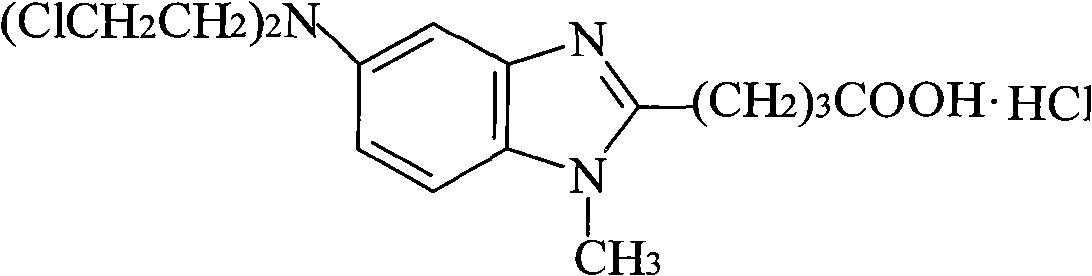
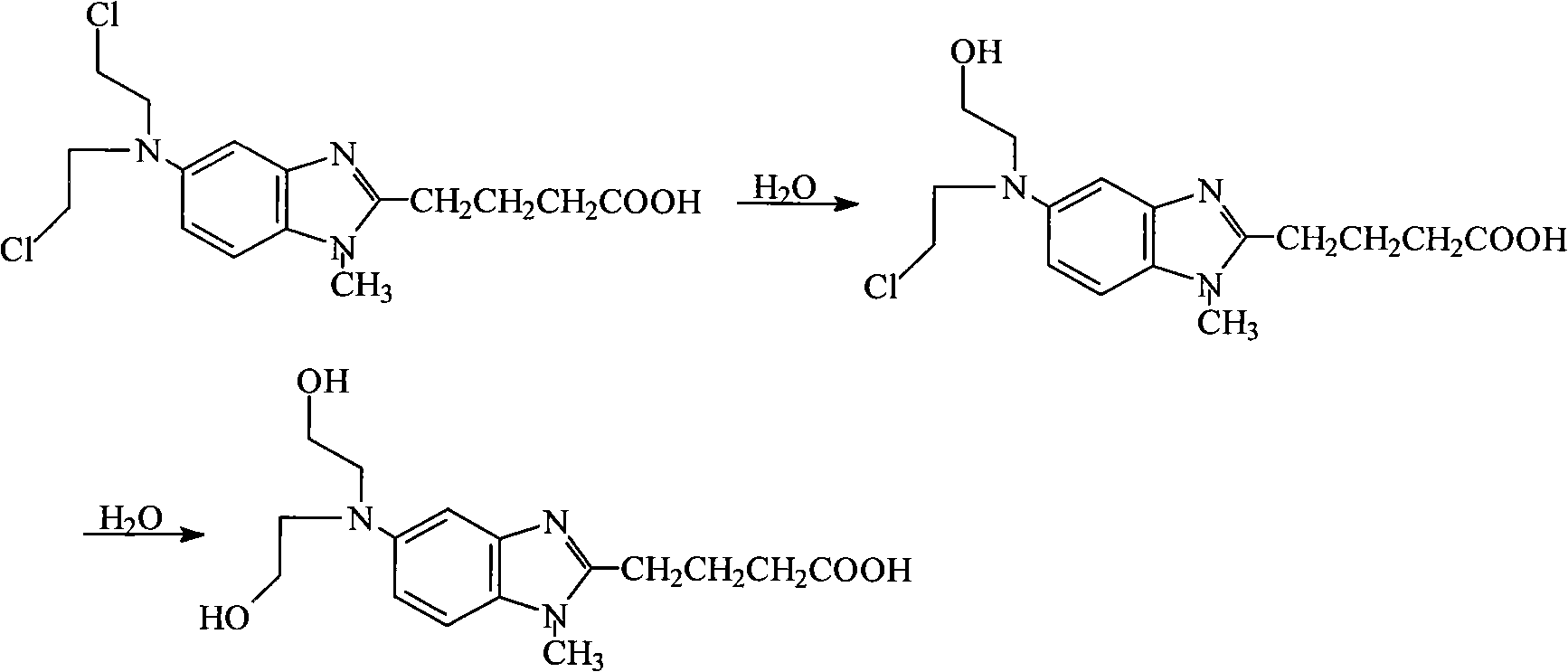
Refining method of bendamustine hydrochloride
A technology of bendamustine hydrochloride and a refining method, which is applied in the field of refining bendamustine hydrochloride, can solve the problems of cumbersome operation, difficult removal of impurities, and influence on the quality of finished products, and achieve simple process, low adverse reactions, Ease of process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of crude bendamustine hydrochloride
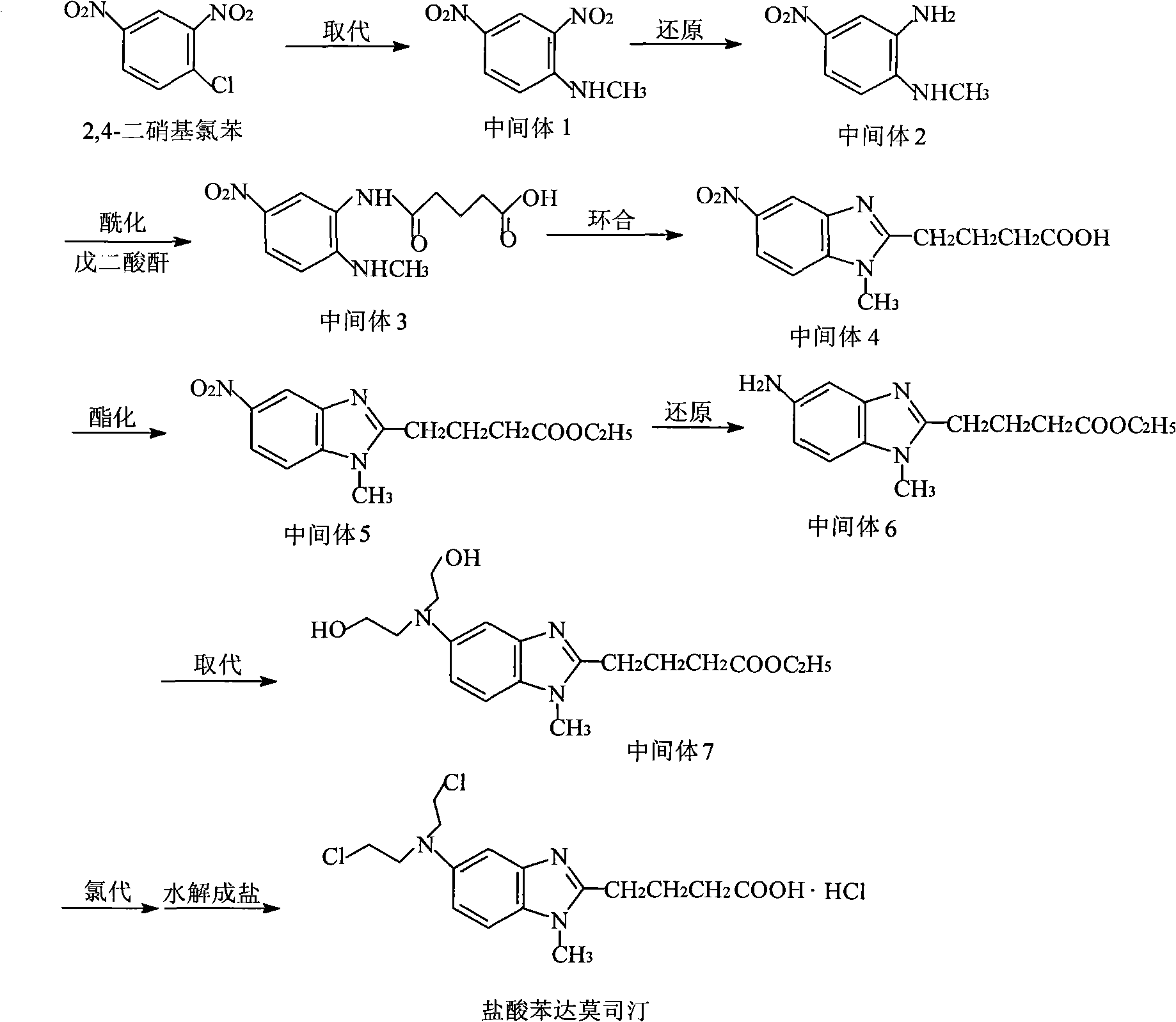
[0027] The crude bendamustine hydrochloride can be prepared by referring to the literature Journal fur praktischeChemie.4.Reihe.Band 20.1963 (178-186), or by referring to the following method:
[0028] (1) Preparation of N-methyl-(2,4-dinitro)aniline (Intermediate 1)
[0029] Add 438g of 2,4-dinitrochlorobenzene, 141g of methylamine hydrochloride, 573g of sodium acetate trihydrate and 1050ml of N,N-dimethylformamide into a 3L three-necked flask, heat to reflux, stir and react for 3h, TLC Stop the reaction after detecting no raw material. The reaction solution was quickly poured into 3000ml of ice water after being slightly cooled, and allowed to stand for crystallization. After filtering, the filter cake is washed with an appropriate amount of water to remove DMF, and the filter cake is dried at 80°C by blowing. A yellow solid 397.3g (Intermediate 1) was obtained, yield: 93.3%, mp: 178.6-179°C.
[0030] (2) Preparat...
Embodiment 2
[0053] Put 5g of bendamustine hydrochloride crude product prepared according to Example 1 (purity 93.2%, maximum single impurities 4.16%) into the reaction flask, add 20g 1mol / L hydrochloric acid solution, heat to 80°C, stir to dissolve, while hot After filtering, the filtrate was cooled to 10°C to crystallize for 12h, filtered, and dried at 45°C for 6h to obtain 4.6g of white solid with a yield of 92%. The purity is 99.84%, the maximum single impurity is 0.05%, and the HPLC purity detection conditions are the same as in Example 1.
Embodiment 3
[0055] Place 9g of crude bendamustine hydrochloride (purity 94.7%, maximum single impurities 4.49%) prepared according to Example 1 in a reaction flask, add 60g of 0.09mol / L hydrochloric acid solution, heat to 50°C, stir to dissolve, add 0.5g of activated carbon was decolorized for 5 minutes, filtered while hot, the filtrate was cooled to 0°C for 5h to crystallize, filtered, and dried at 30°C for 10h to obtain 8.5g of white solid, with a yield of 94.4%. The purity is 99.87%, the maximum single impurity is 0.08%, and the HPLC purity detection conditions are the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com