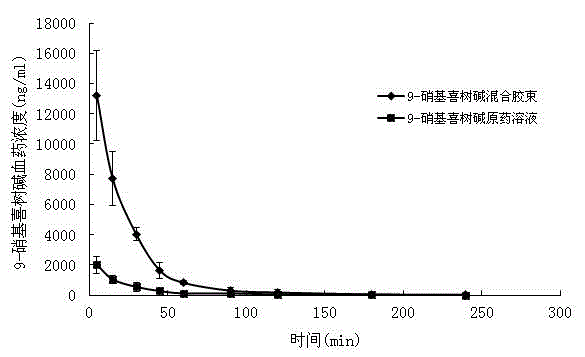
9-nitrocamptothecin mixed micelle freeze-dried powder injection and preparation method thereof
A technology of nitrocamptothecin and freeze-dried powder injection, which is applied in the field of mixed micellar freeze-dried powder injection of 9-nitrocamptothecin and its preparation, which can solve the problems of poor solubility and low oral bioavailability, etc. Achieve the effects of alleviating toxicity, facilitating clinical use, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Weigh 60mg of soybean lecithin and place it in a 100ml round bottom flask, add 10ml of ethanol solution of sodium deoxycholate (10mg / ml), fully dissolve to obtain stock solution 1, dissolve 6mg of 9-nitrocamptothecin In 50ml of acetone, the stock solution 2 was obtained, and the two were mixed evenly, and the organic solvent was spin-dried in a water bath at 40°C, protected from light and depressurized, to obtain a yellow film, and then added 2.0ml of normal saline to disperse evenly to obtain a drug-containing mixed micellar solution , adjust the pH=6.0 with sodium hydroxide and hydrochloric acid, centrifuge at 13000 rpm / min for 5 min, and take the supernatant to obtain 9-nitrocamptothecin mixed micelles injection.
[0047] Sterile filter with a 0.22 μm microporous membrane filter. Put it into a vial, add 10% glucose as a lyoprotectant, pre-freeze at 40°C for 4h, and then freeze-dry (-50°C, 24h).
[0048] Take the freeze-dried finished product, observe that the appear...
Embodiment 2
[0050] Accurately weigh 85mg of egg yolk lecithin into a 100ml round bottom flask, add 11ml of ethanol solution of sodium glycocholate (10mg / ml), fully dissolve to obtain stock solution 1, dissolve 2mg of 9-nitrocamptothecin In 25ml of methanol, the stock solution 2 was obtained, and the two were mixed evenly, and the organic solvent was spin-dried in a water bath at 40°C, protected from light and decompressed, to obtain a yellow film, and then added 2.0ml of water for injection to disperse evenly to obtain a drug-containing mixed micelle solution, adjusted to pH=5.5 with sodium hydroxide and hydrochloric acid, centrifuged at 13,000 rpm / min for 5 minutes, and the supernatant was taken to obtain 9-nitrocamptothecin mixed micelles injection.
[0051] Sterile filter with a 0.22 μm microporous membrane filter. Put it into a vial, add 5% sucrose as a lyoprotectant, pre-freeze at 40°C for 4h, and then freeze-dry (-50°C, 24h).
[0052] Take the freeze-dried finished product, observe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com