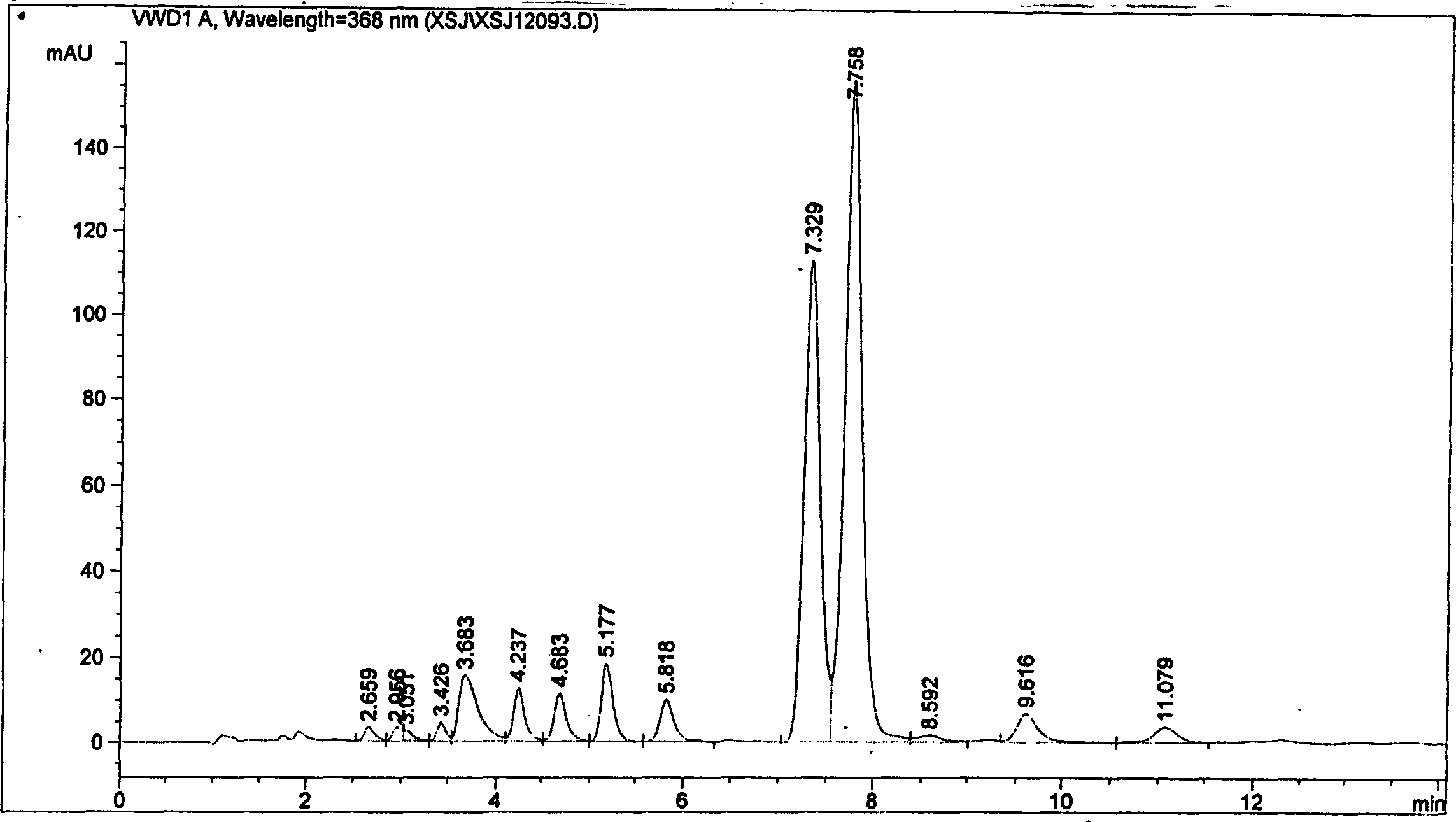
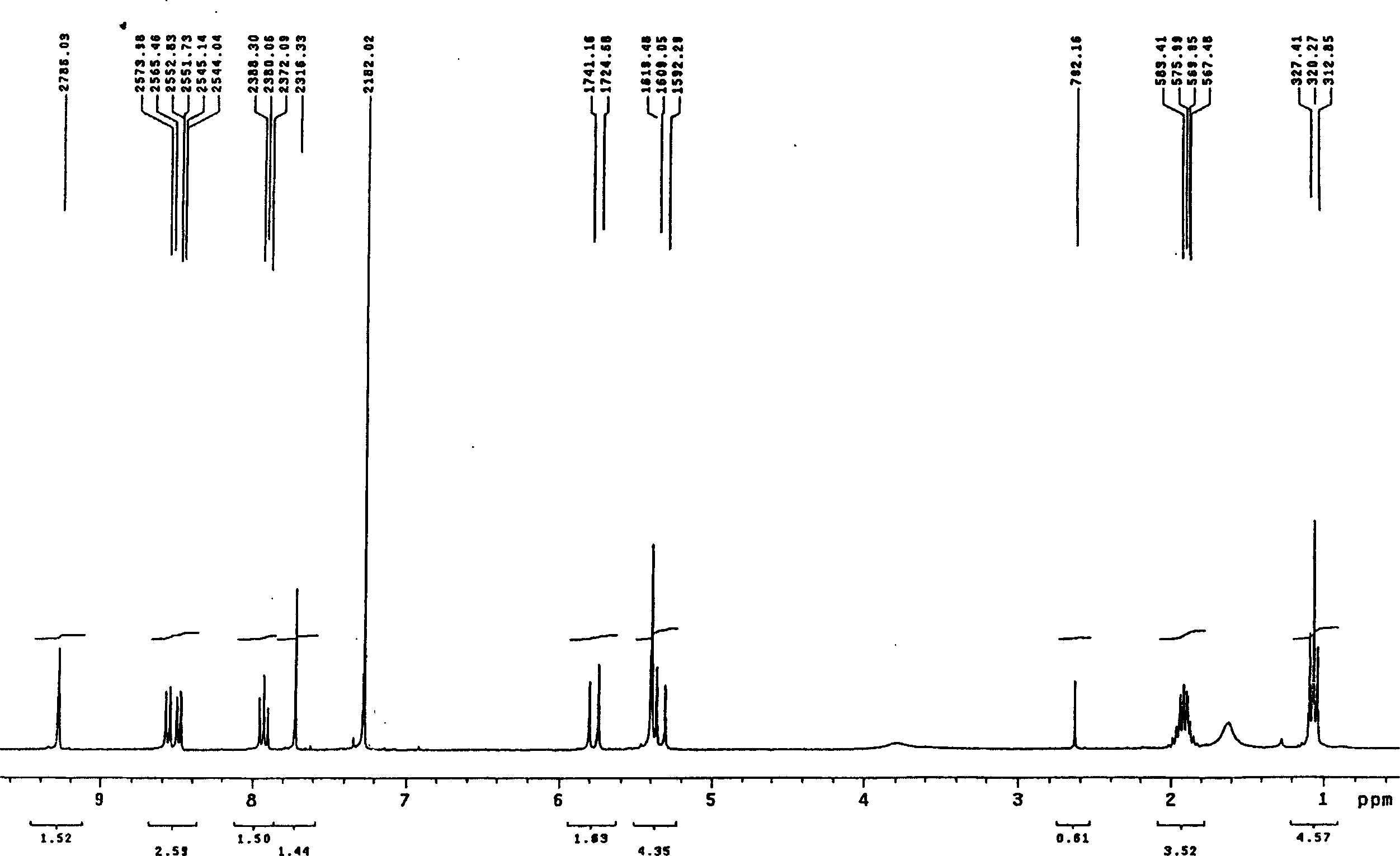
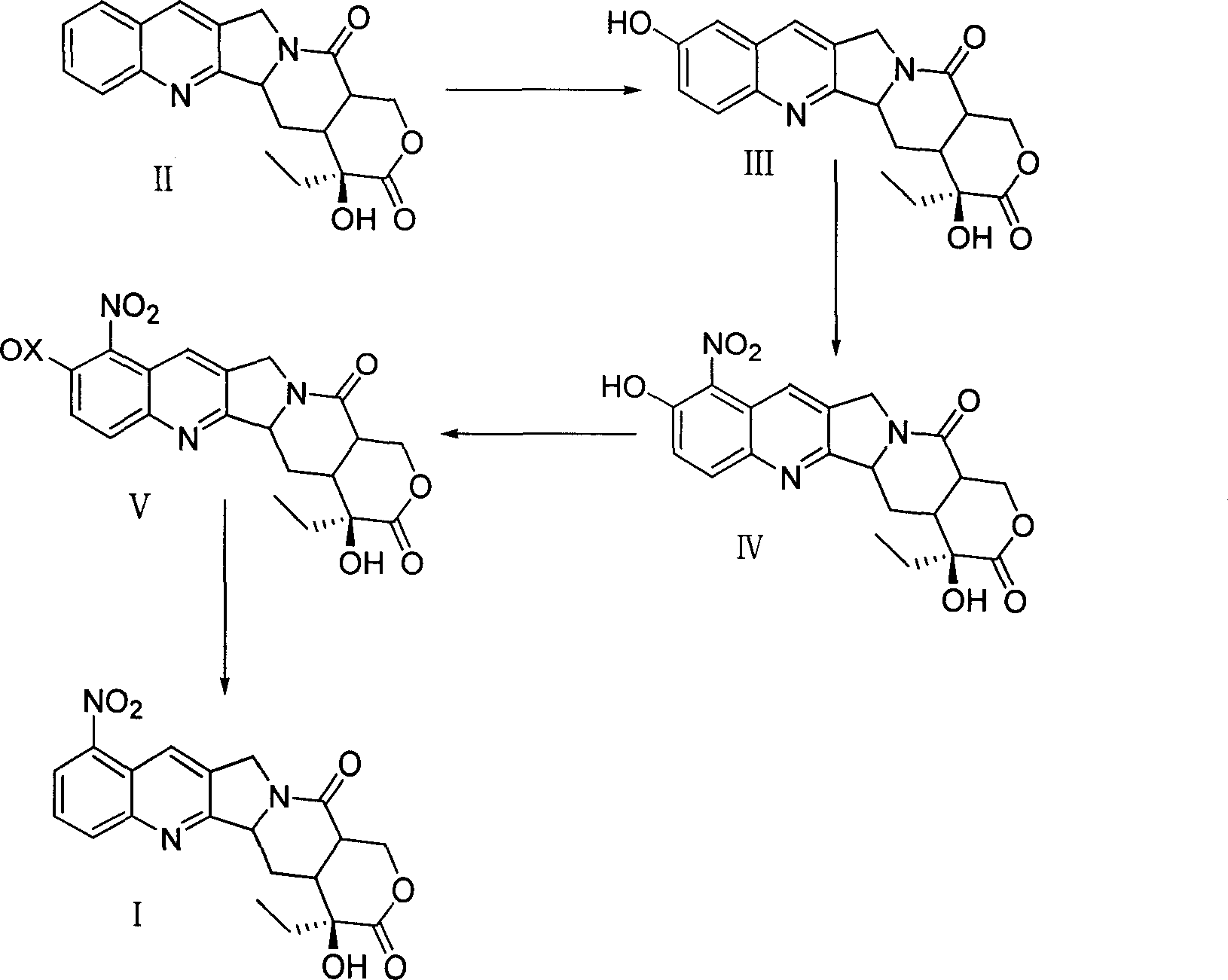
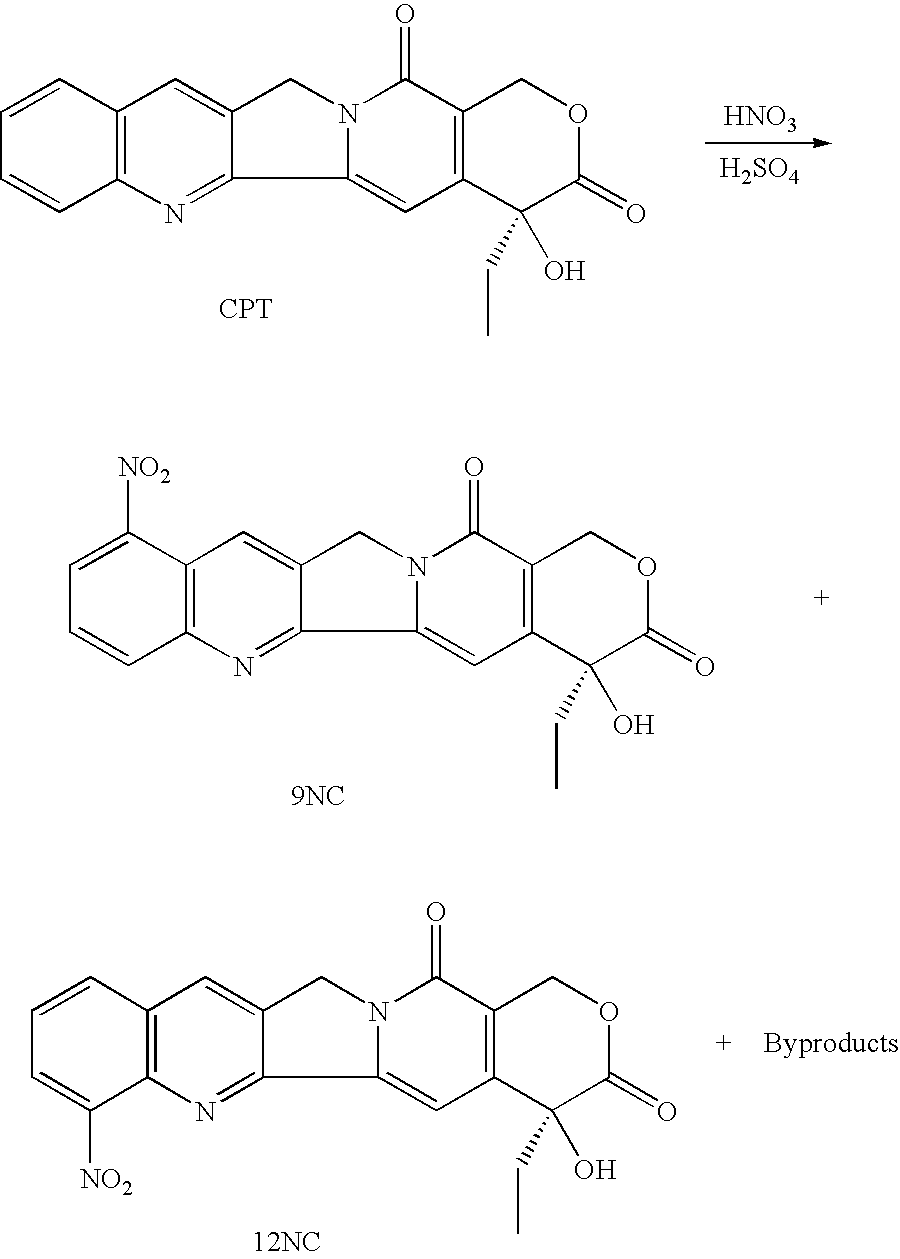
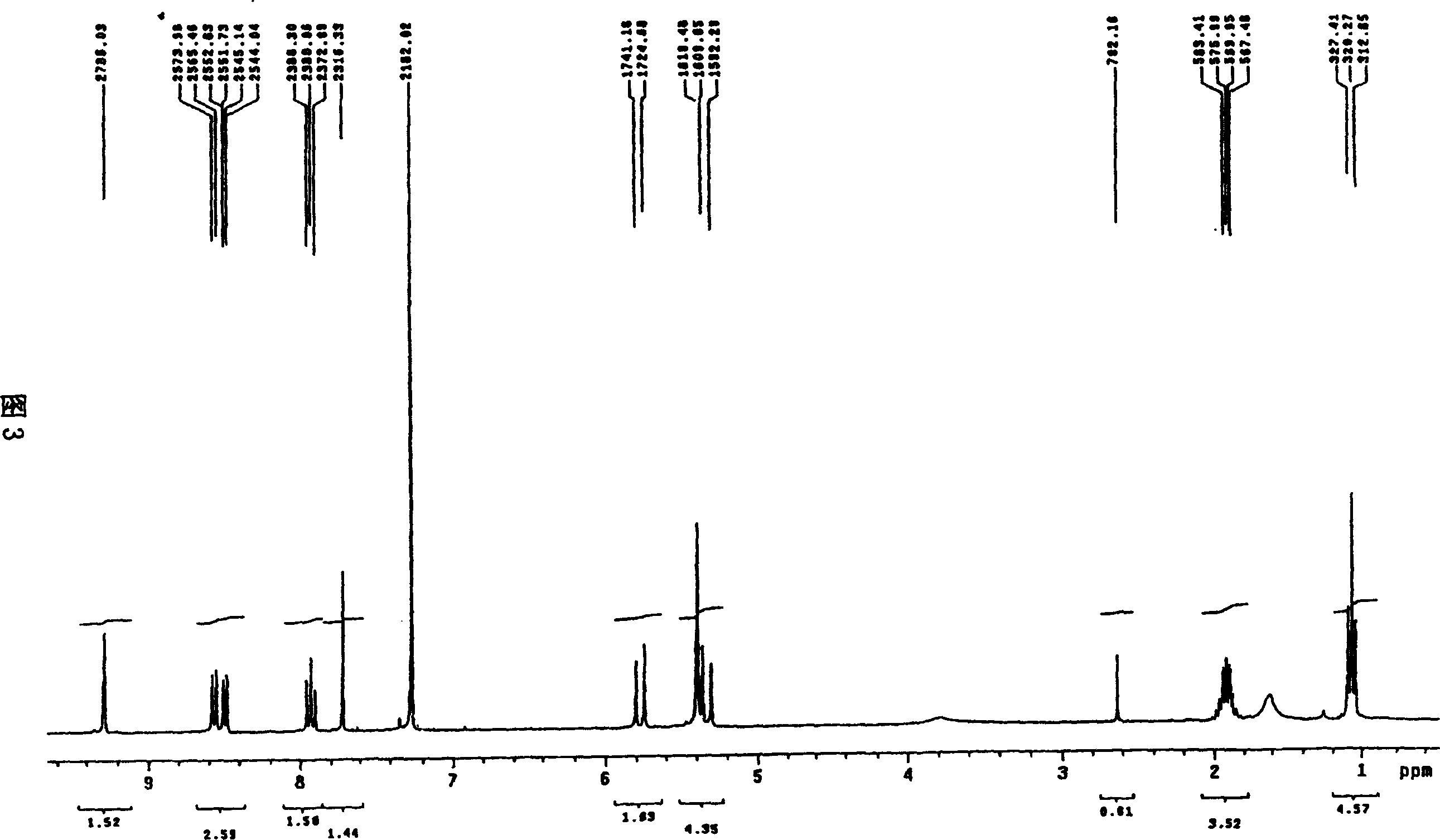
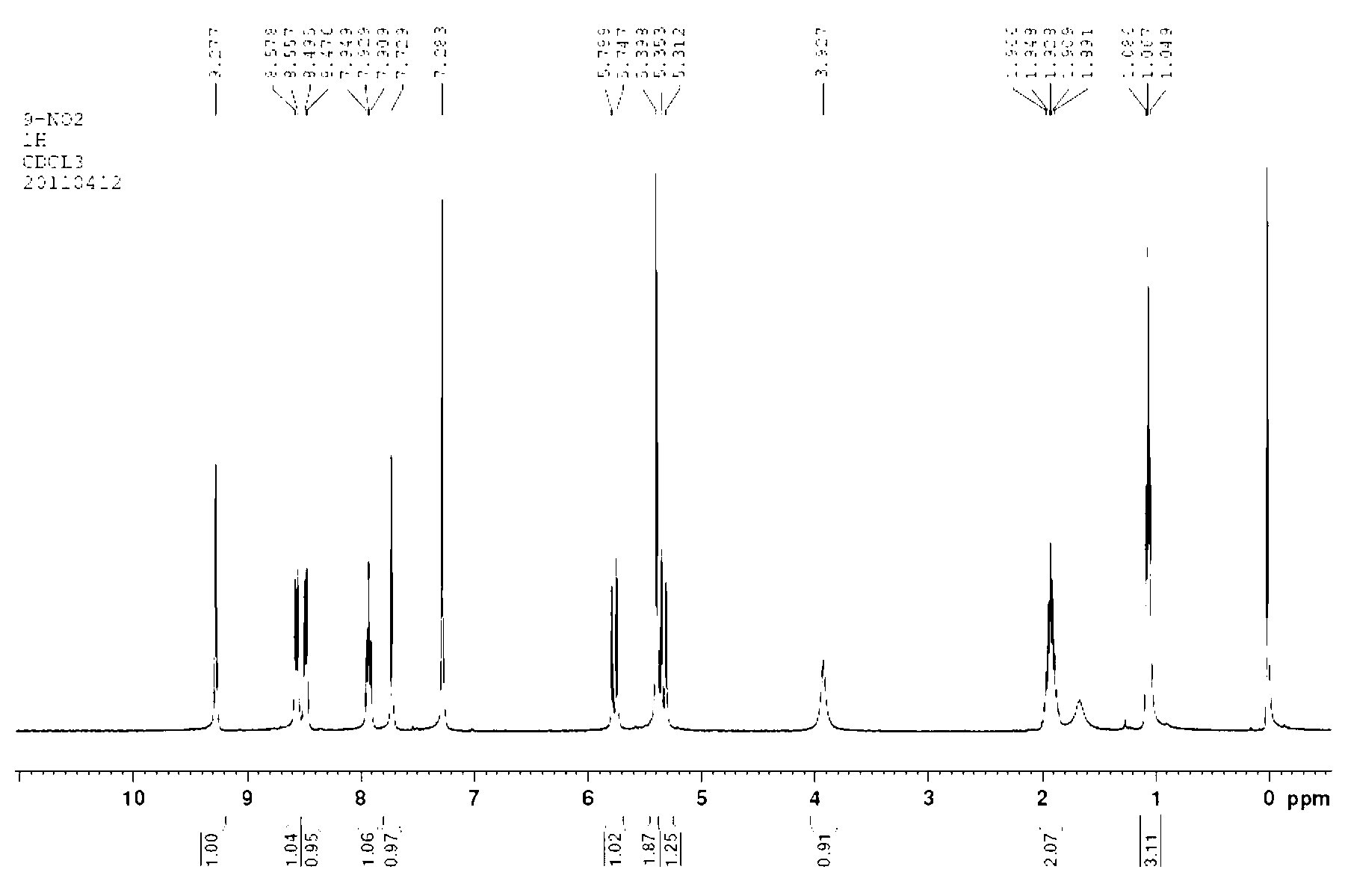
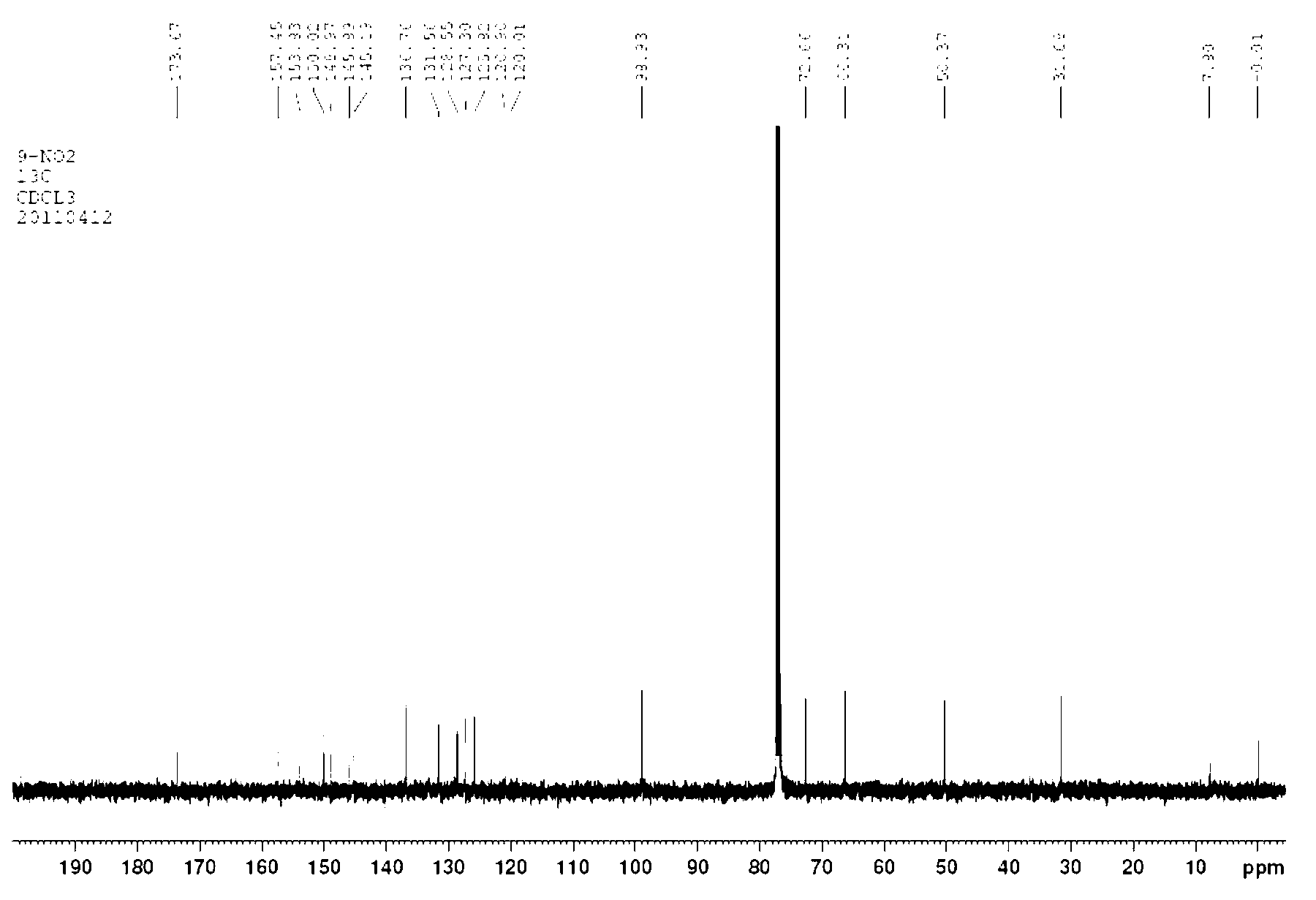
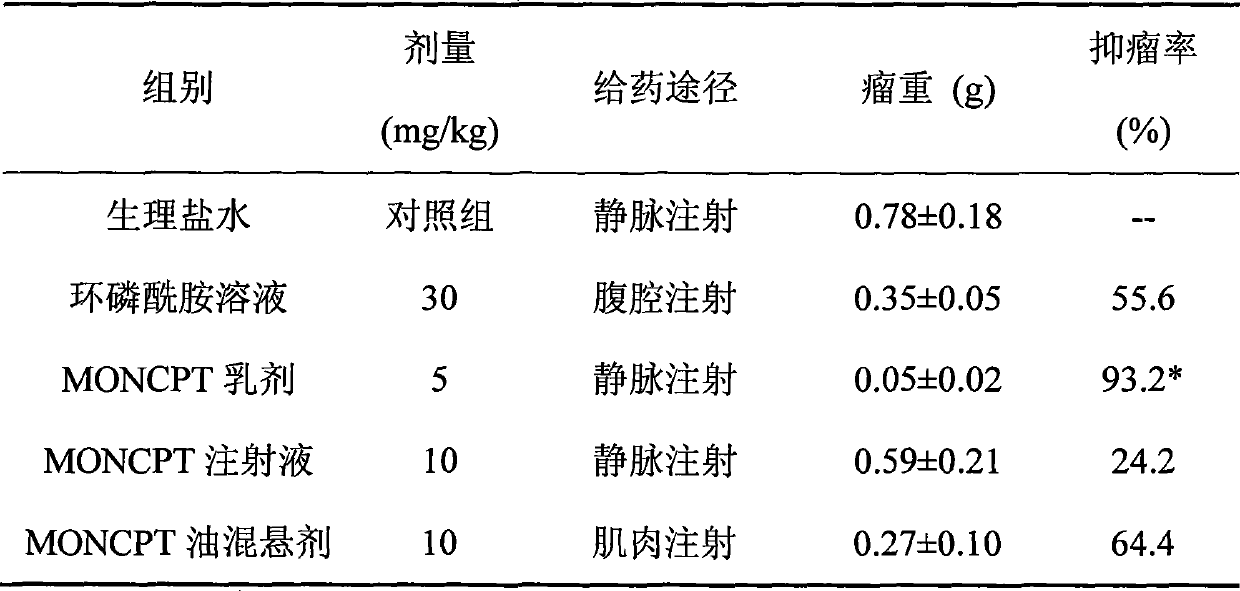
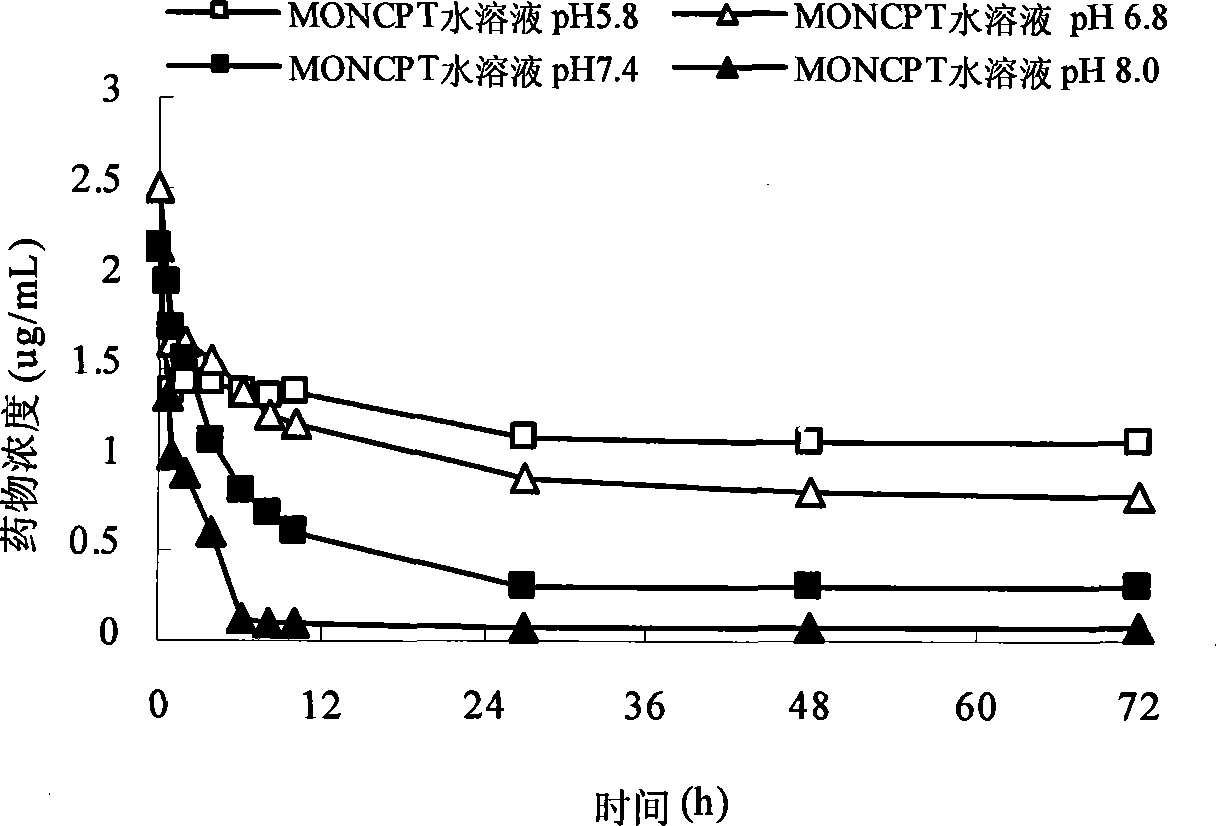
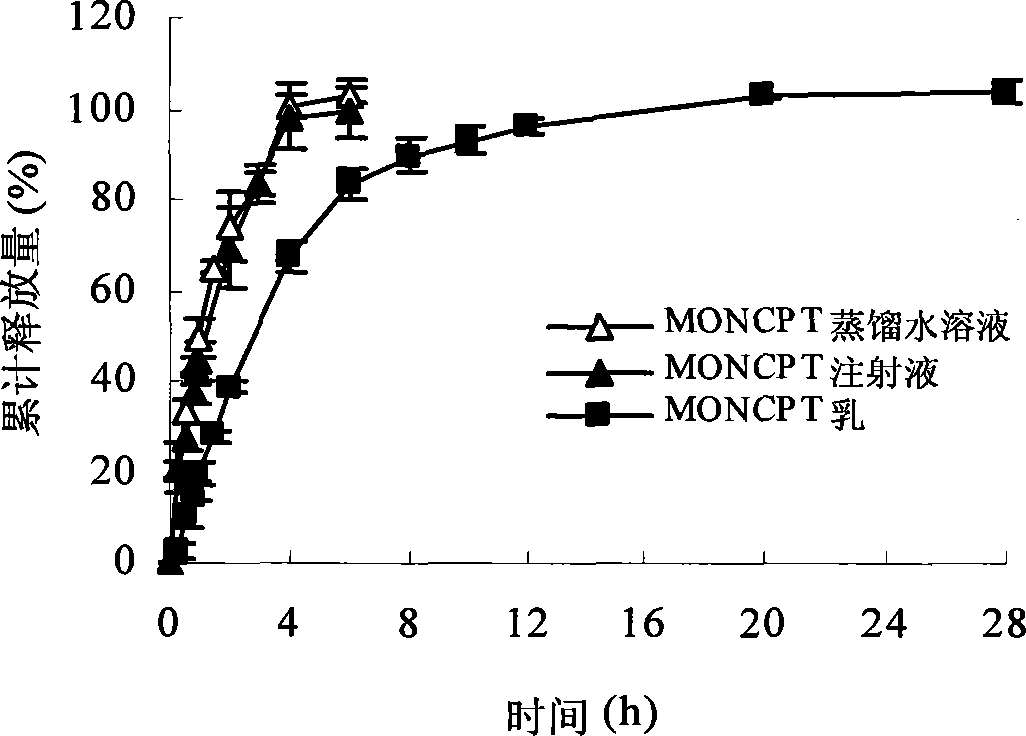
Patents
Literature
31 results about "Nitrocamptothecin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A semisynthetic agent related to camptothecin with potent antitumor and antiviral properties. Rubitecan binds to and inhibits the enzyme topoisomerase I and induces protein-linked DNA single-strand breaks, thereby blocking DNA and RNA synthesis in dividing cells; this agent also prevents repair of reversible single-strand DNA breaks. Check for http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=42528&idtype=1 active clinical trials or http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=42528&idtype=1&closed=1 closed clinical trials using this agent. (http://nciterms.nci.nih.gov:80/NCIBrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&code=C1485 NCI Thesaurus)
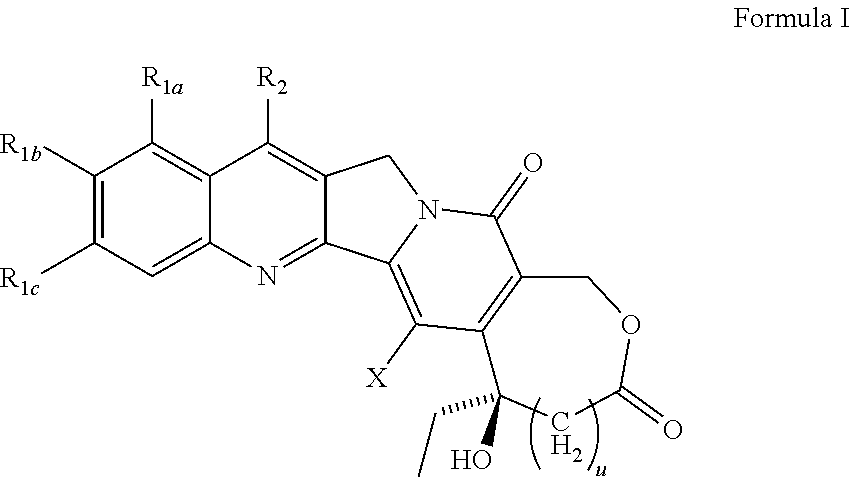
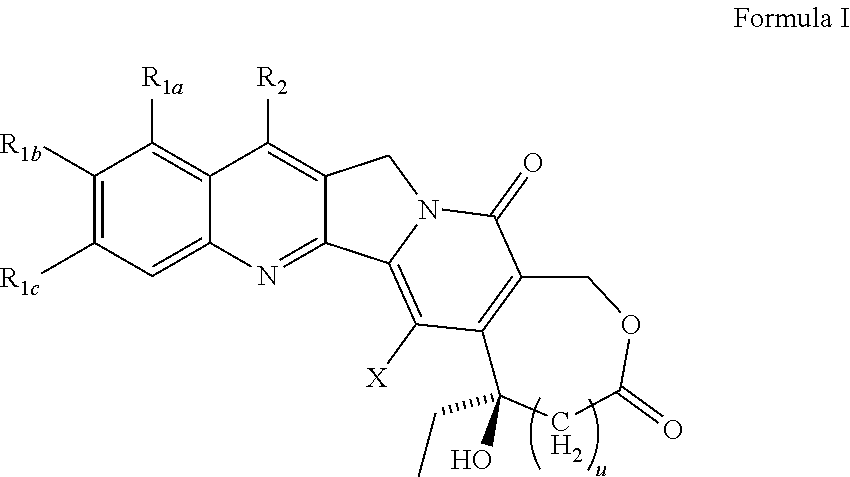
Compositions and formulations of 9-nitrocamptothecin polymorphs and methods of use therefor
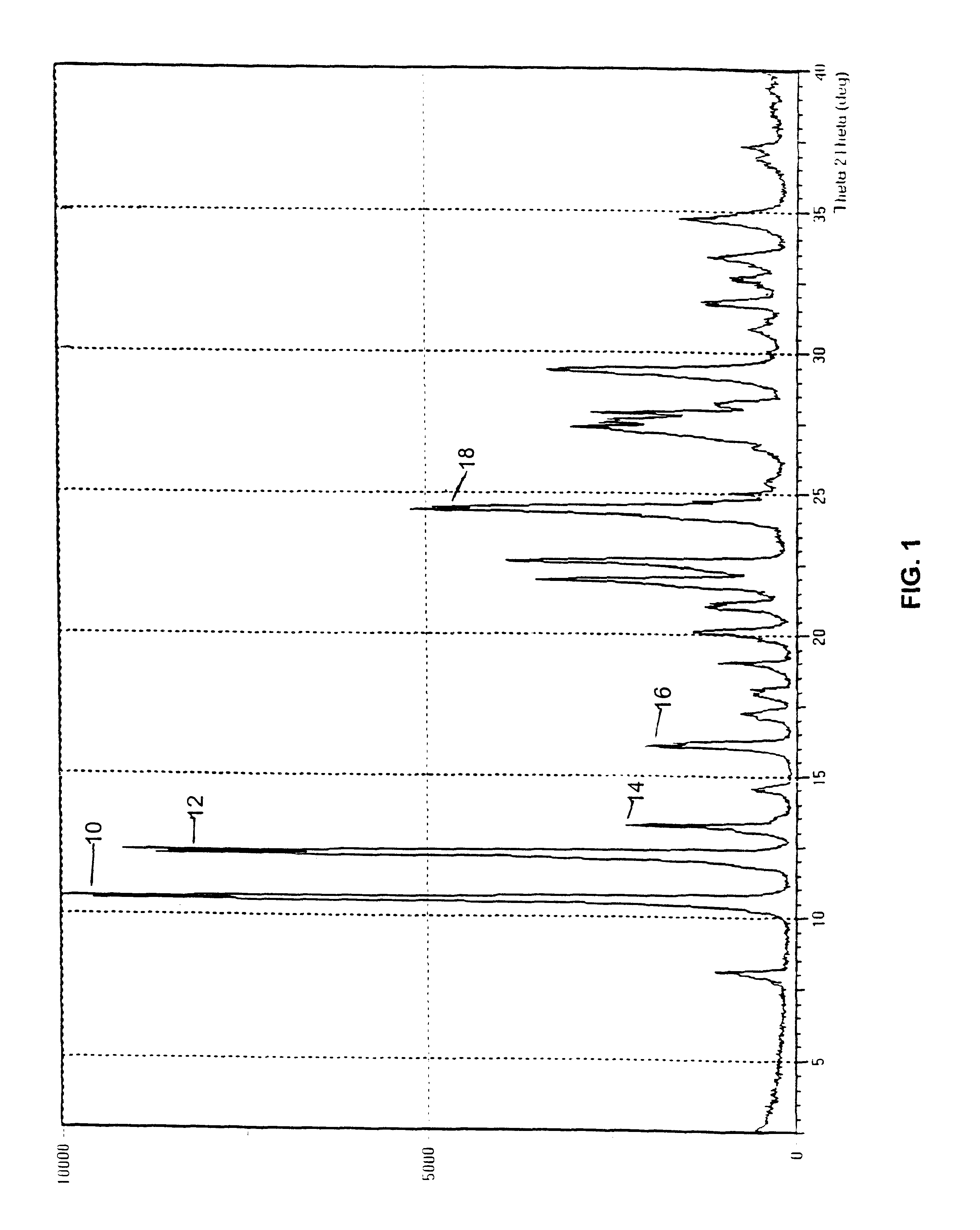
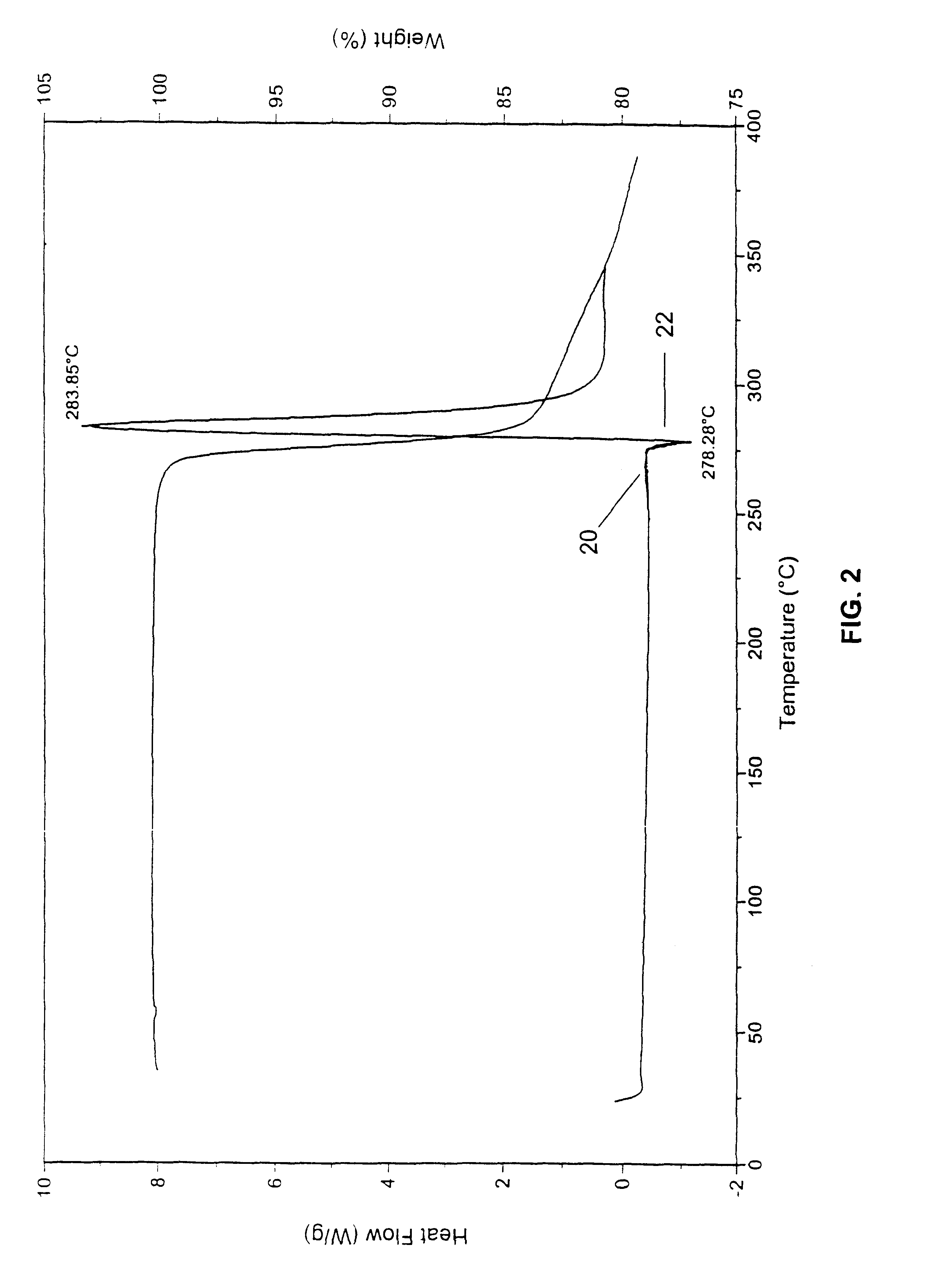
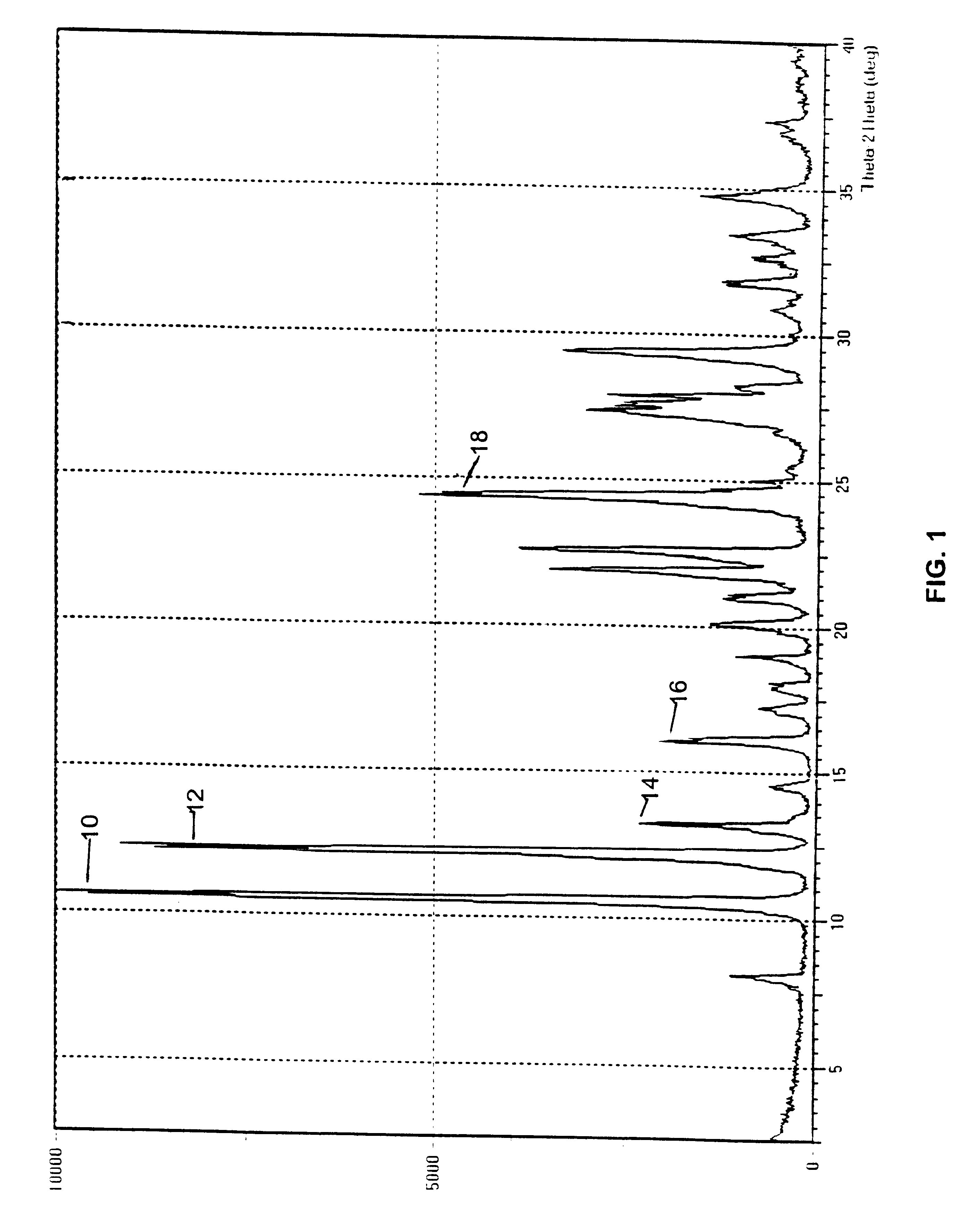
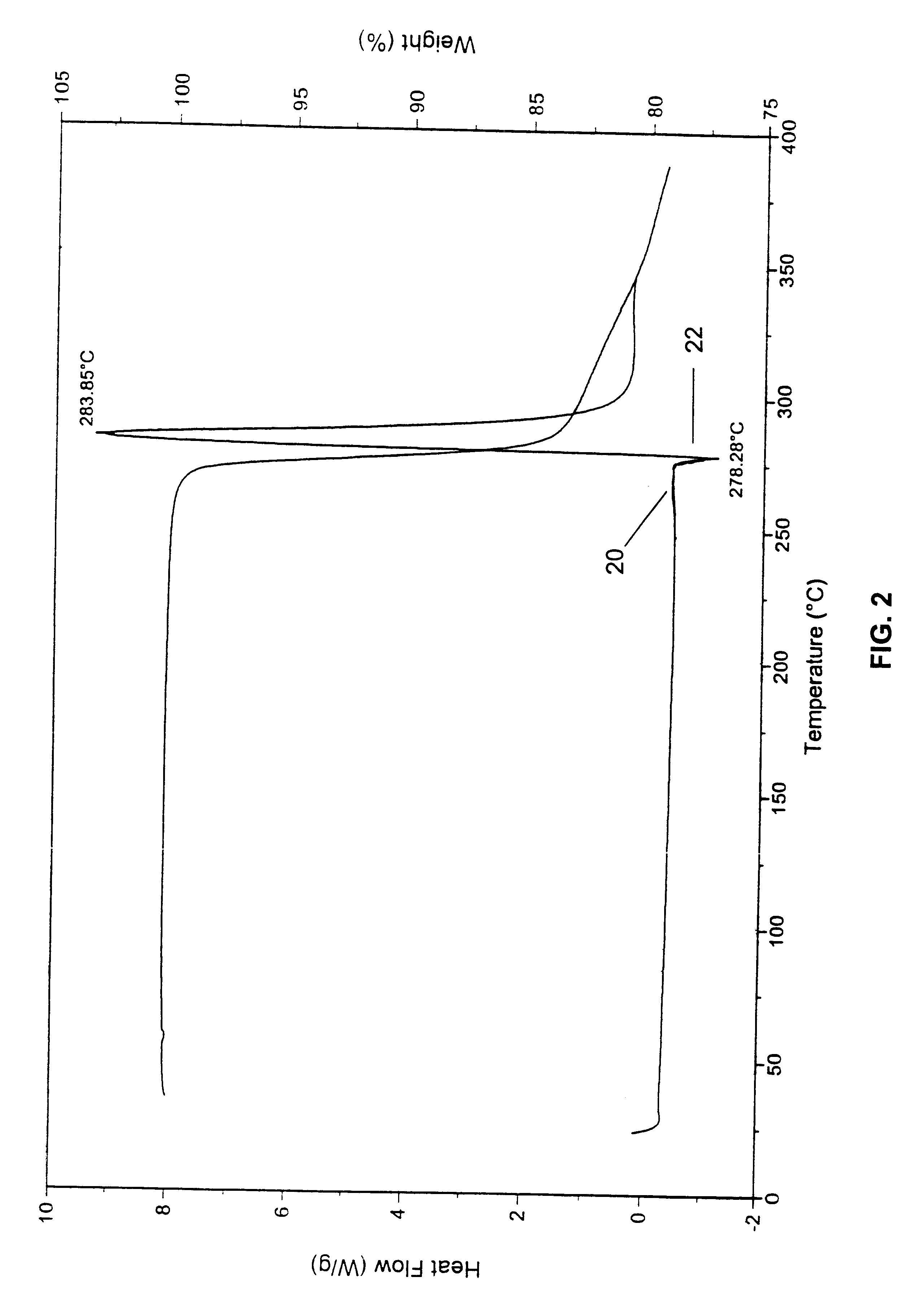
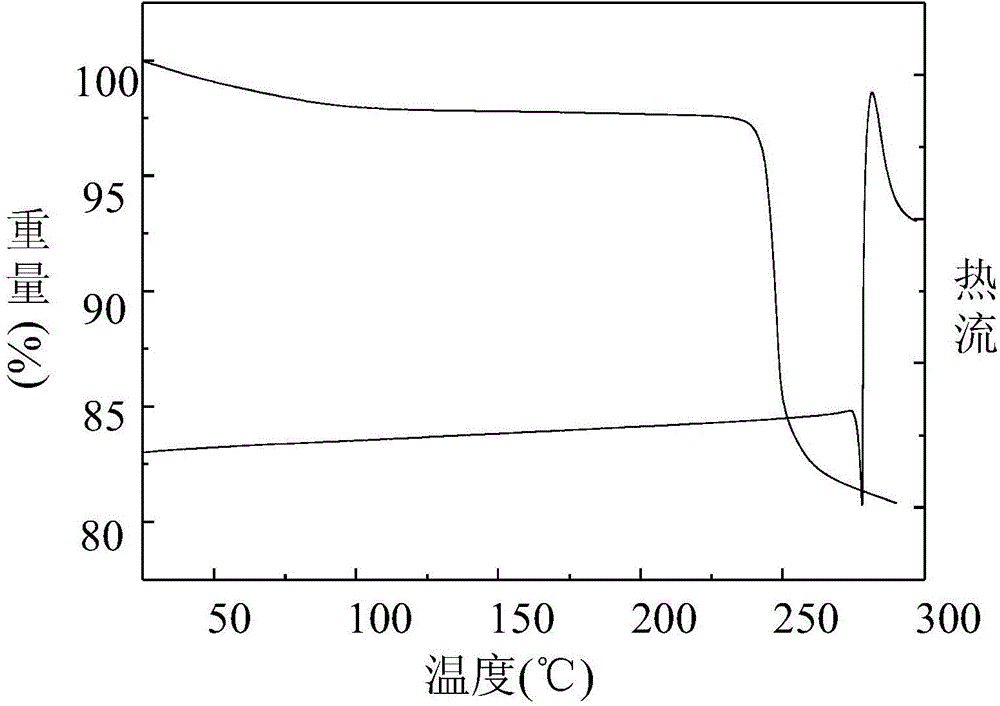
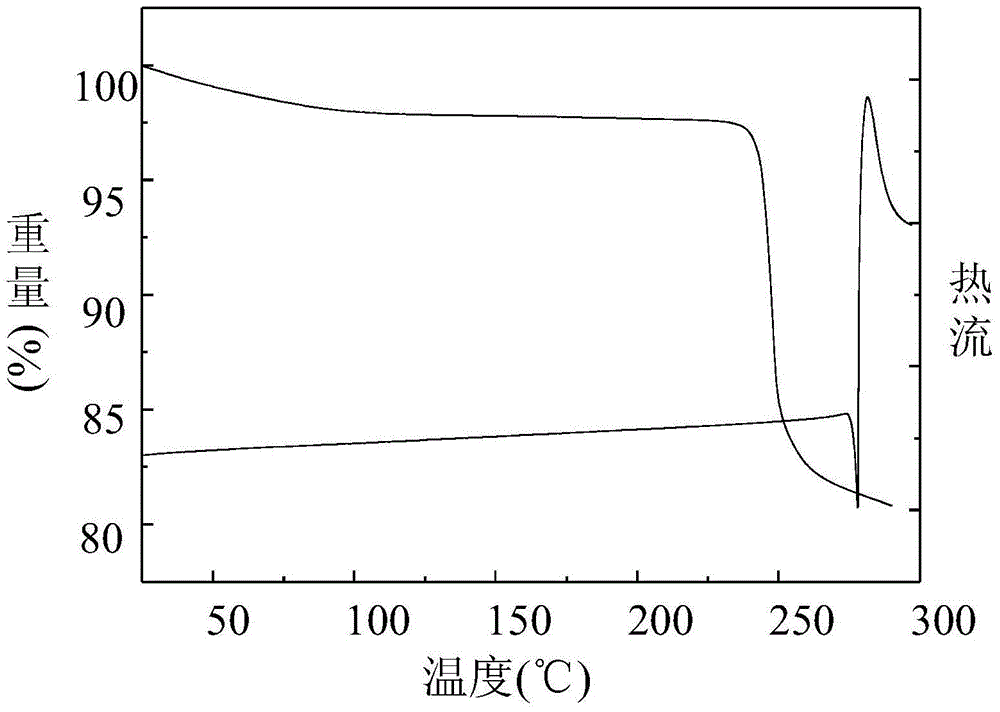
A polymorphic form of 9-nitrocamptothecin is provided, the polymorph being characterizable as having, by differential scanning calorimetry, an endotherm at between 149.2 and 151.2° C., an exotherm at between 162.6 and 164.6° C., and an exotherm at between 272 and 274° C.
Owner:SUPERGEN
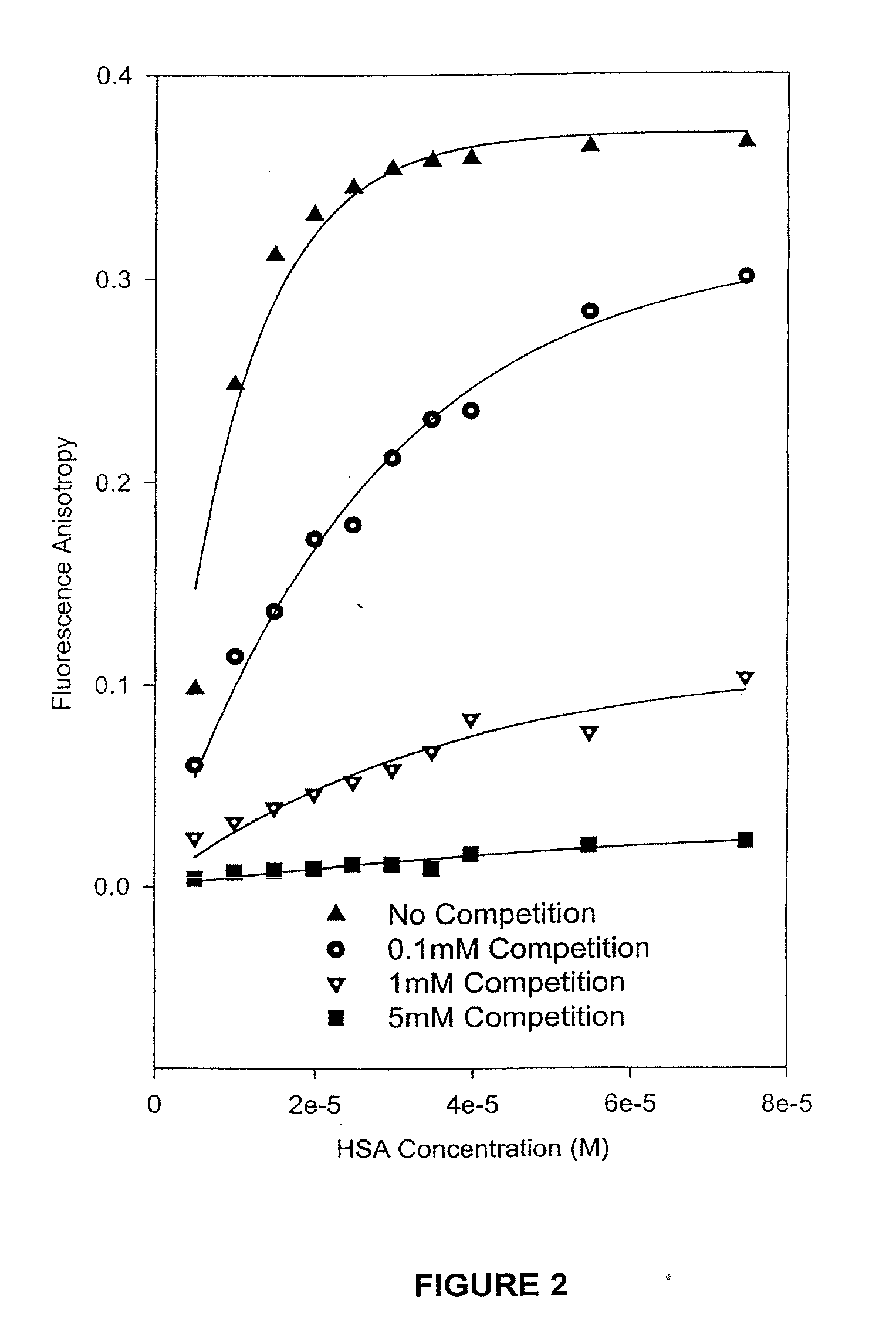
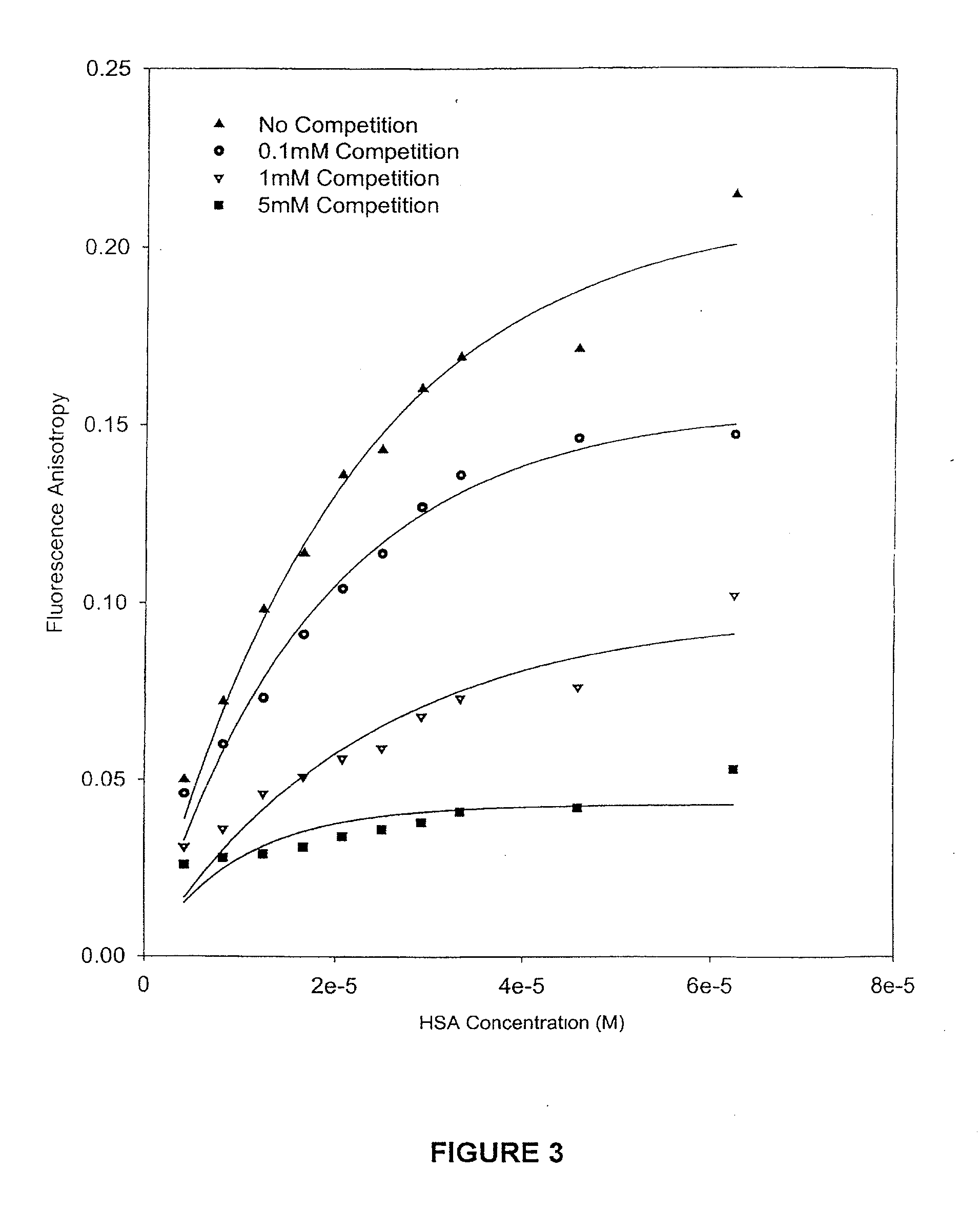
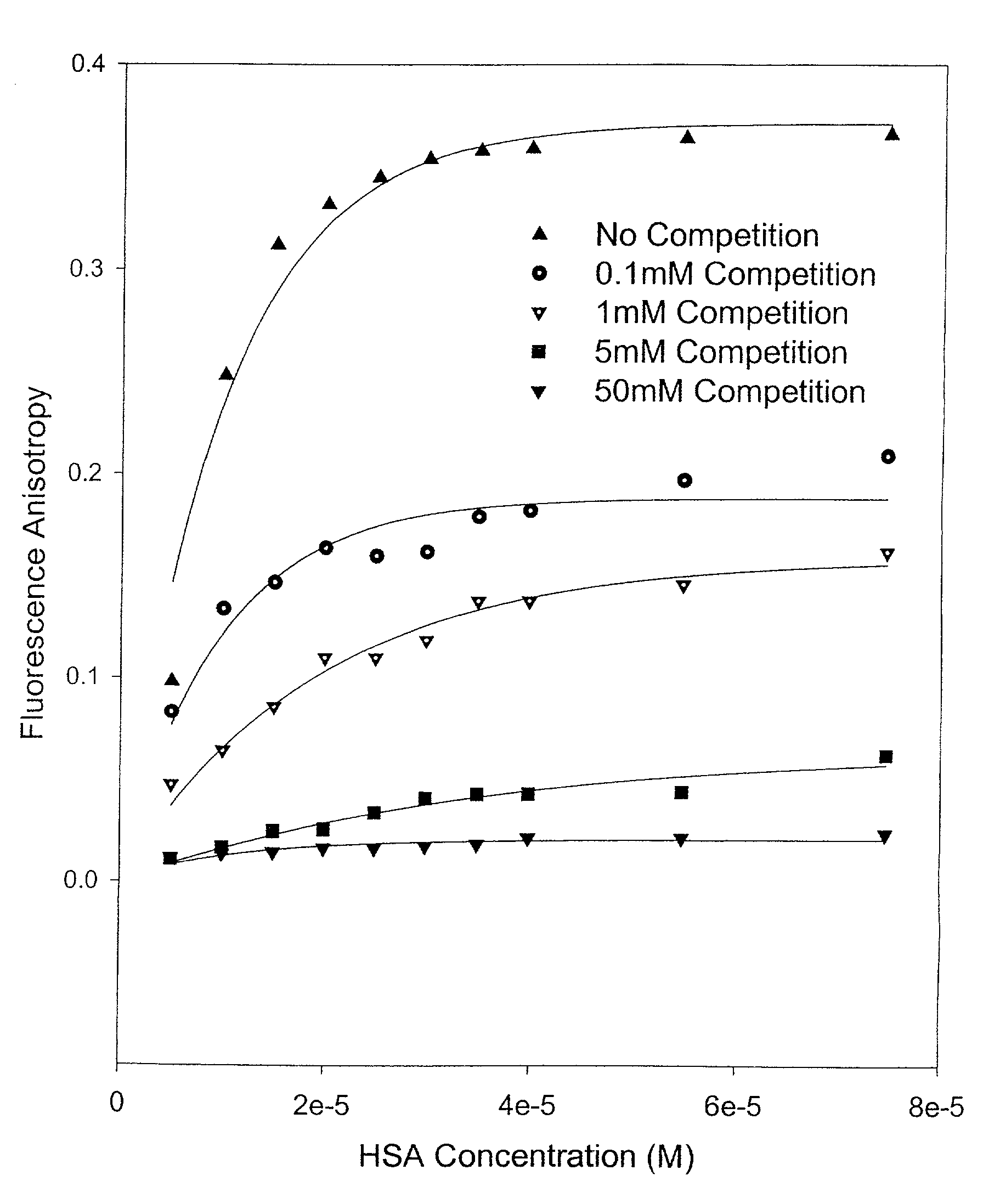
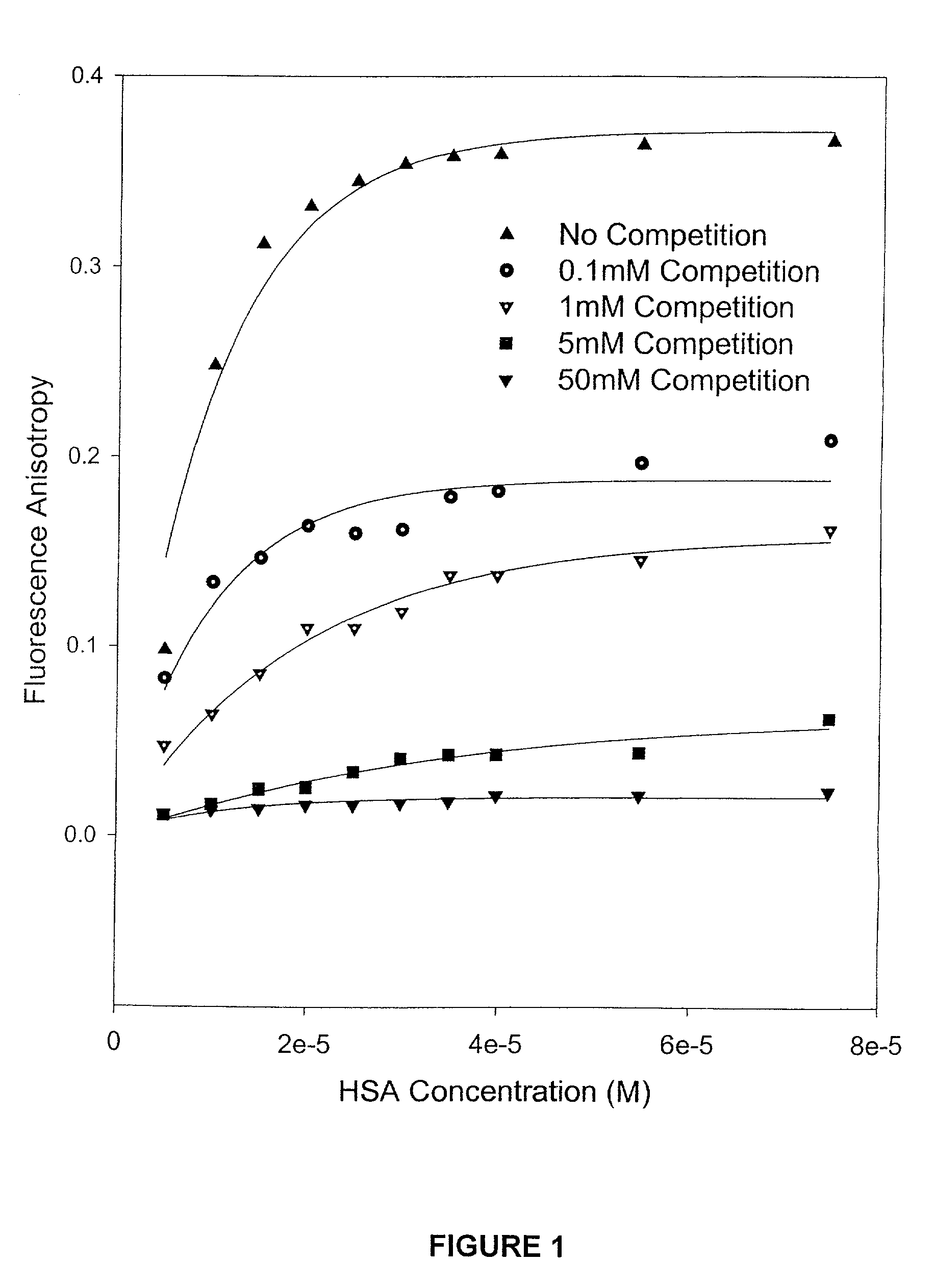
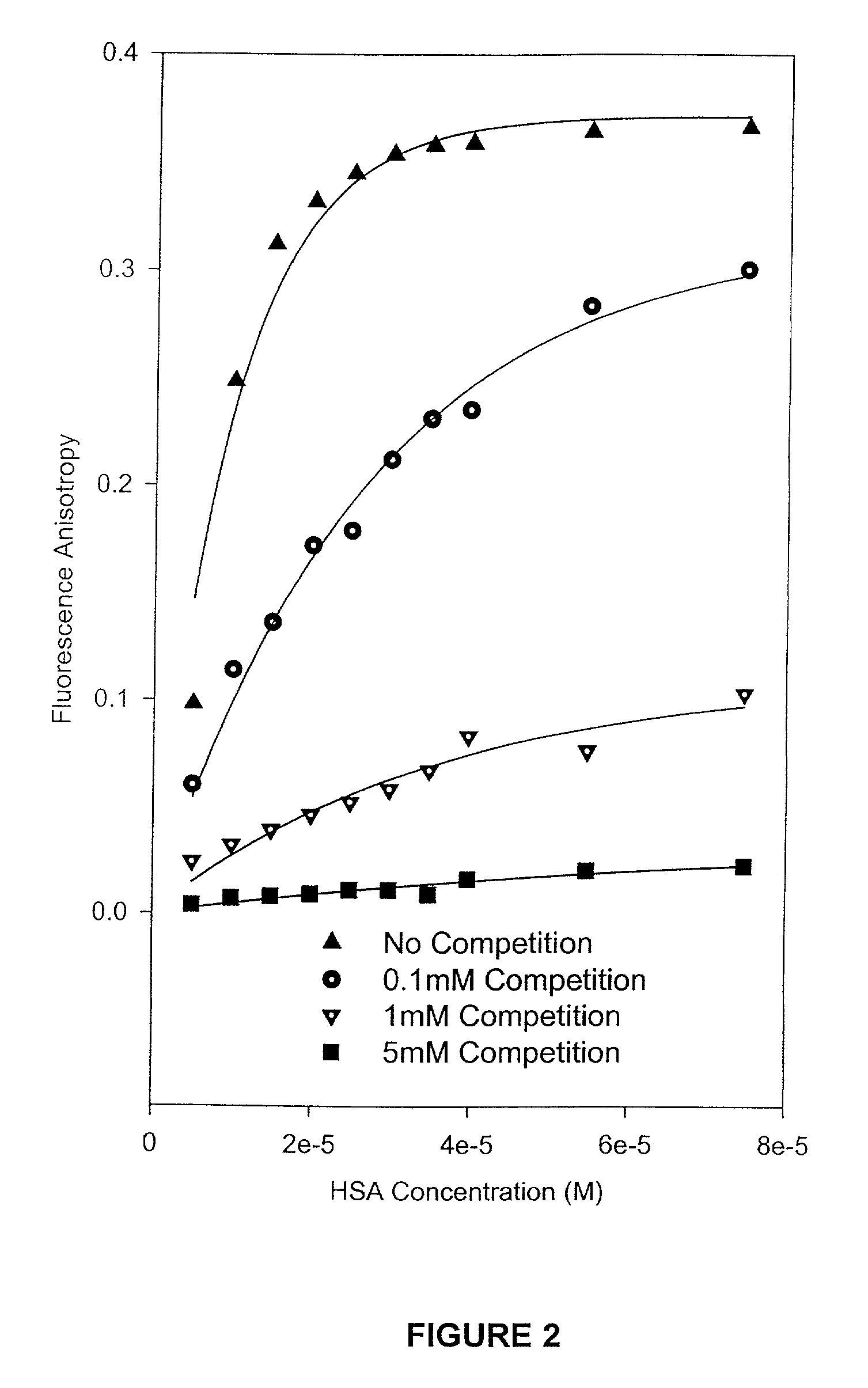
Methods and compositions for optimizing blood and tissue stability of camptothecin and other albumin-binding therapeutic compounds
InactiveUS20100240602A1Improve stabilityHigh affinitySalicyclic acid active ingredientsBiocideNitrocamptothecinHIV positives
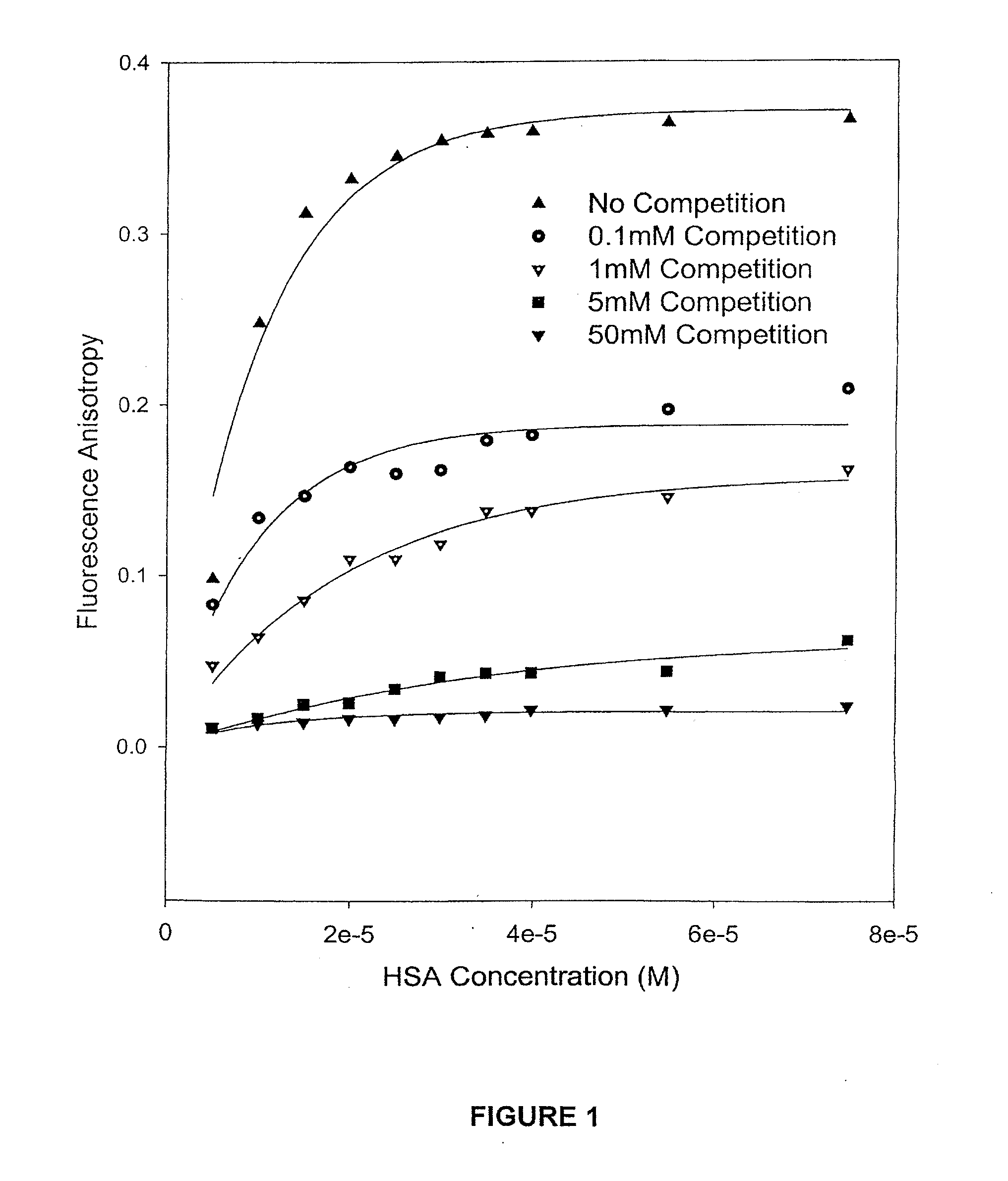
The present invention provides methods and formulations for optimizing the anti-cancer and anti-HIV activities of a camptothecin drug, including camptothecin and its related analogs including 9-aminocamptothecin and 9-nitrocamptothecin. The invention involves methodologies and formulations that limit human serum albumin-mediated reduction of the anti-cancer and anti-HIV effects of the camptothecins, and the methods and formulations provide combination therapies in which binding of the camptothecin agent to human serum albumin can be modulated by the administration of a competing agent such as ibuprofen, clofibrate or clofibric acid that also binds human serum albumin. Reduced camptothecin drug binding to human serum albumin can result in elevated camptothecin free drug levels and thus improve the effectiveness of treatment regimens involving these drugs. Further agents such as methotrexate and AZT can also be used in cancer and HIV-positive patients employing camptothecin drugs.
Owner:BURKE THOMAS G +1
Camptothecin derivatives
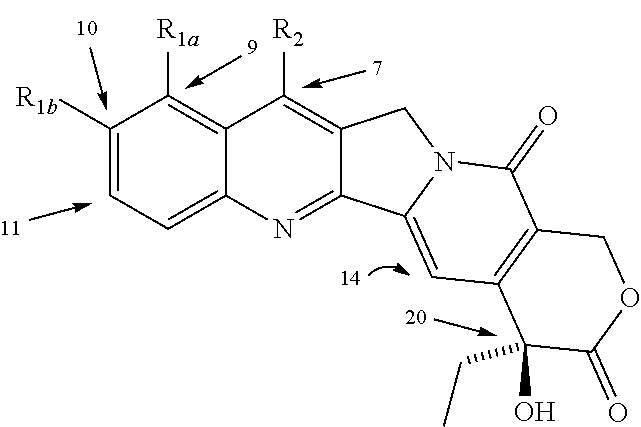
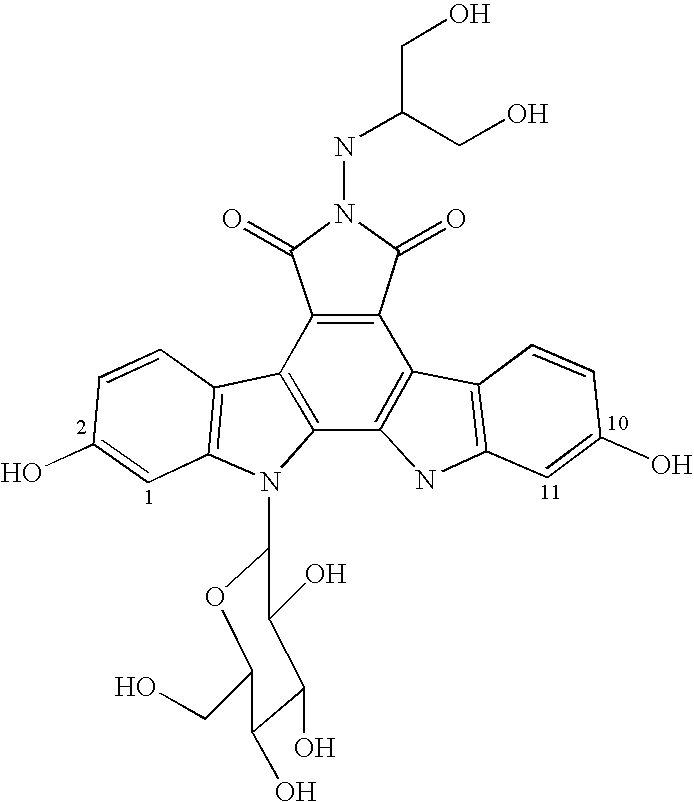
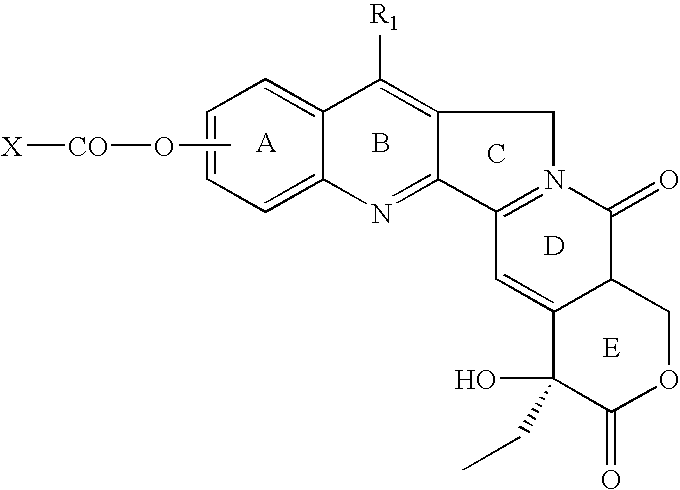
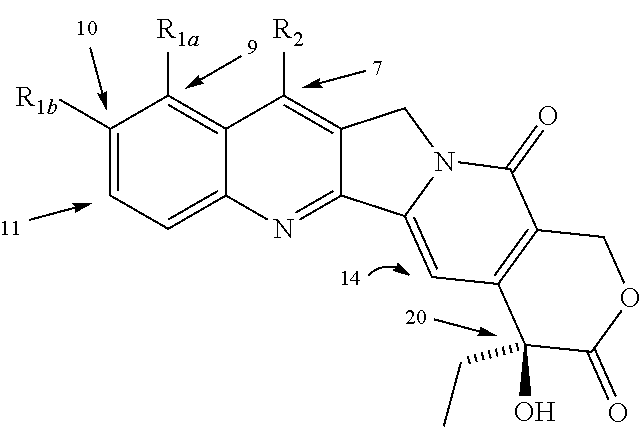
Various 14-Nitro, 14-amino, and 14-substituted amino camptothecin derivatives are useful in the treatment of cancer and other hyperproliferative diseases. Various 14-nitro camptothecin derivatives are conveniently prepared by reacting a camptothecin derivative with fuming nitric acid, optionally employing acetic anhydride as a solvent.
Owner:MOLECULAR TEMPLATES
9-nitrocamptothecin solid dispersant and preparation method
InactiveCN1422618AImprove solubilityDissolution rate is fastOrganic active ingredientsPharmaceutical delivery mechanismSolubilityNitrocamptothecin
The present invention relates to a compound 9-nitrocamptothecine solid dispersion and its preparation method. Said invention adopts 9-nitrocamptothecine as medicine active componnet, and adds carrier material, and separately adopts grinding method, freeze-drying method, melting method and solvent method to prepare the invented product which can be made into capsule, table and drip pill. It can raise solubility of medicine, as compared with raw material medicine its resolution speed can be raised by 4-80 times, it can promote absorption, can reduce irritation of medicine to stomach and intestine and can raise stability of the medicine.
Owner:FUDAN UNIV +1
Compositions and formulations of 9-nitrocamptothecin polymorphs and methods of use therefor
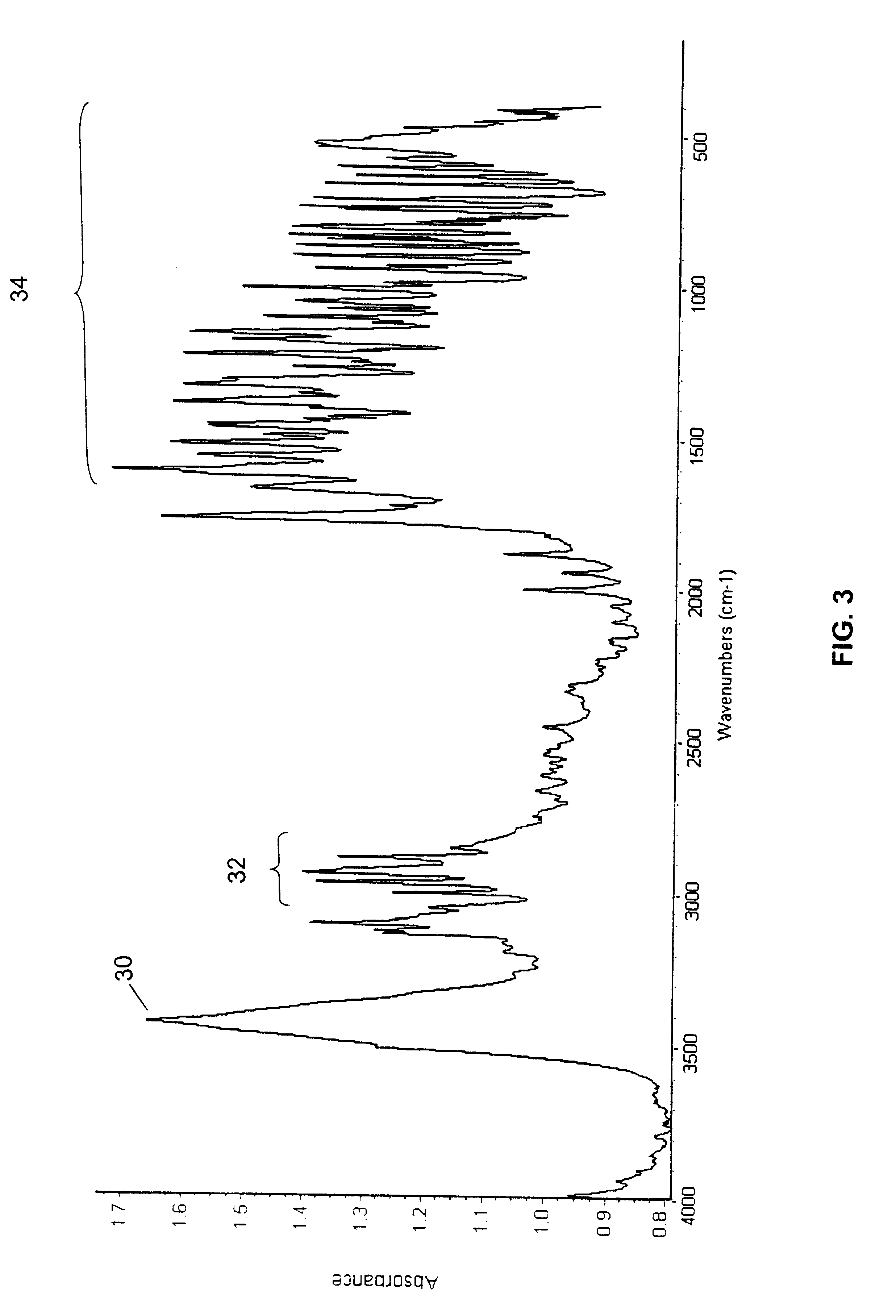
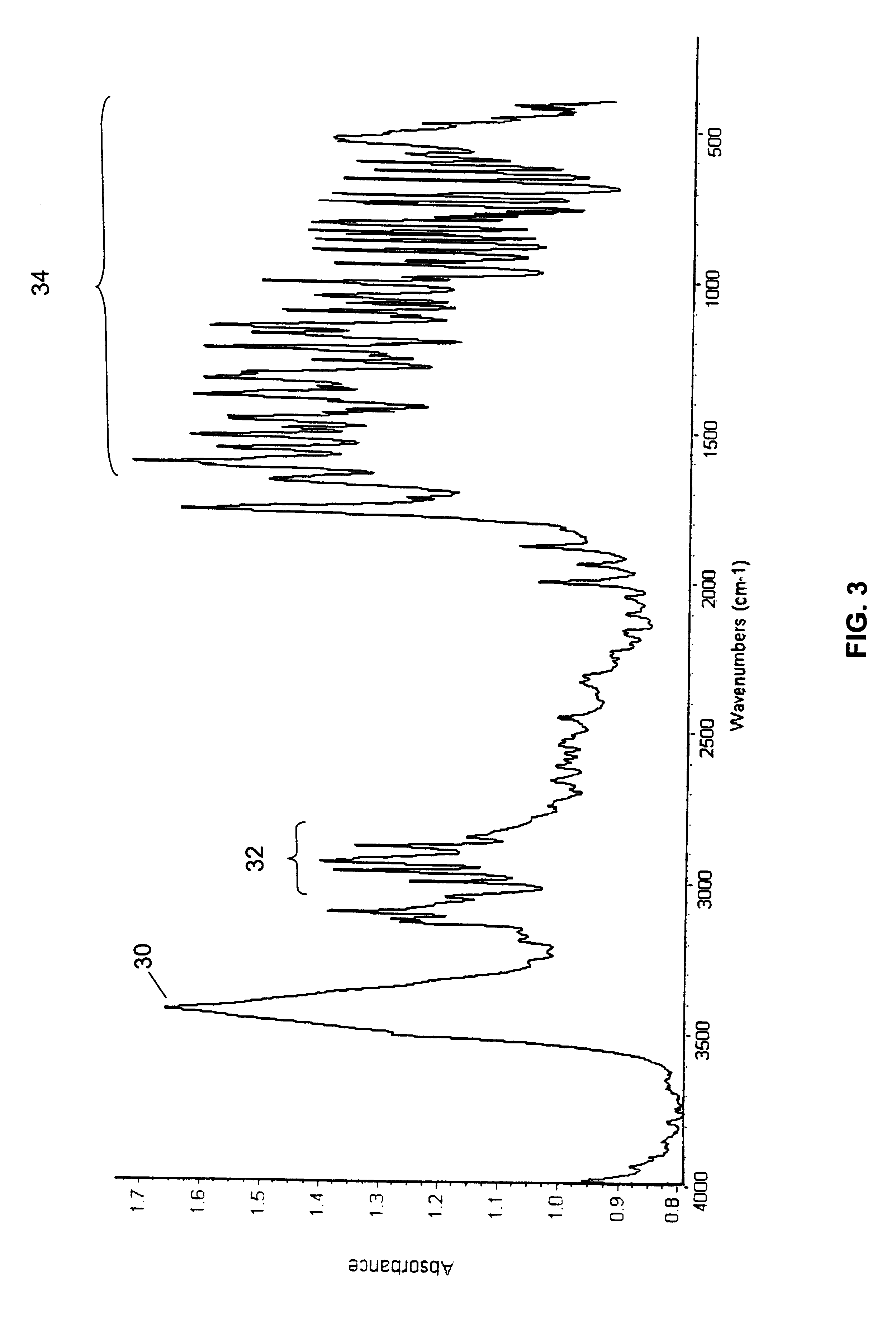
A polymorphic form of 9-nitrocamptothecin is provided, the polymorph being characterizable as having, by differential scanning calorimetry, an endotherm at between 175.5 and 177.5° C., an exotherm at between 181.7 and 183.7° C., and an IR spectrum with no absorption centered between 3625 cm-1 and 3675 cm-1.
Owner:SUPERGEN
Method for treating abnormal cell growth
InactiveUS20050267140A1Ease of detectabilityEasy to prepareBiocideAnimal repellantsNitrocamptothecinSN-38
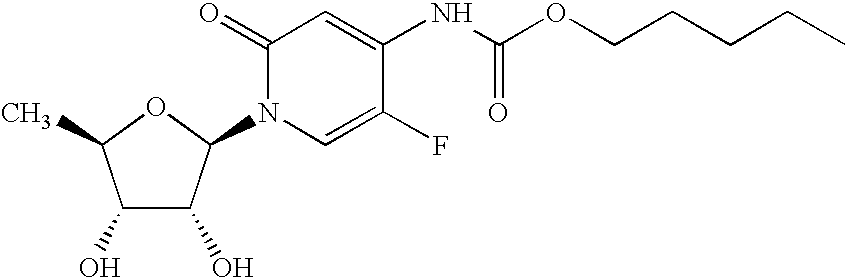
Therapeutic pharmaceutical compositions and methods of treatment of abnormal cell growth comprising a pyrimidine derivative or a pharmaceutically acceptable salt, solvate or prodrug thereof in combination with an oral camptothecin, an oral camptothecin derivative, an indolopyrrocarbazole derivative or a pharmaceutically acceptable salt, solvate or prodrug thereof for the treatment of cancer are described. In one embodiment of the present invention the oral camptothecin derivative is selected from the group consisting of 10-hydroxycamptothecin, 9-aminocamptothecin, 9-nitrocamptothecin, irinotecan, irinotecan salt, SN-38, CPT-11, and topotecan and the indolopyrrocarbazole derivative is edotecarin. In one embodiment the pyrimidine derivative is selected from the group consisting gemcitabine, MTA and capecitabine. In one preferred embodiment, the pyrimidine derivative is capecitabine and the camptothecin derivative is CPT-11.
Owner:PFIZER INC
Camptothecin derivatives
Various 14-nitro, 14-amino, and 14-substituted amino camptothecin derivatives are useful in the treatment of cancer and other hyperproliferative diseases. 14-Nitro camptothecin derivatives are conveniently prepared by reacting a camptothecin derivative with fuming nitric acid, optionally employing acetic anhydride as a solvent.
Owner:MOLECULAR TEMPLATES
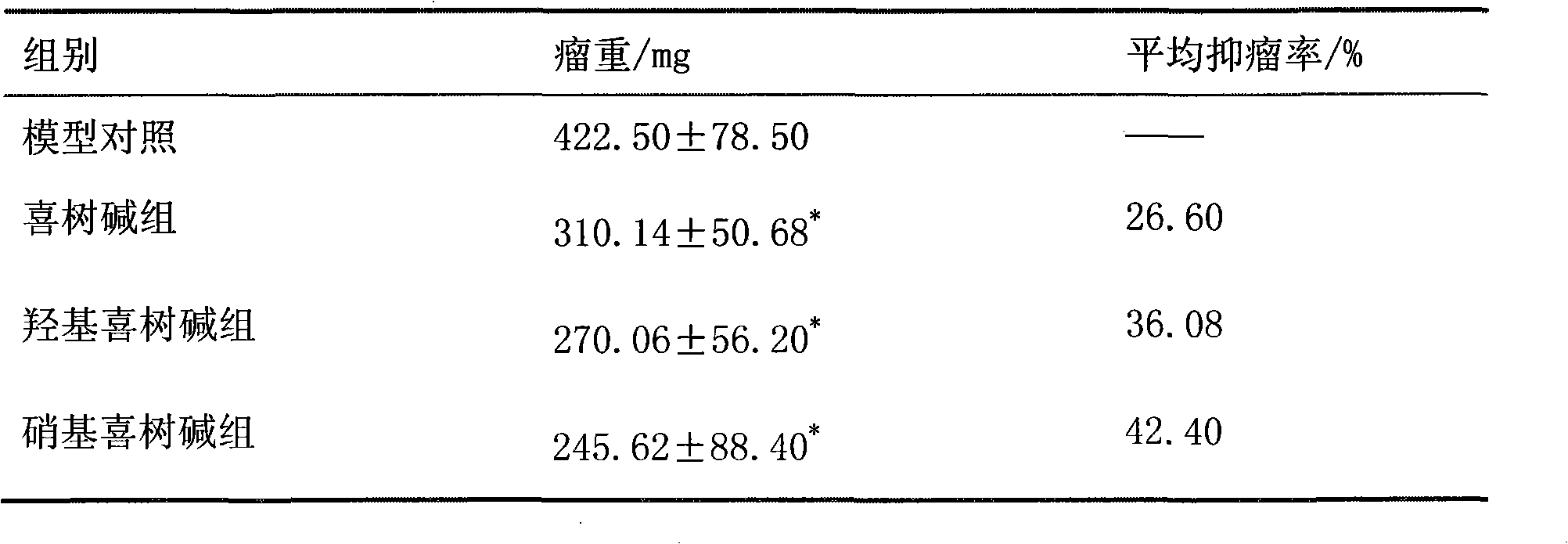
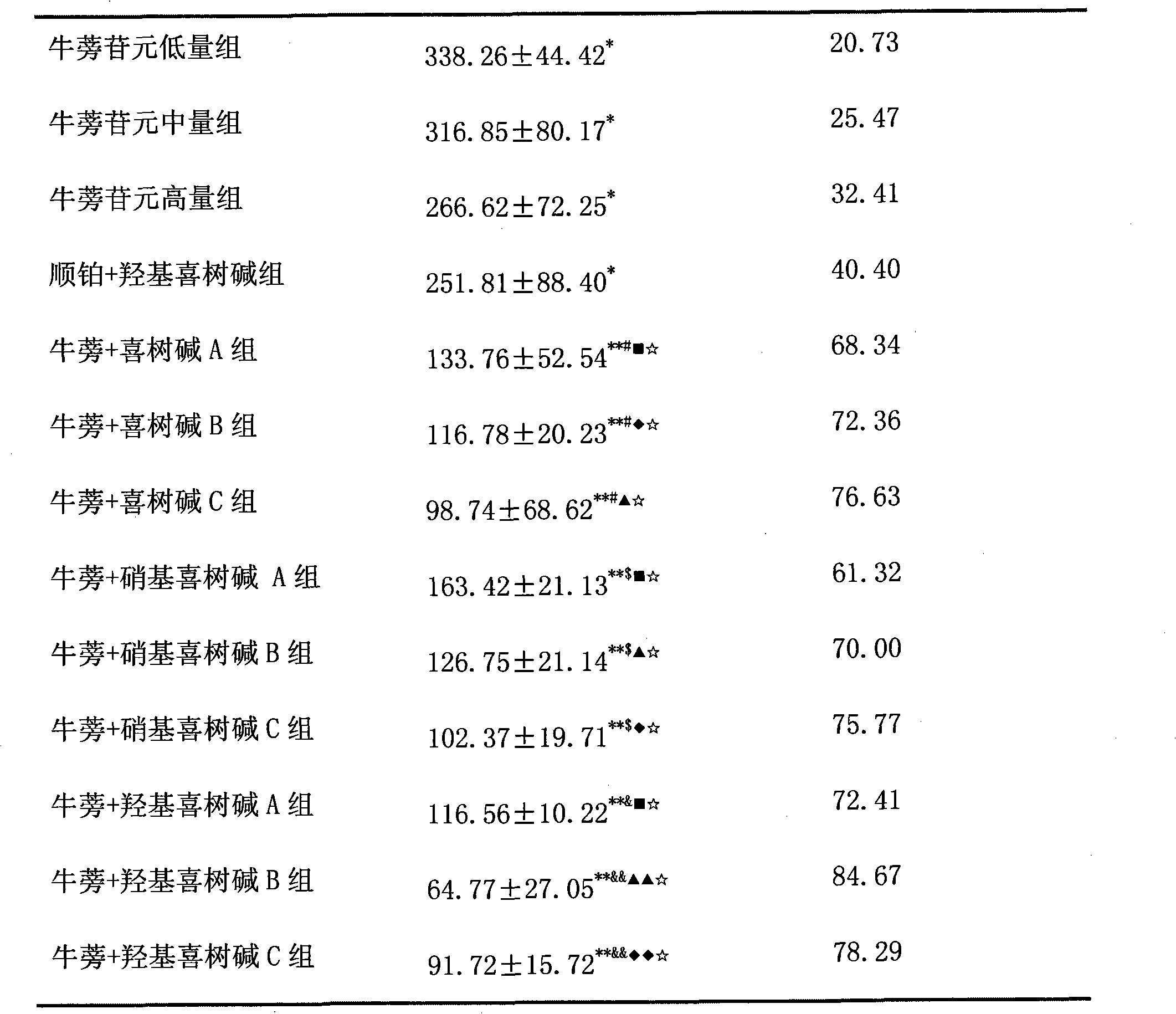
Anticancer drug composition
ActiveCN102370645ASignificant synergistic anticancer effectLow toxicityOrganic active ingredientsEmulsion deliveryNitrocamptothecinMicroemulsion
The invention belongs to the field of medicine, and specifically relates to an anticancer drug composition. The anticancer drug composition contains arctigenin and camptothecine drugs. The camptothecine drugs of the present invention comprise camptothecine, hydroxy camptothecine or nitrocamptothecine. The present invention provides a microemulsion preparation containing the drug composition and an injection containing the drug composition. Compared to the single drug, the drug composition of the present invention provides a synergistic effect for inhibition of non-small cell lung cancer.
Owner:SHANDONG NEWTIME PHARMA
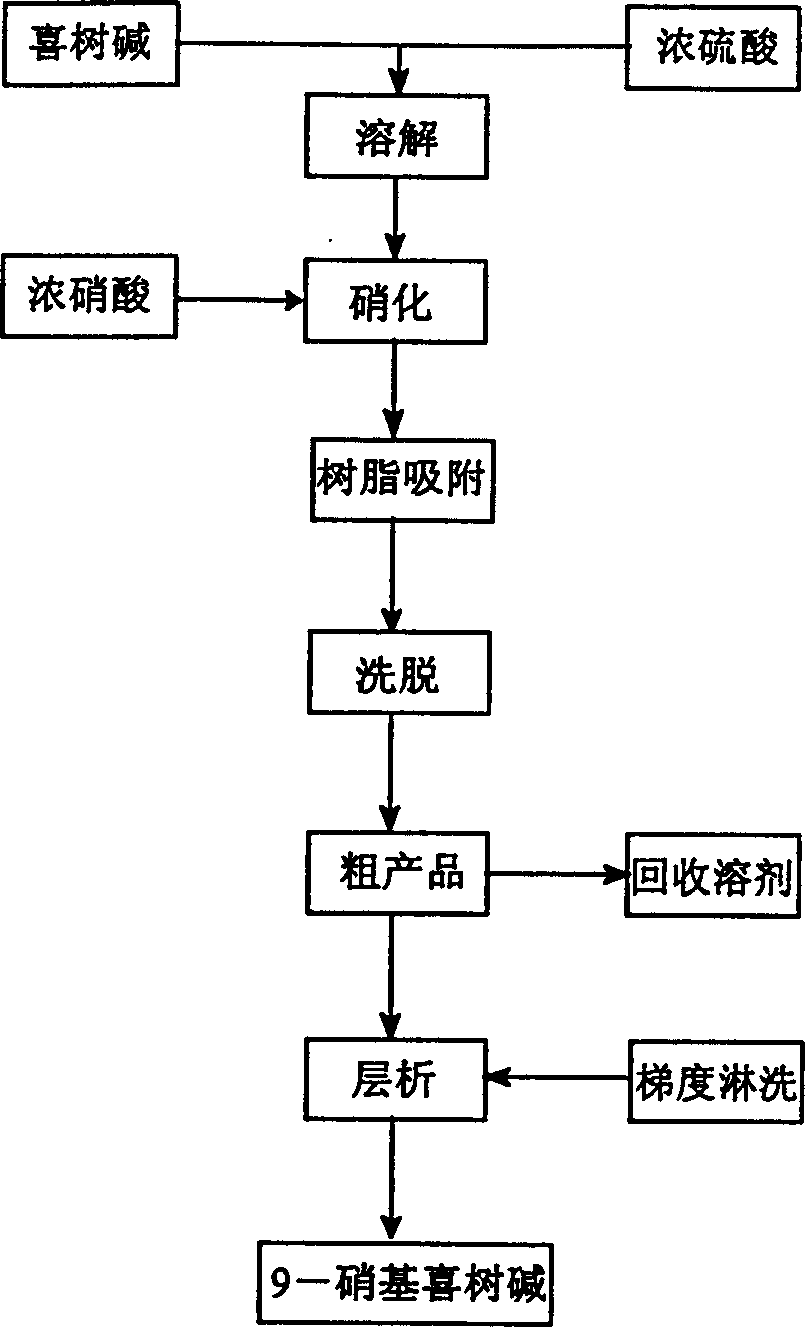
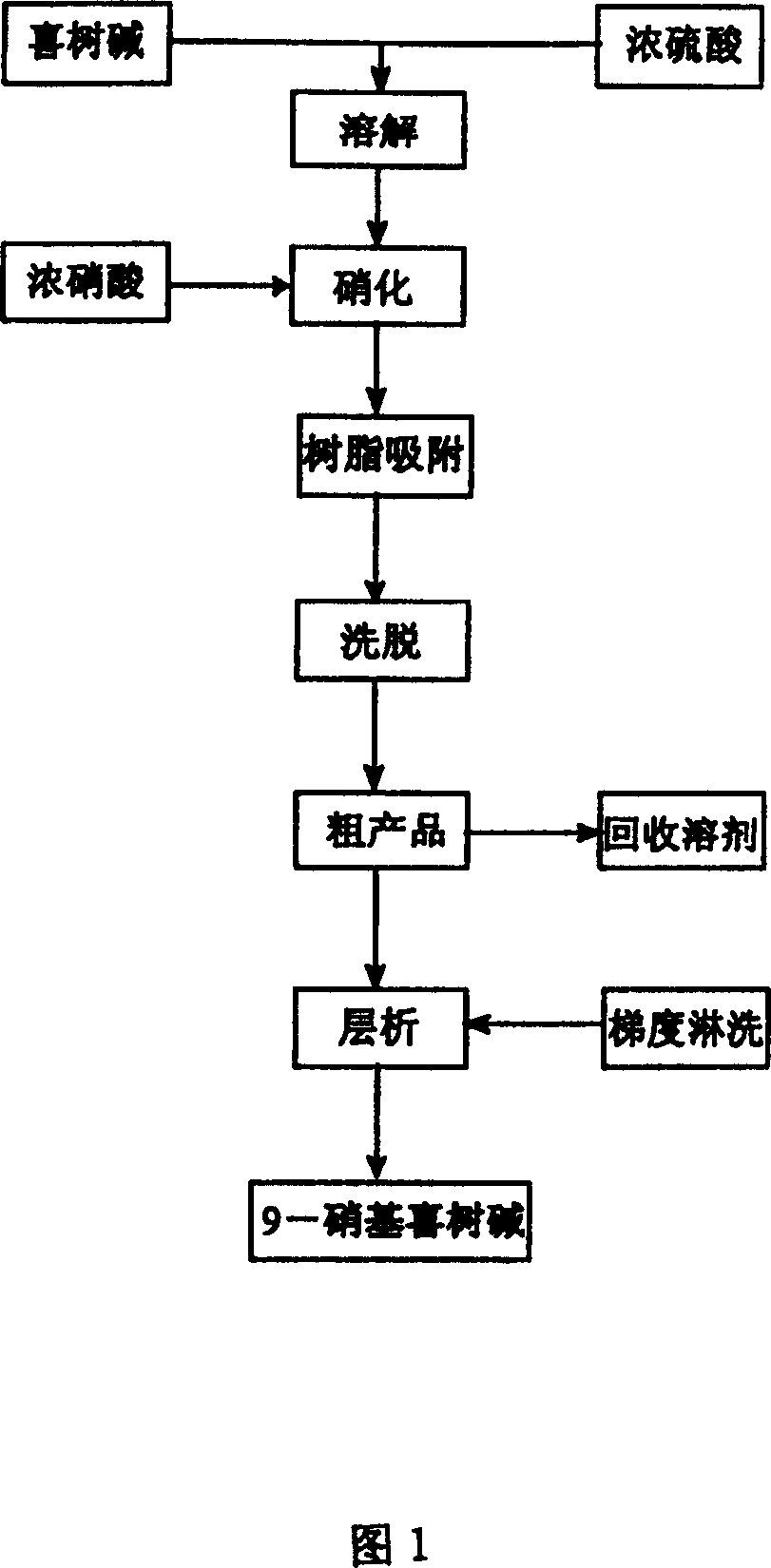
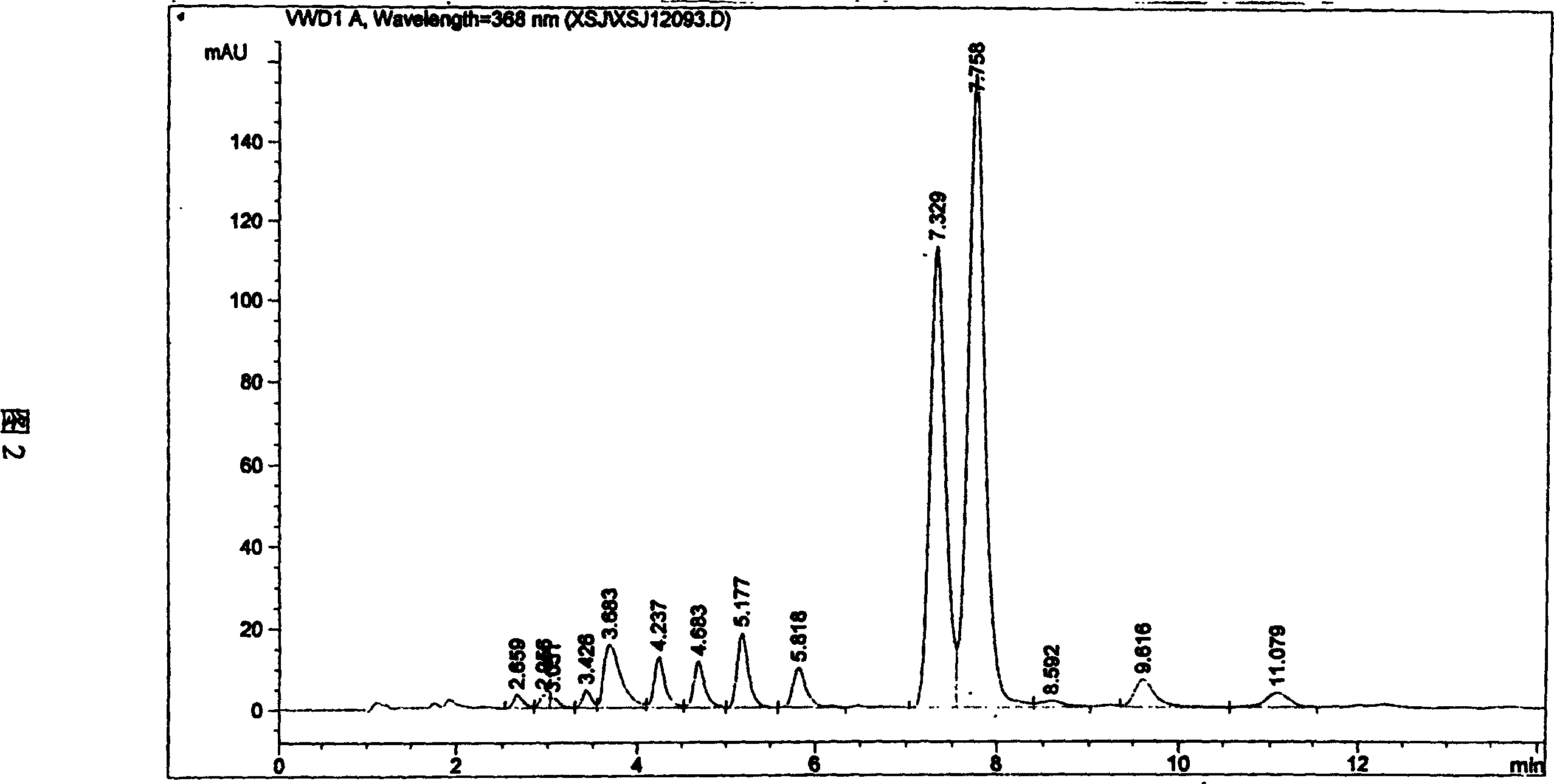
Method for separating and purifying 9-nitro camptothecin
A process for separating and purifying 9-nitro camptothecine includes such steps as nitrifying the camptothecine by concentrated sulfuric acid or nitric acid, adsorbing the acid liquid containing coarse product by macroreticular resin, desorbing to obtain coarse product, chromatography by silicon gel column, eluting by n-hexane, ethyl acetate, or their mixture, rotation evaporating to recover solvent, and vacuum drying. Its purity is 99.5-99.9%.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Method for preparing 9-nitro-20(s)-camptothecine
InactiveCN1781923ASolve non-selective problemsHigh yieldOrganic chemistryChromatographic separationNitrocamptothecin
The present invention provides a practical method of preparing 9-nitro camtothecine and the method has high selectivity and no need of chromatographic separation. The new anticancer medicine 9-nitro camtothecine is obtained in high selectivity by means of controlling the amount of the reductant, the reaction time, the reaction temperature and material feeding mode. The present invention also provides the intermediate and the destination product purifying method.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Methods of preparing and purifying 9-nitro-20-camptothecin
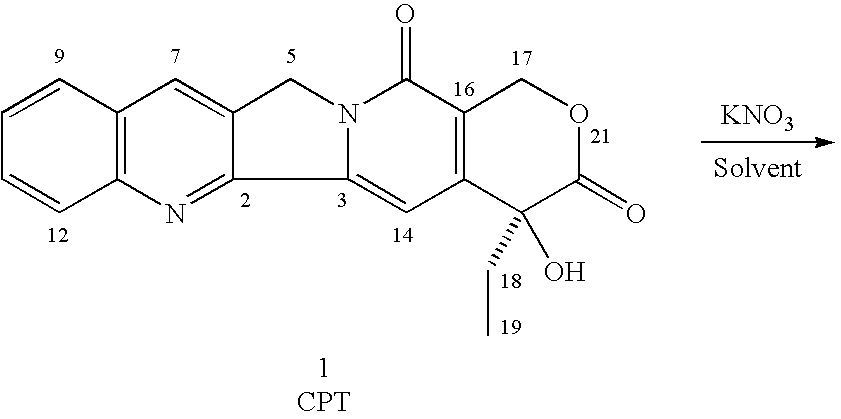
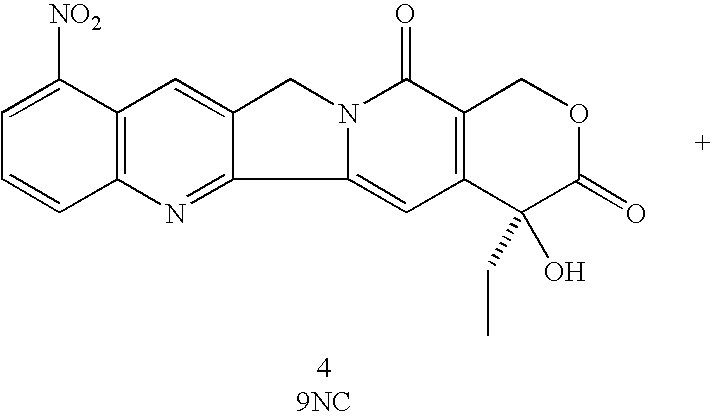
A method is disclosed for the preparation of 9-nitrocamptothecin which involves reacting 20-camptothecin with at least one inorganic nitrate salt and at least one acid effective in catalyzing the formation of a nitronium ion, where the reaction occurs at a temperature and for a time sufficient to form the 9-nitrocamptothecin. Also, methods of further purifying the 9-nitrocamptothecin by column chromatography or by reprecipitation is also disclosed.
Owner:THE STEHLIN FOUND FOR CANCER RES
Production method of 9-nitro camptothecin
The invention provides a process for preparing 9-nitrocamptothecine which consists of the following steps, oxidizing camptothecine raw material to obtain N-oxidized camptothecine, nitrating the N-oxidized camptothecine, -dioxyhexacyclic inversing flow and concentrating to obtain 9-nitro camptothecine crude product, finally carrying out recrystallization to obtain the refined product, the final yield of the 9-nitrocamptothecine can reach over 50%, the product purity can reach over 97%.
Owner:王洋 +1
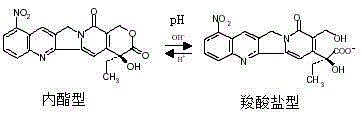
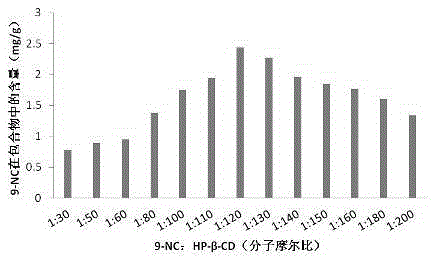
9-nitrocamptothecin-cyclodextrin inclusion compound and preparation method thereof as well as pharmaceutical composition containing cyclodextrin inclusion compound
InactiveCN103611166AHigh dissolution rateImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityFreeze-drying
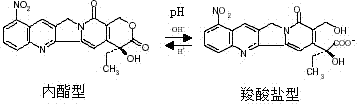
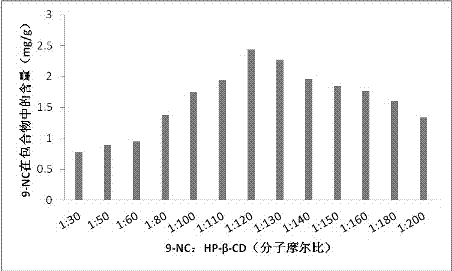
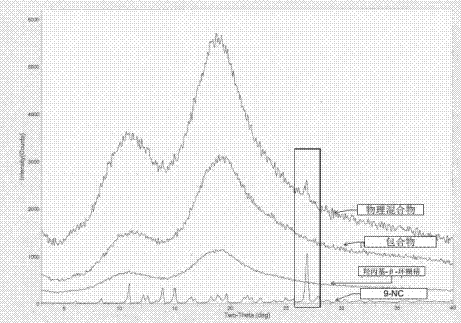
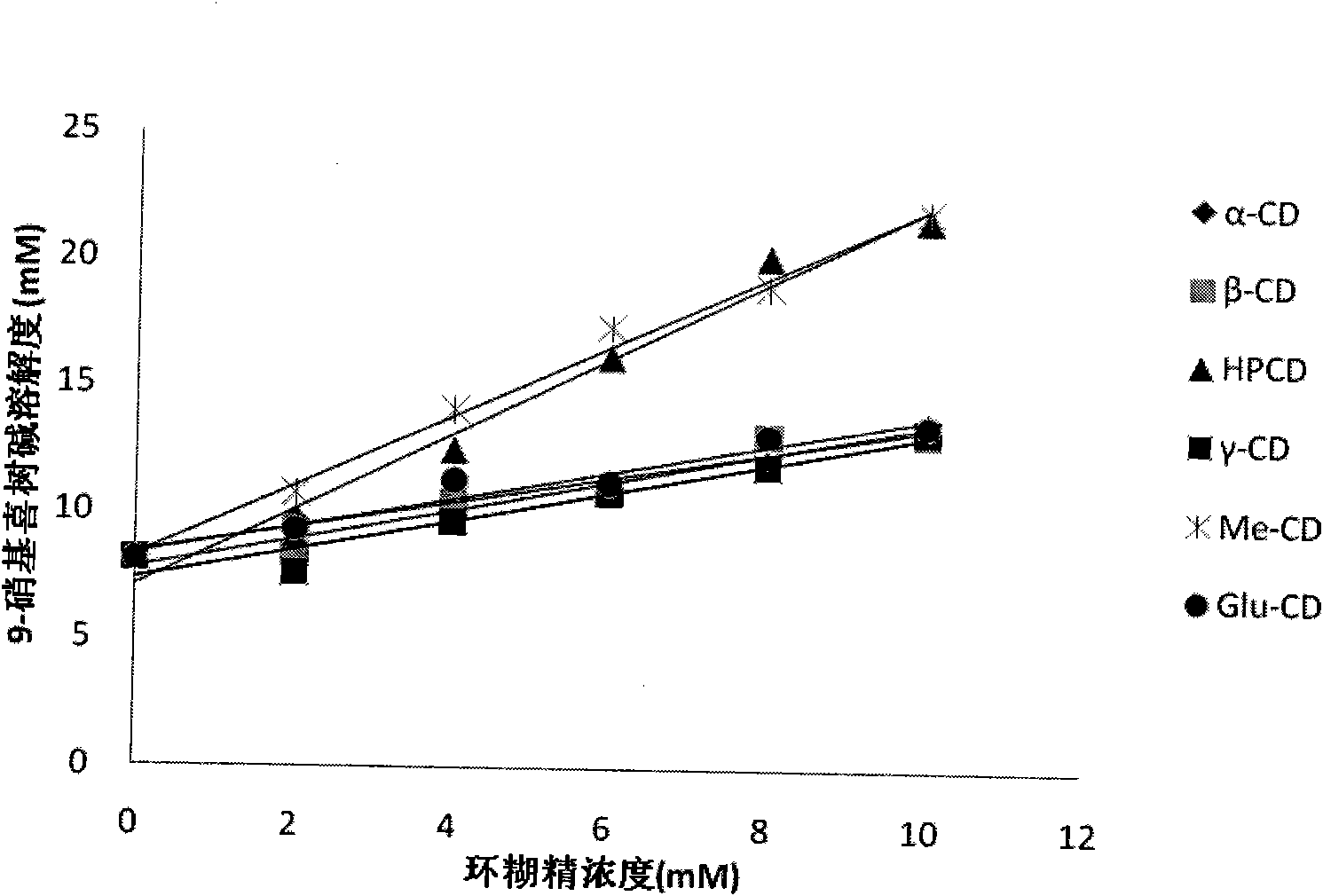
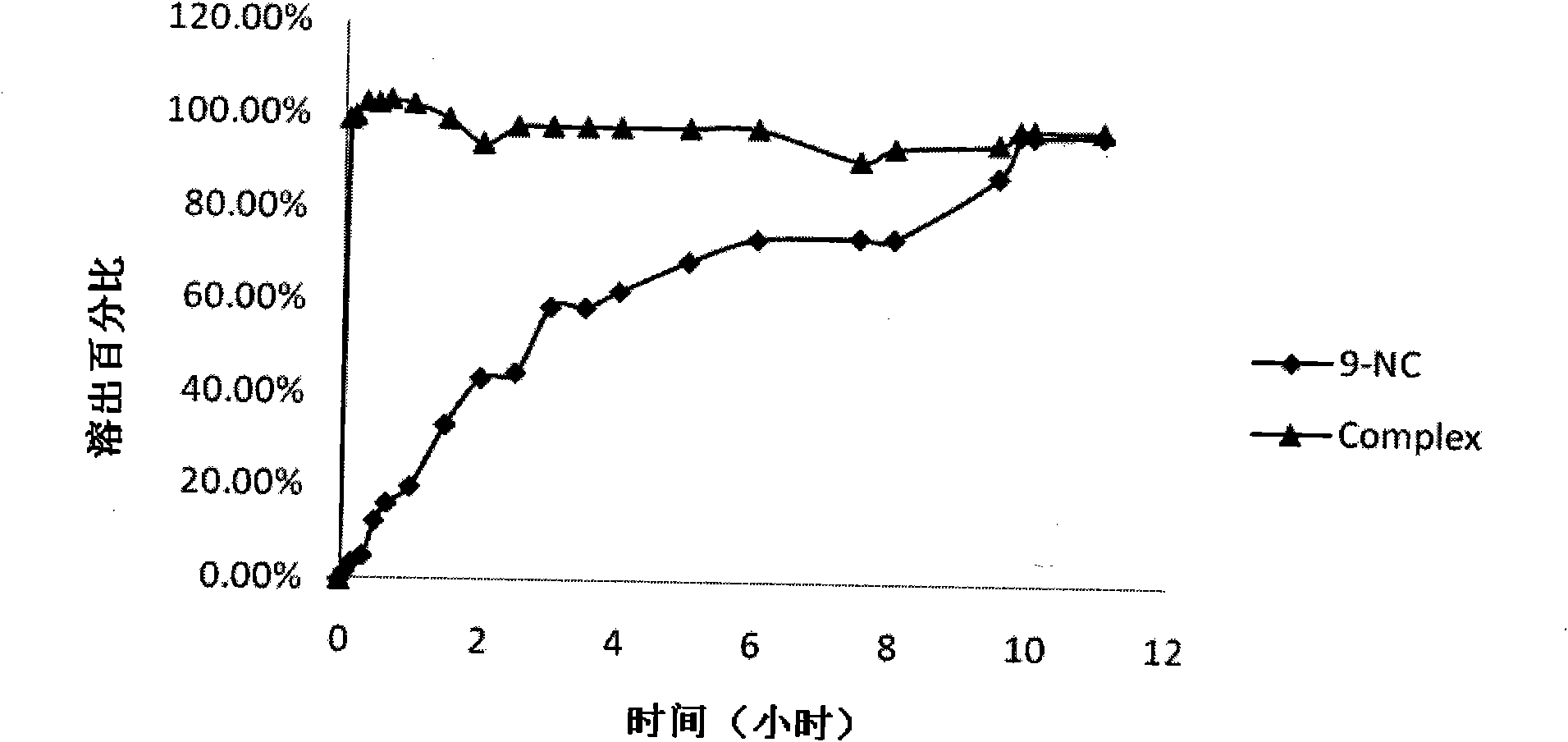
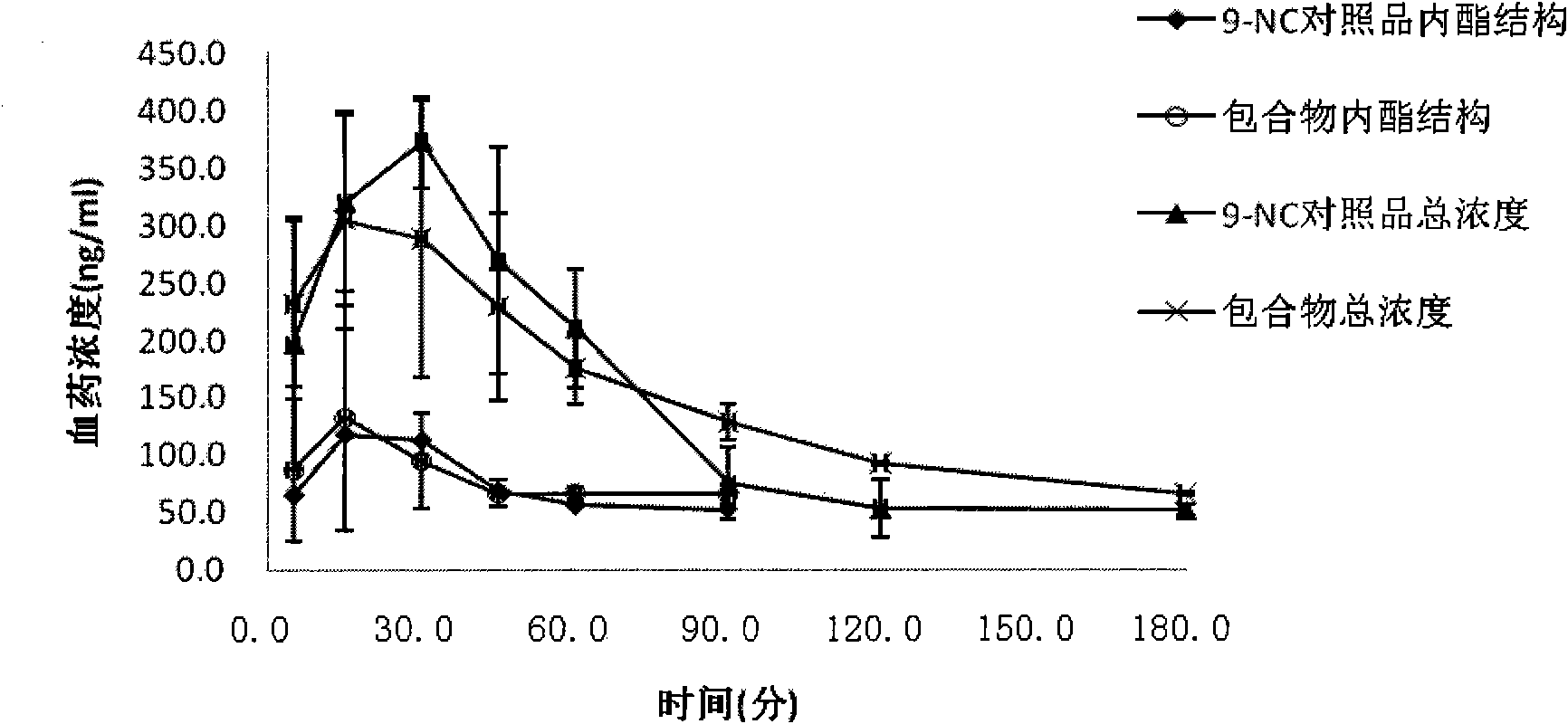
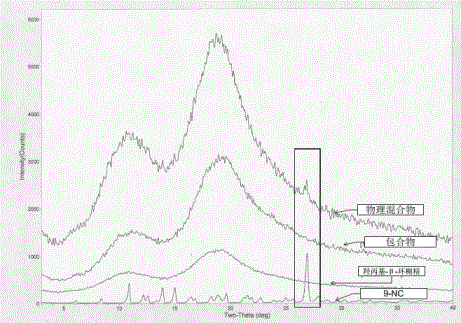
The invention provides a 9-nitrocamptothecin (NC)-cyclodextrin inclusion compound aiming at the technical problems of poor solubility and low bioavailability of 9-nitrocamptothecin in the prior art. The 9-NC-cyclodextrin inclusion compound comprises an active ingredient 9-NC of which the molecular mole ratio is 1:(30-200), an inclusion agent beta-CD or a derivant thereof. A concrete preparation method comprises the following steps: dropwise adding a 9-NC-acetone saturated solution to a CD solution; magnetically stirring at 25-60 DEG C until acetone completely volatilizes; taking supernate to carry out freeze drying after centrifuging the obtained suspension, so as to obtain 9-NC-CD inclusion compound powder. Meanwhile, the invention provides a pharmaceutical composition containing the 9-NC-CD inclusion compound and a pharmaceutically acceptable excipient. Compared with a 9-NC free drug, the solubility of the inclusion compound provided by the invention is increased by over 300 times; meanwhile, the inclusion compound displays higher lactone stability and in-vitro slow-release effect. An X-ray diffraction analysis method and a thermal analysis method indicate that 9-NC in the inclusion compound is completely included, and the reliability of the technology is further verified. Therefore, the inclusion compound can be developed into a liquid preparation, also can improve in vivo absorption of a 9-NC solid preparation, and the bioavailability is improved.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
9-nitrocamptothecin freeze-dried powder injection and preparation method thereof
InactiveCN102151248AAvoid residueImprove securityPowder deliveryOrganic active ingredientsSolubilityNitrocamptothecin
The invention belongs to the field of chemical pharmacy, and relates to a compound 9-nitrocamptothecin freeze-dried powder injection and a preparation method thereof. In the invention, 9-nitrocamptothecin is used as the active pharmaceutical ingredient, is mixed with an inclusion material, and is respectively treated by an unsaturated water solution method, a grinding method and a suspension method, thus obtaining the 9-nitrocamptothecin freeze-dried powder injection. The invention overcomes the defects of extremely low water solubility, poor chemical stability, low absolute bioavailability for oral administration, strong stimulus to the gastrointestinal tract and the like of the 9-nitrocamptothecin in the prior art; and the prepared freeze-dried powder injection can be completely dissolved in clinical application and has obviously improved bioavailability. The method has the advantages of simple operation process, low cost and no pollution; and the inclusion compound is easy to store, and is nontoxic and harmless.
Owner:FUDAN UNIV
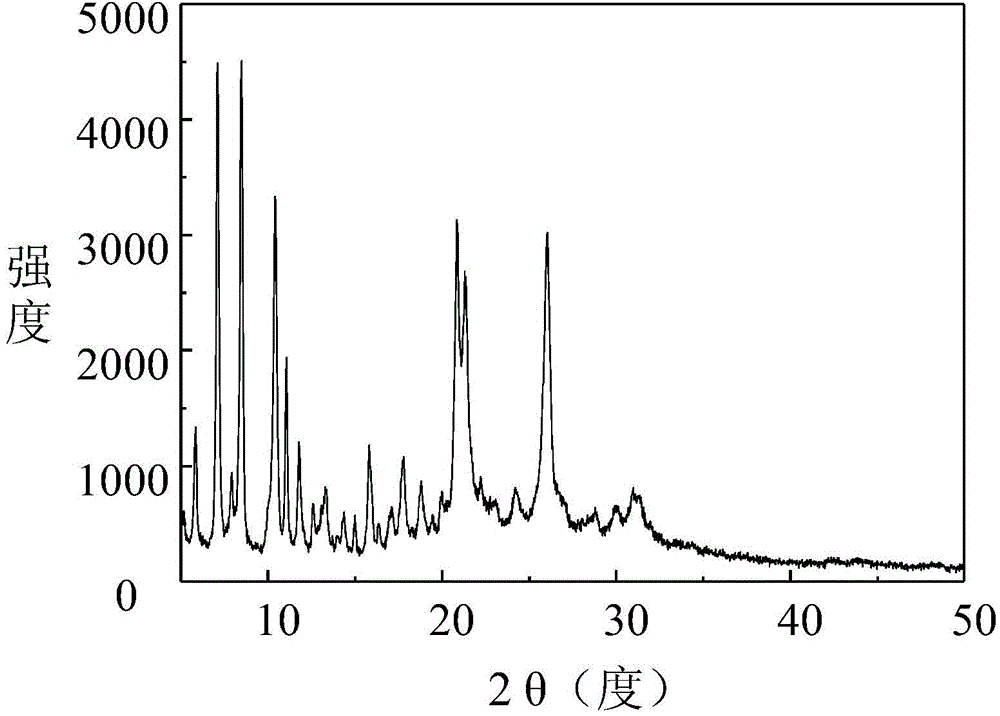
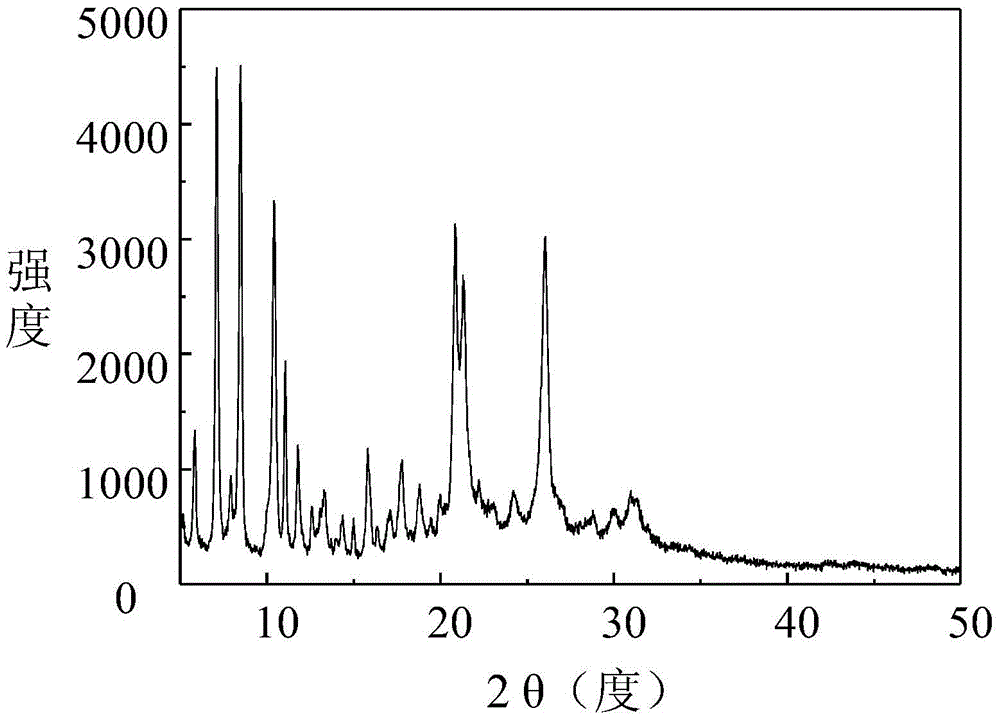
Two novel crystal forms of 9-nitrocamptothecin and preparation methods thereof
ActiveCN104628737AImprove thermal stabilitySmall particle sizeOrganic chemistry methodsBulk chemical productionSolubilitySupercritical anti solvent
The invention discloses two novel crystal forms of 9-nitrocamptothecin and preparation methods thereof. In the X-ray powder diffraction pattern with copper target as a radiation source, the crystal form I has characteristic diffraction peaks at the diffraction angle 2theta of 5.8+ / -0.1 degrees, 7.4+ / -0.1 degrees, 8.8+ / -0.1 degrees, 10.5+ / -0.1 degrees, 11.3+ / -0.2 degrees, 11.7+ / -0 degrees, 15.7+ / -0.2 degrees, 17.8+ / -0.1 degrees, 18.7+ / -0.1 degrees, 21.1+ / -0.1 degrees, 24.1+ / -0.1 degrees and 26.2+ / -0.1 degrees and the crystal form II has the characteristic diffraction peaks at the diffraction angle 2theta is 6.1+ / -0.2 degrees, 6.7+ / -0.1 degrees, 7.5+ / -0.2 degrees, 9.4+ / -0.1 degrees, 11.4+ / -0.2 degrees, 12.4+ / -0.1 degrees, 14.1+ / -0.1 degrees, 15.1+ / -0.1 degrees, 17.6+ / -0.1 degrees, 19.7+ / -0.1 degrees, 22.6+ / -0.1 degrees, 23.8+ / -0.1 degrees, 25.2+ / -0.1 degrees, 27.3+ / -0.1 degrees and 29.2+ / -0.2 degrees. The preparation methods of the two novel crystal forms of 9-nitrocamptothecin are based on a supercritical anti-solvent process. The solubility of the prepared novel crystal forms I and II of 9-nitrocamptothecin is higher than that of the existing crystal forms of 9-nitrocamptothecin, and the thermal stability of the prepared novel crystal forms I and II of 9-nitrocamptothecin is more excellent than that of the existing crystal forms of 9-nitrocamptothecin.
Owner:SOUTH CHINA UNIV OF TECH
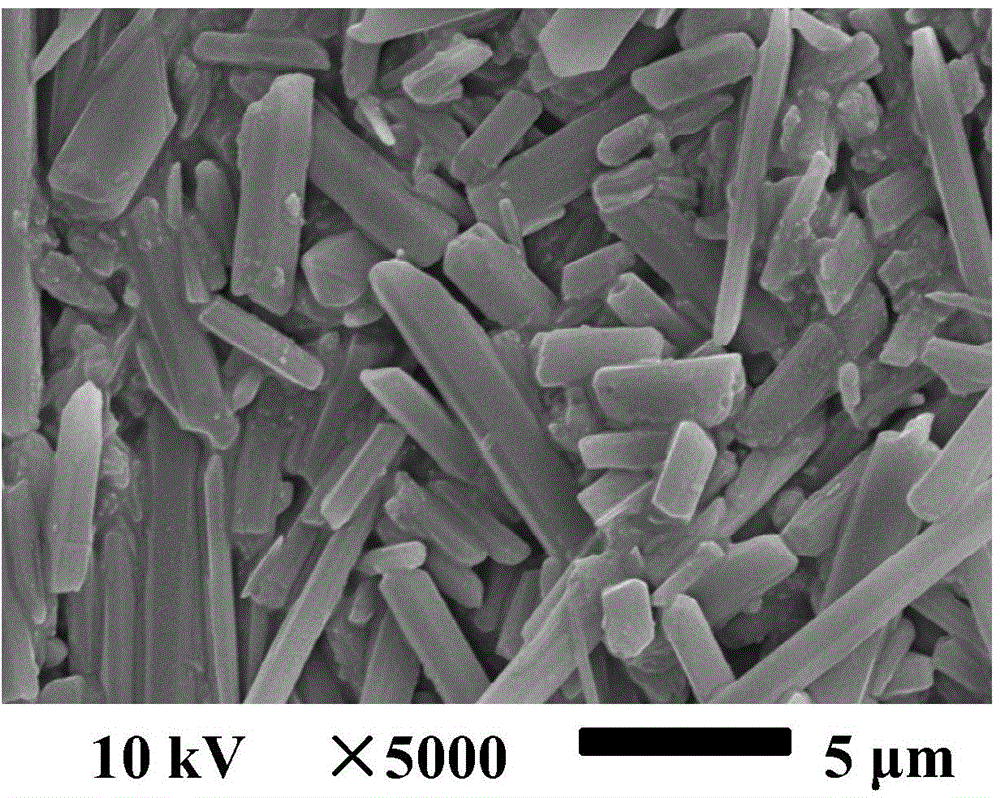
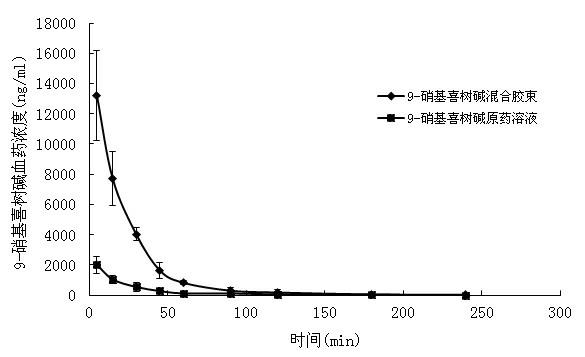
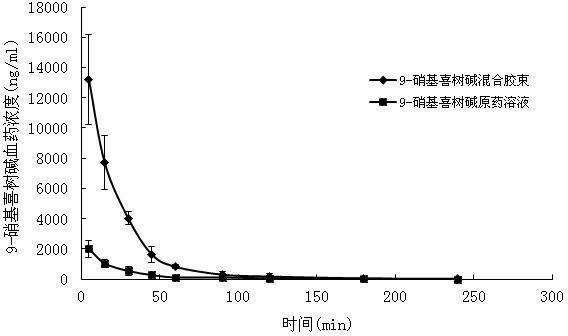
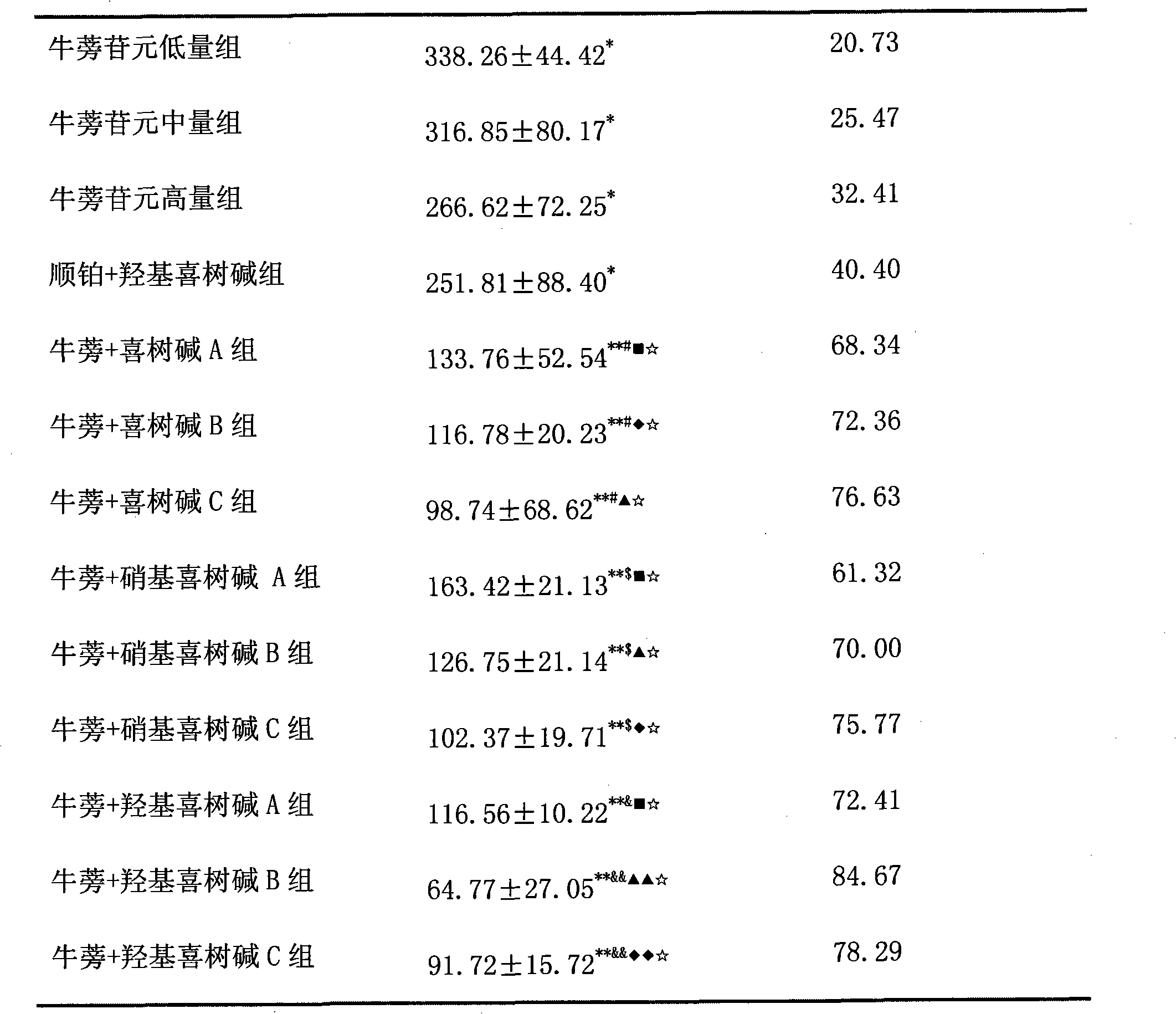
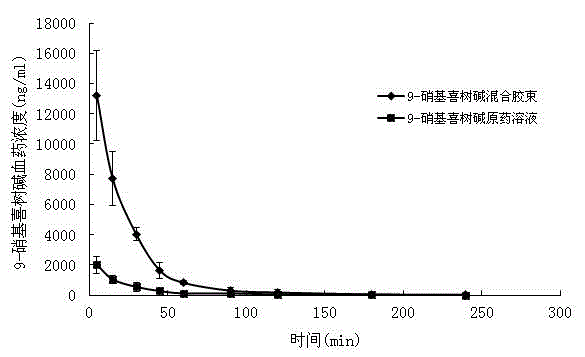
9-nitrocamptothecin mixed micelle freeze-dried powder injection and preparation method thereof
ActiveCN102552185AImprove solubilityGood storage stabilityOrganic active ingredientsPowder deliveryPhospholipinMixed micelle
The invention relates to a 9-nitrocamptothecin mixed micelle freeze-dried powder injection and a preparation method thereof. The preparation adopts 9-nitrocamptothecin as an effective component, and comprises phospholipid, bile salts, a freeze-drying protective agent, and other auxiliary materials. The ratio of 9-nitrocamptothecin, phospholipid, bile salts, the freeze-drying protective agent, andother auxiliary materials is 1:1-45:5-70:10-150:a proper amount. The freeze-dried powder injection prepared by the invention not only can effectively solve the problem of insolubility of 9-nitrocamptothecin in water, but also can significantly improve drug bioavailability. The preparation process of the invention is simple, and applicable to industrial production, and the product is good in stability, and good in curative effect.
Owner:SICHUAN UNIV
Anticancer drug composition
ActiveCN102370645BSignificant synergistic anticancer effectLow toxicityOrganic active ingredientsEmulsion deliveryNitrocamptothecinMicroemulsion
Owner:SHANDONG NEWTIME PHARMA
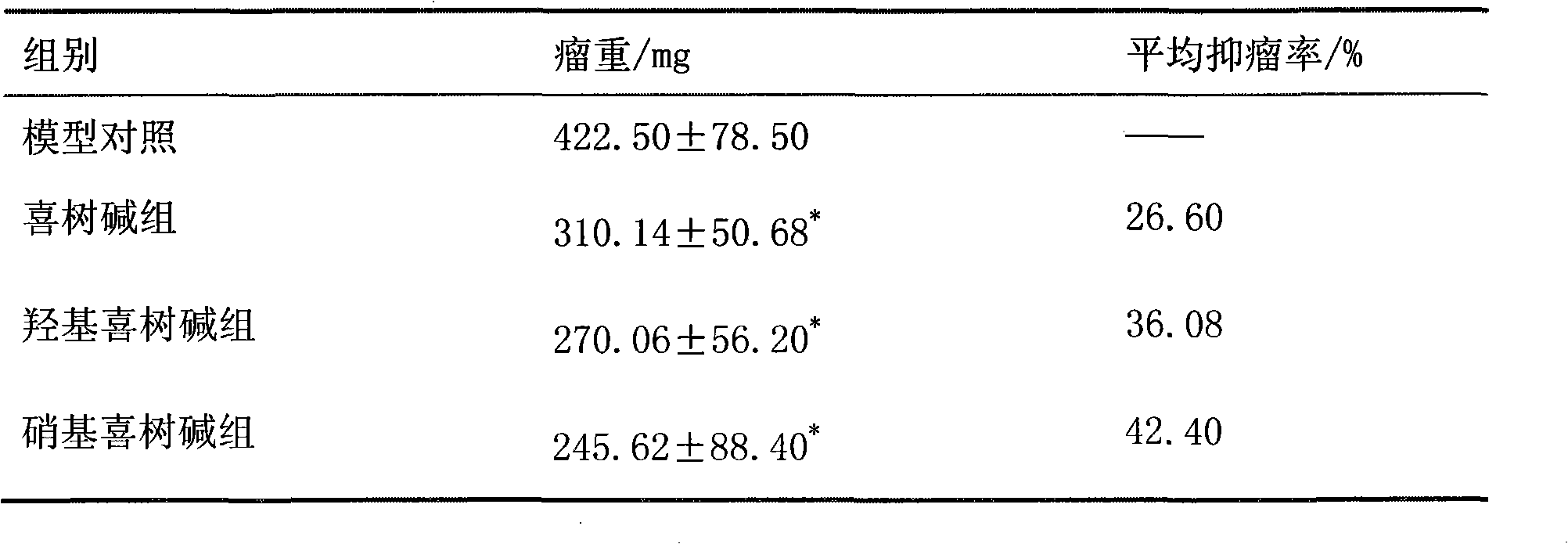
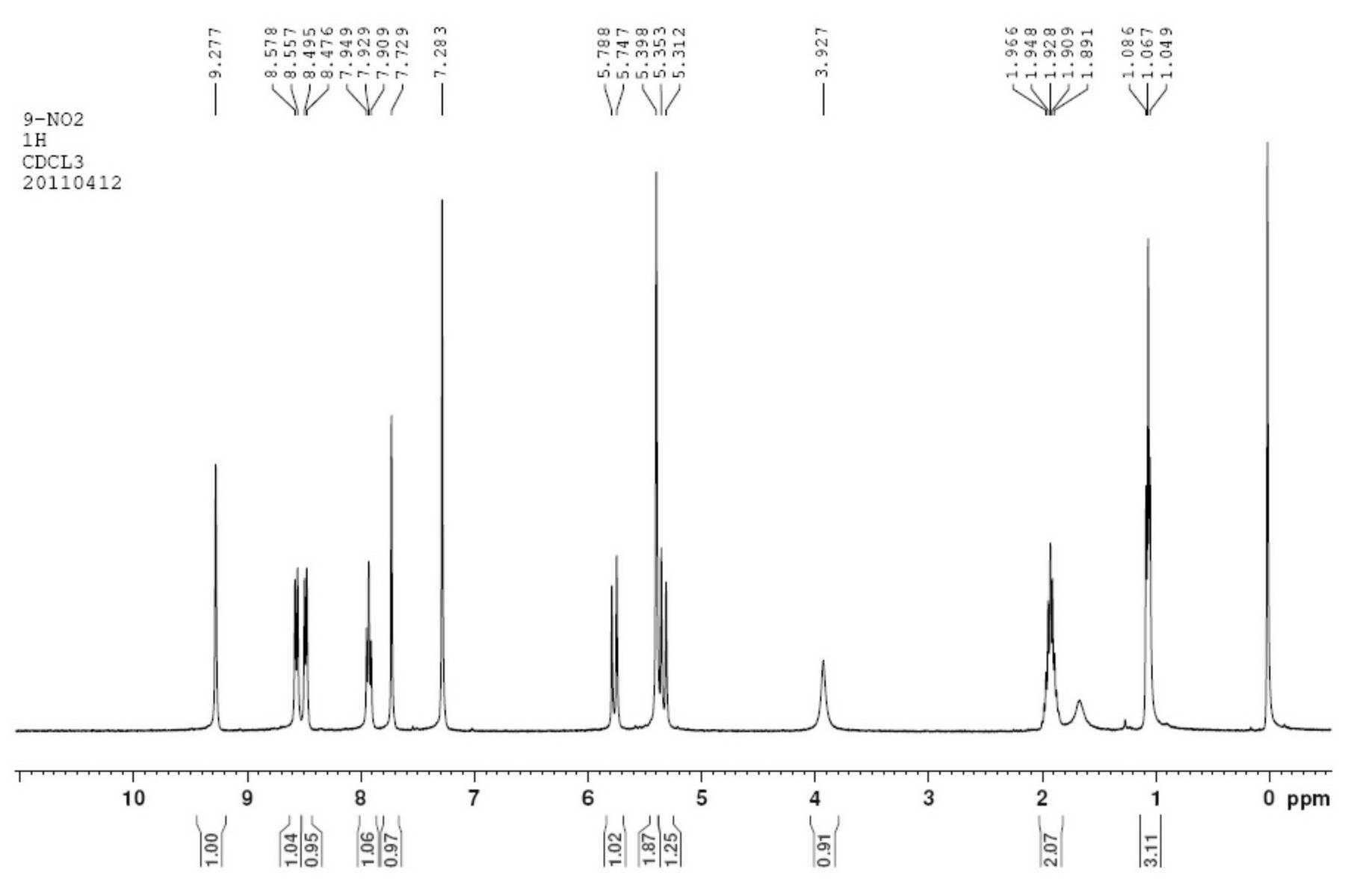
Method for synthesizing 9-nitrocamptothecin
ActiveCN102229615AUnlimited outputTo satisfy the market's needsOrganic chemistryNitrocamptothecinNitro reduction
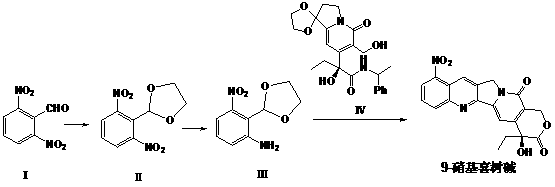
The invention relates to a method for synthesizing 9-nitrocamptothecin. The method solves the technical problem that the 9-nitrocamptothecin cannot be industrially synthesized. The method comprises the following steps of: performing single nitro reduction on 2,6-dinitrobenzaldehyde serving as a raw material to obtain 2-amino-6-nitrobenzaldehyde glycol acetal, and performing condensation reaction on the2-amino-6-nitrobenzaldehyde glycol acetal and alpha-(S)-tricyclic amide to obtain the 9-nitrocamptothecin. The method has the advantages of mild reaction condition, simplicity and convenience inoperation, low cost, high product yield and good product purity, overcomes the defects of the prior art, and is suitable for industrialized batch synthesis production.
Owner:HENAN DONGTAI PHARM
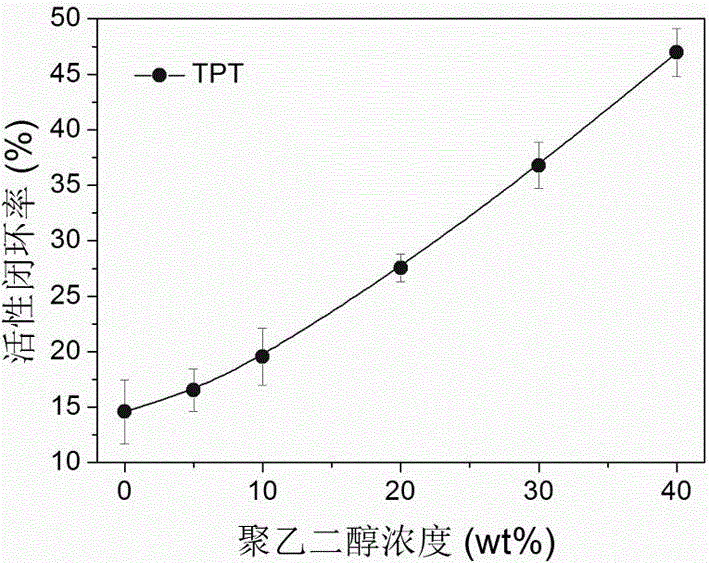
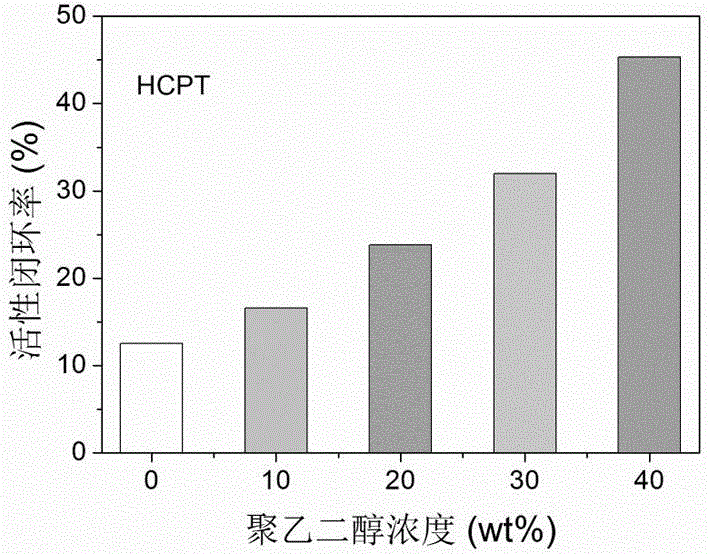
Liquid preparation for improving active ring-closing rate of camptothecin compounds and its preparation method and application
InactiveCN102961323BImprove active loop closure rateOrganic active ingredientsPharmaceutical delivery mechanismRubitecanNitrocamptothecin
The invention relates to a preparation for increasing the active closed-loop rate of camptothecin compounds through simply adding polyethylene glycol. The liquid preparation is prepared from polyethylene glycol, camptothecin derivatives and a dispersion medium, wherein the polyethylene glycol is taken as an additive, the camptothecin derivatives are taken as medicament molecules, the dispersion medium is taken as a solvent, and the main body of the dispersion medium is water. The main additive is polyethylene glycol, and the medicament molecules are one or more of camptothecin, 10-hydroxycamptothecine, 9-nitrocamptothecin, 9-aminocamptothecin, 7-ethyl-10-hydroxycamptothecine, Topotecan, Irinotecan, Rubitecan, Exatecan, Belotecan, Karenitecin, Chimmitecan, Gimatecan, and the like. Through simply adding polyethylene glycol, the preparation can significantly improve the active closed-loop rate of camptothecin drugs, enhances the medicament effect, and can effectively reduce the general reaction of medicament, and therefore, the preparation is expected to be used in the cancer treatment of different stages.
Owner:FUDAN UNIV
9-nitrocamptothecin solid dispersant and preparation method
InactiveCN1235587CDissolution rate is fastImprove solubilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityNitrocamptothecin
Owner:FUDAN UNIV +1
Methods and compositions for optimizing blood and tissue stability of camptothecin and other albumin-binding therapeutic compounds
InactiveUS7691872B2Enhance drug levelImprove biological effectBiocideAntiviralsNitrocamptothecinHIV positives
The present invention provides methods and formulations for optimizing the anti-cancer and anti-HIV activities of a camptothecin drug, including camptothecin and its related analogs including 9-aminocamptothecin and 9-nitrocamptothecin. The invention involves methodologies and formulations that limit human serum albumin-mediated reduction of the anti-cancer and anti-HIV effects of the camptothecins, and the methods and formulations provide combination therapies in which binding of the camptothecin agent to human serum albumin can be modulated by the administration of a competing agent that also binds human serum albumin. Reduced camptothecin drug binding to human serum albumin can result in elevated camptothecin free drug levels and thus improve the effectiveness of treatment regimens involving these drugs. Further agents such as methotrexate and AZT can also be used in cancer and HIV-positive patients employing camptothecin drugs.
Owner:UNIV OF KENTUCKY RES FOUND +1
Nano composition of 9-nitrocamptothecin lipoid and its preparation
A composite 9-nitrocamptothecin lipid nanoparticle used for preparing injection is prepared from 9-nitrocamptothecin, lipid and surfactant.
Owner:FUDAN UNIV
Methods of preparing and purifying 9-nitro-20-camptothecin and 9-nitro-20-camptothecin and its compositions
A method is disclosed for the preparation of 9-nitrocamptothecin which involves reacting 20-camptothecin with at least one inorganic nitrate salt and at least one acid effective in catalyzing the formation of a nitronium ion, where the reaction occurs at a temperature and for a time sufficient to form the 9-nitrocamptothecin. Also, methods of further purifying the 9-nitrocamptothecin by column chromatography or by reprecipatation is also disclosed.
Owner:THE STEHLIN FOUND FOR CANCER RES
Two new crystal forms of 9-nitrocamptothecin and preparation method thereof
ActiveCN104628737BImprove thermal stabilitySmall particle sizeOrganic chemistry methodsBulk chemical productionSupercritical anti solventNitrocamptothecin
The invention discloses two novel crystal forms of 9-nitrocamptothecin and preparation methods thereof. In the X-ray powder diffraction pattern with copper target as a radiation source, the crystal form I has characteristic diffraction peaks at the diffraction angle 2theta of 5.8+ / -0.1 degrees, 7.4+ / -0.1 degrees, 8.8+ / -0.1 degrees, 10.5+ / -0.1 degrees, 11.3+ / -0.2 degrees, 11.7+ / -0 degrees, 15.7+ / -0.2 degrees, 17.8+ / -0.1 degrees, 18.7+ / -0.1 degrees, 21.1+ / -0.1 degrees, 24.1+ / -0.1 degrees and 26.2+ / -0.1 degrees and the crystal form II has the characteristic diffraction peaks at the diffraction angle 2theta is 6.1+ / -0.2 degrees, 6.7+ / -0.1 degrees, 7.5+ / -0.2 degrees, 9.4+ / -0.1 degrees, 11.4+ / -0.2 degrees, 12.4+ / -0.1 degrees, 14.1+ / -0.1 degrees, 15.1+ / -0.1 degrees, 17.6+ / -0.1 degrees, 19.7+ / -0.1 degrees, 22.6+ / -0.1 degrees, 23.8+ / -0.1 degrees, 25.2+ / -0.1 degrees, 27.3+ / -0.1 degrees and 29.2+ / -0.2 degrees. The preparation methods of the two novel crystal forms of 9-nitrocamptothecin are based on a supercritical anti-solvent process. The solubility of the prepared novel crystal forms I and II of 9-nitrocamptothecin is higher than that of the existing crystal forms of 9-nitrocamptothecin, and the thermal stability of the prepared novel crystal forms I and II of 9-nitrocamptothecin is more excellent than that of the existing crystal forms of 9-nitrocamptothecin.
Owner:SOUTH CHINA UNIV OF TECH
Method for separating and purifying 9-nitro camptothecin
A process for separating and purifying 9-nitro camptothecine includes such steps as nitrifying the camptothecine by concentrated sulfuric acid or nitric acid, adsorbing the acid liquid containing coarse product by macroreticular resin, desorbing to obtain coarse product, chromatography by silicon gel column, eluting by n-hexane, ethyl acetate, or their mixture, rotation evaporating to recover solvent, and vacuum drying. Its purity is 99.5-99.9%.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Production method of 9-nitro camptothecin
The invention provides a process for preparing 9-nitrocamptothecine which consists of the following steps, oxidizing camptothecine raw material to obtain N-oxidized camptothecine, nitrating the N-oxidized camptothecine, -dioxyhexacyclic inversing flow and concentrating to obtain 9-nitro camptothecine crude product, finally carrying out recrystallization to obtain the refined product, the final yield of the 9-nitrocamptothecine can reach over 50%, the product purity can reach over 97%.
Owner:王洋 +1
9-nitrocamptothecin mixed micelle freeze-dried powder injection and preparation method thereof
ActiveCN102552185BImprove solubilityGood storage stabilityOrganic active ingredientsPowder deliveryPhospholipinMixed micelle
The invention relates to a 9-nitrocamptothecin mixed micelle freeze-dried powder injection and a preparation method thereof. The preparation adopts 9-nitrocamptothecin as an effective component, and comprises phospholipid, bile salts, a freeze-drying protective agent, and other auxiliary materials. The ratio of 9-nitrocamptothecin, phospholipid, bile salts, the freeze-drying protective agent, and other auxiliary materials is 1:1-45:5-70:10-150:a proper amount. The freeze-dried powder injection prepared by the invention not only can effectively solve the problem of insolubility of 9-nitrocamptothecin in water, but also can significantly improve drug bioavailability. The preparation process of the invention is simple, and applicable to industrial production, and the product is good in stability, and good in curative effect.
Owner:SICHUAN UNIV
Method for synthesizing 9-nitrocamptothecin
ActiveCN102229615BUnlimited outputTo satisfy the market's needsOrganic chemistryState of artNitrocamptothecin
Owner:HENAN DONGTAI PHARM
9-nitrocamptothecin-cyclodextrin inclusion compound, its preparation method and pharmaceutical composition containing the inclusion compound
InactiveCN103611166BHigh dissolution rateImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityNitrocamptothecin
The invention provides a 9-nitrocamptothecin (NC)-cyclodextrin inclusion compound aiming at the technical problems of poor solubility and low bioavailability of 9-nitrocamptothecin in the prior art. The 9-NC-cyclodextrin inclusion compound comprises an active ingredient 9-NC of which the molecular mole ratio is 1:(30-200), an inclusion agent beta-CD or a derivant thereof. A concrete preparation method comprises the following steps: dropwise adding a 9-NC-acetone saturated solution to a CD solution; magnetically stirring at 25-60 DEG C until acetone completely volatilizes; taking supernate to carry out freeze drying after centrifuging the obtained suspension, so as to obtain 9-NC-CD inclusion compound powder. Meanwhile, the invention provides a pharmaceutical composition containing the 9-NC-CD inclusion compound and a pharmaceutically acceptable excipient. Compared with a 9-NC free drug, the solubility of the inclusion compound provided by the invention is increased by over 300 times; meanwhile, the inclusion compound displays higher lactone stability and in-vitro slow-release effect. An X-ray diffraction analysis method and a thermal analysis method indicate that 9-NC in the inclusion compound is completely included, and the reliability of the technology is further verified. Therefore, the inclusion compound can be developed into a liquid preparation, also can improve in vivo absorption of a 9-NC solid preparation, and the bioavailability is improved.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Camptothecinum derivative emulsion agent and preparation thereof
InactiveCN101259103BImprove stabilitySolve the problem of insoluble in waterOrganic active ingredientsEmulsion deliveryEmulsionDrug content
The invention provides an emulsion of camptothecin derivative which comprises the components by weight: 0.01 to 0.1 percent of 10-methyl-9 nitryl camptothecin, 5 to 20 percent of oil for injection, 1-4 percent of emulsifier, 1-4 percent of co-emulsifier, 0 to 60 percent of solubilizer, 2 to 3 percent of glycerin of isotonizing agent and Vitamin E and the rest is water for injection; the sum of the percentages of all components is 100 percent. The emulsifier and a co-surfactant are dissolved in the oil for injection, heated and added with the solubilizer and 10-methyl-9 nitryl camptothecin to be dissolved, ant then added with the glycerin of isotonizing agent and the water for injection and made into primary emulsion by means of high speed homogenization or ultrasound, then further made into the emulsion by means of high pressure homogenization or micro jet. The prepared emulsion of the invention has uniform particle diameter and high content of drug and the appearance of the emulsion is a milky white or blue opalescence homogeneous system; meanwhile, compared with other preparation methods, the micro jet technique is adopted by the invention to prepare the emulsion, so as to ensure less loss of drug and better stability of the emulsion. The emulsion of camptothecin derivative of the invention has reasonable design and easy operation, which is suitable for practical use.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com