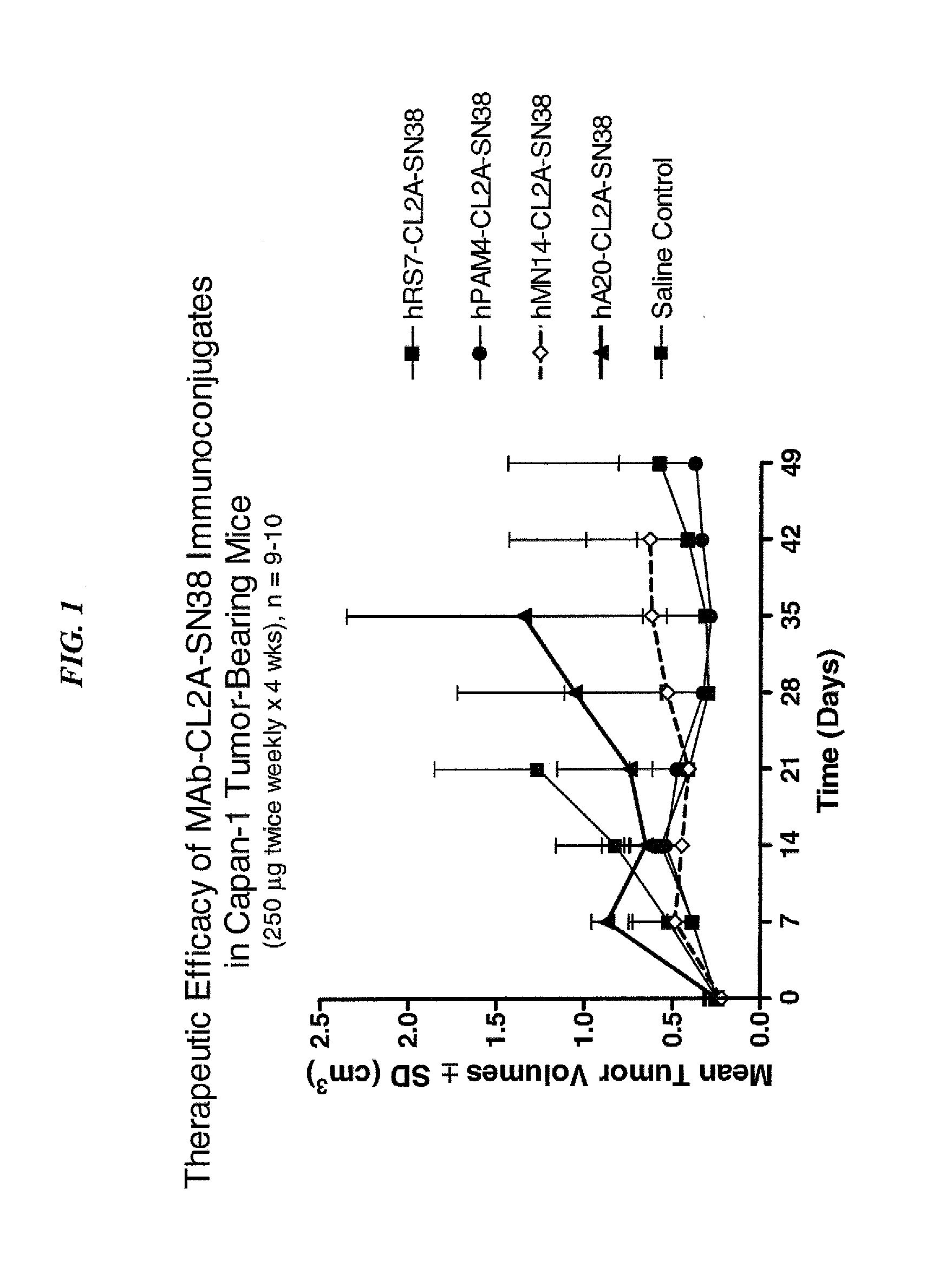
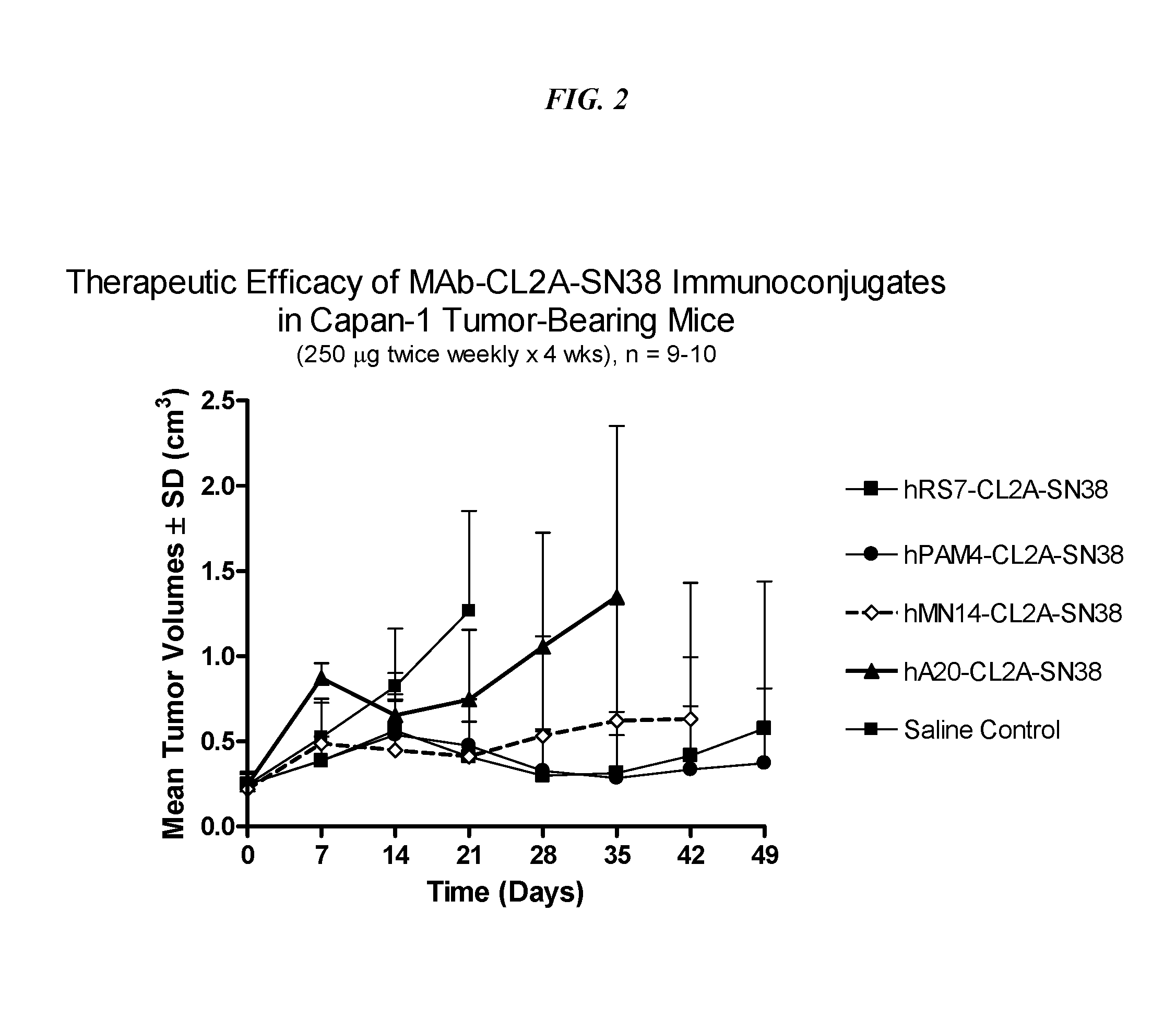
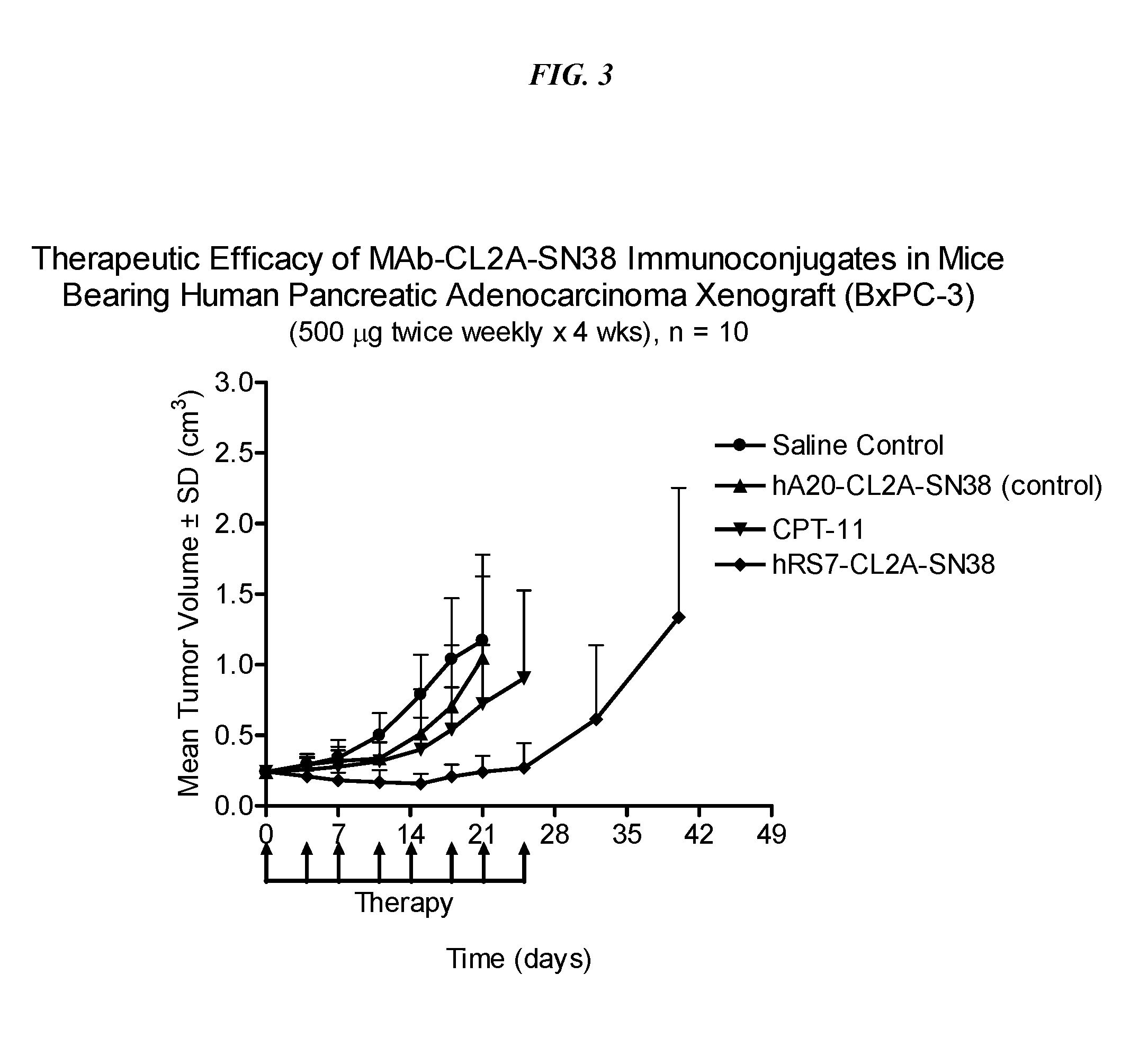
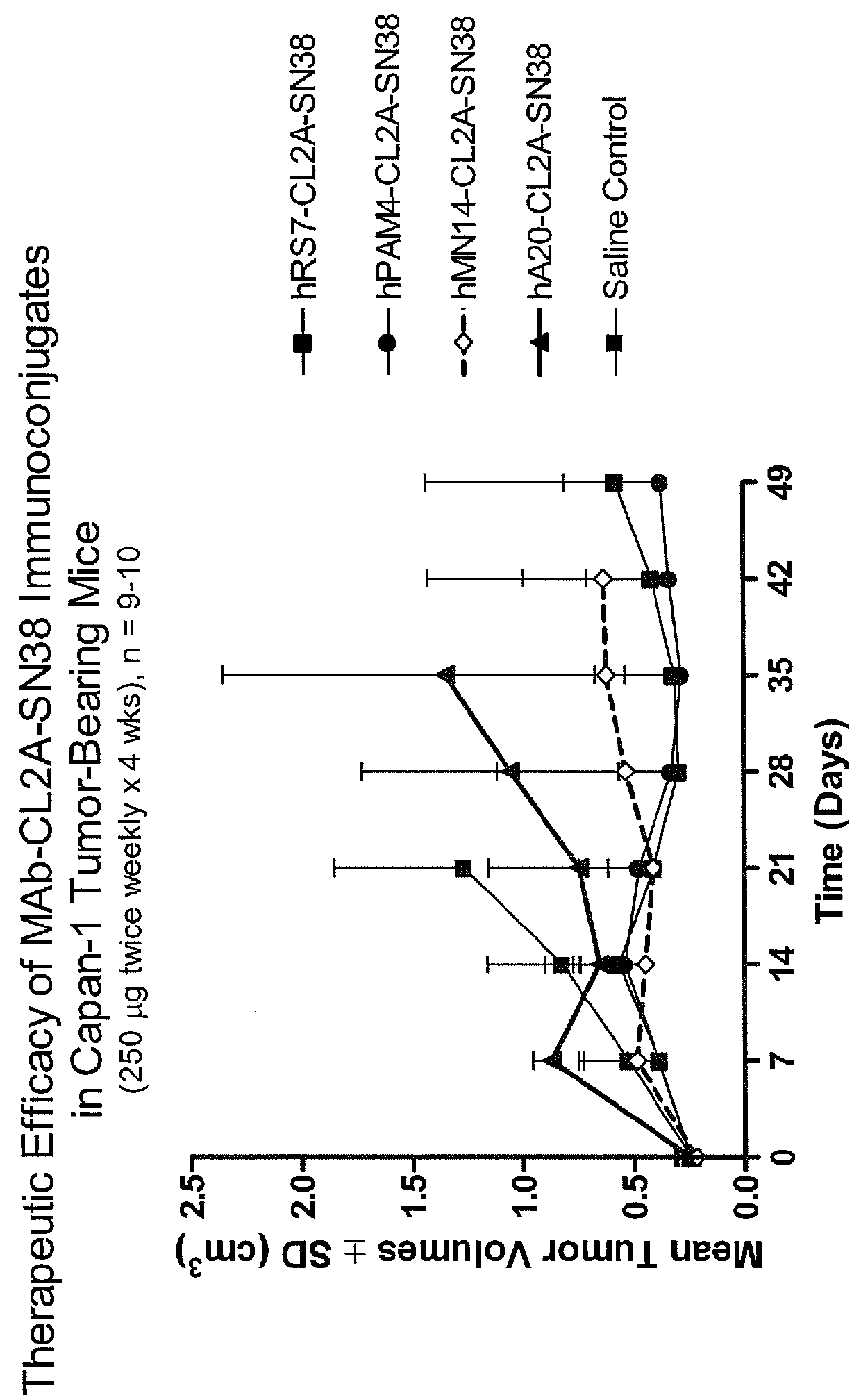
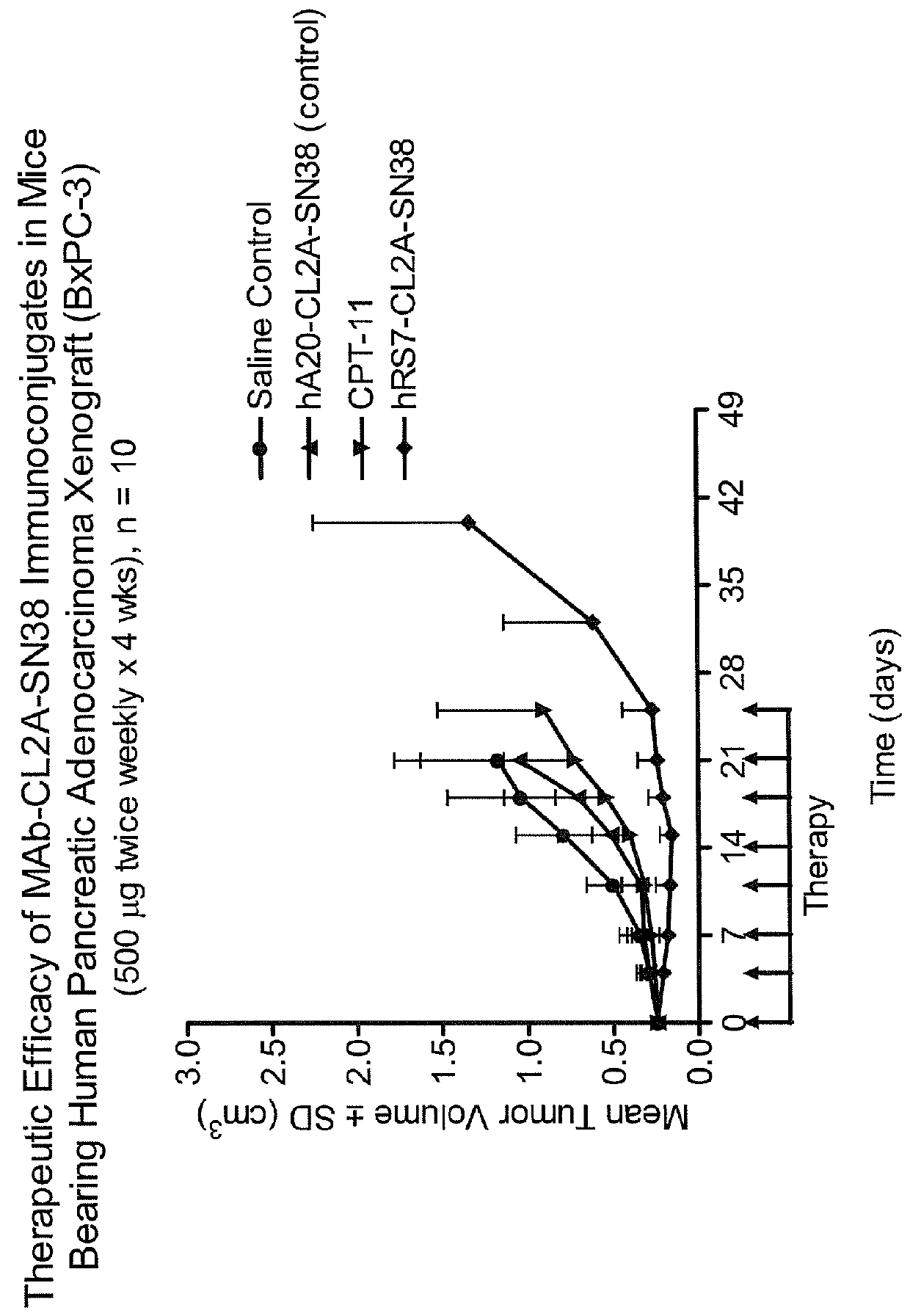
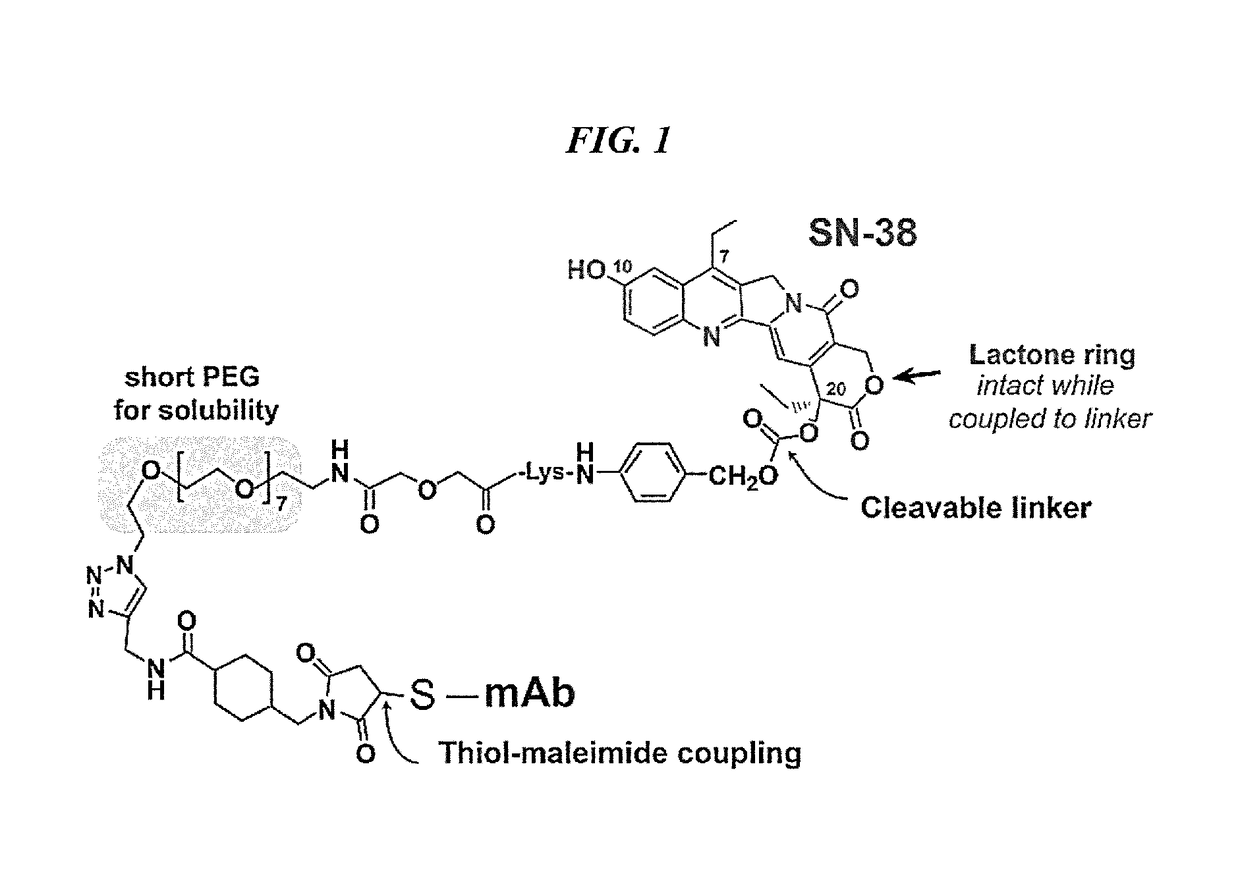
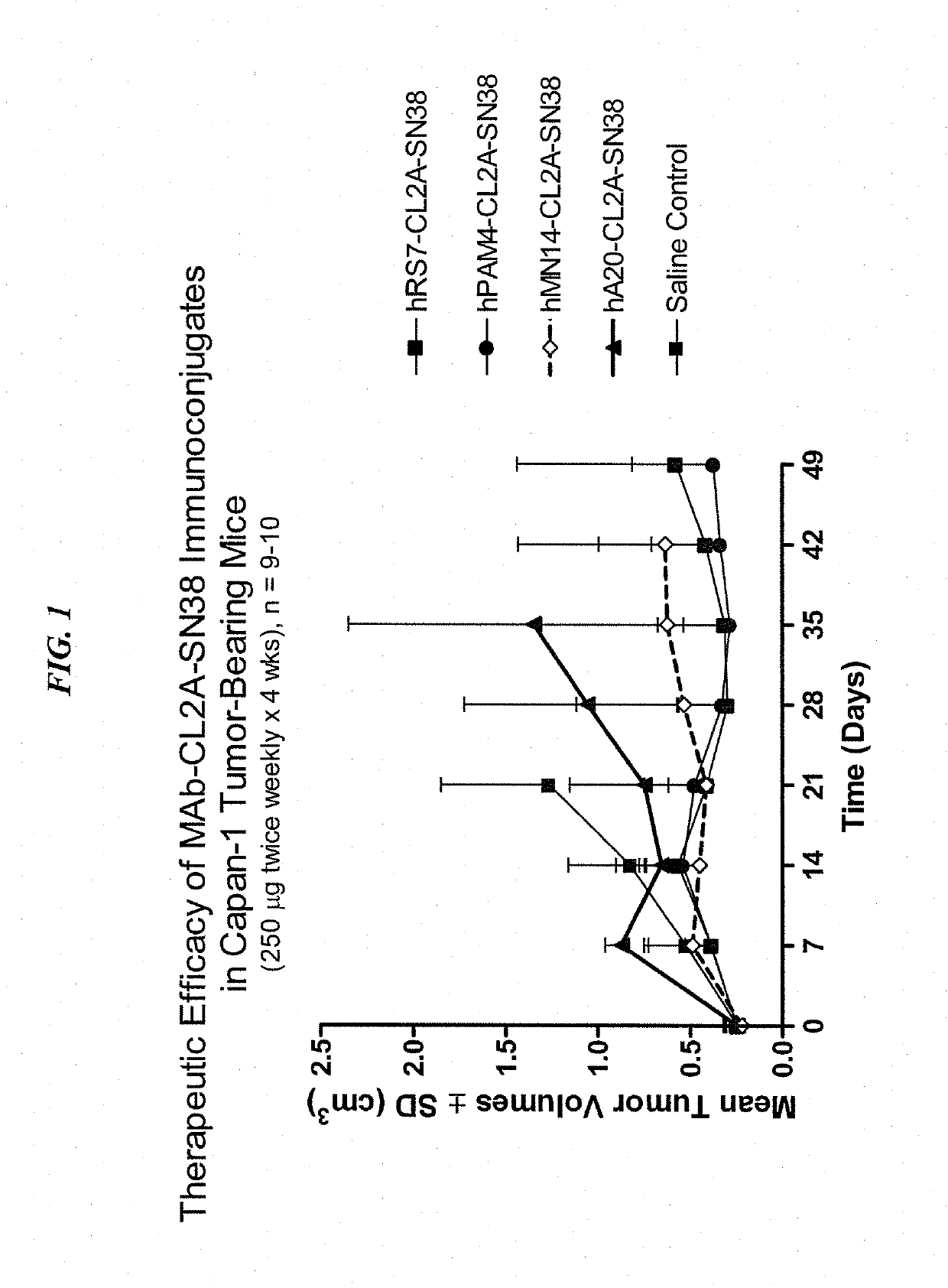
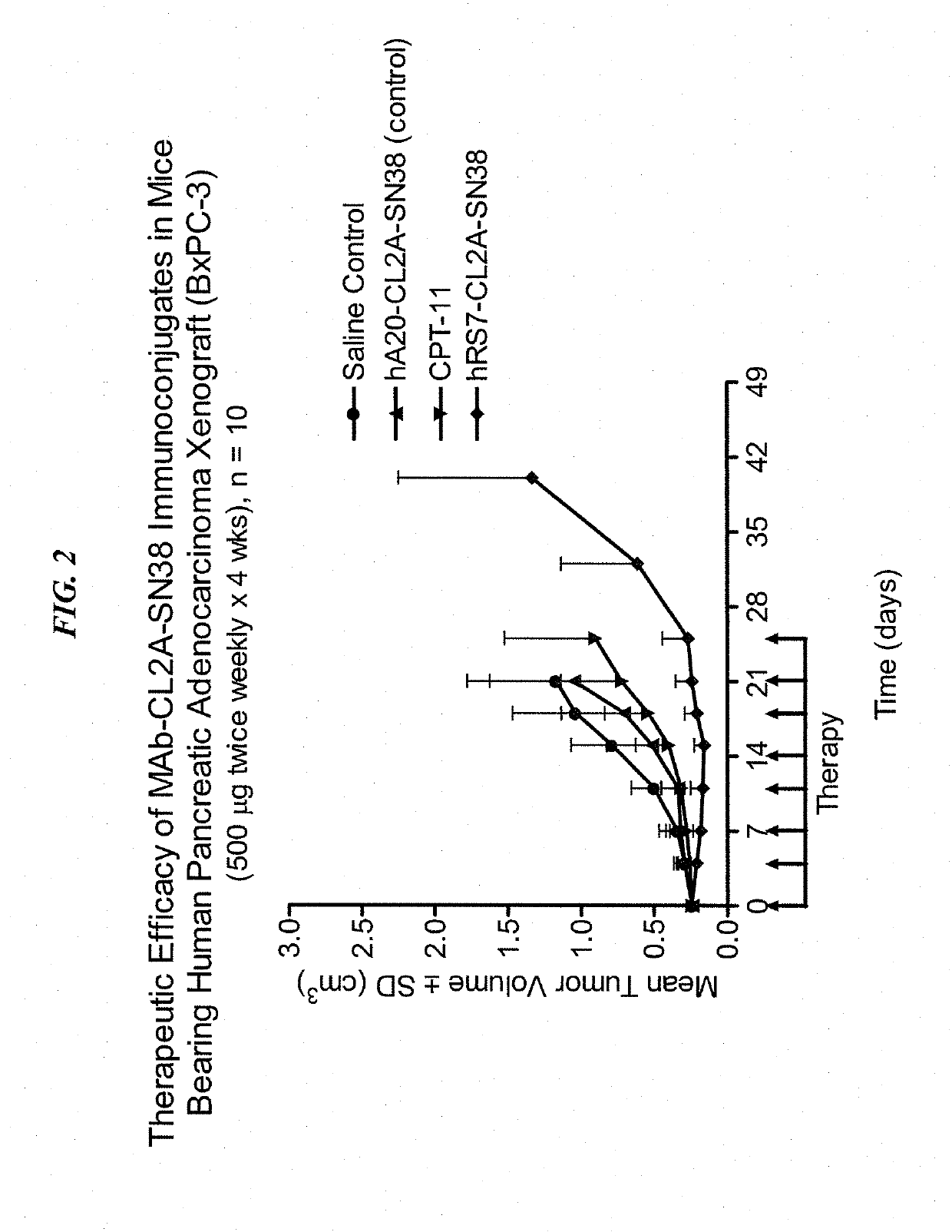
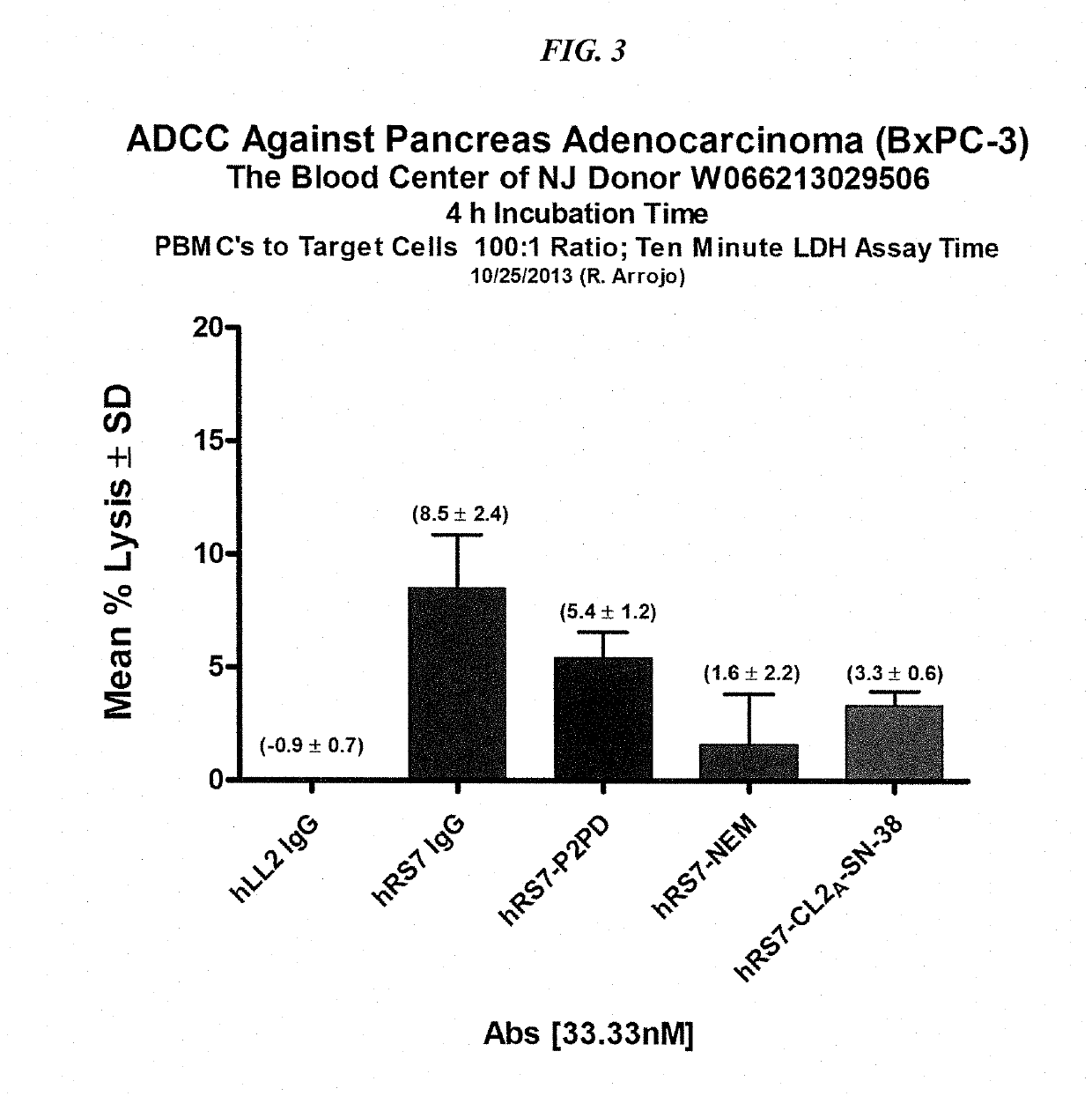
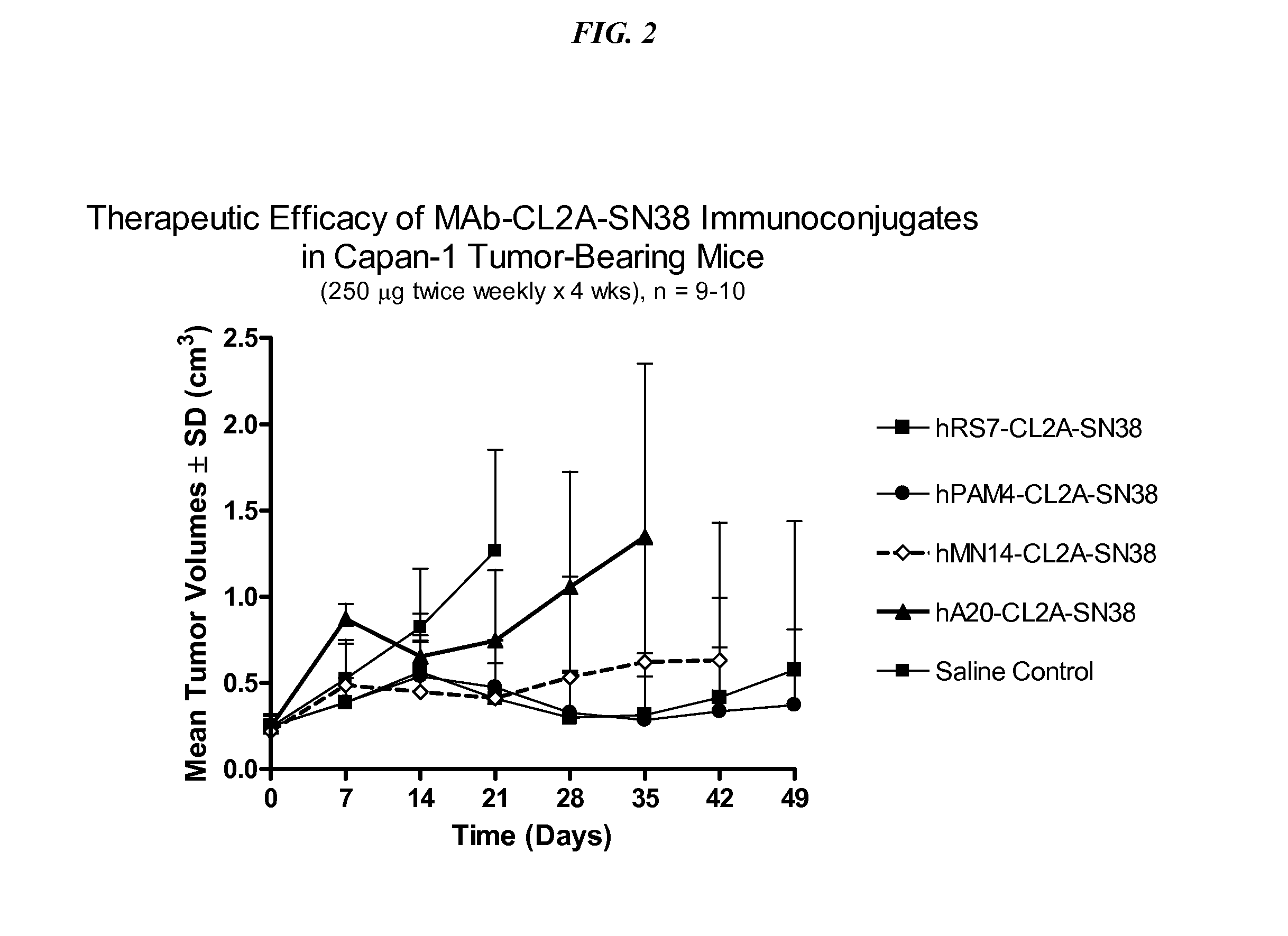
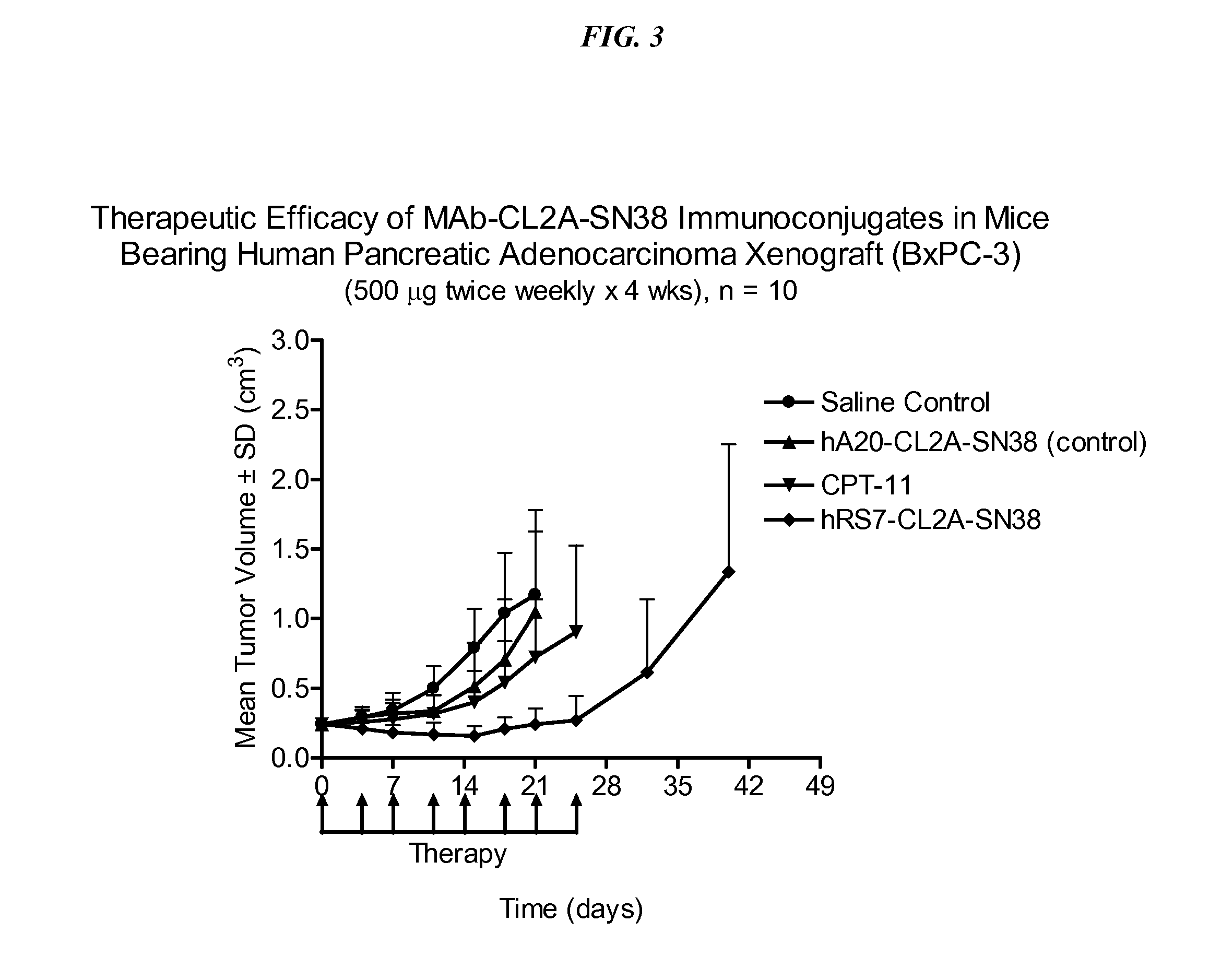
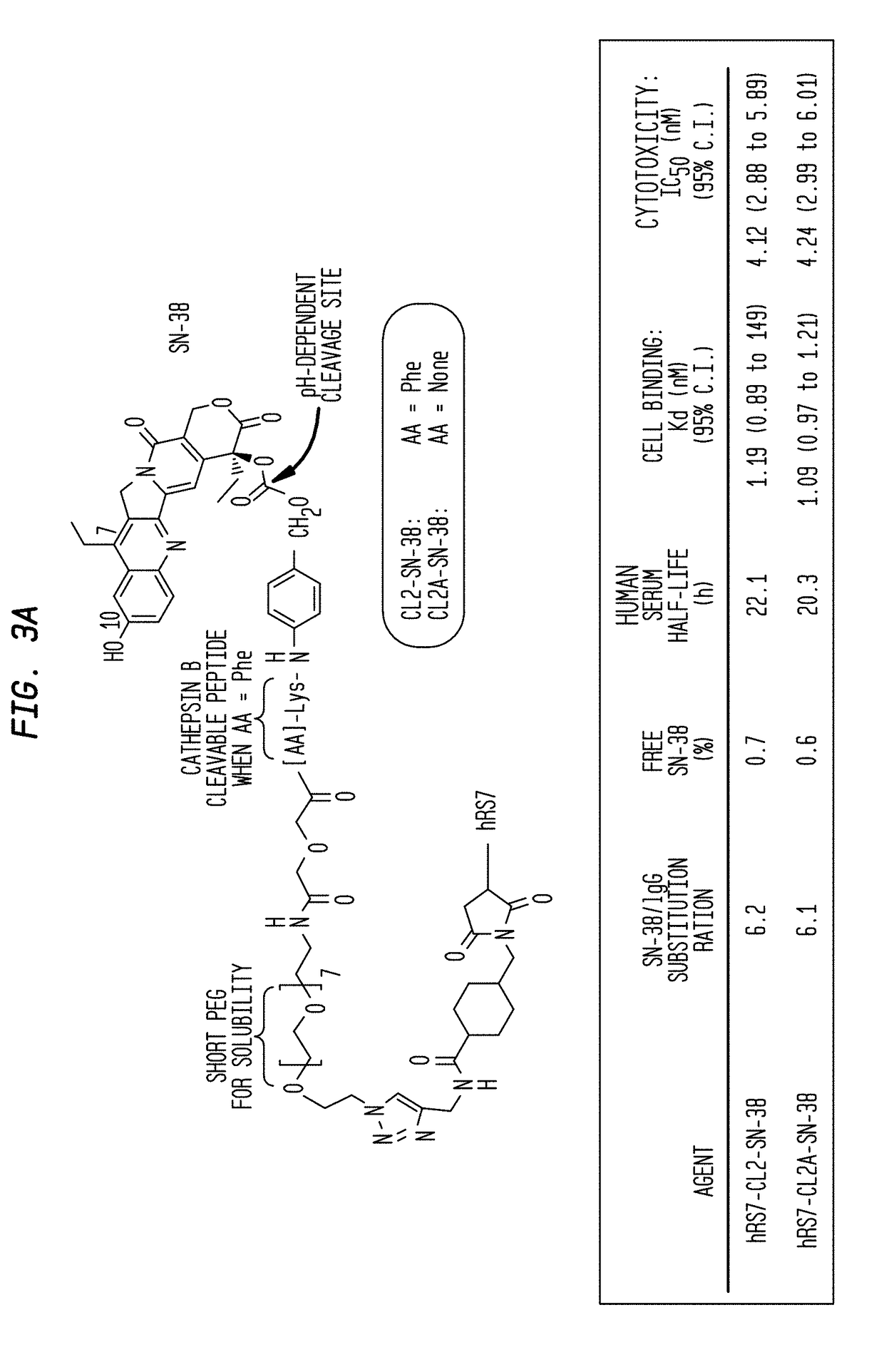
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
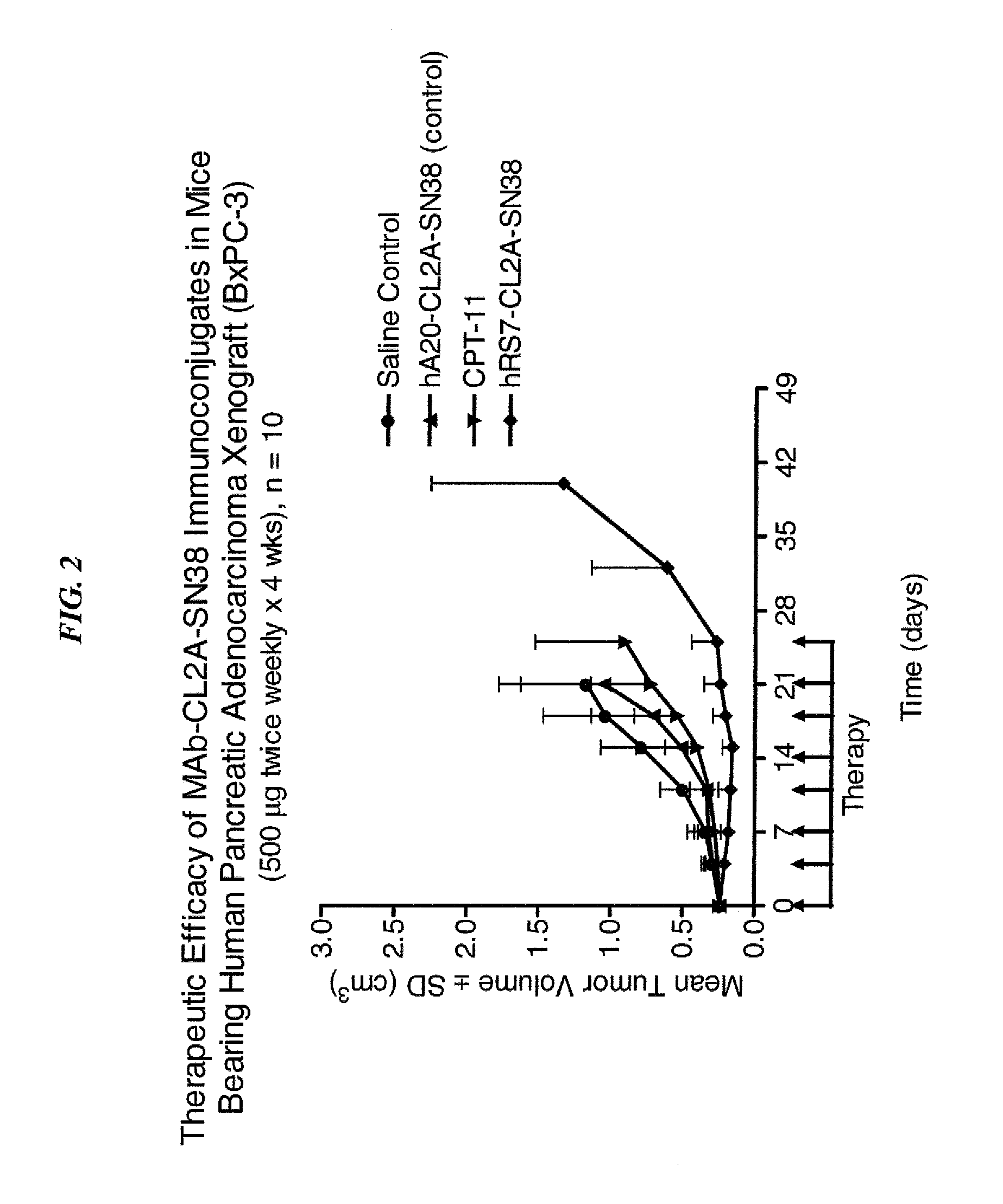
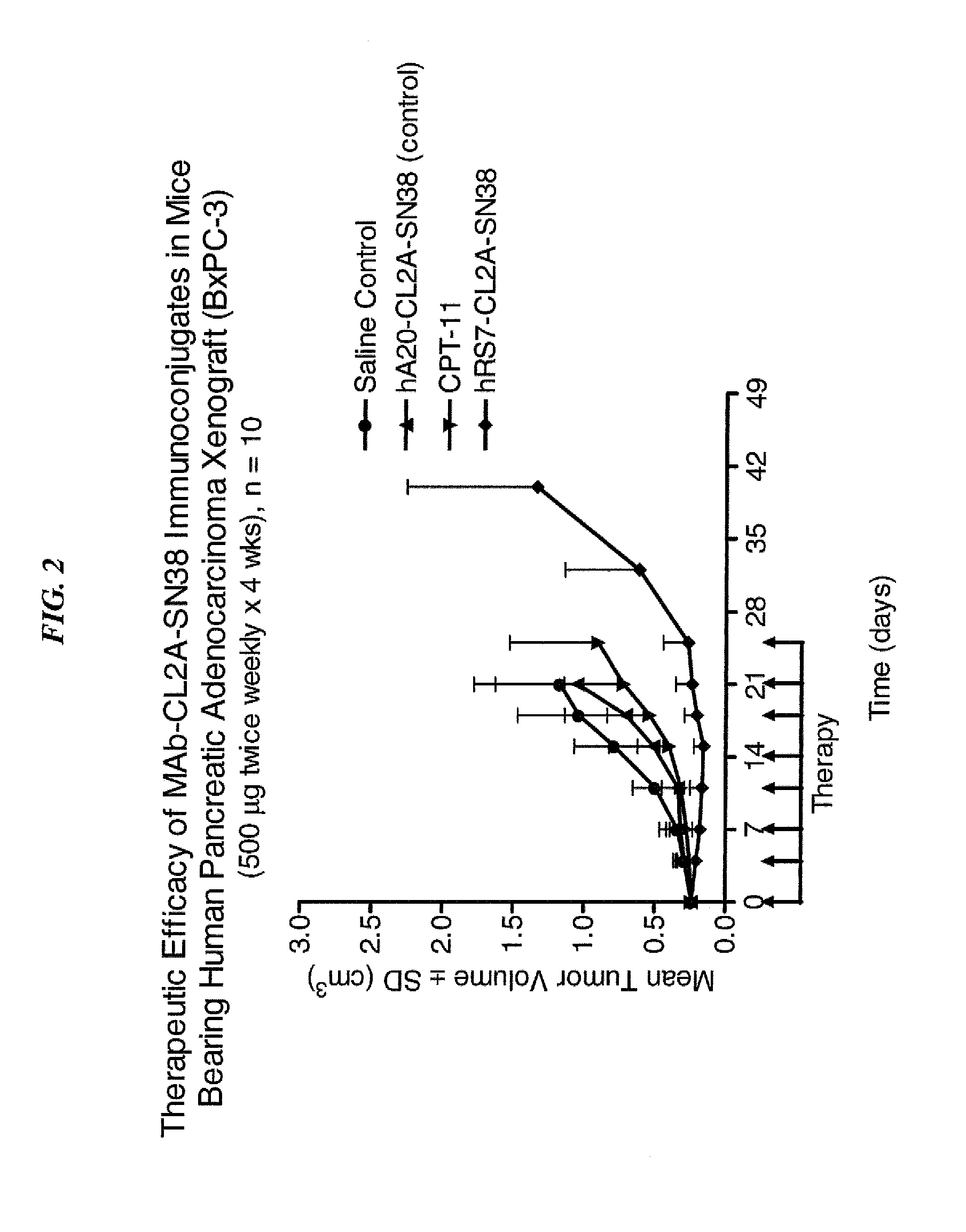
ActiveUS20140170063A1Overcome tumorImprove targetingHeavy metal active ingredientsOrganic active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 ROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
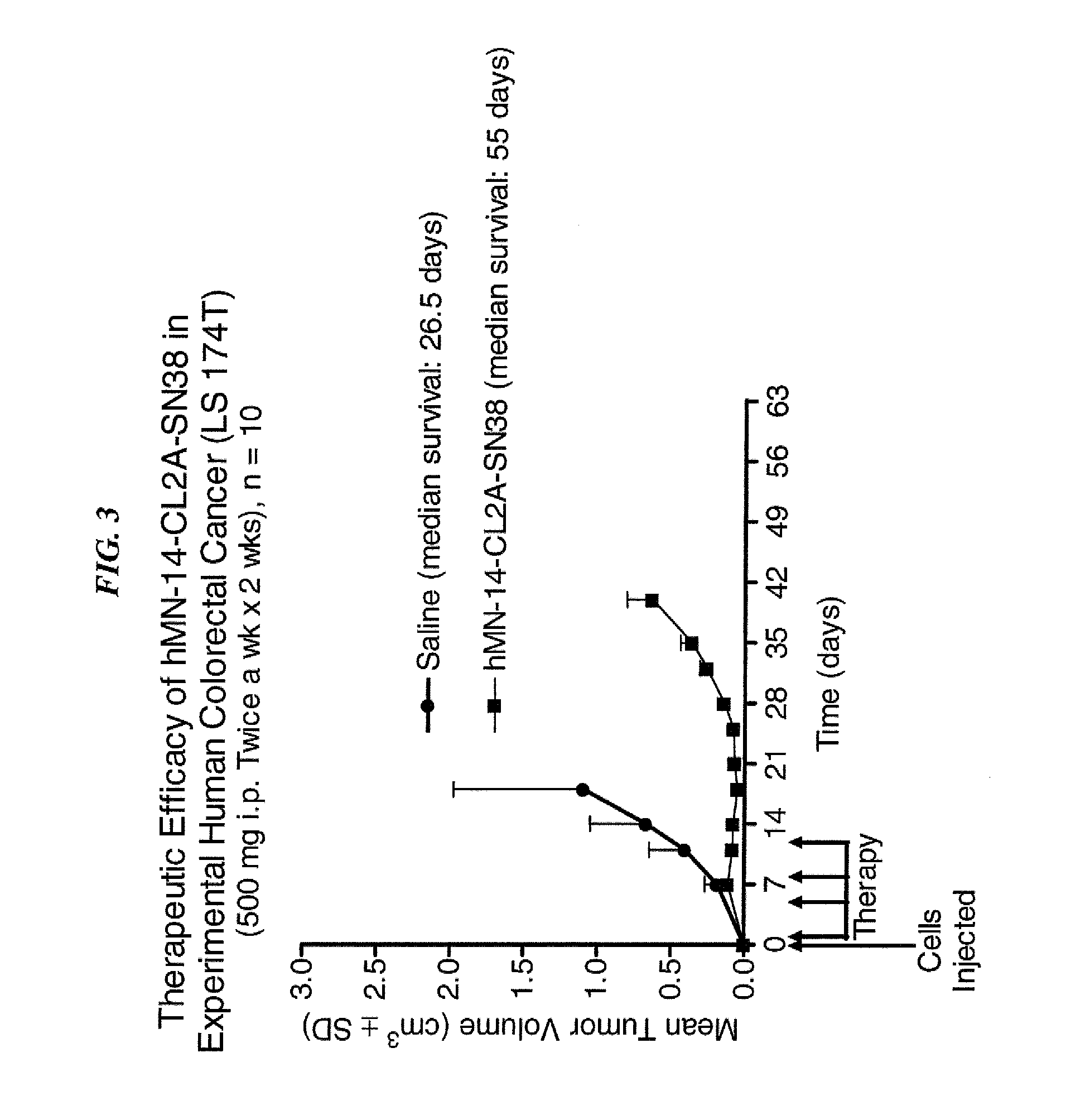
ActiveUS20140219914A1Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
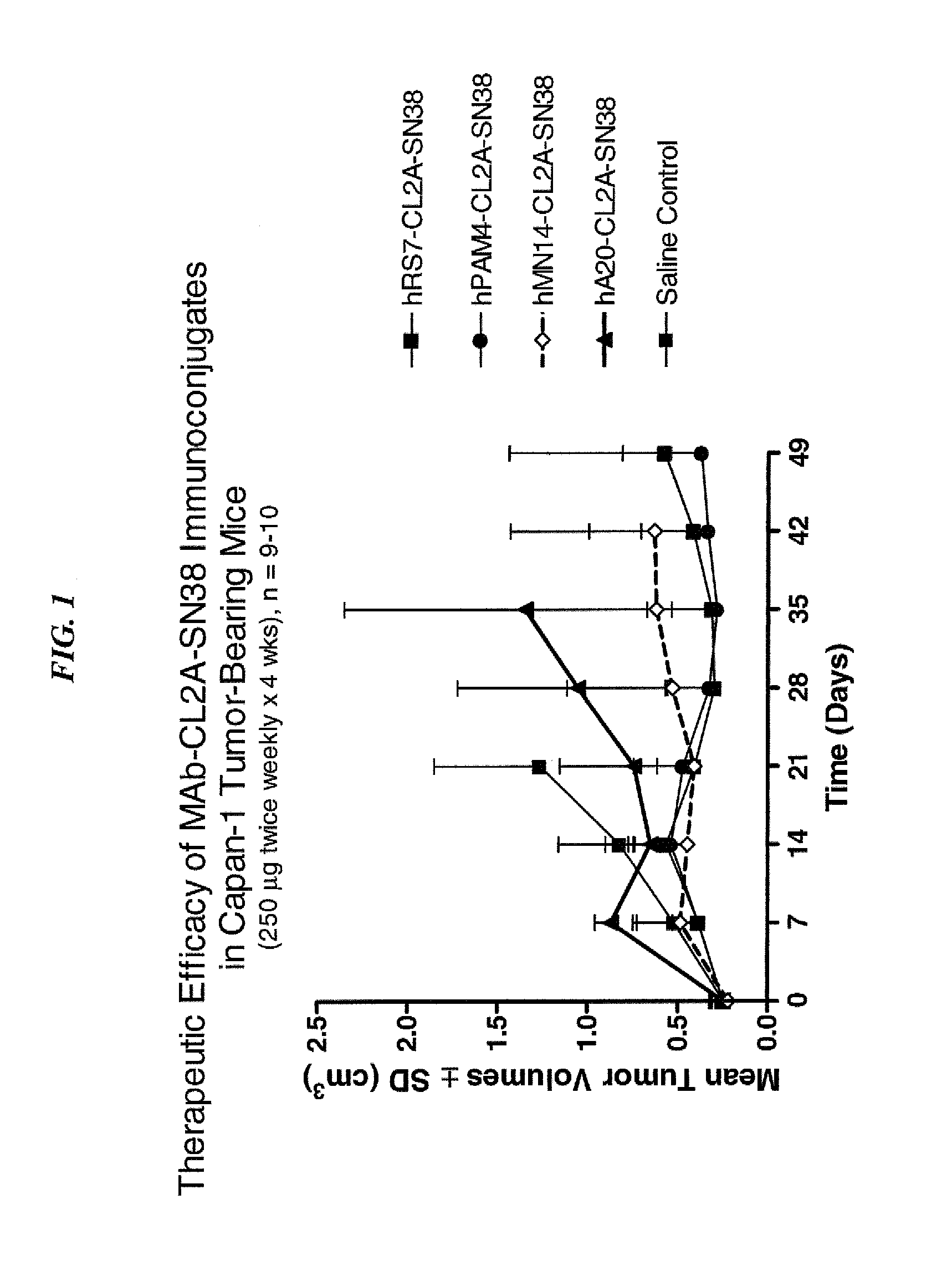
ActiveUS9028833B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
SN-38 lipid complexes and their methods of use
The present invention is for novel compositions and methods for treating diseases caused by cellular proliferation, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include SN-38 lipid complexes in which the complexes can contain any of a variety of neutral or charged lipids and, desirably, cardiolipin. The compositions are capable of efficiently incorporating SN-38 into complexes and are capable of solubilizing relatively high concentrations of SN-38.
Owner:NEOPHARMA INC
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS20160296633A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadTreatment effect
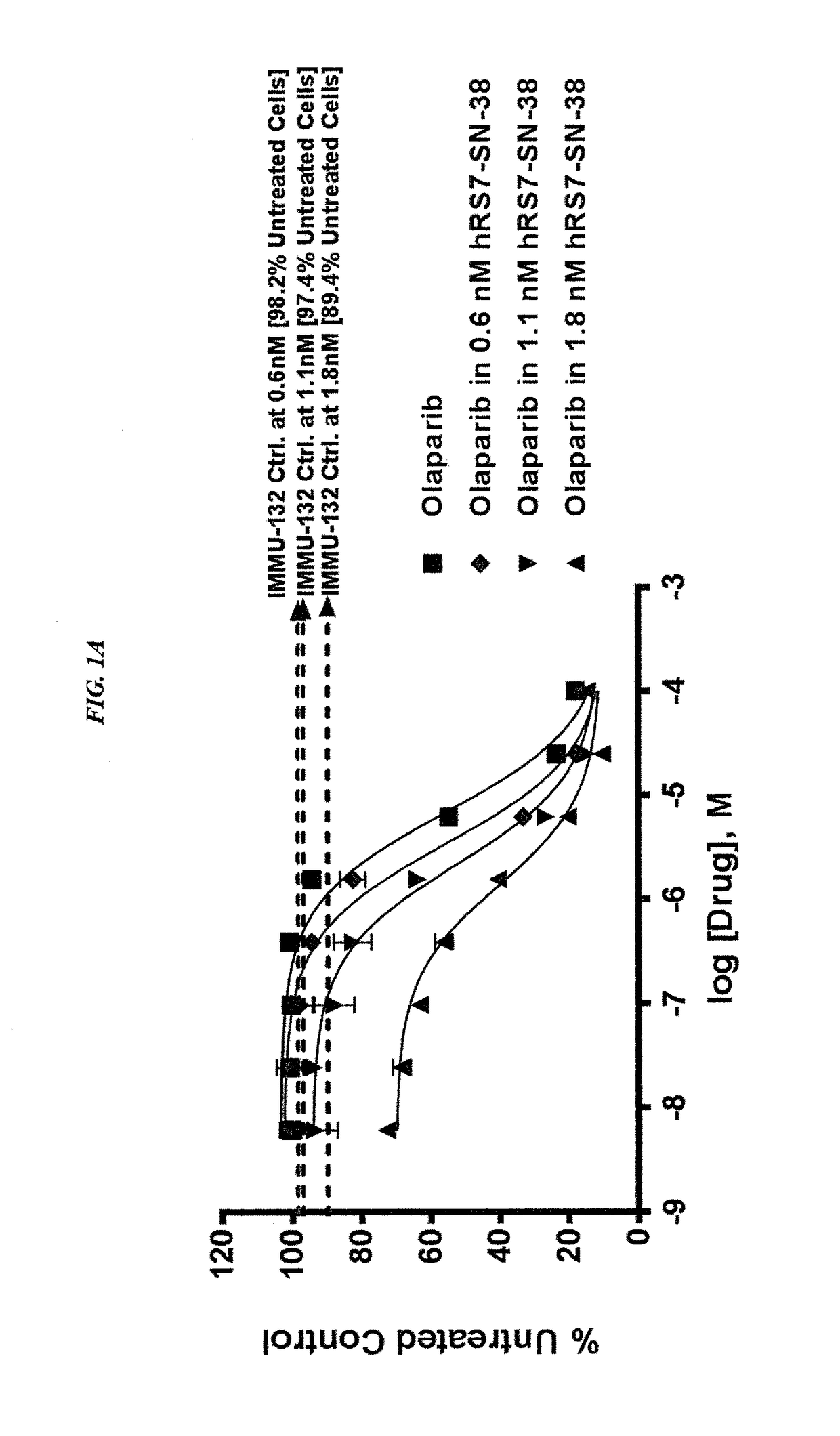
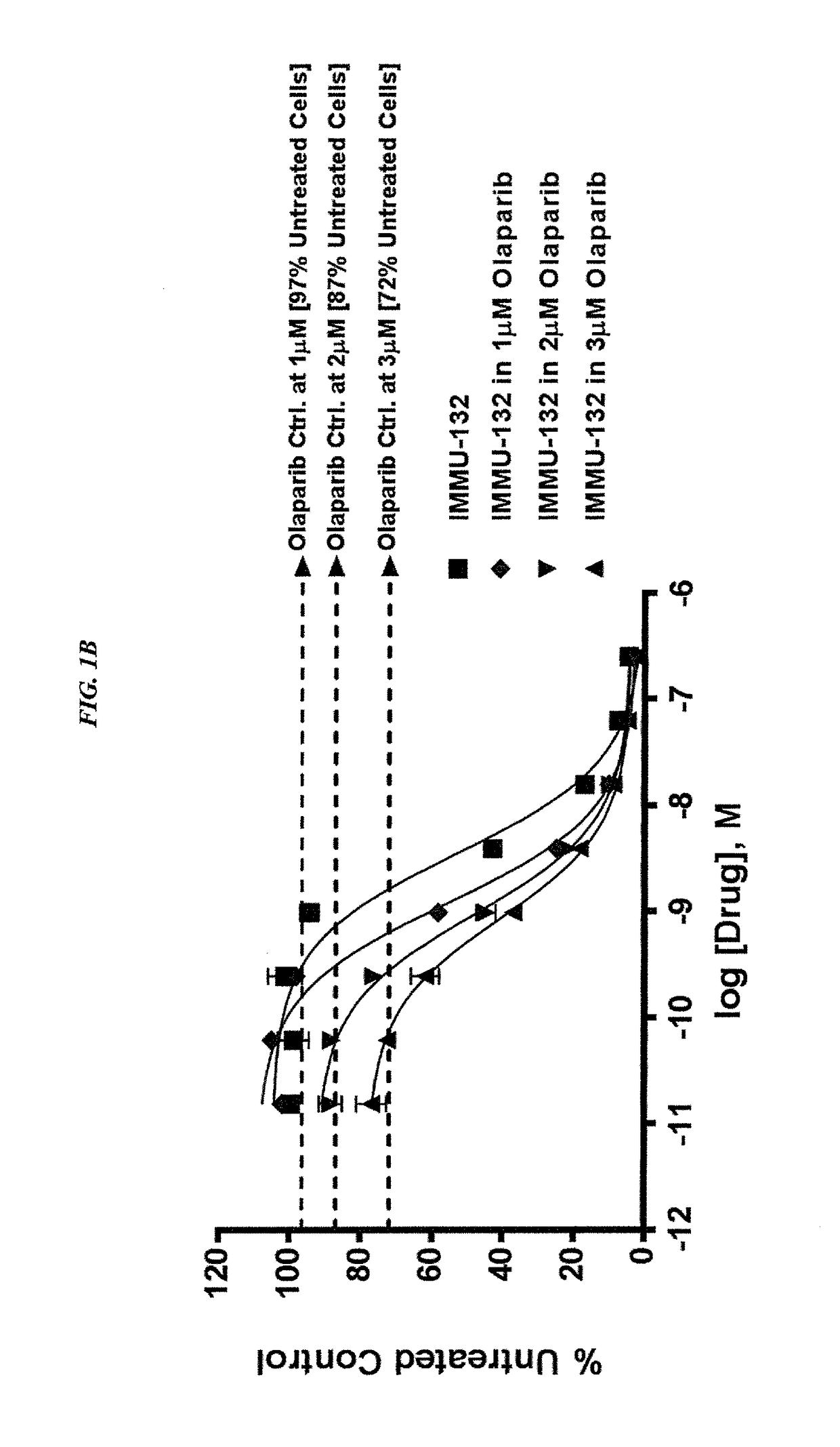
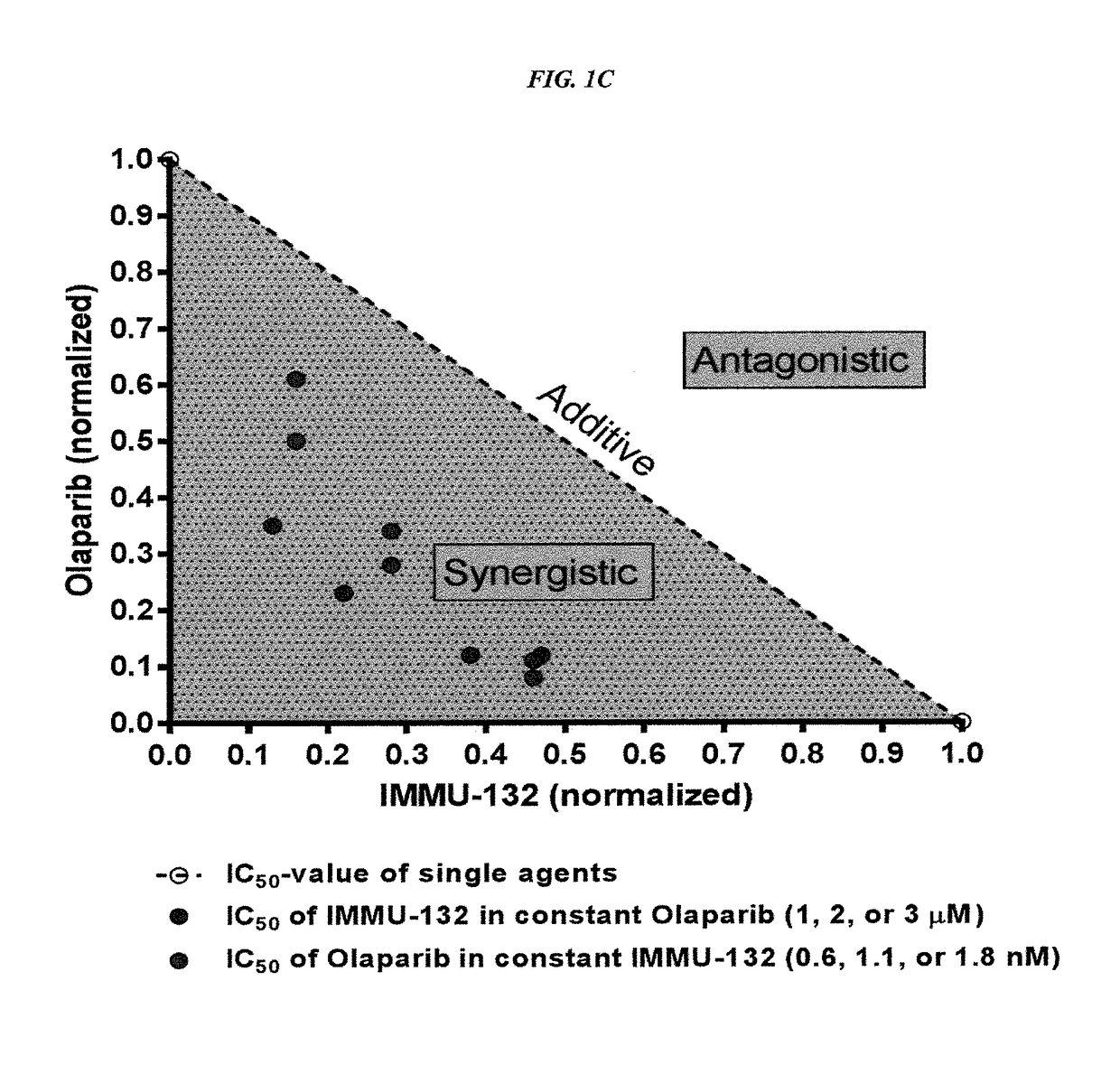
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Amphiphilic prodrug of 7- ethyl-10-hydroxycamptothecin and preparation method thereof
InactiveCN102060991AControl release speedPromote degradationOrganic chemistryPharmaceutical delivery mechanismSolubility7-ethyl-10-hydroxycamptothecin
The invention discloses an amphiphilic prodrug of 7- ethyl-10-hydroxycamptothecin, which is prepared by connecting hydroxyl at the site 10 and / or 20 site of the 7-ethyl-10- hydroxycamptothecin and a hydrophilic group through a scissionable chemical bond. The amphiphilic prodrug is self-assembled in water to form a micelle or vesicle structure. Therefore, on one hand, the solubility of SN-38 in water is greatly increased, and the stability of SN-38 lactone rings is improved; and on the other hand, the drug loading rate is high and the SN-38 can be quickly released in cells, consequently the defect of low drug loading rate of traditional drugs is overcame. Moreover, with the nano-micelles or the nano-vesicles of the prodrug, EPR (enhanced permeability and retention) effect targeted cancer tissues of tumors can be effectively utilized.
Owner:ZHEJIANG UNIV
Anticancer composition
InactiveCN1961864AOrganic active ingredientsPharmaceutical delivery mechanismAdjuvantTreatment effect
Disclosed is an anti-cancer composition slow release injection which comprises slow release microspheres and dissolvent, the slow release microspheres comprise slow release auxiliary materials, Epothilone derivatives, glyoxaline piperazidine, Topo enzyme inhibitor combination selected from SN-38, CPT-11, HCPT, Topotecan, Irinotecan, Etoposide, Teniposide, Amrubicin, Valtaxin, XK469, AD312, ICRF-187 or ICRF-193, the dissolvent being specific dissolvent containing suspension adjuvant, the slow release auxiliary materials are selected from polylactic acid and its copolymer, polyethylene / polylactic acid, glycollic acid copolymer, aliphatic acid and sebacylic acid copolymer, the viscosity of the suspension adjuvant is 80-3000cp (at 20-30 deg C), and is selected from carboxymethylcellulose, The slow release microspheres can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a release period of about 40 days. The slow release injection and slow release implanting agent can be used independently for effectively suppressing tumor accretion, or used in combination with non-operative methods such as chemotherapy and / or radiotheraphy with the function of improving their treatment effects.
Owner:JINAN SHUAIHUA PHARMA TECH
Pharmaceutically active lipid based formulation of SN-38
SN38, camptothecin derivatives are poorly water soluble, highly lipophilic camptothecin derivatives and are very active against a variety of human cancers. Because of their very poor water solubility, SN38 has not been used to treat human patients with cancer due to the inability to administer sufficient quantities of dissolved in a pharmaceutical formulation. This invention overcomes these limitations by teaching novel pharmaceutically acceptable SN38 liposome complex formulation for the direct administration of the formulation to human patients with cancer. The claimed invention also describes the methods to prepare liposomal SN38 complexes and antitumor compositions of liposomal SN38 complexes to allow the administration in sufficient amounts to treat various types of cancer and as antiviral agents. This invention is also directed to injectable sterile solutions, antitumor compositions, liposomes. The present invention is for novel compositions and methods for treating diseases caused by cellular proliferation, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include SN38 lipid complexes in which the complexes can contain any of a variety of neutral or charged lipids and, desirably, cardiolipin. The compositions are capable of efficiently incorporating SN38 into complexes and are capable of solubilizing relatively high concentrations of SN38.
Owner:NEOPHARMA INC
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
InactiveUS20170274093A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsOrganic active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Humanized Anti-ceacam5 antibody and uses thereof
InactiveUS20150125386A1Overcome tumorImprove targetingImmunoglobulins against animals/humansRadioactive preparation carriersDrug conjugationWhole body
The present invention concerns compositions and methods of use of a humanized Class III anti-CEA antibody, comprising the heavy and light amino acid sequences SEQ ID NO:1 and SEQ ID NO:2. The antibody is effective to treat CEACAM5-expressing tumors, either alone or in combination with one or more therapeutic agents. Drug conjugated Class III anti-CEA antibodies, such as SN-38 or P2PDox immunoconjugates, are particularly efficacious. Surprisingly, the antibody-drug conjugates (ADCs) exhibit high anti-cancer efficacy, while exhibiting low levels of systemic toxicity that are readily treated with standard amelioration techniques. Antibodies and / or immunoconjugates comprising the amino acid sequences SEQ ID NO:1 and SEQ ID NO:2 are surprisingly efficacious for therapy of solid tumors, even when the tumor has proven resistant to standard anti-cancer therapies.
Owner:IMMUNOMEDICS INC
Combining anti-HLA-DR or anti-Trop-2 antibodies with microtubule inhibitors, PARP inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS9707302B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Delivery system for cytotoxic drugs by bispecific antibody pretargeting
InactiveUS8435539B2Reducing certain severe side effectsReceive treatment wellBiocidePeptide/protein ingredientsDiseaseBinding site
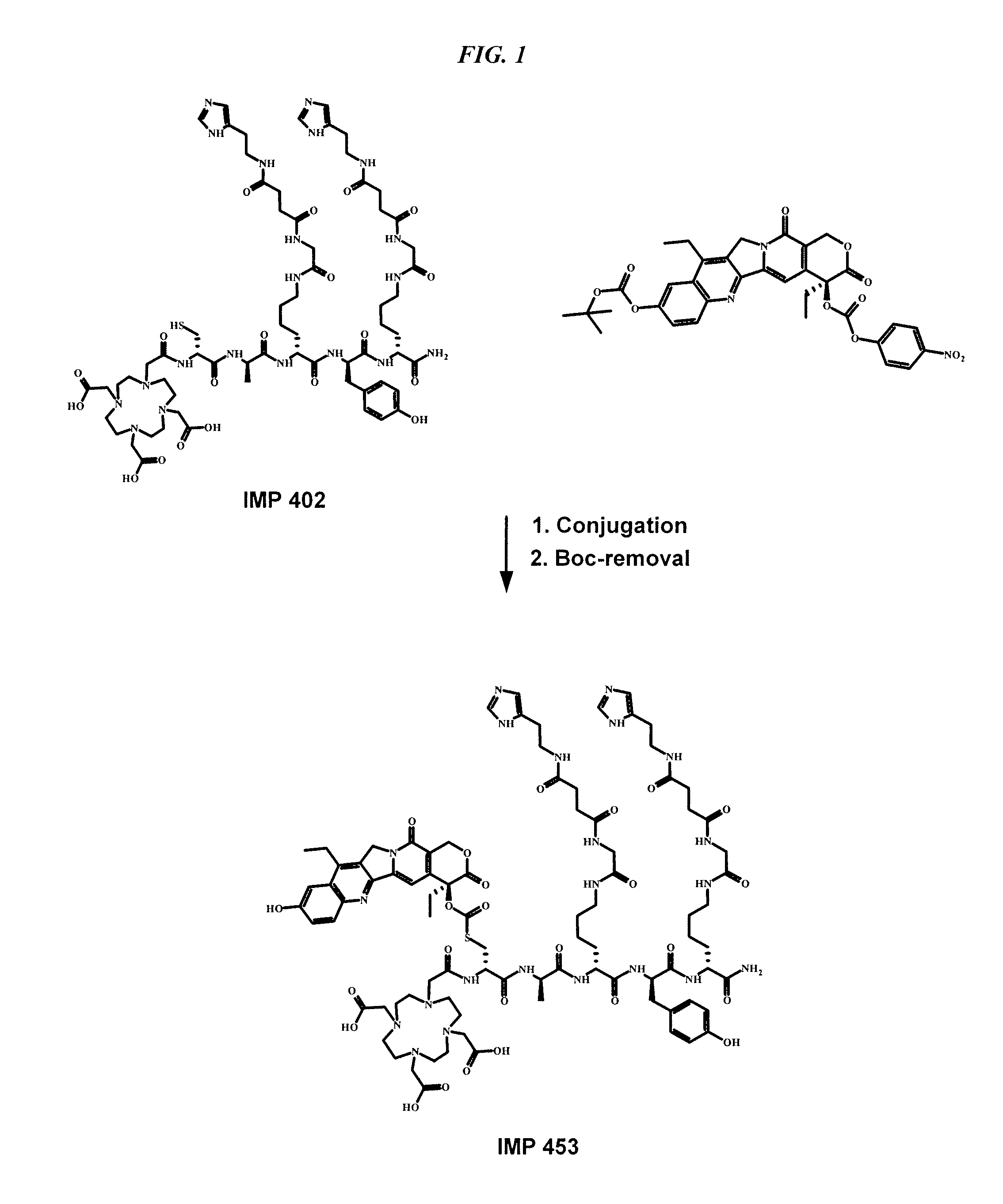
The present invention relates to methods and compositions for pretargeting delivery of therapeutic agents. In preferred embodiments, the pretargeting method comprises: a) administering a bispecific antibody with a first binding site for a disease-associated antigen and a hapten on a targetable construct; b) administering a targetable construct comprising at least one therapeutic agent. In preferred embodiments, the bispecific antibody is made by the dock-and-lock (DNL) technique. In a more preferred embodiment, the targetable construct comprises one or more SN-38 moieties.
Owner:IMMUNOMEDICS INC
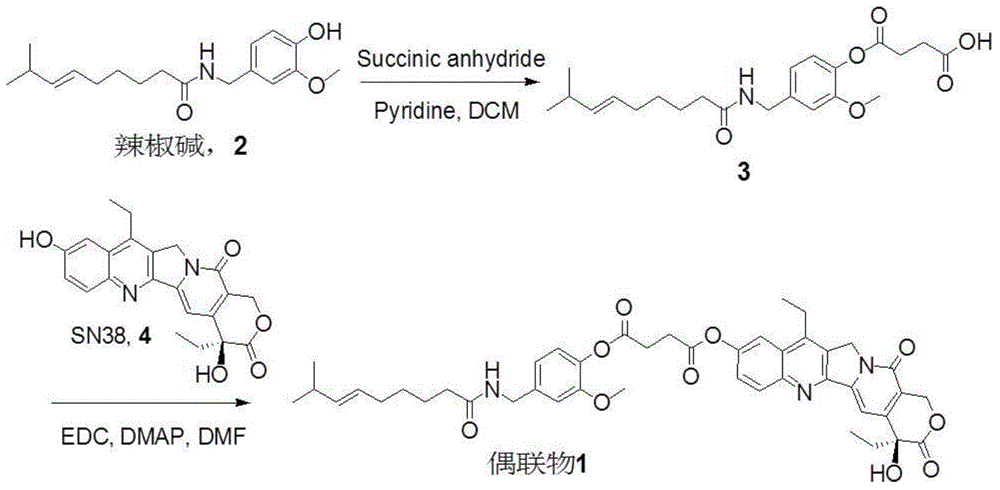
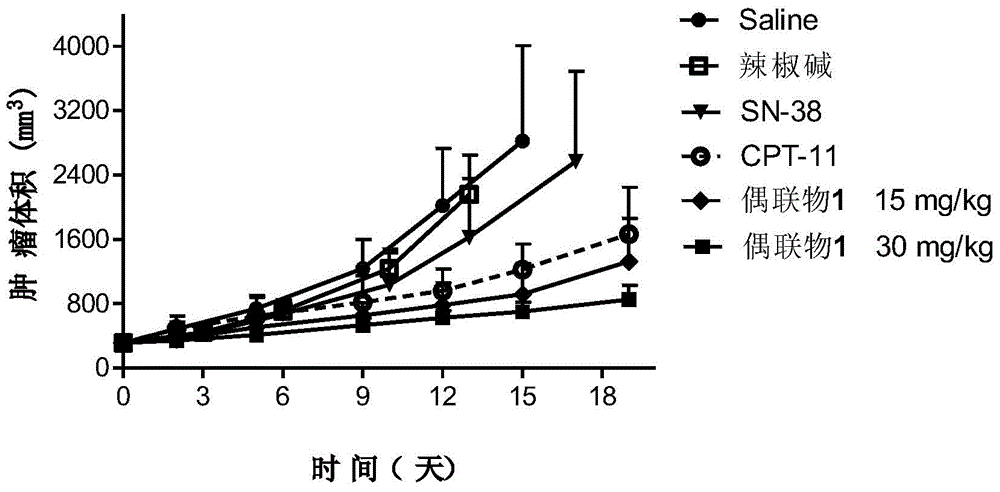
Capsicine-camptothecin anti-cancer drug conjugate and preparation method and application thereof
InactiveCN104447777AImprove solubilityGood antitumor activityOrganic active ingredientsOrganic chemistrySN-38Solvent
The invention discloses a capsicine-camptothecin anti-cancer drug conjugate and a preparation method and application thereof. In the preparation method disclosed by the invention, chemical modification is performed on capsicine, the modified capsicine is coupled to camptothecin anti-cancer drug through ester bonds, and the obtained capsicine-camptothecin anti-cancer drug conjugate can be dissolved in pharmacy solvents which can be accepted in clinic, for example, Tween, so that the conjugate can be directly used for oral medication in clinic; coupling occurs on C20 hydroxy of the camptothecin or on C10 or C20 hydroxy of SN-38. Through the coupling of the ester bonds, the conjugate can directly release two kinds of activated components in a body in a hydrolytic manner. On one hand, the camptothecin or the SN-38 can be released without the catalytic hydrolysis of carboxylesterase, so that the bioavailability of the SN-38 can be effectively improved; on the other hand, the capsicine has a certain anti-cancer activity and can take synergistic action with the SN-38 after being released in the body, so that the anti-cancer effect of the SN-38 is intensified.
Owner:ZHEJIANG UNIV
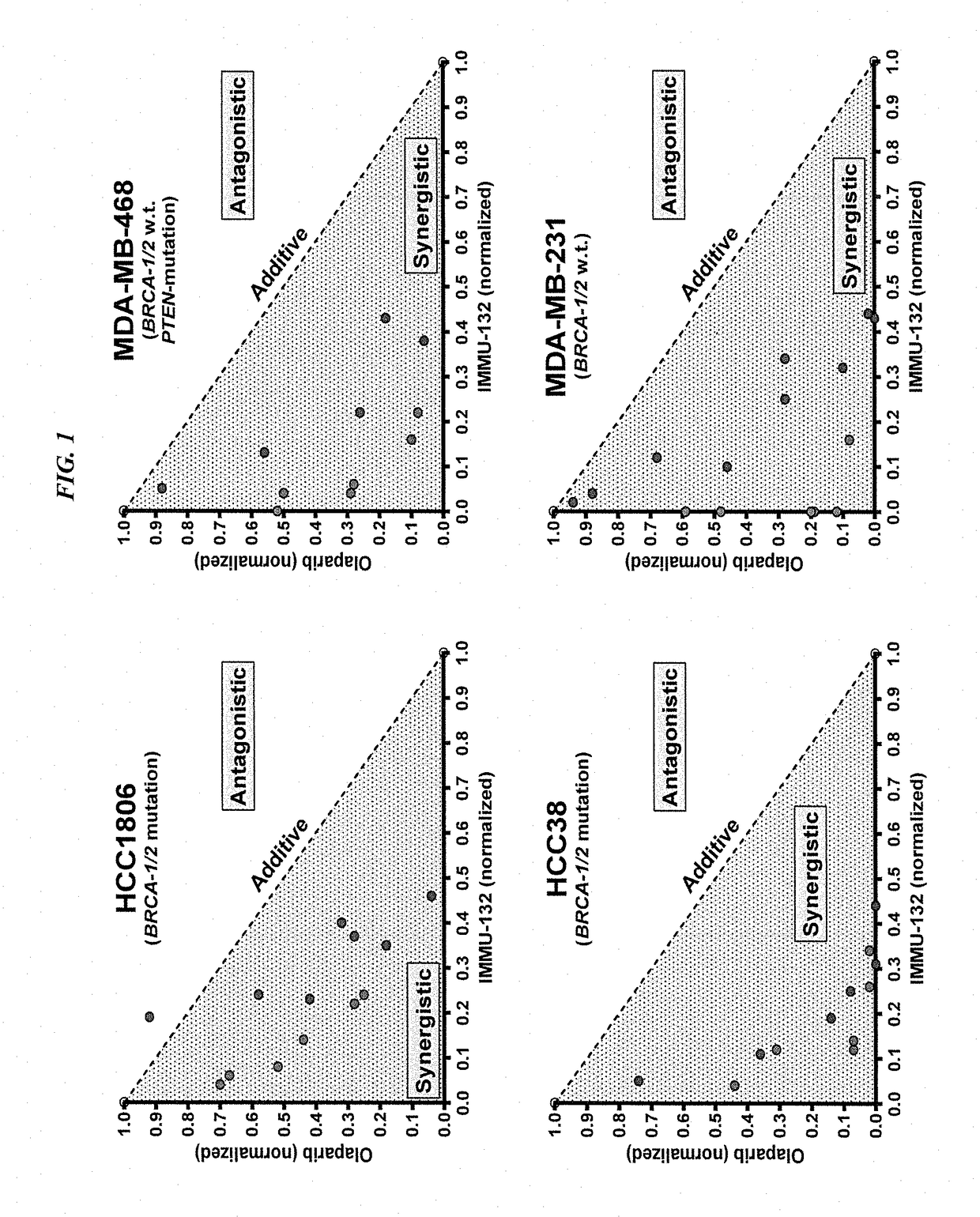
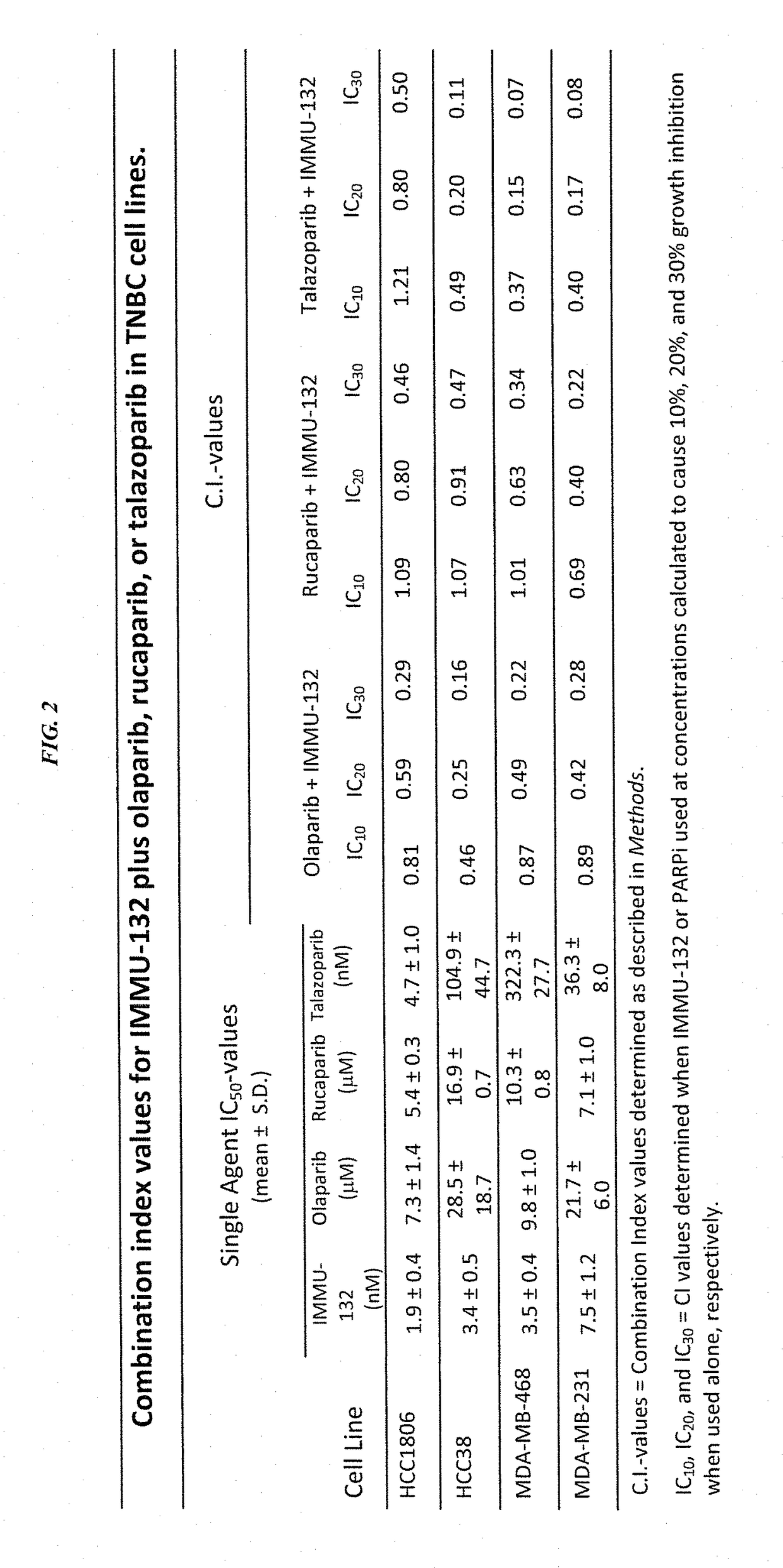
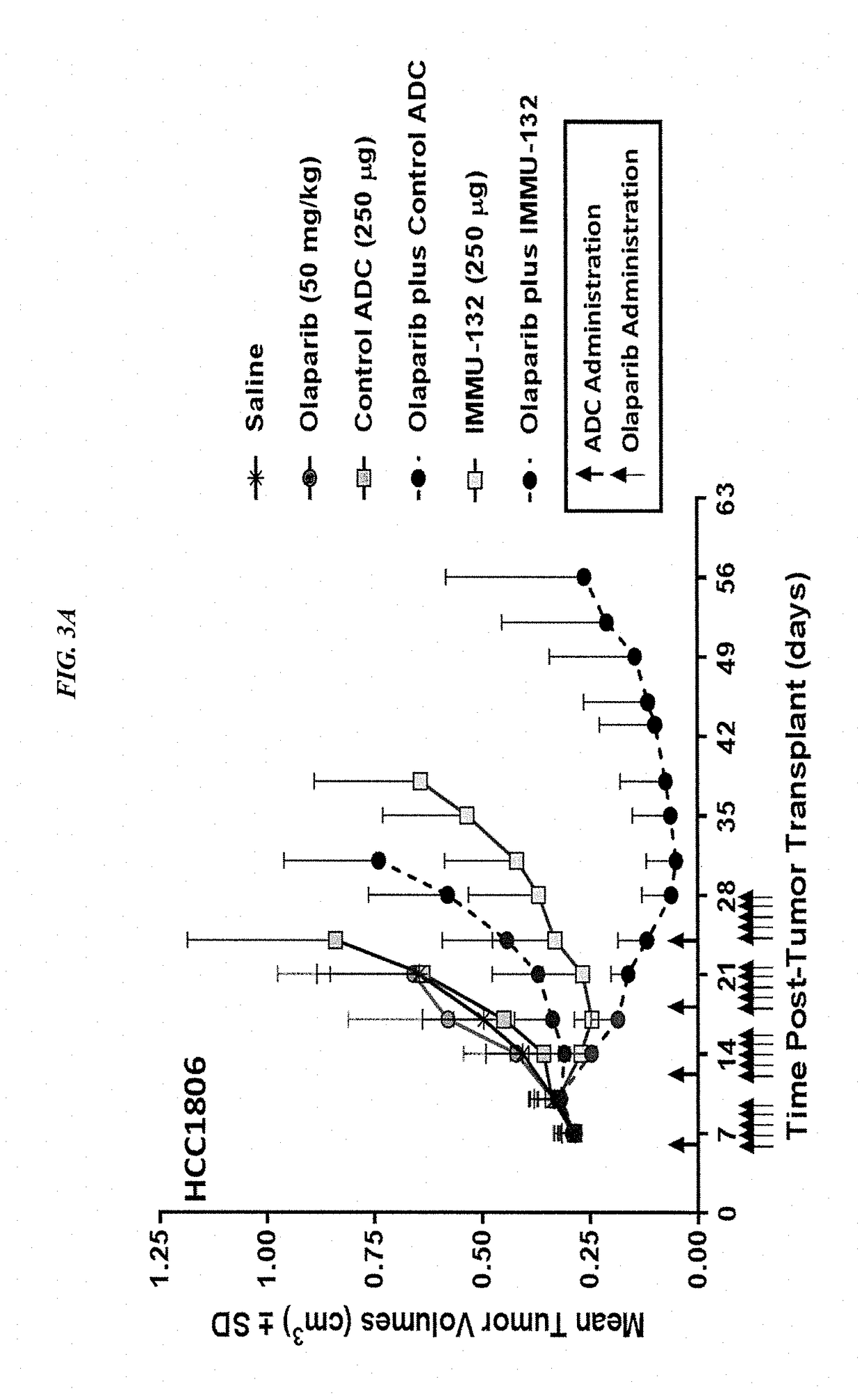
Synergistic effect of anti-Trop-2 antibody-drug conjugate in combination therapy for triple-negative breast cancer when used with microtubule inhibitors or PARP inhibitors
ActiveUS10195175B2Heavy metal active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCombined Modality TherapySN-38
The present invention relates to combination therapy with ADCs against a tumor-associated antigen, such as Trop-2, and drugs, such as microtubule inhibitors and / or PARP inhibitors. Where ADCs are used, they preferably incorporate SN-38 or another drug that induces DNA strand breaks. Preferably, the combination of ADC and PARPi or microtubule inhibitor exhibits synergistic effects against the cancer. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
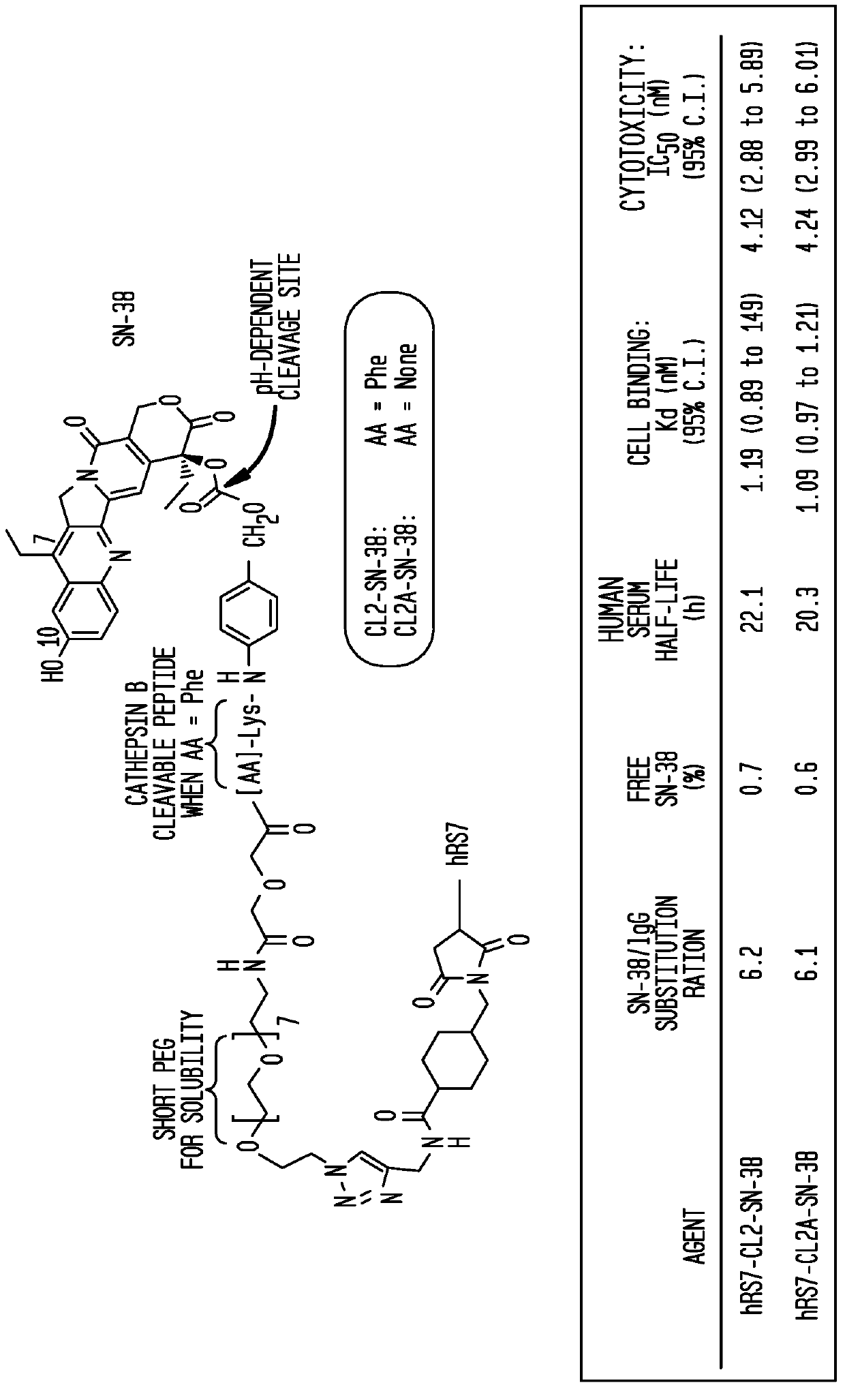
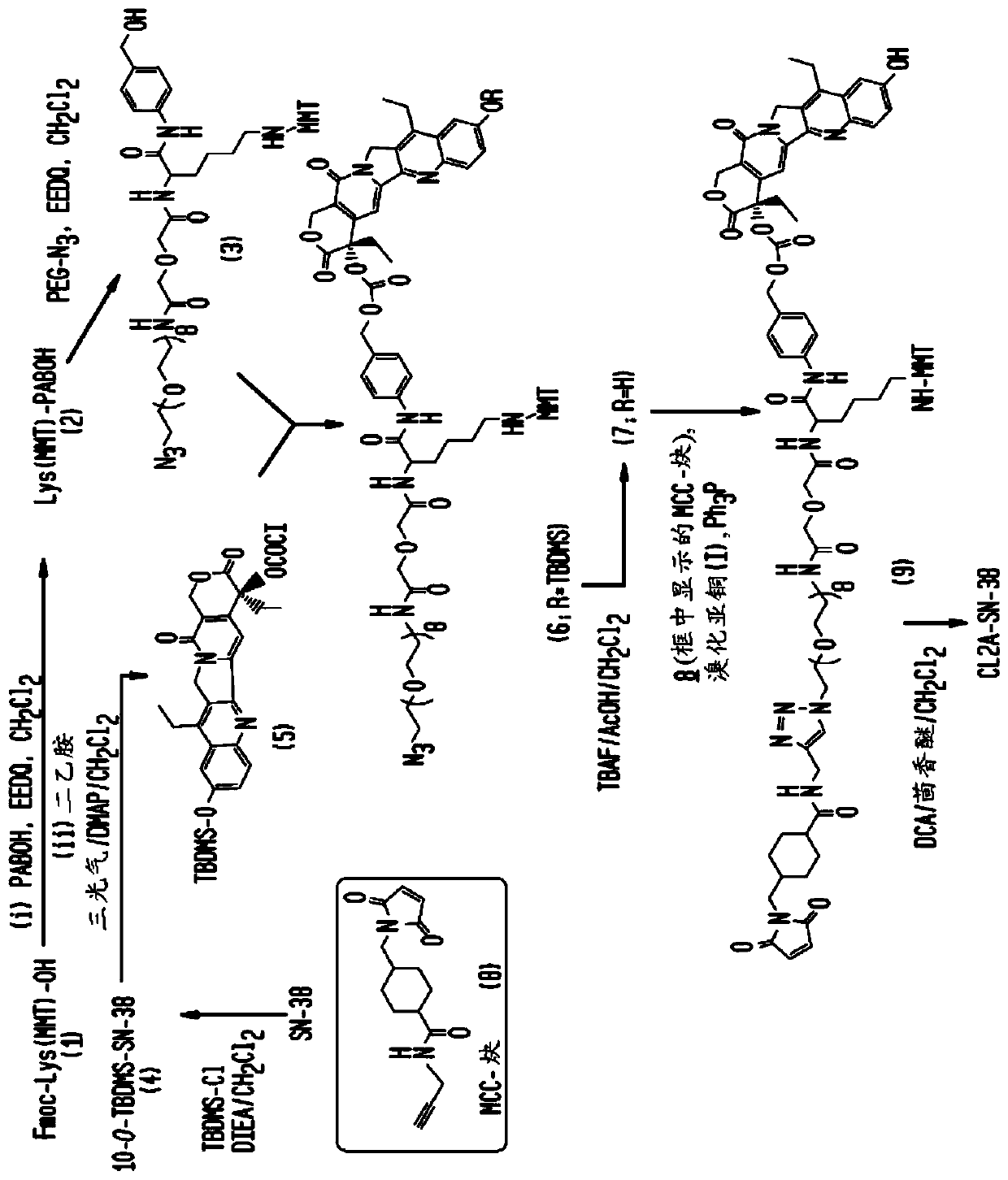
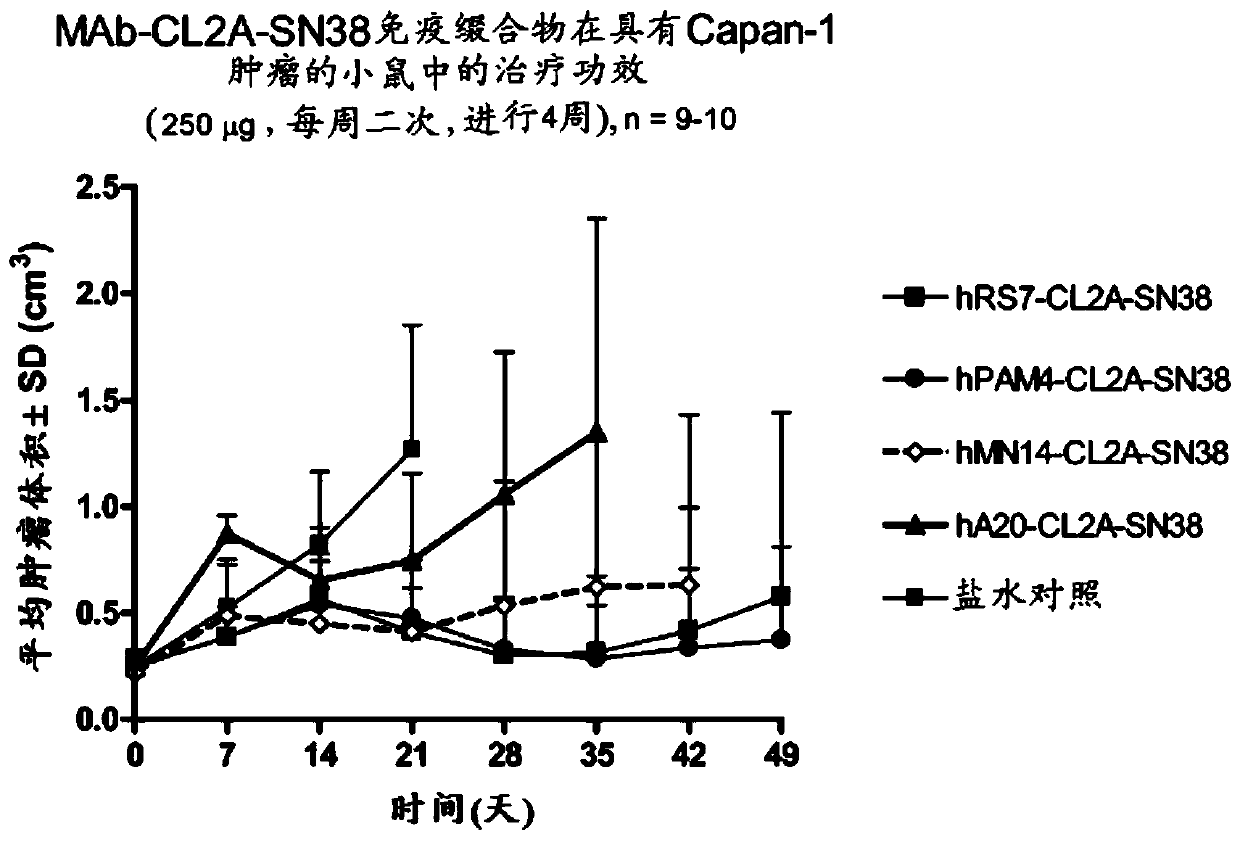
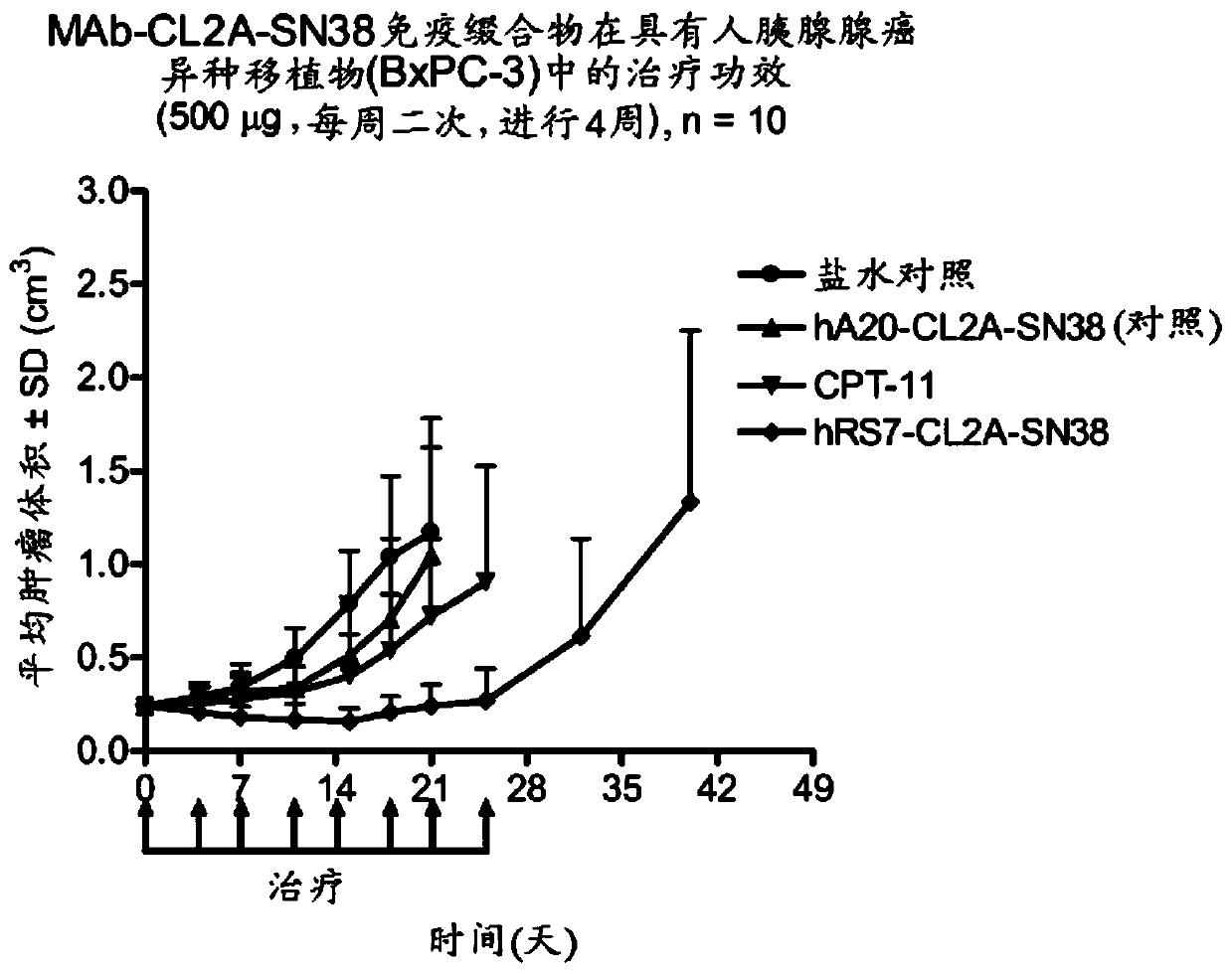
Antibody-sn-38 immunoconjugates with a cl2a linker
ActiveUS20160303253A1High protein recoveryImprove efficiencyPowder deliveryOrganic active ingredientsDiseaseSide effect
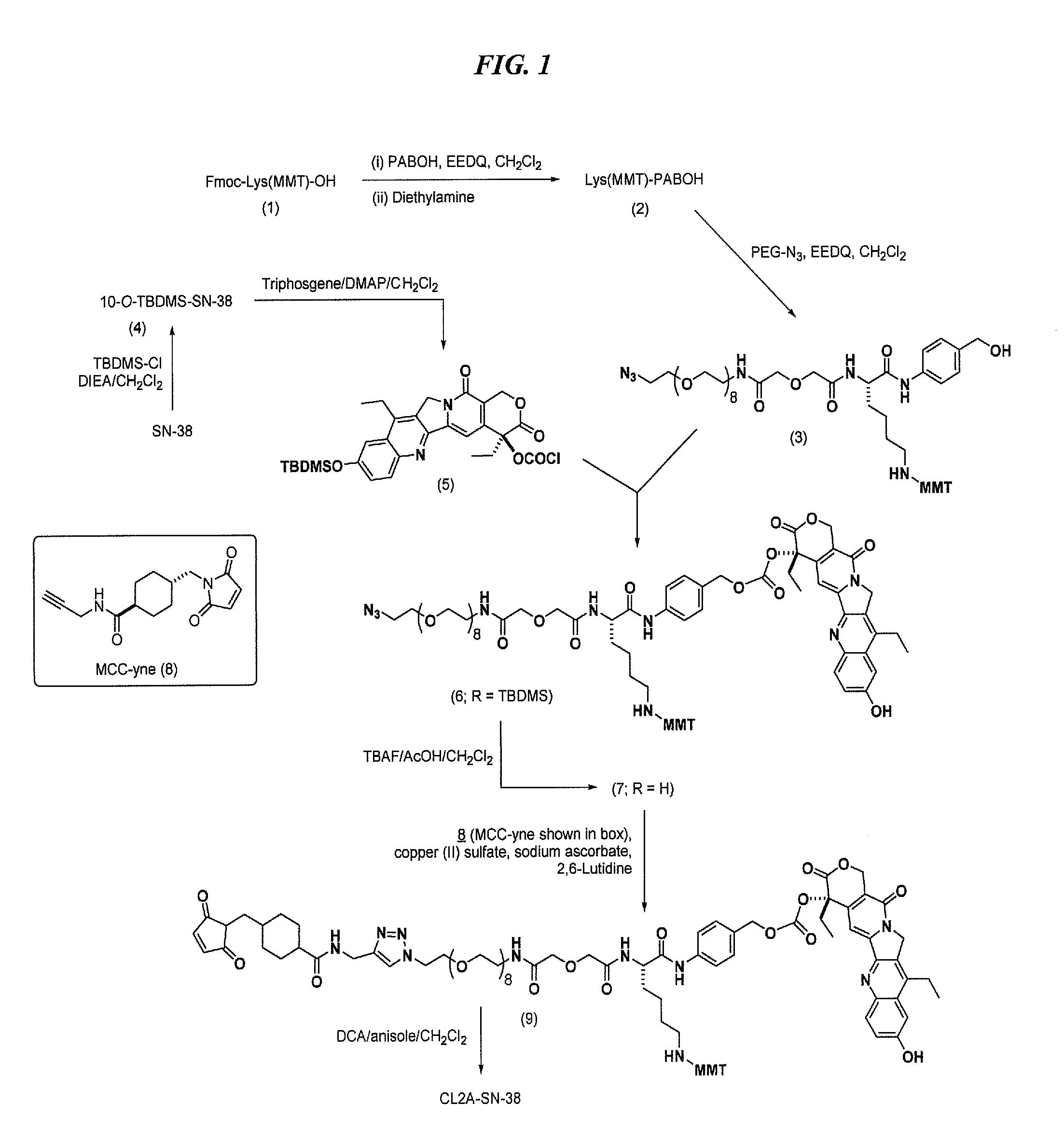
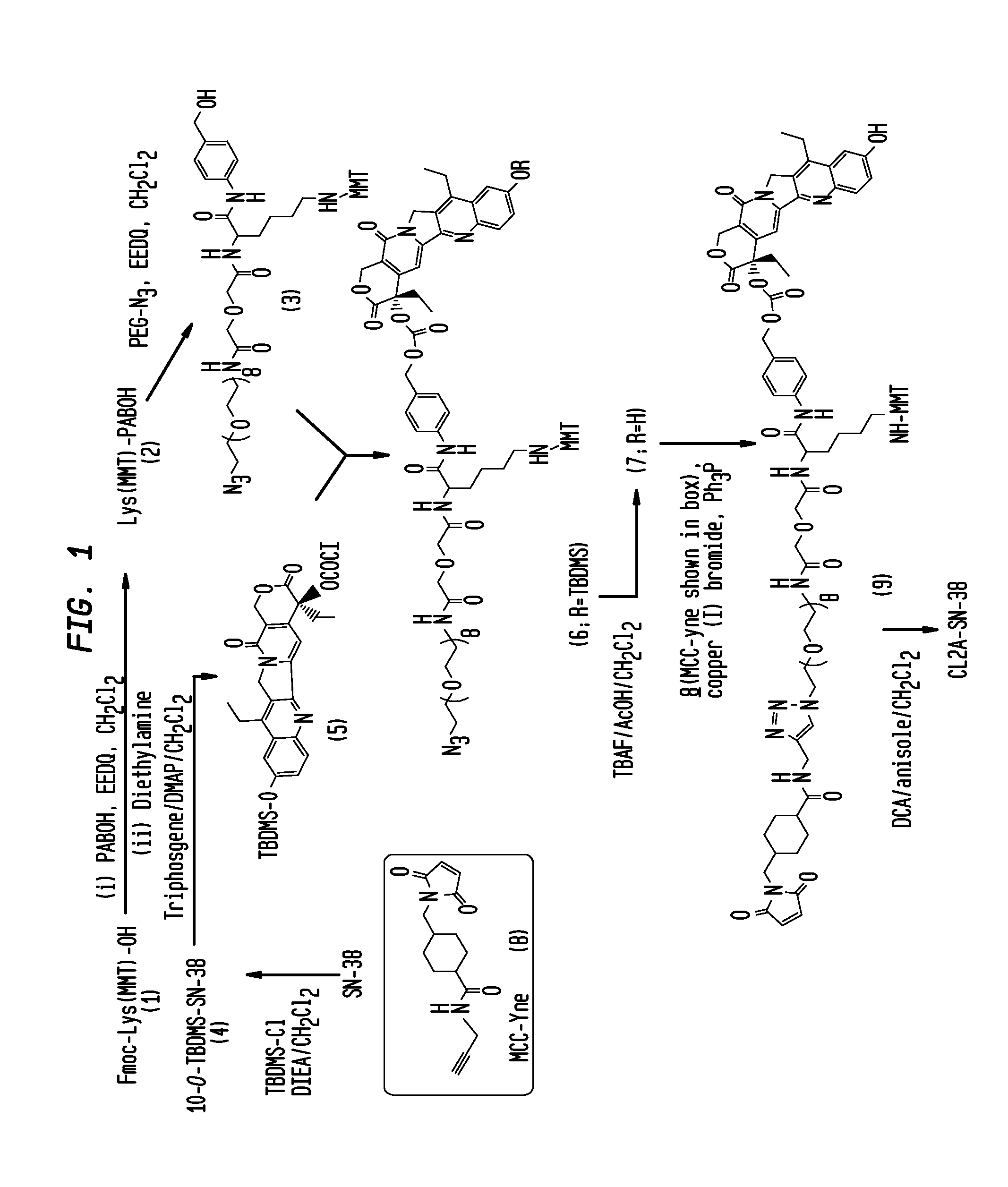
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6-8, alternatively 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme disclosed herein to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
Treatment of high trop-2 expressing triple negative breast cancer (TNBC) with sacituzumab govitecan (immu-132) overcomes homologous recombination repair (HRR) rescue mediated by rad51
ActiveUS20180271992A1Good effectLow toxicityHeavy metal active ingredientsOrganic active ingredientsRAD51Lymphatic Spread
The present invention relates to treatment of Trop-2 positive cancers with the combination of anti-Trop-2 ADC and a Rad51 inhibitor. Preferably the drug conjugated to the antibody is SN-38, and the ADC is sacituzumab govitecan. The ADC may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg. When administered at specified dosages and schedules, the combination of ADC and Rad51 inhibitor can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the combination is effective to treat cancers that are refractory to or relapsed from irinotecan or topotecan.
Owner:IMMUNOMEDICS INC
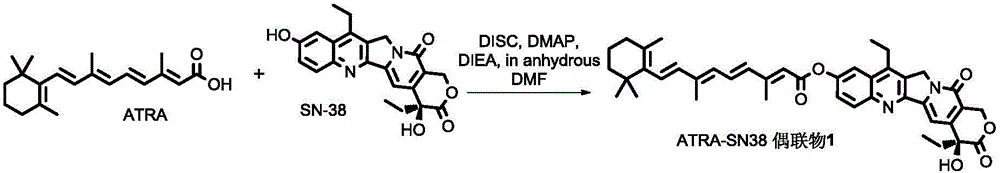
All-trans retinoic acid-camptothecin anticancer drug conjugate as well as preparation method and application thereof
ActiveCN104478890AImprove solubilityExpand the scope of clinical applicationOrganic active ingredientsOrganic chemistryPolyoxyethylene castor oilSolubility
The invention discloses an all-trans retinoic acid-camptothecin anticancer drug conjugate as well as a preparation method and application thereof. The structural formula of the all-trans retinoic acid-camptothecin anticancer drug conjugate is shown in a formula (I), (II), (III), (IV), (V) or (VI). The all-trans retinoic acid-camptothecin anticancer drug conjugate has good solubility in Tween, polyoxyethylene castor oil, a Poly(ethylene adipate)-polylactic acid copolymer and a Poly(ethylene adipate)-poly (lactic acid-glycollic acid) copolymer, can be self assembled into nanometer grains in water, can be directly injected or taken orally or processed into other dosage forms. According to the all-trans retinoic acid-camptothecin anticancer drug conjugate disclosed by the invention, as all-trans retinoic acid and SN-38 or camptothecin take synergistic effect, compared with a conjugate only containing one of irinotecan, SN-38 and all-trans retinoic acid, the all-trans retinoic acid-camptothecin anticancer drug conjugate has good tumor suppression effect.
Owner:ZHEJIANG UNIV
Imaging of drug accumulation as a guide to antitumor therapy
InactiveUS7175830B2Effective judgmentAvoid the needX-ray constrast preparationsRadioactive preparation carriersDocetaxel-PNPDocetaxel
The use of radio-labeled antitumor drugs in the treatment of solid tumors by the method of administering a radio-labeled anticancer drug to a patient and imaging at least a part of the patient using Positron Emission Tomography imaging is described. The method can be used to monitor delivery of antitumor drugs to tumors, to predict the effectiveness of therapy with a particular antitumor drug or combination of antitumor drugs, to assess the effectiveness of modulators of cellular accumulation, to individualize therapy and to evaluate the effectiveness of antitumor drugs with respect to particular cancers. Particularly preferred drugs are labeled taxanes, e.g., 11C-paclitaxel and 11C-docetaxel, labeled anthracyclines, e.g., 11C-doxorubicin and 11C-epirubicin, and other radiolabeled drugs, e.g. 11C-topotecan, 11C-SN-38, and 11C-imatinib. The invention further describes antitumor drugs labeled with the radioactive label 11C and methods of preparing radio-labeled drugs.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US REPRESENTED BY THE SEC
Thermal sensitive liposome preparation containing camptothecin antineoplastic agents
InactiveCN101744767AOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention relates to a thermal sensitive liposome preparation containing camptothecin antineoplastic agents, which contains the camptothecin antineoplastic agents, common lipoid, a phase-change regulator and an optional long-circulating material, wherein the phase-change regulator accounts for 6-100% of the weight of liposome, the long-circulating material accounts for 0-40% of the weight of the liposome, and the weight ratio of the amptothecin antineoplastic agents to the liposome is 1:0.5-1:100. Since the liposome preparation is characterized by thermal sensitivity, when the tissues of a tumor are heated partially, the liposome is targeted to the position of the tumor and releases a lot of medicine at the position of the tumor, thereby improving the curative effect and reducing the overall toxic and side effect. The camptothecin antineoplastic agents of the inveition can include water-solubility derivatives, such as irinotecan and topotecan, and water-insolubility derivatives, such as hydroxycamptothecine, SN-38, 9-NC, and the like.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
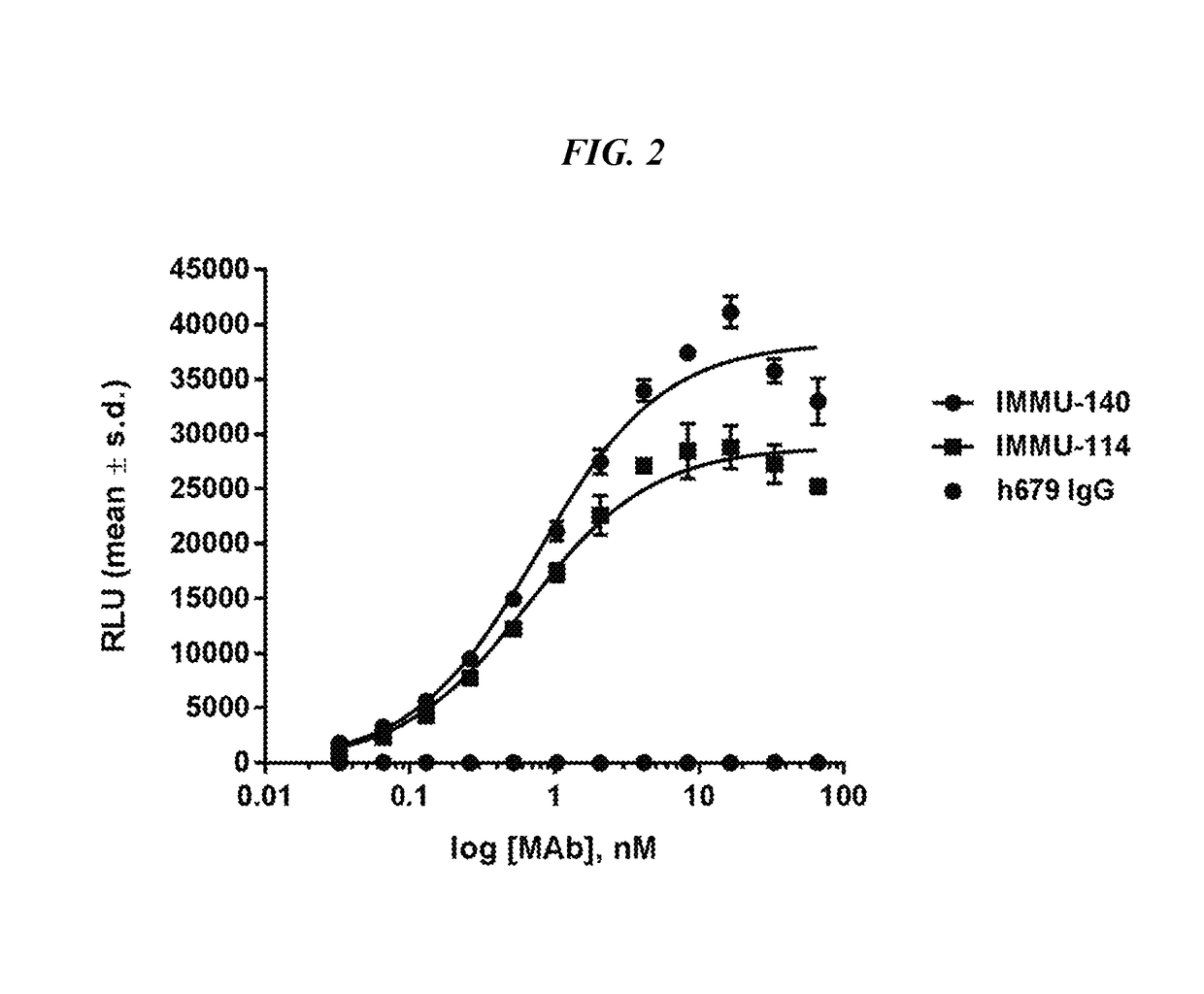
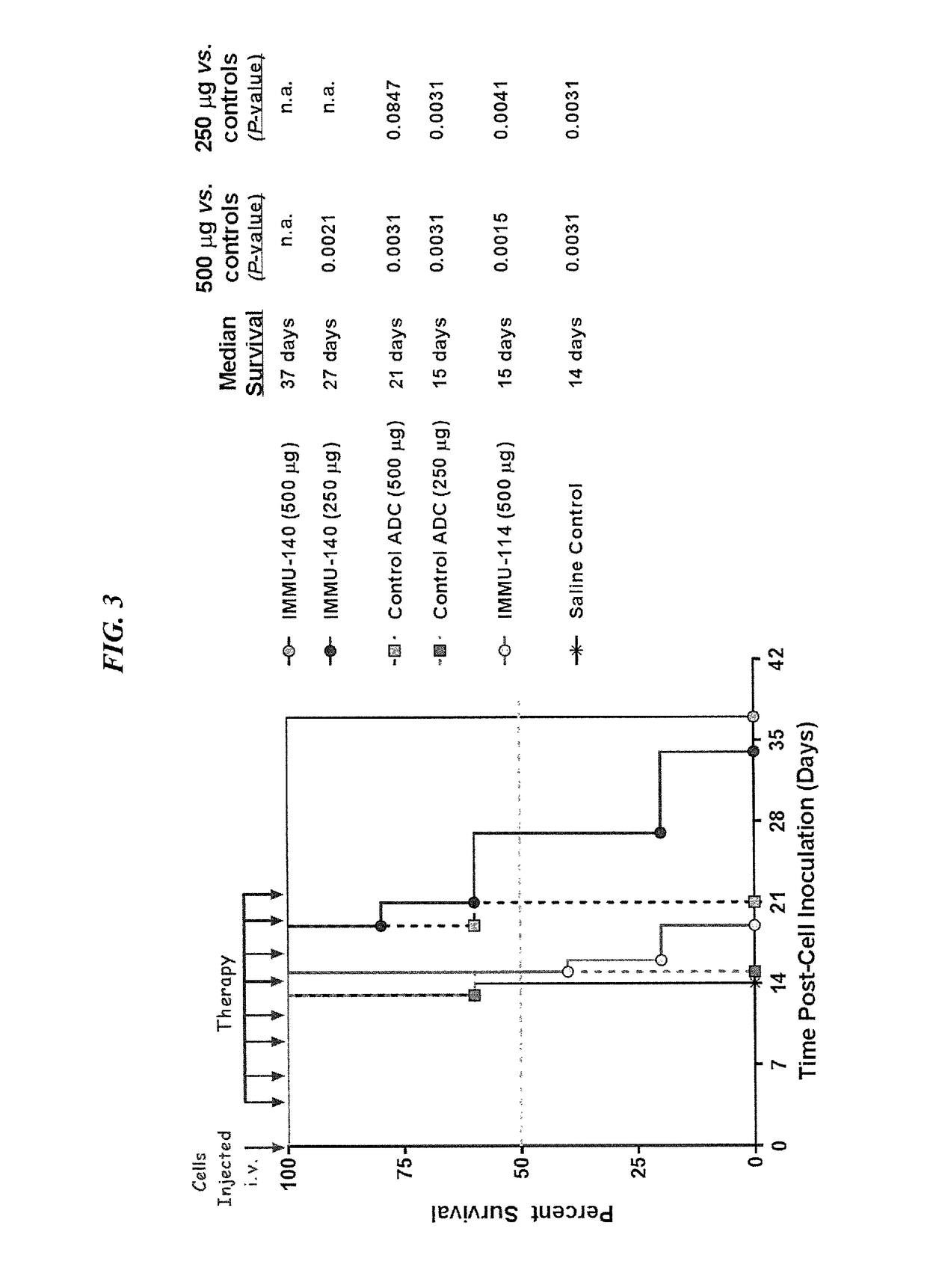
Efficacy of anti-HLA-DR antiboddy drug conjugate IMMU-140 (hL243-CL2A-SN-38) in HLA-DR positive cancers
ActiveUS10206918B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-HLA-DR antibody or antigen-binding antibody fragment. The immunoconjugate may be administered at a dosage of between 3 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8, 10 or 12 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. The methods and compositions are particularly useful for treating AML, ALL or multiple myeloma.
Owner:IMMUNOMEDICS INC
Method for treating abnormal cell growth
InactiveUS20050267140A1Ease of detectabilityEasy to prepareBiocideAnimal repellantsNitrocamptothecinSN-38
Therapeutic pharmaceutical compositions and methods of treatment of abnormal cell growth comprising a pyrimidine derivative or a pharmaceutically acceptable salt, solvate or prodrug thereof in combination with an oral camptothecin, an oral camptothecin derivative, an indolopyrrocarbazole derivative or a pharmaceutically acceptable salt, solvate or prodrug thereof for the treatment of cancer are described. In one embodiment of the present invention the oral camptothecin derivative is selected from the group consisting of 10-hydroxycamptothecin, 9-aminocamptothecin, 9-nitrocamptothecin, irinotecan, irinotecan salt, SN-38, CPT-11, and topotecan and the indolopyrrocarbazole derivative is edotecarin. In one embodiment the pyrimidine derivative is selected from the group consisting gemcitabine, MTA and capecitabine. In one preferred embodiment, the pyrimidine derivative is capecitabine and the camptothecin derivative is CPT-11.
Owner:PFIZER INC
Combination of abcg2 inhibitors with sacituzumab govitecan (immu-132) overcomes resistance to sn-38 in trop-2 expressing cancers
ActiveUS20190248917A1Receive treatment wellGood effectOrganic active ingredientsRadioactive preparation carriersCombined Modality TherapyAntibody fragments
Owner:IMMUNOMEDICS INC
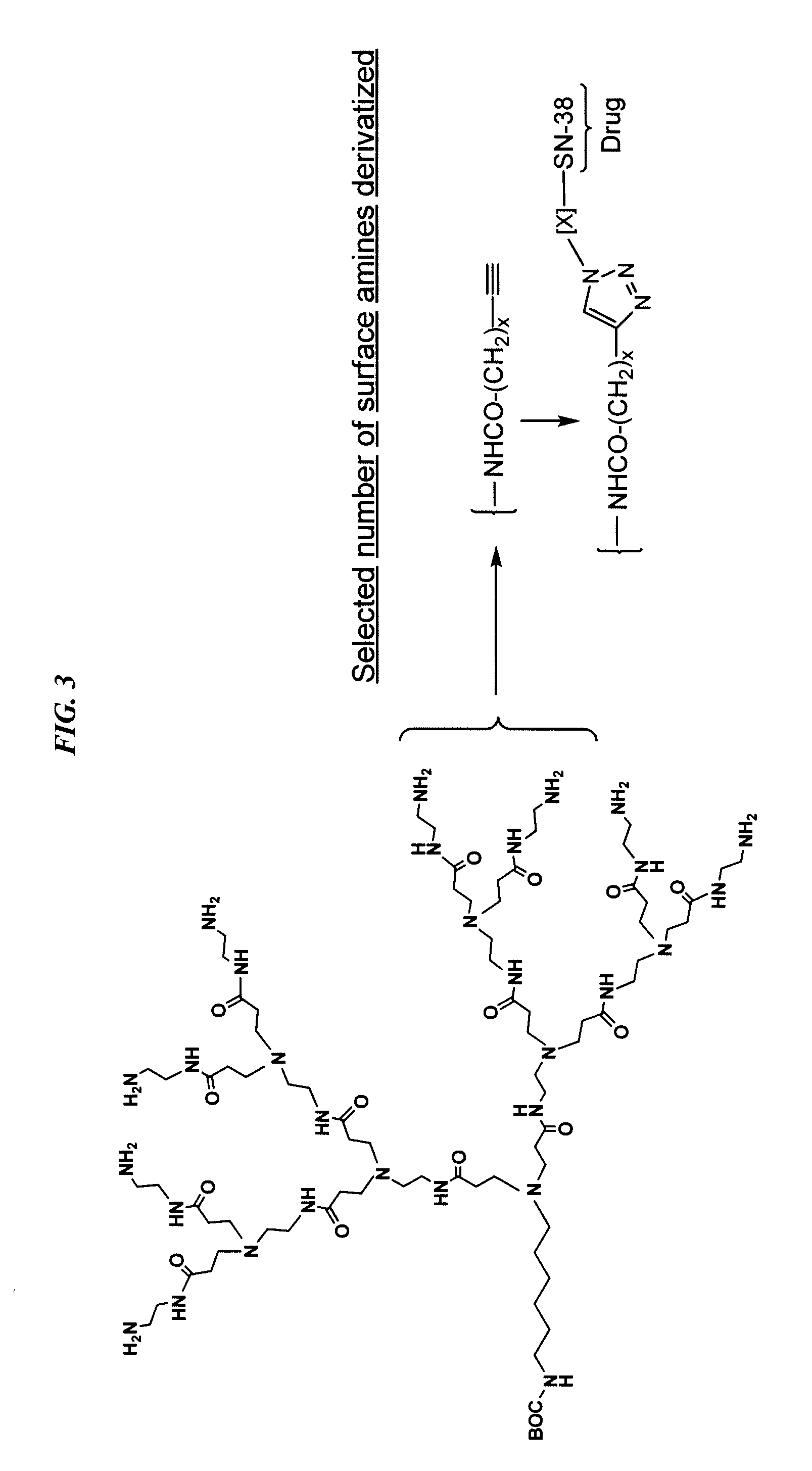
Antibody-sn-38 immunoconjugates with a cl2a linker
InactiveUS20150306243A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
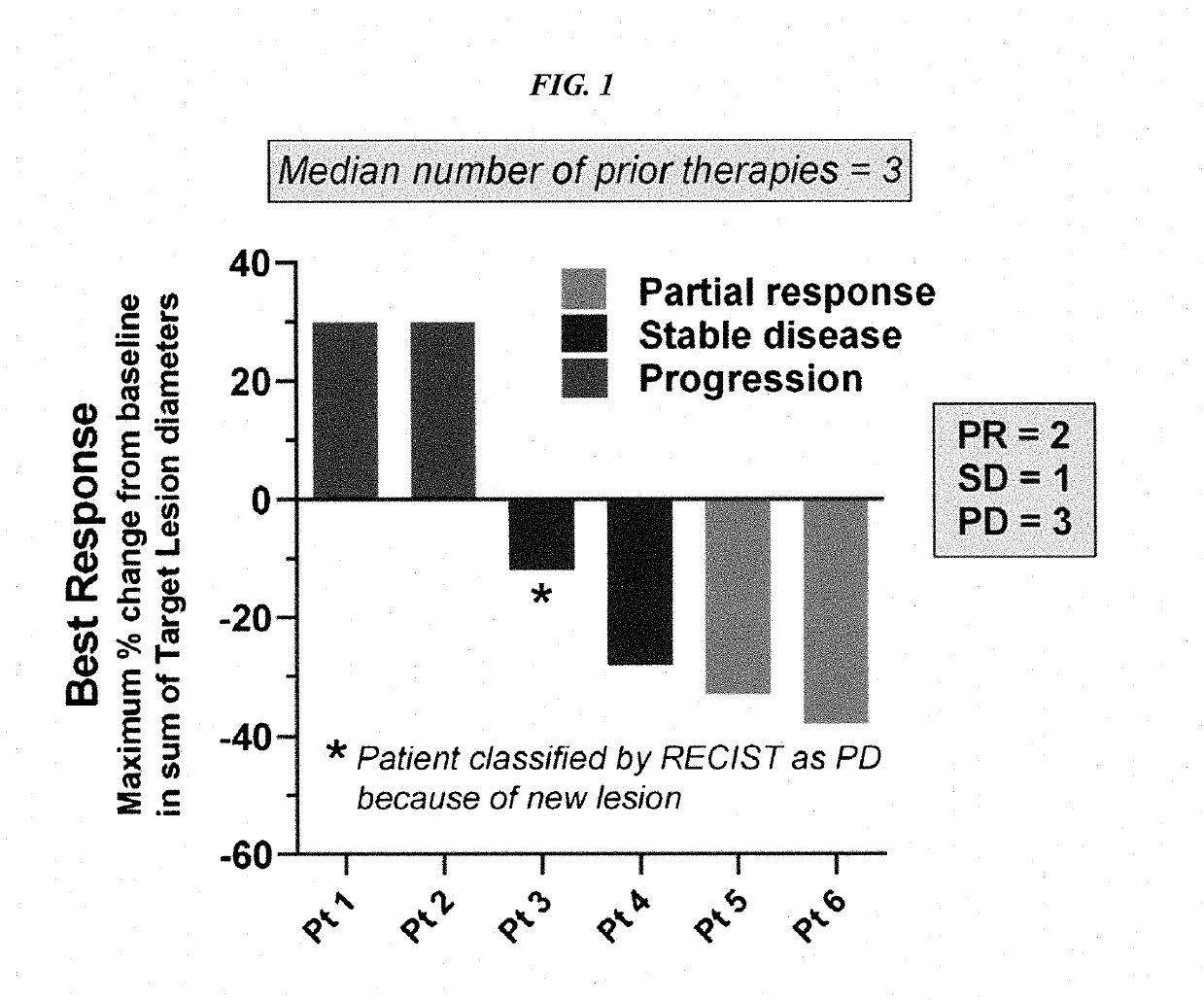
Therapy for metastatic urothelial cancer with the antibody-drug conjugate, sacituzumab govitecan (IMMU-132)
ActiveUS10413539B2Reducing certain severe side effectsReceive treatment wellImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. The ADC may be administered at a dosage of between 4 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, most preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the ADC is administered in combination with one or more other therapeutic agents, such as a PARP inhibitor, a microtubule inhibitor, a Bruton kinase inhibitor or a PI3K inhibitor. Most preferably, the ADC is of use for treating a Trop-2 expressing cancer, such as metastatic urothelial cancer.
Owner:IMMUNOMEDICS INC
Subcutaneous administration of antibody-drug conjugates for cancer therapy
ActiveUS20180280532A1Good effectLow toxicityInorganic non-active ingredientsPharmaceutical delivery mechanismCD20Lymphatic Spread
The present invention relates to methods of cancer therapy using subcutaneous administration of antibody-drug conjugates (ADCs). Preferably, the ADC comprises an antibody that binds to Trop-2, CEACAM5, CEACAM6, CD20, CD22, CD30, CD46, CD74, Her-2, folate receptor, or HLA-DR. More preferably, the drug is SN-38. Subcutaneous administration is at least as effective as intravenous administration of the same ADC. Surprisingly, subcutaneous administration can be used without inducing unmanageable adverse local toxicity at the injection site. Subcutaneous administration is advantageous in requiring less frequent administration, substantially reducing the amount of time required for intravenous administration, and reducing the levels of systemic toxicities observed with intravenous administration. When administered at specified dosages and schedules, the ADCs can reduce solid tumors in size, reduce or eliminate metastases and are effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
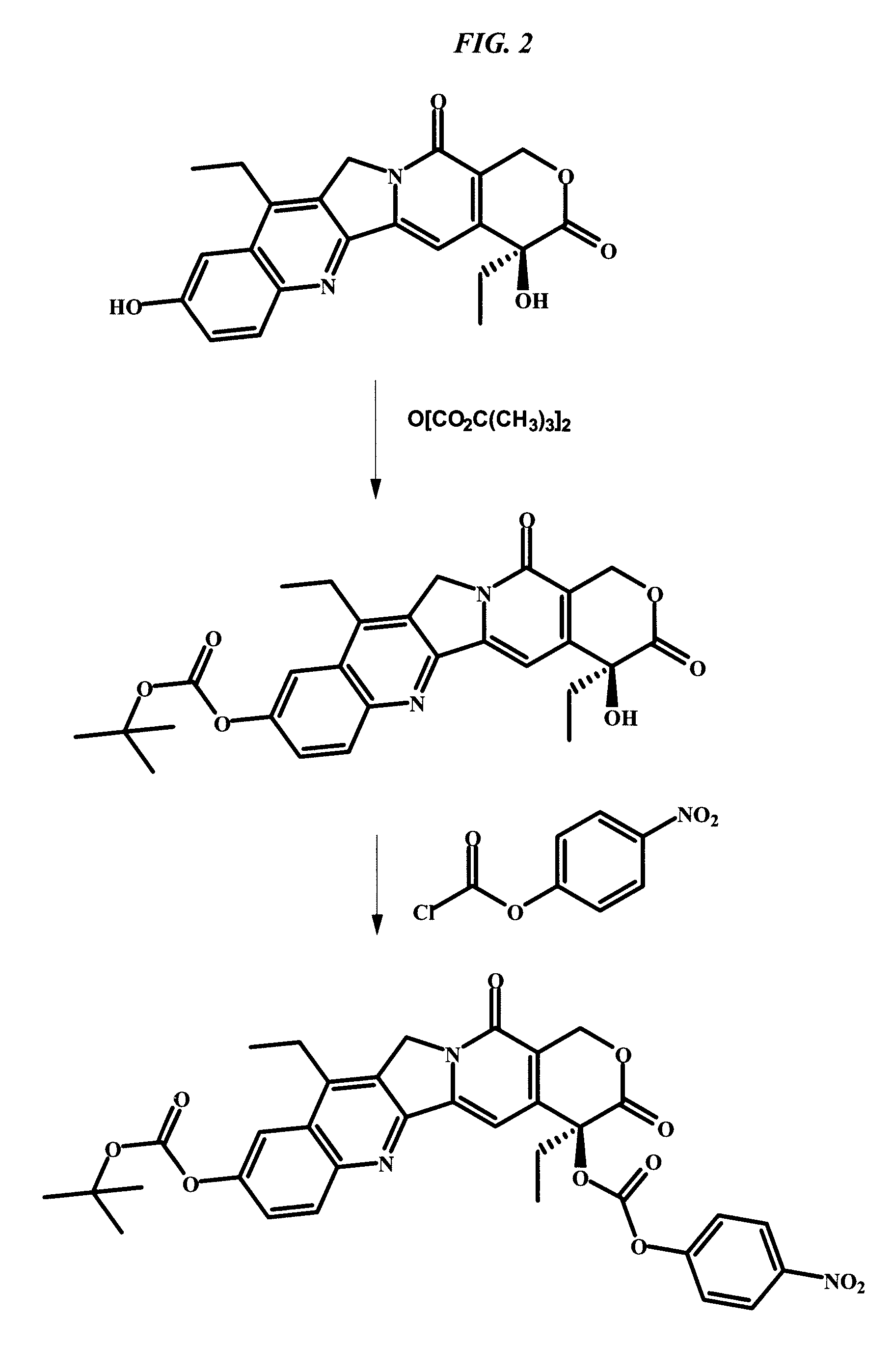
Antibody-SN-38 immunoconjugates with CL2A linker
Owner:IMMUNOMEDICS INC
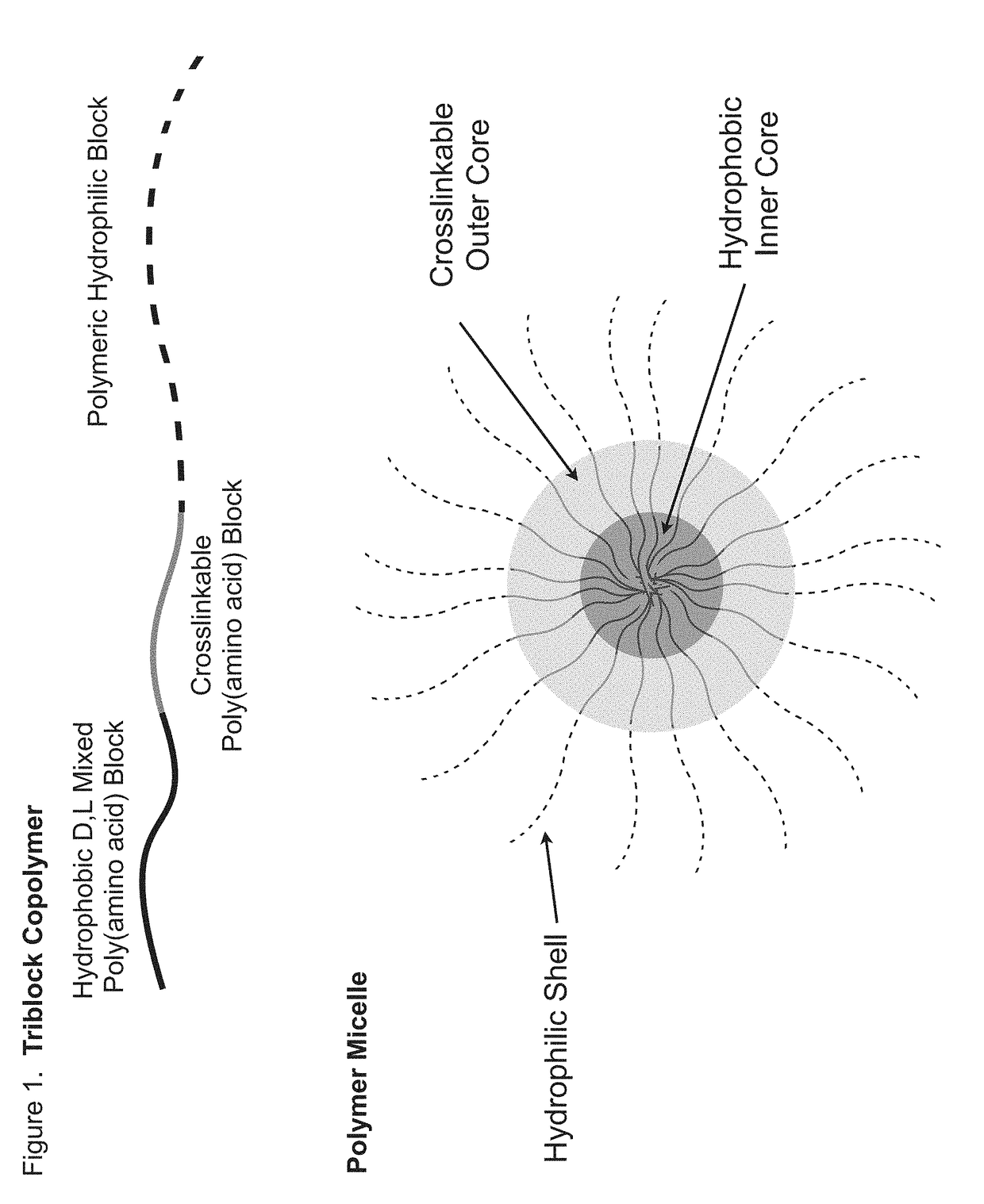
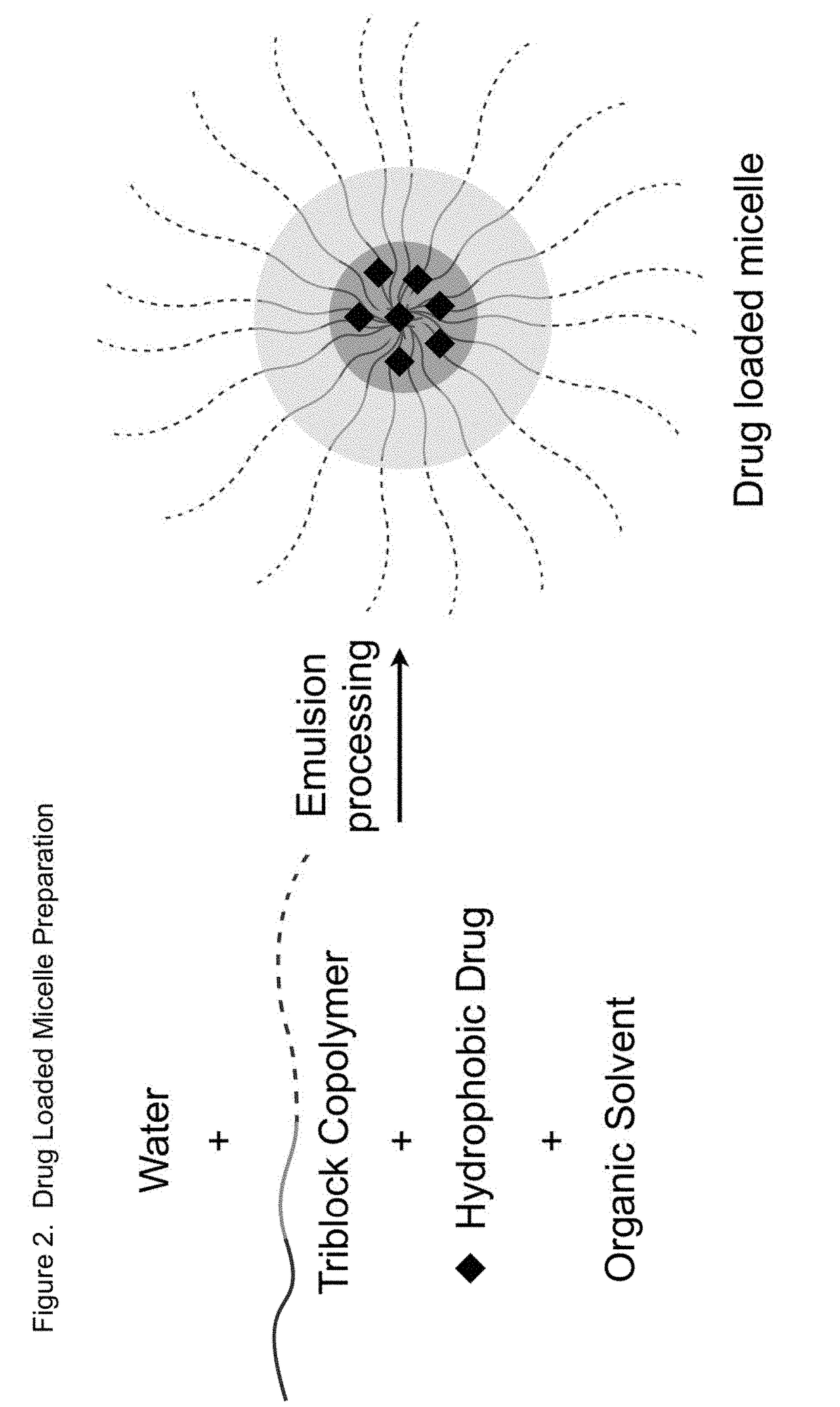
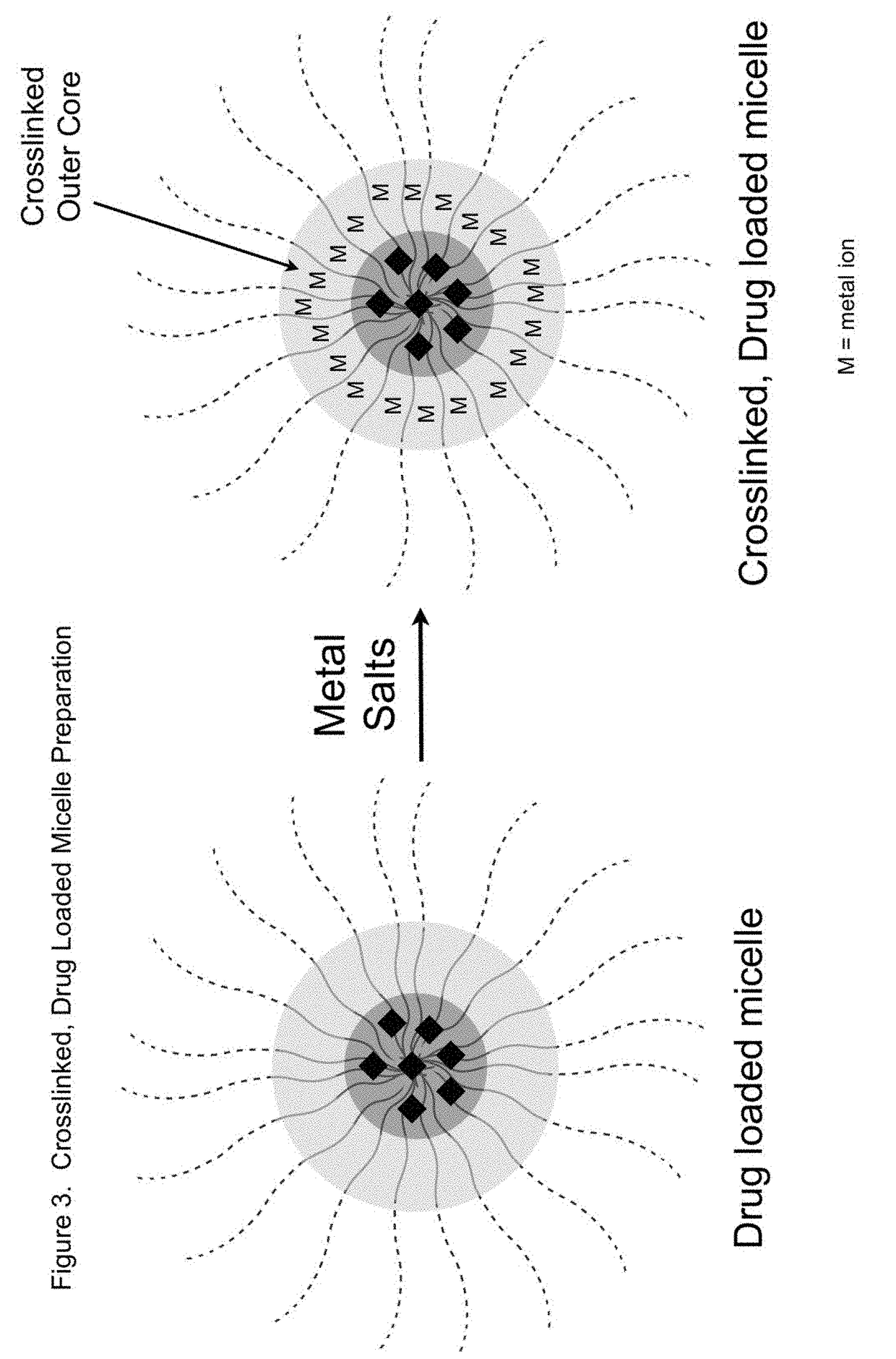
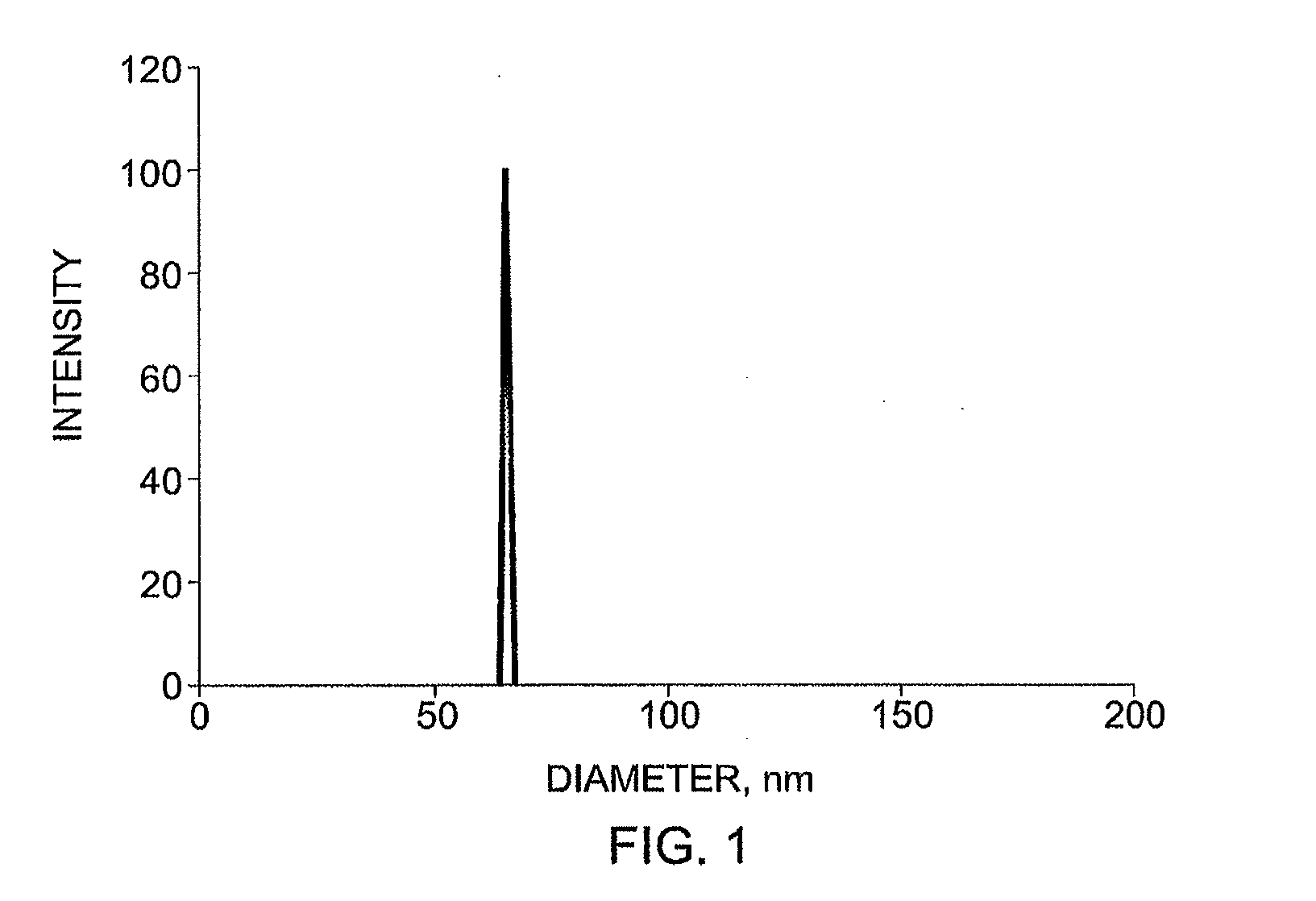
Sn-38 loaded iron crosslinked micelle and methods thereof
Owner:INTEZYNE TECH INC
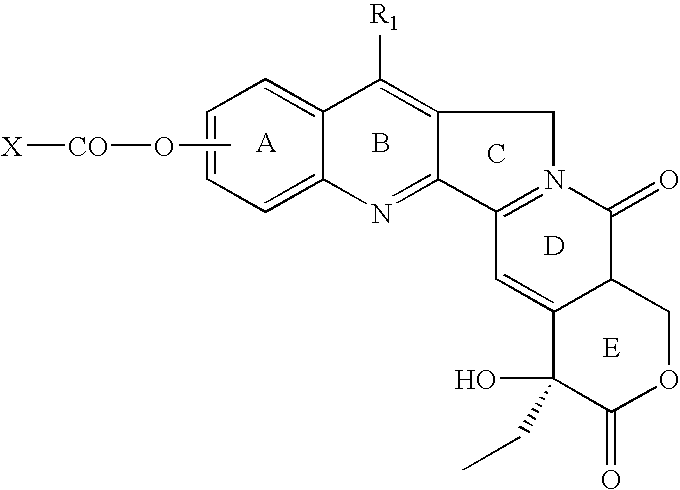
Novel pro- and codrug derivatives for nanoparticle delivery of select anticancer agents formed using rapidly cleavable phenolic ester bridges
An ester of ArOH according to the formula R—X—CO—OAr, wherein ArOH is a pharmaceutically active compound selected from the group consisting of SN-38, PI-103, etoposide and fenretinide, wherein a) R is a residue of cholesterol, sitosterol, SN-38, PI-103, etoposide or fenretinide and X is O—CO-L, wherein L is either a direct bond or a linking group including a branched or unbranched hydrocarbyl moiety that may optionally include in-chain or pendant heteroatom substituents and / or cyclic moieties; b) R—X—CO-0 is an all-trans retinoate radical or the 9-cis or 13-cis isomer thereof; or c) R—X— is a branched or unbranched, saturated or unsaturated hydrocarbyl moiety comprising at least 5 carbon atoms and optionally including at least one in-chain or pendant heteroatom substituent and / or cyclic moiety. A dispersion of nanoparticles in an aqueous medium includes nanoparticles including an ester of ArOH according to the formula R—X—CO—OAr wherein ArOH is a pharmaceutically active compound in which Ar is a substituted or unsubstituted aryl or heteroaryl radical, and wherein R is as defined above or R—X—CO-0 is as defined above. The ester or dispersion may be used to treat a diagnosed medical condition in a patient.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
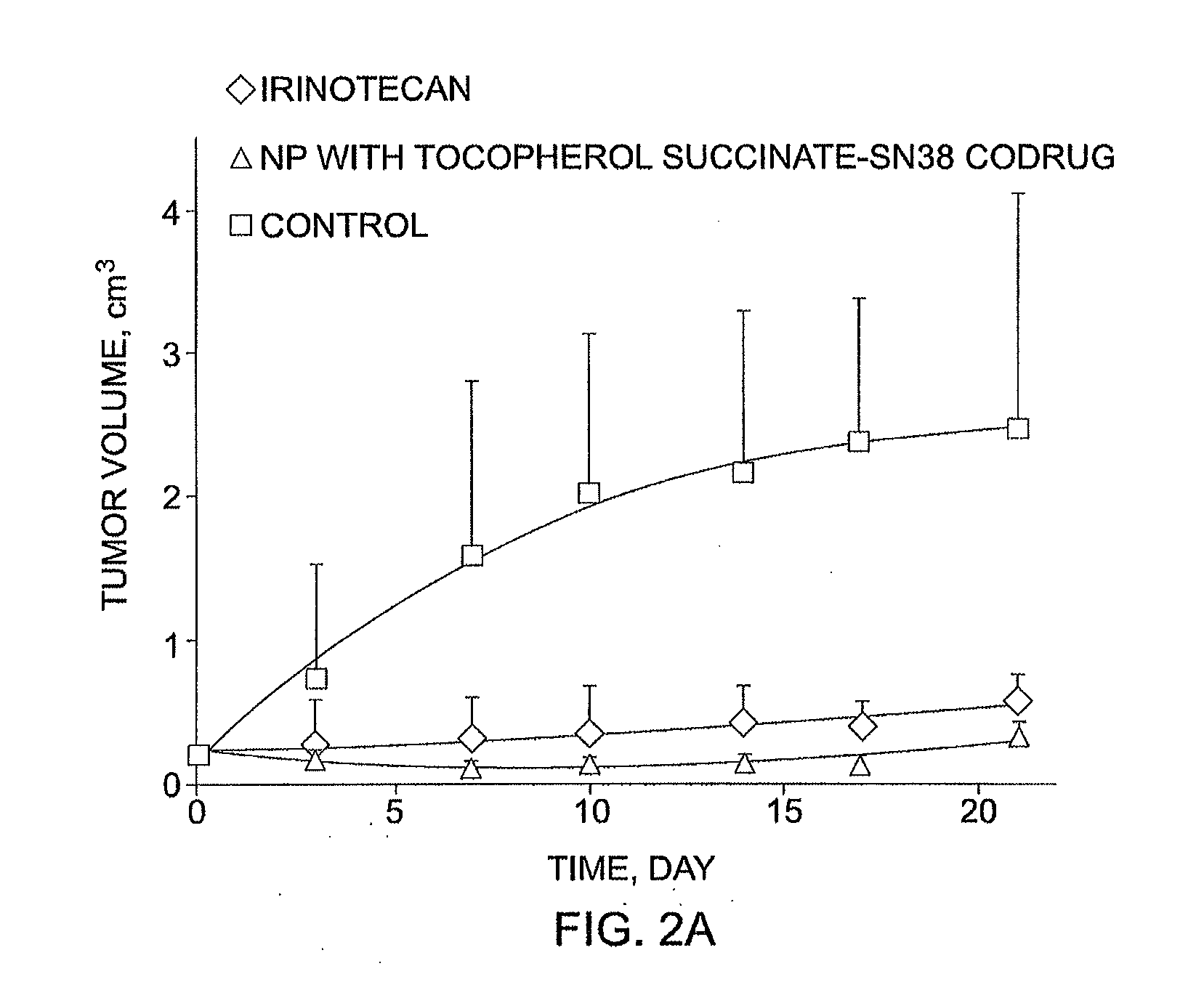
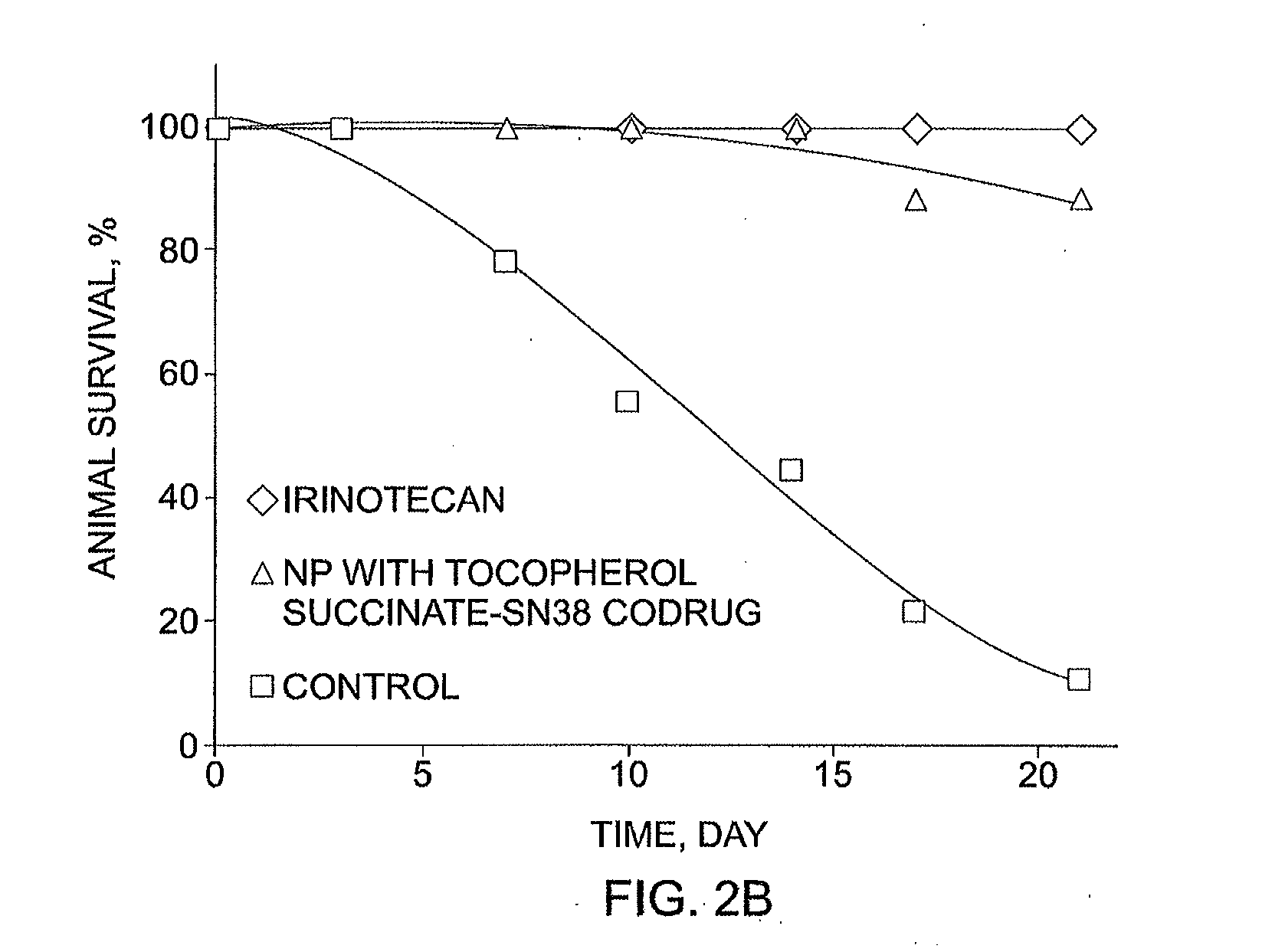
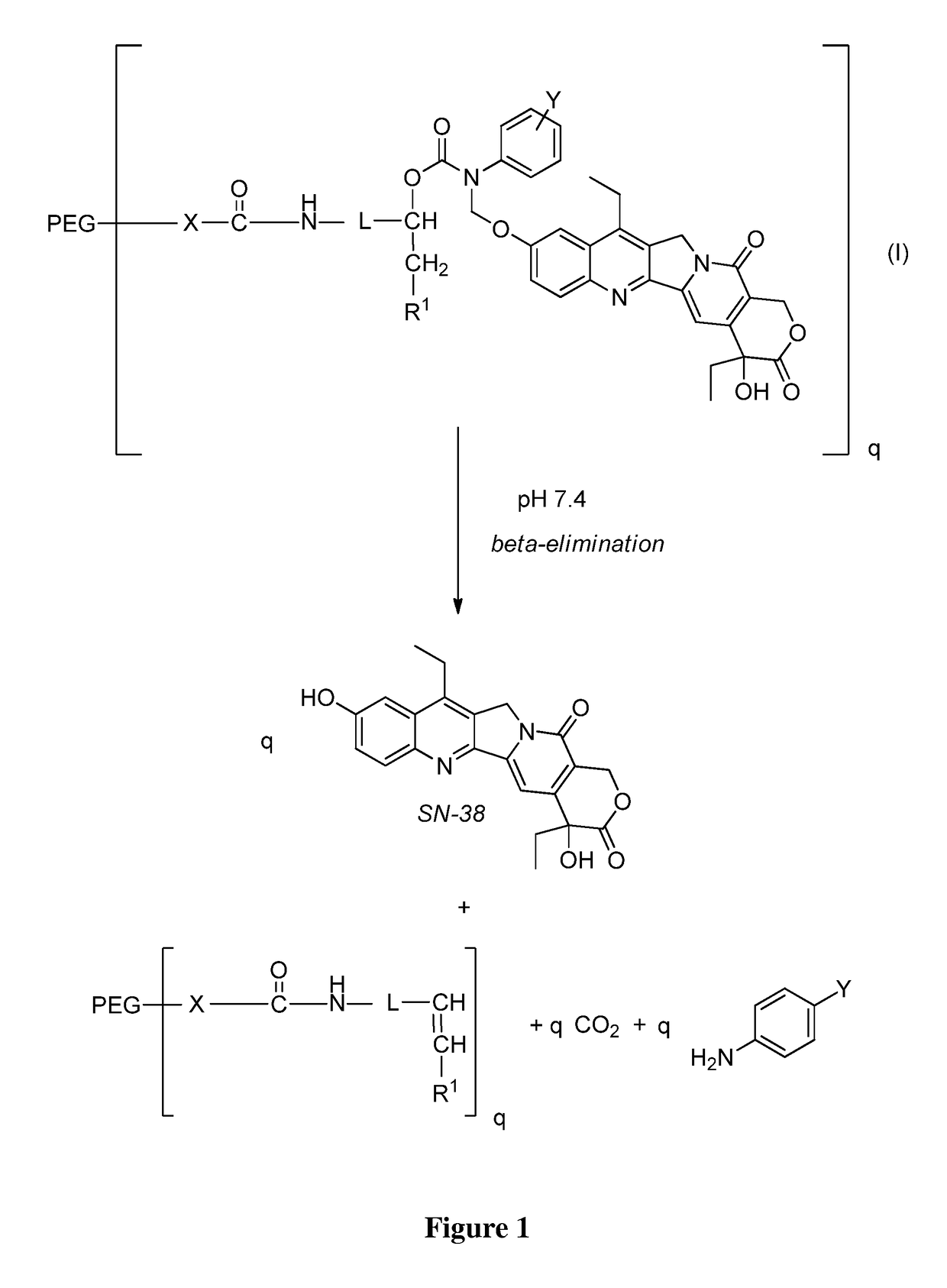
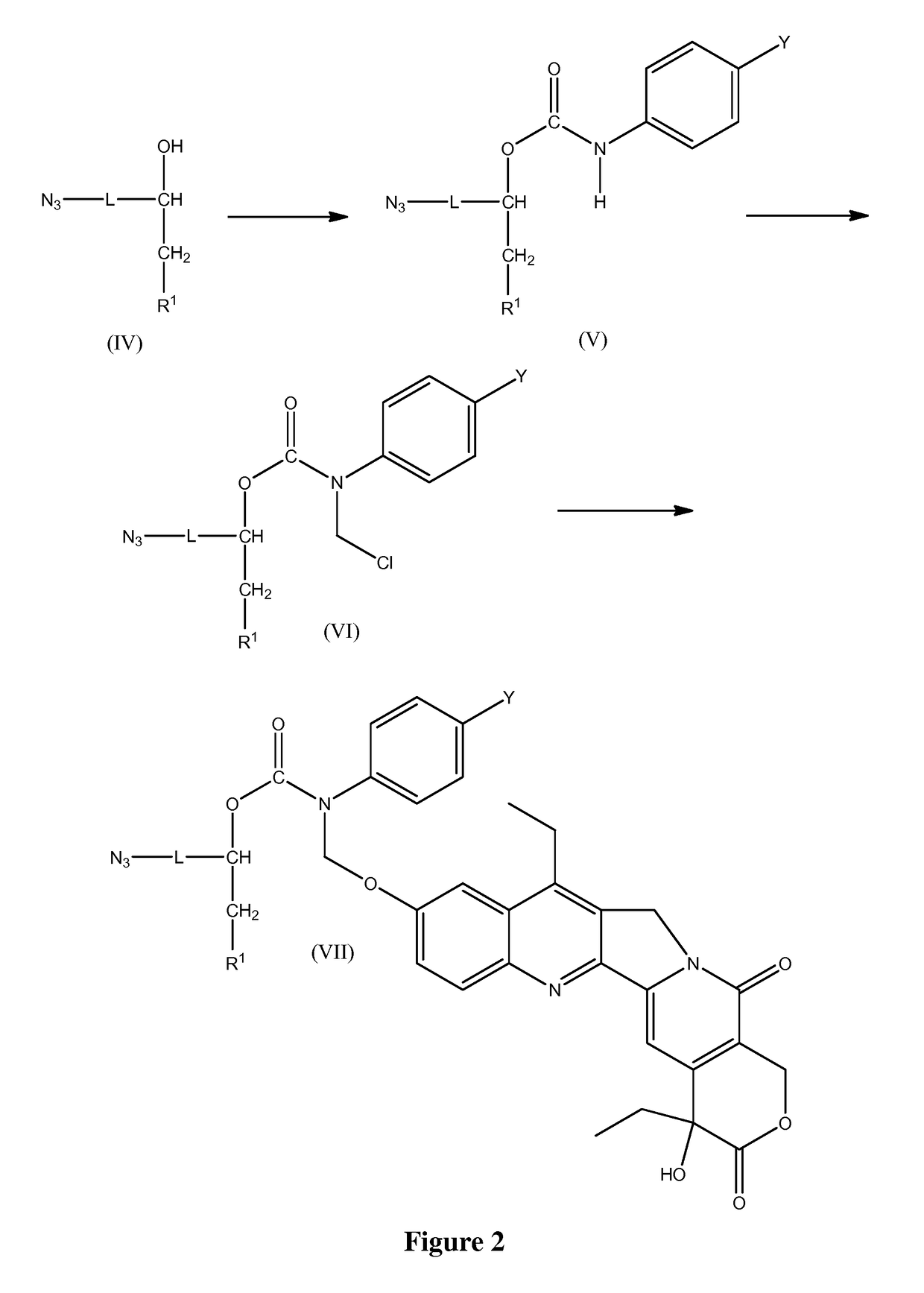
Slow-release conjugates of SN-38
ActiveUS10016411B2Quantity minimizationOrganic active ingredientsOrganic chemistryDrug release rateGlucuronate
Conjugates of SN-38 that provide optimal drug release rates and minimize the formation of the corresponding glucuronate are described. The conjugates release SN-38 from a polyethylene glycol through a β-elimination mechanism.
Owner:PROLYNX LLC
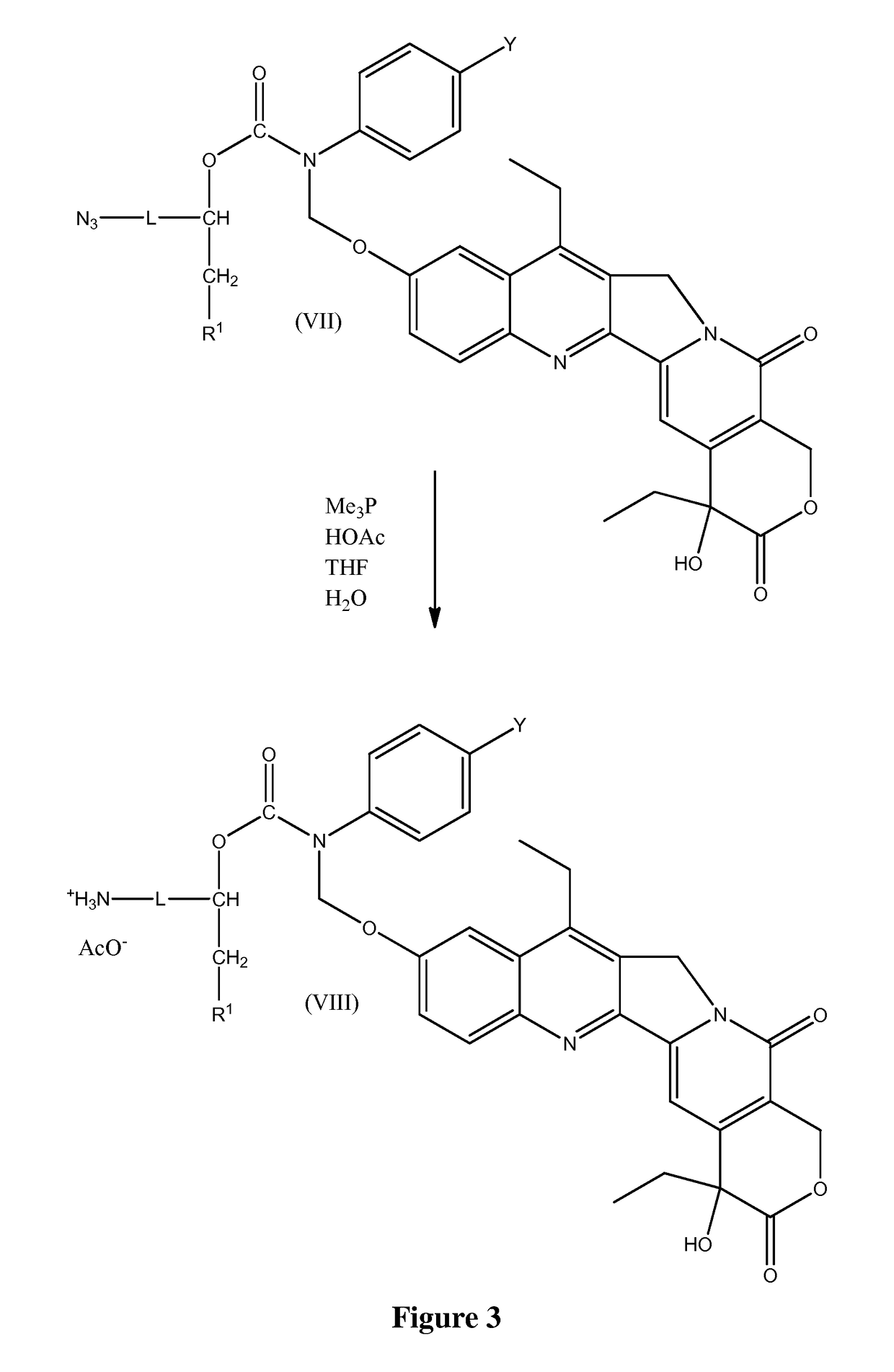
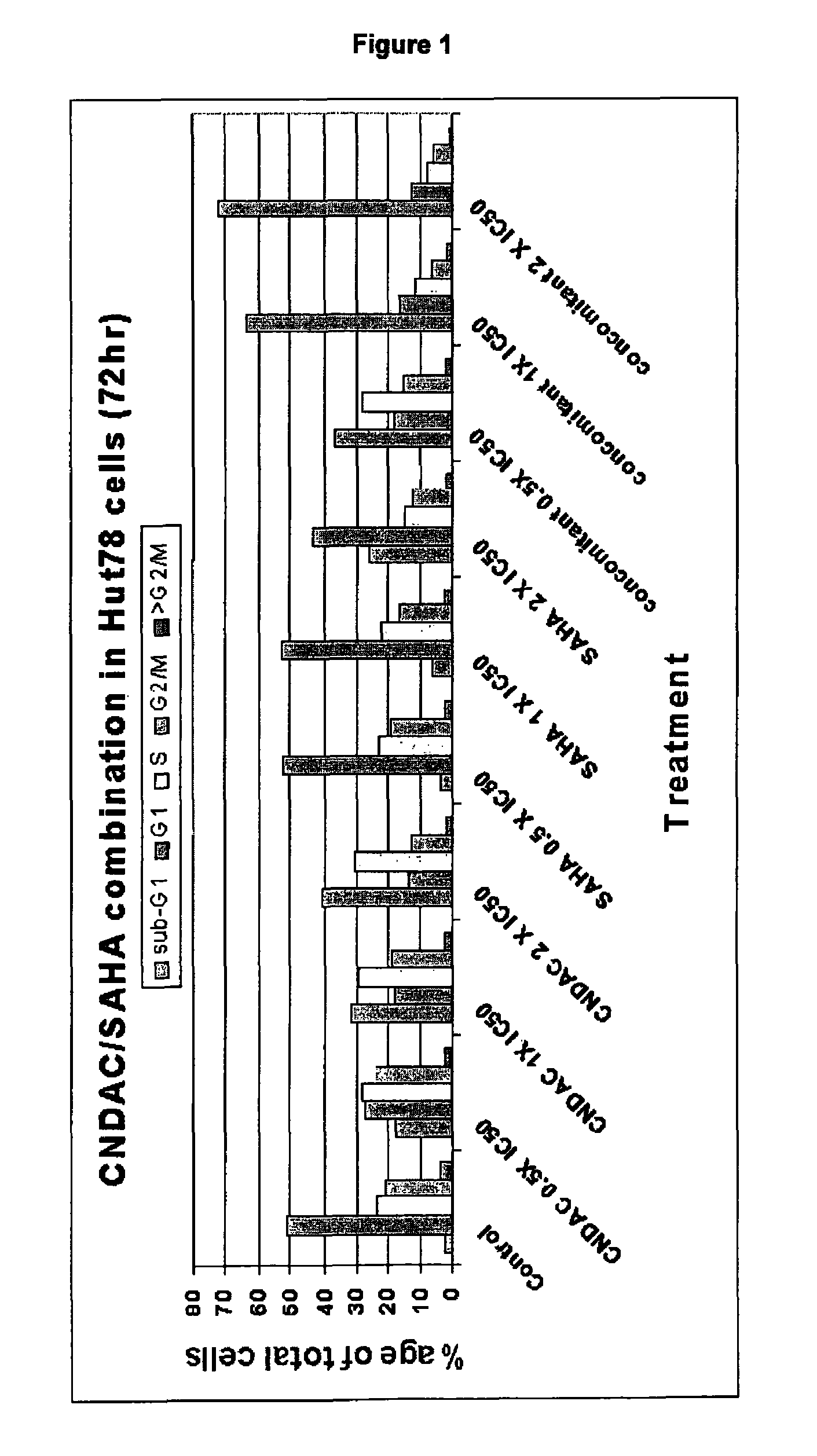
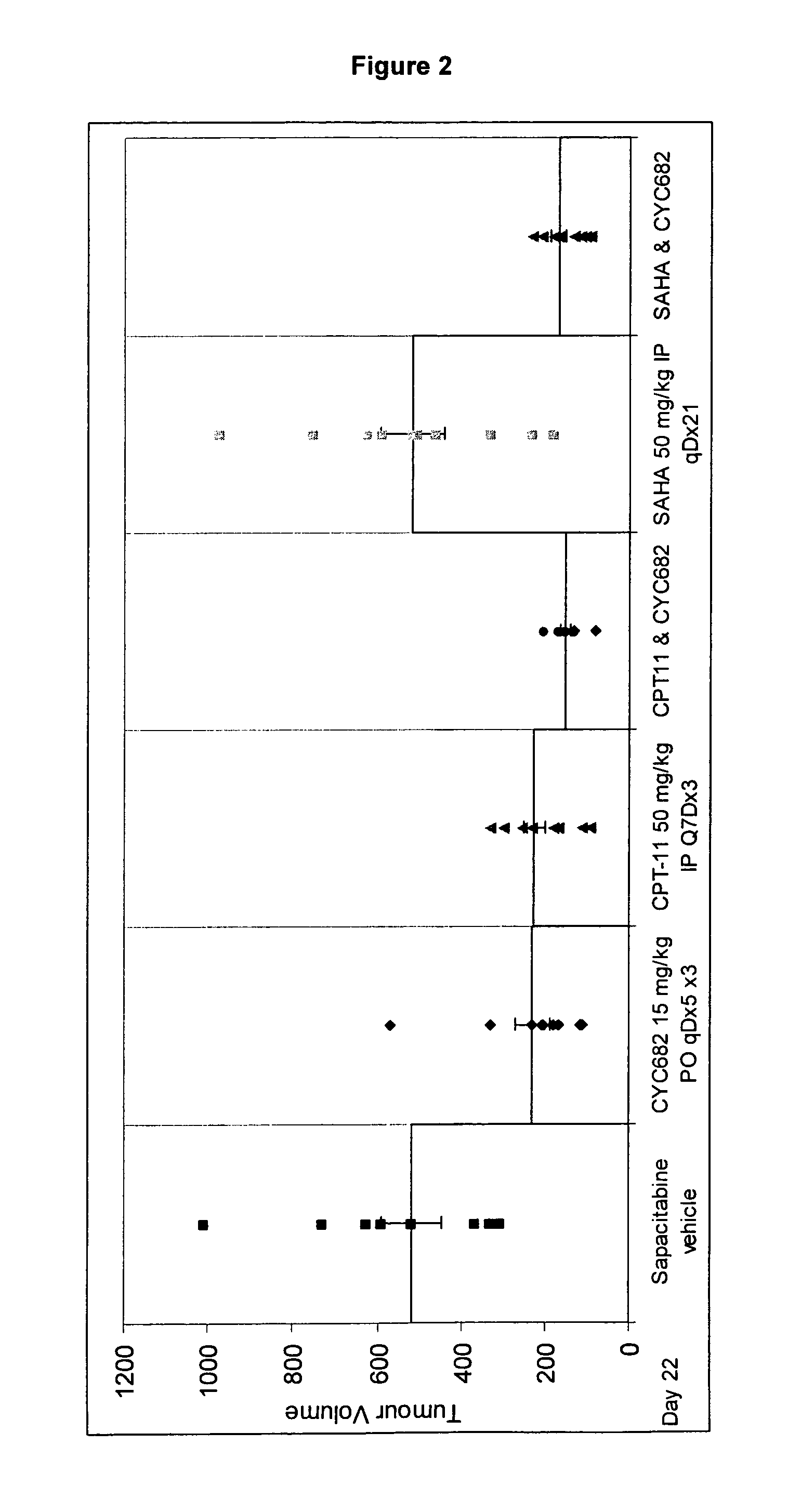
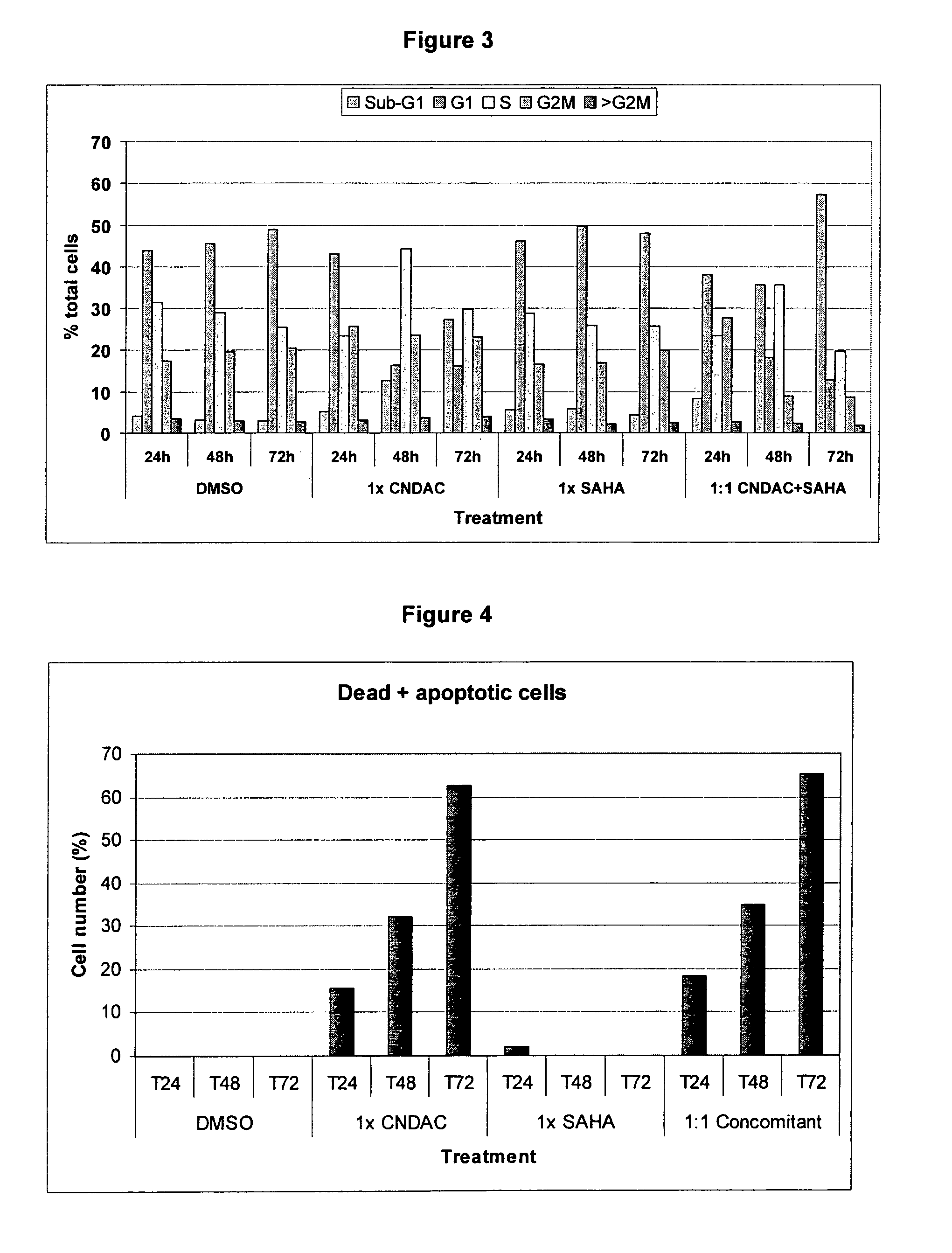
Combination comprising CNDAC (2′-cyano-2′-deoxy-N4-palmitoyl-1-beta-D-arabinofuranosyl-cytosine) and a cytotoxic agent
A first aspect of the invention relates to a combination comprising 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof. A second aspect relates to a pharmaceutical product comprising (i) 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and (ii) a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof, as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect relates to a method of treating a proliferative disorder, said method comprising simultaneously, separately or sequentially administering to a subject 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof. A fourth aspect of the invention relates to the use of a subject 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, in the preparation of a medicament for treating cutaneous T-cell lymphoma (CTCL).
Owner:CYCLACEL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com