Process for making manganese dioxide and its polymorphs reversible
A manganese dioxide, electrochemical technology, applied in secondary batteries, electrochemical generators, active material electrodes, etc., can solve the problem of destroying the reversibility of manganese dioxide electrodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
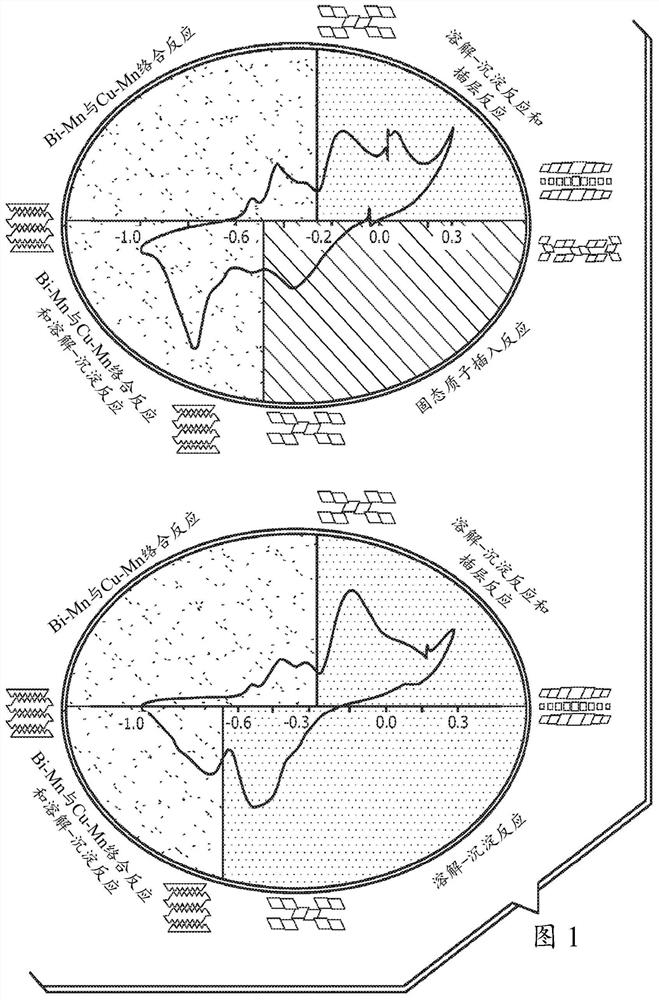
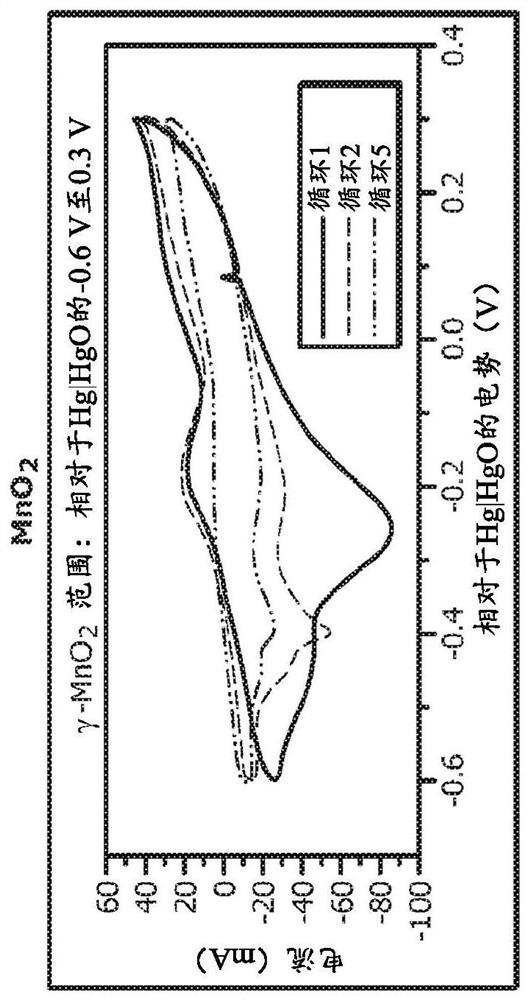
[0055] In a first example, a set of combined experiments was performed to show the beneficial effects of the additives. Cyclic voltammetry (CV) curves are shown in FIG. 3 . A 5 wt.% loading of manganese dioxide (EMD) was used in the mixture, with the remainder being balanced by graphite. Figure 3 (panels a-d) clearly shows that manganese dioxide is irreversible when cycled alone. It degrades to form Mn 3 o 4 the inactive phase. Figure 3 (panels e-h) shows the beneficial effect of adding bismuth oxide (1 wt.%) to the mixture. Figure 3 (panels e and f) shows that the mere presence of bismuth oxide is sufficient to make manganese dioxide rechargeable, as shown in Figure 3 (panels g and h), activation is not necessary. However, activation at a lower potential between -0.6 V and -1 V vs. Hg|HgO is necessary for maximum capacity retention of theoretical capacity. Figure 3 (panels i-l) shows the beneficial effect of adding only copper to the mixture. In this example, copper wa...
example 2
[0057] In a second example, both bismuth oxide and copper were added to the mixture with manganese dioxide. The loading weight percent of manganese dioxide and additives is the same as Example 2. As shown in Figure 4, the presence of two additives is very beneficial in achieving higher capacity retention much faster than simply having additives independent of each other. When manganese dioxide was cycled between -1 V and 0.3 V vs. Hg|HgO with both additives, the capacity retention was 100% of the theoretical capacity of manganese dioxide.
example 3
[0059] In a third example, as shown in FIG. 5 , manganese dioxide, bismuth oxide, and copper were subjected to constant current cycling with the same loading weight % as in the previous examples. A combined approach is also used in this example. In the absence of additives, the capacity retention of manganese dioxide is very poor. Addition of bismuth oxide imparted rechargeability; however, capacity retention was much better when cycled between -1 V and 0.3 V vs. Hg|HgO. The added copper was shown to be the most important additive since copper alone imparts reversibility and more excellent capacity retention when cycled to −0.6 V or −1 V vs. Hg|HgO. The theoretical full capacity retention is much faster when both bismuth oxide and copper are used together.
[0060] Having described various methods and apparatus, certain embodiments may include, but are not limited to:
[0061] In a first embodiment, an electrode comprises: a manganese oxide compound; selected from the group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com