High performance liquid chromatographic analysis method of bendamustine hydrochloride and its related substances
A technology of bendamustine hydrochloride and high performance liquid chromatography, which is applied in the detection field of antitumor drug bendamustine hydrochloride and related substances, can solve the problems such as no detailed reports and no standards, and achieves analytical results. The effect of short time period, effective method and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Determination of related substances of bendamustine hydrochloride (1)
[0050] 1) Determination of related substances of bendamustine hydrochloride - system specificity experiment
[0051] Chromatographic conditions: mobile phase: A: acetonitrile; B: 0.02mol / l potassium hexafluorophosphate solution (phosphoric acid to adjust the pH value to 3.0); A: B = 425: 575 (V: V)
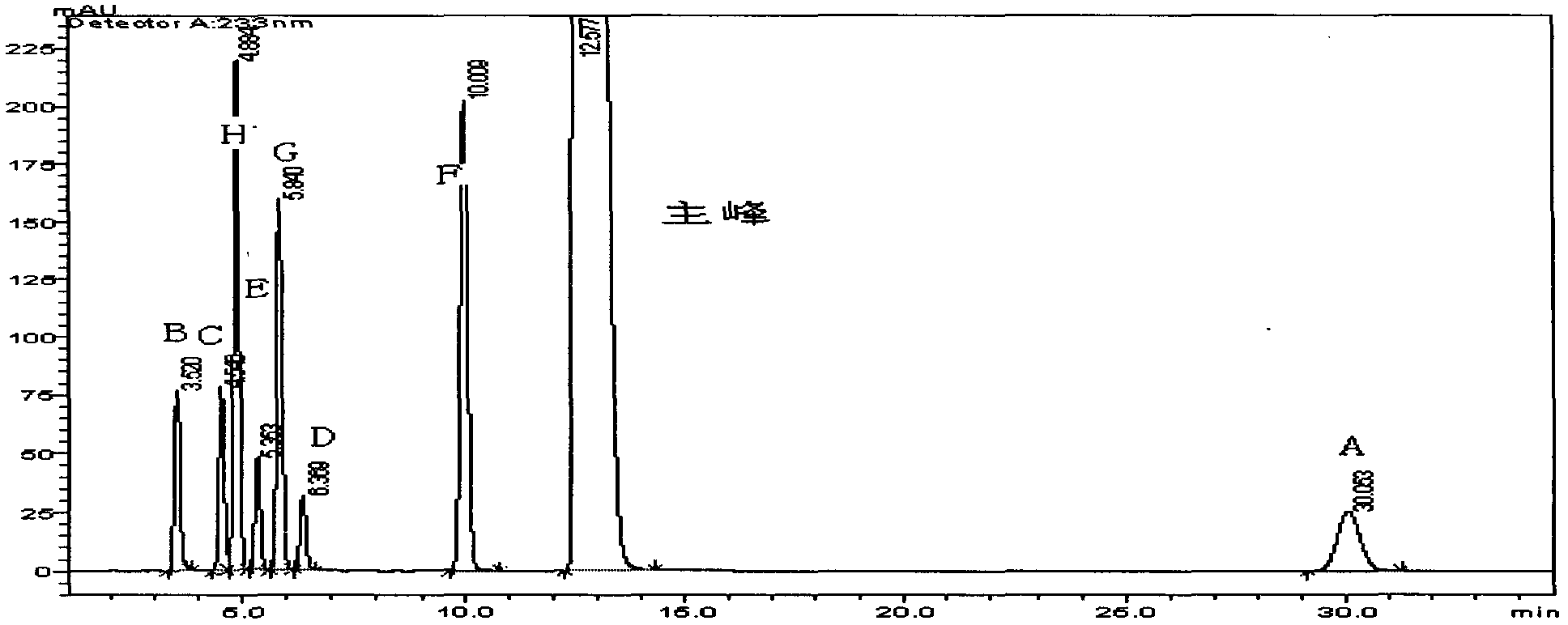
[0052] Preparation of sample solution: Weigh the appropriate amount of each impurity standard (A~H) and finished product, weigh them accurately, put them in the same measuring bottle, add diluent to the appropriate concentration, shake well, and filter to get ready. Inject the sample and record the chromatogram.
[0053] Experimental results: see attached figure 1 , the retention time of the main peak is 12.3min, the separation effect of the main peak and each impurity peak is good, and each impurity peak can be baseline-separated.
[0054] 2) Quantitation limit and detection limit dete...
Embodiment 2~9
[0066] Embodiments 2-9: Determination of Related Substances of Bendamustine Hydrochloride (2-9)
[0067] Mobile phase: A: acetonitrile; B: 0.02mol / l potassium hexafluorophosphate solution (phosphoric acid to adjust the pH value);
[0068]
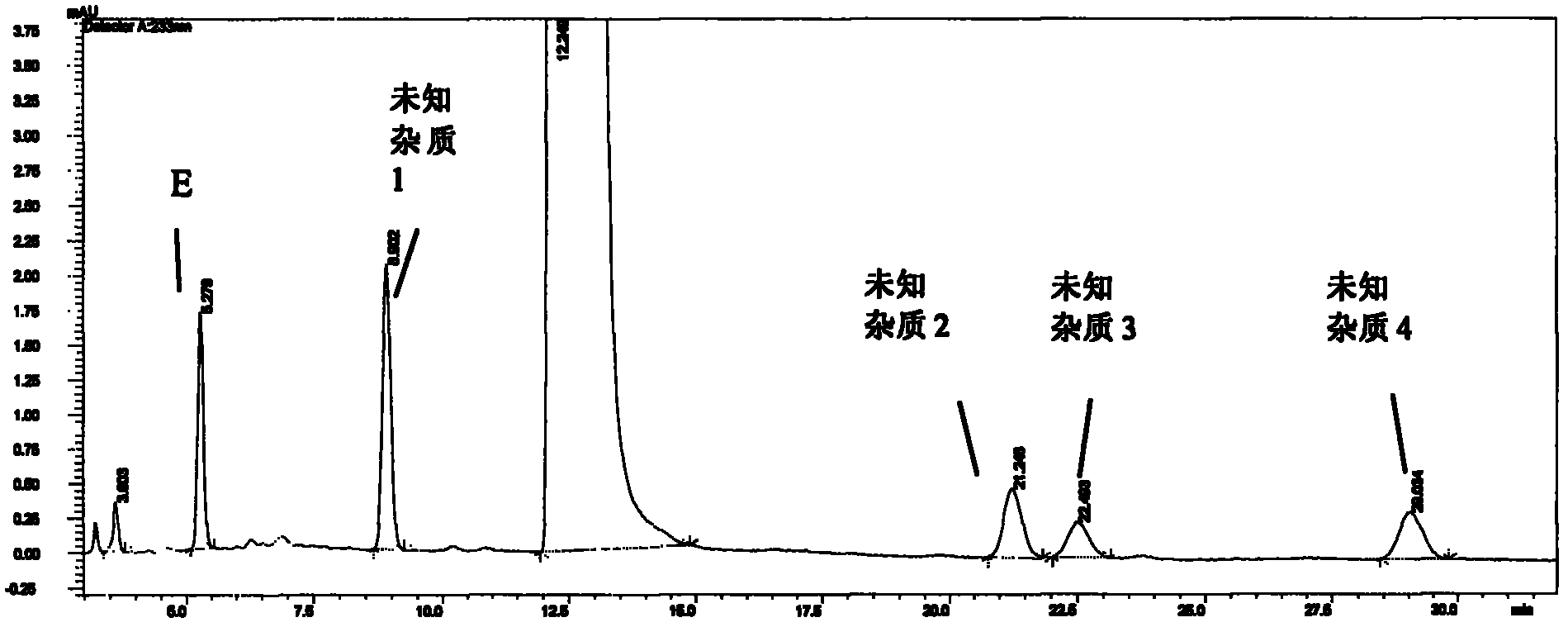
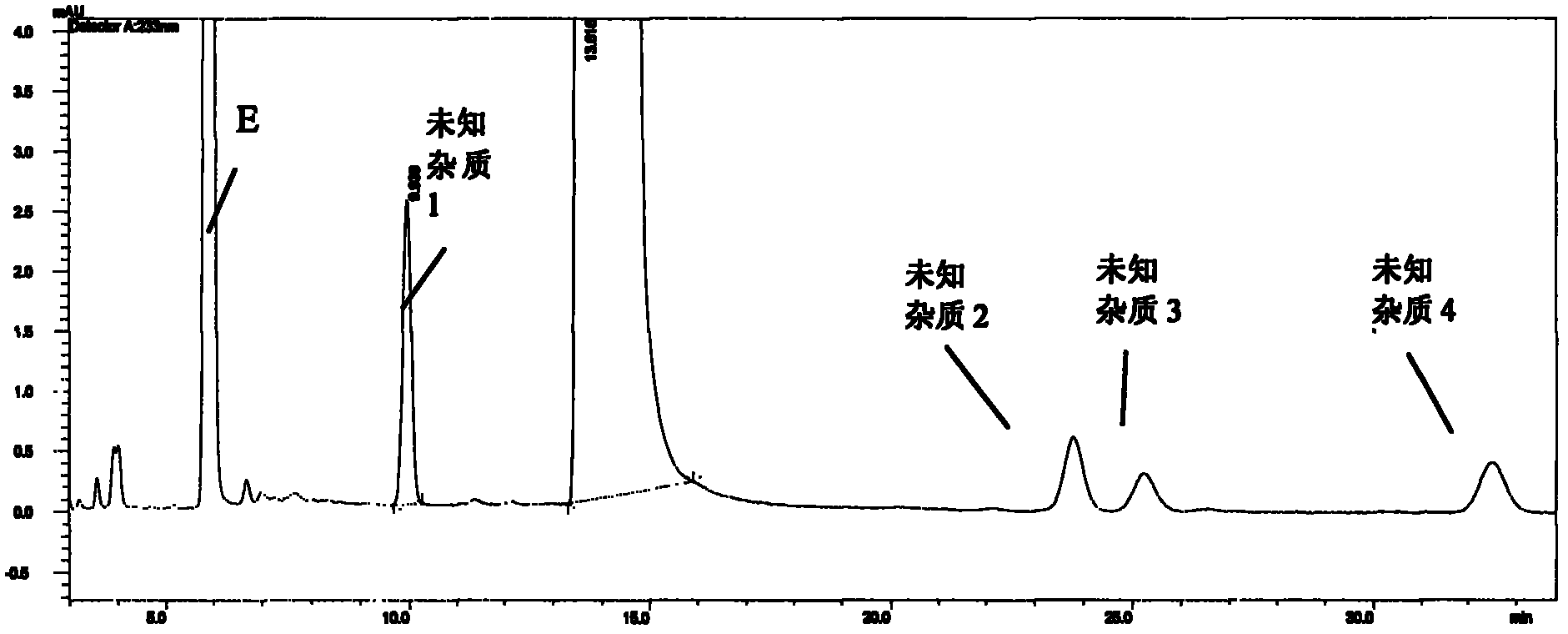
[0069] Sample solution preparation: Weigh an appropriate amount of the finished product, weigh it accurately, put it in the same measuring bottle, add mobile phase to the appropriate concentration, shake well, and you get it. Inject the sample and record the chromatogram.
[0070] Experimental results: attached Figure 3-10 , each impurity peak is well separated from the main peak, and each impurity peak (unknown impurity 1-4) can be baseline-separated.
Embodiment 10~13
[0071] Embodiments 10-13: Determination of Related Substances of Bendamustine Hydrochloride (10-13)
[0072] Instrument: Agilent 1200 high performance liquid chromatography (DAD detector)
[0073] Mobile phase: A: acetonitrile; B: 0.02mol / l potassium hexafluorophosphate solution (phosphoric acid to adjust the pH value);
[0074]
[0075] Sample solution preparation: Weigh an appropriate amount of the finished product, weigh it accurately, put it in the same measuring bottle, add mobile phase to the appropriate concentration, shake well, and you get it. Inject the sample and record the chromatogram.
[0076] Experimental results:
[0077] 1) by attached Figure 11 , the extracted ultraviolet spectrum shows that each impurity is the homologue of the finished product, and the maximum ultraviolet absorption wavelength is the same. When the detection wavelengths are 220nm, 233nm and 245nm respectively, the levels of impurities are basically the same.
[0078] 2) by attached...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com