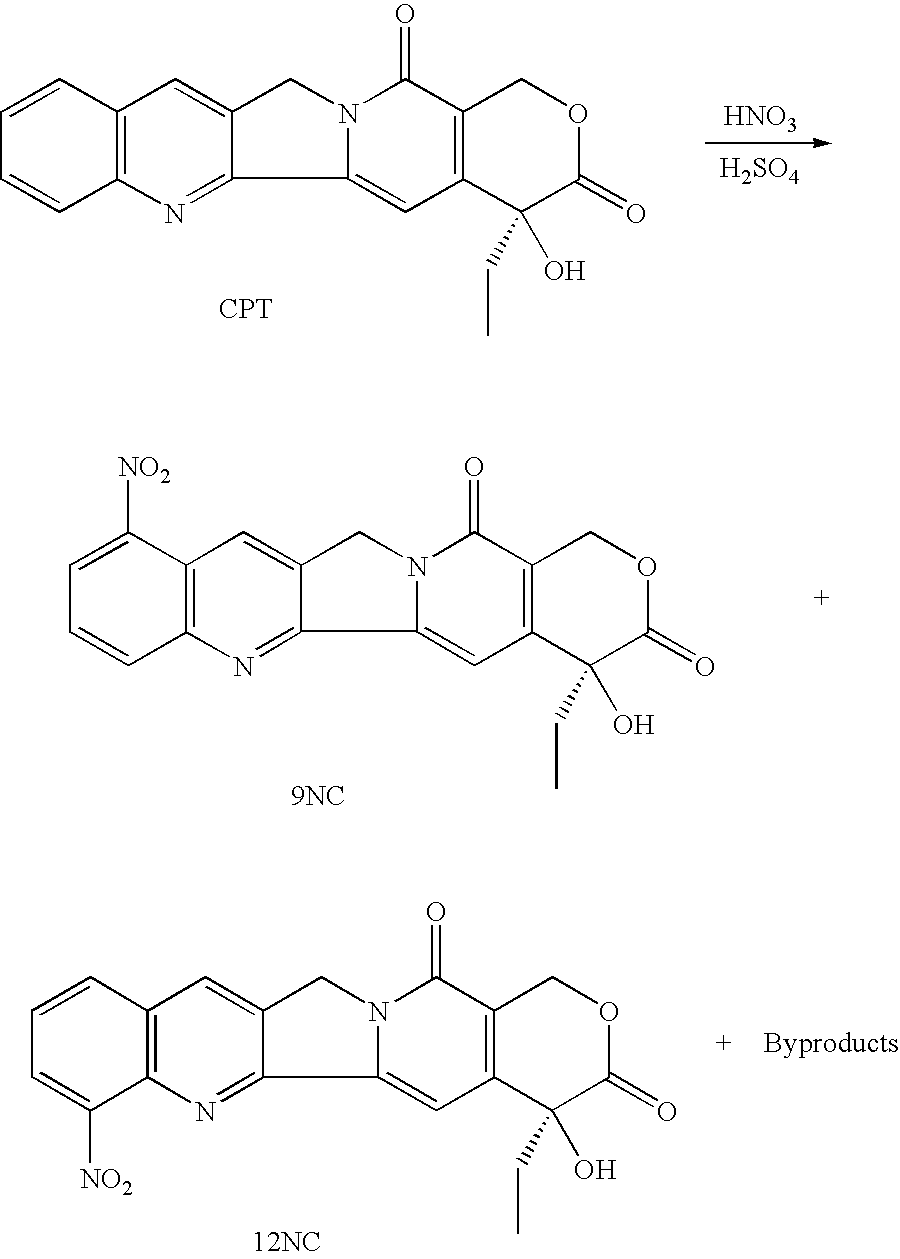
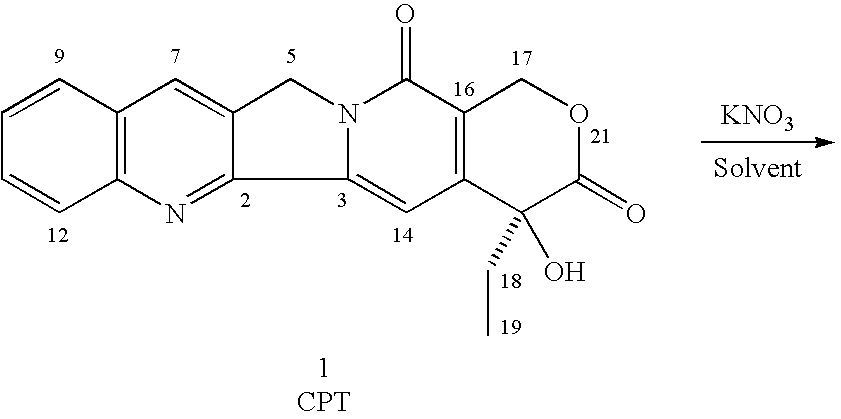
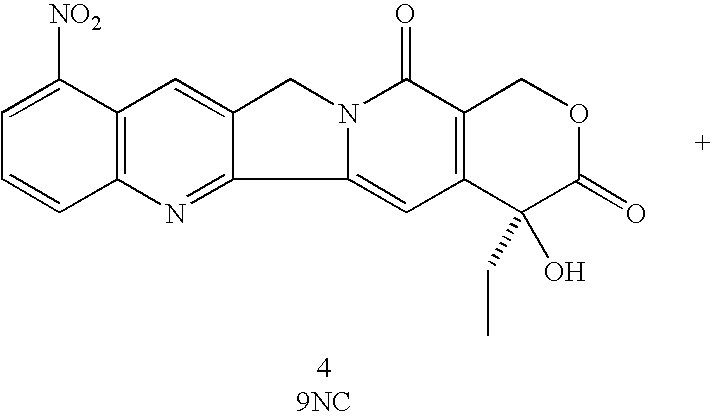
Methods of preparing and purifying 9-nitro-20-camptothecin
a technology of nitro-20-camptothecin and purification method, which is applied in the field of preparing and purifying 9nitro-20-camptothecin, can solve the problems of further decrease of 9nc yield and 60% yield, and achieve the effect of increasing the yield of 9-nitrocamptothecin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reaction of Camptothecin With KNO.sub.3 in Acetic Acid
To 30 ml acetic acid in a 100 ml round-bottomed flask equipped with a magnetic stirrer were added 0.50 g (0.0014 mol) 20(S)-camptothecin and 0.50 g KNO.sub.3 (0.0050 mol). The mixture was stirred at room temperature for 24 hours and poured onto 500 ml ice-water in several portions while stirring. The suspension was extracted three times with 200 ml methylene chloride each time (200 ml.times.3). The combined extracts were dried over 200 g anhydrous sodium sulfate for 6 hours. After removal of methylene chloride by a rotary evaporator, the residue was refluxed in petroleum ether for 4 hours. After filtration and drying in air for 4 hours the product obtained was a gray white powder. HPLC analysis showed no nitration indicative of camptothecin with KNO.sub.3 in acetic acid. The starting camptothecin was recovered 100% (Table 1).
example 2
Reaction of Camptothecin With KNO.sub.3 in Acetic Anhydride
Camptothecin was nitrated and worked-up in the same manner as the reaction in Example 1. The HPLC analysis of the reaction product showed 100% recovery of the starting camptothecin (Table 1).
example 3
Reaction of Camptothecin With KNO.sub.3 in Trifluoroacetic Anhydride
Camptothecin was nitrated and work-up in the same manner as in Example 1. The HPLC analysis data for the reaction mixture is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com