Preparation method of highly pure bendamustine hydrochloride
A technology of bendamustine hydrochloride and high purity is applied in the field of preparation of bendamustine hydrochloride to achieve the effects of simple method, low adverse reaction and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
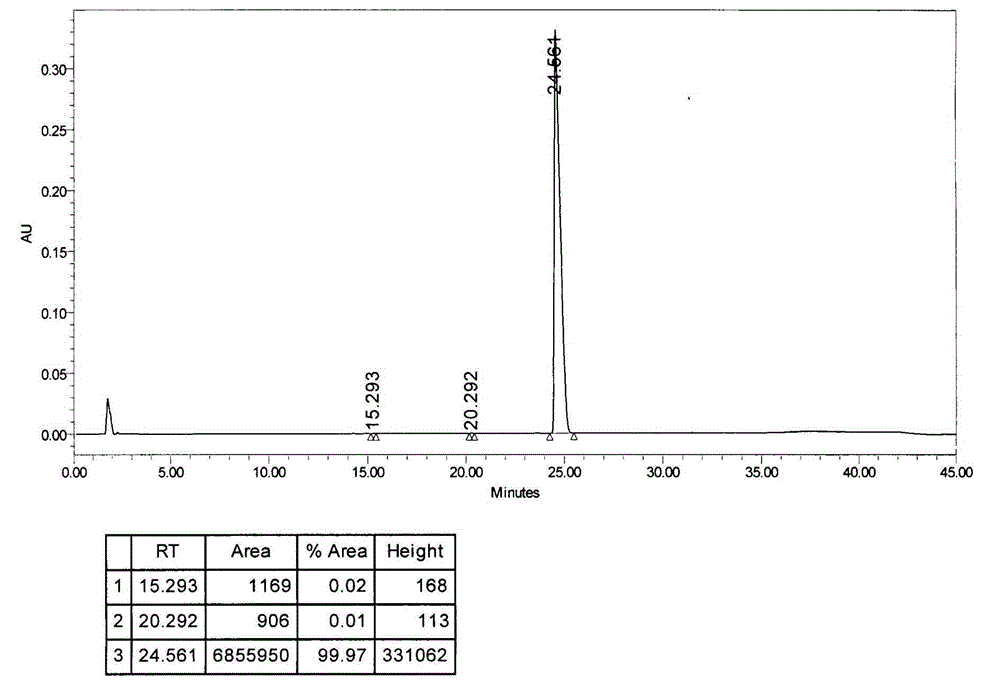
[0036] Put 5 grams of crude bendamustine hydrochloride in a 250ml three-necked flask, add 10g of dimethyl sulfoxide to dissolve at about 25°C, add 50ml of purified water, recrystallize at about 5°C, and filter to obtain a filter cake. The filter cake is a solid wet material. Add 3 times the volume of acetone to the filter cake to make a slurry, filter to obtain the undried product of bendamustine hydrochloride, and dry it at 30°C under vacuum -0.09MPa for 24 hours to obtain high-purity bendamustine hydrochloride Ting finished product 3.7g, HPLC (high performance liquid chromatography) content: 99.97%, the map is as follows figure 1 As shown, impurity I: 0.02%, other impurities 0.01%, moisture 4.66%.
[0037] 1 H-NMR(DMSO-d6,400MHz): δ12.23(bs,1H), δ7.72(d,1H),δ7.11(dd,1H),δ6.69(d,1H),δ3.89 (s,3H),δ3.81(m,4H),δ3.76(m,4H),δ3.14(t,2H),δ2.40(t,2H),δ2.00(m,2H) .
[0038] Adopt Carlo Erba 1106 elemental analyzer, elemental analysis result: C 16 h 21 Cl 2 N 3 o 2 .HCl.H 2 ...
Embodiment 2
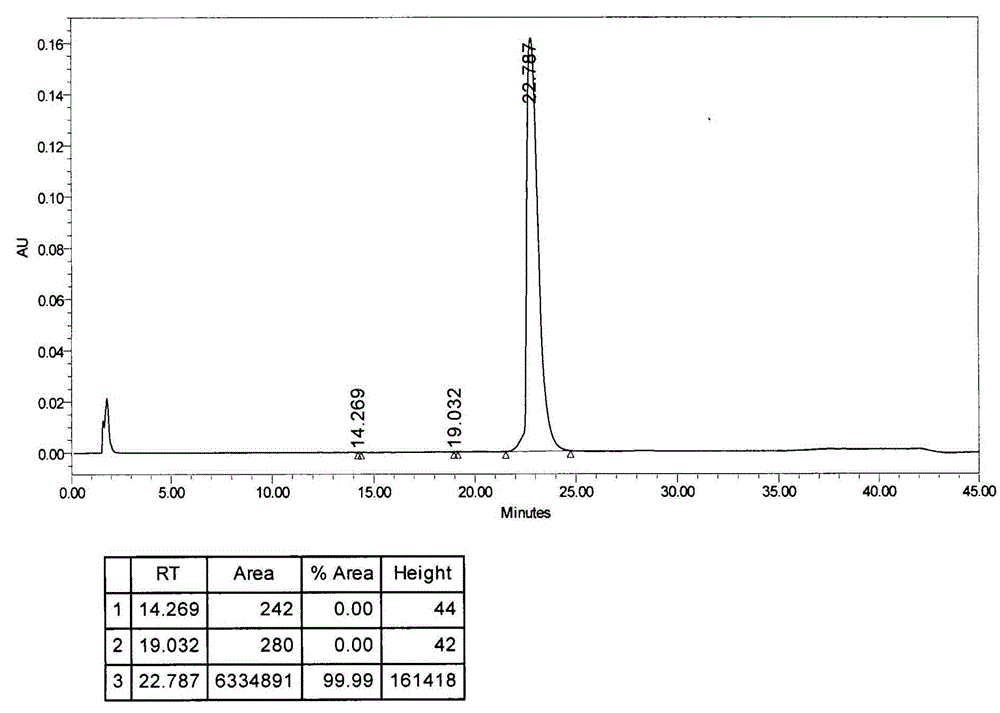
[0040] Put 5 grams of bendamustine hydrochloride crude product in a 250ml three-necked flask, add 9g of dimethyl sulfoxide and 1g of a mixed solvent with a mass fraction of 36% concentrated hydrochloric acid and dissolve it at about 25°C, add 50ml of purified Water, recrystallize at about 10°C, filter to obtain a filter cake, the filter cake is a solid wet material, add 3 times the volume of tetrahydrofuran to the filter cake to make a slurry, filter to obtain the undried product of bendamustine hydrochloride, vacuum -0.09 at 40°C Dry at MPa for 24 hours to obtain 3.5 g of high-purity bendamustine hydrochloride finished product, HPLC (high performance liquid chromatography) content: 99.99%, and the spectrum is as follows figure 2 As shown, impurity I: less than 0.01%, other impurities less than 0.01%, moisture 4.81%.
Embodiment 3
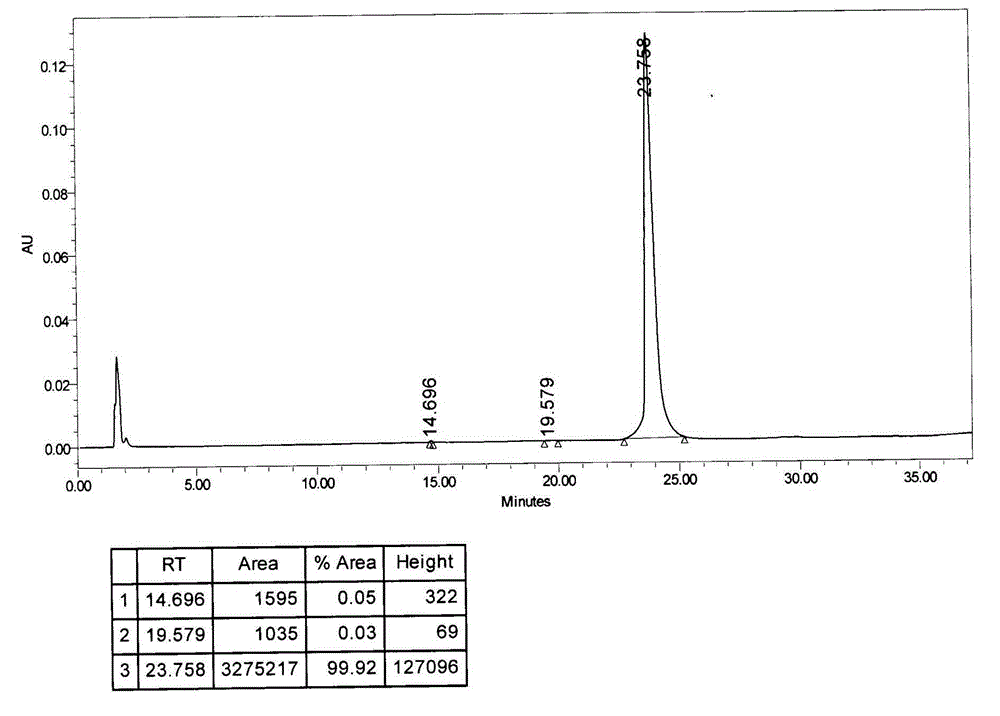
[0042]Put 5 grams of crude bendamustine hydrochloride in a 250ml three-neck flask, add 10g of dimethyl sulfoxide to dissolve at about 40°C, add 50ml of acetone, recrystallize at about 20°C, filter to obtain a filter cake, filter The cake is a solid wet material. Add 5 times the volume of acetonitrile to the filter cake to make a slurry, filter to obtain the undried product of bendamustine hydrochloride, and dry it under vacuum at -0.09MPa at 60°C for 18 hours to obtain a high-purity bendamustine hydrochloride product 4.3g, HPLC (high performance liquid chromatography) content: 99.92%, the map is as follows image 3 As shown, impurity I: 0.05%, other impurities 0.03%, moisture 4.51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com