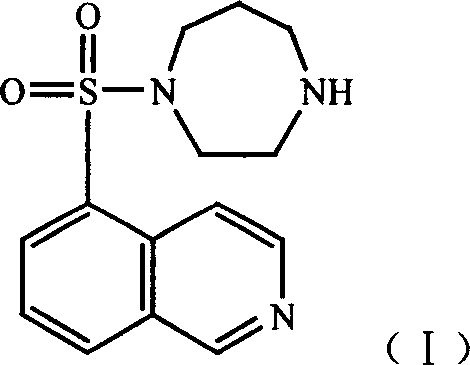
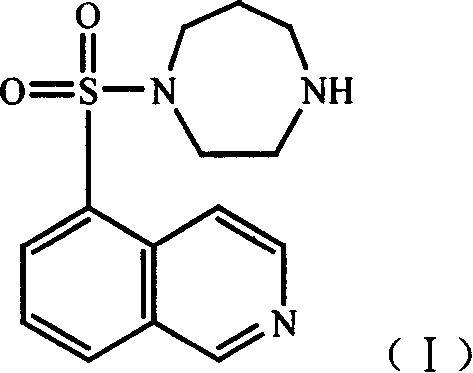
Hydrate of medicinal salt of Fasudil
A technology of fasudil and medicinal salts, which is applied in the directions of drug combinations, medical preparations containing active ingredients, medicinal formulas, etc., can solve the problems of limited clinical application, instability to humidity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 Fasudil
[0052] (1) Weigh 100g (0.48mol) of 5-isoquinolinesulfonic acid and add it to a 2000ml four-necked bottle equipped with reflux condensation and a constant pressure dropping funnel, add 400ml of thionyl chloride, stir and heat up, and drop Add 80 ml of dimethylformamide (DMF). After the addition was completed, the temperature was raised to 80° C., and the reaction was carried out with heat preservation, the color gradually changed to dark yellow, and the reaction was completed in 2 hours. Concentrate the reaction solution to dryness, add 1000ml of dichloromethane, stir evenly, freeze for 4 hours, filter with suction, and vacuum-dry the filter cake to obtain 110g of white powder, which is 5-isoquinolinesulfonyl chloride hydrochloride.
[0053] (2) Add the 5-isoquinolinesulfonyl chloride hydrochloride obtained in the previous step into ice water, adjust the pH value to 5-6 with saturated sodium bicarbonate solution under stirring,...
Embodiment 2
[0055] Example 2 Preparation and Structure Confirmation of Fasudil Nitrate and Its Hydrate
[0056] 2.1 Preparation and structure confirmation of fasudil nitrate
[0057] Take by weighing 20 g (about 69 mmol) of the pale yellow oil obtained in Example 1, be dissolved in 50 ml of absolute ethanol, and under ice bath and stirring condition, adjust the pH value to 3 with 10% nitric acid ethanol solution, and the obtained The solution was frozen for 4 hours and filtered, the filter cake was washed once with a small amount of absolute ethanol, filtered, and the filter cake was dried at 120°C for 8 hours to obtain 22.1 g of fasudil nitrate anhydrous white solid, yield: 88.6%, melting point: 218~220℃.
[0058] Elemental analysis (C 14 h 17 N 3 o2 S·HNO 3 ): C, 47.39%; H, 5.17%; N, 15.80%; S, 9.02%; Theoretical: C, 47.45%; H, 5.12%; N, 15.81%; S, 9.05%.
[0059] IR(KBr)cm -1 : 3378, 2915, 2538, 1620, 1466, 1324, 1150, 1133, 1015, 950, 824, 759, 711.
[0060] 2.2 Preparation ...
Embodiment 3
[0066] Example 3 Preparation and Structure Confirmation of Fasudil Sulfate and Its Hydrate
[0067] 3.1 Preparation and structure confirmation of fasudil nitrate
[0068] Take by weighing 20g (about 69mmol) of light yellow oily matter gained in Example 1, be dissolved in the dehydrated alcohol of 50ml, under ice-bath and stirring condition, adjust pH value to 3 with 10% sulfuric acid ethanol solution, obtain The solution was frozen for 4 hours and filtered, the filter cake was washed once with a small amount of absolute ethanol, filtered, and the filter cake was dried at 120° C. for 8 hours to obtain 20.3 g of a white solid, namely fasudil anhydrous sulfate. Yield: 84.7%, melting point: 215-220°C.
[0069] Elemental analysis (C 14 h 17 N 3 o 2 S 1 / 2H 2 SO 4 ): C, 49.35%; H, 5.37%; N, 12.33%; S, 14.12%; Theoretical: C, 49.40%; H, 5.33%; N, 12.34%; S, 14.13%.
[0070] IR(KBr)cm -1 : 3384, 2918, 2543, 1625, 1460, 1330, 1159, 1140, 1017, 956, 831, 765, 718.
[0071] 3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com