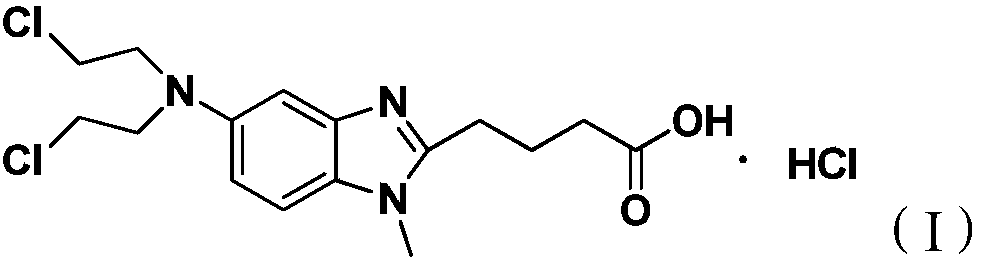
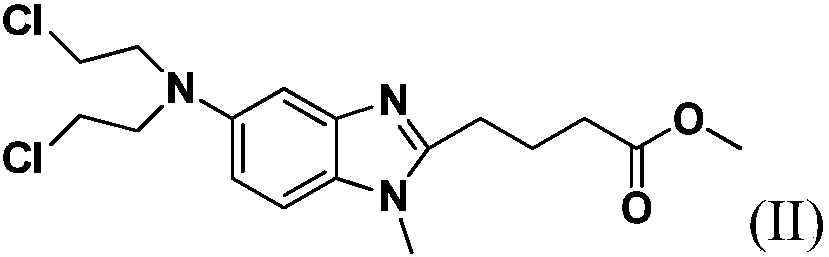
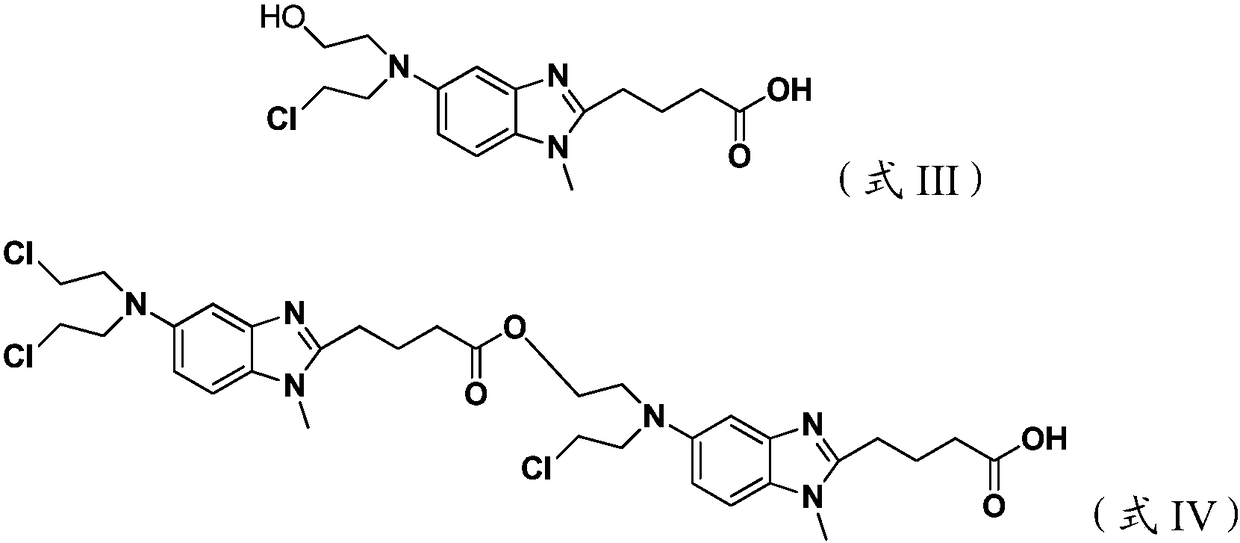
Preparation method for bendamustine hydrochloride crude product
A technology of bendamustine hydrochloride crude product and concentrated hydrochloric acid, applied in the field of medicine, can solve the problems of unfavorable large-scale industrial production, increase production cost, potential safety hazard, etc. in the concentration process, achieve high yield and product purity, and reduce the environment. Pollution, safety and health effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 10 g of 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester to 100 g of 36% concentrated hydrochloric acid After stirring and dissolving, add 0.5g of activated carbon, stir and raise the temperature to 95±5°C for 4 hours, lower to 15-30°C, filter, add 450g of purified water to the filtrate, continue to cool down to 2°C, stir for 1.5h, then filter, The filter cake was rinsed once with 10 g of ice-purified water and 10 g of ice-based acetone, and the filter cake was vacuum-dried at 40-50° C. for 12 hours to obtain 7.8 g of a white solid with a yield of 76.3%. The HPLC chromatographic purity of the sample was 99.43%. The content of {5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester is 0.18%, and the maximum other impurities are 0.10%.
[0028] HPLC purity detection method
[0029] Take about 20mg of this product, put it into a 25ml volumetric flask, add a small amount of methanol to dissolve ...
Embodiment 2
[0033] Add 10 g of 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester to 100 g of 36% concentrated hydrochloric acid After stirring and dissolving, add 0.5g of activated carbon, stir and raise the temperature to 95±5°C for 4 hours, lower to 15-30°C, filter, add 400g of saturated sodium chloride solution to the filtrate, continue to cool down to 3°C, and stir for 1.5h , filtered, the filter cake was rinsed once with 10 g of ice-purified water and 10 g of ice-based acetone, and the filter cake was vacuum-dried at 40-50 ° C for 12 hours to obtain 8.3 g of a white solid, with a yield of 81.2%, and a sample chromatographic purity of 99.24%. 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester content 0.39%, formula III compound content 0.05% , the content of the compound of formula IV is 0.07%, the maximum other impurities are 0.14%, and the total impurities are 0.76%. HPLC purity detection condition is ...
Embodiment 3
[0035]Add 10 g of 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester to 100 g of 36% concentrated hydrochloric acid Add 0.5g of activated carbon after stirring and dissolving, stir and heat up to 95±5°C for 4h, lower to 15-30°C, filter, add 420g of saturated potassium chloride solution to the filtrate, continue to cool down to -5°C, and stir for 1.5h After filtering, the filter cake was rinsed once with 10 g of ice purified water and 10 g of ice acetone respectively, and the filter cake was vacuum-dried at 40-50 °C for 12 hours to obtain 8.0 g of white solid, yield 78.3%, sample chromatographic purity 99.20% , 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester content 0.48%, other maximum single hetero 0.11 %. HPLC purity detection condition is the same as embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com