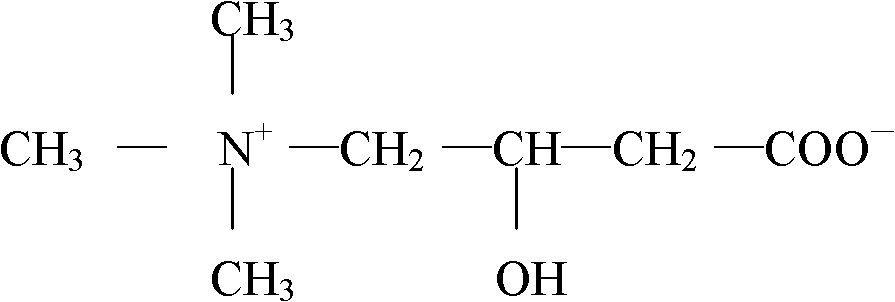
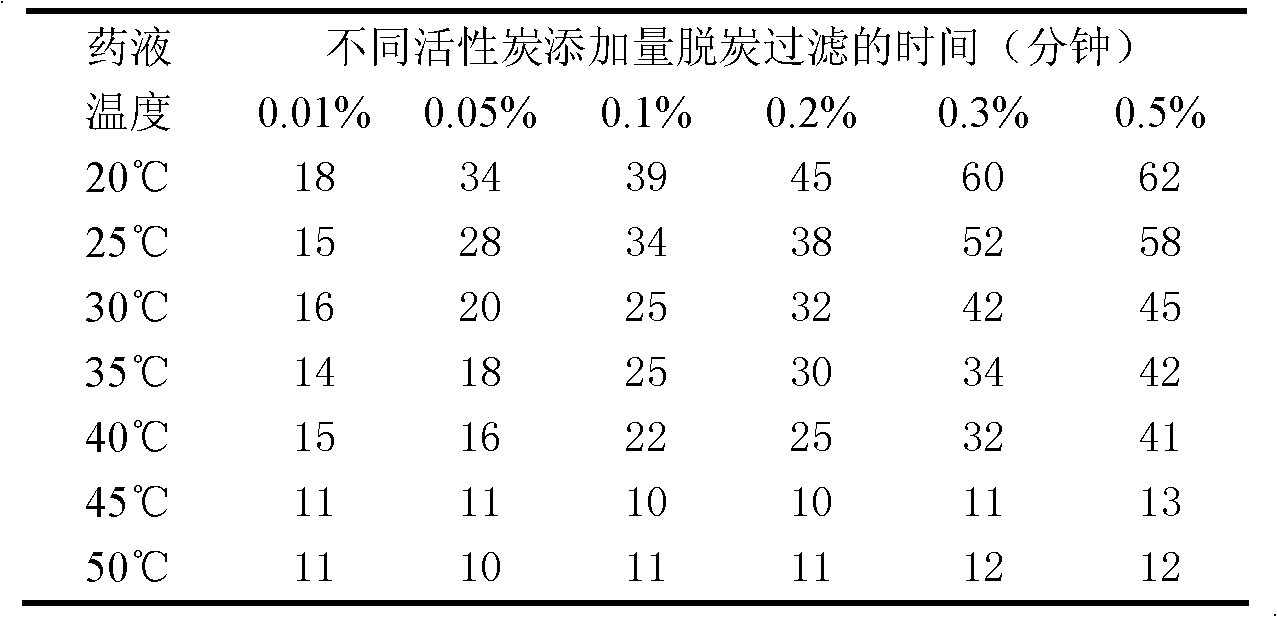
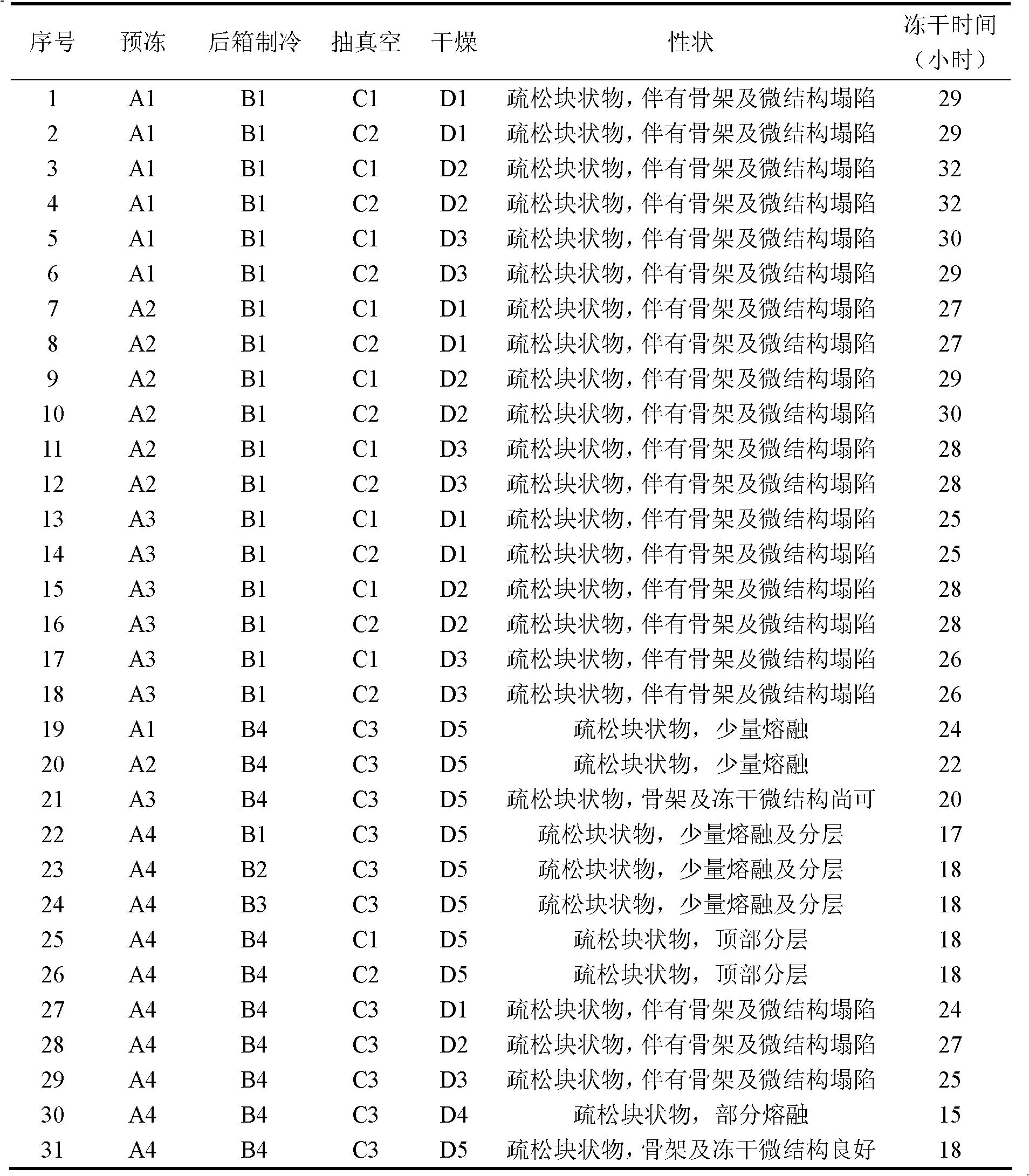
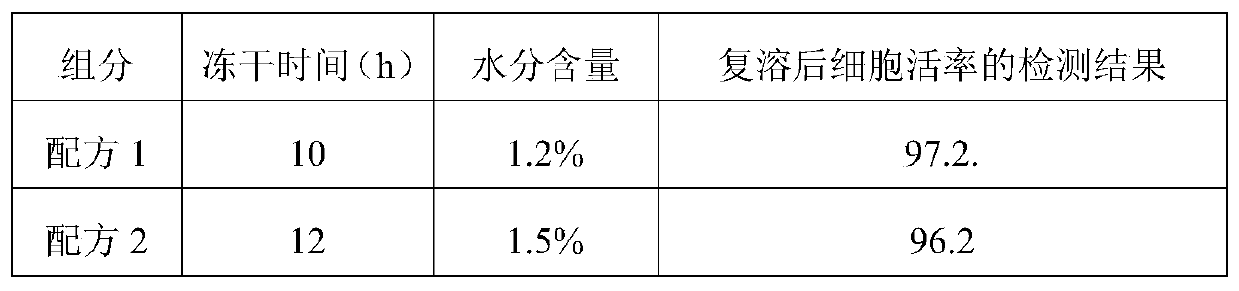
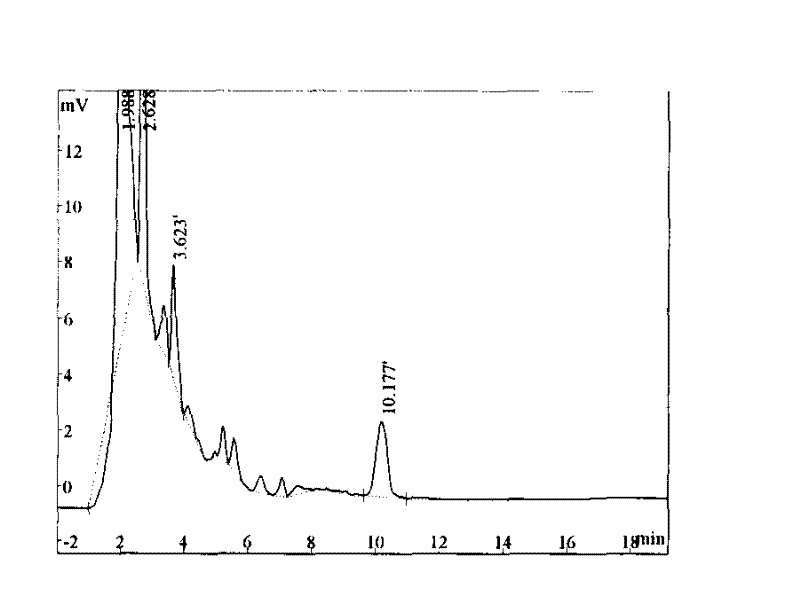
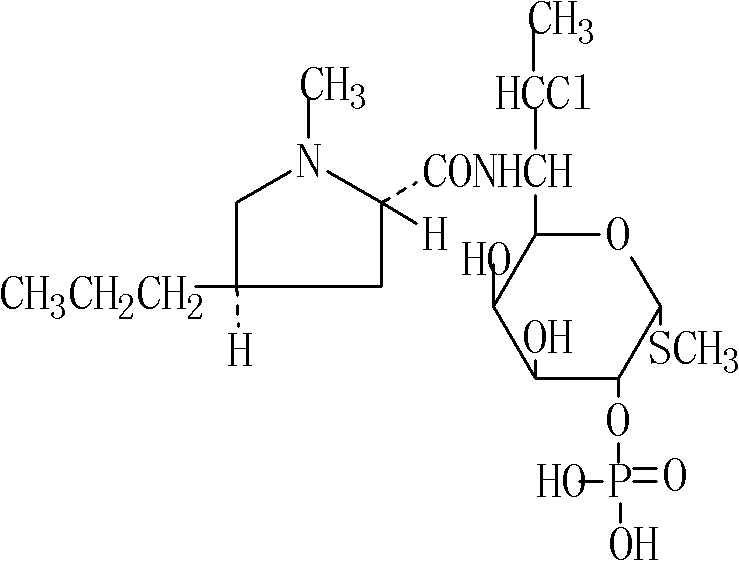
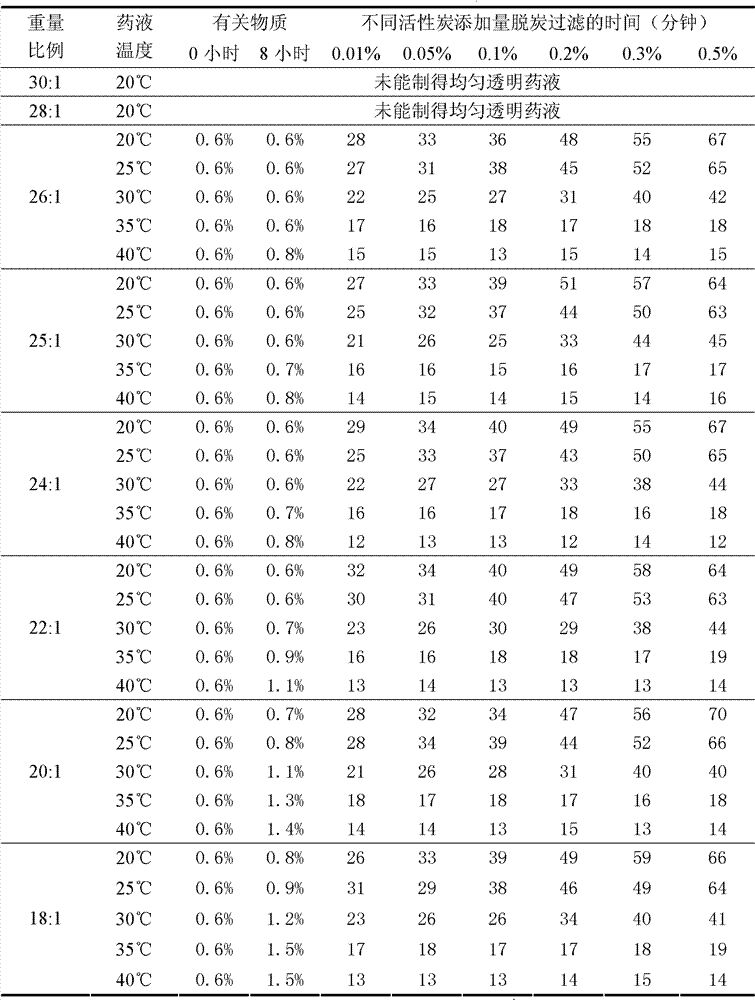
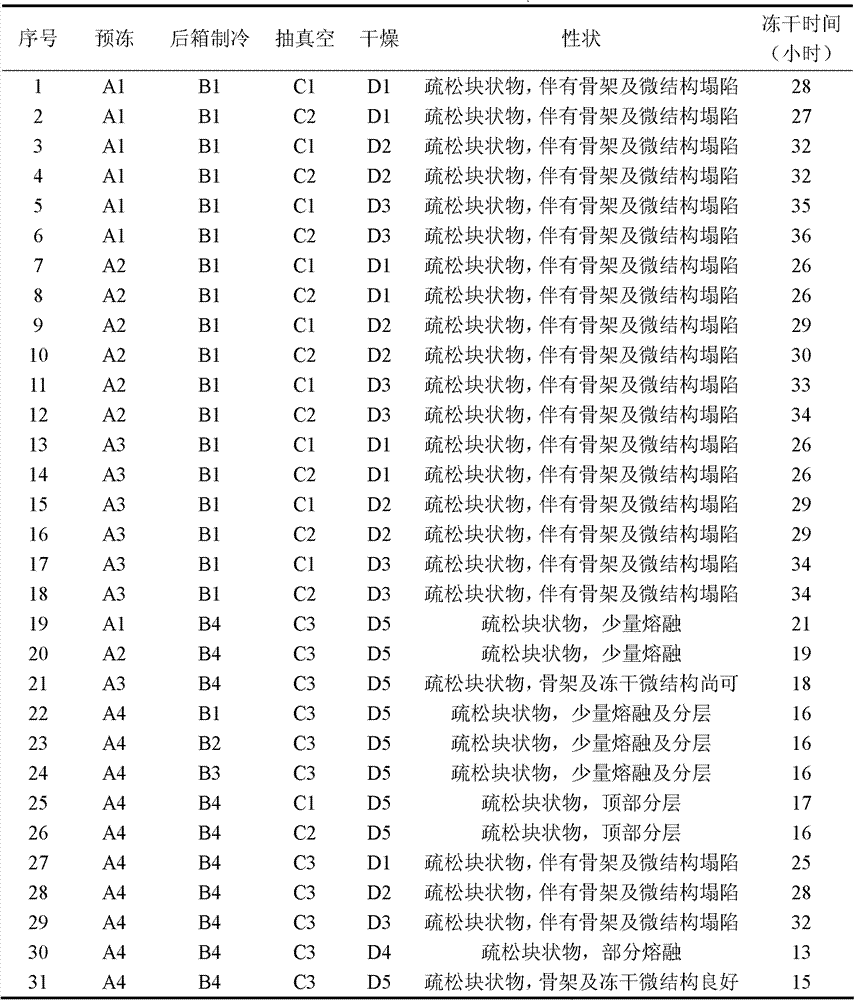
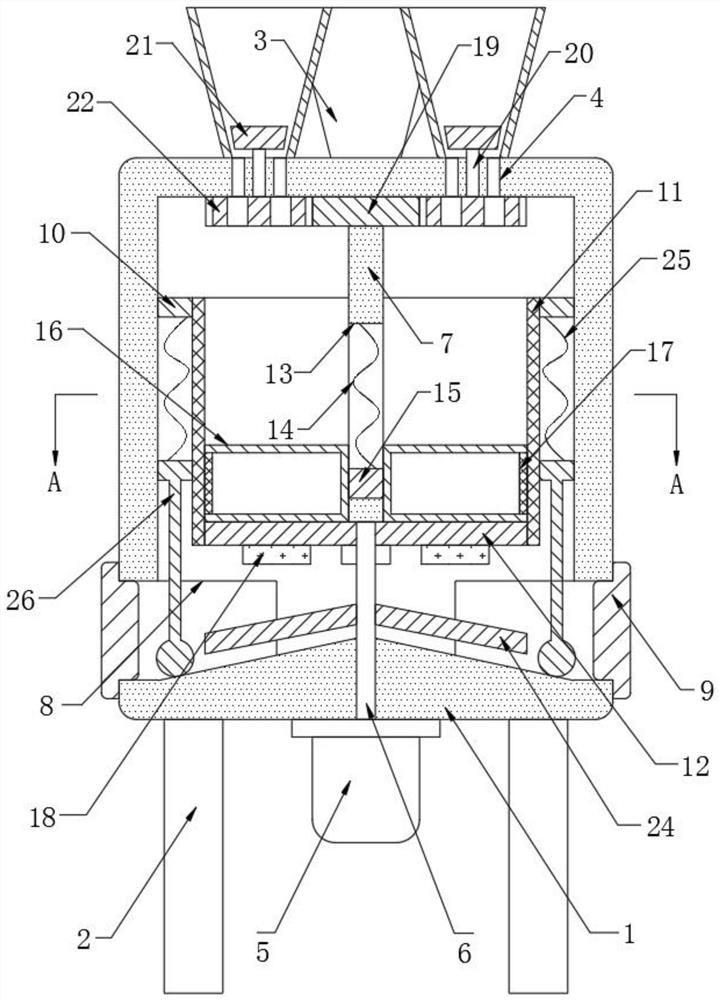
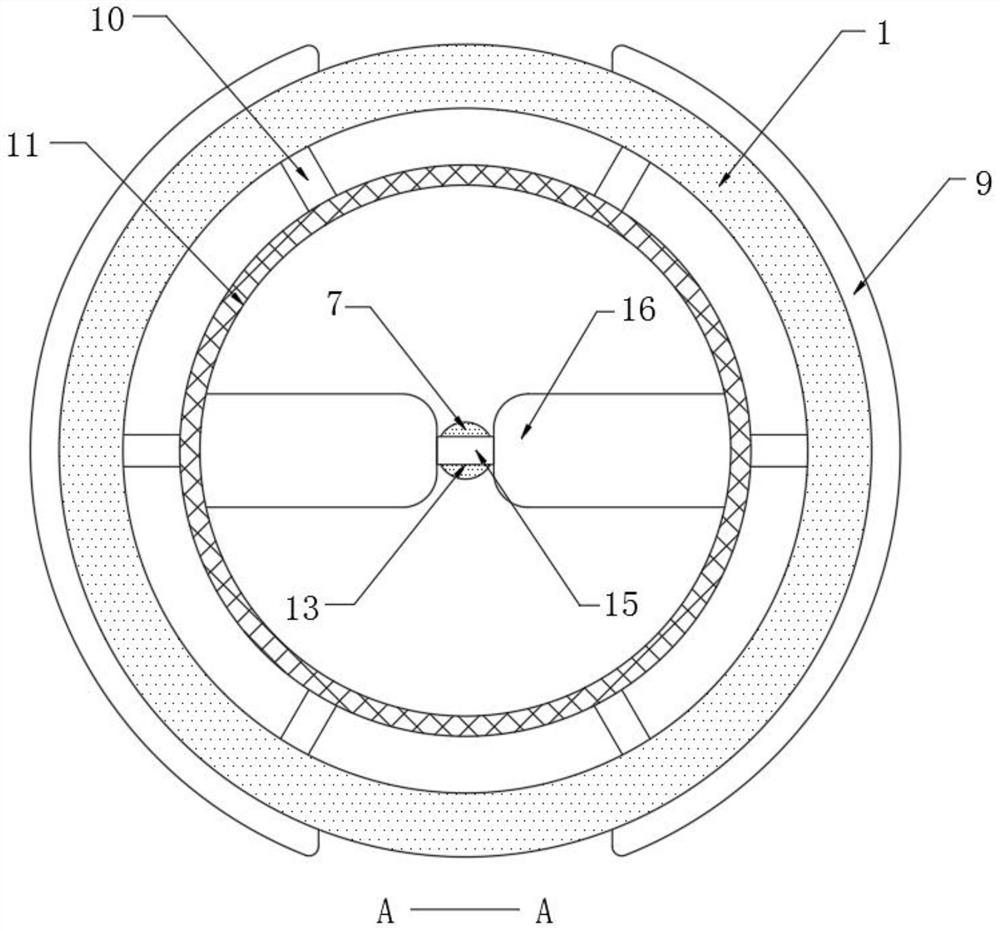
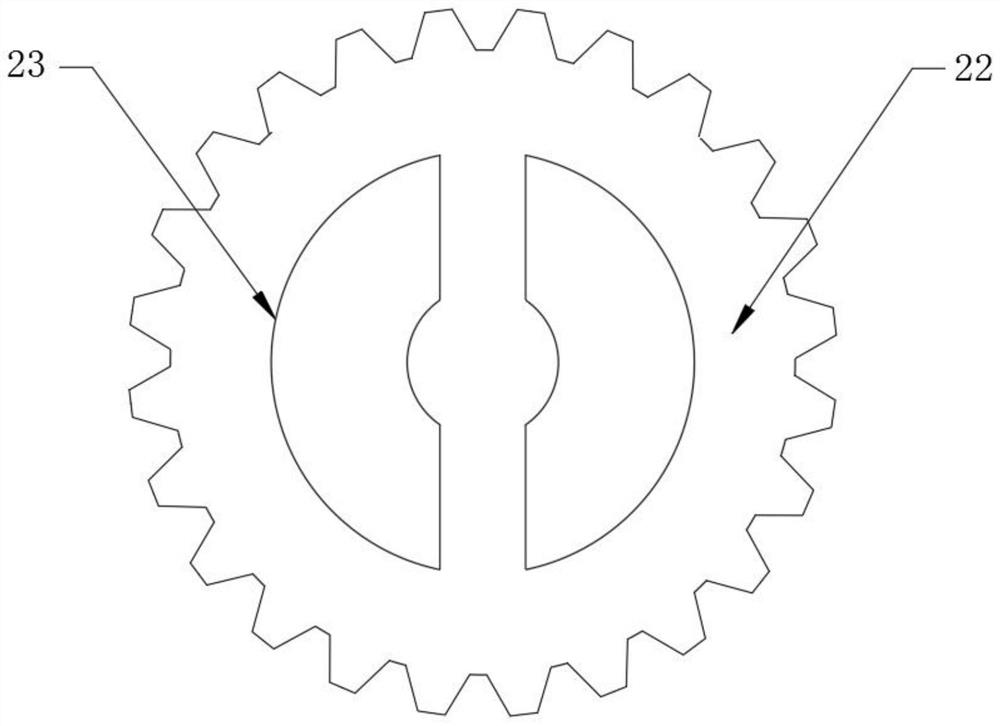
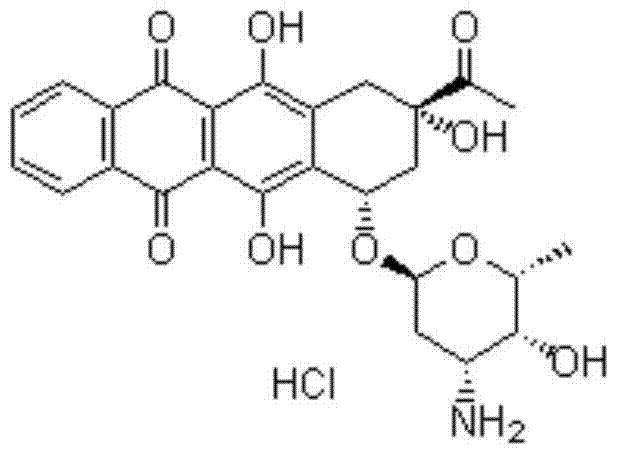
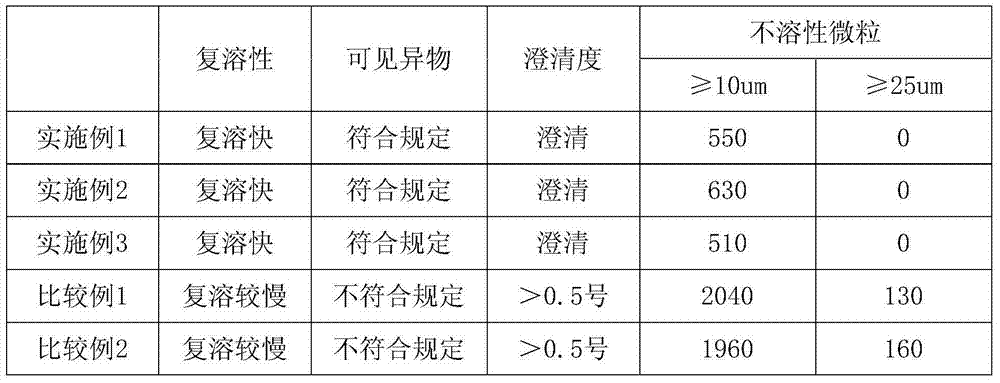
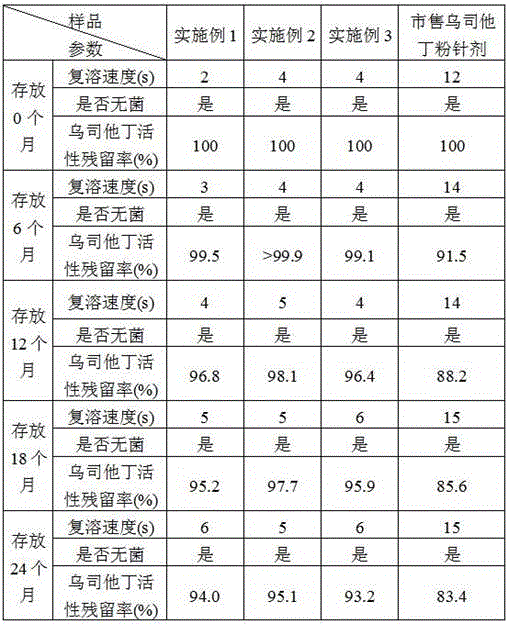
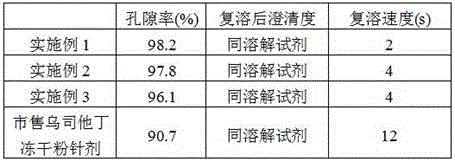
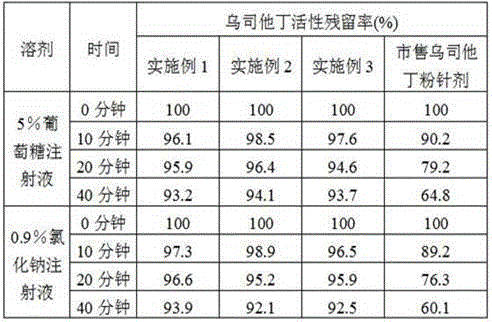
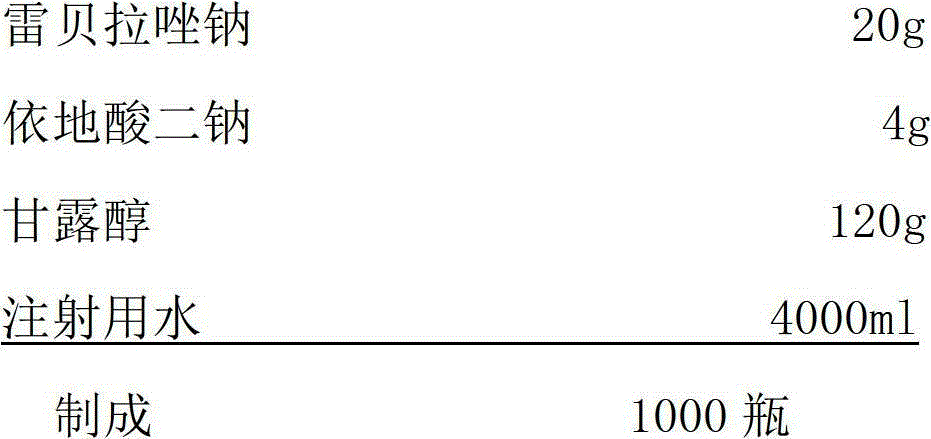Patents
Literature
37results about How to "Improve reconstitution performance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
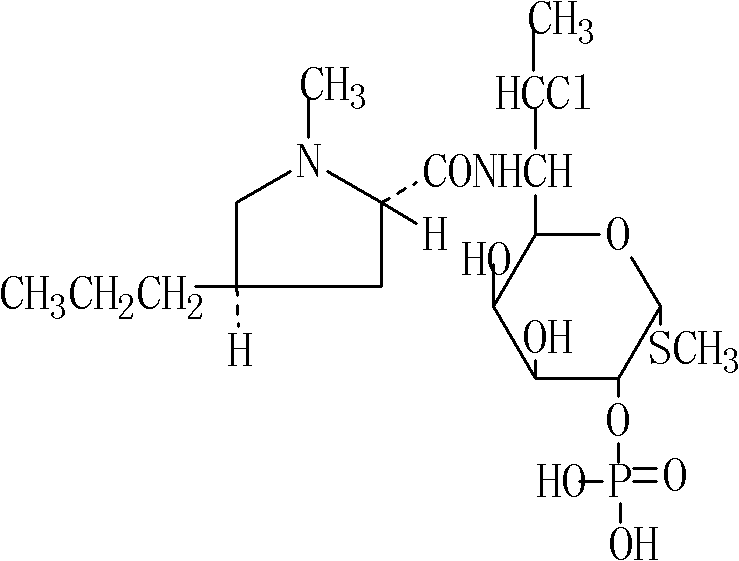
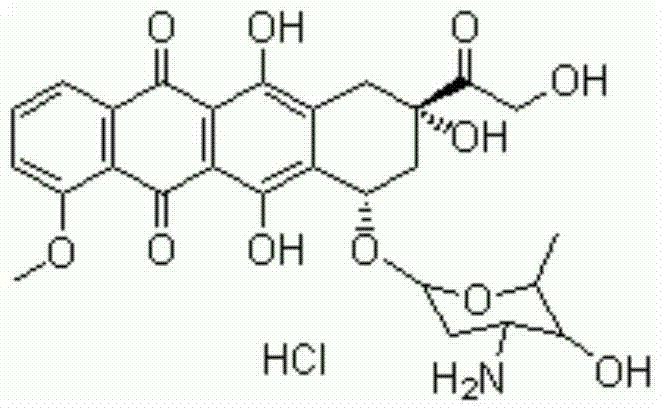
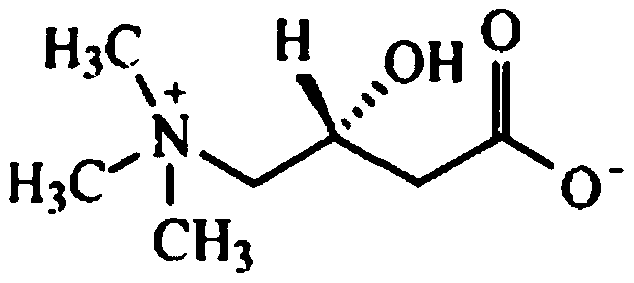
Levocarnitine composition for injection and preparation method of levocarnitine composition
ActiveCN102327238AReduce dosageReduce skeletonOrganic active ingredientsPowder deliveryPorosityFreeze-drying
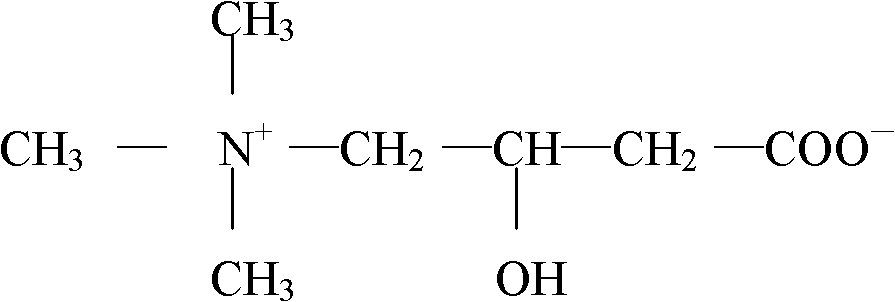
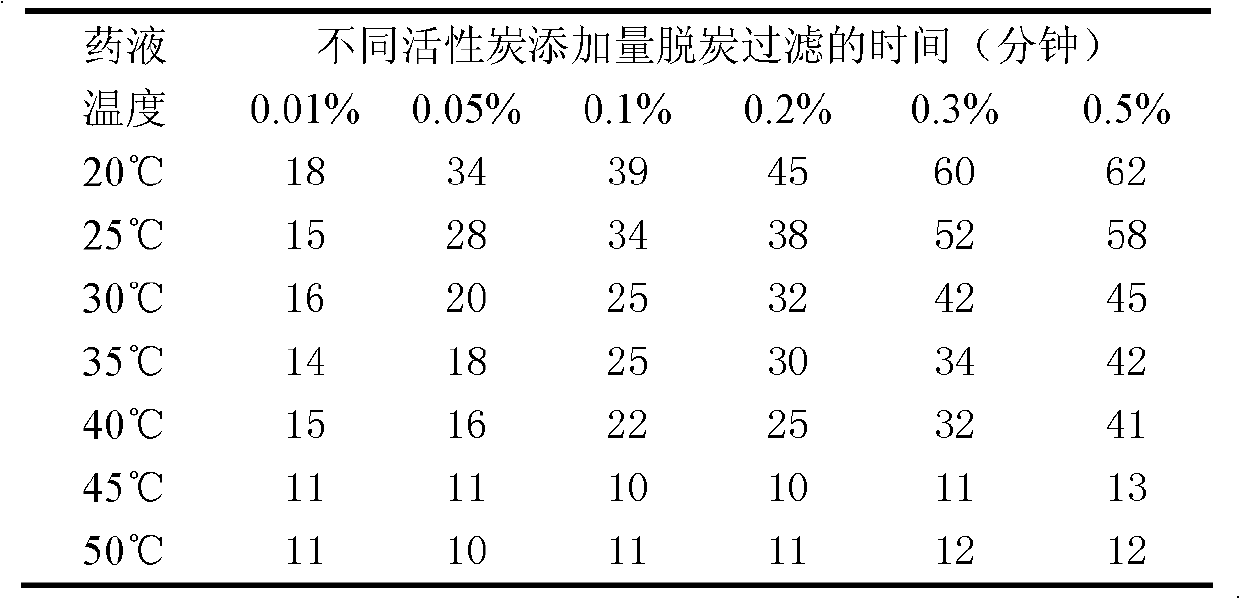
The invention relates to a levocarnitine composition for injection and a preparation method of the levocarnitine composition; the levocarnitine composition is lyophilized powder containing levocarnitine and mannitol, wherein the weight ratio of the levocarnitine to the mannitol is 1:(0.75-1.25); the average particle diameter of the lyophilized powder is 90-130 nm, and the porosity is 94-98%. The preparation method comprises the steps of: 1) preparing: weighing levocarnitine and mannitol, putting the levocarnitine and the mannitol in a preparing tank, adding injection water, agitating to enable the levocarnitine and the mannitol to be completely dissolved and uniformly mixing, regulating the pH to 5.7-6.3 through a 0.1mol / L hydrochloric acid solution; 2) decarburizing and sterile filtering; 3) sterile packaging; 4) vacuum freeze drying to obtain the levocarnitine composition . The levocarnitine composition has the advantages of simple preparation, advanced technique, uniform quality, excellent stability, better redissolution capability and clinic medicine application safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Skin external composition and application thereof
InactiveCN110339147AAvoid harmAvoiding the Potential Hazards of AllergiesCosmetic preparationsToilet preparationsCellular componentCytotoxic substances
The present invention relates to the field of skin repair, and provides a skin external composition. The skin external composition comprises mesenchymal stem cell exosome lyophilized powder, wherein the lyophilized powder contains at least 25.0-35.0 ng / g of fibronectin, 5.0-14.5 ng / g of a vascular endothelial growth factor, 5.5-15.5 ng / g of a fibroblast growth factor, 5.0-10.0 ng / g of an epithelial cell growth factor, 25.0-30.0 ng / g of a hepatocyte growth factor, 7.5-17.5 ng / g of a transforming growth factor, 0.5-5 ng / g of 1-sphingosine phosphate, and 0.5-5 ng / g of ceramide-1-phosphoric acid.The skin external composition provided by the invention comprises the mesenchymal stem cell exosome lyophilized powder having a repairing effect on the skin, does not contain medium components or cellular components, avoids damage to the human body and the skin by adding a cytotoxic substance and avoids the potential hazard of skin allergies. The product is more stable after being prepared as lyophilized powder so as to exert biological activity for a longer period of time.
Owner:浙江梵泊细胞工程有限公司
A kind of clindamycin phosphate composition for injection and preparation method thereof
ActiveCN102258488AExcellent freeze-dried structureExcellent average particle sizeAntibacterial agentsPowder deliveryPorosityClindamycin Phosphate
The invention relates to a clindamycin phosphate composition for injection. The composition is freeze-dried powder which consists of clindamycin phosphate and sodium hydroxide, wherein the weight ratio of the clindamycin phosphate to the sodium hydroxide is (24-26):1; the average grain diameter of the freeze-dried powder is 70-130nm; and the porosity is 92-98 percent. A preparation method of the composition comprises the following steps of: (1) preparing: weighing the clindamycin phosphate and the sodium hydroxide, filling in a preparation tank, adding water for injection, stirring to fully dissolve the clindamycin phosphate and the sodium hydroxide and uniformly mixing; (2) decarbonizing and performing sterile filtration; (3) performing sterile subpackaging; and (4) freeze-drying under vacuum. The composition has the advantages of simple formula, advanced process, uniform quality and superior stability and meanwhile has better redissolving performance and clinical medication safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Zirconium chelate-modified compound alkali soluble emulsion as well as high-resolubility waterborne ink prepared from emulsion and method
The invention discloses zirconium chelate-modified compound alkali soluble emulsion and high-resolubility waterborne ink prepared based on the same. Epoxy propane, diisocyanate, acrylic monomers and corresponding functional monomers are taken as raw materials, high-molecular alkali soluble emulsion with similar bimodal particle size distribution is synthesized by adopting suspension polymerization and ring-opening polymerization principles and serves as a novel waterborne ink link material; the emulsion is high in solid content and high in resolubility, and has water-resistant performance; zirconium chelate is added in a preparation process of the emulsion, and central atoms of zirconium chelate are cross-linked with hydrophilic groups on high-molecular resin, so that water-resistant performance of an emulsion system is further improved under high resolubility; the obtained emulsion is mixed with pigment, an assistant, a co-solvent and the like, the high-resolubility waterborne ink is obtained with a technological method of ball milling and mixing, and the ink is high in resolubility, strong in water resistance and moderate in viscosity and overcomes shortcomings of low resolubility, poor water resistance and the like of existing waterborne ink.
Owner:HUBEI GOLDEN THREE GORPRINTING IND
Pharmaceutical composition comprising lipid-soluble vitamin for injection and preparation method of pharmaceutical composition
ActiveCN103520186AImprove complianceIncrease fat solubilityPowder deliveryMetabolism disorderSolubilityFreeze-drying
The invention relates to a pharmaceutical composition comprising lipid-soluble vitamin for injection, which improves water solubility, and a preparation method of the pharmaceutical composition. The pharmaceutical composition comprises vitamin A palmitate, vitamin D2, vitamin E, vitamin K1, water-soluble carboxylation chitosan and mannite. The preparation method comprises the steps of medicine liquid preparation, activated carbon adsorption, decarburization, filtering sterilization, filling, freeze-drying and the like. The pharmaceutical composition comprising lipid-soluble vitamin for injection utilizes the water-soluble carboxylation chitosan as a solubilizer, and has the advantages of few accessories, good water solubility, stable and controllable quality, high safety, simple preparation method, easiness in industrialization and the like.
Owner:HAISCO PHARMA GRP INC
Octreotide acetate freeze-dried powder injection for injection and preparation method thereof
InactiveCN107510837AImprove stabilityExtended storage timePowder deliveryPeptide/protein ingredientsOctreotide acetateSodium bicarbonate
The invention discloses an octreotide acetate freeze-dried powder injection for injection, which comprises octreotide acetate, mannitol, trehalose, L-cysteine, lactic acid and sodium bicarbonate, wherein the mass ration of the octreotide acetate to the mannitol to the trehalose to the L-cysteine is 1 to (200 to250) to (50 to 100) to (5 to 10). According to the octreotide acetate freeze-dried powder injection for injection provided by the invention, by adding the mannitol, the trehalose and the L-cysteine with a certain ratio to combine a freeze-drying protective additive, the stability of octreotide acetate is greatly improved, and the storage time of the octreotide acetate is prolonged.
Owner:国药集团成都信立邦生物制药有限公司
High-temperature aluminum foil environment-friendly black ink and preparation method thereof
The invention discloses a high-temperature aluminum foil environment-friendly black ink and a preparation method thereof, and belongs to the technical field of ink. The high-temperature aluminum foil environment-friendly black ink is composed of the following components by the mass percentage: 35%-40% of composite resin, 30%-40% of a composite solvent, 15%-25% of a pigment, and 3%-5% of an auxiliary agent. The composite resin can significantly improve the thermal viscosity and resolubility of the aluminum foil ink, has excellent adhesive force on a metal surface, has good film-forming performance, has certain thermal endurance, and can improve flexibility, impact resistance and heat sealing property. The ink prepared by the composite resin enables the adhesive force to reach more than or equal to 98% at the temperature of 80-100 DEG C on an aluminum foil; the composite resin has good compatibility with resin and has good solvent release property, and the problem of solvent residues is greatly solved; non-benzene raw materials are adopted, so that the benzene residues are eliminated completely, and the ink is green and environmentally friendly; and at the same time, the preparation method is sample and feasible, low in preparation cost and easy to popularize and use.
Owner:CHENGDU PRESSTER NEW MATERIALS
Irinotecan hydrochloride pharmaceutical composition and preparation method thereof
InactiveCN103655491AImprove reconstitution performanceUniform and stable qualityOrganic active ingredientsPowder deliveryChemistryIrinotecan Hydrochloride
The invention discloses irinotecan hydrochloride pharmaceutical composition and a preparation method thereof. The composition contains irinotecan hydrochloride and mannitol. The preparation method comprises the following steps: 1), preparation is performed: ambroxol hydrochloride and mannitol are placed into a preparation tank in a weight ratio of 1:(1-20), water for injection is added, and the mixture is stirred until complete dissolution and uniform mixing are achieved; 2), sterile filtration, subpackaging and half stoppering are performed; and 3), vacuum freeze drying is performed to obtain a product. The composition and the preparation method have the advantages that the formula is simple, the technology is advanced, the quality is uniform and stable, moisture drying is thorough, and the redissolution performance is better.
Owner:BEIJING KEYUAN CHUANGXIN TECH
Sodium valproate crystal form as well as preparation method and application thereof
ActiveCN102603510AQuality improvementSignificant effectNervous disorderAnhydride/acid/halide active ingredientsX-rayCurative effect
The invention discloses a sodium valproate crystal form II as well as a preparation method and application thereof, wherein characteristic absorption peaks appear at diffraction angles of (2theta)=6.3-6.5 DEG, 7.12-7.32 DEG, 7,3-7.5 DEG, 16.95-17.15 DEG, 18.15-18.35 DEG, 18.88-19.08 DEG and 19.17-19.37 DEG in a powder x-ray diffraction pattern of the crystal. The sodium valproate crystal form II provided by the invention has the advantages that the hygroscopicity is significantly reduced and lower water content of the product is guaranteed to ensure that the quality of the sodium valproate is more stable in storage period; while the hygroscopicity is reduced, the dissolution time of the product is significantly shortened and the dissolvability of the product is improved to ensure that the curative effect and very good safety of the sodium valproate are guaranteed in clinical application, the injection pain is also significantly relieved, compliance of patients is increased during treatment and very good clinical effect of the treatment is obtained.
Owner:SICHUAN CREDIT CHEMWERTH PHARMACEUTICAL CO LTD
Pharmaceutical composition of fat-soluble vitamin for injection and preparation method thereof
ActiveCN103520186BImprove complianceIncrease fat solubilityPowder deliveryMetabolism disorderSolubilityFreeze-drying
The invention relates to a pharmaceutical composition comprising lipid-soluble vitamin for injection, which improves water solubility, and a preparation method of the pharmaceutical composition. The pharmaceutical composition comprises vitamin A palmitate, vitamin D2, vitamin E, vitamin K1, water-soluble carboxylation chitosan and mannite. The preparation method comprises the steps of medicine liquid preparation, activated carbon adsorption, decarburization, filtering sterilization, filling, freeze-drying and the like. The pharmaceutical composition comprising lipid-soluble vitamin for injection utilizes the water-soluble carboxylation chitosan as a solubilizer, and has the advantages of few accessories, good water solubility, stable and controllable quality, high safety, simple preparation method, easiness in industrialization and the like.
Owner:HAISCO PHARMA GRP INC
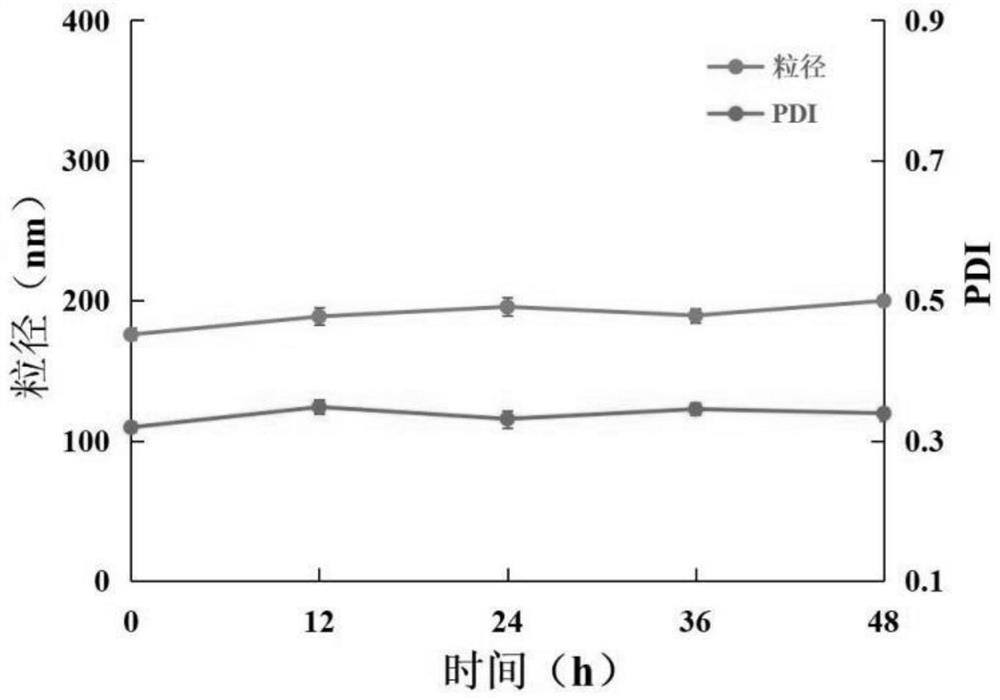
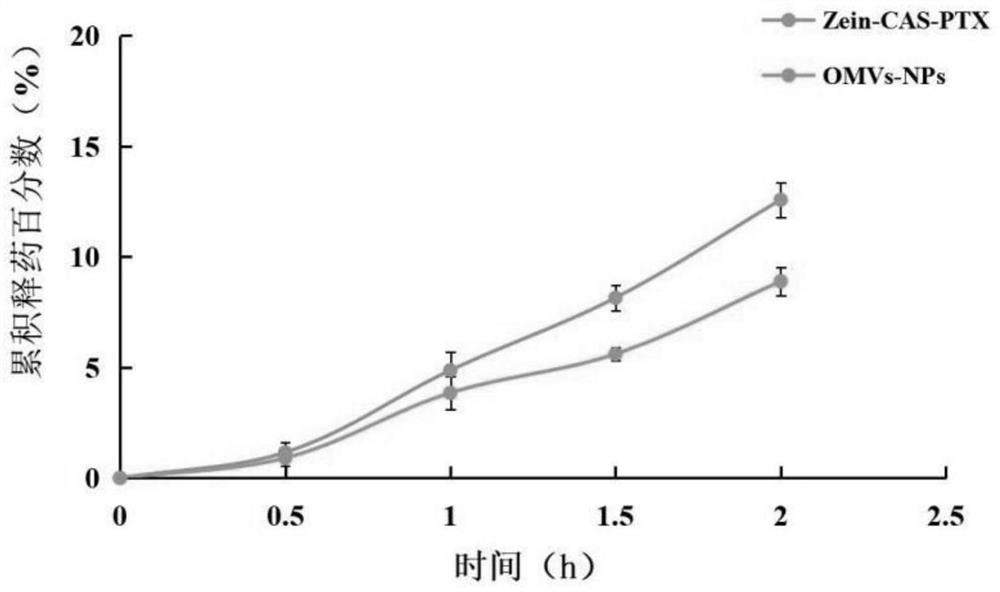
Modified zein nano-drug delivery system wrapped by outer membrane vesicles of bacteria as well as preparation method and application of modified zein nano-drug delivery system
PendingCN114588127AImprove stabilityHighly concentrated particle sizeOrganic active ingredientsMacromolecular non-active ingredientsPharmaceutical drugMicrobiology
The invention discloses a modified zein nano drug delivery system wrapped by bacterial outer membrane vesicles as well as a preparation method and application of the modified zein nano drug delivery system. The medicine is dissolved in absolute ethyl alcohol, deionized water is added after ultrasonic dissolution, zein is added, and ultrasonic treatment is carried out; quickly adding a sodium caseinate solution into the obtained mixed solution while stirring, and stirring to obtain a modified zein drug-loaded nanoparticle solution; the preparation method comprises the following steps: uniformly mixing bacterial outer membrane vesicles with a modified zein drug-loaded nanoparticle solution, extruding the mixed solution for 2-8 times by using high-pressure nitrogen as an extrusion pressure source through a 200nm polycarbonate film, centrifuging the obtained product, and collecting the precipitate, thereby obtaining the bacterial outer membrane vesicle wrapped modified zein drug-loaded nanoparticle system. The nano drug-loading system provided by the invention is narrow in particle size distribution range and good in stability, and can load hydrophobic drugs which can be dissolved in ethanol, so that the solubility of the hydrophobic drugs in water is enhanced, and the stability and bioavailability of the drugs are improved.
Owner:LIAONING UNIVERSITY
Potassium magnesium aspartate freeze-dried powder preparation for injection and preparation method of preparation
ActiveCN103110616ASimple prescriptionFewer ingredient stepsOrganic active ingredientsPowder deliveryFreeze-dryingLactose
The invention relates to potassium magnesium aspartate freeze-dried powder preparation for injection. The potassium magnesium aspartate freeze-dried powder preparation for injection is prepared from the following materials in parts by weight: 400-600 parts of potassium aspartate, 400-600 parts of magnesium aspartate and 320-480 parts of lactose. The freeze-dried powder preparation disclosed by the invention has the advantages of being simple in prescription and scientific and reasonable in preparation method, so that the freeze-dried product is more fluffy and porous, thereby remarkably improving the re-dissolution of the freeze-dried powder preparation during the clinical use process; meanwhile, the stability of the preparation is further improved on the condition of low auxiliary material dosage and less categories, so that the medication safety of the patient is ensured.
Owner:SHANXI PUDE PHARMA CO LTD
Doxorubicin hydrochloride pharmaceutical composition and preparation method thereof
InactiveCN103655588AReduce or even eliminate the phenomenon of pumping awayEnsure safetyOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLMedicine
The invention discloses doxorubicin hydrochloride pharmaceutical composition and a preparation method thereof. The composition contains doxorubicin hydrochloride and mannitol. The preparation method comprises the following steps: 1), preparation: the ambroxol hydrochloride and the mannitol at a weight ratio of 1:(1-20) are placed into a preparation tank, water for injection is added, and the mixture is stirred until the mixture is completely dissolved and uniformly mixed 2), sterile filtration, subpackaging and half stoppering; and 3), vacuum freeze drying, thereby obtaining the composition. The composition and the preparation method have the advantages that the formula is simple, the technology is advanced, the quality is uniform and stable, moisture drying is thorough, and the redissolution performance is better.
Owner:BEIJING KEYUAN CHUANGXIN TECH
High-adhesion ink for water-based gravure plastic films and preparation method of high-adhesion ink
The invention relates to the technical field of printing ink, and discloses high-adhesion ink for water-based gravure plastic films. The high-adhesion ink is prepared from the following components inparts by weight: 50 to 70 parts of water-based acrylic resin, 10 to 15 parts of a coloring agent, 15 to 25 parts of a solvent and 5 to 6 parts of auxiliary agents, wherein the base materials of the water-based acrylic resin are dimethyl ester, butyl acrylate and acrylic acid. The preparation method comprises the following steps: weighing the raw materials according to mass parts; then mixing the water-based acrylic resin, the coloring agent, the solvent and the defoaming agent by stirring, and then grinding the mixture until fineness of the raw materials is 15 microns or lower; then adding auxiliary agents except the defoaming agent, and carrying out uniform mixing and stirring again until the viscosity reaches 24-28 s / 25 DEG C of Zahn 4# cup; and finally carrying out filtration and packaging. The ink is safe and environmentally friendly, and is low in volatile organic compound (VOC) content and small in smell; flowability is good; a printed product is high in color concentration, excellent in color and luster and good in adhesion fastness; the printed product has good anti-blocking, anti-migration and anti-friction properties; and the high-adhesion ink can replace solvent-based ink for gravure plastic films.
Owner:佛山市红树水性印涂材料有限公司
Compound lumbricus extract, and preparation process and composition thereof
ActiveCN102309538AReduce moisture contentLoose textureAnthropod material medical ingredientsCardiovascular disorderReflux extractionLumbricus
The invention relates to a compound lumbricus extract, and a preparation process and a composition thereof. The compound lumbricus extract comprises, by weight, 25 parts of a fresh product of lumbricus, 3.5 parts of Ligusticum wallichii, 3 parts of Radix Astragali and 2 parts of achyranthes root. The preparation process comprises the following steps: 1) preparing lumbricus powder, that is, taking 25 parts of the fresh product of lumbricus, cleaning and draining the fresh product of lumbricus, adding water with a weight equal to that of the fresh product of lumbricus, carrying out stirring and homogenization at a temperature of 37 DEG C, carrying out centrifugation, taking a supernatant for membrane filtration, condensing obtained filtrate, and carrying out freeze drying on obtained concentrate; 2) preparing extracts of Ligusticum wallichii, achyranthes root and Radix Astragali, that is, taking Ligusticum wallichii, achyranthes root and Radix Astragali by above-mentioned corresponding weight percentage, adding 6 to 10 times of an ethanol solution with a concentration of 50 to 90%, carrying out reflux extraction twice, each lasting 2 hours, carrying out filtration, merging alcohol liquid, recovering the alcohol liquid, condensing the alcohol liquid until relative density of the alcohol liquid is about 1.1 to 1.5, and drying the alcohol liquid; 3) preparing the compound lumbricus extract, that is, uniformly mixing the lumbricus powder prepared in step 1) and extracts of Ligusticum wallichii, achyranthes root and Radix Astragali prepared in step 2).
Owner:南京易亨制药有限公司
Composite ink and preparation method thereof
The invention relates to a composite ink and a preparation method thereof. The composite ink is prepared by mass percentages, and comprises: polyurethane resin ingredient A, urethane resin ingredient B and cross-linking auxiliaries, wherein the polyurethane resin ingredient A is prepared from, 10-30% of no benzene, no ketone-polyurethane resin, 1-10% of UK resin, 1-20% of pigments, 0.5-5% of wax powder, 10-30% of propyl acetate, 1-20% of propyl alcohol; the urethane resin ingredient B is prepared from, 1-20% of no benzene, no ketone-polyurethane resin, 5-25% of ethyl acetate, 1-10% of anhydrous ethanol and 1-6% of distilled water mixture; the cross-linking auxiliaries include 0.5-5% of cross-linking auxiliaries. The main materials of the composite ink adopt the no benzene, no ketone-polyurethane resin which is tested to find no halogen or heavy metal, so as to meet the environmental protection requirements, thereby not polluting the environment in the process of the terminal treatment. Since the no benzene, no ketone-polyurethane resin has good solubility, when the composite ink is diluted by 10%, the printing can reach the concentration of other existing ink, so that the transfer rate of the customer printing is increased by 10%, and the ink redissolving property can be fully improved.
Owner:张春明
Clindamycin phosphate composition for injection and preparation method thereof
ActiveCN102258488BHigh porosityImprove reconstitution performancePowder deliveryLyophilised deliveryPorosityFreeze-drying
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Mulberry leaf black tea solid beverage preparation method and processing equipment
PendingCN113796439AImprove coldnessImprove bitternessRotary stirring mixersTea substituesFreeze-dryingGlycemic
The present invention discloses a mulberry leaf black tea solid beverage preparation method and processing equipment. The mulberry leaf black tea solid beverage preparation method mainly comprises the following processing steps of freeze drying, mechanical crushing, constant temperature extraction, low temperature concentration, secondary freeze drying, blending and mixing, wet granulation, and drying and packaging. The processing equipment comprises a processing box, a plurality of supports are fixed to the bottom of the processing box, a plurality of feeding barrels are fixed to the upper end of the processing box, a plurality of feeding openings correspondingly communicating with the feeding barrels are formed in the top of the processing box in a penetrating mode, and a plurality of pre-grinding mechanisms are arranged at the inner top of the processing box. The mulberry leaf black tea solid beverage preparation method has the advantages that the prepared mulberry leaf black tea solid beverage is good in taste, good in resolubility, small in size, convenient to carry, instant to drink and capable of being drunk after being brewed with warm water, the condition restriction problem that mulberry leaf black tea can be drunk after being brewed with hot water is solved, and the mulberry leaf black tea solid beverage can serve as an auxiliary hypoglycemic dietary therapy product to be used for daily postprandial blood sugar control of diabetics.
Owner:安庆麦宝瑞生物科技有限公司
Idarubicin hydrochloride pharmaceutical composition and preparation method thereof
InactiveCN103655490AImprove stabilityStable and uniform qualityOrganic active ingredientsPowder deliveryFreeze-dryingIdarubicin Hydrochloride
The invention discloses idarubicin hydrochloride pharmaceutical composition and a preparation method thereof. The composition contains idarubicin hydrochloride and mannitol. The preparation method comprises the following steps: 1), preparation is performed: ambroxol hydrochloride and mannitol are placed into a preparation tank in a weight ratio of 1:(1-20), water for injection is added, and the mixture is stirred to be completely dissolved and uniformly mixed; 2), sterile filtration, subpackaging and half stoppering are performed; and 3), vacuum freeze drying is performed to obtain a product. The composition and the preparation method have the advantages that the formula is simple, the technology is advanced, the quality is uniform and stable, moisture is thoroughly dried, and the redissolution performance is better.
Owner:BEIJING KEYUAN CHUANGXIN TECH
Tyvek water-based flexographic printing material and preparation method thereof
InactiveCN112796125AImprove reconstitution performanceIncrease drying speedDyeing processAcrylic resinSolvent based
The invention is suitable for the technical field of printing material preparation, and provides a Tyvek water-based flexographic printing material. The Tyvek water-based flexographic printing material comprises the following raw material components in percentage by weight of 55-65% of a diluent which comprises the following raw material components in percentage by weight of 60-80% of polyurethane resin, 0-35% of acrylic resin, 1-5% of an adhesion promoter, 1-4% of a wear-resistant agent, 0-1% of a wetting agent, 0-8% of a stabilizing agent, 0.1-0.5% of a defoaming agent, 30-40% of deionized water, and 35-45% of water-based color paste. Therefore, when a flexographic printing machine is used for printing a Tyvek material, the printing material has the characteristics of clear printed patterns, good adhesive force, high drying speed, low odor and capability of meeting the latest environmental protection requirement, and can completely replace the existing high-VOC solvent type printing ink.
Owner:山东德创精化科技有限公司
A kind of ulinastatin freeze-dried powder preparation and preparation method thereof
ActiveCN105596302BAvoid failureGood resolubilityPowder deliveryNervous disorderSolubilityMANNITOL/SORBITOL
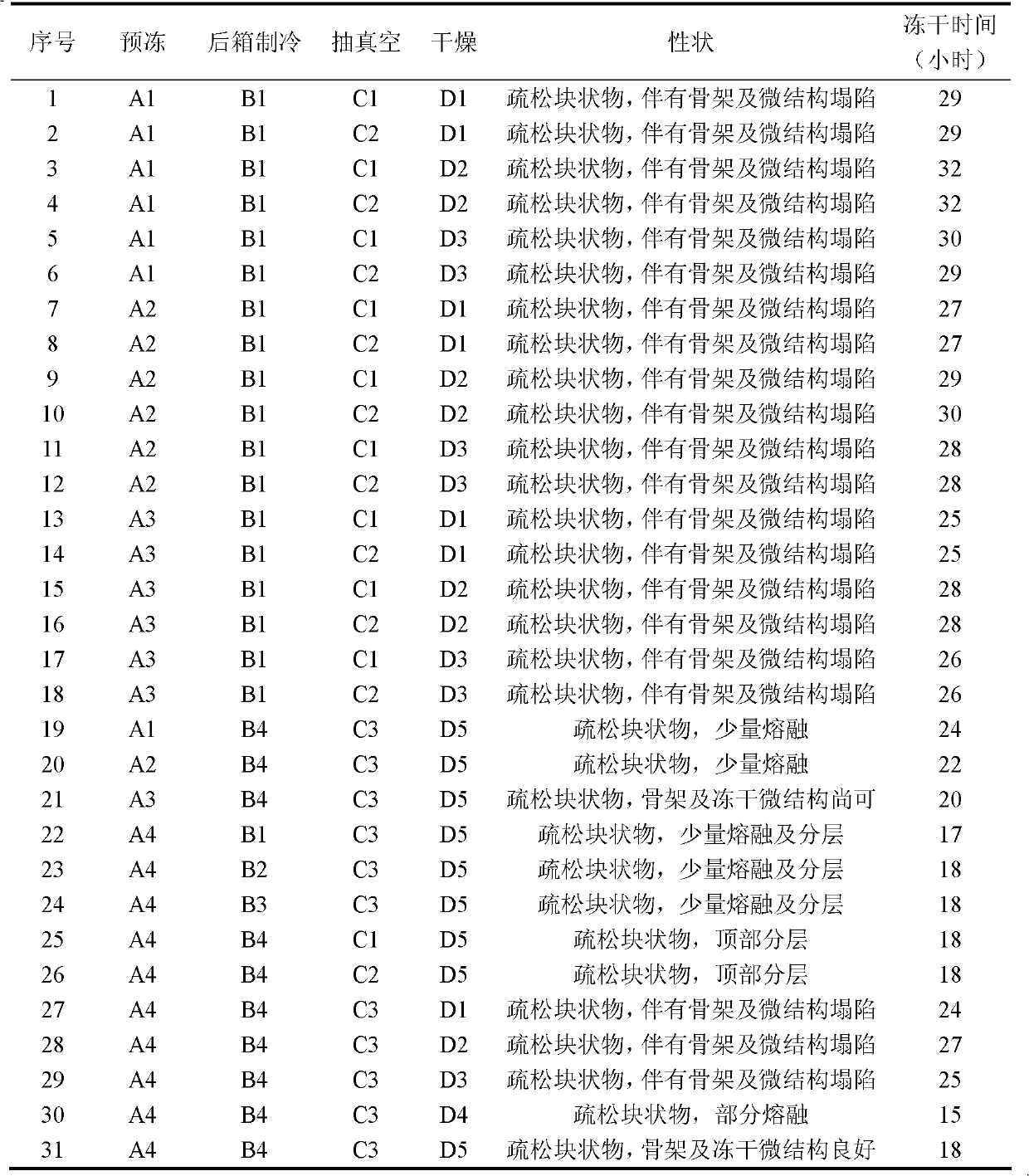
The invention relates to an ulinastatin freeze-dried powder preparation and a preparation method thereof. Each 1,000 ulinastatin freeze-dried powder preparations are prepared from 25,000,000-100,000,000 units of ulinastatin, 1-30g of mannitol, 0-10g of sodium chloride, 0-5g of sodium hydrogen phosphate and 0-5g of sodium dihydrogen phosphate. The preparation method of the ulinastatin freeze-dried powder preparation provided by the invention has the characteristics that the ulinastatin and auxiliary materials are separately dissolved, and the active component ulinastatin is prepared when used; a freeze-dried former solution is prepared in a low-temperature environment of 2-4 DEG C, and the temperature range is kept in the overall configuration process, so that the stability of the ulinastatin is ensured; and a 'fast-slow-fast' three-step pre-freezing method is adopted in the freeze-drying pre-freezing process, so that the drying rate is improved, and the solubility is improved. The ulinastatin freeze-dried powder preparation prepared by the formula and the preparation method is relatively excellent in solubility and stability.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
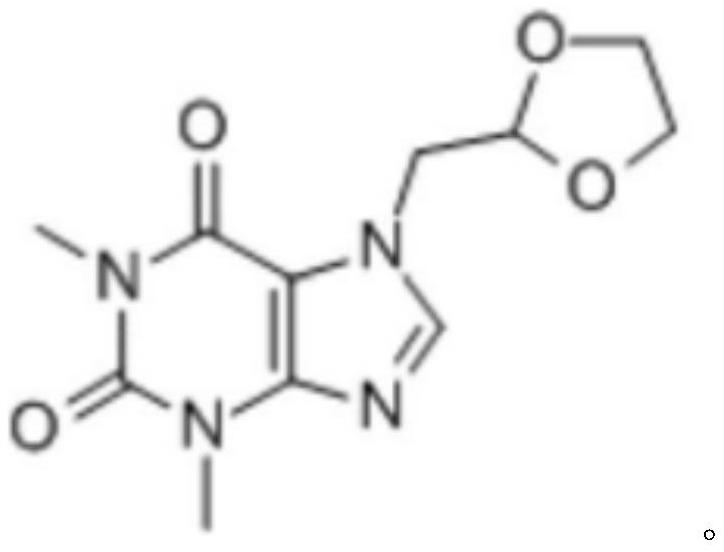
Doxofylline injection and preparation method thereof
PendingCN111840235AImprove uniformityImprove reconstitution performancePowder deliveryPharmaceutical non-active ingredientsDoxofyllineDrugs preparations
The invention discloses a doxofylline injection and a preparation method thereof, and relates to the field of pharmaceutical preparations. The doxofylline injection is prepared from the following components in parts by weight: 0.8 to 1.2 parts of doxofylline, 2.5 to 3.5 parts of mannitol and 40 to 60 parts of water for injection, and the doxofylline injection is produced by adopting an injection bottle with the specification of 10mL. The doxofylline injection has the advantages that by improving the process, especially the freeze-drying process, the bottle breaking number is reduced, the lossis reduced, meanwhile, the uniformity, the redissolving property, the stability and other properties of the doxofylline injection are also improved by improving the freeze-drying process, and other auxiliary agents do not need to be added. In addition, a glass tube injection bottle with the specification of 10mL is adopted for production, so that the income is greatly increased.
Owner:武汉人福药业有限责任公司
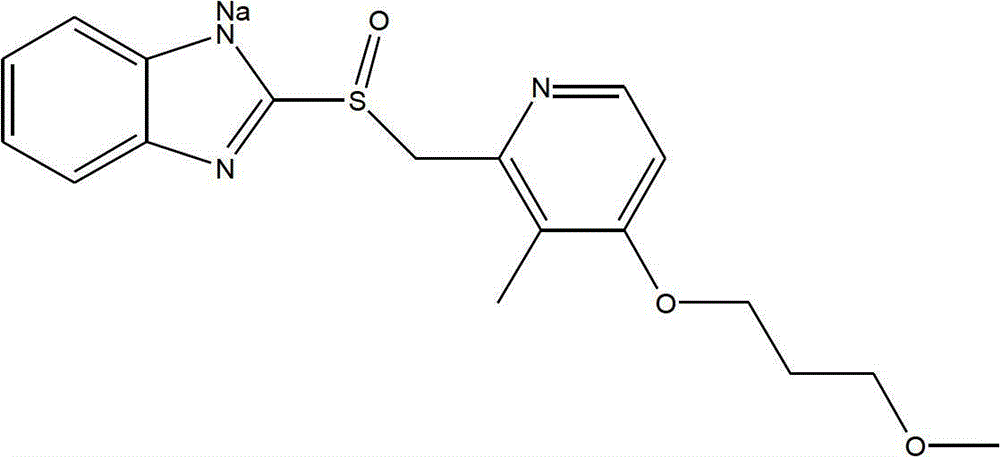
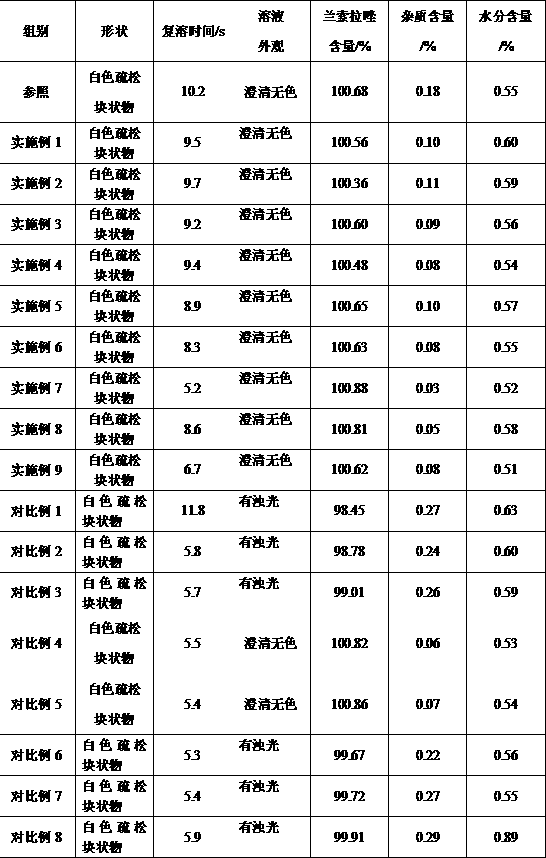
Sodium rabeprazole composition for injection
ActiveCN102949354BIncrease moisture contentAvoid degradationAntibacterial agentsPowder deliveryFreeze-dryingDisodium Edetate
The present invention provides a sodium rabeprazole composition for injection. The composition comprises sodium rabeprazole and disodium edetate, wherein a weight ratio of the sodium rabeprazole to the disodium edentate is 1:0.15-1. The sodium rabeprazole composition does not contain mannitol and other fillers. According to the sodium rabeprazole composition, under a certain premise, freeze drying rate of the product is significantly improved so as to avoid additional sublimation time, such that production efficiency is improved, and production energy consumption is reduced, wherein the premise comprises that a product appearance is ensured and reconstituting property meets injection requirements. In addition, the method is suitable for industrial mass production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
A kind of lansoprazole freeze-dried agent for injection and preparation method thereof
ActiveCN110538155BImprove resolubility and stabilityImprove stabilityOrganic active ingredientsPowder deliveryFreeze dryInfusion solution
The present invention discloses a lansoprazole freeze-dried agent for injection. The lansoprazole freeze-dried agent comprises the following components: lansoprazole, pullulan, histidine and hydroxypropyl-beta-cyclodextrin. A preparation method comprises the following steps: S1, preparing a solution: adding water for injection into lansoprazole, pullulan, histidine and hydroxypropyl-beta-cyclodextrin according to a formula amount, and after stirring and dissolving, adjusting a pH value by using a sodium hydroxide solution to obtain a dissolved solution; S2, conducting sterilizing and filtering: filtering and sterilizing the dissolved solution obtained in the step S1 to obtain sterile solution; S3, conducting filling and semi-plug-pressing; S4, conducting freeze-drying: freeze-drying the sample solution obtained in the step S3 to obtain a freeze-dried sample; and S5, conducting plug-pressing and capping. The prepared lansoprazole freeze-dried agent for injection fully exerts synergisticinteraction effects among the raw materials, improves stability and re-solubility of the lansoprazole, and improves stability of infusion compatibility in clinical use.
Owner:双鹤药业(海南)有限责任公司
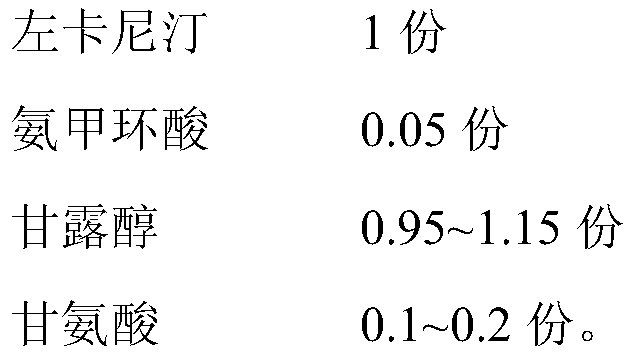
Levocarnitine composition for injection and its preparation method
ActiveCN106265544BImprove freeze-drying efficiencyReduce adverse effectsOrganic active ingredientsPowder deliveryPorosityGlycine
The invention belongs to the technical field of medicine and particularly relates to a levocarnitine composition for injection and a preparation method thereof. The levocarnitine composition for injection comprises levocarnitine, tranexamic acid, mannitol and glycine. The levocarnitine composition for injection has the remarkably-excellent freeze-drying structure, average grain diameter and porosity, the redissolution performance of the levocarnitine composition for injection can be effectively improved, and the stability and consistency of the performance within the period of validity of a preparation are guaranteed.
Owner:REYOUNG PHARMA
Process for producing pigment dispersion composition
InactiveUS9018317B2High densityGood dispersibilityMaterial nanotechnologyInksEnd-groupPigment dispersion
A method for producing a pigment dispersion composition having excellent image density, dispersibility, and storage stability, has high resolubility, and forms an ink film having excellent marker resistance and scratch resistance. The method includes bringing a pigment (I) having a surface acidic group and a basic compound (II) having two or more ammo groups selected from a primary amino group and a secondary amino group in its molecule, into contact with each other in an aqueous medium to prepare a pigment having an unreacted surface amino group, bringing the pigment into contact with a polyisocyanate polyurethane resin (III) having two or more isocyanate end groups so that the pigment and the polyisocyanate polyurethane resin are bonded via a urea bond to prepare a dispersion of a polyurethane resin-bonded pigment (A), and heating the dispersion of the polyurethane resin-bonded pigment (A) at 40 to 100° C. for 1 to 30 days.
Owner:TOKAI CARBON CO LTD
Ganciclovir composition for injection and preparation method thereof
ActiveCN102274197BHigh porosityImprove reconstitution performancePowder deliveryInorganic non-active ingredientsPorosityFreeze-drying
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
A kind of octreotide acetate freeze-dried powder injection and preparation method thereof
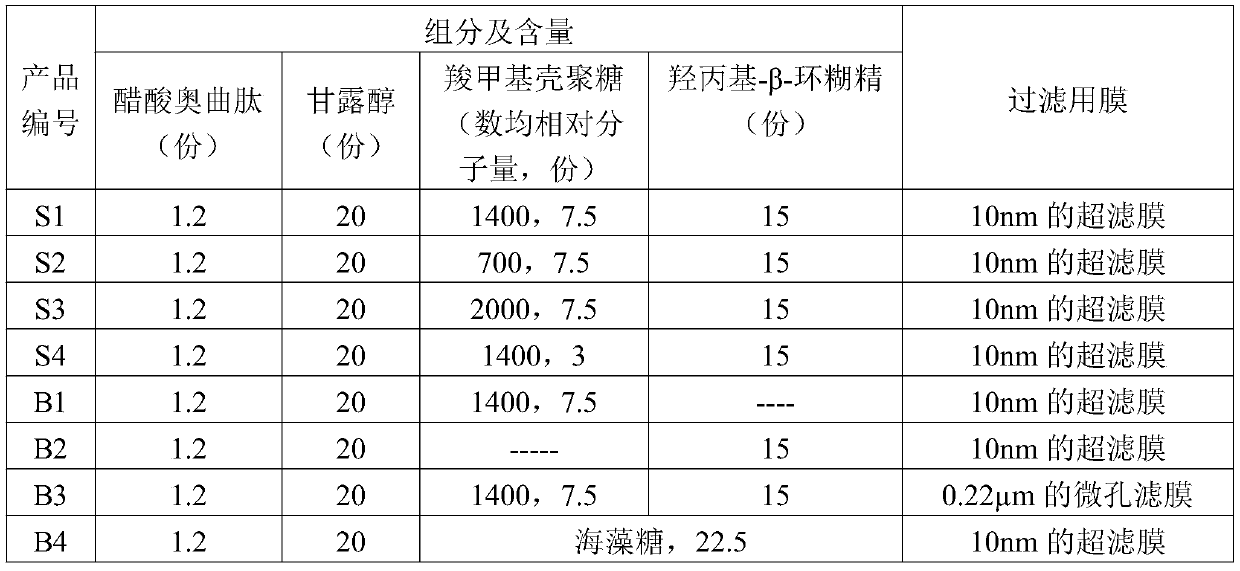
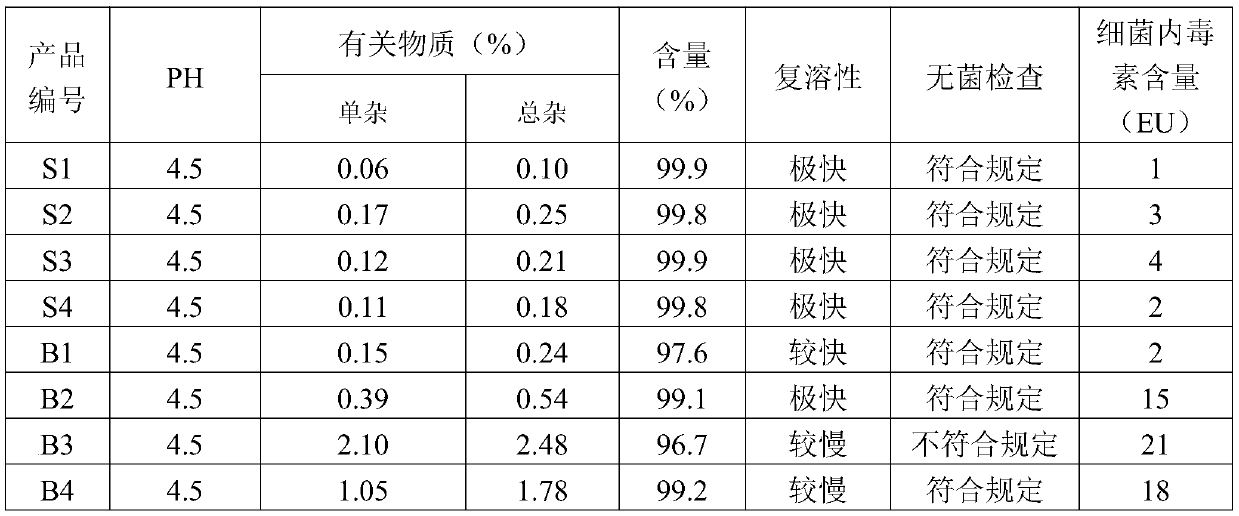
ActiveCN109876136BInhibition of bacteria contentImprove reconstitution performancePowder deliveryPeptide/protein ingredientsOctreotide acetateAcetic acid
The invention belongs to the field of pharmaceutical preparations, and particularly relates to an octreotide acetate lyophilized powder injection and a preparation method thereof. The octreotide acetate lyophilized powder injection includes carboxymethyl chitosan and hydroxypropyl-beta-cyclodextrin, and a pH buffering agent is acetic acid and glycinamide. The carboxymethyl chitosan can inhibit thegrowth of bacteria in a product; hydroxypropyl-beta-cyclodextrin can improve the resolubility of the product, and can play the role of a lyophilization protectant together with mannitol to improve the stability of the product; In addition, the carboxymethyl chitosan and hydroxypropyl-beta-cyclodextrin can form a weak gel in a solution, which plays a role in sustained release of octreotide acetate. The octreotide acetate lyophilized powder obtained by the invention has low bacteria and impurities content, and the stability and resolubility are also superior to that of the traditional products.
Owner:国药集团成都信立邦生物制药有限公司
Methylphenidate hydrochloride pharmaceutical composition and preparation method thereof
InactiveCN103655488AStable and uniform qualityUniform and accurate contentOrganic active ingredientsPowder deliveryMethylphenidate HydrochlorideMANNITOL/SORBITOL
The invention discloses methylphenidate hydrochloride pharmaceutical composition and a preparation method thereof. The composition contains methylphenidate hydrochloride and mannitol. The preparation method comprises the following steps: 1), preparation is performed: ambroxol hydrochloride and mannitol are placed into a preparation tank in a weight ratio of 1:(1-20), water for injection is added, and the mixture is stirred until complete dissolution and uniform mixing are achieved; 2), sterile filtration, subpackaging and half stoppering are performed; and 3), vacuum freeze drying is performed to obtain a product. The composition and the preparation method have the advantages that the formula is simple, the technology is advanced, the quality is uniform and stable, moisture drying is thorough, and the redissolution performance is better.
Owner:BEIJING KEYUAN CHUANGXIN TECH
Levocarnitine composition for injection and preparation method of levocarnitine composition
ActiveCN102327238BHigh porosityImprove reconstitution performancePowder deliveryOrganic active ingredientsPorosityFreeze-drying
The invention relates to a levocarnitine composition for injection and a preparation method of the levocarnitine composition; the levocarnitine composition is lyophilized powder containing levocarnitine and mannitol, wherein the weight ratio of the levocarnitine to the mannitol is 1:(0.75-1.25); the average particle diameter of the lyophilized powder is 90-130 nm, and the porosity is 94-98%. The preparation method comprises the steps of: 1) preparing: weighing levocarnitine and mannitol, putting the levocarnitine and the mannitol in a preparing tank, adding injection water, agitating to enable the levocarnitine and the mannitol to be completely dissolved and uniformly mixing, regulating the pH to 5.7-6.3 through a 0.1mol / L hydrochloric acid solution; 2) decarburizing and sterile filtering; 3) sterile packaging; 4) vacuum freeze drying to obtain the levocarnitine composition . The levocarnitine composition has the advantages of simple preparation, advanced technique, uniform quality, excellent stability, better redissolution capability and clinic medicine application safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com