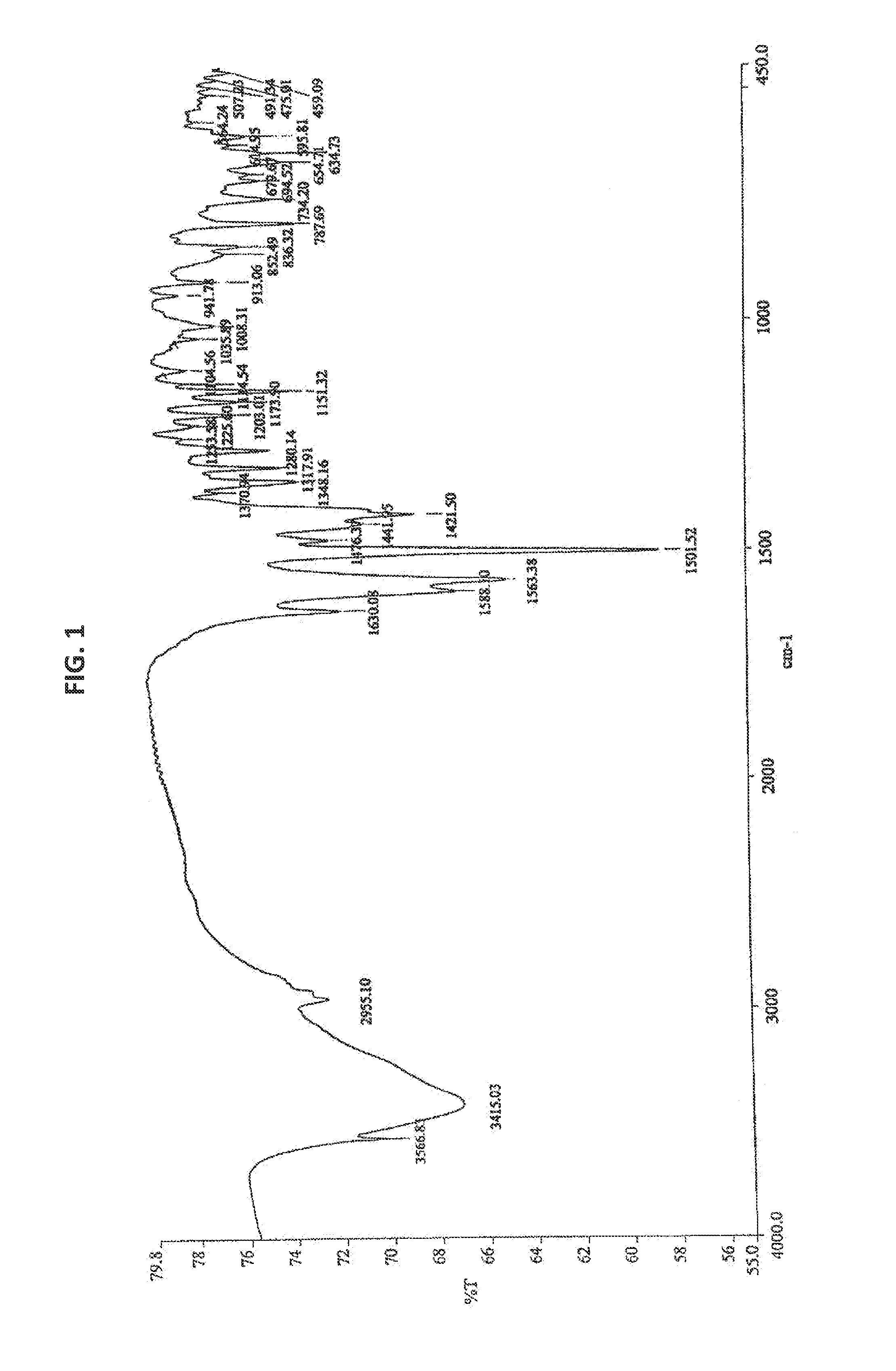
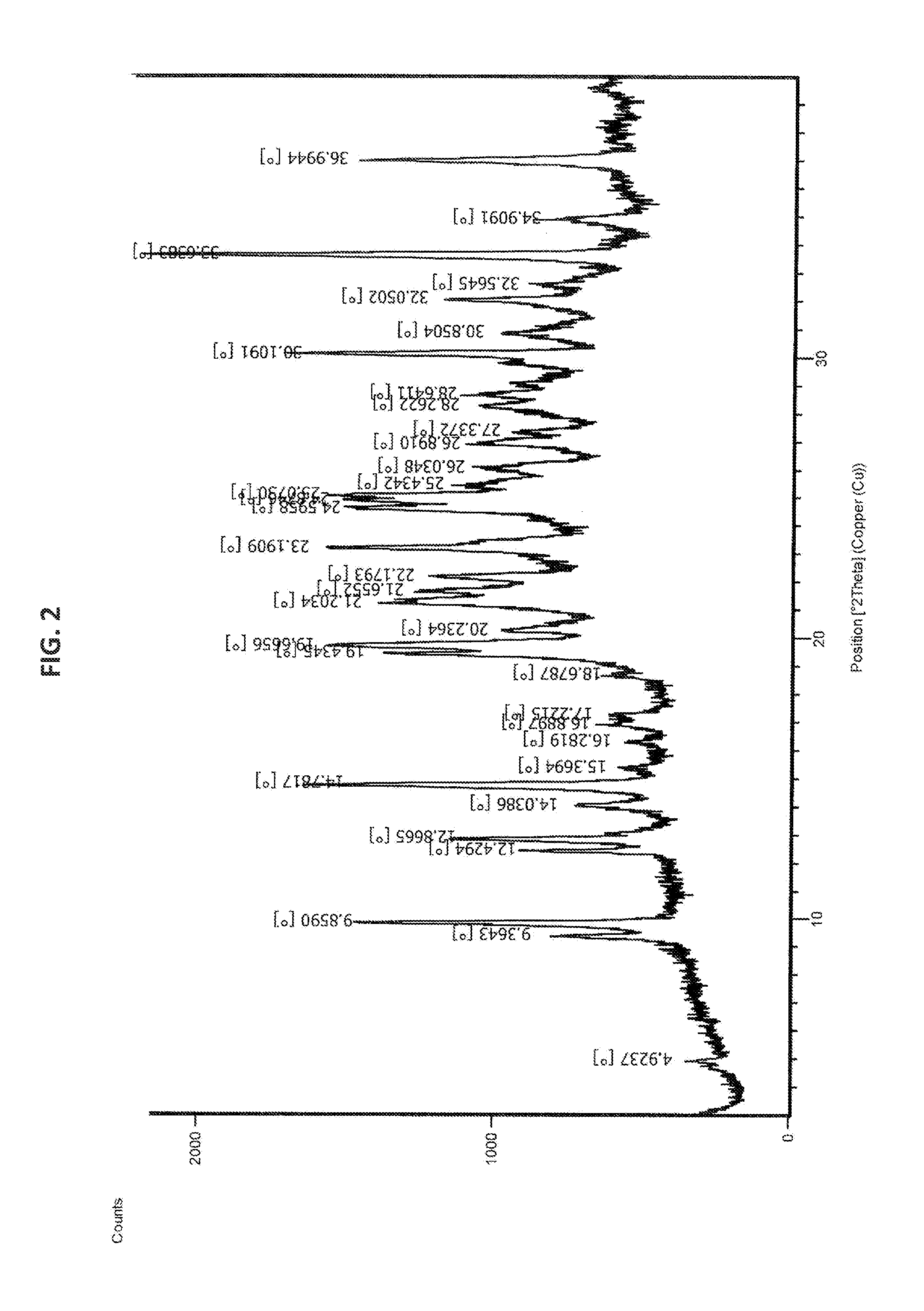
Process for the preparation of bendamustine hydrochloride
a technology of bendamustine and hydrochloride, which is applied in the field of process for the preparation of bendamustine hydrochloride, can solve the problems of increasing the level of various process-related impurities, affecting the economic viability of the process, and difficulty in its preparation and administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example-1
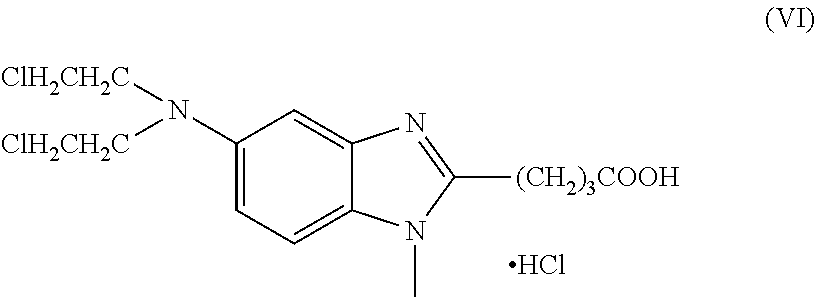
Preparation of Bendamustine Hydrochloride as Per Patent No. DD159877
[0085]Ethyl 4-[1-methyl-5-bis-(2-hydroxyethyl)-amino-benzimidazolyl-2]butanoate (4, 4.305 g) was added to chloroform (36 mL) and agitated till clear solution is formed. The solution was cooled to 0° C. Thionyl chloride (2.175 g) was added to the above solution within 40 minutes maintaining the temperature of the solution to 0-5° C. by cooling. The reaction mixture was agitated at 0-5° C. for 1 hour. The temperature was raised slowly to room temperature by removing cooling within 2.5 to 3 hrs and subsequently agitated at room temperature for 15 to 16 hrs. The solution was dispersed by agitating in 37.5 mL concentrated hydrochloric acid whereby the excessive thionyl chloride was decomposed under increased hydrochloric acid and SO2 development. The chloroform was distilled away and further stirred for 3 hrs at around 95° C. Activated carbon (0.78 g) was added to the solution and stirred for further 30 minutes at around...
example-1
Preparation of Ethyl 4-{5-[bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butanoate (III)
[0086]Ethyl 4-[5-amino-1-methyl-1H-benzimidazol-2-yl)butanoate (II, 40.0 g, 0.153 mol) was added to 2-bromoethanol (80 mL) and agitated for 15-30 minutes. Acetonitrile (80 mL) and calcium carbonate (61.3 g, 0.61 mol) were added to the reaction mixture. The reaction mixture was heated to 80-90° C. within 2 hours and refluxed at 80-90° C. for 34-38 hours. The reaction mixture was cooled to below 70° C. and acetonitrile (80.0 mL) was added. The reaction mixture was further cooled to 20-30° C. and filtered through celite prewashed with acetonitrile. The filtrate was concentrated at 50-60° C. under vacuum till viscous mass is obtained.
[0087]The viscous mass was cooled to 20-30° C. Dichloromethane (320.0 mL) was added to the viscous mass under stirring and washed with potassium carbonate solution (32.0 g in 200 mL water). The organic layer was washed with DM water twice. The organic layer (Di...
example-2
Preparation of Ethyl 4-{5-[bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butanoate (IV)
[0088]4-{5-[bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butanoate (III, 90.0 g, 1.15 mole) was added to dichloromethane (6.24 L) and agitated till clear solution is formed. A solution of thionyl chloride (292.3 g, 2.52 mol) in dichloromethane (1.56 L) was added slowly in 2 to 3 hours. After complete addition of thionyl chloride solution, reaction mixture was refluxed at 35-45° C. for 6 hours. The reaction mixture was cooled to 20-30° C. and 1.95 L dichloromethane was added. Potassium carbonate solution (351.0 g in 1.95 L water) was added to the reaction mixture slowly to control the evaluation of effervescence. The layers were separated. The organic (dichloromethane) layer was washed with brine solution. The organic layer was concentrated at 30-35° C. under vacuum till viscous mass is obtained. The viscous mass was dissolved in acetone (1.95 L) and DM water (1.365 L) was slowl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com