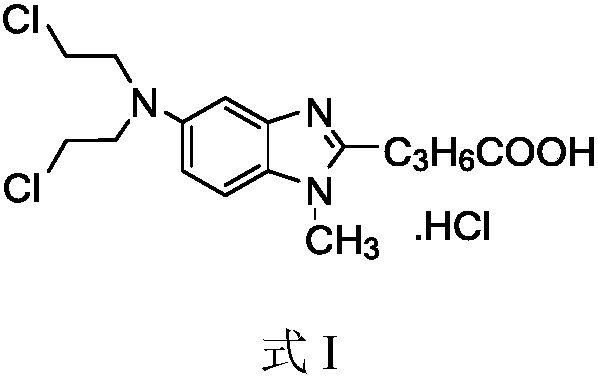
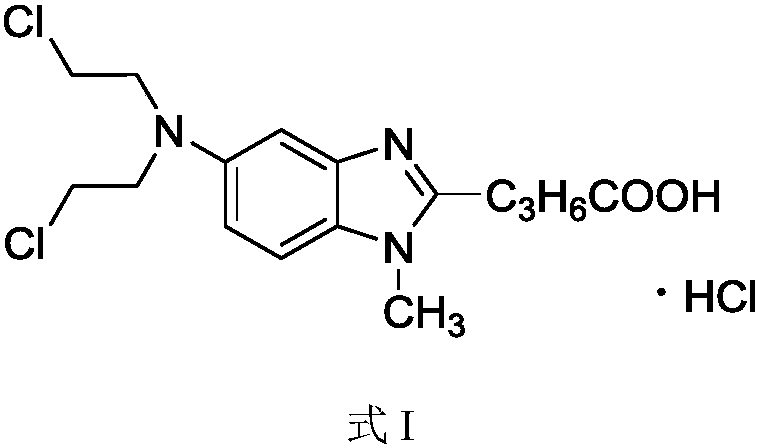
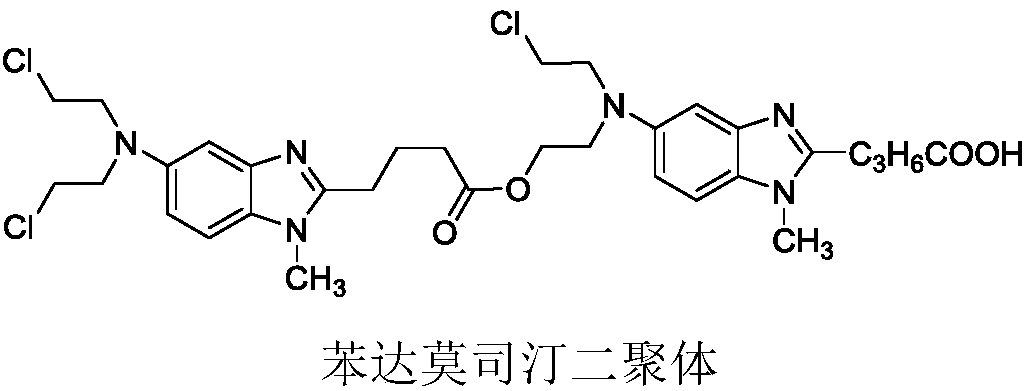
Method for preparing bendamustine hydrochloride
A technology of hydrochloric acid and concentrated hydrochloric acid, which is applied in the field of preparation of bendamustine hydrochloride, can solve the problems of accumulation, low refining yield, cumbersome operation, etc., and achieve the effects of short production cycle, high refining efficiency and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 100 g of the crude product of bendamustine hydrochloride in 300 g of concentrated hydrochloric acid (mass fraction is 37%, concentration is about 12 mol / L), the dissolution temperature is 25°C, dissolve in 15 min, filter while it is hot, add 2.1 L to pre-cool to 10 ℃ purified water, cooled and crystallized, filtered and dried to obtain bendamustine hydrochloride white crystals with a yield of 82%. Using the area normalization method, the HPLC purity detection results are shown in the table below, the purity of bendamustine is 99.79%, and the maximum simple impurity content is 0.048%.
[0024] components HP1 NP1 Bendamustine Hydrochloride Bendamustine hydrochloride methyl ester Bendamustine hydrochloride ethyl ester content 0.028% 0.022% 99.79% 0.021% 0.048%
Embodiment 2
[0026] Dissolve 100 g of the crude product of bendamustine hydrochloride in 100 g of concentrated hydrochloric acid (mass fraction is 37%, the concentration is about 12 mol / L), the dissolution temperature is 40 ° C, 25 min to dissolve, filter while it is hot, add 900 mL to pre-cool to 10 ° C Cool and crystallize in purified water, filter and dry to obtain bendamustine hydrochloride white crystals with a yield of 89.4%. Using the area normalization method, the HPLC purity detection results are shown in the following table, the purity of bendamustine is 99.86%, and the maximum simple impurity content is 0.044%.
[0027] components HP1 NP1 Bendamustine Hydrochloride Bendamustine hydrochloride methyl ester Bendamustine hydrochloride ethyl ester content 0.042% 0.008% 99.86% 0.044% /
Embodiment 3
[0029] Dissolve 100 g of the crude product of bendamustine hydrochloride in 150 g of concentrated hydrochloric acid (mass fraction is 37%, concentration is about 12 mol / L), dissolve at a temperature of 30°C, dissolve in 20 minutes, filter while hot, add 700mL to pre-cool to 10°C Cool and crystallize in purified water, filter and dry to obtain bendamustine hydrochloride white crystals with a yield of 91.6%. Using the area normalization method, the HPLC purity detection results are shown in the following table, the purity of bendamustine is 99.92%, and the maximum simple impurity content is 0.029%.
[0030] components HP1 NP1 Bendamustine Hydrochloride Bendamustine hydrochloride methyl ester Bendamustine hydrochloride ethyl ester content 0.025% / 99.92% / 0.029%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com