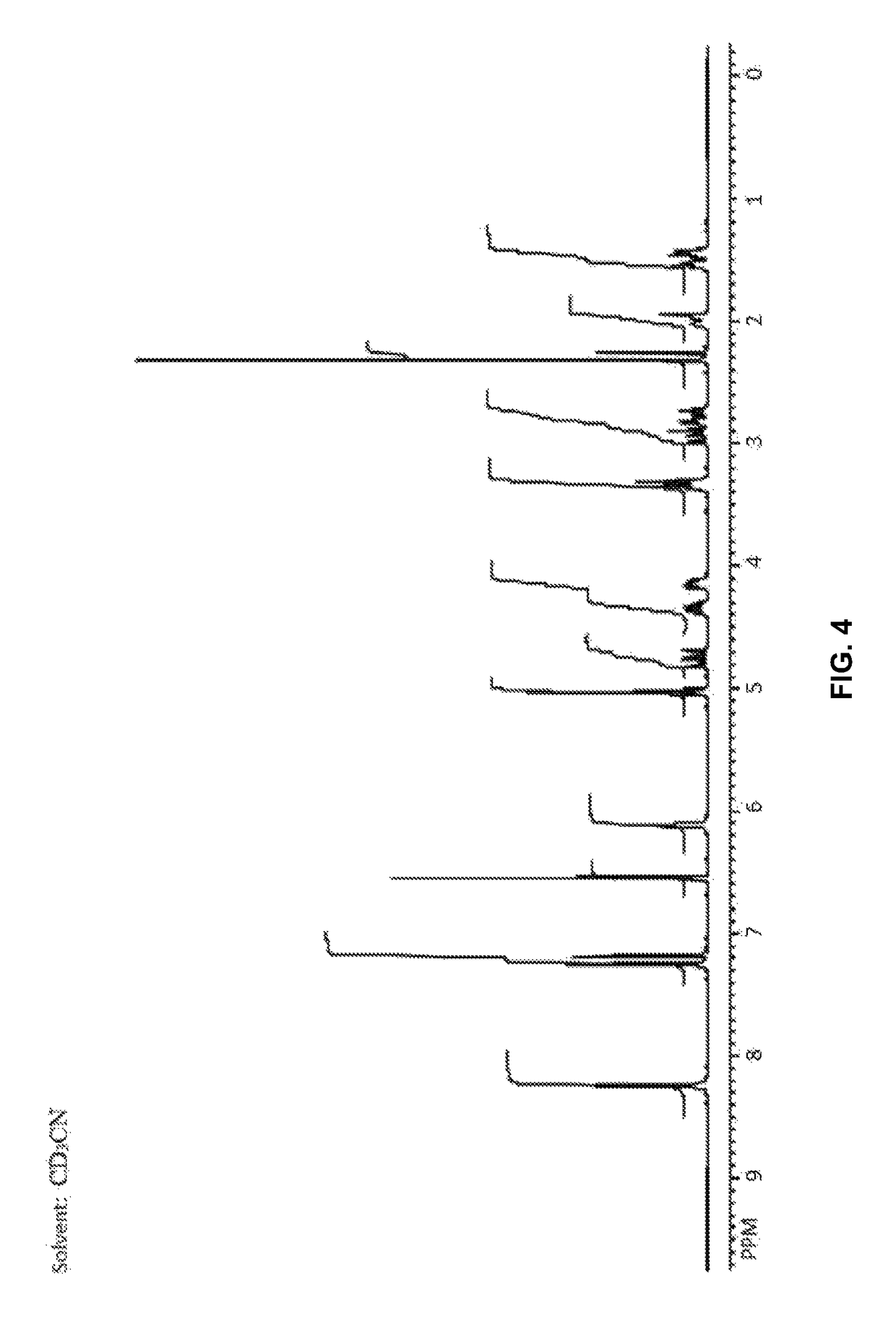
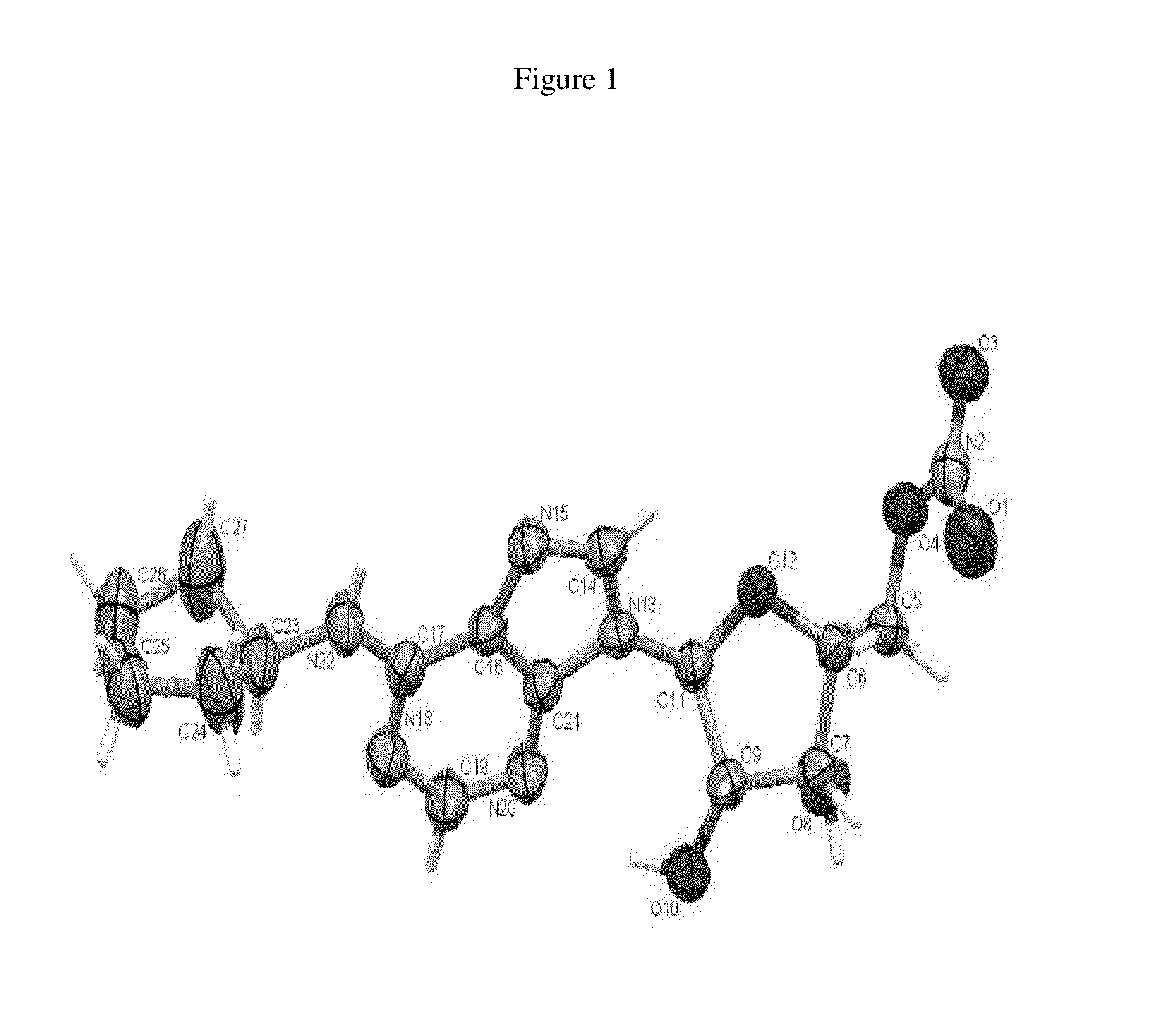
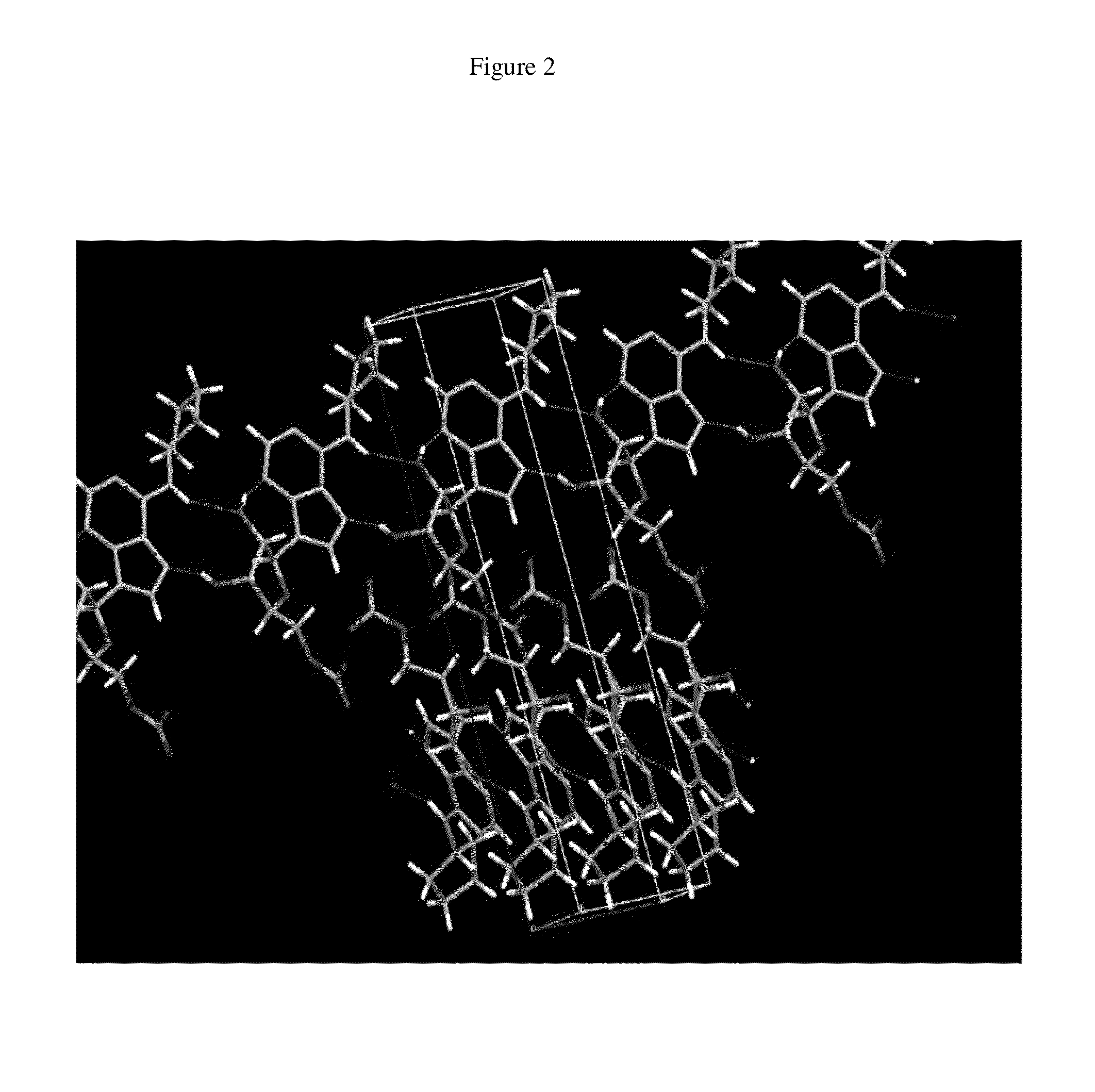
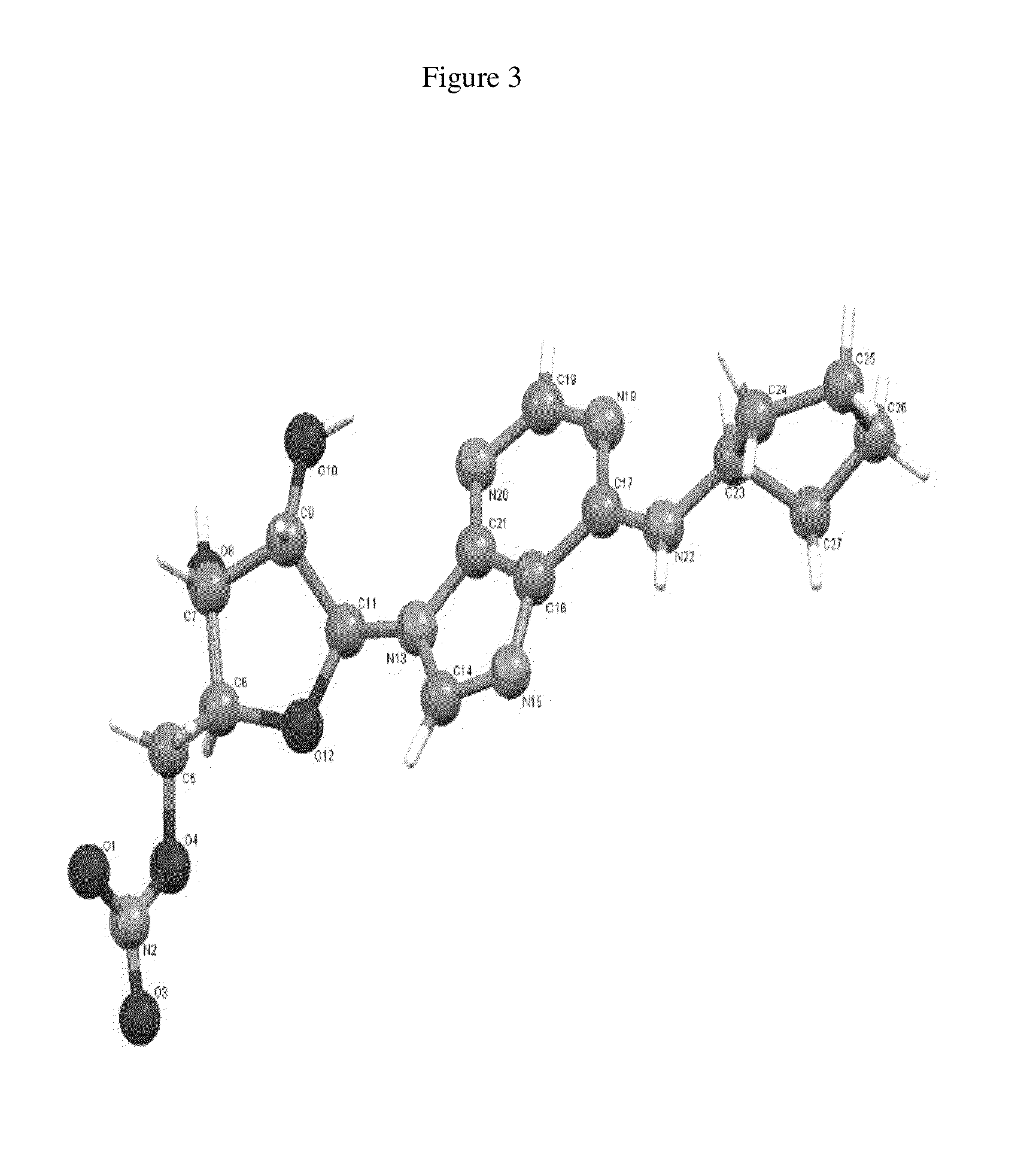
Patents
Literature
115 results about "Polymorphs of silicon carbide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Many compound materials exhibit polymorphism, that is they can exist in different structures called polymorphs. Silicon carbide (SiC) is unique in this regard as more than 250 polymorphs of silicon carbide had been identified by 2006, with some of them having a lattice constant as long as 301.5 nm, about one thousand times the usual SiC lattice spacings.
Polymorphs of abiraterone acetate and preparation method thereof
The invention discloses polymorphs A, B, C and D of abiraterone acetate. A preparation method of the polymorphs comprises the step of re-crystallizing the abiraterone acetate subjected to the separation and the purification of a chromatographic column in different solvents. Through stable investigation, four polymorphs have favorable stability and flowability, can be used as raw materials for storage and transportation and are suitably applied to antitumor medicinal preparations.
Owner:深圳万乐药业有限公司
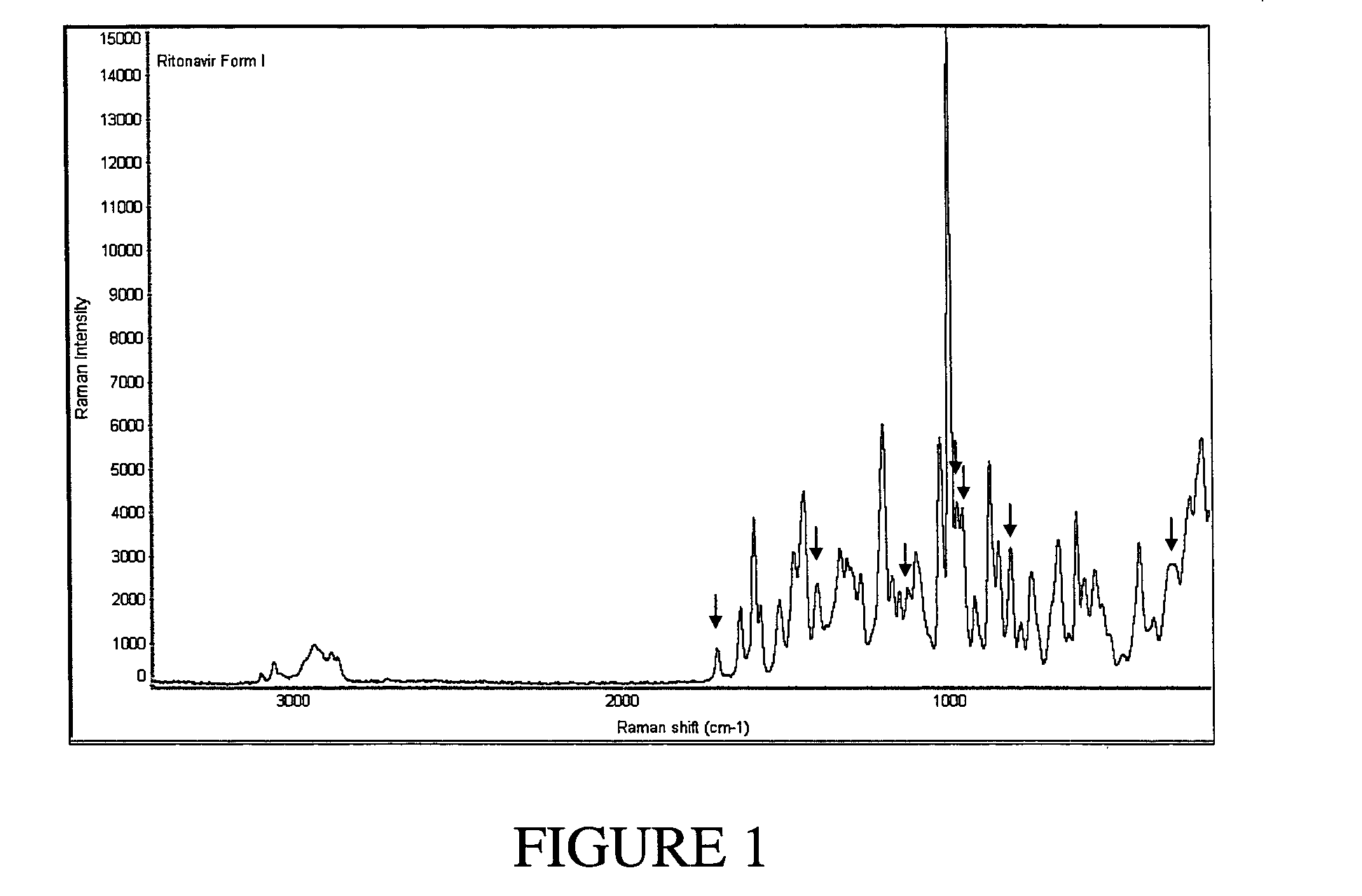
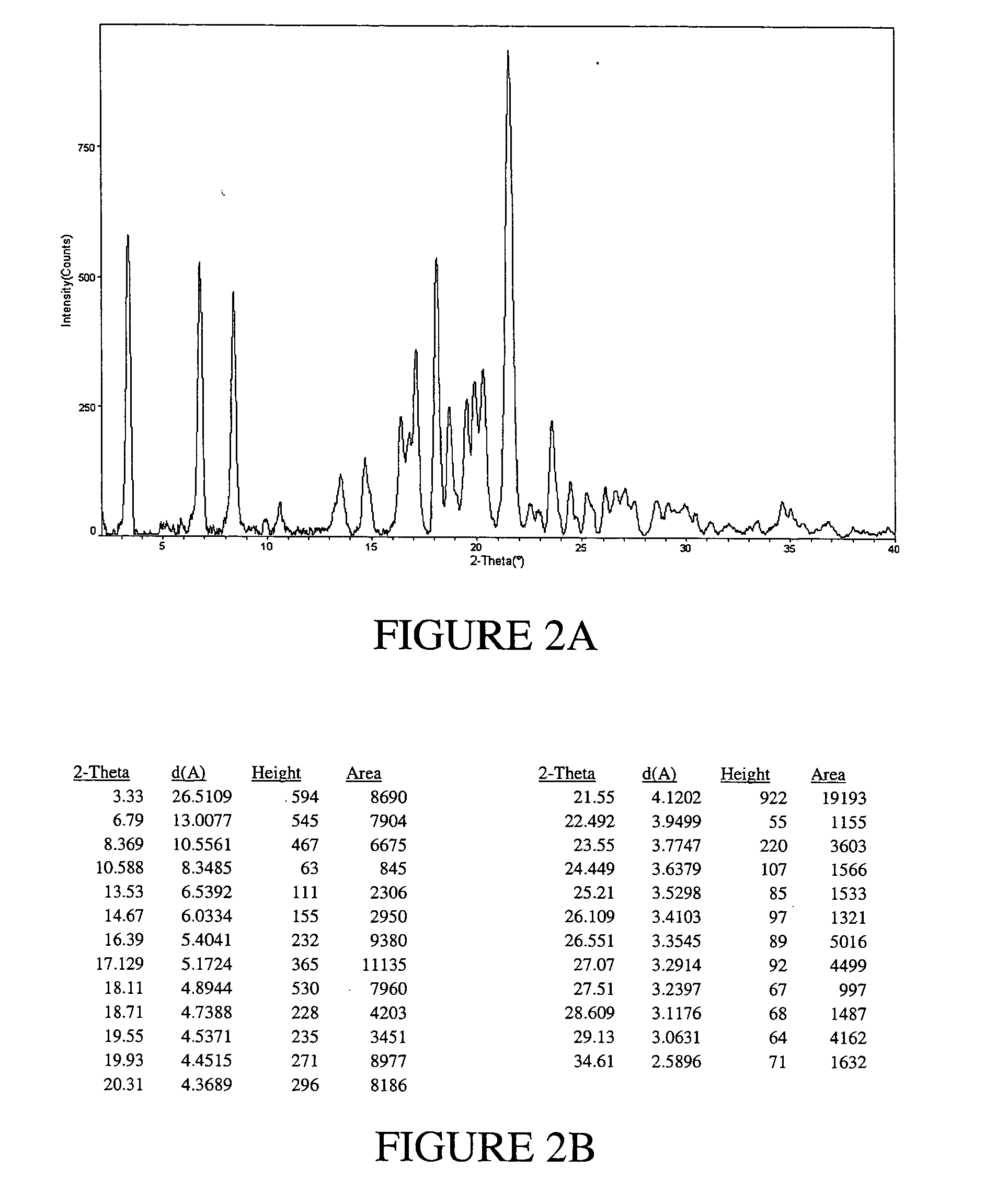
Solvates and polymorphs of ritonavir and methods of making and using the same
ActiveUS20040024031A1Improved pharmacokinetic profileOrganic active ingredientsBiocideDiseaseMedicinal chemistry
Novel solvates and crystal polymorphs of Ritonavir are disclosed, as well as methods of making them. Specific solvates of the compound include a formamide solvate and a partially desolvated solvate. Also disclosed are methods of making previously known forms of Ritonavir. Methods of using the novel forms of Ritonavir for the treatment of diseases, such as HIV-infection, are disclosed, as are pharmaceutical compositions and unit dosage forms comprising the novel forms of Ritonavir.
Owner:CENTOCOR ORTHO BIOTECH
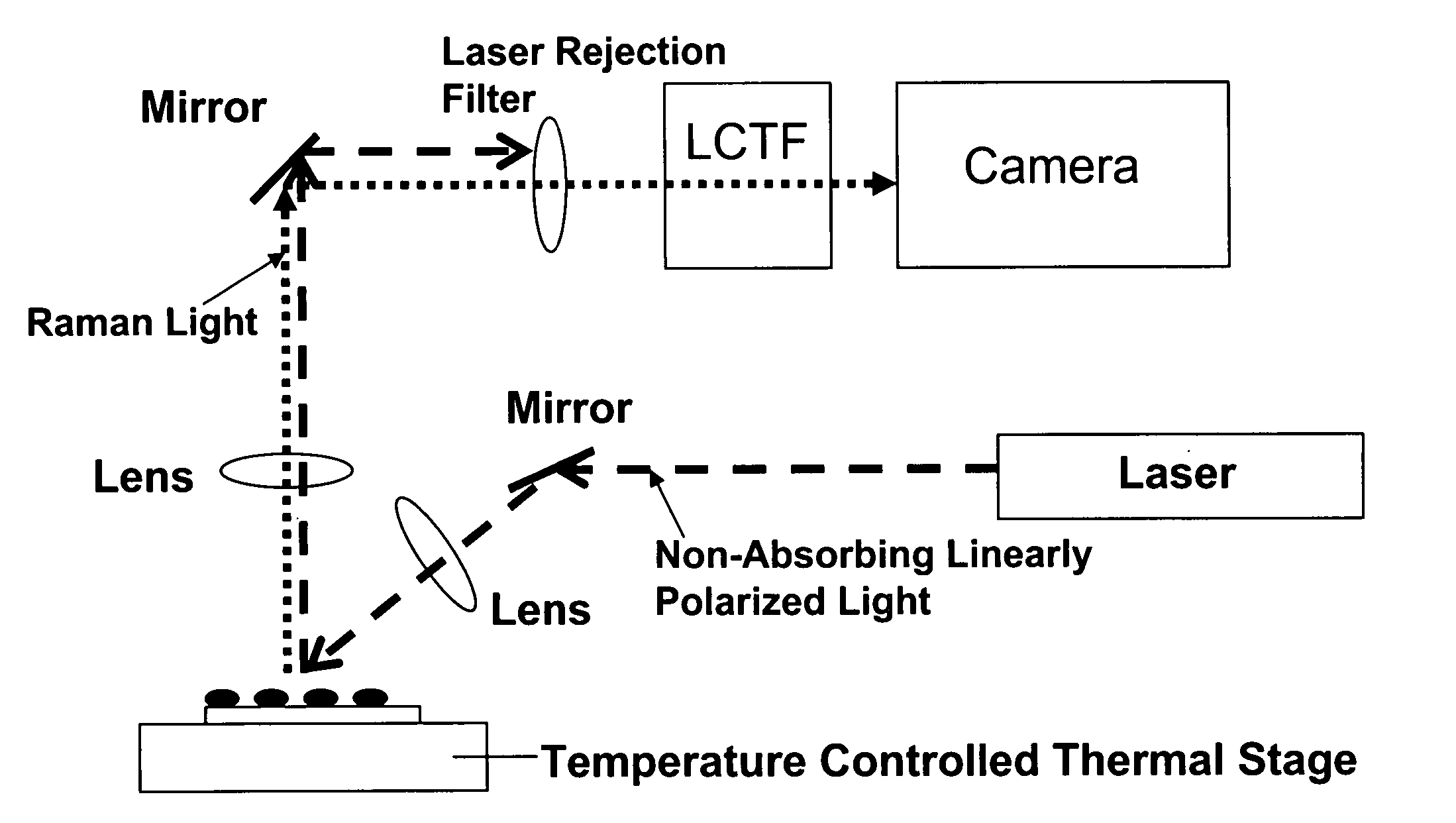
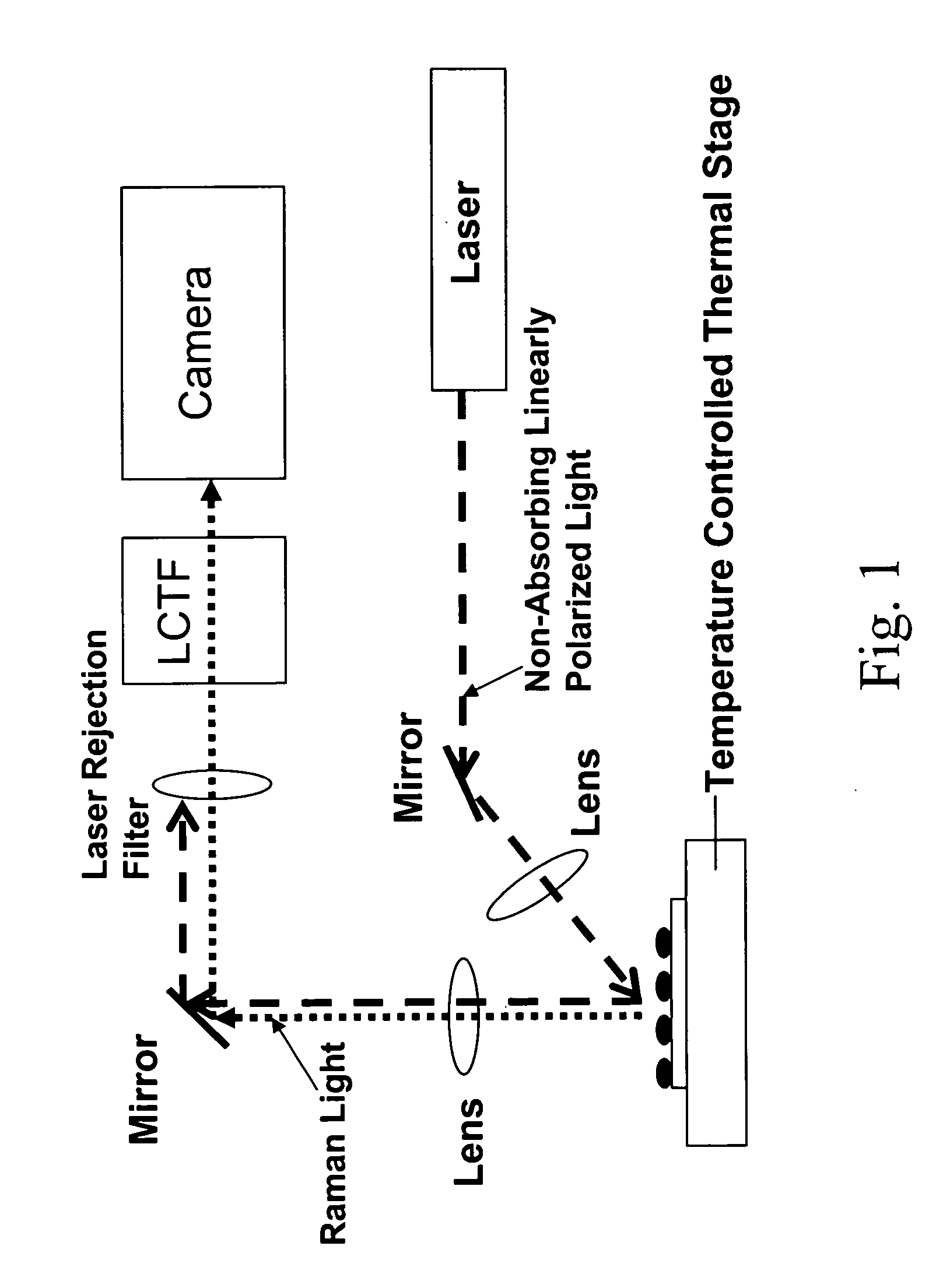
Control and monitoring of non-resonant radiation-induced nucleation, crystallization, and polymorph formation
InactiveUS20060124443A1Crystallization conditions screeningOrganic compound preparationOptical radiationEngineering
The invention relates to methods of assessing the polymorphic form of a substance by assessing Raman-shifted radiation scattered by a particle of the substance. The method is useful, for example, for assessing particle sizes and size distributions in mixtures containing both particles of the substance and other materials. The invention also relates to methods of selecting and controlling polymorph formation by illuminating a material with non-resonant (i.e., non-absorbed) laser radiation as it is thermally driven through a phase transition temperature.
Owner:CHEMIMAGE
Compositions and formulations of 9-nitrocamptothecin polymorphs and methods of use therefor
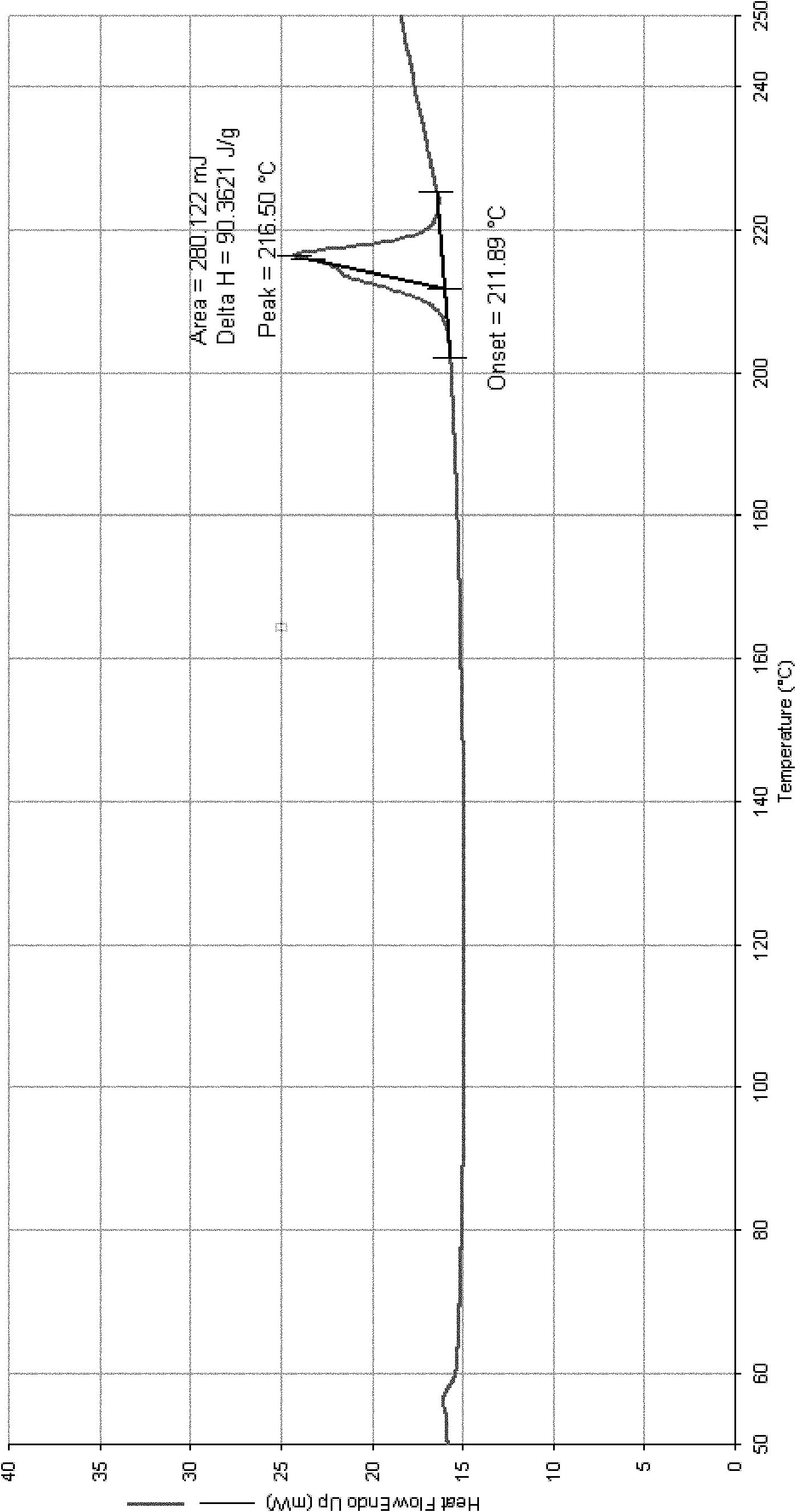
A polymorphic form of 9-nitrocamptothecin is provided, the polymorph being characterizable as having, by differential scanning calorimetry, an endotherm at between 149.2 and 151.2° C., an exotherm at between 162.6 and 164.6° C., and an exotherm at between 272 and 274° C.
Owner:SUPERGEN
Dexlansoprazole process and polymorphs
Processes for the preparation of dexlansoprazole, an amorphous form of dexlansoprazole, a solid dispersion of amorphous dexlansoprazole and a pharmaceutically acceptable carrier, and processes for their preparation are provided. Also provided are crystalline compounds 2-[(R)-[(4-chloro-3-methyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole and 2-[(R)-[(4-nitro-3-methyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, and methods for their preparation.
Owner:DR REDDYS LAB LTD
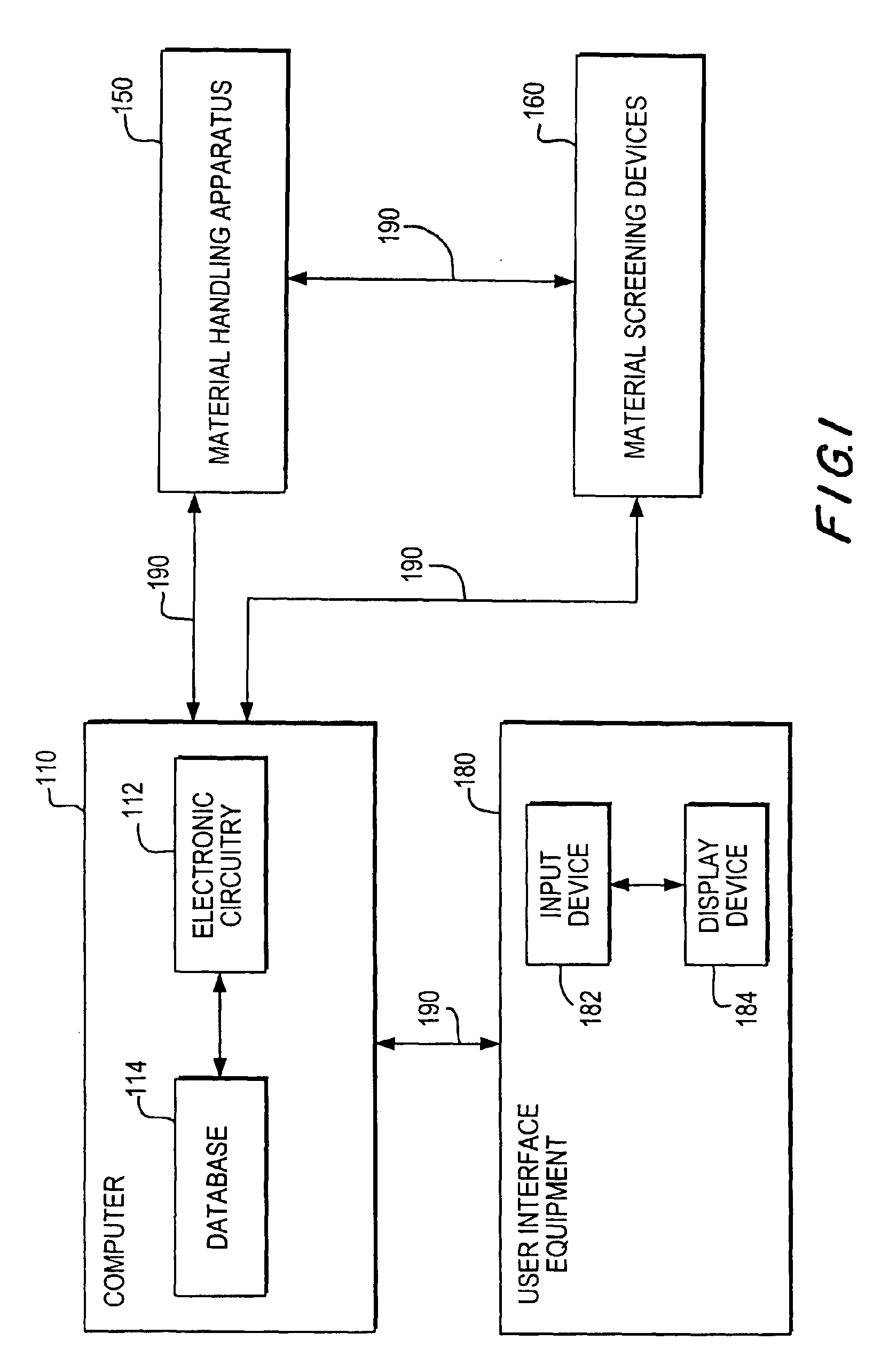
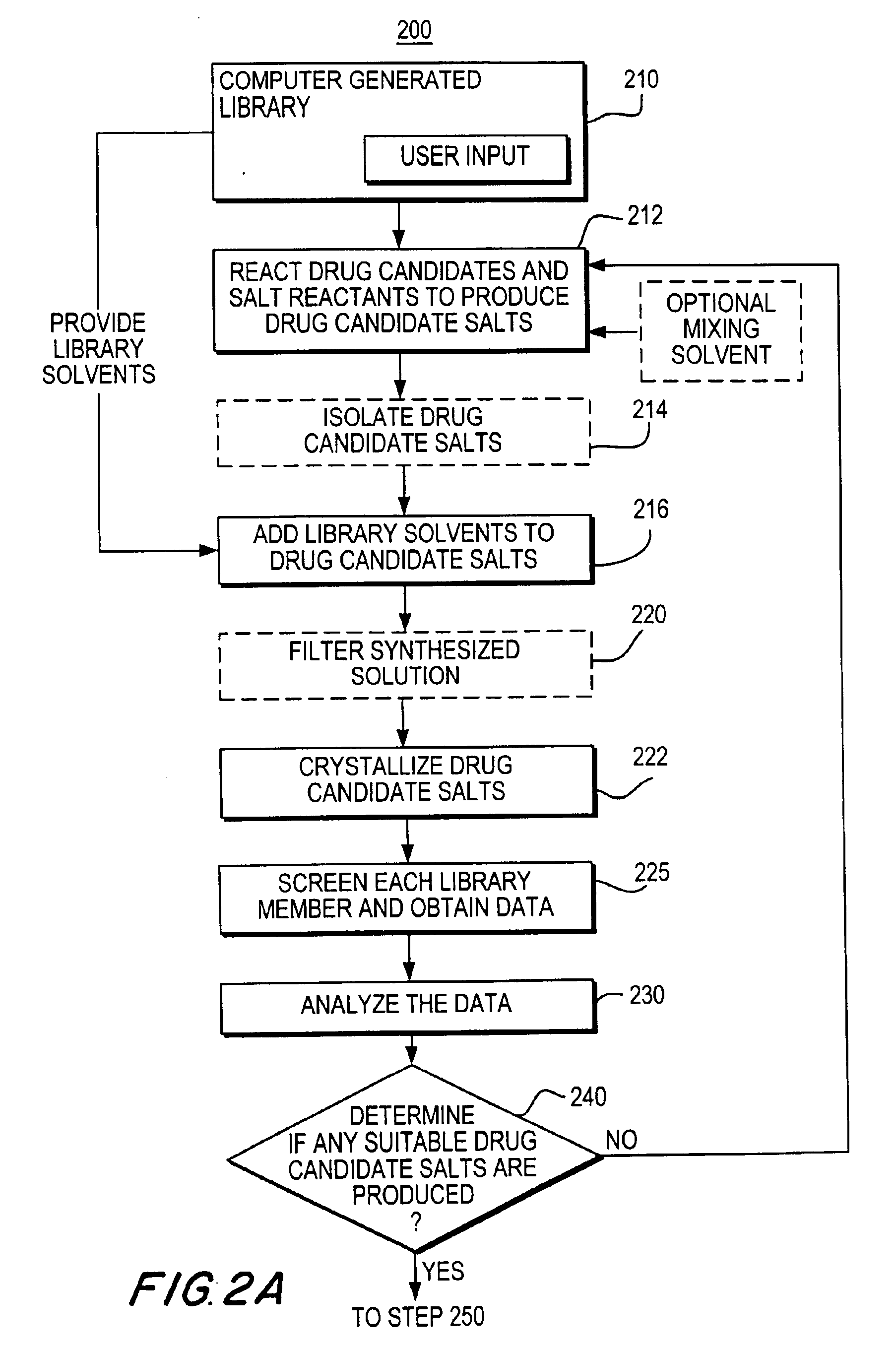
Apparatuses and methods for creating and testing pre-formulations and systems for same
InactiveUS6939515B2Shorten the timeReduce riskSequential/parallel process reactionsOrganic chemistry methodsMedicineComputerized system
The invention provides methods, apparatus, and systems for performing high-throughput preparation and screening of salts and polymorphs of drug candidates. The invention is directed towards enhancing the pre-formulation discovery process used for drug development. In particular, processes that determine suitable salts and processes that discover substantially every polymorph that can form from a particular drug candidate are provided. The processes are performed using several apparatuses that are specifically configured to carry-out various steps in a high-throughput characterization process. One such apparatus is configured for synthesizing a plurality of library members based on, for example, a library model generated by a computer system. Another apparatus may filter the synthesized solution to provide a substantially pure mixture that can be subjected to salt or polymorph testing. Yet another apparatus may be used to crystallize mixtures on a substrate such that the crystallized mixture can be screened by one or more screening devices.
Owner:FREESLATE
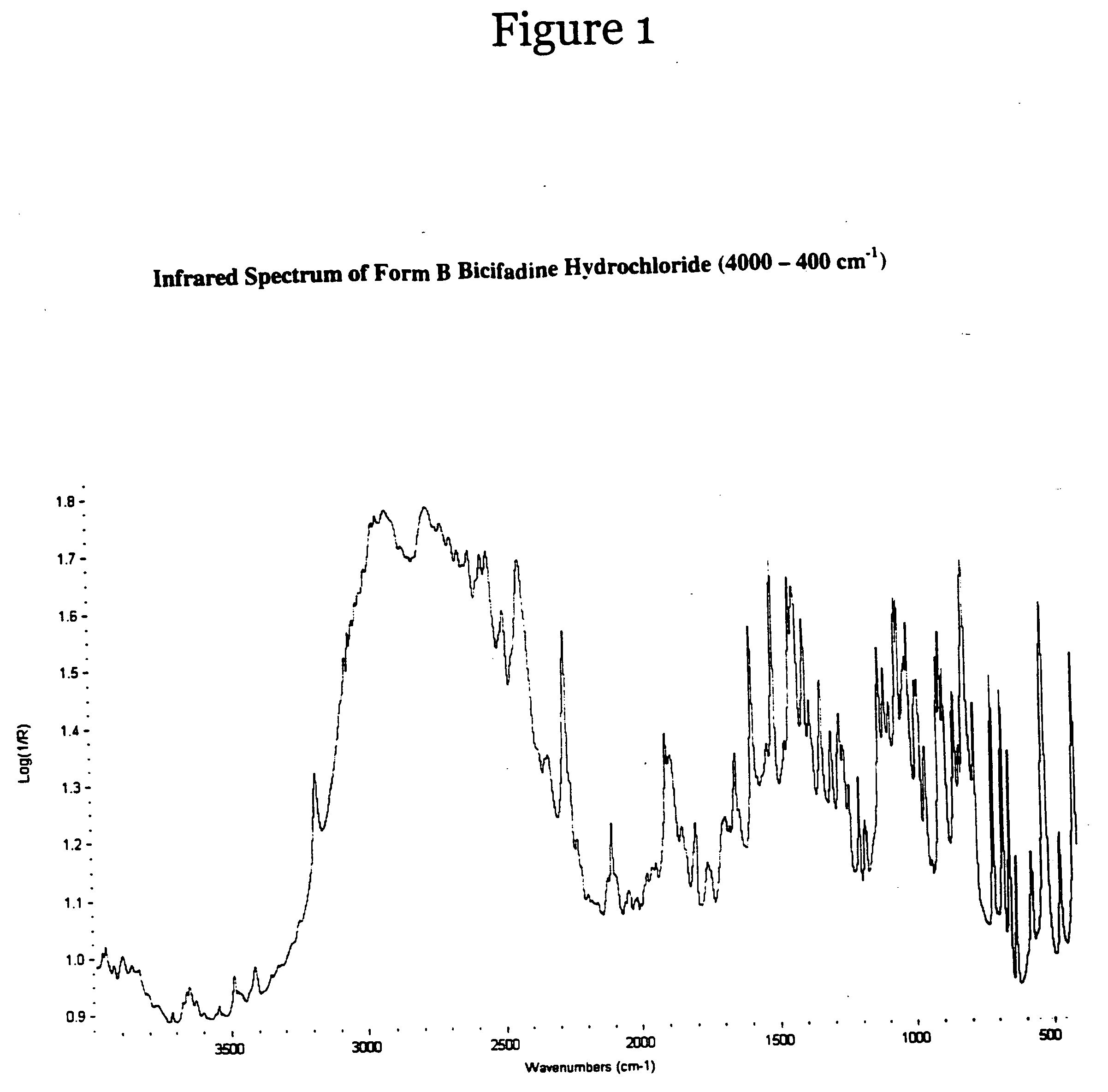
Polymorphs of bicifadine hydrochloride
A new polymorphic crystalline form of bicifadine hydrochloride, designated form B, which is more thermodynamically stable than the previously known polymorphic form of bicifadine hydrochloride, designated as form A, methods for preparing said crystalline form B and pharmaceutical compositions containing said crystalline form B.
Owner:EBI LIFE SCI INC
Method for using a static electric field to induce crystallization and to control crystal form
InactiveUS20050256300A1From normal temperature solutionsPeptide/protein ingredientsTesting tubesDc voltage
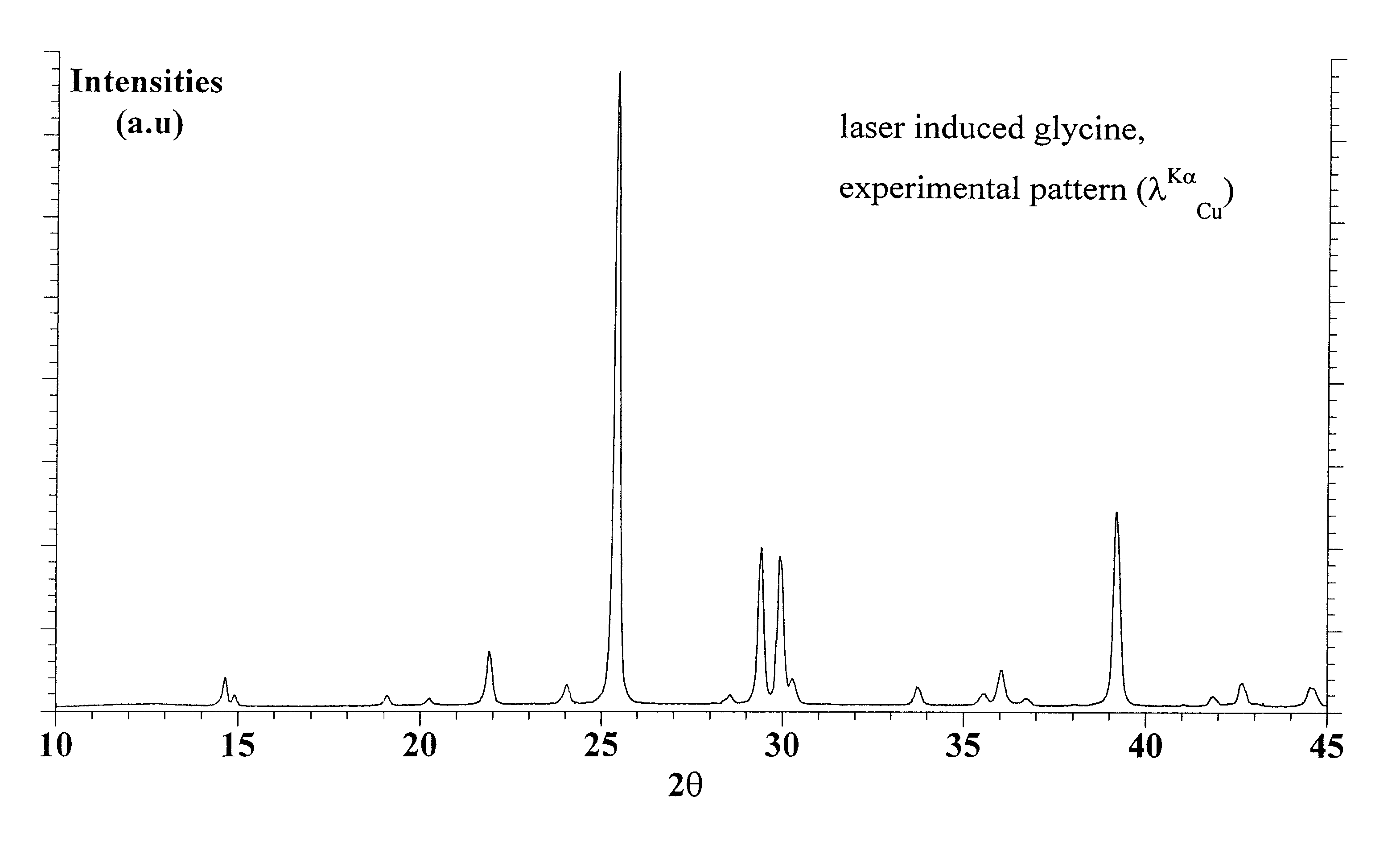
Applying a strong static DC electric field to supersaturated aqueous glycine solutions resulted in the nucleation of the γ polymorph attributed to the electric-field induced orientation of the highly polar glycine molecules in large preexisting solute clusters, helping them organize into a crystalline structure. A method to induce crystallization and to prepare polymorphs and / or morphologies of materials by using a static electric field to cause nucleation and crystal growth to occur in a supersaturated solution in such a way as to obtain a crystal structure that would not normally appear without the use of the static electric field. Aqueous glycine solutions were prepared by combining solid glycine and water. Supersaturated solutions were generated by heating the tubes to 62-64° C. and holding them at that temperature in an ultrasonicator overnight. Once the glycine was completely dissolved, the solutions were slowly cooled to room temperature. A chamber was constructed consisting of two brass electrodes separated by a 5 mm insulating gap, with a hole drilled down through the center, parallel to the gap-electrode interface, with a diameter large enough to accommodate the test tube. A DC voltage was applied across the electrodes, large enough to produce electric fields in the range of 400,000 to 800,000 V / m. Tests tubes containing the aged solutions were placed in the high-voltage chamber. Exposure of the aged solutions to fields of 600,000 V / m resulted in crystallization typically within 30-90 min. The onset of nucleation was observed visually by the formation of a needle-shaped crystallite.
Owner:INTELLECTUAL VENTURES HLDG 19
Amorphous polymorph for pemetrexed disodium and preparation method thereof
The invention discloses an amorphous polymorph for pemetrexed disodium and a preparation method thereof. An X-ray powder diffraction pattern of the amorphous polymorph does not contain an identifiable diffraction peak form. For not containing crystal water, the amorphous polymorph for the pemetrexed disodium is simple to dry on production, does not need special equipment, has low production cost, and is suitable for industrial production.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Imatinib mesylate polymorph and pharmaceutical composition
ActiveCN102070605ANot suitable for the needs of large-scale and stable productionSimple preparation processOrganic active ingredientsSenses disorderImatinib mesylateMedicine
The invention discloses an imatinib mesylate polymorph I, which is a typical X-ray diffraction pattern and has a diffraction peak at 2theta of 17.7+ / -0.2 degrees, 18.1+ / -0.2 degrees, 18.6+ / -0.2 degrees, 19.1+ / -0.2 degrees, 19.7+ / -0.2 degrees and 20.4+ / -0.2 degrees. In addition, the invention also discloses a preparation method thereof and a pharmaceutical composition. The imatinib mesylate polymorph I has the advantages of high purity, excellent physicochemical property and high stability, and is more suitable for industrial mass production.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Method for using laser light to control crystal form
InactiveUS6426406B1Organic chemistry methodsCarboxylic acid esters preparationCrystal structureLaser light
A method to prepare new or unexpected polymorphs of materials which have not been observed, or to obtain a known polymorph under different conditions than those in which it is usually made, by using a laser to cause nucleation and crystal growth to occur in a supersaturated solution in such a way as to obtain a crystal structure which would not normally appear without the use of the laser.
Owner:INTELLECTUAL VENTURES HLDG 19
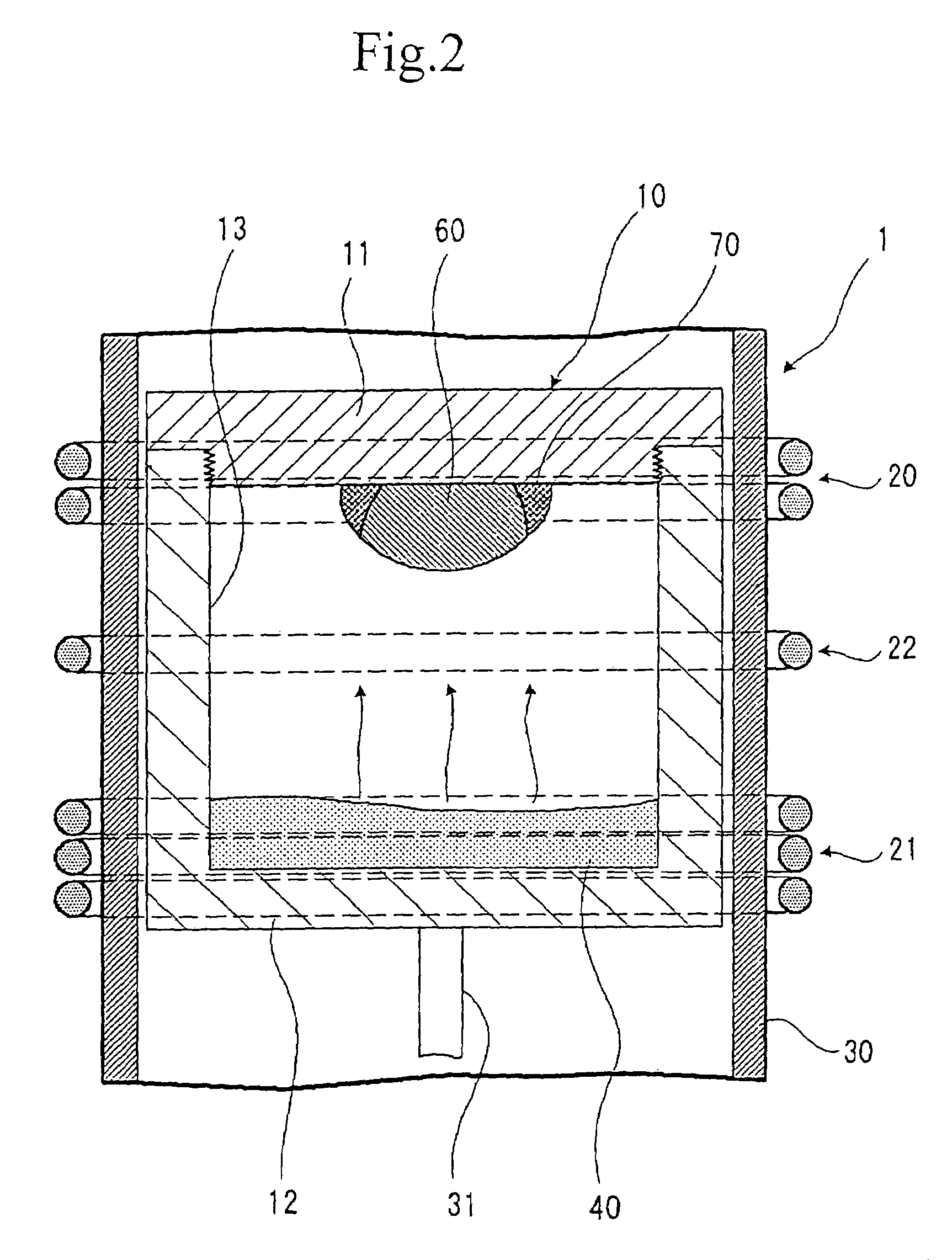
Silicon carbide single crystal and method and apparatus for producing the same
InactiveUS7048798B2Maintain good propertiesIncrease resistancePolycrystalline material growthSemiconductor/solid-state device manufacturingSeed crystalElectron
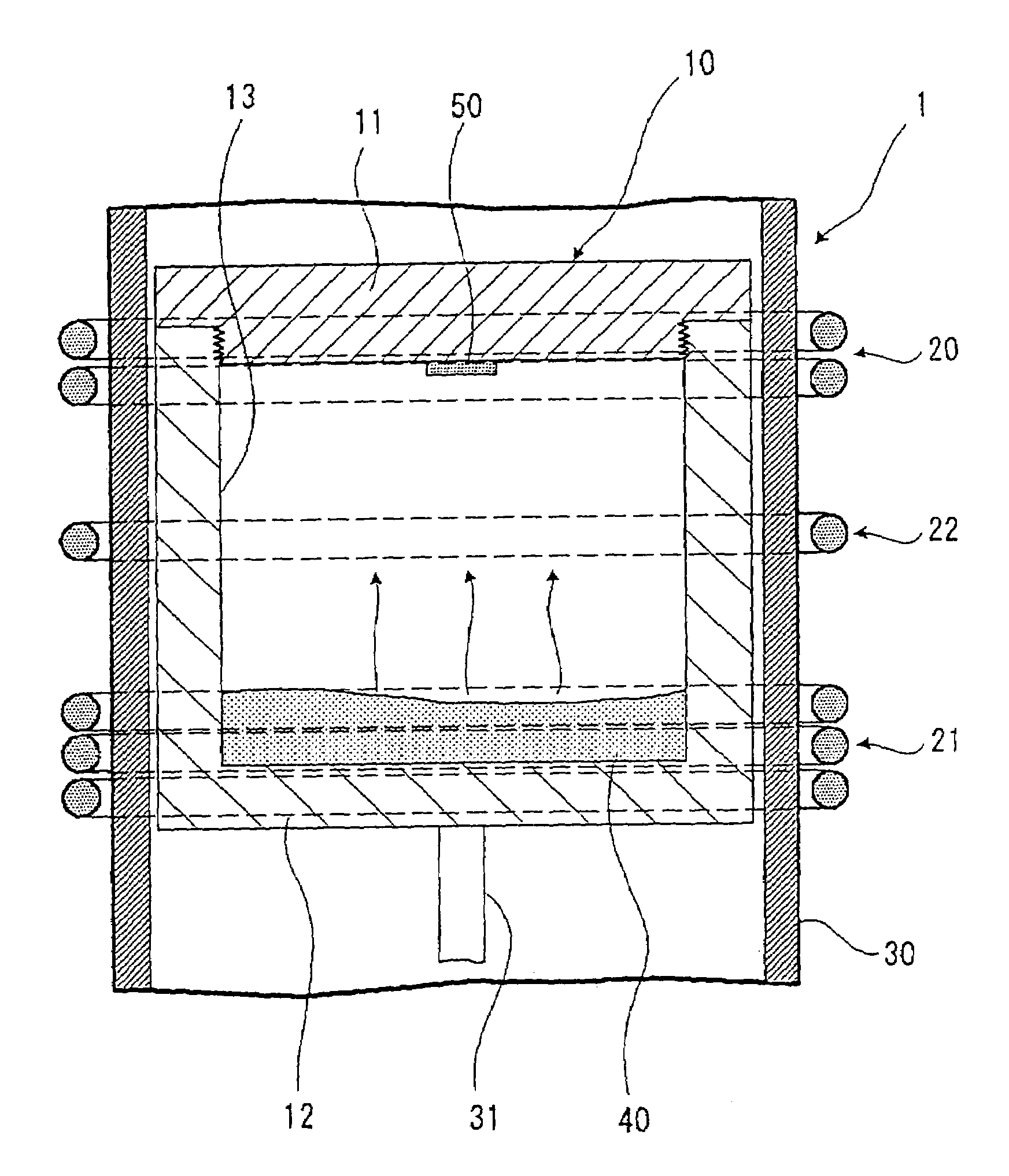
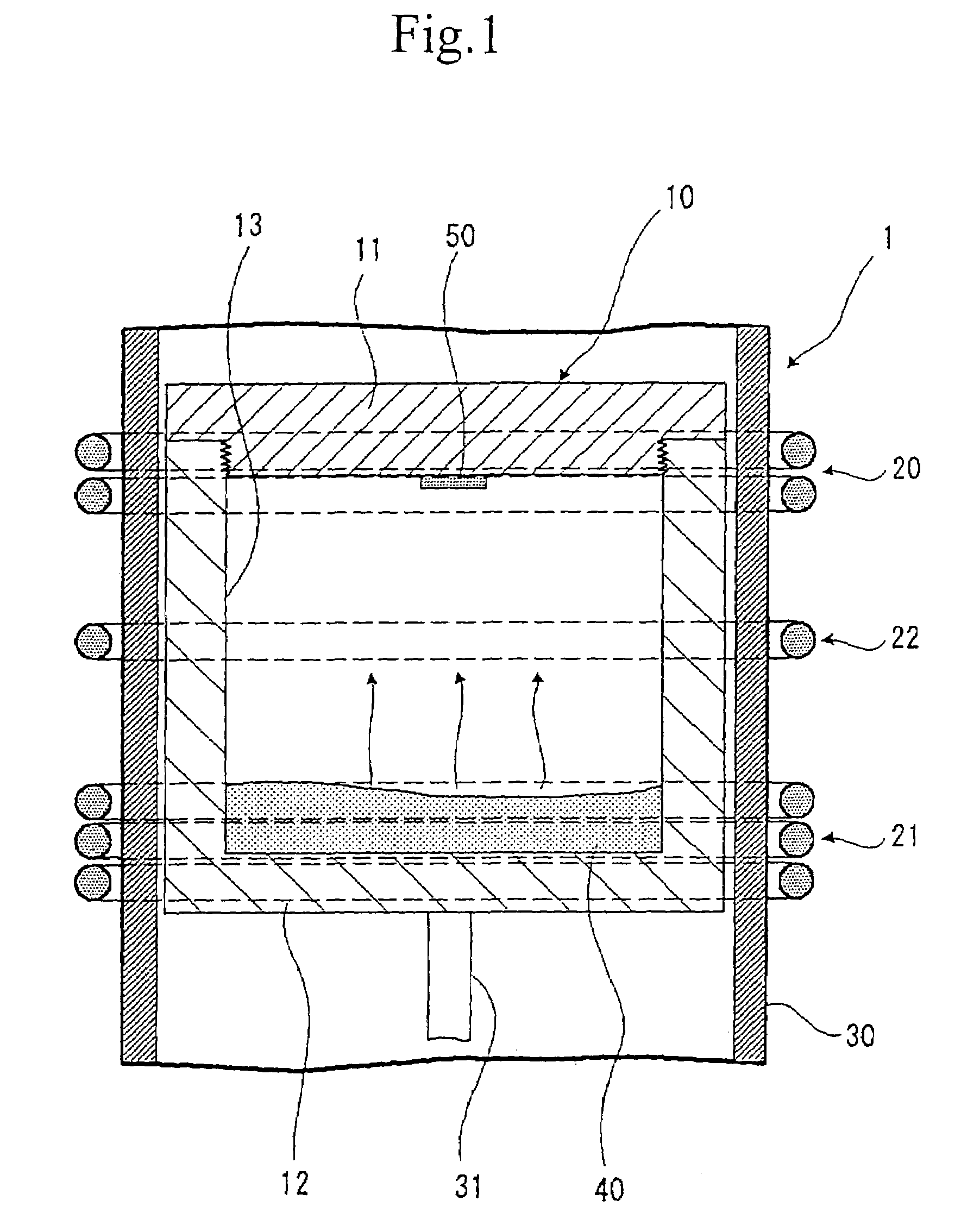
A method of producing a silicon carbide single crystal in which a sublimation raw material 40 is accommodated at the side of vessel body 12 in a graphite crucible 10, placing a seed crystal of a silicon carbide single crystal at the side of cover body 11 of the graphite crucible 10, the sublimation raw material 40 is sublimated by a first induction heating coil 21 placed at the side of sublimation raw material 40, a re-crystallization atmosphere is form by a second induction heating coil 20 placed at the side of cover body 11 so that the sublimation raw material 40 sublimated by the first induction heating coil 21 is re-crystallizable only in the vicinity of the seed crystal of a silicon carbide single crystal, and the sublimation raw material 40 is re-crystallized on the seed crystal of a silicon carbide single crystal, and a silicon carbide single crystal 60 is grown while keeping the whole surface of its growth surface in convex shape through the all growth processes.A high quality silicon carbide single crystal with large diameter excellent in dielectric breakdown property, heat resistance, radiation resistance and the like, suitable for electronic and optical devices and the like, and showing no contamination of polycrystals and polymorphs, no defect of micropipes and the like can be produced efficiently without cracking and the like.
Owner:RESONAC HOLDINGS CORPORATION
Bendamustine hydrochloride crystal and preparation method thereof
InactiveCN102351799ASimple preparation processSimple and fast operationOrganic chemistrySolubilityBendamustine hydrochloride
The invention discloses a new bendamustine hydrochloride crystal and a preparation method thereof. On a characteristic X-ray powder diffraction pattern, the polymorph I of bendamustine hydrochloride disclosed by the invention has one or more characteristic peaks represented by 2theta at the positions as follows: 10.6+ / -0.2, 15.0+ / -0.2, 18.7+ / -0.2, 20.0+ / -0.2, 22.9+ / -0.2, and 26.5+ / -0.2. The new crystal of bendamustine hydrochloride has the characteristics of good solubility, good stability and the like. The operation method is simple and easy to operate, and is easy to industrially produce.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Test kit for the quantitative determination of narcotic drugs
A test kit for the quantitative determination of narcotic drugs comprising (A) series of sealed vessels, each vessel containing a deuterium free isotopologue of a narcotic drug in exactly defined concentrations and quantities, wherein the isotopologue differs from vessel to vessel and—wherein the quantities of the isotopologue differ from vessel to vessel or are the same for all vessels; and / or (B) series of sealed vessels, each vessel containing in exactly defined concentrations and quantities the same isotopologue in quantities which differ from vessel to vessel; wherein the free isotopologues are selected from narcotic drugs; prodrugs, salts, solvates, hydrates and polymorphs and contain at least three stable isotopes selected from the group consisting of 13C, 15N and 18O in the molecule with a degree of labeling of at least 95 mol-%; the use of the test kit and a method for quantitatively determining narcotic drugs.
Owner:CHIRON CORP
Ingavirin polymorph and its preparation method
InactiveCN103382179AGood physical and chemical propertiesImprove stabilityOrganic chemistryEngineeringIn vivo
The invention belongs to the field of chemical pharmaceutical technology and relates to an ingavirin polymorph and its preparation method. The invention aims to provide the ingavirin polymorph in allusion to insufficiencies existing in the prior art. The invention provides ingavirin polymorphs A and B which have excellent physicochemical properties and good stability and are used in the research on in vivo study of ingavirin. Meanwhile, the invention provides a preparation method of the polymorphs A and B.
Owner:SICHUAN BAILI PHARM CO LTD
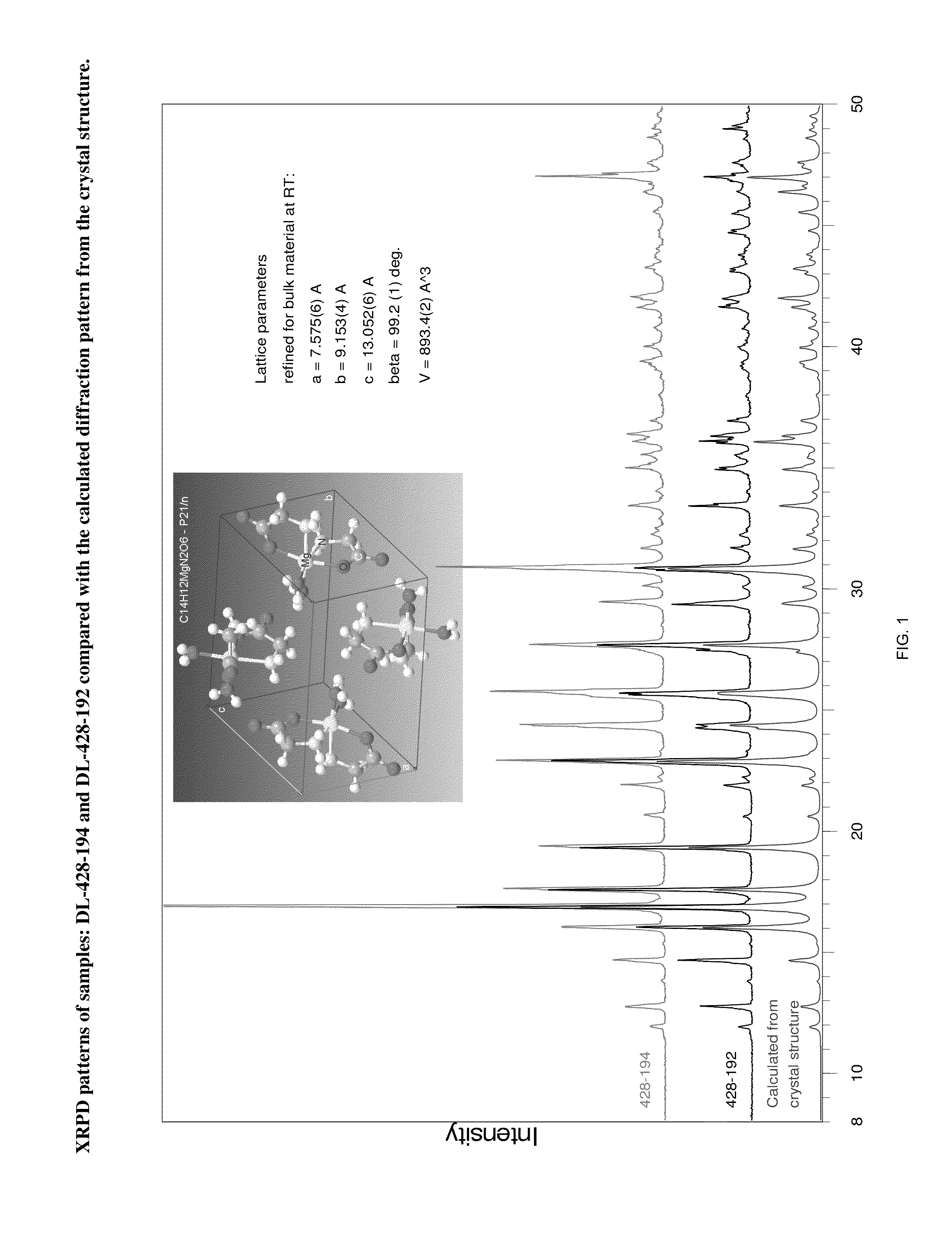
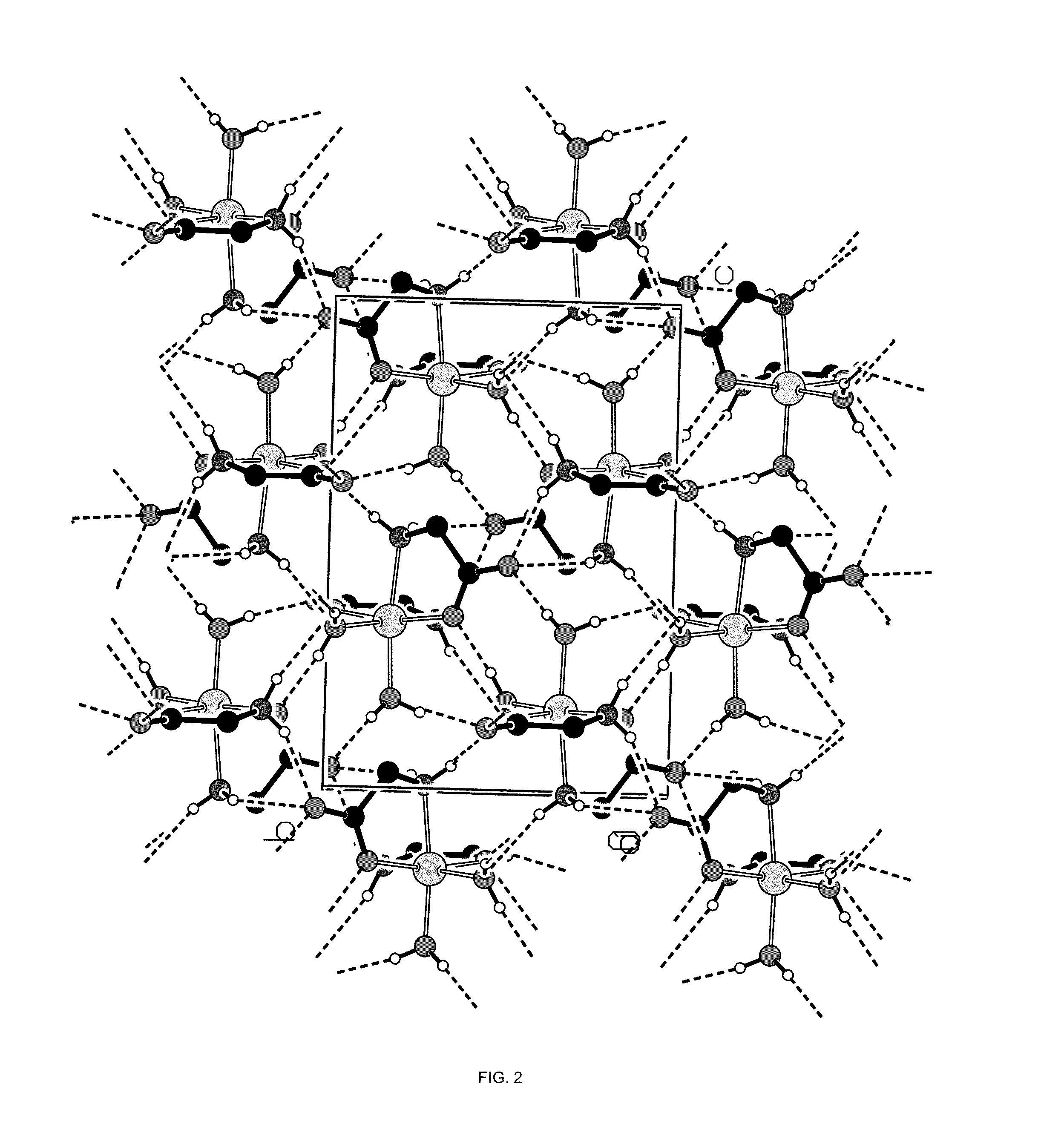
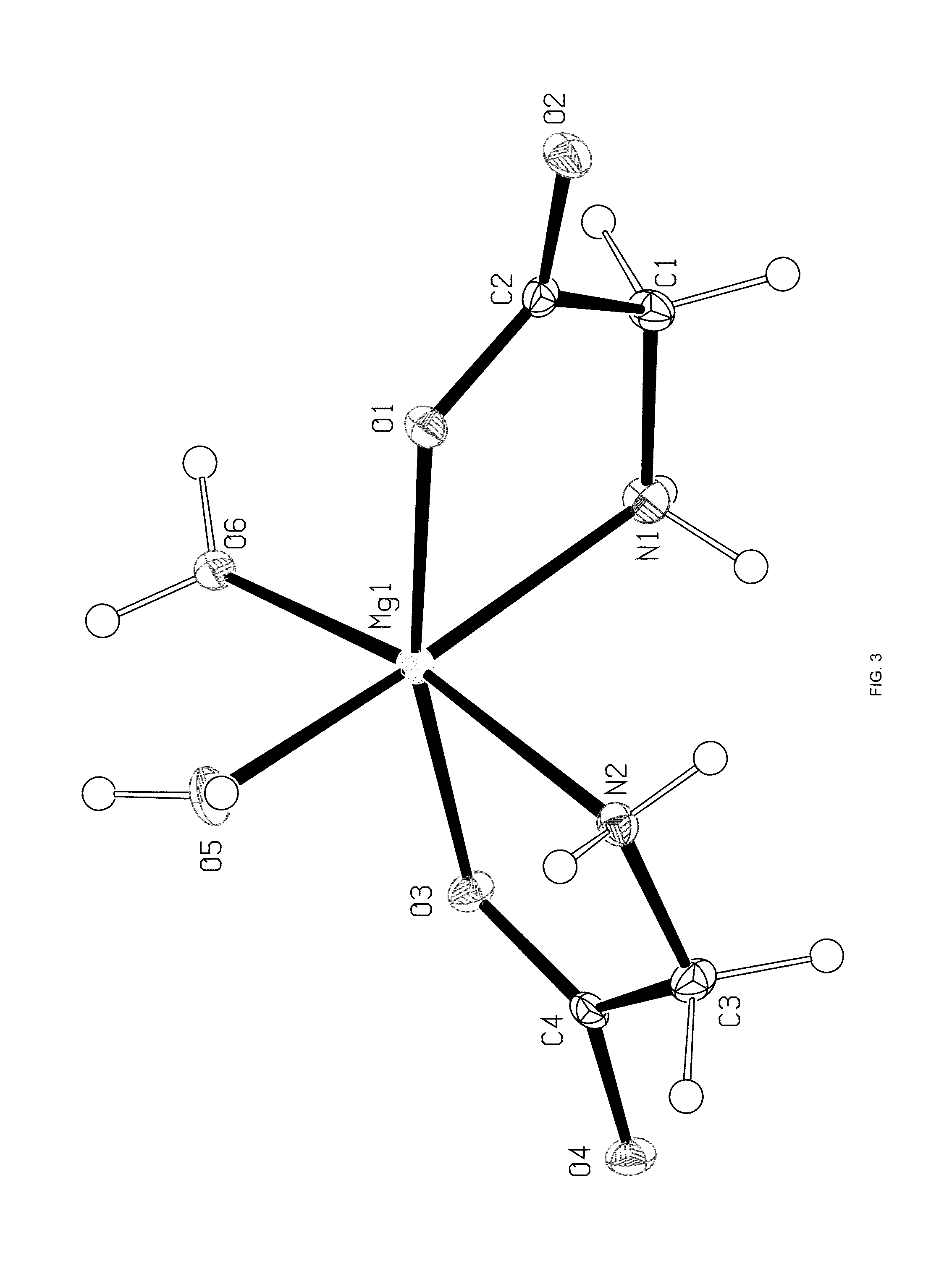
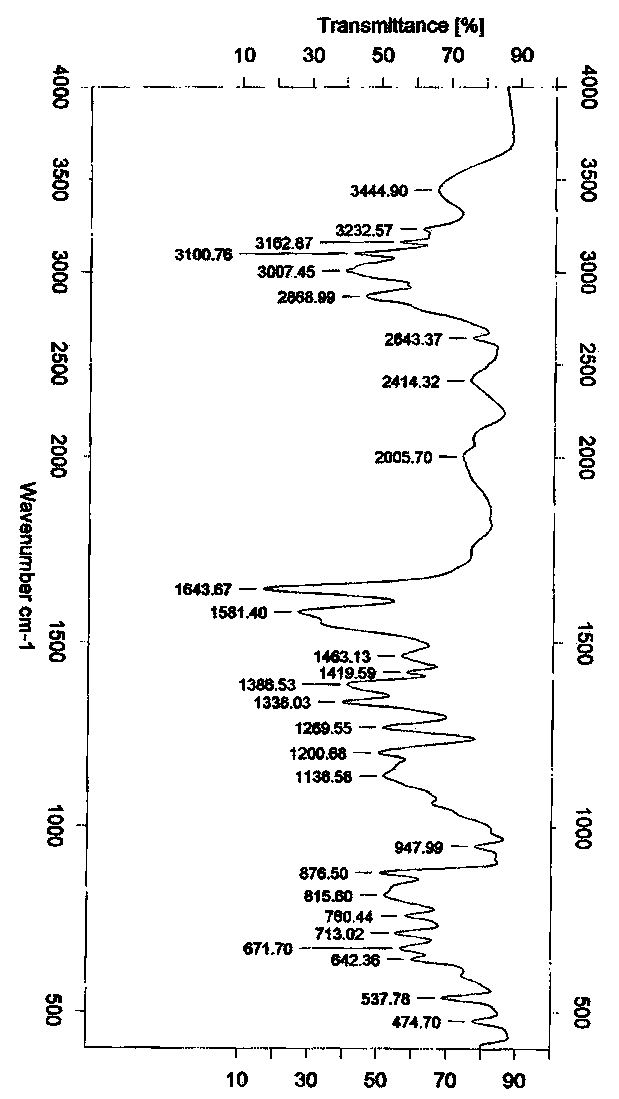
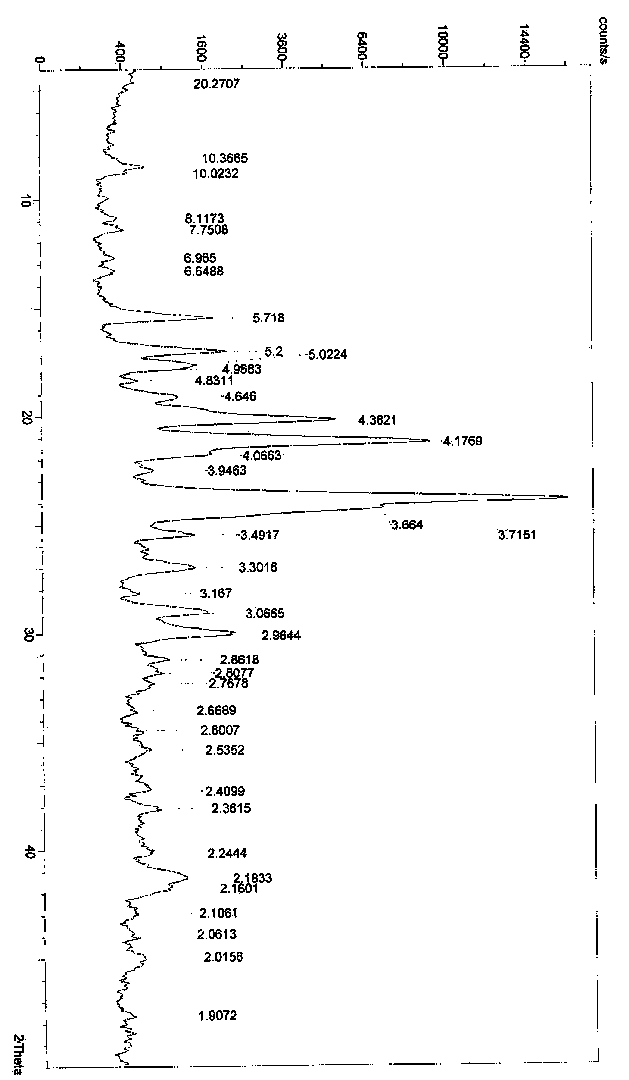
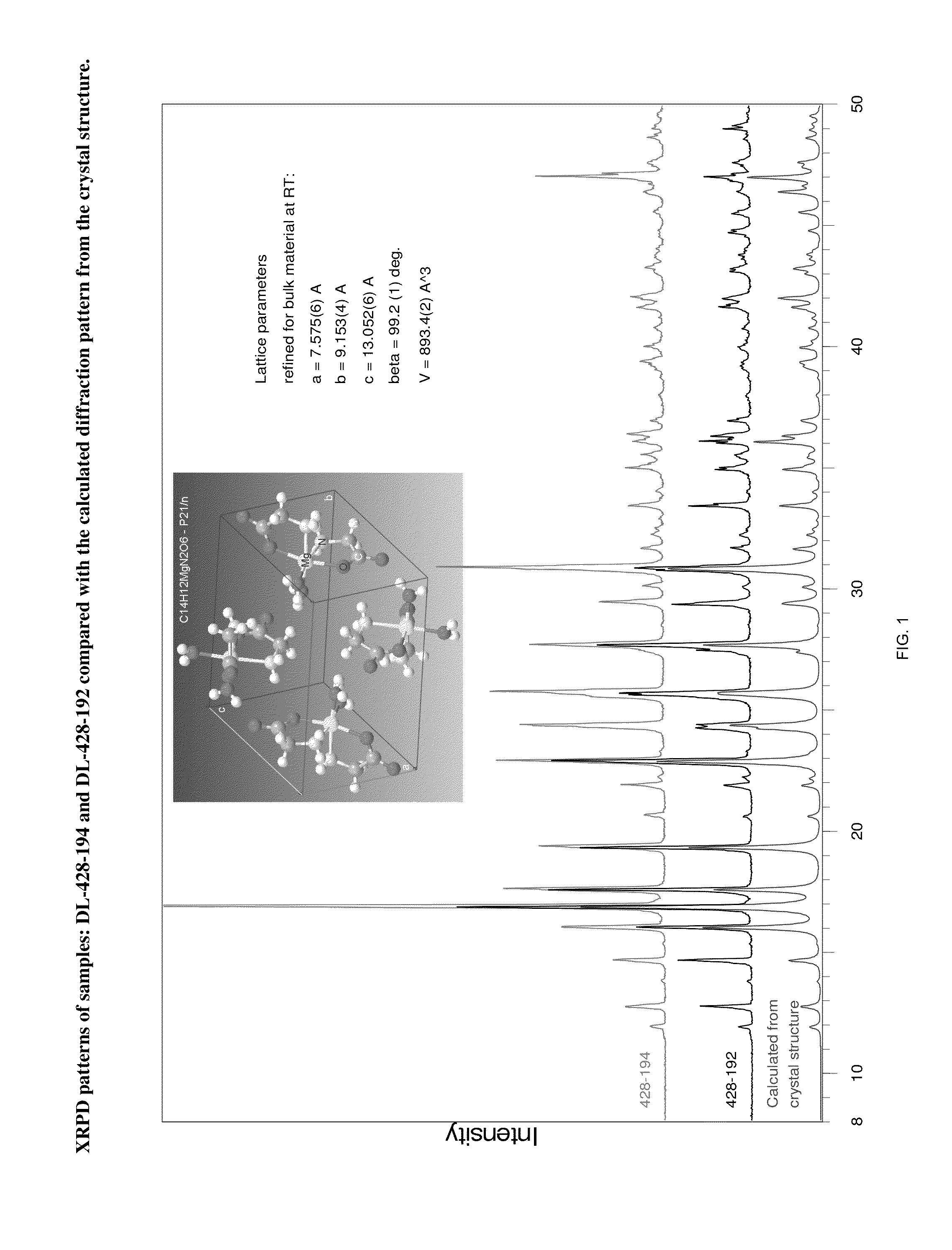
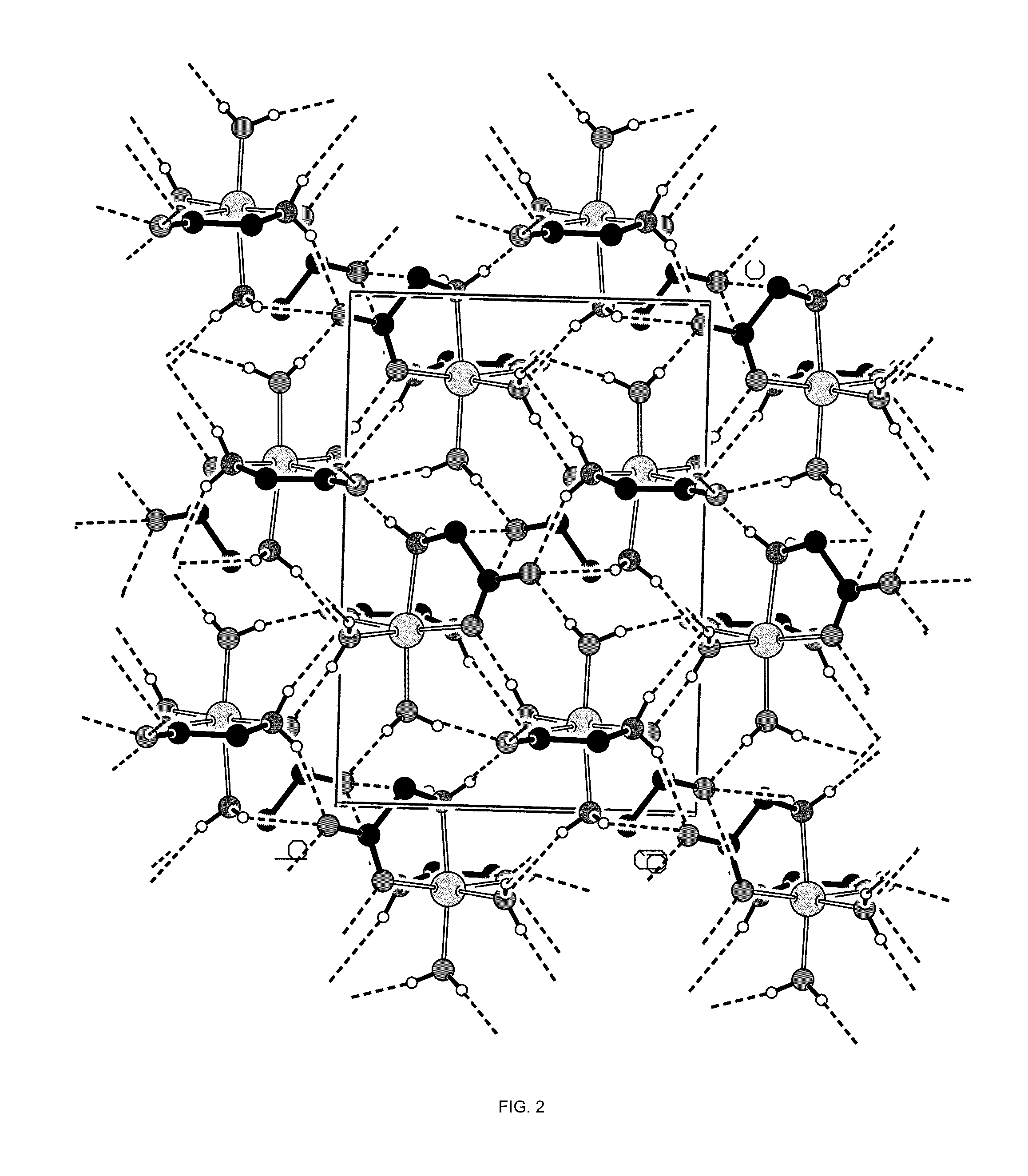
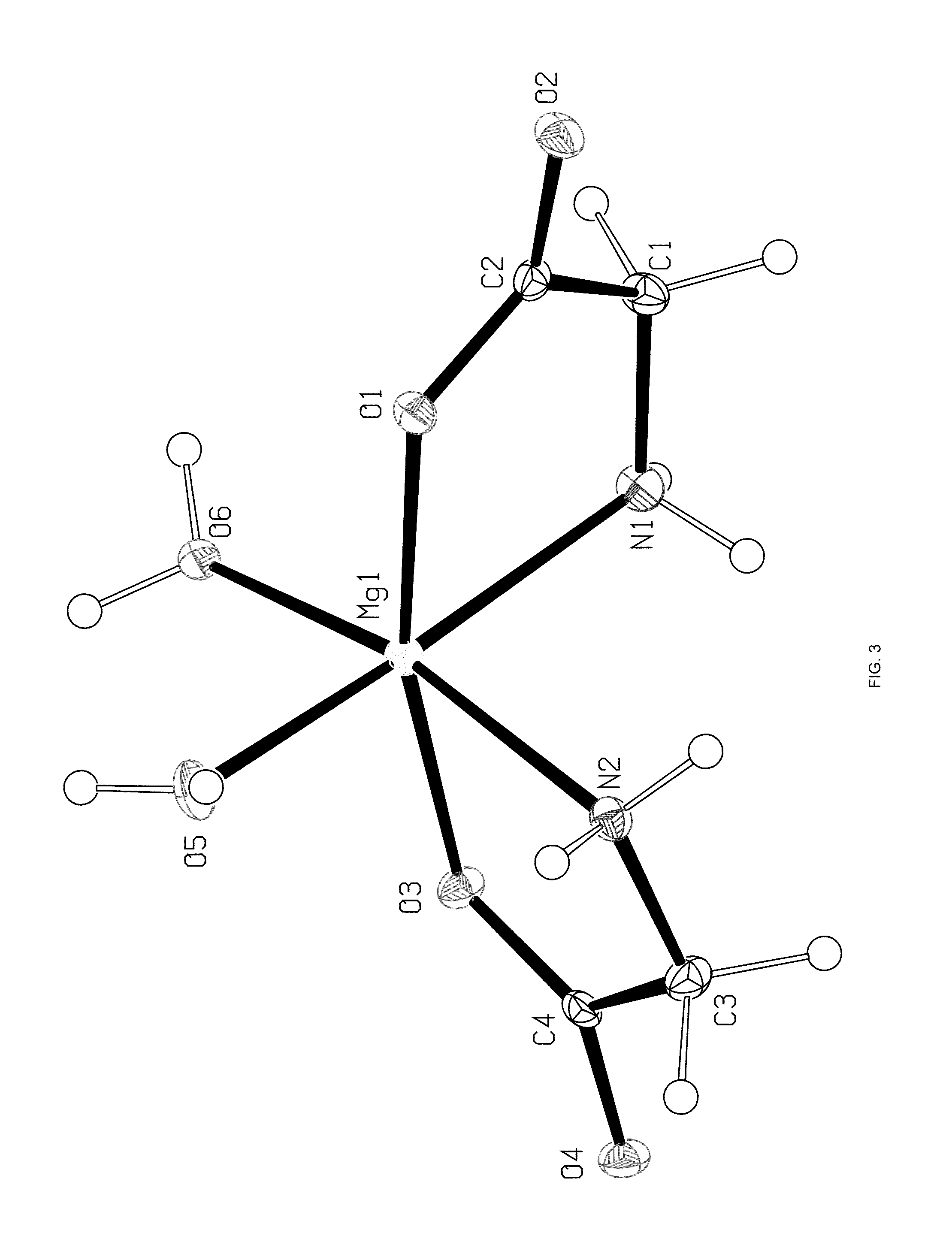
Crystal polymorph of magnesium glycinate dihydrate and process for its preparation
Embodiments of the invention provide solid forms of magnesium glycinate dihydrate and compositions thereof, which are useful for treating hyperphosphatemia and which exhibit desirable characteristics for the same. The invention further provides processes for the production of solid forms of magnesium glycinate dihydrate.
Owner:CURRAX PHARMA LLC
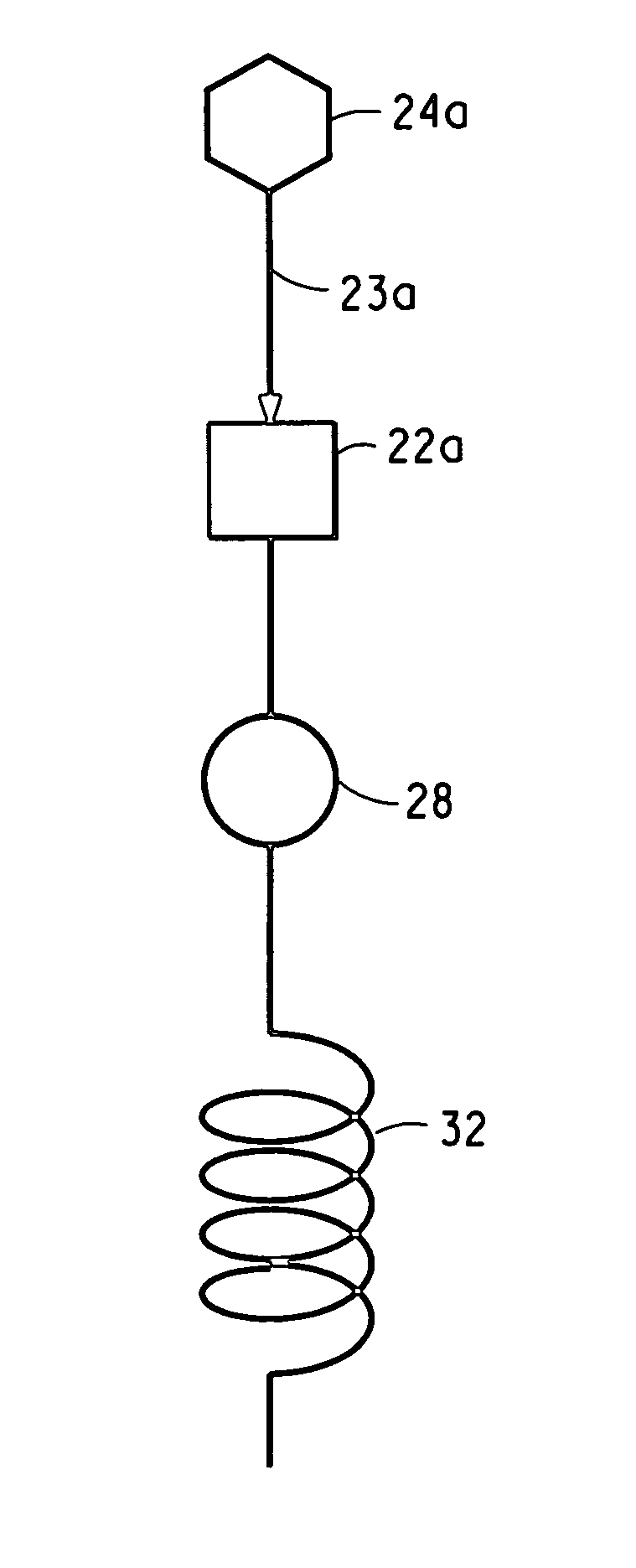
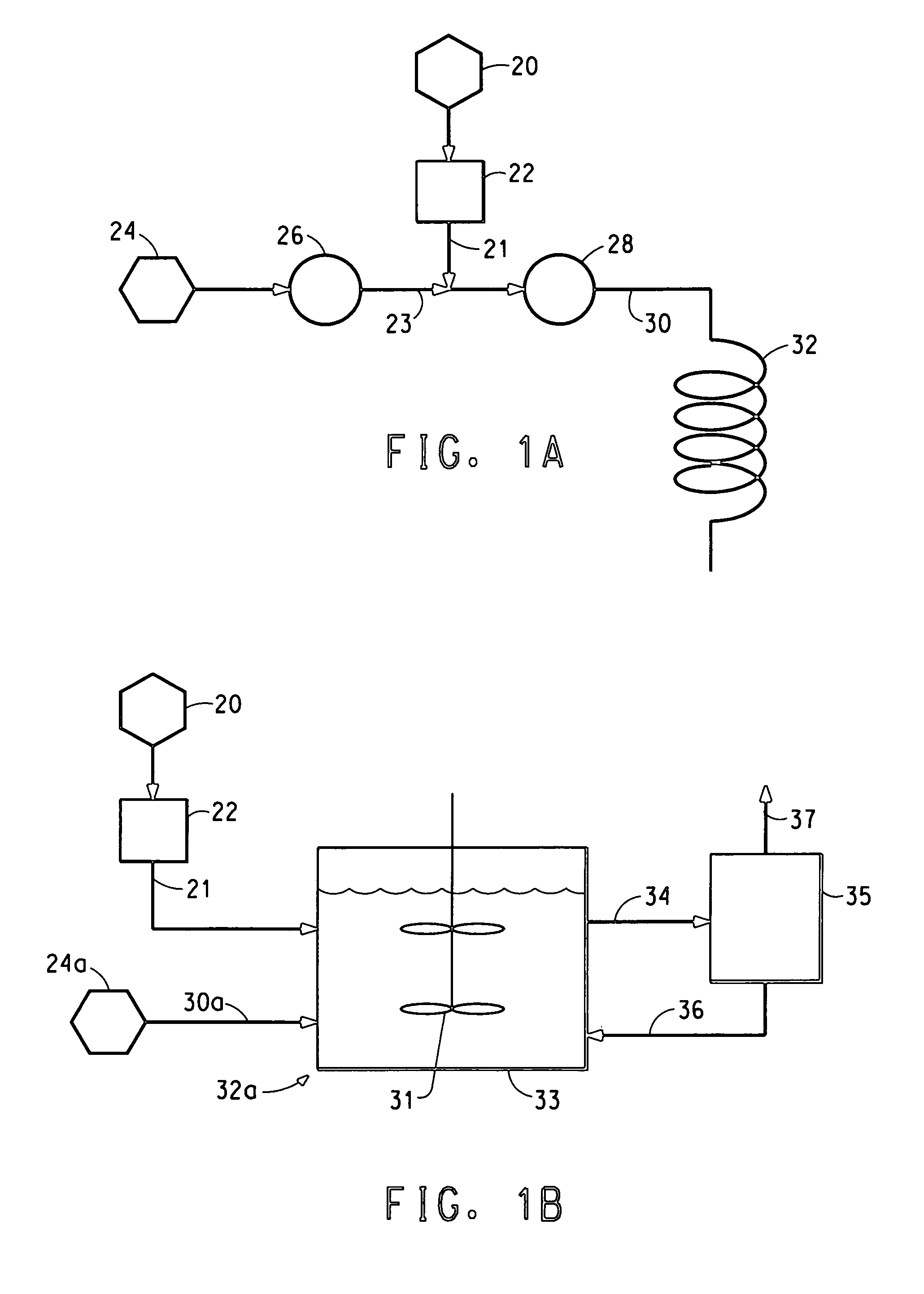
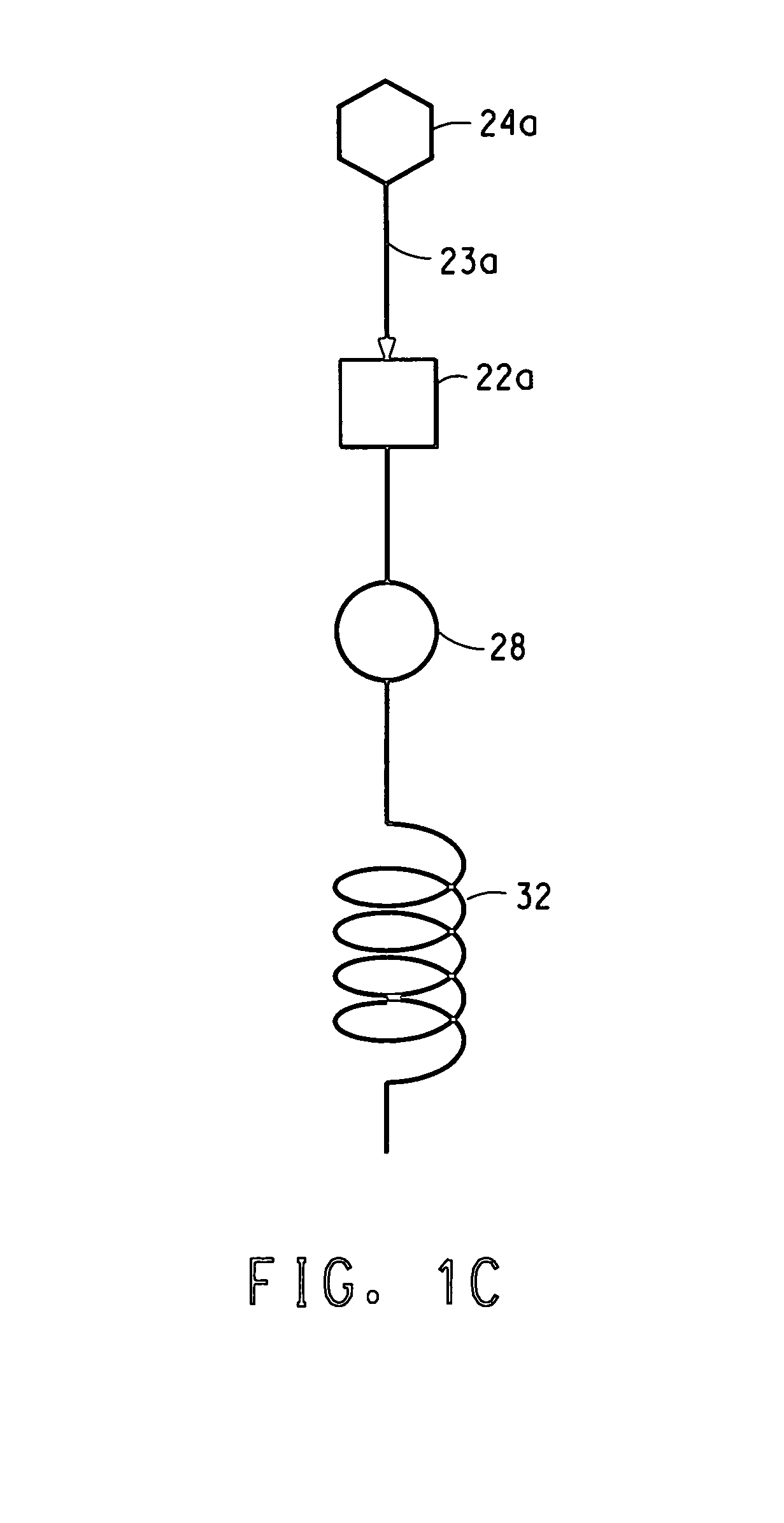
Apparatus and process used in growing crystals
InactiveUS7122083B2Material nanotechnologyFrom normal temperature solutionsCompound (substance)Crystal structure
This invention relates to a novel system for making uniform crystals. The system, by virtue of the nature of its crystal product, is useful in various chemical, pharmaceutical, agricultural, and biotechnology applications. The invention features physically separated and controlled crystal nucleation and growth zones useful in industrially scaled crystallization processes. The invention also provides a method to preferentially nucleate and crystallize a desired category of crystal structure (enantiomer, solvate, polymorph) of non-chiral and chiral compounds.
Owner:DUPONT POLYMERS INC
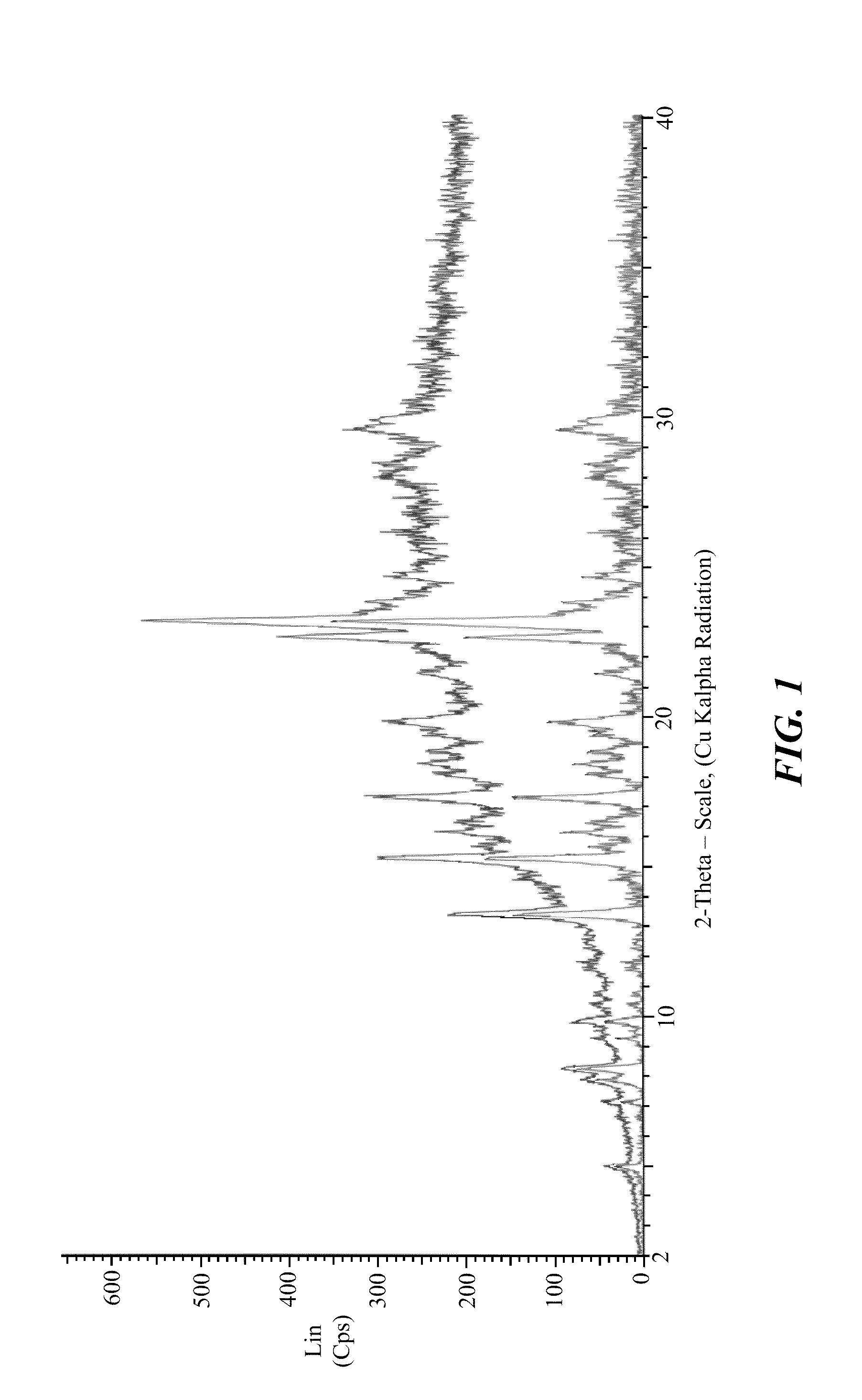
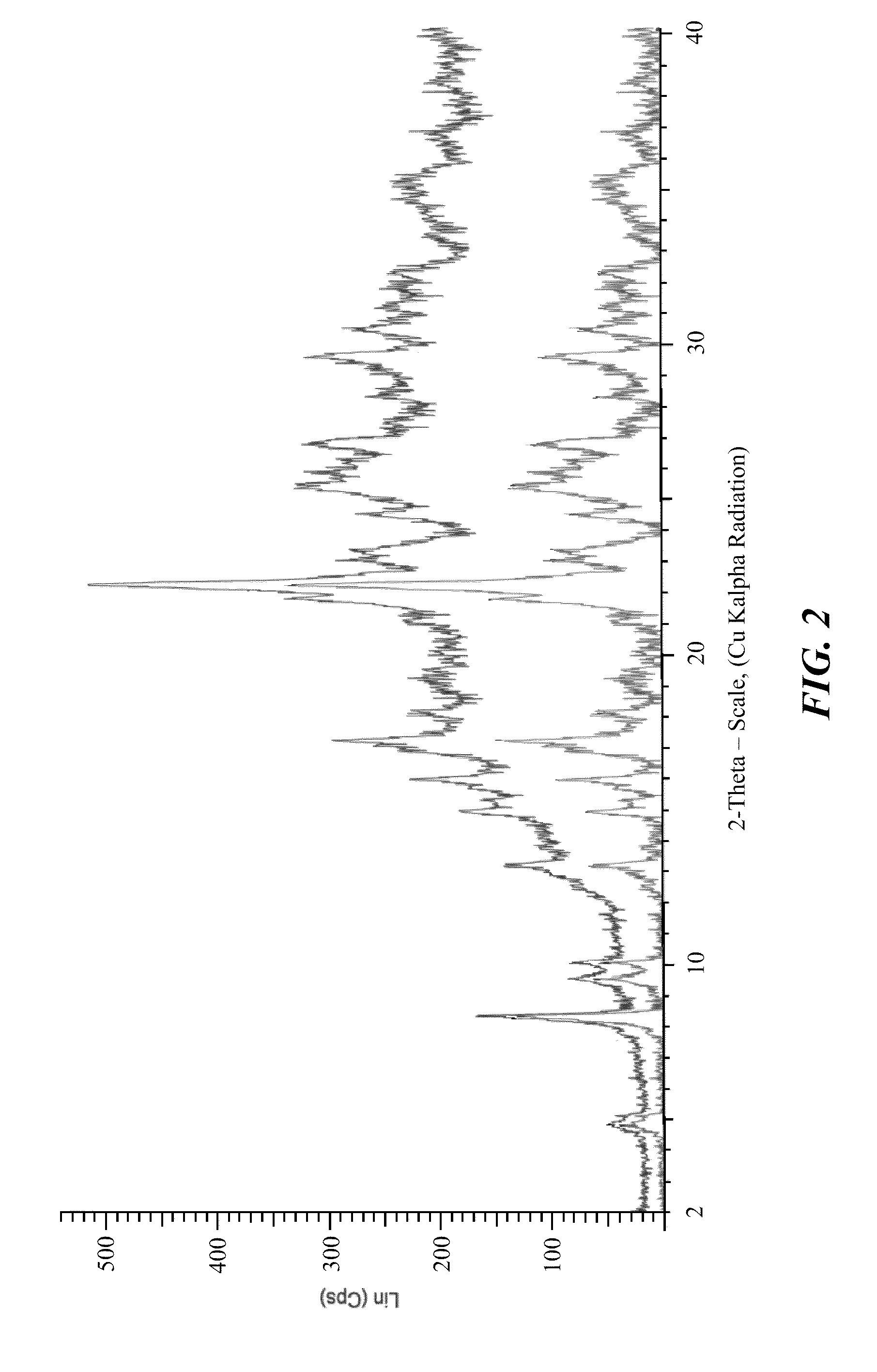
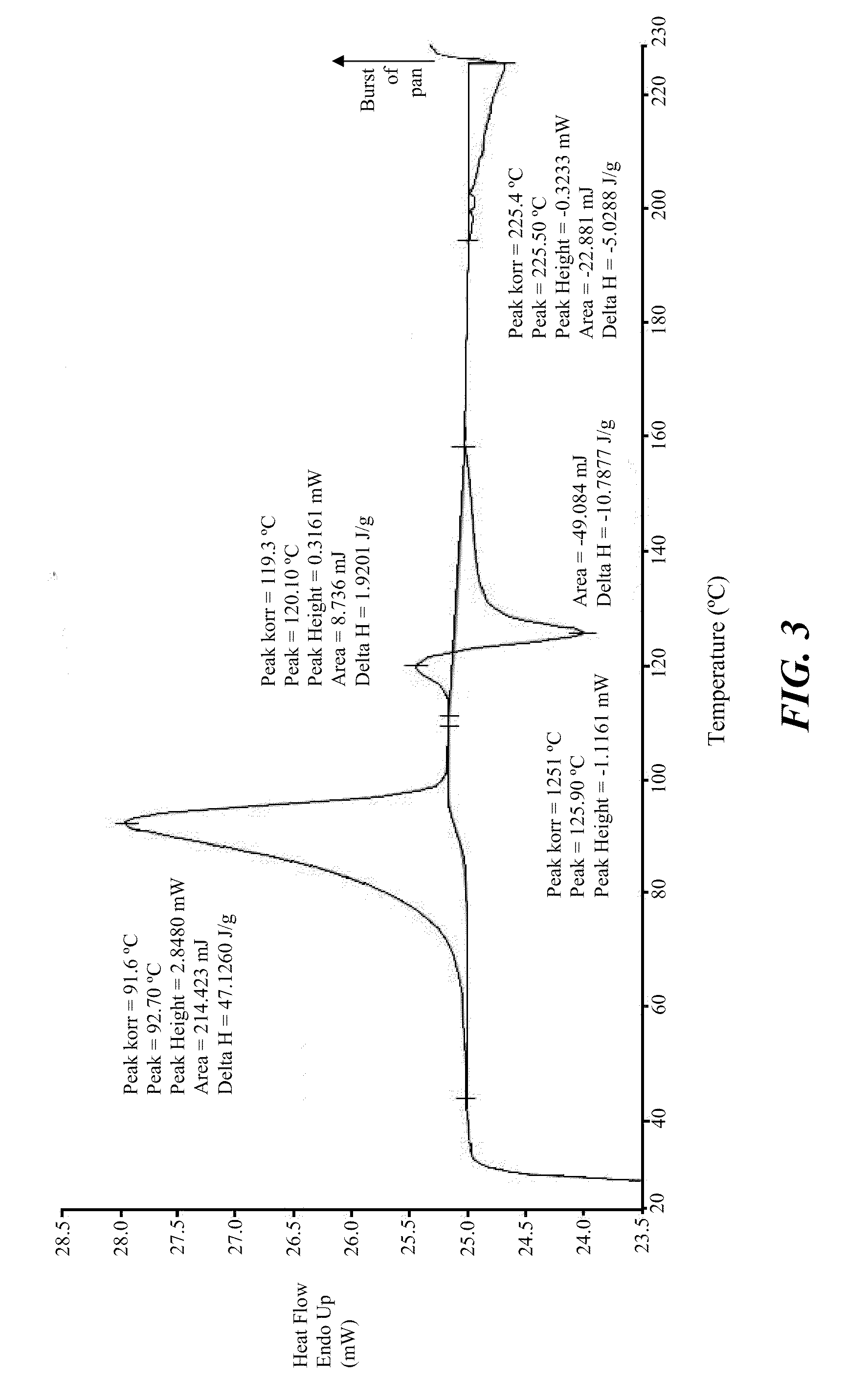
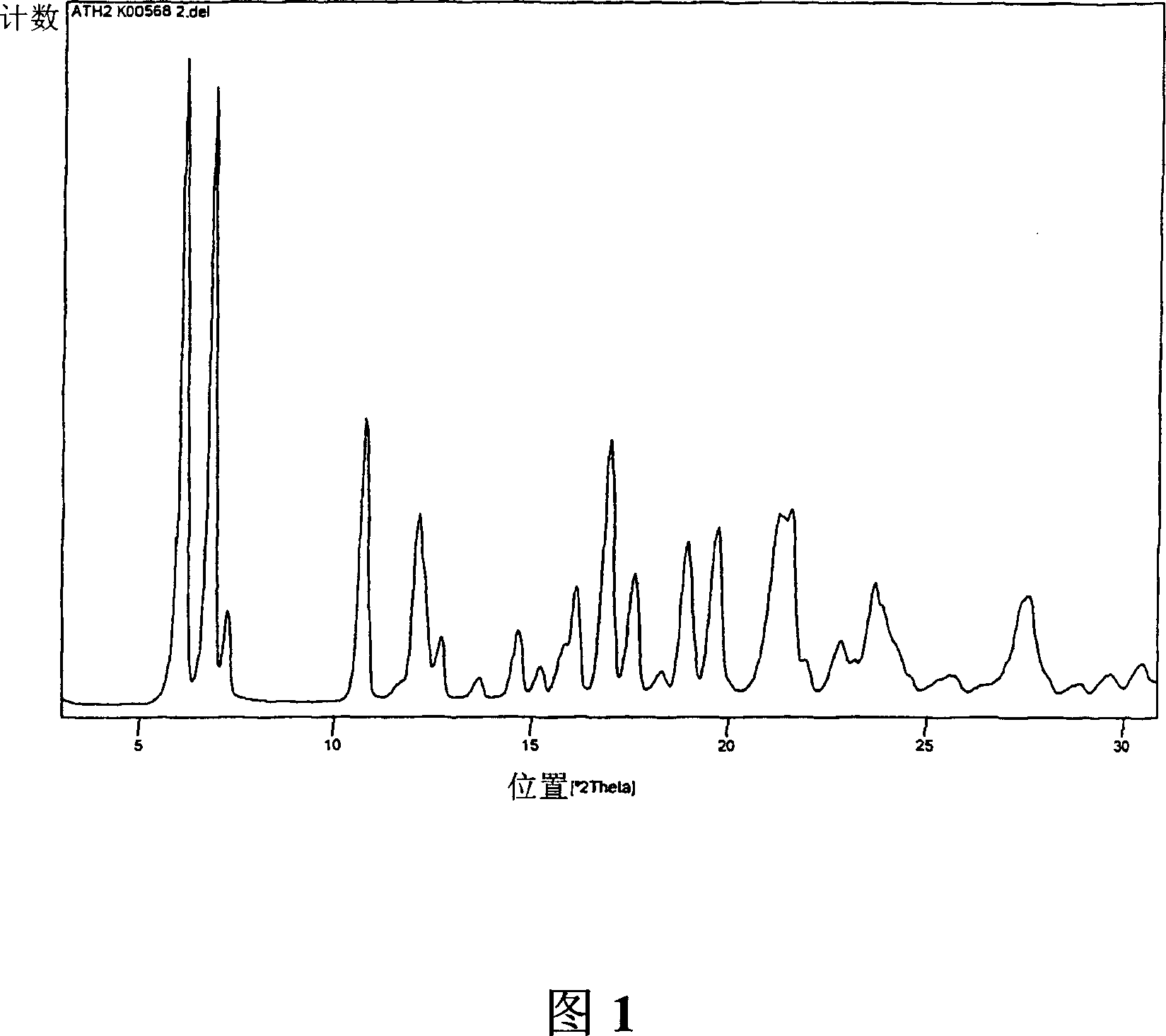
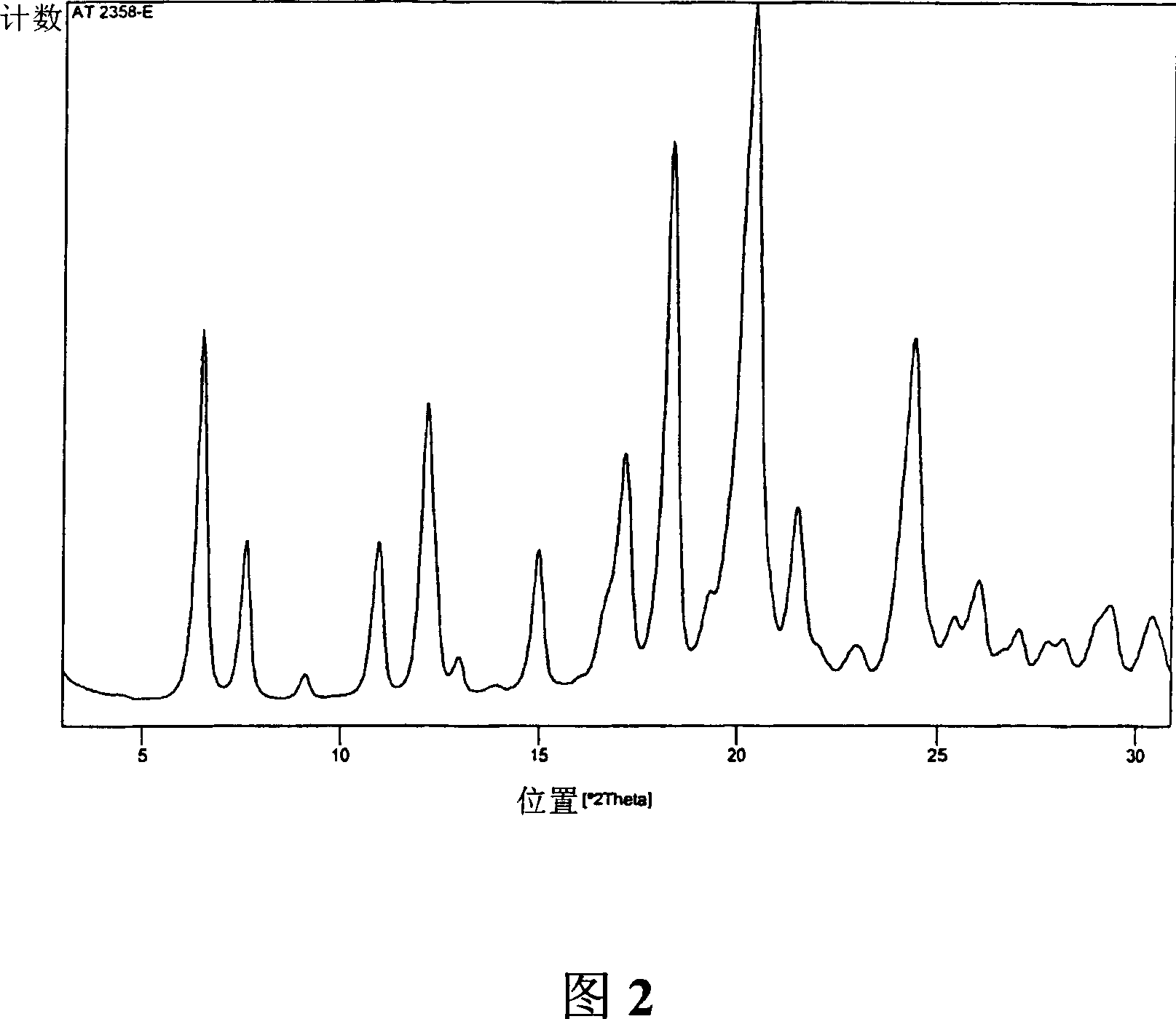
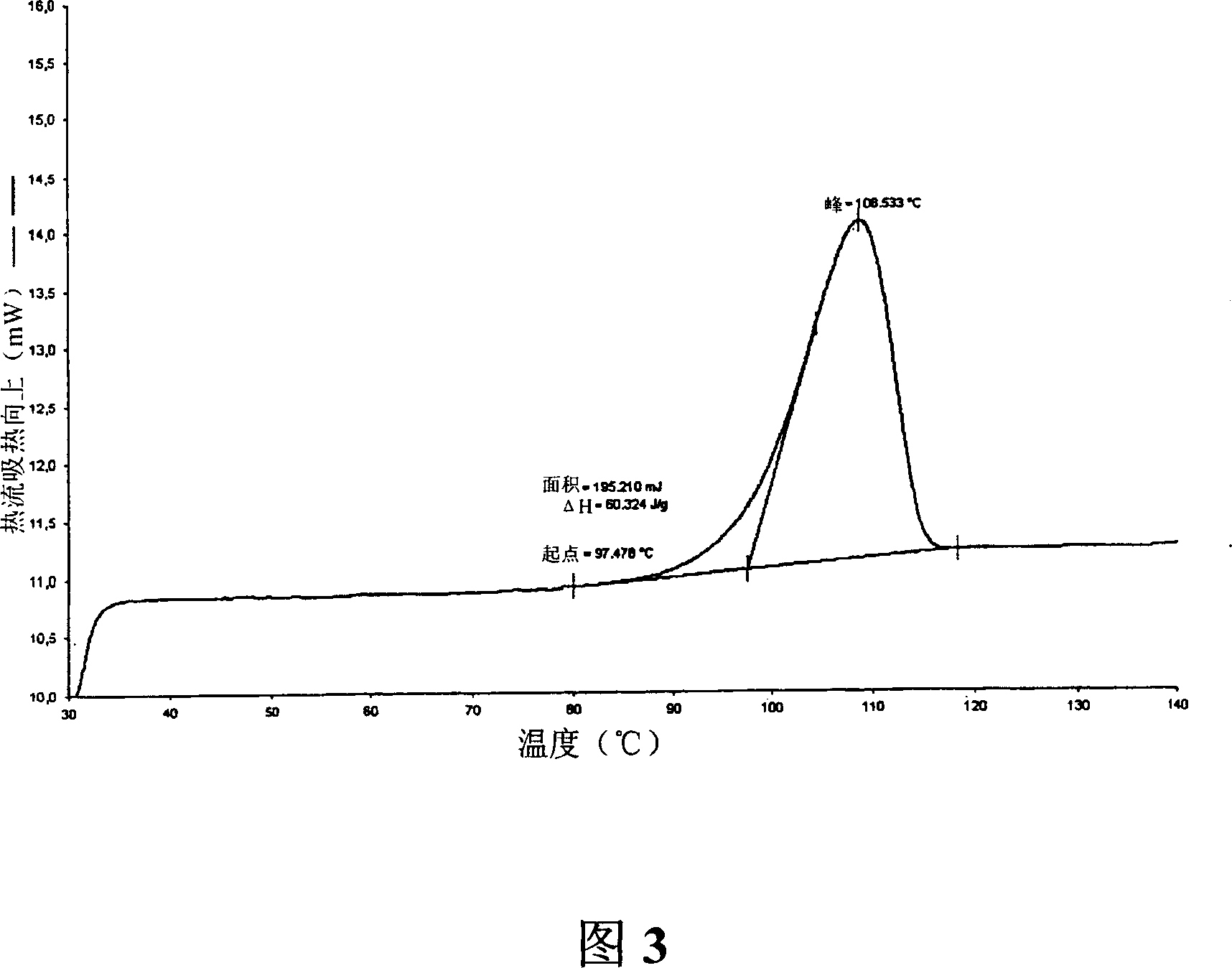
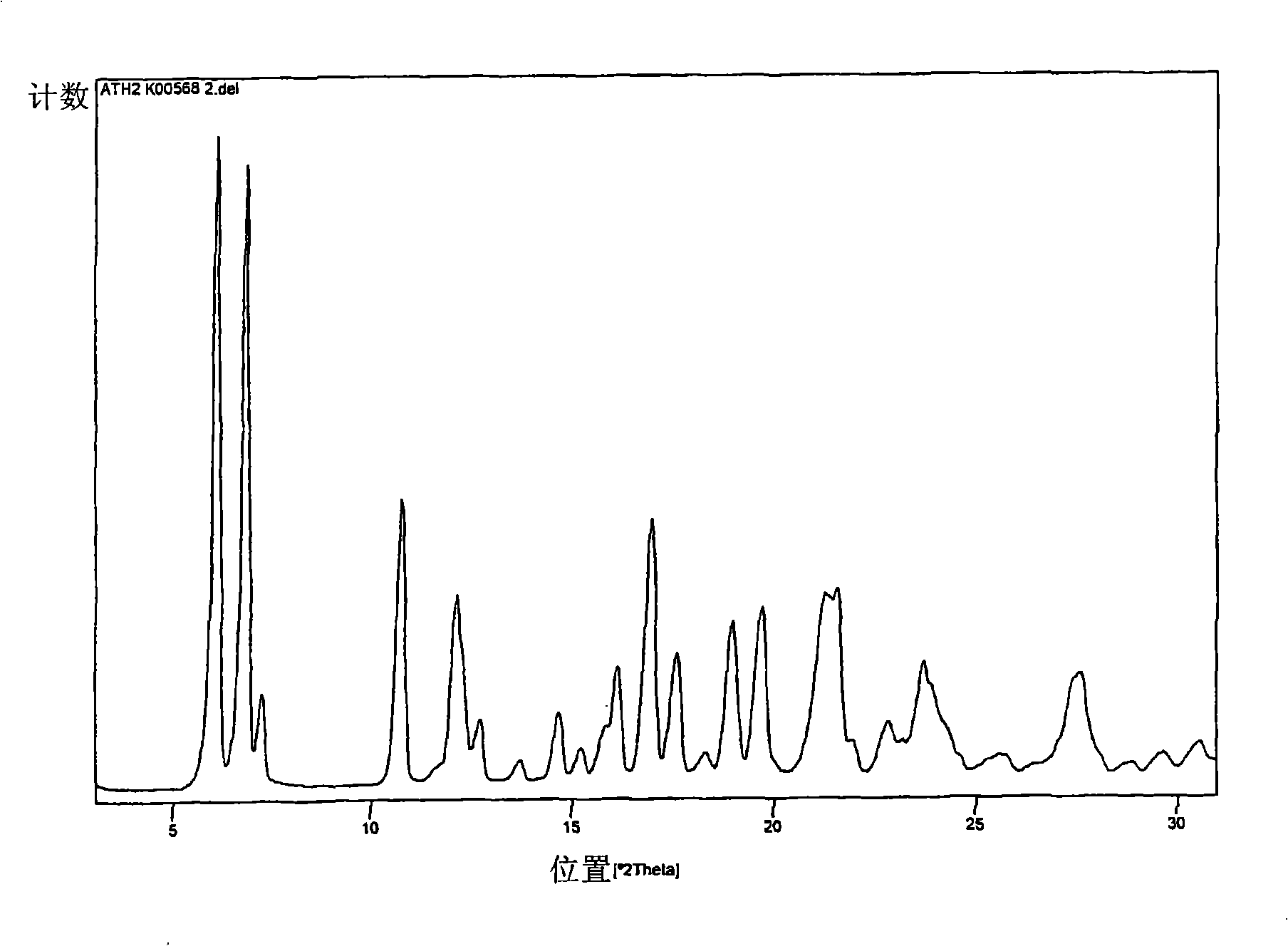
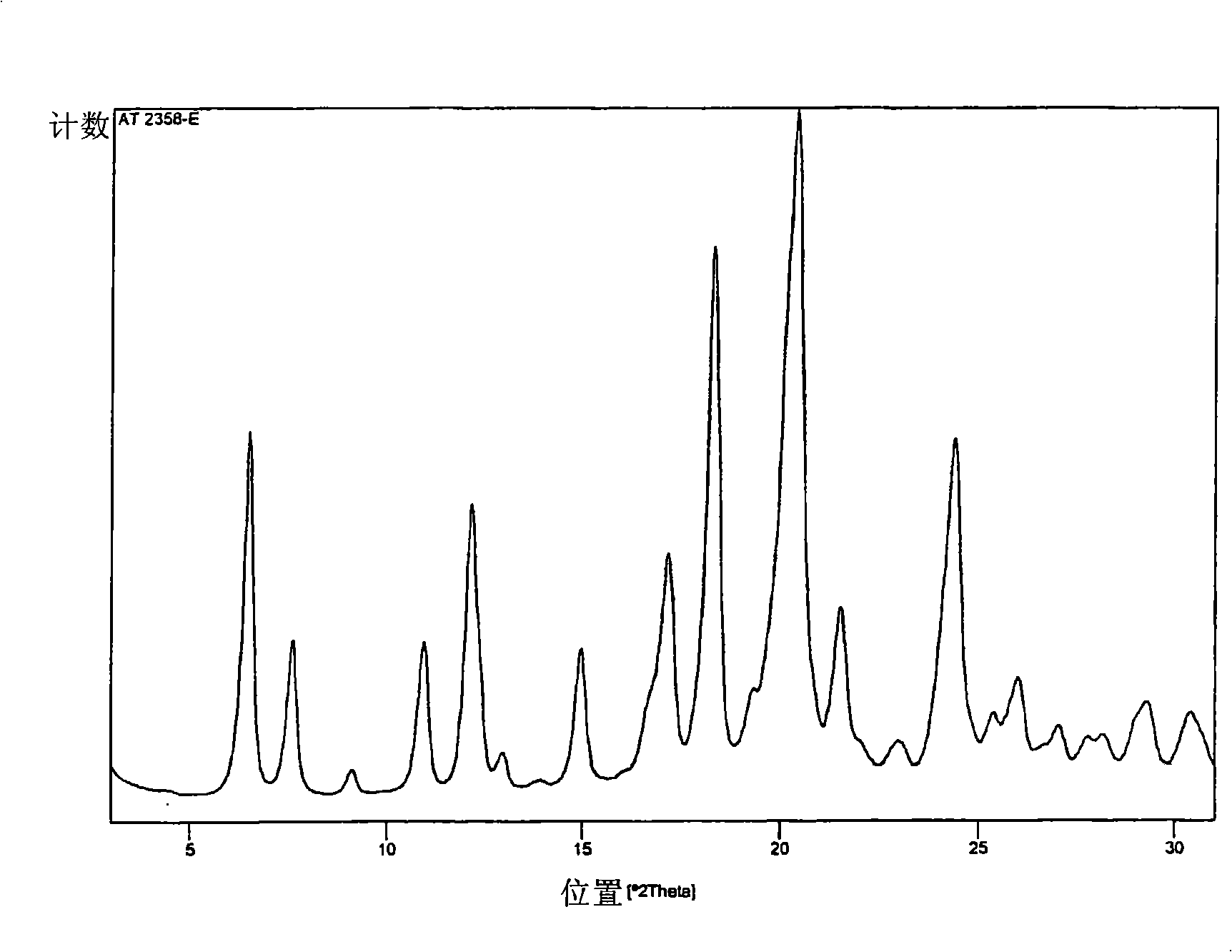
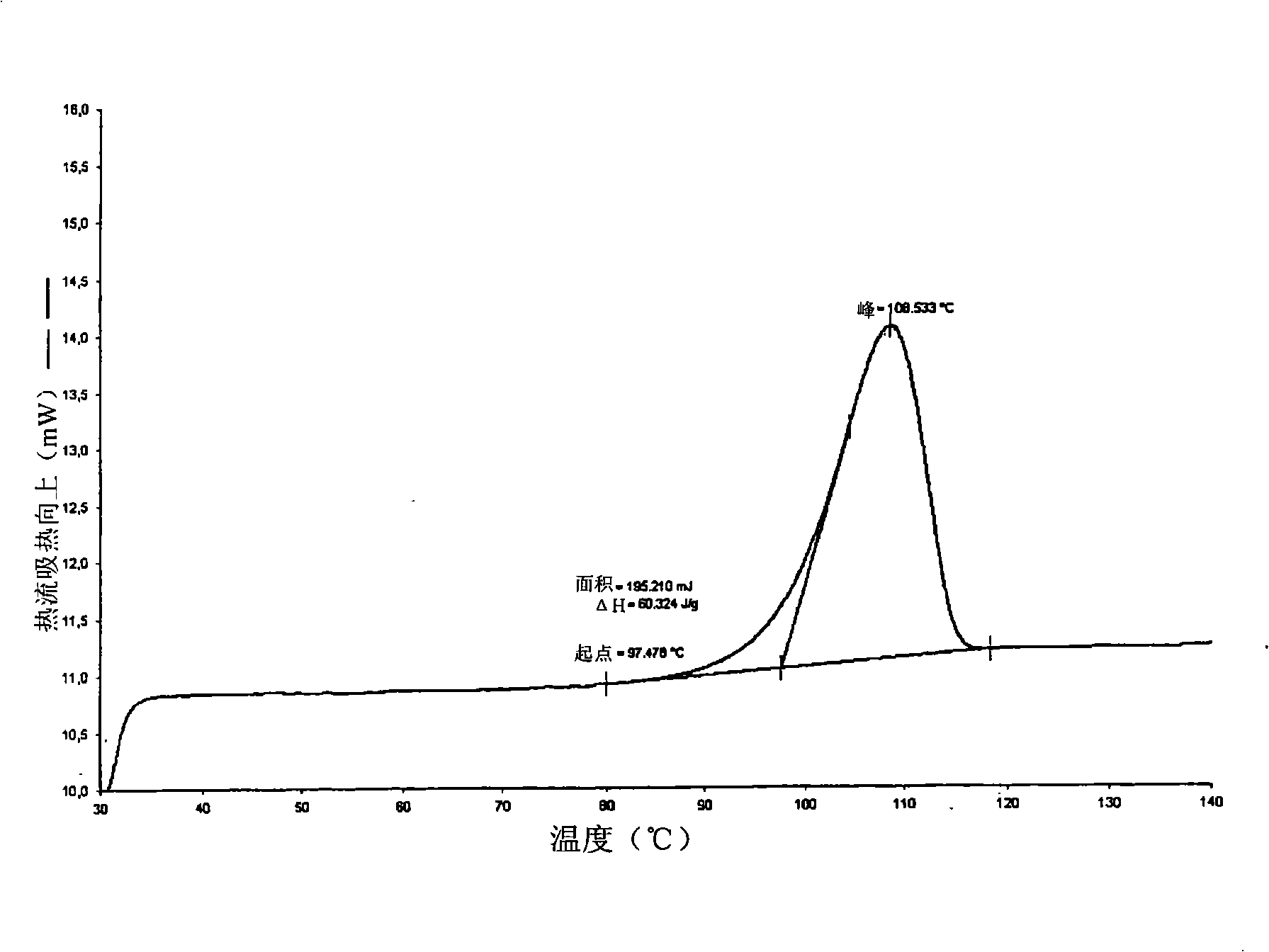
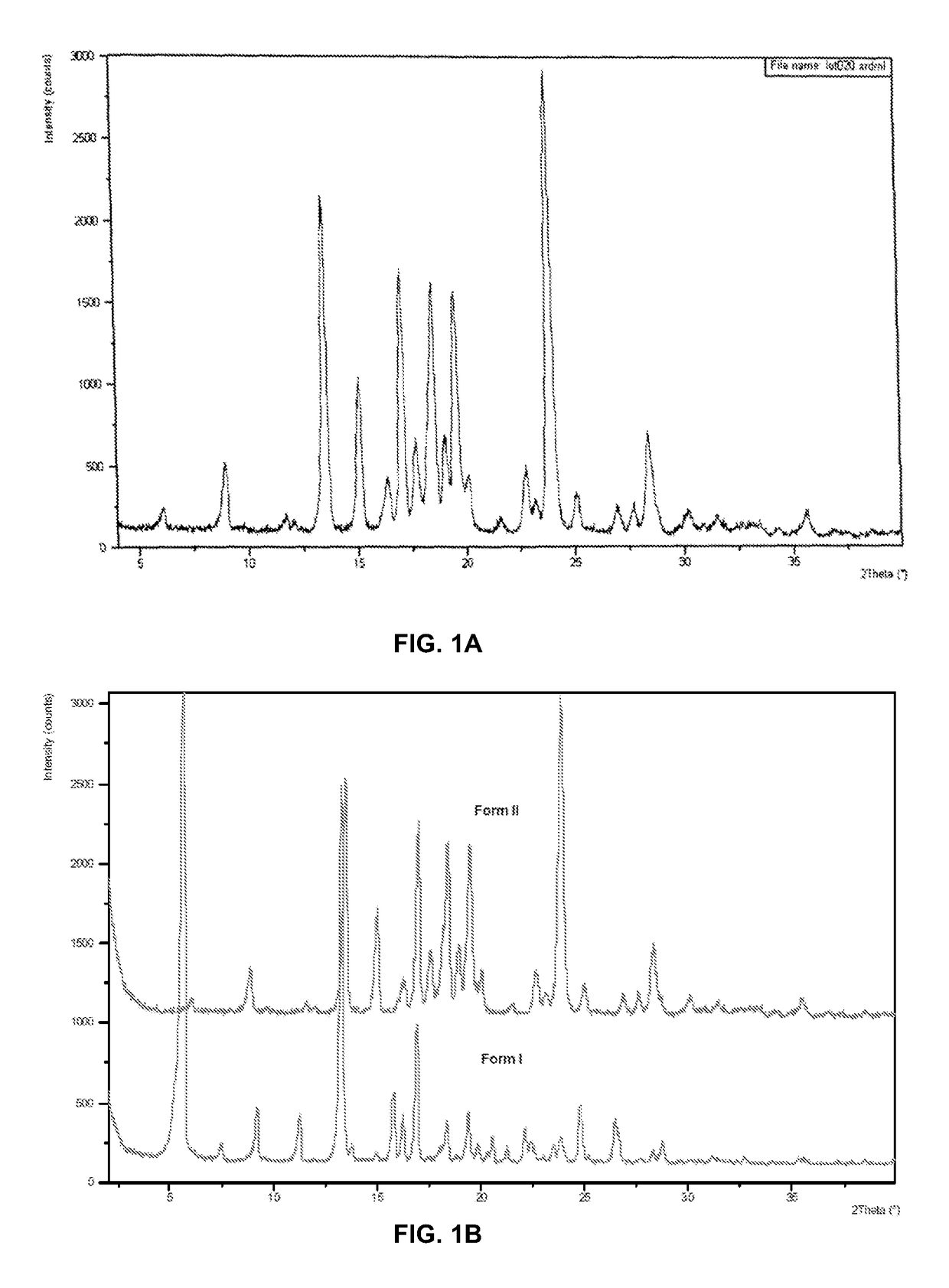
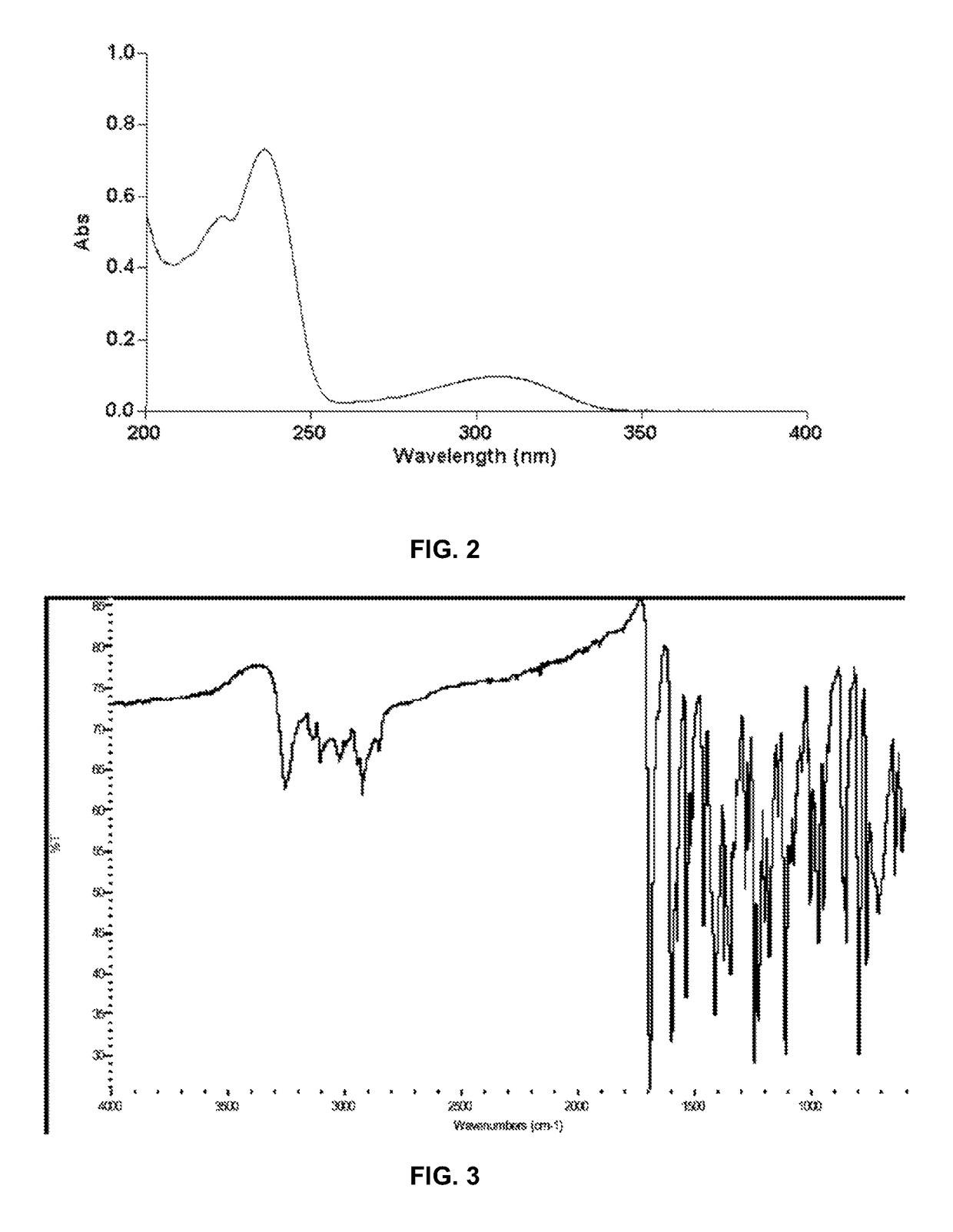
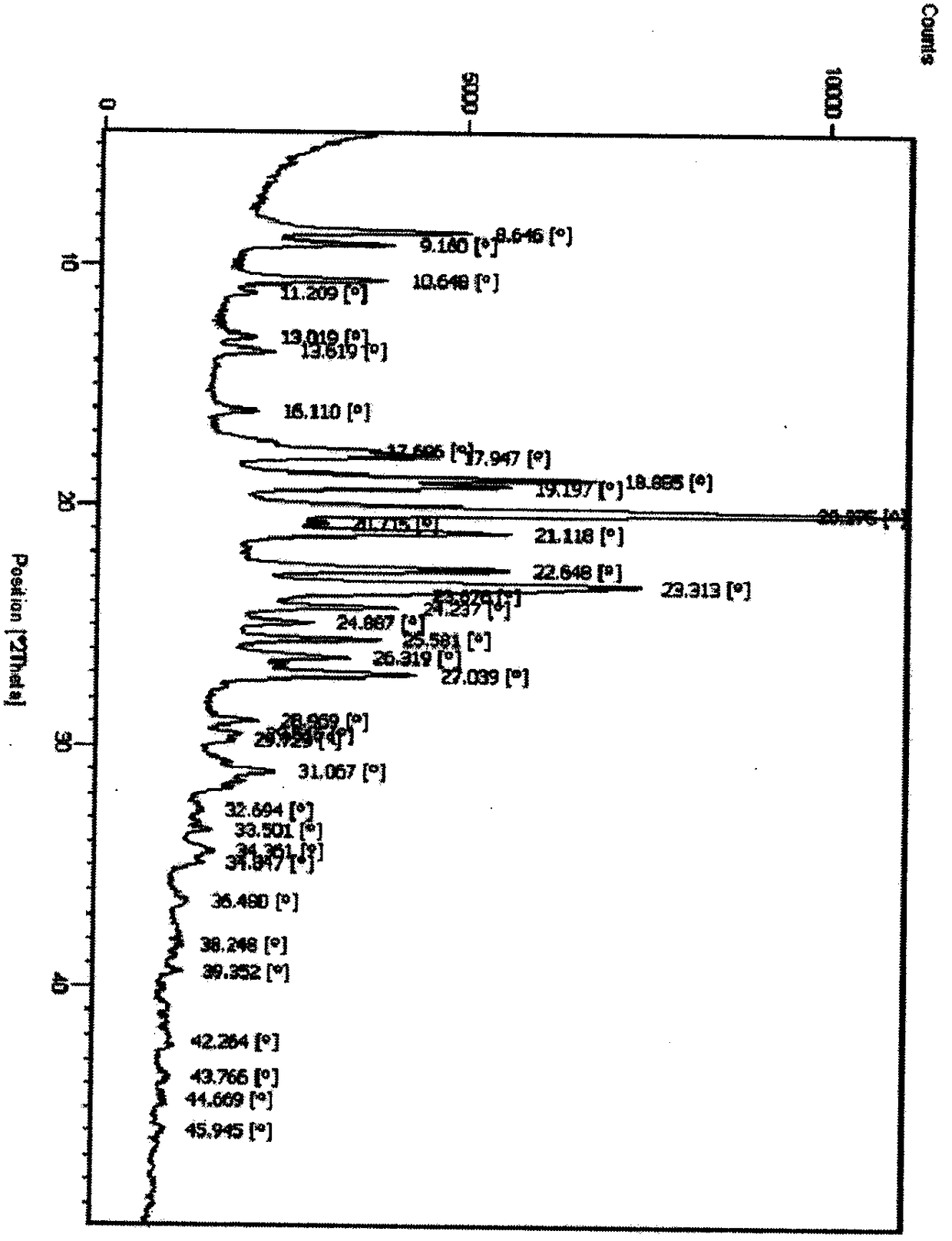
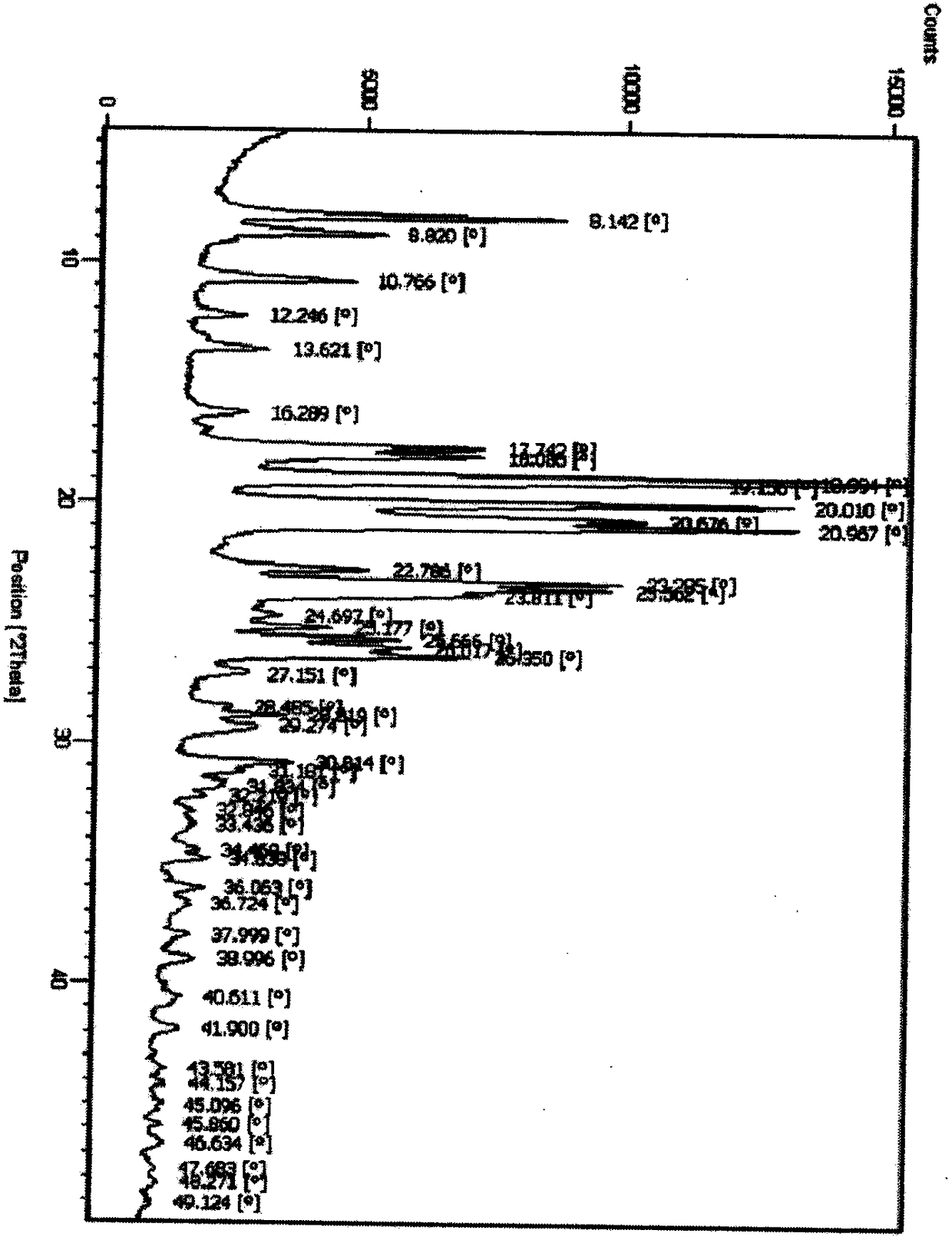
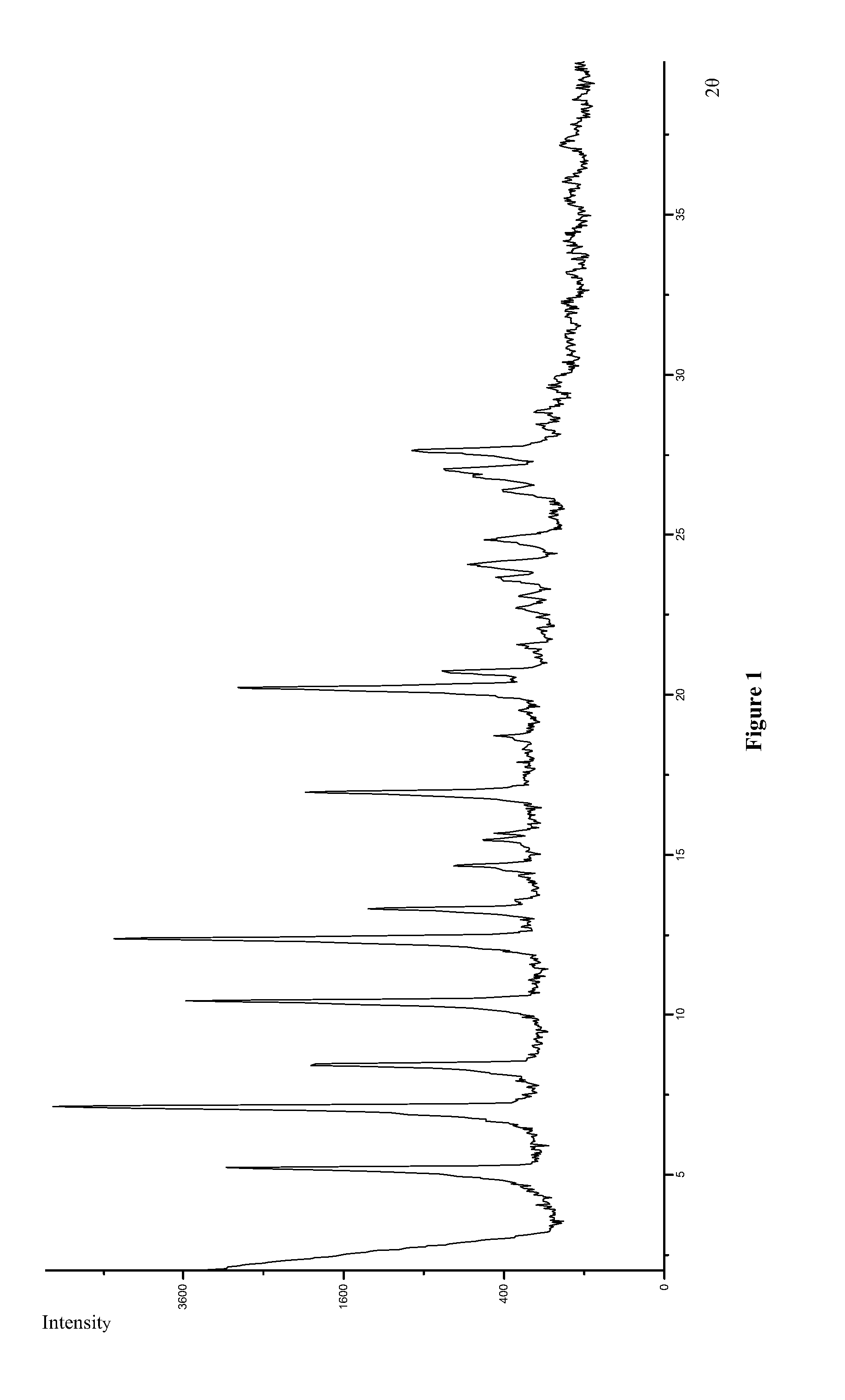
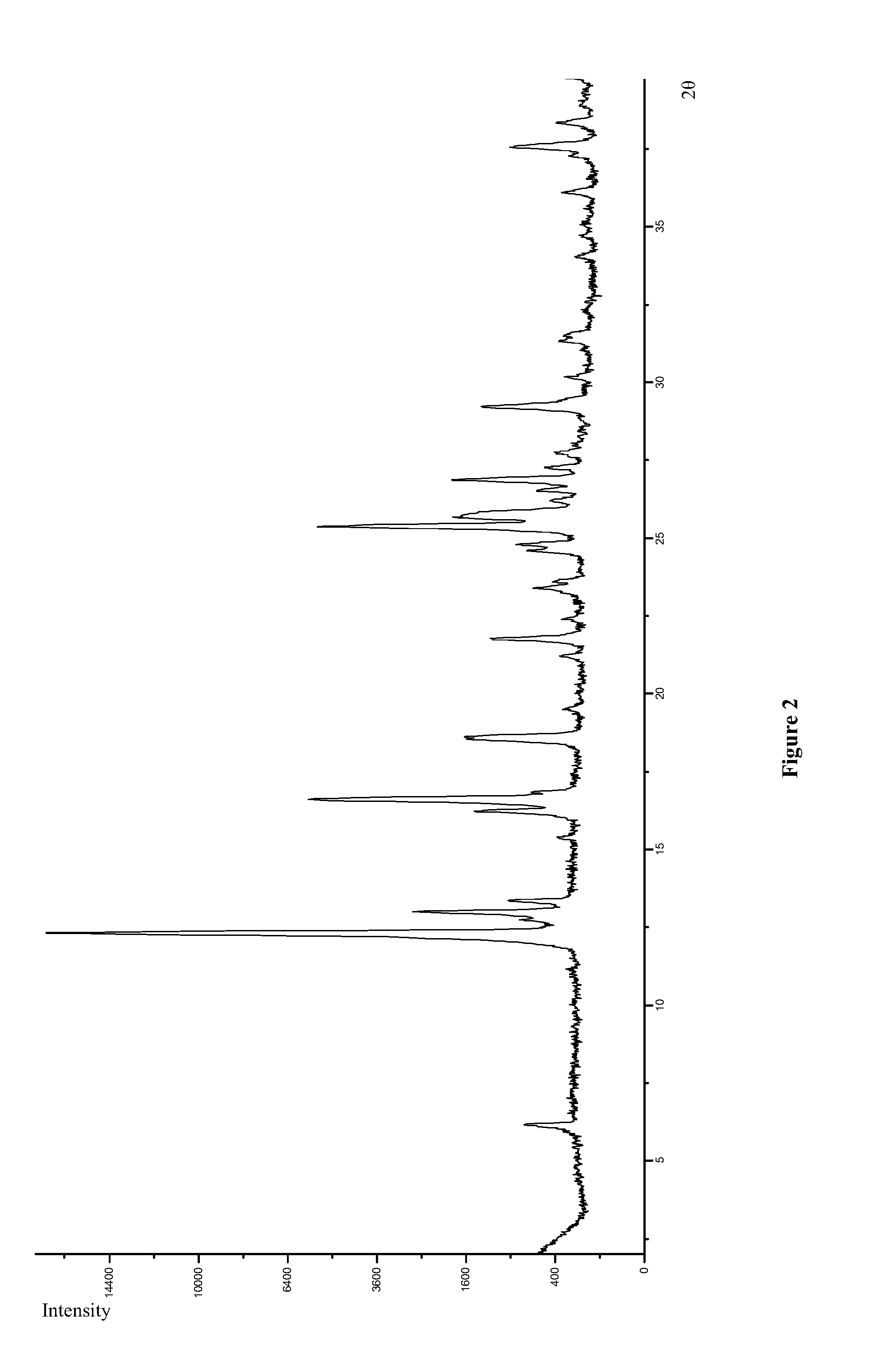
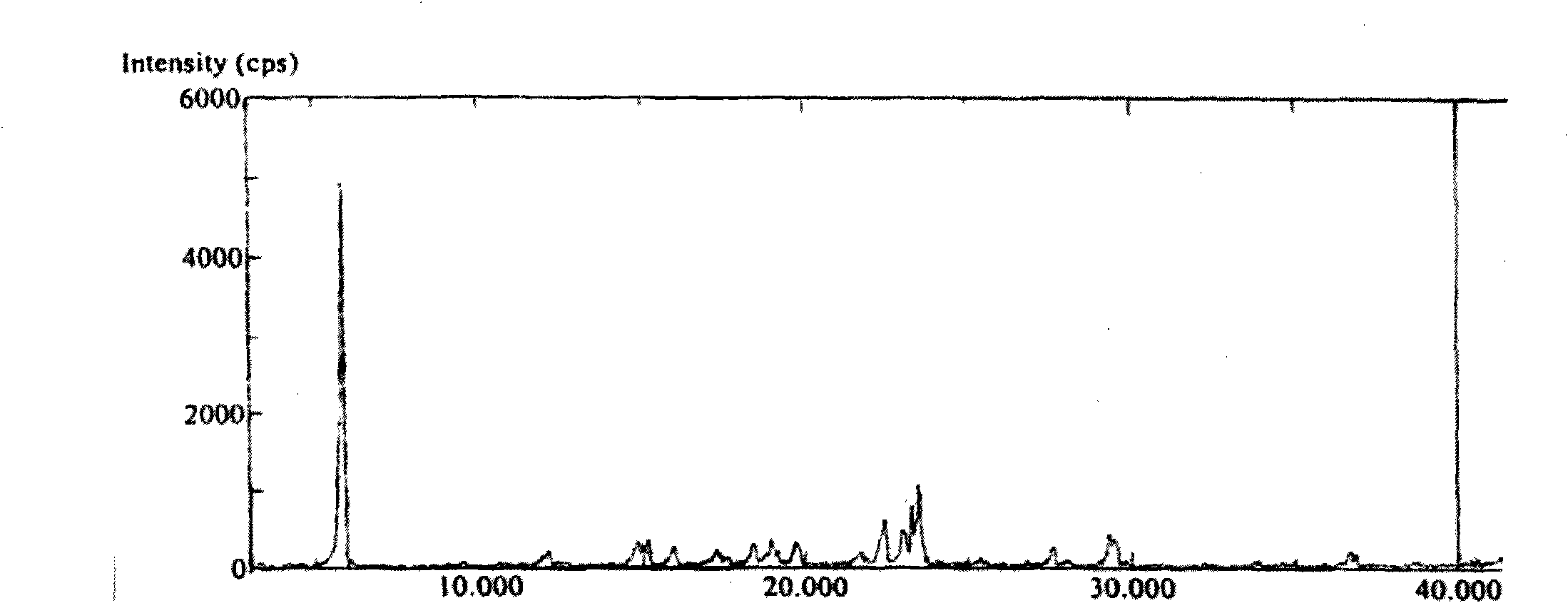
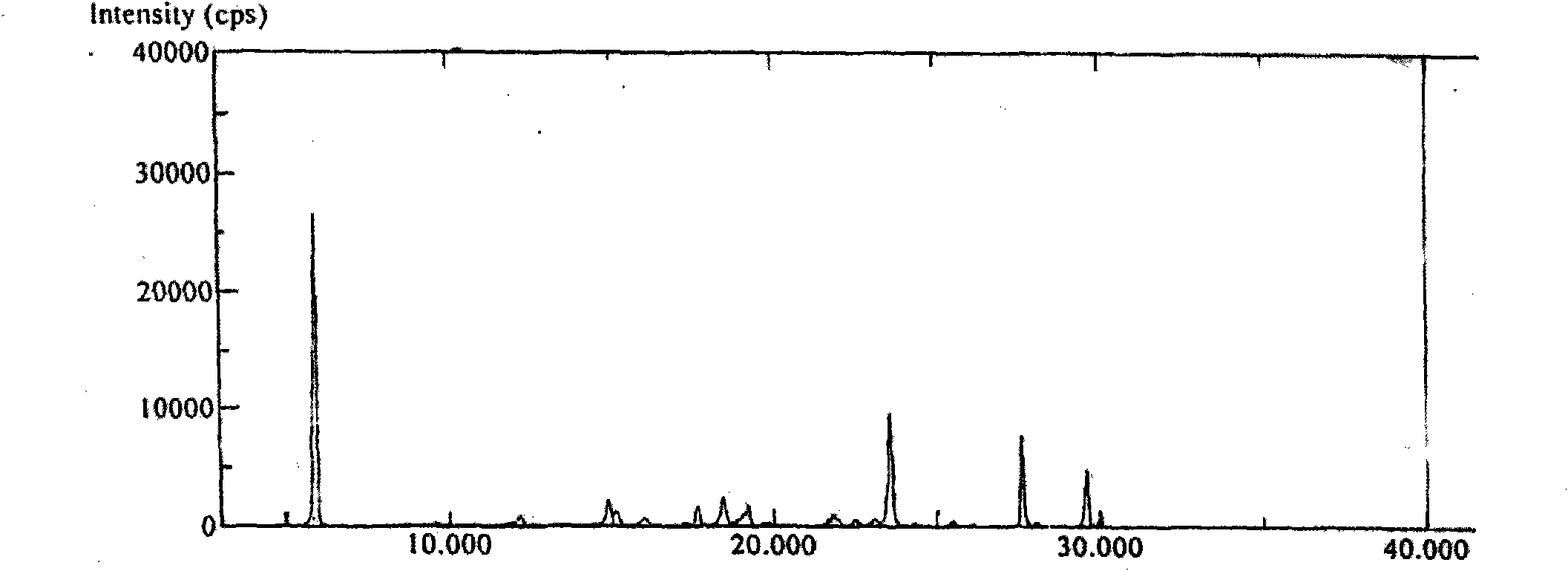
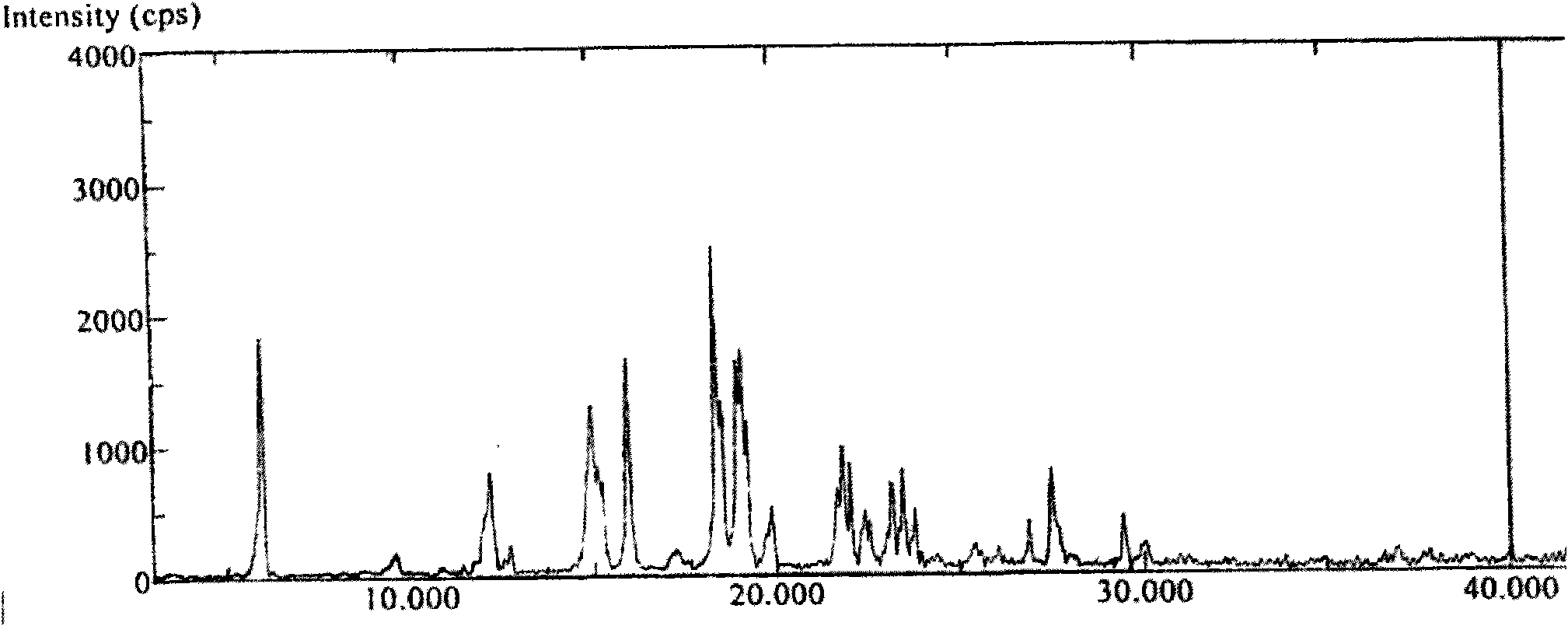
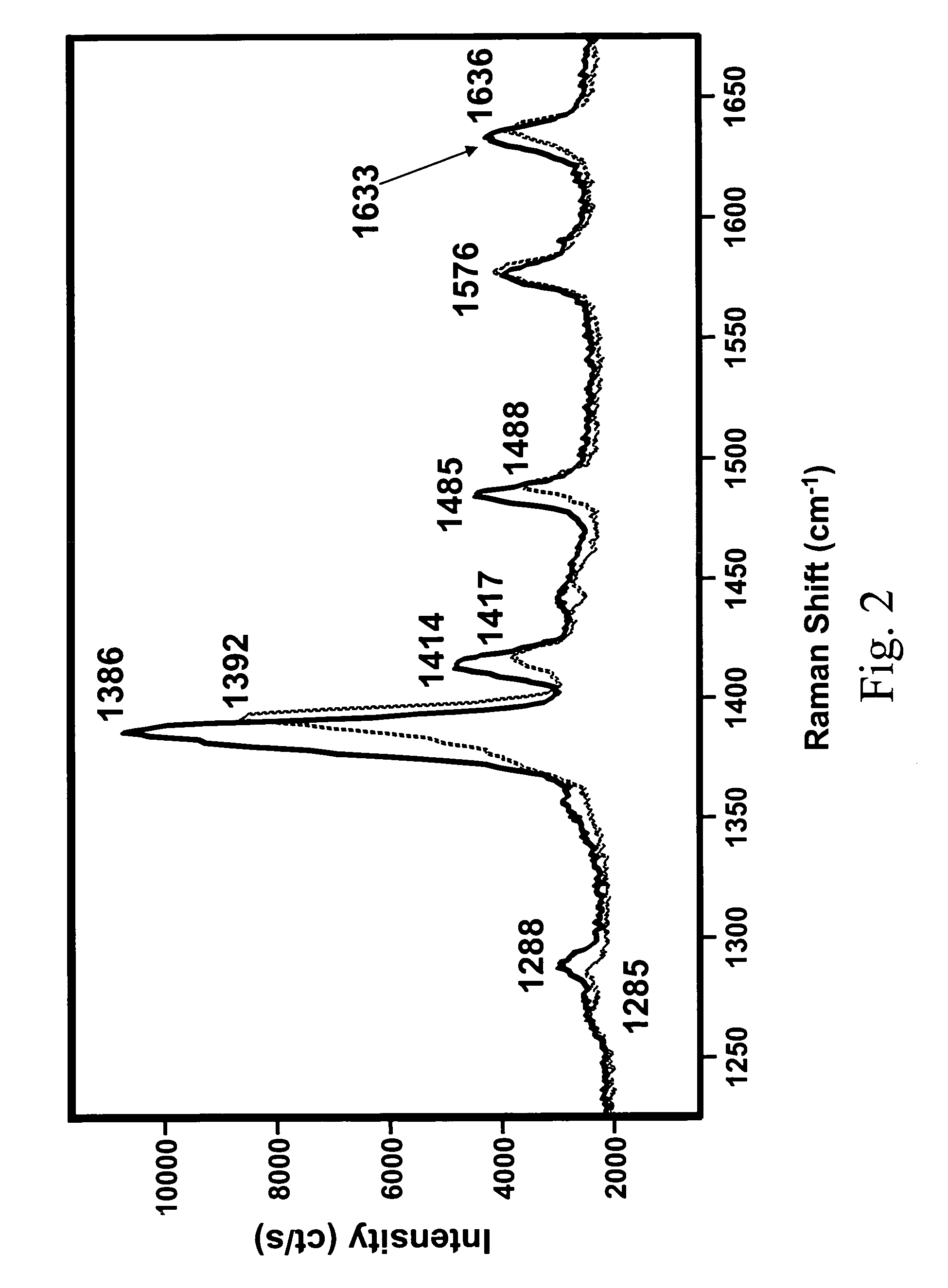
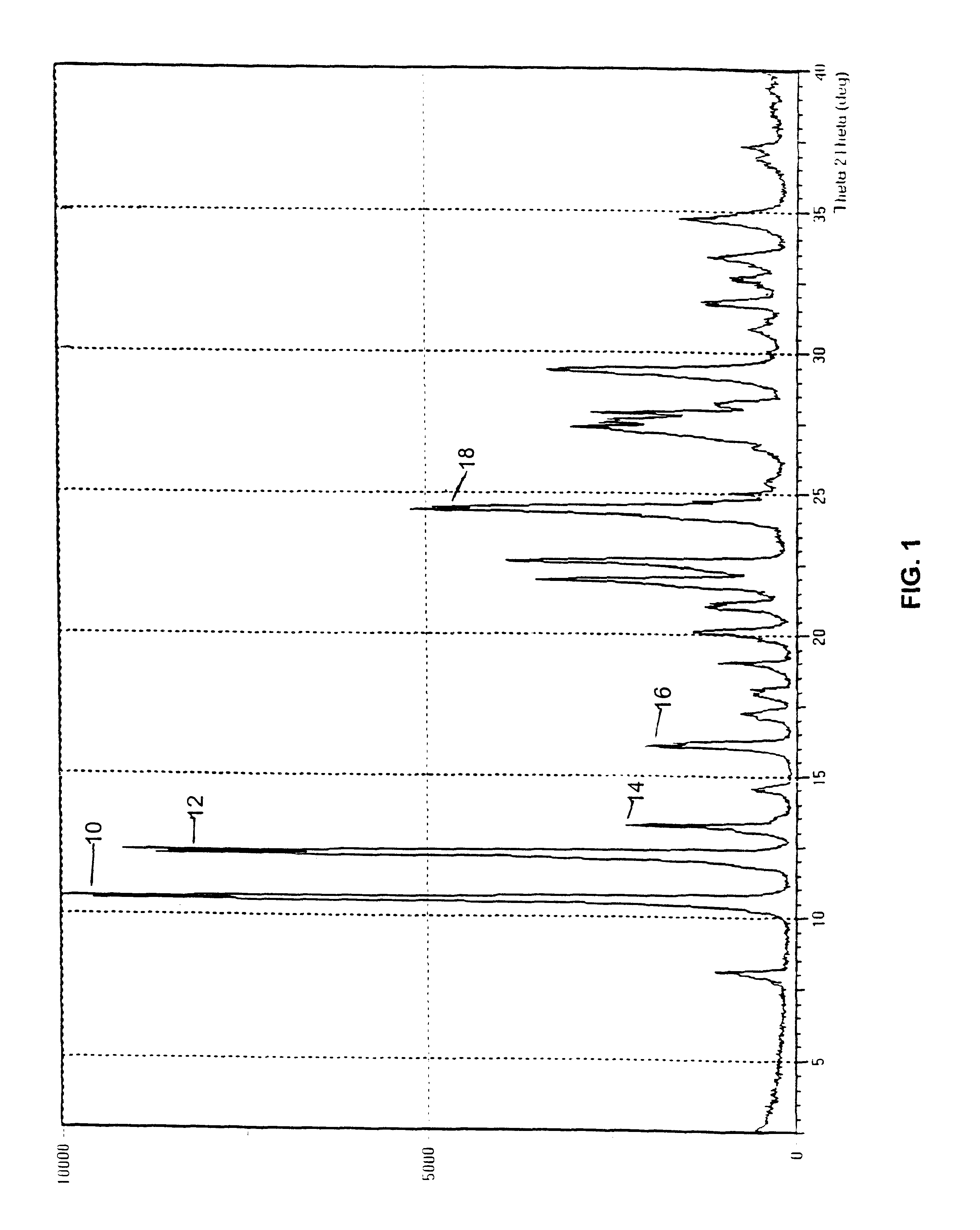
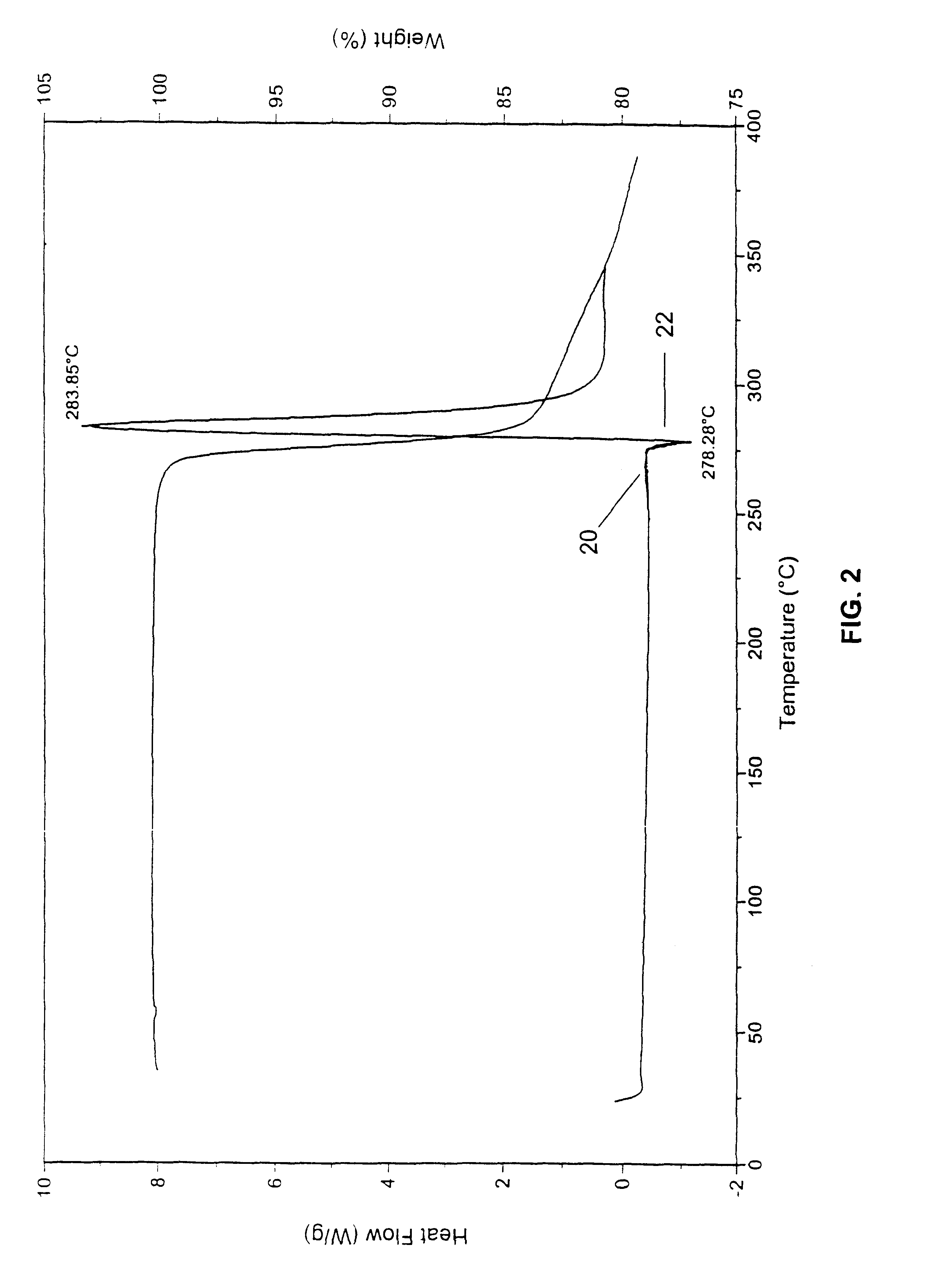
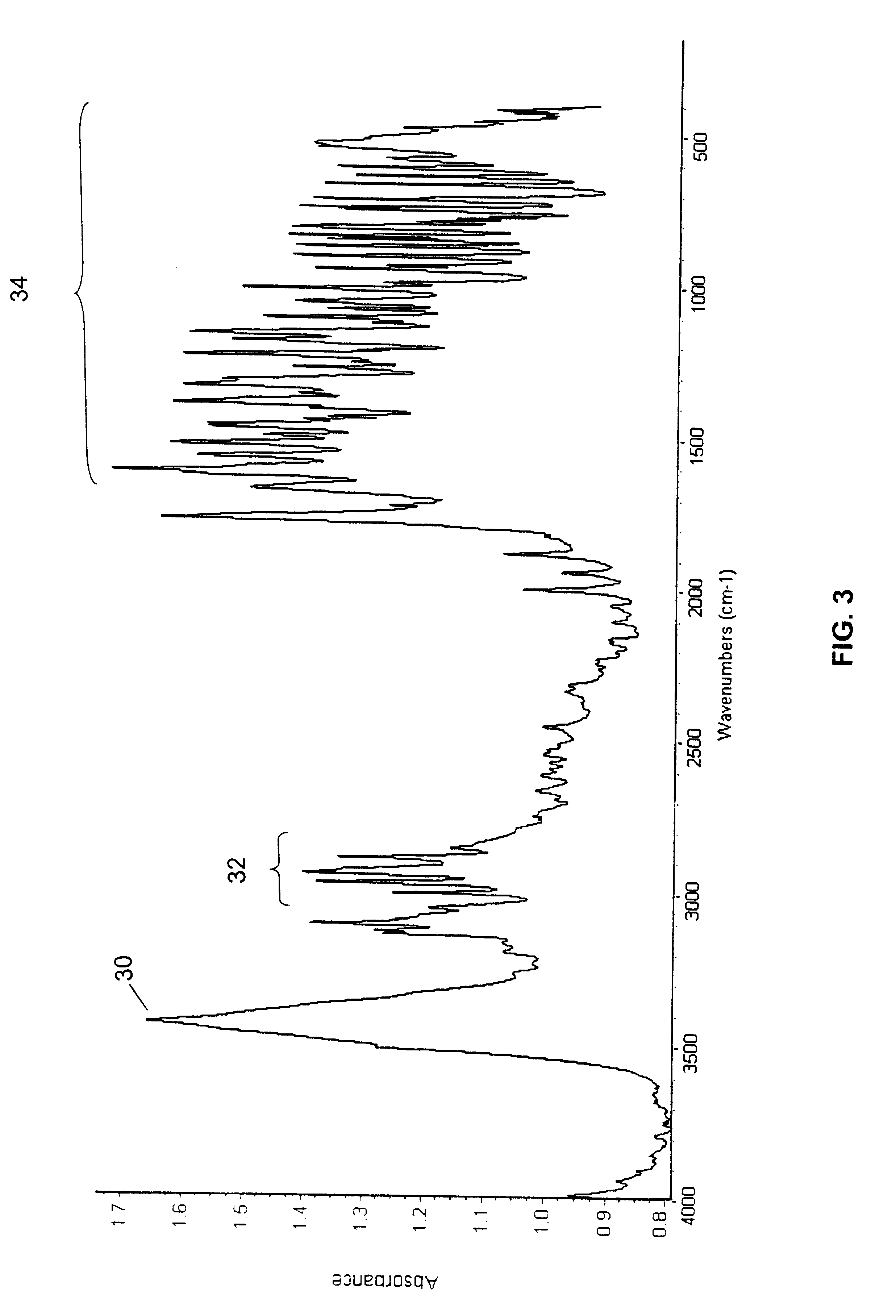
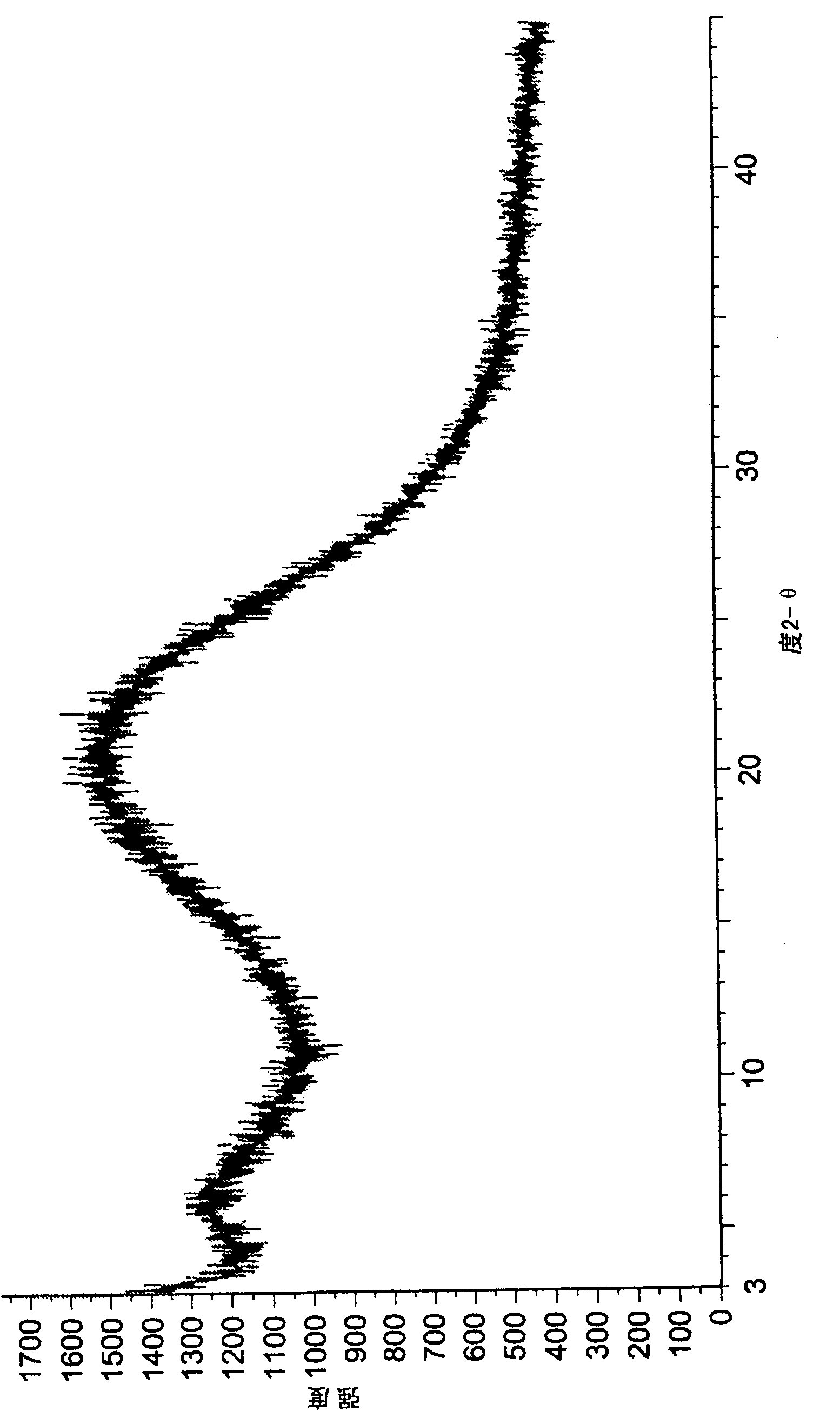
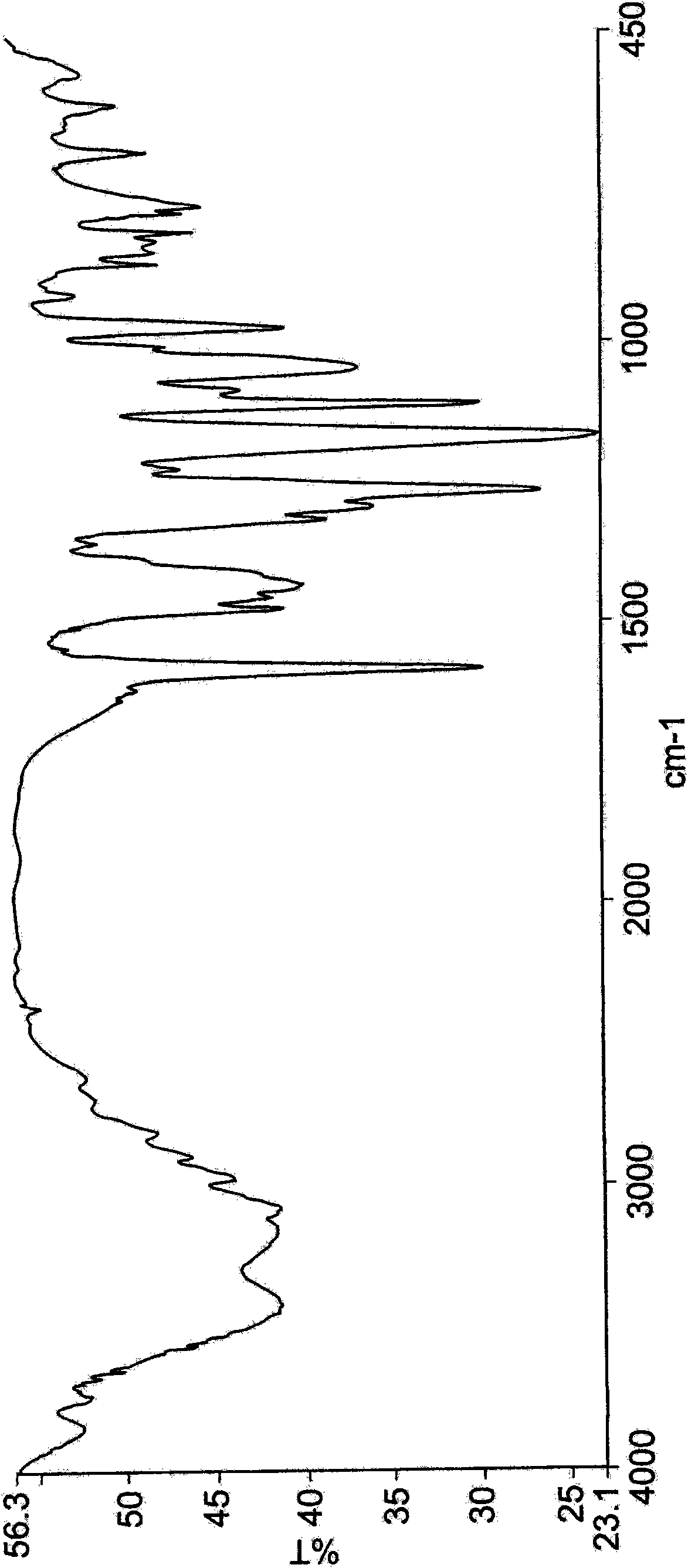
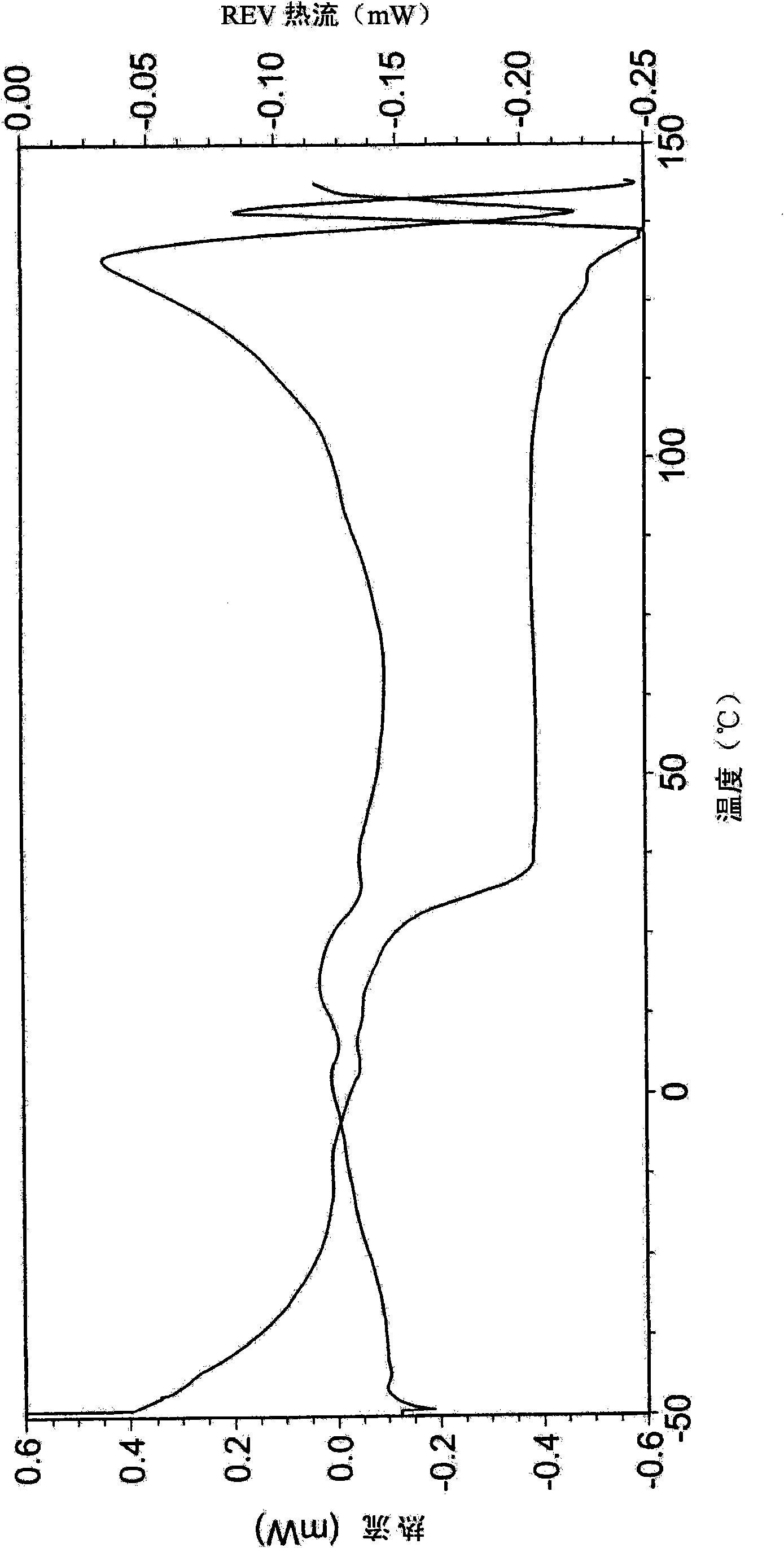
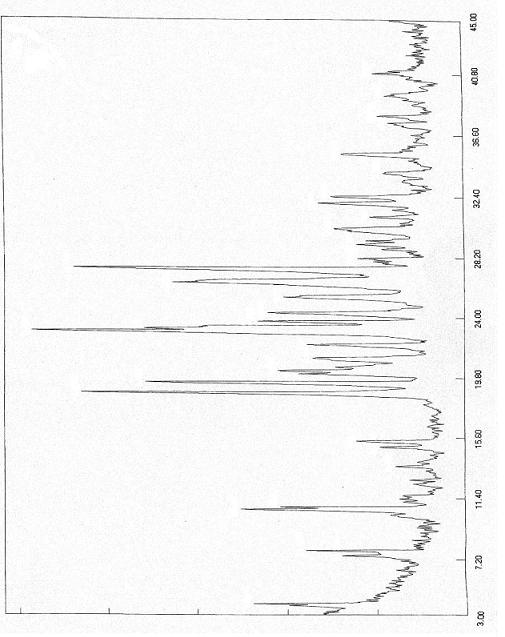
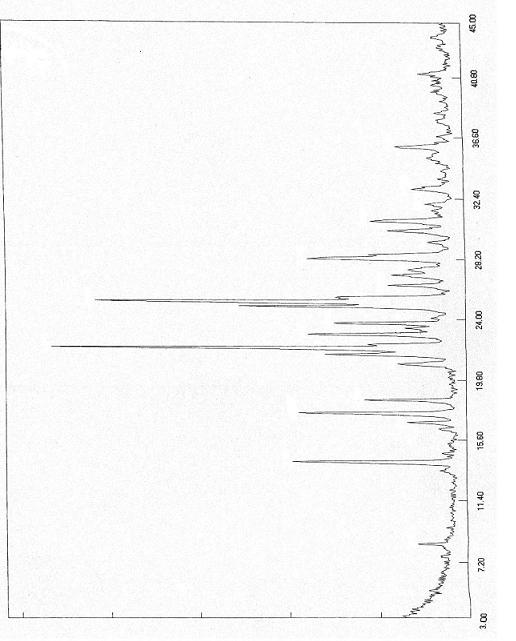
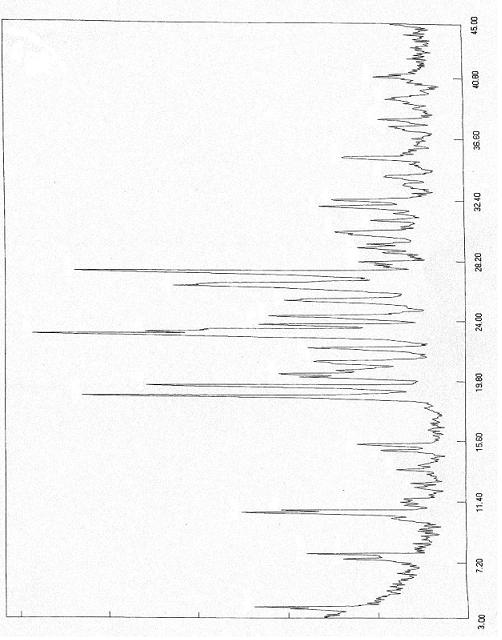
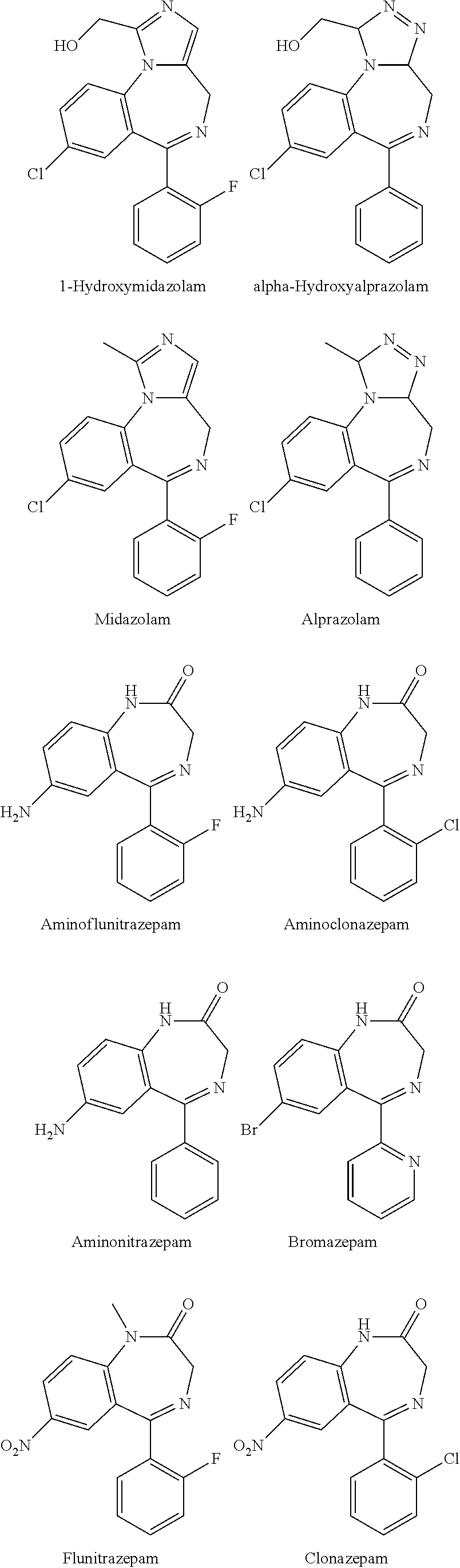
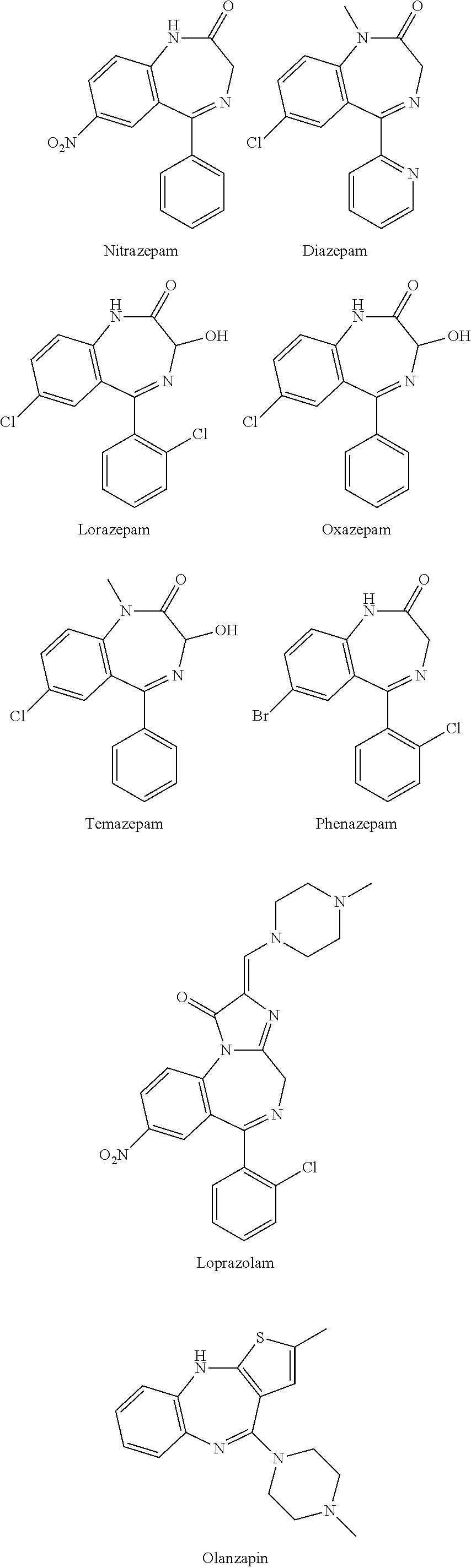
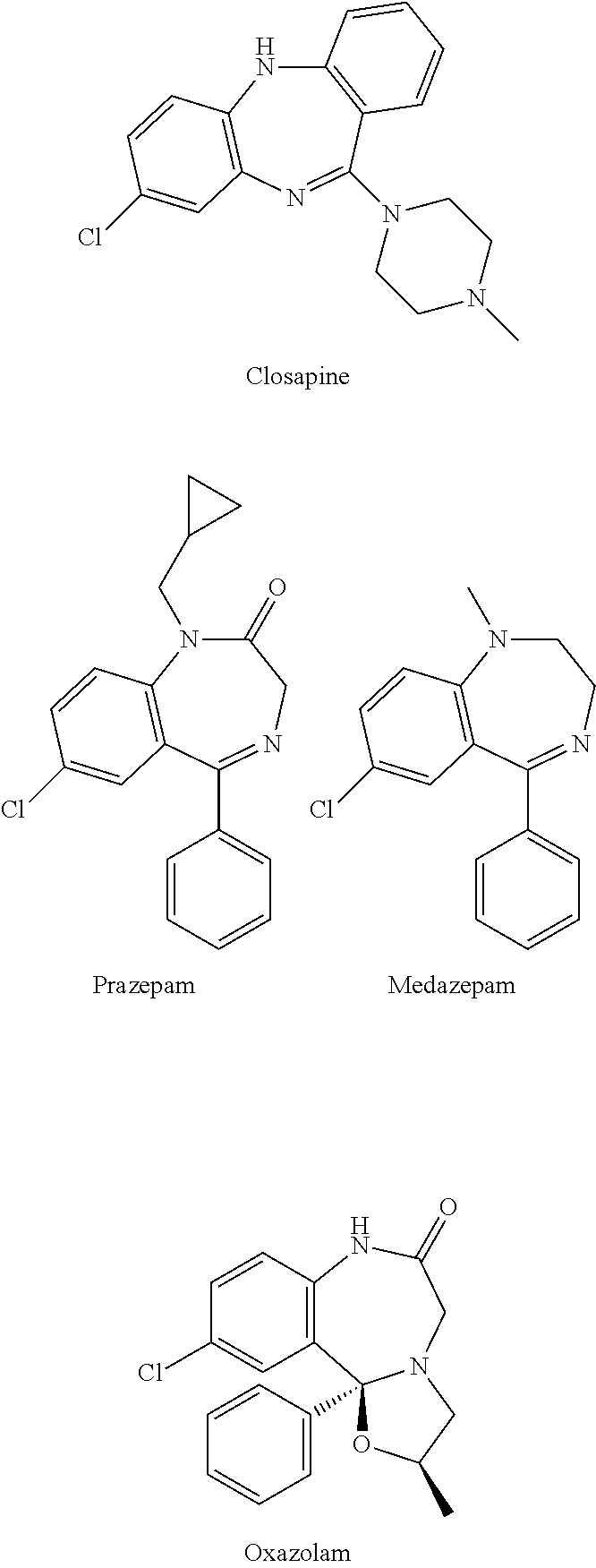
Polymorphs of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsufonyl)amino]-n-methyl-1-benzofuran-3-carboxamide and methods of making the same
Substantially pure polymorphic forms of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsulfonyl)amino]-N-methyl-1-benzofuran-3-carboxamide are produced by recrystallization in an organic solvent.
Owner:WYETH LLC +1
Stable crystalline salts of antifolate compounds
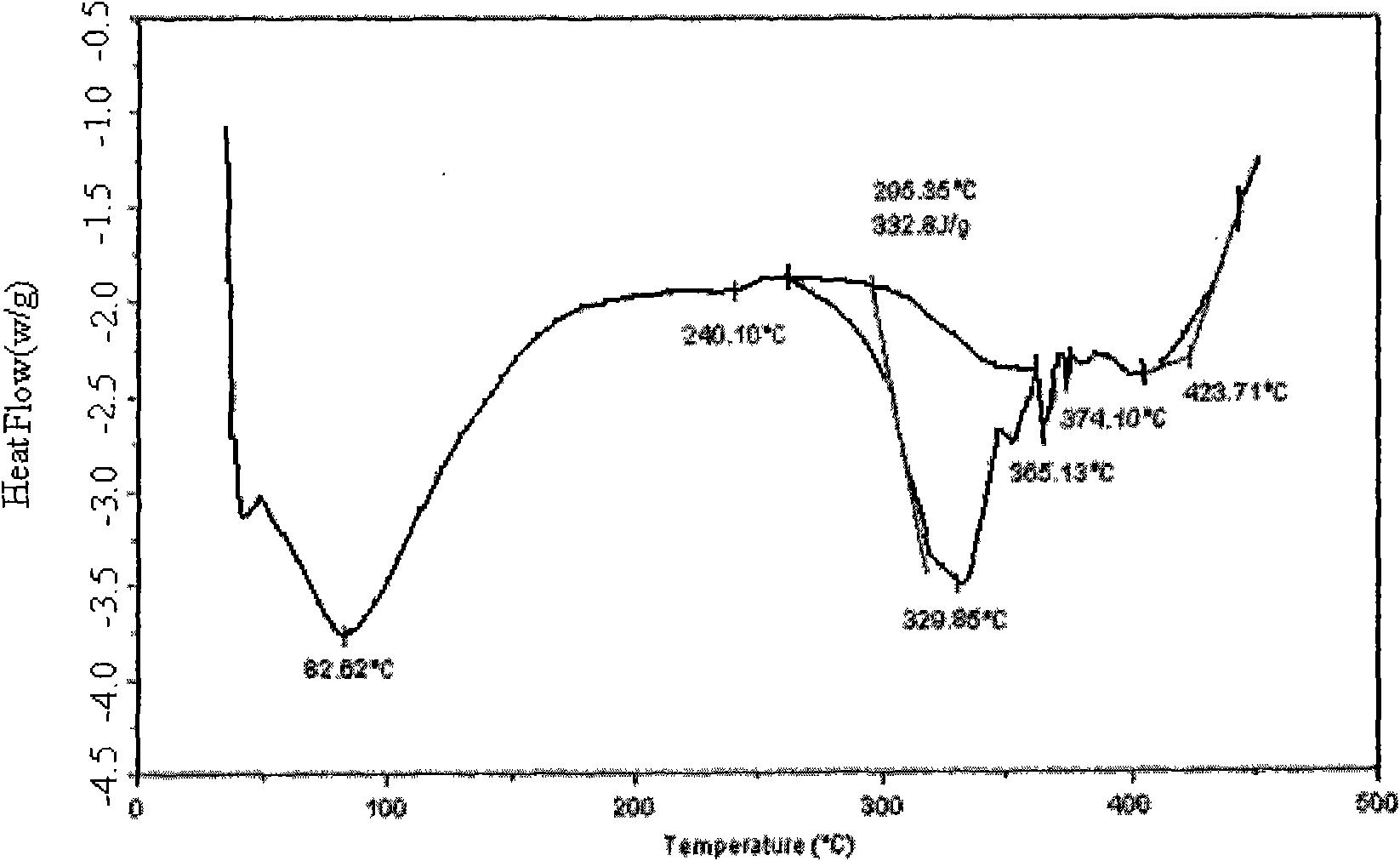
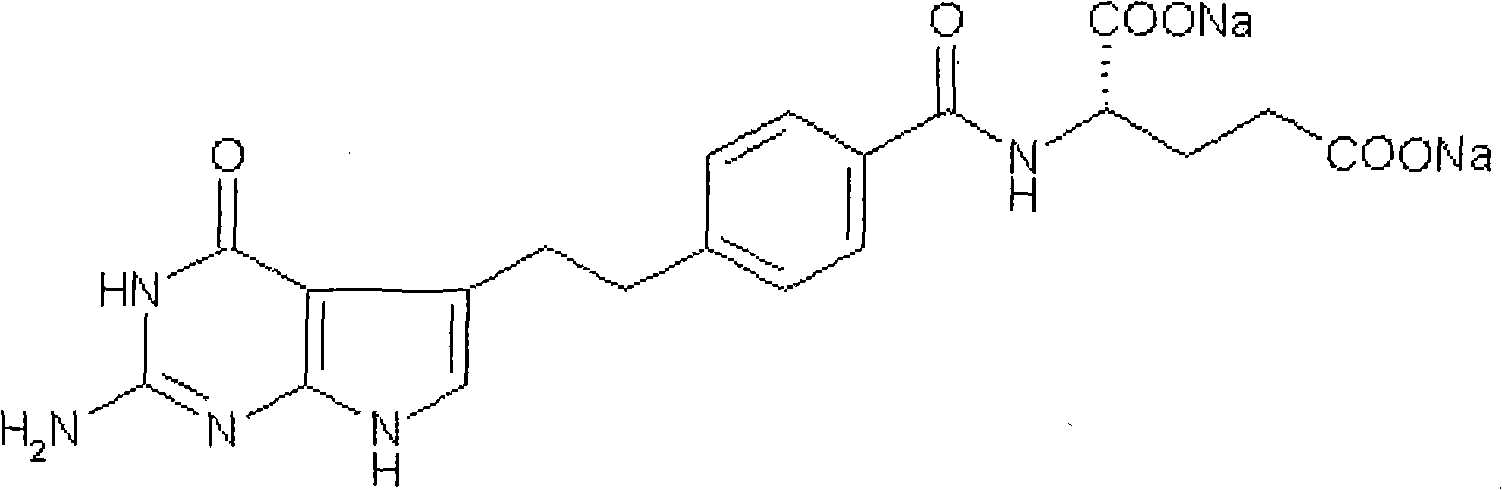
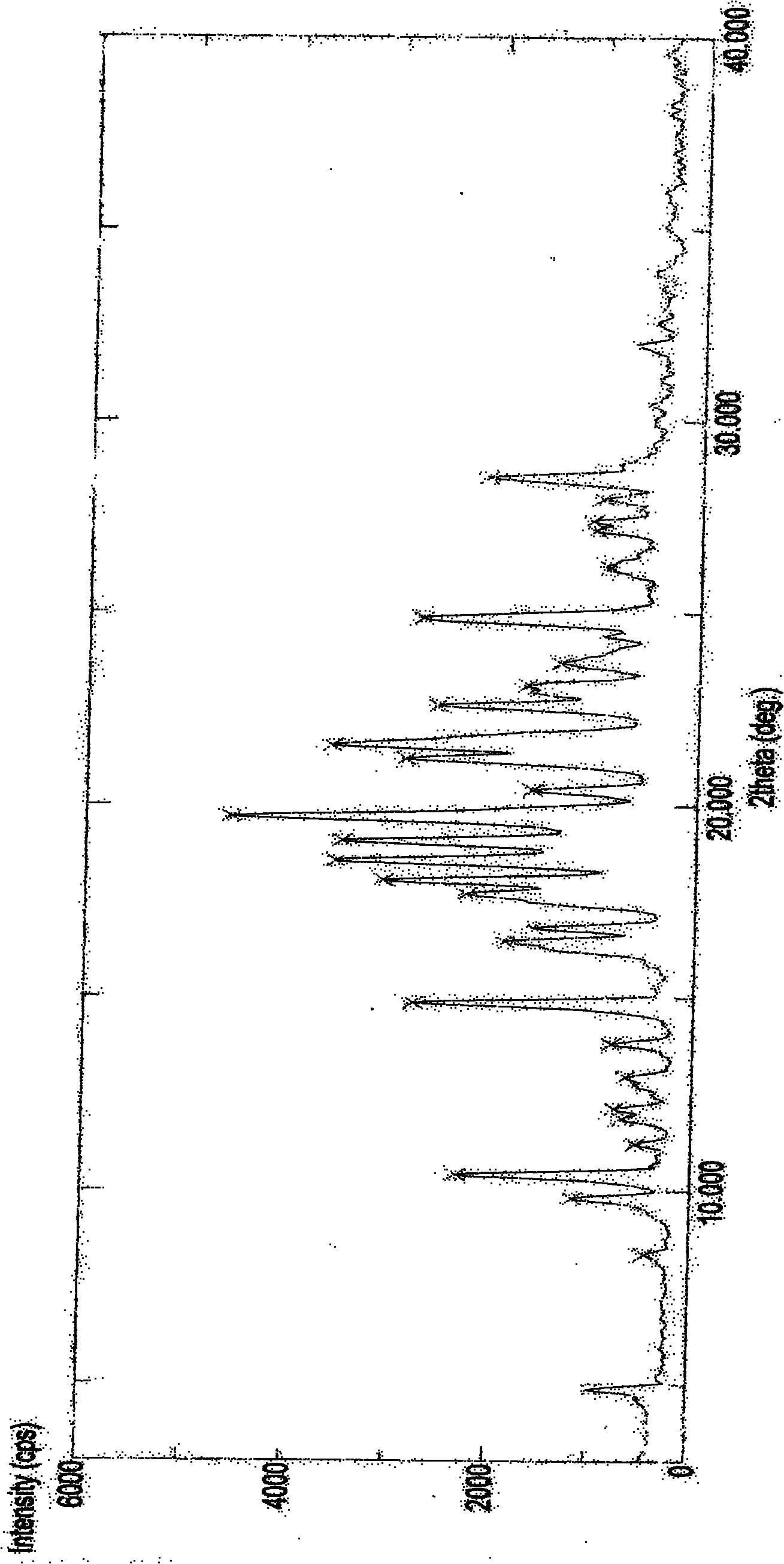
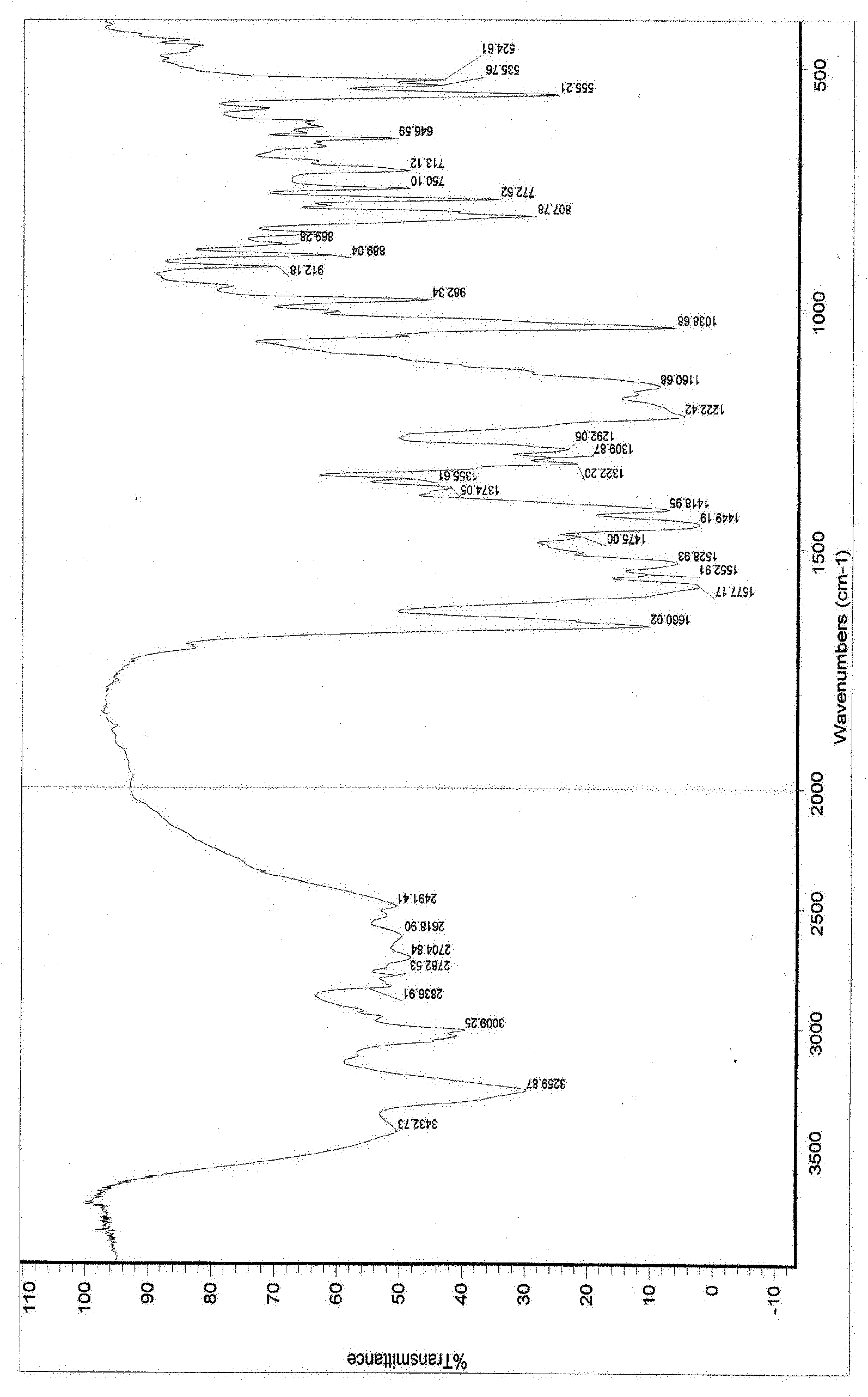
The present invention provides stable crystalline polymorphic forms of antifolate compounds, particularly (S)-2-{4-[2-(2,4-diamino-quinazolin-6-yl)-ethyl]-benzoylamino}-4-methylene-pentanedioic acid, dipotassium salt, and methods of preparation thereof The polymorphs may be in the form of hydrates. The invention further provides pharmaceutical compositions comprising the polymorphs and methods of treatment using the polymorphs. The polymorphs are useful in the treatment of multiple conditions, including abnormal cell proliferation, inflammatory diseases, asthma, and arthritis, and the polymorphs may be administered alone in or combination with on or more further active agents.
Owner:CHELSEA THERAPEUTICS
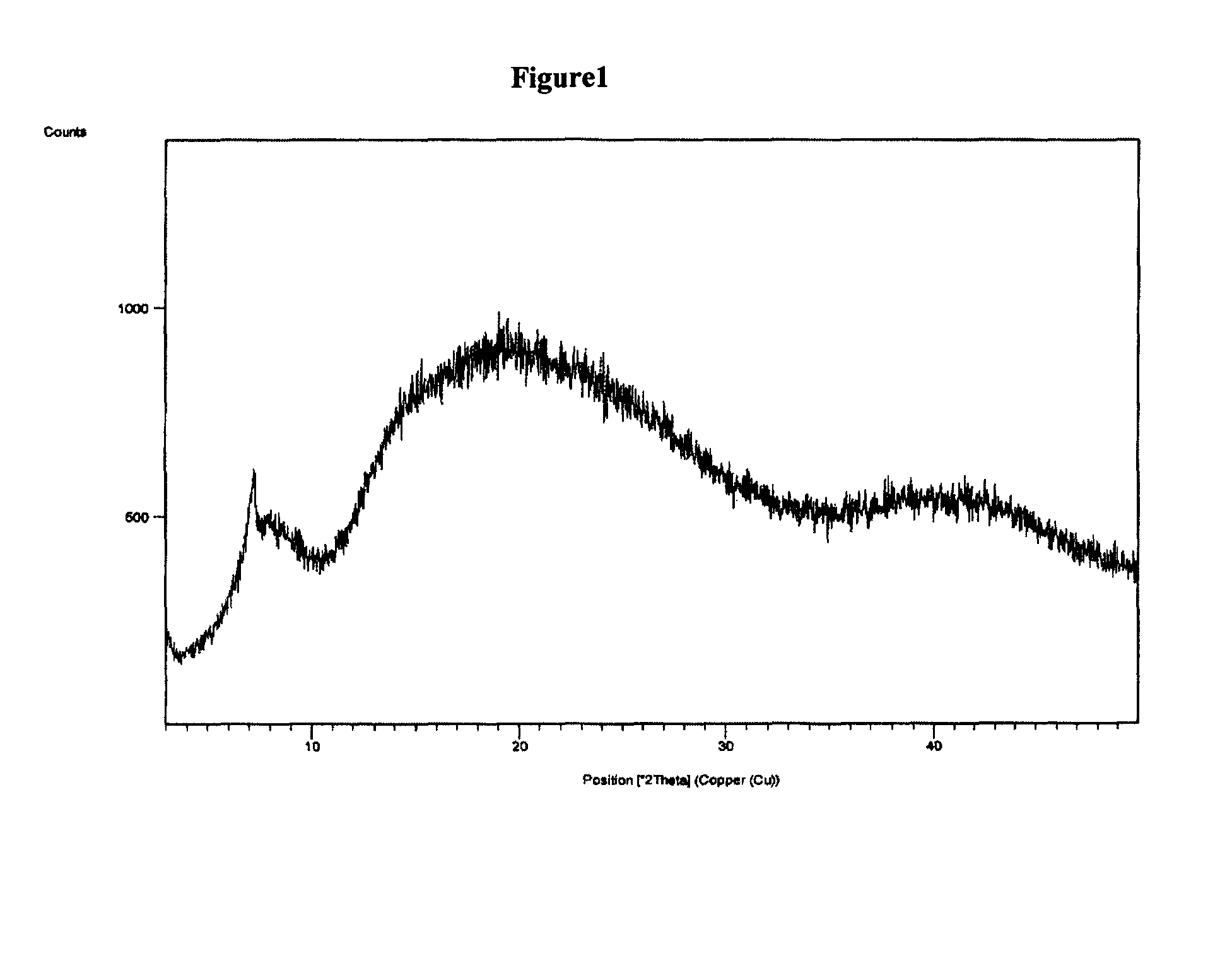
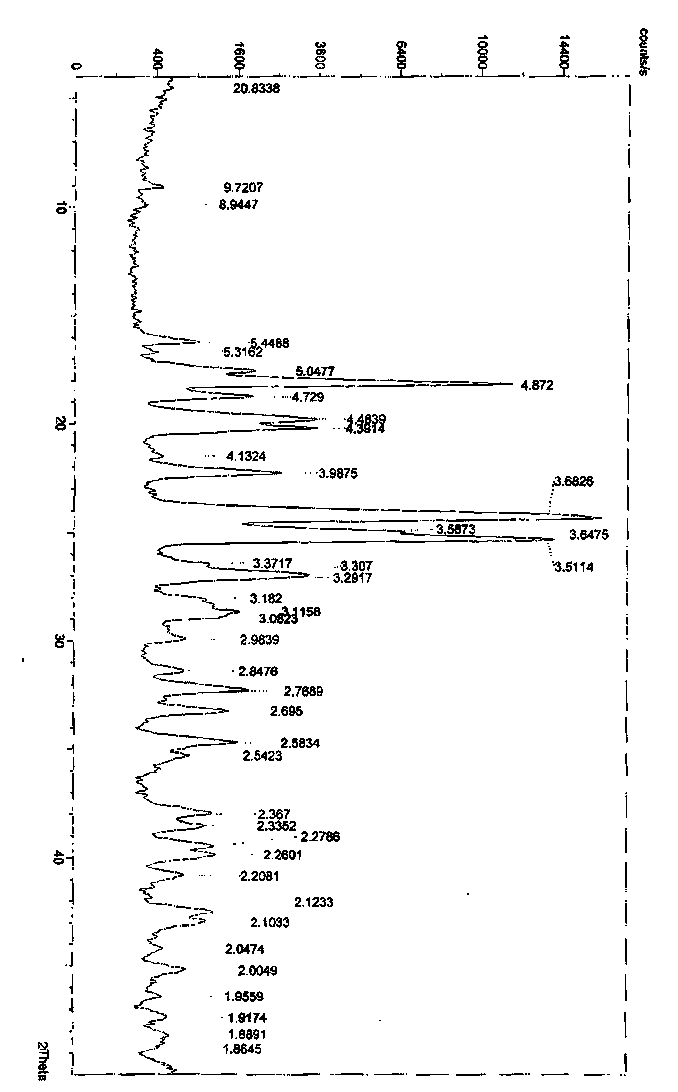
Polymorphic form of rifaximin and process for its preparation
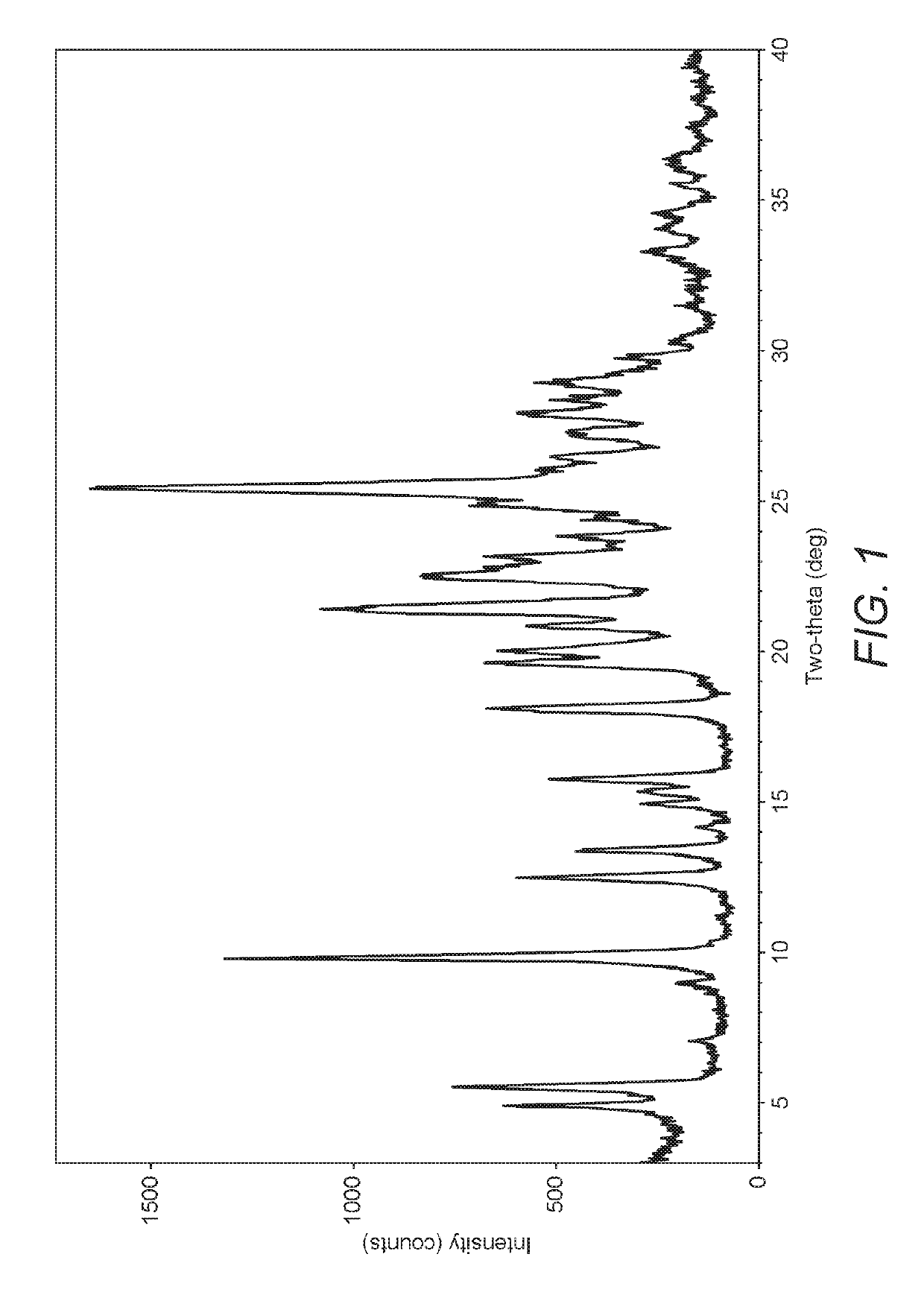
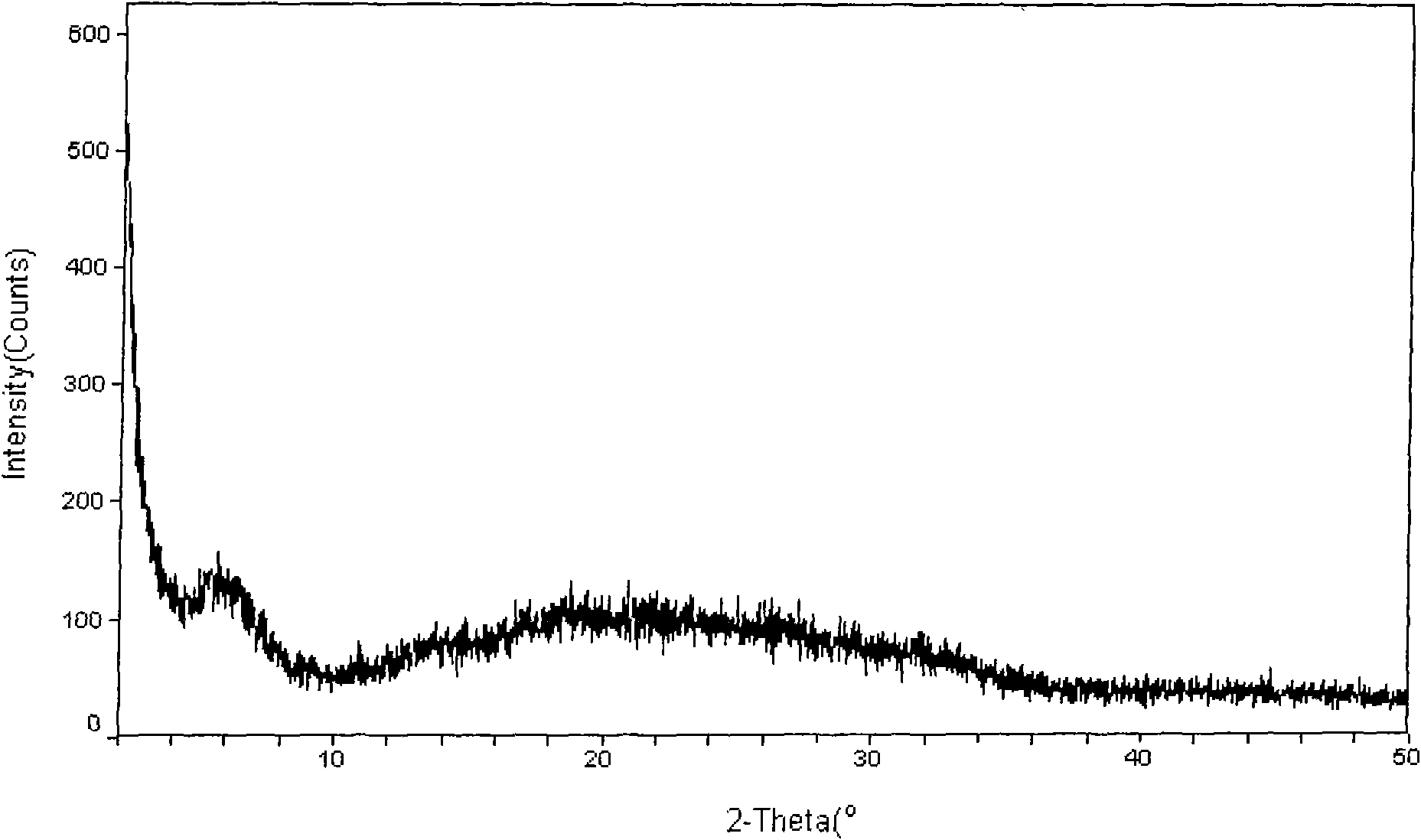
The present invention discloses a stable amorphous form of Rifaximin characterized by having X-ray powder diffraction pattern as given in FIG. 1, having a 2θ peaks at 7.2 and having moisture content in the range of 3% to 4% preferably, 3.4% to 3.7%. This invention also discloses a novel process for its preparation.
Owner:SEQUENT SCI LTD
Polymorphs of acyl sulfonamides
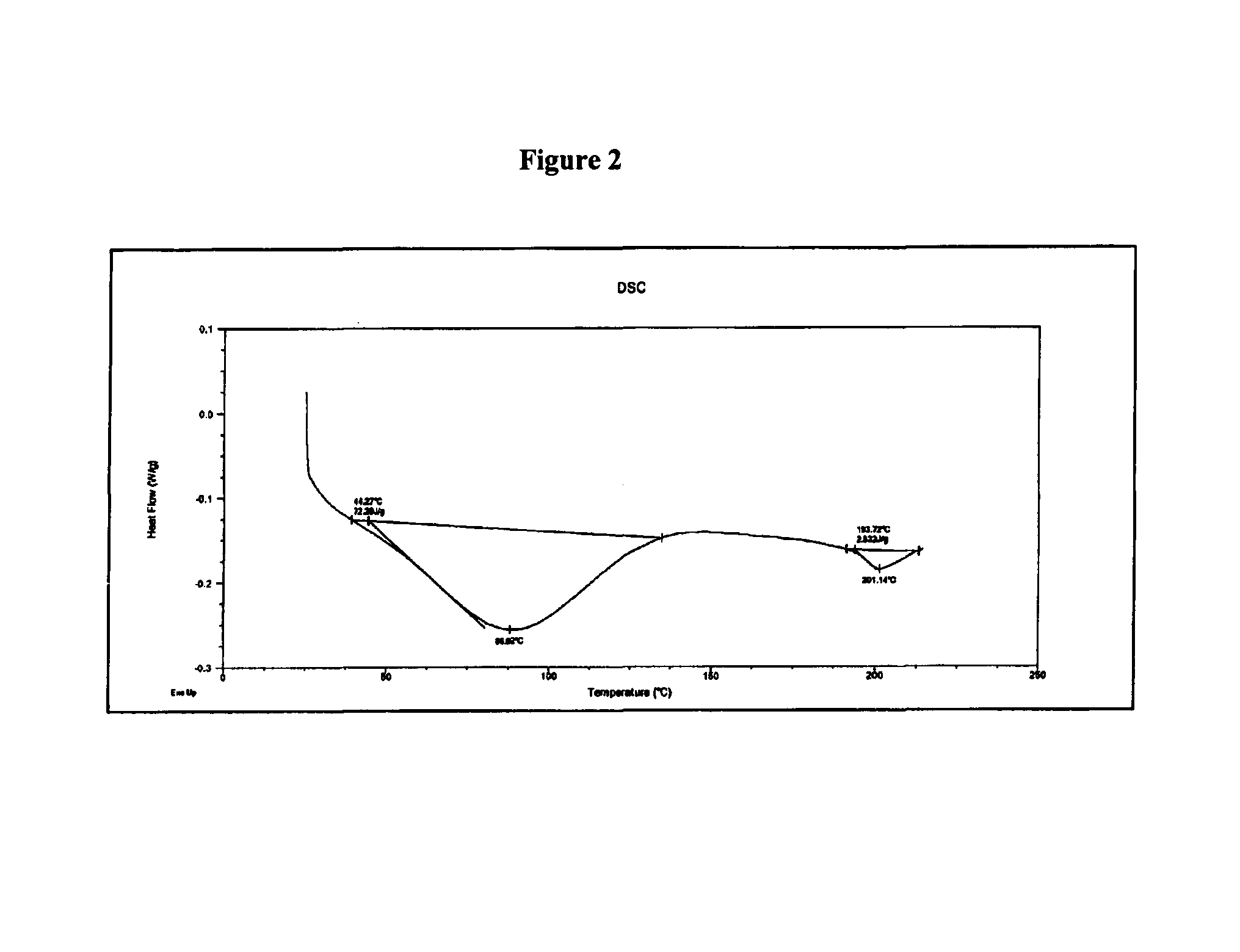
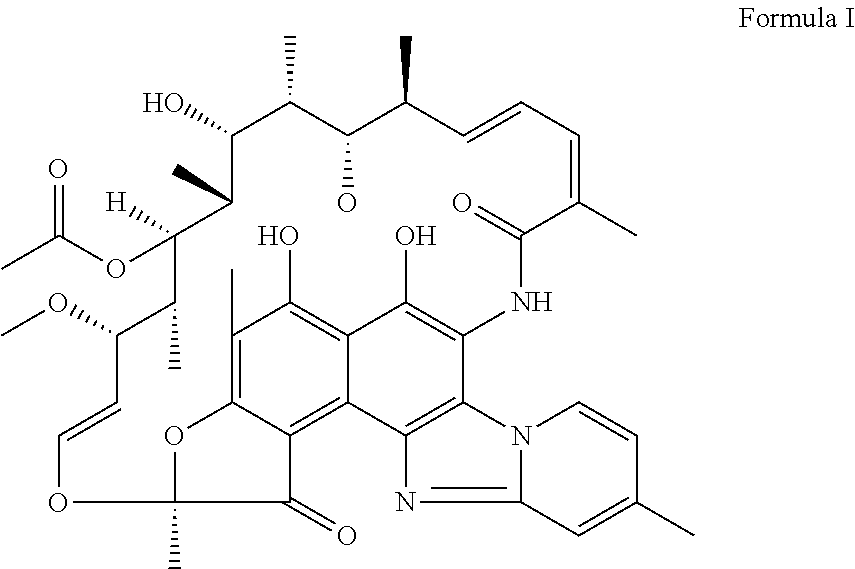
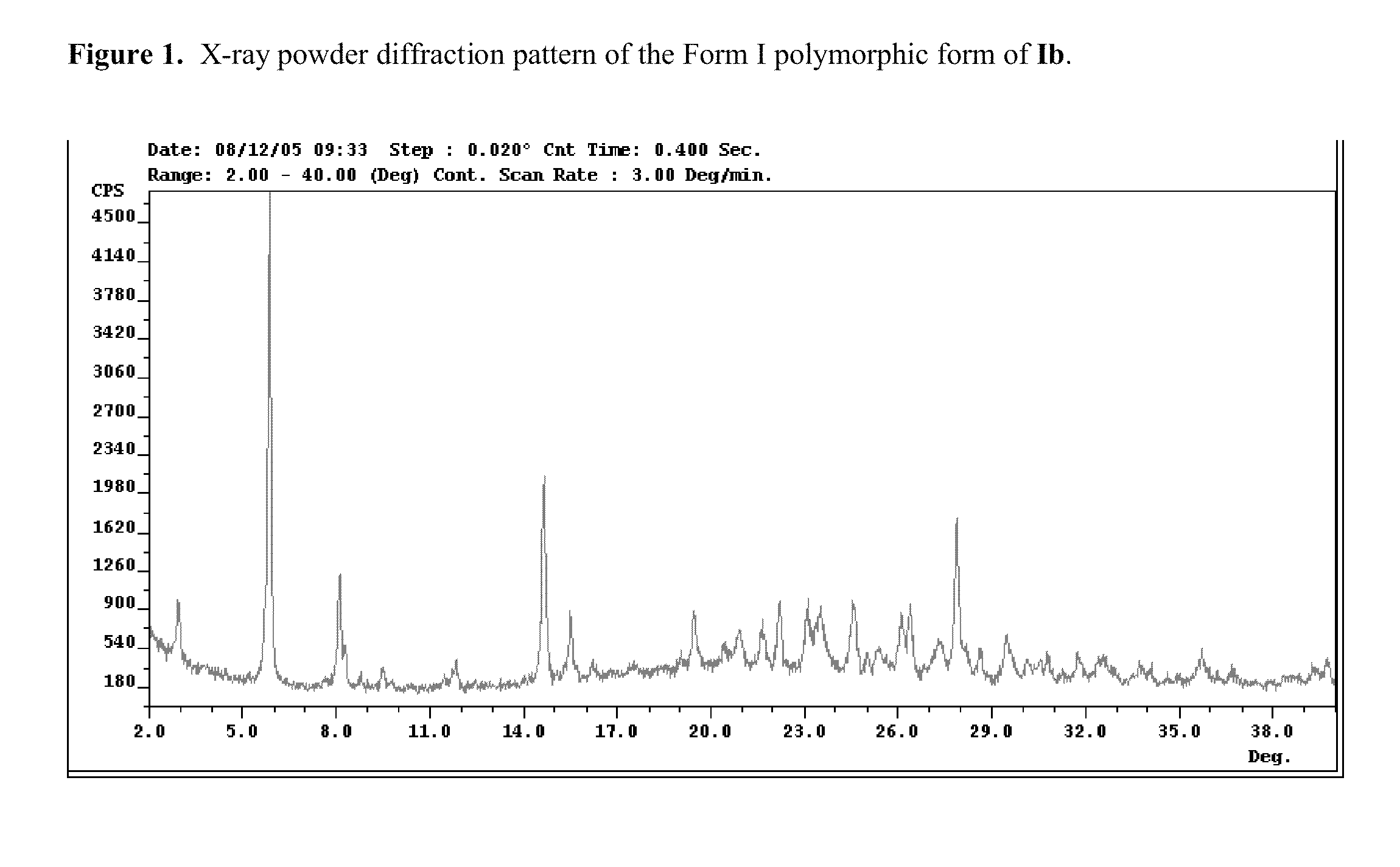
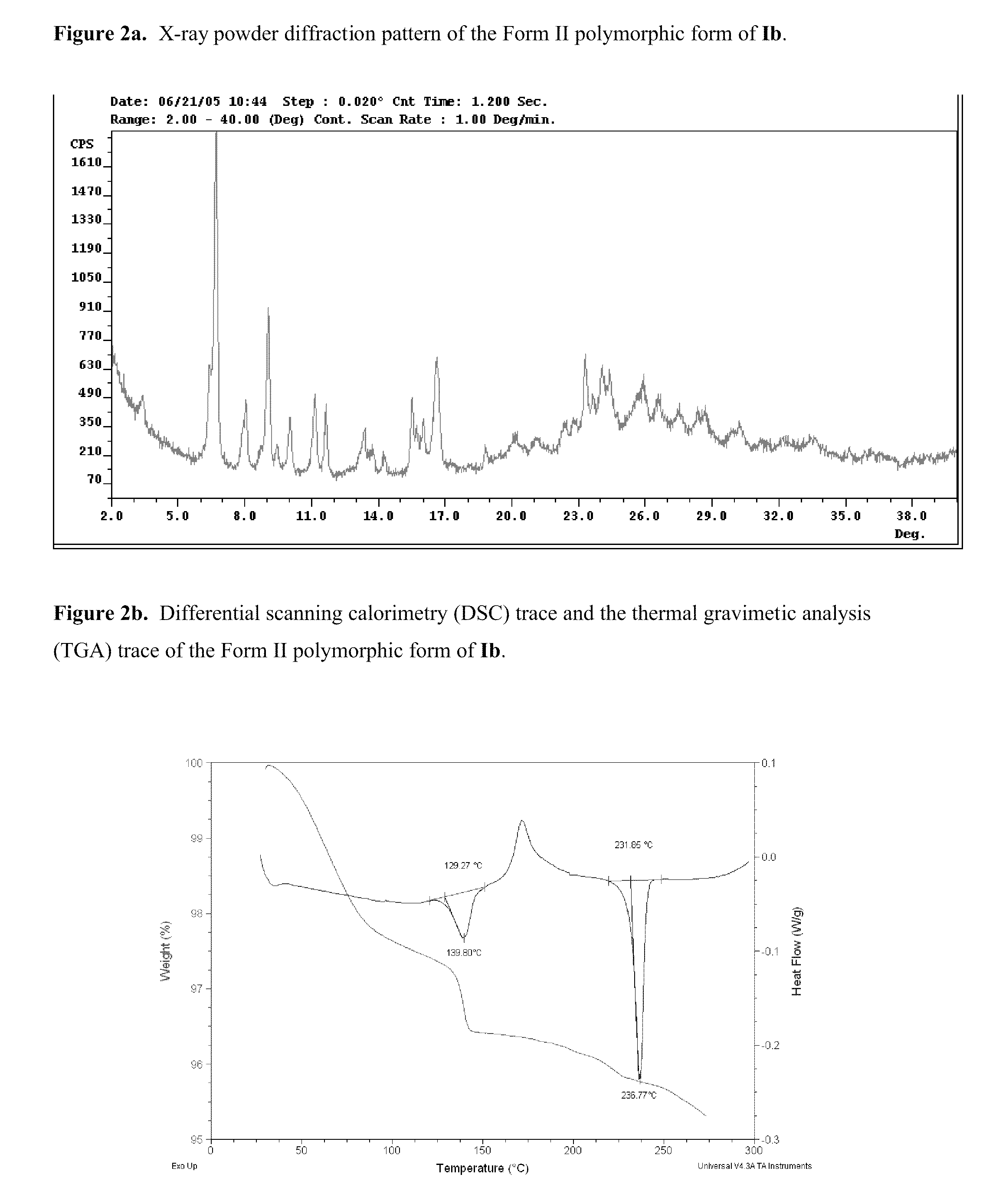
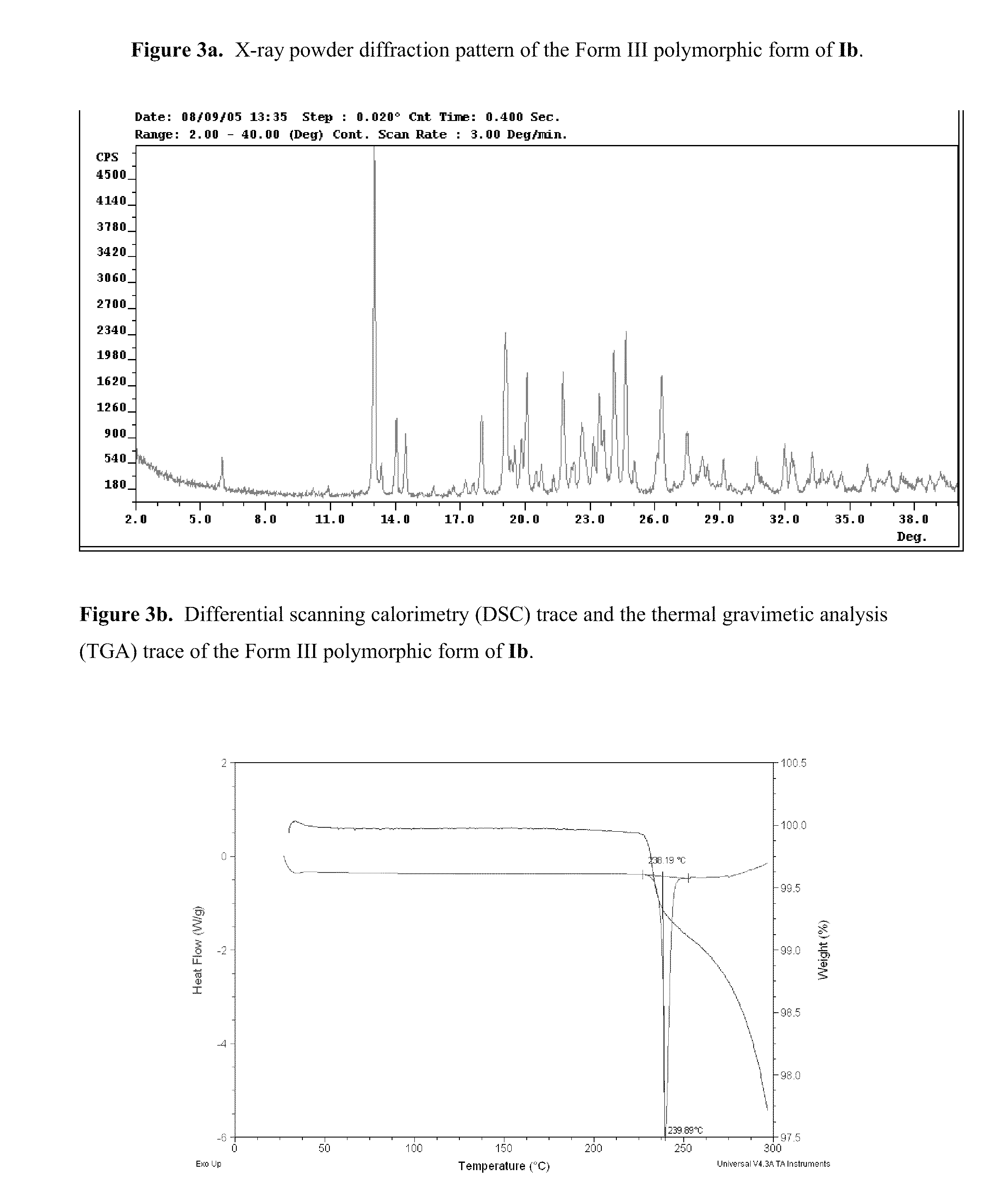
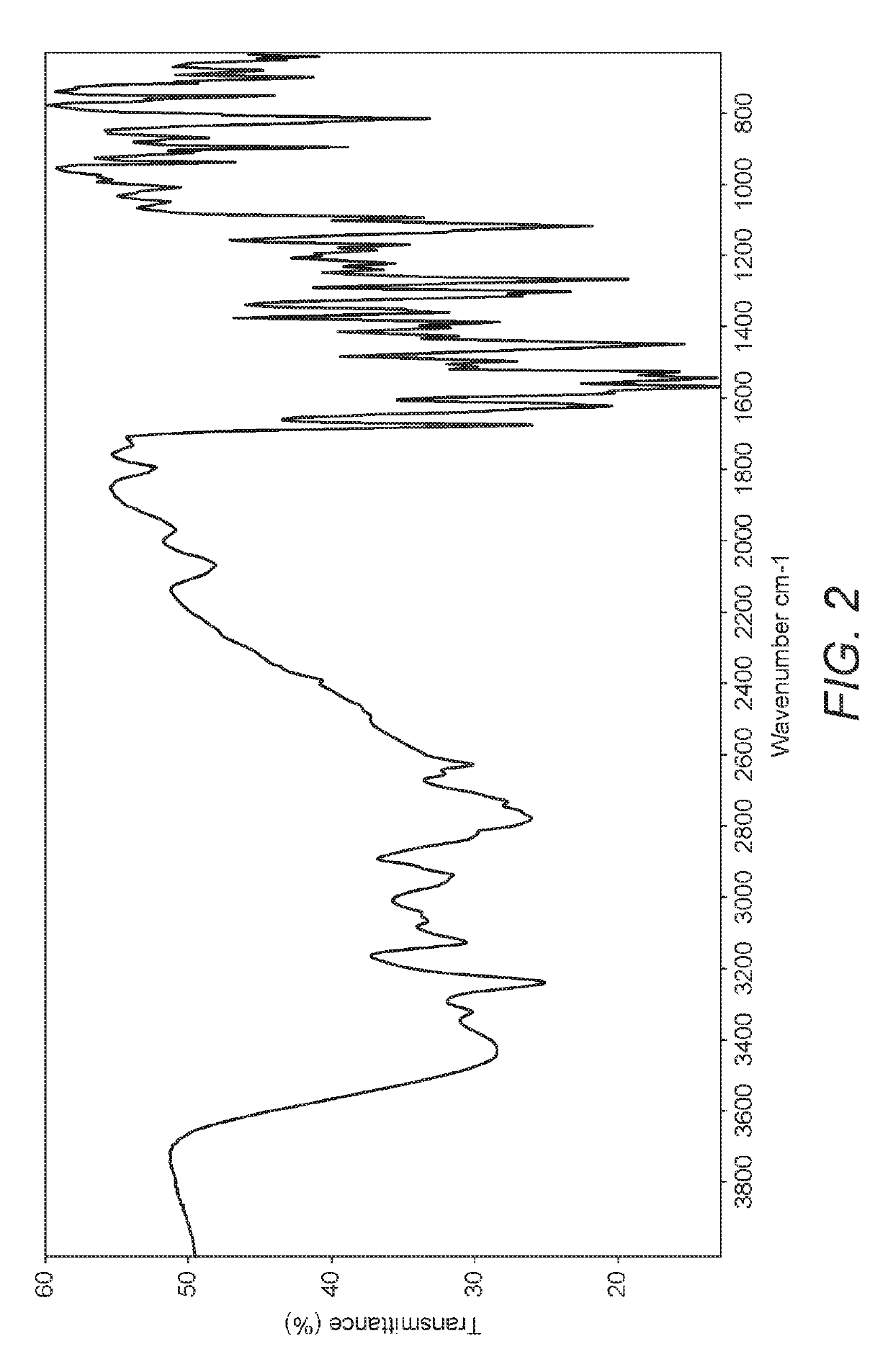
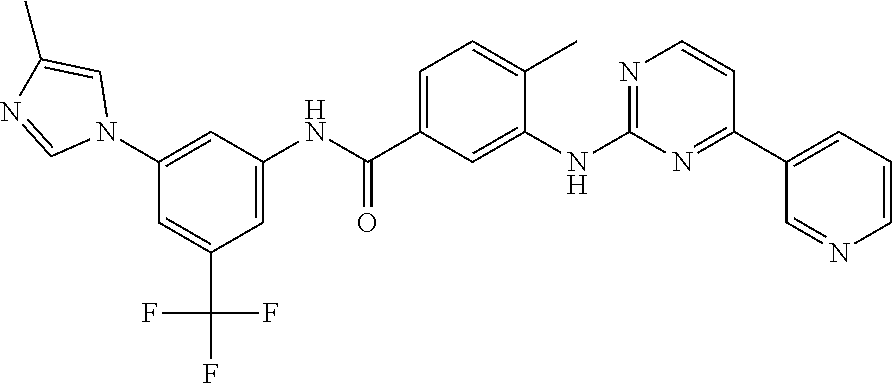
The application discloses novel polymorphic crystalline forms of 2-[4-Bromo-3-(3-chloro-5-cyano-phenoxy)-2-fluoro-phenyl]-N-(2-chloro-4-propionylsulfamoyl-phenyl)-acetamide, sodium salt (Ib)with improved stability and physical properties which facilitate manufacturing, handling and formulating for treatment or prophylaxis of HIV mediated diseases, AIDS or ARC, in monotherapy or in combination therapy.
Owner:ROCHE PALO ALTO LLC
Polymorphic form X of nilotinib dihydrochloride hydrate
InactiveUS10301282B2Improve permeabilityImprove solubilityOrganic chemistry methodsAntineoplastic agentsMedicinal chemistryNILOTINIB HYDROCHLORIDE
The present invention relates to a novel polymorph of nilotinib hydrochloride (Form X), to processes for its preparation, to pharmaceutical compositions containing the same and to its use in medicine.
Owner:CIPLA LTD
Polymorphs of atorvastatin tert.-butylester and use as intermediates for the preparation of atorvastatin
The invention relates to crystalline forms and 1 and 2 of atorvastatin tert.-butyl ester (Formula (II)), processes for their preparation and their conversion to highly pure atorvastatin hemi calcium in non-crystalline, in particular amorphous form.
Owner:新梅斯托卡尔卡托瓦纳兹德拉韦尔公司
Polymorphs of atorvastatin tert.-butylester and use as intermediates for the preparation of atorvastatin
The invention relates to crystalline forms 2 of atorvastatin tert.-butyl ester (Formula (II)), processes for their preparation and their conversion to highly pure atorvastatin hemi calcium in non-crystalline, in particular amorphous form.
Owner:新梅斯托卡尔卡托瓦纳兹德拉韦尔公司
Compounds, compositions and methods
ActiveUS10202363B2Suitable for bulk preparation and handlingMore thermodynamically stableOrganic active ingredientsSenses disorderMedicineChemical compound
The disclosed subject matter provides certain polymorphic forms of Compound (I) as well as pharmaceutical compositions comprising Compound (I) or such polymorphic forms, and methods of using or making such compounds and pharmaceutical compositions. It has now been discovered that Compound (I) can exist in multiple crystalline forms (polymorphs). One particular crystalline form, Form II, has been found to be more thermodynamically stable and, thus, likely more suitable for bulk preparation and handling than other polymorphic forms. Efficient and economic methods have been developed to prepare Compound (I) and Form II in high purity on a large scale. In animal studies, Form II has demonstrated safety and efficacy in treating depressive disorders and, when micronized, improved absorption compared to non-micronized Form II.
Owner:MERCK SHARP & DOHME CORP +1
Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)} methyl nitrate and processes of preparation thereof
The present invention provides novel anhydrous polymorph forms of 2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl nitrate (Compound A). The present invention also provides processes for preparation of the anhydrous polymorphic forms of compound A.
Owner:INOTECK PHARMA CORP
Process for making manganese dioxide and its polymorphs reversible
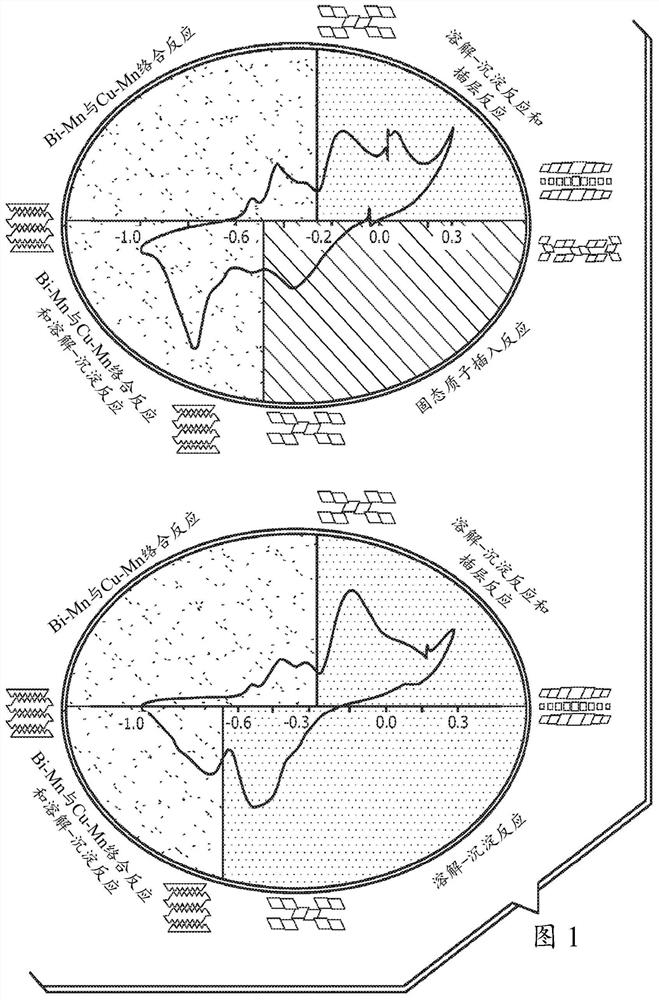
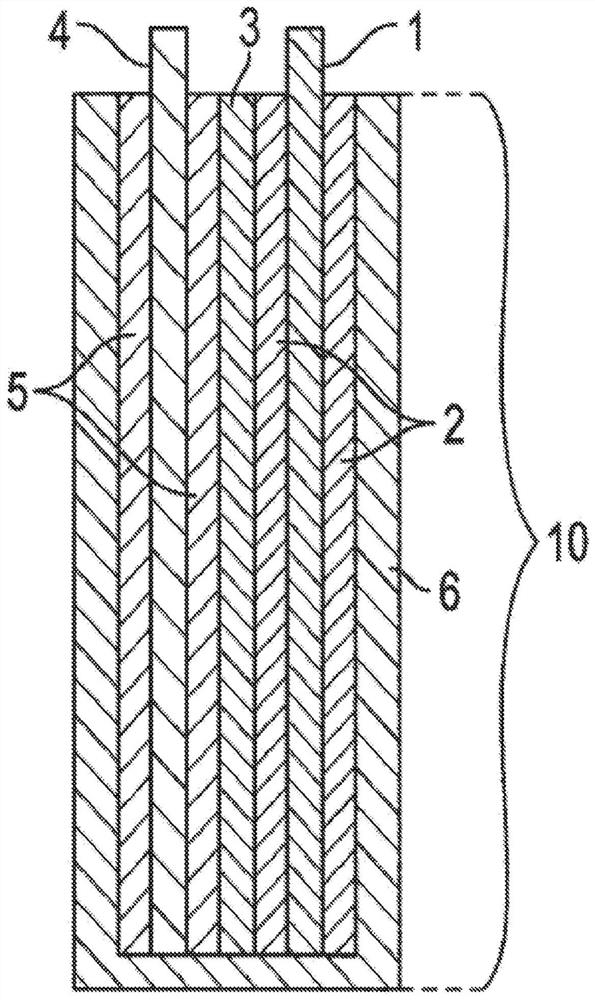
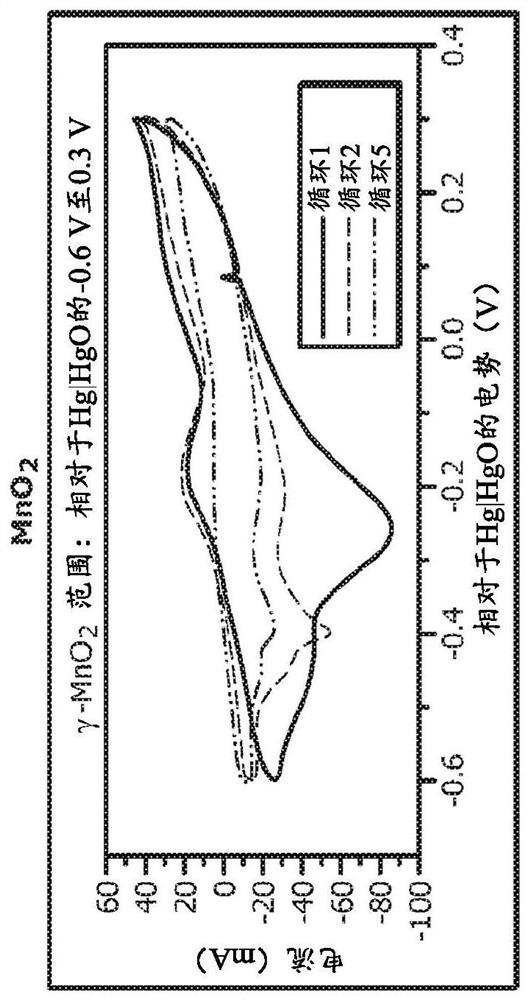
PendingCN112106234AElectrochemical processing of electrodesFinal product manufactureElectrical batteryEngineering
A method of forming a layered manganese dioxide for use in a cathode of a battery comprises disposing a cathode into a housing of an electrochemical cell, disposing an anode into the housing, disposing a polymeric separator between the anode and the cathode such that the anode and the cathode are electrically separated, adding an alkaline electrolyte to the housing, cycling the electrochemical cell into the 2nd electron capacity of the manganese dioxide, and forming a layered manganese dioxide having a layered manganese dioxide structure with the one or more additives incorporated into the layered manganese dioxide structure. The cathode comprising a cathode material comprising: a manganese dioxide compound, one or more additives selected from the group consisting of bismuth, copper, tin,lead, silver, cobalt, nickel, magnesium, aluminum, potassium, lithium, calcium, gold, antimony, iron, zinc, and combinations thereof, and a conductive carbon.
Owner:RES FOUND THE CITY UNIV OF NEW YORK
BTK inhibitor polymorph and preparation method thereof
Owner:SHOUYAO HLDG BEIJING CO LTD
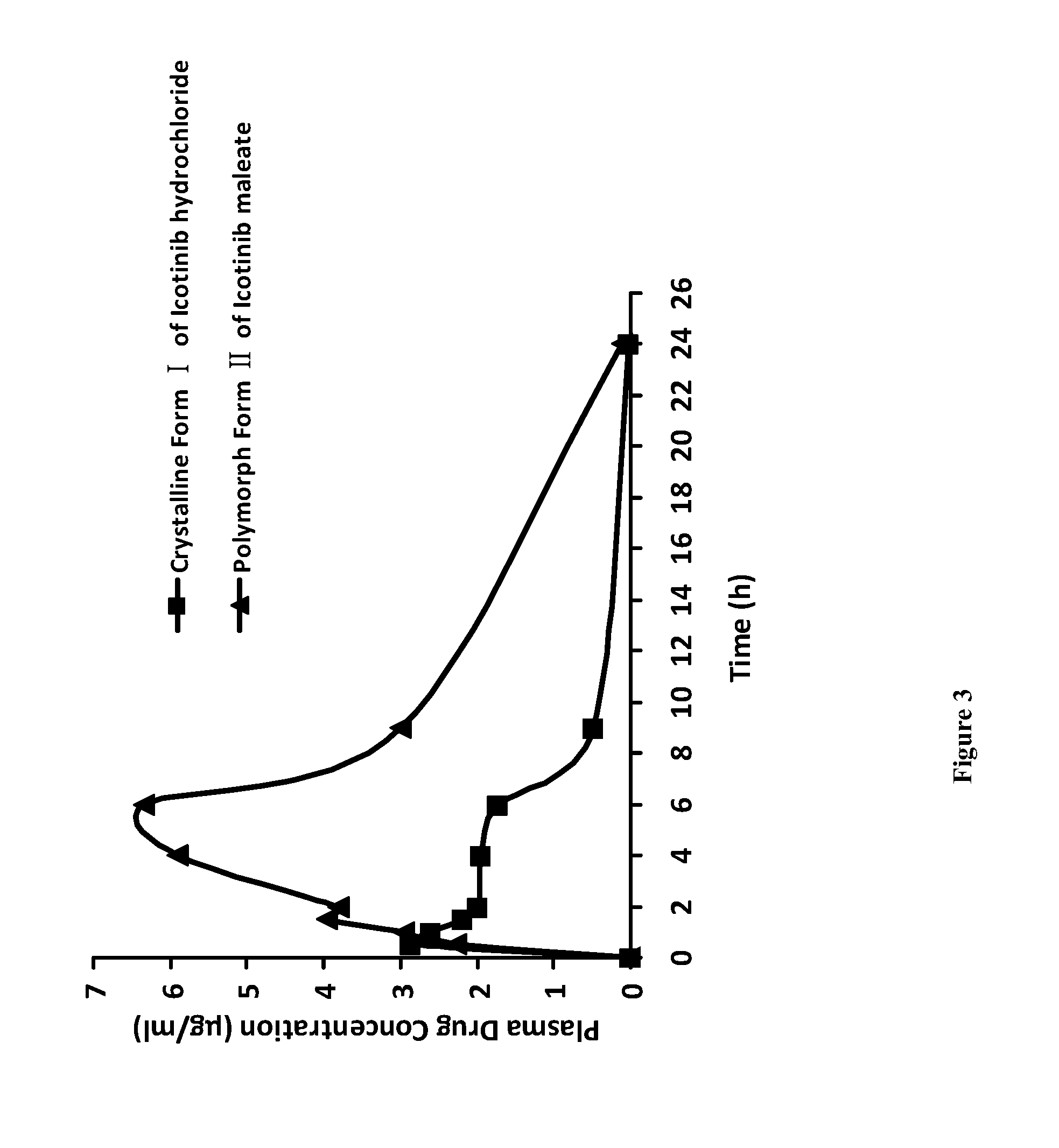
Polymorphic forms of icotinib maleate and uses thereof
Owner:BETTA PHARM CO LTD
Crystal polymorph of magnesium glycinate dihydrate and process for its preparation
ActiveUS20140186408A1Useful in treatmentReduce severityBiocideOrganic active ingredientsPhysical chemistryCombinatorial chemistry
Embodiments of the invention provide solid forms of magnesium glycinate dihydrate and compositions thereof, which are useful for treating hyperphosphatemia and which exhibit desirable characteristics for the same. The invention further provides processes for the production of solid forms of magnesium glycinate dihydrate.
Owner:CURRAX PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Polymorphs of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsufonyl)amino]-n-methyl-1-benzofuran-3-carboxamide and methods of making the same Polymorphs of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsufonyl)amino]-n-methyl-1-benzofuran-3-carboxamide and methods of making the same](https://images-eureka.patsnap.com/patent_img/f15fb06f-5102-4d17-9266-539684ff24dd/US20070265335A1-20071115-D00000.png)
![Polymorphs of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsufonyl)amino]-n-methyl-1-benzofuran-3-carboxamide and methods of making the same Polymorphs of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsufonyl)amino]-n-methyl-1-benzofuran-3-carboxamide and methods of making the same](https://images-eureka.patsnap.com/patent_img/f15fb06f-5102-4d17-9266-539684ff24dd/US20070265335A1-20071115-D00001.png)
![Polymorphs of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsufonyl)amino]-n-methyl-1-benzofuran-3-carboxamide and methods of making the same Polymorphs of 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsufonyl)amino]-n-methyl-1-benzofuran-3-carboxamide and methods of making the same](https://images-eureka.patsnap.com/patent_img/f15fb06f-5102-4d17-9266-539684ff24dd/US20070265335A1-20071115-D00002.png)