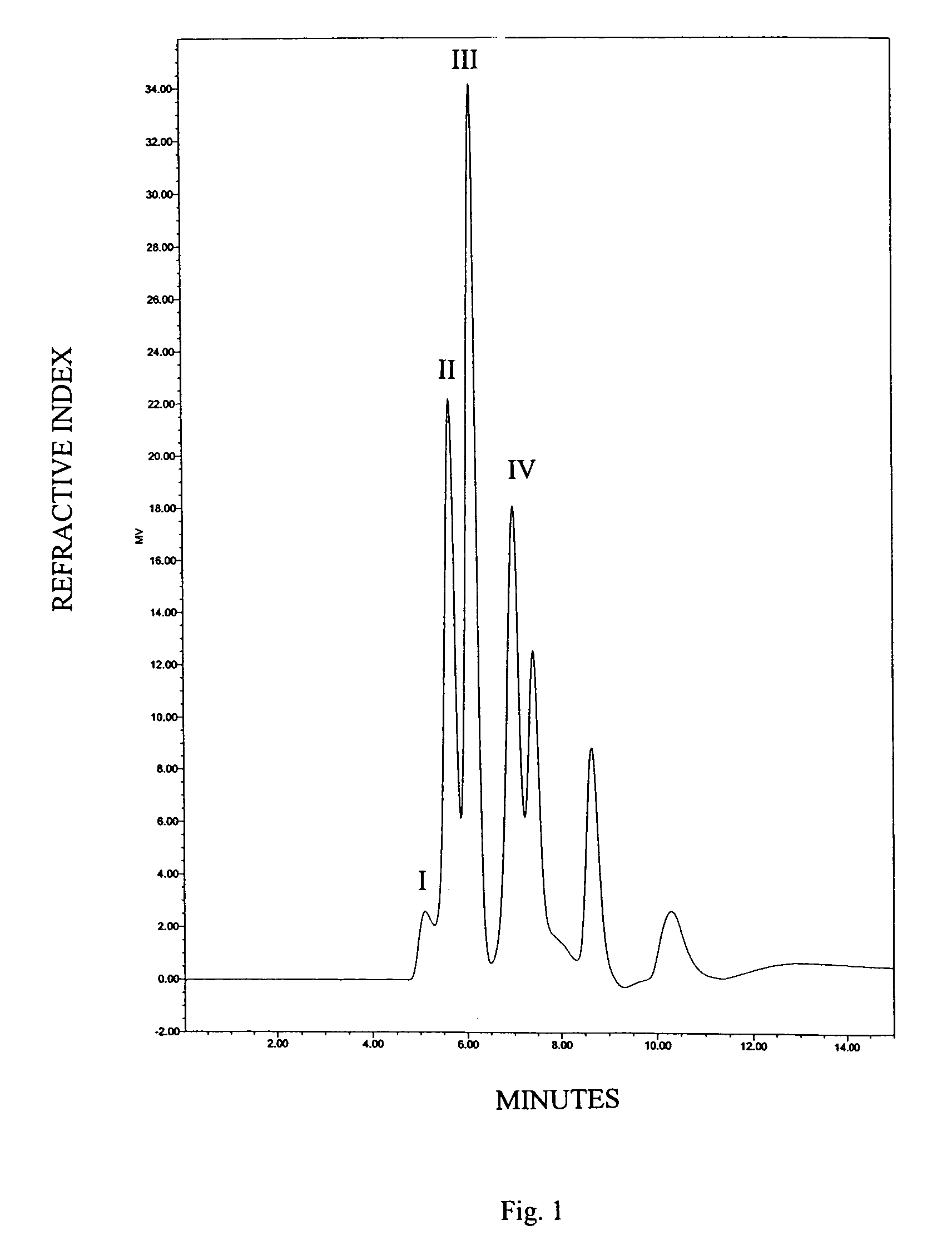
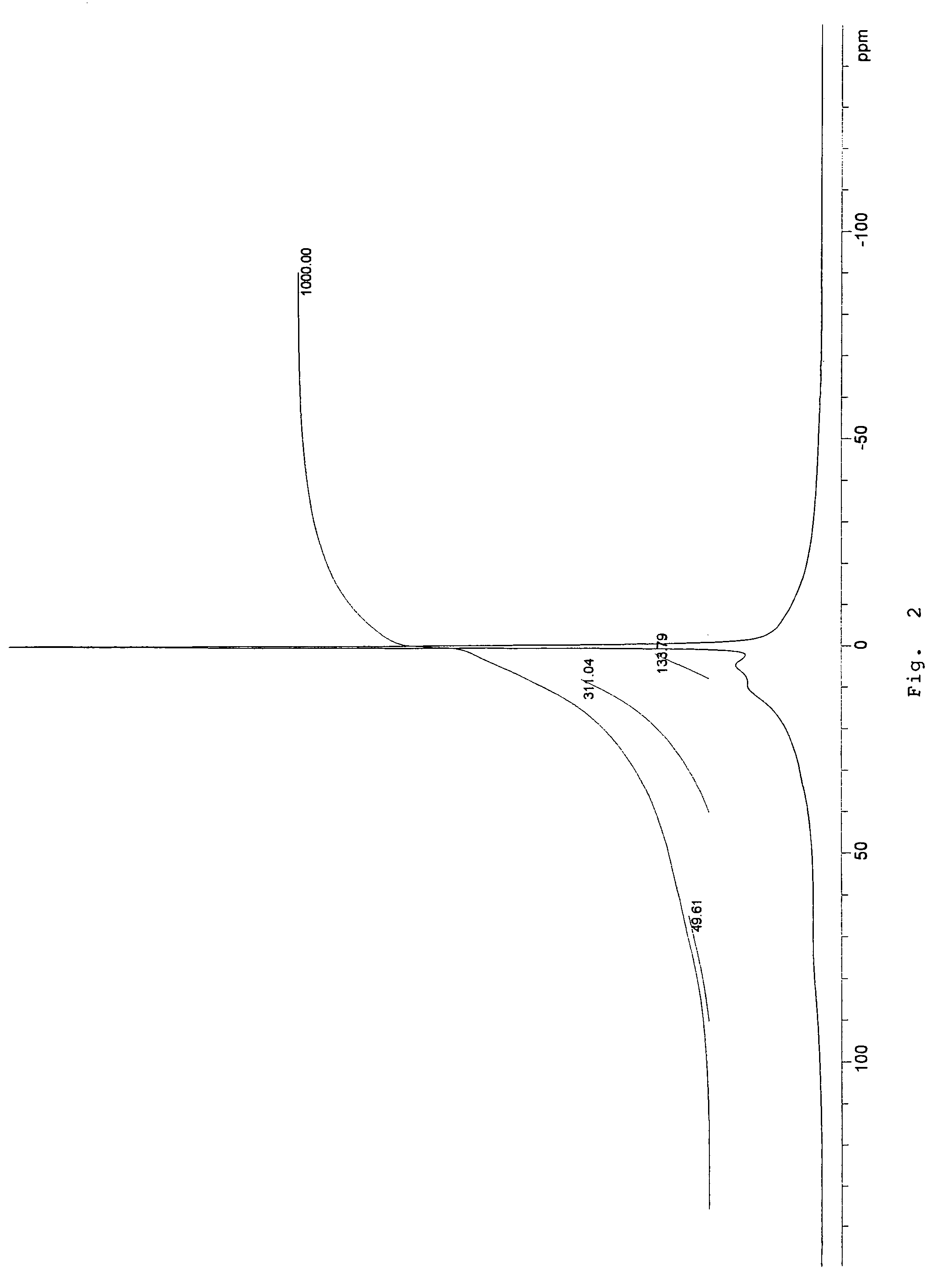
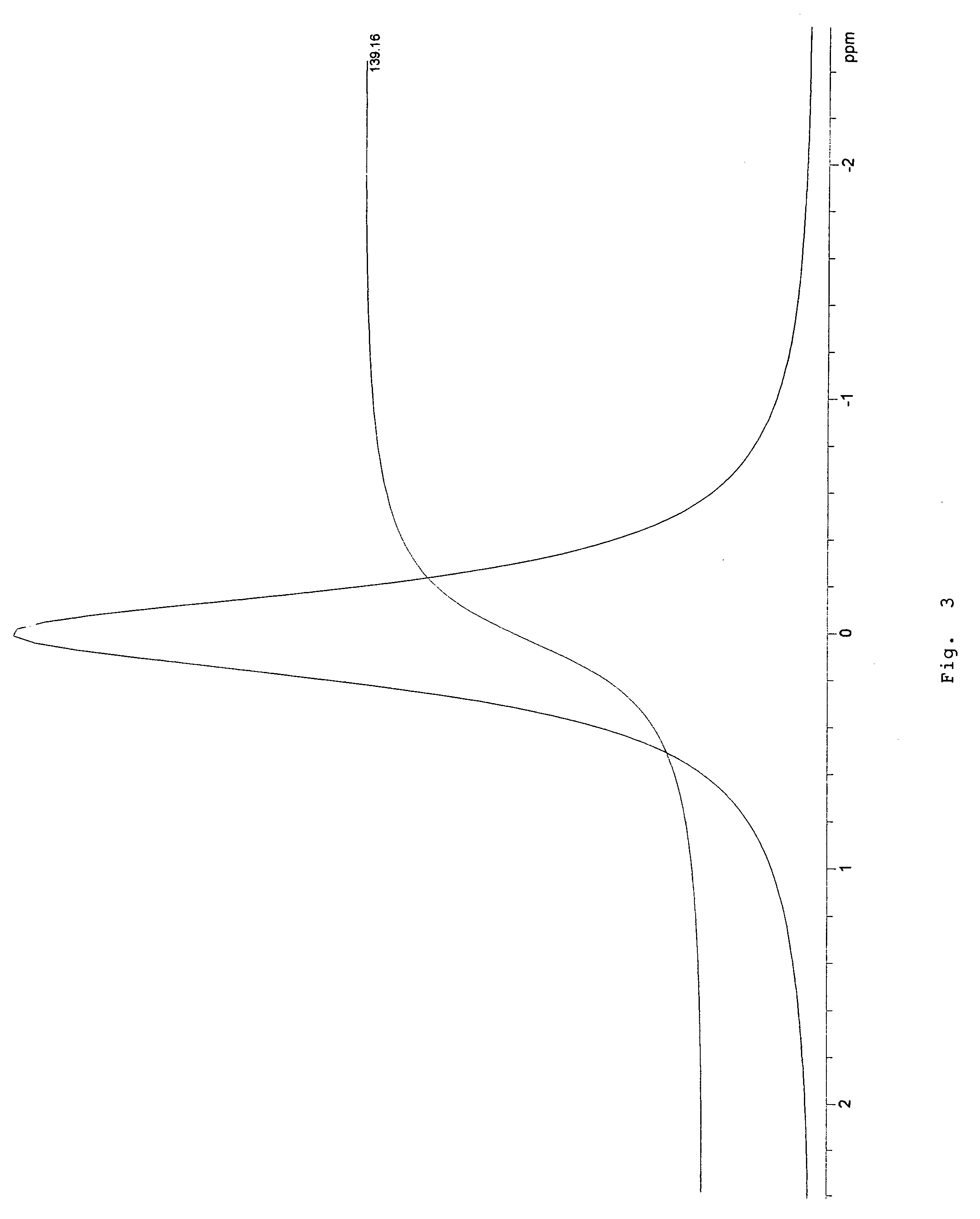
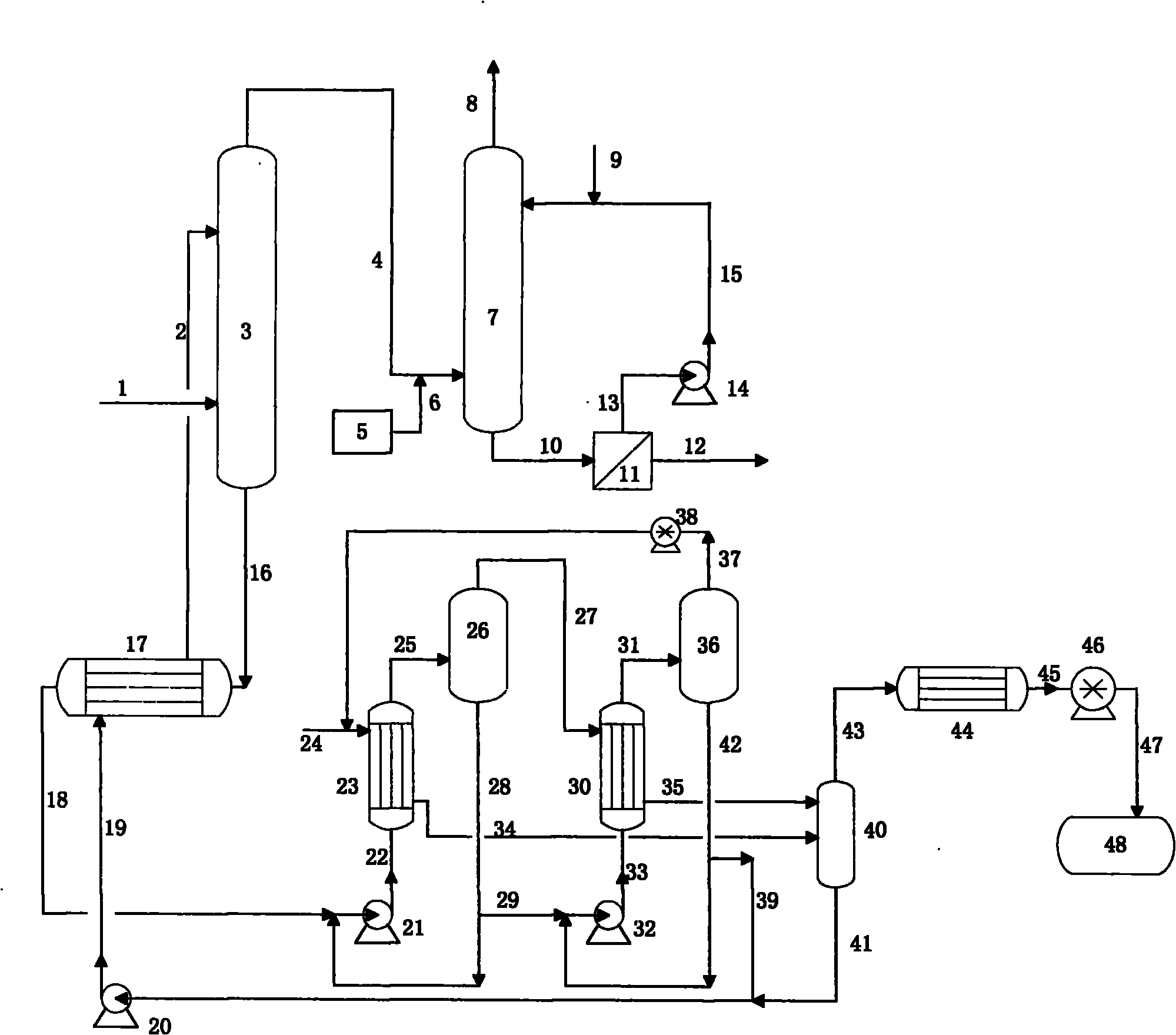
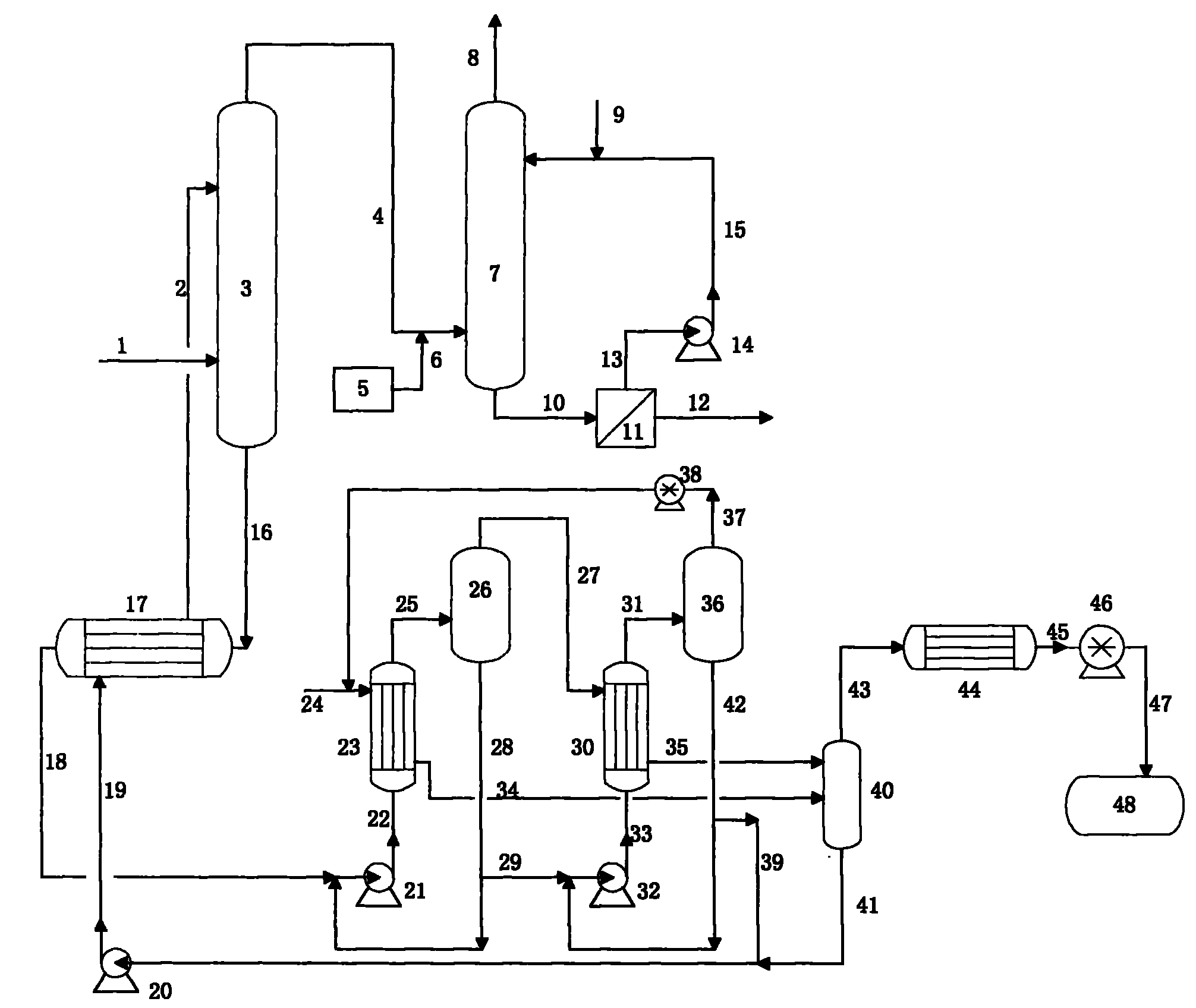
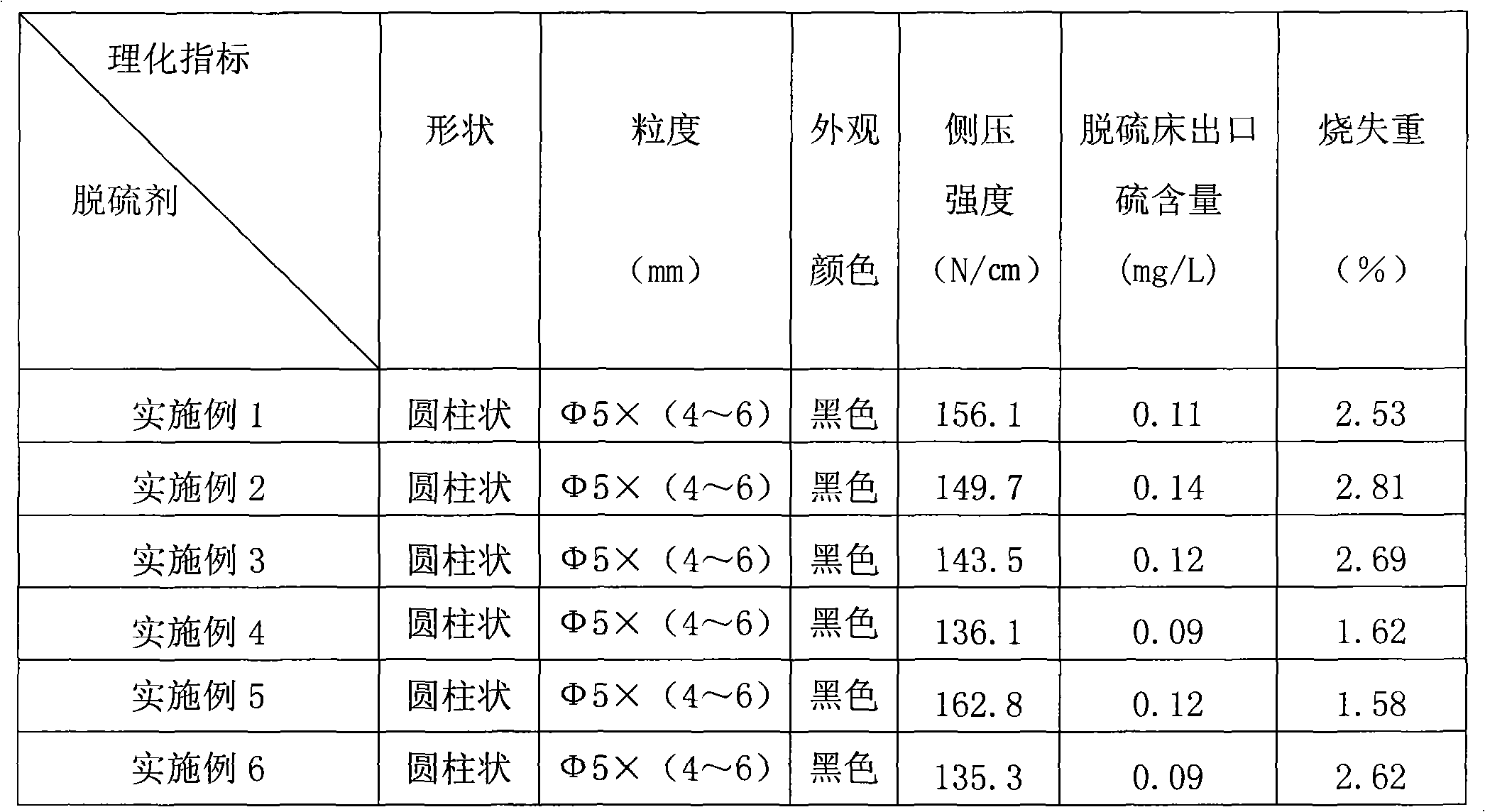
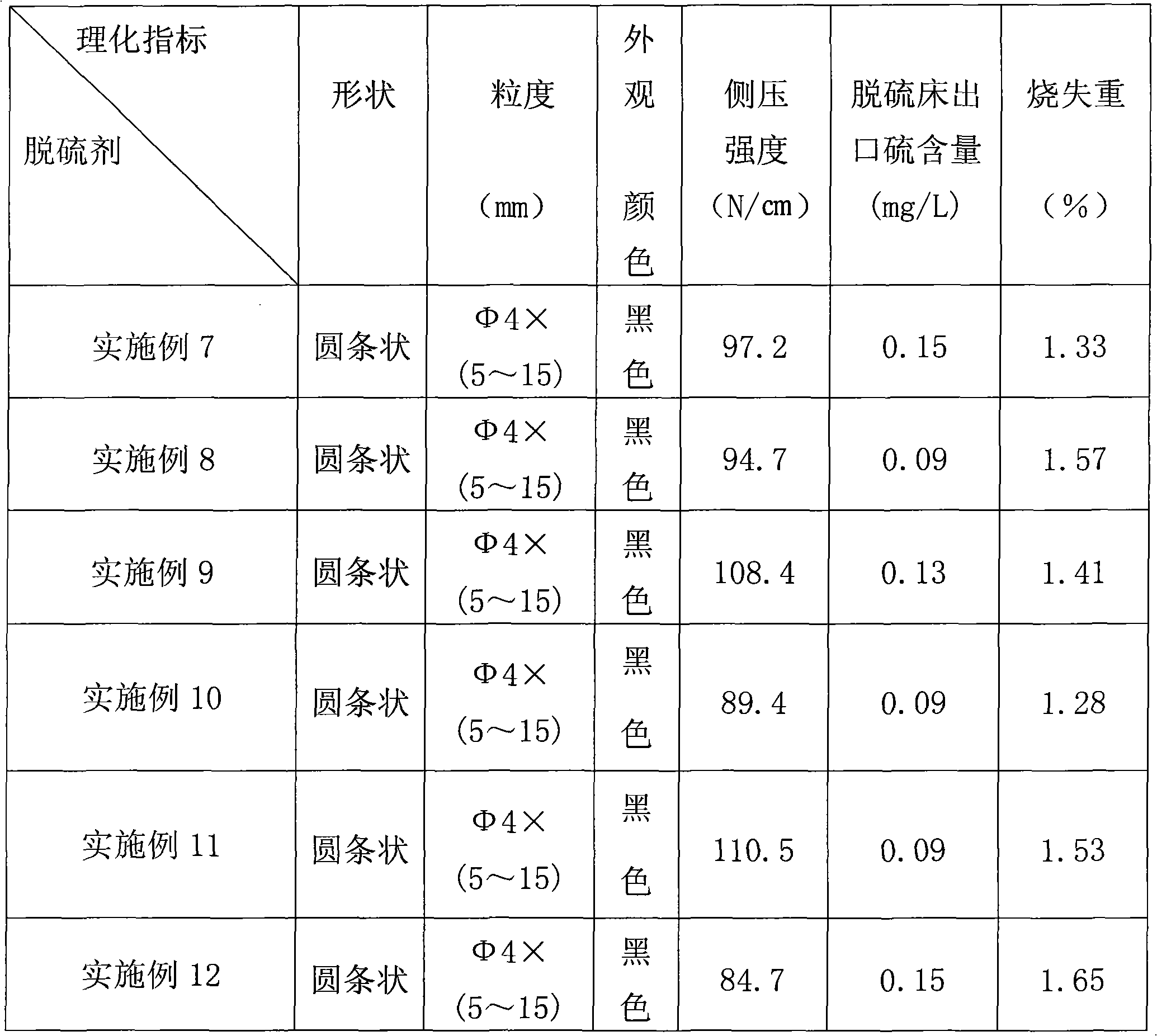
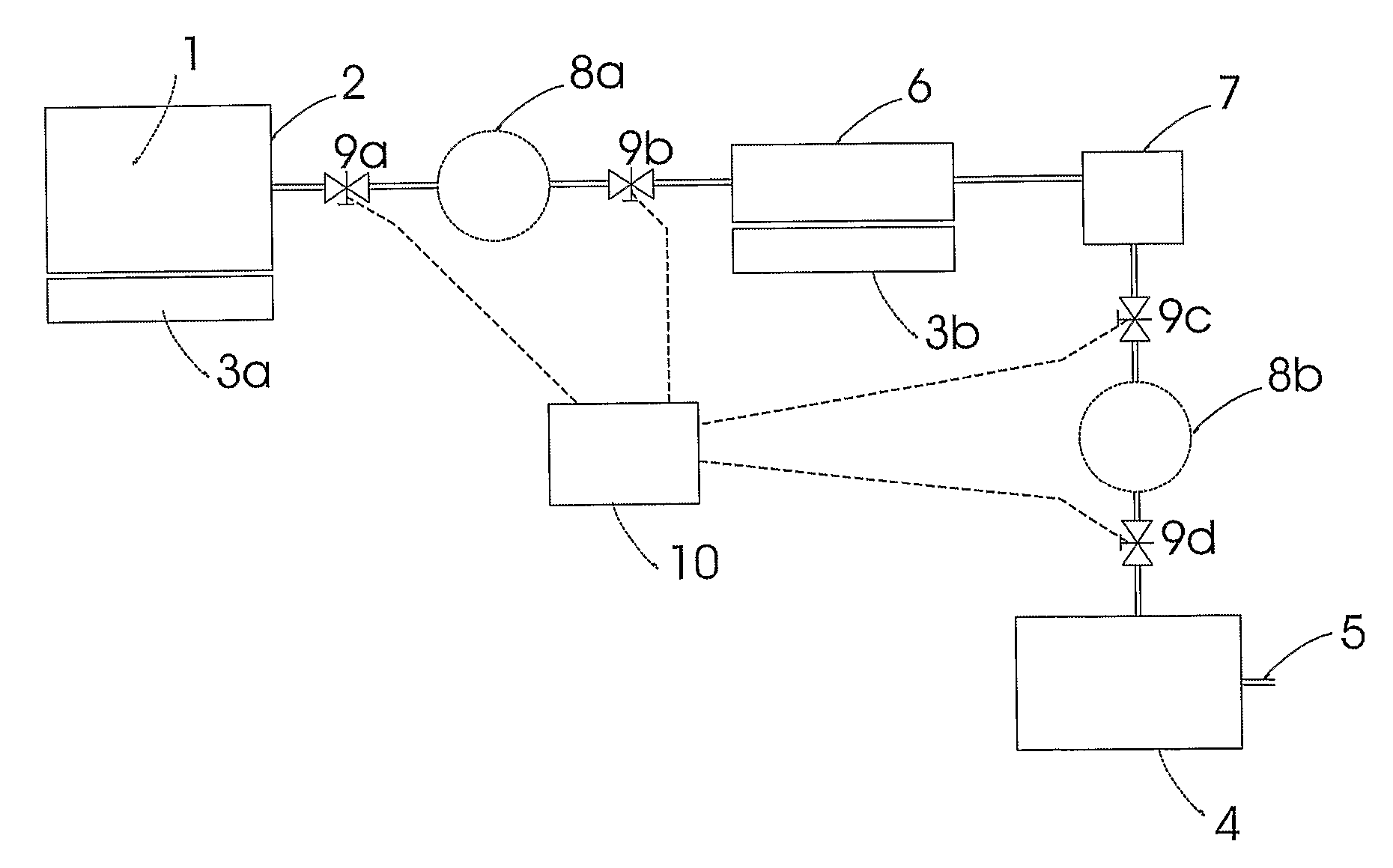
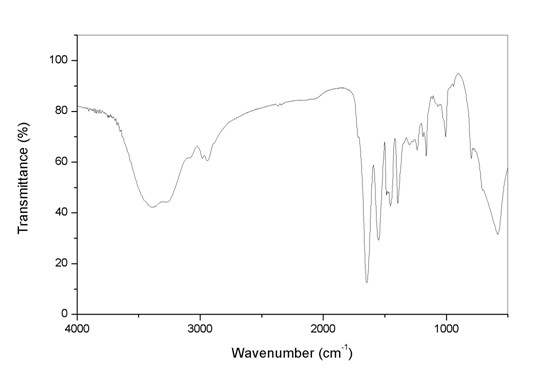
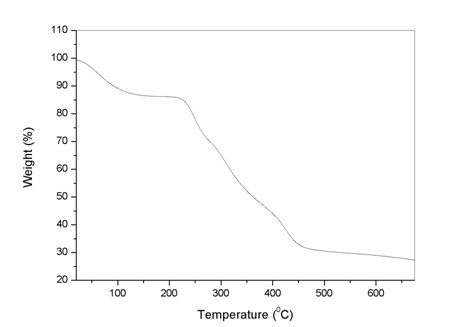
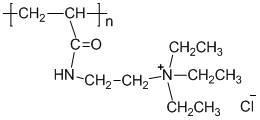
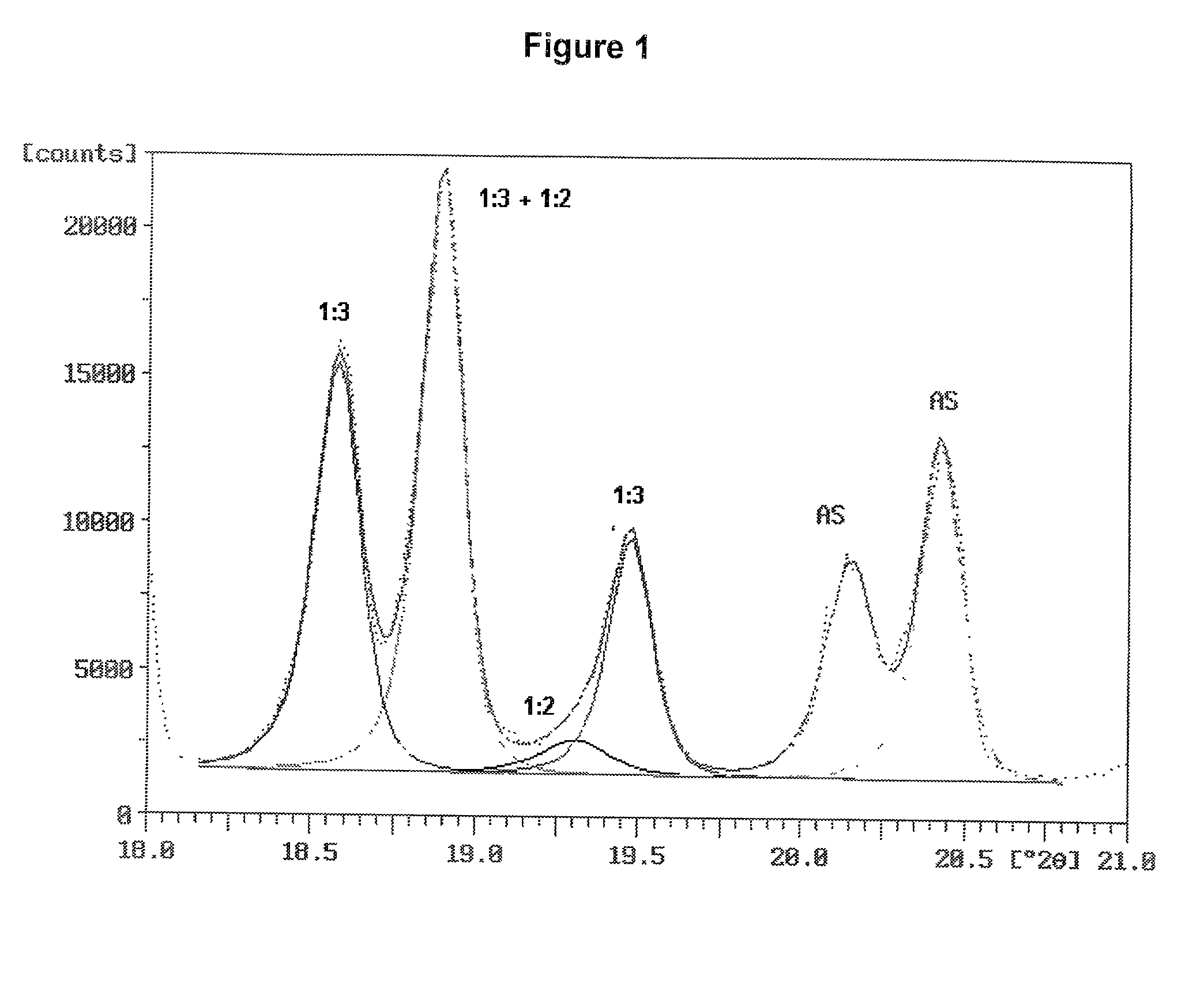
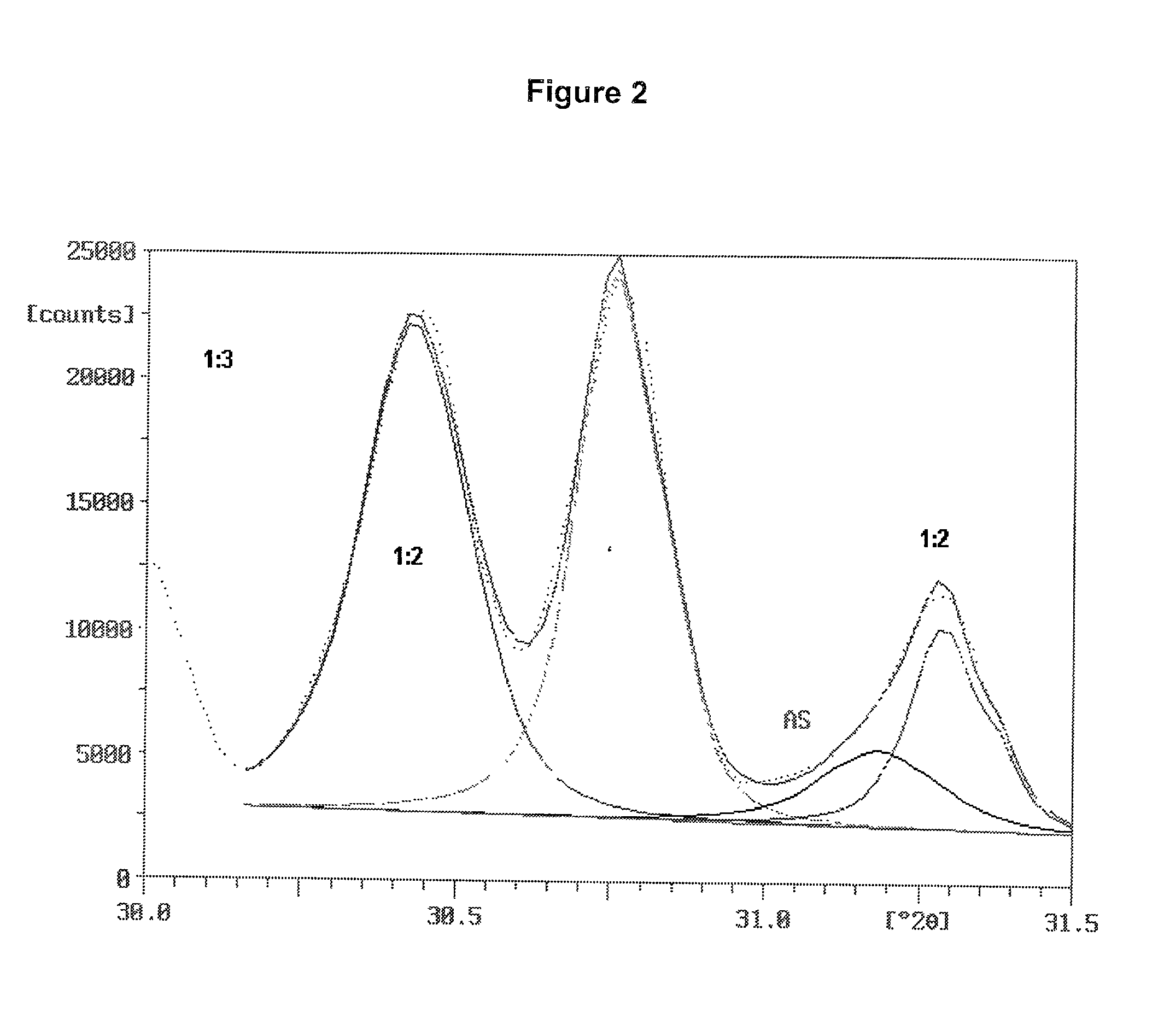
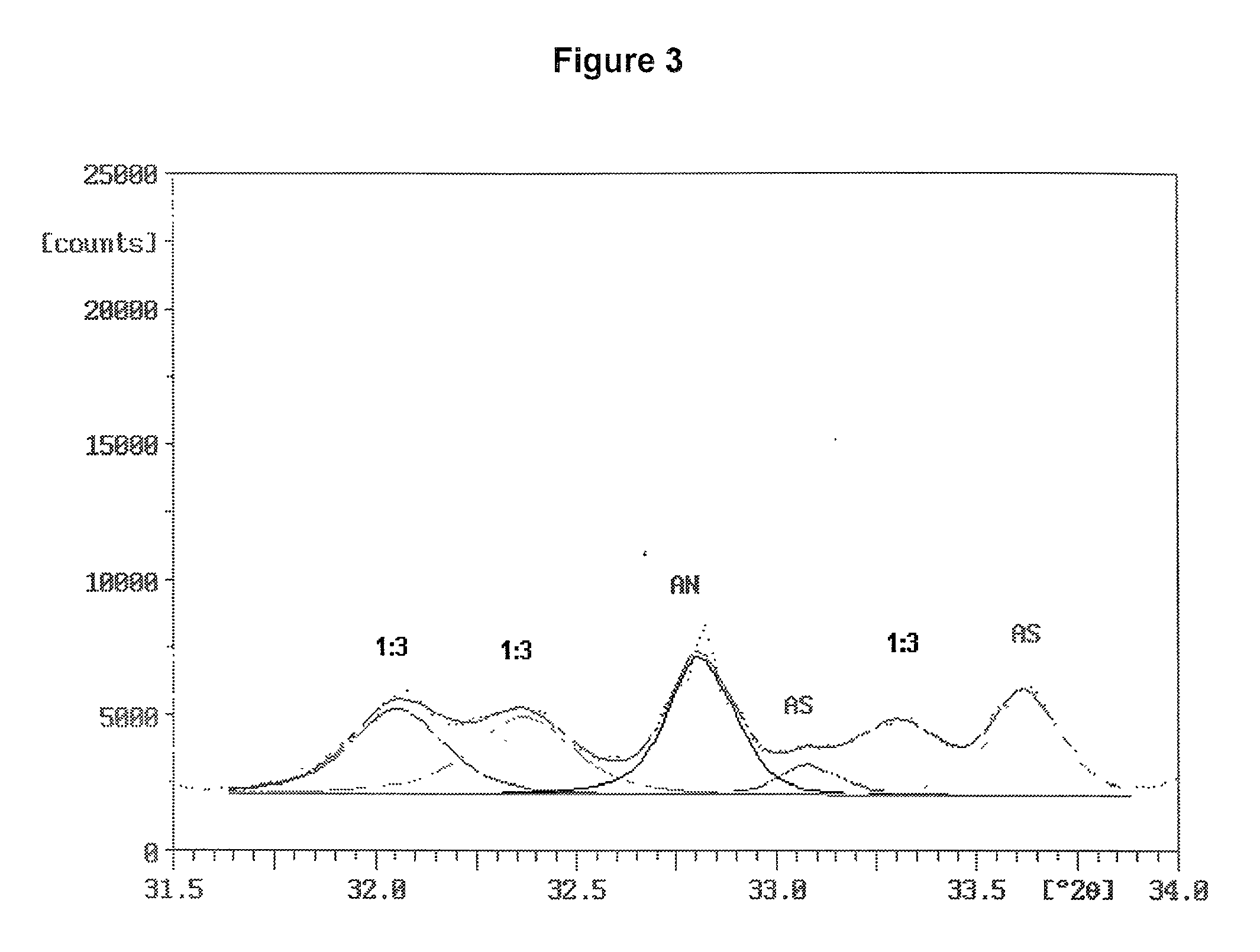
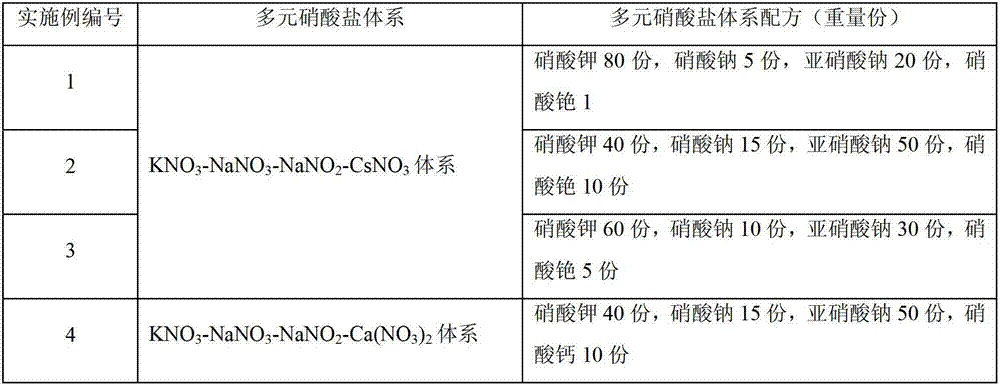
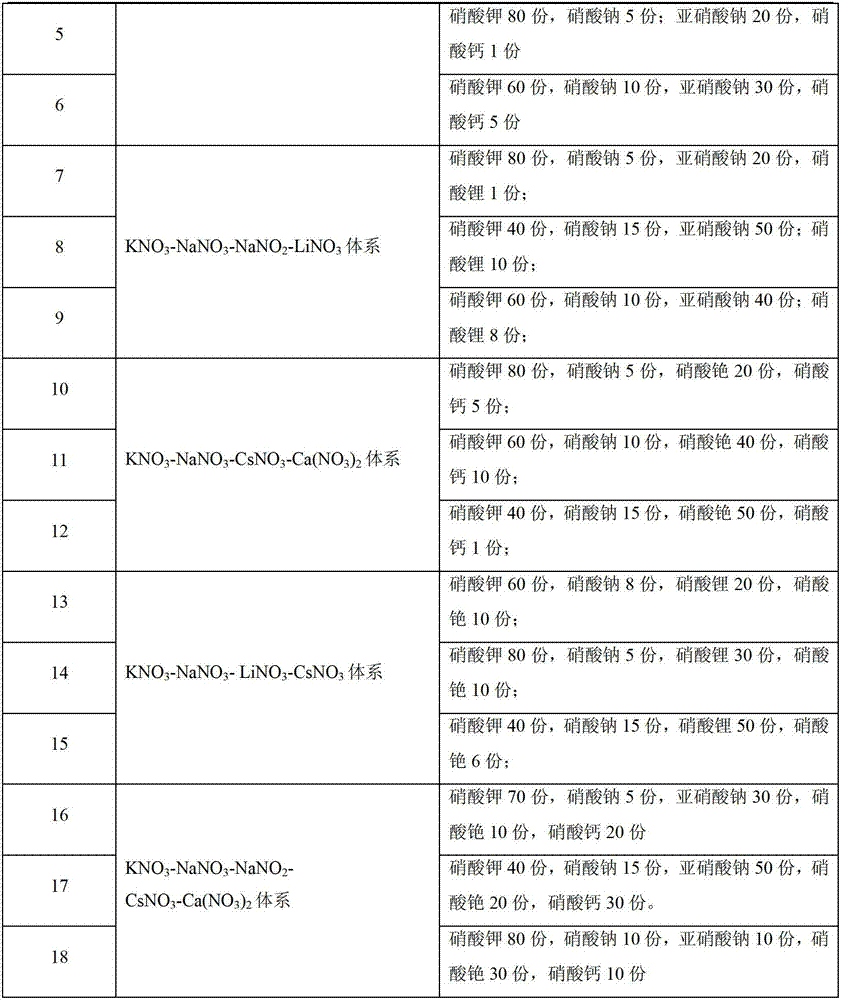
Patents
Literature
2450 results about "Nitrate salts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Salts of nitric acid containing the group NO3- are called nitrate salts. Nitrate anions form salts with a wide range of elements in the periodic table. Examples of nitrates are ammonium nitrate (NH4NO3), sodium nitrate (NaNO3) and potassium nitrate (KNO3).
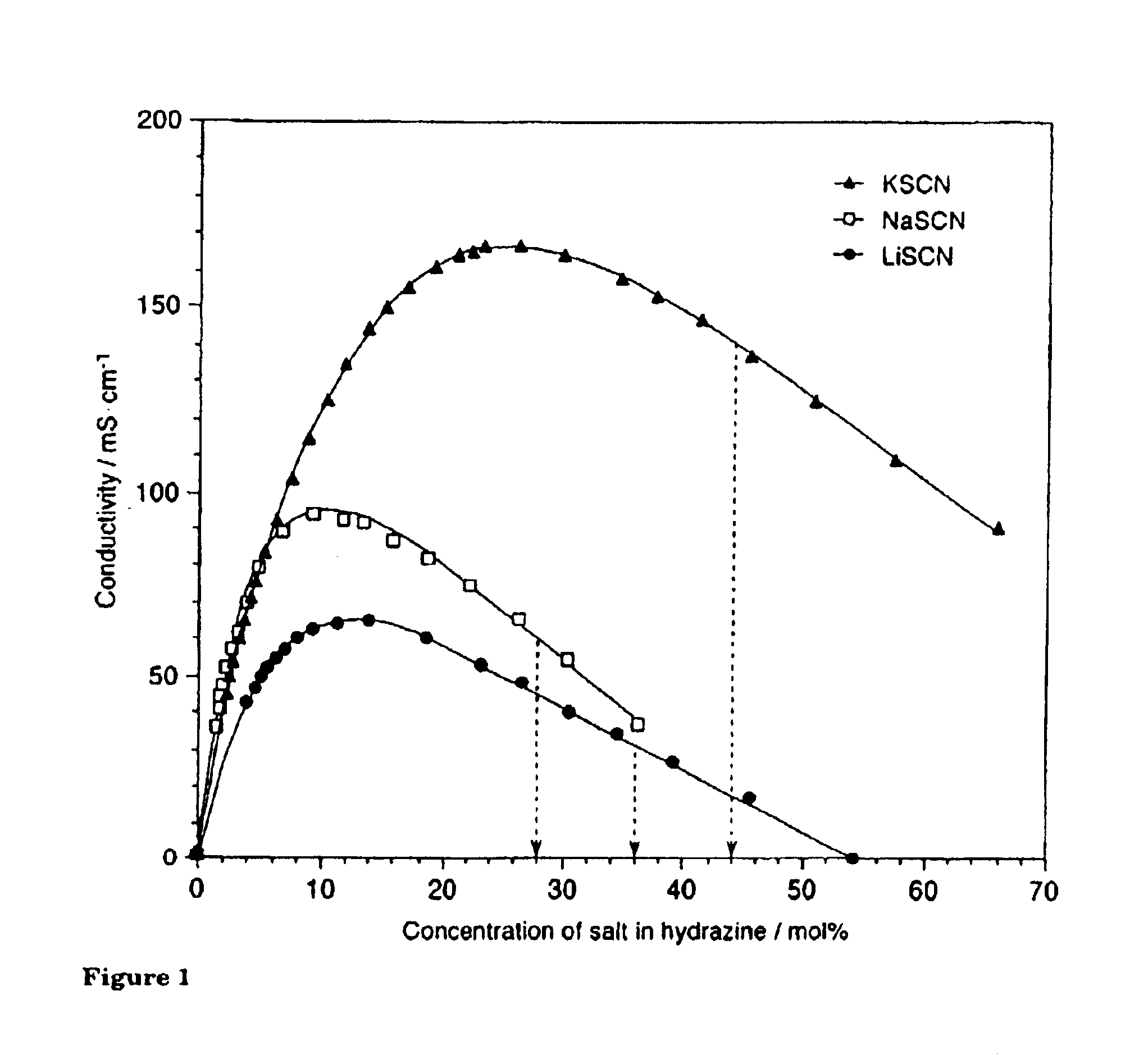
Low-melting point inorganic nitrate salt heat transfer fluid
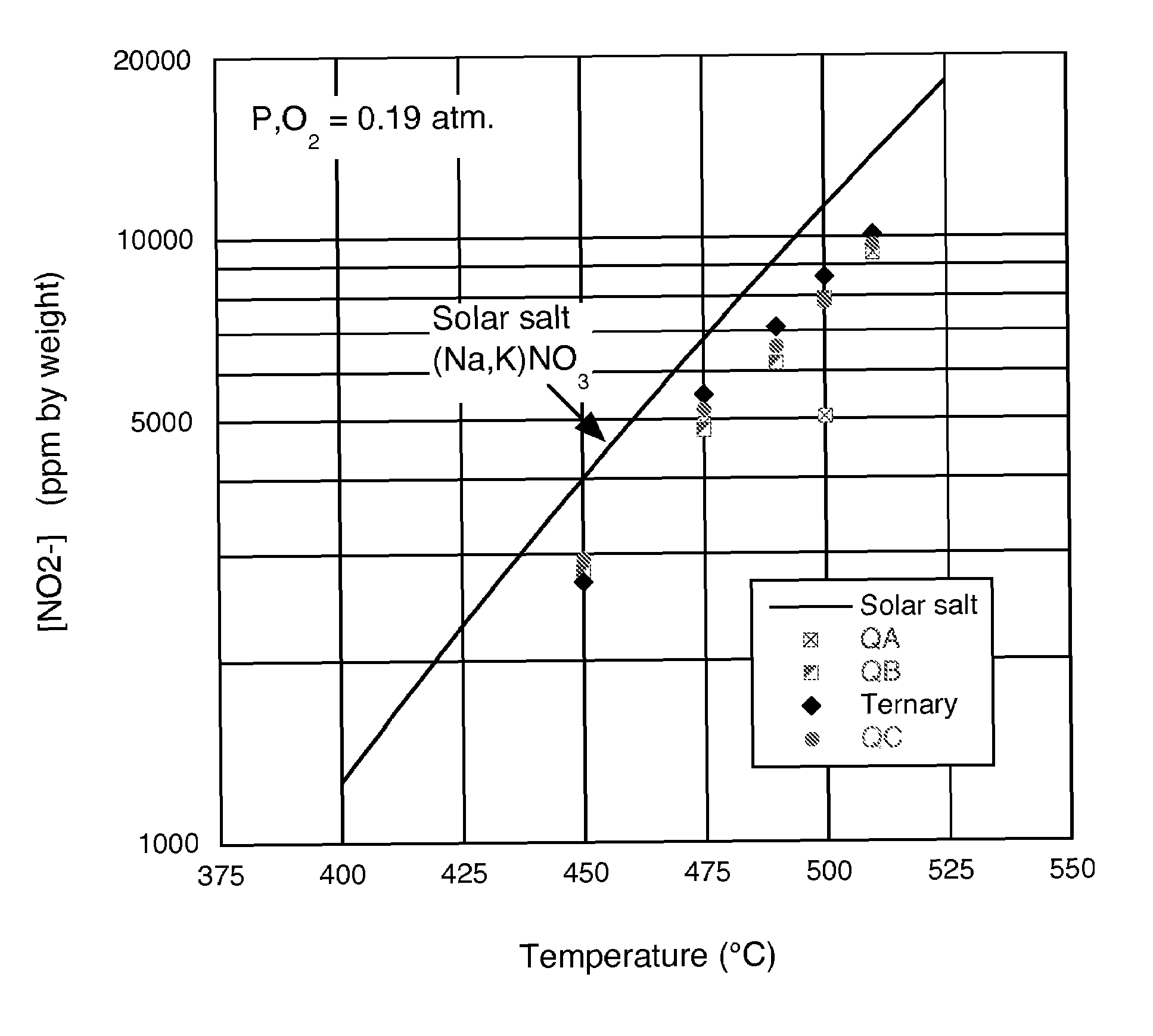
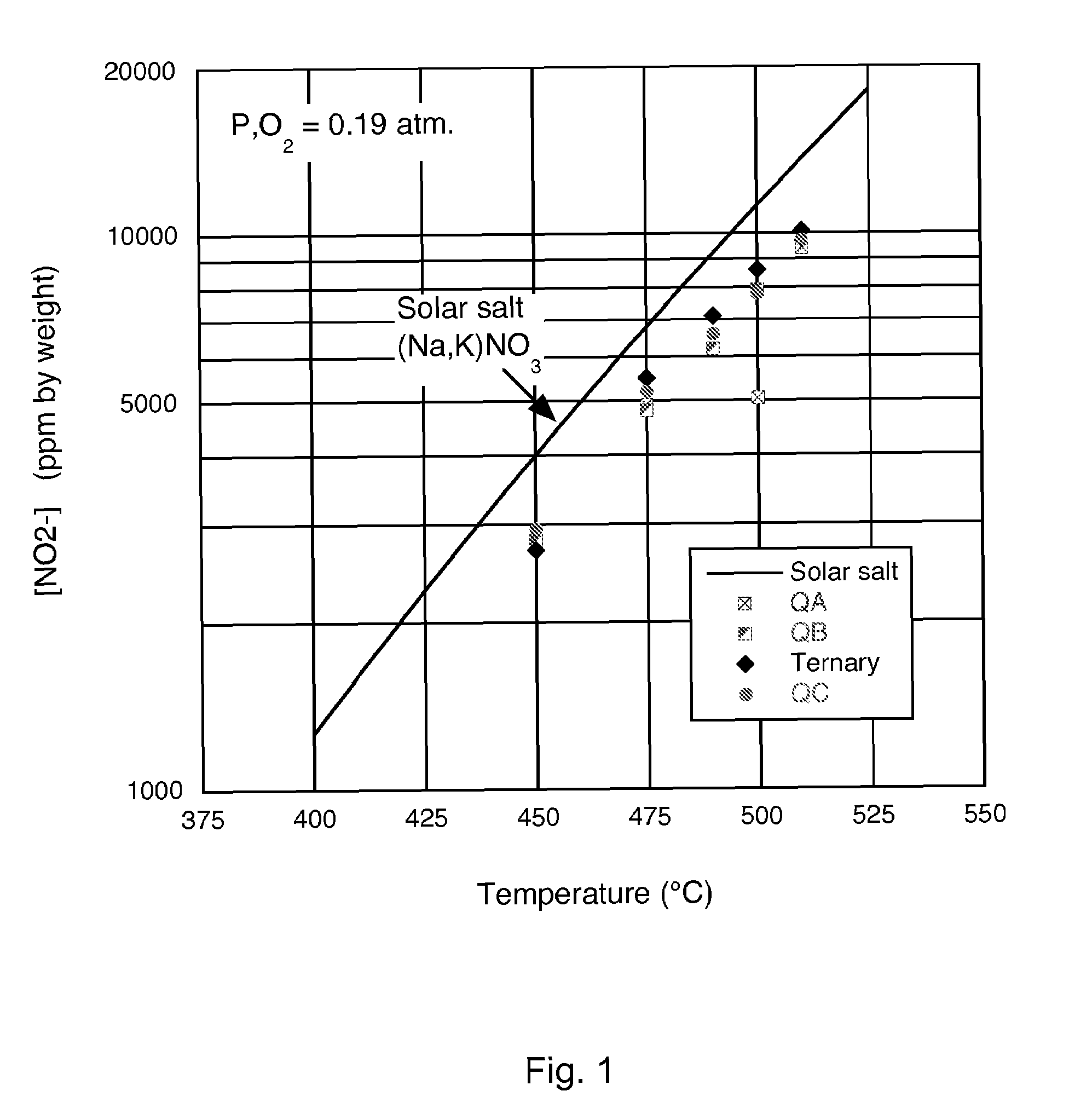
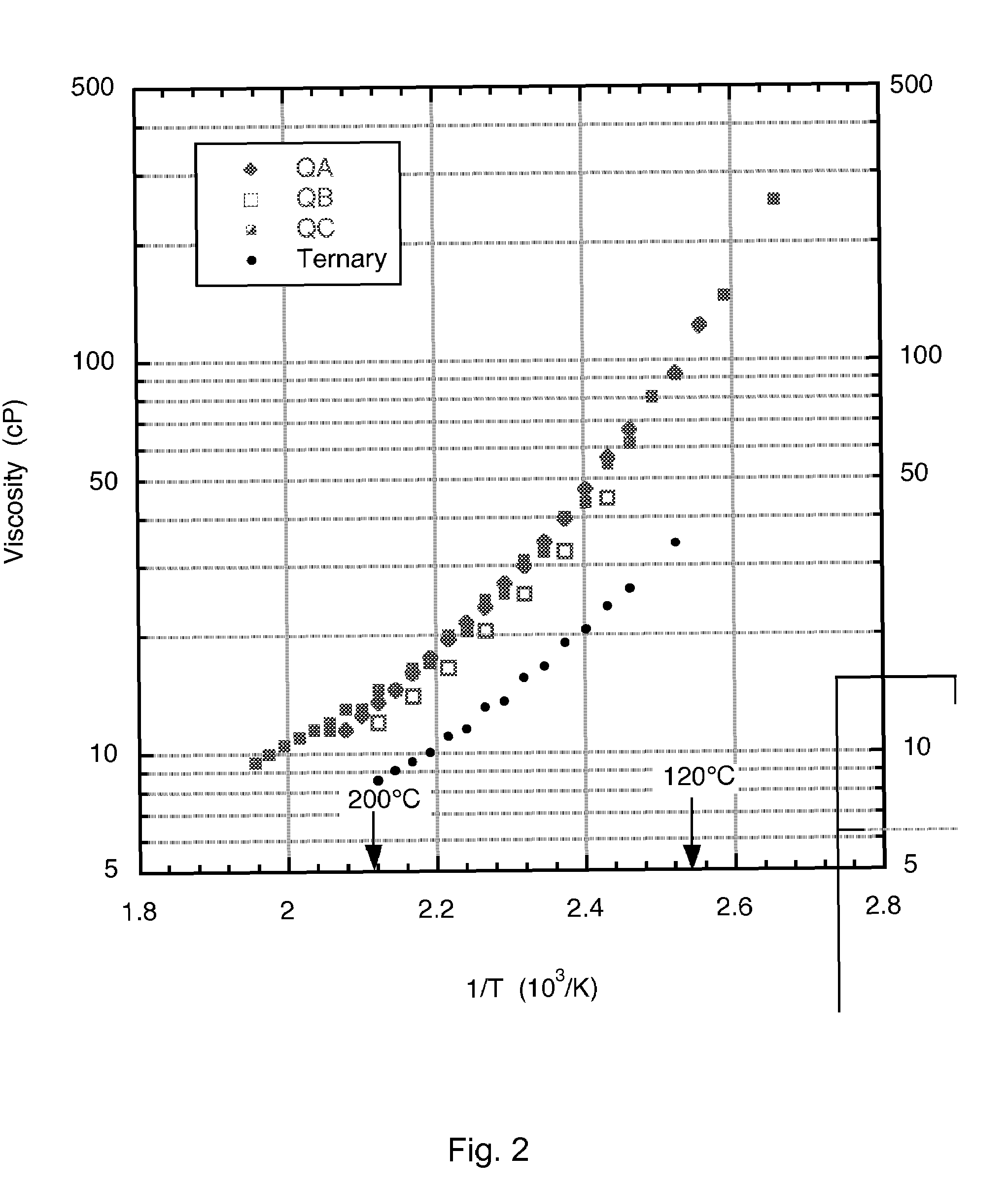
A low-melting point, heat transfer fluid made of a mixture of four inorganic nitrate salts: 9-18 wt % NaNO3, 40-52 wt % KNO3, 13-21 wt % LiNO3, and 20-27 wt % Ca(NO3)2. These compositions can have liquidus temperatures less than 100 C; thermal stability limits greater than 500 C; and viscosity in the range of 5-6 cP at 300 C; and 2-3 cP at 400 C.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Methanol diesel oil manufacturing mode
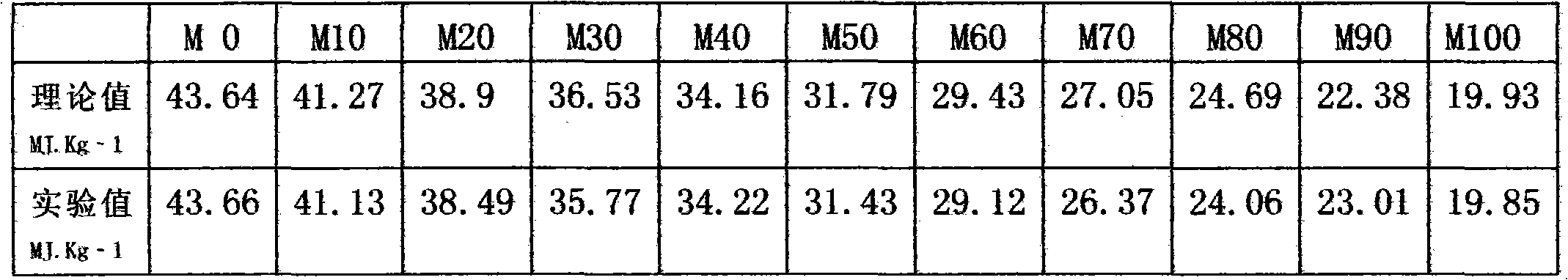
The invention relates to a manufacturing mode of environment-friendly and energy-saving M15_M85 methanol diesel oil. The methanol diesel oil is composed of diesel oil, methanol, and additives. The methanol diesel oil is prepared with operations under normal temperatures and normal pressures. The invention discloses a plurality of common additives used for preparing methanol diesel oil in modern times. If explosives and aviation fuels are appropriately utilized, methanol defalcated heat value can be well compensated. The theory is tentatively considered as a gaseous detonation theory. The additives can be selected from nitric acid esters, nitrates, nitro compounds, non-aromatic compounds, peroxides, and the like.
Owner:陈若歆
Nanosilver-containing antibacterial and antifungal granules and methods for preparing and using the same
The present invention relates to nanosilver-containing antibacterial and antifungal granules ("NAGs"). The NAGs have longlasting inhibitory effect on a broad-spectrum of bacteria and fungi, which include, but are not limited to, Escherichia coli, Methicillin resistant Staphylococcus aureus, Chlamydia trachomatis, Providencia stuartii, Vibrio vulnificus, Pneumobacillus, Nitrate-negative bacillus, Staphylococcus aureus, Candida albicans, Bacillus cloacae, Bacillus allantoides, Morgan's bacillus (Salmonella morgani), Pseudomonas maltophila, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bacillus subtilis, Bacillus foecalis alkaligenes, Streptococcus hemolyticus B, Citrobacter, and Salmonella paratyphi C. The NAGs contain ground stalk marrow of the plant Juncus effuses L. which has been dispersed with nanosilver particles. The nanosilver particles are about 1-100 mn in diameter. Each of the nanosilver particles contain a metallic silver core which is surrounded by silver oxide. The present invention also provides a process for making the NAGs. The NAGs can be used in a variety of healthcare and industrial products. Examples of the healthcare products include, but are not limited to, ointments or lotions to treat skin trauma, soaking solutions or cleansing solutions for dental or women hygiene, medications for treating gastrointestinal bacteria infections, sexual related diseases, and eye diseases. Examples of industrial products include, but are not limited to, food preservatives, water disinfectants, paper disinfectants, construction filling materials (to prevent mold formation).
Owner:LEGEND WIN FINANCE
Aerobic denitrifying Paracoccus denitrificans and application thereof
ActiveCN102465104ABiologically active and stableImprove denitrification effectBacteriaTreatment with aerobic and anaerobic processesSynechococcusPyrococcus
The invention relates to aerobic denitrifying paracoccus denitrificans and application thereof. A bacterial strain provided in the invention is paracoccus denitrificans DN-3CGMCC No. 3658; the Paracoccus denitrificans can carry out aerobic denitrification by using nitrate nitrogen under both aerobic and oxygen-limited conditions and can also carry out heterotrophic nitrification-aerobic denitrification by using ammonia-N, and a total nitrogen removal rate on above mentioned occasions is greater than 90%. The paracoccus denitrificans provided in the invention has stable hereditary features, isapplicable to treatment of a variety of nitrogen-containing waste water and produces a good nitrogen removal effect.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nitrate molten salt heat transferring and reserving medium and preparation method and application thereof
InactiveCN102533226AWide operating temperature rangeImprove thermal stabilityHeat-exchange elementsDecompositionInstability
The invention discloses a nitrate molten salt heat transferring and reserving medium and a preparation method and application thereof. The nitrate molten salt heat transferring and reserving medium is prepared with 5 to 40 percent of potassium nitrate, 5 to 25 percent of sodium nitrate, and 10 to 70 percent of calcium nitrate. The melting point of the nitrate molten salt heat transferring and reserving medium can be as low as 120 DEG C, and the upper limit of temperature for use can reach 550 DEG C, the nitrate molten salt heat transferring and reserving medium has a wide temperature scope of application, can work normally at the temperature scope of 180 DEG C to 550 DEG C, and has good thermal stability; the shortcoming of high melting point of binary nitrate molten salt system can be overcome, and the problem of instability caused by easy oxidative decomposition at high temperature of NaNO2 in the Chinese patent 200110027954.1 and ternary nitrate salt system can be solved, and the problems of corrosion and cost increase caused by the existing LiNO3 in the Chinese patent 00111406.9 and the American patent US007588694B1 can also be solved.
Owner:SUN YAT SEN UNIV +1
Method for preparing high-alkali value (TBN400) synthesized calcium alkyl benzene sulfonate
ActiveCN101318915AImprove cleanlinessGood dispersionAdditivesSulfonic acid preparationTotal Base NumberAlkaline earth metal
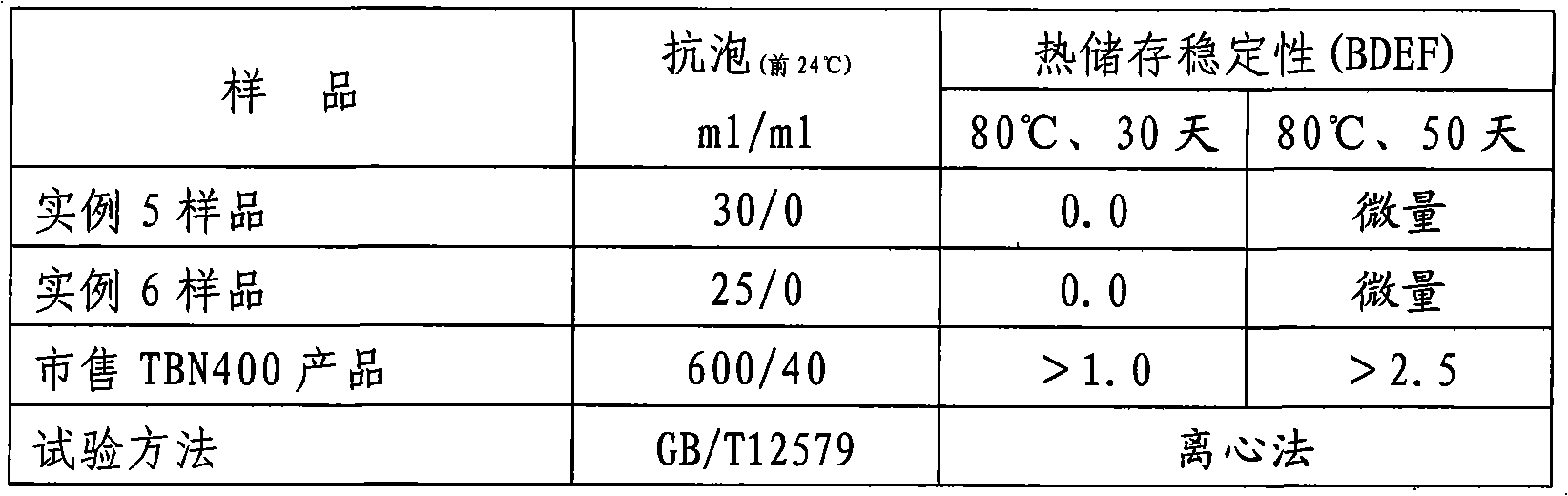
The invention provides a method for preparing high base number (TBN400) synthetic calcium alkyl-benzene sulfonate. The method comprises the following steps of: adopting a mixed acid of long-chain linear alkyl-benzene sulfonic acid and high-boiling heavy alkyl-benzene sulfonic acid, calcium oxide and / or calcium hydroxide, low-carbon alcohol, alkaline-earth metal halide or nitrate, and a mixture of alkaline-earth metal alkylphenol or alkaline-earth metal alkylphenate and polyisobutylene succinic anhydride for a neutralization reaction in the presence of a solvent and cutback oil at a temperature of between 40 and 80 DEG C; then, passing through carbon dioxide to a product of the neutralization reaction at a temperature of between 40 and 60 DEG C for a carbonation reaction; and producing high base synthetic alkyl-benzene sulfonate with a total base number (TBN) of 400mgKOH / g by adopting a process of a one-step method. The product is divided into high-base number (TBN400) synthetic alkyl-benzene sulfonate containing chlorine and high-base number (TBN400) synthetic alkyl-benzene sulfonate without the chlorine. The product produced by adopting the method with low viscosity, small turbidity, easy filtration, light color and no skin formation has the advantages of excellent high-temperature detergency, excellent anti-foaming property and excellent heat storage stability.
Owner:JINZHOU DPF TH CHEM CO LTD
Lithium ion battery cathode material embedded nano metal loaded carbon nanosheet as well as preparation method and application thereof
InactiveCN104538595AUniform sizeRegular shapeMaterial nanotechnologyCell electrodesChemistryCarbon source
The invention discloses a lithium ion battery cathode material embedded nano metal loaded carbon nanosheet as well as a preparation method and an application thereof. By taking sugar (chitosan, cane sugar, fructose and glucose) as a carbon source, nitrate of transitional metals (Fe, Co and Ni) as a metal source and a catalyst and inert salts (sodium sulfate, sodium chloride, potassium chloride and cesium chloride) as a template and a dispersant, a two-dimensional carbon nanosheet which uniformly loads embedded nickel (or iron or cobalt) nanoparticles is prepared by a pyrolytic method in one step. The two-dimensional carbon nanosheet which uniformly loads embedded nickel (or iron or cobalt) nanoparticles prepared by the method is high in degree of graphitization, relatively high in specific surface area and high in conductivity, uniform in distribution and easy to separate, and the surface of the nanosheet is in a porous structure. The nanosheet as the lithium ion battery cathode material has relatively good circulating and rate performances.
Owner:NANJING NORMAL UNIVERSITY
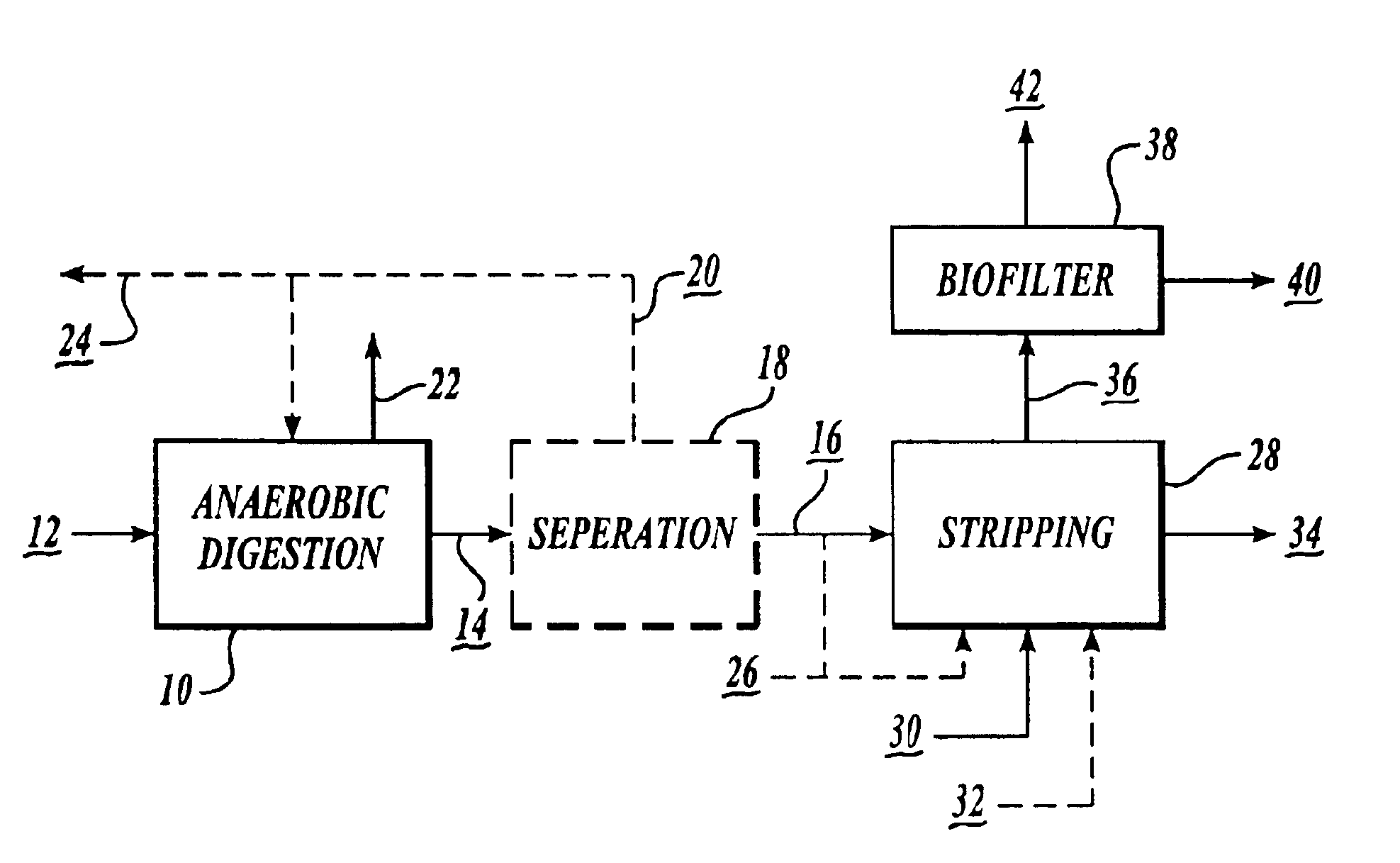
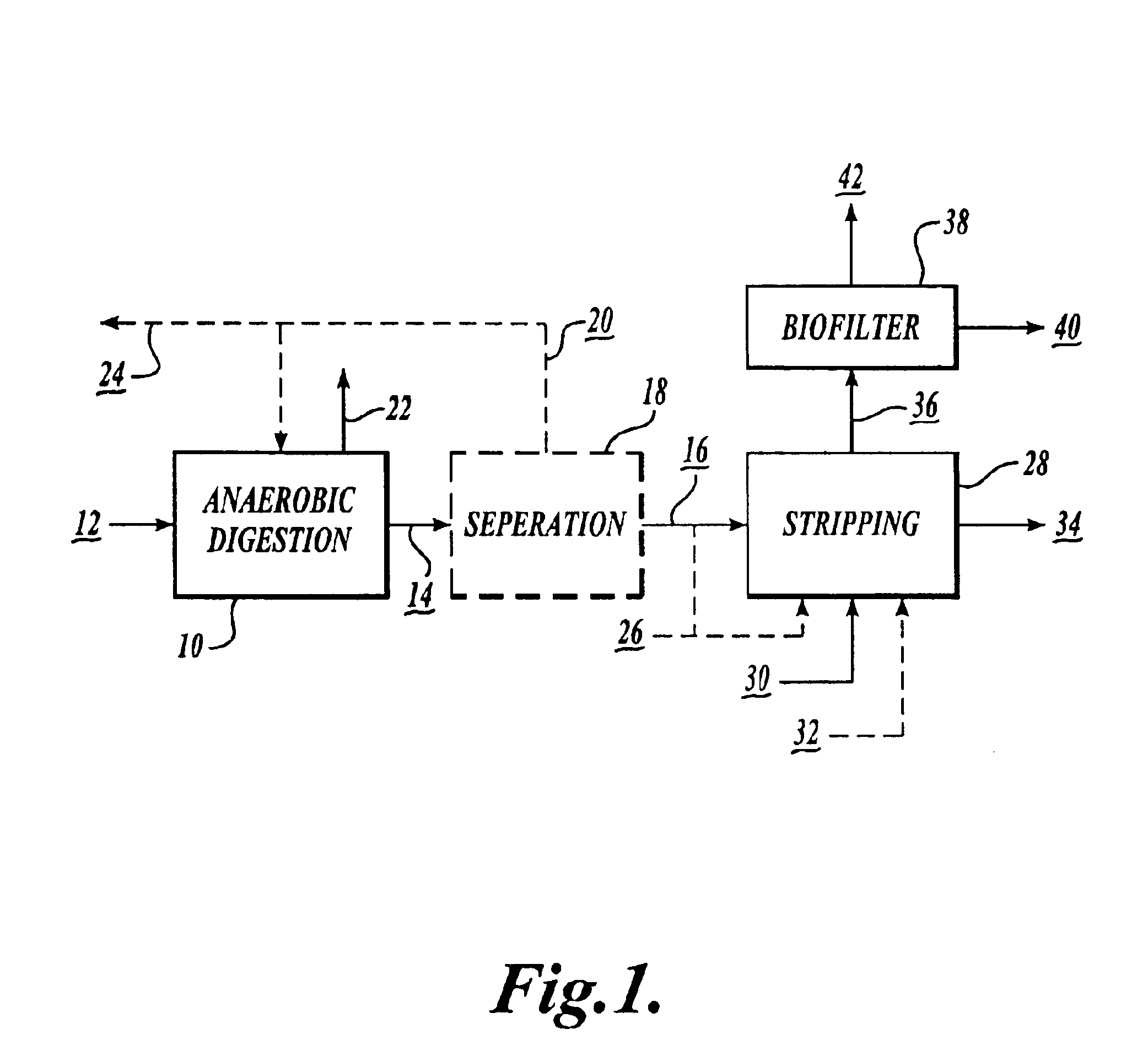
Nitrogen recovery system and method
InactiveUS6866779B1Less greenhouse gasGood health benefitsBio-organic fraction processingCyanogen compoundsLiquid wasteNitrate
A process for the recovery of nitrogen from anaerobically digested liquid waste and for the collection of the nitrogen as nitrate compounds that can be used to produce fertilizer and compost, includes stripping ammonia from anaerobically digested liquid waste, and converting the ammonia into nitrates via nitrification.
Owner:ENVIRONMENTAL ENERGY & ENG
Magnetic Metal Oxide Biochar Composite Particles, and Their Use in Recovering Pollutants From Aqueous Solution
InactiveUS20180016162A1Cheap manufacturingEconomical to useBio-organic fraction processingWaste water treatment from animal husbandryPhosphateAgricultural runoff
Composite particles are disclosed comprising magnesium oxide, iron oxide, and biochar; and methods of making and using the composite particles. The composite particles may be used to recover solutes including phosphate, nitrate, ammonium, and organic compounds from aqueous solution, and the resulting solute-loaded particles may be used as a fertilizer to enhance plant growth. The composites be used to remove pollutants from agricultural runoff, wastewater, and surface water. The particles possess magnetic properties that enhance their recovery following solute adsorption.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Calcium carbonate precipitation method
InactiveUS6036933ACalcium/strontium/barium carbonatesMagnesium carbonatesNitratePrecipitated calcium carbonate
PCT No. PCT / GB96 / 00488 Sec. 371 Date Oct. 22, 1997 Sec. 102(e) Date Oct. 22, 1997 PCT Filed Mar. 1, 1996 PCT Pub. No. WO96 / 26902 PCT Pub. Date Sep. 6, 1996A method for producing precipitated calcium carbonate by reacting an aqueous solution of calcium nitrate [Ca(NO3)2] with an aqueous solution of ammonium carbonate [(NH4)2CO3] and allowing calcium carbonate to precipitate from the resultant mixture containing nitrate [NH4NO3] in the mother liquor, the process being characterized in that: (i) the calcium nitrate [Ca(NO3)2)] solution utilized in the processes is prepared by slaking lime [CaO] in water in the presence of ammonium nitrate [NH4NO3] to form calcium nitrate [Ca(NO3)2] and ammonium hydroxide [NH4OH] in solution, filtering the solution to render it solids free, and heating the filtrate to dissociate the ammonium hydroxide [NH4OH] and to drive ammonia gas [NH3] from the solution; (ii) the ammonium carbonate (NH4)2CO3 solution utilized is prepared by absorbing ammonia gas [NH3] and carbon dioxide gas [CO2] in water, the ammonia gas preferably being derived from the step in (i) above in which the Ca(NO3)2 solution is heated; and (iii) the ammonium nitrate used is derived from the precipitation phase during which calcium carbonate is precipitated from the mother liquor containing ammonium nitrate.
Owner:PRETORIA PORTLAND CEMENT COMPANY +1
High pH antiperspirant compositions of enhanced efficacy
InactiveUS7087220B2Good curative effectIncrease valueCosmetic preparationsToilet preparationsIrritationStrontium
Aluminum and aluminum-zirconium antiperspirant compositions of enhanced efficacy and a pH value of at least 3.5 are provided that are made by reaction with insoluble, strongly alkaline strontium or calcium salts. The aluminum and aluminum-zirconium strontium or calcium compositions show high pH values with characteristic HPLC Band III to Band II ratios of at least 0.5. The basic aluminum halohydrate (or nitrate) solutions typically have aluminum to anion ratio of less that 1.9. The solution compositions are stable with respect to both HPLC Band III to Band II ratio and viscosity at concentrations of about 20% to about 40% by weight of anhydrous solid. The solid state compositions form hard sticks with low irritation, at low metal to chloride ratios of about 0.9 to about 1.2.
Owner:SUMMIT RES LAB
Recycled flue gas desulfurization and denitration method
The invention relates to a recycled flue gas desulfurization and denitration method, which sequentially comprises the following steps of: introducing flue gas of SO2 and NOx into a desulfurizing tower, and absorbing the SO2 with a desulfurizing agent (barren liquor) in the desulfurizing tower; desorbing the desulfurizing agent (rich liquor) which absorbs the SO2 through a multi-effect evaporator,releasing the SO2 gas, and concentrating the SO2 gas into liquid SO2 through condensation drying; making the desulfurized flue gas enter a denitration tower, injecting gaseous ozone from an ozone generator, oxidizing the NO in the flue gas, absorbing the oxidized NO by using a denitrifier to form nitrate; crystallizing and separating out the nitrate in the solution after the nitrate reaches certain concentration, and filtering and drying to obtain the nitrate product. The method turns the wastes into wealth, and the SO2 and NOx in the flue gas are recycled by higher-additional value liquid SO2 and nitrate products, so that the recycling and value maximization in the desulfurization and denitration process is realized. Due to the adoption of the technical scheme, the high desulfurization and denitration rate can be achieved, the desulfurization rate is over 96 percent, the denitration rate is more than 90 percent, and the purity of the nitrate product is over 96 percent. The method hasthe advantages of simple desulfurization and denitration process, low investment, and low operation cost for desulfurization and denitration, and solves the problems that the conventional desulfurization and denitration process has high cost, generates a side product of a mixture of sulfuric acid (sulfate) and nitric acid (nitrate), and has low additional value.
Owner:EAST CHINA UNIV OF SCI & TECH
Desulfurizing agent for reforming stock oil and preparation method thereof
InactiveCN101643664AExpand the range of raw materialsLow raw material costRefining with metal oxidesAluminiumSulfide
The invention relates to a desulfurizing agent for reforming stock oil and a preparation method thereof, in particular to the desulfurizing agent used for desorbing sulfides in the reforming stock oilsuch as H2S and the like. The desulfurizing agent is characterized by consisting of 10%-40% of ZnO, 15%-22% of NiO, 10%-17% of Al2O3, 5%-22% of SiO2 and the balance inevitable impurities. The desulfurizing agent takes zinc and nickel as active components and bentonite (or diatomite, or white clay, or zeolite, or high-alumina cement and or common cement) as a carrier, and active components thereofare directly present in the form of oxides of zinc and nickel or oxides transformed by corresponding carbonate (basic carbonate) and nitrate. In the invention, the desulfurizing agent has enlarged raw material range, thus effectively reducing raw material cost, simplifying production process of the desulfurizing agent, lowering production cost, improving strength to more than twice, and prolonging service life of the desulfurizing agent.
Owner:JINDUICHENG MOLYBDENUM CO LTD
Use Of An Ammonia Storage Device In Production Of Energy
InactiveUS20070207351A1Reactant parameters controlMagnesium halidesAlkaline earth metalAmmonia storage
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power is useful for large stationary energy producing facilities, but also for use for is useful for large stationary energy producing facilities, but also for use for small rechargeable and / or replaceable power supply units for micro-fabricated or miniaturized ammonia decomposition reactors for use in mobile units and portable devices may be used for large energy producing facilities, and by use of small rechargeable and / or replaceable ammonia storage decomposition reactors, it is also possible to provide energy for mobile units and portable devices.
Owner:AMMINEX
Extended engine coolant lifetime through polymeric polycarboxylate secondary silicate stabilization
InactiveUS6203719B1Inhibiting cavitation-erosion corrosionOther chemical processesHeat-exchange elementsPhosphateNitrate salts
The present invention provides corrosion inhibition formulations and compositions for inhibiting mineral scale and the corrosion of metals particularly the cavitation corrosion of aluminum in the presence of aqueous liquids. The combination of a mixture of polymeric polycarboxylates, azoles, nitrate salts, phosphates, stabilized silicates and transition metal compounds provide a synergistic protective effect against the cavitation corrosion of aluminum in aqueous liquids reducing the corrosion rate and is effective at relatively low concentrations and varying pH ranges. The addition of selected polymeric polycarboxylates not only significantly reduces glycol based coolant cavitation erosion-corrosion, heat rejecting aluminum corrosion, and hard water precipitates and scale, it has been discovered that polymeric polycarboxylates in combination with siloxane stabilized silicates enhance secondary silicate stabilization leading to improvement in aluminum corrosion protection and coolant life when utilized with selected amounts of the above-identified additives. The formulations are particularly suitable for automotive applications.
Owner:VALVOLINE LICENSING & INTPROP LLC
Kocuria palustris strain and applications thereof
ActiveCN103103141AFast growthIncrease productionBacteriaMicroorganism based processesBiotechnologyHigh concentration
The invention relates to a Kocuria palustris strain and applications thereof. The strain is Kocuria palustris FSDN-A, and is preserved in the China General Microbiological Culture Collection Center on July 14, 2011, wherein a preservation number is CGMCCNO.5061. According to the present invention, the strain can adopt nitrite nitrogen or nitrate nitrogen as a substrate to complete a denitrification process; when the Kocuria palustris FSDN-A is adopted to treat ammonia-containing wastewater, characteristics of simple process, high denitrogenation activity, and high concentration organic carbon source tolerance are provided; after the strain is poured, the system is rapidly started; and the strain has broad application prospects in wastewater denitrogenation treatment processes, and is especially suitable for treatment of wastewater containing nitrogen oxides and organic pollutants.
Owner:CHINA PETROLEUM & CHEM CORP +1
Normal temperature formaldehyde removal catalysis material
InactiveCN104741130ALow costEfficient enrichmentDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsAlkaline earth metalCatalytic oxidation
The invention relates to a formula and a corresponding preparation technology of a normal temperature formaldehyde removal catalysis material, and belongs to the technical fields of normal temperature adsorption catalysis and air pollution treatment. The catalysis material is prepared by adopting a one-step co-precipitation process or a step-by-step dipping process to load active components with granular, cylindrical, spherical or honeycomb high-specific surface area shell activated carbon as a carrier, one or more non-noble metal salts as main active components, such as nitrate, sulfate or chloride of manganese, iron, cobalt, nickel, copper, zinc, lanthanum or cerium, one or more alkali metal / alkaline earth metal salts as an inorganic additive, such as oxalate, nitrate, sulfate, acetate, carbonate and bicarbonate of sodium, potassium, magnesium, calcium, and ethylenediamine tetracetic acid / ethylenediamine tetraacetic acid salt, basic amino acid, polyamide monomer and polyurethane monomer as a nitrogen-containing organic additive. The normal temperature formaldehyde removal catalysis material has the characteristics of rapid capture, high efficiency catalytic oxidation and long working cycle, and can meet requirements of long-time effective removal of formaldehyde in indoor real environment.
Owner:江苏瑞丰科技实业有限公司
Polishing composition for noble metals
ActiveUS20060024967A1Other chemical processesSemiconductor/solid-state device detailsPhosphateOxidation state
The invention provides a polishing composition and a method of chemically-mechanically polishing a substrate comprising a noble metal, the polishing composition comprising (a) an oxidizing agent that oxidizes a noble metal, (b) an anion selected from the group consisting of sulfate, borate, nitrate, and phosphate, and (c) a liquid carrier. The invention further provides a polishing composition and a method of chemically-mechanically polishing a substrate comprising ruthenium, the polishing composition comprising (a) an oxidizing agent that oxidizes ruthenium above the +4 oxidation state, (b) a polishing additive selected from the group consisting of metal sequestering polymers, metal chelators, organic thiols, compounds that reduce ruthenium tetraoxide, lactones, and α-hydroxycarbonyl compounds.
Owner:CMC MATERIALS INC
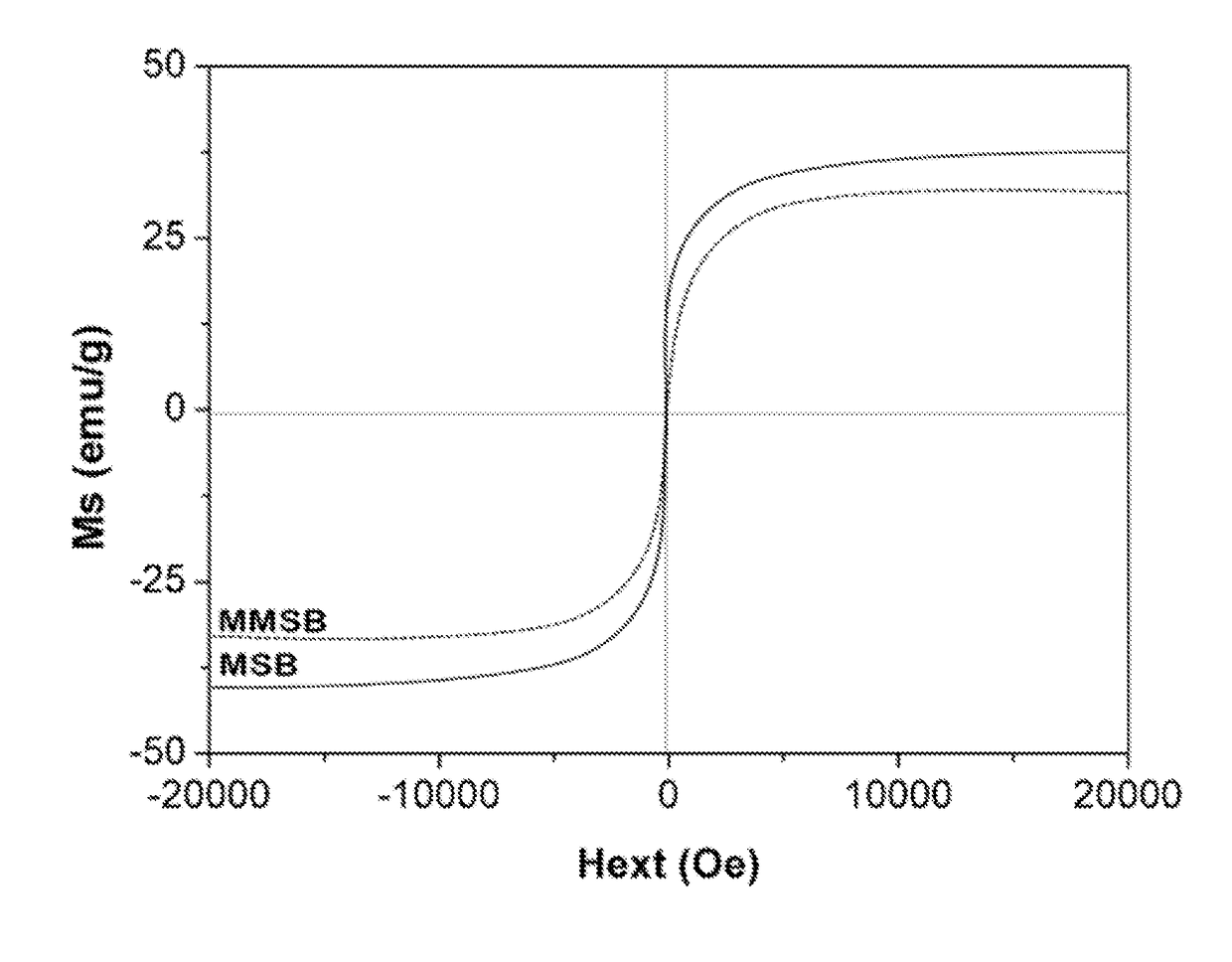
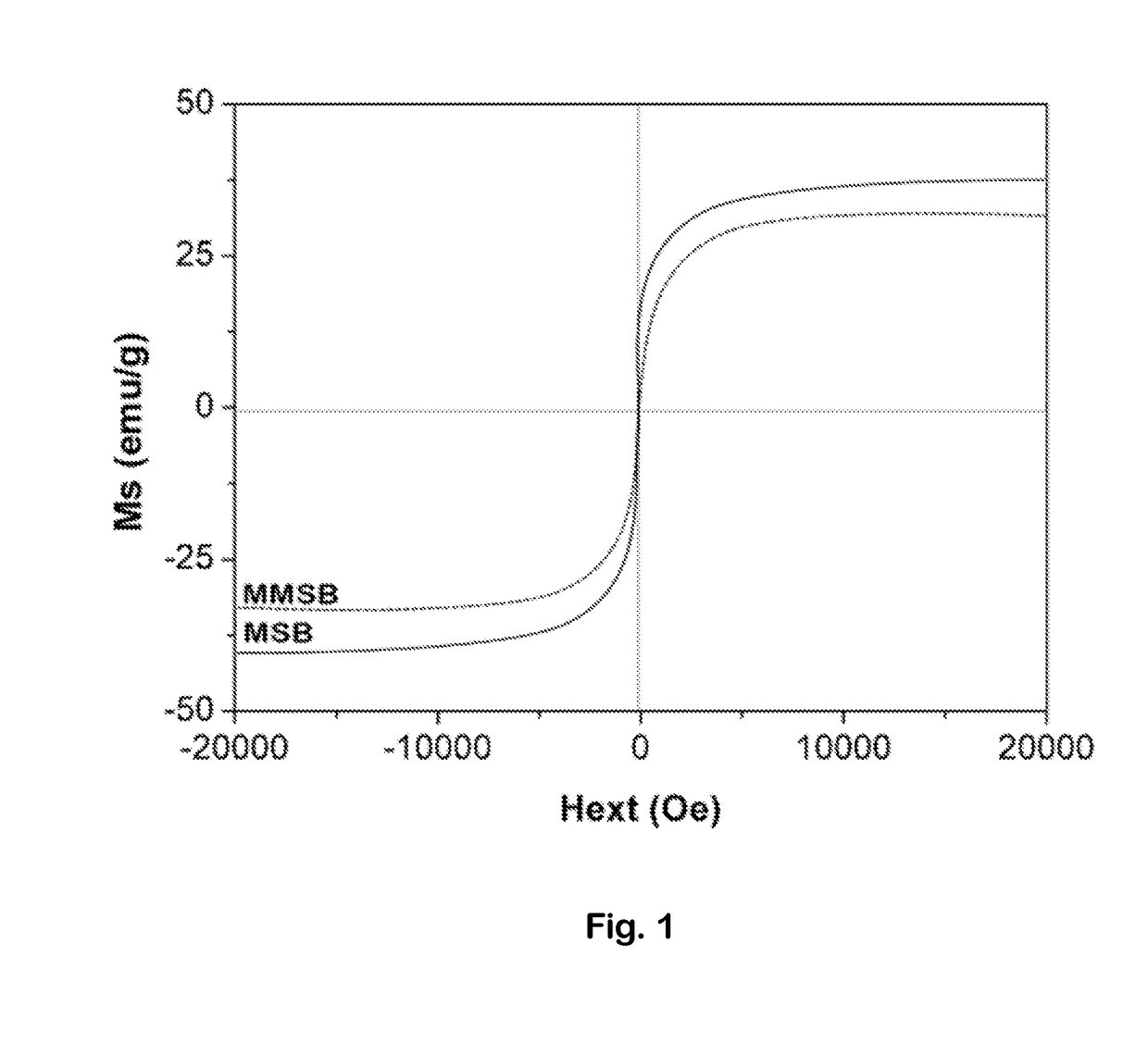
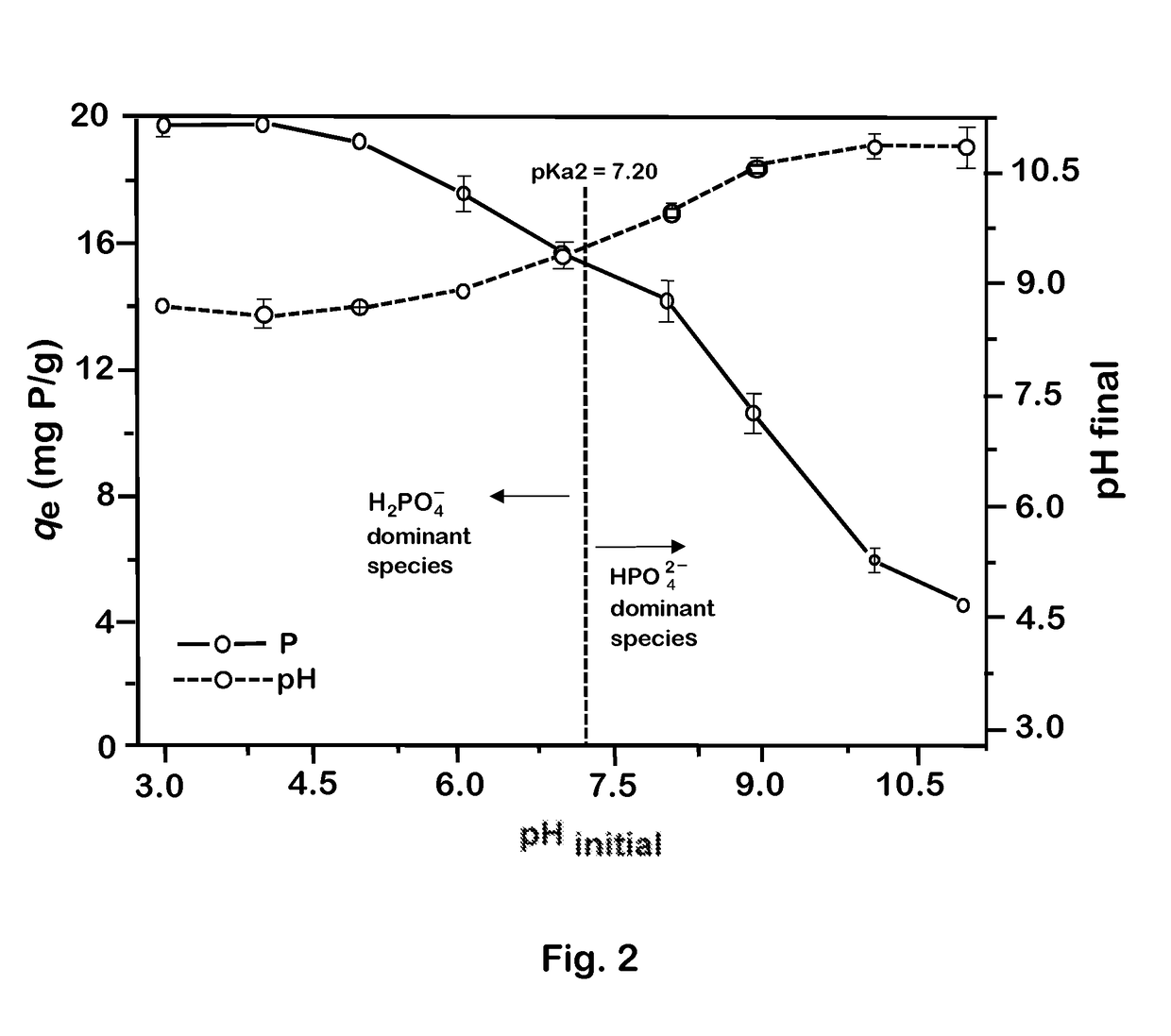
Magnetic microsphere resin for removing nitrate nitrogen selectively, and preparation method thereof
ActiveCN102430433AHigh base exchange capacityAccelerated settlementWater/sewage treatment by ion-exchangeAnion exchangersOil phaseStructural formula
The invention discloses magnetic microsphere resin for removing nitrate nitrogen selectively, and a preparation method thereof, and belongs to the field of ion exchange resin. The resin consists of a resin skeleton, and magnetic granules wrapped in the resin skeleton; and the resin skeleton has a basic structural formula shown in the specification, wherein B is a quaternary ammonium salt group for adsorbing the nitrate nitrogen selectively, the saturation magnetization intensity of the quaternary ammonium salt group is between 5 and 30 emu / g, the exchange capacity of strong base is between 3.0 and 4.5 mmol / g, the exchange capacity of weak base is between 0.5 and 1.5 mmol / g, and the average grain diameter of the resin is between 50 and 500 micrometers. The resin is synthesized by a suspension polymerization method; acrylate monomers are mixed with a pore-forming agent and an initiator to form an oil phase; after being mixed with the magnetic granules uniformly, the mixture is subjectedto suspension polymerization with an aqueous phase mixed with a dispersing agent to form the magnetic polymer granules, and the magnetic polymer granules are subjected to aminolysis and alkylation toform the resin serving as a finished product. The resin can adsorb negative ions such as nitrates, nitrites and the like in a water body selectively, so the magnetic microsphere resin has a bright application prospect in fields of drinking water treatment, groundwater remediation and the advanced treatment of urban domestic sewage.
Owner:NANJING UNIV +1
Ammonium sulfate nitrate
Ammonium sulfate nitrate composite materials useful as fertilizers having desirable levels of nitrate ions, superior stability against detonation, higher density, greater resistance to moisture, and a method for their manufacture. The ammonium sulfate nitrate composites have as essential constituents ammonium sulfate and the NH4SO4.2(NH4NO3) double salt with less than 5 wt. % in combined total of the more hazardous NH4SO4.3(NH4NO3) double salt and ammonium nitrate. The composites of the invention are formed by reacting ammonium sulfate with ammonium nitrate in a molar ratio of about 0.9:1 to about 1.1:1 in the presence of a small amount of water in a narrow range of temperatures and then cooling to solidification at a sufficiently rapid rate to prevent macroscopic segregation of the reaction products.
Owner:ADVANSIX RESINS & CHEM LLC
Heat-transfer heat-accumulation medium prepared by combining quartz sand and multi-composition molten nitrate salt and preparation method
ActiveCN103923618AStable chemical propertiesSlightly corrosiveEnergy inputHeat-exchange elementsNitrate saltsMolten salt
The invention relates to a heat-transfer heat-accumulation medium prepared by combining quartz sand and a multi-composition molten nitrate salt and a preparation method, and belongs to the field of solar photo-thermal power generation. The heat-transfer heat-accumulation medium is composed of a multi-composition molten nitrate salt system and quartz sand. The invention also provides the preparation method of the heat-transfer heat-accumulation medium. The heat-transfer heat-accumulation medium disclosed by the invention keeps the low melting point of the multi-composition molten nitrate salt, and also helps to improve the upper limit operating temperature of the multi-composition molten nitrate salt, and the upper limit operating temperature is up to 700 DEG C. The heat-transfer medium is low in cost, wide in operating temperature range, good in heat stability and high in thermal conductivity, and is extremely suitable for a solar thermal power generation system and a heat-accumulation heat-transfer system based on solar photo-thermal power generation.
Owner:QINGHAI ENESOON NEW MATERIAL TECH & SCI CO LTD
Formation of superparamagnetic particles
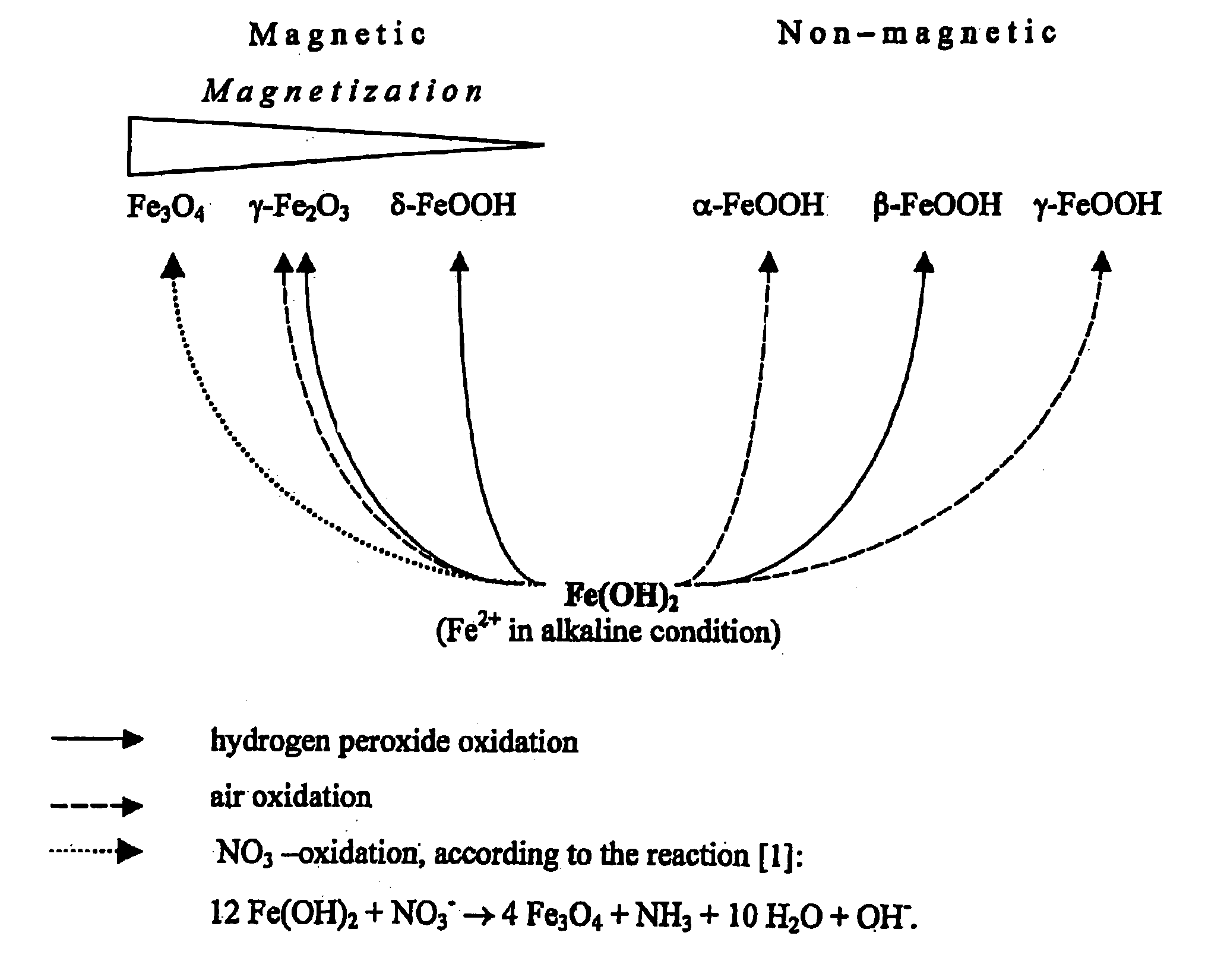
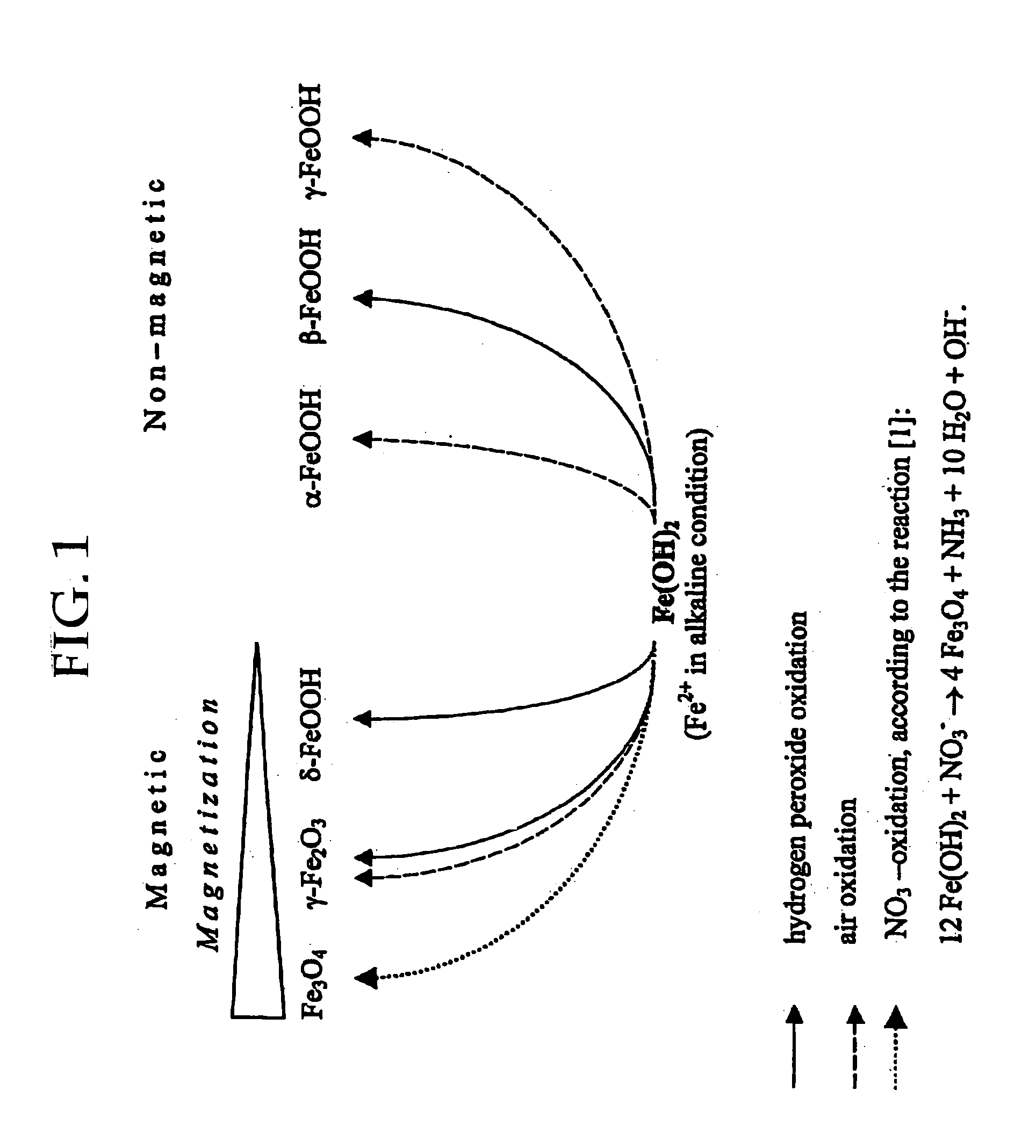
InactiveUS20050019755A1Efficient removalImprove conductivityNanomagnetismMicrobiological testing/measurementCross-linkNitrate
The present invention features a method for preparing superparamagnetic iron particles by the in situ formation of these particles in a cross-linked starch matrix or by the formation of a superparamagnetic chitosan material. The superparamagnetic materials are formed by mild oxidation of ferrous ion, either entrapped into a cross-linked starch matrix or as a chitosan-Fe(II) complex, with the mild oxidizing agent, nitrate, under alkaline conditions. The present invention further features superparamagnetic iron compositions prepared by the method of the invention. The compositions of the invention are useful for the separation, isolation, identification, or purification of biological materials.
Owner:MARCHESSAULT ROBERT H +5
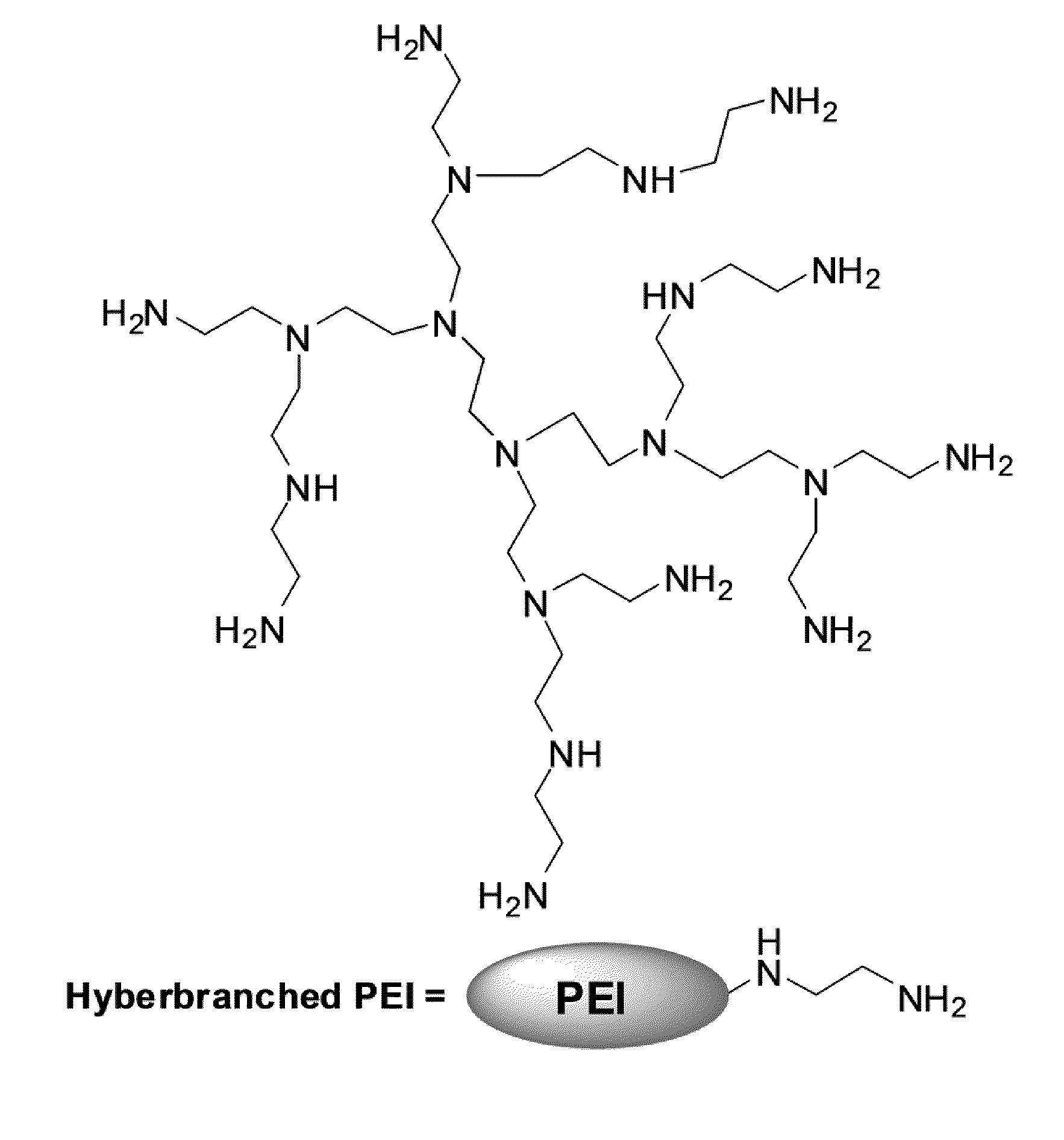
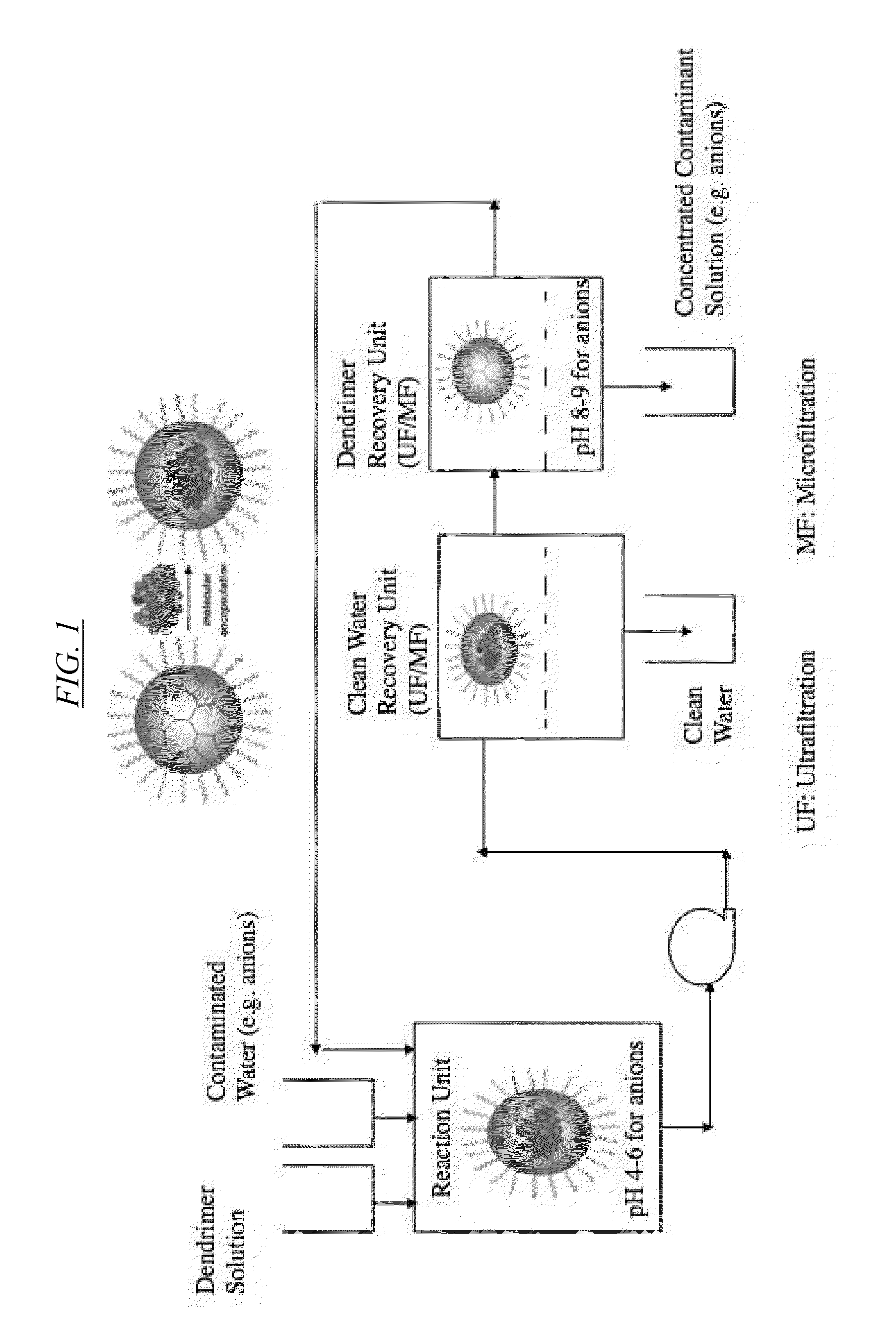
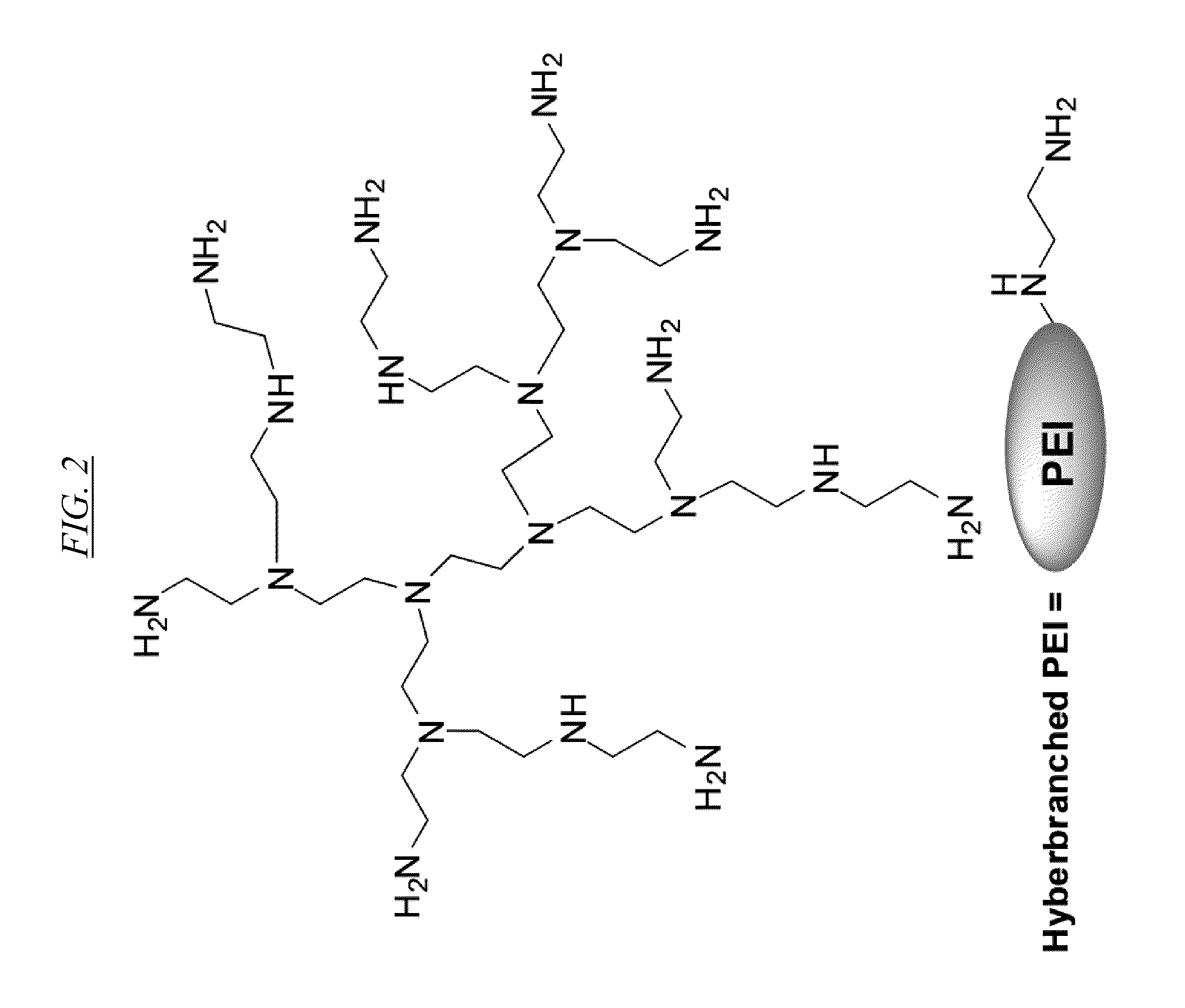
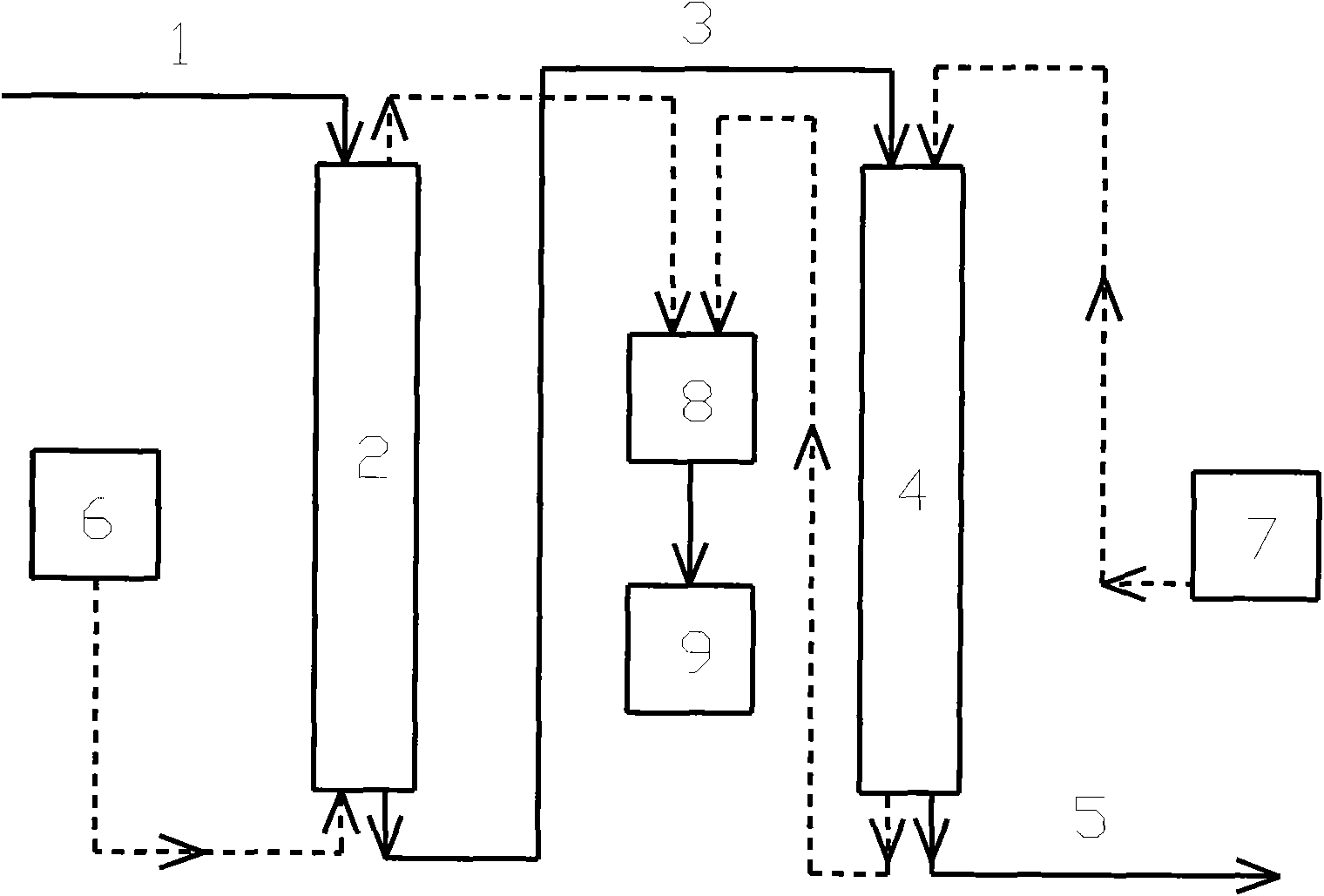
Extraction Of Anions From Solutions And Mixtures Using Hyperbranched Macromolecules
Hyperbranched macromolecules and methods are described for selectively filtering contaminants such as anions from water and non-aqueous solutions, particularly in the presence of competing contaminants including other anions. The hyperbranched macromolecules may contain alkyl, 2-hydroxyalkyl, 2-methyl-2-hydroxylalkyl, 2-hydroxy-2-phenylalkyl, and other groups, which may be hydrophilic or hydrophobic. The molecules may preferentially bind to the contaminant at interest at low pH, and release the contaminant at a pH of about 9. The molecules may be used to filter contaminants including perchlorate and nitrate even in the presence of high sulfate concentrations.
Owner:CALIFORNIA INST OF TECH +1
Catalyst for water vapor reformation of ethanol to prepare hydrogen and its prepn and use
ActiveCN101020141ASources are cheap and readily availableEasy to prepareHydrogenChemical recyclingHydrogenWater vapor
The present invention is catalyst for water vapor reformation of ethanol to prepare hydrogen and its preparation process and use. The catalyst is composite alumina and silica carrier supported Ni-Fe, Ni-Co, Ni-Cu, Ni-Zn, Cu-Zn, Cu-Co, Ni-Zr, NiO-LaOx, NiO-CeOx, NiO-YOx, NiO-MgOx or NiO-SrOx. It is prepared through the following steps: preparing catalyst carrier; compounding mixed solution of two kinds of nitrate; prepared turbid liquid of catalyst carrier; adding the mixed solution into the turbid liquid through stirring, evaporating water, stoving, grinding and baking. The catalyst has simple preparation process, low use temperature, high ethanol converting rate, and other advantages.
Owner:YUNNAN ZHENGBANG SCI & TECH
Process for treating nitrogen-containing wastewater by ion exchange and reclaiming ammonium nitrate
InactiveCN101891316AAchieve reuseFlexible operation and managementAmmonium nitratesWater/sewage treatment by ion-exchangeHigh concentrationStrong acids
The invention discloses a process for treating nitrogen-containing wastewater by ion exchange and reclaiming ammonium nitrate, and belongs to the technical field of environmental protection and wastewater treatment. The process is characterized in that: a strong acid cation exchanger and a weak alkali anion exchanger are connected in series to realize denitrification of the nitrogen-containing wastewater of a nitrogen fertilizer plant; the process is used for treating the high-concentration ammonia nitrogen and median-concentration nitrate nitrogen containing wastewater of the nitrogen fertilizer plant; strong acid cation exchange resin and weak alkali anion exchange resin are regenerated by adopting nitric acid and aqueous ammonia respectively; and the regenerated waste liquid ammonium nitrate is evaporated and concentrated to reclaim the ammonium nitrate. Compared with the traditional wastewater denitrification method, the process can remove ammonia nitrogen and nitrate nitrogen from the wastewater at the same time, reclaim the ammonium nitrate and recycle production water, does not produce secondary pollution, reclaims products from the pollutants, has obvious environmental benefit and economic benefit, and is a new path for treating the high-concentration ammonia nitrogen and median-concentration nitrate nitrogen containing wastewater of the nitrogen fertilizer plant.
Owner:TAIYUAN UNIV OF TECH
Cellulose solvent compositions and methods of making and employing same
InactiveUS6827773B2Duplicating/marking methodsMonocomponent cellulose artificial filamentEthylenediamineCellulose
Cellulose solvents, cellulose compositions, and methods of making and using the same. For example, a cellulose composition including cellulose dissolved in the following solvent: an amine-based composition, provided however, the amine-based composition is not ammonia; and a salt selected from the group including a thiocyanate salt, a halide salt, and a nitrate salt. Representative amine-based compositions include hydrazine, hydrazine hydrate, and ethylenediamine.
Owner:NORTH CAROLINA STATE UNIV
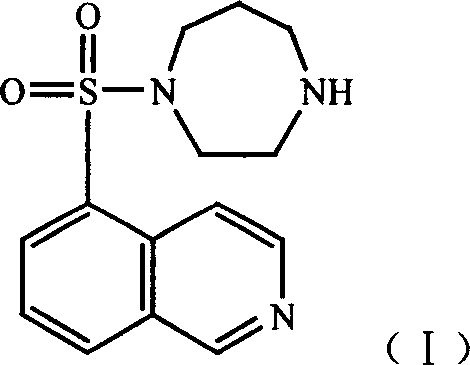
Hydrate of medicinal salt of Fasudil
InactiveCN101092413AImprove stabilityImprove solubilityOrganic active ingredientsOrganic chemistryHydrobromideDisease
This invention discloses hydrates of pharmaceutical salts of hexahydro-1-(5-sulfonylisoquinoline)-1H-1,4-diaza, i.e., fasudil, especially semi-hydrates of nitrate, sulfate, hydrobromide and mesylate of fasudil, their preparation method, and their application in drugs for preventing and / or treating cardio-cerebrovascular diseases. The hydrates of pharmaceutical salts of fasudil have such advantages as high stability and high solubility, and can be manufactured into various preparations with other active components and / or pharmaceutically acceptable carriers. The method has such advantages as simple process, high drug purity, high yield, and stable product quality, and is suitable for industrial production.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Layered catalyst for selectively oxidizing 4-methylguaiacol-to-vanillin, and preparation method thereof
ActiveCN104162444AHigh selectivityEasy to separate and purifyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIndiumCerium
The invention relates to a layered catalyst for selectively oxidizing 4-methylguaiacol-to-vanillin, and a preparation method thereof, and belongs to the technical field of catalysts. A coactive site is added to change the electronic structure of a main catalytic active site Co; and anions with different selective dear anion effect by inserting different hydrophilic and hydrophobic properties are inserted into an interlayer in order to influence the selectivity on products. One or more of magnesium, zinc, copper, aluminum, iron, nickel, manganese, titanium, samarium, calcium, cerium and chromium, gallium, indium, tin and other elements is / are added as a coactive component. The preparation method comprises the following steps: preparing a solution by using nitrates or chlorides of active components and the coactive component(s), adding a precipitating agent, carrying out a heating reaction, washing a solid substance, and drying; adding the prepared layered hydrotalciteinto a solution containing anions to be exchanged, and carrying out an ion exchange process to obtain the intercalated hydrotalcite catalyst. The prepared catalyst can be applied to the oxidation reaction of 4-methylguaiacol. The catalyst has high activity and has high selectivity to vanillin.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing perovskite type catalyst for methane or methanol recapitalization
InactiveCN101185885AEliminates sinterable propertiesHigh catalytic activityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsWater bathsMetal nitrate
The invention discloses a preparation method of a perovskite catalyst used for reforming methane or methanol, which comprises the following steps: the metal nitrates of A, A', B and B' are added with a complexing agent and heated to dissolute; ammonia regulation solution with pH=5-7 is added; gel is formed after dehydrating in a constant temperature water bath at the temperature of sixty to ninety DEG C; catalyst powders are obtained after the gel is dried and roasted; the general formula of the catalyst composition is A1-xA'XB1-yB'yO3, wherein x is more than or equal to 0 and less than or equal to1,y is more than or equal to 0 and less than or equal to 1, A or A' is La, Ce, Pr, Gd or Sm; B or B' is Cr, Zr, Zn, Ni, Co, Mn, Fe, Ru, Rh, Pt or Pd. The technique of the invention is simple; the cost is low; the catalyst has the high activity and the high stability; the sulfur tolerance is good; the conversion is high; the service life is long; at the same time, the invention has the advantages of being aseismatic, the small resistance and being easy to be replaced, etc. after being coated on a honeycomb structure and is suitable for mass production. The invention belongs to the field of the catalyst reforming the methane or methanol.
Owner:HANERGY TECH
Chromium-free treating liquid for preparing erosion-resisting oxide film on aluminum alloy surface, treating and using method thereof
ActiveCN101054664AImprove corrosion resistanceGolden colorMetallic material coating processesSodium acetateChromium free
The present invention discloses a chromium-free treatment liquid for preparing corrosion resistant oxidation film at aluminium alloy surface as well as the processing and use method thereof, characterized in that said treatment fluid utilizes a cobalt (III) salt as a main salt and a hypermanganate and a nitrate as a composite oxidizing compound to prepare a composite Co-Mn oxidation film having favorable corrosion resistance performances at aluminium alloy surface. The formula for said treatment liquid is that each litre solution contains cobalt salt of 5 g / L(-1)-20 g / L(-1), kalii permangana of 1 g / L(-1)-4 g / L(-1), sodium nitrate of 1 g / L(-1)-4 g / L(-1), promotor (chlorides and fluorides) of 1 g / L(-1)-4 g / L(-1), sodium acetate of 20 g / L(-1)-50 g / L(-1) and wetting agents (sodium dodecylbenzene sulfonate, OP-10) of 0.5 g / L(-1)-1 g / L(-1). Saic processing and use method comprises (1)sanding the aluminium alloy until its surface is smooth and even; (2) pretreating the aluminium alloy surface; (3) performing a conversion processing by using the treatment fluid; (4) cleaning-up using water and atmospheric-drying naturally. Said treatment fluid in accordance with the present invention possesses the advantages of environmental protection due to its hexavalent chromium-free, fast film-forming speed, simple process, uniform rete, strong corrosion resistance, few environment pollution, and the like.
Owner:GUANGYA ALUMINUM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com