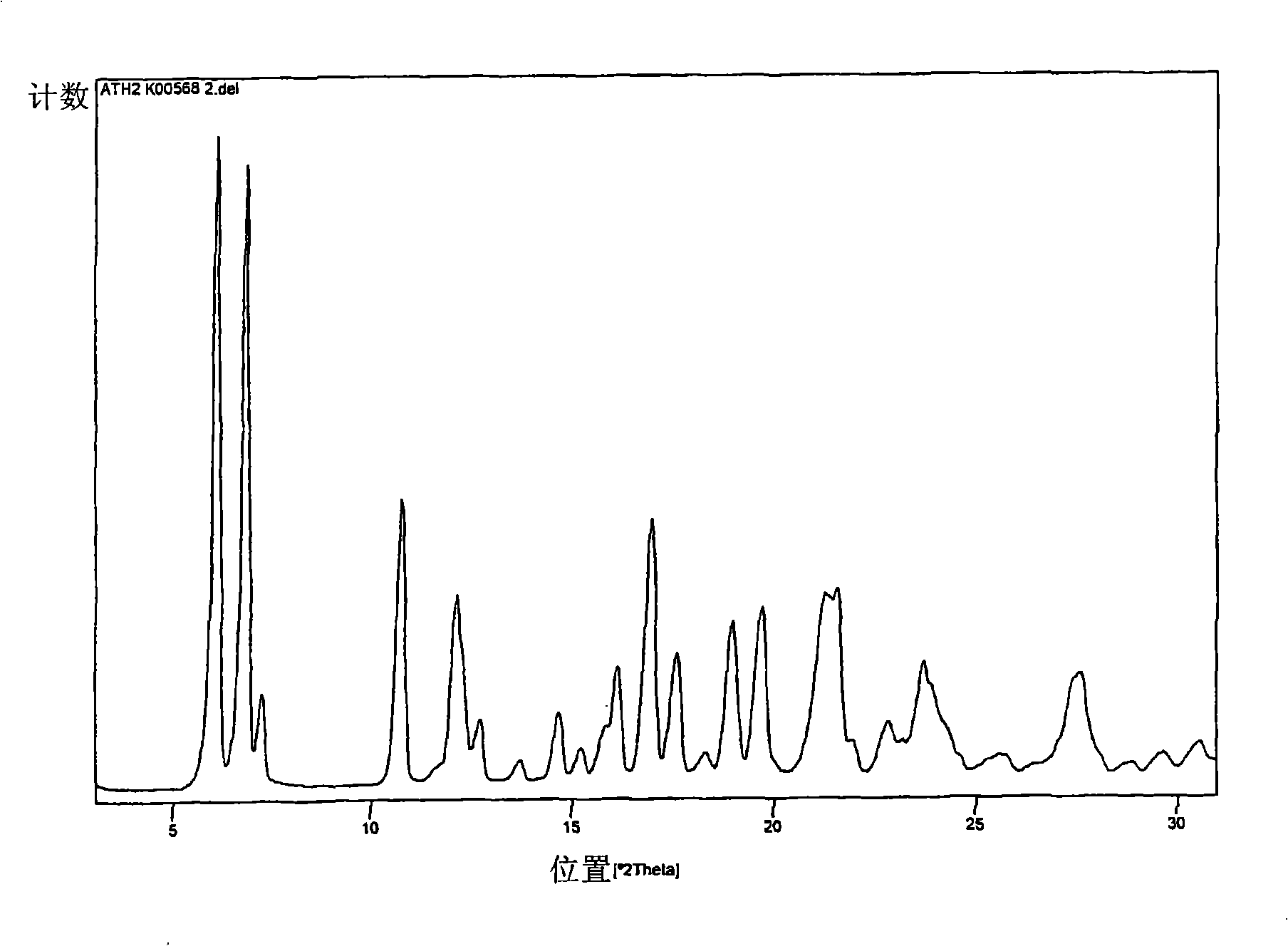
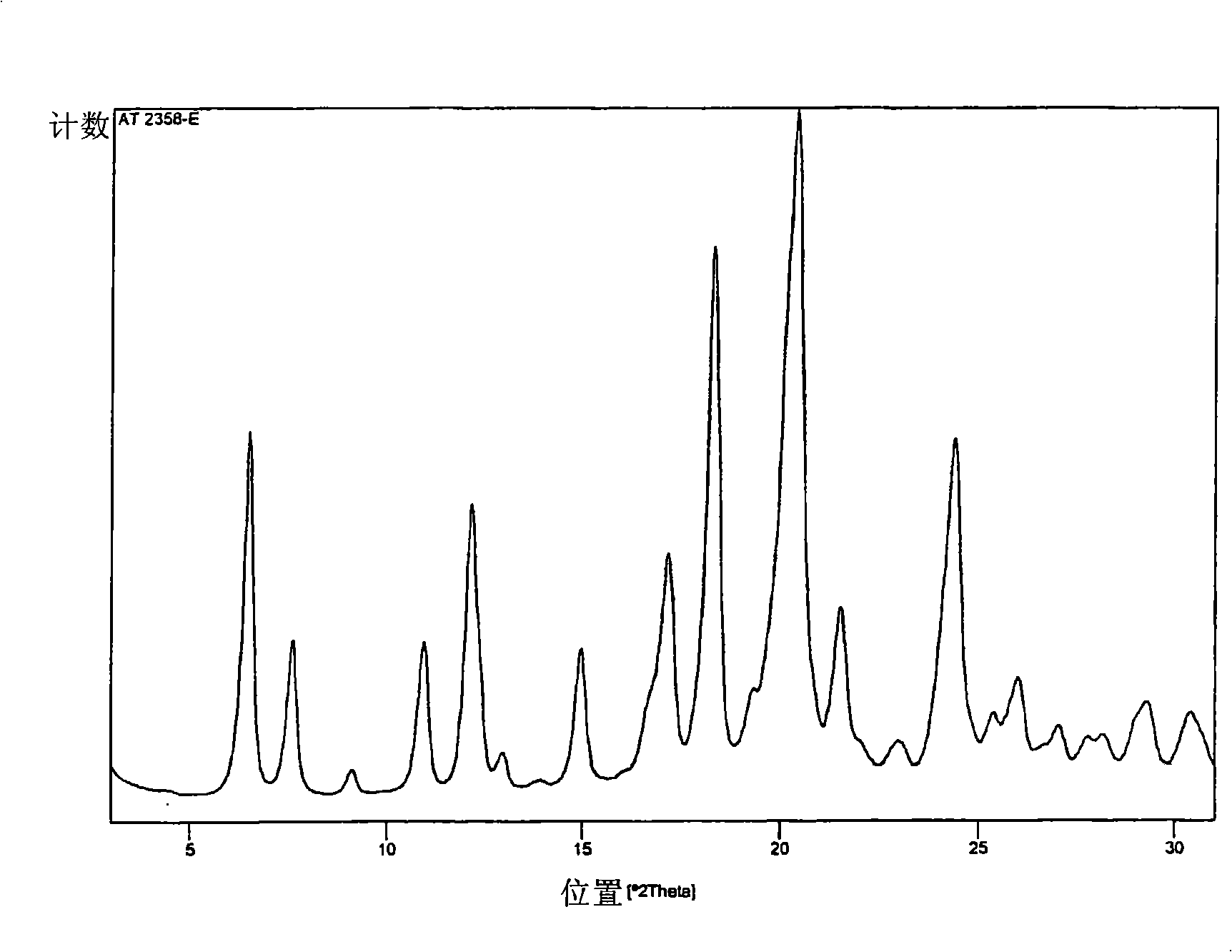
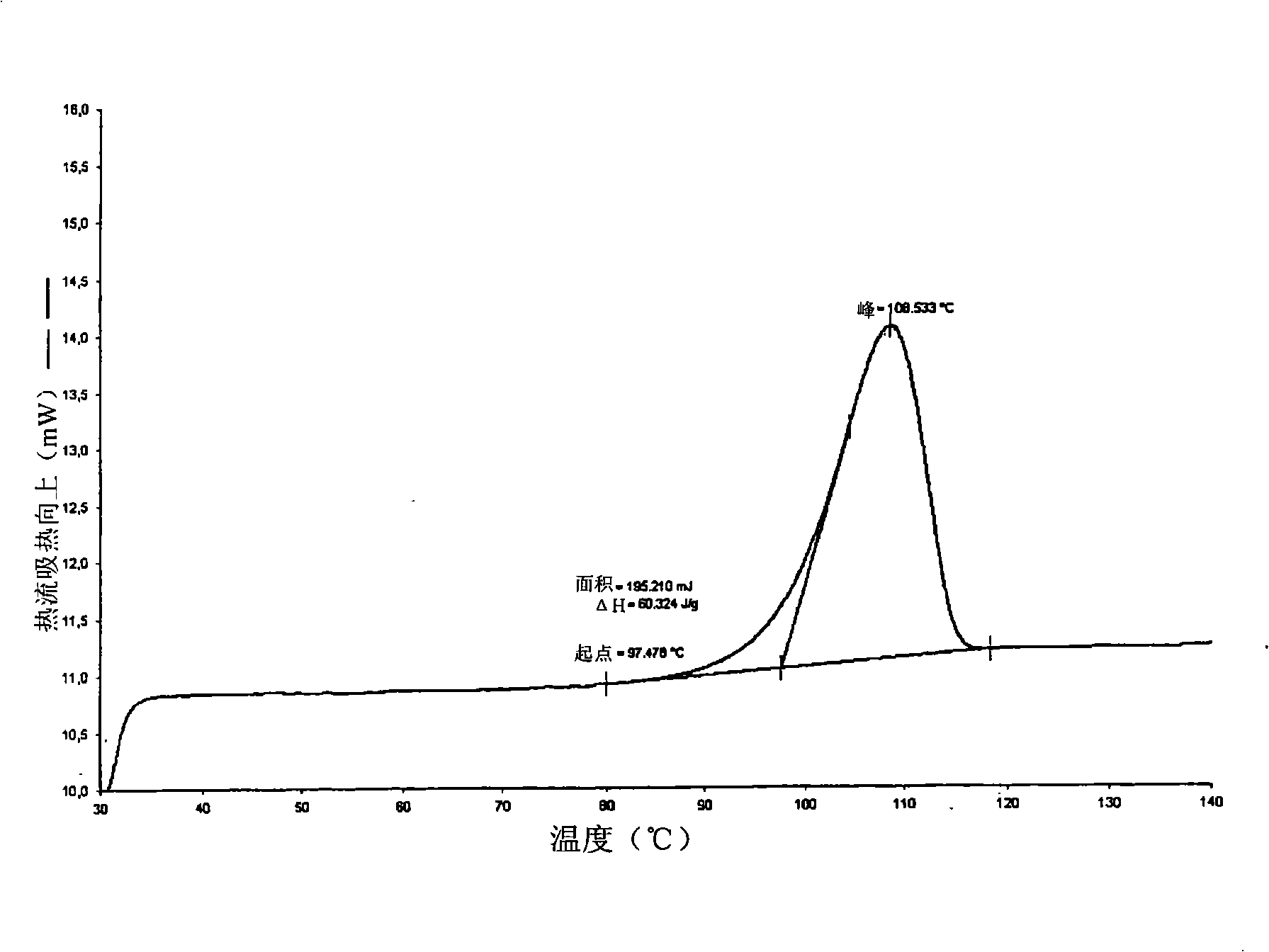
Polymorphs of atorvastatin tert.-butylester and use as intermediates for the preparation of atorvastatin
A technology of atorvastatin tert-butyl ester and atorvastatin, which is applied to the polymorphic form of atorvastatin tert-butyl ester and its application field as an intermediate in the preparation of atorvastatin, which can Solve the problems of inability to provide high purity, inability to obtain crystalline form of atorvastatin tert-butyl ester, inability to obtain filterability and purity of atorvastatin hemi-calcium, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] In the preparation process of crystal form 1 and crystal form 2, the dimethyl ketone atorvastatin tert-butyl ester (I) used as a starting material can be in any form, for example, it can be a reaction solution, a filtrate, any crude , polymorphs, anhydrates, solvates, or hydrated forms, or mixtures thereof, and may be prepared according to any of the following methods known to the expert or described in the prior art.
[0067] The water-miscible solvent is preferably an organic solvent, especially acetonitrile, 1,2-dimethoxyethane, tetrahydrofuran, lower alcohols such as methanol, ethanol, propanol, isopropanol and butanol, or ethyl acetate. The solvent is most preferably acetonitrile. Furthermore, the acid is preferably HCl.
[0068] It is also preferred that during the preparation of Form 1 and Form 2 of atorvastatin tert-butyl ester (II), the aqueous solution of water miscible solvent and acid is 10:1, more preferably 6:1 and most preferably 4 : 1 volume ratio appl...
Embodiment 1
[0089] To 1.32g (4R-cis)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester (4.8mmol, 1.2 equivalents) in To a solution in a 9:1 mixture of heptane and toluene (30 mL), 0.49 g of 2-methylbutyric acid (0.52 mL, 4.8 mmol, 1.2 equivalents) and 1.66 g of 4-fluoro-α-[2-methyl -1-oxypropyl]-γ-oxy-N,β-diphenylbenzenebutyramide (4.0 mmol). The heterogeneous mixture was stirred at reflux for 22 h under argon atmosphere. The resulting yellow solution was cooled to room temperature, diluted with 30 mL tert-butyl methyl ether and washed sequentially with 50 mL 1M NaOH, 30 mL 1M HCl, and finally brine. Evaporation of the solvent gave a light brown viscous residue which was dissolved in 12 mL of acetonitrile, 2.2 mL of water and 0.6 mL of 1M HCl. The resulting clear solution was stirred overnight, during which time a precipitate appeared. 10 mL of water was added followed by 20 mL of acetonitrile, the latter being preferred for reducing the semi-solid precipitate to...
Embodiment 2
[0092] To 1.32g (4R-cis)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester (4.8mmol) in 9:1 To a solution in a mixture of heptane and toluene (30 mL), 0.245 g of 2-methylbutanoic acid (0.26 mL, 2.4 mmol) and 1.66 g of 4-fluoro-α-[2-methyl-1-oxypropane were added successively base]-γ-oxyl-N,β-diphenylbenzenebutyramide (4.0 mmol). The heterogeneous mixture was stirred at reflux for 48 h under argon atmosphere. The resulting yellow solution was cooled to room temperature, diluted with 30 mL tert-butyl methyl ether and washed sequentially with 50 mL 1M NaOH, 30 mL 1M HCl, and finally brine. Evaporation of the solvent gave an orange sticky residue which was dissolved in 12 mL of acetonitrile, 2.2 mL of water and 0.6 mL of 1M HCl. The resulting mixture was heated and stirred at 45-49° C. for 5.5 h until the intermediate of general formula (I) was almost completely consumed according to HPLC analysis. The mixture was cooled and stirring was continued at 20-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com