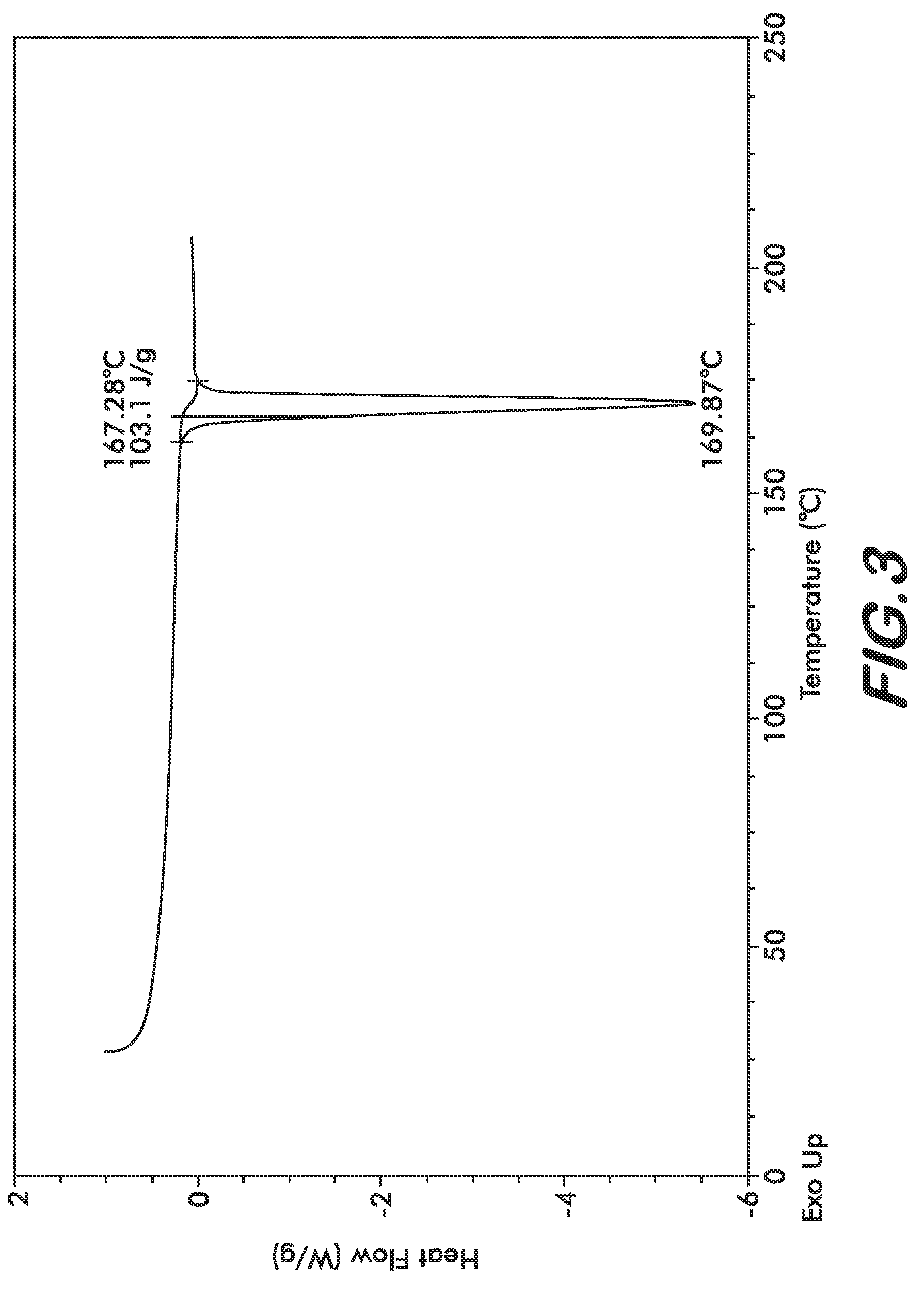
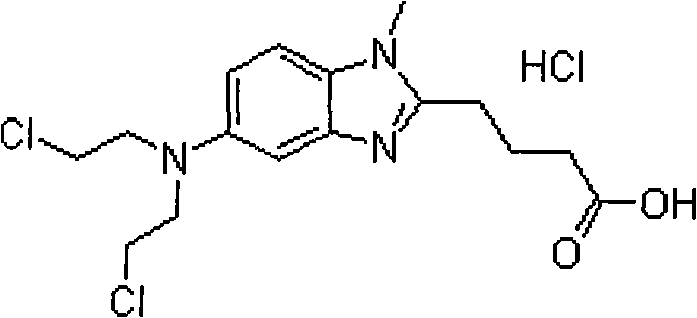
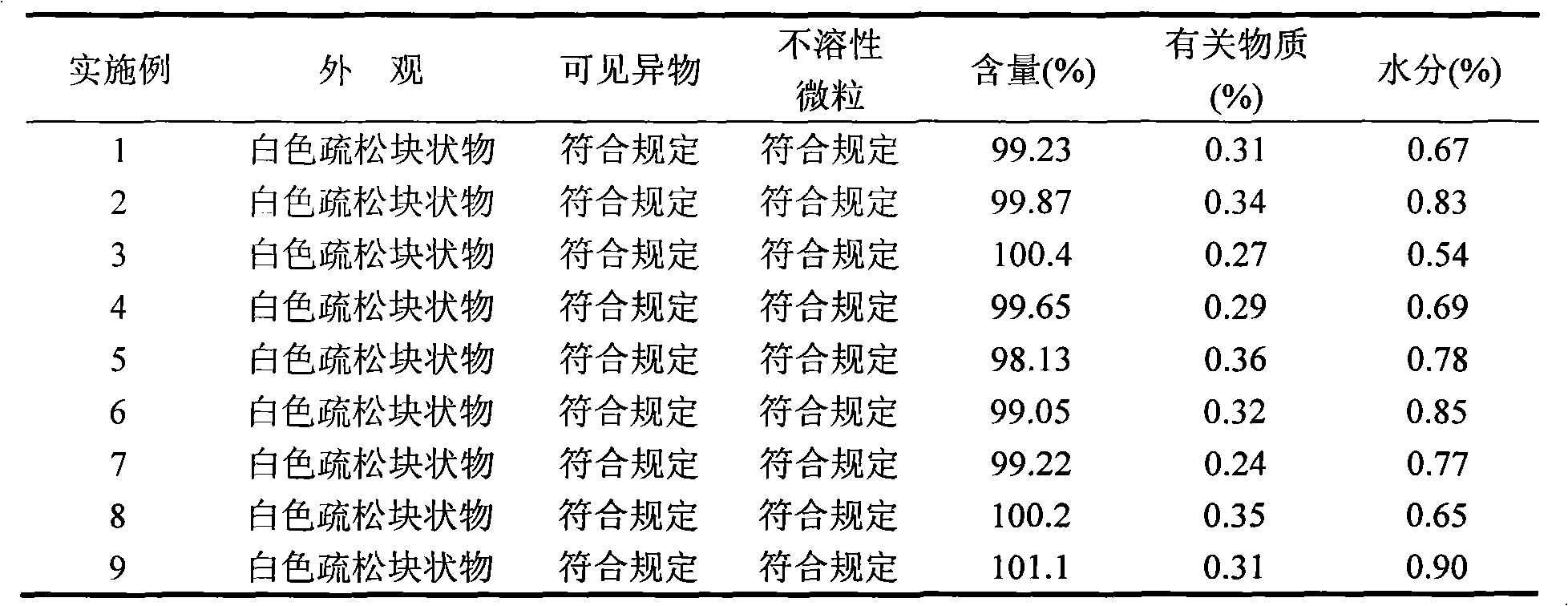
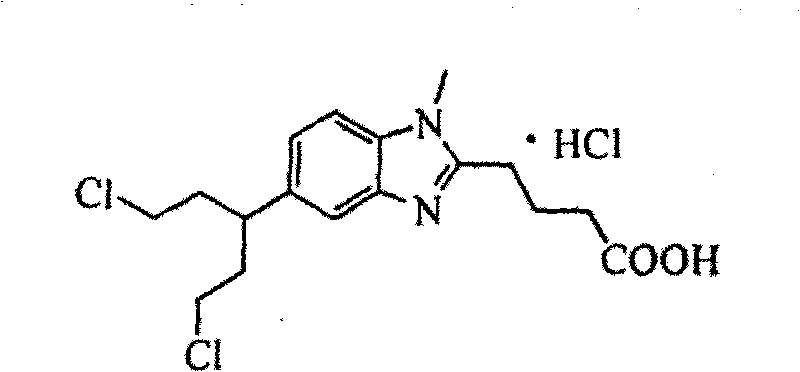
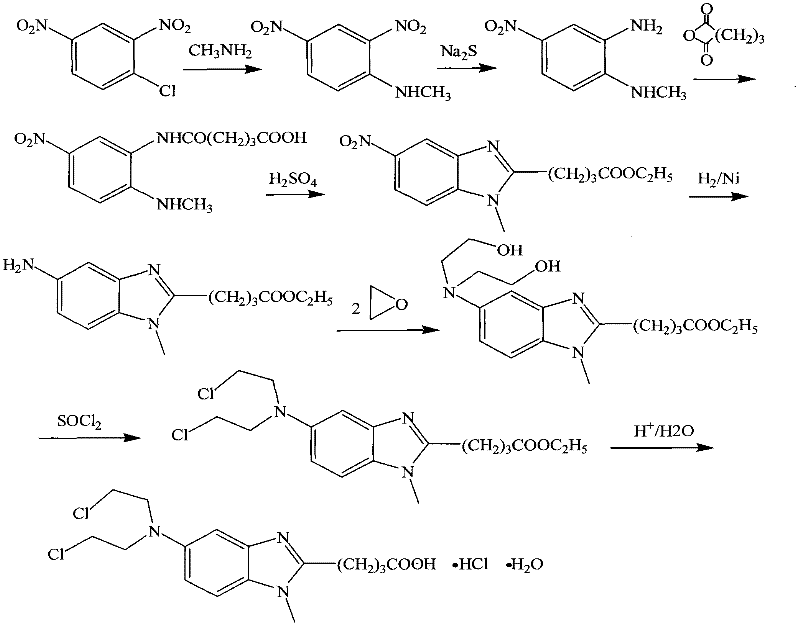
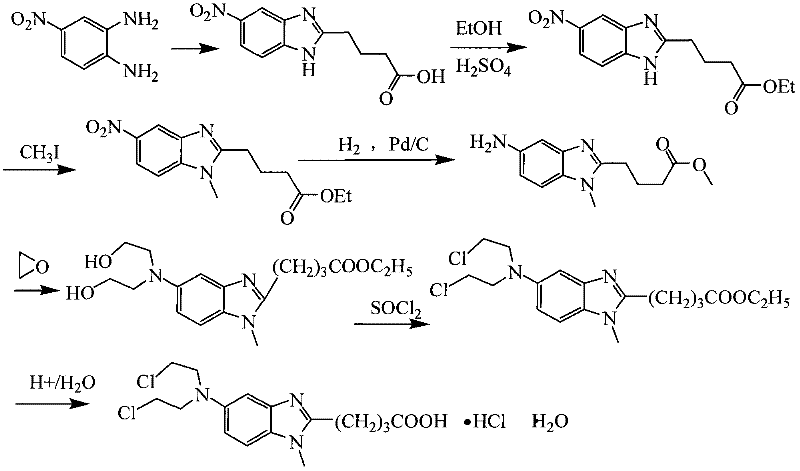
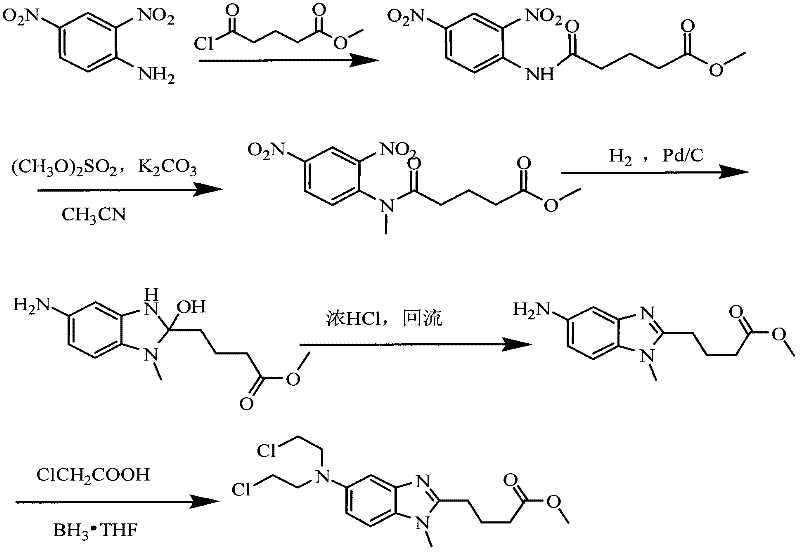
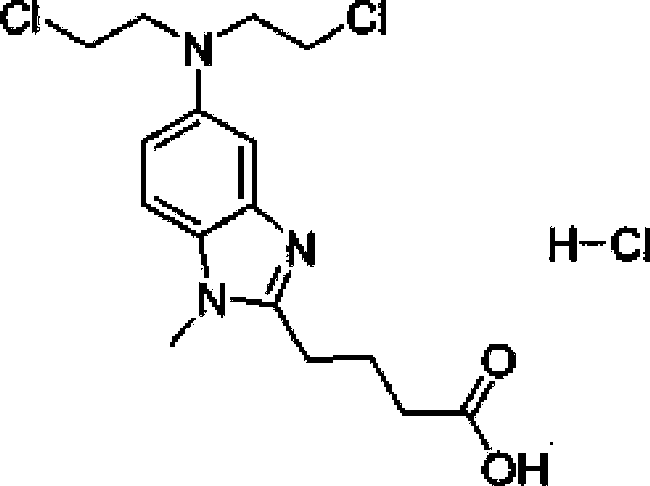
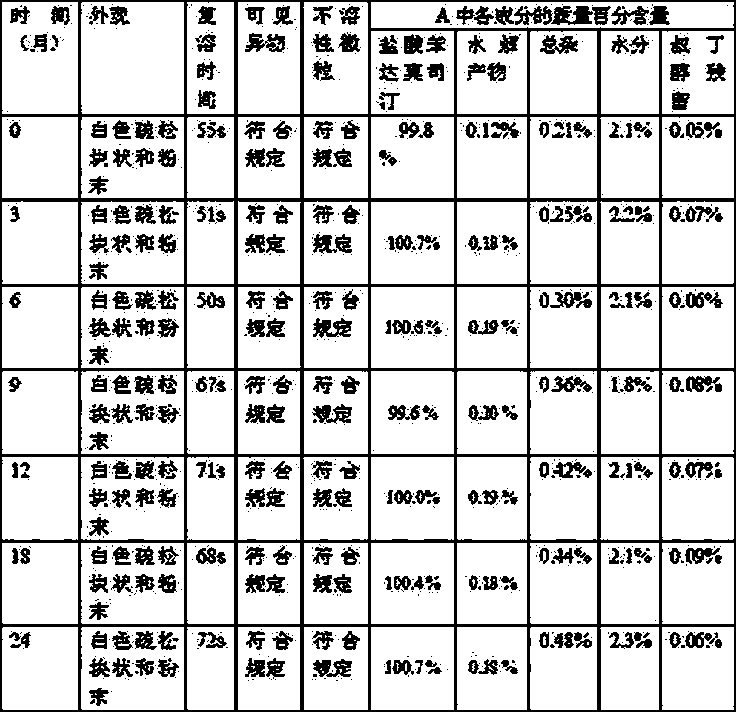
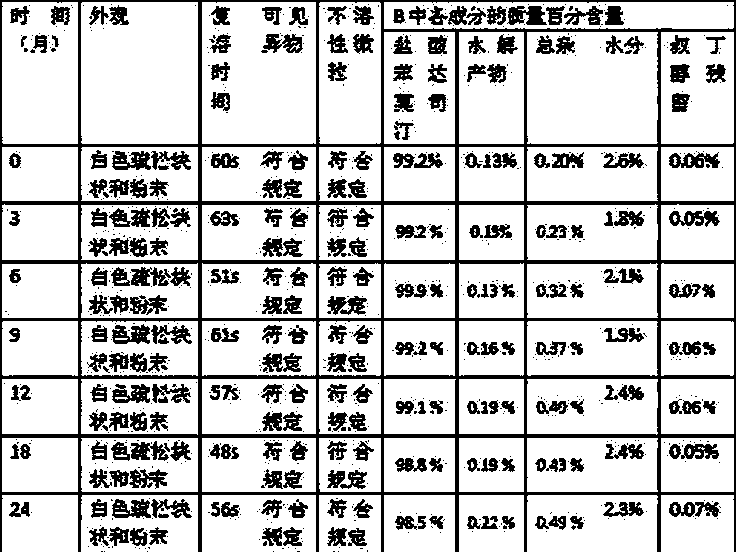
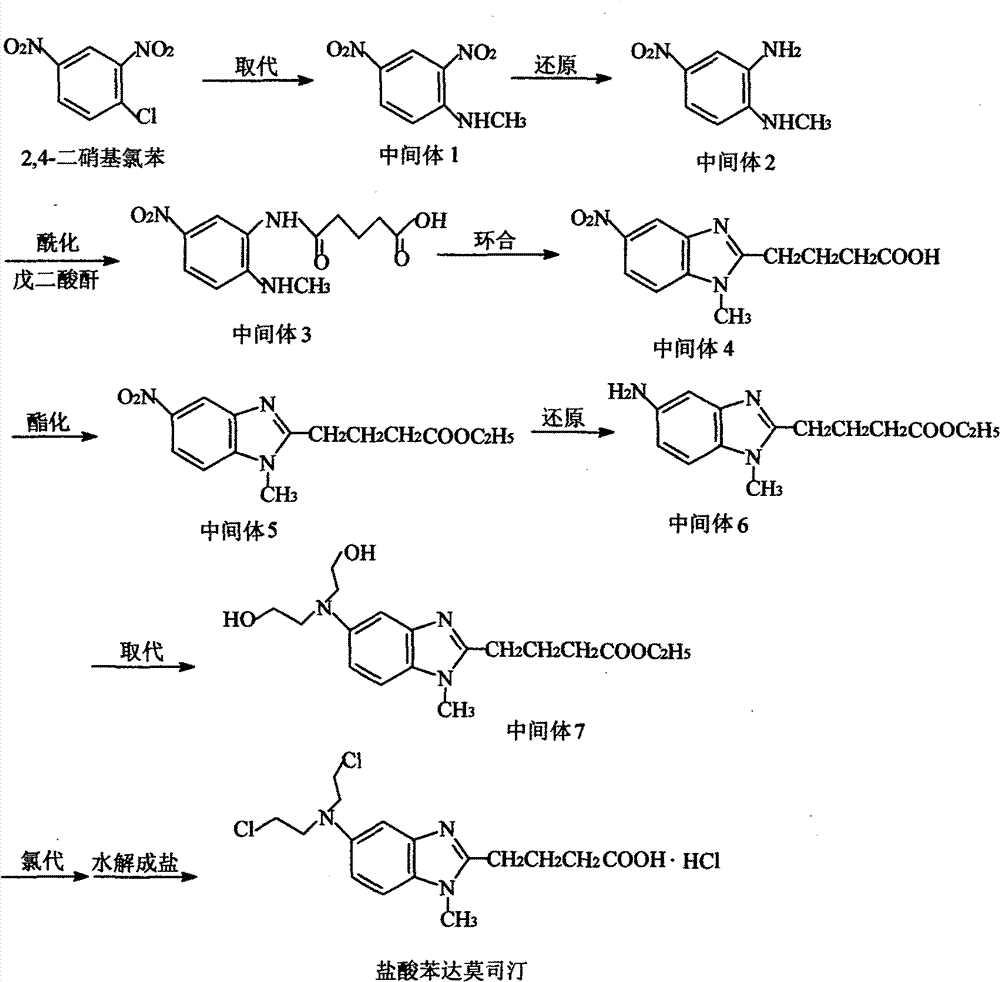
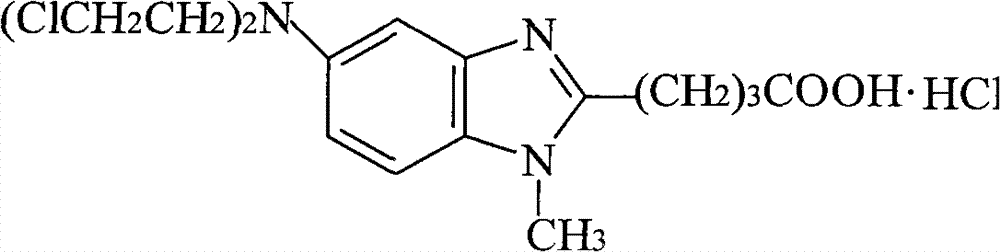
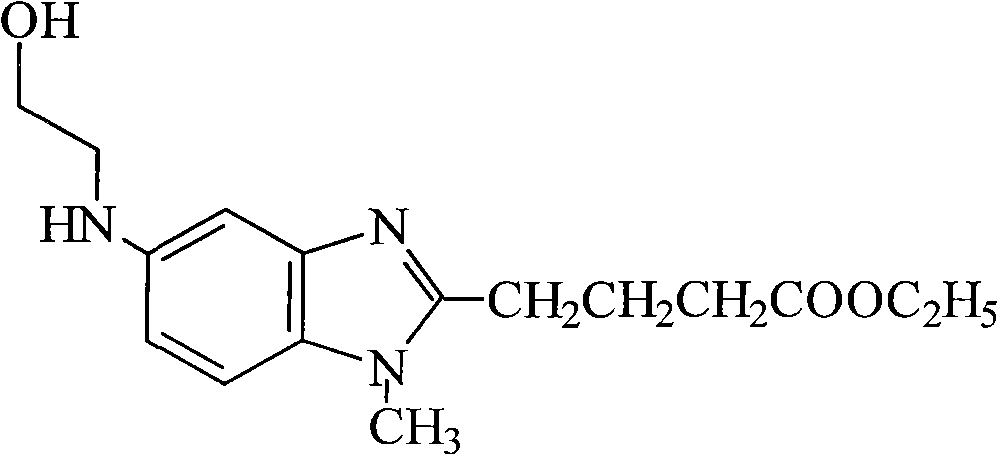
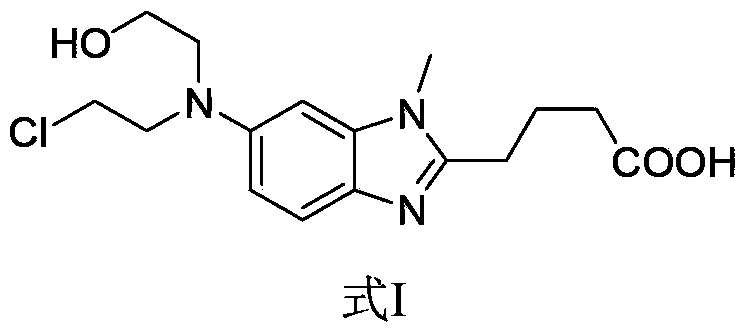
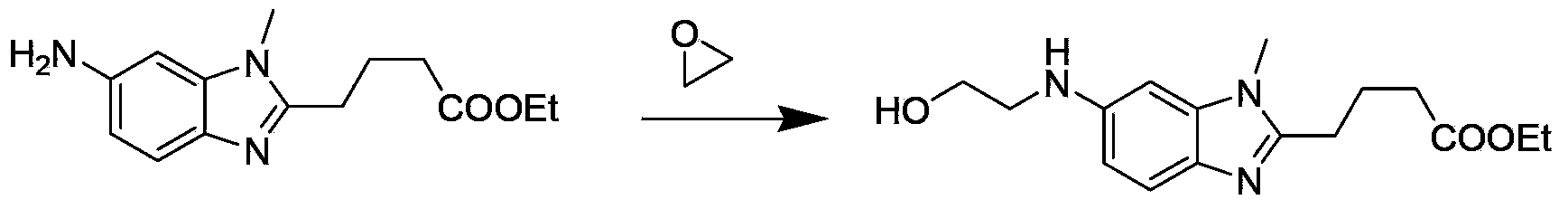
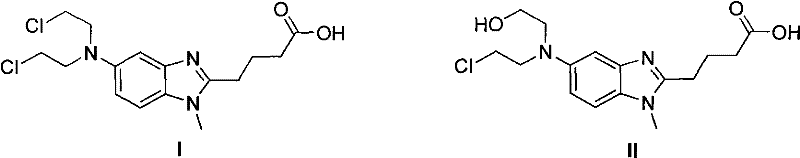Patents
Literature
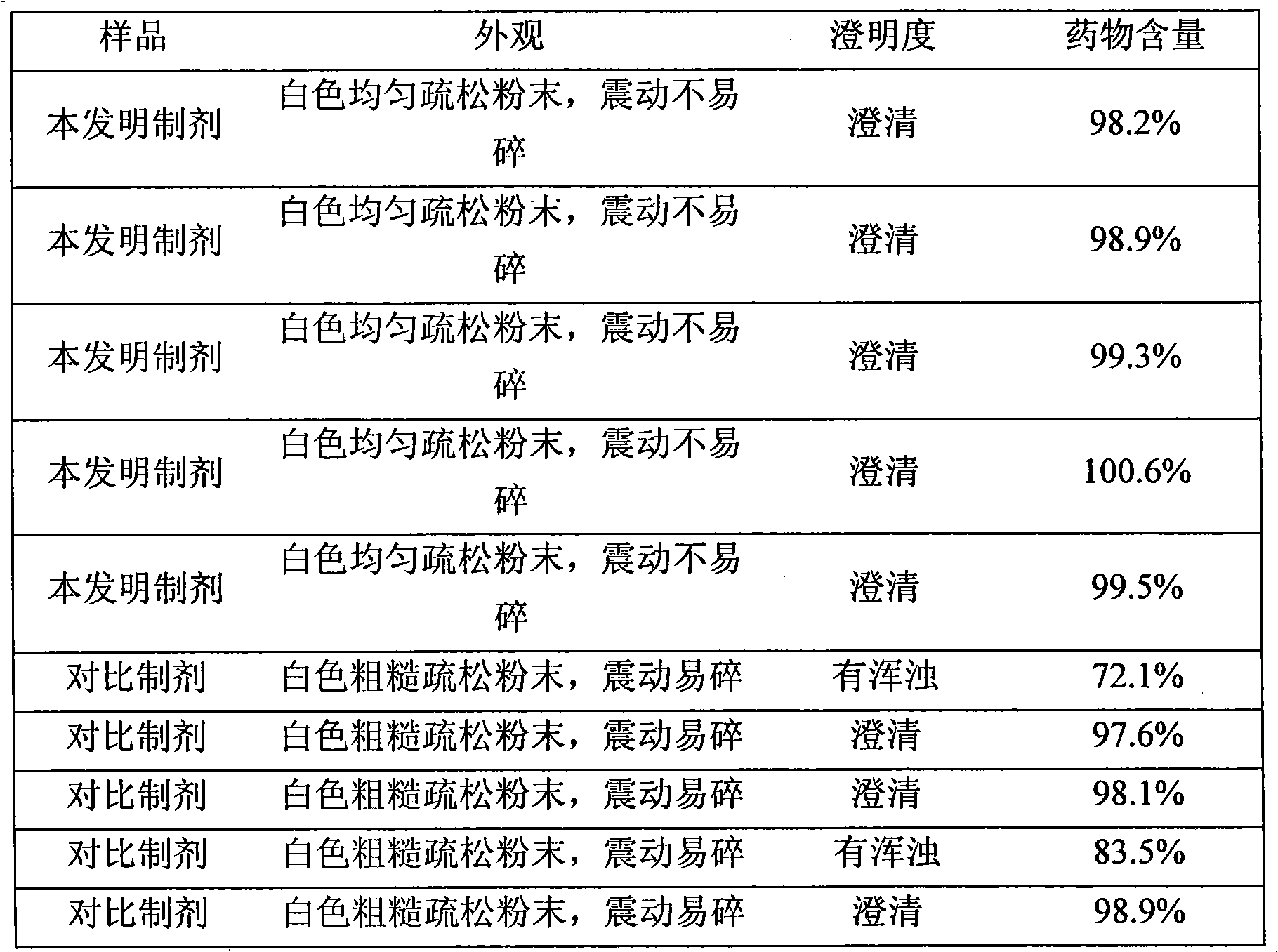
74 results about "Bendamustine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
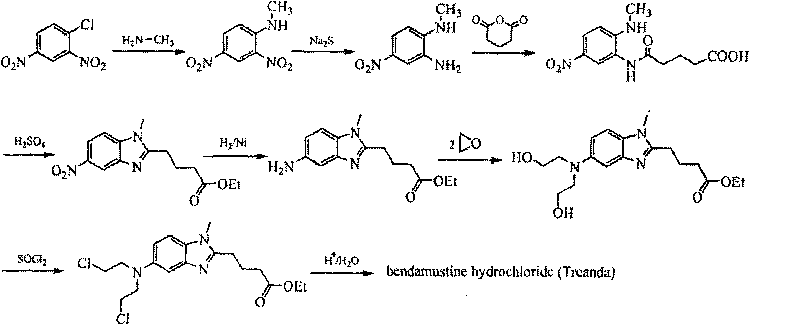
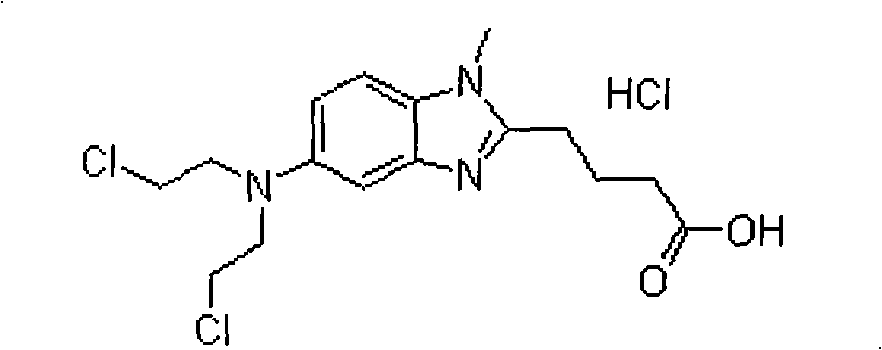
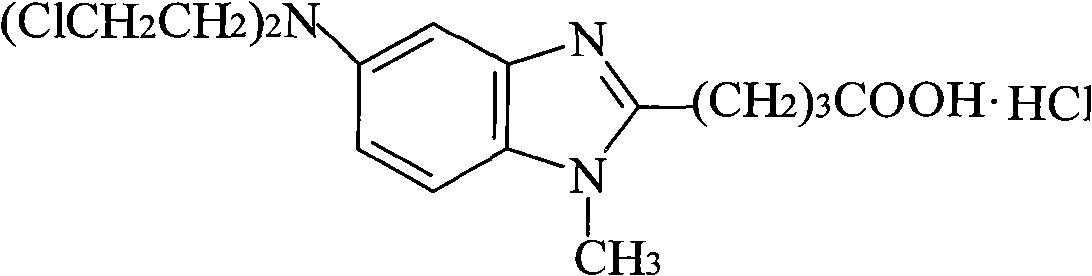
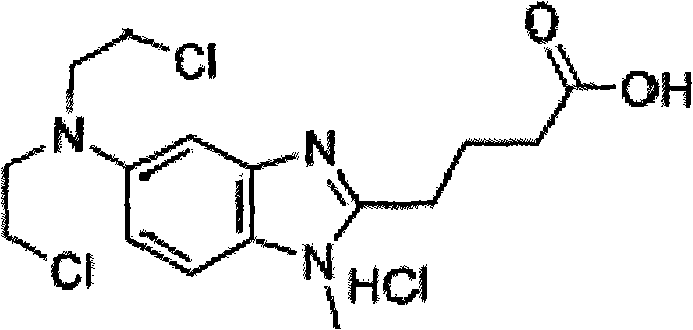
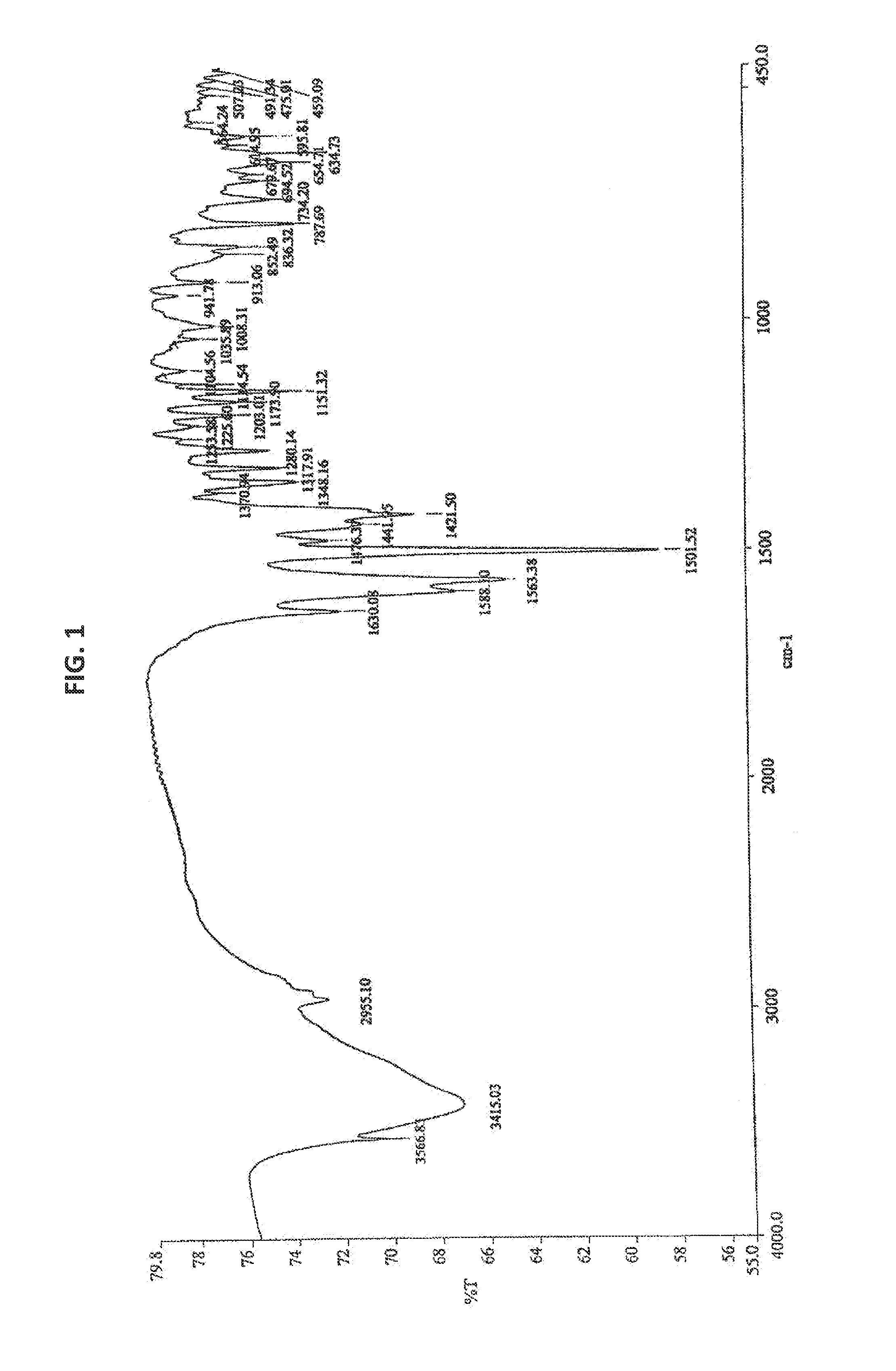
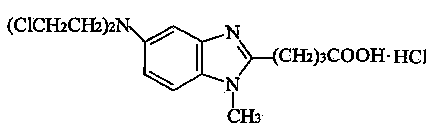
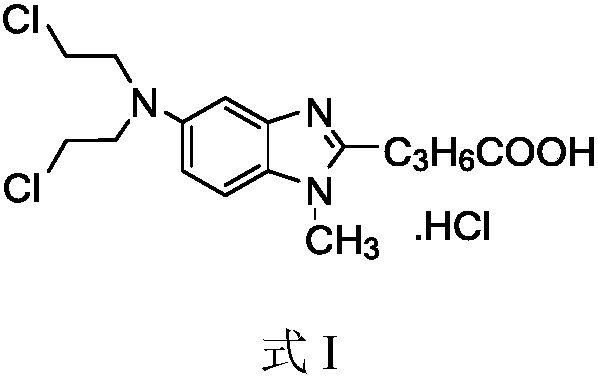
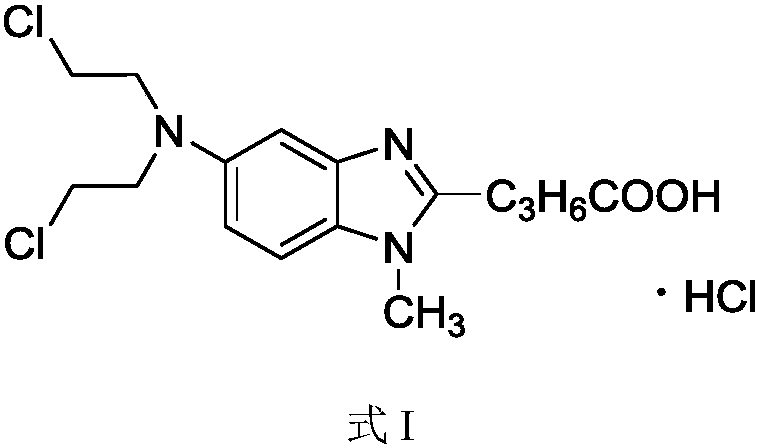
Bendamustine Hydrochloride is the hydrochloride salt of bendamustine, a bifunctional mechlorethamine derivative with alkylator and antimetabolite activities. Bendamustine possesses three active moieties: an alkylating group; a benzimidazole ring, which may act as a purine analogue; and a butyric acid side chain.
Novel solid forms of bendamustine hydrochloride
InactiveUS20090264488A1Organic active ingredientsBiocideBendamustine hydrochlorideMedicinal chemistry
Novel solid forms of bendamustine hydrochloride are described, as well as methods of their preparation and use.
Owner:CEPHALON INC
Novel solid forms of bendamustine hydrochloride
Novel solid forms of bendamustine hydrochloride are described, as well as methods of their preparation and use.
Owner:CEPHALON LLC
Solid forms of bendamustine hydrochloride
Novel solid forms of bendamustine hydrochloride are described, as well as methods of their preparation and use.
Owner:CEPHALON LLC
Bendamustine hydrochloride freeze-dried powder injection
InactiveCN101584668AImprove stabilityReduced stabilityPowder deliveryOrganic active ingredientsBendamustine hydrochlorideFreeze-drying
The invention relates to a bendamustine hydrochloride freeze-dried powder injection and its preparing method. The prepared bendamustine hydrochloride freeze-dried powder injection is used for chronic lymphocytic leukemia (CLL) and in the treating process of rituximab / MabThera or containing the same, or for inert B cell non hodgkin lymphoma (B-NHL) patients whose illness state still deteriorates during the treatment period in six months. The bendamustine hydrochloride freeze-dried powder injection contains bendamustine hydrochloride and uses the mixed solvent composed of the tertiary butanol and the injection water in the preparation process. The concentration of the bendamustine hydrochloride in the mixed solvents is 5-10 mg / ml. The volume ratio of the solvents is: 5-50% of tertiary butanol and the balance of injection water. The preparation process is that: measuring the tertiary butanol, adding injection water, mixing evenly, cooling to 2-15 DEG C, heat preserving, then adding the bendamustine hydrochloride, stirring to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing the plug, out box, tying and packing.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for synthesizing highly-pure bendamustine hydrochloride
ActiveCN101691359AComply with drug quality standardsShort synthesis cycleOrganic chemistryBendamustine hydrochlorideEthylene oxide
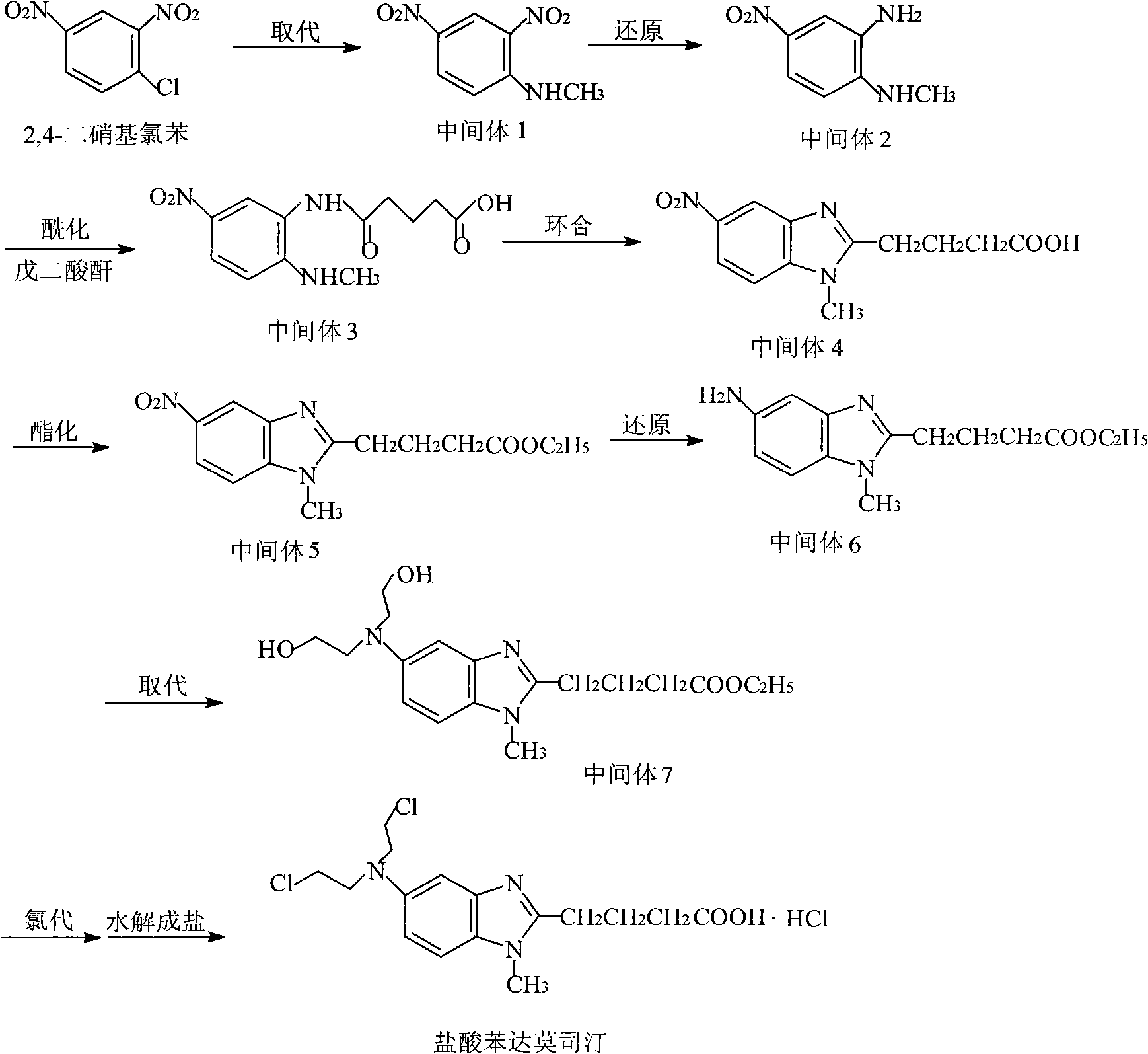
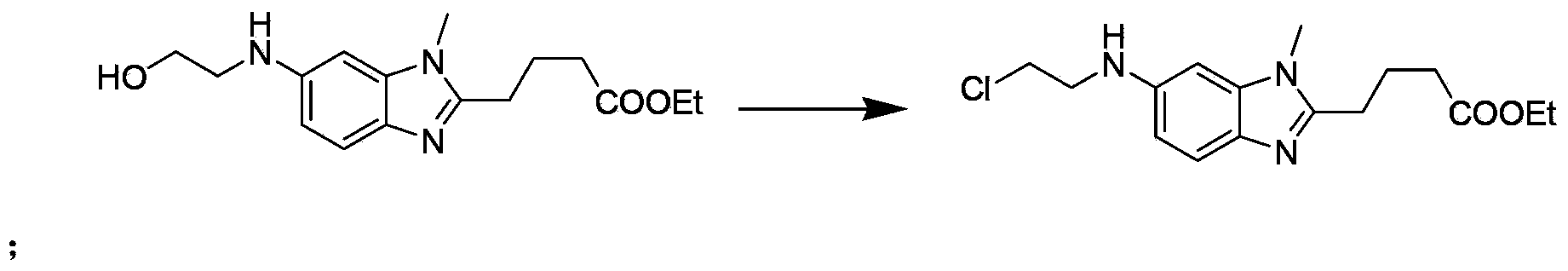
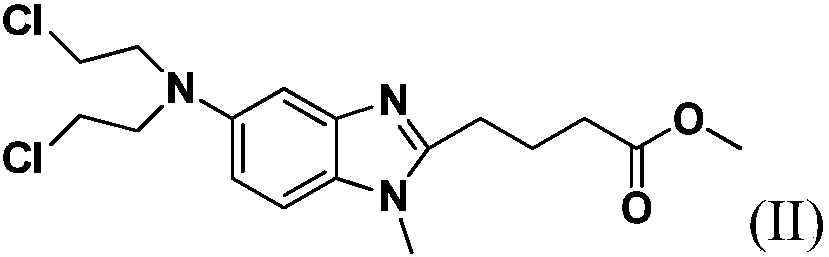
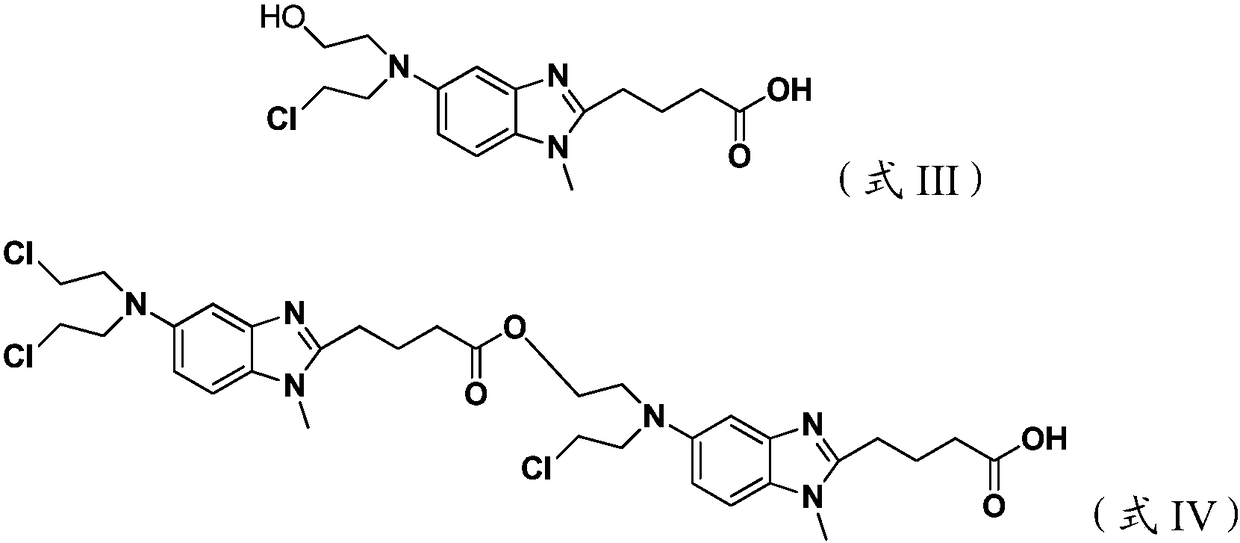
The invention discloses a method for synthesizing highly-pure bendamustine hydrochloride, which comprises the following steps: taking [1-methy-2(4'-ethyl butyrate)-5-amino]-1H-benzimidazole as a raw material; and orderly reacting the [1-methy-2(4'-ethyl butyrate)-5-amino]-1H-benzimidazole with ethylene oxide and phosphorus oxychloride through the four steps of substitution, hydrolysis, salification and refinement so as to produce the bendamustine hydrochloride. The method in the invention is mild in condition, simple in operation, short in synthesis period and suitable for scaled-up industrial production; and the purity of the synthesized bendamustine hydrochloride is over 99.5 percent, while monomeric impurity is below 0.1 percent, which meet the quality standards of raw material medicament.
Owner:深圳万乐药业有限公司
Bendamustine hydrochloride compound
InactiveCN101606934AStable and uniform qualityImprove stabilityOrganic active ingredientsPharmaceutical product form changeBendamustine hydrochlorideFreeze-drying
The invention relates to a bendmustine hydrochloride compound and a method for preparing the same. The bendmustine hydrochloride compound prepared by the method can be used for treating chronic lymphocytic leukemia (CLL) and inertia B-cell non-Hodgkin's lymphoma (B-cell NHL). The bendmustine hydrochloride compound contains bendmustine hydrochloride and hydroxyproyl beta-cyclodextrin, wherein the mass ratio of bendmustine hydrochloride to hydroxyproyl beta-cyclodextrin is 1:1-20. During the preparation process, mixed solvent of tertiary butanol and water for injection can be used, wherein the concentration of bendmustine hydrochloride in the mixed solvent is 5-10mg / mL; the ratio in the solvent (100%) by volume is as follows: 5-50% of tertiary butanol and the balance of water. The preparation process comprises the following steps of: weighing the tertiary butanol, sequentially adding the solvent and the hydroxyproyl beta-cyclodextrin, dissolving, evenly mixing and cooling the mixture to 2-15 DEG C, maintaining the temperature, then adding the bendmustine hydrochloride, stirring until bendmustine hydrochloride dissolves, filtering, filling, stoppering, dishing-up, freeze-drying, corking, out-box, covering and packaging, and finally, the bendmustine hydrochloride compound is obtained.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Bendamustine hydrochloride freeze-dried powder injection for injection and preparation method thereof
InactiveCN101966158AQuality is easy to controlSimple production processOrganic active ingredientsPowder deliverySolubilityBendamustine hydrochloride
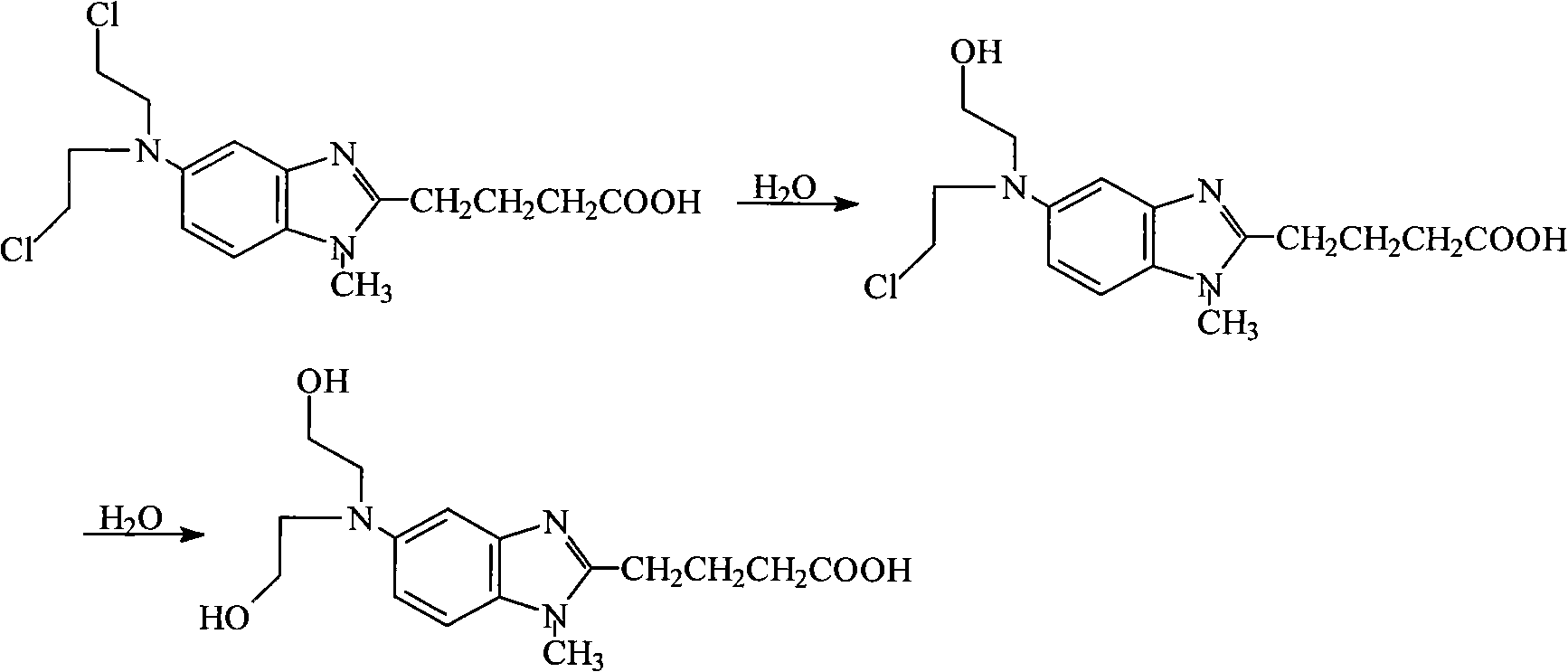
The invention relates to the technical field of medicinal preparations. Bendamustine hydrochloride is easy to degrade in the preparation process, so in order to prevent degradation products from generating, the aqueous solution of tertiary butanol with certain concentration is used as a solvent; therefore, medicaments and relevant auxiliary materials are dissolved, and the dissolved mixture is subjected to freeze-drying so as to obtain the bendamustine hydrochloride; however, the tertiary butanol is harmful to human bodies when inhaled or orally taken, the conventional literature indicates that the tertiary butanol can cause the oxidative injury of deoxyribonucleic acid (DNA), and experiments of rats and mice prove that the tertiary butanol has the carcinogenicity. The invention provides a bendamustine hydrochloride freeze-dried powder injection for injection and a preparation method thereof. In the preparation method, an organic solvent and particularly the tertiary butanol are not added in the processes of preparation and freeze-drying, so the safety of production and clinical medication is improved greatly. The bendamustine hydrochloride freeze-dried powder injection for the injection has the high stability and water solubility, has the characteristics of stability, controlled quality, safety and reliability, and particularly does not have organic solvent residues.
Owner:上海丽思化工科技有限公司
Method for preparing high-purity bendamustine hydrochloride
InactiveCN101948436AHigh purityMeet quality requirementsOrganic chemistryBendamustine hydrochlorideEthyl butyrate
The invention provides a method for preparing high-purity bendamustine hydrochloride. The method comprises the following steps of: (1) completely dissolving oily or colloidal 4-{5-[bis-(2-ethoxyl)amino]-1-methyl-2 benzimidazole}ethyl butyrate in 0.1 to 0.5g / ml solution of C1 to C4 alkyl acetate, wherein the dissolution temperature is 0 to 40 DEG C; (2) adding C5 to C8 hydrocarbons into the solution obtained in the step (1) dropwise, stirring the mixed solution at the temperature of between 10 DEG C below zero and 40 DEG C for crystallization, filtering the mixed solution to obtain solids of 4-{5-[bis-(2-ethoxyl)amino]-1-methyl-2 benzimidazole}ethyl butyrate; (3) performing chlorination on the solids of 4-{5-[bis-(2-ethoxyl)amino]-1-methyl-2 benzimidazole}ethyl butyrate obtained in the step 2 and thionyl chloride, performing hydrolysis by using concentrated hydrochloric acid to obtain salts, and purifying the salts to obtain the crude products of bendamustine hydrochloride; and (4) refining the crude products of bendamustine hydrochloride obtained in the step 3 by using water to obtain the finished products of bendamustine hydrochloride. The purity of the products prepared by the method of the invention is over 99.5 percent, and the content of single impurity is below 0.1 percent; and the method has the advantages of high yield, high product stability and suitability for industrialized production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Refining method of bendamustine hydrochloride
InactiveCN101948437AImprove stabilityEfficient removalOrganic chemistryBendamustine hydrochlorideDissolution
The invention provides a refining method of bendamustine hydrochloride, which comprises the following steps: completely dissolving 1 part by weight of bendamustine hydrochloride crude product in 3-10 parts by weight of hydrochloric acid solution with the concentration of 0.01-2mol / L, wherein the temperature is 30-100 DEG C during the dissolution process; and filtering when being hot, cooling, crystallizing, filtering, and drying for obtaining a bendamustine hydrochloride white crystal. The method can effectively remove impurities in the bendamustine hydrochloride crude product, lead the purity of the product to be more than 99.5%, lead single impurity to be not more than 0.1%, and lead the refining yield to be not lower than 90%; in addition, the refining method has simple process, low cost and easy industrial production of the process; and a preparation produced by taking the raw material has few impurities, good efficacy and few adverse reactions, and can bring the greatest benefit to patients with tumors.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Process for preparing bendamus tine hydrochloride monohydrate
InactiveUS20130217888A1Organic active ingredientsOrganic compound preparationPurification methodsBendamustine hydrochloride
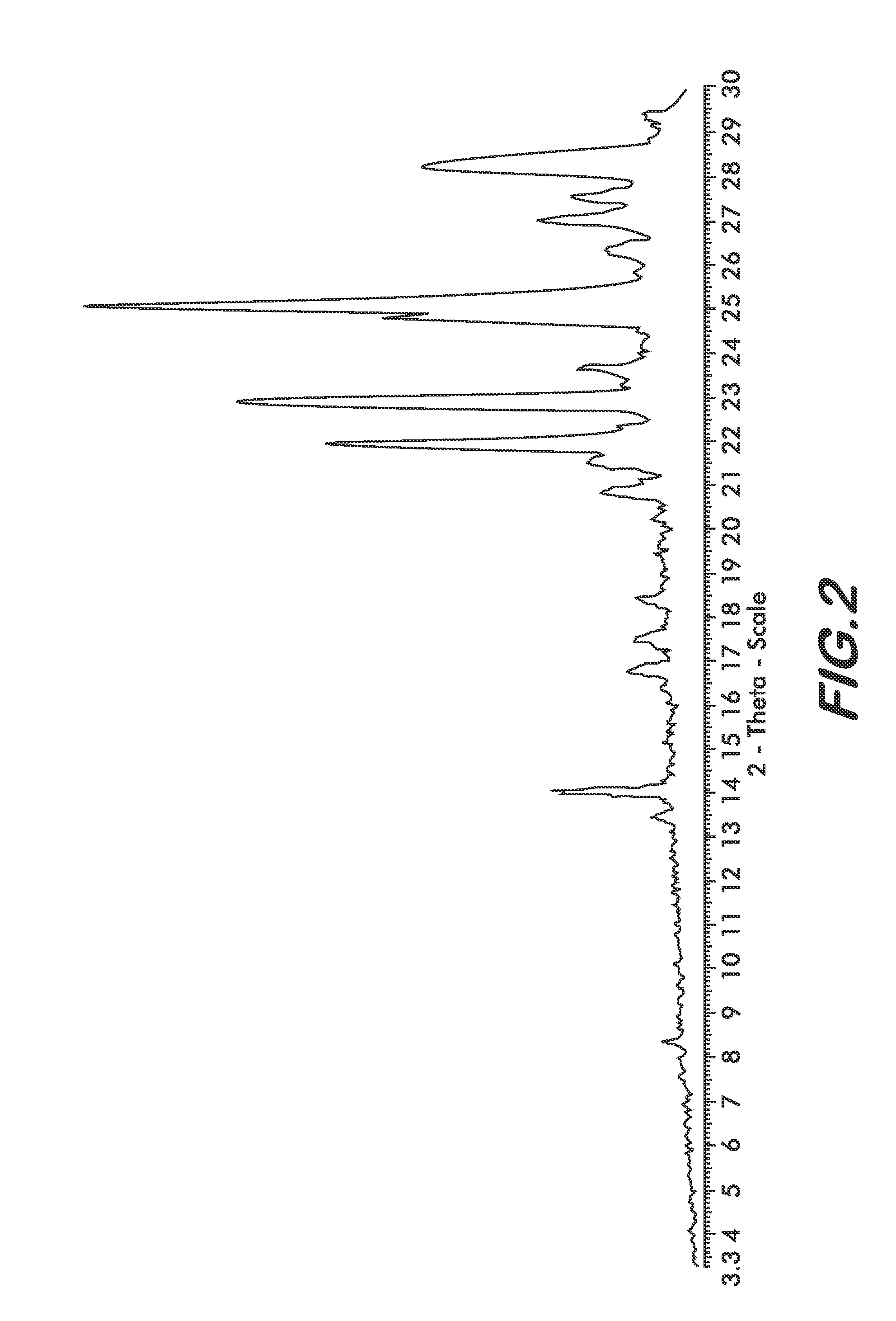
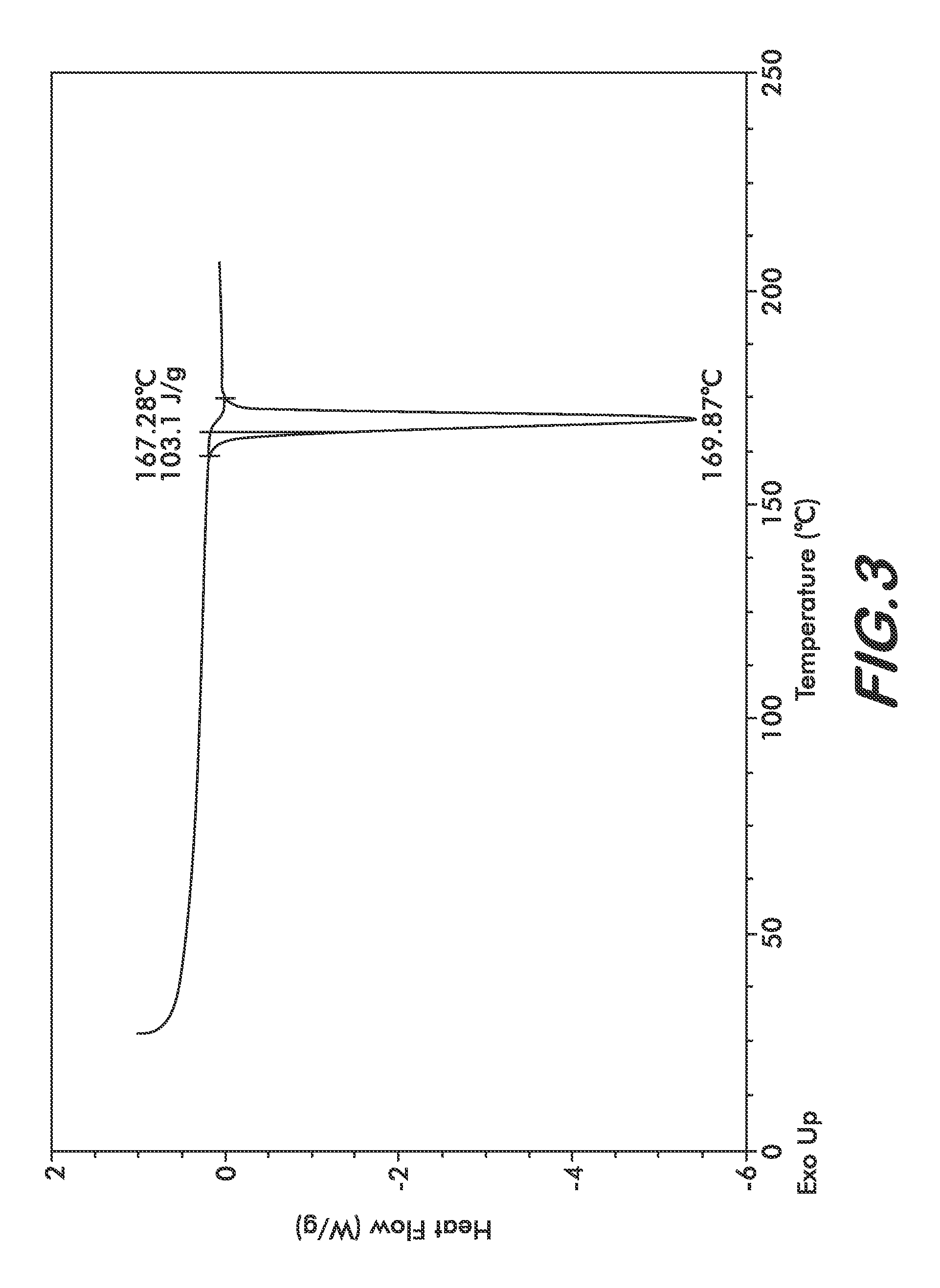
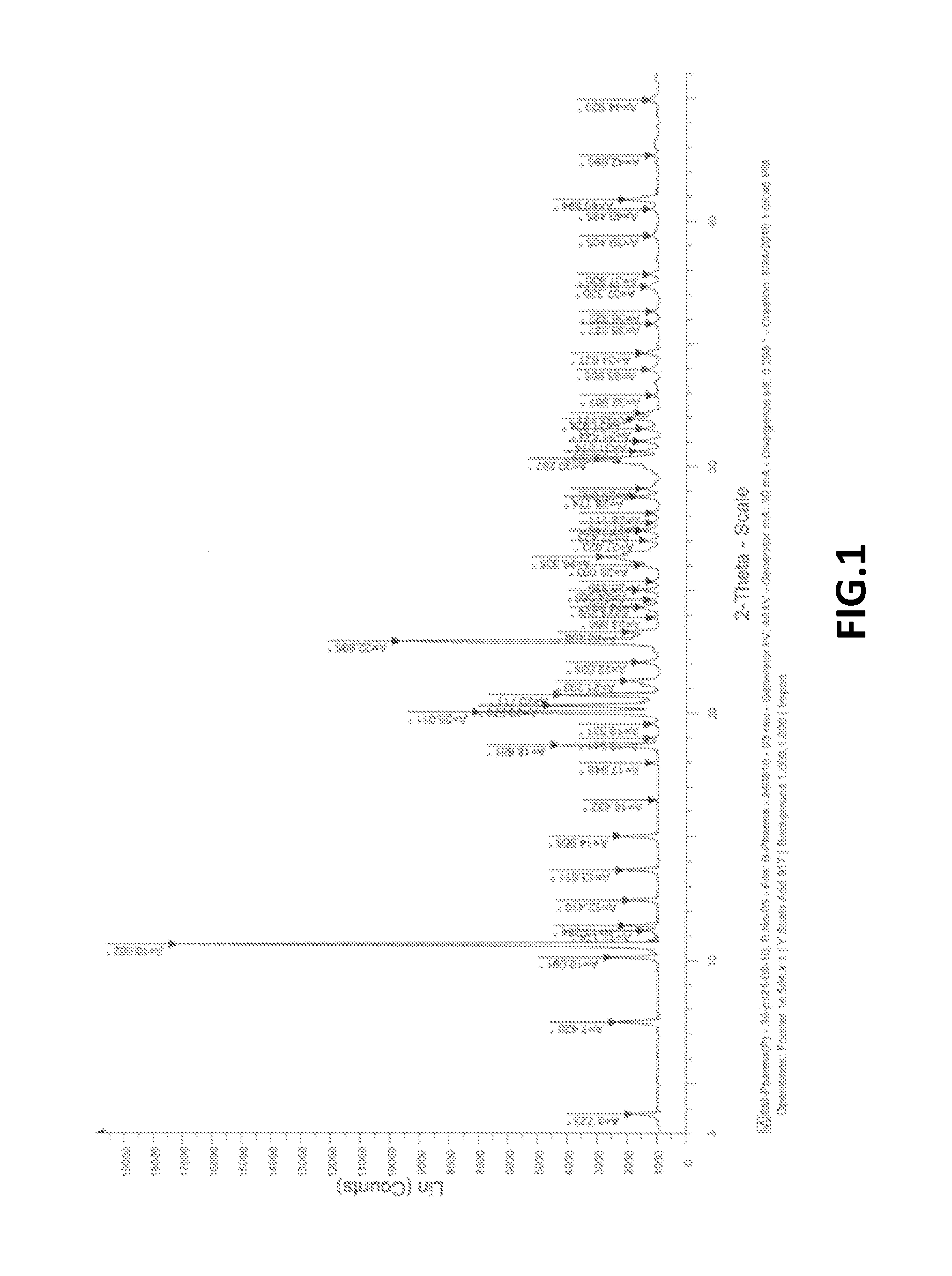
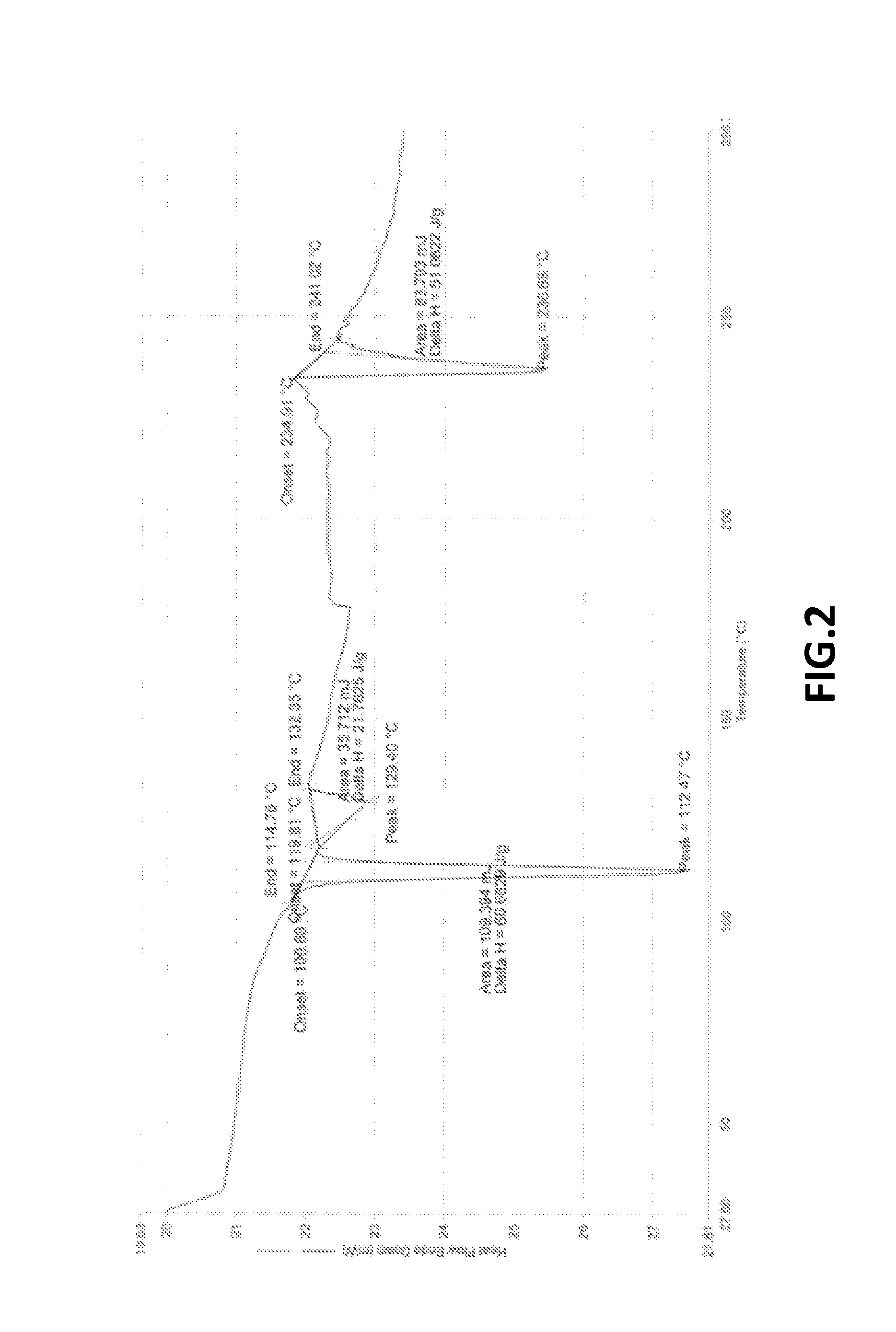
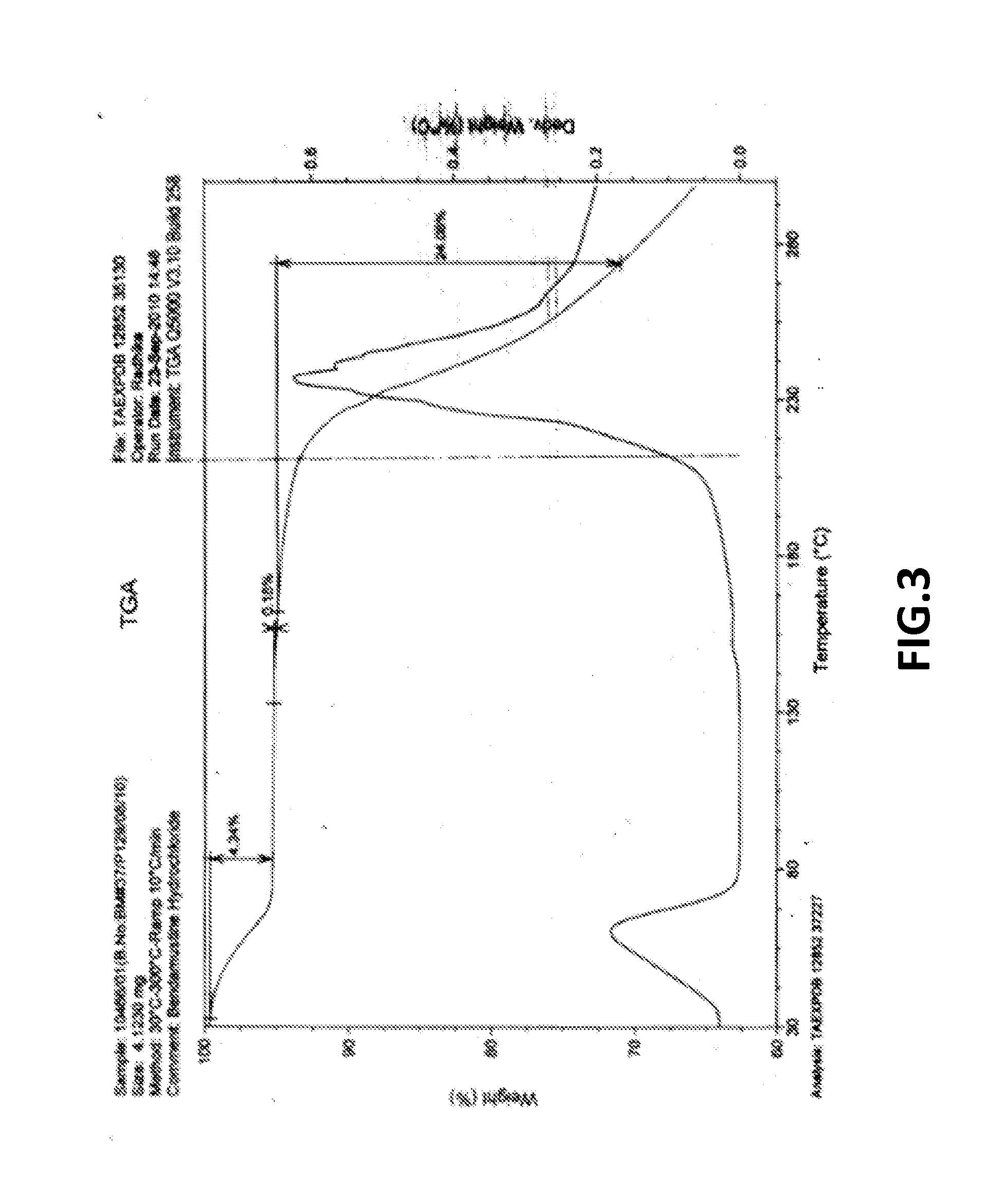
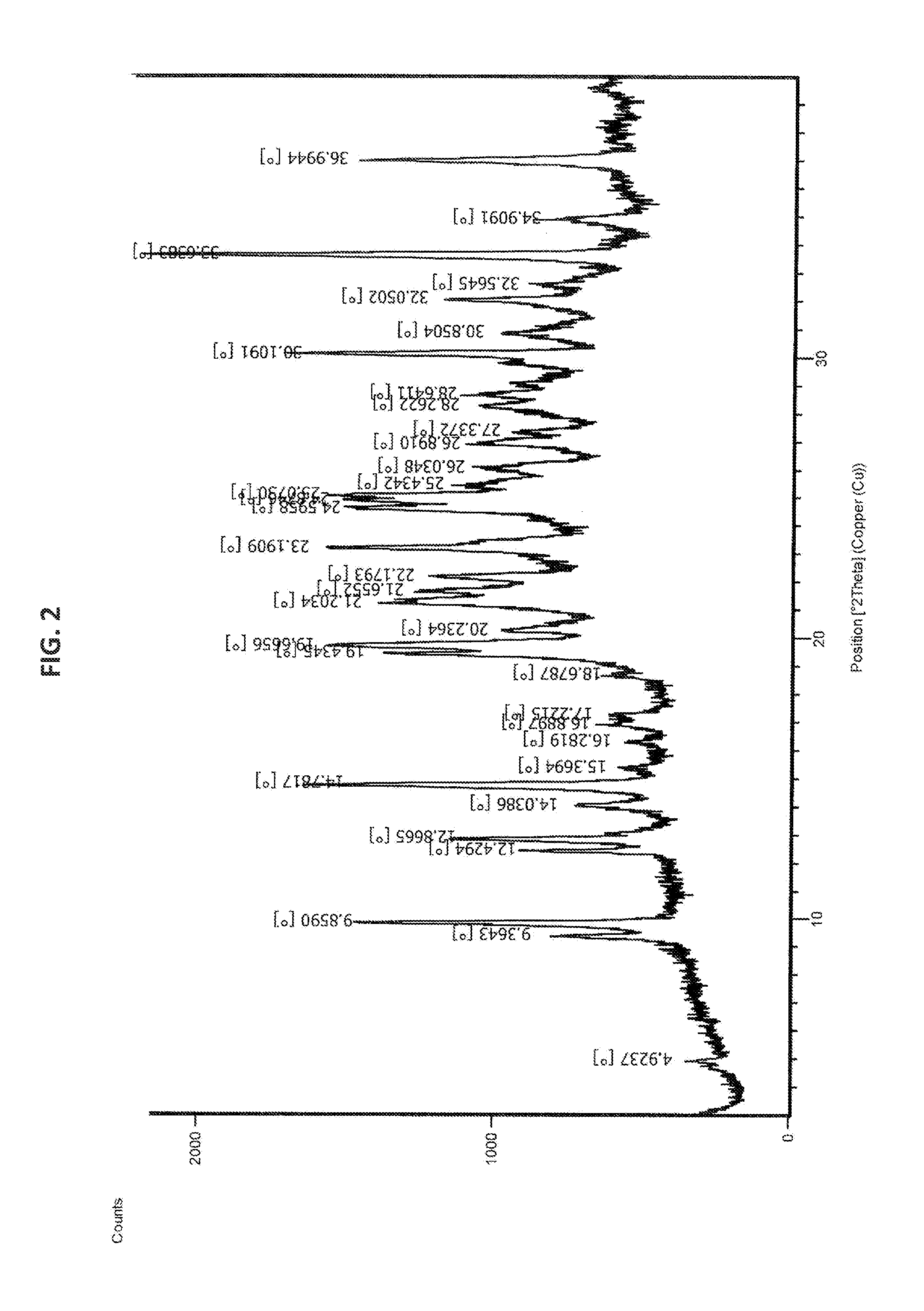
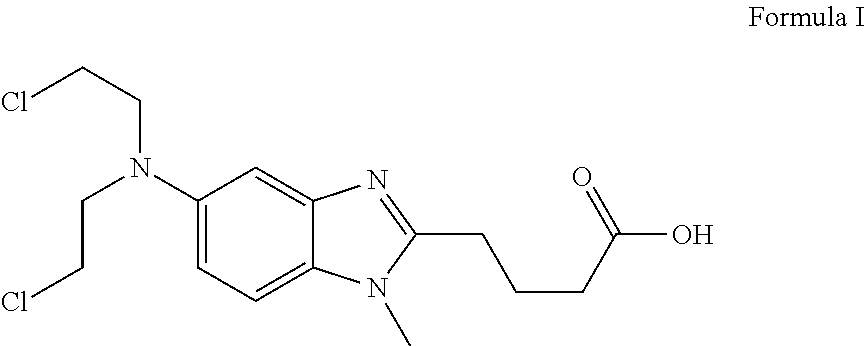
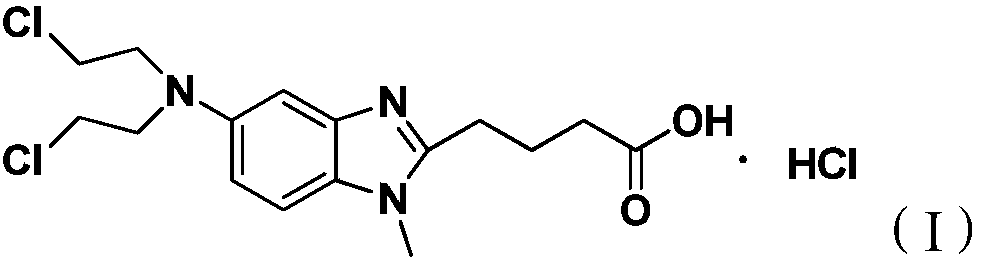
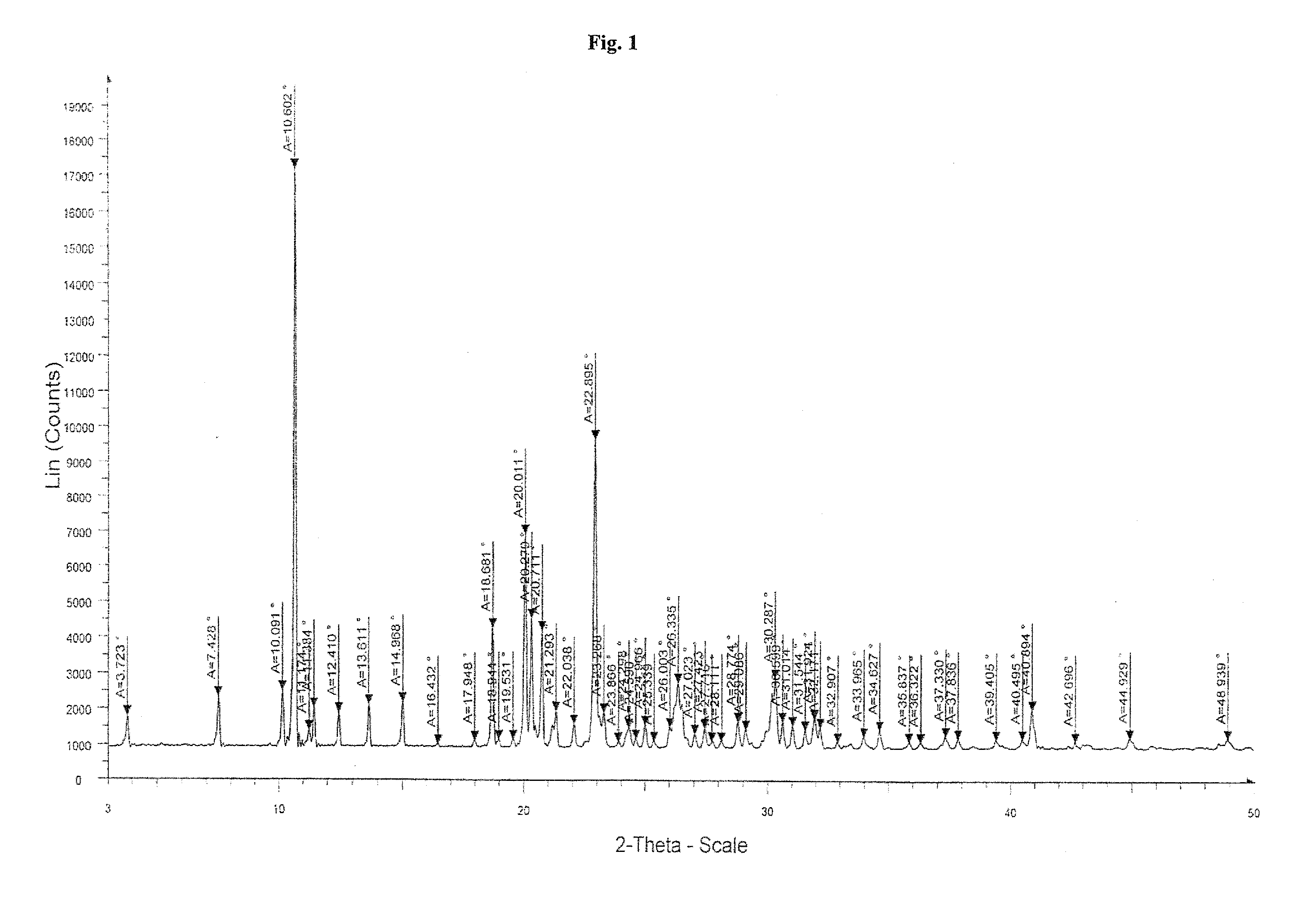
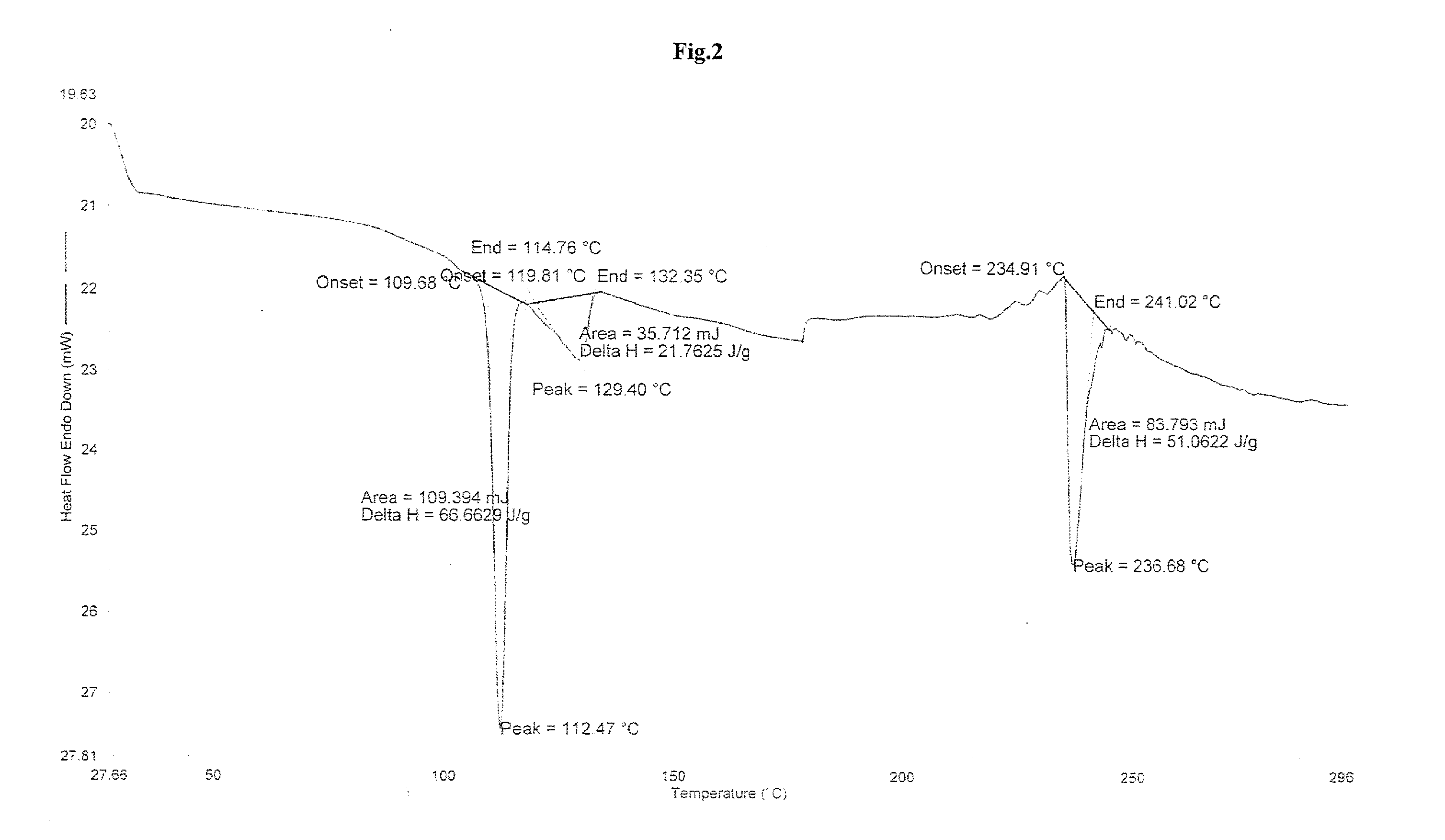
The present invention provide processes for the preparation of Bendamustine hydrochloride monohydrate of formula (I)The present application also provides a process of purification of Bendamustine hydrochloride or monohydrate to get substantially pure Bendamustine hydrochloride monohydrate crystalline Form-SM. The said Bendamustine hydrochloride monohydrate crystalline Form-SM is characterized by X-ray powder diffraction pattern comprising at least 5 characteristic peaks selected from the XRPD 2 theta degrees peaks at 7.42, 10.60, 11.17, 16.43, 17.94, 22.89, 26.33, 28.77, 30.28, 31.92, 40.89±0.1 2θ°.The present application also provides a process for the preparation of Bendamustine hydrochloride monohydrate crystalline Form-SM useful in making pharmaceutical composition for the treatment of cancer or similar proliferative disorders.
Owner:SHILPA MEDICARE LTD
Method for preparing bendamustine hydrochloride freeze-dried powder injection
InactiveCN101836962ALow content of related substancesSafe and non-flammablePowder deliveryOrganic active ingredientsBendamustine hydrochlorideOrganic solvent
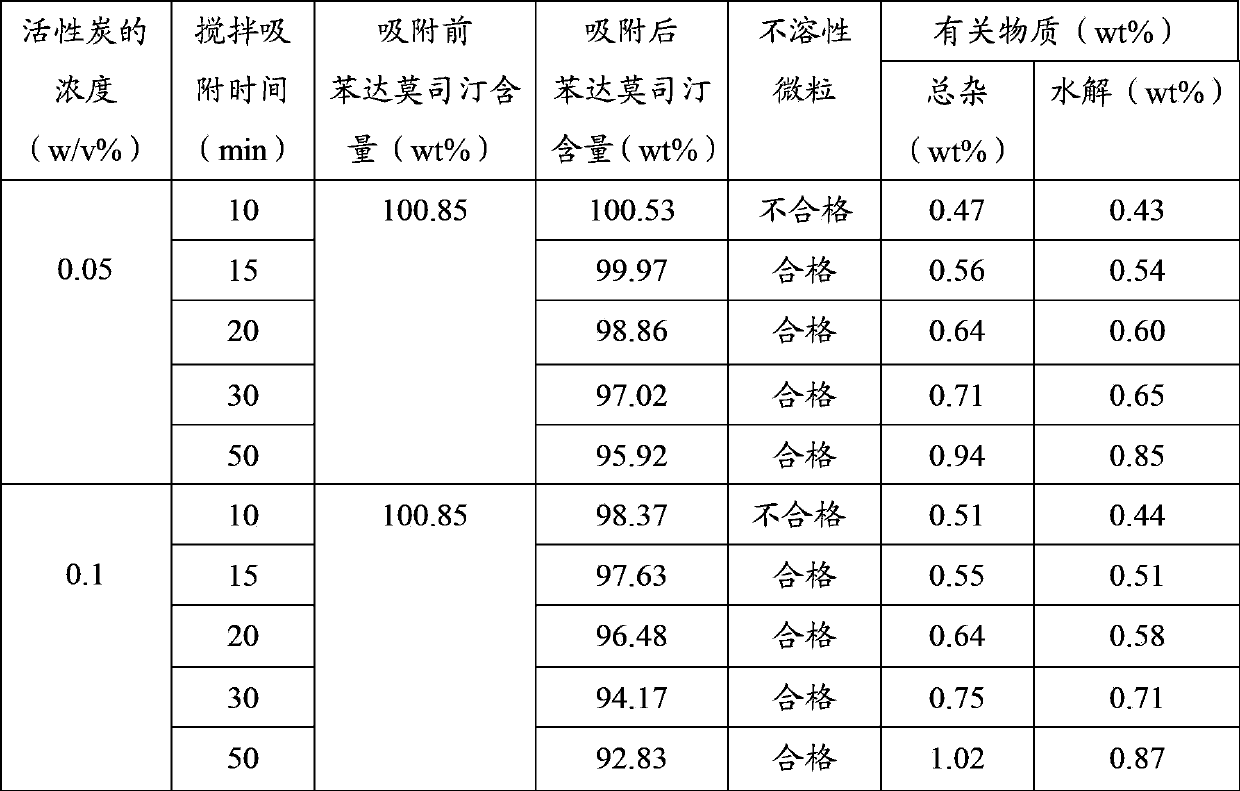
The invention discloses a method for preparing bendamustine hydrochloride freeze-dried powder injection, which is characterized by comprising the following steps of: adding the bendamustine hydrochloride and a freeze-dried excipient into water for injection with the temperature of between 30 and 50 DEG C, dissolving, and rapidly adding the water for injection with the temperature of between 0 and10 DEG C; mixing uniformly, starting a cooling system, and rapidly cooling the liquid medicament to the temperature of between 0 and 10 DEG C; adjusting the pH value to between 2.0 and 3.0, adding active carbon, stirring, filtering the active carbon, filtering, filling, and freeze-drying to obtain the freeze-dried powder injection. In the technical method, organic solvents are not added and only water is used as the solvent; the pH value and temperature are controlled; and the preparation-related substance has low content.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Method for purifying bendamustine hydrochloride
The invention discloses a method for purifying bendamustine hydrochloride, which comprises the following steps of: dissolving a bendamustine hydrochloride crude product into hydrochloric acid solution for recrystallization, and performing filtration to obtain pure bendamustine hydrochloride. The method belongs to the fields of organic chemistry and medicinal chemistry, and has the advantages of simple process, short cycle, high yield, high quality of product, suitability for industrial production and the like.
Owner:浙江凯普化工有限公司
Bendamustine hydrochloride crystal and preparation method thereof
InactiveCN102351799ASimple preparation processSimple and fast operationOrganic chemistrySolubilityBendamustine hydrochloride
The invention discloses a new bendamustine hydrochloride crystal and a preparation method thereof. On a characteristic X-ray powder diffraction pattern, the polymorph I of bendamustine hydrochloride disclosed by the invention has one or more characteristic peaks represented by 2theta at the positions as follows: 10.6+ / -0.2, 15.0+ / -0.2, 18.7+ / -0.2, 20.0+ / -0.2, 22.9+ / -0.2, and 26.5+ / -0.2. The new crystal of bendamustine hydrochloride has the characteristics of good solubility, good stability and the like. The operation method is simple and easy to operate, and is easy to industrially produce.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing bendamustine hydrochloride freeze-dried preparation
ActiveCN101912369AReduce the rate of hydrolysisLow impurity contentOrganic active ingredientsPowder deliveryBendamustine hydrochlorideOrganic solvent
The invention discloses a method for preparing bendamustine hydrochloride freeze-dried preparation. In the method, the bendamustine hydrochloride is dissolved in medicinal ethanol first and then mixed with water-containing solution, and the mixture is stored at low temperature, so long-time contact between the bendamustine hydrochloride and water is prevented, and the hydrolyzation speed of the bendamustine hydrochloride is reduced because of the low-temperature storage. Moreover, for improving the situation that stable freeze-dried preparation with high re-dissolubility and beautiful appearance cannot be obtained by using organic solvent ethanol in the prior art, the inventor, through deep study, finds that the stable freeze-dried preparation with high re-dissolubility and beautiful appearance can be realized by using the safe medicinal ethanol. The bendamustine hydrochloride freeze-dried preparation prepared by the method has the advantages of low impurity content, low organic solvent residue content, high stability, beautiful appearance, high re-dissolubility and applicability to clinical application and industrial production.
Owner:深圳万乐药业有限公司
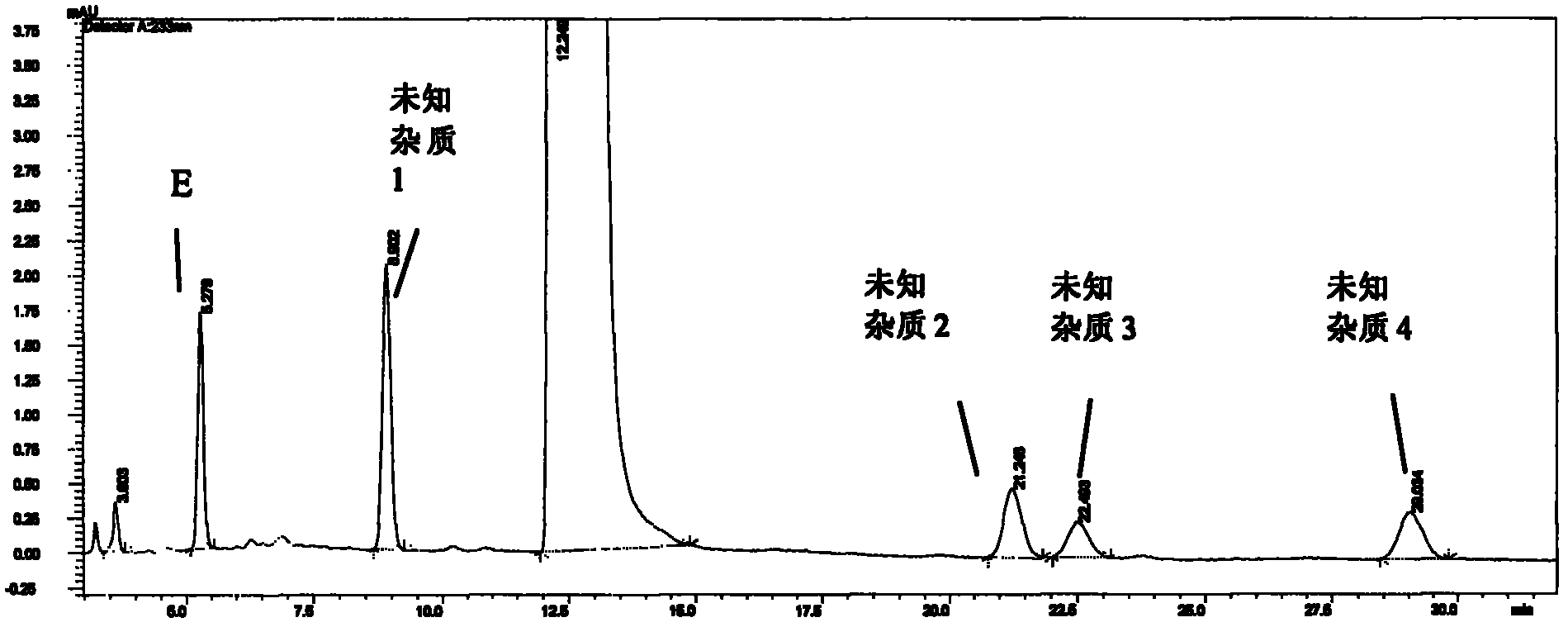
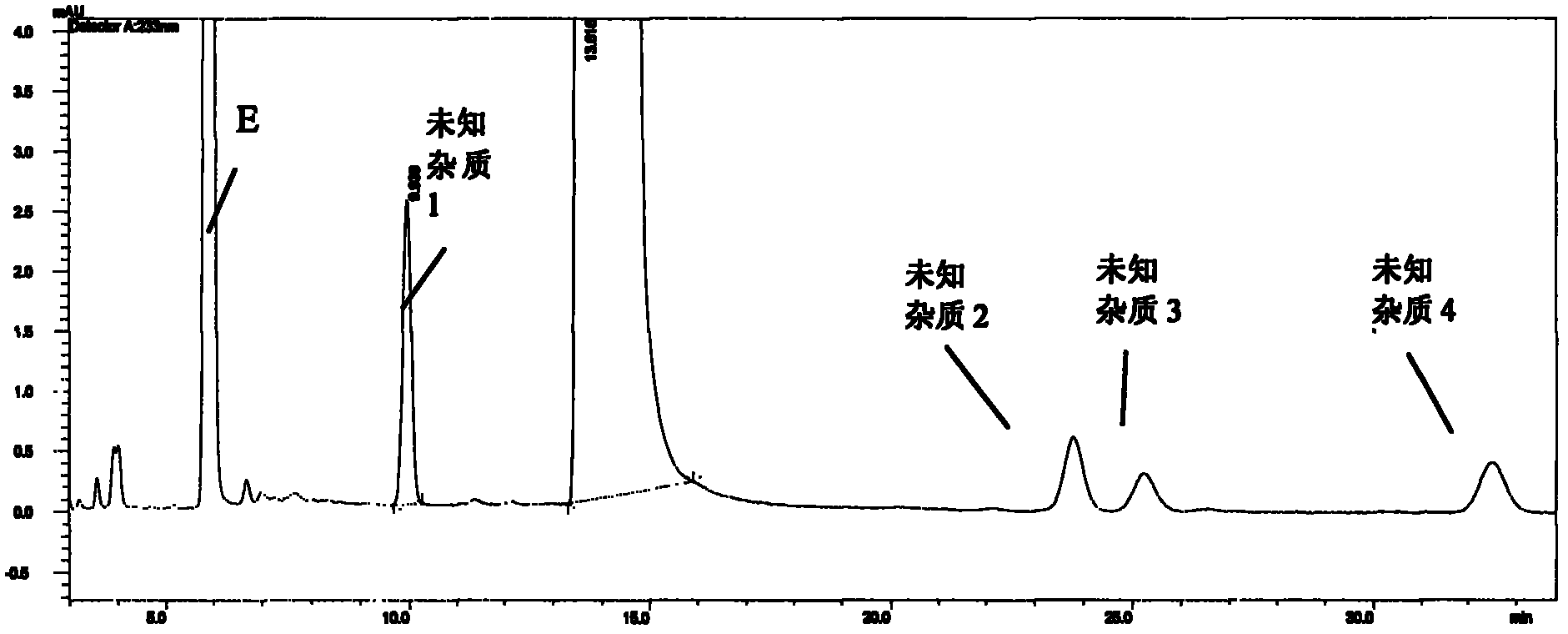
High performance liquid chromatographic analysis method of bendamustine hydrochloride and its related substances
ActiveCN102375033AEfficient separationEfficient methodComponent separationOrganic solventBendamustine hydrochloride
The invention relates to a method for determining related substances of bendamustine hydrochloride through high performance liquid chromatography. In the method, a sample to be detected is prepared into a sample solution with a mobile phase, and then reversed isocratic elution is carried out on an octadecylsilane bonded silica gel chromatographic column, with an organic solvent and a buffer solution as the mobile phase. The method of the invention effectively solves the problems in separation of related substances from bendamustine hydrochloride bulk drugs and detection thereof. The method provided in the invention has the advantages of simplicity, feasibility, and short analysis time cycle.
Owner:CHONGQING HUABANGSHENGKAI PHARM
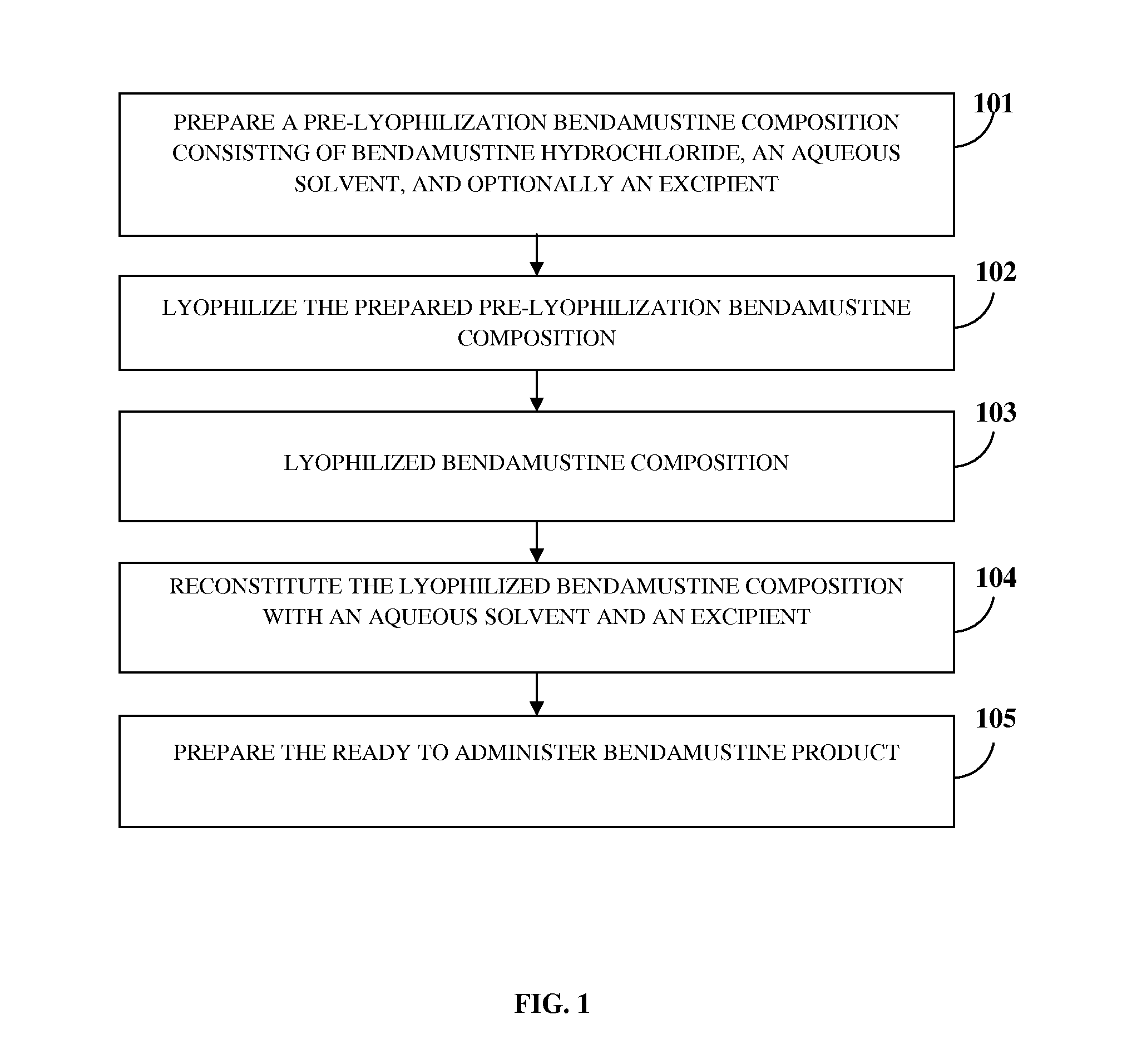
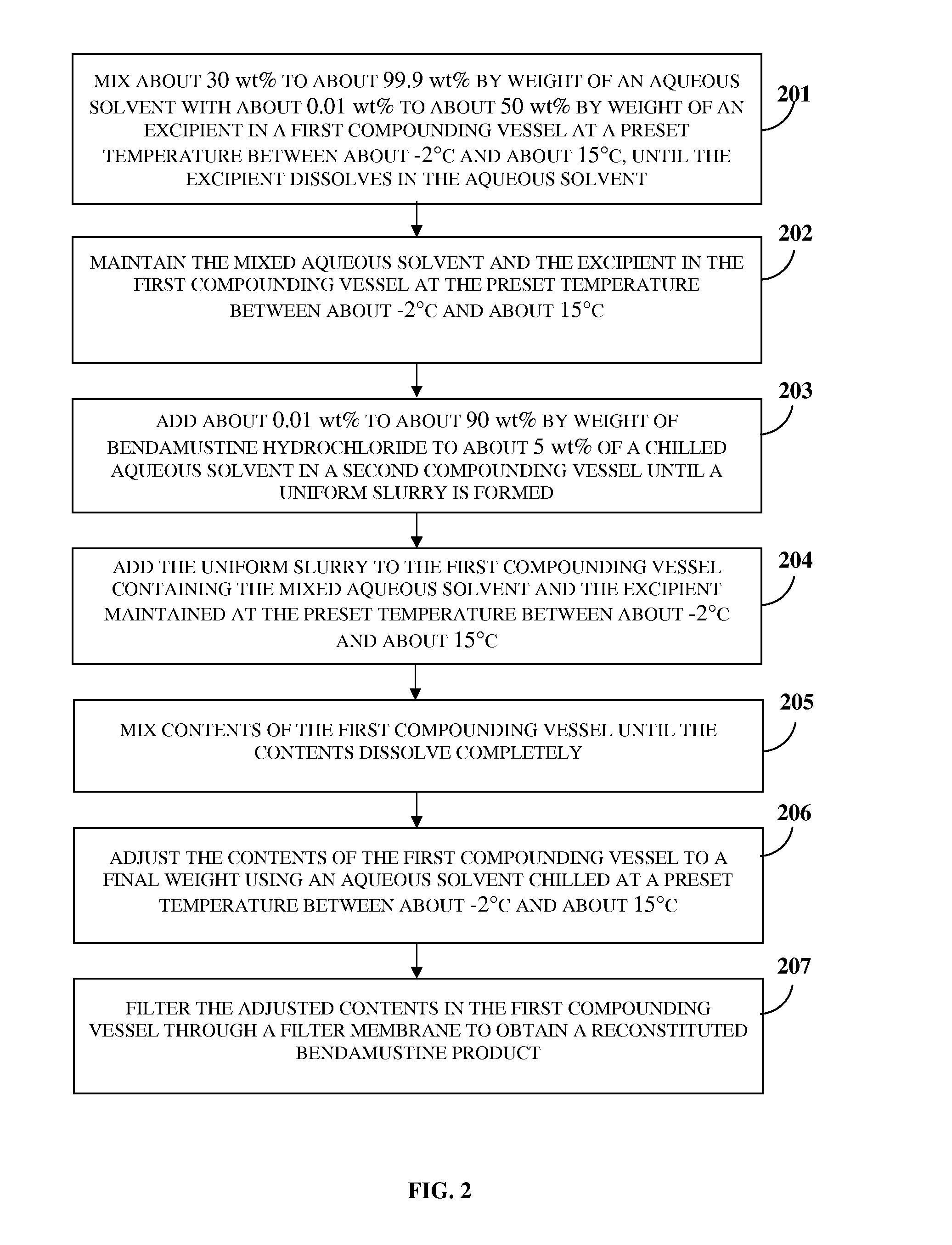
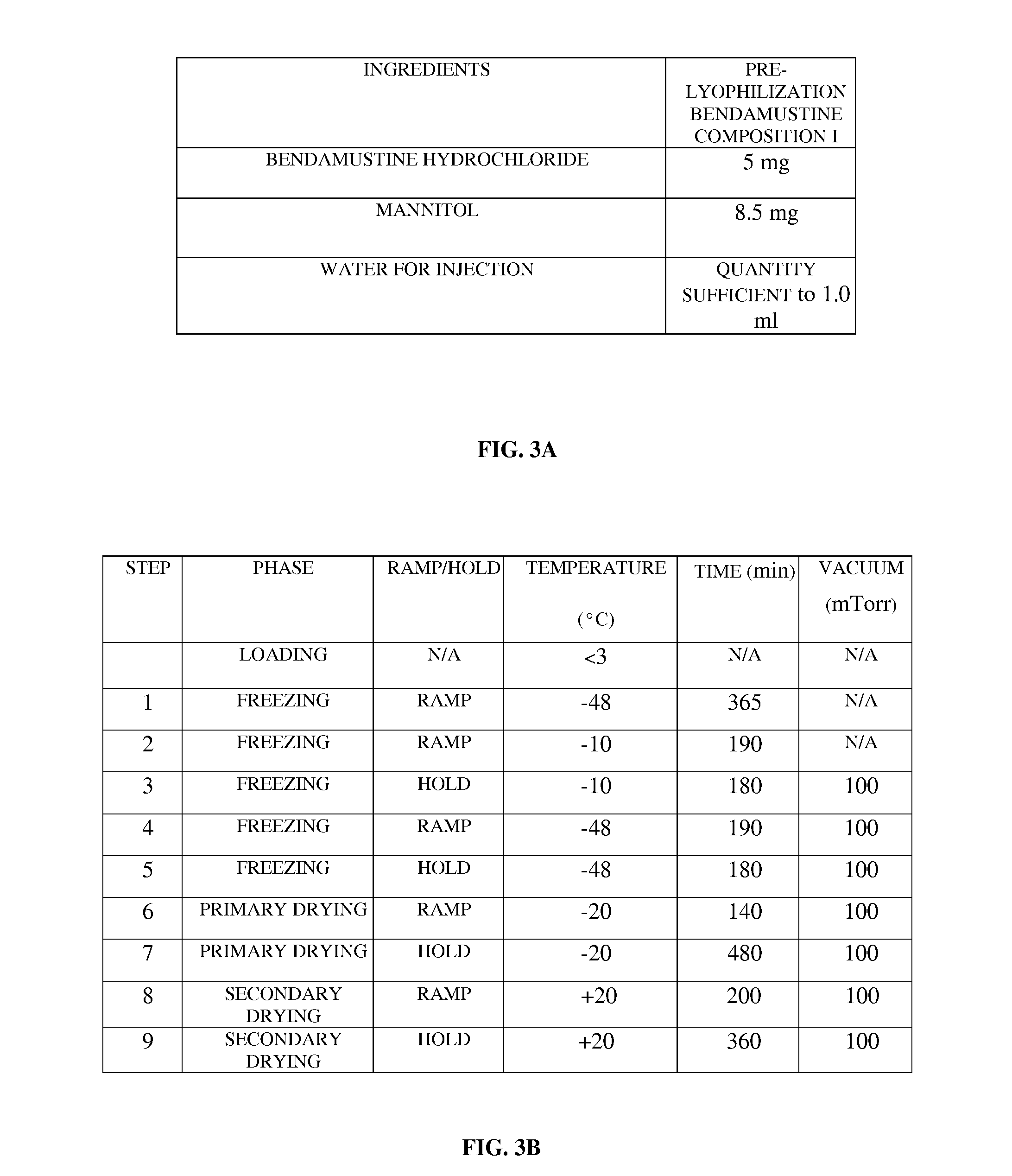
Bendamustine Formulations
A lyophilized bendamustine composition consisting of bendamustine hydrochloride, an aqueous solvent, and optionally an excipient, and a method of preparation thereof for treating a condition in a subject are provided. The aqueous solvent includes, for example, water, an acid, a base, or a salt, etc. The excipient includes at least one cryoprotectant, for example, sucrose, trehalose, mannitol, etc. A pre-lyophilization bendamustine composition is lyophilized, for example, by freezing, primary drying, annealing, secondary drying, condensed cooling and evacuation at preset temperatures to obtain the lyophilized bendamustine composition as a cake, a powder, or a solid concentrate. The lyophilized bendamustine composition is free of a non-aqueous solvent. Reconstitution is performed by mixing the lyophilized bendamustine composition with an aqueous solvent for about 30 seconds to 300 seconds. The reconstituted bendamustine product containing about 0.2 wt % to about 2.5 wt % by weight of impurities is administered to the subject in need thereof.
Owner:INNOPHARMA LICENSING
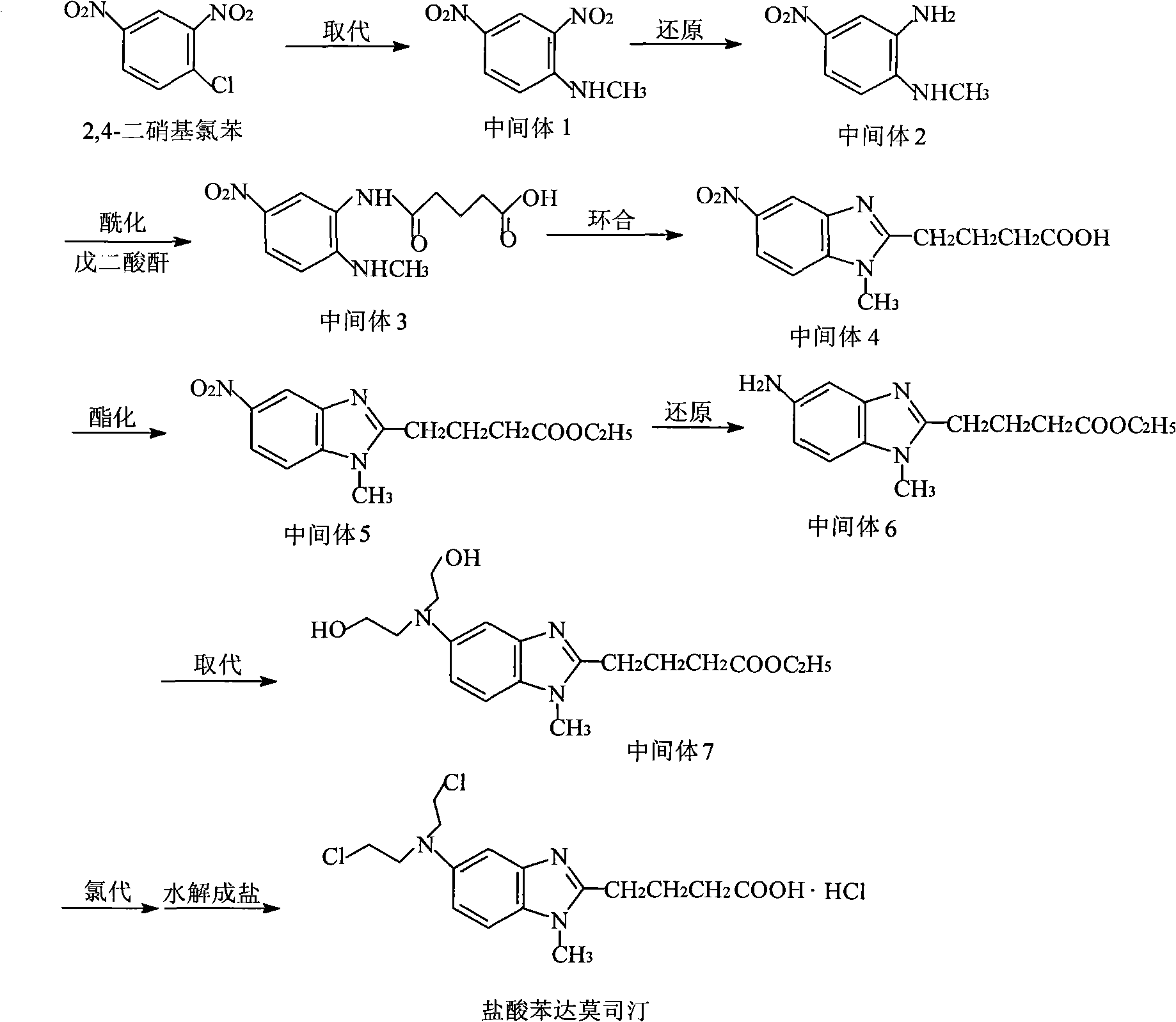
Process for the preparation of bendamustine hydrochloride
ActiveUS20140121383A1Easy to handleReduce the amount requiredOrganic chemistryAntineoplastic agentsLithiumBendamustine hydrochloride
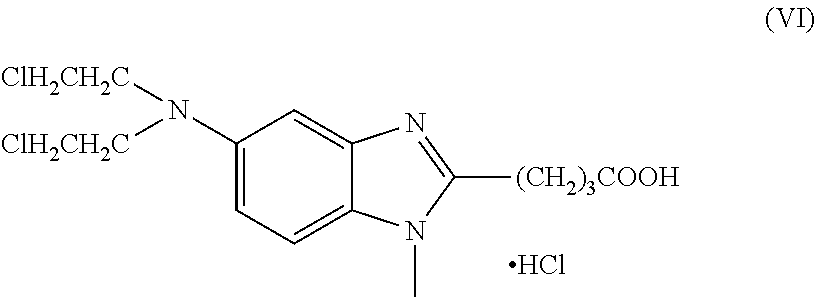
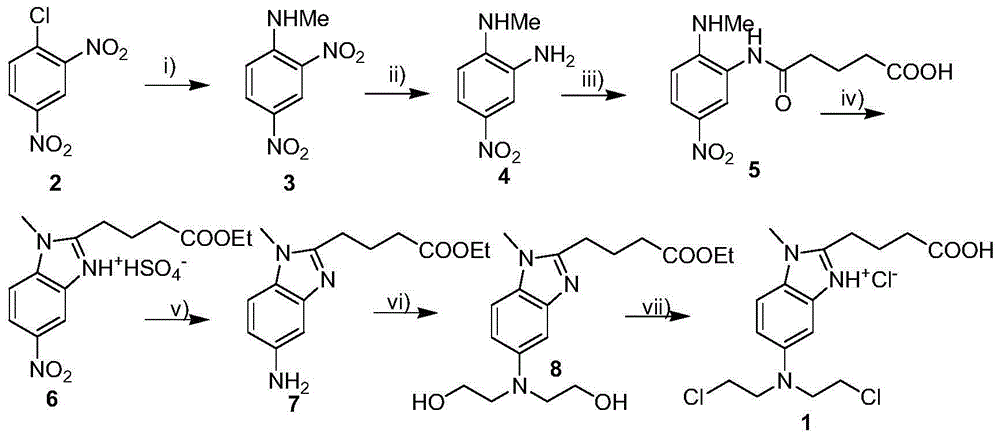
The present invention relates to an improved process for the synthesis of bendamustine, in particular, bendamustine hydrochloride of the formula (VI) and its intermediate 1-Methyl-5-[bis(2-chloroethyl)amino]-1H-benzimidazol-2-yl]lithium butanoate of formula (V), both having a purity of ≧99%, which is simple, convenient, economical, does not use hazardous chemicals and industrially viable.
Owner:FRESENIUS KABI ONCOLOGY LTD
Method for preparing intermediate in process of preparing bendamustine hydrochloride
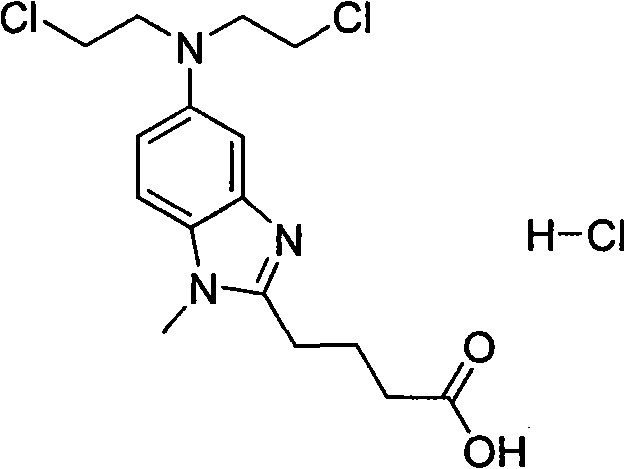
The invention provides a method for preparing an intermediate in the process of preparing bendamustine hydrochloride. The prepared intermediate is [1-methyl-2-(4'-ethyl butyrate)-5-N,N-bis(2'-chloroethyl)]-1H-benzimidazole or [1-methyl-2-(4'-methyl butyrate)-5-N,N-bis(2'-chloroethyl)]-1H-benzimidazole. The method comprises the following steps of: (1) putting a compound shown as a formula I into a reactor, adding an organic solvent for dissolving, adding a reducing agent into the reactor, reacting at the temperature of lower than 25 DEG C, regulating the pH value until a solution is neutral, and filtering, washing and drying a solid precipitate to obtain a crude product; and (2) dissolving the crude product in a mixture of methanol and water, stirring in ice water to precipitate a solid, and filtering, washing and drying the solid precipitate to obtain a target product. The yield is over 74 percent, the prepared key intermediate has the purity of over 99.5 percent, the content of a single impurity is less than 0.1 percent, and the method has the advantages of high yield and high product stability and is easy to operate and suitable for industrial production.
Owner:武汉长联来福制药股份有限公司
Bendamustine hydrochloride freeze-dried powder injection preparation method, product prepared through method, and use of product
ActiveCN103860482AGood formabilityImprove reconstitution performanceOrganic active ingredientsPowder deliveryDepyrogenationBendamustine hydrochloride
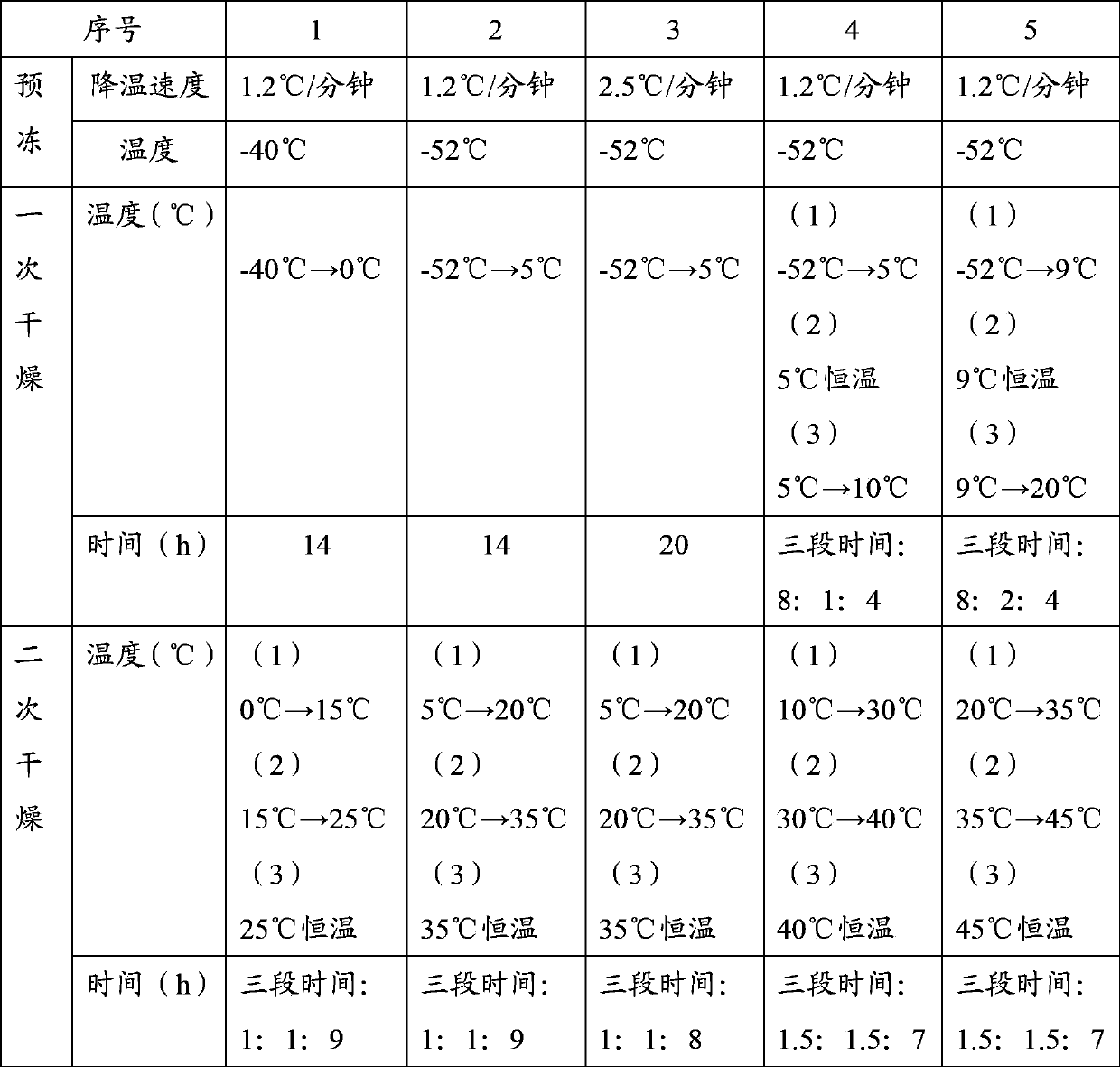
The invention provides a preparation method of a bendamustine hydrochloride freeze-dried powder injection. The preparation method is characterized in that the preparation method comprises the following steps: 1, dissolving: weighing bendamustine hydrochloride and an excipient, adding injection water, stirring for dissolving, and preserving the obtained solution in an ice bath; 2, depyrogenation: adding active carbon to the solution obtained in step 1, stirring in the ice bath, filtering for decarbonization, and carrying out fine filtration to obtain a soup; 3, pre-freezing: cooling the soup to -53 - -50DEG C to obtain a pre-frozen soup; 4, primary drying: evacuating the pre-frozen soup to 12- 20Pa, uniformly heating to 8-10DEG C, and uniformly heating to 18- 22DEG C to obtain a primary-dried soup; and 5, secondary drying: uniformly heating the primary-dried soup to 35DEG C, then uniformly heating to 45DEG C, and cooling the obtained powder to room temperature under vacuum conditions in order to obtain the bendamustine hydrochloride freeze-dried powder injection.
Owner:HAIKOU PHARMA FACTORY
Bendamustine HCL Stable Lyophilized Formulations
InactiveUS20150087681A1Impurities increaseImproved stability profileBiocideOrganic active ingredientsDiseaseBendamustine hydrochloride
The present invention provides a lyophilized bendamustine hydrochloride (HCL) pharmaceutical composition. The present invention further provides methods of producing the lyophilized bendamustine HCL composition from a composition including bendamustine HCL, mannitol, formic acid, and water. The pharmaceutical formulation can be used for any disease that is sensitive to treatment with bendamustine, such as neoplastic diseases.
Owner:NAVINTA
Preparation method of bendamustine hydrochloride composition for injection
InactiveCN103989641ARapid reconstitutionPowder deliveryOrganic active ingredientsBendamustine hydrochlorideFreeze-drying
The invention discloses a preparation method of a pharmaceutical composition, and in particular relates to a preparation method bendamustine hydrochloride composition for injection. The method comprises the following steps: (1) dissolving a filling agent into water of injection, adjusting pH with hydrochloric acid until the pH of the filling agent solution is 0.5 to 1.5, and then storing under 2 to 15 DEG C; (2) dispersing bendamustine hydrochloride into tert butyl alcohol to obtain bendamustine hydrochloride suspension; (3) mixing the filling agent solution and bendamustine hydrochloride suspension, agitating and dissolving, adding water of injection under 2 to 15 DEG C until the desired volume to obtain bendamustine hydrochloride composition solution, storing under 2 to 15 DEG C, filtering and sterilizing, and then freeze-drying to obtain the bendamustine hydrochloride composition for injection. The preparation method is simple and easy to operate, and the prepared bendamustine hydrochloride composition for injection is high in stability and low in impurity content.
Owner:SICHUAN HUIYU PHARMA
Method for preparing high-purity bendamustine hydrochloride
InactiveCN101948436BHigh purityMeet quality requirementsOrganic chemistryBendamustine hydrochlorideEthyl butyrate
The invention provides a method for preparing high-purity bendamustine hydrochloride. The method comprises the following steps of: (1) completely dissolving oily or colloidal 4-{5-[bis-(2-ethoxyl)amino]-1-methyl-2 benzimidazole}ethyl butyrate in 0.1 to 0.5g / ml solution of C1 to C4 alkyl acetate, wherein the dissolution temperature is 0 to 40 DEG C; (2) adding C5 to C8 hydrocarbons into the solution obtained in the step (1) dropwise, stirring the mixed solution at the temperature of between 10 DEG C below zero and 40 DEG C for crystallization, filtering the mixed solution to obtain solids of 4-{5-[bis-(2-ethoxyl)amino]-1-methyl-2 benzimidazole}ethyl butyrate; (3) performing chlorination on the solids of 4-{5-[bis-(2-ethoxyl)amino]-1-methyl-2 benzimidazole}ethyl butyrate obtained in the step 2 and thionyl chloride, performing hydrolysis by using concentrated hydrochloric acid to obtain salts, and purifying the salts to obtain the crude products of bendamustine hydrochloride; and (4) refining the crude products of bendamustine hydrochloride obtained in the step 3 by using water to obtain the finished products of bendamustine hydrochloride. The purity of the products prepared by the method of the invention is over 99.5 percent, and the content of single impurity is below 0.1 percent; and the method has the advantages of high yield, high product stability and suitability for industrialized production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
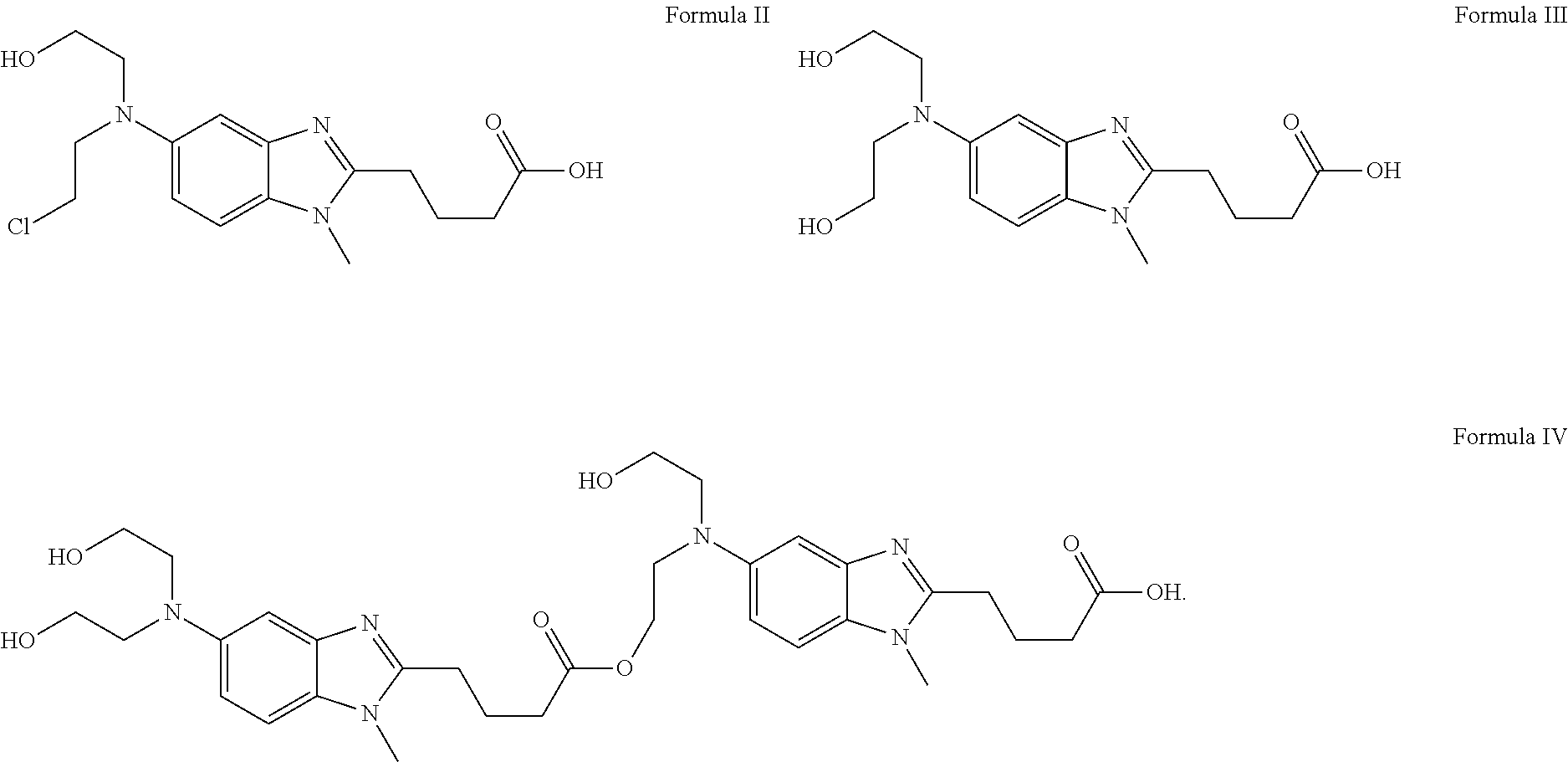
Preparation method of impurity HP1 in bendamustine hydrochloride
InactiveCN103351346AEasy to prepareHigh purityOrganic chemistryBendamustine hydrochlorideMethyl group
The invention discloses a preparation method of an impurity HP1 in bendamustine hydrochloride. The preparation method comprises the step of using [1-methyl-2-(4'-ethyl carbethoxy)-5-amino]-1H-benzimidazole as a starting raw material to prepare [1-methyl-2-(4'-acidyl)-5-N-(2'-hydroxy-ethyl)-N'-(2'-chloro-ethyl)]-1H-benzo imidazole, namely the HP1. The preparation method of the impurity HP1 in the bendamustine hydrochloride is simple, the prepared product has the advantage of high purity, and a qualified reference is provided for the quality control of the bendamustine hydrochloride.
Owner:SOUTHEAST UNIV
Method for purifying bendamustine hydrochloride
The invention discloses a method for purifying bendamustine hydrochloride, which comprises the following steps of: dissolving a bendamustine hydrochloride crude product into hydrochloric acid solution for recrystallization, and performing filtration to obtain pure bendamustine hydrochloride. The method belongs to the fields of organic chemistry and medicinal chemistry, and has the advantages of simple process, short cycle, high yield, high quality of product, suitability for industrial production and the like.
Owner:浙江凯普化工有限公司
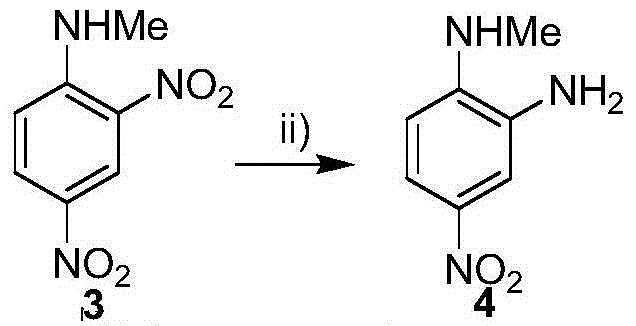
Selective reduction method for nitro
InactiveCN104402738AOrganic compound preparationCarboxylic acid amides preparationNitro compoundBendamustine hydrochloride
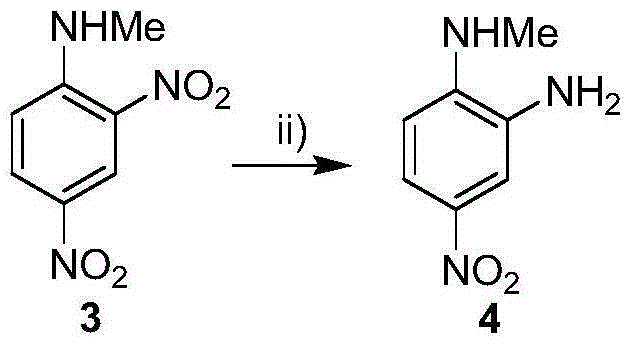
The invention relates to an intermediate compound of bendamustine hydrochloride obtained by selective reduction of polynitro compound. The method uses an active hydrogen donor under palladium-carbon catalysis, then the active hydrogen donor is gradually added in reduced amino ortho nitro to obtain the corresponding compound. The compound can conveniently synthesize bendamustine hydrochloride through the method in the invention.
Owner:NANJING UNIV
Bendamustine hydrochloride freeze-dried powder injection and preparation method thereof
ActiveCN108078931AImprove stabilityImprove solubilityPowder deliveryOrganic active ingredientsOrganic solventBendamustine hydrochloride
The invention discloses a bendamustine hydrochloride freeze-dried powder injection. The freeze-dried liquid medicine is prepared from the following raw materials: 1 to 2 parts of bendamustine hydrochloride, 2 to 3 parts of freeze-dried excipients, 14 to 50 parts of organic solvents and 45 to 83 parts of water. The use of organic solvent formulations, low-temperature liquids, filling and freeze-drying techniques can effectively control the level of impurities in products.
Owner:KINDOS PHARM CO LTD +1
Method for preparing bendamustine hydrochloride
ActiveCN110759867AGood reproducibilityImprove stabilityOrganic chemistryBendamustine hydrochlorideDrugs synthesis
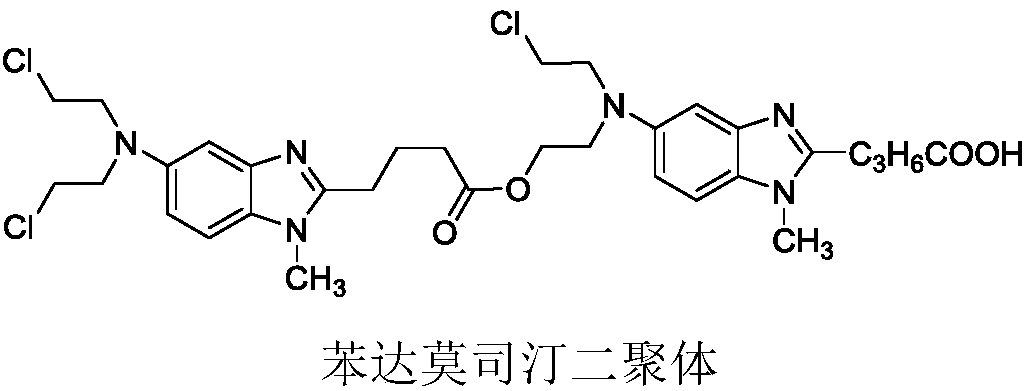
The invention belongs to the field of drug synthesis and particularly relates to a method for preparing bendamustine hydrochloride. According to the preparation method, concentrated hydrochloric acidis adopted as a refining solvent, thus, the bendamustine hydrochloride can be rapidly dissolved in the concentrated hydrochloric acid at the temperature of 20 DEG C to 45 DEG C, and the production cycle is short; the method is applicable to enlarged-scale industrial production, the refining efficiency is high, the yield is high, the reproducibility is good, and the obtained bendamustine hydrochloride is high in stability; and proven by high-temperature, high-humidity, accelerated and long-term tests, related substances and content thereof are free of obvious changes, the content of each singleimpurity is lower than 0.1%, and particularly, an impurity, i.e., a bendamustine dimer is undetectable, so that the bendamustine hydrochloride meets the technical requirements of ICH European Union (EU) quality research technique governing principles on limit of impurities.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Preparation method of highly pure bendamustine hydrochloride
InactiveCN104693125AImprove stabilityNot easy to decomposeOrganic chemistryBendamustine hydrochlorideCombinatorial chemistry
The invention discloses a purification method in the organic chemistry field, and especially relates to a preparation method of highly pure bendamustine hydrochloride. The preparation method comprises the following steps: 1, dissolving: dissolving crude bendamustine hydrochloride in a solvent at -20-60DEG C to obtain a crude bendamustine hydrochloride solution; 2, re-crystallizing: adding the crude bendamustine hydrochloride solution into a bendamustine hydrochloride-semisoluble-to-insoluble solvent, re-crystallizing at -20-60DEG C, filtering to obtain a bendamustine hydrochloride wet solid, adding the bendamustine hydrochloride-semisoluble-to-insoluble solvent to the wet solid, beating, and filtering to obtain an undried bendamustine hydrochloride product; and 3, drying. The preparation method has a high purification efficiency, the highest purity of a purified product reaches 99.99%, and the method has the advantages of simplicity, mild condition, repetition rate reaching 100%, and easy industrial production.
Owner:SICHUAN HUIYU PHARMA
Preparation method for bendamustine hydrochloride crude product
ActiveCN109422695AReduce pollutionStrengthen labor protectionOrganic chemistryBendamustine hydrochlorideVacuum drying
The invention discloses a preparation method for a bendamustine hydrochlorichloride crude product. The preparation method comprises the steps of dissolving methyl 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}butyrate or ethyl 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}butyrate in concentrated hydrochloric acid, adding activated carbon, carrying out a reaction, performing cooling to 15-30 DEG C, and performing filtering; and adding purified water or a solution of alkali metal salts containing chloride ions to the obtained filtrate, performing cooling under stirring to -5 DEG C to 5 DEG C, continuing performing stirring for 0.5-2h, performing filtering, separately washing the obtained filtrate cake with icy purified water and icy acetone once, and performing vacuum drying at 40-50 DEG C to obtain the bendamustine hydrochlorichloride crude product. The crude product obtained by the preparation method of the invention has a purity of 99.2% or above and a yield of 75% or above. In addition, the preparation method has mild conditions, low pollution, and high yield and product purity, and is more suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Highly pure bendamustine hydrochloride monohydrate
InactiveUS20150175554A1Organic active ingredientsOrganic compound preparationBendamustine hydrochloridePharmaceutical drug
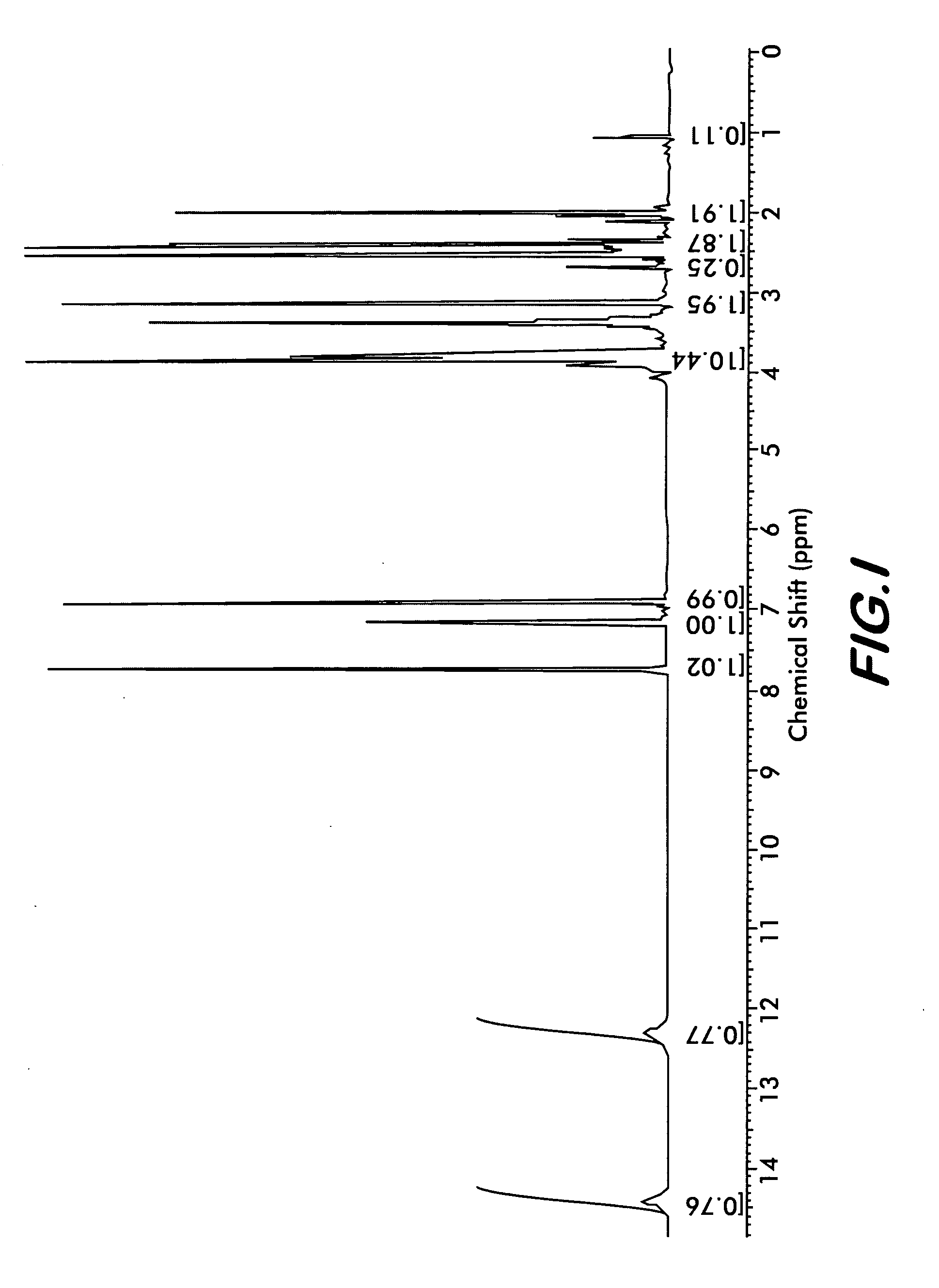
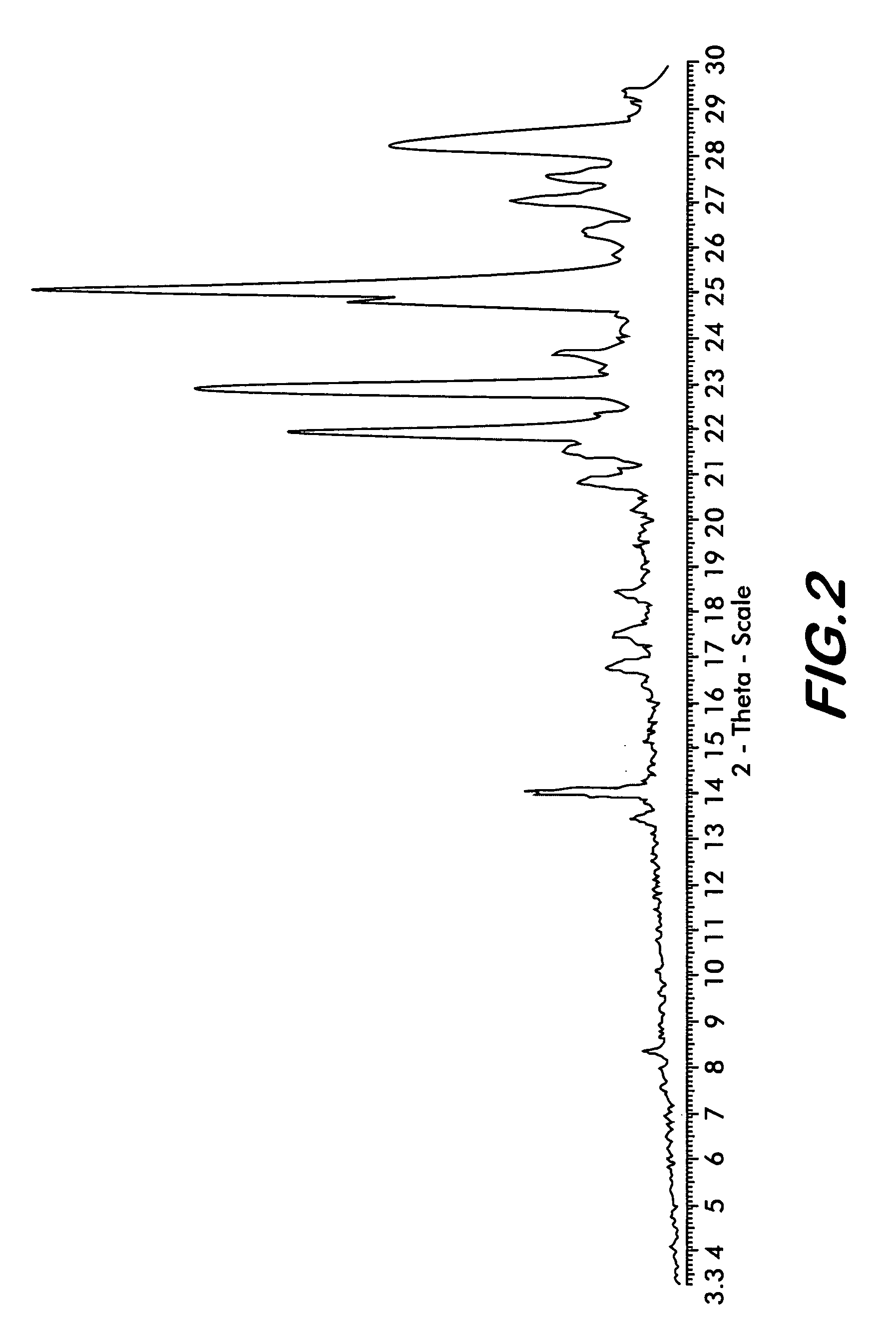
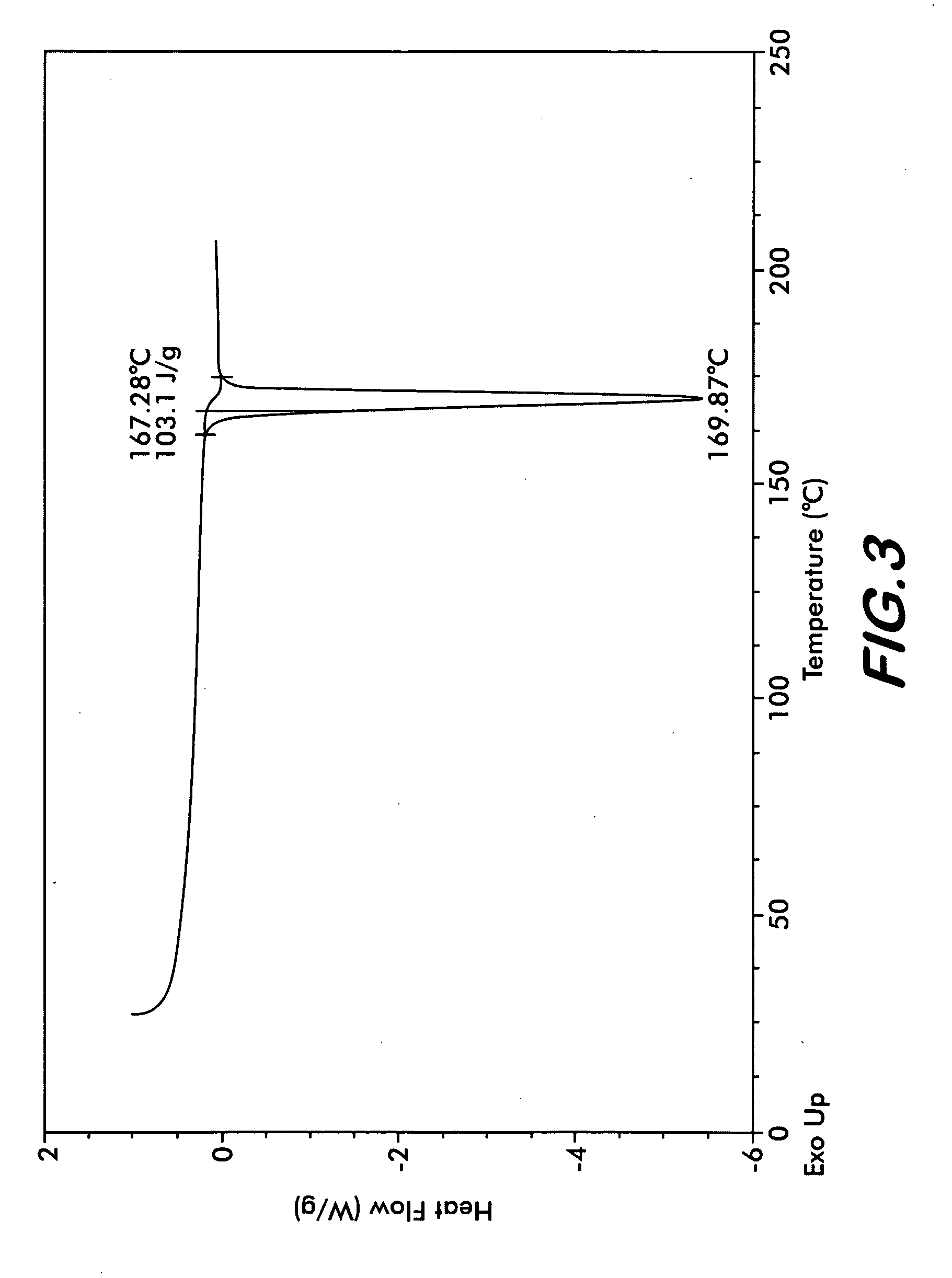
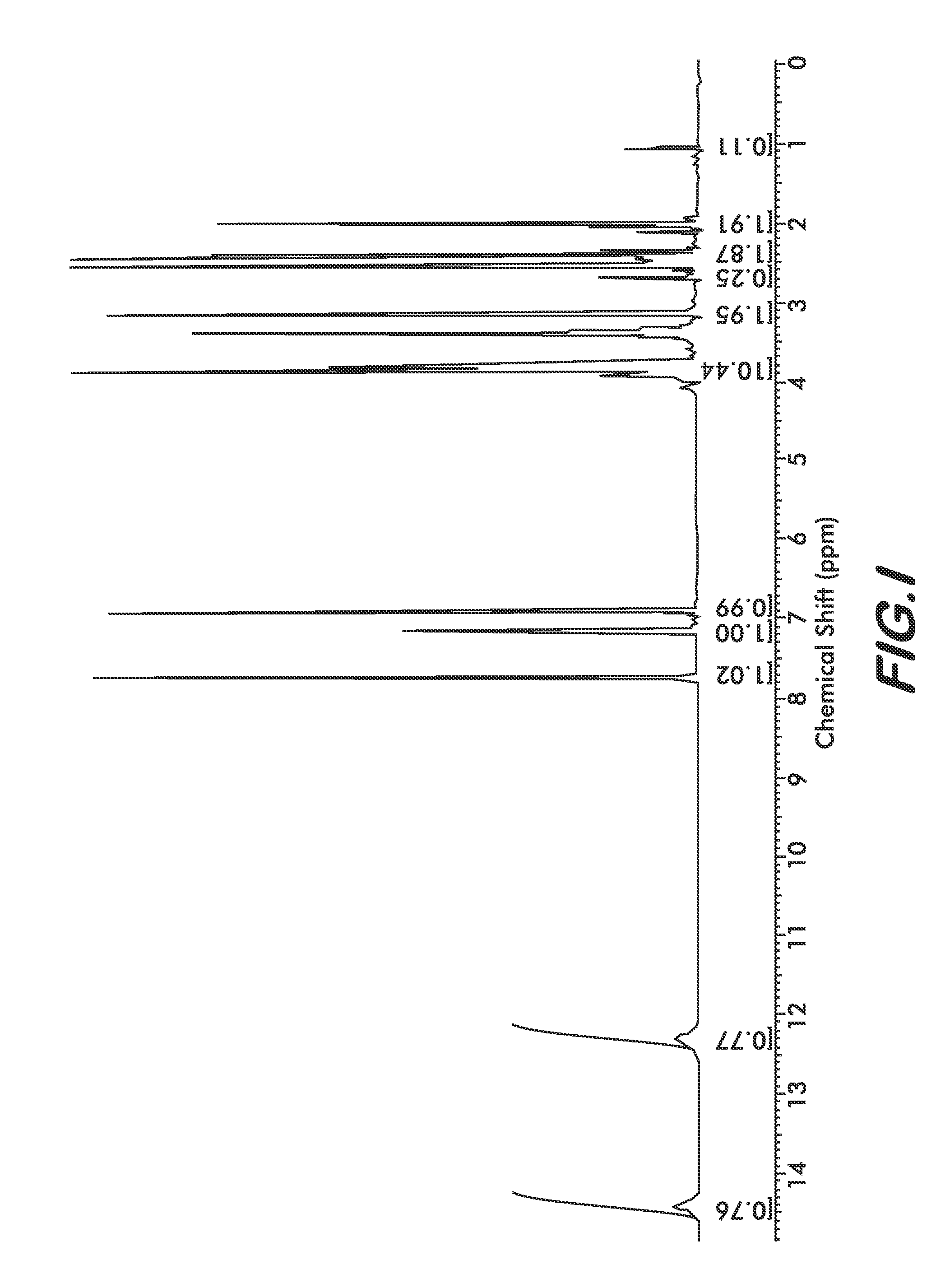
The present invention provide processes for the preparation of highly pure Bendamustine hydrochloride monohydrate of formula (I)The present application relates to Bendamustine hydrochloride monohydrate crystalline Form-SM characterized by X-ray powder diffraction pattern as depicted in FIG. 1 consisting peaks selected from the XRPD 2 theta degrees peaks at 7.42, 10.60, 11.17, 16.43, 17.94, 22.89, 26.33, 28.77, 30.28, 31.92, and 40.89±0.1 2θ° having a purity of greater than 99.5% (by HPLC).The present application also provides a process for the preparation of highly pure Bendamustine hydrochloride monohydrate crystalline Form-SM useful in making pharmaceutical composition for the treatment of cancer or similar proliferative disorders.
Owner:SHILPA MEDICARE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com